Abstract
Omega-3 (n-3) fatty acids are emerging as bioactive agents protective against cardiovascular disease. However, their cellular delivery pathways are poorly defined. Here we questioned whether the uptake of n-3 TGRP is mediated by cell surface proteoglycans (PG) using LDLR+/+ and LDLR−/− cell models. LDLR+/+ but not LDLR−/− cells showed higher n-6 over n-3 TGRP uptake. Removal of cell surface proteins and receptors by pronase markedly enhanced the uptake of n-3 but not n-6 TGRP. Lactoferrin blockage of apoE-mediated pathways decreased the uptake of n-6 TGRP by up to 85% (p < 0.05) but had insignificant effect on n-3 TGRP uptake. PG removal by sodium chlorate in LDLR+/+ cells substantially reduced n-3 TGRP uptake but had little effect on n-6 TGRP uptake. Thus, while n-6 TGRP uptake is preferentially mediated by LDLR-dependent pathways, the uptake of n-3 TGRP depends more on PG and non- LDLR cell surface anchoring.
Supplementary key words: omega-3, omega-6, triglyceride-rich particles, apolipoprotein E, proteoglycan, LDL receptor, scavenger receptor-B1
Introduction
Accumulating evidence in humans and animal studies suggest beneficial roles of EPA and DHA in diminishing risk of cardiovascular diseases [1, 2], improving insulin resistance and diabetes [3]. These n-3 fatty acids also have positive effects on fetal and infant visual function and brain development [4, 5, 6], prevention of age-related neuronal atrophy [7, 8, 9], ameliorating allergic responses [10], and lowering the incidences of certain cancers [11, 12]. EPA and DHA alter the balance of eicosanoid synthetic pathways, resulting in reduction of potent pro-inflammatory mediators from arachidonic acid. Our previous study showed that n-3 polyunsaturated fatty acids are associated with significant down-regulation of SRE mediated gene expression [13] -- changes that would suppress de novo endogenous synthesis of fatty acids and TG in organs such as liver.
TGRP rich in with n-3 vs. n-6 fatty acids have different metabolic properties. In vitro studies demonstrated that the hydrolysis of n-6 TGRP by lipoprotein lipase (LpL) was much higher than that of n-3 TGRP, consistent with the in vivo mouse data that while n-3 TGRP has faster blood clearance as compared to n-6 TGRP, such effect is not associated with LpL activity [14]. The removal of n-6 but not n-3 TGRP from blood was modulated by apoE, LDL-R, and lactoferrin-sensitive pathways. However, mechanisms of n-3 TGRP clearance are still not well-defined.
In other studies, we showed that at physiological triglyceride-rich particle (TGRP) concentrations, cell surface proteoglycans (PG) can be a predominant non-LDL receptor mechanism for binding and internalizing IDL-sized TGRP made with triolein [15]. Our previous studies in cultured peritoneal macrophages suggested a role for cell surface PG in the uptake of n-3 TGRP. However, in that study, no comparison were made between n-6 vs. n-3 rich particles nor was the potential role of the LDLR considered [16].
The aim of the present study was to evaluate potential mechanisms for n-6 and n-3 TGRP clearance in vitro using LDLR +/+ and LDLR −/− fibroblasts. Our data indicates that n-6 TGRP, utilizes LDLR-mediated uptake whereas PG pathways play an important role in the cell uptake of n-3 TGRP.
Materials and methods
Materials
[1α, 2α (n)-3H] cholesteryl oleoyl ether was purchased from Nu-chek Prep Inc (Elysian, MN). Lactoferrin, aprotinin, sodium chlorate (NaClO3) and human α2-macroglobulin (α2m), bovine serum albumin (BSA) fraction V were from Sigma-Aldrich (St. Louis, MO). Heparinase and heparitinase were purchased from Seikagaku American Corp. (Rockville, MD). Purified recombinant apoE3 produced in Escherichia coli was kindly provided by Dr. Tikva Vogel (Biotechnology General Inc., Rehovot, Israel) and this apoE has been shown to exhibit the same in vitro binding and in vivo plasma behavior as does native plasma apoE3 [17].
Cells culture
LDLR+/+ (HS68) [16] and LDL−/− (GM01915C) [18] human skin fibroblasts were plated in monolayer at densities of approximately 2.5×104 or 5×104 cells/well in plates of 12 or 6 wells, respectively. Cells were maintained to 80% confluency in a humidified incubator (5% CO2) at 37 °C in Dulbecco’s modified Eagle’s medium supplemented with 10% (v/v) fetal or standard calf serum, penicillin (100 units/mL), glutamine (292 ug/mL), and streptomycin (100 units/mL) as described by Al-Haideri et al. [16].
Preparation of triglyceride-rich particles (TGRP)
Phospholipid-stabilized n-6 and n-3 TGRP were provided by B. Braun Melsungen AG (Melsungen, Germany) [15, 19]. TGRP were labeled with non-degradable [3H] CEt (0.2 mCi/100 mg of TG) to trace particle uptake using previously described methods [19–21]. Labeled TGRP was characterized for triglyceride content to determine specific activities using an enzymatic kit (GPO-PAP test) from Boehringer Mannheim (Indianapolis, IN) and stored at 4 °C under argon. Each batch of TGRP was used for experiments within 10 days of preparation.
Determination of n-6 and n-3 TGRP uptake
LDLR+/+ and LDLR−/− fibroblasts were incubated with lipoprotein-deficient serum (LPDS) to deplete cellular cholesterol two days before experiments. On the day of the experiments, cells were incubated with DMEM containing 1% BSA with n-6 and n-3 TGRPs in the presence or absence of 5% apolipoprotein E (w/w, apoE/TG). When apoE was used, radiolabeled TGRP were pre-incubated with apoE at 25°C with gentle agitation for 20 min before mixing with experimental medium as described above to allow sufficient time for equilibrium binding [20]. All experiments were carried out on a rocker (Lab Line Instruments, Inc., Melrose Park, IL) at 37 °C for 4 h. In some experiments, cells were pre-incubated with NaClO3 or pronase to inhibit proteoglycan sulfation and removal of cell surface proteins, respectively, as previously described [18, 22, 23]. Trypan blue and Thiazolyl Blue Tetrazolium Bromide (MTT) assays [24] were used to ascertain cell viability after these treatments and >80% of cells were viable after these treatments. At the end of each experiment, cells were chilled on ice and washed twice for 5 min with cold PBS containing 0.2% BSA and then quickly washed twice with PBS.
To measure TGRP uptake, cells were first treated with heparin (1400 units/mL of PBS) for 1 h at 4 °C to release surface bound TGRP [15]. After heparin treatment, cells were washed twice with PBS. For cell-associated [3H]CEt, cells were hydrolyzed by incubating with 0.1 N NaOH. Cell lysates were used for measurements of radioactivity and cell protein. Based on radioactivity measurements, cell protein and specific activity, uptake was expressed as micrograms of TG per mg of cell protein unless otherwise specified. Data are expressed as the mean ± SD of triplicate incubations. All experiments were repeated at least three times and showed similar results.
Statistics
Student t-tests were performed to evaluate the differences between mean values of two groups (with apoE vs. without apoE or with vs. without inhibitors, i.e. sodium chlorate and pronase). Results are expressed as mean ± SD of triplicate experimental determinations. Significant differences were determined at p <0.05 level.
Results
The role of LDLR in cellular uptake of n-6 vs. n-3 TGRP
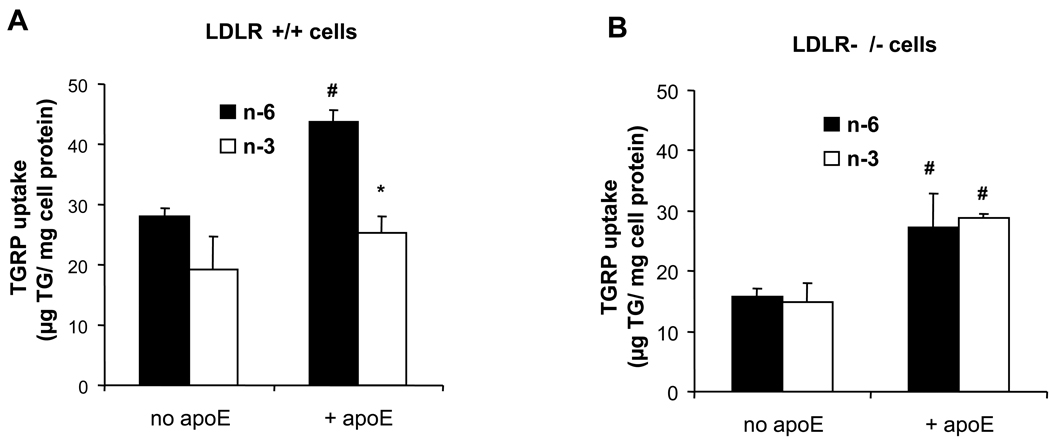
We first compared the uptake of n-3 and n-6 TGRP in LDLR+/+ and LDLR−/− human fibroblasts (Figure 1). When n-6 or n-3 TGRP was incubated in the absence of apoE in LDLR+/+ cells, n-6 TGRP uptake was slightly higher (not statistically different) than n-3 TGRP uptake (Figure 1A). There was no difference in n-6 vs. n-3 TGRP uptake in LDLR−/− cells (Figure 1B). Interestingly, in LDLR +/+ cells, TGRP co-incubation with recombinant human apoE (5%, w/w), stimulated n-6 TGRP uptake by over 50% (p< 0.05) but showed no significant effect on n-3 TGRP uptake (Figure 1A). In the absence of LDLR, there was no difference between the uptake of n-3 and n-6 TGRPs regardless of the presence of apoE (Figure 1B). Nevertheless, apoE was equally potent in stimulating n-3 and n-6 uptake in the absence of LDLR (Figure 1B). Since n-3 TGRP cellular uptake was increased more by apoE in LDLR−/− cells, these results provide evidence that n-3 uptake pathways are less dependent on LDLR, and indicate the presence of alternative pathways for n-3 TGRP cell uptake.
Figure 1. Effects of apoE and LDLR on n-6 and n-3 TGRP uptake.
(A) LDL receptor positive HS68 fibroblasts (LDLR+/+) and (B) LDL receptor negative (LDLR−/−) 01915C fibroblasts were incubated with 200 µg/mL n-6 (closed bars) or n-3 TGRP (open bars) in the absence or presence of 5% apoE (w/w) for 4 h at 37 °C. Results are the means for TG uptake in triplicate determination ± SD and expressed as micrograms of TG per milligram of cell protein. # p < 0.05, significant increase of TGRP uptake in the presence apoE. * p < 0.05, significant difference in the uptake of n-3 vs. n-6 particles.
Cells were then treated with pronase to further investigate the roles of cell surface protein in n-6 and n-3 particle uptake. Cells were pre-incubated with pronase to remove pronase-susceptable proteins prior to TGRP treatment. Table 1 shows that removal of cell surface proteins had little effects on n-6 uptake but markedly enhanced the uptake of n-3 TGRP at 4-hr incubation.
Table 1.
Pronase treatment affects the uptake of n-3 but not n-6 TGRP.
| TGRP uptake (4hrs) (µg TG/mg cell protein) |
||
|---|---|---|
| n-6 | n-3 | |
| Pronase (−) | 7.15 ± 0.39 | 6.66 ± 0.52 |
| Pronase (+) | 5.79 ± 0.72 | 27024* ± 5.61 |
| Pronase (+/+) | 5.85 ± 0.98 | 16.853# ± 3.92 |
Pronase (−): control without pronase treatment
Pronase (+):pronase pre-treatment
Pronase (+/+):pronase pre-treatment plus co-incubation with TGRP
p<0.05;
p=0.05
Effects of lactoferrin and α2-macroglobulin on the uptake of n-3 and n-6 TGRPs
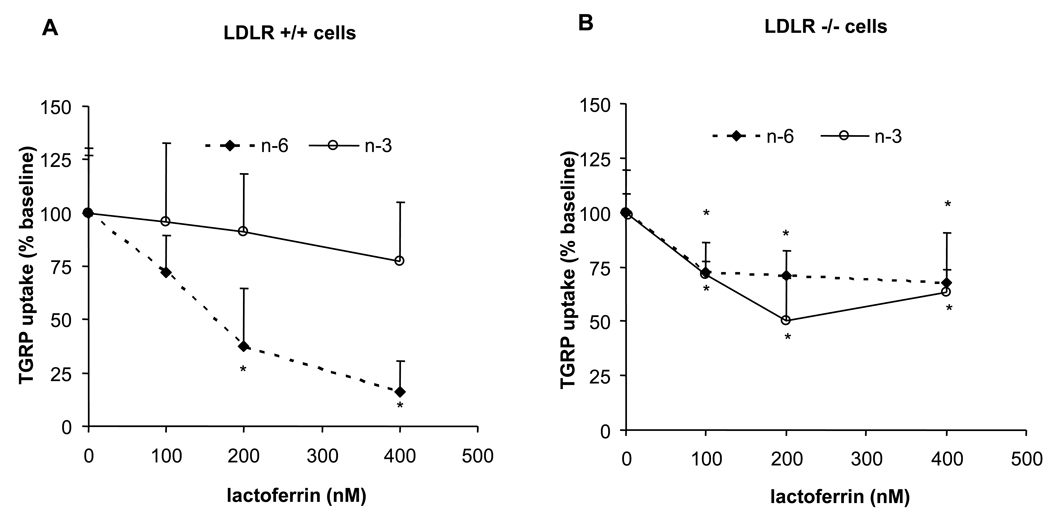
We previously demonstrated that lactoferrin inhibits blood clearance and cell uptake of n-6 TGRPs by apoE-mediated pathways in vivo and in vitro [14]. Also, LDLR-related protein (LRP) can have a role in TGRP uptake [25]. When α2-macroglobulin was incubated with n-6 or n-3 TGRPs, α2m did not affect the uptake of n-6 or n-3 TGRP in either cell line at all concentrations (data not shown). This indicates that under conditions herein LRP is not involved in differentiating the uptake of n-3 and n-6 TGRPs. We incubated n-6 and n-3 TGRP with lactoferrin to further substantiate the role of apoE-mediated pathways in the uptake of n-6 or n-3 TGRPs (Figure 2). Lactoferrin inhibited n-6 TGRP uptake in a dose dependent manner, and at the highest lactoferrin concentration (400 nM), n-6 TGRP uptake was reduced by 85% in LDLR+/+ cells (Figure 2A). Lactoferrin minimally reduced uptake of n-3 TGRP in LDLR+/+ cells. On the contrary, LDLR−/− cells showed a comparable 30% decrease in the uptake of n-6 and n-3 TGRP (Figure 2B), suggesting that lactoferrin-sensitive, LDLR-independent pathways mediate the uptake of n-6 and n-3 TGRPs to a similar extent, a finding that likely related to the ability of lactoferrin to inhibit apoE binding to multiple sites on the cell surface. These results are consistent with those in Figure 1, which they show that apoE-mediated uptake via the LDLR is responsible more for n-6 than n-3 TGRP uptake. On the other hand, in the absence of the LDLR, lactoferrin-sensitive mechanisms contribute equally to the uptake of n-6 TGRPs as well as n-3 TGRPs.
Figure 2. Effects of lactoferrin on the uptake of n-6 and n-3 TGRPs.
Concentration-dependent effect of lactoferrin on TGRP uptake in LDLR+/+ (A) and (B) LDLR−/− fibroblasts. Cells were incubated in the presence of 1% BSA, [3H]CEt-TGRP (200 µg/ml), and in the presence of apoE (5%w/w) and lactoferrin (0–400 nM), at 37 °C for 4 h to measure apoE-mediated pathways. Results are the mean ± SD of triplicate determinations expressed percentage (%). Baseline value of no lactoferrin treatment was set at 100%. * p < 0.05, significant decrease from baseline.
Effects of cell surface proteoglycans on n-6 and n-3 TGRP uptake
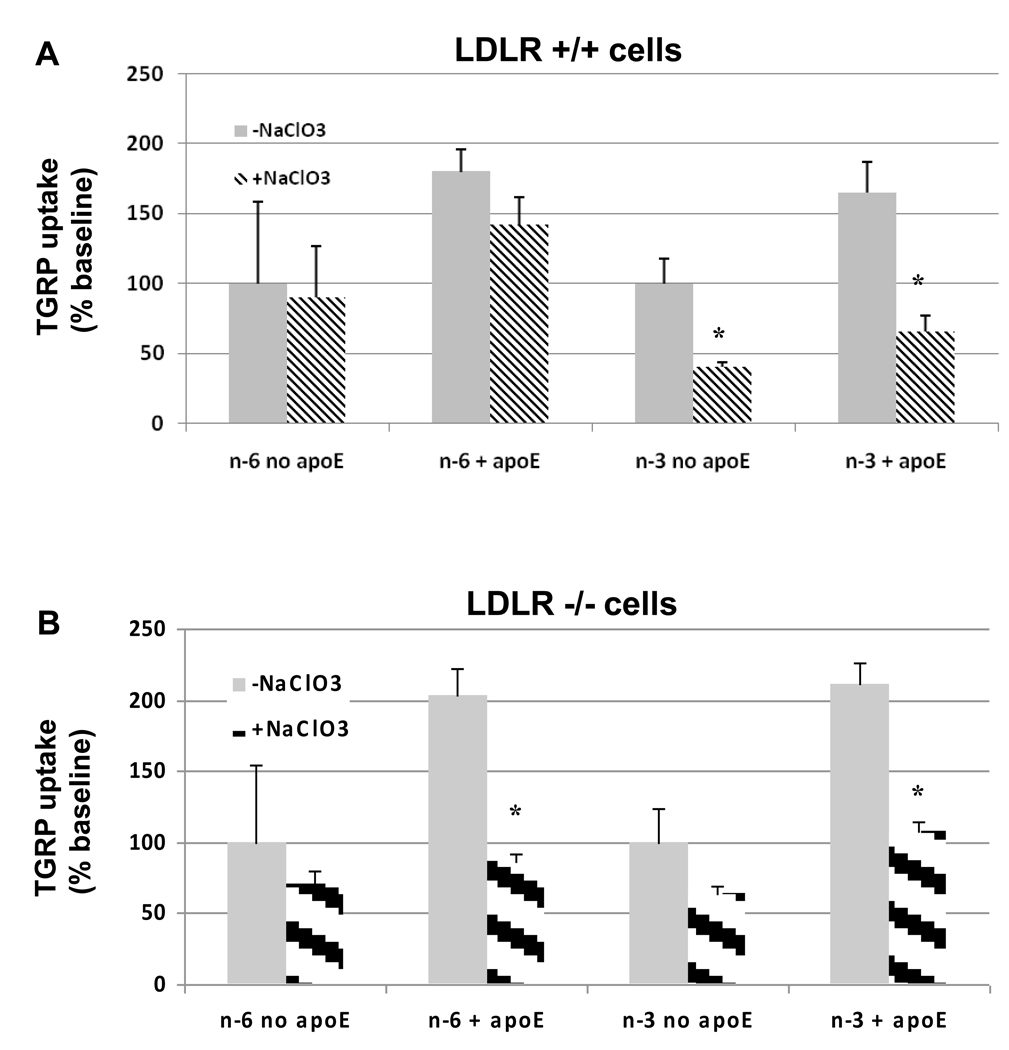
We assessed the role of proteoglycan pathways using sodium chlorate which inhibits sulfation of proteoglycans, as well as heparitinase to remove cell surface heparan sulfate glycosaminoglycans. As shown in Figure 3, chlorate had little or no effects on n-6 TGRP uptake in the absence of apoE in LDLR+/+ (Figure 3A) or LDLR−/− (Figure 3B) cells. Incubation with NaClO3 did not significantly reduce n-6 TGRP uptake in the presence of apoE in LDLR+/+ cells, consistent with a major role of the LDLR in mediating the uptake of n-6 particles (Figure 3A). However, in the absence of LDLR, n-6 TGRP uptake also became sensitive to NaClO3 treatment (Figure 3B). On the other hand, n-3 TGRP uptake was significantly reduced by chlorate in both LDLR+/+ and LDLR−/− cells, in the presence and absence of apoE. Heparitinase was used for specific studies on heparan sulfate specific proteoglycans. The effect of heparitinase was similar in that it reduced n-3 TGRP uptake substantially more than n-6 TGRP uptake (data not shown). These data support the role of PG rather than the LDLR in mediating n-3 TGRP uptake.
Figure 3. Effect of sodium chlorate on n-6 and n-3 TGRP uptake.
LDLR+/+ (A) and LDLR−/− (B) fibroblasts were incubated in 10% LPDS and NaClO3 (50mM) for 14 h prior to the experiments. On the day after experiment, cells were incubated with 1% BSA containing in the absence (grey bars) or presence (hatched bars) of 50 mM NaClO3 and radiolabeled TGRPs for 4 h in order to inhibit the sulfation of newly synthesized proteoglycans. Results are the mean ± SD of triplicate determinations expressed percentage (%). Baseline uptake value of no apoE treatment was set at 100%. * p < 0.05, significant difference between NaClO3-treated and non- NaClO3-treated groups‥
Discussion
We previously demonstrated that cell uptake pathways of TGRP differs depending on triglyceride composition in vitro and in vivo [14, 26]. Although these studies clearly showed that n-6 TGRP uptake was mediated by both LDLR- and apoE-dependent pathways, little is known regarding pathways responsible for n-3 TGRP uptake. Our current studies describe potential mechanisms for n-3 TGRP cell uptake that are distinct from those of n-6 TGRP. The results herein demonstrate that while apoE enhances both n-6 and n-3 TGRP uptake, it acts more to increase n-6 TGRP uptake in LDLR+/+ cells. Our pronase results imply that the removal of cell surface protein alleviates the suppressive mechanisms for cellular n-3 TGRP uptake but does not affect n-6 TGRP uptake. We also found that lactoferrin, an agent competes with cell surface apoE binding, substantially reduced n-6, but not n-3 TGRP uptake in LDLR+/+ cells, in keeping with previous studies that n-6 TGRP uptake is more LDLR dependent as compared to n-3 TGRP [14, 15, 21]. Finally, inhibition of the PG pathways markedly reduced n-3 TGRP uptake with little change in n-6 TGRP uptake in LDLR+/+ cells. These differences were not seen in LDLR−/− cells, supporting that LDLR has a greater contribution to n-6 than to n-3 TGRP uptake. The uptake of n-3 TGRP is largely mediated by cell surface proteoglycans.
Pathways of n-6 TGRP uptake have been studied extensively. These particles are cleared through “classical” pathways such as the LDLR have shown that n-6 TGRP clearance and cell uptake are dependent on lactoferrin-sensitive and apoE mediated pathways [15]. Moreover, membrane anchoring was seen with n-3 TGRP in cell culture, which is consistent with margination of n-3 TGRP in human studies [26]. n-3 TG contains EPA and DHA, which has been shown to significantly increase margination volumes [27]. LpL, different from its catalytic activity, also increases anchoring of lipoprotein particles to cell surfaces via its binding functions [28, 29]. While our studies did not investigate n-3 clearance with regards to LpL metabolism, Park et al has shown that margination of n-3-rich particles correlates with LpL activity [27]. These observations justified further elucidation of the cellular mechanisms mediating n-3 TGRP uptake.
α2-macroglobulin (α2M) was used to examine the binding sites of the lipoprotein receptor-related protein (LRP). Although Kowal et al. [25] showed that α2M/LRP can mediate apoE- enriched TGRP uptake, our data do not indicate a major role of LRP on TGRP uptake at least in fibroblasts. LRP possesses an apoE-dependent recognition site [30] and it is known to have different binding domains for α2M and apoE. As a result, it is possible that while the α2M binding domain is inhibited, the apoE-binding domain is mediating TGRP uptake.
Our previous studies have demonstrated disparate metabolic effects of n-6 and n-3 TGRP in vitro and in vivo [14, 16]. This can explain the different effects on protein expression and disease outcomes such as chronic and acute inflammatory diseases (i.e. CVD). Further understanding of the metabolic differences between n-3 and n-6 TGRPs is of interest since modulation of TG composition provides vehicles for potential pharmaceutical targeting with tissue-specific delivery. Additional pathways need to be examined to further delineate n-3 TGRP clearance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Calder PC. n-3 Fatty acids and cardiovascular disease: evidence explained and mechanisms explored. Clin. Sci. (London) 2004;107:1–11. doi: 10.1042/CS20040119. [DOI] [PubMed] [Google Scholar]
- 2.Von Schacky C. Omega-3 fatty acids and cardiovascular disease. Curr. Opin. Clin. Nutr. Metab. Care. 2004;7:131–136. doi: 10.1097/00075197-200403000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Pan DA, Lillioja S, Milner MR, Kriketos AD, Baur LA, Bogardus C, Storlien LH. Skeletal muscle membrane lipid composition is related to adiposity and insulin action. J. Clin. Invest. 1995;96:2802–2808. doi: 10.1172/JCI118350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Connor DL, Hall R, Adamkin D, Auestad N, Castillo M, Connor WE, Connor SL, Fitzgerald K, Groh-Wargo S, Hartmann EE, Jacobs J, Janowsky J, Lucas A, Margeson D, Mena P, Neuringer M, Nesin M, Singer L, Stephenson T, Szabo J, Zemon V. Growth and development in preterm infants fed long-chain polyunsaturated fatty acids: a prospective, randomized controlled trial. Pediatrics. 2001;108:359–371. doi: 10.1542/peds.108.2.359. [DOI] [PubMed] [Google Scholar]
- 5.Birch EE, Hoffman DR, Uauy R, Birch DG, Prestidge C. Visual acuity and the essentiality of docosahexaenoic acid and arachidonic acid in the diet of term infants. Peditr. Res. 1998;44:201–209. doi: 10.1203/00006450-199808000-00011. [DOI] [PubMed] [Google Scholar]
- 6.Birch EE, Garfield S, Hoffman DR, Uauy R, Birch DG. A randomized controlled trial of early dietary supply of long-chain polyunsaturated fatty acids in term infants. Dev. Med. Child. Neurol. 2000;42:174–181. doi: 10.1017/s0012162200000311. [DOI] [PubMed] [Google Scholar]
- 7.Salem N, Jr., Niebylski CD. The nervous system has an absolute molecular species requirement for proper function. Mol. Membr. Biol. 1995;12:131–134. doi: 10.3109/09687689509038508. [DOI] [PubMed] [Google Scholar]
- 8.Hgyes E, Nyakas C, Kiliaan A, Farkas T, Penke B, Luiten PGM. Neuroprotective effect of developmental docosahexaenoic acid supplement against excitotoxic brain damage in infant rats. Neuroscience. 2003;119:999–1012. doi: 10.1016/s0306-4522(03)00198-2. [DOI] [PubMed] [Google Scholar]
- 9.Nunzi MG, Milan F, Guidolin D, Polato P, Toffano G. Effects of phosphatidylserine administration of aged-related structural changes in the rat hippocampus and septal complex. Pharmacopsychiatry. 1989;22 Suppl 2:125–128. doi: 10.1055/s-2007-1014632. [DOI] [PubMed] [Google Scholar]
- 10.Hilkens CM, Vermeulen H, Joost van Neerven RJ, Snijdewint FGM, Wierenga EA, Kapsenber ML. Differevtial modulation of T helper type 1 (Th1) and T helper type 2 (Th2) cytokine secretion by prostaglandin E2 critically depends on interleukin-2. Eur. J. Immonol. 1995;25:59–63. doi: 10.1002/eji.1830250112. [DOI] [PubMed] [Google Scholar]
- 11.Larsson SC, Kumlin M, Ingelman-Sundberg M, Wolk A. Dietary long-chain n-3 fatty acids for the prevention of cancer: a review of potential mechanisms. Am. J. Clin. Nutr. 2004;79:935–945. doi: 10.1093/ajcn/79.6.935. [DOI] [PubMed] [Google Scholar]
- 12.Roynette CE, Calder PC, Dupertuis YM, Pichard C. n-3 Polyunsaturated fatty acids and colon cancer prevention. Clin. Nutr. 2004;23:139–151. doi: 10.1016/j.clnu.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 13.Worgall TS, Sturley SL, Seo T, Osborne TF, Deckelbaum RJ. Polyunsaturated fatty acids decrease expression of promoters with sterol regulatory elements by decreasing levels of mature sterol regulatory element-binding protein. J. Biol. Chem. 1998;273:25537–25540. doi: 10.1074/jbc.273.40.25537. [DOI] [PubMed] [Google Scholar]
- 14.Qi K, Seo T, Al-Haideri M, Worgall TS, Vogel T, Carpentier YA, Deckelbaum RJ. Omega-3 triglycerides modify blood clearance and tissue targeting pathways of lipid emulsions. Biochemistry. 2002;41:3119–3127. doi: 10.1021/bi015770h. [DOI] [PubMed] [Google Scholar]
- 15.Al-Haideri M, Goldberg IJ, Galeano NF, Gleeson A, Vogel T, Gorecki M, Sturley SL, Deckelbaum RJ. Heparan sulfate proteoglycan-mediated uptake of apolipoprotein E-triglyceride-rich lipoprotein particles: a major pathway at physiological particle concentrations. Biochemistry. 1997;36:12766–12772. doi: 10.1021/bi9631024. [DOI] [PubMed] [Google Scholar]
- 16.Densupsoontorn N, Carpentier YA, Racine R, Seo T, Murray FM, Deckelbaum RJ. Non-classical pathways for n-3 triglyceride-rich particle blood clearance in mouse models in vivo and peritoneal macrophages in vitro. J. Nutrition. 2008;138:257–261. doi: 10.1093/jn/138.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vogel T, Weisgraber KH, Zeevi MI, Ben-Artzi H, Levanon AZ, Rall SC, Jr., Innerarity TL, Hui DY, Taylor JM, Kanner D. Human apolipoprotein E expression in Escherichia coli: Structural and function identity of the bacterially produced protein with plasma apolipoprotein E. Proc. Natl. Acad. Sci. 1985;82:8698–8700. doi: 10.1073/pnas.82.24.8696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seo T, St. Clair RW. Heparan sulfate proteoglycans mediate internalization and degradation of B-VLDL and promote cholesterol accumulation by pigeon macrophages. J. Lipid Res. 1997;38:765–779. [PubMed] [Google Scholar]
- 19.Treskova E, Carpentier YA, Ramakrishnan R, Al-Haideri M, Seo T, Deckelbaum RJ. Blood clearance and tissue uptake of intravenous lipid emulsions containing long-chain and medium-chain triglycerides and fish oil in a mouse model. JPEN J Parenter Enteral Nutr. 1999;23:253–257. doi: 10.1177/0148607199023005253. [DOI] [PubMed] [Google Scholar]
- 20.Schweigelsohn B, Presley JF, Gorecki M, Vogel T, Carpentier YA, Maxfield FR, Deckelbaum RJ. Effects of apoprotein E on intracellular metabolism of model triglyceride-rich particles are distinct effects on cell particle uptake. J. Biol. Chem. 1995;270:1761–1769. doi: 10.1074/jbc.270.4.1761. [DOI] [PubMed] [Google Scholar]
- 21.Granot E, Schweigelsohn B, Tabas I, Gorecki M, Vogel T, Carpentier YA, Deckelbaum RJ. Effects of particle size on cell uptake of model triglyceride-rich particles with and without apoprotein E. Biochem. 1994;33:15190–15197. doi: 10.1021/bi00254a030. [DOI] [PubMed] [Google Scholar]
- 22.Galeano NF, Al-Haideri M, Keyserman F, Rumsey SC, Deckelbaum RJ. Small dense low density lipoprotein has increased affinity for LDL receptor-independent cell surface binding sites: a potential mechanism for increased atherogenicity. J. Lipid Res. 1998;39:1263–1273. [PubMed] [Google Scholar]
- 23.Ho YY, Al-Haideri M, Mazzone T, Vogel T, Presley JF, Sturley SL, Deckelbaum RJ. Endgenously expressed apolipoprotein E has different effects on cell Lipid metabolism as compared to exogenous apolipoprotein E carried on triglyceride- rich particles. Biochem. 2000;39:4756–4754. doi: 10.1021/bi992294a. [DOI] [PubMed] [Google Scholar]
- 24.Carmichael J, Degraff WG, Gazdar AF, Minna JD, Mitchell JB. Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of chemosensitivity testing. Cancer Res. 1987;47:936–942. [PubMed] [Google Scholar]
- 25.Kowal RC, Herz J, Goldstein JL, Esser V, Brown MS. Low Density receptor-related protein mediates uptake of cholesteryl esters derived from apoprotein E-enriched lipoproteins. Proc. Natl. Acad. Sci. 1989;86:5810–5814. doi: 10.1073/pnas.86.15.5810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qi K, Seo T, Jiang Z, Carpentier YA, Deckelbaum RJ. Triglycerides in fish oil affect the blood clearance of lipid emulsions containing long- and medium-chain triglycerides in mice. J. Nutr. 2006;136:2766–2772. doi: 10.1093/jn/136.11.2766. [DOI] [PubMed] [Google Scholar]
- 27.Park Y, Jones PG, Harris WS. Triaclyglycerol-rich lipoprotein margination: a potential surrogate for whole-body lipoprotein lipase activity and effects of eicosapentaenoic and docosahexaenoic acids. Am. J. Clin. Nutr. 2004;80:45–50. doi: 10.1093/ajcn/80.1.45. [DOI] [PubMed] [Google Scholar]
- 28.Williams KJ, Fless GM, Petrie KA, Synder ML, Brocia RW, Swenson TL. Mechanisms by which lipoprotein lipase alters cellular metabolism of lipoprotein (a), low density lipoprotein, and nascent lipoproteins. Roles for low density lipoprotein receptors and heparan sulfate proteoglycans. J. Biol. Chem. 1992;267:13284–13292. [PubMed] [Google Scholar]
- 29.Rumsey SC, Obunike JC, Arad Y, Deckelbaum RJ, Goldberg IJ. Lipoprotein lipase-mediated uptake and degradation of low density lipoproteins by fibroblasts and macrophages. J. Clin. Invest. 1992;90:1504–1512. doi: 10.1172/JCI116018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vash B, Phung N, Zein S, DeCamp D. Three complement-type repeats of the low-density lipoprotein receptor-related define a common binding site for RAP, PAI-1, and lactoferrin. Blood. 1998;92:3277–3285. [PubMed] [Google Scholar]