Abstract
Regulation of metabolism at the level of transcription and its corollary metabolite-mediated regulation of transcription are well-documented mechanisms by which plants adapt to circumstance. That said the function of only a minority of transcription factor networks are fully understood and it seems likely that we have only identified a subset of the metabolites that play a mediator function in the regulation of transcription. Here we describe an integrated genomics approach in which we perform combined transcript and metabolite profiling on Arabidopsis (Arabidopsis thaliana) plants challenged by various environmental extremes. We chose this approach to generate a large variance in the levels of all parameters recorded. The data was then statistically evaluated to identify metabolites whose level robustly correlated with those of a particularly large number of transcripts. Since correlation alone provides no proof of causality we subsequently attempted to validate these putative mediators of gene expression via a combination of statistical analysis of data available in publicly available databases and iterative experimental evaluation. Data presented here suggest that, on adoption of appropriate caution, the approach can be used for the identification of metabolite mediators of gene expression. As an exemplary case study we document that in plants, as in yeast (Saccharomyces cerevisiae) and mammals, leucine plays an important role as a regulator of gene expression and provide a leucine response gene regulatory network.
Biological systems have to react to environmental and/or developmental changes by adjusting their biochemical and cellular machineries on numerous levels. In many cases small molecules play a crucial role in mediating such adjustments. A prominent example of this is the much discussed role of sugar sensing in plants (Jang and Sheen, 1994; Koch, 1996; Smeekens, 2000; Rolland et al., 2006). Important functions have been long ascribed to Suc (see, for example, Johnson and Ryan, 1990; Cheng et al., 1992; Chiou and Bush, 1998) and Glc (see, for example, Brouquisse et al., 1991; Loreti et al., 2000) and these have been more recently supported by a range of genetic, transcriptional, and biochemical evidence (Dijkwel et al., 1997; Zhou et al., 1998; Yanagisawa et al., 2003; Price et al., 2004; Solfanelli et al., 2006). Particularly well described in plants are the roles of hexokinase (Jang et al., 1997; Dai et al., 1999; Moore et al., 2003) and of the SNF1 complex (Zhang et al., 2001; Halford and Paul, 2003; Tiessen et al., 2003). That said recent evidence has pointed to a role for the PII protein as a transcriptional regulator that senses the energy status of the cell (Mizuno et al., 2007; Beez et al., 2009; Bourrellier et al., 2009). Recently evidence has also been accrued implicating trehalose and trehalose 6-P as signal molecules (Muller et al., 1999; Lunn et al., 2006) and of energy signaling mediated either by extracellular ATP levels or by the AKIN protein kinases (Baena-González et al., 2007; Roux and Steinebrunner, 2007). Hormones aside, the above arguably lists the best-characterized metabolic mediators of gene expression. Given that new hormone classes are still being discovered (see, for example, the recent identification of strigolactones; Gomez-Roldan et al., 2008; Umehara et al., 2008) and that simple perusal of the vast literature on transgenic perturbation of metabolism reveals that compensatory mechanisms are commonplace, it would seem reasonable to suggest that we have, to date, only identified a small subset of the regulatory loops linking metabolism and gene expression.
Most studies aimed at identifying metabolic mediators of gene expression have, to date, relied on the external application of putative mediators of expression. However, such approaches are, by nature, limited by the potential of the compounds to be taken up into the cell and can additionally be complicated by interaction of molecules with apoplastic receptors (Fernie et al., 2001; Lalonde et al., 2001; Roux and Steinebrunner, 2007). Here we present a set of data resulting from a large experiment where the response of a plant system (leaves of Arabidopsis [Arabidopsis thaliana]) to wide-ranging environmental variance has been followed by both RNA expression and metabolite-profiling techniques in parallel. While many studies integrate data from transcript and metabolite-profiling experiments (see, for example, Goossens et al., 2003; Urbanczyk-Wochniak et al., 2003; Hirai et al., 2004; Nikiforova et al., 2005; Fu et al., 2009) these tend to focus either on gene annotation (Tohge et al., 2005) or on a specific biotic or abiotic stress (Cho et al., 2008; Depuydt et al., 2009) or developmental process (Carrari et al., 2006; Howell et al., 2009). Here our focus was rather to look for parameters that were strongly correlated across a wide range of conditions in the hope that such a strategy would allow us to identify novel metabolite-mediated gene regulation. When analyzing the dataset obtained for correlations between changes in metabolites and those in transcript abundance we observed a surprisingly large number of highly statistically significant correlations. In many cases several hundred up to several thousand transcripts correlate with a single metabolite. These high numbers of transcripts correlating with one metabolite are unlikely to be caused by the fact that several hundred genes influence the formation (and/or depletion) of this metabolite but rather suggest the opposite situation, i.e. that this metabolite is a potential mediator directly and/or indirectly influencing the expression of several hundred genes. We discuss examples that we believe support both this concept and the notion that we probably have only uncovered a very small number of those small molecules exhibiting a mediator function in plants and other biological systems.
RESULTS AND DISCUSSION
Whereas a decade ago essentially only classical hormones were regarded as small molecule metabolite signals, data of recent years suggests that signaling function is not limited to these compounds. Metabolites have been demonstrated to interact with protein factors (see, for example, Sellick and Reece, 2005) and riboswitches (for review, see Mandal and Breaker, 2004) to regulate gene expression. Thus metabolites can mediate gene expression both in conjuncture with and independently of proteins. Manipulation of signal transduction cascades has proven effective in optimizing cellular productivity of biotechnologically important compounds (Attfield, 1997) and to be a highly efficient strategy for the suppression of a range of deleterious medical conditions (O'Shea et al., 2004; O'Neill, 2006). Therefore, their elucidation, and that of other forms of metabolite-mediated gene regulation, is of immense importance for human circumstance. Starting from the hypothesis that there are more small molecule mediators of gene expression than currently described we present here a strategy for their detection. This strategy was established to circumvent limitations imposed by the classical approach for the detection of such mediator molecules and is based on three simple criteria: (1) the ability to modulate the cellular content of the putative regulator, (2) the ability to detect and quantify putative regulators, and (3) the ability to monitor the cellular response to putative regulators. For this purpose we designed an experiment in which Arabidopsis plants were subjected to a diverse range of environmental perturbations (for a full list see “Materials and Methods” and Table I) and applied transcriptomics and metabolomic analysis to monitor the changes induced. We believe that a metabolomics-based approach has distinct advantages since it circumvents complications arising from external application of metabolites (uptake, compartmentation, interaction with apoplastic receptors, etc.). Following collection of this data we performed a coresponse analysis between metabolites and transcripts following the hypothesis that signal metabolites would be identified based on the criteria that they show a robust correlation to transcript changes. To allow robust correlation analysis, from the total of more than 22,000 transcripts and 800 metabolites, we first removed data with insufficient data points (detected in less than two-thirds of the conditions). This resulted in a dataset containing approximately 560 metabolites and more than 12,500 transcripts (Fig. 1; Supplemental Table S1). At the global level we observed many highly significant metabolite-transcript correlations, with around 30% of the approximately 7 million possible being significant (P < 0.05). However, to work with only the most significant/robust examples, we used Spearman nonparametric correlation and applied a Bonferoni P value correction (see “Materials and Methods”). This resulted in a total of around 175,000 significant correlations (adjusted P < 0.05). In total, 50% of metabolites were correlated with more than 50 transcripts.
Table I. Environmental conditions.
| Treatment | Transfer to Experimental Condition Before Harvest | Comments |
| Cold | 12°C; 100 μE light for 1 d | Transfer to cold incubator |
| Cold | 12°C; 100 μE light for 4 d | Transfer to cold incubator |
| Cold | 5°C; dark for 1 d | Transfer to cold incubator |
| Cold | 5°C; dark for 4 d | Transfer to cold incubator |
| Drought | Drought for 3 d | No watering for 3 d before harvesting |
| Drought | Drought for 4 d | No watering for 4 d before harvesting |
| Freezing | Freezing; −5°C for 2 h | Transfer to cold incubator |
| Freezing | Freezing; −5°C for 8 h | Transfer to cold incubator |
| Heat | 26°C; 220 μE (light) for 1 d | Transfer to 26°C phytotrone before harvesting |
| Heat | 26°C; 220 μE (light) for 4 d | Transfer to 26°C phytotrone before harvesting |
| Short day | Short day growth conditions | Growth under short day (8 h light, 16 h dark) |
| Developmental | Wild-type flowering | |
| Developmental | 14-d-old plants | |
| Diurnal rhythm | Diurnal rhythm; harvest 7 am | |
| Diurnal rhythm | Diurnal rhythm; harvest 12 pm | |
| Diurnal rhythm | Diurnal rhythm; harvest 5 pm | |
| Diurnal rhythm | Diurnal rhythm; harvest 8 pm | |
| Extended night | Extended night (18 h continuous dark) | Extended night before harvest |
| Extended night + cold | Extended night (18 h continuous dark) + 12°C for 2 h | Extended night before harvest |
| Light | Intermediate light conditions (100 μE light) for 4 d | Shading before harvest |
| Light | Low light conditions (40 μE light) for 4 d | Shading before harvest |
| Nutrient supply | Null soil + Hoagland solution | Nutrient treatment before harvest |
| Nutrient supply | Watering with 12 mm nitrate for 2 d | Nutrient treatment before harvest |
| Nutrient supply | Watering with 12 mm potassium for 2 d | Nutrient treatment before harvest |
| Nutrient supply | Watering with Hoagland solution for 2 d | Nutrient treatment before harvest |
| Nutrient supply | Watering with Hoagland solution for 4 d | Nutrient treatment before harvest |
Figure 1.
Experimental approach.
To validate our approach we first tested whether or not known signaling compounds would be identified as compounds that regulate gene expression based on the above-mentioned criteria. The plant hormones salicylic acid (SA) and abscisic acid (ABA), both reliably detected in our dataset, being obvious candidates. SA positively correlates with 69 transcripts in our dataset (Supplemental Table S1). Of these 16 were revealed to also be responsive to external SA treatment in publically available datasets (see “Materials and Methods” for details; compare with Supplemental Table S1), since these are all induced they are in perfect agreement with respect to direction of change. Accumulation of SA precedes the onset of system acquired resistance (SAR) in response of pathogen attack by inducing the nuclear translocation of the transcriptional cofactor NPR1 to activate many genes required for disease resistance like the pathogenesis-related proteins PR (Kinkema et al., 2000). Among the overlapping genes we identify two PR proteins (i.e. PR-1 and PNP-A; Kinkema et al., 2000; Meier et al., 2008) as well as an oxireductase (At3g284809) already reported to be involved in SAR. In addition to SAR response, other transcripts known to be involved in the hypersensitive response like CTR3 and LPT are also identified in both datasets as target of SA.
Similar results are obtained with ABA, however, the number of correlating transcripts was much lower. That said, importantly, both of these overlaps are significant (P = 10−14 and 0.02, respectively [Fisher exact test]). Among those genes, the transcription factor ATHB-7 previously described to be regulated strictly via ABA (Söderman et al., 1996) is identified in both datasets. However, in contrast to the hundreds of transcripts that respond to the external addition of these hormones, our approach identifies many fewer. The reasons for this are likely complex however major reasons are probably the fact that external application of hormones may induce genes in tissue types unrelated to the in vivo situation, while equally there are likely considerable tissue- and developmental-specific differences between the seedlings often used in comparison to the leaf tissue investigated here.
Second, we asked whether or not transcriptional responses we predict using correlation analysis are in agreement with independent genome-wide expression-profiling data for the perturbation of nonhormonal small molecule mediators of gene expression. We identified three suitable studies: Suc or Glc addition to seedlings, and the application of the carotenoid biosynthesis inhibitor norflurazon. As Suc and Glc are signals that regulate gene expression in plants (Gibson, 2005) and are routinely detected via gas chromatography-mass spectrometry (MS), they are ideal to test our approach. In our data matrix 785 transcripts correlate positively and 524 negatively to endogenous Suc. We compared these to transcripts reported to be induced or repressed 6 h after Suc addition (Solfanelli et al., 2006). By chance alone, we would expect only 51 Suc-induced transcripts among genes positively correlated with Suc and just 28 Suc-repressed transcripts would be among the negatively correlated genes. However, we identified 149 and 158 overlapping transcripts, respectively, in these comparisons. Moreover, the reciprocal comparisons (i.e. positive/repressed and negative/induced) are massively underrepresented as compared to expected. These comparisons are extremely significant in all cases (Fig. 2, top section, P = 10−11–10−79). Glc showed lower connectivity in our dataset, with 196 significantly correlated transcripts. We compared these data to the 741 genes reported to be up- or down-regulated following a 3 h Glc treatment (Price et al., 2004). As shown in Figure 2 (bottom section), the induced/positive and repressed/negative pairings showed highly significant overlap (P = 10−9–10−23). Furthermore, all overlapping genes were in quantitative agreement with no overlap between the reciprocal pairings. Note that due to the lower number of genes correlating with Glc respectively responding by up- or down-regulation to Glc (196 genes correlate; 741 respond positive or negative) as compared to the Suc case (1,309 versus 1,480) a lower number of overlaps is already significant. The Fisher exact test gives the probability of a certain configuration in a contingency test inherently correcting already for the sample size.
Figure 2.
Validation of sugar candidate metabolite mediators of gene expression. Summary tables showing the overlap between transcripts significantly correlated with a given metabolite (top) and those up- and down-regulated in response to its perturbation (left). See text for details. P values from Fisher exact tests are shown, with red and blue indicating over- and underrepresentation, respectively.
Carotenoid derivatives are widely implicated in plant signaling with ABA, a classical plant hormone, the best known (Kende and Zeevaart, 1997). There is also considerable evidence that carotenoid derivatives such as strigolactones act as signals in the regulation of shoot branching and in the control of root and shoot architecture (Booker et al., 2004; Van Norman et al., 2004; Klee, 2008; Leyser, 2009). Norflurazon inhibits phytoene desaturase and is widely used as an inhibitor of carotenoid biosynthesis (see, for example, Bartels and Watson, 1978; Van Norman et al., 2004). Twelve carotenoids are present in our dataset and several of these are significantly correlated with each other (data not shown). We reasoned that one of these carotenoids, or a correlated but undetected derivative, may represent a mediator of gene expression. Among the carotenoids we measured, cryptoxanthin, zeaxanthin, and lutein are significantly correlated to large numbers of transcripts. As norflurazon treatment will lead to a reduction in carotenoids we predicted that transcripts down-regulated by norflurazon should positively correlate with a carotenoid-derived signal, while induced genes should negatively correlate. For all three carotenoids, there was highly significant (Fig. 3, P = 10−30–10−46) overlap between positively correlated genes and those repressed by norflurazon. In addition, for zeaxanthin and lutein the induced/negative comparison was also significant (P = 0.001 and 0.04). As with the sugars, the reciprocal overlaps (i.e. repressed/negative and induced/positive) were underrepresented.
Figure 3.
Validation of carotenoid candidate metabolite mediators of gene expression. Summary tables showing the overlap between transcripts significantly correlated with a given metabolite (top) and those up- and down-regulated in response to its perturbation by norflurazon (left). See text for details. P values from Fisher exact tests are shown, with red and blue indicating over- and underrepresentation, respectively.
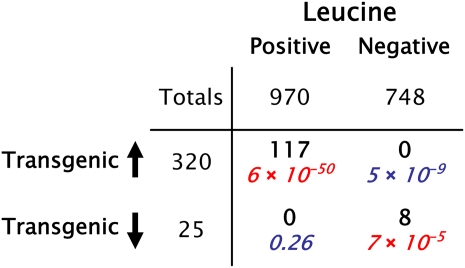
As a third validation we investigated whether these correlations could be used to predict transcript changes when we altered the endogenous levels of candidate metabolites. For this approach we performed transcript profiling to identify genes strongly up- or down-regulated in a transgenic Arabidopsis line exhibiting a reproducible change in Leu but few other metabolic consequences (further details for this line compare with “Materials and Methods”). We compared these transcripts to those significantly correlated with Leu and found highly significant overlap (Fig. 4). Worthy of mention is the very low number of transcripts that differ in their qualitative pattern.
Figure 4.
Validation of Leu candidate metabolite mediators of gene expression. Summary tables showing the overlap between transcripts significantly correlated with a given metabolite (top) and those up- and down-regulated in response to its perturbation by transgenesis (left). See text for details. P values from Fisher exact tests are shown, with red and blue indicating over- and underrepresentation, respectively.
Overrepresentation analysis of those transcripts revealed significant enrichment of the following functional categories: amino acid degradation, trehalose metabolism TPS/TPP, RNA regulation of WRKY transcription factors, calcium signaling, and development categories. Overrepresentation analysis of the down-regulated genes in the Leu dataset indicated a significant enrichment of genes involved in GDSL motif lipase (Table II).
Table II. Overrepresentation analysis of genes regulated in the Leu dataset.
| Bin | Bin Name | P Value |
| Up-regulated | ||
| 3 | Minor CHO metabolism | 0.00153 |
| 3.2.3 | Minor CHO metabolism.trehalose.potential TPS/TPP | 0.00177 |
| 13.2 | Amino acid metabolism.degradation | 0.00209 |
| 13.2.2 | Amino acid metabolism.degradation.Glu family | 0.00234 |
| 13.2.2.2 | Amino acid metabolism.degradation.Glu family.Pro | 0.00026 |
| 13.2.3 | Amino acid metabolism.degradation.Asp family | 0.00371 |
| 27.3.32 | RNA.regulation of transcription.WRKY domain transcription factor family | 0.00011 |
| 30.3 | Signalling.calcium | 0.00142 |
| 33 | Development | 0.0017 |
| 33.99 | Development.unspecified | 0.00121 |
| Down-regulated | ||
| 26 | Misc | 0.00044 |
| 26.28 | Misc.GDSL-motif lipase | 0.00027 |
To get further insights into the potential signaling function of Leu, we visualized the 117 up-regulated transcripts in the meta network of coexpressed gene cluster from AraGenNet (http://aranet.mpimp-golm.mpg.de/aranet; Mutwil et al., 2009). We could classify those transcripts into 33 clusters (Supplemental Table S2). For 10 clusters we found an overrepresentation of Leu-regulated transcripts (Fig. 5; Supplemental Table S3). Interestingly those 10 clusters are grouped into three subclusters. The first group is formed by interconnected clusters 52, 63, 95, 128, 135, and 153 that are enriched for categories like signaling transduction, biotic stress response, secondary metabolism, lipid metabolism, and development (Supplemental Table S4). The second group connected the clusters 100, 140, and 179 involved in the processes of amino acids, lipids, and nucleotide degradation. The third group is represented by the single cluster 85 related to protein degradation processes. Interestingly, it is possible to identify genes involved in defense mechanism associated to senescence response in all three subclusters, like the senescence regulator transcription factor ANAC092 (cluster 153; Kim et al., 2009) and the senescence-associated SEN1 (cluster 85; Hanaoka et al., 2002).
Figure 5.
Meta network of coexpressed gene clusters and the Leu candidate network. Transcripts that were up-regulated in the Leu overexpression line and significantly correlated with Leu were visualized in the meta network of AraGenNet (Mutwil et al., 2009). Numbers represent clusters, and lines indicate connection between clusters. Gray circles highlighted clusters in which Leu candidates display coexpression pattern (false discovery rate < 0.05) and black lines (i.e. blue or red) indicate the connection between those clusters.
The potential signaling function of Leu has not been described in plants but is well established in other biological systems such as Escherichia coli (Hung et al., 2002) or yeast (Saccharomyces cerevisiae), and mammals where it is thought to activate the target of rapamycin pathway that stimulates protein synthesis (Dann and Thomas, 2006). To provide additional support for this finding we additionally checked the transcript response of feeding Leu to cell suspension cultures derived from Arabidopsis leaf tissue. For this purpose we incubated the cell suspension culture in buffered solution in which Leu (50 μm) was added exogenously for an interval of 90 min, after which the sample was centrifuged and snap frozen, subsequently determining the transcript levels of the 44 most highly responsive genes by quantitative reverse transcription-PCR. Results presented in Figure 6 show a considerable overlap with those found both in our experiments studying environmental variance and those performed on the Leu overproducer. Indeed some 23 of the 44 chosen transcripts (those demarcated with a blue arrow) showed a significant up-regulation on the addition of Leu and only one revealed an unexpected down-regulation in comparison to the results from the experiment described in Figure 1.
Figure 6.
Expression of transcripts after Leu feeding. Arabidopsis suspension cell cultures derived from leaves (Pauly et al., 2001) were treated without (control) or with 50 μm Leu for 90 min. Transcript levels were normalized to UBQ10 as reference control (ΔΔCT). Fold change (−ΔΔCT) was calculated by subtracting ΔCT Leu treated from ΔCT control samples. Arrows indicate the transcripts that confirm our prediction. Values represented the mean of three independent biological replicates ± se. [See online article for color version of this figure.]
CONCLUSION
In this article we have revealed that by exploiting broad environmental perturbation of the Arabidopsis leaf system we were able to generate considerable variance in multiple parameters, thus allowing us to uncover novel but robust associations between these parameters. Our specific focus was the identification of novel metabolite mediators of gene regulation. After performing an extensive analysis of metabolite to transcript correlations we were able to identify a number of canonical mediators including the phytohormones ABA and SA and the sugars Glc and Suc. In addition we were able to identify that carotenoids correlated with the expression of an extensive number of genes and that the expression of many of these genes could also be altered by the inhibition of phytoene desaturase following application of norflurazon. The exact mechanism linking norflurazon treatment with alterations in gene expression is unknown. While this was formerly thought to be largely caused by retrograde signaling between the plastid and nucleus (Mochizuki et al., 2001), the identification of carotenoid-derived strigolactones as a new class of phytohormones (Gomez-Roldan et al., 2008; Umehara et al., 2008) suggests another possible mode of action. Irrespective of the means by which modulation of gene expression is achieved, the close correlation between the levels of these metabolites and many genes and the fact that similar qualitative and quantitative changes in gene expression occur on chemical alteration of these metabolites, suggests that they play an important role in systems regulation. In addition to being able to identify such canonical and recently reported regulators our study also defined Leu as an important mediator molecule. There are several lines of evidence for this assertion. First, in our environmental perturbation experiment it was strongly correlated to the expression of several hundred genes, interestingly, its pattern of correlation was distinct from that of the majority of other metabolites. Second, transgenic Arabidopsis plants overproducing Leu exhibited a considerable number of genes that were over- and underexpressed that overlapped with genes that we identified as highly correlating with Leu in our environmental perturbation experiment. Third, a large proportion of tested genes that were positively correlated with Leu also increased in expression following exogenous application of the metabolite. While the responses in the various experiments did not exactly mirror one another there are several reasons that could explain this, including the influence of plasma membrane-bound receptors, subcellular distribution of metabolites, and/or uptake effects of the endogenously supplied substrates. These caveats notwithstanding we believe that the broad profiling approach that we undertook here, when taken together with iterative statistical and experimental approaches, represents a powerful means to identify novel metabolite mediators of gene expression. We propose Leu to represent a metabolite with such a function. Although the route by which Leu mediates gene expression in yeast and mammals is well defined future research is required to define the exact mechanisms responsible for this process in plants.
MATERIALS AND METHODS
Plant Material
Arabidopsis (Arabidopsis thaliana) accession C24 as well as ecotypes Columbia, Kas-1, Landsberg erecta, and Mt-0 were grown under a 16-h-day/8-h-dark growth regime. Standard greenhouse conditions were 20°C (day)/18°C (night), 400 μmol s−1 m−2, and 70% humidity. Applied treatments and variations to these standard conditions are provided in Table I.
Metabolite Dataset
Metabolite profiling by gas chromatography-MS and liquid chromatography-MS was performed as previously described (Gibon et al., 2006; Lisec et al., 2006). Pooled samples of C24 grown under standard phytotron conditions were run in all metabolite-profiling batches as a common reference. Results were expressed as mean log2 ratios between triplicate biological samples and this common reference. Only metabolites that were detected in at least two-thirds of conditions were retained for analysis, resulting in a dataset containing 562 analytes.
Transcript Dataset
Transcript profiling was performed using the Arabidopsis Affymetrix ATH1 array as previously described (Redman et al., 2004). The triplicate biological samples for each condition were pooled and hybridized to a single ATH1 array. Ten biological replicates of C24 grown under standard phytotron conditions were hybridized to 10 ATH1 arrays. All arrays were normalized together using RMA algorithm (Irizarry et al., 2003) implemented in the affy package (Gautier et al., 2004) for bioconductor (Gentleman et al., 2004). Expression data were log2 ratios versus the median of the 10 reference samples. A subset of probesets, all matching a single nuclear gene (TAIR6 annotation, www.arabidopsis.org), was used. Where multiple probesets matched the same gene, either the original design probeset, or, in the few cases where none matched the design, the first (alphanumeric) was retained. To determine if a given transcript was detected in each sample we used the P value from the MAS5 detection algorithm as implemented in the affy package. Only probesets that received a P value <0.05 in at least two-thirds of the conditions were kept, giving a final expression set of 12,513 probesets (Supplemental Table S1).
Statistical Analysis
Correlation analysis was performed by calculating the Spearman rank correlation coefficient and statistical significance using a C script as described (Steinhauser et al., 2004). P values for each metabolite were corrected across all transcript correlations for that metabolite using a Bonferroni adjustment. Overrepresentation analysis was performed using Fisher exact tests. Specifically, this tested the significance of the contingency table (Figs. 2–4) for the overlap between the number of correlated transcripts and the number of regulated transcripts in the independent dataset, considering the total number of 12,513 transcripts. P value adjustment and Fisher tests were performed using R (www.r-project.org).
Validation Datasets
For all validation datasets we only retained those regulated genes that were in our dataset, i.e. those present among the 12,513 bijective mapping genes present in at least two-thirds of all conditions.
Suc
The data of Solfanelli et al. (2006) were used. Briefly, 3-d-old Arabidopsis seedlings were treated for 6 h in the presence or absence of 90 mm Suc, with two biological replicates. As described the data were analyzed and filtered using the standard MAS5 software criteria; appropriate P/A call, increase or decrease call, and showing at least a 2-fold change in both replica.
Glc
The data of Price et al. (2004) were used. In brief, 6-d-old Arabidopsis seedlings were treated for 3 h in the presence or absence of 167 mm Glc, with four biological replicates. Analysis details are provided in the article, but essentially MAS5 normalized data were analyzed and genes showing a significant change of at least 3-fold were classified as regulated.
Norflurazon, ABA, SA
Raw data in the form of .CEL files were obtained for these treatments from http://affymetrix.arabidopsis.info/narrays/experimentbrowse.pl experiment IDs 51, 176, and 192, respectively. The norflurazon treatment was performed by growing seedlings for 6 d in the presence or absence of 5 μm norflurazon. The hormone treatments consisted of applying a mock treatment or the hormone (10 μm) to 7-d-old seedlings. All experiments had two biological replicates. Data were normalized using the RMA algorithm as described above. Genes that showed >2-fold change in both replicates were classified as regulated.
Leu
Construction of the Leu overexpressor line: The Escherichia coli gene b 1708 encoding a lipoprotein was fused to a chimeric promoter containing a trimer of the ocs upstream activating sequence fused to the mannopine synthase promoter (Ni et al., 1995) and used for transforming Arabidopsis C24 via vacuum infiltration using Agrobacterium tumefaciens. Transgenic lines were selected on the seed level. One line showing one insert was used for further metabolic characterization. Metabolic profiling of this line revealed few consistent changes. Specifically, this line displayed around a 2-fold increase in Leu, Ile, and Glc and a 5-fold increase in Fru. In our data matrix the metabolite pairs Leu-Ile and Glc-Fru are highly correlated (Spearman rho = 0.92 and 0.95, respectively), therefore we just considered the metabolites with more transcript correlations for validation, i.e. Leu and Glc. We then performed transcript profiling as previously described on the transgenic line and three wild-type controls. Data were RMA normalized and genes that showed >2-fold change in all three comparisons were classified as regulated.
Transcripts that overlapped with those significantly correlated with Leu were classified into functional categories using PageMan with Benjamin Hochberg correction (Usadel et al., 2006) and coexpression network was visualized using AraGenNet (http://aranet.mpimp-golm.mpg.de/aranet; Mutwil et al., 2009). The frequency of the Leu putative target genes in assigned clusters were statistically compared against the frequencies of all genes found in the AraGenNet meta network using Fisher test with multiple testing corrections.
In a further approach we supplied Leu to cell suspension cultures derived from Arabidopsis leaves (Pauly et al., 2001) via exogenous feeding of 50 μm Leu in buffered Murashige and Skoog medium applied for 90 min and assessed the effect on the levels of various transcripts. Total RNA extraction, cDNA synthesis, and quantitative reverse transcription-PCR were performed as described (Czechowski et al., 2004, 2005; Caldana et al., 2007). PCR reactions were conducted in an ABI PRISM 7900 HT sequence detection system (Applied Biosystems). Primer sequences are provided in Supplemental Table S5.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Table S1. Metabolite-transcript correlations.
Supplemental Table S2. Classification of Leu-regulated transcripts into clusters.
Supplemental Table S3. Classification of Leu candidate transcripts.
Supplemental Table S4. Enrichment of Leu candidate genes.
Supplemental Table S5. Primers used for quantitative reverse transcription transcripts.
Supplementary Material
Acknowledgments
We thank Stefan Henkes, Ralf Looser, and Richard Trethewey at Metanomics GmbH, Berlin, for performing some of the metabolite analysis.
References
- Attfield PV. (1997) Stress tolerance: the key to effective strains of industrial baker's yeast. Nat Biotechnol 15: 1351–1357 [DOI] [PubMed] [Google Scholar]
- Baena-González E, Rolland F, Thevelein JM, Sheen J. (2007) A central integrator of transcription networks in plant stress and energy signalling. Nature 448: 938–942 [DOI] [PubMed] [Google Scholar]
- Bartels PG, Watson CW. (1978) Inhibition of carotenoid synthesis by fluridone and norflurazon. Weed Sci 26: 198–203 [Google Scholar]
- Beez S, Fokina O, Hermann C, Forchhammer K. (2009) N-acetyl-L-glutamate kinase (NAGK) from oxygenic phototrophs: P-II signal transduction across domains of life reveals novel insights in NAGK control. J Mol Biol 389: 748–758 [DOI] [PubMed] [Google Scholar]
- Booker J, Auldridge M, Wills S, McCarty D, Klee H, Leyser C. (2004) The Arabidopsis MAX pathway controls shoot branching by regulating auxin transport. Curr Biol 14: 1232–1238 [DOI] [PubMed] [Google Scholar]
- Bourrellier F, Belen A, Ferrario-Mery S, Vidal J, Hodges M. (2009) Metabolite regulation of the interaction between Arabidopsis thaliana PII and N-acetyl-I-glutamate kinase. Biochem Biophys Res Commun 387: 700–704 [DOI] [PubMed] [Google Scholar]
- Brouquisse R, James F, Raymond P, Pradet A. (1991) Study of glucose starvation in excised maize root tips. Plant Physiol 96: 619–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldana C, Scheible WR, Mueller-Roeber B, Ruzicic S. (2007) A quantitative RT-PCR platform for high-throughput expression profiling of 2500 rice transcription factors. Plant Methods 3: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrari F, Baxter C, Usadel B, Urbanczyk-Wochniak E, Zanor MI, Nunes-Nesi A, Nikiforova V, Centeno D, Ratzka A, Pauly M, et al. (2006) Integrated analysis of metabolite and transcript levels reveals the metabolic shifts that underlie tomato fruit development and highlight regulatory aspects of metabolic network behaviour. Plant Physiol 142: 1380–1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CL, Acedo GN, Cristinsin M, Conkling MA. (1992) Sucrose mimics the light induction of Arabidopsis nitrate reductase gene transcription. Proc Natl Acad Sci USA 89: 1861–1864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiou TJ, Bush DR. (1998) Sucrose is a signal molecule in assimilate partitioning. Proc Natl Acad Sci USA 95: 4784–4788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho K, Shibato J, Agrawal GK, Jung YH, Kubo A, Jwa NS, Tamogami S, Satoh K, Kikuchi S, Higashi T, et al. (2008) Integrated transcriptomics, proteomics and metabolomics analyses to survey ozone responses in the leaves of rice seedling. J Proteome Res 7: 2980–2998 [DOI] [PubMed] [Google Scholar]
- Czechowski T, Bari RP, Stitt M, Scheible WR, Udvardi MK. (2004) Real-time RT-PCR profiling of over 1400 Arabidopsis transcription factors: unprecedented sensitivity reveals novel root- and shoot-specific genes. Plant J 38: 366–379 [DOI] [PubMed] [Google Scholar]
- Czechowski T, Stitt M, Altmann T, Udvardi MK, Scheible WR. (2005) Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant Physiol 139: 5–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai N, Schaffer A, Petreikov M, Shahak Y, Giller Y, Ratner K, Levine A, Granot D. (1999) Overexpression of Arabidopsis hexokinase in tomato plants inhibits growth, reduces photosynthesis, and induces rapid senescence. Plant Cell 11: 1253–1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dann SG, Thomas G. (2006) The amino acid sensitive TOR pathway from yeast to mammals. FEBS Lett 580: 2821–2829 [DOI] [PubMed] [Google Scholar]
- Depuydt S, Trenkamp S, Fernie AR, Elftieh S, Renou JP, Vuylsteke M, Holsters M, Vereecke D. (2009) An integrated genomics approach to define niche establishment by Rhodococcus fascians. Plant Physiol 149: 1366–1386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkwel PP, Huijser C, Weisbeek PJ, Chua NH, Smeekens SCM. (1997) Sucrose control of phytochrome A signalling in Arabidopsis. Plant Cell 9: 583–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernie AR, Roessner U, Geigenberger P. (2001) The sucrose analog palatinose leads to a stimulation of sucrose degradation and starch synthesis when supplied to discs of growing potato tubers. Plant Physiol 125: 1967–1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J, Keurentjes JJB, Bouwmeester H, America T, Verstappen FWA, Ward JL, Beale MH, de Vos RCH, Dijkstra M, Scheltema RA, et al. (2009) System-wide molecular evidence for phenotypic buffering in Arabidopsis. Nat Genet 41: 166–167 [DOI] [PubMed] [Google Scholar]
- Gautier L, Cope L, Bolstad BM, Irizarry RA. (2004) affy—analysis of Affymetrix GeneChip data at the probe level. Bioinformatics 20: 307–315 [DOI] [PubMed] [Google Scholar]
- Gibon Y, Usadel B, Blaesing OE, Kamlage B, Hoehne M, Trethewey R, Stitt M. (2006) Integration of metabolite with transcript and enzyme activity profiling during diurnal cycles in Arabidopsis rosettes. Genome Biol 7: R76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson SI. (2005) Control of plant development and gene expression by sugar signaling. Curr Opin Plant Biol 8: 93–102 [DOI] [PubMed] [Google Scholar]
- Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S, Ellis B, Gautier L, Ge YC, Gentry J, et al. (2004) Bioconductor: open software development for computational biology and bioinformatics. Genome Biol 5: R80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Roldan V, Fermas S, Brewer PB, Puech-Pages V, Dun EA, Pillot JP, Letisse F, Matusova R, Danoun S, Portais JC, et al. (2008) Strigolactone inhibition of shoot branching. Nature 455: 189–195 [DOI] [PubMed] [Google Scholar]
- Goossens A, Hakkinen ST, Laakso I, Seppanen-Laakso T, Biondi S, De Sutter V, Lammertyn F, Nuutila AM, Soderlund H, Zabeau M, et al. (2003) A functional genomics approach toward the understanding of secondary metabolism in plant cells. Proc Natl Acad Sci USA 100: 8595–8600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halford NG, Paul MJ. (2003) Carbon metabolite sensing and signalling. Plant Biotechnol J 1: 381–398 [DOI] [PubMed] [Google Scholar]
- Hanaoka H, Noda T, Shirano Y, Kato T, Hayashi H, Shibata D, Tabata S, Ohsumi Y. (2002) Leaf senescence and starvation-induced chlorosis are accelerated by the disruption of an Arabidopsis autophagy gene. Plant Physiol 129: 1181–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai MY, Yano M, Goodenowe DB, Kanaya S, Kimura T, Awazuhara M, Arita M, Fujiwara T, Saito K. (2004) Integration of transcriptomics and metabolomics for understanding of global responses to nutritional stresses in Arabidopsis thaliana. Proc Natl Acad Sci USA 101: 10205–10210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell KA, Narsai R, Carroll A, Ivanova A, Lohse M, Usadel B, Millar AH, Whelan J. (2009) Mapping metabolic and transcript temporal switches during germination in rice highlights specific transcription factors and the role of RNA instability in the germination process. Plant Physiol 149: 961–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung SP, Baldi P, Hatfield GW. (2002) Global gene expression profiling in Escherichia coli K12—the effects of leucine-responsive regulatory protein. J Biol Chem 277: 40309–40323 [DOI] [PubMed] [Google Scholar]
- Irizarry RA, Bolstad BM, Collin F, Cope LM, Hobbs B, Speed TP. (2003) Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res 31: e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang JC, Leon P, Zhou L, Sheen J. (1997) Hexokinase as a sugar sensor in higher plants. Plant Cell 9: 5–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang JC, Sheen J. (1994) Sugar sensing in higher plants. Plant Cell 6: 1665–1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R, Ryan CA. (1990) Wound-inducible potato inhibitor II genes—enhancement of expression by sucrose. Plant Mol Biol 14: 527–536 [DOI] [PubMed] [Google Scholar]
- Kende H, Zeevaart J. (1997) The five “classical” plant hormones. Plant Cell 9: 1197–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Woo HR, Kim J, Lim PO, Lee IC, Choi SH, Hwang D, Nam HG. (2009) Trifurcate feed-forward regulation of age-dependent cell death involving miR164 in Arabidopsis. Science 323: 1053–1057 [DOI] [PubMed] [Google Scholar]
- Kinkema M, Fan W, Dong X. (2000) Nuclear localization of NPR1 is required for activation of PR gene expression. Plant Cell 12: 2339–2350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klee HJ. (2008) Plant biology—hormones branch out. Nature 455: 176–177 [DOI] [PubMed] [Google Scholar]
- Koch KE. (1996) Carbohydrate-modulated gene expression in plants. Annu Rev Plant Physiol Plant Mol Biol 47: 509–540 [DOI] [PubMed] [Google Scholar]
- Leyser O. (2009) The control of shoot branching: an example of plant information processing. Plant Cell Environ 32: 694–703 [DOI] [PubMed] [Google Scholar]
- Lisec J, Schauer N, Kopka J, Willmitzer L, Fernie AR. (2006) Gas chromatography mass spectrometry-based metabolite profiling in plants. Nat Protoc 1: 387–396 [DOI] [PubMed] [Google Scholar]
- Loreti E, Alpi A, Perata P. (2000) Glucose and disaccharide-sensing mechanisms modulate the expression of alpha-amylase in barley embryos. Plant Physiol 123: 939–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunn JE, Feil R, Hendriks JHM, Gibon Y, Morcuende R, Osuna D, Scheible WR, Carillo P, Stitt M. (2006) Sugar-induced increases in trehalose 6-phosphate are correlated with redox activation of ADPglucose pyrophosphorylase and higher rates of starch synthesis in Arabidopsis thaliana. Biochem J 397: 139–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal M, Breaker RR. (2004) Gene regulation by riboswitches. Nat Rev Mol Cell Biol 5: 451–463 [DOI] [PubMed] [Google Scholar]
- Meier S, Bastian R, Donaldson L, Murray S, Bajic V, Gehring C. (2008) Co-expression and promoter content analyses assign a role in biotic and abiotic stress responses to plant natriuretic peptides. BMC Plant Biol 8: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno Y, Moorhead GBG, Ng KKS. (2007) Structural basis for the regulation of N-acetylglutamate kinase by PII in Arabidopsis thaliana. J Biol Chem 282: 35733–35740 [DOI] [PubMed] [Google Scholar]
- Mochizuki N, Brusslan JA, Larkin R, Nagatani A, Chory J. (2001) Arabidopsis genomes uncoupled 5 (GUN5) mutant reveals the involvement of Mg-chelatase H subunit in plastid-to-nucleus signal transduction. Proc Natl Acad Sci USA 98: 2053–2058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore B, Zhou L, Rolland F, Hall Q, Cheng WH, Liu YX, Hwang I, Jones T, Sheen J. (2003) Role of the Arabidopsis glucose sensor HXK1 in nutrient, light, and hormonal signalling. Science 300: 332–336 [DOI] [PubMed] [Google Scholar]
- Muller J, Wiemken A, Aeschbacher R. (1999) Trehalose metabolism in sugar sensing and plant development. Plant Sci 147: 37–47 [Google Scholar]
- Mutwil M, Usadel B, Schütte M, Loraine A, Ebenhöh O, Persson S. (2009) Assemby an interactive correlation network from the Arabidopsis genome using a novel heuristic clustering algorithm. Plant Physiol 152: 29–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni M, Cui D, Einstein J, Narasimhulu S, Vergara CE, Gelvin SB. (1995) Strength and tissue-specificity of chimeric promoters derived from the octopine and mannopine synthase genes. Plant J 7: 661–667 [Google Scholar]
- Nikiforova VJ, Kopka J, Tolstikov V, Fiehn O, Hopkins L, Hawkesford MJ, Hesse H, Hoefgen R. (2005) Systems rebalancing of metabolism in response to sulphur deprivation, as revealed by metabolome analysis of Arabidopsis plants. Plant Physiol 138: 304–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill LAJ. (2006) Targeting signal transduction as a strategy to treat inflammatory diseases. Nat Rev Drug Discov 5: 549–563 [DOI] [PubMed] [Google Scholar]
- O'Shea JJ, Pesu M, Borie DC, Changelian PS. (2004) A new modality for immunosuppression: targeting the JAK/STAT pathway. Nat Rev Drug Discov 3: 555–564 [DOI] [PubMed] [Google Scholar]
- Pauly M, Eberhard S, Albersheim P, Darvill A, York WS. (2001) Effects of the mur1 mutation on xyloglucans produced by suspension-cultured Arabidopsis thaliana cells. Planta 214: 67–74 [DOI] [PubMed] [Google Scholar]
- Price J, Laxmi A, St Martin SK, Jang JC. (2004) Global transcription profiling reveals multiple sugar signal transduction mechanisms in Arabidopsis. Plant Cell 16: 2128–2150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman JC, Haas BJ, Tanimoto G, Town CD. (2004) Development and evaluation of an Arabidopsis whole genome Affymetrix probe array. Plant J 38: 545–561 [DOI] [PubMed] [Google Scholar]
- Rolland F, Baena-Gonzalez E, Sheen J. (2006) Sugar sensing and signaling in plants: conserved and novel mechanisms. Annu Rev Plant Biol 57: 675–709 [DOI] [PubMed] [Google Scholar]
- Roux SJ, Steinebrunner I. (2007) Extracellular ATP: an unexpected role as a signaller in plants. Trends Plant Sci 12: 522–527 [DOI] [PubMed] [Google Scholar]
- Sellick CA, Reece RJ. (2005) Eukaryotic transcription factors as direct nutrient sensors. Trends Biochem Sci 30: 405–412 [DOI] [PubMed] [Google Scholar]
- Söderman E, Mattsson J, Engström P. (1996) The Arabidopsis homeobox gene ATHB-7 is induced by water deficit and by abscisic acid. Plant J 10: 375–381 [DOI] [PubMed] [Google Scholar]
- Smeekens S. (2000) Sugar-induced signal transduction in plants. Annu Rev Plant Physiol Plant Mol Biol 51: 49–81 [DOI] [PubMed] [Google Scholar]
- Solfanelli C, Poggi A, Loreti E, Alpi A, Perata P. (2006) Sucrose-specific induction of the anthocyanin biosynthetic pathway in Arabidopsis. Plant Physiol 140: 637–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhauser D, Usadel B, Luedemann A, Thimm O, Kopka J. (2004) The CSBDB a comprehensive systems biology database. Bioinformatics 20: 3647–3651 [DOI] [PubMed] [Google Scholar]
- Tiessen A, Prescha K, Branscheid A, Palacios N, McKibbin R, Halford NG, Geigenberger P. (2003) Evidence that SNF1-related kinase and hexokinase are involved in separate sugar-signalling pathways modulating post-translational redox activation of ADP-glucose pyrophosphorylase in potato tubers. Plant J 35: 490–500 [DOI] [PubMed] [Google Scholar]
- Tohge T, Nishiyama Y, Hirai MY, Yano M, Nakajima J, Awazuhara M, Inoue E, Takahashi H, Goodenowe DB, Kitayama M, et al. (2005) Functional genomics by integrated analysis of metabolome and transcriptome of Arabidopsis plants over-expressing an MYB transcription factor. Plant J 42: 218–235 [DOI] [PubMed] [Google Scholar]
- Umehara M, Hanada A, Yoshida S, Akiyama K, Arite T, Takeda-Kamiya N, Magome H, Kamiya Y, Shirasu K, Yoneyama K, et al. (2008) Inhibition of shoot branching by new terpenoid plant hormones. Nature 455: 195–201 [DOI] [PubMed] [Google Scholar]
- Urbanczyk-Wochniak E, Luedemann A, Kopka J, Selbig J, Roessner-Tunali U, Willmitzer L, Fernie AR. (2003) Parallel analysis of transcript and metabolic profiles: a new approach in systems biology. EMBO Rep 4: 989–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usadel B, Nagel A, Steinhauser D, Gibon Y, Blasing O, Redestig H, Sreenivasulu N, Krall L, Hannah M, Poree F, et al. (2006) PageMan: an interactive ontology tool to generate, display, and annotate overview graphs for profiling experiments. BMC Bioinformatics 7: 535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Norman JM, Frederick RL, Sieburth LE. (2004) BYPASS1 negatively regulates a root-derived signal that controls plant architecture. Curr Biol 14: 1739–1746 [DOI] [PubMed] [Google Scholar]
- Yanagisawa S, Yoo SD, Sheen J. (2003) Differential regulation of EIN3 stability by glucose and ethylene signalling in plants. Nature 425: 521–525 [DOI] [PubMed] [Google Scholar]
- Zhang YH, Shewry PR, Jones H, Barcelo P, Lazzeri PA, Halford NG. (2001) Expression of antisense SnRK1 protein kinase sequence causes abnormal pollen development and male sterility in transgenic barley. Plant J 28: 431–441 [DOI] [PubMed] [Google Scholar]
- Zhou L, Jang JC, Jones TL, Sheen J. (1998) Glucose and ethylene signal transduction crosstalk revealed by an Arabidopsis glucose-insensitive mutant. Proc Natl Acad Sci USA 95: 10294–10299 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.