Abstract
Recent evidence suggests that the amygdala central nucleus (CeA) and midbrain-striatal dopamine systems are critically involved in the alteration of attentional and emotional processing of initially neutral stimuli by associative learning. In rats, the acquisition of learned orienting responses to visual cues paired with food is impaired by lesions of the CeA, and by lesions that disconnect CeA from the dorsolateral striatum, a region traditionally implicated in elevated responsiveness to sensory stimuli. Similarly, the acquisition of emotional significance to cues paired with food also depends on the function of CeA and of the ventral striatal nucleus accumbens, a region often considered crucial to acquired reward and motivation. For example, the ability of a cue previously paired with food to increase the rate of food-reinforced instrumental responding (Pavlovian-instrumental transfer) is eliminated by lesions of the CeA or the accumbens core. In this experiment, we found that lesions that functionally disconnected CeA from the substantia nigra pars compacta impaired the acquisition of conditioned orienting to auditory cues paired with food, but had no effect on their ability to enhance instrumental responding, relative to the effects of unilateral lesions of that region. By contrast, lesions that disconnected CeA from the ventral tegmental area had no effect on the acquisition of conditioned orienting, but facilitated Pavlovian-instrumental transfer relative to unilateral midbrain lesions, rescuing that function to sham-lesion control levels. Otherwise, unilateral lesions of either midbrain region impaired transfer. Implications of these results for circuit models of amygdalo-striatal interactions in associative learning are discussed.
Keywords: amygdala central nucleus, substantia nigra, ventral tegmental area, orienting, Pavlovian-instrumental transfer, rat
Associative learning procedures result not only in the acquisition of behavioral conditioned responses to conditioned stimuli (CSs) but also changes in attentional and emotional processing of those stimuli (Holland, 1997). Recent evidence suggests that many of these latter changes depend on the amygdala central nucleus (CeA) and striatal dopamine systems. For example, rats’ the acquisition of learned orienting responses (ORs) to visual CSs paired with food is impaired by lesions of the CeA (Gallagher et al., 1990) and by lesions that disconnect CeA from the dorsolateral striatum (DLS; Han et al., 1997), a region traditionally implicated in elevated responsiveness to sensory stimuli (Chevalier & Deniau, 1990; Schultz, 1992). Similarly, CSs often acquire incentive-motivational properties, giving them the ability to modulate ongoing motivated behavior. CSs previously paired with food can increase the rate of food-reinforced instrumental responding (Pavlovian-instrumental transfer, PIT). Notably, PIT is absent in rats with lesions of the CeA or the ventral striatal nucleus accumbens (ACB), a region often considered crucial to acquired reward and motivation (Berridge & Robinson, 1998; Cardinal et al., 2002). In this article we examined whether CeA’s influence on these products of associative learning is mediated relatively independently by its connections with the substantia nigra pars compacta (SNc) and the mesostriatal dopamine system in the case of learned orienting, and by its interactions with the ventral tegmental area (VTA) and the mesolimbic dopamine system in PIT.
Previous data support the claim that conditioned ORs are mediated by CeA’s influence on the mesostriatal dopamine system, which in turn may broadly influence sensory-motor responsiveness. In a Fos + Flurogold double-labeling study, Lee et al. (2005) showed that CeA neurons that are selectively activated by a visual CS paired with food, project to dopaminergic neurons of the SNc, which in turn innervate the DLS (Gonzalez & Chesselet, 1990). Furthermore, inactivation of SNc (El-Amamy & Holland, 2006) or lesions that disconnected CeA from either SNc (Lee et al., 2005) or DLS (Han et al., 1997) prevented the acquisition of conditioned ORs.
In the experiment reported here, we first extended Lee et al.’s (2005) findings by examining the effects of functionally disconnecting CeA from either SNc or VTA on the acquisition of conditioned ORs to auditory CSs paired with food. The acquisition of this OR, a startle-like response, to auditory CSs shares many behavioral properties with the acquisition of visual ORs (Holland, 1977) and is similarly dependent on CeA function (Gallagher et al., 1990; Groshek et al., 2005). We anticipated that removing CeA’s influence on the mesostriatal dopamine system via SNc would prevent the acquisition of conditioned ORs, whereas disconnecting CeA from the VTA would have relatively little effect on those responses. In subsequent phases, we examined the effects of CeA-SNc or CeA-VTA disconnection on PIT. By contrast to our expectations for the acquisition of ORs, we anticipated that the ability of the auditory CSs to modulate instrumental responding would be substantially affected by altering interactions between CeA and the VTA (another region whose function is critical to PIT, Murschall & Hauber, 2006), and hence the mesolimbic dopamine system, but relatively unaffected by disconnecting CeA from SNc.
Methods
Subjects
The subjects were 64 male naive Long-Evans strain rats (Charles River Laboratories, Raleigh, NC, USA), which weighed between 275-325 g when they arrived at the vivarium. They had free access to lab chow (2018 Rodent Diet, Harlan, Madison, WI, USA) for a week before their food was restricted to maintain them at 85% of their free-feeding weights. The rats were caged individually with constant access to water in a colony room illuminated from 6:00am to 6:00pm. All experiments were conducted according to the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals, and the protocols were approved by the Johns Hopkins University Animal Care and Use Committee.
Apparatus
There were eight test chambers (22.9 × 20.3 × 20.3 cm) with aluminum front and back walls and clear acrylic side walls and top. An infrared activity monitor (Coulbourn Instruments, Allentown, PA, USA) and a panel of infrared lights used to illuminate the chamber for video recording were placed on the top of each chamber. An illuminated clear acrylic food cup, with a capacity of about 1.7 ml, was placed behind a square hole in the center the front wall. A photocell beam in the food cup was used to detect head entries and time spent in the cup. An aluminum lever (2.0 × 2.0 cm) was mounted on each side of the food cup, centered between the cup and the side walls; throughout the Pavlovian training sessions they were covered with aluminum boxes (3.0 × 2.0 × 3.0 cm). A speaker, which was used to present auditory cues, was placed on the back wall of a double-walled sound-resistant shell that enclosed each experimental chamber. A television camera was placed 18 cm above the speaker to record each rat’s behavior; except in passing, video data are not presented in this article.
Surgery
All rats received surgery prior to behavioral training. The rats were first anesthetized with isoflurane gas (Abbott Laboratories, Chicago, IL, USA) and their heads were secured in a stereotaxic frame (Kopf Instruments, Tejunga, CA, USA). Forty rats received unilateral lesions of the CeA, half in the right hemisphere and half in the left. CeA lesions were made with two injections of 0.2μl of ibotenic acid (BioSearch Technologies, Novato, CA, USA), using a 2-μl Hamilton syringe, 2.3 mm and 2.7 mm posterior to bregma, both 4.4 mm lateral of the midline and 7.9 mm ventral to the skull surface. Each 0.2-μl volume was injected over 2 minutes, and the syringe needle was left in place for 6 min after the injection. In addition to the CeA lesions, 14 rats in Group VTA-contra received a contralateral lesion of VTA, 12 rats in Group SNc-contra received a contralateral lesion of SNc, and 7 rats each received ipsilateral lesions of either VTA or SNc. Because connections between CeA and both SNc and VTA are predominantly ipsilateral (Swanson, 1982), contralateral lesions of CeA and SNc or VTA prevent communication between those two regions. Notably, these “disconnection” lesions spare functions subserved by each region unilaterally, except for those that require communication between them. By contrast, ipsilateral lesions destroy the same amount of tissue in each region as the contralateral lesions, but leave communication between CeA and SNc or VTA intact in one hemisphere. Thus, the ipsilaterally-lesioned rats served as appropriate controls for assessing the effects of CeA-SNc/VTA disconnection in the contralaterally-lesioned rats. The VTA and SNc lesions were made using 6 μg/μl 6-hydroxydopamine (6-OHDA; Sigma, St. Louis, MO, USA) in a PBS/0.1% w/v ascorbic acid vehicle, infused over a 1-min period, 5.0 mm posterior to bregma, 0.8 mm lateral of the midline and 8.2 mm ventral to the skull surface (VTA) or 5.3 mm posterior to bregma, 2.4 mm lateral of the midline and 7.4 mm ventral to the skull surface (SNc). In the first replication of this study (n = 24), a volume of 1.0 μl of 6-OHDA was used to make the SNc and VTA lesions; because several of these lesions were too large (see Results), this volume was reduced to 0.75 μl for the last two (ns = 16 and 24) replications.
The remaining 24 rats received vehicle infusions into CeA in one hemisphere. Of these rats, 10 received unilateral VTA lesions (5 contralateral and 5 ipsilateral to the sham CeA lesion) and 6 received unilateral SNc lesions (3 and 3, respectively). These rats served as controls to evaluate the effects of CeA damage ipsilateral to SNc/VTA lesions. Finally, 8 rats received contralateral vehicle injections into either SNc (4) or VTA (4). These rats served as sham-lesioned controls for comparison with all of the other lesion groups.
After surgery, all rats were given a single 0.03-mg/kg subcutaneous injection of buprenorphine hydrochloride (Sigma) for amelioration of pain, and were allowed to recover from surgery for 14 days before behavioral training began.
Pavlovian OR training
Rats were first given one 64-min session of pre-exposure to the two auditory CSs to assess unconditioned ORs. Each CS, a 10-s 80-dB white noise and a 10-s 1,500-hz 80-db tone, was presented eight times in this session, randomly intermixed; no food was delivered. Next, the rats received two 64-min sessions to train them to drink from the liquid cup; in each of these sessions, 16 1-s, 0.2-ml deliveries of a 4% sucrose solution were delivered into the liquid cup at random intervals. All rats then received 3 64-min sessions designed to establish conditioned responding to one of the auditory stimuli. In each of these sessions, there were 16 10-s presentations of either the noise or tone CS+ (counterbalanced) followed immediately by the delivery of the sucrose US. Finally, all rats received 10 64-min discrimination training sessions, each of which included 8 reinforced presentations of the CS+ and 8 nonreinforced presentations of the other auditory stimulus cue (CS−).
PIT: Pavlovian training
In the previous phase, we used short-duration CSs (10 s) to study the acquisition of ORs. These responses are much less frequent when longer CS-food intervals are used (Holland, 1980). By contrast, most studies of PIT have used longer duration (60-120-s) CSs. Therefore, to prepare rats for PIT testing, we extended the durations of the original tone and noise CSs and retrained them. The rats received 8 32-min sessions of Pavlovian discriminative conditioning, each with four 2-min CS+ and four 2-min CS− trials, randomly intermixed. The identities of CS+ and CS− remained as they were in Exp. 1; only their durations were changed. Four reinforcers were delivered at random times during each 2-min CS+ trial. Levers were covered in this phase.
PIT: Operant training
After the Pavlovian training, one of the levers in each chamber was uncovered, and the rats were shaped to perform an instrumental lever press response for sucrose delivery. In the first session, a mash of food pellets mixed with sucrose and water was smeared on the lever to encourage the rats to perform the instrumental response for the first time. Each lever press produced the delivery of 0.1 ml of 4% sucrose. The shaping session was terminated after 50 lever presses; rats that failed to press the lever at least 50 times in the first session were given up to 2 additional shaping sessions. In some rats, the lever-pressing behavior was shaped by successive approximations. Shaping was followed by 4 30-min sessions of instrumental training with a random interval 30-s schedule, and 6 sessions of training with a random interval 30-s schedule.
PIT testing
After a single Pavlovian reminder session, identical to the training sessions described earlier (with levers covered), the rats received a final 32-min test session, in which 4 presentations each of the CS+ and CS− were presented at random intervals while the rats had access to the lever. Half of the rats received the test trials in order +−−++−+− and half received them in the order −++−−+−+. There was no delivery of sucrose during the test. Lever presses were recorded during the 2-min CS+, CS−, and empty 1-min pre-CS intervals.
Response measures
Two primary measures of Pavlovian conditioning are reported in this article. First, during the initial, OR, phase of the experiment, ORs were indexed by the rate of activity counts emitted by the infrared activity monitors during the first 5 s of each 10-s auditory Cs presentation, corresponding to the period when the startle-like ORs occur. Previous studies show that this measure correlates well with observers’ judgments of startle behavior (Groshek et al., 2005). Comparable observer judgments of startle were made from video tapes in the present study, but are not reported systematically here. Second, food cup responses were indexed by the photocells in the recessed liquid cup; as in earlier studies, we reported the percentage of time each rat spent with its head in the liquid cup during the last 5s of each 10-s CS presentation, near the time of food delivery, when anticipatory food cup behavior is maximal (Holland, 1980, 2000).
Activity counts and food cup times in the 5-s period prior to each CS presentation were reported, as a measure of baseline responding. Finally, in the initial OR phase, we also recorded activity responding in the second half of the CS intervals and food cup responding in the first half of the CS; summary values for these two measures are reported.
In the Pavlovian training for the PIT portion of the experiment, sucrose was delivered at random times throughout the CS. The measure of conditioning used in this phase was the percentage of time spent in the food cup in each CS period before the first sucrose delivery. ORs to these lengthy CSs were infrequent, and were not reported. The measure of instrumental conditioning in the PIT instrumental training and test phases of the experiment was the rate of lever press responding.
Histology
At the end of the experiment, the rats were deeply anesthetized with isoflurane gas and perfused transcardially with 0.9% saline followed by 4% paraformaldehyde in 0.1M phosphate buffer (PB). Brains were removed and stored overnight in a solution of 4% paraformaldehyde in 0.1 M PB containing 12% sucrose. The brains were then frozen with powdered dry ice and stored at −80°C until they were sliced on a freezing microtome.
Sections (40 μm) were taken from the amygdala and midbrain regions and Nissl-stained for evaluation of the CeA and midbrain lesions. CeA lesion borders were outlined on images taken from Nissl-stained sections at three anterior-posterior levels of CeA, and the percentage of CeA area damage calculated based on CeA borders estimated from Swanson (2004).
Additional sections from the midbrain were processed for tyrosine hydroxylase (TH) immunoreactivity, for the principal evaluation of midbrain lesions. The sections were washed in 0.3% hydrogen peroxide for 30 minutes, then washed three times with PBS. They were then blocked in 5% normal horse serum in 0.3% Triton X-100 in 0.1 M PBS (PBST) for 1 hour. The sections were then incubated in a 1:5000 solution of TH primary antibody (Immunostar, Hudson, WI) in 5% normal horse serum in PBST for 72 hours. After being washed 3 times with PBS, they were incubated in a 1:250 solution of horse anti-mouse secondary antibody (Vector Laboratories, Burlingame, CA) in PBST for 1 hour, and again washed 3 times with PBS. Next the sections were incubated in avidin-biocytin complex (ABC; Vectastain kit, Vector Laboratories) for 45 minutes. They were washed 3 times with PBS, then SG (Vectastain) was used for color development. After staining was complete, the sections were washed with PBS, mounted and coverslipped. These slides were used to evaluate midbrain lesions and dopaminergic depletion of the striatum. Damage to midbrain regions was calculated by counting neurons visible in TH-stained sections in three coronal sections of VTA and SNc.
Results
Histology
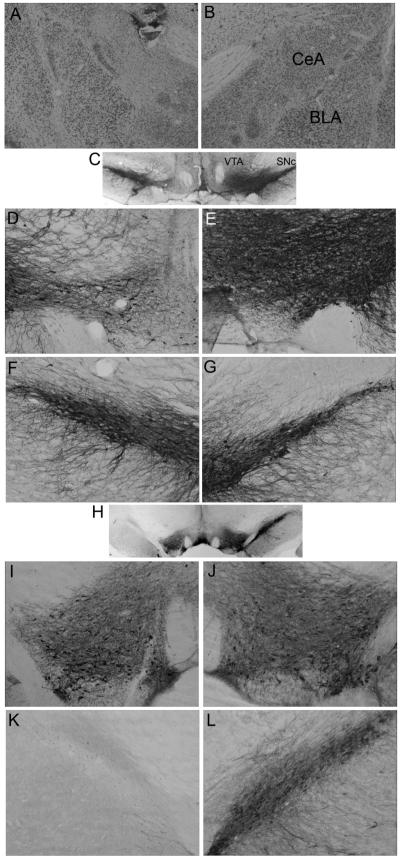
Figure 1 shows photomicrographs of sample lesion and control sections for CeA, SNc, and VTA. Rats with less than 40% damage to medial CeA (n = 6) were eliminated from the study; the mean ± sem CeA damage for the rats that met all lesion selection criteria was 61.5 ± 6.6%. Rats were also removed if the ratio of the number of TH+ neurons in the target region on the lesioned side to the number visible on the unlesioned side was greater than 0.40 (n=4), or if a comparable ratio constructed for TH+ neurons in the midbrain region not targeted by the lesions was less than 0.70 (n = 10). All of the latter discards were cases of combined SNc and VTA damage after a lesion targeted to VTA, and either ipsilateral or sham lesions of CeA. Mean ± sem damage ratios for accepted SNc-lesioned rats were 0.08 ± 0.02 for SNc and 0.99 ± 0.02 for VTA; damage ratios for VTA-lesioned rats were 0.83 ± 0.03 for SNc and 0.24 ± 0.20 for VTA. Inspection of Nissl-stained sections adjacent to those stained for TH led to similar exclusion decisions, but did not permit judgments about loss of non-dopaminergic neurons.
Figure 1.
Sample lesions. The top two panels show Nissl-stained coronal sections of the amygdala in the lesioned (panel A) and unlesioned (panel B) hemispheres. Panel A shows substantial neuron loss and gliosis in amygdala central nucleus (CeA), with little loss in the adjoining basolateral amygdala (BLA). Panels C and H show sections stained for tyrosine hydroxylase in brains with lesions of the ventral tegmental area (VTA; panel C) or substantia nigra pars compacta (SNc; panel H). Panels D-G show higher magnifications of the section shown in panel C and panels I-L show higher magnifications of the section shown in panel H. Panels D, F, I, and J show the lesioned side and panels E, G, J, and L show the unlesioned side. Panels D, E, I, and J show VTA and Panels F, G, K, and L show SNc.
The data from some rats were discarded on multiple lesion criteria; data from a total of 23 of the 64 rats were discarded. The final totals for each lesion subgroup were 9 SNc-contra, 4 SNc-ipsi, 4 SNc-uni, 8 VTA-contra, 5 VTA-ipsi, 3 VTA-uni, and 8 sham lesion controls (CTL).
Although 6-OHDA lesions that targeted SNc were quite specific to that region, with little neuron loss noted in VTA, it was difficult to obtain selective VTA lesions. In general, lesions that produced nearly complete loss of TH+ neurons also produced extensive damage to SNc. Nearly half of the lesions that targeted VTA produced damage to SNc that was nearly as substantial as that found in SNc-targeted lesions; we discarded these rats’ data from consideration. However, even in the rats with accepted VTA lesions, some loss in SNc neurons was observed, although our selection criteria guaranteed that the amount of SNc damage was substantially lower in the accepted VTA-lesioned brains than in the SNc-lesioned brains. Nevertheless, our observation of double dissociations in the behavioral effects of SNc-contra and VTA-contra lesions relative to their unilateral controls (to be described later) justifies our description of them as selective “SNc” and “VTA” lesions.
Behavioral data: initial analysis and data pooling
In each phase that included the Pavlovian CSs, each response measure during the appropriate CS and pre-CS periods was subjected to a separate analysis of variance (ANOVA). Individual contrasts for these and all subsequent analyses used the Tukey honestly significant difference (HSD) procedure. Initially, we conducted ANOVAs of the results of each phase, including only data from the ipsilateral and unilateral midbrain lesion control groups. These midbrain lesion site (SNc or VTA) X CeA lesion type (ipsilateral or sham) ANOVAs revealed no additional effects of the unilateral CeA lesion, Fs < 1. Therefore, for all subsequent analyses, we pooled the data from the ipsilateral and unilateral lesion subgroups within each lesion type, leaving five lesion groups, Group VTA-contra (n = 8), Group VTA-uni (n = 8), Group SNc-contra (n =9), Group SNc-uni (n = 8), and Group CTL (n = 8). However, Table 1 provides summary data for each of the original subgroups as well as the five major groups, for both Phase 1 Pavlovian conditioning and the PIT test.
Table 1.
Summary responding of each group and subgroup
| Phase 1 activity (OR) | Phase 1 food cup (CR) | PIT lever presses | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Group/Subgroup (n) | CS+ | CS− | pre-CS | CS+ | CS− | pre-CS | CS+ | CS− | pre-CS |
| VTA-Contra (8) | 99.5±18.3 | 59.6±11.4 | 41.0±9.3 | 72.8±4.9 | 11.4±2.7 | 3.8±1.0 | 4.5±0.6 | 1.4±0.8 | 3.5±0.5 |
| VTA-Uni (8) | 94.1±11.9 | 67.2±12.0 | 42.0±9.2 | 73.9±5.9 | 13.7±3.0 | 5.1±1.0 | 1.8±0.6 | 0.7±0.7 | 3.4±0.8 |
| VTA-Ipsi (5) | 96.1±16.1 | 68.0±18.1 | 40.5±10.5 | 76.5±8.5 | 16.1±4.3 | 5.7±1.4 | 1.8±0.7 | 0.3±0.8 | 3.2±0.3 |
| VTA-Uni (3) | 90.8±15.2 | 65.8±12.1 | 44.4±20.3 | 69.5±9.6 | 9.6±1.9 | 4.1±0.7 | 1.8±1.3 | 1.4±1.3 | 3.7±2.2 |
| SNC-Contra (9) | 63.9±9.4 | 35.3±8.8 | 44.3±4.0 | 80.5±5.7 | 16.0±4.0 | 8.3±3.2 | 2.1±0.5 | 1.3±0.9 | 3.3±0.9 |
| SNC-Uni (8) | 122.1±9.8 | 67.6±12.7 | 46.9±6.0 | 77.9±3.9 | 20.0±5.7 | 3.0±0.7 | 2.1±1.0 | 1.9±0.5 | 3.7±0.2 |
| SNC-Ipsi (4) | 119.7±20.1 | 64.3±20.1 | 50.1±6.2 | 79.0±6.6 | 18.3±10.5 | 3.7±1.3 | 1.8±1.6 | 1.6±0,3 | 3.2±0.3 |
| SNC-Uni (4) | 124.6±6.2 | 70.9±18.5 | 43.7±10.2 | 76.8±4.6 | 21.7±6.4 | 2.3±0.8 | 2.4±1.0 | 2.2±1.2 | 4.1±0.1 |
| CTL (8) | 100.4±12.3 | 54.6±11.4 | 23.9±5.6 | 72.4±5.4 | 6.4±1.5 | 2.0±0.6 | 4.6±0.7 | 1.1±0.7 | 3.3±0.6 |
Note. Table 1 shows responding averaged over the final four sessions of Phase 1 (first six columns of data) and during the Pavlovian-instrumental transfer (PIT) test (last 3 columns), for each of the groups and subgroups of rats. Entries for the activity measure (orienting response, OR) are mean±sem counts/min recorded during the first 5-s of the reinforced (CS+) and nonreinforced (CS−) conditioned stimuli and during the 5-s pre-CS intervals. Entries for the food cup conditioned response (CR) measure are mean±sem percentage of the time spent in the food cup during the last 5-s of the CSs and during the 5-s pre-CS intervals. Entries for lever presses during the CSs are the mean±sem elevation in lever presses/min relative to the pre-CS rates, which are given in the final column. The group labels are defined in the text.
The observation that unilateral CeA lesions had no additional effect on either conditioned ORs or PIT, which led us to combine midbrain-alone and ipsilateral CeA-midbrain lesions, has precedent. In previous studies that included unilateral CeA lesion groups (Han et al., 1997, 1999) we found no effects of that lesion on conditioned ORs, compared to sham-lesioned rats. Similarly, unpublished evidence suggests that unilateral CeA lesions also have little effect on PIT. In the present experiment, all 3 rats discarded because they had unilateral CeA lesions but no dopamine lesions, displayed normal PIT (described in El-Amamy, 2006), as did both rats with unilateral CeA lesions that were discarded from a previous study (Holland & Gallagher, 2003).
Phase 1 conditioned ORs
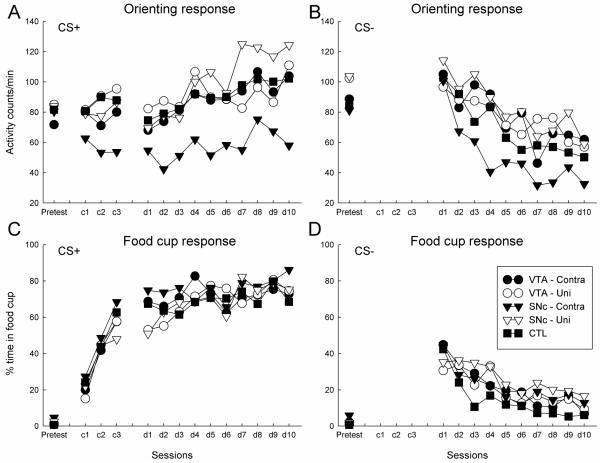
Figures 2A shows ORs during the pretest, conditioning, and discrimination training phases. ORs habituated rapidly during the pretest session; there were no effects of lesions, either over the whole session (shown) or on a trial-by-trial basis. Over the course of both the initial, nondiscriminative training sessions and the extended discriminative conditioning phase, acquisition of conditioned ORs was impaired by the contralateral CeA-SNc lesions, but not by the contralateral CeA-VTA lesions, relative to performance of both the appropriate unilaterally lesioned control groups (which did not differ from each other) and the sham-lesioned controls. Thus, as with visual CSs paired with food (Lee et al., 2005), disruption of communication between CeA and SNc impaired the acquisition of conditioned ORs to auditory CSs. By contrast, disruption of communication between CeA and VTA had no significant effect on the acquisition of conditioned ORs to the auditory CS.
Figure 2.
Orienting responses (panels A and B) and food cup (panels C and D) responses to the reinforced auditory conditioned stimulus (CS+; panels A and C) and the nonreinforced stimulus (CS−; panels B and D) in the first three experimental phases. Rats first received nonreinforced presentations of noise and tone stimuli in the pretest session, then pairings of one of those stimuli with sucrose during 3 nondiscriminative conditioning sessions (c1-c3), and finally continued pairings of that stimulus with sucrose, along with nonreinforced presentations of the other stimulus, in 10 discriminative conditioning sessions (d1-d10). Prior to training, some rats received lesions that disconnected amygdala central nucleus (CeA) from either the substantia nigra pars compacta (Group SNc-contra) or the ventral tegmental area (Group VTA-contra). Two unilateral lesion control groups received lesions of SNc or VTA in one hemisphere and either neurotoxic or sham lesions of CeA in the same hemisphere (Groups SNc-uni and VTA-uni, respectively). Lesion control rats (Group CTL) received sham lesions of both CeA and either SNc or VTA. Relative to all other groups, disconnection of CeA and SNc (Group SNc-Contra) significantly impaired the acquisition of conditioned ORs (A) but not food cup responses (C), and had no effect on unconditioned ORs (A). There were no significant between-group differences in the loss of generalized responding to the nonreinforced cue (CS−) over the course of discrimination training.
There were no significant differences in ORs to CS− (Figure 2B) across the lesion groups. ORs to CS− early in discrimination training likely reflected a combination of unconditioned ORs to the relatively novel CS− and generalization of conditioning from CS+. The pattern of ORs to CS− observed in Group SNc-contra is consistent with this possibility, because whereas conditioned ORs to CS+ were reduced in that group, unconditioned ORs (in the pretest session) were not.
An ANOVA of ORs in the pretest session, with lesion group and identity of cue (tone or noise) as variables showed only a significant effect of cue identity (more activity counts with tone than with noise), F(1, 31) = 6.22, p = 0.018. More important, neither the effect of lesion group nor the lesion X cue interaction was significant, Fs < 1, ps > 0.712. Thus, unconditioned ORs were unaffected by the lesions. ANOVA of pre-CS (baseline) activity in the pretest session also showed an effect of subsequent CS+ identity, F(1, 32) = 5.49, p = 0.026. These effects indicate that the overall activity levels of the rats that were to be trained with tone as CS+ were higher at the outset of training.
For the 3 nondiscriminative training sessions, a lesion group X CS+ identity X session ANOVA of OR scores to the reinforced CS+ showed a marginally significant effect of CS identity (higher responding in rats for which the tone was CS+), F(1, 31) = 4.09, p = 0.052, and a significant three-way interaction, F(8, 62) = 2.14, p = 0.045. The three-way interaction in part reflected the observation that activity rates during the tone CS+ increased in all groups except SNc- contra, which showed a decrease, whereas activity rates during the noise showed smaller changes over sessions in all of the groups. Post-hoc comparisons indicated that Group SNc- contra displayed significantly lower OR scores than Groups SNc-uni, VTA-uni, and CTL, ps < 0.045, in sessions 2 and 3; no other contrasts were significant, ps > 0.153.
A comparable ANOVA of ORs to CS+ in the discrimination training phase showed significant effects of reinforced CS identity (more activity during the tone CS+), F(1, 31) = 6.82, p = 0.014, and sessions, F(9, 279) = 12.32, p < 0.001, and a significant lesion group X session interaction (the deficit in Group SNc relative to the other groups grew over sessions), F(36, 279) = 1.47, p = 0.050. Individual contrasts showed that ORs of Group SNc-contra were significantly lower than those in each other group, ps < 0.047; no other contrasts were reliable, ps > 0.170.
Similar ANOVAS of ORs during CS− in the discrimination phase showed significant effects of CS− identity (more activity during the tone), F(1, 31) = 25.125, p < 0.001, sessions, F(9, 279) = 12.36, p < 0.001, and a significant CS− identity X sessions interaction (the tone superiority declined as responding to both CS− cues decreased), F(9, 279) = 2.84, p = 0.003. Notably, neither the main effect of lesion groups nor any of its interactions was significant, Fs < 1, ps > 0.436.
ANOVAs of pre-CS activity in the two conditioning phases yielded no significant effects or interactions with any factor, except in the discrimination training phase, in which the main effect of sessions, F(9, 279) = 2.32, p = 0.016, was significant (pre-CS activity declined over sessions). The ranges of pre-CS activity counts/min over the 6 groups were 55.0 ± 10.3 to 58.2 ± 10.1 in the nondiscriminative phase, and 26.4 ± 6.6 to 44.1 ± 9.3 in the discriminative training phase.
In our previous studies of lesion effects on conditioned ORs to auditory cues (e.g., Gallagher et al., 1990) we reported the occurrence of a startle-like response, defined as a sudden movement including a change in position, as scored by observers of video-taped sessions. Comparable observations in this study (not shown) showed the same pattern of results and statistical significance as the automated activity measure, consistent with Groshek et al., (2005), who also recorded both OR measures.
Phase 1 conditioned food cup responses
As in previous experiments, the deficit induced by CeA-SNc disconnection was confined to the conditioned OR; conditioned food cup responding was not significantly impaired by the lesions (Figure 2C). Thus, the lesions did not seem to produce general impairment in learning or motivation. ANOVAs of food cup CRs to CS+, similar to those just described for ORs, showed no significant effects or interactions in the pretest session, and only effects of sessions in the nondiscriminative, F(2, 62) = 75.47, p < 0.001, and discriminative, F(9, 279) = 6.11, p < 0.001, conditioning phases. ANOVA of food cup CRs to CS− (Figure 2D) in the discrimination training phase also yielded only a significant effect of sessions, F(9, 279) = 19.05, p < 0.001. Finally, lesion group and lesion group X session ANOVAs of pre-CS food cup responding during the pretest and nondiscriminative conditioning phases (respectively) showed no significant effects, ps > 0.298, and a lesion group X sessions ANOVA of pre-CS responding in the discriminative conditioning phase showed only a significant effect of sessions (pre-CS responding decreased over sessions), F(9, 279) = 1.94, p = 0.046. Among the groups, the overall pre-CS food cup responding ranged from 12.0 ± 1.5% to 16.4 ± 3.6% in the nondiscriminative conditioning phase and from 5.0 ± 0.8% to 8.4 ± 2.0% in the discriminative conditioning phase.
Temporal distribution of learned responses in Phase 1
As noted in Methods, in intact rats, conditioned ORs occur at CS onset, and food cup CRs occur more frequently as the time of food delivery approaches. Given that several investigators (e.g., Buhusi & Meck, 2005; Jahanshahi et al., 2006; Mattel & Meck, 2004) have posited a role for striatal dopamine systems (and SNc in particular) in the timing of intervals of this duration, we examined the temporal distribution of activity and food cup responses. Table 2 shows mean ± sem responding during the first and second 5-s interval of CS+ presentations over the final four sessions of discrimination training. Table 2 also displays a timing ratio (responding in first half / total responding) for each measure. The value of this ratio is greater than 0.5 if responding occurs mostly in the first 5-s interval and is less than 0.5 if responding occurs mostly in the second 5-s interval. The temporal distributions of both responses were similar to those observed in previous studies, and were not affected by the lesions. Notably, activity responding in the second 5-s interval, which likely reflected aspects of motion other than the startle-like OR, did not differ across groups, F < 1, p = 0.461, nor did the activity timing ratio, F < 1, p = 0.727. Likewise, food cup responding during the first 5-s interval did not differ significantly across groups, F(1, 4) = 1.89, p = 0.134; post-hoc Tukey HSD tests revealed no significant individual comparisons, ps > 0.145.
Table 2.
Temporal distribution of responding in Phase 1
| Group | Activity | Food cup | ||||
|---|---|---|---|---|---|---|
| first 5 s | second 5 s | Ratio | first 5 s | second 5 s | Ratio | |
| VTA-X | 99.5 ± 18.3 | 64.7 ± 20.5 | 0.65 ± 0.03 | 48.7 ± 4.1 | 72.8 ± 4.9 | 0.40 ± 0.03 |
| VTA-U | 94.1 ± 11.9 | 74.7 ± 13.7 | 0.59 ± 0.05 | 41.6 ± 6.2 | 73.9 ± 5.9 | 0.35 ± 0.03 |
| SNC-X | 63.9 ± 9.4 | 53.1 ± 8.7 | 0.58 ± 0.05 | 57.3 ± 5.0 | 80.5 ± 5.7 | 0.41 ± 0.01 |
| SNC-U | 122.1 ± 9.8 | 89.9 ± 13.9 | 0.60 ± 0.03 | 48.7 ± 4.7 | 77.9 ± 3.9 | 0.38 ± 0.01 |
| CTL | 100.4 ± 12.3 | 75.4 ± 14.2 | 0.59 ± 0.03 | 41.0 ± 4.1 | 72.4 ± 5.4 | 0.36 ± 0.01 |
Note. Entries for the activity measure are mean ± sem counts/min; entries for the food cup measure are mean ± sem percentage of the 5-s interval spent in the food cup. Ratios for each measure are first 5-s response / (first 5-s response + second 5-s response). The group labels are defined in the text.
Pavlovian-instrumental transfer
Pavlovian retraining with longer CSs and variable times of food delivery was unaffected by the lesions. Across the five groups, mean ± sem food cup responding before the delivery of the first US during CS+ ranged between 40.2 ± 5.5% and 50.8 ± 3.4%, during the equivalent periods in CS−, 11.1 ± 2.4% and 20.4 ± 6.3%, and in the pre-CS periods, 6.6 ± 1.6 and 13.7 ± 4.0%. A lesion group X CS+ identity X contingency (CS+ or CS−) X sessions ANOVA showed only an effect of contingency, F(1, 31) = 145.93, p < 0.001, and a contingency X sessions interaction, F(7, 217) = 3.64, p < 0.001.
The acquisition of instrumental lever pressing preparatory to PIT testing was comparable in all groups. A lesion group X session ANOVA showed only a significant effect of sessions, F(9, 324) = 20.90, p < 0.001. Across the 5 groups, the overall lever press rates ranged from 6.1 ± 1.1 to 7.1 ± 0.9 responses/min.
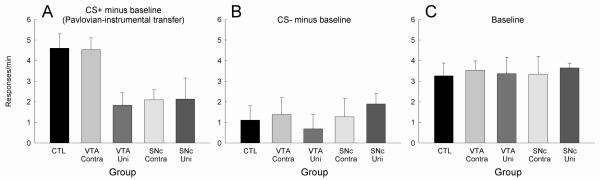
Figure 3 shows the results of the Pavlovian-instrumental transfer test. Overall, most rats showed PIT; the rate of lever pressing was significantly higher during presentations of the CS+ than during the baseline or CS− periods. The effects of the lesions on PIT were complex. First, relative to sham-lesioned control rats, unilateral midbrain dopamine lesions, of either SNc or VTA, reduced the size of the PIT effect, as indexed by the difference between responding during CS+ and baseline periods. Second, in the case of SNc lesions, disconnection of that region from CeA had no additional effect on the magnitude of PIT. Third, disconnection of CeA and VTA apparently rescued the deficit produced by unilateral VTA lesions; the performance of rats with those lesions did not differ significantly from PIT performance of the sham-lesioned control rats.
Figure 3.
Results of the test of Pavlovian-instrumental transfer. Panel A shows the elevation in lever press rate over baseline produced by the auditory conditioned stimulus that previously was paired with sucrose (CS+). Panel B shows the elevations produced by the auditory stimulus that was previously nonreinforced (CS−). Panel C shows the baseline rates of lever pressing from which the elevation scores in panels A and B were derived.
A lesion group X CS identity (tone or noise) X test period (CS+, CS−, or baseline) ANOVA of PIT test performance showed an overall effect of test period, F(2, 62) = 45.28, p < 0.001, an interaction of test period with CS identity (greater enhancement with the noise), F(2, 62) = 11.27, p < 0.001, and most important, an interaction of test period with lesion group, F(8, 62) = 2.38, p = 0.026. Separate ANOVAs contrasting CS+ responding with baseline or CS− responding (that is limiting the test period factor to CS+ and CS− or CS+ and baseline) each yielded significant effects of test period, Fs(1, 31) = 117.03 and 25.08, respectively, ps < 0.001, and lesion group X test period interactions, F(4, 31) = 4.55, p = 0.005, and F(4, 31) = 2.68, p = 0.049, respectively. In addition, the CS+ identity X test period interaction was significant for the contrast of CS+ and CS− responding, F(1, 31) = 16.93, p < 0.001, and approached significance for the CS+ vs baseline contrast, F(1, 31) = 3.20, p = 0.083. These latter effects reflect greater responding in the presence of the noise than during the tone across the board, which inflates the apparent size of the PIT effect when CS+ was the noise and deflates it when CS+ was the tone. Notably, this effect did not interact with lesion group in either of these ANOVAs or the overall ANOVA, Fs < 1.27, ps > 0.305.
Tukey HSD comparisons showed that PIT (as measured by the elevation CS+ produced over baseline responding) was significantly (p < 0.050) reduced in Groups SNc-uni, SNc-contra, and VTA-uni, relative to each of Groups CTL and VTA-contra. There were no other significant differences among the groups.
ANOVAs showed no significant lesion effects on responding in either CS− or pre-CS periods, Fs < 1.20, ps > 0.330, during the PIT test.
Discussion
We found a double dissociation in the effects of disconnection of CeA from either SNc or VTA on the acquisition of conditioned ORs and the display of PIT to auditory CSs paired with food. Relative to the effects of unilateral midbrain lesions, contralateral lesions that disconnected CeA from SNc impaired the acquisition of conditioned ORs, but did not alter the display of PIT, whereas CeA-VTA disconnection lesions enhanced PIT but did not affect conditioned ORs. Notably, although in the absence of contralateral damage to CeA, unilateral midbrain lesions had no effect on conditioned ORs, those lesions alone significantly impaired PIT. Thus, although CeA-VTA disconnection lesions augmented PIT relative to unilateral VTA lesions, that enhancement might be better described as rescue to the level of sham-lesioned control rats.
These observations are consistent with previous suggestions that the roles of CeA in modulating attention and in assigning motivational significance to events in associative learning are anatomically distinct. Within this framework, the emergence of conditioned ORs reflects enhanced attentional processing of CSs, mediated by a circuit that includes CeA, SNc, and DLS, whereas PIT reflects acquisition of incentive motivation by CSs, within a system that includes CeA, VTA, and ACB. However, combined with the results of previous studies that show impairments of PIT after lesions or inactivation of CeA, VTA, or ACB lesions on PIT, the present observations of the effects of CeA and midbrain dopamine lesions on PIT indicate that the interactions among components of circuitry responsible for PIT are more complex than has been suggested previously.
Conditioned ORs
Our observation that the acquisition of auditory ORs was impaired by lesions that disconnected CeA and SNc is consistent with, and extends, Lee et al.’s (2005) findings with visual CSs. The observed interaction of CeA with mesostriatal dopamine systems in the acquisition of conditioned ORs in response to cues of both modalities supports the assertion that conditioned ORs reflect heightened attention to events that acquire significance through associative learning, rather than the direct facilitation of particular, sense-specific S-R reflexes via descending projections of CeA (e.g., Meloni & Davis, 1999; Rosen et al., 1991). Similarly, the lack of an effect of these lesions on unconditioned ORs in the pretest shows that their effects on conditioned ORs were not due to deficits in motor or sensory-motor performance induced by dopamine depletion. Furthermore, the normal acquisition of learned food cup responses regardless of the status of connections between CeA and SNc shows that the reduced learning of ORs in rats with CeA-SNc disconnection lesions did not reflect general impairments in learning or motivation. Finally, it is unlikely that the OR deficit was the result of impairments in the CSs’s acquisition of emotional significance; in that case, we would have anticipated effects of CeA-VTA disconnection lesions as well.
It is informative to contrast the lack of effects of CeA-VTA disconnection lesions on conditioned ORs observed here with lesion effects reported in studies of the conditioning of approach responses to visual cues with autoshaping procedures. Many investigators have suggested that these responses reflect the CS’s acquisition of positive incentive motivation, such that orientation and approach to that CS, even attempting to contact it, resembles approach to and contact with food itself (Cardinal et al., 2002; Holland, 1977; Parkinson, et al., 2000). Cardinal et al. (2002) suggested that this acquisition of incentive value to CSs requires the modulation of mesolimbic reward circuitry by CeA, specifically, via projections of CeA to VTA dopamine neurons, which in turn project to the ACB core. In support of that claim, ibotenic acid lesions of CeA or 6-OHDA lesions of ACB (which presumably damaged VTA neurons whose terminal fields were in ACB) prevented the acquisition of rats’ approach responses to visual cues in an autoshaping procedure (Parkinson et al., 2000, 2002). Taken together with Lee et al.’s (2005) findings, those results suggest that orienting and approach to localizable visual CSs paired with food reflect both attentional and incentive motivational processes, and thus such responses might demand contributions of both mesostriatal and mesolimbic dopamine systems.
However, it seems less likely that in the present experiment the startle response would also depend on the auditory cue’s acquisition of incentive motivational properties. Although it seems intuitively plausible that the transfer of positive value to a localizable visual stimulus might result in orienting, approach and attempting to contact that cue, it is less obvious that the acquisition of incentive value by an auditory cue would result in an undirected startle response. All together, these results are consistent with the hypotheses that the conditioning of both visual and auditory ORs involves the enhancement of dorsolateral striatal sensory-motor function, but that visual approach also depends on the engagement of ventral striatal incentive motivational systems in a way auditory ORs do not. It is interesting to speculate that variation in the localizability of both visual and auditory stimuli might influence both the likelihood of learning approach responses and the relative importance of mesolimbic dopamine circuits in that learning.
Although most discussions of the role of dopamine signaling in associative learning focus on its role in reinforcement or reward processes, some investigators have noted that a key function of dopamine is to enhance or focus attention to significant events (Haber et al., 2000; Schultz & Dickinson, 2000). Notably, dopamine responses are observed to novel, intense and “intrinsically interesting” stimuli (Horvitz, 2000; Shultz & Dickinson, 2000). Several authors have described striatal and cortical systems whereby processing of such signals can be maintained or enhanced at the expense of the processing of weaker or background stimuli (Cepeda & Levine, 1998; Horvitz, 2002; Schultz & Dickinson, 2000). These responses, however, often habituate with repeated stimulus exposure as the stimuli prove insignificant. Nevertheless, even events that do not initially evoke dopamine responses may come to do so when paired with primary rewards (Schultz & Dickinson, 2000). Although this acquired dopamine response is typically described as the conditioning of reward properties to the CS, it may also reflect (or produce) enhancement of orienting or other aspects of attention to the CS, as we have described earlier. Thus, dopamine neurons may provide “attention signals” as well as “reward signals” (Redgrave et al., 2000). In support of this view, elsewhere we have demonstrated the involvement of CeA-SNc connections in another aspect of attention in associative learning, the surprise-induced enhancement of CS associability, the rate at which a CS may enter into new associations (Lee et al., 2006). Similarly, Rogers et al. (2001) found that quinolinic acid lesions of dorsal striatum impaired performance in the 5-choice serial reaction time task, used to assess sustained attention. Moreover, deficits in switching and maintaining attention have been noted in patients with Parkinson’s disease (Ravizza & Ivry, 2001), which is characterized by progressive loss of dopamine function and degeneration of the substantia nigra and other neuron groups.
Pavlovian Instrumental Transfer
The PIT test showed two major outcomes, each of which was somewhat unexpected. First, relative to control rats with sham lesions of SNc or VTA, rats with unilateral lesions of either SNc or VTA, combined with ipsilateral excitotoxic or sham lesions of CeA, showed reduced PIT, despite normal instrumental baseline response rates. Second, contralateral excitotoxic lesions of CeA rescued this impairment in rats with VTA lesions, but not in those with SNc lesions. We consider each of these findings in turn.
First, what is the origin of the reduction in PIT produced by unilateral dopamine lesions? Except for Group VTA-contra, all groups with unilateral dopamine lesions showed equivalent disruption of PIT. A simple possibility is that these deficits are the results of motor impairment produced by dopamine depletion. Unilateral 6-OHDA lesions of SNc have been reported to produce motor deficits in rotorod, paw reaching, and other such tasks, leading to the use of rats with such lesions as a model of Parkinson’s disease (Iancu et al., 2005; Moore et al., 2001). Furthermore, damage to the VTA can add to the motor deficits resulting from SNc damage (Moore et al., 2001). It could be argued that rats with unilateral dopamine lesions might have motor deficits that render them less able to display higher rates of instrumental behavior. Indeed, it is worth mentioning that the 10 rats that were discarded from this study because their unilateral dopamine lesions encompassed both SNc and VTA showed significantly lower operant response rates in both training and testing than the rats with lesions confined to SNc or VTA alone (El-Amamy, 2006).
However, other aspects of our data make purely motor deficits a less plausible contributor to the PIT deficits. First, effects of these lesions were not observed with other response measures that might also be expected to be sensitive to motor deficits, including activity and food cup entry levels during baseline and CS periods in previous phases of the study. Second, because the PIT tests were conducted in extinction, baseline responding was reduced in all groups relative to lever press rates observed during training. The lever press rates observed on CS+ trials in the PIT test, although considerably higher than baseline responding in the absence of CS+ in that test, were no higher than were observed in the instrumental training sessions, when no effects of the unilateral lesions were observed. Thus, performance of lever presses at these rates alone did not unduly tax the motor abilities of the lesioned rats.
Alternately, the unilateral mid-brain dopamine lesions may affect PIT by removing dopaminergic innervation of ACB, and consequently altering learned incentive motivational function. Complete absence of PIT was observed by Hall et al. (2001) after bilateral quinolinic acid lesions of ACB core, and by Murschall and Hauber (2006) after bilateral inactivation of VTA by infusions of high doses of muscimol (a GABAA agonist) and baclofen (a GABAB agonist). Given that both SNc and VTA have dopaminergic projections to ACB (Swanson, 1982; Brog et al., 1993), the observation of partial impairment in PIT after unilateral dopamine lesions would not be surprising. Our observation that rats in Group SNc-contra and Group SNc-uni performed similarly indicates that if SNc innervation of ACB is important to PIT, that innervation is not modulated by SNc’s connections with CeA. This outcome stands in contrast to our previous observations that in the acquisition of conditioned ORs, SNc’s innervation of DLS is modulated by CeA. It seems likely that different subpopulations of SNc neurons innervate these two striatal regions (Haber & Fudge, 1997).
The second question raised by our findings is how the debilitating effects of unilateral VTA lesions on PIT are ameliorated by contralateral lesions of CeA. This observation is inconsistent with a simple serial CeA-VTA-ACB circuit for PIT suggested previously (e.g., Cardinal et al., 2002; Hall et al., 2001; Parkinson et al., 2001). These investigators suggested that CeA modulates ACB function via its projections to VTA. Consistent with that suggestion, bilateral lesions of either CeA or ACB (Hall et al., 2001; Holland & Gallagher, 2003), and bilateral inactivation of VTA (Murschall & Hauber, 2006) each depresses PIT. From these observations it would be reasonable to expect that disconnecting CeA and VTA would also abolish PIT, rather than enhance it. Although this account might be reconciled with our observations by asserting that the lengthy period between the lesion surgery and the PIT test permitted the sprouting, regrowth, and consequent hyperfunction of dopamine fibers projecting to ACB (e.g., Acheson et al., 1980; Blanchard, et al., 1996; Finkelstein et al., 2000; Zigmond & Stricker, 1973), it seems unlikely that such effects alone could account for our observation of enhanced PIT. Notably, unilateral dopamine lesions alone reduced PIT whereas the addition of a contralateral CeA lesion enhanced it. Thus, all in all, our observation that disconnection of CeA and VTA restores PIT that would normally be suppressed by unilateral VTA lesions indicates that more complex circuitry is involved in CeA’s modulation of processes responsible for PIT.
Whereas other studies show that CeA and VTA each apparently have net excitatory influences on the processes responsible for PIT, the data reported here suggest that the normal consequences of CeA-VTA interaction are inhibitory. Given the many ways each of these structures might influence each other and the striatum, in terms of both circuit function (DiChiara et al., 1994; Morris, et al., 2004; Swanson & Petrovich, 1998) and neurochemical action (Gonzaelz-Hernandez & Rodriguez, 2000; Grace, 2002; Sun & Cassell, 1993), it is too early to speculate on the nature of this interaction. Regardless of the nature of this interaction, however, it seems unlikely that CeA’s excitatory influence on PIT is mediated by VTA. Indeed, although significant CeA-VTA projections have been reported in monkey (Amaral et al., 1992; Fudge & Haber, 2000; Price & Amaral, 1981), those projections have been characterized as relatively light (e.g., Gonzalez & Chesselet, 1990; Rosen et al., 1991; Wallace et al., 1989) or insignificant (Zahm et al., 1999) in most rat studies (but see Lee et al., 2006a).
In the absence of studies of the effects of either CeA-ACB or VTA-ACB disconnections on PIT, we can not distinguish between the possibility that each of these structures contributes to PIT independently and the possibility that they act together. However, observations that stimulation or inactivation of CeA alters ACB dopamine activity in response to food (e.g., Ahn & Phillips, 1999, 2002) are consistent with CeA-ACB interaction. Notably, CeA has important afferents to several other brain regions that innervate ACB, including the lateral hypothalamus, the peduncular pontine tegmental system, the bed nucleus of the stria terminalis, the retrorubral cell field, and many of the midline thalamic nuclei (Brog et al., 1993; Krettek & Price, 1978; McDonald, 1987; Pitkänan, 2000; Rosen et al., 1991). Holland and Gallagher (2003) favored a role for the centromedial and medial dorsal nuclei of thalamus on anatomical grounds, because those regions project directly to the accumbens core (which has been found to be critical to PIT with single response/reinforcr procedures like those used here, Hall et al., 2001), whereas the other regions just noted project primarily to the shell or ventral pole of the ACB. We are currently exploring many of these possibilities.
Finally, the pattern of lesion effects on both conditioned ORs and PIT in this study has important implications for understanding the organization of mesolimbic and mesostriatal dopamine functions, and the role of CeA in modulating those functions. Modern views of striatal-midbrain interaction (e.g., Everitt & Robbins, 2005; Haber et al., 2000) include the idea that ventral striatal regions influence more dorsal striatal regions via “spirals” of ascending and descending connections between striatum and midbrain, which may provide the anatomical basis for the integration of sensory, motor, and emotional components of learning (Nauta & Domesick, 1978). From this perspective, serial circuits involved in conditioned ORs and PIT might be broken by interrupting these spirals at any of a number of midbrain or striatal loci, and hence lesions of any of those regions might have similar effects. Consistent with that view, in our study, unilateral lesions of either VTA or SNc impaired PIT, and neither of those lesions affected conditioned ORs. Furthermore, it is important to recognize that in our disconnection lesion preparation, such loops or spirals are intact in one hemisphere in both contralateral and ipsilateral/unilateral lesion conditions. However, in rats with contralateral lesions, the intact spirals are not subject to modulation by CeA in learning. Thus, within this perspective, the dissociation in the effects of disconnecting CeA from different midbrain cell groups is most likely to reflect differences in the manner by which CeA modulates the action of these striatal/midbrain spirals. It is clear that a fuller understanding of the interaction of these brain regions in controlling the motivational and attentional processes responsible for PIT, learned orienting, and other related phenomena will require examination of the effects of functional disconnections among a number of brain regions.
Acknowledgements
This research was supported in part by grant MH53667 from the National Institutes of Health.
Abbreviations
- 6-OHDA
6-hydroxydopamine
- ACB
Nucleus accumbens
- ANOVA
Analysis of variance
- CeA
Amygdala central nucleus
- CS
Conditioned stimulus
- DLS
Dorsolateral striatum
- GABA
Gamma-aminobutyric acid
- HSD
Honestly significant difference
- OR
Orienting response
- PIT
Pavlovian-instrumental transfer
- SNc
Substantia nigra pars compacta
- TH
Tyrosine hydroxylase
- VTA
Ventral tegmental area
References
- Acheson AL, Zigmond MJ, Stricker EM. Compensatory increase in tyrosine hydroxylase activity in rat brain after intraventricular injections of 6-hydroxydopamine. Science. 1980;207:537–540. doi: 10.1126/science.6101509. [DOI] [PubMed] [Google Scholar]
- Ahn S, Phillips AG. Dopaminergic correlates of sensory-specific satiety in the medial prefrontal cortex and nucleus accumbens of the rat. J. Neurosci. 1999;19:1–6. doi: 10.1523/JNEUROSCI.19-19-j0003.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn S, Phillips AG. Modulation by central and basolateral amygdalar nuclei of dopaminergic correlates of feeding to satiety in the rat nucleus accumbens and medial prefrontal cortex. J. Neurosci. 2002;22:10958–10965. doi: 10.1523/JNEUROSCI.22-24-10958.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral DG, Price JL, Pitkänen A, Carmichael ST. Anatomical organization of the primate amygdaloid complex. In: Aggleton JP, editor. The Amygdala: Neurobiological aspects of emotion, memory, and mental dysfunction. Wiley-Liss; New York: 1992. pp. 1–66. [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res. Rev. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Blanchard V, Anglade P, Dziewczapolski G, Savasta M, Agid Y, Raisman-Vozari R. Dopaminergic sprouting in the rat striatum after partial lesion of the substantia nigra. Brain Res. 1995;709:319–325. doi: 10.1016/0006-8993(95)01391-1. [DOI] [PubMed] [Google Scholar]
- Brog JS, Salyapongse, Deutch AY, Zahm DS. The patterns of afferent innervation of the core and shell in the “accumbens” part of the rat ventral striatum: Immunohistochemical detection of retrogradely transported fluro-gold. J. Comp. Neurol. 1993;338:255–278. doi: 10.1002/cne.903380209. [DOI] [PubMed] [Google Scholar]
- Buhusi CV, Meck WH. What makes us tick? Functional and neural mechanisms of interval timing. Nature Rev Neurosci. 2005;6:755–765. doi: 10.1038/nrn1764. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci. Biobehav. Rev. 2002;26:321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- Cepeda C, Levine MS. Dopamine and N-Methyl-D-Aspartate receptor interactions in the neostriatum. Dev. Neurosci. 1998;20:1–18. doi: 10.1159/000017294. [DOI] [PubMed] [Google Scholar]
- Chevalier G, Deniau JM. Disinhibition as a basic process in the expression of striatal function. Trends Neurosci. 1990;13:277–280. doi: 10.1016/0166-2236(90)90109-n. [DOI] [PubMed] [Google Scholar]
- DiChiara G, Morelli M, Consolo S. Modulatory functions of neurotransmitters in the striatum: Ach/dopamine/NMDA interactions. Trends Neurosci. 1994;17:228–232. doi: 10.1016/0166-2236(94)90005-1. [DOI] [PubMed] [Google Scholar]
- El-Amamy H. The role of connections between the central nucleus of the amygdala, the substantia nigra, and the ventral tegmental area in the production of the conditioned orienting response. 2006. Unpublished manuscript submitted to the Johns Hopkins University in partial fulfillment of the requirements for the B.A./M.S.degrees. [Google Scholar]
- El-Amamy H, Holland PC. Substantia nigra pars compacta is critical to both the acquisition and expression of learned orienting of rats. Eur. J. Neurosci. 2006;24:270–276. doi: 10.1111/j.1460-9568.2006.04896.x. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to compulsions. Nature Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Finkelstein DI, Stanic D, Parish CL, Tomas D, Dickson K, Horne MK. Axonal sprouting following lesions of the rat substantia nigra. Neurosci. 2000;97:99–112. doi: 10.1016/s0306-4522(00)00009-9. [DOI] [PubMed] [Google Scholar]
- Fudge JL, Haber SN. The central nucleus of the amygdala projection to dopamine subpopulations in primates. Neurosci. 2000;97:479–494. doi: 10.1016/s0306-4522(00)00092-0. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Graham PW, Holland PC. The amygdala central nucleus and appetitive Pavlovian conditioning: lesions impair one class of conditioned behavior. J. Neurosci. 1990;10:1906–1911. doi: 10.1523/JNEUROSCI.10-06-01906.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales C, Chesselet M-F. Amygdalonigral pathway: An anterograde study in the rat with phaseolus vulgaris leucoagglutinin (PHA-L) J. Comp. Neurol. 1990;297:182–200. doi: 10.1002/cne.902970203. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Hernandez T, Rodriguez M. Compartmental organization and chemical profile of dopaminergic and GABAergic neurons in the substantia nigra of the rat. J. Comp. Neurol. 2000;421:107–135. doi: 10.1002/(sici)1096-9861(20000522)421:1<107::aid-cne7>3.3.co;2-6. [DOI] [PubMed] [Google Scholar]
- Grace AA. Dopamine. In: Davis KL, Charney D, Nemoroff C, editors. Neuropsychopharmacology: The fifth generation of progress. Lippincott/Williams & Wilkens; Philadelphia, PA: 2002. pp. 119–132. J.T. [Google Scholar]
- Groshek F, Kerfoot EC, McKenna V, Polackwich AS, Gallagher M, Holland PC. Amygdala central nucleus function is necessary for learning, but not expression, of conditioned auditory orienting. Behav. Neurosci. 2005;119:202–212. doi: 10.1037/0735-7044.119.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Fudge JL. The primate substantia nigra and VTA: integrative circuitry and function. Crit. Rev. Neurobiol. 1997;11:323–342. doi: 10.1615/critrevneurobiol.v11.i4.40. [DOI] [PubMed] [Google Scholar]
- Haber SN, Fudge JL, McFarland NR. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J. Neurosci. 2000;20:2369–2382. doi: 10.1523/JNEUROSCI.20-06-02369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J, Parkinson JA, Connor TM, Dickinson A, Everitt BJ. Involvement of the central nucleus of the amygdala and nucleus accumbens core in mediating Pavlovian influences on instrumental behaviour. Eur. J. .Neurosci. 2001;13:1984–1992. doi: 10.1046/j.0953-816x.2001.01577.x. [DOI] [PubMed] [Google Scholar]
- Han JS, McMahn RW, Holland PC, Gallagher M. The role of an amygdala-nigrostriatal pathway in associative learning. J. Neurosci. 1997;17:3913–3919. doi: 10.1523/JNEUROSCI.17-10-03913.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J-S, Holland PC, Gallagher M. Disconnection of the amygdala central nucleus and substantia innominata/nucleus basalis disrupts increments in conditioned stimulus processing in rats. Behav. Neurosci. 1999;113:143–151. doi: 10.1037//0735-7044.113.1.143. [DOI] [PubMed] [Google Scholar]
- Holland PC. Conditioned stimulus as a determinant of the form of the Pavlovian conditioned response. J. Exp. Psychol. Anim. Behav. Proc. 1977;3:77–104. doi: 10.1037//0097-7403.3.1.77. [DOI] [PubMed] [Google Scholar]
- Holland PC. CS-US interval as a determinant of the form of Pavlovian appetitive conditioned responses. J. Exp. Psychol. Anim. Behav. Proc. 1980;6:155–174. [PubMed] [Google Scholar]
- Holland PC. Brain mechanisms for changes in processing of conditioned stimuli in Pavlovian conditioning: Implications for behavior theory. Anim. Learn. Behav. 1997;25:373–399. [Google Scholar]
- Holland PC. Trial and intertrial durations in appetitive conditioning in rats. Anim. Learn. . Behav. 2000;28:121–135. [Google Scholar]
- Holland PC, Gallagher M. Double dissociation of the effects of lesions of basolateral and central amygdala on CS-potentiated feeding and Pavlovian-instrumental transfer. Eur. J. Neurosci. 2003;17:1680–1694. doi: 10.1046/j.1460-9568.2003.02585.x. [DOI] [PubMed] [Google Scholar]
- Horvitz JC. Mesolimbocortical and nigrostriatal dopamine responses to salient non-reward events. Neurosci. 2000;96:651–656. doi: 10.1016/s0306-4522(00)00019-1. [DOI] [PubMed] [Google Scholar]
- Horvitz JC. Dopamine gating of glutamatergic sensorimotor and incentive motivational input signals to the striatum. Behav. Brain Res. 2002;137:65–74. doi: 10.1016/s0166-4328(02)00285-1. [DOI] [PubMed] [Google Scholar]
- Iancu R, Mohapel P, Brundin P, Paul G. Behavioral characterization of a unilateral 6-OHDA-lesion model of Parkinson’s disease in mice. Behav. Brain Res. 2005;162:1–10. doi: 10.1016/j.bbr.2005.02.023. [DOI] [PubMed] [Google Scholar]
- Jahanshahi M, Jones CRG, Dirnberger G, Frith CD. The substantia nigra pars compacta and temporal processing. J. Neurosc.i. 2006;26:12266–12273. doi: 10.1523/JNEUROSCI.2540-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krettek JE, Price JL. Amygdaloid projections to subcortical structures within the basal forebrain and brainstem in the rat and cat. J. Comp. Neurol. 1978;178:225–253. doi: 10.1002/cne.901780204. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Groshek F, Petrovich GD, Cantalini JP, Gallagher M, Holland PC. Role of amygdala-nigral circuitry in conditioning of a visual stimulus paired with food. J. Neurosci. 2005;25:3881–3888. doi: 10.1523/JNEUROSCI.0416-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Gallagher M, Holland PC. The role of central amygdala connections to different midbrain dopamine systems for conditioned stimulus processing. Society for Neuroscience Abstract. 2006a:749.1. [Google Scholar]
- Lee HJ, Youn JM, O MJ, Gallagher M, Holland PC. Role of substantia nigra-amygdala connections in surprise-induced enhancement of attention. J. Neurosci. 2006b;26:6077–6081. doi: 10.1523/JNEUROSCI.1316-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matell MS, Meck WH. Neuropsychological mechanisms of interval timing behavior. BioEssays. 2000;22:94–103. doi: 10.1002/(SICI)1521-1878(200001)22:1<94::AID-BIES14>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Organization of amygdaloid projections to the mediodorsal thalamus and prefrontal cortex: a fluorescence retrograde transport study in the rat. J. Comp. Neurol. 1987;262:46–58. doi: 10.1002/cne.902620105. [DOI] [PubMed] [Google Scholar]
- Meloni EG, Davis M. Muscimol in the deep layers of the superior colliculus/mesencephalic reticular formation blocks expression but not acquisition of fear-potentiated startle in rats. Behav. Neurosci. 1999;113:1152–1160. doi: 10.1037//0735-7044.113.6.1152. [DOI] [PubMed] [Google Scholar]
- Moore AE, Cicchetti F, Hennen J, Isacson O. Parkinsonian motor deficits are reflected by proportional A9/A10 neuron degeneration in the rat. Exp. Neurol. 2001;172:363–376. doi: 10.1006/exnr.2001.7823. [DOI] [PubMed] [Google Scholar]
- Morris G, Aradir D, Nevet A, Vaadia E, Bergman H. Coincident but distinct messages of midbrain and striatal tonically active neurons. Neuron. 2004;43:133–143. doi: 10.1016/j.neuron.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Murschall A, Hauber W. Inactivation of the ventral tegmental area abolished the general excitatory influence of Pavlovian cues on instrumental performance. Learn. Mem. 2006;13:123–126. doi: 10.1101/lm.127106. [DOI] [PubMed] [Google Scholar]
- Nauta WJH, Domesick VB. Crossroads of limbic and striatal circuitry: hypothalamic-nigral connections. In: Livingston KE, Hornykiewicz O, editors. Limbic mechanisms. Plenum; New York: 1978. pp. 75–93. [Google Scholar]
- Parkinson JA, Dalley JW, Cardinal RN, Bamford B, Fehnert B, Lachenal G, Rudarakanchana N, Halkerson KM, Robbins TW, Everitt BJ. Nucleus accumbens dopamine depletion impairs both acquisition and performance of appetitive Pavlovian approach behaviour: implications for mesoaccumbens dopamine function. Behav. Brain Res. 2002;137:149–163. doi: 10.1016/s0166-4328(02)00291-7. [DOI] [PubMed] [Google Scholar]
- Parkinson JA, Robbins TW, Everitt BJ. Dissociable roles of the central and basolateral amygdala in appetitive emotional learning. Eur. J. Neurosci. 2000;12:405–413. doi: 10.1046/j.1460-9568.2000.00960.x. [DOI] [PubMed] [Google Scholar]
- Pitkänen A. Connectivity of the rat amygdaloid complex. In: Aggleton J, editor. The amygdala: a functional analysis. Oxford University Press; Oxford: 2000. pp. 31–115. [Google Scholar]
- Price JL, Amaral DG. An autoradiographic study of the projections of the central nucleus of the monkey amygdala. J. Neurosci. 1981;11:1242–1259. doi: 10.1523/JNEUROSCI.01-11-01242.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravizza SM, Ivry RB. Comparison of the basal ganglia and cerebellum in shifting attention. J. Cog. Neurosci. 2001;13:285–297. doi: 10.1162/08989290151137340. [DOI] [PubMed] [Google Scholar]
- Redgrave P, Prescott TJ, Gurney K. Is the short-latency dopamine response too short to signal reward error? Trends Neurosci. 2000;22:146–151. doi: 10.1016/s0166-2236(98)01373-3. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Baunez C, Everitt BJ, Robbins TW. Lesions of the medial and lateral striatum in the rat produce differential deficits in attentional performance. Behav. Neurosci. 2001;115:799–811. doi: 10.1037//0735-7044.115.4.799. [DOI] [PubMed] [Google Scholar]
- Rosen JB, Hitchcock JM, Sananes CB, Miserendino MJD, Davis M. A direct projection from the central nucleus of the amygdala to the acoustic startle pathway: Anterograde and retrograde tracing studies. Behav. Neurosci. 1991;105:817–825. doi: 10.1037/0735-7044.105.6.817. [DOI] [PubMed] [Google Scholar]
- Schultz W. Activity of dopamine neurions in the behaving primate. Sem. Neurosci. 1992;4:129–138. [Google Scholar]
- Schultz W, Dickinson A. Neuronal coding of prediction errors. Ann. Rev. Neurosci. 2000;23:473–500. doi: 10.1146/annurev.neuro.23.1.473. [DOI] [PubMed] [Google Scholar]
- Sun N, Cassell MD. Intrinsic GABAergic neurons in the rat central extended amygdala. J. Comp. Neurol. 1993;330:381–404. doi: 10.1002/cne.903300308. [DOI] [PubMed] [Google Scholar]
- Swanson LW. The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res. Bull. 1982;9:321–353. doi: 10.1016/0361-9230(82)90145-9. [DOI] [PubMed] [Google Scholar]
- Swanson LW. Brain Maps: Structure of the rat brain. 3rd edition Academic Press; San Diego, CA: 2004. [Google Scholar]
- Swanson LW, Petrovich GD. What is the amygdala? Trends Neurosci. 1998;21:323–331. doi: 10.1016/s0166-2236(98)01265-x. [DOI] [PubMed] [Google Scholar]
- Wallace DM, Magnuson DJ, Gray TS. The amygdalo-brainstem pathway: selective innervation of dopaminergic, noradrenergic and adrenergic cells in the rat. Neurosci. Let. 1989;97:252–258. doi: 10.1016/0304-3940(89)90606-x. [DOI] [PubMed] [Google Scholar]
- Zahm DS, Jensen SL, Williams ES, Martin JR., III Direct comparison of projections from the central amygdaloid region and nucleus accumbens shell. Eur. J. Neurosci. 1999;11:1119–1126. doi: 10.1046/j.1460-9568.1999.00524.x. [DOI] [PubMed] [Google Scholar]
- Zigmond MJ, Stricker EM. Recovery of feeding and drinking by rats after intraventricular 6-hydroxydopamine or lateral hypothalamic lesions. Science. 1973;182:717–720. doi: 10.1126/science.182.4113.717. [DOI] [PubMed] [Google Scholar]