Abstract
The present research investigated the hypothesis that the hippocampus is involved with the control of appetitive behavior by interoceptive “hunger’ and “satiety” signals. Rats were trained to solve a food deprivation intensity discrimination problem in which stimuli produced by 0-h and 24-h food deprivation served as discriminative cues for the delivery of sucrose pellets. For Group 0+, sucrose pellets were delivered at the conclusion of each 4-min session that took place under 0-h food deprivation, whereas no pellets were delivered during sessions that took place when the rats had been food deprived for 24-h. Group 24+ received the reverse discriminative contingency (i.e., they received sucrose pellets under 24-h but not under 0-hr food deprivation). When asymptotic discrimination performance was achieved (indexed by greater incidence of food magazine approach behavior on reinforced compared to nonreinforced sessions), half the rats in each group received hippocampal lesions and the remaining rats in each group were designated as sham- or nonlesioned controls. Following recovery from surgery, food deprivation discrimination performance was compared for lesioned and control rats in both Groups 0+ and 24+. Discriminative responding was impaired for rats with hippocampal lesions relative to their controls. This impairment was based largely on elevated responding to nonreinforced food deprivation cues. In addition, hippocampal damage was associated with increased body weight under conditions of ad libitum feeding. The results suggest that the inhibition of appetitive behavior by energy state signals may depend, in part, on the hippocampus.
Keywords: memory, energy regulation, obesity, satiety, learning
Although much attention has been focused on the hypothalamus as the site of detection for physiological (e.g., hormonal, metabolic) signals relating to energy balance, a number of findings suggest that the hippocampus may be involved with utilization of the information that is provided by those signals. For example, rats with selective neurotoxic lesions of the hippocampus exhibit increased food intake, body weight gain, metabolic activity, and appetitive behavior compared to rats with an intact hippocampus and to rats with damage confined to the medial prefrontal cortex (Davidson et al., 2009). These findings complement recent results from (a) neuroanatomical studies (e.g.,Cenquizca & Swanson, 2006, 2007) which identified direct projections in rats from hippocampal cell fields to brain regions (e.g., the lateral hypothalamus) that are known to be involved in feeding; (b) studies using functional magnetic resonance imaging (fMRI) with humans and rats that revealed changes in hippocampal activation in response to food stimulation (Thanos et al., 2008) and to feeding and gastric manipulations (e.g., DelParigi et al., 2004; Wang et al., 2006) that were designed to give rise to interoceptive signals of satiety and; (c) studies which showed that humans suffering from severe amnesia associated with hippocampal damage also exhibited a reduced ability to suppress food intake when given repeated opportunities to consume meals (e.g., Hebben, Corkin, Eichenbaum, & Shedlack, 1985; Rozin, Dow, Moscovitch, & Rajaram, 1998).
The goal of the present study was to examine the effects of damage to the hippocampus on the ability of rats to utilize their interoceptive energy state signals to control their appetitive behavior. In this study, rats were trained to solve a deprivation intensity discrimination problem (Davidson, 1987) in which cues arising from 0-h food deprivation (produced by ad libitum access to food for approximately 24 h prior to a training session) and 24-h food deprivation (no access to food for approximately 24 h prior to the training session) served as discriminative signals for the delivery of sucrose pellets. When asymptotic discrimination performance was achieved, the effects of selective removal of the complete hippocampus on retention of the previously learned discrimination were assessed. If rats rely on the hippocampus to utilize the information provided by their interoceptive energy state signals to anticipate the delivery of sucrose, then retention of deprivation intensity discrimination performance should be impaired for rats with hippocampal lesions compared to controls. The nature or pattern of the impairment exhibited by lesioned rats (e.g., increased or decreased appetitive responding under 0-h compared to 24-h food deprivation) will also be informative about the functional role of the hippocampus in utilizing energy state information.
Previous research found that rats with selective ibotenate lesions were impaired in learning to use interoceptive cues arising from different levels of food deprivation as discriminative cues for a brief shock (Davidson & Jarrard, 1993). These same rats were unimpaired in learning to use brief auditory conditioned stimuli (CSs) as signals for shock. Thus, the results of this “food deprivation intensity discrimination” training indicated that hippocampal damage interfered with either the detection or the utilization of energy state signals. This basic outcome was also obtained in another study with a shock unconditioned stimulus (US), when rats with lesions confined to the ventral and dorsal hippocampus, respectively, were compared to controls (Hock & Bunsey, 1998).
One thing that is unclear based on these findings, is whether or not the hippocampus is needed for rats to use their energy state cues to anticipate appetitive, as well as aversive outcomes. Recent research (Thibaudeau, Dore, & Goulet, 2009) indicates that hippocampal damage impairs Pavlovian trace conditioning with punctate exteroceptive CSs when learning is based on an aversive, but not when it is based on an appetitive US. Thus, it would be important to know whether the effects of hippocampal damage on discrimination performance with interoceptive food deprivation intensity cues, is similarly dependent on the type of US (appetitive or aversive) that is used to reinforce learning. If the hippocampus is needed for rats to use interoceptive stimuli that correspond to “hunger” and “satiety” as signals for appetitive USs, this would support the idea that interfering with hippocampal functioning could also interfere with the regulation of energy intake and body weight as reported by Davidson et al., (2009).
The study by Davidson & Jarrard (1993) assessed the effects of hippocampal damage that occurred prior to the beginning of food deprivation intensity discrimination training. Energy state signals corresponding to “hunger” and “satiety” are presumably present early in life. Therefore, animals (including humans) would have ample opportunity to learn about these cues before problems with intake and body weight regulation emerge. Thus, if interference with hippocampal functioning contributes to impaired energy and body weight regulation, it is more likely that this effect would be based on interfering with the retention or utilization of previous learning about energy state signals, rather than the acquisition of new learning about those cues. The present experiment also set out to assess the effects of ibotenate lesions of the complete hippocampus on the ability of rats to use prior learning that established interoceptive cues corresponding to “hunger’ and “satiety” as discriminative signals for the availability of an appetitive US.
Following the general procedures and experimental design used previously to establish appetitive food deprivation intensity discrimination learning in non-lesioned animals (Davidson, Kanoski, Tracy et al., 2005; Kanoski, Walls, & Davidson, 2007), we trained two groups of rats to use cues arising from 24 h (i.e., 24 h without food) and 0 h food deprivation (i.e., 0 h without food following a 24 h period with food freely available) as discriminative stimuli. For rats in Group 0+, sucrose pellets were delivered at the end of each 4 min session that took place under 0-h food deprivation and no pellets were delivered at the end of sessions that took place when the rats were food deprived for 24 h. Rats in Group 24+ received the reversed deprivation level-sucrose pellet contingency. After asymptotic discrimination performance was achieved, half the rats in each group received hippocampal lesions and half were assigned to control conditions. Following recovery from surgery, deprivation intensity discrimination performance was tested under the same conditions that were used in original training.
Previous studies using this basic design confirmed stimulus control by interoceptive food deprivation intensity cues by showing that neurohormonal manipulations known to promote food intake (e.g., systemic ghrelin administration) generalize to cues produced by a high level of food deprivation (Davidson, Kanoski, Tracy et al., 2005) whereas treatment with hormones known to suppress intake (e.g., CCK-8) generalize to cues produced by a low level of food deprivation (Kanoski, Walls et al., 2007).
In the present study, if hippocampal lesions impair the ability of rats to utilize their deprivation cues as discriminative stimuli, then the difference in responding between hippocampal lesioned rats in Groups 0+ and 24+ should be smaller compared to controls, whether testing occurs under 0-h or under 24-h food deprivation. If removing the hippocampus impairs the ability of deprivation cues to excite appetitive responding, then during testing rats with hippocampal lesions in both Groups 0+ and 24+ should exhibit reduced responding under their rewarded food deprivation level relative to controls. On the other hand, if hippocampal lesions cause rats in these two groups to exhibit increased tendencies to respond under their nonrewarded, but not their rewarded, food deprivation level, this would suggest that the hippocampus is involved with the inhibition of responding to nonreinforced cues. This latter outcome would be consistent with our view that the hippocampus is involved with inhibiting memories of the reinforcing postingestive consequences of eating (e.g., Davidson et al., 2009; Davidson, Kanoski, Schier, Clegg, & Benoit, 2007) and other appetitive USs (e.g., Chan, Morell, Jarrard, & Davidson, 2001; Davidson, Jarrard, & Jarrard, 2004).
Methods
Subjects
The subjects were 32 naïve, male, Sprague–Dawley, albino rats that weighed between 375 and 430 g upon arrival in the laboratory from Harlan Inc., Indianapolis, IN. The rats were housed individually in stainless steel cages under a reverse 12 h light dark cycle (lights off at 0700 h) and given access to standard laboratory chow (Laboratory Rodent Diet; Constant Nutrition 5001) and water ad libitum for 2 weeks prior to training. Water was available to the rats throughout the experiment except when the rats were in the apparatus for training and test sessions. All procedures for the care and treatment of the rats during this experiment were approved by the Purdue Animal Care and Use Committee.
Apparatus
All training and test sessions were conducted in eight identical conditioning chambers, constructed of aluminum end walls and clear Plexiglas sidewalls. A recessed food magazine was in the center of one end wall of each chamber. A white noise at approximately 60 dB was used during all training and testing sessions to mask extraneous background sounds. A computer-controlled infrared monitoring system was used to record food magazine entries. One infrared photo transmitter and one receiver were located on each side wall immediately in front of the recessed food magazine, such that rats would have to break the beam to gain entry to the food magazine.
Procedures
Training
The rats were assigned to two groups (n = 16 each), matched on body weight (see Table 1). For both groups, food deprivation levels alternated each day between 0 h or nondeprived and 24 h food deprivation. On 0 h food deprivation days, all rats had free access to food for approximately 24 h before the beginning of a training session. On 24 h food deprivation days, rats had no access to food for approximately 24 h prior to the beginning of the training session. Rats in Group 0+ received five sucrose pellets (45 mg sucrose pellets, P.J. Noyes Company Inc., Lancaster, NH) at the conclusion of each training session that took place under 0 h food deprivation and received no pellets during training sessions that took place under 24 h food deprivation. Group 24+ received the opposite contingency between food deprivation level and presentation of sucrose pellets. Although training sessions were always held at the same time of day (1500 h), the sessions did not occur every day to prevent the pellets from being delivered according to a single-alternating schedule. The schedule was also designed so that the number of transitions from 0 h to 24 h and from 24 h to 0 h food deprivation was equated during training. All of the rats were trained and tested in four squads of eight animals, with each rat in a squad assigned to a different conditioning chamber. When the rats were trained under their rewarded level of food deprivation they were placed in the conditioning chambers for 4 min before the sucrose pellets were delivered. During sessions in which rats were trained under their non-rewarded deprivation condition, the feeders operated at the end of 4 min but no pellets were delivered. On both rewarded and non-rewarded training sessions, the rats were removed from the conditioning chambers and returned to their home cages approximately 2 min after feeder operation. Initial training consisted of 88 sessions, with 44 training days each under 0 h and 24 h food deprivation, respectively. Throughout the experiment, the 4 min period that ended with feeder activation was further subdivided into twenty four, 10 s intervals. The percent of these intervals during which the photo beam was interrupted was calculated over the last 1, 2, 3, and all 4 min of each session prior to feeder activation.
Table 1.
Conditions for Pre-operative Training and Post-operative Testing
| Training Deprivation Level | Testing Deprivation Level | ||||
|---|---|---|---|---|---|
| Group | 0-hr | 24-hr | Lesion | 0-hr | 24-hr |
| 0+ | Suc+ | Suc- | HIP 0+ | Suc+ | Suc- |
| CON 0+ | Suc+ | Suc- | |||
| 24+ | Suc- | Suc+ | HIP 24+ | Suc- | Suc+ |
| CON 24+ | Suc- | Suc+ | |||
Suc+ = sucrose reward; sue- = no reward: 0+ = rewarded under0–hr but not under 24-hr food deprivation; 24+ = rewarded under 24-hr but not under 0-hr food deprivation: HIP = lesions of the complete hippocampus; CON = sham and unoperated controls
Testing
At the conclusion of training (see Table 1), the rats were assigned to hippocampal-lesioned and control groups matched on terminal level of discrimination performance based on the last two training sessions under each food deprivation level. Procedures for lesioning are described below. Half the rats in Group 0+ (HIP 0+) and half in Group 24+ (HIP 24+) were assigned to receive complete lesions of the hippocampus. The remaining rats in Group 0+ were assigned to control conditions (CON 0+) with half the rats receiving sham-lesions (n = 4) and half designated as unoperated controls (n = 4). The same procedure was used to assign the remaining rats in Group 24+ to control conditions (CON 24+). Testing began 20 days after surgery when the mean weight of Groups HIP 0+ and HIP 24+ returned to a level equal to that of their respective controls (CON 0+ and CON 24+). When this criterion was achieved postoperative recovery was deemed complete for the hippocampal lesioned rats. Retention of deprivation intensity discrimination training was assessed in four test sessions, with two sessions each under 0 h and 24 h food deprivation, respectively in the order 24-h, 0-h, 24-h, 0-h. The procedures used for these test sessions were the same as those described for training. The rats were weighed prior to each test session and for 10 additional days of alternating 24- and 0-h food deprivation after testing was completed.
Surgical and histological procedures
Rats in the hippocampus lesioned group (CHip) had the hippocampus removed using multiple, focal injections of small amounts of the selective neurotoxin, ibotenic acid (IBO: Biosearch Technologies). The IBO was dissolved in phosphate buffered saline (pH 7.4) at a concentration of 10 mg/ml. The rats were anesthetized with intraperitoneal injections of equithesin (a combination of pentobarbital and chloral hydrate) and were placed in a Kopf stereotaxic apparatus. Following the procedure described in detail in Jarrard (1989, 2002), an incision was made in the scalp, and the bone overlying the area to be lesioned was removed. Injections of IBO were made with a 5-μl Hamilton syringe mounted on the stereotaxic frame and held in a Kopf microinjector unit (Model 5000). A small diameter glass micropipette was glued onto the end of the needle of the syringe in order to minimize damage to the cortex overlying the area to be lesioned. Injections were made over approximately 1 min at each site and the pipette was left in place for approximately 1 min to prevent spread of the neurotoxin up the tract.
The surgical procedures were generally the same as those described in our previous experiments (Jarrard, 1989; Jarrard, Davidson, & Bowring, 2004; Jarrard & Meldrum, 1993). The stereotaxic coordinates used for the CHip lesions involved injections at 30 sites, totaling 2.08 ul of IBO, can be found at the website (http://hippocampus2.wlu.edu). At the end of experiment, all rats were administered an overdose of the anesthetic and were perfused transcardially with buffered physiological saline followed by 10% formaldehyde solution. The brains were removed, embedded in egg yolk, cryoprotected in a 30% solution of sucrose-formalin, and subsequently cut on a cryostat into 40-μm sections. Every fifth section from rats in the CHip group was saved for histology and stained. A cresyl violet cell-body stain was used to determine cell loss and gliosis resulting from the lesions. Evaluation of the nature and extent of the lesions was carried out by L.E.J.
Results
Histology
The nature and extent of the damage to hippocampus resulting from the surgical procedures described above was quite similar for all rats. Generally, the loss of cells that comprise the hippocampus is similar to that found in other experiments where these stereotaxic coordinates were employed (Jarrard, 1989; Jarrard & Meldrum, 1993). The images presented in Figure 1 are from a rat with a representative lesion of hippocampus. This image was chosen because the damage it shows is similar to that present in the brains of the other lesioned rats and because of the relative absence of tissue tears, uneven darkness of staining, and other artifacts that are sometimes associated with histology. The damage depicted in the figure extends throughout the hippocampus and includes all cell fields. Especially apparent, and for the most part complete, is the loss of cells in the anterior/dorsal and intermediate two-thirds of the hippocampus. The only sparing of hippocampus at this level is the lower blade of the dentate gyrus in the most medial, anterior/dorsal level. With damage this extensive and with the long survival time there is obvious atrophy of any remaining hippocampus together with a proliferation of glial cells and slight distortion of the ventricles and adjacent brain areas. Also apparent in the figure is the sparing of fibers-of–passage in the fimbria-fornix, projections that were shown in earlier research to be functional (Jarrard, 1989). In several animals there were small ‘patches’ of normal appearing cells (usually posterior/ventral dentate granule cells and CA1 pyramidals) but these were usually unilateral and small in extent. There was minimal extrahippocampal involvement for rats with hippocampus completely removed. The exception was occasional damage to subiculum but this was usually small in extent and usually unilateral.
Figure 1. Experiment 2 histology.
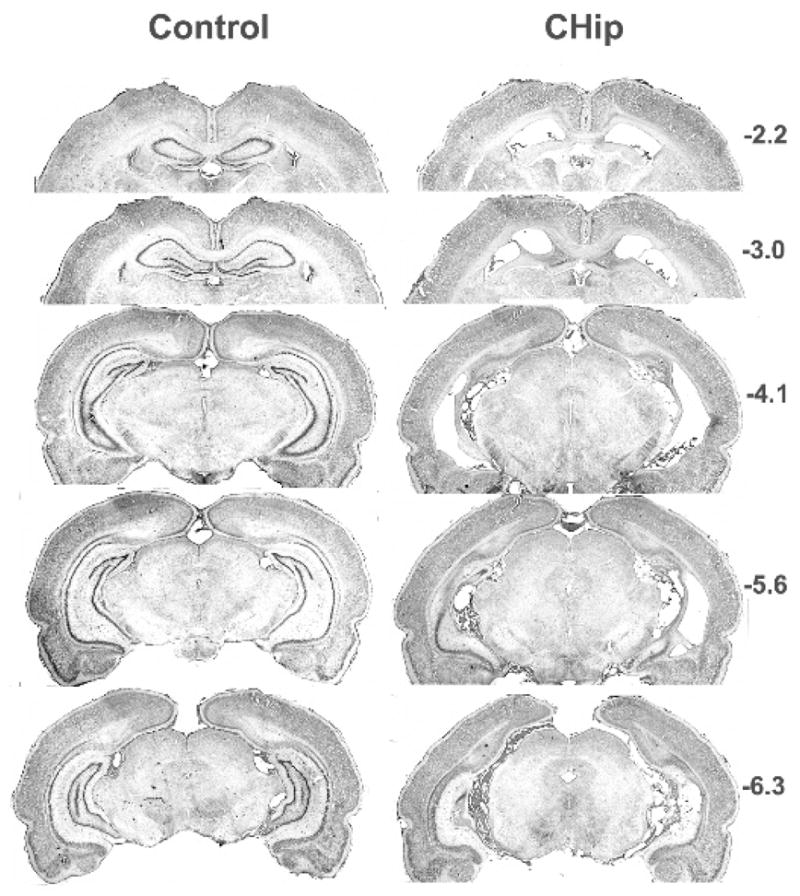
Photomicrographs of cresyl violet-stained coronal sections taken from a representative rat in the Control (CON) and Complete Hippocampal (CHip) Groups in Experiment 2. Approximate stereotaxic coordinates of the coronal sections shown on the right are with reference to bregma.
Training
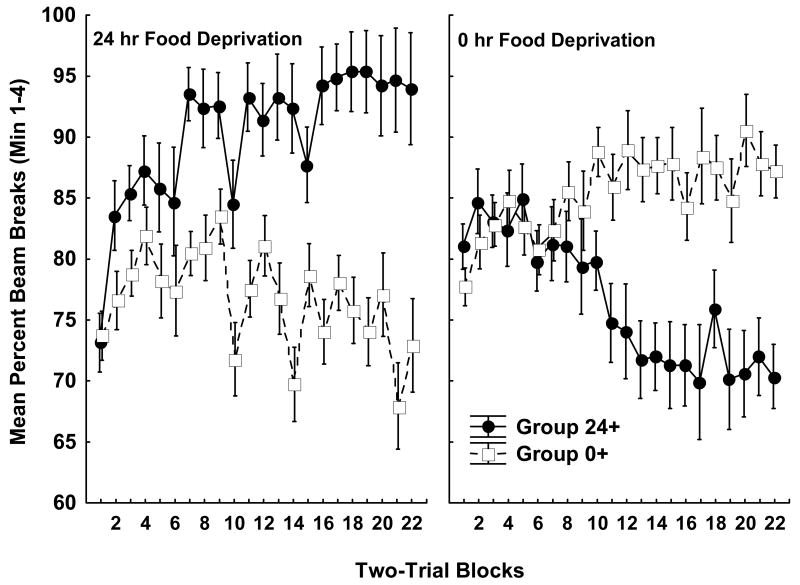
Rats solved the food deprivation intensity discrimination problem. Figure 2 shows the mean number of beam breaks for each group over each two session block of training under 24 h and 0 h food deprivation. Group 24+ learned to respond more than Group 0+ when trials occurred under 24-h food deprivation (left panel), whereas Group 0+ came to exhibit more responding than Group 24+ on trials under 0-h food deprivation (right panel). In addition to these between-subjects differences, a within-subjects comparison showed that both groups learned to respond more under their reinforced compared to their nonreinforced food deprivation level. This pattern of results yielded significant main effects of Deprivation Level (F(1, 20) = 6.35, p < .05), and Blocks (F(21, 420) = 1.99, p < .01), as well as significant Deprivation Level × Group (F(1, 20) = 128.58, p < .01), and Deprivation Level × Block × Group (F(21, 420) = 9.45, p < .01) interactions. Neither the main effect of Group nor other interactions involving Group achieved significance. Post-hoc Newman-Keuls tests that were used to evaluate the Deprivation Level × Group interaction showed that Group 24+ responded significantly more than Group 0+ when both groups were food deprived for 24 h, whereas Group 0+ responded more than Group 24+ when both groups were under 0 h food deprivation (ps < .01). Furthermore, Group 24+ responded significantly more under 24 compared to 0 h food deprivation. Significantly more responding occurred under 0 h compared to 24 h food deprivation for Group 0+ (ps < .01).
Figure 2. Food deprivation intensity discrimination training.
The data shown depict mean photobeam interruptions throughout each four-trial block of 4-min training sessions. All data were collected prior to surgical treatment. The left panel depicts data from 24-h food deprivation training sessions, while the right panel depicts data from sessions when the rats were 0-h food deprived. Data for rats trained with sucrose pellets delivered at the end of sessions under 24-h food, and not delivered under 0-h food deprivation are designated Group 24+ (filled symbols) whereas rats trained with the reversed deprivation level-sucrose pellet contingency are designated Group 0+ (open symbols). Error bars represent S.E.M.
Testing
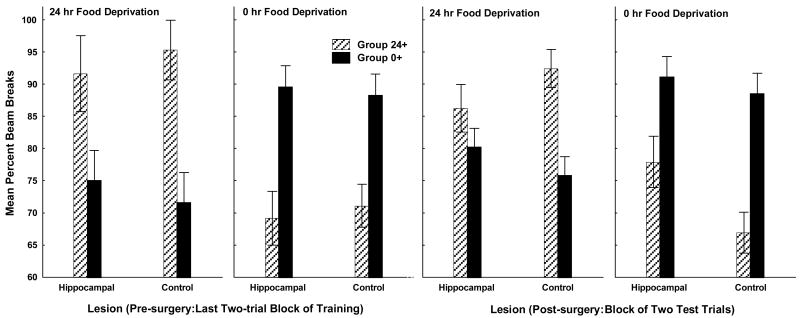
Prior to the beginning of testing, food deprivation intensity discrimination performance did not differ for rats that were assigned to subsequently receive lesions of the complete hippocampus compared to controls. The two leftmost panels of Figure 3 shows that on the last two-trial block of training under 24 and 0 h food deprivation there was little difference in discrimination performance for rats that later had the hippocampus removed compared to rats that were subsequently tested with the hippocampus intact. Mean beam breaks over the entire 4-min session were higher under 24 h food deprivation for Group 24+ than Group 0+ and were higher under 0 h food deprivation for Group 0+ than for Group 24+. In addition, both groups responded more under their reinforced compared to their nonreinforced food deprivation level, independent of whether or not the rats in each group were assigned to receive hippocampal lesions prior to subsequent testing.
Figure 3. Deprivation intensity discrimination testing.
Leftmost two panels show mean photobeam interruption over the last two-trial block of training (pre-surgery) under 24-h and 0-h food deprivation for rats that were to subsequently receive lesions of the complete hippocampus (Hippocampal) and the combined operated and unoperated controls (Control). The two rightmost panels of show data collected for the Hippocampal and Control rats after surgery. Error bars represent S.E.M.
ANOVA provided statistical confirmation for these conclusions by obtaining a significant Deprivation Level × Training Contingency interaction (F(1, 25) = 52.38, p < .01) and a nonsignificant Deprivation Level × Training Contingency × Surgery interaction (F(1, 25) < 1), indicating that the effects of the training contingency on responding under each level of food deprivation did not vary for hippocampal-lesioned compared to control animals.
Following surgery, retention of food deprivation intensity discrimination learning was impaired for rats with lesions of the complete hippocampus (HIP) relative to controls (CON). The two rightmost panels of Figure 3 shows that when testing occurred either under 24 h or 0 h food deprivation the magnitude of the difference between reinforced and nonreinforced trials was smaller for rats with hippocampal lesions than for controls. Furthermore, under both deprivation levels, rats with damage to hippocampus tended to respond more than controls on their nonreinforced trials. That is, when tested under 24 h food deprivation, rats that had been trained to anticipate sucrose pellets under 0 but not under 24 h food deprivation (Group 0+) were less able to refrain from responding if they had received hippocampal lesions. Likewise, when tested under 0 h food deprivation, rats that had been trained to approach the food magazine when 24 but not 0 h food deprived (Group 24+) appeared to have more difficulty suppressing their responding during testing under 0 h food deprivation when their hippocampus had been removed, compared to control rats without hippocampal damage. This pattern of results produced significant Deprivation Level × Training contingency (F(1, 25) = 67.46, p < .01), Deprivation Level × Surgery (F(1, 25) = 4.08, p < .05), and Deprivation Level × Training contingency × Surgery (F(1, 25) = 7.36, p < .05) interactions.
Newman-Keuls tests evaluating the Deprivation Level × Training contingency interaction revealed that when tested under 24 h food deprivation Group 24+ responded more than Group 0+ and responded less than Group 0+ when tested under 0 h food deprivation (ps < .05). Moreover, both of these groups responded more under their reinforced compared to their nonreinforced level of food deprivation (ps < .05). In evaluating the Deprivation Level × Surgery interaction, Newman-Keuls tests also showed that collapsed across Training Contingency, control rats responded significantly less when food deprived for 0 h compared to 24 h, whereas rats with hippocampal lesions did not respond significantly less when nondeprived relative to the deprived condition. Further, using Newman-Keuls tests to breakdown the Deprivation Level × Training contingency × Surgery interaction showed that for hippocampal lesioned rats in Group 24+ the difference in responding on trials under 0 h compared to 24 h food deprivation did not achieve significance, whereas this difference was highly significant (p < .001) for control rats. For Group 0+, both lesioned rats and controls responded significantly more under 0 h compared to 24 h food deprivation (ps < .05). However, an ANOVA comparing mean responding on reinforced (+) relative to nonreinforced (-) trials, collapsed across test deprivation levels, obtained a significant Surgery × Trial type (+/-) interaction (F(1, 25) = 7.51, p < .05). Newman-Keuls tests showed that rats with hippocampal lesions responded significantly more on nonreinforced trials than did controls. This difference did not approach significance on reinforced trials (p > .84).
Body weight
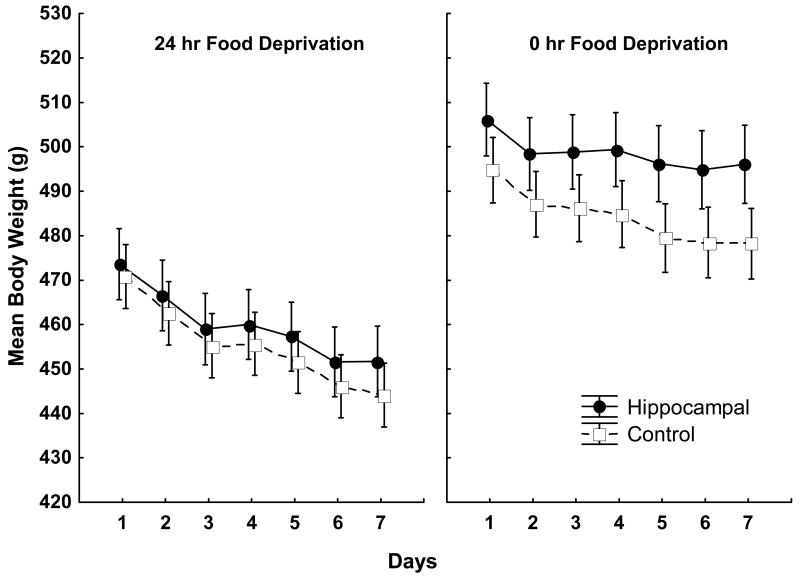
Body weight was measured for each rat during test trials (two each) under 0 h and 24 h food deprivation and for 10 additional days under alternating 0h and 24 h food deprivation after these test trials were completed. Figure 4 shows mean body weight for hippocampal lesioned and control rats on both 24 h (left panel) and 0 h (right panel) food deprivation days. As can be seen, not surprisingly rats in both lesioned and control groups weighed less under 24 h compared to 0 h food deprivation. However, while there appeared to be little effect of lesion treatment on body weight when the rats were food deprived, body weight for rats with hippocampal lesions began to exceed that for controls over days under 0-h food deprivation. Although food intake was not measured, the results suggest that rats with hippocampal lesions may have consumed more when given ad lib food than did control rats.
Figure 4. Body weight under 24- and 0-h food deprivation.
Mean body weight for rats with Hippocampal lesions (filled symbols) and Controls (open symbols) under alternating 24-h (left panel) and 0-h (right panel) food deprivation. Error bars represent S.E.M.
An ANOVA with Deprivation Level and Days as within-subjects factors and Surgery as a between subjects factor obtained significant main effects of Deprivation Level (F(1, 27) = 1132.30, p< .05) and Days (F(6, 162) = 127.75, p < .01) and a significant Surgery × Days (F(6, 162) = 3.15, p < .01) and Surgery × Deprivation Level (F(1, 27) = 22.86, p < .01) interactions. A separate ANOVA evaluating the effects of Surgery and Days on body weight differences under only 0 h deprivation obtained a significant main effect of Days (F(6, 162) = 42.62, p <.01) and a significant Surgery × Days interaction (F(6, 162) = 3.03, p < .01), indicating that body weight decreased significantly over days under 0 h deprivation and that the difference in body weight between rats with the hippocampus removed and controls grew larger over that period. In contrast, when the rats were food deprived for 24 h, an ANOVA obtained a significant main effect of Days (F(6, 162) = 186.21, p < .01) but no significant Surgery × Days interaction (F(6, 162) = 1.59, p > .15).
Discussion
The results showed that following achievement of asymptotic food deprivation intensity discrimination performance, ibotenate lesions of the hippocampus impaired the ability of rats to use cues arising from 0 and 24 h food deprivation as discriminative signals for sucrose pellets. This outcome adds to previous findings which showed that the same type of hippocampal lesions impaired acquisition of food deprivation intensity discrimination learning based on an aversive US (Davidson & Jarrard, 1993; Hock & Bunsey, 1998). Furthermore, the present results showed that rats with hippocampal lesions were less able than controls to refrain from responding in the presence of food deprivation cues that signaled nonreinforcement, and this effect was especially pronounced when cues arising from 0 h food deprivation signaled the absence of the sucrose US. Rats with hippocampal damage and controls did not differ significantly with respect to their responding to food deprivation cues that signaled reinforcement.
The results also confirm that hippocampal damage alters body weight regulation. Hippocampal damage was associated with higher body weight relative to controls when rats were on an alternating schedule of 24 h and 0 h food deprivation. Specifically, hippocampal-lesioned rats came to weigh significantly more than controls under 0 h food deprivation. Confirming an earlier result (Davidson et al., 2009), this finding indicates that the hippocampus is involved with controlling body weight when food-deprived rats are given free access to food.
This pattern of retention test responding we observed is informative about the role played by the hippocampus in mediating appetitive behavior. Excessive responding in the presence of signals that the US will not be forthcoming is consistent with the idea that the ability of these cues to inhibit the memory of the appetitive US depends on the hippocampus (see Davidson et al., 2007; Davidson, Kanoski, Walls, & Jarrard, 2005). Conversely, the finding that removing the hippocampus failed to alter responding in the presence of signals for sucrose reinforcement suggests that simple excitation of the US memory is hippocampal-independent. Previous research indicates that while memory inhibition, such as that involved with suppression of responding to trained and extinguished cues, requires an intact hippocampus, memory excitation does not (for reviews see Chan et al., 2001; Davidson & Jarrard, 2004).
The current findings may also shed light on how energy state signals might participate in this type of memory inhibition. A variety of data suggest that the hippocampus is needed to resolve what has been described as “predictable ambiguity” (Morris, 2006). For example, under conditions where a single target stimulus is associated with both reinforcement and nonreinforcement, animals will attempt to identify other cues (e.g. contextual or discrete stimuli) that resolve this ambiguity by predicting when the target stimulus will and will not be followed by the US. Interference with hippocampal functioning has been shown to impair performance on many learning and memory problems that require animals to resolve predictable ambiguities, and this impairment often takes the form of increased responding to nonreinforced target cues.
The food deprivation intensity discrimination problem can be seen as requiring the resolution of predictable ambiguity. In that problem, the relation between stimuli in the training apparatus and delivery of sucrose pellets is ambiguous in that these cues are paired with reinforcement on some occasions, but not others. The problem is designed so that animals can use their energy state cues to resolve this ambiguity. The finding that rats with hippocampal lesions are impaired at solving this problem has implications for understanding why these animals might overeat and show increased body weight gain.
We (Davidson et al., 2007) proposed previously that the decision to eat or to refrain from eating outside of the laboratory requires animals, including humans, to resolve a predictable ambiguity, in that food and cues related to food are associated with reinforcing or appetitive postingestive stimulation on some occasions (e.g., following several hours without eating), but not at other times (e.g., after consuming a large meal). Animals can resolve the ambiguity and can inhibit their appetitive and consummatory responses by using their interoceptive energy state signals to anticipate when food cues will not be accompanied by a strong appetitive postingestive US. Consistent with this model, a popular conceptualization of the physiological controls of energy regulation suggests that eating is initiated in response, not to energy deficit or hunger cues, but to environmental stimuli that are associated with the rewarding consequences of eating (e.g., Berthoud, 2004; Woods & Seeley, 2000). Within this framework, food and environmental cues related to food will evoke eating behavior until that behavior is suppressed by the onset and continued presence of meal-related satiety signals. Thus, satiety signals can be seen as resolving predictable ambiguity by informing animals when food related stimuli are not predictive of reinforcing postingestive consequences. The present results suggest that the ability to use energy state signals to solve this type of problem and to inhibit appetitive behavior may depend on the structural integrity of the hippocampus.
One implication of the present findings is that it may be important to identify factors in the current food environment that may promote energy and body weight dysregulation by interfering with hippocampal functioning. Recent research in our laboratory and elsewhere suggests that, in addition to promoting weight gain, intake of energy-rich diets (i.e., diets high in processed sugars and saturated fats), may impair hippocampal-dependent learning and memory processes (e.g., Kanoski, Meisel, Mullins, & Davidson, 2007; Molteni, Barnard, Ying, Roberts, & Gomez-Pinilla, 2002; Murray et al., 2009). Reciprocal links between the effects of these diets on energy regulation and on learning and memory processes merit further experimental attention.
Acknowledgments
The authors thank Lindsey Schier, Andrea Tracy, Ashley Martin, and Elwood Walls for discussions that helped to develop and refine many of the ideas that are presented in this paper. Funding in support of this work was provided by Grants R01 HD29792 and P01 HD052112 from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/bne
References
- Berthoud HR. Mind versus metabolism in the control of food intake and energy balance. Physiology & Behavior. 2004;81(5):781–793. doi: 10.1016/j.physbeh.2004.04.034. [DOI] [PubMed] [Google Scholar]
- Cenquizca LA, Swanson LW. Analysis of direct hippocampal cortical field CA1 axonal projections to diencephalon in the rat. Journal of Comparative Neurology. 2006;497(1):101–114. doi: 10.1002/cne.20985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenquizca LA, Swanson LW. Spatial organization of direct hippocampal field CA1 axonal projections to the rest of the cerebral cortex. Brain Res Rev. 2007 doi: 10.1016/j.brainresrev.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan KH, Morell JR, Jarrard LE, Davidson TL. Reconsideration of the role of the hippocampus in learned inhibition. Behavioural Brain Research. 2001;119(2):111–130. doi: 10.1016/s0166-4328(00)00363-6. [DOI] [PubMed] [Google Scholar]
- Davidson TL. Learning about deprivation intensity stimuli. Behavioral Neuroscience. 1987;101(2):198–208. doi: 10.1037//0735-7044.101.2.198. [DOI] [PubMed] [Google Scholar]
- Davidson TL, Chan K, Jarrard LE, Kanoski SE, Clegg DJ, Benoit SC. Contributions of the hippocampus and medial prefrontal cortex to energy and body weight regulation. Hippocampus. 2009;19(3):235–252. doi: 10.1002/hipo.20499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson TL, Jarrard LE. A role for hippocampus in the utilization of hunger signals. Behav Neural Biol. 1993;59(2):167–171. doi: 10.1016/0163-1047(93)90925-8. [DOI] [PubMed] [Google Scholar]
- Davidson TL, Jarrard LE. The hippocampus and inhibitory learning: a ‘Gray’ area? Neuroscience & Biobehavioral Reviews. 2004;28(3):261–271. doi: 10.1016/j.neubiorev.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Davidson TL, Jarrard LE, Jarrard LE. The hippocampus and inhibitory learning: a ‘Gray’ area? Neuroscience & Biobehavioral Reviews. 2004;28(3):261–271. doi: 10.1016/j.neubiorev.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Davidson TL, Kanoski SE, Schier LA, Clegg DJ, Benoit SC. A potential role for the hippocampus in energy intake and body weight regulation. Current Opinion in Pharmacology. 2007;7(6):613–616. doi: 10.1016/j.coph.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson TL, Kanoski SE, Tracy AL, Walls EK, Clegg D, Benoit SC. The interoceptive cue properties of ghrelin generalize to cues produced by food deprivation. Peptides. 2005;26(9):1602–1610. doi: 10.1016/j.peptides.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Davidson TL, Kanoski SE, Walls EK, Jarrard LE. Memory inhibition and energy regulation. Physiology & Behavior. 2005;86(5):731–746. doi: 10.1016/j.physbeh.2005.09.004. [DOI] [PubMed] [Google Scholar]
- DelParigi A, Chen K, Salbe AD, Hill JO, Wing RR, Reiman EM, et al. Persistence of abnormal neural responses to a meal in postobese individuals. International Journal of Obesity & Related Metabolic Disorders: Journal of the International Association for the Study of Obesity. 2004;28(3):370–377. doi: 10.1038/sj.ijo.0802558. [DOI] [PubMed] [Google Scholar]
- Hebben N, Corkin S, Eichenbaum H, Shedlack K. Diminished ability to interpret and report internal states after bilateral medial temporal resection: case H.M. Behavioral Neuroscience. 1985;99(6):1031–1039. doi: 10.1037//0735-7044.99.6.1031. [DOI] [PubMed] [Google Scholar]
- Hock BJ, Jr, Bunsey MD. Differential effects of dorsal and ventral hippocampal lesions. Journal of Neuroscience. 1998;18(17):7027–7032. doi: 10.1523/JNEUROSCI.18-17-07027.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrard LE. On the use of ibotenic acid to lesion selectively different components of the hippocampal formation. Journal of Neuroscience Methods. 1989;29(3):251–259. doi: 10.1016/0165-0270(89)90149-0. [DOI] [PubMed] [Google Scholar]
- Jarrard LE. Use of excitotoxins to lesion the hippocampus: update. Hippocampus. 2002;12(3):405–414. doi: 10.1002/hipo.10054. [DOI] [PubMed] [Google Scholar]
- Jarrard LE, Davidson TL, Bowring B. Functional differentiation within the medial temporal lobe in the rat. Hippocampus. 2004;14(4):434–449. doi: 10.1002/hipo.10194. [DOI] [PubMed] [Google Scholar]
- Jarrard LE, Meldrum BS. Selective excitotoxic pathology in the rat hippocampus. Neuropathology & Applied Neurobiology. 1993;19(5):381–389. doi: 10.1111/j.1365-2990.1993.tb00458.x. [DOI] [PubMed] [Google Scholar]
- Kanoski SE, Meisel RL, Mullins AJ, Davidson TL. The effects of energy-rich diets on discrimination reversal learning and on BDNF in the hippocampus and prefrontal cortex of the rat. Behav Brain Res. 2007;182(1):57–66. doi: 10.1016/j.bbr.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanoski SE, Walls EK, Davidson TL. Interoceptive “satiety” signals produced by leptin and CCK. Peptides. 2007;28(5):988–1002. doi: 10.1016/j.peptides.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molteni R, Barnard RJ, Ying Z, Roberts CK, Gomez-Pinilla F. A high-fat, refined sugar diet reduces hippocampal brain-derived neurotrophic factor, neuronal plasticity, and learning. Neuroscience. 2002;112(4):803–814. doi: 10.1016/s0306-4522(02)00123-9. [DOI] [PubMed] [Google Scholar]
- Morris RGM. Theories of hippocampal function. In: Andersen P, Morris R, Amaral D, Bliss T, O'Keefe J, editors. The Hippocampus Book. Oxford, UK: Oxford University Press; 2006. pp. 581–713. [Google Scholar]
- Murray AJ, Knight NS, Cochlin LE, McAleese S, Deacon RM, Rawlins JN, et al. Deterioration of physical performance and cognitive function in rats with short-term high-fat feeding. FASEB J. 2009 doi: 10.1096/fj.09-139691. [DOI] [PubMed] [Google Scholar]
- Rozin P, Dow S, Moscovitch M, Rajaram S. What causes humans to begin and end a meal? A role for memory for what has been eaten, as evidenced by a study of multiple meal eating in amnesic patients. Psychological Science. 1998;9(5):392–396. [Google Scholar]
- Thanos PK, Michaelides M, Gispert JD, Pascau J, Soto-Montenegro ML, Desco M, et al. Differences in response to food stimuli in a rat model of obesity: in-vivo assessment of brain glucose metabolism. International Journal of Obesity. 2008;32(7):1171–1179. doi: 10.1038/ijo.2008.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GJ, Yang J, Volkow ND, Telang F, Ma Y, Zhu W, et al. Gastric stimulation in obese subjects activates the hippocampus and other regions involved in brain reward circuitry. Proc Natl Acad Sci U S A. 2006;103(42):15641–15645. doi: 10.1073/pnas.0601977103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SC, Seeley RJ. Adiposity signals and the control of energy homeostasis. Nutrition. 2000;16(10):894–902. doi: 10.1016/s0899-9007(00)00454-8. [DOI] [PubMed] [Google Scholar]