Abstract
IL-1 receptor antagonist (IL-1Ra), a natural inhibitor of IL-1β, has been shown to regulate the progression of a variety of inflammatory diseases. Although experimental studies and clinical trials have demonstrated the importance of IL-1Ra in chronic inflammatory diseases, the cellular mechanisms responsible for regulating the endogenous production of IL-1Ra by innate immune cells are currently unresolved. In the present study, we identify that glycogen-synthase kinase 3 (GSK3) regulates the production of the anti-inflammatory cytokine IL-1Ra via its ability to regulate the MAPK ERK1/2 in TLR-stimulated cells. Elucidation of the cell-signaling pathway by which GSK3 controlled ERK activity demonstrated that GSK3 inhibition resulted in an abrogation in the levels of the inhibitory residue serine 71 on Rac1 and increased the ability of Rac1 to interact with and activate p21-activated protein kinase. siRNA-mediated knockdown of Rac1 attenuated the ability of GSK3 inhibition to augment phopsho-ERK1/2 levels in LPS-stimulated immune cells. Moreover, inhibiting the ability of GSK3 to augment ERK1/2 activity abrogated enhanced IL-1Ra production by GSK3-inhibited cells. Our findings identify that GSK3 negatively regulates the levels of IL-1Ra produced by LPS-stimulated innate immune cells.
TLRs are a family of type I transmembrane receptors that have been shown to play a fundamental role in the recognition and regulation of the host inflammatory response against microbial products (1, 2). TLRs are primarily expressed on APCs and exhibit a high degree of specificity in their ability to discriminate among a wide array of conserved molecular patterns associated with numerous pathogens (3). Microbial ligand interactions with the TLR complex lead to the induction of several signal transduction pathways that can dictate both the nature and magnitude of the host inflammatory response (4). In this regard, the balance of inflammatory (pro- vs anti-inflammatory) cytokines is a critical parameter in determining not only whether excessive host inflammation will occur, but also the degree of subsequent host cell damage and associated toxicity (5–7). Indeed, it is well documented that the relative levels of the proinflammatory cytokine IL-1β and the endogenous inhibitor of IL-1β, IL-1 receptor antagonist (IL-1Ra),4 correlate with disease severity in many different chronic inflammatory diseases (5). Animal models assessing the importance of IL-1Ra have shown that the genetic deletion of IL-1Ra results in the onset of spontaneous polyarthritis (8, 9). Conversely, studies have shown that the genetic transfer of IL-1Ra within inflamed joints of animals reduces or prevents the onset of bone erosion, cartilage erosion, joint inflammation, and cellular infiltration associated with the disease process (10, 11). Additional studies assessing the role of exogenously administered IL-1Ra have demonstrated that IL-1Ra exhibits potent anti-inflammatory properties in vivo and can mediate protective effects against inflammatory diseases (12–14). Due to the large body of experimental evidence supporting the role of IL-1Ra in rheumatoid arthritis and other chronic inflammatory diseases, as well as its potential therapeutic usage, subsequent studies have been performed in human subjects. Indeed, controlled clinical trials using recombinant human IL-1Ra have reported reduced clinical signs and symptoms associated with rheumatoid arthritis in human subjects (15–18).
Although these findings clearly document the importance of IL-1Ra, little is known regarding the cellular mechanisms regulating the endogenous production of IL-1Ra. In this regard, past studies have identified that the PI3K pathway is intricately involved in the production of IL-1β (19, 20) and IL-1Ra (19, 21, 22) by innate immune cells. Activation of PI3K has been shown to negatively regulate the production of the proinflammatory cytokine IL-1β by innate immune cells in response to TLR-stimulation (23). Studies by Molnarfi et al. (19) have additionally shown that inhibition of PI3K activity exhibits a differential effect on the levels of IL-1β and IL-1Ra in which the levels of IL-1β and IL-1Ra are increased and decreased, respectively, in PI3K-inhibited cells. These studies highlighted the importance of the PI3K pathway in influencing the production of IL-1Ra. However, it remains to be elucidated how the PI3K pathway positively regulates IL-1Ra production by innate immune cells. Thus, the aim of the current study was to characterize the downstream cellular mechanism responsible for the ability of the PI3K pathway to control IL-1Ra production by innate immune cells.
Materials and Methods
Reagents
Ultrapure Escherichia coli 0111:B4 LPS was purchased from Invivogen. RPMI 1640 medium was obtained from the American Type Culture Collection (ATCC) and supplemented with 2 mM l-glutamine and 100 IU/ml penicillin–100 µg/ml streptomycin solution (Mediatech) with 10% FBS (HyClone). The glycogen-synthase kinase 3 (GSK3) inhibitors lithium chloride (LiCl) (24) and SB216763 (25) were purchased from EMD Chemical and Tocris Bioscience, respectively. Experimental controls included an osmolality control for LiCl (NaCl at 10 mM) or DMSO (0.1%) as the organic solvent control for the GSK3 inhibitor SB216763 and the MEK1/2 inhibitor U0126. The MEK1/2 inhibitor U0126 was obtained from LC Laboratories. Rabbit phospho-p21-activated protein kinase (PAK) 1 (Ser199/204)/PAK2 (Ser192/197), phospho-c-Raf (Ser338), phospho-p44/42 MAPK (Thr202/Tyr204) and phospho-p44/42 MAPK (Thr202/Tyr204) (E10) mouse mAb (Alexa Fluor 488 conjugate) Abs were purchased from Cell Signaling Technology. The anti-IL-1Ra FITC Ab used for flow cytometry was purchased from eBioscience. The phospho-MEK1/2 (pSpS218/222)/(pSpS222/226) Ab was obtained from Invitrogen. Phospho-Rac1 (Ser71) and anti-Rac1 Abs were from Santa Cruz Biotechnology and Millipore, respectively. The active Rac/cdc42 pull-down assay reagent (PAK1 PBD, agarose) was obtained from Millipore. At the indicated time point, whole-cell lysates obtained from 5 × 106 monocytes were assayed for the levels of phospho-GSK3-β (serine 9) using the DuoSet IC human phospho-GSK3-β (serine 9) kit from R&D Systems. The Raf1 kinase inhibitor I (26) was purchased from EMD Chemicals. Lipofectamine RNAiMAX reagent was obtained from Invitrogen. The GSK3β and Rac1 SMARTpool siRNA and control siRNA reagents were purchased from Dharmacon. IL-1Ra and IL-1β cytokine levels were determined in cell-free supernatants by ELISA following protocols recommended by the manufacturer (R&D Systems).
Cell culture
PBMC were obtained from healthy donors as per protocols approved by the University of Louisville, Institutional Review Board, Human Subjects Protection Program, study number 503.05. Monocytes were isolated by negative selection using the human monocyte isolation kit II from Miltenyi Biotec. The purity of monocytes was > 90% as determined by flow cytometry using FITC-labeled anti-CD14. To assess the role of MEK1/2 and GSK3 in IL-1Ra and IL-1β production, 5 × 105 monocytes were incubated with the MEK1/2 inhibitor U0126 (50 µM) and/or one of the GSK inhibitors SB216763 (10 µM) or LiCl (10 mM) for 2 h in a 96-well plate before stimulation with LPS (1 µg/ml). Experimental controls included an osmolality control for LiCl (NaCl at 10 mM) or DMSO (0.1%) as the organic solvent control for the GSK3 inhibitor SB216763 and the MEK1/2 inhibitor U0126. Cell-free supernatants were assayed for IL-1Ra and IL-1β levels by ELISA 20 h post stimulation.
Cell transfections
In brief, 2.5 × 106 monocytes were transfected with 6.5 nM GSK3β, Rac1, or control siRNA using the lipofectamine reagent as per the protocol established by Invitrogen. Seventy-two hours post transfection, cells were seeded in either a 96- or 6-well plate, and used for the indicated studies. siRNA-mediated knockdown in Rac1 levels were determined by Western blot. The cellular knockdown in GSK3-β levels after siRNA transfection using control or GSK3-β specific siRNA was assessed by flow cytometry.
Western blot analysis
Human monocytes (1.5–3.0 × 106 cells) were seeded in 6-well plates and pretreated with medium, LiCl (10 mM), SB216763 (10 µM), or U0126 (50 µM) for 2 h before the addition of LPS. At the indicated time points, cells were washed with ice-cold PBS and whole-cell lysates were prepared as previously described (27). As determined by the microBCA method (28) (Pierce), equal concentrations of total cellular protein were suspended in lithium dodecyl sulfate buffer, heated for 10 min at 70°C, resolved by lithium dodecyl sulfate-PAGE, and then transferred to polyvinylidene difluoride membranes using the Novex system (Invitrogen). Probing and visualization of immunoreactive bands were performed using the ECL plus kit (Amersham Pharmacia) following the manufacturer’s protocol. Blots were scanned for phosphorylated and total proteins using the Kodak Image Station 4000MM system (Eastman Kodak). The molecular imaging software version 4.0.5f2 (Eastman Kodak) was used to determine the mean intensity ratio of phosphorylated proteins to total levels of a loading control.
Flow cytometric analysis
Purified monocytes were plated at 2.5 × 105 cells/well in a 96-well flat-bottom plate. Cells were pretreated with the indicated pharmacological inhibitor or siRNA. For the detection of intracellular phospho-ERK levels, cells were harvested at the given time point, and transferred to 5 ml polystyrene round-bottom tubes. Cells were washed twice with 2 ml of FACS buffer (PBS containing 2% FBS and 0.01% sodium azide) and then fixed by adding 500 µl of formaldehye to a final concentration of 4% in PBS for 10 min at room temperature. Cells were washed once in PBS and resuspended in 500 µl of 90% methanol and incubated on ice for 10 min. Cells were washed in PBS containing 2% FBS, and then resuspended in PBS containing 2% FBS and an anti-phospho-ERK Ab (Alexa Fluor 488 conjugate). Cells were incubated at room temperature for 30 min. Cells were then washed twice in PBS containing 2% FBS and analyzed immediately by flow cytometry. For the detection of intracellular IL-1Ra levels, the levels of IL-1Ra were assessed by adding monensin during the last 8 h of a 20 h stimulation. Cells were fixed in 4% paraformaldehdye, permeabilized using eBioscience Perm buffer, and incubated with anti-human IL-1Ra-FITC Ab for 30 min. Samples were washed twice with Perm buffer and analyzed immediately by flow cytometry.
Statistical analysis
Statistical significance between groups was evaluated by the ANOVA and the Tukey multiple comparison test using the InStat program (GraphPad). Differences between groups were considered significant at the level of p values < 0.05.
Results
GSK3 inhibition augments IL-1Ra production by LPS-stimulated monocytes
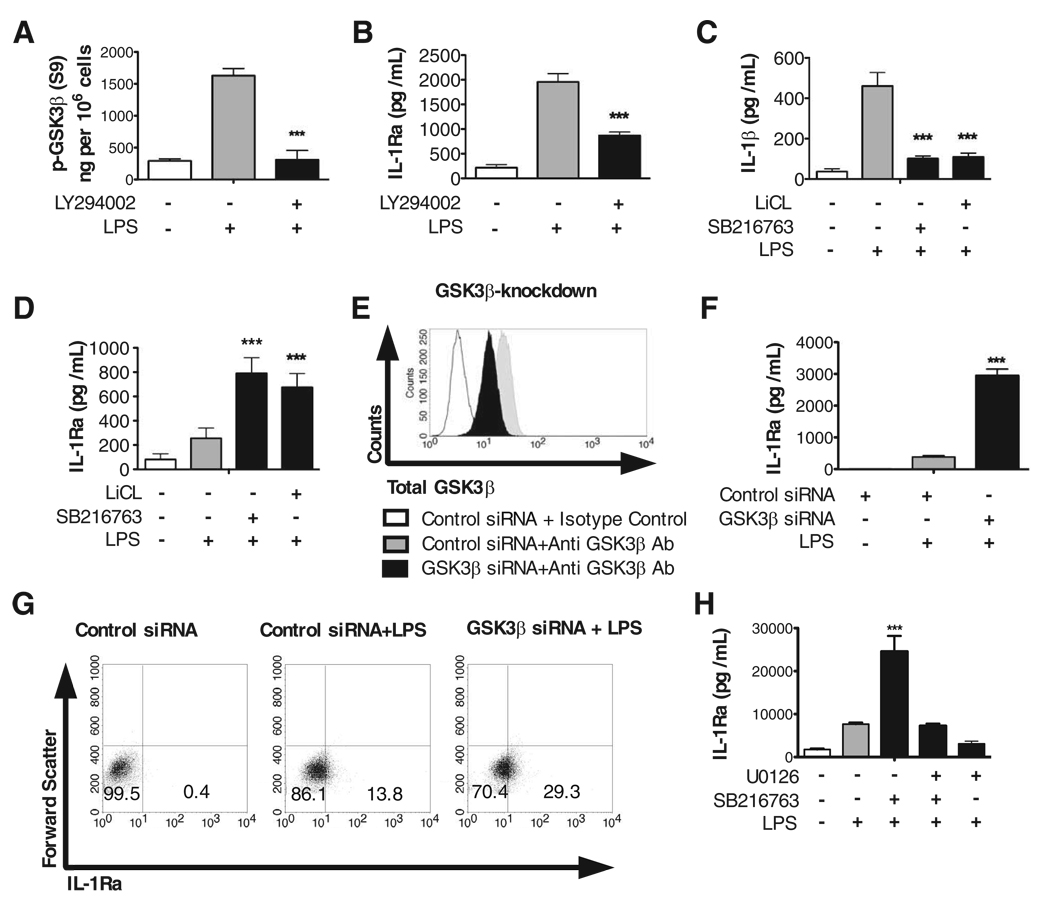
Molnarfi et al. (19) recently demonstrated that the PI3K pathway differentially regulates IL-1Ra and IL-1β production by TLR-stimulated immune cells. Taken in conjunction with previous studies demonstrating that the constitutively active serine/threonine kinase, GSK3, is downstream of PI3K and that inhibition of GSK3 results in suppressed IL-1β levels (27), we next investigated whether GSK3 was the downstream kinase within the PI3K pathway responsible for the ability of PI3K to regulate IL-1Ra production. To initially test this possibility, human monocytes were stimulated with LPS for 30 min and the levels of phospho-GSK3-β (Serine 9) were determined by ELISA (Fig. 1A). Monocytes stimulated with LPS exhibited over a 3-fold increase in phospho-GSK3-β (Serine 9) levels, as compared with nonstimulated monocytes (Fig. 1A). Moreover, inhibition of PI3K using LY294002 resulted in the reduction of phospho-GSK3-β (Serine 9) levels to near those observed in nonstimulated control monocytes (Fig. 1A), as well as a concurrent reduction (***, p < 0.001) in the levels of IL-1Ra produced by LPS-stimulated monocytes (Fig. 1B). To directly determine whether GSK3 was the downstream kinase within the PI3K pathway that differentially regulated the levels of IL-1Ra and IL-1β, we next inhibited GSK3 with the aid of the pharmacological inhibitors, LiCl or SB216763, as well as siRNA to knockdown cellular levels of GSK3-β (Fig. 1, C–G). Inhibition of GSK3 in LPS-stimulated monocytes using either lithium chloride or SB216763 significantly (***, P < 0.001) reduced the levels of IL-1β, whereas the levels of IL-1Ra were significantly (***, p < 0.001) augmented, as compared with monocytes stimulated with LPS alone (Fig. 1, C and D). Furthermore, as compared with siRNA control cells stimulated with LPS, siRNA-mediated knockdown in the cellular levels of GSK3-β (Fig. 1E) significantly (***, p < 0.001) increased the production of IL-1Ra by LPS-stimulated monocytes (Fig. 1, F and G). Taken together, these data demonstrate that GSK3-β inactivation enhances IL-1Ra levels by LPS-stimulated monocytes while concurrently suppressing the levels of IL-1β.
FIGURE 1.
Inactivation of GSK3 augments IL-1Ra levels, and this effect is attenuated upon ERK inhibition. A, Phospho-GSK3-β levels were determined from whole-cell lysates obtained from monocytes (5 × 106 cells) that were pretreated for 2 h with the PI3K inhibitor LY294002 (25 µM) and then stimulated for 30 min with or without LPS (1 µg/ml). B, Monocytes were pretreated with the PI3K inhibitor LY294002 (25 µM) for 2 h and then stimulated with LPS. C and D, Human monocytes were pretreated for 2 h with the GSK3 inhibitor SB216763 (10 µM) or LiCl (10 mM) and then stimulated with LPS. E, siRNA-mediated knockdown in GSK3β levels was determined by flow cytometry 96-h post transfection. F and G, Human monocytes were transfected with GSK3β-specific or control siRNA for 72 h followed by the addition of LPS (1 µg/ml). H, Human monocytes were pretreated for 2 h with the GSK3 inhibitor SB216763 (10 µM) and/or the MEK1/2 inhibitor U0126 (50 µM) followed by stimulation with LPS (1 µg/ml). Unstimulated cells were treated with NaCl (10 mM) or DMSO (0.1%) as the osmolality or organic solvent controls for LiCl or SB216763, respectively. Cell-free supernatants were collected 20-h post LPS-stimulation and assayed for IL-1Ra or IL-1β levels by ELISA. ***, Statistically significant differences at p < 0.001, as compared with LPS-stimulated cells. Data represent the mean ± SD of three separate experiments.
Ability of GSK3 to regulate IL-1Ra levels by LPS-stimulated cells is dependent upon ERK1/2
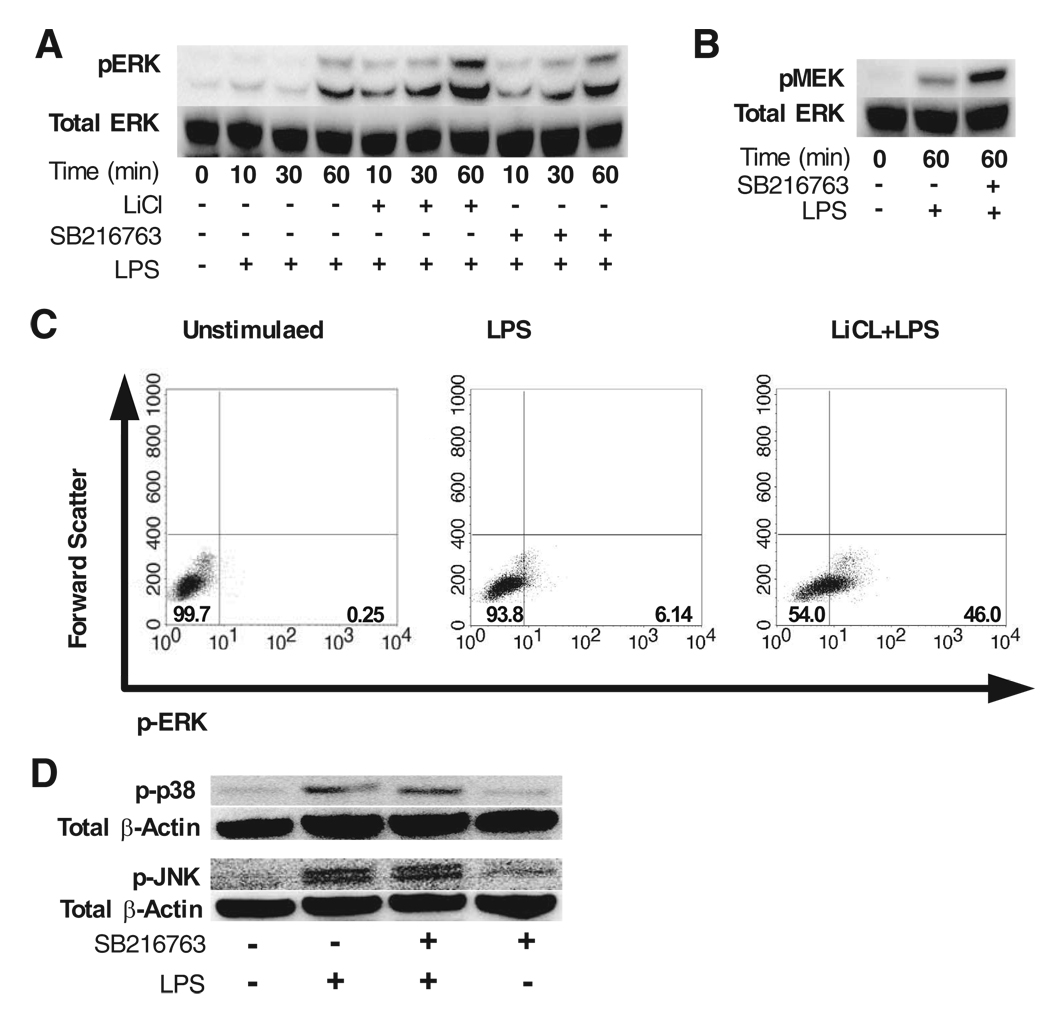
Previous studies have shown that the PI3K pathway can regulate the MAPK ERK1/2 (29) and that ERK1/2 is involved in controlling IL-1Ra production by innate immune cells (30). Because GSK3 can be inactivated by PI3K (Ref. 31, 32 and Fig. 1A), we next wanted to determine whether the ability of GSK3-inhibition to augment IL-1Ra levels was dependent upon ERK1/2, and if so, did GSK3 inhibition influence the levels of phospho-ERK1/2 in LPS-stimulated cells. For these studies, human monocytes were pretreated with the indicated GSK3 inhibitor in the presence or absence of the ERK1/2 (MEK1/2) inhibitor U0126, and stimulated with LPS. In this regard, the ability of GSK3 to augment IL-1Ra production by LPS-stimulated monocytes was abrogated by the use of the ERK1/2 inhibitor, U0126 (Fig. 1H). Because these data demonstrated that the ability of GSK3 to augment IL-1Ra levels by LPS-stimulated cells was abrogated by inhibiting the direct up-stream kinase that can phosphorylate ERK1/2, i.e., MEK1/2, we next assessed whether inhibiting GSK3 increased phospho-MEK1/2 and phospho-ERK1/2 levels in human human monocytes. As observed in Fig. 2, A and B, GSK3-inhibition using either the GSK3 inhibitor SB216763 (25) or LiCl (24) enhanced both phospho-ERK1/2 and phospho-MEK1/2 (S218/221) levels, as compared with monocytes stimulated with LPS alone. In contrast, blocking the kinase activity of MEK1/2 abrogated the augmented phospho-ERK1/2 levels observed in GSK3-inactivated cells (data not shown).
FIGURE 2.
Inactivation of GSK3 augments phospho-ERK levels in LPS-stimulated monocytes. Human monocytes were pretreated with the GSK3 inhibitor SB216763 (10 µM) or LiCl (10 mM) for 2 h before stimulation with LPS (1 µg/ml). Unstimulated cells were treated with NaCl (10 mM) or DMSO (0.1%) as the osmolality or organic solvent controls for LiCl or SB216763, respectively. Immunoblots (30 µg total protein) were probed for phospho-ERK (A) or phospho-MEK (B) levels and subsequently reprobed for total ERK to ensure equal protein loading. C, The percent of monocytes expressing phospho-ERK in the presence or absence of LiCl (10 mM) or osmolality control NaCl (10 mM) for unstimulated or LPS-stimulated groups (60 min). D, Immunoblots showing the levels of phospho-p38 or phospho-JNK in monocytes stimulated with LPS (1 µg/ml) in the presence or absence of the GSK3 inhibitor SB216763 (10 µM). Data are representative of three separate experiments.
We next investigated how GSK3 regulates phospho-ERK levels in LPS-stimulated monocytes at the single cell level by flow cytometry. As shown in Fig. 2C, LPS stimulation increased the percent of monocytes expressing phospho-ERK to ~6%, as compared with nonstimulated controls (0.25%). Moreover, GSK3-inhibition increased the frequency of phospho-ERK positive cells to 46%, as compared with monocytes stimulated with LPS alone (6%) (Fig. 2C). Taken together, these data demonstrate that GSK3 negatively regulates the levels of phospho-ERK and that the ability of GSK3 to modulate ERK levels is critical for its ability to regulate IL-1Ra levels by LPS-stimulated monocytes.
In contrast to the observed increases in phospho-ERK levels, inhibition of GSK3 in LPS-simulated monocytes did not discernibly affect the levels of phospho-p38 or phospho-JNK1/2, as compared with cells stimulated with LPS alone (Fig. 2D). Taken together, these findings show that the ability of GSK3 to increase IL-1Ra levels is mediated via its ability to augment phospho-ERK1/2 levels.
GSK3 inactivation suppresses phospho-Rac1 (S71) levels and augments the ability of Rac1 to interact with the PAK binding domain of PAK
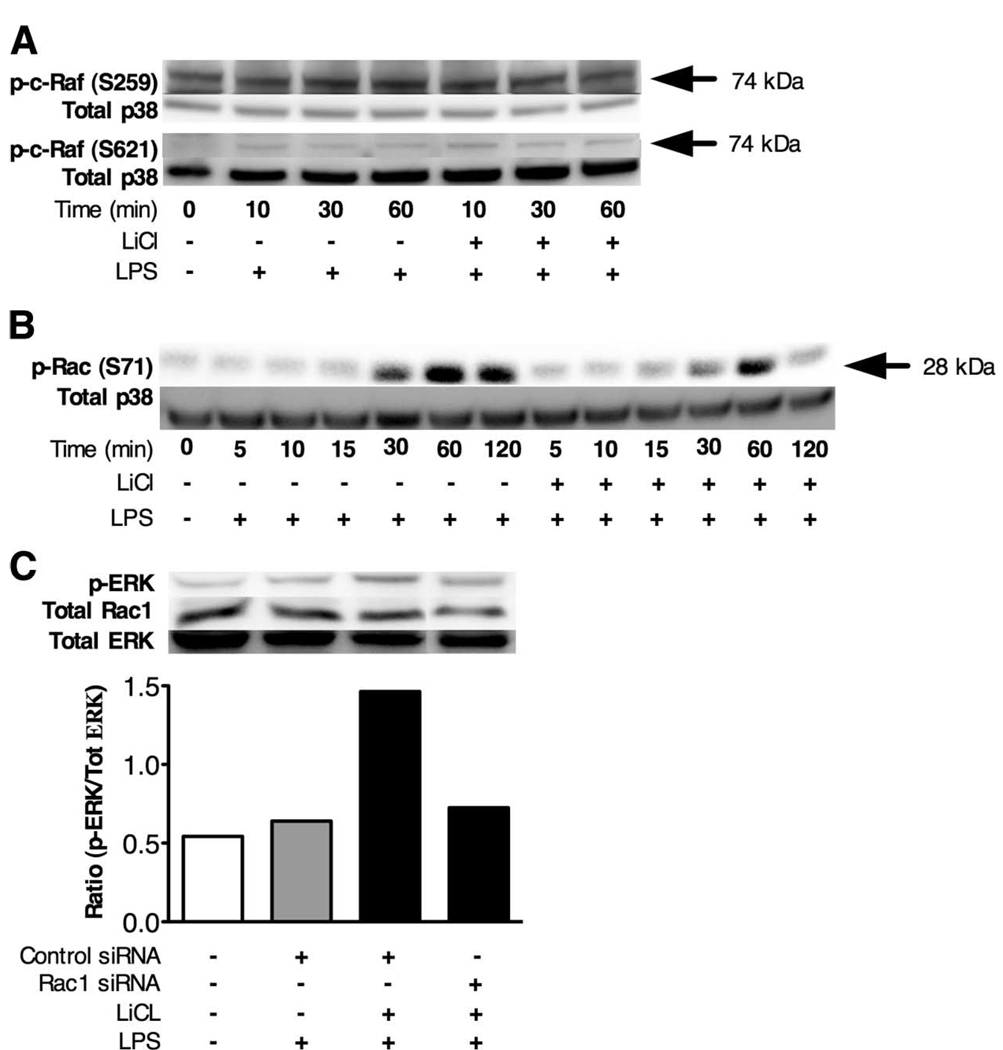
We next characterized the cellular mechanism by which GSK3 inhibition regulated ERK1/2 activity in LPS-stimulated cells. Because GSK3 is a constitutively active serine/threonine kinase, it could be predicted that GSK3 phosphorylates an intermediate kinase within the ERK1/2 pathway that results in the negative regulation of its activity. Using the web-based software Scansite, (www.scansite.mit.edu), it was identified that GSK3 could potentially phosphorylate several kinases that have been reported to negatively regulate ERK1/2 activity, including c-Raf (S259) (33), c-Raf (S621) (34), and Rac1 (S71) (35). As shown in Fig. 3A, GSK3 inhibition did not discernibly affect the phosphorylated levels of c-Raf (S259) or c-Raf (S621) in LPS-stimulated monocytes, as compared with monocytes stimulated with LPS alone. In contrast, GSK3 inhibition reduced the levels of phosphorylated Rac1 (S71) in LPS-stimulated monocytes, as compared with cells stimulated with LPS alone (Fig. 3B). To next examine the functional role of Rac1 in GSK3-mediated ERK activation, we used the use of siRNA targeting Rac1. As shown in Fig. 3C, inhibition of GSK3 increased phospho-ERK1/2 levels in LPS-stimulated monocytes, as compared with monocytes transfected with control siRNA and stimulated with LPS. In contrast, the ability of GSK3 to augment phospho-ERK1/2 levels in LPS-stimulated monocytes was markedly reduced in cells exhibiting a knockdown in Rac1 levels (Fig. 3C). Taken together, these data demonstrate an essential role for Rac1 in the ability of GSK3 to mediate ERK phosphorylation.
FIGURE 3.
Ability of GSK3 to regulate phospho-ERK levels in monocytes is dependent upon Rac1. A, Immunoblots showing GSK3-inhibition (LiCl at 10 mM) does not affect the levels of phospho- c-Raf (S259 or S621) in LPS-stimulated (1 µg/ml) cells. B, Immunoblot demonstrating LiCl (10 mM) mediated GSK3-inhibition suppressed the levels of phospho-Rac1 (S71) in LPS-stimulated (1 µg/ml) monocytes. C, siRNA-mediated knockdown of Rac1 inhibits the ability of GSK3 inhibition (LiCl at 10 mM) to increase phospho-ERK levels in LPS-stimulated (1 µg/ml) monocytes. siRNA-mediated knockdown of Rac1 levels was determined by Western blot 72 h post transfection. A–C, Immunoblots were reprobed for total p38 or total ERK to ensure equivalent protein loading. Data are representative of three experiments.
The PAK is a serine/threonine-associated kinase that is a target of Rac-mediated phosphorylation (36). Moreover, the ability of PAK to interact with Rac1 has been shown to be negatively regulated by the phosphorylation of Rac1 (S71) (37). Thus, we next determined if reductions in phospho-Rac1 (S71) levels observed in GSK3-inactivated cells augmented Rac1-PAK interactions. For this, monocytes were stimulated with LPS in the presence or absence of GSK3 inhibition, and the association of PAK with Rac1 was monitored by coimmunoprecipitation (Fig. 4A). As demonstrated in Fig. 4A, GSK3-inhibition enhanced the levels of Rac1 binding to the PAK binding domain of PAK1, as compared with monocytes stimulated with LPS alone (Fig. 4A). To further examine how GSK3 inhibition affects PAK1 activity, cells were stimulated with LPS in the presence or absence of the GSK inhibitor and probed by Western blot for the activation of PAK by monitoring phospho-PAK1 (Ser199/204) levels. GSK3-inhibition increased phospho-PAK1 (S199/204) levels, as compared with monocytes stimulated with LPS alone (Fig. 4B). These findings demonstrate that GSK3 inactivation increases the association of Rac1 to PAK1 and augment the levels of phospho-PAK1 (S199/204).
FIGURE 4.
GSK3 inactivation increases the association of active Rac1 to the p21-binding domain (PBD) of PAK. A, Active Rac1 in cellular lysates was pulled down by agarose-coupled PAK1-PBD. Equal protein concentrations (20 µg of total cellular protein) were resolved by SDS-PAGE and probed for active Rac1 levels by Western blot. B, Monocytes were incubated in the presence or absence of the GSK3 inhibitor LiCl (10 mM) or SB216763 (10 µM) for 2 h followed by stimulation with LPS (1 µg/ml). Whole-cell lysates were obtained from monocytes at the indicated time points and probed for phospho-PAK (S199/204) levels by Western blot. Immunoblots were reprobed for total β-actin to ensure equivalent loading. Data are representative of three experiments.
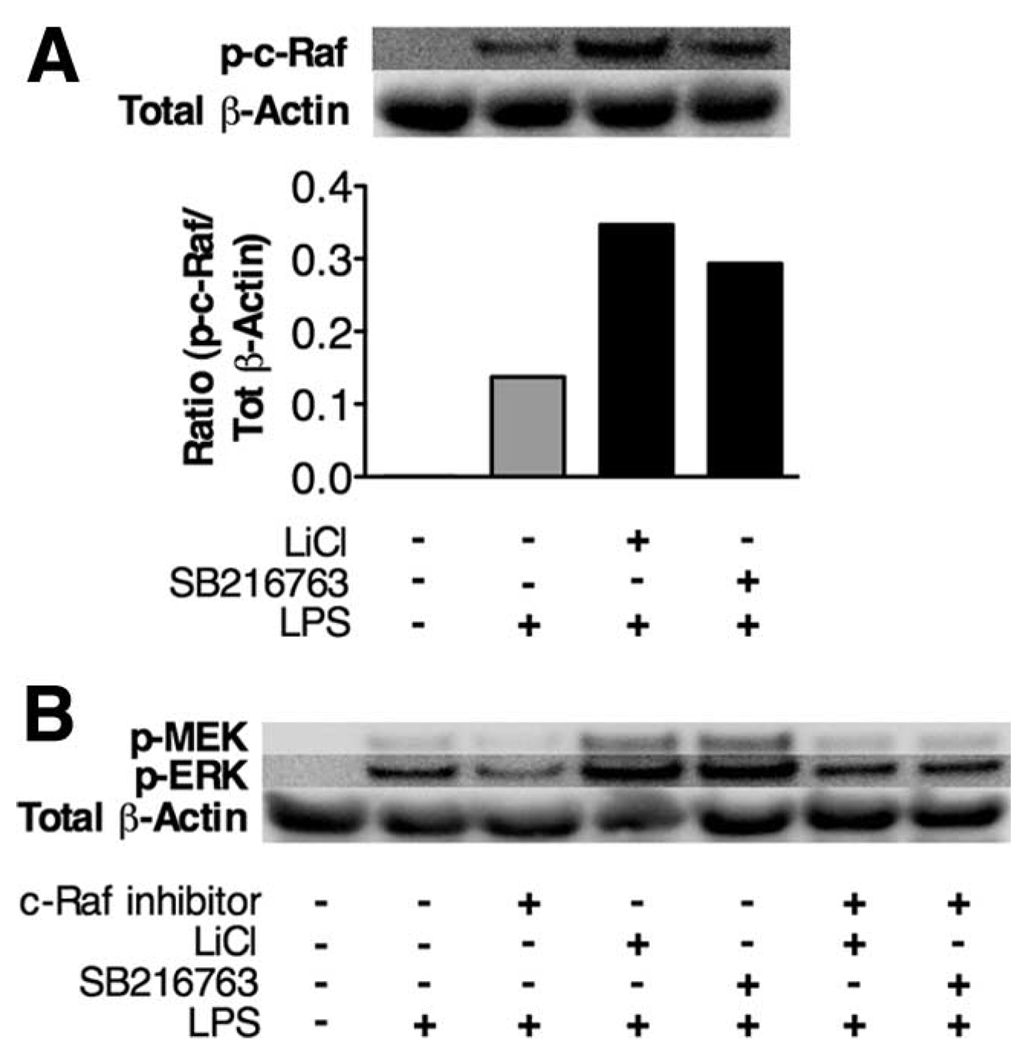
PAK has previously been shown to phosphorylate several downstream kinases, including c-Raf (S338) (38), a known MEK activator. Based on our data demonstrating that GSK3 regulates PAK activity, we next examined whether GSK3 inhibition affected the activation of the downstream target of PAK, c-Raf, by monitoring phosphorylated levels of c-Raf (S338). As shown in Fig. 5A, GSK3 inhibition using either LiCl or SB216763 enhanced c-Raf (S338) levels, as compared with the levels of c-Raf (S338) in monocytes stimulated with only LPS. To define whether the enhanced phospho-c-Raf (S338) levels played a role in GSK3’s ability to control ERK1/2 activation, we used the use of a specific c-Raf inhibitor (26). As demonstrated in Fig. 5B, inhibition of c-Raf reduced the levels of phospho-ERK induced by LPS-stimulated monocytes. Moreover, c-Raf inhibition abrogated the ability of GSK3 inactivation to increase phospho-MEK and phospho-ERK levels by LPS-stimulated monocytes (Fig. 5B). These data demonstrate that the increased c-Raf activity observed in GSK3-inactivated cells is critical for the ability of GSK3 to augment phospho-ERK levels in LPS-stimulated cells.
FIGURE 5.
Phospho-c-Raf (S338) levels are enhanced by GSK3 inhibition and are critical for the ability of GSK3-inactivation to augment phospho-ERK levels. A, Monocytes were pretreated with the GSK3 inhibitor SB216763 (10 µM) or LiCl (10 mM) followed by the addition of LPS (1 µg/ml) for 60 min. The levels of phospho-c-Raf (S338) in monocytes were determined by probing 20 µg of total cellular protein by Western blot. The immunoblot was reprobed for total p38 and the mean intensity ratio of phospho-Raf S338 to total p38 was calculated by densitometry. B, Monocytes were preincubated with the GSK3 inhibitor SB216763 (10 µM) or LiCl (10 mM) with or without the Raf-1 inhibitor (100 nM) for 90 min followed by the addition of LPS (1 µg/ml). Whole cell lysates were prepared 60 min post stimulation and 30 µg protein was probed by Western blot for phospho-MEK and phospho-ERK levels. The blot was reprobed for total β-actin levels to ensure equivalent loading. Data are representative of three experiments.
Discussion
Due to the biological importance of IL-1Ra in counteracting the inflammatory properties of IL-1β (5, 9, 19, 39), as well as the findings by several laboratories demonstrating that the PI3K pathway was involved in the differential regulation of IL-1Ra (19, 21, 22) and IL-1β (19, 20), a major aim of the current study was to identify the downstream signaling component within the PI3K pathway that was involved in regulating IL-1Ra production. Our results demonstrate that the downstream kinase within the PI3K pathway, GSK3, regulates the production of the anti-inflammatory cytokine IL-1Ra by LPS-stimulated innate immune cells due to its ability to modulate the activity of the MAPK ERK1/2 (Fig. 6).
FIGURE 6.
Model of how GSK3 regulates IL-1Ra production by LPS-stimulated monocytes. Inhibition of GSK3 suppressed phospho-Rac1 (S71) levels and augmented the ability of active Rac1 to associate with PAK. siRNA-mediated knockdown in Rac1 levels attenuated the ability of GSK3 inhibition to increase phospho-ERK levels in LPS-stimulated cells. GSK3 inhibition increased phospho-c-Raf levels, in which c-Raf inhibition blocked the increased levels of phospho-MEK and ERK1/2. Inhibition of MEK1/2 demonstrated that the ability of GSK3 to augment IL-1Ra production was dependent upon ERK1/2 activity.
The PI3K pathway has been shown to regulate the production of the cytokine IL-1β (19, 20) and IL-1Ra (19, 21, 22). Studies by Learn et al. (21) demonstrated that inhibition of PI3K in septic whole blood or LPS-adapted THP-1 cells abrogated LPS-induced IL-1Ra secretion. Although this study did not examine the regulation of IL-1Ra by nonseptic or nonendotoxin tolerized cells, subsequent studies by Molnarfi et al. (19) demonstrated that PI3K-inhibition also regulated the production of IL-1Ra by monocytes from normal human donors. Furthermore, these studies demonstrated that inhibition of the PI3K pathway augmented the secretion of IL-1β by LPS-stimulated innate immune cells (19) while suppressing IL-1Ra production (19, 21). Taken together, these findings demonstrated that PI3K activity positively regulates the secretion of IL-1Ra in human monocytes, while concurrently suppressing Il-1β levels (19). The ability of the PI3K pathway to suppress the production of IL-1β was subsequently shown to be dependent upon the PI3K/Akt-mediated inactivation of the downstream kinase, GSK3 in TLR-stimulated monocytes (27). Our present study extends these findings by demonstrating that GSK3 negatively regulates IL-1Ra levels by LPS-stimulated human monocytes. Because the relative levels of IL-1Ra to that of IL-1β are believed to be a critical factor in the ability of IL-1β to mediate its inflammatory properties (5, 9, 12, 14, 39), the identification that GSK3 differentially regulates the levels of IL-1β and IL-1Ra by LPS-stimulated innate cells could be of significant therapeutic importance for the treatment of inflammatory diseases where increased and decreased levels of IL-1Ra and IL-1β, respectively, are beneficial.
The PI3K pathway has been shown to regulate the activity of the MAPK ERK1/2 to a variety of different cellular stimuli (29, 40–42). Our laboratory previously demonstrated that Porphyromonas gingivalis LPS-mediated activation of ERK in human monocytes was severely reduced upon inhibition of PI3K (29). In contrast, the levels of phospho-p38 and phospho-JNK1/2 were not affected by PI3K blockage (29). The data shown herein extend these observations by demonstrating that the downstream kinase of PI3K, GSK3, is responsible for regulating ERK1/2 activity in LPS-stimulated monocytes. These findings agree with those of Wang et al. (43) that identified GSK3 negatively regulates ERK activity in the human cell lines HT29 and Caco-2. However, in contrast to our present findings that GSK3 regulated ERK1/2 activity in a Rac1-dependent manner, the study by Wang et al. (43) showed that the ability of GSK3 to regulate ERK1/2 activity was via a PKCδ-dependent mechanism. Thus, although GSK3 can negatively affect ERK1/2 activation in different cell types, the cell-signaling pathway by which this occurs is likely dependent upon the cell-type and/or cellular stimulus.
Past studies have identified that the activity of the MAPK ERK1/2 is critical for the production of IL-1Ra by LPS-stimulated cells (19, 30). A study by Rabehi et al. (30) demonstrated that inhibition of MEK1/2, the kinase directly upstream of ERK1/2, abrogated the levels of IL-1Ra produced by monocytes stimulated with LPS isolated from Neisseria meningitides. Subsequent studies by others have also highlighted the importance of ERK1/2 activity in positively regulating IL-1Ra by human monocytes stimulated with E. coli LPS (19). Our current data are in agreement with these findings demonstrating that ERK activity is needed to augment IL-1Ra production by LPS-stimulated monocytes or PBMCs. The identification that GSK3 inhibition increased the levels of phospho-ERK and the levels of IL-1Ra, which could be blocked by inhibiting ERK activity, further confirms the importance of ERK in the regulation of IL-1Ra by LPS-stimulated human monocytes. However, although the present study, in conjunction with previously published findings (19, 27), identified that GSK3 inhibition negatively and positively regulates IL-1β and IL-1Ra levels, respectively, it is unlikely that the ability of GSK3 to control ERK activation is responsible for directly controlling both of these cytokines. In support of this, studies assessing IL-1Ra and IL-1β production by human monocytes have demonstrated that a loss of ERK activity results in the reduction of both IL-1Ra and IL-1β (19). Thus, the ability of GSK3 to suppress IL-1 β levels by LPS-stimulated monocytes must involve an additional regulatory pathway. In this regard, the attenuation of IL-1β levels upon GSK3 inhibition in LPS-stimulated monocytes has been shown to be due to a loss of NF-κB p65 transcriptional activity (44). In contrast to the ability of NF-κB p65 to regulate IL-1β production, several studies have shown that IL-1Ra production does not appear to be dependent upon NF-κB activity (44, 45). Therefore, the ability of GSK3 to augment ERK activity while concurrently suppressing NF-κB activation is likely responsible for its ability to differentially regulate IL-1β and IL-1Ra levels.
The anti-inflammatory cytokine IL-1Ra has been demonstrated to be important in regulating the progression and severity of several inflammatory diseases (5). The present study elucidated how IL-1Ra production is regulated by LPS-stimulated cells. Our identification that GSK3 is a central kinase involved in mediating IL-1Ra production may help elucidate novel therapeutic targets in the treatment of inflammatory diseases where increased IL-1Ra levels are beneficial.
Acknowledgments
We thank Dr. M. Benakanakere for assistance with manuscript preparation, Dr. P. Stathopoulou for technical assistance, and Dr. D. Scott for critical reading of the manuscript.
Footnotes
The work was supported by a grant from the National Institute for Dental and Craniofacial Research (1R01DE017680–01A1) to MM.
K.R., H.W., and M.M. designed and performed research, analyzed and interpreted, data and wrote the manuscript. C.A.G. and D.F.K. performed research and interpreted data.
Abbreviations used in this paper: IL-1Ra, IL-1 receptor antagonist; GSK3, glycogen-synthase kinase 3; PAK, phospho-p21-activated protein kinase.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 2.Yang RB, Mark MR, Gray A, Huang A, Xie MH, Zhang M, Goddard A, Wood WI, Gurney AL, Godowski PJ. Toll-like receptor-2 mediates lipopolysaccharide-induced cellular signalling. Nature. 1998;395:284–288. doi: 10.1038/26239. [DOI] [PubMed] [Google Scholar]
- 3.Dinarello CA. Proinflammatory cytokines. Chest. 2000;118:503–508. doi: 10.1378/chest.118.2.503. [DOI] [PubMed] [Google Scholar]
- 4.Hu X, Chen J, Wang L, Ivashkiv LB. Crosstalk among Jak-STAT, Toll-like receptor, and ITAM-dependent pathways in macrophage activation. J. Leukocyte Biol. 2007;82:237–243. doi: 10.1189/jlb.1206763. [DOI] [PubMed] [Google Scholar]
- 5.Arend WP. The balance between IL-1 and IL-1Ra in disease. Cytokine Growth Factor Rev. 2002;13:323–340. doi: 10.1016/s1359-6101(02)00020-5. [DOI] [PubMed] [Google Scholar]
- 6.Schnare M, Barton GM, Holt AC, Takeda K, Akira S, Medzhitov R. Toll-like receptors control activation of adaptive immune responses. Nat. Immunol. 2001;2:947–950. doi: 10.1038/ni712. [DOI] [PubMed] [Google Scholar]
- 7.Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, Takeda K, Akira S. Differential roles of TLR2 and TLR4 in recognition of Gram-negative and Gram-positive bacterial cell wall components. Immunity. 1999;11:443–451. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- 8.Ma Y, Thornton S, Boivin GP, Hirsh D, Hirsch R, Hirsch E. Altered susceptibility to collagen-induced arthritis in transgenic mice with aberrant expression of interleukin-1 receptor antagonist. Arthritis Rheum. 1998;41:1798–1805. doi: 10.1002/1529-0131(199810)41:10<1798::AID-ART11>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 9.Horai R, Saijo S, Tanioka H, Nakae S, Sudo K, Okahara A, Ikuse T, Asano M, Iwakura Y. Development of chronic inflammatory arthropathy resembling rheumatoid arthritis in interleukin 1 receptor antagonist-deficient mice. J. Exp. Med. 2000;191:313–320. doi: 10.1084/jem.191.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bandara G, Mueller GM, Galea-Lauri J, Tindal MH, Georgescu HI, Suchanek MK, Hung GL, Glorioso JC, Robbins PD, Evans CH. Intraarticular expression of biologically active interleukin 1-receptor-antagonist protein by ex vivo gene transfer. Proc. Natl. Acad. Sci. USA. 1993;90:10764–10768. doi: 10.1073/pnas.90.22.10764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hung GL, Galea-Lauri J, Mueller GM, Georgescu HI, Larkin LA, Suchanek MK, Tindal MH, Robbins PD, Evans CH. Suppression of intra-articular responses to interleukin-1 by transfer of the interleukin-1 receptor antagonist gene to synovium. Gene Ther. 1994;1:64–69. [PubMed] [Google Scholar]
- 12.Schwab JH, Anderle SK, Brown RR, Dalldorf FG, Thompson RC. Pro- and anti-inflammatory roles of interleukin-1 in recurrence of bacterial cell wall-induced arthritis in rats. Infect. Immun. 1991;59:4436–4442. doi: 10.1128/iai.59.12.4436-4442.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuiper S, Joosten LA, Bendele AM, Edwards CK, 3rd, Arntz OJ, Helsen MM, Van de Loo FA, Van den Berg WB. Different roles of tumour necrosis factor α and interleukin 1 in murine streptococcal cell wall arthritis. Cytokine. 1998;10:690–702. doi: 10.1006/cyto.1998.0372. [DOI] [PubMed] [Google Scholar]
- 14.Van Lent PL, Van De Loo FA, Holthuysen AE, Van Den Bersselaar LA, Vermeer H, Van Den Berg WB. Major role for interleukin 1 but not for tumor necrosis factor in early cartilage damage in immune complex arthritis in mice. J. Rheumatol. 1995;22:2250–2258. [PubMed] [Google Scholar]
- 15.Pascual V, Allantaz F, Arce E, Punaro M, Banchereau J. Role of interleukin-1 (IL-1) in the pathogenesis of systemic onset juvenile idiopathic arthritis and clinical response to IL-1 blockade. J. Exp. Med. 2005;201:1479–1486. doi: 10.1084/jem.20050473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campion GV, Lebsack ME, Lookabaugh J, Gordon G, Catalano M. Dose-range and dose-frequency study of recombinant human interleukin-1 receptor antagonist in patients with rheumatoid arthritis: the IL-1Ra Arthritis Study Group. Arthritis Rheum. 1996;39:1092–1101. doi: 10.1002/art.1780390704. [DOI] [PubMed] [Google Scholar]
- 17.Cunnane G, Madigan A, Murphy E, FitzGerald O, Bresnihan B. The effects of treatment with interleukin-1 receptor antagonist on the inflamed synovial membrane in rheumatoid arthritis. Rheumatology. 2001;40:62–69. doi: 10.1093/rheumatology/40.1.62. [DOI] [PubMed] [Google Scholar]
- 18.Cohen S, Hurd E, Cush J, Schiff M, Weinblatt ME, Moreland LW, Kremer J, Bear MB, Rich WJ, McCabe D. Treatment of rheumatoid arthritis with anakinra, a recombinant human interleukin-1 receptor antagonist, in combination with methotrexate: results of a twenty-four-week, multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2002;46:614–624. doi: 10.1002/art.10141. [DOI] [PubMed] [Google Scholar]
- 19.Molnarfi N, Gruaz L, Dayer JM, Burger D. Opposite regulation of IL-1β and secreted IL-1 receptor antagonist production by phosphatidylinositide-3 kinases in human monocytes activated by lipopolysaccharides or contact with T cells. J. Immunol. 2007;178:446–454. doi: 10.4049/jimmunol.178.1.446. [DOI] [PubMed] [Google Scholar]
- 20.Takano Y, Yamauchi K, Hayakawa K, Hiramatsu N, Kasai A, Okamura M, Yokouchi M, Shitamura A, Yao J, Kitamura M. Transcriptional suppression of nephrin in podocytes by macrophages: roles of inflammatory cytokines and involvement of the PI3K/Akt pathway. FEBS Lett. 2007;581:421–426. doi: 10.1016/j.febslet.2006.12.051. [DOI] [PubMed] [Google Scholar]
- 21.Learn CA, Boger MS, Li L, McCall CE. The phosphatidylinositol 3-kinase pathway selectively controls sIL-1RA not interleukin-1β production in the septic leukocytes. J. Biol. Chem. 2001;276:20234–20239. doi: 10.1074/jbc.M100316200. [DOI] [PubMed] [Google Scholar]
- 22.Molnarfi N, Hyka-Nouspikel N, Gruaz L, Dayer JM, Burger D. The production of IL-1 receptor antagonist in IFN-β-stimulated human monocytes depends on the activation of phosphatidylinositol 3-kinase but not of STAT1. J. Immunol. 2005;174:2974–2980. doi: 10.4049/jimmunol.174.5.2974. [DOI] [PubMed] [Google Scholar]
- 23.Weinstein SL, Finn AJ, Dave SH, Meng F, Lowell CA, Sanghera JS, DeFranco AL. Phosphatidylinositol 3-kinase and mTOR mediate lipopolysaccharide-stimulated nitric oxide production in macrophages via interferon-β. J. Leukocyte Biol. 2000;67:405–414. doi: 10.1002/jlb.67.3.405. [DOI] [PubMed] [Google Scholar]
- 24.Stambolic V, Ruel L, Woodgett JR. Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signalling in intact cells. Curr. Biol. 1996;6:1664–1668. doi: 10.1016/s0960-9822(02)70790-2. [DOI] [PubMed] [Google Scholar]
- 25.Cross DA, Culbert AA, Chalmers KA, Facci L, Skaper SD, Reith AD. Selective small-molecule inhibitors of glycogen synthase kinase-3 activity protect primary neurones from death. J. Neurochem. 2001;77:94–102. doi: 10.1046/j.1471-4159.2001.t01-1-00251.x. [DOI] [PubMed] [Google Scholar]
- 26.Lackey K, Cory M, Davis R, Frye SV, Harris PA, Hunter RN, Jung DK, McDonald OB, McNutt RW, Peel MR, et al. The discovery of potent cRaf1 kinase inhibitors. Bioorg. Med. Chem. Lett. 2000;10:223–226. doi: 10.1016/s0960-894x(99)00668-x. [DOI] [PubMed] [Google Scholar]
- 27.Martin M, Rehani K, Jope RS, Michalek SM. Toll-like receptor-mediated cytokine production is differentially regulated by glycogen synthase kinase 3. Nat. Immunol. 2005;6:777–784. doi: 10.1038/ni1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kang DE, Soriano S, Xia X, Eberhart CG, De Strooper B, Zheng H, Koo EH. Presenilin couples the paired phosphorylation of β-catenin independent of axin: implications for β-catenin activation in tumorigenesis. Cell. 2002;110:751–762. doi: 10.1016/s0092-8674(02)00970-4. [DOI] [PubMed] [Google Scholar]
- 29.Martin M, Schifferle RE, Cuesta N, Vogel SN, Katz J, Michalek SM. Role of the phosphatidylinositol 3 kinase-Akt pathway in the regulation of IL-10 and IL-12 by Porphyromonas gingivalis lipopolysaccharide. J. Immunol. 2003;171:717–725. doi: 10.4049/jimmunol.171.2.717. [DOI] [PubMed] [Google Scholar]
- 30.Rabehi L, Irinopoulou T, Cholley B, Haeffner-Cavaillon N, Carreno MP. Gram-positive and gram-negative bacteria do not trigger monocytic cytokine production through similar intracellular pathways. Infect. Immun. 2001;69:4590–4599. doi: 10.1128/IAI.69.7.4590-4599.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stambolic V, Woodgett JR. Mitogen inactivation of glycogen synthase kinase-3 β in intact cells via serine 9 phosphorylation. Biochem. J. 1994;303(Pt 3):701–704. doi: 10.1042/bj3030701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 33.Zimmermann S, Moelling K. Phosphorylation and regulation of Raf by Akt (protein kinase B) Science. 1999;286:1741–1744. doi: 10.1126/science.286.5445.1741. [DOI] [PubMed] [Google Scholar]
- 34.Roy S, McPherson RA, Apolloni A, Yan J, Lane A, Clyde-Smith J, Hancock JF. 14–3-3 facilitates Ras-dependent Raf-1 activation in vitro and in vivo. Mol. Cell Biol. 1998;18:3947–3955. doi: 10.1128/mcb.18.7.3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kwon T, Kwon DY, Chun J, Kim JH, Kang SS. Akt protein kinase inhibits Rac1-GTP binding through phosphorylation at serine 71 of Rac1. J. Biol. Chem. 2000;275:423–428. doi: 10.1074/jbc.275.1.423. [DOI] [PubMed] [Google Scholar]
- 36.Eblen ST, Slack JK, Weber MJ, Catling AD. Rac-PAK signaling stimulates extracellular signal-regulated kinase (ERK) activation by regulating formation of MEK1-ERK complexes. Mol. Cell Biol. 2002;22:6023–6033. doi: 10.1128/MCB.22.17.6023-6033.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nosaka Y, Arai A, Kanda E, Akasaki T, Sumimoto H, Miyasaka N, Miura O. Rac is activated by tumor necrosis factor α and is involved in activation of Erk. Biochem. Biophys. Res. Commun. 2001;285:675–679. doi: 10.1006/bbrc.2001.5222. [DOI] [PubMed] [Google Scholar]
- 38.King AJ, Sun H, Diaz B, Barnard D, Miao W, Bagrodia S, Marshall MS. The protein kinase Pak3 positively regulates Raf-1 activity through phosphorylation of serine 338. Nature. 1998;396:180–183. doi: 10.1038/24184. [DOI] [PubMed] [Google Scholar]
- 39.Perrier S, Darakhshan F, Hajduch E. IL-1 receptor antagonist in metabolic diseases: Dr. Jekyll or Mr. Hyde? FEBS Lett. 2006;580:6289–6294. doi: 10.1016/j.febslet.2006.10.061. [DOI] [PubMed] [Google Scholar]
- 40.Jiang K, Zhong B, Gilvary DL, Corliss BC, Hong-Geller E, Wei S, Djeu JY. Pivotal role of phosphoinositide-3 kinase in regulation of cytotoxicity in natural killer cells. Nat. Immunol. 2000;1:419–425. doi: 10.1038/80859. [DOI] [PubMed] [Google Scholar]
- 41.Robertson LK, Mireau LR, Ostergaard HL. A role for phosphatidylinositol 3-kinase in TCR-stimulated ERK activation leading to paxillin phosphorylation and CTL degranulation. J. Immunol. 2005;175:8138–8145. doi: 10.4049/jimmunol.175.12.8138. [DOI] [PubMed] [Google Scholar]
- 42.Rommel C, Clarke BA, Zimmermann S, Nunez L, Rossman R, Reid K, Moelling K, Yancopoulos GD, Glass DJ. Differentiation stage-specific inhibition of the Raf-MEK-ERK pathway by Akt. Science. 1999;286:1738–1741. doi: 10.1126/science.286.5445.1738. [DOI] [PubMed] [Google Scholar]
- 43.Wang Q, Zhou Y, Wang X, Evers BM. Glycogen synthase kinase-3 is a negative regulator of extracellular signal-regulated kinase. Oncogene. 2006;25:43–50. doi: 10.1038/sj.onc.1209004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bondeson J, Browne KA, Brennan FM, Foxwell BM, Feldmann M. Selective regulation of cytokine induction by adenoviral gene transfer of IκBα into human macrophages: lipopolysaccharide-induced, but not zymosan-induced, proinflammatory cytokines are inhibited, but IL-10 is nuclear factor-κB independent. J. Immunol. 1999;162:2939–2945. [PubMed] [Google Scholar]
- 45.Amos N, Lauder S, Evans A, Feldmann M, Bondeson J. Adenoviral gene transfer into osteoarthritis synovial cells using the endogenous inhibitor IκBα reveals that most, but not all, inflammatory and destructive mediators are NFκB dependent. Rheumatology. 2006;45:1201–1209. doi: 10.1093/rheumatology/kel078. [DOI] [PubMed] [Google Scholar]