Abstract
Although dynorphin A-(1–17) has been characterized in vitro as a high efficacy κ-opioid receptor agonist, functional studies of dynorphin A-(1–17) following central or systemic administration indicate the involvement of both opioid and non-opioid components. The aim of this study was to investigate whether local administration of dynorphin-related analogs can attenuate capsaicin (8-methyl-N-vanillyl-6-nonenamide)-induced nociception and what type of opioid receptor mediates the local action of dynorphin A-(1–17) in monkeys. Capsaicin (100 μg) was used to evoke a nociceptive response, thermal allodynia, which was manifested as a reduced tail-withdrawal latency in normally innocuous 46°C warm water. Co-administration of dynorphin A-(1–17) (0.3–10 μg) with capsaicin in the tail dose-dependently inhibited thermal allodynia; however, both non-opioid fragments dynorphin A-(2–17) (10–300 μg) and dynorphin A-(2–13) (10–300 μg) were ineffective. Local antiallodynia of dynorphin A-(1–17) was antagonized by a small dose (100 μg) of an opioid receptor antagonist, quadazocine, applied s.c. in the tail. Pretreatment with a selective κ-opioid receptor antagonist, nor-binaltorphimine (nor-BNI), s.c. 320 μg in the tail also reversed local antiallodynia of dynorphin A-(1–17). Both locally effective doses of antagonists, when applied s.c. in the back, did not antagonize local dynorphin A-(1–17), indicating that peripheral κ-opioid receptors selectively mediated the local action of dynorphin A-(1–17) in the tail. In addition, a much larger dose of dynorphin A-(1–17) (1000 μg), when administered s.c. in the back or i.m. in the thigh, did not cause sedative or diuretic effects. These results suggest that in vivo opioid actions of dynorphin-related peptides can be differentiated locally in this procedure. They also indicate that local application of peptidic ligands may be a useful medication for localized pain.
Keywords: Allodynia, Antinociception, Diuresis, Dynorphin, κ-Opioid receptors, Sedation
1. Introduction
Dynorphin A-(1–17) is a heptadecapeptide that is an endogenous ligand for κ-opioid receptors (Goldstein et al., 1981; Chavkin et al., 1982). Dynorphin A-(1–17) has a relatively high binding selectivity for κ-opioid receptors in cloned opioid receptors and in primate cortex membranes (Meng et al., 1993; Raynor et al., 1994; Kim et al., 1996; Butelman et al., 1998), although recent in vitro studies suggest that dynorphin A-(1–17) may interact with μ- and δ-opioid receptors only at higher concentrations (Alt et al., 1998; Zhang et al., 1998). Nevertheless, dynorphin A-(1–17) has very high efficacy in activating G proteins in a cell line expressing human κ-opioid receptors (Zhu et al., 1997; Remmers et al., 1999).
On the other hand, the in vivo characterization of dynorphin A-(1–17) is controversial. For instance, intrathecal injection of dynorphin A-(1–17), dynorphin A-(1–13), or the non-opioid dynorphin A-(2–17) all caused severe motor dysfunction in rats as shown by flaccid extension of hindlimbs and loss of muscle tone (e.g., Faden and Jacobs, 1984; Stevens and Yaksh, 1986; Caudle and Isaac, 1988; Long et al., 1988). This motor impairment was not reversed by opioid receptor antagonists and was not observed with synthetic κ-opioid receptor agonists such as U50,488 [(trans)-3,4-dichloro-N-methyl-N-[2-(1- pyrrolidinyl)-cyclohexyl]benzeneacetamide]. However, it can be partially reduced or blocked by N-methyl-D-aspartate (NMDA) receptor antagonists (Caudle and Isaac, 1988; Shukla and Lemaire, 1994; Shukla et al., 1997). Intravenous administration of dynorphin A-(1–17) in monkeys decreased food-reinforced responding, but dynorphin A-(2–17) was ineffective. The operant inhibitory effects of dynorphin A-(1–17) were not reversed by an opioid receptor antagonist, but stimulation of serum prolactin release by intravenous dynorphin A-(1–17) was mediated by κ-opioid receptors in monkeys (Butelman et al., 1999). Therefore, dynorphin A-(1–17) and its related analogs following central or systemic administration have complex in vivo profiles including opioid and non-opioid components of actions.
Few studies have characterized the effects of dynorphin-related analogs following local administration. Only one rodent study has reported that intraplantar dynorphin A-(1–17) produced a naloxone-reversible antinociception in an inflammatory pain model, but dynorphin A-(2–17) was ineffective. However, this study did not evaluate what opioid receptor subtypes mediated the local action of dynorphin A-(1–17) (Beyer et al., 1997). Considering that local administration of peptides may minimize distribution factors and adverse effects produced by central or systemic administration, it is valuable to explore the local antinociception of dynorphin-related analogs in different experimental pain models. Previously, we found that s.c. capsaicin in the tail of monkey evoked a nociceptive response, thermal allodynia, which was manifested as a reduced tail-withdrawal latency in normally innocuous warm water (Ko et al., 1998). In this procedure, small, systemically inactive doses of κ-opioid receptor agonists can locally attenuate capsaicin-induced thermal nociception, which has been demonstrated as a peripheral action of κ-opioid receptors (Ko et al., 1999a).
The aim of this study was therefore to examine whether local administration of dynorphin A-(1–17) and non-opioid fragments dynorphin A-(2–17) and dynorphin A-(2–13) can attenuate capsaicin-induced nociception in monkeys. Antagonism studies were performed to characterize opioid receptor subtypes mediating local actions of dynorphin A-(1–17). In addition, intramuscular (i.m.) administration of dynorphin A-(1–17) and a prototypic κ-opioid receptor agonist U50,488 was conducted in order to compare potential diuretic effects, which can be produced following i.m. administration of non-peptidic κ-opioid receptor agonists (Leander, 1983; Dykstra et al., 1987).
2. Materials and methods
2.1. Subjects
Eleven adult male and female rhesus monkeys (Macaca mulatta) with body weights ranging between 7.1 and 12.9 kg were used (their mean weight during this study was 9.7 kg). They were housed individually with free access to water and were fed approximately 25–30 biscuits (Purina Monkey Chow; Ralston Purina, St. Louis, MO) and fresh fruit daily. Six monkeys were previously trained in the warm water tail-withdrawal procedure and all monkeys did not have exposure to opioids or capsaicin for one month before the present study. Animals used in this study were maintained in accordance with the University Committee on the Use and Care of Animals in the University of Michigan, and the Guide for the Care and Use of Laboratory Animals (7th edn). by the Institute of Laboratory Animal Resources (Natl. Acad. Press, Washington, DC, revised 1996).
2.2. Procedure
Thermal antinociception was measured by a warm water tail-withdrawal procedure, which has been described previously (Ko et al., 1998). Briefly, monkeys were seated in restraint chairs and the lower part of the shaved tail (approximately 15 cm) was immersed into warm water maintained at temperatures of 42°C, 46°C, or 50°C. Tail-withdrawal latencies were recorded by an experimenter who did not know experimental conditions. A maximum cutoff latency (20 s) was used in order to prevent tissue damage. Each experimental session began with control determinations at three temperatures in a varying order. Subsequent tail-withdrawal latencies were determined at 5, 15, 30, 45, and 60 min after injection. A single-dosing procedure was used in all test sessions. Experimental sessions were conducted only once per week.
2.3. Experimental design
After capsaicin was administered s.c. in the tail, it dose-dependently caused thermal allodynia for approximately 30 min following injection (Ko et al., 1998). Based on these previous results, we choose 100 μg of capsaicin in 46°C water as a standard noxious stimulus for the present studies.
2.3.1. Antinociceptive effects of dynorphin-related analogs
Dynorphin A-(1–17) (0.3–10 μg), dynorphin A-(2–17) (10–300 μg), or dynorphin A-(2–13) (10–300 μg) was co-administered with capsaicin in the tail to evaluate their antiallodynic effects. The maximum locally effective dose was administered s.c. in the back (i.e., around the scapular region) against capsaicin. In addition, this locally effective dose was administered s.c. in the tail to evaluate whether it changed the thermal nociceptive threshold in the absence of capsaicin. For comparison, systemic antinociceptive effects of dynorphin A-(1–17) (3–300 μg/kg) were studied by s.c. administration in the back immediately after capsaicin injection.
2.3.2. Antagonism of dynorphin A-(1–17)-induced local antiallodynia
Considering that onset and distribution factors may be minimized with local administration, an opioid receptor antagonist, quadazocine (10–100 μg), was co-administered with capsaicin and dynorphin A-(1–17) in the tail to evaluate local antagonist effects. Similarly, the maximum locally effective dose of quadazocine was injected s.c. in the back to verify whether the antagonism was localized in the tail.
In addition, a selective κ-opioid receptor antagonist, nor-binaltorphimine (nor-BNI) was used to confirm local antiallodynic actions of dynorphin A-(1–17). Previously, we have demonstrated that pretreatment with nor-BNI 320 μg in the tail significantly antagonized U50,488 [(trans)-3,4-dichloro-N-methyl-N-[2-(1-pyrrolidinyl)-cyclohexyl]-benzeneacetamide]-induced local antinociception against capsaicin, but it did not antagonize the local antinociception of μ-opioid receptor agonists (Ko et al., 1998, 1999a). By using the same procedure, nor-BNI was administered s.c. in the marked tail. The tail-withdrawal latencies in 42°C, 46°C, and 50°C water were measured for 1 h after injection, in order to verify whether nor-BNI alone interfered with normal tail-withdrawal responses. Then, local antiallodynic effects of dynorphin A-(1–17) against capsaicin were determined 24 h after local nor-BNI pretreatment. Likewise, nor-BNI 320 μg was administered s.c. in the back with the same pretreatment time to verify whether the antagonism was localized in the tail.
2.3.3. Diuretic effects of U50,488 and dynorphin A-(1–17)
Urine outputs were collected in the home cage over 3 h after i.m. administration of U50,488 (100–1000 μg) or dynorphin A-(1–17) (10–1000 μg). These dose ranges were chosen, starting from their locally effective doses, which produced local antinociception against capsaicin. A fixed injection volume (0.5 ml) was used in order to compare diuretic effects, which may be interfered by differential distribution factors of non-peptidic versus peptidic ligands. Experimental sessions were conducted typically between 9 am to noon; and they were performed twice per week with at least a 3-day interval,. The result with each dose was replicated two to three times in each monkey (n = 5).
2.4. Data analysis
Individual tail-withdrawal latencies were converted to percent of maximum possible effect (%MPE) by the following formula: %MPE = [(test latency − control latency)/(cutoff latency, 20 s − control latency)] × 100. The 15-min time point was used for data analysis because this was the time of peak effects of capsaicin and peptidic opioid agonists following local administration (Ko et al., 1998, 1999a). Mean ED50 values were obtained from individual ED50 values, which were calculated by least-squares regression using the portion of the dose–effect curves spanning 50% MPE, and 95% confidence limits were also determined. Similarly, the mean ID50 value of quadazocine was determined in the same manner by defining the dose that inhibited 50% MPE of dynorphin A-(1–17). Considering the mean body weight of monkeys was approximately 10 kg during this study, an attempt was made to compare doses of s.c. injection in the tail (μg) versus in the back (μg/kg) based on the mean weight of monkeys (i.e., 10 μg/kg corresponds to 100 μg, given an approximate monkey weight of 10 kg). In addition, dose-dependent effects were analyzed with one-way analysis of variance followed by the Newman–Keuls test (P < 0.01).
2.5. Drugs
Dynorphin A-(1–17) and its related analogs (Department of Chemistry, University of Arizona, Tucson, AZ), U50,488 HCl (Upjohn, Kalamazoo, MI), quadazocine methanesulfonate (Sanofi, Malvern, PA), and nor-BNI (provided by Dr. H.I. Mosberg, Division of Medicinal Chemistry, College of Pharmacy, University of Michigan, Ann Arbor, MI) were dissolved in sterile water. For systemic administration, all compounds were administered s.c. in the back (i.e., around the scapular region) with 0.1 ml/kg volume. Capsaicin (Sigma, St. Louis, MO) was dissolved in a solution of Tween 80/ethanol/saline in a ratio of 1:1:8. For local antinociceptive assay, all compounds were mixed in the capsaicin solution and were injected s.c. in the terminal 1 to 4 cm of the tail with constant 0.1 ml volume. For diuretic assay, all compounds were injected intramuscularly in either lateral side of thighs with constant 0.5 ml volume.
3. Results
Monkeys used in this study displayed a consistent profile in tail-withdrawal responses, which were similar to what we have reported previously in different groups of monkeys (Ko et al., 1998, 1999a). Normally, they kept their tails in 42°C and 46°C water for 20 s (cutoff latency) and removed their tails from 50°C water rapidly (within 1–3 s). As noted, the thermal pain threshold in monkeys in this study is similar to other primate studies. For instance, it has been reported that monkeys frequently escaped the 51°C stimulus, but almost never from the 43°C and 47°C temperatures; human subjects have described 43°C as slightly warm, 47°C as distinctly warm but not painful, and 51°C as a clearly painful stimulus (Kupers et al., 1997). After capsaicin 100 μg was injected s.c. in the monkey’s tail, it evoked a nociceptive response, thermal allodynia, which was manifested as a reduced tail-withdrawal latency of approximately 2–3 s in 46°C water. This thermal allodynic response peaked at 5 to 15 min and gradually disappeared within 1 h after injection (Ko et al., 1998).
3.1. Antinociceptive effects of dynorphin-related analogs
Fig. 1 compares the antinociceptive effects of dynorphin A-(1–17) against capsaicin-induced thermal allodynia following s.c. administration in the tail and in the back. Co-administration of dynorphin A-(1–17) (0.3–10 μg) with capsaicin (100 μg) in the tail dose-dependently attenuated allodynia in 46°C water (Fig. 1, top). However, when the locally effective dose of dynorphin A-(1–17) 10 μg was administered s.c. in the back, it was not effective against capsaicin. The ED50 value of dynorphin A-(1–17)-induced local antinociception in this procedure was 3.3 μg (95% C.L.: 1.9–5.8 μg). In contrast, when dynorphin A-(1–17) (3–300 μg/kg) was administered s.c. in the back, it did not attenuate capsaicin-induced allodynia (Fig. 1, bottom). Given that the mean weight of monkeys was 9.7 kg during this study, 300 μg/kg of dynorphin A-(1–17) approximately corresponded to 3000 μg total dose for a monkey (see Fig. 1, the second abscissa of bottom panel). The antiallodynic potency of s.c. dynorphin A-(1–17) in the tail was at least 300- to 1000-fold higher than s.c. dynorphin A-(1–17) in the back. It is worth noting that s.c. dynorphin A-(1–17) in the tail and in the back at these doses did not cause any notable behavioral change, such as sedation, through the entire test session after injection.
Fig. 1.
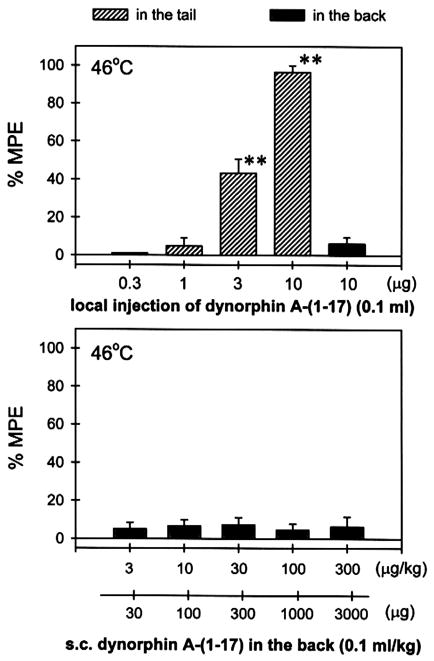
Antinociceptive effects of dynorphin A-(1–17) against capsaicin-induced thermal allodynia in 46°C water. Hashed bars indicate dynorphin A-(1–17) was co-administered with capsaicin (100 μg) in the tail and filled bars indicate dynorphin A-(1–17) was administered s.c. in the back. Each value represents the mean±S.E.M. (n = 3–6). Asterisks represent a significant difference from control (**P < 0.01). Abscissae: doses of dynorphin A-(1–17). Ordinates: percent of maximum possible effect (%MPE). Each data point was obtained at 15 min after injection. See Materials and methods for other details.
Fig. 2 illustrates tail-withdrawal responses of monkeys at 15 min following local injection in the tail. The injection procedure itself (i.e., vehicle injection) did not interfere with normal tail-withdrawal responses. Although 50°C water alone is a noxious stimulus, s.c. dynorphin A-(1–17) 10 μg in the tail did not produce antinociception against 50°C water in this procedure. This dose of dynorphin A-(1–17) alone did not cause any swelling in the tail. It should be noted that neither vehicle nor dynorphin A-(1–17) injected in the tail changed tail-withdrawal latencies through the entire test session (i.e., 5–60 min). On the other hand, both dynorphin A-(2–17) and dynorphin A-(2–13), when co-administered with capsaicin in the tail, did not attenuate capsaicin-induced allodynia in 46°C water, and this ineffectiveness was observed up to a dose of 300 μg (Fig. 3).
Fig. 2.
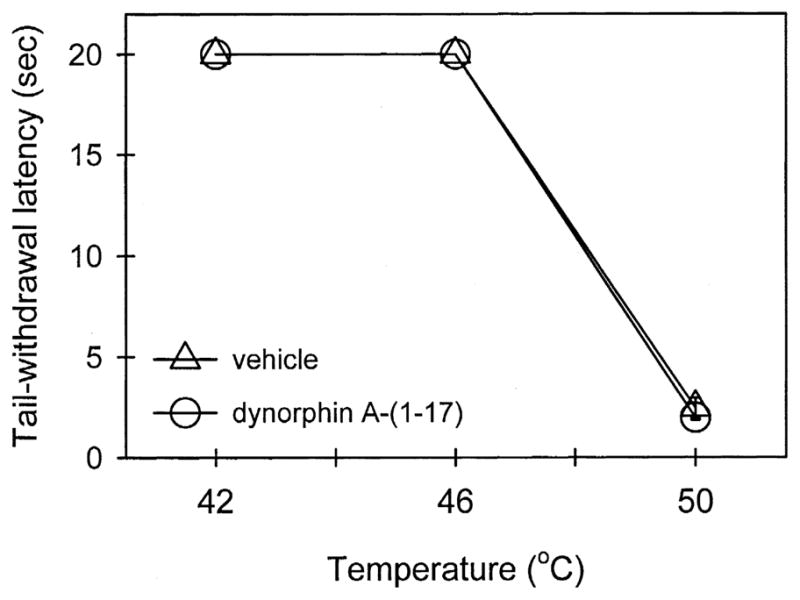
Thermal nociceptive threshold in the absence and presence of dynorphin A-(1–17). Open circles represent tail-withdrawal latencies following s.c. administration of dynorphin A-(1–17) 10 μg in the tail. See Materials and methods and Fig. 1 for other details.
Fig. 3.
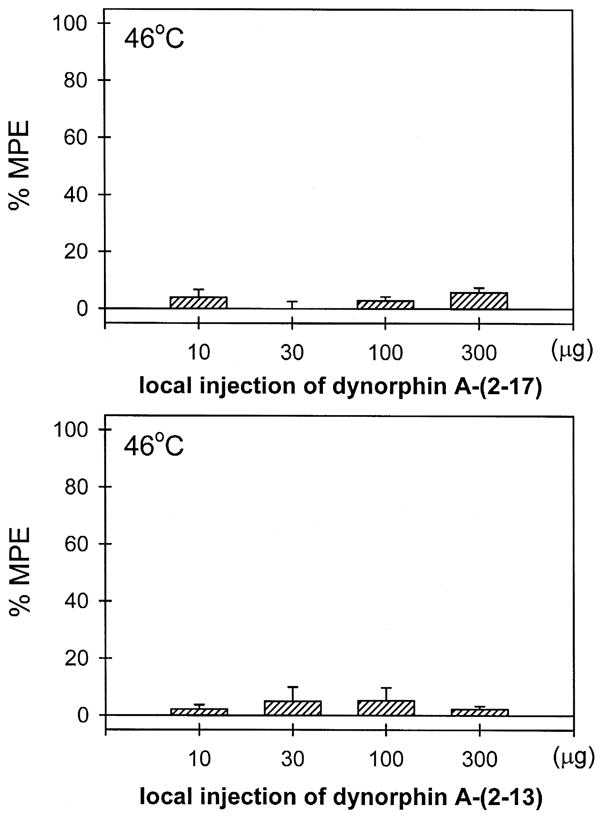
Lack of local antinociception of dynorphin A-(2–17) and dynorphin A-(2–13) against capsaicin-induced thermal allodynia in 46°C water. Both peptides were co-administered with capsaicin in the tail. See Materials and methods and Fig. 1 for other details.
3.2. Antagonism of dynorphin A-(1–17)-induced local antiallodynia
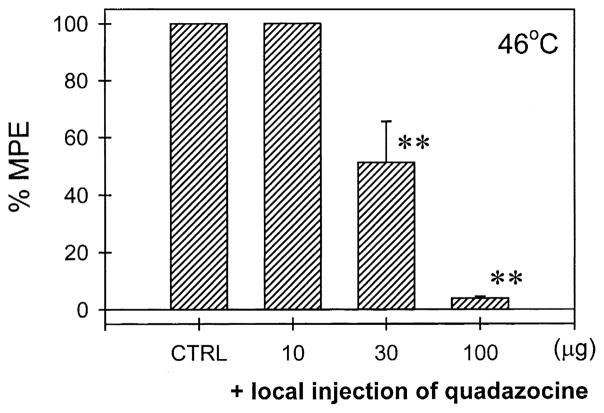
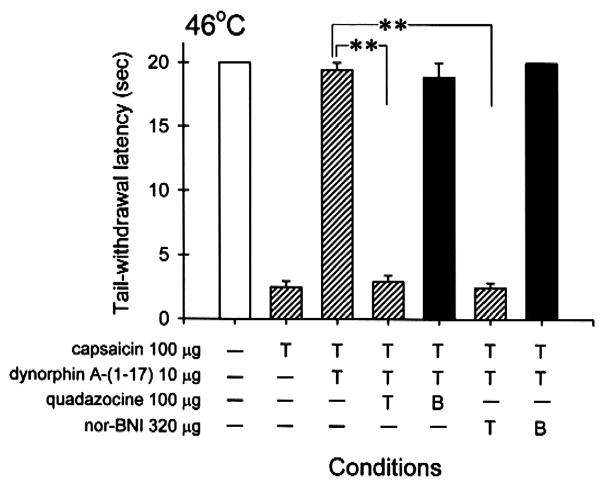
Fig. 4 illustrates the local quadazocine antagonism of dynorphin A-(1–17)-induced antiallodynia in the tail. Co-administration of quadazocine (10–100 μg) with capsaicin (100 μg) and dynorphin A-(1–17) (10 μg) in the tail dose-dependently antagonized the local inhibition of dynorphin A-(1–17) in capsaicin-induced allodynia in 46°C water. The ID50 value of quadazocine against dynorphin A-(1–17) in this procedure was 30.6 μg (95% C.L.: 13.7–68.2 μg). However, when the locally effective dose of quadazocine 100 μg was administered s.c. in the back, it did not antagonize local dynorphin A-(1–17) (Fig. 5). Furthermore, pretreatment with nor-BNI 320 μg in the tail also significantly antagonized dynorphin A-(1–17)-induced local antinociception against capsaicin (Fig. 5). In the same pretreatment procedure, when nor-BNI 320 μg was administered s.c. in the back, it did not antagonize local dynorphin A-(1–17). As noted, following s.c. injection of nor-BNI in the tail or in the back, there were no changes of tail-withdrawal latencies in both 46 and 50°C water (data not shown). Administration of nor-BNI also did not change monkeys’ thermal nociceptive threshold after 24 h and beyond.
Fig. 4.
Antagonism of quadazocine in local antiallodynic effects of dynorphin A-(1–17) in 46°C water. CTRL represents the effects of co-administration of capsaicin 100 μg and dynorphin A-(1–17) 10 μg in the tail. Quadazocine was co-administered with capsaicin and dynorphin A-(1–17) in the tail. See Materials and methods and Fig. 1 for other details.
Fig. 5.
Comparison of quadazocine and nor-BNI antagonism when they were administered s.c. in the tail and in the back against dynorphin A-(1–17)-induced local antiallodynia. The symbol “T” indicates the corresponding compound was administered s.c. in the tail. The symbol “B” indicates the corresponding antagonist was administered s.c. in the back. The symbol “−” represents the absence of conditions. See Materials and methods and Fig. 1 for other details.
3.3. Diuretic effects of U50,488 and dynorphin A-(1–17)
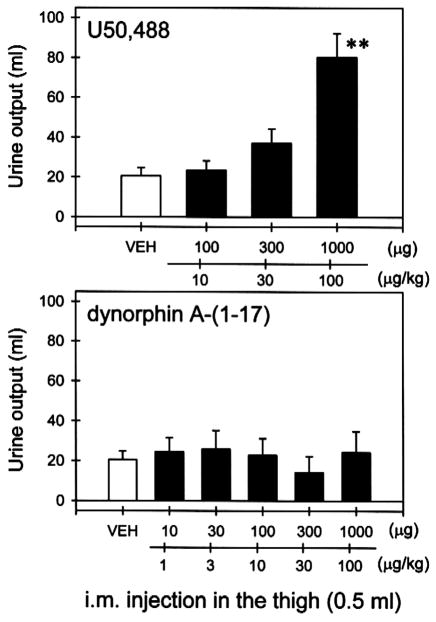
Fig. 6 compares the diuretic effects of U50,488 and dynorphin A-(1–17) following i.m. administration. The mean value of urine output during 3 h period is approximately 20.6 ±4.2 ml (S.E.M.). i.m. injection of U50,488 (100–1000 μg) in the thigh dose-dependently increased urine output (Fig. 6, top). In contrast, i.m. injection of dynorphin A-(1–17) (10–1000 μg) did not increase urine output in the same group of monkeys (Fig. 6, bottom).
Fig. 6.
Comparison of diuretic effects of U50,488 and dynorphin A-(1–17). Each value represents the mean ± S.E.M. (n = 5). VEH indicates the effects of i.m. injection of water in the thigh. Asterisks represents a significant difference from VEH (**P < 0.01). Abscissae: doses of compounds (μg). Ordinates: urine output (ml) over 3 h after injection. See Materials and methods for other details.
4. Discussion
This study demonstrates that local administration of dynorphin A-(1–17) was very potent in attenuating capsaicin-induced thermal allodynia in rhesus monkeys and the non-opioid fragments, dynorphin A-(2–17) and dynorphin A-(2–13), were not effective in the procedure. Dynorphin A-(1–17)-induced local antiallodynia was mediated by peripheral κ-opioid receptors. These observations provide the functional evidence of selective κ-opioid receptor-mediated actions of dynorphin A-(1–17) following local administration in non-human primates.
4.1. Antinociceptive effects of dynorphin-related analogs
This is the first study to demonstrate that local administration of dynorphin A-(1–17) dose-dependently inhibited capsaicin-induced thermal allodynia in monkeys (Fig. 1). The locally effective dose of dynorphin A-(1–17), when applied s.c. in the back, did not inhibit capsaicin-induced nociception. This indicates that the site of action of locally administered dynorphin A-(1–17) may be located in the tail. Dynorphin A-(1–17) was approximately 10-fold more potent than dynorphin A-(1–13) in the same procedure (i.e., ED50: 3 μg vs. 28 μg) (Ko et al., 1999a). Interestingly, this potency difference is related to in vitro studies, in which dynorphin A-(1–17) was approximately 30-fold more potent than dynorphin A-(1–13) in stimulating the [35S]GTPγS binding in a κ-opioid receptor cell line (i.e., ED50: 13 vs. 414 nM) (Remmers et al., 1999).
Although dynorphin A-(1–17) was characterized in vitro as a high efficacy κ-opioid receptor agonist, systemic dynorphin A-(1–17) did not display such a profile. Intravenous administration of dynorphin A-(1–17) only produced slight antinociception against a noxious stimulus of 50°C water, in which a non-peptide κ-opioid receptor agonist U69,593 produced full antinociception (Butelman et al., 1999). Similarly, s.c. dynorphin A-(1–17) in the back did not produce antinociception against capsaicin (Fig. 1). In particular, the antiallodynic potency of s.c. dynorphin A-(1–17) in the tail was at least 300- to 1000-fold higher than s.c. dynorphin A-(1–17) in the back. Such a large difference in potency was only observed with peptidic ligands, but not with non-peptidic compounds (Ko et al., 1998, 1999a). It could be explained in part by pharmacokinetic factors of peptides such as rapid degradation or poor distribution (Yu et al., 1996; Gambus et al., 1998).
Thus, locally administered dynorphin A-(1–17) (i.e., s.c. or i.m.) may minimize the centrally mediated effects. As noted, even s.c. dynorphin A-(1–17) in the back at a dose up to 300 μg/kg did not produce any overt behavioral change, such as sedation, which has been demonstrated as part of central actions of κ-opioid receptors (Ko et al., 1999b). Furthermore, i.m. dynorphin A-(1–17) in the thigh at a dose up to 1000 μg did not produce diuretic effects, but i.m. U50,488 1000 μg significantly increased urine output in the same monkeys (Fig. 6). This dose of U50,488 is 10-fold higher than its locally effective dose (100 μg) in producing antiallodynic effects (Ko et al., 1999a). In contrast, i.m. dynorphin A-(1–17) 1000 μg is 100-fold higher than its antiallodynic dose (10 μg). This suggests that s.c. or i.m. dynorphin A-(1–17) has a wider window between local and central/systemic actions. For some localized pain (e.g., dental pulp or knee joint), local administration of dynorphin A-(1–17) or other κ-opioid receptor peptides may provide pain relief without untoward side effects.
It should be noted that the antiallodynic dose of dynorphin A-(1–17) alone in the tail did not modify the thermal nociceptive threshold (Fig. 2). Dynorphin A-(1–17)-induced local antinociception was only observed under hyperalgesic- or allodynic-like states (Beyer et al., 1997; present study). This observation is similar to those from previous studies, which characterized local antinociceptive actions of μ- and κ-opioid receptor agonists in non-human primates (Ko et al., 1998, 1999a). Given that dynorphin A-(1–17) is present at the site of inflammation (Hassan et al., 1992), dynorphin A-(1–17) may modulate the activity of peripheral sensory fibers, which are dynamically regulated by a variety of mediators following tissue injury and inflammation. Nevertheless, the role of dynorphin A-(1–17) in the central nervous system is more complicated, as several studies have speculated that dynorphin-related peptides may contribute to the development or maintenance of neuropathic pain (e.g., Vanderah et al., 1996; Claude et al., 1999).
Capsaicin evoked pain sensations by activating C-fibers and stimulating the release of neuropeptides such as substance P and calcitonin gene-related peptide from primary nociceptive afferents (Winter et al., 1995; Caterina et al., 1997; Szallasi and Blumberg, 1999). Some studies have suggested that activation of peripheral κ-opioid receptors inhibits the excitability of nociceptive neurons and reduces the release of substance P from primary afferent fibers (Yonehara et al., 1992; Andreev et al., 1994). Considering that capsaicin-sensitive nerve fibers are involved in a variety of nociceptive conditions (Bartho et al., 1990; Kim et al., 1995; Winter et al., 1995), local administration of κ-opioid receptor agonists may be effective for reducing pain derived from different nociceptive origins (Junien 1995; Nagasaka et al., 1996; Wilson et al., 1996; Ko et al., 1999a). However, higher concentration of capsaicin-induced nociceptor-desensitization limits the opportunity of studying different pain intensities with this capsaicin pain model in non-human primates. It will be important to develop other types of pain models in monkeys to evaluate the antinociceptive efficacy of κ-opioid analgesics.
4.2. Antagonism of dynorphin A-(1–17)-induced local antiallodynia
Local administration of an opioid receptor antagonist, quadazocine, dose-dependently antagonized local antiallodynia of dynorphin A-(1–17) (Fig. 4). This local antagonist potency of quadazocine against dynorphin A-(1–17) is similar to its potency against a selective κ-opioid receptor agonist U50,488 in the same procedure (i.e., ID50 31 μg/tail vs. 28 μg/tail), but it is less potent than the quadazocine dose in antagonizing effects of μ-opioid receptor agonists (i.e., ID50 4 μg/tail) (Ko et al., 1998, 1999a). In particular, local administration of quadazocine 10 μg was effective in antagonizing local actions of fentanyl (Ko et al., 1998), but it was not effective in antagonizing local actions of dynorphin A-(1–17) in this study. Moreover, local pretreatment with a selective κ-opioid receptor antagonist, nor-BNI, also reversed local antiallodynic effects of dynorphin A-(1–17) (Fig. 5). Both locally effective doses of quadazocine and nor-BNI, when applied s.c. in the back, did not antagonize local effects of dynorphin A-(1–17) (Fig. 5). These antagonism studies confirm the site of action of locally administered dynorphin A-(1–17), indicating that peripheral κ-opioid receptors mediated local antinociception of dynorphin A-(1–17) against capsaicin-induced thermal allodynia in the tail.
Both dynorphin-related fragments dynorphin A-(2–17) and dynorphin A-(2–13) did not produce local antinociception against capsaicin (Fig. 3). This observation further supports the notion that κ-opioid receptors selectively mediated local antinociceptive effects of dynorphin A-(1–17) in this procedure. More importantly, it provides functional evidence that tyrosine at position one of dynorphin A-(1–17) is an essential requirement for binding to opioid receptors (Meng et al., 1993; Naqvi et al., 1998). Several rodent studies have indicated that central or systemic administration of dynorphin A-(2–17) or dynorphin A-(2–13) produced non-opioid antinociceptive effects (Przewlocki et al., 1983; Hooke et al., 1995; Beyer et al., 1997). The involvement of NMDA receptors has been suggested to explain some of the non-opioid effects of dynorphin A-(1–17) and related peptides (Shukla and Lemaire, 1994; Shukla et al., 1997). However, the dissociation of local antinociception between dynorphin A-(1–17) and dynorphin A-(2–17) in the present study suggests that this capsaicin-induced thermal allodynia is a selective behavioral endpoint to study the inhibitory effects of locally administered opioid receptor agonists in monkeys.
4.3. General summary
This study demonstrates that peripheral κ-opioid receptors mediated dynorphin A-(1–17)-induced local antinociception against capsaicin-induced thermal allodynia in monkeys. Dynorphin A-(1–17) has been characterized as a high efficacy κ-opioid receptor agonist in vitro (Zhu et al., 1997; Remmers et al., 1999). However, central or systemic administration of dynorphin A-(1–17) displayed both opioid and non-opioid effects in different species and systemic dynorphin A-(1–17) did not have strong antinociceptive effects in non-human primates (e.g., Faden and Jacobs, 1984; Shukla and Lemaire, 1994; Butelman et al., 1999). The present study indicates that local administration of dynorphin A-(1–17) provides an opportunity to study selective κ-opioid receptor-mediated antinociception of dynorphin A-(1–17). In addition, it suggests another approach for pain management by activating peripheral κ-opioid receptors (Junien 1995; Nagasaka et al., 1996; Wilson et al., 1996; Ko et al., 1999a). These results strengthen the notion that local application of peptidic ligands without untoward side effects may be a useful medication for localized pain.
Acknowledgments
The authors would like to thank Mark Johnson, Michael Song, and John Busenbark for excellent technical assistance. This study was supported by USPHS grants DA00254 (J.H.W.) and DA04248 (V.J.H.).
References
- Alt A, Mansour A, Akil H, Medzihradsky F, Traynor JR, Woods JH. Stimulation of guanosine-5′-O-(3-[35S]thio)triphosphate binding by endogenous opioids acting at a cloned mu receptor. J Pharmacol Exp Ther. 1998;286:282–288. [PubMed] [Google Scholar]
- Andreev N, Urban L, Dray A. Opioids suppress spontaneous activity of polymodal nociceptors in rat paw skin induced by ultraviolet irradiation. Neuroscience. 1994;58:793–798. doi: 10.1016/0306-4522(94)90456-1. [DOI] [PubMed] [Google Scholar]
- Bartho L, Stein C, Herz A. Involvement of capsaicin-sensitive neurones in hyperalgesia and enhanced opioid antinociception in inflammation. Naunyn-Schmiedeberg’s Arch Pharmacol. 1990;342:666–670. doi: 10.1007/BF00175710. [DOI] [PubMed] [Google Scholar]
- Beyer A, Schafer M, Stein C. Antinociceptive effects of dynorphin peptides in a model of inflammatory pain. Pain. 1997;70:141–147. doi: 10.1016/s0304-3959(97)03327-7. [DOI] [PubMed] [Google Scholar]
- Butelman ER, Ko MC, Sobczyk-kojiko K, Mosberg HI, Van Bemmel B, Zernig G, Woods JH. Kappa-opioid receptor binding populations in rhesus monkey brain: relationship to an assay of thermal antinociception. J Pharmacol Exp Ther. 1998;285:595–601. [PubMed] [Google Scholar]
- Butelman ER, Harris TJ, Perez A, Kreek MJ. Effects of systemically administered dynorphin A(1–17) in rhesus monkeys. J Pharmacol Exp Ther. 1999;290:678–686. [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Caudle RM, Isaac L. Influence of dynorphin (1–13) on spinal reflexes in the rat. J Pharmacol Exp Ther. 1988;246:508–513. [PubMed] [Google Scholar]
- Chavkin C, James IF, Goldstein A. Dynorphin is a specific endogenous ligand of the kappa opioid receptor. Science. 1982;215:413–415. doi: 10.1126/science.6120570. [DOI] [PubMed] [Google Scholar]
- Claude P, Gracia N, Wagner L, Hargreaves KM. Effect of dynorphin on iCGRP release from capsaicin-sensitive fibers. Conference Abstract of the 9th World Congress on Pain; IASP Press; 1999. p. 160. [Google Scholar]
- Dykstra LA, Gmerek DE, Winger G, Woods JH. Kappa opioids in rhesus monkeys: I. Diuresis, sedation, analgesia and discriminative stimulus effects. J Pharmacol Exp Ther. 1987;242:413–420. [PubMed] [Google Scholar]
- Faden AI, Jacobs TP. Dynorphin-related peptides cause motor dysfunction in the rat through a non-opiate action. Br J Pharmacol. 1984;81:271–276. doi: 10.1111/j.1476-5381.1984.tb10074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambus PL, Schnider TW, Minto CF, Youngs EJ, Billard V, Brose WG, Hochhaus G, Shafer SL. Pharmacokinetics of intravenous dynorphin A(1–13) in opioid-naive and opioid-treated human volunteers. Clin Pharmacol Ther. 1998;64:27–38. doi: 10.1016/S0009-9236(98)90019-4. [DOI] [PubMed] [Google Scholar]
- Goldstein A, Fischli W, Lowney LI, Hunkapiller M, Hood L. Porcine pituitary dynorphin: complete amino acid sequence of the biologically active heptadecapeptide. Proc Natl Acad Sci U S A. 1981;78:7219–7223. doi: 10.1073/pnas.78.11.7219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan AHS, Pzewlocki R, Herz A, Stein C. Dynorphin, a preferential ligand for k-opioid receptors, is present in nerve fibers and immune cells within inflamed tissue of the rat. Neurosci Lett. 1992;140:85–88. doi: 10.1016/0304-3940(92)90688-4. [DOI] [PubMed] [Google Scholar]
- Hooke LP, He L, Lee NM. [Des-Tyr1]dynorphin A-(2–17) has naloxone-insensitive antinociceptive effect in the writhing assay. J Pharmacol Exp Ther. 1995;273:802–807. [PubMed] [Google Scholar]
- Junien JL. Role of peripheral kappa receptors in the modulation of pain. In: Galmiche JP, Fraitag B, editors. Sensitive Gastrointestinal Disorders. John Libbery Eurotext; Paris: 1995. pp. 23–30. [Google Scholar]
- Kim YI, Na HS, Han JS, Hong SK. Critical role of the capsaicin-sensitive nerve fibers in the development of the causalgic symptoms produced by transecting some but not all of the nerves innervating the rat tail. J Neurosci. 1995;15:4133–4139. doi: 10.1523/JNEUROSCI.15-06-04133.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KW, Eun YA, Soh SM, Eun JS, Cho KP. Ligand binding profiles of U-69,593-sensitive and -insensitive sites in human cerebral cortex membranes: evidence of kappa opioid receptors heterogeneity. Life Sci. 1996;58:1671–1679. doi: 10.1016/0024-3205(96)00142-7. [DOI] [PubMed] [Google Scholar]
- Ko MC, Butelman ER, Woods JH. The role of peripheral mu opioid receptors in the modulation of capsaicin-induced thermal nociception in rhesus monkeys. J Pharmacol Exp Ther. 1998;286:150–156. [PMC free article] [PubMed] [Google Scholar]
- Ko MC, Butelman ER, Woods JH. Activation of peripheral kappa opioid receptors inhibits capsaicin-induced thermal nociception in rhesus monkeys. J Pharmacol Exp Ther. 1999a;289:378–385. [PMC free article] [PubMed] [Google Scholar]
- Ko MCH, Johnson MD, Butelman ER, Willmont KJ, Mosberg HI, Woods JH. Intracisternal nor-binaltorphimine distinguishes central and peripheral kappa-opioid antinociception in rhesus monkeys. J Pharmacol Exp Ther. 1999b;291:1113–1120. [PMC free article] [PubMed] [Google Scholar]
- Kupers RC, Chen CC, Bushnell MC. A model of transient hyperalgesia in the behaving monkey induced by topical application of capsaicin. Pain. 1997;72:269–275. doi: 10.1016/s0304-3959(97)00052-3. [DOI] [PubMed] [Google Scholar]
- Leander JD. A kappa opioid effect: increased urination in the rat. J Pharmacol Exp Ther. 1983;224:89–94. [PubMed] [Google Scholar]
- Long JB, Petras JM, Mobley WC, Holaday JW. Neurological dysfunction after intrathecal injection of dynorphin A (1–13) in the rat: II. Nonopioid mechanisms mediate loss of motor, sensory and autonomic function. J Pharmacol Exp Ther. 1988;246:1167–1174. [PubMed] [Google Scholar]
- Meng F, Xie GX, Thompson RC, Mansour A, Goldstein A, Watson SJ, Akil H. Cloning and pharmacological characterization of a rat kappa opioid receptor. Proc Natl Acad Sci U S A. 1993;90:9954–9958. doi: 10.1073/pnas.90.21.9954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasaka H, Awad H, Yaksh TL. Peripheral and spinal actions of opioids in the blockade of the autonomic response evoked by compression of the inflamed knee joint. Anesthesiology. 1996;85:808–816. doi: 10.1097/00000542-199610000-00016. [DOI] [PubMed] [Google Scholar]
- Naqvi T, Haq W, Mathur KB. Structure–activity relationship studies of dynorphin A and related peptides. Peptides. 1998;19:1277–1292. doi: 10.1016/s0196-9781(98)00042-4. [DOI] [PubMed] [Google Scholar]
- Przewlocki R, Shearman GT, Herz A. Mixed opioid/non-opioid effects of dynorphin and dynorphin related peptides after their intrathecal injection in rats. Neuropeptides. 1983;3:233–240. doi: 10.1016/0143-4179(83)90019-7. [DOI] [PubMed] [Google Scholar]
- Raynor K, Kong H, Chen Y, Yasuda K, Yu L, Bell GI, Reisine T. Pharmacological characterization of the cloned κ-, δ-, and μ-opioid receptors. Mol Pharmacol. 1994;45:330–334. [PubMed] [Google Scholar]
- Remmers AE, Clark MJ, Mansour A, Akil H, Woods JH, Medzihradsky F. Opioid efficacy in a C6 glioma cell line stably expressing the human kappa opioid receptor. J Pharmacol Exp Ther. 1999;288:827–833. [PubMed] [Google Scholar]
- Shukla VK, Lemaire S. Non-opioid effects of dynorphins: possible role of the NMDA receptor. Trends Pharmacol Sci. 1994;15:420–424. doi: 10.1016/0165-6147(94)90091-4. [DOI] [PubMed] [Google Scholar]
- Shukla VK, Prasad JA, Lemaire S. Nonopioid motor effects of dynorphin A and related peptides: structure dependence and role of the N-methyl-D-aspartate receptor. J Pharmacol Exp Ther. 1997;283:604–610. [PubMed] [Google Scholar]
- Stevens CW, Yaksh TL. Dynorphin A and related peptides administered intrathecally in the rat: a search for putative kappa opiate receptor activity. J Pharmacol Exp Ther. 1986;238:833–838. [PubMed] [Google Scholar]
- Szallasi A, Blumberg PM. Vanilloid (Capsaicin) receptors and mechanisms. Pharmacol Rev. 1999;51:159–212. [PubMed] [Google Scholar]
- Vanderah TW, Laughlin T, Lashbrook JM, Nichols ML, Wilcox GL, Ossipov MH, Malan TP, Jr, Porreca F. Single intrathecal injections of dynorphin A or des-Tyr-dynorphins produce long-lasting allodynia in rats: blockade by MK-801 but not naloxone. Pain. 1996;68:275–281. doi: 10.1016/s0304-3959(96)03225-3. [DOI] [PubMed] [Google Scholar]
- Wilson JL, Nayanar V, Walker JS. The site of anti-arthritic action of the k-opioid, U-50,488H, in adjuvant arthritis: importance of local administration. Br J Pharmacol. 1996;118:1754–1760. doi: 10.1111/j.1476-5381.1996.tb15601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter J, Bevan S, Campbell EA. Capsaicin and pain mechanisms. Br J Anaesth. 1995;75:157–168. doi: 10.1093/bja/75.2.157. [DOI] [PubMed] [Google Scholar]
- Yonehara N, Imai Y, Chen JQ, Takiuchi S, Inoki R. Influence of opioids on substance P release evoked by antidromic stimulation of primary afferent fibers in the hind instep of rats. Reg Pept. 1992;38:13–22. doi: 10.1016/0167-0115(92)90068-6. [DOI] [PubMed] [Google Scholar]
- Yu J, Butelman ER, Woods JH, Chait BT, Kreek MJ. In vitro biotransformation of dynorphin A (1–17) is similar in human and rhesus monkey blood as studied by matrix-assisted laser desorption/ionization mass spectrometry. J Pharmacol Exp Ther. 1996;279:507–514. [PubMed] [Google Scholar]
- Zhang S, Tong Y, Tian M, Dehaven RN, Cortesburgos L, Mansson E, Simonin F, Kieffer B, Yu L. Dynorphin A as a potential endogenous ligand for four members of the opioid receptor gene family. J Pharmacol Exp Ther. 1998;286:136–141. [PubMed] [Google Scholar]
- Zhu J, Luo LY, Li JG, Chen C, Liu-Chen LY. Activation of the cloned human kappa opioid receptor by agonists enhances [35S]GTPγS binding to membranes: determination of potencies and efficacies of ligands. J Pharmacol Exp Ther. 1997;282:676–684. [PubMed] [Google Scholar]