Abstract
Native flower visitors removed less nectar from trypsin proteinase inhibitor (TPI)-silenced Nicotiana attenuata plants (ir-pi) than from wild-type plants in four field seasons of releases, even when the nectar repellant, nicotine, was also silenced. Analysis of floral chemistry revealed no differences in the emission of the floral attractants benzylacetone and benzaldehyde or in the concentrations of nectar sugar and nicotine between wild-type and ir-pi flowers, suggesting that these two lines are equally able to attract insect visitors. TPI activity was found in all wild-type flower parts and was highest in anther heads, while TPI activity was not found in any parts of ir-pi flowers. The nectar of ir-pi flowers contained 3.6-fold more total proteins than the nectar of wild-type flowers. Proteomics analysis and hydrogen peroxide (H2O2) measurements revealed that ir-pi nectar contained more nectarins and nectar germin-like proteins and about 1.5-fold more H2O2 compared with wild-type nectar. Field experiments with wild-type flowers supplemented with a solution containing sugar and glucose oxidase demonstrated a causal association between the accumulation of H2O2 and the reduction in nectar removal. These results showed that silencing TPI expression increases the accumulation of nectar proteins and H2O2 levels, which in turn reduces nectar removal by native insect floral visitors. The effect of silencing TPIs on nectar protein accumulation suggests an endogenous regulatory function for TPIs in N. attenuata flowers. The repellency of H2O2 to floral visitors raises new questions about the qualities of nectar that make it attractive for pollinators.
Floral nectar is an innovative feature of plants that is thought to have evolved as a reward for pollen-transporting floral visitors. Sugars (e.g. Glc, Fru, and Suc), amino acids, and lipids (Baker and Baker, 1982, 1986) provide nutritional rewards that are essential for many pollinators. But nectar is also known to contain other compounds, such as volatile organic compounds (VOCs), alkaloids, phenolics, and nonprotein amino acids (Baker, 1977, 1978; Raguso, 2004; Kessler and Baldwin, 2007), which do not increase the nutritional value of nectar. Nectar is also exploited as a food source by nectar robbers and nectar-infesting microorganisms, which do not provide mutualistic services to the plant and are known to directly reduce a plant's fitness either by competing with pollinators or by infesting reproductive organs (Traveset et al., 1998; Irwin and Brody, 1999; Maloof and Inouye, 2000; Farkas et al., 2007). Therefore, flowers must solve the dilemma of repelling nectar thieves or florivores that provide no pollination services while simultaneously attracting fitness-enhancing pollinators.
Most of the defensive compounds in nectar have been reported to act selectively (i.e. only on antagonists). For example, the floral nectar of Catalpa speciosa contains iridoid glycosides that fend off nectar robbers but not the plant's specific pollinators (Stephenson, 1981). Similarly, the presence of phenols in the floral nectar of Aloe vryheidensis lowers its palatability to generalist floral visitors like sunbirds or honey bees while not affecting the attractiveness of the nectar to a specialist bird, the dark-capped bulbul (Johnson et al., 2006). In its native habitat, Nicotiana attenuata (Solanaceae) maximizes its maternal and paternal reproductive success while repelling herbivores, florivores, and nectar robbers by producing a sophisticated blend of both repellants (nicotine) and attractants (benzylacetone) in its nectar and floral head space (Kessler et al., 2008) as well as by changing its floral phenology in response to herbivore attack, so as to switch from the use of night-active hawkmoth pollinators, which oviposit herbivores on the plants they pollinate, to day-active hummingbird pollinators, which do not (Kessler et al., 2010). While this sophisticated use of chemical attractants and repellants is likely a common solution to the dilemma, very little is known about the function of most secondary metabolites found in nectar (Thornburg, 2007).
Similar chemically mediated strategies are used to solve a similar problem when plants use a combination of direct and indirect defenses to protect their leaves from herbivore attack (Halitschke et al., 2008). In N. attenuata, attack by the specialist herbivore Manduca sexta elicits a remarkable array of direct and indirect defenses, most of which are elicited by the jasmonate signaling pathway in response to herbivore-specific elicitors (Baldwin, 2001; Kessler and Baldwin, 2002; Wu and Baldwin, 2009). These herbivory-elicited responses include the accumulation of toxins and digestibility reducers, which function as direct defenses, as well as the release of a complicated blend of VOCs (Gaquerel et al., 2009), which repel further oviposition by M. sexta moths and attract predacious bugs that feed on M. sexta eggs or larvae, thereby functioning as an indirect defense (Kessler and Baldwin, 2001).
Once herbivores start feeding on N. attenuata leaves, they are frequently repelled by a suite of locally and systemically elicited direct defenses (Steppuhn et al., 2008). Trypsin protease inhibitors (TPIs) are an effective component of this inducible defensive system that reduces the performance of folivores by targeting their main proteolytic digestive enzymes and is strongly induced by herbivore attack (van Dam et al., 2000; Glawe et al., 2003; Zavala et al., 2004b, 2008; Horn et al., 2005). However, in N. attenuata, the biosynthesis of TPIs incurs substantial fitness costs (Zavala et al., 2004a); silencing the TPI gene in N. attenuata abolishes the plant's capacity to produce TPIs and allows it to grow faster, flower earlier, and produce more seed capsules compared with TPI-producing genotypes (Zavala et al., 2004a). Similarly, restoring TPI production by transforming an ecotype of N. attenuata naturally deficient in TPI production (Wu et al., 2007) reduces lifetime seed production (Zavala et al., 2004a). TPIs are not only restricted to leaves but accumulates in reproductive organs, where they may protect these fitness-valuable tissues against attack from florivores and microbes. Atkinson et al. (1993) and Johnson et al. (2007) elegantly demonstrated that TPIs dramatically accumulate in Nicotiana alata stigmas to become the most abundant protein in these tissues. PIs have been reported to accumulate in Solanum americanum seeds, where they were shown to play an important role in seed development (Suk-Fong et al., 2006). These studies highlight that while it is clear that TPIs occur at high levels in reproductive organs, their role in floral function has not been thoroughly explored.
As part of a research program to study the defensive functions of nicotine and TPIs against folivores, we planted N. attenuata plants that had been transformed with RNA interference constructs to silence their nicotine (ir-pmt), TPI (ir-pi), or both (ir-pmt/pi) in the plant's native habitats in Utah during four field seasons. Serendipitously, we noticed that the amount of nectar removed by the native community of floral visitors from ir-pmt/pi plants did not differ from that removed from wild-type plants, although we had recently discovered that silencing nicotine alone (ir-pmt) consistently increased nectar removal (Kessler and Baldwin, 2007). These observations suggested that silencing TPIs alone might impede nectar removal by the native community of floral visitors. During two field seasons (2007 and 2009), we compared the amount of nectar removed from wild-type plants and from TPI-silenced plants (ir-pi) and found that, indeed, less nectar was consistently removed from ir-pi plants. To understand these observations, we compared the floral chemistry of wild-type and ir-pi plants, including floral volatiles, nectar sugar, nicotine, and proteomes. We found that silencing TPIs increased the accumulation of nectar proteins, especially the nectar germin-like proteins (GLPs) and nectarins, which are known to participate in the nectar redox cycle and generate hydrogen peroxide (H2O2; Carter and Thornburg, 2000). Consistent with data on nectar proteins, we also found significantly higher levels of H2O2 in the nectar of ir-pi plants compared with those of wild-type plants. To test whether the differences in the accumulation of H2O2 in the nectar of ir-pi and wild-type plants could explain the nectar removal observations in the field, we experimentally increased H2O2 in the nectar of wild-type plants to the levels found in ir-pi nectar using a mixture of Glc oxidase (GOX) and Glc and compared nectar removal by the native community of floral visitors.
RESULTS
Response of Floral Visitors to ir-pi N. attenuata Plants
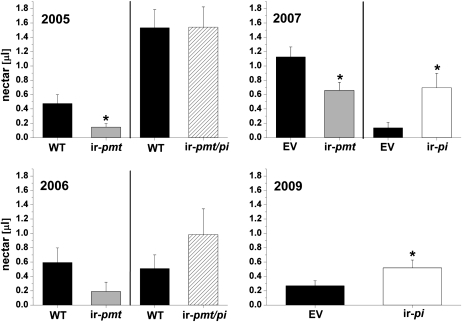
We found in three field seasons (2005, 2006 [Kessler and Baldwin, 2007], and 2007 [Kessler et al., 2008]) that flowers of plants silenced in nicotine production (ir-pmt) had significantly more nectar removed by the native community of floral visitors than flowers from wild-type plants or empty vector (EV) plants. In 2005 and 2006, the same experiments were conducted, as well as with plants transformed for both nicotine and proteinase inhibitors (ir-pmt/pi) to test the consequences of the combined lack of nicotine and proteinase inhibitors on the native populations of flower visitors. We found that nectar levels in ir-pmt/pi plants did not differ regardless of whether plants were bagged with mesh to exclude flower visitors but allow for normal evapotranspiration (Supplemental Fig. S1; wild type versus ir-pmt/pi, Student's t test, t13 = −0.27, P = 0.79 [2005]; wild type versus ir-pmt/pi, t11 = 1.62, P = 0.13 [2006]) or allowed to be visited by floral visitors (t13 = −0.26, P = 0.80 [2005]; t12 = 1.17, P = 0.27 [2006]; Fig. 1).
Figure 1.
Nectar removal from flowers of wild-type, EV, ir-pmt, ir-pmt/pi, and ir-pi plants grown in a field plantation and exposed to native floral visitors during the 2005 to 2007 and 2009 field seasons. Mean ± se standing nectar volume is given in flowers of N. attenuata wild-type plants (WT) or EV plants and plants silenced in proteinase inhibitors (ir-pi), nicotine (ir-pmt), or both proteinase inhibitors and nicotine (ir-pmt/pi) production. Nectar volume was measured between 6:00 and 7:30 am in flowers that had opened for the first time the previous night. Significant differences were determined by Student's t test (* P < 0.05) in 2005, 2006, and 2009 and by paired Student's t test (* P < 0.05) in 2007.
Inspired by these findings, we compared the consequences of only silencing proteinase inhibitors (ir-pi) on the native community of floral visitors. We compared nectar levels in EV and ir-pi plants, both with and without visitors, in 2007 and 2009. No differences in nectar volume between EV and ir-pi plants were found when inflorescences were enclosed in mesh bags overnight during the time of peak flower visitation (Supplemental Fig. S1; EV versus ir-pi, t14 = −0.9, P = 0.4 [2007]; EV versus ir-pi, t43 = 0.3, P = 0.8 [2009]). Significantly less nectar was removed by floral visitors in uncovered ir-pi plants compared to EV plants (paired t test, t19 = 2.9, P = 0.009 [2007]; Student's t test, t62 = −1.07, P = 0.05 [2009]; Fig. 1).
Analysis of Flower Volatiles, Nectar Nicotine, and Nectar Sugar in Wild-Type and ir-pi Plants
To determine the cause of the differences in nectar removal between wild-type and ir-pi flowers, we examined the volatile profiles from these two genotypes for compounds known to attract or repel flower visitors. Single flowers from field-grown N. attenuata wild-type and ir-pi plants were trapped overnight from 8 pm until 10 am. No significant quantitative differences were detected in the emission of the two most abundant floral VOCs, benzylacetone and benzaldehyde (Student's t test, t13 = −0.36, P = 0.72; Supplemental Fig. S2) as well as in the emission of other VOCs that are trace constituents of the floral head space of field-grown plants, namely α-pinene, β-pinene, d-limonene, ethylbenzoate, benzylalcohol, and β-myrcene (P > 0.05).
Furthermore, the nectar nicotine concentration, which averaged 18.5 ± 2.2 μm, did not differ between wild-type and ir-pi plants (Student's t test, t13 = 1.00, P = 0.33; Supplemental Fig. S3). Similarly, nectar sugar concentrations did not differ between EV and ir-pi plants (Student's t test, t14 = 0.04, P = 0.84; Supplemental Fig. S4).
TPI Activity in Flower Parts and Nectar of Wild-Type and ir-pi N. attenuata Plants
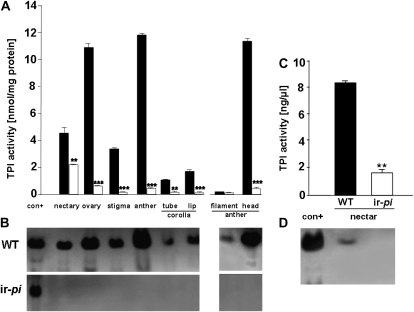
To determine whether or not active TPIs present in flower parts of both genotypes (wild-type and ir-pi N. attenuata) might explain the differences in nectar removal, we measured TPI activity in all flower parts and in nectar of both lines (Fig. 2). In wild-type flowers, active TPIs were present in all parts, but at different levels. Anther heads together with ovaries exhibited the highest TPI activity among all wild-type flower parts. TPI levels in the anther heads (12.7 ± 0.4 nmol mg−1 protein) and ovaries (12.14 ± 0.7 nmol mg−1 protein) were not significantly different (P = 0.21); these were followed by the TPI levels in the nectary (5.08 ± 0.8 nmol mg−1 protein), stigma and styles (3.7 ± 0.04 nmol mg−1 protein), corolla limbs (1.9 ± 0.1 nmol mg−1 protein), corolla tubes (1.16 ± 0.004 nmol mg−1 protein), and anther tubes (0.22 ± 0.04 nmol mg−1 protein). TPI activity was also detected in wild-type nectar (8.3 ± 0.4 ng μL−1; Fig. 2C). In contrast, TPI activity was not detected in all parts of ir-pi flowers by gel activity assays (Fig. 2B). The presence of trace levels of TPI activity in all parts of ir-pi flowers using the radial diffusion assays (Fig. 2A) is likely due to the presence of secondary metabolites such as phenolic compounds that inhibit the trypsin in the assay.
Figure 2.
Two independent assays of TPI activity (means ± se) in different flower parts (A and B) and nectar (C and D) of three replicate pooled samples of wild-type (WT) and ir-pi flower parts: x-ray film contact print technique (B and D) and radial diffusion assay (A and C). These two techniques revealed that nectar and all flower parts of N. attenuata (wild type) contain active TPIs, whereas in ir-pi plants, TPI activity was absent. The highest level of TPI activity in N. attenuata (wild-type) flowers was detected in anther heads and ovaries. A and C, Radial diffusion assays (Van Dam et al., 2001). Asterisks indicate significant differences of TPI activity in wild-type flower parts compared with those of ir-pi (unpaired t test, *** P < 0.0001, ** P < 0.005). No significant difference in TPI activity was found between anther heads and ovaries of wild-type flower parts; however, anther heads and ovaries had significantly higher TPI activity than other wild-type flower parts. B and D, X-ray film assay (Zavala et al., 2008). Forty micrograms of total protein (determined by the Bradford assay) from each flower part was loaded on a 12% native gel for electrophoresis. After electrophoresis, the gel was incubated for 15 min in 0.02% trypsin solution and washed three times with 0.1 m Tris-HCl (pH 7.8). TPI spots were visualized after washing the films with tap water as unhydrolyzed gelatin on the x-ray film. In C and D, nectar samples collected in the morning (from 7 to 9 am) from flowers that had opened for the first time the previous night were centrifuged, dialyzed, and concentrated, after which their protein concentrations were estimated by the Bradford method. Sixty micrograms of protein from each nectar sample was loaded on a 12% native gel. Significant differences between TPI activity in wild-type nectar and ir-pi nectar are indicated: ** P < 0.005.
Proteomic Analysis of Wild-Type and ir-pi Nectar
The analysis of nectar proteomes of wild-type and ir-pi plants revealed that the latter contains quantitatively more proteins than the former. Bradford protein measurements (Bradford, 1976) showed that the concentration of proteins in ir-pi nectar (0.17 ± 0.01 μg μL−1) was 3-fold higher than in wild-type nectar (0.047 ± 0.002 μg μL−1).
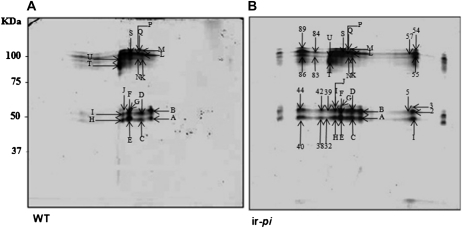
Nectar proteins were identified by matrix-assisted laser-desorption ionization time of flight (MALDI-TOF) and nanoflow liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis performed on both common and differentially expressed spots separated by two-dimensional (2D) gel electrophoresis for both ir-pi and wild-type nectar (Fig. 3). In contrast to other Nicotiana species, N. attenuata flowers only produce up to 5 μL of nectar; therefore, the nectar of more than 400 flowers was required to be pooled for the proteomics analysis of each line. The common spots, which accumulated at a similar position (Supplemental Table S2), were mostly nectar GLPs and nectarins (I, II, and III). In the nectar of ir-pi plants, a number of additional spots were observed that yielded MS/MS de novo peptide sequences leading to further identification as nectar GLPs and nectarins (Supplemental Table S1). The spots identified as ribulose 1,5-bisphosphate carboxylase proteins on the 2D gels are likely contaminations originating from the corolla tube when the glass capillary was pushed down the corolla tubes of more than 400 flowers to collect the pooled nectar samples required for the proteome analysis. The spot numbers, peptide sequences, names, accession numbers, and sequences of the identified proteins are summarized in Supplemental Tables S1 and S2. Details of the alignments of sequenced peptides with the identified proteins are presented in Supplemental Table S3.
Figure 3.
Proteomic maps of 2D separated wild-type (A) and ir-pi (B) nectar proteins. Nectar (2 mL) was collected from N. attenuata flowers between 7 and 9 am, centrifuged, dialyzed, and concentrated. Nectar protein concentration was estimated by the Bradford method. Approximately 250 μg of protein was resolved on 11-cm IPG strips of pH range 3 to 10 (isoelectric focusing), further separated on 12% acrylamide SDS-PAGE gels, and stained with Bio-Safe Coomassie Brilliant Blue G-250 stain. Gels were scanned and images analyzed using PDQuest software. Representative protein profiles of pooled wild-type (WT) nectar and ir-pi nectar from 30 plants from each genotype were analyzed. Numbers indicate protein spots that are different on the two gels, and letters indicate protein spots that are similar on the two gels. For identities of the protein spots, see Supplemental Tables S1 and S2.
H2O2 Measurement in Wild-Type and ir-pi Nectar
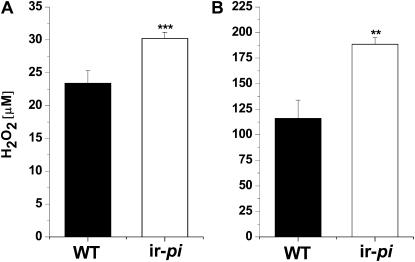
To determine whether the higher protein content of ir-pi nectar is correlated with the higher concentrations of H2O2, we measured H2O2 in the nectar using two different quantification procedures. The measurement of H2O2 using a fluorescence method revealed that ir-pi nectar contains 30.2 μm H2O2, which is 1.3-fold higher than in wild-type nectar (23.4 μm; Student's t test, t6 = −6.1, degrees of freedom = 10, P = 0.0001; Fig. 4A), whereas the H2O2 measurement using the luminescence method resulted in higher total values (188.6 and 116.2 μm for ir-pi and the wild type, respectively); however, similar differences were found in H2O2 nectar contents between the two genotypes (1.6-fold in ir-pi compared with the wild type; t7 = −4.3, degrees of freedom = 12, P = 0.001; Fig. 4B).
Figure 4.
H2O2 concentrations (means ± se) in wild-type (WT) and ir-pi N. attenuata nectar. Nectar was collected in the early morning from newly opened flowers of seven replicate plants (one plant for each). H2O2 levels were immediately measured by the Amplex Red H2O2/peroxidase assay (A) and the luminol chemiluminescence method (B). Significant differences between ir-pi and wild-type plants were determined with Student's t test (** P = 0.001, *** P = 0.0001).
H2O2 in Nectar and Its Influence on Nectar Removal from N. attenuata Flowers in Nature
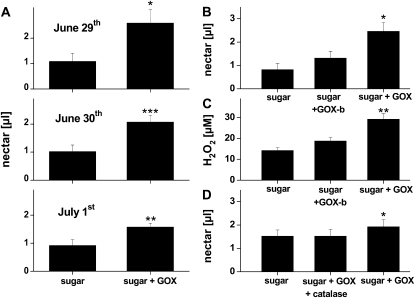
To determine whether nectar H2O2 levels observed in ir-pi flowers are responsible for the lack of nectar removal, we measured nectar removal from flowers that had been supplemented with sugar solutions or a solution containing sugar and GOX on three consecutive nights. Significantly less nectar was removed by floral visitors in flowers supplemented with both sugar and GOX than in flowers supplemented only with the sugar solution (paired t test, t17 = −3.01, P = 0.02 [June 29]; t18 = −4.95, P = 0.001 [June 30]; t18 = 4.45, P = 0.002 [July 1]; Fig. 5A). To clarify which of the two, H2O2 or the GOX protein, was responsible for the reduction in nectar removal, we conducted the same experiment with two additional treatments, first by supplementing the nectar of wild-type flowers with sugar and boiled GOX (boiling denatures the GOX and prevents it from producing H2O2 from Glc), and second by supplementing wild-type nectar with a solution containing sugar, GOX, and catalase, which enzymatically degrades the H2O2 produced by the GOX. Each of these two treatments was tested in separate experiments against sugar and sugar plus GOX treatments (Fig. 5, C and D). H2O2 levels in nectar supplemented with the sugar and GOX solution were nearly double those of the nectar supplemented with only the sugar solutions or the nectar supplemented with sugar and boiled GOX (ANOVA, F2,12 = 16.08, P = 0.0004; Fig. 5B). H2O2 levels in GOX-supplemented nectar resembled those measured in the nectar of ir-pi flowers (Fig. 4A). Native floral visitors removed significantly less nectar from flowers supplemented with sugar plus GOX than from flowers supplemented with only sugar or sugar and boiled GOX (ANOVA, F2,26 = 7.79, P = 0.002; Fig. 5C). The repellent effect of GOX was also reversed by adding catalase to the GOX solution, which resulted in an increased nectar removal by nearly 20% (Mann-Whitney U test, U = 84, P = 0.05, n = 14; Fig. 5D).
Figure 5.
The addition of GOX and the resulting increase in nectar H2O2 levels impede nectar removal in N. attenuata flowers. Rates of nectar removal by the native community of flower visitors were estimated by the standing nectar volume at dawn of wild-type flowers that had opened the previous night and were supplemented with sugar, GOX, boiled GOX, or catalase in various combinations to manipulate nectar H2O2 levels. A, Mean ± se (n = 10) nectar volume per flower at dawn in wild-type flowers that had 10 μL of a sugar and GOX (sugar + GOX) or a sugar-only solution added to their nectar the previous evening when they opened. B, Mean ± se (n = 10) nectar volume per flower in wild-type flowers that had 10 μL of a sugar and GOX (sugar + GOX), a sugar and boiled GOX (GOX-b), or a sugar-only solution added to their nectar the previous evening when they opened. C, Mean ± se (n = 5) nectar H2O2 levels after treating flowers as described in B. D, Mean ± se (n = 14) nectar volume per flower in wild-type flowers that had 10 μL of a sugar and GOX (sugar + GOX), a sugar, GOX, and catalase, or a sugar-only solution added to their nectar the previous evening when they opened. Significant differences in A were determined by a paired Student's t test; significant differences in B, C, and D were determined by ANOVA and compare treatments with the “sugar controls.” Asterisks indicate significant differences among treatments (* P < 0.05, ** P < 0.01, *** P < 0.001).
Floral Visitors
Using black light traps, we examined the community of potential floral visitors in the experimental area during N. attenuata’s flowering period (middle of May to the end of June) from 2005 to 2009. While the population size of day-active hummingbird (Archilochus alexandrii) was comparable among the years, the size and composition of the night-active moth populations differed substantially among the field seasons. The tobacco hornworm (M. sexta) was rare during all years (less than one moth trapped per week), but the tomato hornworm (Manduca quinquemaculata) was present in all years, with at least one moth trapped per night (except during 2009, when this species was completely absent). 2007 was an outbreak year for M. quinquemaculata, and at least four moths trapped per night. The most abundant hawkmoth species, the white-line sphinx moth (Hyles lineata), was particularly abundant in 2005, when more than 100 moths could be trapped per night. From 2006 to 2008, an average of five to 10 H. lineata were observed per night, but in 2009, only one moth of this species could be found per week. A fourth hawkmoth species, the ash sphinx (Sphinx chersis), was only consistently observed in 2007, when one moth could be trapped per night. The tobacco budworm (Heliothis species) and antlions (Paranthaclisis species) were recorded only between 2007 and 2009, and the populations of both species varied greatly. In 2007, more than 10 Heliothis individuals could be found per night, in 2008, five individuals, and in 2009, only one individual per night could be found. Adult antlions were abundant in 2009, when at least 30 individuals were observed per night, in 2007, around 15 individuals, and in 2008, less than 10 per night were observed.
DISCUSSION
In four field seasons, we found that less nectar was removed by the native community of floral visitors from the flowers of N. attenuata plants silenced in TPI production compared with the flowers of wild-type plants. The effects on nectar removal were consistent, although the insect community that visited the flowers varied significantly among the different field seasons, suggesting that the entire community of nectar feeders, rather than just select members, was affected by changes in nectar quality that resulted from the silencing of TPIs. While TPIs are absent from the nectar of TPI-silenced plants, the total protein content as well as the number of proteins and H2O2 levels are increased compared with nectar of wild-type plants. We demonstrate, by supplementing nectar with Glc as a substrate and Glc oxidase as an enzyme to produce H2O2, that the elevated H2O2 levels found in the nectar of ir-pi flowers is sufficient to account for the reduction in nectar removal by the native nectar feeders.
High-resolution bottom-up mass spectrometric analysis of wild-type and ir-pi nectar proteins, separated on 2D polyacrylamide gels (Fig. 3), revealed that both lines contained proteins identified as nectarins (nectarins I–III) and nectar GLPs (Supplemental Table S2). Nectarins have been also identified in ornamental tobacco's floral nectar by Carter et al. (1999), who reported the presence of an array of proteins (nectarins I–IV). They also demonstrated that nectarin I has dismutase activity, which converts superoxide to H2O2 in the nectar redox cycle. GLPs are also known to have an oxalate oxidase activity (Lane et al., 1993; Lane, 2000) and superoxide dismutase activity (Bernier and Berna, 2001), both of which can produce H2O2 at levels that are toxic to microbes (Peng and Kuc, 1992) and herbivores (Ramputh et al., 2002). The sequence analysis of the differentially accumulated protein spots in ir-pi N. attenuata nectar revealed the presence of additional nectarins and nectar GLPs, but with different protein sequences (Supplemental Table S1) from those that are common between the two lines (Supplemental Table S2); these proteins also migrated to different positions on the 2D gel. Therefore, the structures of these proteins also likely differ, suggesting that silencing TPIs in N. attenuata influences the posttranslational modification of proteins in nectar. The involvement of protease inhibitors in protein processing and secretion has been shown in various organisms, but not in plants. For example, in mammals, Ser proteases inhibitors are known to inhibit protein secretion in kidneys (Iwashita et al., 2003). In general, PIs are thought to affect protein processing, especially in bacterial systems that require proteases for protein maturation and processing (Coutte et al., 2001). How TPIs affect the processing and the secretion of nectar protein in N. attenuata is not clear, but our data clearly show that silencing TPIs increases nectar protein accumulation, which in turn suggests that proteases might also be required in the maturation and/or secretion of nectar proteins. It would be particularly interesting to examine if endogenous PIs influence the system of PI targeting to vacuoles and extracellular locations that has been elegantly described for the stigmas of N. alata (Johnson et al., 2007).
The analysis of TPI activity in nectar and in different flower parts of ir-pi plants revealed that none of these contain any active TPIs, which is also true for the leaves of ir-pi plants (Steppuhn and Baldwin, 2007), suggesting that either all active TPIs in both leaves and flowers are expressed by a single gene (Wu et al., 2006) or that the RNA interference construct used for this line also affects the expression of TPI-related genes located in the flowers. In wild-type flower parts, most TPI activity is found in the anther heads and ovaries (Fig. 2); here, TPIs have been hypothesized to function as protective agents against fungal infection and insect damage, as demonstrated by Heath et al. (1997). The presence of active TPIs in pollen suggests a possible defensive role in protecting the anther heads of N. attenuata plants against pollen-collecting insects.
Given that only the nectar proteomic analysis and H2O2 measurements revealed differences between the nectar of wild-type and ir-pi flowers, several hypotheses that might explain the differences in nectar removal in the field could be excluded, but two remain. The differences in nectar removal could be due to either the direct repellent effects on nectar feeders of either (1) the increased levels of nectarins and GLPs or (2) the H2O2 levels produced by these proteins. The first hypothesis seems unlikely, since several studies suggest that nectar proteins are nutritionally important for some pollinating insects such as butterflies and dung flies; on the other hand, however, the presence of proteins that have an anti-herbivore effect cannot be excluded (Peumans et al., 1997). Falsification of this hypothesis would require the ability to produce plants with high or low levels of nectarins and/or the GLPs that do not produce H2O2 or to heterologously express and purify these proteins in sufficient quantities so that they could be added to the nectar of wild-type plants in active and denatured forms. The experiments conducted here are consistent with a direct repellency of H2O2.
The H2O2 levels in N. attenuata were in the range of those observed by Carter and Thornburg (2000), who showed that nectar from Nicotiana tabacum, Nicotiana plumbaginifolia, and N. alata had H2O2 concentrations of 20 to 4,000 μm. At these concentrations, H2O2 might have an antibacterial effect, as these are significantly higher than the concentrations known to be lethal to cells (10–100 μm; Halliwell and Gutteridge, 1999). While keeping nectar sterile may have clear advantages for the plant, it is not clear why high levels of H2O2 are repellant to nectar-feeding insects. Perhaps nectar with “mouthwash” qualities could have antimicrobial effects on the beneficial microflora of the insect's mouth and digestive parts. Plants have many means of enhancing the “shelf life” of their nectar; antimicrobial properties are known for terpenoids (Deans and Watermann, 1993) and proteins such as lipases (Oh et al., 2005) and lectins (Peumans et al., 1997), and these may allow plants to keep their nectar from fermenting without repelling pollinators.
Several years ago, we identified a population of N. attenuata growing in Arizona that was naturally deficient in TPI production (Glawe et al., 2003; Zavala et al., 2004a; Wu et al., 2008). This ecotype, in which a frame-shift mutation in the TPI gene causes it to be destroyed by nonsense-mediated decay of the TPI transcripts (Wu et al., 2007), completely abolishes its ability to accumulate transcripts or active TPIs. Interestingly, recent field studies have demonstrated that this Arizona genotype accumulates very low levels of nectar (only one-third of that of the TPI-producing Utah genotype). We hypothesize that this very low rate of nectar accumulation is an adaptation to the loss of this ecotype's ability to produce TPIs and the resulting high levels of H2O2 in its nectar, with its associated deterrent effect on pollinators. While nectar constituents that deter nectar removal can have a positive effect on plant fitness by altering pollinator behavior, as has been shown for nectar nicotine (Kessler et al., 2008), different nectar repellants may elicit different responses in their flower visitors, and the only solution to the deterrent effect of high nectar H2O2 levels may simply be not to produce nectar. The success of such “cheating” strategies will be frequency dependent, and much more work needs to be done in natural populations in which the Arizona ecotype occurs to measure the frequency of the low-nectar genotypes, the H2O2 levels of their nectar, and their outcrossing rates to evaluate this evolutionary scenario.
This study has demonstrated that the loss of an important defense gene, such as TPI, substantially alters how plants interact with floral insect visitors. Before this study, TPIs were known for their antidigestive activity in guts of herbivores (Zavala et al., 2004b), for their antimicrobial function against insect pathogens (Heath et al., 1997), and for playing a role in seed development (Suk-Fong et al., 2006). Here, we provide evidence for a role for TPIs in the processing and secretion of nectar proteins, which, in turn, results in higher levels of nectar H2O2. In conclusion, we would like to point out that this work underscores the value of analyzing the phenotypes of transformed plants in natural habitats. Had the floral visitors not told us that there was something unusual about the nectar of ir-pi plants, we would never have started this line of research.
MATERIALS AND METHODS
Plant Material and Growth
Nicotiana attenuata (Solanaceae) seeds were originally collected in a native population growing at the DI Ranch near Santa Clara, Utah (Baldwin and Ohnmeiss, 1993). All experiments were performed with the 14th (for EV, ir-pi, and ir-pmt/pi) or 22nd generation inbred line of wild-type N. attenuata plants. N. attenuata wild-type and EV plants are comparable for all ecologically relevant traits and thus can both be used as control plants for other transformed N. attenuata lines (Schwachtje et al., 2008). Line A-04-186-1 was used as ir-pi, and line A-04-103-3 was used as ir-pmt/pi; these lines are fully characterized (Steppuhn et al., 2004; Zavala et al., 2004a, 2004b; Steppuhn and Baldwin, 2007). Plants used for glasshouse experiments were germinated and grown as described previously by Kruegel et al. (2002). N. attenuata seeds were sterilized, induced to germinate by a 1-h treatment with 0.1 m GA3 and 50× (v/v) diluted liquid smoke (House of Herbs; Baldwin et al., 1994), and then germinated on sterile phytagel agar with Gamborg B5 medium (Duchefa; www.duchefa.com) with 26°C/16 h of light and 24°C/8 h of dark. Ten-day-old seedlings were planted into soil in Teku pots and, once established, transferred to 1-L pots and grown in the glasshouse at 26°C to 28°C under 16 h of light supplemented by Philips Sun-T Agro 400 Na lights (www.philips.com). Plants grown in natural populations at the Lytle Ranch Preserve research station were germinated as described above. Seedlings were transferred to Jiffy 703 pots (www.jiffypot.com) and grown in a shade house for 30 d. Young seedlings were watered every other day and fertilized twice with an iron solution (stock solution of 2.78 g of FeSO4·7H2O and 3.93 g of Triplex in 1 L of water, 100-fold diluted). Young wild-type and ir-pi/ir-pmt plants (for the 2005 and 2006 field seasons) or EV and ir-pi plants (2007 and 2009 field seasons) were planted in a paired design in a field plot. All pairs were at least 3 m apart, and plants within a pair were at least 1.5 m apart.
Nectar Volume and Nectar Sugar Measurement
To determine if the different genotypes differed in the amount of nectar they accumulated in their flowers and whether the native floral visitors exhibited preferences for the nectar produced by the different genotypes, we measured the standing nectar volume from the flowers of field-grown plants the morning after the first night the flowers were open. To determine the nectar accumulation without the influence of floral visitors, plants were covered with mesh bags (Breather plant bags; www.kleentest.com), which allowed for evapotranspiration but excluded floral visitors. The same plants remained uncovered on another day to measure the effect of floral visitors on standing nectar volume. Nectar volume was measured between 5:00 and 7:00 am with a 25-μL glass capillary as described by Kessler and Baldwin (2007). The volume of two flowers per plant was measured, and the average was taken to calculate standing nectar volume per plant. Similarly, we measured nectar sugar concentrations of two flowers per plant (mesh bagged) with a portable refractometer (Kessler and Baldwin, 2007).
Measurement of Floral Volatiles
Single flowers that were about to open were covered with plastic cups connected to coconut-charcoal air-sampling traps (ORBO-32; Sigma; sigmaaldrich.com). Air was drawn from the cup through the charcoal traps in an open-flow trapping system from 6:00 pm when flowers had just opened until 10:00 am the following morning. Traps were stored at −20°C until elution. Compounds were eluted by spiking each trap with 4 μL of tetralin (2 ng μL−1 dichlormethane) as an internal standard and subsequently eluted with 350 μL of dichlormethane into gas chromatography (GC) vials.
Volatile analysis was performed by ultra-high-resolution 2D GCxGC-TOF analyses as described by Gaquerel et al. (2009). An Agilent 6890N gas chromatograph equipped with an Agilent 7683 autoinjector (http://www.agilent.com) and a 4D thermal modulator (LECO; http://leco.com) was used to conduct the 2D GCxGC separations. This gas chromatograph was coupled with a LECO Pegasus III TOF mass spectrometer. The GC inlet and transfer line were held at 250°C. Splitless injections of 1 μL were made onto an RTX-5MS column (20 m × 250 μm i.d. × 0.1 μm; Restek; http://www.restek.com) for the first dimension separation (i.e. column 1 [C1]) of the GCxGC system. The collected C1 effluent was transferred to a DB-17 column 2 (C2) of the GCxGC system (0.89 m × 100 μm i.d. × 0.1 μm; Agilent Technologies) every 6 s (modulation time). C1 was held at 40°C for 5 min and then increased at 5°C per minute to 190°C and finally increased at 25°C per minute to 250°C, where it was held for 5 min. C2 was initially set at 45°C and followed the same temperature program as C1, giving a total run time of 45 min. The modulator was maintained at a temperature 30°C higher than C1. The ion source was maintained at 250°C. Data were collected, after a solvent peak delay of 120 s, in the mass-to-charge ratio range 50 to 300, at a rate of 200 spectra per second. Data were processed as described by Gaquerel et al. (2009).
Nicotine Concentration of Nectar
Nectar was collected in the morning (6:00–8:00 am) from newly opened flowers. Nectar of five flowers was collected in one GC vial. Ten microliters of nectar was transferred to a 1.5-mL Eppendorf tube and dissolved in 50 μL of methanol and 0.04% (v/v) acetic acid spiked with 1 ng μL−1 nicotine-D3 as an internal standard. Particulate matter was removed by centrifugation (10 min at 12,000g). Ten microliters of the solution was injected into an Agilent 1100 HPLC system connected with a Bruker-MicroToF (www.bruker.com) mass spectrometer operated in electrospray ionization positive mode with a capillary exit voltage of 130 V. Separations were achieved with a gradient solvent program (solvent A: 10 mm ammonium bicarbonate, pH 10; solvent B: acetonitrile) on a Phenomenex Gemini NX, 3-μm, 2- × 50-mm C-18 reverse-phase column with the following HPLC program: 10% B isocratic for 2 min, linear gradient to 80% B in 5 min, and isocratic at 80% B for 3 min. The column was reconditioned for 7 min between injections.
Protein Extraction and TPI Activity Determination in Flower Parts
To determine whether TPI activity varied among flower parts and between N. attenuata wild-type and ir-pi flowers, we extracted protein from different flower parts pooled from three replicate wild-type and ir-pi N. attenuata plants. Briefly, the plant material was weighed in an Eppendorf tube (300 mg fresh weight), crushed in liquid N2, and thawed on ice; then, 250 μL of protein extraction buffer (Jongsma et al., 1994) was used for every 100 mg of tissue. After vortexing, flower part tissues were completely suspended and centrifuged at 4°C for 20 min at 12,000g. The clear supernatants were transferred to fresh Eppendorf tubes and kept on ice until analysis. Protein concentration of the flower part samples was determined by the Bradford method (Bradford, 1976) with bovine serum albumin (Sigma) as a standard.
Nectar collected (approximately 2 mL obtained from about 400 flowers) for proteomic analysis was dialyzed for 8 h using benzoylated dialysis tubing (Sigma-Aldrich; www.sigmaaldrich.com) and subsequently concentrated using an Eppendorf concentrator 5301 (http://www.eppendorf.de). Finally, nectar protein concentration was estimated by the Bradford method with bovine serum albumin as a standard.
TPI activities were measured using the radial diffusion assay described by Van Dam et al. (2001) using bovine trypsin (Sigma) dissolved in agar. For TPI activities, different solutions of increasing concentrations of soybean trypsin inhibitor (Boehringer Mannheim) were prepared and used to obtain a standard curve. In addition to the radial diffusion assay, TPI activities were also determined after native gel electrophoresis with an x-ray film assay (Konica x-ray film; Zavala and Baldwin, 2006) as follows. Protein from the different flower parts (40 μg) was loaded on an 18- × 16-cm (12%) native polyacrylamide gel. The gels were then subjected to electrophoresis for 3 h at 22°C at 40 mA using a vertical electrophoresis tank (SE 600; Hoefer). After electrophoresis, the gels were equilibrated three times in 0.1 m Tris-HCl (pH 7.8) for 10 min and then incubated in 0.02% bovine trypsin for 15 min under shaking. The gels were then washed twice to remove the excess of trypsin solution and blotted for 15 min on an x-ray film. TPI spots were visible after washing the films with tap water, and the bands with TPI activity were visualized as unhydrolyzed gelatin on the x-ray film. Finally, the films were dried and photographed.
Identification of Floral Nectar Proteins by 2D Gel Electrophoresis
We analyzed the protein contents in N. attenuata wild-type and ir-pi nectar to reveal the effects of silencing TPIs on N. attenuata nectar proteome. For this, we collected 2 mL of nectar of each genotype, from which an equal amount (250 μg) of nectar proteins (obtained as described previously) was used for the 2D gel analysis.
The 2D gel electrophoresis procedure was conducted as described (Giri et al., 2006). For the first dimension separation, 12-cm immobilized pH gradient (IPG) strips (pH3-10 NL; Bio-Rad; www.bio-rad.com/amplification) were rehydrated (200 mL of rehydration buffer) with 250 μg of nectar protein from each genotype. After being rehydrated for 12 to 14 h at room temperature, proteins were focused on a Protean IEF Cell (Bio-Rad) at 20°C and 50 mA current per IPG strip. Before the second dimension separation, the IPG strips were equilibrated with a DTT buffer (3.6 g of urea, 5 mg of dithiothreitol, 1 mL of glycerol, 4.4 mL of 10% SDS, and 1 mL of 0.6 m Tris-HCl buffer, pH 6.8, in 10 mL) followed by an iodoacetamide buffer (similar to DTT buffer, with 0.25 g of iodoacetamide instead of dithiothreitol), 20 min each at room temperature under shaking. SDS-PAGE gels (12%) were used in the second dimension separation and stained with Bio-Safe Coomassie Brilliant Blue G-250 (Bio-Rad). Gel images were taken using a GS-800 calibrated densitometer (Bio-Rad). The spots that were differentially expressed between wild-type and ir-pi nectar samples were excised and subjected to trypsin protein digestion for protein sequence analysis.
MS and Bioinformatic Analysis of Protein Spots
Nectar proteins were processed on 96-well microtiter plates using an Ettan TA Digester (Amersham Biosciences; www.amershambiosciences.com). The manually excised gel plugs were destained four times with 50 μL of acetonitrile/50 mm ammonium bicarbonate and two times with 50 μL of 70% acetonitrile for 20 min each. The gel plugs were subsequently air dried for 1 h and digested overnight with 75 ng of trypsin (sequencing-grade porcine trypsin; Promega), and the reaction was carried out in 10 μL of 25 mm ammonium bicarbonate at 37°C. The digested protein solutions were mixed with 50 μL of extraction buffer (50% acetonitrile and 0.1% trifluoroacetic acid), incubated for 20 min, and transferred to a 96-well plate. The extraction was repeated twice. Finally, the extracted peptides were vacuum dried at 30°C and stored for analysis.
The MALDI-TOF/MS tryptic peptide fingerprint analysis was carried out on a MALDI Micro MX mass spectrometer (Waters; www.waters.com) used in positive reflectron mode using previously reported settings (Giri et al., 2006). The MALDI-TOF spectra were searched against a taxon-specific National Center for Biotechnology Information subdatabase defined by the term “viridiplantae.” PLGS version 2.1.5 bioinformatic software was used for all searches (Waters; www.waters.com) with the following parameters: peptide tolerance of 80 ppm, one missed cleavage, carbamidomethyl modification of Cys, variable Met oxidation. An estimated calibration error of 0.05 D and a minimum of four peptide matches were used as the criteria for obtaining positive database hits.
Due to the dearth of protein sequences from N. attenuata public databases, the MALDI-TOF/MS fingerprint analysis revealed only a few proteins. To reveal the character of the many unknown proteins, we used the high accuracy and fragmentation capabilities of a Q-TOF-MS/MS-type mass spectrometer (Q-Tof Ultima; Waters; www.waters.com) to obtain de novo sequences and additional rounds of p-BLASTing. The chromatographic and MS analysis parameters have been published elsewhere (Giri et al., 2006). The ProteinLynx Global Server Browser version 2.1.5 was used for the bioinformatic data processing (deconvolution, baseline subtraction, smoothing), de novo sequence characterization, and database searches. The fragment peptide data were searched against the National Center for Biotechnology Information subdatabase defined by the viridiplantae term, mentioned previously, with the following parameters: peptide tolerance of 20 ppm, fragment tolerance of 0.05 D, estimated calibration error of 0.005 D, one missed cleavage, carbamidomethylation of Cys, and possible oxidation of Met. Amino acid sequences from de novo sequencing were compared for similarities with the UniProt nonredundant (≤100%) database (www.uniprot.org) using the MS-BLAST program (http://dove.embl-heidelberg.de/Blast2/msblast.html) and an in-house-based search engine. Due to the short amino acid sequence character of the unknown peptides, we batch BLASTed all the peptide sequences obtained from each spot with the protein database. The combined matching probabilities of these peptides increased the chances of identifying target proteins. A consolidated database (sp_nrdb) containing all nonredundant protein databases (Swiss-Prot, Swiss-ProtNew, SptremblNew, Sptrembl) and the PAM30MS matrix with default settings were used for searches (Shevchenko et al., 2001).
Quantification of H2O2 Concentration in Nectar
To quantify the H2O2 produced within the nectar of wild-type and ir-pi flowers, we measured H2O2 in nectar collected in the early morning from bulk collections of newly opened flowers of seven replicate plants. H2O2 levels were immediately estimated using two methods.
(1) The Amplex Red H2O2/peroxidase assay kit (A22188) using the TECAN-infinite M200 instrument (http://www.tecan.com). Briefly, the nectar was diluted (1:1, v/v) in 1× reaction buffer, and then 1 μL of the diluted nectar was incubated for 30 min at room temperature under dark conditions, together with 1× reaction buffer and a working solution containing 100 μm Amplex Red reagent and 0.2 units mL−1 horseradish peroxidase. Fluorescence was then measured with a fluorescence microplate reader equipped for excitation in the range of 530 to 560 nm and fluorescence detection at 590 nm. The standard curve was made using a different concentration of H2O2 provided in the kit (Supplemental Fig. S5A).
(2) The luminol chemiluminescence method described by Bezzi et al. (2008). Briefly, 100 μL of luminol (0.56 mm, dissolved in a borate buffer, pH 9) was mixed together with 100 μL of a solution containing cobalt (0.73 mm) and EDTA (2.3 mm). This mixture was then added to diluted nectar dissolved in acetone:ethanol (2:1, v/v); the luminescence emitted from this reaction was then measured using a Labsystems Luminoskan instrument (http://www.gmi-inc.com). The standard curve was made using different concentrations of commercial H2O2 (Sigma; Supplemental Fig. S5B).
Experimental Manipulation of H2O2 Levels of Wild-Type Nectar in the Field
Nectar H2O2 levels were increased by adding either a 40% sugar solution (containing 80% Glc and 20% Suc) alone for control or a sugar solution with added GOX (3.2 units per treatment; type VII from Aspergillus niger; Sigma) to natural nectar in wild-type N. attenuata flowers. Two flowers per plant were treated (one per treatment) by pipetting 10 μL of each solution into the corolla tube. All other flowers were removed. For all data in Figure 5 (except A, June 29, and C), plants were covered by mesh tents (4 × 4 × 2 m) to avoid nectar robbing by Xylocopa species bees. Black lights were placed inside the tents for 2 h after dusk to attract flower visitors into the tents before closure. Nectar volume was measured the next morning between 6:00 and 8:00 am. The same experiment was conducted for data presented in Figure 5, B and D, with two additional treatments: first, by adding sugar and boiled GOX to the nectar of wild-type flowers, and second, by adding sugar, GOX, and catalase (250 units per treatment; from bovine liver; Sigma) to the nectar. GOX was boiled for 2 h in a water bath and allowed to cool down before being added to the sugar solutions.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Standing nectar volume from flowers of wild-type, EV, ir-pmt, ir-pmt/pi, and ir-pi plants that were mesh bagged to exclude native floral visitors during the 2005, 2006, 2007, and 2009 field seasons (related to Fig. 1).
Supplemental Figure S2. VOCs released from individual flowers of plants growing in the field plot at Lytle Ranch Preserve (Utah) from wild-type and ir-pi N. attenuata plants.
Supplemental Figure S3. Nicotine concentration in nectar.
Supplemental Figure S4. Nectar sugar concentration in field-grown plants.
Supplemental Figure S5. H2O2 standard curves using Amplex Red H2O2/peroxidase assay (A) or luminol chemiluminescence assay (B).
Supplemental Table S1. Identified proteins accumulated only in ir-pi nectar.
Supplemental Table S2. Identified proteins accumulated in both wild-type and ir-pi nectar.
Supplemental Table S3. Alignment details of sequenced peptides (Query) with the identified proteins (Sbjct).
Supplementary Material
Acknowledgments
We thank Dr. Anke Steppuhn for help with nectar removal measurements in the 2005 field season and Brigham Young University for use of its field station, the Lytle Ranch Preserve.
References
- Atkinson AH, Heath RL, Simpson RJ, Clarke AE, Anderson MA. (1993) Proteinase inhibitors in Nicotiana alata stigmas are derived from a precursor protein which is processed into five homologous inhibitors. Plant Cell 5: 203–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker H, Baker I. (1986) The occurrence and significance of amino acids in floral nectar. Plant Syst Evol 151: 175–186 [Google Scholar]
- Baker HG. (1977) Non-sugar chemical constituents of nectar. Apidologie (Celle) 8: 349–356 [Google Scholar]
- Baker HG. (1978) Chemical aspects of the pollination of woody plants in the tropics. Tomlinson PB, Zimmermann M, , Tropical Trees as Living Systems. Cambridge University Press, New York, pp 57–82 [Google Scholar]
- Baker HG, Baker I. (1982) Chemical constituents of nectar in relation to pollination mechanisms and phylogeny. Nitecki M, , Biochemical Aspects of Evolutionary Biology. University of Chicago Press, Chicago, pp 131–171 [Google Scholar]
- Baldwin IT. (2001) An ecologically motivated analysis of plant-herbivore interactions in native tobacco. Plant Physiol 127: 1449–1458 [PMC free article] [PubMed] [Google Scholar]
- Baldwin IT, Ohnmeiss TE. (1993) Alkaloidal responses to damage in Nicotiana native to North America. J Chem Ecol 19: 1143–1153 [DOI] [PubMed] [Google Scholar]
- Baldwin IT, Staszak-Kozinski L, Davidson R. (1994) Up in smoke. I. Smoke-derived germination cues for postfire annual, Nicotiana attenuata Torr. ex. Watson. J Chem Ecol 20: 2345–2371 [DOI] [PubMed] [Google Scholar]
- Bernier F, Berna A. (2001) Germins and germin-like proteins: plant do-all proteins. But what do they do exactly? Plant Physiol Biochem 39: 545–554 [Google Scholar]
- Bezzi S, Loupassaki S, Petrakis C, Kefalas P, Calokerinos A. (2008) Evaluation of peroxide value of olive oil and antioxidant activity by luminol chemiluminescence. Talanta 77: 642–646 [Google Scholar]
- Bradford MM. (1976) A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254 [DOI] [PubMed] [Google Scholar]
- Carter C, Graham RA, Thornburg RW. (1999) Nectarin I is a novel, soluble germin-like protein expressed in the nectar of Nicotiana sp. Plant Mol Biol 41: 207–216 [DOI] [PubMed] [Google Scholar]
- Carter C, Thornburg RW. (2000) Tobacco nectarin I: purification and characterization as a germin-like, manganese superoxide dismutase implicated in the defense of floral reproductive tissues. J Biol Chem 275: 36726–36733 [DOI] [PubMed] [Google Scholar]
- Coutte L, Antoine R, Drobecq H, Locht C, Jacob-Dubuisson F. (2001) Subtilisin-like autotransporter serves as maturation protease in a bacterial secretion pathway. EMBO J 20: 5040–5048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deans SG, Watermann PG. (1993) Biological activity of volatile oils. Hay RKM, Watermann PG, , Volatile Oil Crops: Their Biology, Biochemistry, and Production. Longman Scientific and Technical, Essex, UK, pp 97–111 [Google Scholar]
- Farkas A, Orosz-Kovacs Z, Deri H, Chauhan SVS. (2007) Floral nectaries in some apple and pear cultivars with special reference to bacterial fire blight. Curr Sci 92: 1286–1289 [Google Scholar]
- Gaquerel E, Weinhold A, Baldwin IT. (2009) Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphigidae) and its natural host Nicotiana attenuata. VIII. An unbiased GCxGC-ToF-MS analysis of the plant's elicited volatile emissions. Plant Physiol 149: 1408–1423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giri AP, Wunsche H, Mitra S, Zavala JA, Muck A, Svatos A, Baldwin IT. (2006) Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata. VII. Changes in the plant's proteome. Plant Physiol 142: 1621–1641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glawe GA, Zavala JA, Kessler A, Van Dam NM, Baldwin IT. (2003) Ecological costs and benefits correlated with trypsin protease inhibitor production in Nicotiana attenuata. Ecology 84: 79–90 [Google Scholar]
- Halitschke R, Stenberg JA, Kessler D, Kessler A, Baldwin IT. (2008) Shared signals: ‘alarm calls’ from plants increase apparency to herbivores and their enemies in nature. Ecol Lett 11: 24–34 [DOI] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JMC. (1999) Free Radicals in Biology and Medicine, Ed 3. Oxford University Press, Oxford [Google Scholar]
- Heath RL, McDonald G, Christeller JT, Lee M, Bateman K, West J, vanHeeswijck R, Anderson MA. (1997) Proteinase inhibitors from Nicotiana alata enhance plant resistance to insect pests. J Insect Physiol 43: 833–842 [DOI] [PubMed] [Google Scholar]
- Horn M, Patankar AG, Zavala JA, Wu JQ, Doleckova-Maresova L, Vujtechova M, Mares M, Baldwin IT. (2005) Differential elicitation of two processing proteases controls the processing pattern of the trypsin proteinase inhibitor precursor in Nicotiana attenuata. Plant Physiol 139: 375–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin RE, Brody AK. (1999) Nectar-robbing bumble bees reduce the fitness of Ipomopsis aggregata (Polemoniaceae). Ecology 80: 1703–1712 [Google Scholar]
- Iwashita K, Kitamura K, Narikiyo T, Adachi M, Shiraishi N, Miyoshi T, Nagano J, Tuyen DG, Nonoguchi H, Tomita K. (2003) Inhibition of prostasin secretion by serine protease inhibitors in the kidney. J Am Soc Nephrol 14: 11–16 [DOI] [PubMed] [Google Scholar]
- Johnson E, Miller E, Anderson M. (2007) Dual location of a family of proteinase inhibitors within the stigmas of Nicotiana alata. Planta 225: 1265–1276 [DOI] [PubMed] [Google Scholar]
- Johnson SD, Hargreaves AL, Brown M. (2006) Dark, bitter-tasting nectar functions as a filter of flower visitors in a bird-pollinated plant. Ecology 87: 2709–2716 [DOI] [PubMed] [Google Scholar]
- Jongsma MA, Bakker PL, Visser B, Stiekema WJ. (1994) Trypsin-inhibitor activity in mature tobacco and tomato plants is mainly induced locally in response to insect attack, wounding and virus-infection. Planta 195: 29–35 [Google Scholar]
- Kessler A, Baldwin IT. (2001) Defensive function of herbivore-induced plant volatile emissions in nature. Science 291: 2141–2144 [DOI] [PubMed] [Google Scholar]
- Kessler A, Baldwin IT. (2002) Manduca quinquemaculata’s optimization of intra-plant oviposition to predation, food quality, and thermal constraints. Ecology 83: 2346–2354 [Google Scholar]
- Kessler D, Baldwin IT. (2007) Making sense of nectar scents: the effects of nectar secondary metabolites on floral visitors of Nicotiana attenuata. Plant J 49: 840–854 [DOI] [PubMed] [Google Scholar]
- Kessler D, Diezel C, Baldwin IT. (2010) Changing pollinators as a means of escaping herbivores. Curr Biol 20: 237–242 [DOI] [PubMed] [Google Scholar]
- Kessler D, Gase K, Baldwin IT. (2008) Field experiments with transformed plants reveal the sense of floral scents. Science 321: 1200–1202 [DOI] [PubMed] [Google Scholar]
- Kruegel T, Lim M, Gase K, Halitschke R, Baldwin IT. (2002) Agrobacterium-mediated transformation of Nicotiana attenuata, a model ecological expression system. Chemoecology 12: 177–183 [Google Scholar]
- Lane BG. (2000) Oxalate oxidases and differentiating surface structure in wheat: germins. Biochem J 349: 309–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane BG, Dunwell JM, Ray JA, Schmitt MR, Cuming AC. (1993) Germin, a protein marker of early plant development, is an oxalate oxidase. J Biol Chem 268: 12239–12242 [PubMed] [Google Scholar]
- Maloof JE, Inouye DW. (2000) Are nectar robbers cheaters or mutualists? Ecology 81: 2651–2661 [Google Scholar]
- Oh IS, Park AR, Bae MS, Kwon SJ, Kim YS, Lee JE, Kang NY, Lee S, Cheong H, Park OK. (2005) Secretome analysis reveals an Arabidopsis lipase involved in defense against Alternaria brassicicola. Plant Cell 17: 2832–2847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng M, Kuc J. (1992) Peroxidase-generated hydrogen-peroxide as a source of antifungal activity in vitro and on tobacco leaf-disks. Phytopathology 82: 696–699 [Google Scholar]
- Peumans W, Smeets K, Van Nerum K, Van Leuven F, Van Damme E. (1997) Lectin and alliinase are the predominant proteins in nectar from leek (Allium porrum L.) flowers. Planta 201: 298–302 [DOI] [PubMed] [Google Scholar]
- Raguso RA. (2004) Why are some floral nectars scented? Ecology 85: 1486–1494 [Google Scholar]
- Ramputh AI, Arnason JT, Cass L, Simmonds JA. (2002) Reduced herbivory of the European corn borer (Ostrinia nubilalis) on corn transformed with germin, a wheat oxalate oxidase gene. Plant Sci 162: 431–440 [Google Scholar]
- Schwachtje J, Kutschbach S, Baldwin IT. (2008) Reverse genetics in ecological research. PLoS One 3: e1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevchenko A, Sunyaev S, Loboda A, Shevchenko A, Bork P, Ens W, Standing KG. (2001) Charting the proteomes of organisms with unsequenced genomes by MALDI-quadrupole time-of-flight mass spectrometry and BLAST homology searching. Anal Chem 73: 1917–1926 [DOI] [PubMed] [Google Scholar]
- Stephenson AG. (1981) Toxic nectar deters nectar thieves of Catalpa speciosa. Am Midl Nat 105: 381–383 [Google Scholar]
- Steppuhn A, Baldwin IT. (2007) Resistance management in a native plant: nicotine prevents herbivores from compensating for plant protease inhibitors. Ecol Lett 10: 499–511 [DOI] [PubMed] [Google Scholar]
- Steppuhn A, Gase K, Krock B, Halitschke R, Baldwin IT. (2004) Nicotine's defensive function in nature. PLoS Biol 2: e217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steppuhn A, Schuman MC, Baldwin IT. (2008) Silencing jasmonate signalling and jasmonate-mediated defences reveals different survival strategies between two Nicotiana attenuata accessions. Mol Ecol 17: 3717–3732 [DOI] [PubMed] [Google Scholar]
- Suk-Fong S, Edward CY, Mee-Len C. (2006) Downregulation of Solanum americanum genes encoding proteinase inhibitor II causes defective seed development. Plant J 45: 58–70 [DOI] [PubMed] [Google Scholar]
- Thornburg RW. (2007) Nectar chemistry. Nicolson SW, Nepi M, Pacini E, , Nectaries and Nectar. Springer, Dordrecht, The Netherlands, pp 215–264 [Google Scholar]
- Traveset A, Willson MF, Sabag C. (1998) Effect of nectar-robbing birds on fruit set of Fuchsia magellanica in Tierra Del Fuego: a disrupted mutualism. Funct Ecol 12: 459–464 [Google Scholar]
- van Dam NM, Hadwich K, Baldwin IT. (2000) Induced responses in Nicotiana attenuata affect behavior and growth of the specialist herbivore Manduca sexta. Oecologia 122: 371–379 [DOI] [PubMed] [Google Scholar]
- Van Dam NM, Horn M, Mare M, Baldwin IT. (2001) Ontogeny constrains systemic protease inhibitor response in Nicotiana attenuata. J Chem Ecol 27: 547–568 [DOI] [PubMed] [Google Scholar]
- Wu J, Hettenhausen C, Baldwin IT. (2006) Evolution of proteinase inhibitor defenses in North American allopolyploid species of Nicotiana. Planta 224: 750–760 [DOI] [PubMed] [Google Scholar]
- Wu J, Hettenhausen C, Schuman MC, Baldwin IT. (2008) A comparison of two Nicotiana attenuata accessions reveals large differences in signaling induced by oral secretions of the specialist herbivore Manduca sexta. Plant Physiol 146: 927–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Kang JH, Hettenhausen C, Baldwin IT. (2007) Nonsense-mediated mRNA decay (NMD) silences the accumulation of aberrant trypsin proteinase inhibitor mRNA in Nicotiana attenuata. Plant J 51: 693–706 [DOI] [PubMed] [Google Scholar]
- Wu JQ, Baldwin IT. (2009) Herbivory-induced signalling in plants: perception and action. Plant Cell Environ 32: 1161–1174 [DOI] [PubMed] [Google Scholar]
- Zavala JA, Baldwin IT. (2006) Jasmonic acid signalling and herbivore resistance traits constrain regrowth after herbivore attack in Nicotiana attenuata. Plant Cell Environ 29: 1751–1760 [DOI] [PubMed] [Google Scholar]
- Zavala JA, Giri AP, Jongsma MA, Baldwin IT. (2008) Digestive duet: midgut digestive proteinases of Manduca sexta ingesting Nicotiana attenuata with manipulated trypsin proteinase inhibitor expression. PLoS One 3: e2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala JA, Patankar AG, Gase K, Baldwin IT. (2004a) Constitutive and inducible trypsin proteinase inhibitor production incurs large fitness costs in Nicotiana attenuata. Proc Natl Acad Sci USA 101: 1607–1612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala JA, Patankar AG, Gase K, Hui D, Baldwin IT. (2004b) Manipulation of endogenous trypsin proteinase inhibitor production in Nicotiana attenuata demonstrates their function as antiherbivore defenses. Plant Physiol 134: 1181–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.