Abstract
Background
Cardiac resynchronization therapy (CRT) is the first clinical heart failure treatment that both acutely and chronically improves chamber systolic function while reducing mortality. The mechanical impact of CRT is immediate and well documented, yet its chronic influences on myocyte function and adrenergic modulation that may contribute to its sustained benefits are largely unknown.
Methods and Results
We used a canine model of dyssynchronous heart failure (DHF; left-bundle ablation, atrial tachypacing for 6-wks) and CRT (DHF for 3 weeks, bi-ventricular tachypacing for subsequent 3-wks), contrasting both to non-failing controls. CRT restored contractile synchrony and improved systolic function compared to DHF. Myocyte sarcomere shortening and calcium transients were markedly depressed at rest and after isoproterenol stimulation in DHF (both anterior and lateral walls); and CRT substantially improved both. β1 and β2 stimulation was enhanced, coupled to increased β1 receptor abundance but no change in binding affinity. CRT also augmented adenylate cyclase activity over DHF. Inhibitory G-protein (Gαi) suppression of β-adrenergic stimulation was greater in DHF and reversed by CRT. Gαi expression itself was unaltered; however, expression of negative regulators of Gαi signaling (particularly RGS3) rose uniquely with CRT over DHF and controls. CRT blunted elevated myocardial catecholamines in DHF, restoring levels towards control.
Conclusion
CRT improves rest and β-adrenergic stimulated myocyte function and calcium handling, up-regulating β1 receptors, adenylate cyclase activity, and suppressing Gi-coupled signaling associated with novel RGS upregulation. The result is greater rest and sympathetic reserve despite reduced myocardial neuro-stimulation, as components underlying its net benefit.
Keywords: heart failure; myocytes; pacing; receptors, adrenergic, beta; dyssynchrony; adenylate cyclase, RGS
INTRODUCTION
Congestive heart failure (CHF) is a leading cause of morbidity and mortality world-wide commanding more than 30 billion heath care dollars annually in the United States alone1. Over the past decade, arguably the most significant advance in CHF treatment has been bi-ventricular pacing (cardiac resynchronization therapy, CRT) which improves cardiac function, symptoms, and prognosis in a subgroup of CHF patients with discoordinate contraction due to conduction delay 2–5. CRT abruptly improves systolic chamber function and energetic efficiency by reducing wasted reciprocal stretch of one wall by an otherwise out-of-phase contraction of the opposing region3,6,7. CRT chronically suppresses progressive cardiac dilation8, enhances myocardial gene expression of calcium handling proteins9,10 and blunts fetal gene expression (e.g. BNP)11. CRT is the only heart failure treatment to date that can both acutely and chronically increase systolic function yet prolong survival, something not yet achieved by a drug therapy.
To date, the mechanisms for CRT benefits have been principally studied at the chamber level, and largely in human subjects at rest. Its impact on myocyte rest and reserve function has been little studied, though recent investigations have revealed that CRT acutely and chronically improves cardiac reserve coupled to increasing heart rate12–14. Contractile reserve is also importantly modulated by β-adrenergic signaling, something that is often down-regulated in failing hearts15,16. CRT acutely blunts efferent sympathetic stimulation reflected by peripheral muscle sympathetic nerve activity17, and such changes have been chronically linked to clinical efficacy18. However, whether and how CRT alters β-adrenergic signaling at the cellular level is unknown.
Accordingly, the present study tested whether CRT can ameliorate cardiac myocyte β-adrenergic reserve abnormalities and identified signaling mechanisms for such effects. To achieve this, we employed a recently described canine model of dyssynchronous heart failure with or without CRT treatment19. Here, we reveal that CRT substantially improves both rest and β-AR stimulated myocyte contraction and calcium cycling throughout the ventricle, restoring a more normal balance of reduced myocardial adrenergic stimulation with enhanced cellular responsiveness. Enhanced β-AR signaling is linked to a selective rise in β1 receptors, adenylate cyclase activity, and up-regulation of regulators of G-protein signaling that accompany suppression of inhibitory G-protein modulation.
MATERIALS AND METHODS
Canine model of DHF and CRT
Details of the canine model were recently reported19. Briefly, dogs (n=32) were subjected to left bundle radiofrequency ablation followed by 6-wks of atrial tachypacing (~200 bpm, dyssynchronous heart failure, DHF) or 3-wks atrial pacing (dyssynchrony) and then 3 weeks bi-ventricular pacing (LV lateral and RV antero-apical epiardium) at the same rapid rate (CRT). Sham DHF dogs (n=3) with both surgical ventricular leads placed but not utilized were also studied. Non-instrumented dogs (n=13) served as controls. Echo and tissue Doppler studies were performed at 3 and 6 weeks in conscious animals to assess LV dysynchrony19, chamber dimensions, and ejection fraction.
At terminal study, dogs were anesthetized with pentobarbital, pacing suspended, and a micromanometer (Millar, Houston, TX) advanced to record LV pressures. The chest was opened, hearts rapidly harvested under cold cardioplegia and myocardium frozen for tissue analysis (endocardial and mid/epicardial segments from septum and LV lateral) or for myocyte isolation from anterior-septum and lateral walls. Details of these procedures have been reported20,21 and are also provided in supplemental online methods.
Eight additional animals were chronically instrumented with sonomicrometers to derive left ventricular volume (Sonometrics, WA) and micromanometer (Konigsberg, CA) to measure LV pressure, assigned to CRT or DHF groups, and LV function assessed in the conscious state at both 3 and 6 weeks to obtain paired invasive hemodynamic data.
Myocyte function studies
Myocyte sarcomere shortening and whole cell calcium transients were assessed using an inverted microscope (Ellipse TE2001, Nikon) equipped with an image/fluorescence system (MyoCam, IonOptix, MA). Details are provided in supplemental methods.
Protein and Gene Expression
Myocardium was homogenized in lysis buffer (Cell Signaling), and 50–100µg loaded for gel electrophoresis using standard methods20. Gαi-1/2/3, Gαs, RGS2, RGS3, RGS4, GRK2 (Santa Cruz biotechnology, each at 1:400), and glyceraldhyde-3-phosphate dehydrogenase (GAPDH) (IMGENEX, 1:10000) were probed. Membrane fractions were obtained and probed for some assays, and gene expression assessed by real-time PCR using the SYBR Green PCR master mix (Applied Biosystems) and ABI PRISM7900 (supplemental methods).
β-AR and Adenylyl Cyclase Activity
Adenylyl cyclase activity was determined by timed cAMP synthesis in 30–50 µg per 100 µL membrane preparation aliquots using a commercial assay (Amersham) to measure cAMP22 (online supplemental methods). All samples were assayed in duplicate.
Radioligand-Binding Assay
β-AR radioligand binding studies were performed in myocardial membrane fractions using the nonselective β-AR antagonist [125I]-cyanopindolol (125I-CYP), as described23 (details in supplemental methods).
Myocardial Catecholamines
Myocardial catecholamines were measured in left ventricular myocardium, with samples weighed and homogenized in 4× volume of 0.4 M perchloric acid containing 0.5mM EDTA, and centrifuged at 5000 rpm at 4°C. Catecholamines were extracted from the supernatant using an alumina extraction procedure and quantified by liquid chromatography with electrochemical detection as described24. Concentration was normalized to tissue weight.
Statistical Analysis
Comparisons of results from the three different experimental groups (no repeated measures) were performed by one-way ANOVA using a Tukey multiple comparison test. Cells derived from different regions in the same heart were treated as independent groups, as heart identification per se was not significant in any analysis. Molecular/biochemical analyses contrasting region and group effects were assessed by two-way ANOVA. Echo data obtained in the same heart at 3 and 6 wks was analyzed by repeated measures ANOVA. Data are presented as mean±SEM.
The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
RESULTS
Chamber and regional mechanics in DHF versus CRT Hearts
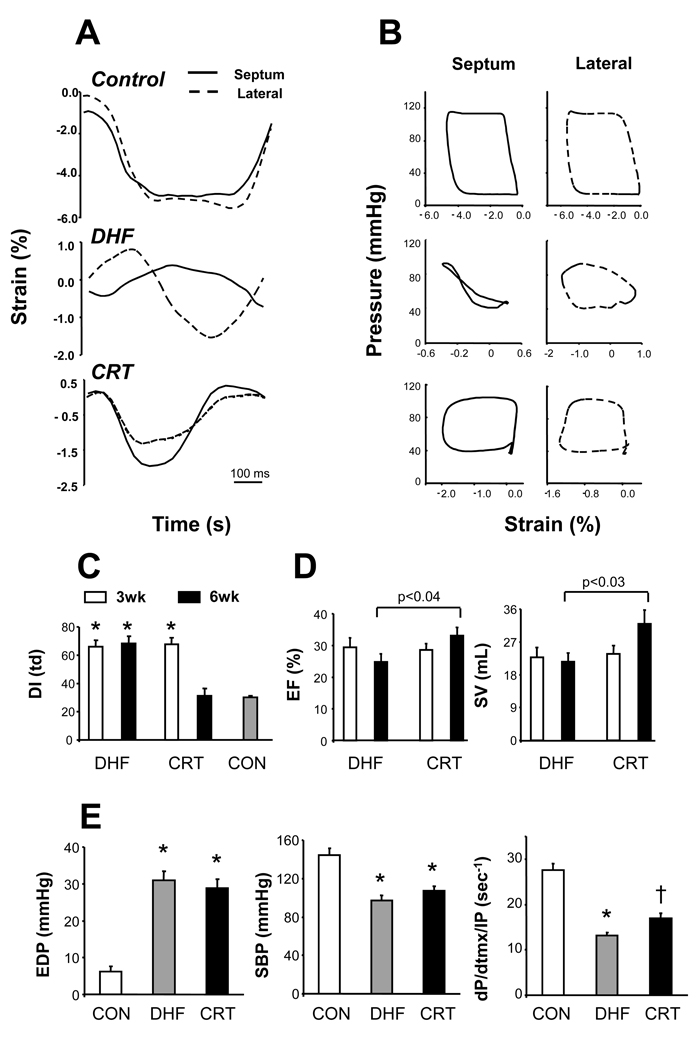
Example myocardial strain-time waveforms and pressure-strain loops from antero-septal and lateral regions are shown in Fig 1A,B for control, DHF, and CRT hearts. In DHF, early septal shortening was accompanied by lateral stretch (vice-versa in late systole), and pressure-strain loops showed marked heterogeneity of regional work (loop area). Synchrony was restored in CRT hearts (summary data in Fig 1C). Group results for echo-derived ejection fraction and stroke volume are displayed in Fig 1D. Both groups were dyssynchronous at 3 wks, and thus the decline in both variables was similar at that time (vs 66% and 35 mL control values, respectively). At 6 wks, however, CRT improved function whereas it worsened in DHF (p<0.05 by unpaired t-test; p<0.01 by two-way repeated measures ANOVA, paired changes). Invasive end-diastolic and systolic pressures were similarly altered at terminal study, though contractile function assessed by dP/dtmax normalized to instantaneous developed pressure (dP/dtmx/IP) was improved by CRT (Fig 1E). CRT hearts also generated nearly twice the mean ventricular power compared with DHF (285 vs 146 watts; p<0.03). Contractile improvement by CRT was also shown by paired analysis in chronically instrumented conscious dogs (Supplemental Table 1).
Fig. 1.
A) Example radial strain-vs-time tracings for septal and lateral walls in a healthy control, dyssynchronous failing heart (DHF), and heart treated with bi-ventricular pacing (CRT). Marked disparities in regional strain in DHF were ameliorated by CRT. B) Corresponding pressure-strain loops show disparities in regional work (loop area) in DHF that is rendered more homogeneous by CRT. C) Group dyssynchrony analysis (standard deviation of time at peak systolic radial strain from multiple segments) shows marked discoordination in both groups at 3-wks that is corrected by CRT but persists in DHF hearts. *p<0.0001 versus 6wk CRT and control (CON). D) Echo-derived ejection fraction (EF) and stroke volume (SV) at 3 and 6 wk time points in each group. Both rose in CRT compared to DHF at 6-wks (p-values shown for unpaired analysis; RMANOVA; p<0.01 for group×time interaction). E) Invasive pressures in both models at 6wk terminal study. EDP-end-diastolic pressure; SBP-systolic pressure. * p<0.01 versus CON; † - p<0.01 vs NL, and p<0.05 vs DHF.
CRT improves basal and β-adrenergic stimulated myocyte function
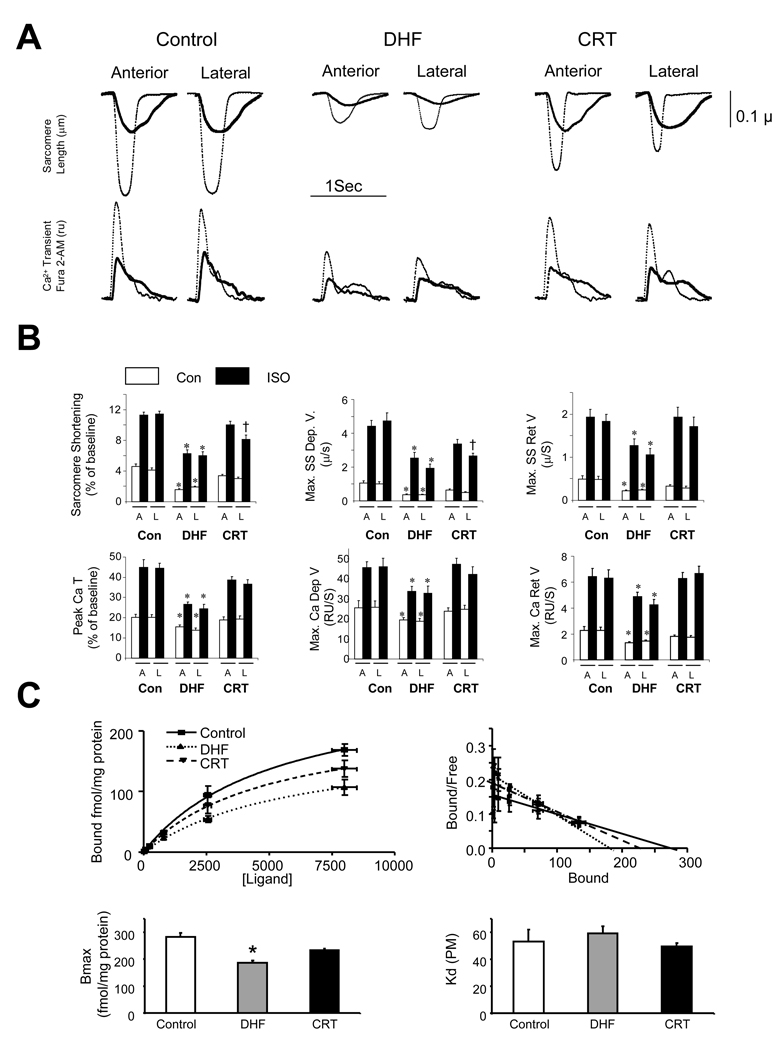
Figure 2a shows example myocyte sarcomere shortening and calcium transient tracings at rest and following ISO stimulation. In DHF cells, rest and ISO-stimulated shortening were markedly depressed compared to controls, and associated with slow systolic and re-lengthening rates. This was mirrored by depressed peak Ca2+ transients and delayed upstroke and decay kinetics under both conditions. CRT myocytes had modestly improved rest function but a markedly improved response to ISO. Summary data are provided in Fig 2b, with results from endocardium and epicardium combined as they were similar. Intriguingly, improved function and adrenergic reserve was observed in myocytes from both early activated antero-septum and late activated lateral wall, indicating a global effect from CRT. These data were further confirmed in studies performed at 27° (0.5 Hz) (Supplemental Fig. 1). DHF-CRT disparities were not due to the surgical preparation, as SHAM-DHF data were identical to those from the primary DHF group (i.e. without surgically placed leads; Supplemental Figure 2). Lastly, we examined β-adrenergic responsiveness in the intact heart. Starting from similar baselines, dobutamine (10 µg/kg/min) enhanced maximal and minimal dP/dt more in CRT than in DHF hearts (36.4±8.6 vs 10.0±5.6%, and 26.1±2.3 vs 5.6±5.0%, respectively; both p<0.04).
Fig. 2.
A) Myocyte sarcomere length-time tracings and Ca2+ transients obtained from control, DHF, or CRT hearts (37°, 1 Hz stimulation). Data are shown at rest (bold) and after ISO stimulation (thin). Both rest and ISO stimulated function and Ca2+ transients were depressed in DHF and improved by CRT. B) Summary results support these examples; showing depressed function and Ca2+ handling was seen in both anterior (A) and lateral (L) walls in DHF, and both were improved by CRT (Mean±SEM; n=10–35 cells from 3 to 6 hearts for each data point). *, P≤0.05 vs. control and CRT; † p<0.05 vs anterior. C) Radiolabeled affinity binding assays for β-AR. Upper panels show raw data and Scatchard plots from which total binding (Bmax, receptor density) and binding affinity (Km) were determined (lower panels). Bmax declined with DHF (* p<0.05 versus con) and was restored towards normal by CRT. Binding affinity was unaltered.
CRT enhances β-AR number not binding affinity
Fig. 2C displays radiolabeled β-AR binding assays data. Concentration-binding curves and corresponding Scatchard plots (upper panels) revealed maximal binding (receptor density, Bmax) was significantly depressed in DHF hearts but increased towards normal in CRT. There were no differences in binding affinity between groups (Kd, Fig 2C, lower right).
CRT improves β1 and β2-AR Responsiveness
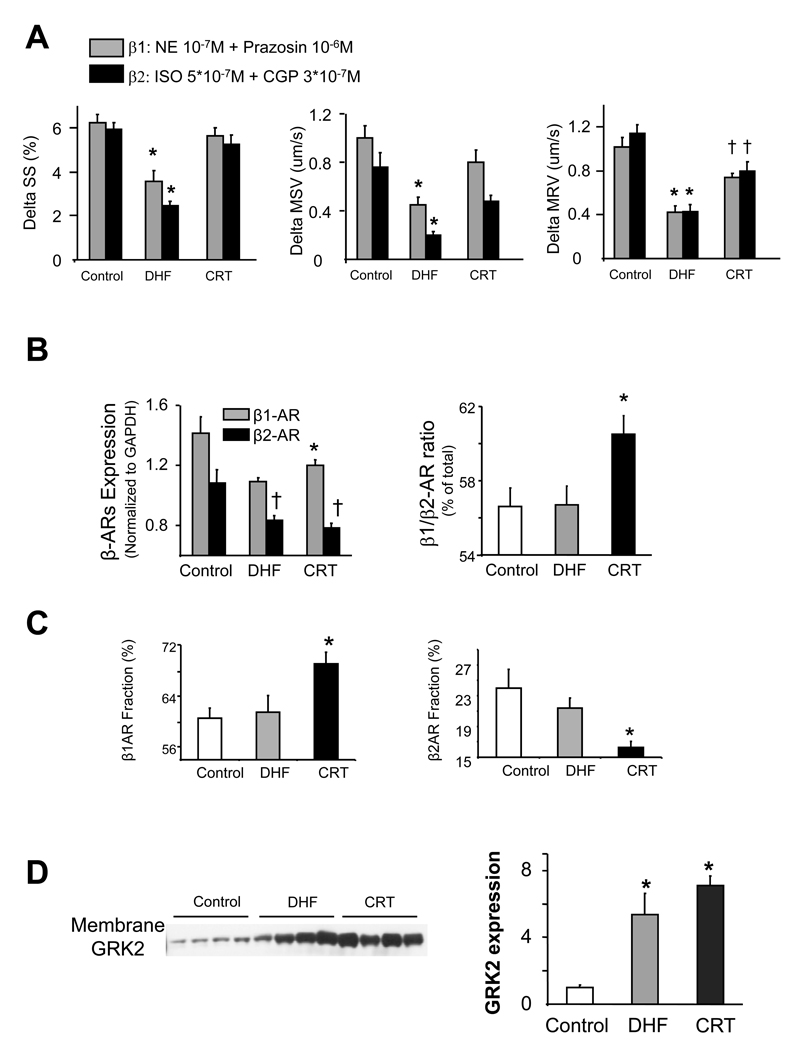
Both β1 and β2-AR regulation were depressed in DHF hearts and improved by CRT (Fig 3a). Reduced responsiveness in DHF was partly related to depression of gene expression for both receptor subtypes; however, β1 but not β2-AR expression rose with CRT, increasing the β1/β2 ratio (Fig 3b). This relative rise in β1 was confirmed by competitive binding assays (Fig 3c). Receptor sub-type binding affinity was also examined, and again no differences were found between controls and either HF group (Supplemental figure 3). β-AR signaling (notably β1) can decline due to phosphorylation by G-receptor kinase 2 (GRK-2), and GRK-2 levels often rise in experimental and human cardiac failure25. GRK-2 protein expression rose in DHF but remained elevated with CRT (Fig 3d); thus it was unlikely to explain the differential β-AR responses.
Fig. 3.
A) Change in sarcomere shortening (SS), mean shortening velocity (MSV) and mean re-lengthening velocity (MRV) with selective stimulation of β1 or β2-AR (data obtained at 27°, 0.5 Hz stimulation, n=15–30 cells/condition from 3–6 different hearts ). Both were depressed in DHF hearts, but became more similar to controls with CRT (* p<0.01 vs CON and CRT; † p≤0.05 vs. CON). B) Both β1 and β2 mRNA expression declined in DHF. β1 increased with CRT whereas β2 remained reduced (* p<0.05 vs DHF and CON; †-p<0.05 versus CON), increasing the net β1/β2 ratio (* p<0.01 vs DHF and CON). C) Receptor number based on competition binding assays with selective inhibitors confirmed differential upregulation of β1 versus β2 by CRT (* p<0.05 versus CON and CRT). D) Immune-blot of GRK-2 from membrane fraction. Expression rose in both DHF and CRT and was similar between groups (* p<0.05 versus CON). Equal protein loading was confirmed by Ponceau stain.
CRT enhances adenylate cyclase activity and myocyte response to forskolin
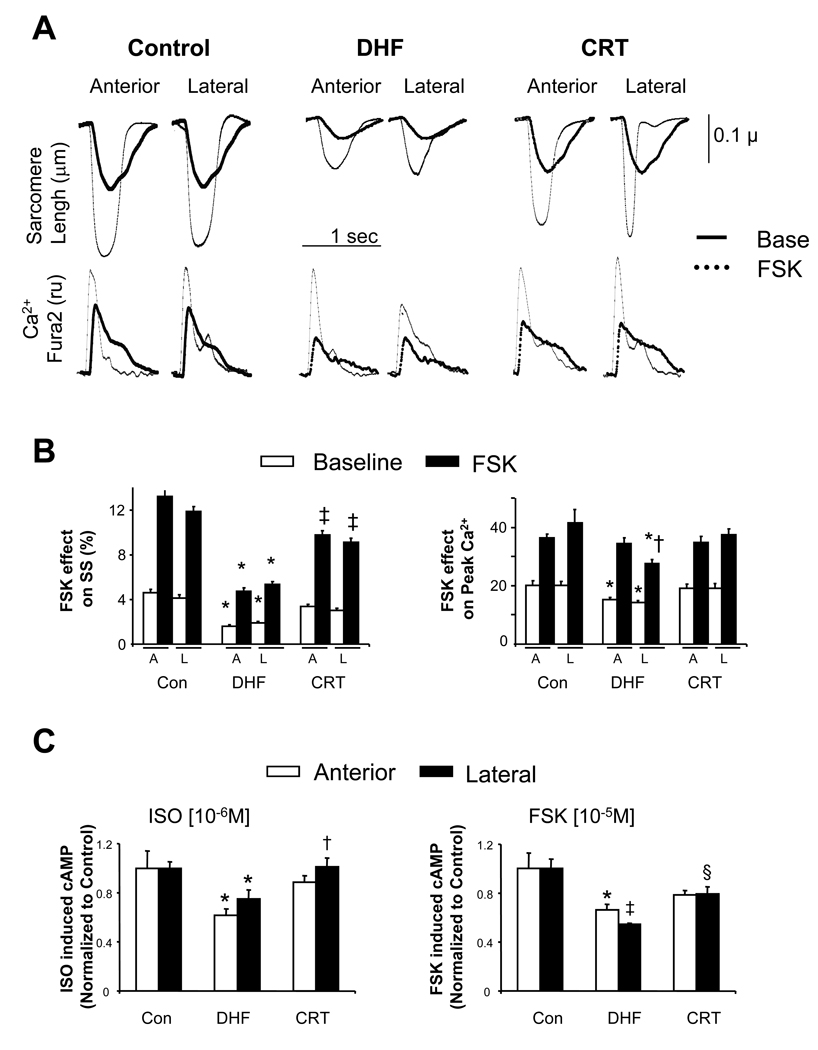
We next tested whether dysregulation of adenylate cyclase may have contributed to the DHF phenotype and its improvement by CRT. Example myocyte shortening and Ca2+ transients are displayed in Fig 4a, and summarized in Fig 4b. In DHF, sarcomere shortening stimulated by forskolin (FSK) was greatly depressed in both regions. However, peak Ca2+-transients were essentially normalized in the anterior-septum despite reduced shortening, whereas in the lateral wall, they remained somewhat depressed. This suggests dysregulation at the myofilament level in DHF, particularly in the antero-lateral wall. CRT improved FSK-shortening responses in both regions, though they remained below controls, yet restored peak Ca2+ transients to control levels. These results indicated that CRT enhanced adenylate cyclase reserve activation. We further tested this in vitro by measuring cAMP generation due to ISO or FSK stimulation (Fig 4c). AC activity was depressed in both regions in DHF hearts, and significantly improved with CRT.
Fig. 4.
A) Examples of influence of adenylate cyclase activation by forskolin (FSK) on sarcomere shortening and Ca2+ transients in myocytes from CON, DHF, and CRT hearts (n=15–30 cells in each group and region from n=3–6 different hearts). B) Summary data. In DHF, FSK stimulated shortening was very depressed, even with peak Ca2+ transients enhanced to control levels in the anterior wall (* p<0.05 versus CON, †-p<0.05 versus lateral). FSK-stimulated shortening was greatly improved in CRT though not quite to control levels († p<0.05 vs CON), while peak Ca2+ was restored to normal response levels. C) Adenylate cyclase activity assessed by cAMP generation assay in response to ISO or FSK. Data are shown normalized to control (15±1 – ISO, 201±14 – FSK, fMol cAMP/mg protein/min). In DHF hearts, AC activity was depressed in both regions and with both stimuli, and this was improved by CRT (* p<0.05 vs CON, †p<0.03 vs DHF; ‡p<0.001 vs CON, §p<0.02 vs DHF).
CRT suppresses Gαi-signaling enhanced in Dyssynchronous heart failure
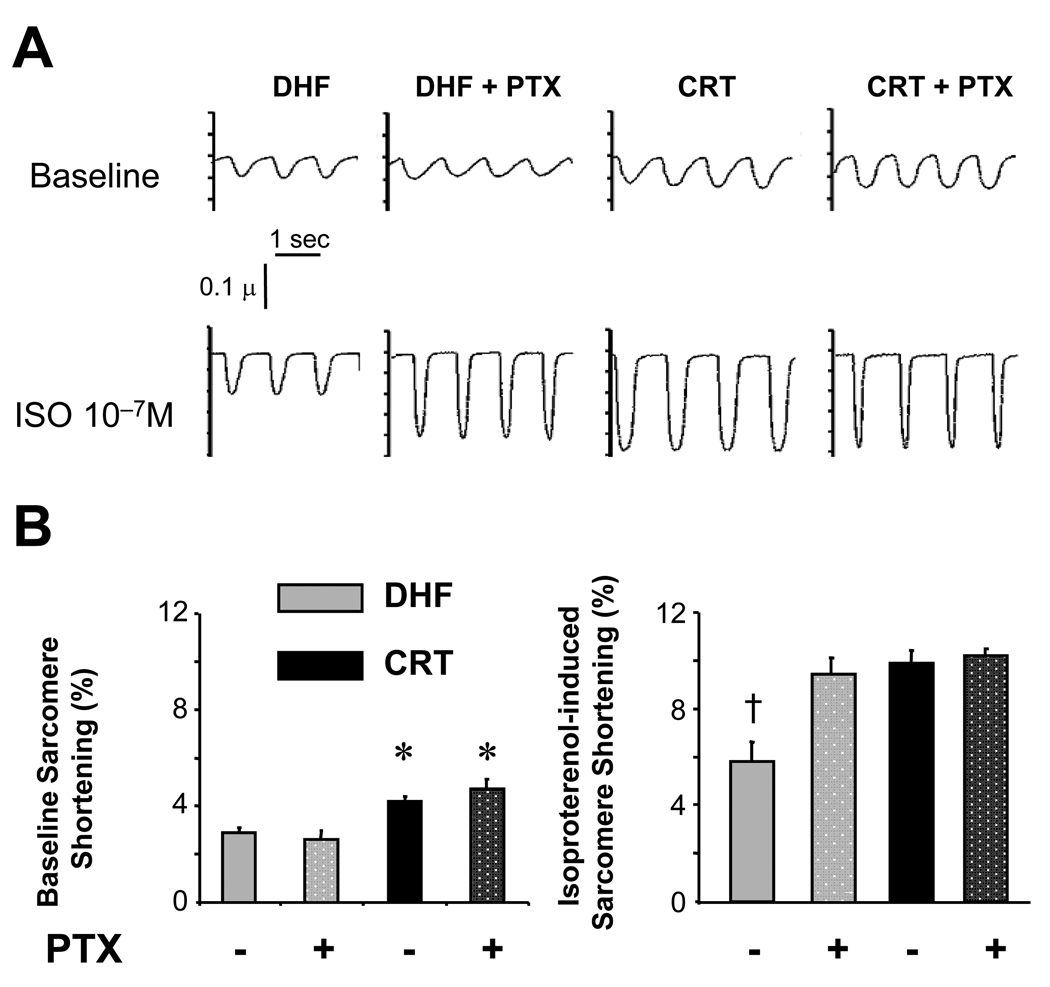
Another important mechanism for downregulated β-AR stimulation is inhibition by Gαi stimulation which can increase in human and experimental heart failure26–28. To test for this, myocytes were pre-treated with pertussis toxin (PTX) to suppress Gαi, followed by ISO stimulation. PTX had no impact on basal contraction in DHF or CRT myocytes (Fig 5a), but markedly enhanced the ISO response in DHF cells, effectively restoring contraction to levels observed in CRT cells without PTX pre-treatment. In contrast, PTX negligibly impacted the ISO response in CRT cells, suggesting CRT itself had resulted in functional Gαi inactivation.
Fig. 5.
A) Sarcomere shortening in DHF and CRT myoctes at rest (Baseline, top panels) and with ISO stimulation (lower panels), with or without pertussis toxin (PTX) pre-treatment. PTX did not alter rest shortening in either group; however, it markedly enhanced the ISO response in DHF myocytes, achieving levels observed in CRT myocytes without PTX. CRT myocytes, by contrast, showed no change in shortening magnitude despite PTX administration. B) Summary data (n=10–30 cells from 3–5 hearts in each condition; *, p<0.05 vs. DHF ± PTX; † p<0.05 vs. all other conditions).
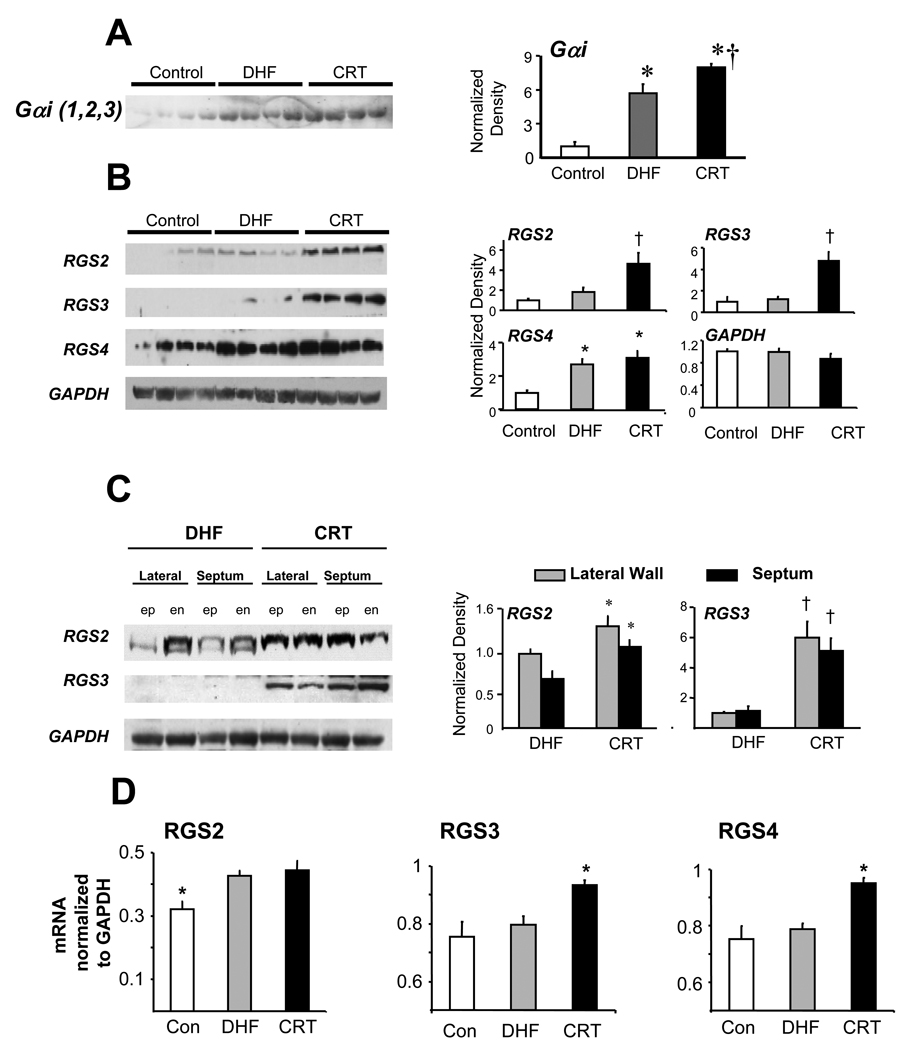
Gαi protein expression (membrane fraction) was increased in DHF hearts, but was if anything even slightly greater in CRT hearts (Fig 6a). Stimulatory G protein (Gαs) was unchanged from control in both groups (data not shown). An alternative mechanism to suppress Gαi-signaling is enhancing negative regulators of G-protein signaling (RGS) proteins, GTP-ases that restore the trimeric G-protein complex to suppress receptor-coupled activation. RGS2, -3, and -4 suppress Gαi in the heart29, and both RGS2 and RGS3 protein expression rose selectively in CRT but not DHF myocardium, the latter being similar to control (Fig 6b). These changes were observed in both regions and myocardial layers (Fig. 6c). Increased RGS3 expression in CRT hearts was confirmed at the mRNA level (Fig 6d), and selective increases in RGS4 mRNA were also observed. Thus, CRT specifically enhanced RGS proteins that can inhibit Gαi signaling.
Fig. 6.
Protein regulation of Gαi and RGS proteins. A) Membrane GαI (1,2,3) increased in DHF (* p<0.01 versus controls), and slightly more in CRT hearts (†, <0.05 versus DHF). Equal loading was conformed by Ponceau stain. B) Differential expression of RGS proteins by DHF versus CRT. Protein is from lateral endocardium with four different animals shown for each group. Both RGS 2 and RGS3 were markedly upregulated in CRT hearts, but not DHF, whereas RGS4 increased in both groups similarly over control. Summary data to the right are normalized to GAPDH. †-p<0.05 versus other two groups. * p<0.05 versus control. C) Differential up-regulation of RGS2 and RGS3 in CRT hearts was similar in both anterior and lateral myocardium, and in endocardial (en) and epicardial (ep) layers. * p<0.05, † p<0.001 versus respective DHF data. D) Gene expression (rtPCR) of RGS proteins shown normalized to GAPDH. All increased in CRT compared to control, with changes in RGS3 and RGS4 only seen in CRT (*p<0.05 vs other groups; †p<0.01 versus other groups).
CRT reduces myocardial catecholamines
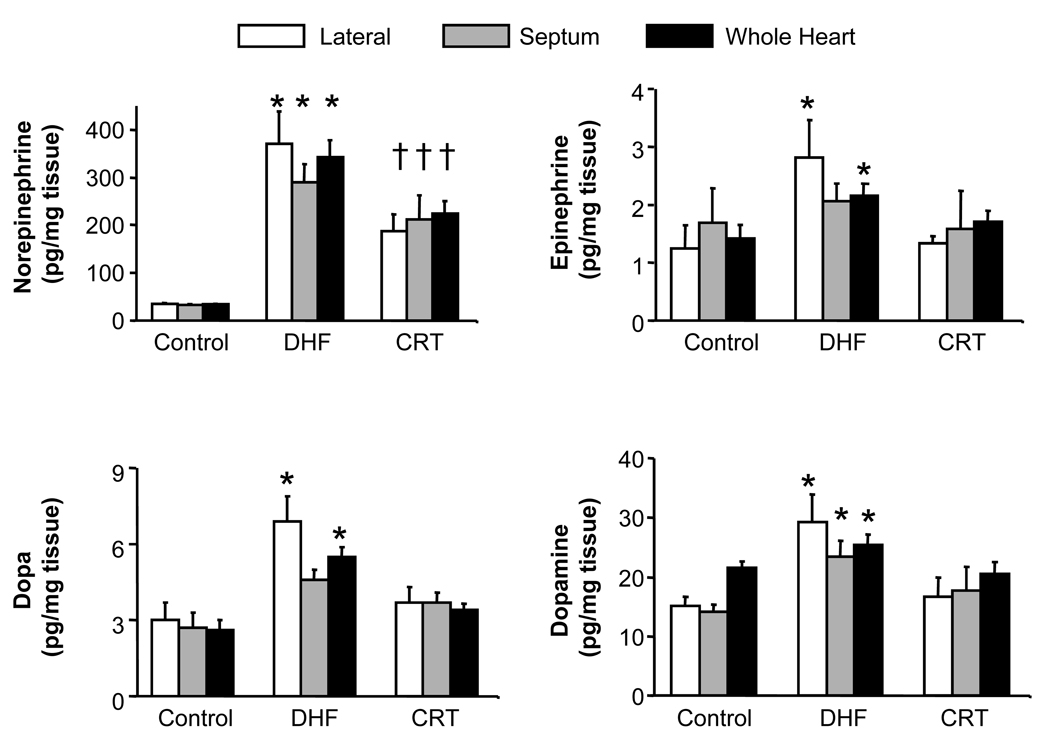
Improved rest and β-AR stimulated function was accompanied by a concomitant withdrawal of myocardial catecholamine stimulation. In DHF hearts, myocardial catecholamine levels rose in both antero-septal and lateral walls (somewhat more in the lateral, Fig 7), yet they declined towards control values with CRT. Thus, CRT restored adrenergic responsiveness while simultaneously reducing the cardiac catecholamine stimulation; analogous to the normal balance.
Figure. 7.
Myocardial catecholamines increase more in DHF than CRT hearts. Data are shown for four different catecholamines measured by HPLC from frozen myocardial tissue. Results are provided for anterior and lateral regions separately, and both combined. There was a tendency for higher levels in the lateral wall, though this did not reach significance for norepinerphrine or dopamine. CRT generally reduced levels in both regions (* p<0.05 vs. control and CRT; †p≤0.05 vs. control).
DISCUSSION
The direct effect of CRT as mediated by bi-ventricular stimulation is to offset conduction delay so both regions of the heart are stimulated and contract more synchronously. This immediately improves function, as the heart no longer transfers blood from one side to the other but rather ejects it into the periphery6. Here, we reveal that over time, CRT potently benefits underlying myocyte function and calcium transients and prominently enhances their β-adrenergic responsiveness. In part, the latter is related to upregulation of β1-AR abundance, enhanced adenylate cyclase activation, and suppression of inhibitory G-protein activity likely linked to up-regulation of RGS proteins such as RGS3. Such RGS changes have not been previously reported in a heart failure therapy, and may reflect a unique feature of CRT.
DHF myocytes displayed depressed rest function and peak and activation/decay kinetics in the Ca2+ transient, and CRT enhanced both. The detailed mechanisms of these resting changes were not explored in the present study, as they are the focus of a companion investigation from our group30. In this other study, we report marked depression of the Ca2+ current-voltage dependence in the lateral wall, and reduced whole cell Ca2+ transients in both regions (as shown here); both being ameliorated by CRT. DHF hearts also have reduced Cavβ2 gene expression (component of ICa) and SERCA2 gene and protein expression that is not observed in CRT, whereas expression of ryanodine receptor and phospholamban is lower and Na+-Ca2+ exchanger higher in both models. These changes were not regional. Other factors for altered rest function and calcium handling maybe the capacity of CRT to reduce cytokine (TNFα), p38 MAP kinase, and calcium-calmodulin kinase expression/activity, as we previously reported in this model19.
Deficits in both rest and ISO (or FSK) stimulated cell shortening and Ca2+ were similar in both early and late activated regions in DHF, and both improved with CRT. This is intriguing, as it highlights a global rather than purely regional impact of dysynchrony and its amelioration by resynchronization. Such global changes are concordant with prior observations regarding cell survival signaling in the DHF and CRT models19, though other modifications, such as those in gap junction and stress kinases proteins have appeared more regional19,20. DHF behavior could reflect an overall failure state made worse by abnormal regional loading. CRT modestly improved global function but largely resolved loading disparities. Since early and late contracting regions adversely load each other, this could impact signaling more generally throughout the heart. CRT also improves chamber efficiency7 that in turn could alter neuro-stimulation, myocyte function and reserve more globally.
The major focus of the present study was on β-AR responsiveness, as depression of this signaling is a common feature of cardiac failure. In addition to the impact of impaired calcium homeostasis, impaired β-adrenergic responsiveness arises from multiple abnormalities, including reduced receptor abundance (expression, internalization, and/or degradation), receptor desensitization, increased Gαi-signaling, and reduced adenylate cyclase activity15,16,31. Therapies such as angiotensin converting enzyme inhibition32 and beta-blockade33, and ventricular assist devices34, enhance β-AR signaling via several of these mechanisms. Though CRT did not target a specific neurohormone, nor profoundly unload the LV while restoring cardiac output (as occurs with assist devices), it markedly improved β-AR reserve. Some mechanisms associated with CRT, such as the rise in β1 expression, and improved AC activity, share similarities to earlier therapies. However, others are novel, such as reduced Gαi signaling associated not with lowered expression per se, but rather increased levels of inhibitory RGS proteins.
Gαi-coupled signaling can play several roles. One is depressing adrenergic stimulation27,35, which is particularly true for the β2-AR36, though Gαi crosstalk between receptor subtypes has shown it can blunt β1 stimulation of L-type Ca2+ currents37. In this regard, DHF hearts displayed evidence of enhanced functional Gαi coupling, whereas this was effectively removed by CRT – despite ameliorated but still persisting heart failure. Enhanced Gαi stimulation can also be cardio-protective effects against apoptosis27,38,39. Yet, chronic suppression of Gαi by CRT does not appear to enhanced cell death but do just the opposite, as previously shown in the current model19 and suggested in a human study40. One key difference is likely the concomitant decline in catecholamine stimulation by CRT, reducing adrenergic-mediated toxicity. CRT acutely lowers sympathetic nerve stimulation17,18 in humans attributed to its increase in stroke volume, while chronic suppression is observed in CRT responders18. Here we show this downregulation at the myocardial level, restoring a more normal balance between catecholamine stimulation and myocyte adrenergic responsiveness.
CRT upregulated both RGS2 and RGS3 expression, which is intriguing given their role in blunting adrenergic responses by enhancing β2-AR-Gαi modulation31,41. To our knowledge, this is the first example of RGS up-regulation by a heart failure therapy and one further reflected by suppressed Gi-coupled signaling. Neither RGS species was enhanced in DHF hearts, similar to human heart failure data42, whereas RGS4 protein increased in both, consistent with findings in human CHF43. RGS3 and RGS4 regulate both Gi and Gq signaling44,45 whereas RGS2 more specifically targets Gq29,46, and its up-regulation with CRT may also blunt this signaling (e.g. angiotensin, endothelin). RGS3 upregulation may have additional effects, such as redirecting the activation of Rho GTPases via Gi-coupled receptors to switch from Rac1 to RhoA activation47.
Our study has some limitations. First, the model shortens the time course of CRT usually studied in humans, much as tachypacing itself abbreviates the time course of heart failure. However, as previously reported19 and examined here more fully, the DHF model mimics many pathophysiologic features of the dysynchronous failure, and improvements with CRT similarly share many characteristics seen in humans. Second, animals were not co-treated with ACE inhibitors or adrenergic blockers (e.g. carvedelol). While such therapies can themselves enhance adrenergic reserve, the fact that CRT improves heart function and exercise capacity in patients already on such treatments, and assists in the use of β-blockers in previously intolerant patients48, supports independent mechanisms. Our goal here was to identify changes related to CRT itself.
When CRT was first introduced, there was broad skepticism that such an intervention would prove beneficial given the complexity of underlying abnormal signaling in heart failure. Subsequent trials, that revealed its utility, raised suspicions that more may be transpiring beneath the surface. The current findings come nearly 7 years after CRT was first approved for human use in the United States, and show such suspicions were warranted. Enhancement of β-AR responsiveness along with reduced cardiac catecholamine stimulation, are likely important mechanisms whereby CRT improves systolic function and reserve, and chronic mortality.
CLINICAL SUMMARY
Cardiac resynchronization therapy (CRT) is an effective treatment for patients with heart failure and dyssynchrony due to conduction delay, and is to date the only therapy that can both acutely and chronically improve chamber systolic function yet also reduce long-term mortality. The chamber mechanical impact of CRT is well documented, and occurs rapidly. However, more chronic influences on both myocyte function and adrenergic modulation that may underlie sustained benefits are largely unknown. To address this, we developed an experimental canine model of dyssynchronous heart failure (DHF, tachypacing with a left bundle branch block) with or without subsequent resynchronization (biventricular tachypacing, CRT). Both models display global heart failure, though CRT did improve systolic function as observed in humans. Here we show marked global reductions in both resting and beta-adrenergic stimulated myocyte function and whole-cell calcium-handling in dyssynchronous HF, and that both markedly improved (β-adrenergic reserve to near normal levels) by CRT. Changes involved calcium homeostasis, increased adrenergic receptor (β1) density and adenylate cyclase activity, and novel suppression of inhibitory G-protein signaling. Accompanying this adrenergic upregulation was a decline in myocardial catecholamines from the higher levels observed in DHF hearts. Thus, CRT effectively restored a more normal balance of greater cellular adrenergic responsiveness with reduced chronic sympathetic stimulation. This may play an important role in the long-term efficacy of CRT on clinical symptoms and survival, and its interaction with concurrent pharmacologic neuro-blockade treatment
Acknowledgments
Funding Sources
Supported by NHLBI Grant: PO1-HL077180 (Drs. Kass and Tomaselli), RO1:HL-089297, The Peter Belfer Laboratory and Abraham and Virginia Weiss Professorship (Dr. Kass), and Intramural Research Program of the NIH, National Institute on Aging (Dr. Xiao).
Footnotes
Disclosures
None.
REFERENCES
- 1.Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, Hailpern SM, Ho M, Howard V, Kissela B, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O'Donnell C, Roger V, Sorlie P, Steinberger J, Thom T, Wilson M, Hong Y. Heart disease and stroke statistics--2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–e146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 2.Abraham WT. Cardiac resynchronization therapy. Prog Cardiovasc Dis. 2006;48:232–238. doi: 10.1016/j.pcad.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 3.Spragg DD, Kass DA. Pathobiology of left ventricular dyssynchrony and resynchronization. Prog Cardiovasc Dis. 2006;49:26–41. doi: 10.1016/j.pcad.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 4.Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T, Carson P, DiCarlo L, DeMets D, White BG, DeVries DW, Feldman AM. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–2150. doi: 10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]
- 5.Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, Tavazzi L. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–1549. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 6.Kass DA, Chen CH, Curry C, Talbot M, Berger R, Fetics B, Nevo E. Improved left ventricular mechanics from acute VDD pacing in patients with dilated cardiomyopathy and ventricular conduction delay. Circulation. 1999;99:1567–1573. doi: 10.1161/01.cir.99.12.1567. [DOI] [PubMed] [Google Scholar]
- 7.Nelson GS, Berger RD, Fetics BJ, Talbot M, Hare JM, Spinelli JC, Kass DA. Left ventricular or biventricular pacing improves cardiac function at diminished energy cost in patients with dilated cardiomyopathy and left bundle-branch block. Circulation. 2000;102:3053–3059. doi: 10.1161/01.cir.102.25.3053. [DOI] [PubMed] [Google Scholar]
- 8.John Sutton MG, Plappert T, Abraham WT, Smith AL, Delurgio DB, Leon AR, Loh E, Kocovic DZ, Fisher WG, Ellestad M, Messenger J, Kruger K, Hilpisch KE, Hill MR. Effect of cardiac resynchronization therapy on left ventricular size and function in chronic heart failure. Circulation. 2003;107:1985–1990. doi: 10.1161/01.CIR.0000065226.24159.E9. [DOI] [PubMed] [Google Scholar]
- 9.Vanderheyden M, Mullens W, Delrue L, Goethals M, De Bruyne B, Wijns W, Geelen P, Verstreken S, Wellens F, Bartunek J. Myocardial gene expression in heart failure patients treated with cardiac resynchronization therapy responders versus nonresponders. J Am Coll Cardiol. 2008;51:129–136. doi: 10.1016/j.jacc.2007.07.087. [DOI] [PubMed] [Google Scholar]
- 10.Mullens W, Bartunek J, Wilson Tang WH, Delrue L, Herbots L, Willems R, De Bruyne B, Goethals M, Verstreken S, Vanderheyden M. Early and late effects of cardiac resynchronization therapy on force-frequency relation and contractility regulating gene expression in heart failure patients. Heart Rhythm. 2008;5:52–59. doi: 10.1016/j.hrthm.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 11.Iyengar S, Haas G, Lamba S, Orsinelli DA, Babu GJ, Ferketich AK, Yamokoski L, Periasamy M, Abraham WT. Effect of cardiac resynchronization therapy on myocardial gene expression in patients with nonischemic dilated cardiomyopathy. J Card Fail. 2007;13:304–311. doi: 10.1016/j.cardfail.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 12.Vanderheyden M, Mullens W, Delrue L, Goethals M, Verstreken S, Wijns W, De Bruyne B, Bartunek J. Endomyocardial upregulation of beta1 adrenoreceptor gene expression and myocardial contractile reserve following cardiac resynchronization therapy. J Card Fail. 2008;14:172–178. doi: 10.1016/j.cardfail.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 13.Hay I, Melenovsky V, Fetics BJ, Judge DP, Kramer A, Spinelli J, Reister C, Kass DA, Berger RD. Short-term effects of right-left heart sequential cardiac resynchronization in patients with heart failure, chronic atrial fibrillation, and atrioventricular nodal block. Circulation. 2004;110:3404–3410. doi: 10.1161/01.CIR.0000148177.82319.C7. [DOI] [PubMed] [Google Scholar]
- 14.Vollmann D, Luthje L, Schott P, Hasenfuss G, Unterberg-Buchwald C. Biventricular pacing improves the blunted force-frequency relation present during univentricular pacing in patients with heart failure and conduction delay. Circulation. 2006;113:953–959. doi: 10.1161/CIRCULATIONAHA.105.579987. [DOI] [PubMed] [Google Scholar]
- 15.Tilley DG, Rockman HA. Role of beta-adrenergic receptor signaling and desensitization in heart failure: new concepts and prospects for treatment. Expert Rev Cardiovasc Ther. 2006;4:417–432. doi: 10.1586/14779072.4.3.417. [DOI] [PubMed] [Google Scholar]
- 16.Feldman DS, Carnes CA, Abraham WT, Bristow MR. Mechanisms of disease: beta-adrenergic receptors--alterations in signal transduction and pharmacogenomics in heart failure. Nat Clin Pract Cardiovasc Med. 2005;2:475–483. doi: 10.1038/ncpcardio0309. [DOI] [PubMed] [Google Scholar]
- 17.Hamdan MH, Barbera S, Kowal RC, Page RL, Ramaswamy K, Joglar JA, Karimkhani V, Smith ML. Effects of resynchronization therapy on sympathetic activity in patients with depressed ejection fraction and intraventricular conduction delay due to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 2002;89:1047–1051. doi: 10.1016/s0002-9149(02)02273-7. [DOI] [PubMed] [Google Scholar]
- 18.Najem B, Unger P, Preumont N, Jansens JL, Houssiere A, Pathak A, Xhaet O, Gabriel L, Friart A, De Roy L, Vandenbossche JL, van de BP. Sympathetic control after cardiac resynchronization therapy: responders versus nonresponders. Am J Physiol Heart Circ Physiol. 2006;291:H2647–H2652. doi: 10.1152/ajpheart.00373.2006. [DOI] [PubMed] [Google Scholar]
- 19.Chakir K, Daya SK, Tunin RS, Helm RH, Byrne MJ, Dimaano VL, Lardo AC, Abraham TP, Tomaselli GF, Kass DA. Reversal of global apoptosis and regional stress kinase activation by cardiac resynchronization. Circulation. 2008;117:1369–1377. doi: 10.1161/CIRCULATIONAHA.107.706291. [DOI] [PubMed] [Google Scholar]
- 20.Spragg DD, Leclercq C, Loghmani M, Faris OP, Tunin RS, DiSilvestre D, McVeigh ER, Tomaselli GF, Kass DA. Regional alterations in protein expression in the dyssynchronous failing heart. Circulation. 2003;108:929–932. doi: 10.1161/01.CIR.0000088782.99568.CA. [DOI] [PubMed] [Google Scholar]
- 21.O'Rourke B, Kass DA, Tomaselli GF, Kaab S, Tunin R, Marban E. Mechanisms of altered excitation-contraction coupling in canine tachycardia-induced heart failure, I: experimental studies. Circ Res. 1999;84:562–570. doi: 10.1161/01.res.84.5.562. [DOI] [PubMed] [Google Scholar]
- 22.Tanaka R, Fulbright BM, Mukherjee R, Burchell SA, Crawford FA, Zile MR, Spinale FG. The cellular basis for the blunted response to beta-adrenergic stimulation in supraventricular tachycardia-induced cardiomyopathy. J Mol Cell Cardiol. 1993;25:1215–1233. doi: 10.1006/jmcc.1993.1134. [DOI] [PubMed] [Google Scholar]
- 23.Zhou YY, Yang D, Zhu WZ, Zhang SJ, Wang DJ, Rohrer DK, Devic E, Kobilka BK, Lakatta EG, Cheng H, Xiao RP. Spontaneous activation of beta(2)- but not beta(1)-adrenoceptors expressed in cardiac myocytes from beta(1)beta(2) double knockout mice. Mol Pharmacol. 2000;58:887–894. doi: 10.1124/mol.58.5.887. [DOI] [PubMed] [Google Scholar]
- 24.Eisenhofer G, Goldstein DS, Stull R, Keiser HR, Sunderland T, Murphy DL, Kopin IJ. Simultaneous liquid-chromatographic determination of 3,4-dihydroxyphenylglycol, catecholamines, and 3,4-dihydroxyphenylalanine in plasma, and their responses to inhibition of monoamine oxidase. Clin Chem. 1986;32:2030–2033. [PubMed] [Google Scholar]
- 25.Petrofski JA, Koch WJ. The beta-adrenergic receptor kinase in heart failure. J Mol Cell Cardiol. 2003;35:1167–1174. doi: 10.1016/s0022-2828(03)00243-8. [DOI] [PubMed] [Google Scholar]
- 26.Rau T, Nose M, Remmers U, Weil J, Weissmuller A, Davia K, Harding S, Peppel K, Koch WJ, Eschenhagen T. Overexpression of wild-type Galpha(i)-2 suppresses beta-adrenergic signaling in cardiac myocytes. FASEB J. 2003;17:523–525. doi: 10.1096/fj.02-0660fje. [DOI] [PubMed] [Google Scholar]
- 27.El Armouche A, Zolk O, Rau T, Eschenhagen T. Inhibitory G-proteins and their role in desensitization of the adenylyl cyclase pathway in heart failure. Cardiovasc Res. 2003;60:478–487. doi: 10.1016/j.cardiores.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 28.Kiuchi K, Shannon RP, Komamura K, Cohen DJ, Bianchi C, Homcy CJ, Vatner SF, Vatner DE. Myocardial beta-adrenergic receptor function during the development of pacing-induced heart failure. J Clin Invest. 1993;91:907–914. doi: 10.1172/JCI116312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hao J, Michalek C, Zhang W, Zhu M, Xu X, Mende U. Regulation of cardiomyocyte signaling by RGS proteins: differential selectivity towards G proteins and susceptibility to regulation. J Mol Cell Cardiol. 2006;41:51–61. doi: 10.1016/j.yjmcc.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 30.Aiba T, Hesketh GG, Barth AS, Liu T, Daya S, Chakir K, Dimaano VL, Abraham TP, O’Rourke B, Akar FG, Kass DA, Tomaselli GF. Electrophysioloigcal consequences of dyssynchronous heart failure and its restoration by resynchronization therapy. Circulation. 2008 doi: 10.1161/CIRCULATIONAHA.108.794834. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lohse MJ, Engelhardt S, Eschenhagen T. What is the role of beta-adrenergic signaling in heart failure? Circ Res. 2003;93:896–906. doi: 10.1161/01.RES.0000102042.83024.CA. [DOI] [PubMed] [Google Scholar]
- 32.Yonemochi H, Yasunaga S, Teshima Y, Iwao T, Akiyoshi K, Nakagawa M, Saikawa T, Ito M. Mechanism of beta-adrenergic receptor upregulation induced by ACE inhibition in cultured neonatal rat cardiac myocytes: roles of bradykinin and protein kinase C. Circulation. 1998;97:2268–2273. doi: 10.1161/01.cir.97.22.2268. [DOI] [PubMed] [Google Scholar]
- 33.Sigmund M, Jakob H, Becker H, Hanrath P, Schumacher C, Eschenhagen T, Schmitz W, Scholz H, Steinfath M. Effects of metoprolol on myocardial beta-adrenoceptors and Gi alphaproteins in patients with congestive heart failure. Eur J Clin Pharmacol. 1996;51:127–132. doi: 10.1007/s002280050172. [DOI] [PubMed] [Google Scholar]
- 34.Pandalai PK, Bulcao CF, Merrill WH, Akhter SA. Restoration of myocardial beta-adrenergic receptor signaling after left ventricular assist device support. J Thorac Cardiovasc Surg. 2006;131:975–980. doi: 10.1016/j.jtcvs.2006.01.027. [DOI] [PubMed] [Google Scholar]
- 35.Kompa AR, Gu XH, Evans BA, Summers RJ. Desensitization of cardiac beta-adrenoceptor signaling with heart failure produced by myocardial infarction in the rat. Evidence for the role of Gi but not Gs or phosphorylating proteins. J Mol Cell Cardiol. 1999;31:1185–1201. doi: 10.1006/jmcc.1999.0951. [DOI] [PubMed] [Google Scholar]
- 36.Xiao RP, Zhu W, Zheng M, Cao C, Zhang Y, Lakatta EG, Han Q. Subtype-specific alpha1- and beta-adrenoceptor signaling in the heart. Trends Pharmacol Sci. 2006;27:330–337. doi: 10.1016/j.tips.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 37.He JQ, Balijepalli RC, Haworth RA, Kamp TJ. Crosstalk of beta-adrenergic receptor subtypes through Gi blunts beta-adrenergic stimulation of L-type Ca2+ channels in canine heart failure. Circ Res. 2005;97:566–573. doi: 10.1161/01.RES.0000181160.31851.05. [DOI] [PubMed] [Google Scholar]
- 38.Communal C, Singh K, Sawyer DB, Colucci WS. Opposing effects of beta(1)- and beta(2)-adrenergic receptors on cardiac myocyte apoptosis : role of a pertussis toxin-sensitive G protein. Circulation. 1999;100:2210–2212. doi: 10.1161/01.cir.100.22.2210. [DOI] [PubMed] [Google Scholar]
- 39.Chesley A, Lundberg MS, Asai T, Xiao RP, Ohtani S, Lakatta EG, Crow MT. The beta(2)-adrenergic receptor delivers an antiapoptotic signal to cardiac myocytes through G(i)-dependent coupling to phosphatidylinositol 3'-kinase. Circ Res. 2000;87:1172–1179. doi: 10.1161/01.res.87.12.1172. [DOI] [PubMed] [Google Scholar]
- 40.D'Ascia C, Cittadini A, Monti MG, Riccio G, Sacca L. Effects of biventricular pacing on interstitial remodelling, tumor necrosis factor-alpha expression, and apoptotic death in failing human myocardium. Eur Heart J. 2006;27:201–206. doi: 10.1093/eurheartj/ehi579. [DOI] [PubMed] [Google Scholar]
- 41.Foerster K, Groner F, Matthes J, Koch WJ, Birnbaumer L, Herzig S. Cardioprotection specific for the G protein Gi2 in chronic adrenergic signaling through beta 2-adrenoceptors. Proc Natl Acad Sci U S A. 2003;100:14475–14480. doi: 10.1073/pnas.1936026100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang S, Watson N, Zahner J, Rottman JN, Blumer KJ, Muslin AJ. RGS3 and RGS4 are GTPase activating proteins in the heart. J Mol Cell Cardiol. 1998;30:269–276. doi: 10.1006/jmcc.1997.0591. [DOI] [PubMed] [Google Scholar]
- 43.Mittmann C, Chung CH, Hoppner G, Michalek C, Nose M, Schuler C, Schuh A, Eschenhagen T, Weil J, Pieske B, Hirt S, Wieland T. Expression of ten RGS proteins in human myocardium: functional characterization of an upregulation of RGS4 in heart failure. Cardiovasc Res. 2002;55:778–786. doi: 10.1016/s0008-6363(02)00459-5. [DOI] [PubMed] [Google Scholar]
- 44.Scheschonka A, Dessauer CW, Sinnarajah S, Chidiac P, Shi CS, Kehrl JH. RGS3 is a GTPase-activating protein for g(ialpha) and g(qalpha) and a potent inhibitor of signaling by GTPase-deficient forms of g(qalpha) and g(11alpha) Mol Pharmacol. 2000;58:719–728. doi: 10.1124/mol.58.4.719. [DOI] [PubMed] [Google Scholar]
- 45.Wieland T, Lutz S, Chidiac P. Regulators of G protein signalling: a spotlight on emerging functions in the cardiovascular system. Curr Opin Pharmacol. 2007;7:201–207. doi: 10.1016/j.coph.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 46.Zhang W, Anger T, Su J, Hao J, Xu X, Zhu M, Gach A, Cui L, Liao R, Mende U. Selective loss of fine tuning of Gq/11 signaling by RGS2 protein exacerbates cardiomyocyte hypertrophy. J Biol Chem. 2006;281:5811–5820. doi: 10.1074/jbc.M507871200. [DOI] [PubMed] [Google Scholar]
- 47.Vogt A, Lutz S, Rumenapp U, Han L, Jakobs KH, Schmidt M, Wieland T. Regulator of G-protein signalling 3 redirects prototypical Gi-coupled receptors from Rac1 to RhoA activation. Cell Signal. 2007;19:1229–1237. doi: 10.1016/j.cellsig.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 48.Aranda JM, Jr, Woo GW, Conti JB, Schofield RS, Conti CR, Hill JA. Use of cardiac resynchronization therapy to optimize beta-blocker therapy in patients with heart failure and prolonged QRS duration. Am J Cardiol. 2005;95:889–891. doi: 10.1016/j.amjcard.2004.12.023. [DOI] [PubMed] [Google Scholar]