Abstract
Exposure to ultraviolet B (UVB) irradiation (290–320 nm wavelength) from sunlight induces a variety of medical problems, including sunburn, immunosuppression and skin cancers. However, the molecular mechanisms related to UVB-induced cell damage and/or mutagenic effects have not been fully defined. Here, we demonstrate that one of the catalytic subunits of the IκB kinase complex (IKK), IKKα, plays a critical role in mediation of the UVB-induced G0/G1 cell cycle arrest response by suppressing Cyclin D1 expression. Notably, IKKa-dependent Cyclin D1 regulation is unrelated to IKKβ/NF-κB activity. We further show that IKKα-dependent downregulation of Cyclin D1 expression in the UVB response results from the reduction of ERK1/2-dependent Cyclin D1 transcription coupled with an increase of p38 kinase-dependent Cyclin D1 proteolysis. Thus, our results have identified the novel role of IKKα in regulating cell cycle progression during the cellular UVB response. Targeting IKKα might be promising for the prevention of UVB-induced cell damage and tumorigenic effects.
Keywords: UVB, IKKα, Cyclin D1, cell cycle arrest
Introduction
The ultraviolet (UV) spectrum from sunlight can be separated into three wavelengths, UVA (320–400 nm), UVB (290–320 nm) and UVC (200–290 nm). UVB has been identified as the most harmful environmental stressor, which is implicated in a variety of medical problems, including sunburn and skin cancers [1]. Cell cycle arrest and apoptosis are two major protective responses against UVB-induced cell damage and mutagenic effects, which allow time for repair of the DNA damage or move the severely damaged cells towards apoptosis. Deregulation of either (or both) response contributes largely to the UVB-related pathogenesis [1, 2].
Cyclin D1 plays a crucial role in determining G1 to S cell cycle progression. It can form an active complex with cyclin-dependent kinases 4 and 6 (CDK4 and CDK6) and promote the initiation of the G1/S cell cycle transition by phosphorylating and inactivating the retinoblastoma protein (pRB), a gatekeeper of the G1 phase. Consequently, Cyclin D1 expression levels are tightly controlled, and the deregulated overexpression of this protein has been found to frequently occur in multiple human cancers [3–5].
As a key cell cycle checkpoint protein, the expression of Cyclin D1 is regulated at both the transcriptional and post-translational levels [3–5]. It has been demonstrated that multiple transcriptional factors, such as NF-κB, AP-1, TRE, Oct-1, E2F, CRE, SP-1, ELK-1, c-Ets-1 and c-Ets-2, are able to bind directly to the promoter region of the cyclin d1 gene (CCND1), thereby inducing transcription and mediating cell proliferation in response to a variety of growth stimuli [6–9]. Furthermore, the three mitogen-activated protein kinases (MAPKs), ERKs, JNKs and p38K, are also involved in modulating cyclin d1 transcription by regulating the activities of distinct transcriptional factors [7, 10, 11]. In addition to transcriptional induction, the Cyclin D1 protein is subjected to rapid degradation via the ubiquitin-dependent pathway after performing its action at the G1/S transition [3–5]. The signal for targeting Cyclin D1 to the degradation pathway is largely dependent on phosphorylation at Thr286. GSK-3β, p38K and ERK2 have been defined as the major protein kinases for mediating Cyclin D1 Thr286 phosphorylation and thereby inducing its degradation under different conditions [12–16].
IKKα and IKKβ are catalytic subunits of the IKK complex, which are responsible for the activation of the NF-κB transcription factor and have important implications in cell fate determination under various stress conditions [17]. Although sharing structure similarity, the mechanisms underlying the actions of IKKα and IKKβ in NF-κB activation are quite different. IKKβ is involved in canonical NF-κB pathway activation by triggering the phosphorylation and degradation of the NF-κB inhibitor, I-κB; while IKKα plays a key role in the non-canonical NF-κB pathway activation, which depends on the processing of the NF-κB2/p100 precursor to the p52 subunit [17]. Interestingly, IKKα can also contribute to the canonical NF-κB pathway activation by binding to the promoter regions of NF-κB-responsive genes and mediating the phosphorylation of promoter-associated histone H3 [18, 19]. Moreover, studies of IKKα-null mice have revealed that IKKα possesses some unique functions essential for regulating the development of certain tissues and cancer cells, which cannot be compensated for by IKKβ [8, 20–27]. These results indicate that IKKα may be a multi-functional protein, and its roles may go far beyond its well-known action in NF-κB pathway regulation.
In the current study, we define a novel role for IKKα in initiating the inducible G0/G1 growth arrest response by triggering a rapid downregulation of Cyclin D1 expression in mouse embryonic fibroblasts (MEFs) under UVB exposure, which events are independent of IKKβ/NF-κB activity. We further provide evidence that the effect of IKKα in mediating Cyclin D1 downregulation results from the reduction of ERKs-dependent cyclin d1 gene transcription coupled with an increase of p38 kinase-dependent Cyclin D1 degradation in response to UVB irradiation. Accordingly, we have established the independent function of IKKα in mediating the cell cycle checkpoint under UVB exposure, and this may provide a novel target for the prevention of UVB-induced cell damage effect.
Materials and methods
Cells, plasmids, viruses and reagents
The wild type (WT), IKKβ−/−, IKKα−/− and stably reconstituted IKKα−/−(IKKα) MEFs; the p65+/+ and p65−/− MEFs; and the WT MEFs stably expressing the kinase mutant of IKKβ (IKK-KM) are described in previous studies [28, 29]. The Cyclin D1-luciferase reporter plasmid, in which the transcription of the luciferase reporter gene is driven by the upstream 5’-flanking region of the cyclin d1 gene promoter, has been described previously [30]. The expression plasmid containing the IκBα-AA mutation was provided by Dr. Yong Lin (Lovelace Respiratory Research Institute, USA) and was described previously [31]. WT IKKα and the IKKα kinase mutant (IKKα-KM) adenoviruses were kindly provided by Dr. Yinling Hu (NIH, BA) and were described previously [32]. The antibodies against phospho-JNKs, JNKs, phospho-p38K, p38K, phospho-ERKs, ERKs, phospho-AKT, AKT, phospho-GSK3α/β, GSK3β, phospho-IKKα/β, IKKα, IKKβ, phospho-Cyclin D1 and IκBα were purchased from Cell Signaling Technology (Beverly, MA). The antibodies against Cyclin D1, Cyclin D2, CDK4, p65 and HA were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). The kinase inhibitors PD98059, SP600125, SB202190, LiCl, and the anti-β-actin and anti-FLAG antibodies were obtained from Sigma (St. Louis, MO). Cycloheximide (CHX), MG132 and DAPI were purchased from Calbiochem (San Diego, CA)
UVB apparatus and cell exposure
The UVB light source (CL-1000M Midrange Ultraviolet Crosslinker) was purchased from UVP Inc. (Upland, CA) and emitted 302 nm wavelength UVB light. The Crosslinker is designed to measure and control the UVB radiation within the exposure chamber. A unique UV sensor continually measures the UVB energy and automatically adjusts to variations in UVB intensity that occurs as the UVB tubes age. This same UV sensor feedback measurement system allows us to set UV samples exposures, which automatically deactivates the UV sources when the set UV energy dose has been achieved. Before UVB irradiation, cells seeded in 6-well plates were washed with 2 ml PBS and replaced with 1ml fresh PBS each well. Cells were irradiated at the desired intensity without the plastic lid. After irradiation, cells were returned to incubation in the regular medium for the various time periods prior to harvest.
Cell transfection
All transfections were performed with LipofacTAMINE 2000 reagents (Life Technologies, Inc., Rockville, MD) according to the manufacturer’s instructions. To establish the stable Cyclin D1 luciferase reporter construct-transfected WT and IKKα−/− MEFs, the luciferase reporter plasmid was introduced into the cells together with lacZ-hygro+, a vector conferring hygromycin-resistance. The corresponding hygromycin-resistant stable transfectants were established as a polyclonal mass under hygromycin B selection. These transfectants were then cultured in hygromycin-free medium for at least two passages before subjecting to the various experiments.
Adenovirus infection
IKKα−/− MEFs were infected with 1×108 p.f.u. adenovirus (GFP, WT-IKKα or IKKα-KM) at 70–80% confluence. Virus-containing medium was replaced with fresh medium 24 hrs after infection.
Reverse transcription polymerase chain reaction (RT-PCR)
Total RNA was extracted with Trizol reagent (Gibco) and cDNAs were synthesized with the ThermoScript ™ RT-PCR system (Invitrogen, Carlsbad, CA). To detect cyclin d1 induction, a pair of oligonucleotides (5’- TCC CTT GAC TGC CGA GAA G-3’ and 5’- AGA CCA GCC TCT TCC TCC AC-3’) were synthesized and used as the specific primers to amplify mouse cyclin d1 cDNA. Mouse β-actin cDNA was amplified by the primers 5’-GACGATGATATTGCCGCACT-3’ and 5’-GATACC ACGCTTGCTCTGAG-3’.
Cell cycle assay
Cells seeded in 6-well plates (2×105/well) were starved by replacing medium with 0.1% FBS DMEM 24 hrs prior to UVB radiation. UVB exposure was preformed and the cells were then stained with propidium iodide (PI) solution according to the previous report [33]. Cell cycle distribution was determined by flow cytometry, utilizing a Beckman-Coulter EpicsXL flow cytometer. Twenty thousand events were counted for each analysis, and two to four independent experiments were conducted in each group.
Western-blot assay
Cellular protein extracts were prepared with cell lysis buffer (10 mM Tris-HCl, pH 7.4, 1% SDS, 1 mM Na3VO4) and resolved by SDS-PAGE. The membranes were probed with the indicated primary antibodies and HRP-conjugated secondary antibody. Signals were detected with the ECL western-blotting system as described in our previous reports [28, 29, 33].
Luciferase reporter assay
MEFs stably expressing the luciferase reporter constructs were seeded into 96-well plates (8×103/well) and subjected to UV exposure when the cultures reached 80–90% confluence. Cellular lysates were prepared at the indicated time points, and the luciferase activities were determined by a luminometer (Wallac 1420 Victor 2 multilabel counter system) as described in our previous studies [30, 33]. The results are expressed as relative activity that is normalized to the control cells without treatment.
Immunofluorescence microscopy
Cells were fixed in cold methanol for 15mins and blocked in PBS containing 2% BSA and 0.2% Triton X-100 for 1 hr. The samples were then incubated with anti-Cyclin D1 as the primary antibody, followed by AlexaFluor488 goat anti-mouse IgG (Invitrogen) as the secondary antibody. DAPI was used to stain the nucleus. Images were acquired on an Olympus microscope (Olympus IX71-F22FL/PH).
Results
IKKα is required for the UVB-induced G0/G1 growth arrest response and Cyclin D1 downregulation in MEFs
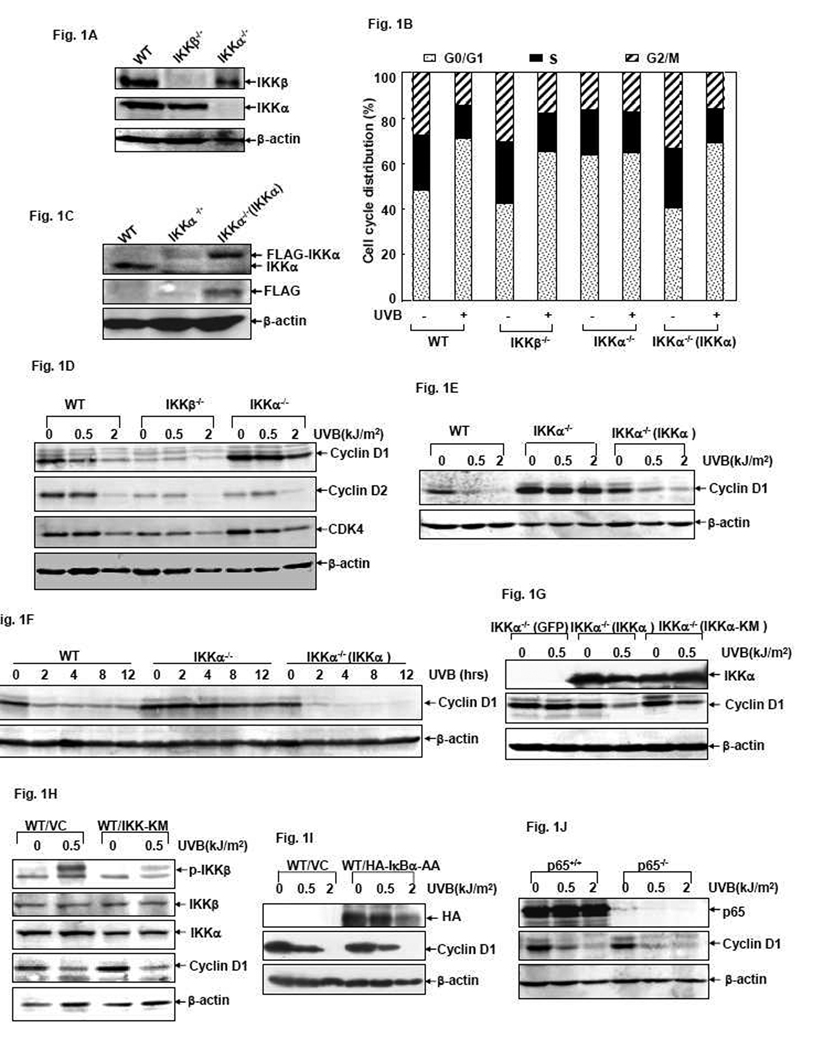
Growth arrest is a cellular protective response against UVB-induced damage [1]. To investigate the roles of IKKα and IKKβ in cellular UVB responses, we first analyzed the cell cycle distribution of WT, IKKα- and IKKβ-gene knockout MEFs (Fig. 1A) in the absence or presence of UVB exposure. As shown in Fig. 1B, UVB irradiation induced a significant growth arrest at the G0/G1 boundary almost identically in the WT and the IKKβ−/− MEFs. However, this response was completely blocked in the IKKα−/− MEFs under the same conditions. To further confirm these data, we reconstituted IKKα−/− MEFs with IKKα (Fig. 1C) and then examined whether the UVB-induced G0/G1 growth arrest response could be rescued in these reconstituted cells. As predicted, the reconstituted IKKα−/−(IKKα) cells displayed the same inducible growth arrest response at G0/G1 phase upon UVB exposure (Fig. 1B). Based on these data, we conclude that the expression of IKKα is required for triggering the UVB-induced G0/G1 growth arrest response in MEFs.
Figure 1. IKKα is required for the UVB-induced G0/G1 growth arrest response and Cyclin D1 downregulation in MEFs.
(A and C) Identification of WT, IKKβ−/−, IKKα−/− and the reconstituted IKKα−/−(IKKα) MEFs. (B) WT, IKKβ−/−, IKKα−/− and IKKα−/− (IKKα) cells were exposed to UVB (1.0 kJ/m2) and cell cycle distribution were determined by flow cytometric assay 12 hrs after exposure. (D) WT, IKKβ−/− and IKKα−/− cells were exposed to different doses of UVB and the expression levels of Cyclin D1, Cyclin D2 and CDK4 were determined by western-blot assay 12 hrs after exposure. (E) WT, IKKα−/− and IKKα−/− (IKKα) cells were exposed to different doses of UVB and the expression levels of Cyclin D1 were detected 12 hrs after exposure. (F) WT, IKKα−/− and IKKα−/− (IKKα) cells were exposed to a single dose (1.0 kJ/m2) of UVB and the expression levels of Cyclin D1 were detected at the indicated time points after exposure. (G) IKKα−/− cells were infected with adenoviruses encoding GFP, wt IKKα or IKKα-KM. The infected cells were exposed to UVB irradiation 48 hrs after infection and then Cyclin D1 levels were determined 6 hrs after exposure. (H) WT cells were transfected with an expression plasmid containing the IKKβ kinase mutant IKK-KM or control vector. 48 hrs after transfection, the cells were exposed to UVB and Cyclin D1 levels were detected 12 hrs after irradiation. (I) WT cells were transfected with the expression plasmid containing HA-IκB-AA or control vector. 36 hrs after transfection, the cells were exposed to different doses of UVB and Cyclin D1 levels were detected 12 hrs after irradiation. (J) p65+/+ and p65−/− MEFs were exposed to different doses of UVB and then Cyclin D1 levels were detected 12 hrs after exposure.
Cyclin D1 is a key factor for promoting the G1/S transition of the cell cycle [3–5]. Recently, we reported that induction of Cyclin D1 downregulation is responsible for the nickel compound-induced growth arrest response and occurs via the IKKα-dependent pathway in human lung carcinoma cells [34]. We therefore investigated whether the IKKα-mediated G0/G1 growth arrest response after UVB exposure is also related to alterations of endogenous Cyclin D1 expression. As shown in Fig. 1D, a dose-dependent downregulation of Cyclin D1 protein expression was clearly detected in the WT cells at 12 hrs after UVB exposure. Moreover, this kind of response was not altered by IKKβ deficiency (although the basal level of Cyclin D1 was extremely low in the IKKβ-null cells), but was significantly suppressed in IKKα-null cells under the same treatment. When IKKα level was recovered in the reconstituted IKKα−/− MEFs, UVB-induced Cyclin D1 downregulation could be efficiently restored, as evidenced by both dose- and time-dependent experiments (Fig. 1E and 1F). These results thus indicate that, as in the action of nickel in the human cells [34], UVB-induced G0/G1 growth arrest response in MEFs is also related to IKKα-dependent reduction of Cyclin D1 expression.
We next examined if deletion of IKKα affected the expression of other D-type Cyclins, as well as one of their functional partners, CDK4, in the UVB response. As shown in Fig. 1D, both Cyclin D2 and CDK4 were downregulated with almost the same manners in WT and IKKα−/− cells, suggesting that genetic ablation of IKKα specifically abolished UVB-induced Cyclin D1 downregulation in MEFs.
The effect of IKKα in mediating UVB-induced Cyclin D1 downregulation is unrelated to IKKα kinase activity and the activation status of IKKβ/NF-κB
To clarify whether IKKα-dependent Cyclin D1 downregulation during the UVB response is related to IKKα kinase activity, we next compared Cyclin D1 levels in the IKKα−/− MEFs reconstituted with WT IKKα or the IKKα kinase mutant IKKα-KM [32], respectively. As shown in Fig. 1G, both WT IKKα and IKKα-KM overexpression could restore the UVB-induced Cyclin D1 downregulation in the IKKα−/− cells. This result indicates that IKKα kinase activity is not required for Cyclin D1 downregulation in the UVB response.
As NF-κB activation is involved in Cyclin D1 expression, and regulation of NF-κB activity is the most important function of IKKα [17], we next sought to determine if the NF-κB signaling pathway is related to the process of UVB-induced Cyclin D1 downregulation in MEFs. To this end, we first introduced the IKKβ kinase mutant IKK-KM into WT MEFs to suppress UVB-induced phosphorylation of IKKβ (Fig. 1H), an upstream event that contributes greatly to NF-κB pathway activation under various conditions [17]. We found that ectopic overexpression of IKK-KM in WT cells significantly inhibited NF-κB transactivation (data not shown) but did not affect the response of Cyclin D1 downregulation induced by UVB irradiation (Fig. 1H). IκBα-AA is an IκBα super-repressor that efficiently blocks IκBα degradation and NF-κB activation in response to a variety of stimuli [17, 31]. To further confirm the role of NF-κB in Cyclin D1 reduction in the UVB response, we then assayed for Cyclin D1 expression in IκBα-AA-transfected WT cells. We found that downregulation of Cyclin D1 expression under UVB exposure was not altered by ectopic overexpression of IκBα-AA (Fig. 1I), although NF-κB activation was dramatically inhibited under the same conditions (data not shown). We further examined Cyclin D1 levels in NF-κB p65 subunit gene knockout MEFs after UVB exposure. The results showed that the UVB-induced Cyclin D1 downregulation was not affected by the absence or presence of p65 (Fig. 1J). Taken together, we propose that the effect of IKKα on suppressing Cyclin D1 expression during the cellular UVB response is unrelated to the IKKα kinase activity and the IKKβ/NF-κB-dependent pathway.
IKKα increases Cyclin D1 degradation via the proteasome during the UVB response
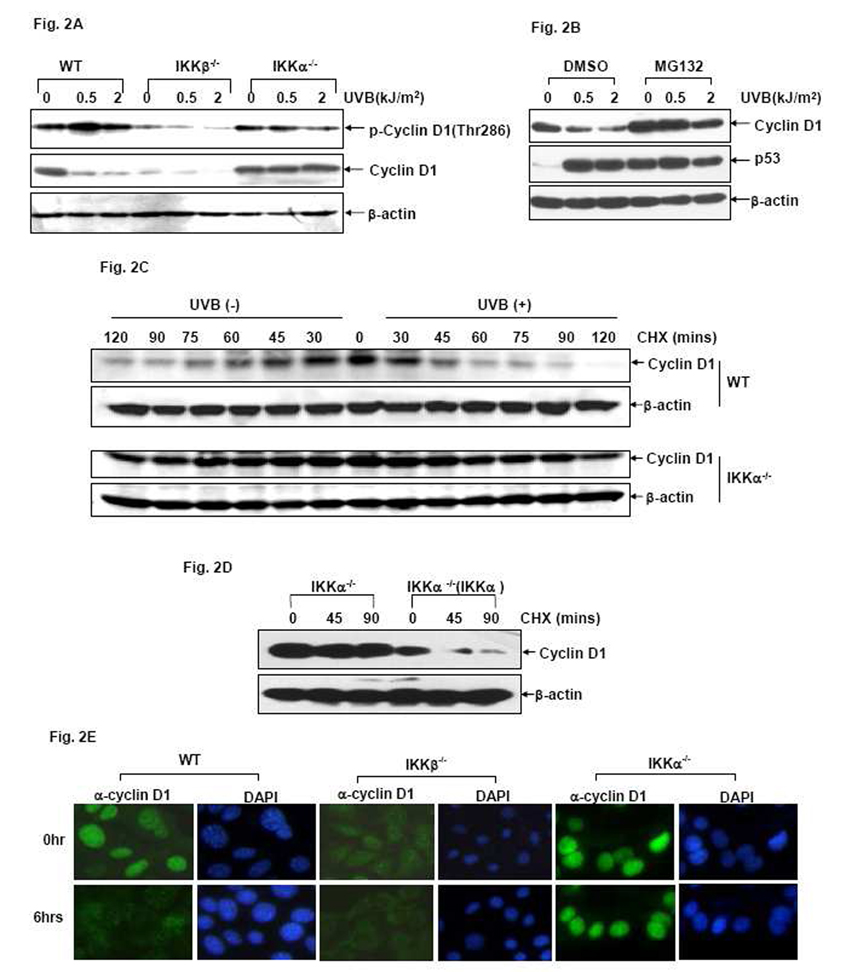
The cellular levels of Cyclin D1 depend largely on the stability of this protein. Phosphorylation of Cyclin D1 at Thr286 serves as a crucial signal for directing Cyclin D1 to the 26S proteasome-dependent degradation pathway [12–16, 35]. To elucidate the molecular mechanism by which IKKα downregulates Cyclin D1 expression during the UVB response, we first examined the phosphorylation status and stability of Cyclin D1 in MEFs after UVB exposure. As shown in Fig. 2A, along with the remarkable downregulation of Cyclin D1 levels, an inducible increase of CyclinD1 phosphorylation at Thr286 was detected in the UVB-treated WT cells. Moreover, pretreatment with the proteasome inhibitor MG132 was able to significantly suppress UVB-induced Cyclin D1 downregulation in WT MEFs (Fig. 2B). These results suggest that induction of Thr286 phosphorylation and subsequent degradation of Cyclin D1 via the proteasome-dependent pathway may constitute at least one molecular mechanism for UVB-induced Cyclin D1 downregulation in MEFs. However, UVB-induced Cyclin D1 phosphorylation at Thr286 was blocked in the IKKα-null cells, which is consistent with the lack of Cyclin D1 downregulation under the same UVB exposure conditions (Fig. 2A). These data indicate that IKKα functions as a critical mediator to trigger the phosphorylation and degradation of Cyclin D1 in the UVB response.
Figure 2. IKKα increases Cyclin D1 degradation during the UVB response.
(A) WT, IKKβ−/− and IKKα−/− cells were exposed to different doses of UVB and the phosphorylation and expression of Cyclin D1 were determined by western-blot assay 6 hrs after exposure. (B) WT cells were pretreated with 10 µM MG132 (10µM) or its vehicle (DMSO) for 1 hr and then exposed to different doses of UVB. The Cyclin D1 levels were detected 6 hrs after UVB exposure. The efficiency of MG132 in disrupting the function of the proteasome in this experiment was verified by the induced expression of p53, the well-known protein that is constitutively degraded via the proteasome-dependent pathway. (C) WT and IKKα−/−cells were left untreated or exposed to a single dose of UVB (1.0 kJ/m2) followed by treatment with 10 µM CHX. The Cyclin D1 levels were detected at the indicated time points after CHX stimulation. (D) IKKα−/− and IKKα−/− (IKKα) cells were exposed to a single dose (1.0 kJ/m2) of UVB followed by treatment with 10 µM CHX. The Cyclin D1 levels were detected at the indicated time points after CHX stimulation. (E) WT, IKKβ−/− and IKKα−/− cells were exposed to UVB (1.0 kJ/m2) and then subcellular distribution of Cyclin D1 was detected by immunofluorescence assays at the indicated time points.
To further confirm the role of IKKα in modulating Cyclin D1 protein stability, we next determined whether IKKα regulated the turnover of endogenous Cyclin D1 under UVB stress conditions. For this purpose, WT and IKKα−/− cells were left untreated or exposed to a signal dose of UVB followed by re-stimulation with cycloheximide (CHX), a protein synthesis inhibitor to block the de novo production of proteins. We found that the half-life of Cyclin D1 in the resting WT MEFs was ~59 mins and was decreased to ~35 mins after UVB exposure (Fig. 2C), indicating an increased degradation of Cyclin D1 protein in the UVB-treated WT cells. However, the half-life of Cyclin D1 in the resting IKKα−/− cells was significantly extended compared to the WT cells and did not show obvious changes after UVB exposure (~93 mins before UVB exposure vs. ~90 mins after UVB exposure) (Fig. 2C). Furthermore, re-introduction of IKKα into the null cells effectively restored the increased turnover of Cyclin D1 induced by UVB (Fig. 2D). Taken together, these results indicate that UVB-induced Cyclin D1 downregulation is accomplished, at least partially, by accelerating the degradation of this protein, which is effectively triggered by IKKα.
It is known that subcellular localization plays a critical role in regulating the protein stability of Cyclin D1 [3, 4, 35]. Therefore, we next analyzed whether IKKα exerted any effect on the subcellular distribution of Cyclin D1 during the UVB response. Results from immunofluorescence (IF) assays showed that endogenous Cyclin D1 displayed the same predominant nuclear distribution in both resting WT and IKKα−/− cells (Fig. 2E). However, UVB stimulation triggered a remarkable nuclear exclusion of Cyclin D1 along with a significant reduction of the Cyclin D1 signal in WT MEFs, but the level and subcellular distribution of Cyclin D1 remained almost unchanged in the IKKα−/− cells after the same UVB treatment (Fig. 2E). The subcellular distribution of Cyclin D1 in IKKβ-null cells was also assayed by IF. Consistent with the low basal level of Cyclin D1 protein detected by western-blotting assay (Fig. 1D), the IF signal for endogenous Cyclin D1 in IKKβ-null cells was also extremely low and could only be captured with overlong exposure. Interestingly, the endogenous Cyclin D1 in the IKKβ-null cells showed a whole-cell distribution under normal conditions and exhibited nuclear exclusive staining identical to WT cells under UVB exposure (Fig. 2E). These results suggest that UVB-induced Cyclin D1 downregulation is predominantly mediated by IKKα, which induces the signaling pathway to upregulate phosphorylation, trigger nuclear exclusion, and increase the degradation of Cyclin D1.
Identification of p38 kinase as the downstream effector of IKKα that mediates increased Cyclin D1 degradation in the cellular UVB response
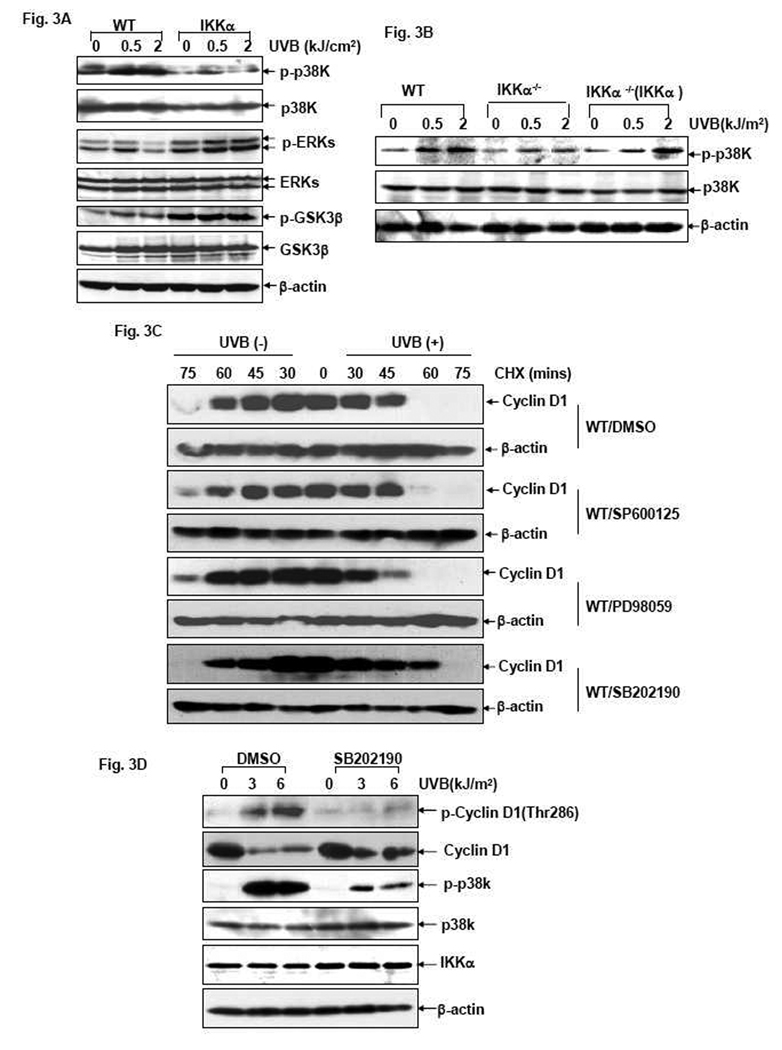
GSK3β, p38K and ERK2 are well-defined protein kinases implicated in mediating Thr286 phosphorylation and degradation of Cyclin D1 under different conditions [12–16]. To determine the signal transduction pathway initiated by IKKα to accelerate Cyclin D1 degradation during the UVB response, we compared the activation status of the aforementioned protein kinases in WT and IKKα−/− cells 1 hr after UVB exposure, at which time the induced Cyclin D1 degradation was only observed in WT cells while significantly inhibited in IKKα-null cells (Fig. 2C). As shown in Fig. 3A, a strong induction of p38K phosphorylation was readily detected in WT MEFs in response to UVB exposure; while the amount of phospho-GSK3β and phospho-ERKs was not obviously changed under the same conditions. Moreover, UVB-induced p38K activation was significantly suppressed by IKKα deficiency, while a slight increase of ERKs activation was observed in the IKKα-null cells under the same conditions. Although GSK3β phosphorylation was dramatically increased by IKKα ablation, such a downregulation of GSK3β activity was not altered after UVB exposure (Fig. 3A). These data thus implicate a major role for p38K, while excluding the involvement of both ERKs and GSK3β, in mediating IKKα-dependent Cyclin D1 proteolysis in the UVB response. To further confirm these results, we next examined the activation status of p38K in the reconstituted IKKα−/−(IKKα) cells. We found that recovering the expression of IKKα in the null cells efficiently restored the strong induction of p38K activation in response to UVB irradiation (Fig. 3B). Therefore, we propose that p38K may be the downstream target of IKKα that is responsible for triggering the degradation of Cyclin D1 after UVB exposure.
Figure 3. p38 kinase functions as the downstream effector of IKKα to mediate increased Cyclin D1 degradation in the UVB response.
(A) WT, IKKβ−/− and IKKα−/− cells were exposed to different doses of UVB and the activation status of p38K, ERKs and GSK3β was determined by western-blot assay 1 hr after UVB exposure. (B) WT, IKKα−/− and IKKα−/− (IKKα) cells were exposed to different doses of UVB and the activation status of p38K was determined by western-blot assay 1 hr after UVB exposure. (C) WT cells were pretreated with the inhibitors of JNKs (10 µM SP600125), ERKs (50 µM PD98059), p38K (20 µM SB202190) or their vehicle control (DMSO) for 1 hr and then left untreated or exposed to UVB irradiation (1.0 kJ/m2). After treatment with 10 µM CHX at the indicated time points, cells were harvested and Cyclin D1 levels were detected by western-blot assay. (D) WT cells were pretreated with 20µM SB202190 or DMSO for 1 hr and then exposed to UVB irradiation (1.0 kJ/m2). The phosphorylation and expression of Cyclin D1 were detected at the indicated time points after UVB exposure.
To determine if the putative IKKα-p38K pathway was indeed responsible for UVB-induced phosphorylation and degradation of Cyclin D1 in MEFs, we compared the kinetics of Cyclin D1 turnover in resting and UVB-treated WT cells in the presence of the p38K inhibitor, SB202190. As shown in Fig. 3C, compared to the control (DMSO-treated) group, pretreatment of resting WT cells with SB202190 did not affect the constitutive degradation of Cyclin D1. However, the increased Cyclin D1 degradation under UVB stimulation was significantly disrupted, as evidenced by the same kinetics of Cyclin D1 turnover with or without UVB exposure in the SB202190-pretreated cells. However, incubation with other protein kinase inhibitors (SP600125 for JNKs and PD98059 for ERKs) had no effect to elongate the half-life of Cyclin D1 in UVB-treated WT MEFs. Furthermore, suppression of p38K activation by SB202190 robustly disrupted UVB-induced Thr286 phosphorylation as well as Cyclin D1 reduction in WT MEFs (Fig. 3D), reminiscent of the similar phenomenon observed in IKKα-null cells under the same UVB stress conditions. Therefore, in combination with all of these data, we propose that p38K is a major downstream effector of IKKα responsible for the phosphorylation of Cyclin D1 at Thr286 and thereby increasing the degradation of this protein during the UVB response.
IKKα suppresses cyclin d1 transcription in response to UVB exposure
In the following experiments, we further explored if IKKα also plays a role in modulating cyclin d1 transcription during the cellular UVB response. To this end, a Cyclin D1-luciferase reporter plasmid, in which the transcription of the luciferase reporter gene is driven by the cyclin d1 promoter [30], was transfected into WT and IKKα-null cells, respectively. The established stable transfectants were then exposed to different doses of UVB, and the luciferase activity was measured 12 hrs after stimulation. As shown in Fig. 4A, a moderate dose-dependent increase of the Cyclin D1 luciferase reporter activity was observed in WT MEFs in response to UVB exposure. RT-PCR assay further confirmed an increase of the endogenous cyclin d1 mRNA transcripts in WT cells under the same UVB irradiation conditions (Fig. 4B). These results indicate that induction of cyclin d1 transcription also constitutes a response in UVB-treated MEFs. Interestingly, the inducible upregulation of cyclin d1 transcription was much more pronounced in IKKα-null cells compared to WT cells, according to the same luciferase reporter and RT-PCR assays (Fig. 4A and 4B). Moreover, reconstitution of IKKα expression in the IKKα-null cells significantly attenuated the induction of Cyclin D1 promoter-driven luciferase activity in response to UVB exposure (Fig. 4C). Thus, these results suggest that in addition to its regulatory role in Cyclin D1 protein stability, IKKα may also possess the ability to suppress UVB-induced cyclin d1 transcription in MEFs.
Figure 4. IKKα suppresses UVB-induced cyclin d1 transcription.
(A) A Cyclin D1 promoter driven-luciferase reporter plasmid was transfected into WT and IKKα-null cells and stable transfectants were established. The cells were then exposed to different doses of UVB and the luciferase activity was measured 12 hrs after stimulation. (B) WT and IKKα-null cells were exposed to different doses of UVB and RT-PCR assay was performed 12 hrs after stimulation to detect cyclin d1 transcripts. The relative expression levels of cyclin d1 mRNA normalized to the β-actin control are presented. (C) WT, IKKα−/− and IKKα−/− (IKKα) cells stably transfected with the Cyclin D1-luciferase reporter plasmid were exposed to different doses of UVB and their luciferase activities were measured 12 hrs after stimulation.
IKKα suppresses UVB-induced cyclin d1 transcription by targeting the ERKs-dependent pathway
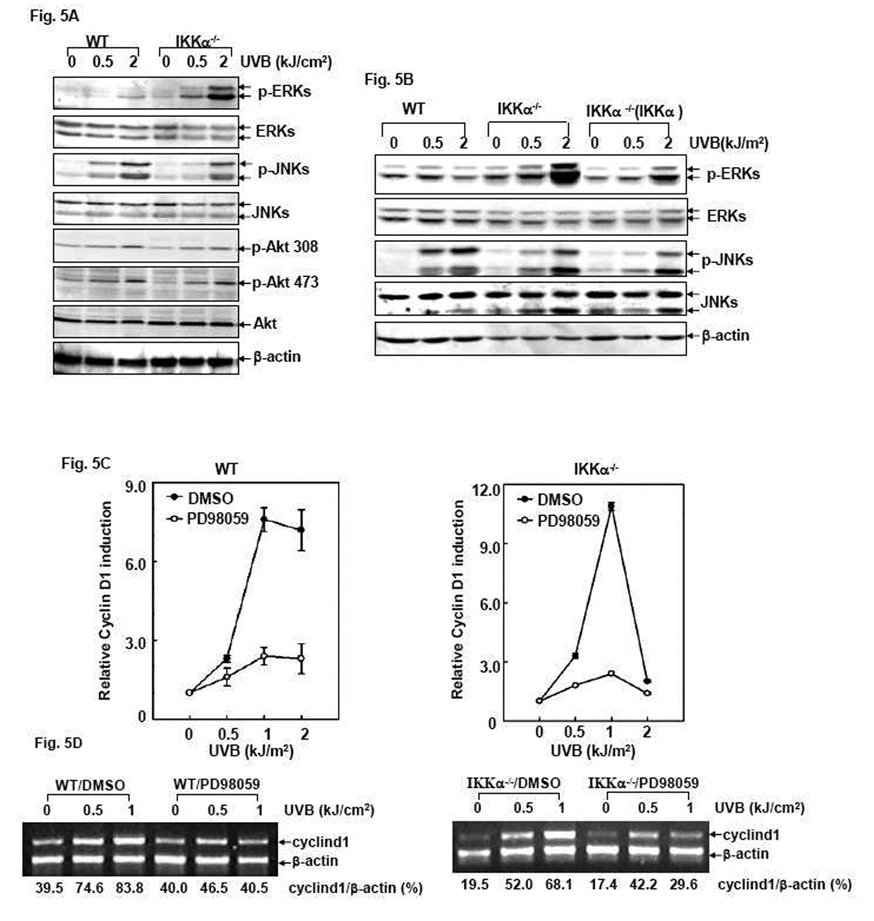
To explore the molecular mechanism by which IKKα suppresses UVB-induced cyclin d1 transcription, we investigated the activation status of the protein kinases possibly involved in cyclin d1 transcription (JNKs, ERKs and Akt) 12 hrs after UVB exposure, at which time the induction of cyclin d1 transcription was observed by the luciferase reporter and RT-PCR assays (Fig. 4). As shown in Fig. 5A, the obvious induction of both JNKs and Akt activation was observed in UVB-treated WT cells, which events were almost identically obtained in the IKKα−/− cells under the same conditions. However, ERKs activation was weak and only discernable under high doses of UVB stimulation in WT cells, but it was significantly upregulated by IKKα deficiency. Furthermore, re-introduction of IKKα into the null cells decreased ERKs activation while without affecting the inducible JNKs phosphorylation (Fig. 5B). These data indicate that IKKα specifically suppresses ERKs activation, which may be the upstream signaling event responsible for downregulating cyclin d1 transcription in the UVB response.
Figure 5. IKKα suppresses cyclin d1 transcription during the UVB response by targeting the ERKs-dependent pathway.
(A) WT, IKKβ−/− and IKKα−/− cells were exposed to different doses of UVB and the activation status of ERKs, JNKs and Akt was determined by western-blot assay 12 hrs after UVB exposure. (B) WT, IKKα−/− and IKKα−/− (IKKα) cells were exposed to different doses of UVB and the activation status of ERKs was determined by western-blot assay 12 hrs after exposure. The detection of JNKs activation under the same conditions was used as a negative control in this assay. (C) WT and IKKα−/− cells stably transfected with the Cyclin D1-luciferase reporter plasmid were pretreated with 50 µM PD98059 or its vehicle (DMSO) for 1 hr followed by exposure to different doses of UVB. The luciferase activity was detected 12 hrs after UVB exposure. (D). WT and IKKα−/− cells were exposed to PD98059 and UVB as in Fig. 5C and RT-PCR assay was performed to detect the cyclin d1 transcripts 12 hrs after UVB exposure. The relative expression levels of cyclin d1 mRNA normalized to the expression of β-actin are presented.
To further address the relevance of ERKs activation to UVB-induced cyclin d1 transcription, we next analyzed Cyclin D1 luciferase reporter activity in both UVB-treated WT and IKKα-null cells after pretreatment with the ERKs inhibitor PD98059. The efficiency of PD98059 on the suppression of UVB-induced ERKs activation was confirmed by western-blot assay (data not shown). The results shown in Fig. 5C and 5D indicate that pretreatment with PD98059 significantly decreased UVB-induced cyclin d1 transcription in both WT and IKKα-null cells, as determined by the luciferase reporter (Fig. 5C) and the RT-PCR (Fig. 5D) assays. These data thus indicate a novel role for IKKα in negatively regulating UVB-induced cyclin d1 transcription by suppressing inducible ERKs activation.
Discussion
IKKα is a multi-functional signaling protein whose activities can be exerted through, or independent of, its action on NF-κB activation [8, 18–26, 36]. The implication of IKKα in the control of cellular growth properties in both normal and tumor cells under physiological and pathological conditions has been well-described in previous reports [8, 25–27, 35–37]. The data in the current study have extended the biological significance of IKKα in cell growth control to the cellular DNA damage response by disclosing a novel function of IKKα in mediating the growth arrest response to UVB irradiation. Moreover, this function of IKKα is unrelated to IKKβ/NF-κB activation status. Along with our recent finding that IKKα is also involved in the growth inhibition response induced by nickel compounds in human cells [34], we thus anticipate that the anti-proliferative function of IKKα might be important for cells to escape from the damage response induced by certain environmental stressors.
In the following study to investigate the molecular mechanism of IKKα in mediating the UVB-induced G0/G1 growth arrest response, we found that the anti-proliferative effect of IKKα during the cellular UVB response is largely related to downregulation of Cyclin D1 expression in MEFs. This result is consistent with our previously published data obtained in human cancer cells that demonstrates a similar role for IKKα in the suppression of Cyclin D1 expression in the nickel compound-induced growth inhibition response [34]. Taken together, these studies thus implicate a general and evolutionarily conserved role for IKKα in the negative regulation of Cyclin D1 levels under stress conditions.
Indeed, as one of the most important downstream mediators of IKKα, Cyclin D1 levels have been proved to be regulated by IKKα via multiple mechanisms. According to a report from Dr. Verma’s group, IKKα directly binds to and phosphorylates Cyclin D1 on Thr286, subsequently promoting the nuclear export and ubiquitin-dependent degradation of Cyclin D1 in resting MEFs [35]. In the current study, we consistently found that the half-life of endogenous Cyclin D1 was extended in untreated IKKα-null cells compared to parental cells (Fig. 2C), further confirming the notion that IKKα contributes to maintaining the turnover and normal cellular levels of Cyclin D1 under physiological conditions. Moreover, IKKα was also shown to possess the activity to rapidly increase the degradation of Cyclin D1 via the proteasome pathway after UVB exposure (Fig. 2B and 2C). In combination of these data, we propose that IKKα is involved in the regulation of Cyclin D1 protein stability under both physiological and stress conditions. However, different from the direct action of IKKα on modulating Cyclin D1 basal levels [35], our data indicate that the activity of IKKα in promoting Cyclin D1 degradation under UVB exposure is indirect and elicited by the induced activation of p38K, as evidenced by reduced Cyclin D1 phosphorylation and rescued Cyclin D1 degradation in SB202190-pretreated WT MEFs with normal expression levels of IKKα (Fig. 3C and 3D). Therefore, we have for the first time defined the functional link between IKKα and p38K in modulating Cyclin D1 protein stability in response to UVB irradiation. In addition, our results also add further evidence to the previous notion that p38K is largely responsible for mediating Cyclin D1 degradation following exposure to environmental stressors or genotoxic insults [14, 15].
Aside from its role in regulating post-translational modification of Cyclin D1, IKKα also has an effect on reducing cyclin d1 transcription in the UVB response. However, in contrast to our results, numerous previous studies have defined a positive role for IKKα in the transcriptional induction of cyclin d1 in response to Wnt, RANK and estrogen signals [7, 8, 37, 38]. Furthermore, investigations of the underlying mechanism have demonstrated that IKKα can associate with the NF-κB responsive promoter and phosphorylates the transcriptional co-activator or histone H3, therefore facilitating the transcription of NF-κB responsive genes, including cyclin d1 [18, 19, 38]. In another case, IKKα has been shown to contribute to cyclin d1 transcription via a Tcf-binding site upon mitogentic signaling [7]. Under UVB stress conditions, we have for the first time identified a negative role for IKKα in the induction of cyclin d1 transcription, which effect is unrelated to NF-κB activity and mediated by suppressing ERKs activation. These data suggest that IKKα has a dual role in the signaling events to trigger cyclin d1 transcription via various mechanisms under different cellular growth conditions. It has been demonstrated that ERKs contribute to cyclin d1 transcription by inducing the binding of the transcriptional factor c-Ets2 to the Ets binding element in the cyclin d1 promoter region [6]. Therefore, whether c-Ets2 is involved in IKKα-mediated cyclin d1 transcriptional suppression will be addressed in future investigations.
Notably, although our data, and that of previous studies [8, 25, 26, 35–37], emphasize the role of IKKα in Cyclin D1 regulation, it does not mean that these effects are absolutely distinct for IKKα and not shared by its structural and functional congener, IKKβ. In fact, although the growth arrest response under UVB exposure was not affected by IKKβ deficiency (Fig. 1C), we were unexpected to found that the protein expression level of Cyclin D1 was extremely low in IKKβ-null cells (Fig. 1D and Fig. 2E). However, we excluded the possibility that low Cyclin D1 expression levels in IKKβ-null cells were due to the deficient transcription, evidenced by the almost identical levels of cyclin d1 mRNA transcripts in WT and IKKβ-null cells (data not shown). In addition, Cyclin D1 protein levels were efficiently recovered in IKKβ-null cells by IKKβ reconstitution. Moreover, MG132 treatment caused Cyclin D1 accumulation in the IKKβ-null cells at levels similar to WT cells (data not shown). These data thus also disfavor the notion of abnormal Cyclin D1 protein stability caused by IKKβ deletion. Therefore, whether IKKβ deficiency affects the translational efficiency of cyclin d1 mRNA is a subject worthy of investigation.
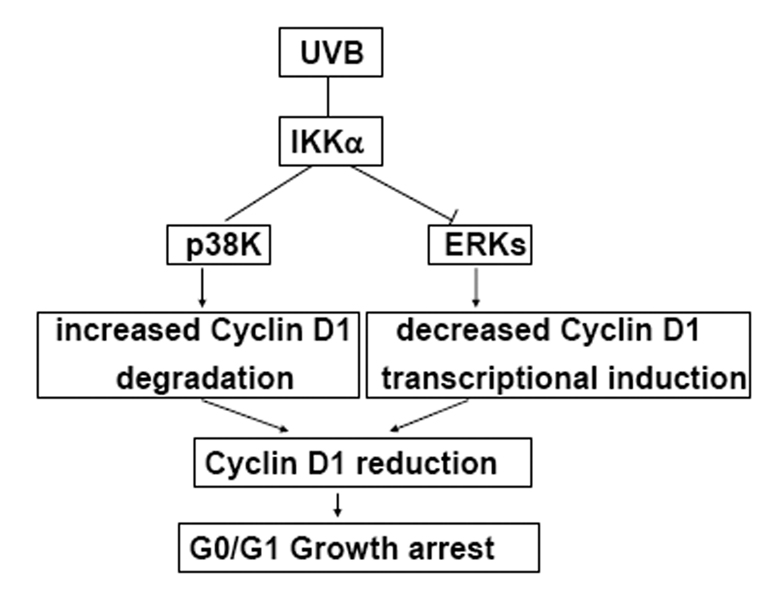
In summary, this study has demonstrated that IKKα is an important factor for initiating the UVB-induced G0/G1 growth arrest response by downregulating Cyclin D1 expression, which is a comprehensive effect, resulted from an increase of p38 kinase-dependent Cyclin D1 proteolysis coupled with the reduction of ERKs-dependent Cyclin D1 transcription (Fig. 6). As UVB has been established as a risk factor for the incidence of skin cancers, further evaluating the relevance of IKKα deregulation in the process of UVB-induced tumorigenesis is therefore of medical importance.
Figure 6. Proposed scheme of the role of IKKα in mediating the UVB-induced G0/G1 cell cycle arrest response by suppressing Cyclin D1 expression.
Under UVB exposure, we demonstrate a dual role of IKKα to selectively activate p38K and suppress ERKs activation in the early and late phases, respectively. The activation of p38K subsequently results in increased phosphorylation and degradation of Cyclin D1; while the inhibition on ERKs activation suppresses the induced transcription of cyclin d1 under UVB irradiation. Due to decreased protein stability and transcriptional suppression, the Cyclin D1 level is downregulated in response to UVB and therefore cell cycle progression is arrested at G0/G1 phase.
Acknowledgement
This project is supported by National Natural Science Foundation of China (No. 30871277, 30970594) and the Beijing Natural Science Foundation (5092022, 5102035) to Dr. Lun Song and NIH/NCI/NIEHS CA094964, CA112557, ES010344, ES012451 and ES000260 to Dr. Chuanshu Huang.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors state no conflict of interest.
References
- 1.Lee E, Koo J, Berger T. UVB phototherapy and skin cancer risk: a review of the literature. Int J Dermatol. 2005;44:355–360. doi: 10.1111/j.1365-4632.2004.02186.x. [DOI] [PubMed] [Google Scholar]
- 2.Johansson M, Persson JL. Cancer therapy: targeting cell cycle regulators. Anticancer Agents Med Chem. 2008;8:723–731. doi: 10.2174/187152008785914833. [DOI] [PubMed] [Google Scholar]
- 3.Kim JK DJ. Nuclear cyclin D1: an oncogenic driver in human cancer. J Cell Physiol. 2009;220:292–296. doi: 10.1002/jcp.21791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alao JP. The regulation of cyclin D1 degradation: roles in cancer development and the potential for therapeutic invention. Mol Cancer. 2007;6:24. doi: 10.1186/1476-4598-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sherr CJ RJ. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 6.Albanese C, Johnson J, Watanabe G, Eklund N, Vu D, Arnold A, Pestell RG. Transforming p21ras mutants and c-Ets-2 activate the cyclin D1 promoter through distinguishable regions. J Biol Chem. 1995;270:23589–23597. doi: 10.1074/jbc.270.40.23589. [DOI] [PubMed] [Google Scholar]
- 7.Albanese C, Wu K, D'Amico M, Jarrett C, Joyce D, Hughes J, Hulit J, Sakamaki T, Fu M, Ben-Ze'ev A, Bromberg JF, Lamberti C, Verma U, Gaynor RB, Byers SW, Pestell RG. IKKalpha regulates mitogenic signaling through transcriptional induction of cyclin D1 via Tcf. Mol Biol Cell. 2003;14:585–599. doi: 10.1091/mbc.02-06-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao Y, Bonizzi G, Seagroves TN, Greten FR, Johnson R, Schmidt EV, Karin M. IKKalpha provides an essential link between RANK signaling and cyclin D1 expression during mammary gland development. Cell. 2001;107:763–775. doi: 10.1016/s0092-8674(01)00599-2. [DOI] [PubMed] [Google Scholar]
- 9.Brown JR, Nigh E, Lee RJ, Ye H, Thompson MA, Saudou F, Pestell RG, Greenberg ME. Fos Family Members Induce Cell Cycle Entry by Activating Cyclin D1. 1998;vol.18:5609–5619. doi: 10.1128/mcb.18.9.5609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lopez-Bergami P, Huang C, Goydos JS, Yip D, Bar-Eli M, Herlyn M, Smalley KS, Mahale A, Eroshkin A, Aaronson S, Ronai Z. Rewired ERK-JNK signaling pathways in melanoma. Cancer Cell. 2007;11:447–460. doi: 10.1016/j.ccr.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lavoie JN, L'Allemain G, Brunet A, Muller R, Pouyssegur J. Cyclin D1 expression is regulated positively by the p42/p44MAPK and negatively by the p38/HOGMAPK pathway. J Biol Chem. 1996;271:20608–20616. doi: 10.1074/jbc.271.34.20608. [DOI] [PubMed] [Google Scholar]
- 12.Diehl JA ZF. Sherr CJ Inhibition of cyclin D1 phosphorylation on threonine-286 prevents its rapid degradation via the ubiquitin-proteasome pathway. Genes Dev. 1997;11:957–972. doi: 10.1101/gad.11.8.957. [DOI] [PubMed] [Google Scholar]
- 13.Diehl JA CM, Roussel MF. Sherr CJ Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis and subcellular localization. Genes Dev. 1998;12:3499–3511. doi: 10.1101/gad.12.22.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Casanovas O, Miro F, Estanyol JM, Itarte E, Agell N, Bachs O. Osmotic stress regulates the stability of cyclin D1 in a p38SAPK2-dependent manner. J Biol Chem. 2000;275:35091–35097. doi: 10.1074/jbc.M006324200. [DOI] [PubMed] [Google Scholar]
- 15.Thoms HC, Dunlop MG, Stark LA. p38-mediated inactivation of cyclin D1/cyclin-dependent kinase 4 stimulates nucleolar translocation of RelA and apoptosis in colorectal cancer cells. Cancer Res. 2007;67:1660–1669. doi: 10.1158/0008-5472.CAN-06-1038. [DOI] [PubMed] [Google Scholar]
- 16.Okabe H, Lee SH, Phuchareon J, Albertson DG, McCormick F, Tetsu O. A critical role for FBXW8 and MAPK in cyclin D1 degradation and cancer cell proliferation. PLoS ONE. 2006;1:e128. [Google Scholar]
- 17.Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 18.Anest V, Hanson JL, Cogswell PC, Steinbrecher KA, Strahl BD, Baldwin AS. A nucleosomal function for IkappaB kinase-alpha in NF-kappaB-dependent gene expression. Nature. 2003;423:659–663. doi: 10.1038/nature01648. [DOI] [PubMed] [Google Scholar]
- 19.Yamamoto Y, Verma UN, Prajapati S, Kwak YT, Gaynor RB. Histone H3 phosphorylation by IKK-alpha is critical for cytokine-induced gene expression. Nature. 2003;423:655–659. doi: 10.1038/nature01576. [DOI] [PubMed] [Google Scholar]
- 20.Kaisho T, Takeda K, Tsujimura T, Kawai T, Nomura F, Terada N, Akira S. IkappaB kinase alpha is essential for mature B cell development and function. J Exp Med. 2001;193:417–426. doi: 10.1084/jem.193.4.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mills DM, Bonizzi G, Karin M, Rickert RC. Regulation of late B cell differentiation by intrinsic IKKalpha-dependent signals. Proc Natl Acad Sci U S A. 2007;104:6359–6364. doi: 10.1073/pnas.0700296104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu Y, Baud V, Delhase M, Zhang P, Deerinck T, Ellisman M, Johnson R, Karin M. Abnormal morphogenesis but intact IKK activation in mice lacking the IKKalpha subunit of IkappaB kinase. Science. 1999;284:316–320. doi: 10.1126/science.284.5412.316. [DOI] [PubMed] [Google Scholar]
- 23.Li Q, Lu Q, Hwang JY, Buscher D, Lee KF, Izpisua-Belmonte JC, Verma IM. IKK1-deficient mice exhibit abnormal development of skin and skeleton. Genes Dev. 1999;13:1322–1328. doi: 10.1101/gad.13.10.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sil AK, Maeda S, Sano Y, Roop DR, Karin M. IkappaB kinase-alpha acts in the epidermis to control skeletal and craniofacial morphogenesis. Nature. 2004;428:660–664. doi: 10.1038/nature02421. [DOI] [PubMed] [Google Scholar]
- 25.Descargues P, Sil AK, Karin M. IKKalpha, a critical regulator of epidermal differentiation and a suppressor of skin cancer. Embo J. 2008;27:2639–2647. doi: 10.1038/emboj.2008.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park E, Zhu F, Liu B, Xia X, Shen J, Bustos T, Fischer SM, Hu Y. Reduction in IkappaB kinase alpha expression promotes the development of skin papillomas and carcinomas. Cancer Res. 2007;67:9158–9168. doi: 10.1158/0008-5472.CAN-07-0590. [DOI] [PubMed] [Google Scholar]
- 27.Zhu F, Park E, Liu B, Xia X, Fischer SM, Hu Y. Critical role of IkappaB kinase alpha in embryonic skin development and skin carcinogenesis. Histol Histopathol. 2009;24:265–271. doi: 10.14670/HH-24.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song L, Li J, Zhang D, Liu ZG, Ye J, Zhan Q, Shen HM, Whiteman M, Huang C. IKKbeta programs to turn on the GADD45alpha-MKK4-JNK apoptotic cascade specifically via p50 NF-kappaB in arsenite response. J Cell Biol. 2006;175:607–617. doi: 10.1083/jcb.200602149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song L, Li J, Hu M, Huang C. Both IKKalpha and IKKbeta are implicated in the arsenite-induced AP-1 transactivation correlating with cell apoptosis through NF-kappaB activity-independent manner. Exp Cell Res. 2008;314:2187–2198. doi: 10.1016/j.yexcr.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ouyang W, Ma Q, Li J, Zhang D, Liu ZG, Rustgi AK, Huang C. Cyclin D1 induction through IkappaB kinase beta/nuclear factor-kappaB pathway is responsible for arsenite-induced increased cell cycle G1-S phase transition in human keratinocytes. Cancer Res. 2005;65:9287–9293. doi: 10.1158/0008-5472.CAN-05-0469. [DOI] [PubMed] [Google Scholar]
- 31.Wang X, Ju W, Renouard J, Aden J, Belinsky SA, Lin Y. 17-allylamino-17-demethoxygeldanamycin synergistically potentiates tumor necrosis factor-induced lung cancer cell death by blocking the nuclear factor-kappaB pathway. Cancer Res. 2006;66:1089–1095. doi: 10.1158/0008-5472.CAN-05-2698. [DOI] [PubMed] [Google Scholar]
- 32.Hu Y, Baud V, Oga T, Kim KI, Yoshida K, Karin M. IKKalpha controls formation of the epidermis independently of NF-kappaB. Nature. 2001;410:710–714. doi: 10.1038/35070605. [DOI] [PubMed] [Google Scholar]
- 33.Song L, Li J, Ye J, Yu G, Ding J, Zhang D, Ouyang W, Dong Z, Kim SO, Huang C. p85alpha acts as a novel signal transducer for mediation of cellular apoptotic response to UV radiation. Mol Cell Biol. 2007;27:2713–2731. doi: 10.1128/MCB.00657-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ouyang W, Zhang D, Li J, Verma UN, Costa M, Huang C. Soluble and insoluble nickel compounds exert a differential inhibitory effect on cell growth through IKKalpha-dependent cyclin D1 down-regulation. J Cell Physiol. 2009;218:205–214. doi: 10.1002/jcp.21590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kwak YT, Li R, Becerra CR, Tripathy D, Frenkel EP, Verma UN. IkappaB kinase alpha regulates subcellular distribution and turnover of cyclin D1 by phosphorylation. J Biol Chem. 2005;280:33945–33952. doi: 10.1074/jbc.M506206200. [DOI] [PubMed] [Google Scholar]
- 36.Moreno-Maldonado R, Ramirez A, Navarro M, Fernandez-Acenero MJ, Villanueva C, Page A, Jorcano JL, Bravo A, Llanos Casanova M. IKKalpha enhances human keratinocyte differentiation and determines the histological variant of epidermal squamous cell carcinomas. Cell Cycle. 2008;7:2021–2029. doi: 10.4161/cc.7.13.6147. [DOI] [PubMed] [Google Scholar]
- 37.Tu Z, Prajapati S, Park KJ, Kelly NJ, Yamamoto Y, Gaynor RB. IKK alpha regulates estrogen-induced cell cycle progression by modulating E2F1 expression. J Biol Chem. 2006;281:6699–6706. doi: 10.1074/jbc.M512439200. [DOI] [PubMed] [Google Scholar]
- 38.Park KJ, Krishnan V, O'Malley BW, Yamamoto Y, Gaynor RB. Formation of an IKKalpha-dependent transcription complex is required for estrogen receptor-mediated gene activation. Mol Cell. 2005;18:71–82. doi: 10.1016/j.molcel.2005.03.006. [DOI] [PubMed] [Google Scholar]