Abstract
Studies in humans and rodents suggest that colon inflammation promotes urinary bladder hypersensitivity and, conversely, that cystitis contributes to colon hypersensitivity, events referred to as cross-organ sensitization. To investigate a potential peripheral mechanism, we examined whether cystitis alters the sensitivity of pelvic nerve colorectal afferents. Male C57BL/6 mice were treated with cyclophosphamide (CYP) or saline, and the mechanosensitive properties of single afferent fibers innervating the colorectum were studied with an in vitro preparation. In addition, mechanosensitive receptive endings were exposed to an inflammatory soup (IS) to study sensitization. Urinary bladder mechanosensitive afferents were also tested. We found that baseline responses of stretch-sensitive colorectal afferents did not differ between treatment groups. Whereas IS excited a proportion of colorectal afferents CYP treatment did not alter the magnitude of this response. However, the number of stretch-sensitive fibers excited by IS was increased relative to saline-treated mice. Responses to IS were not altered by CYP treatment, but the proportion of IS-responsive fibers was increased relative to saline-treated mice. In bladder, IS application increased responses of muscular afferents to stretch, although no differences were detected between saline- and CYP-treated mice. In contrast, their chemosensitivity to IS was decreased in the CYP-treated group. Histological examination revealed no changes in colorectum and modest edema and infiltration in the urinary bladder of CYP-treated mice. In conclusion, CYP treatment increased mechanical sensitivity of colorectal muscular afferents and increased the proportion of chemosensitive colorectal afferents. These data support a peripheral contribution to cross-organ sensitization of pelvic organs.
Keywords: sensitization, pelvic nerve, bladder, colon, inflammatory soup
the referral of visceral pain to nonvisceral, somatic sites has long been appreciated and extensively documented (5, 15, 25). In contrast, the relevance of viscero-visceral referral and sensitization (termed cross-organ sensitization) to visceral disease states has only recently received attention (7, 19, 38) and has been proposed as an important contributor to chronic pelvic pain (CPP; Refs. 38, 49, 55).
Epidemiologic studies support the occurrence of cross-organ sensitization in humans. Thus patients with interstitial cystitis (IC) often present with concomitant chronic diseases, including irritable bowel syndrome (IBS) (2, 22). Conversely, patients with IBS complain of genitourinary problems (2, 23, 62) and CPP (41). Similarly, both acute (49) and chronic (8, 35) colorectal irritation in rodents induce urinary bladder overactivity, increased contraction frequencies, reduced intercontraction intervals (49), and altered micturition reflexes (35). Conversely, urinary bladder inflammation has been shown to increase responses to colorectal distension in rats (49) and mice (8).
The mechanisms leading to cross-organ sensitization are poorly understood. Because second-order spinal neurons receive convergent input from different body targets, including visceral organs, it has been assumed that cross-organ sensitization arises by a central convergence-projection mechanism similar to that advanced for viscerosomatic referral and sensitization (15, 19, 25). However, recent studies point to participation of peripheral mechanisms as well. For example, colorectal irritation in rodents increases urinary bladder afferent (50, 51) as well as detrusor activity (40).
In contrast studies analyzing “bladder-to-colon” peripheral sensitization have not yet been conducted. Thus we examined here the effect of urinary bladder inflammation on single mechanosensitive colorectal afferent fibers. We hypothesized that the mechanosensitivity of colorectal afferents would be altered significantly by cystitis, and that they would exhibit greater sensitization when exposed to an acidic soup of inflammatory mediators.
MATERIALS AND METHODS
Male C57BL/6 mice (Taconic, Germantown, NJ; 7–8 wk old) were used in all experiments. All research protocols adhered to United States Public Health Service policies regarding the care and use of animals in research and were reviewed and approved by the Institutional Animal Care Use Committee of the University of Pittsburgh.
Cyclophosphamide treatment.
In humans, the antineoplastic drug cyclophosphamide (CYP) can induce hemorrhagic cystitis, urgency, increased frequency, and pain (32) through its toxic metabolite, acrolein (16). Accordingly, CYP (8, 10, 11, 36) and acrolein (6, 9, 37) have been used to induce bladder inflammation in rats (11, 36, 37) and mice (6, 8–10).
Mice were injected intraperitoneally on days 1, 3, and 5 with either saline (0.06 ml/g; n = 16) or CYP (100 mg/kg; n = 22) and killed on day 6 for recording of colorectal or bladder afferents or for histological evaluation/myeloperoxidase (MPO) assay.
Recording of single fibers.
Mice were killed by CO2 inhalation (40 kPa), and the colorectum or bladder with associated neurovascular bundle containing the pelvic nerve (PN) was removed and transferred to ice-cold Krebs solution bubbled with carbogen (95% O2, 5% CO2) as described previously (12, 66). The posterior wall of either the colorectum or bladder was opened along the longitudinal axis, pinned flat, mucosal side up, in one chamber of a two-chamber organ bath, and superfused with oxygenated Krebs solution (in mM: 117.9 NaCl, 4.7 KCl, 25 NaHCO3, 1.3 NaH2PO4, 1.2 MgSO4·7H2O, 2.5 CaCl2, 11.1 d-glucose, 2 butyrate, 20 acetate; at ∼32°C). The PN was placed into an adjacent recording chamber filled with light paraffin oil. For the colorectum, the L-type calcium channel antagonist nifedipine (4 μM; to block spontaneous muscle contractions) and the prostaglandin synthesis inhibitor indomethacin (3 μM; to block synthesis of endogenous prostaglandins) were added to the Krebs solution perfusing the organ chamber.
Under a dissection microscope, the nerve sheath was carefully peeled back and the nerve trunk teased into 6–12 bundles from which single fibers were recorded, typically after further splitting. If more than three units were present in a nerve filament, the filament was further divided so one to a maximum of three clearly discriminable units were present. Unit activity was differentially amplified (10,000×), filtered (∼300-to 10,000-Hz band pass), sampled at 20 kHz with a 1401 interface (Cambridge Electronic Design, Cambridge, UK), and stored on a PC. The amplified signal was also used for online audio monitoring. Action potentials (APs) were viewed online, recorded, analyzed off-line with the Spike 2 wavemark function, and discriminated on the basis of distinguishable waveform, amplitude, and duration.
After 60 min of adaptation to bath conditions, recording was initiated. Mechanosensitive receptive fields were identified by systematically stroking the mucosal surface with a camel's hair brush and then tested with mechanical stimuli to enable classification as previously described (12): 1) mucosal stroking with calibrated polyethylene tubing (∼10-, 250-, 500-, and 1,000-mg force, each force applied 10 times, once per second) and 2) circumferential stretch. Stretch was achieved in two different ways: 1) by focal stimulation, using a cantilever system and two hooks made from bent dissection pins 2 mm apart, the latter attached to the tissue adjacent to the mechanosensitive receptive field, and adding different weights (1, 5, 10 and 15 g for 20 s with an interstimulus interval of 20 s) or 2) by utilizing a 22-mm-long, rigid plastic block in which hooks at 1-mm intervals were fixed. The hooks in this “stretcher block” were pinned along the full length of the anti-mesenteric edge of the colorectum, allowing for displacement that was precisely regulated by a servo-controlled force actuator (series 300B dual-mode servo system, Aurora Scientific, Toronto, ON, Canada). In this manner, and with the opposing edge of the colon firmly pinned to the silicon base of the organ bath, the full length of the dissected colorectum was stretched homogeneously in the circumferential (transverse) direction by a slow ramped force (from 0 to 170 mN at 5 mN/s). Circumferential stretch in this controlled manner allowed for the mathematical conversion into simulated intraluminal distension by using the following equation: pressure = 2πforce/(LD), where L is the colon length and D is circumference. In this manner, we were able to convert the 0–170 mN force into 0–45 mmHg of simulated distension.
Chemical activation and sensitization were tested after baseline assessment of mechanosensitivity. This was done by using bronze square tubing (height 1 cm; inner measures 4×4 mm; weight 0.5 g). Briefly, the smooth edges of the tubing were coated with petrolatum jelly and placed over the receptive field. In this way, the receptive field was enclosed and the contact force was minimized (less weight/μm2), the Krebs solution was removed, and 100 μl of an inflammatory soup (IS) was applied directly to the receptive field for 60 s (colorectum or bladder). Subsequently, the IS and the bronze tubing were removed and responses to stroke and stretch were tested 5 min later. As a control for the procedure, we tested some receptive fields after placing the tubing but without replacing the Krebs solution with IS and observed that the response to stretch was not different than when the fibers were tested with no ring at all (unpublished observations). The IS was prepared in aliquots of 20 μl by combining bradykinin, serotonin, and histamine (in distilled water) with prostaglandin E2 (in dimethyl sulfoxide), frozen, and stored at −20°C. On the day of an experiment, an aliquot was diluted to the final concentration (5 μM for all mediators) in freshly oxygenated Krebs solution. The pH of the IS was adjusted to be either neutral (7.4) or acidic (6.0) in different experiments.
Histology and MPO assay.
Colorectum and bladder from naive (n = 6) and saline (n = 6)- and CYP (n = 12)-treated mice were processed for histology and analysis of MPO activity. Pieces of the colorectum as well as one-half of the bladder were immediately placed in buffered 10% formalin and stored for 48 h before embedding in paraffin, sectioning at 4- to 10-μm thickness, and staining with hematoxylin and eosin. Under a bright-field microscope, signs of edema, inflammatory infiltration, and hemorrhage were assessed in 6–12 sections of both organs. Scoring was as previously described (60): 0 = no edema; 1 = edema limited to the submucosa, the width of which did not exceed the width of the detrusor; 2 = edema present in the bladder wall but not the detrusor and the width of the submucosal region greater than, but less than twice than, the width of the detrusor; and 3 = edema present in the bladder wall, possibly including occasional areas of the detrusor, and the width of the submucosal region greater than twice the width of the detrusor. Intermediate scores (0.5 points) were allowed whenever edema was focalized rather than generalized. Infiltration scores were determined as 0 = no infiltration; 1 = <5 inflammatory cells near blood vessels; 2 = >5 inflammatory cells near blood vessels and spread through the organ; and 3 = abundant conglomerates of inflammatory cells through the organ. An additional point was added if hemorrhage was present.
The remaining halves of bladder and colorectum were processed for MPO activity as an index of granulocyte infiltration as previously described (33). Briefly, colorectum and bladder samples were cut in pieces, sonicated for 10 s, and homogenized in a solution of 0.5% hexadecyltrimethylammonium bromide dissolved in phosphate buffer solution (pH 6.0). The homogenized tissues were then freeze-thawed three times and centrifuged at 13,000 rpm for 5 min. Supernatants were added to a buffer supplemented with 1% hydrogen peroxide and o-dianisidine dihydrochloride solution. Optical density readings were taken for 1 min at 30-s intervals at 450 nm.
Data analysis.
Responses to stroking were calculated as the mean number of spikes in the last 5 s of the 10-s stimulus duration. Responses to stretch were taken as the total number of spikes either in 20 s of stretch or during ramped stretch (presented in 15-mmHg bins: 0–15, 16–30, and 31–45 mmHg). Response threshold was determined as the force (converted to pressure) that elicited the first AP (no afferents were spontaneously active). Typically, one or two distinct fibers were recorded simultaneously. On average, three stretches (1 pre- and 2 post-IS) were conducted. In some cases, and for control purposes, stretch-sensitive fibers were challenged with several identical ramped stretches, and we observed that the firing properties were comparable. Responses to the IS are presented both as the average number of spikes during its application plus the following minute as well as the average number of spikes per 10-s bin. In addition, latency to the first AP after IS application and duration (time from the first to the last AP) were also determined. Studies of different fibers were separated by at least 30 min, with not less than a 40-min washout between successive applications of IS. Some mechanosensitive fibers, both sensitive and insensitive to IS, disappeared shortly after the removal of IS. These fibers were only included in the analysis of responses to the IS and were not included when comparing responses to stretch before and after the application of the IS.
Data are plotted throughout as means ± SE. Data on stroke and stretch are normalized for each fiber to the maximum response to 1,000-mg stroking and 10-g or 31- to 45-mmHg stretch, respectively. Two-way analyses of variance (ANOVAs) were performed as appropriate with Prism 5 (GraphPad Software, San Diego, CA). Fisher's exact test as well as t-tests were used to analyze differences between fibers in saline- and CYP-treated groups exhibiting direct responses to IS. Differences were considered significant when P < 0.05.
Data on scores for bladder weight and histology are presented as the median and were statistically analyzed with Kruskal-Wallis and Dunn's multiple comparison tests between CYP- and saline-treated mice.
Bright-field photomicrographs of the urinary bladder and colorectum from naive, control, and CYP-treated mice were taken with a Retiga 2000R Fast CCD camera (Q-Imaging) attached to a Nikon E-600 microscope (Nikon, Tokyo, Japan).
RESULTS
In total, 88 colorectal and 20 urinary bladder mechanosensitive pelvic nerve fibers were studied. No significant differences were observed in the response of colorectal mucosal and muscular-mucosal fibers to mucosal stroking between saline- and CYP-treated mice, either before or after the application of IS. Differences in the responsiveness of colorectal muscular fibers to circular stretch after the application of IS were apparent between saline- and CYP-treated mice. In addition, CYP-treated mice exhibited a greater proportion of IS-activated colorectal afferents than saline-treated mice.
Excitation of colorectal afferents by IS.
Exposure to neutral (pH 7.4) or acidic (pH 6.0) IS evoked APs in a number of mechanosensitive colorectal fibers from both saline- and CYP-treated mice, revealing their chemosensitive nature (responsive fibers; Table 1). A greater proportion of stretch-sensitive chemosensitive fibers was present in CYP- than saline-treated mice, especially evident when receptive endings were exposed to acidic IS (pH 6.0) (Table 1). Fibers giving no direct response to the application of IS were also present in both experimental groups.
Table 1.
Proportion of colorectal fibers responsive to IS at pH 7.4 or 6.0 and effects of application of IS on stretch sensitive fibers in saline- and CYP-treated mice
| Proportion of Fibers Responsive to IS |
IS-Induced Sensitization to Stretch |
||||||
|---|---|---|---|---|---|---|---|
| Mucosal | Muscular | Muscular-Mucosal | Serosal | Muscular + Muscular-Mucosal | Muscular | Muscular-Mucosal | |
| pH 7.4 IS | |||||||
| Saline | 50% (3/6) | 25% (2/8) | 50% (3/6) | 43% (3/7) | 36% (5 of 14) | ↔ | ↔ |
| CYP | 50% (3/6) | 45% (5/11) | 100% (4/4) | 56% (5/9) | 60% (9 of 15) | ↔ | ↔ |
| pH 6.0 IS | |||||||
| Saline | N/A | 0% (0/8) | 33% (2/6) | N/A | 14% (2 of 14) | ↑ | ↑ |
| CYP | N/A | 67%* (6/9) | 37% (3/8) | N/A | 53% (9 of 17) | ⇈ | ↑ |
The proportion of inflammatory soup (IS)-responsive fibers was statistically analyzed with Fisher's exact test
(P < 0.01). IS-produced sensitization to stretch is represented qualitatively in last column: ↔, no sensitization; ↑, sensitization; CYP, cyclophosphamide; N/A, not applicable. See text for details.
Analysis of response characteristics of individual colorectal afferents to IS, grouping them as stretch insensitive (mucosal and serosal) or stretch sensitive (muscular and muscular-mucosal), revealed no significant differences in the total number of APs during 60 s of IS application, latency to effect, or the duration of effect between saline- and CYP treated groups (Supplemental Table S1).1
Responses of colorectal afferents to stretch.
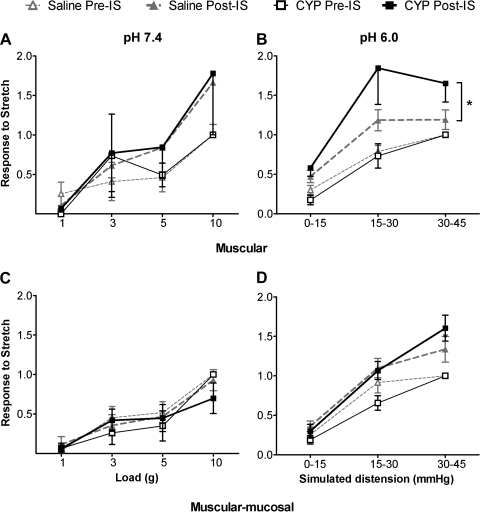
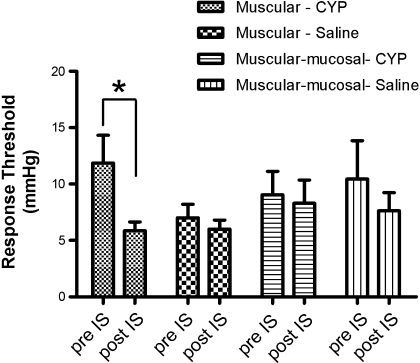
The basal responses (pre-IS) of muscular and muscular-mucosal fibers were not different between CYP- and saline-treated mice (Fig. 1). Application of neutral IS (pH 7.4) did not significantly change their responsiveness to stretch (post-IS) in either saline- or CYP-treated mice (Table 1; Fig. 2, A and C). In contrast, acidic IS (pH 6.0) resulted in significant increases in the response magnitude to stretch in both types of fibers in both saline- and CYP-treated groups (Table 1; Fig. 1, Fig. 2, B and D). Moreover, the sensitization was greater in muscular afferents from CYP- relative to saline-treated mice (Fig. 2B), also reflected by the significant decrease in response threshold after exposure to IS (Fig. 3). No differences in magnitude (Fig. 2D) or response threshold (Fig. 3) were observed in muscular-mucosal fibers in either treatment group.
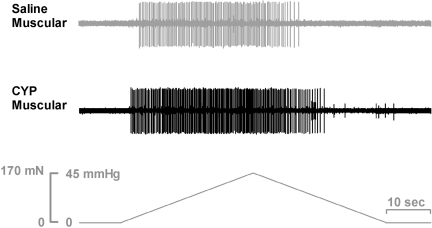
Fig. 1.
Representative examples of muscular and muscular-mucosal colorectal fiber responses to stretch from saline- and cyclophosphamide (CYP)-treated mice. Note the earlier onset of response to stretch (lower response threshold) and greater magnitude of response in the fiber from the CYP-treated mouse.
Fig. 2.
Responses of colorectal muscular (A, B) and muscular-mucosal (C, D) fibers to stretch before (pre) and after (post) application of neutral (pH 7.4; A, C) or acidic (pH 6.0; B, D) inflammatory soup (IS) in saline- and CYP-treated mice. (Note that A and C correspond to the focal stretching protocol and B and D to the homogeneous circumferential stretching protocol; see methods for details.) Basal stretch sensitivity of both types of fibers from saline- and CYP-treated mice were not significantly different, and data were thus normalized (1 = response to maximum stretch) to facilitate comparisons between pre- and post-IS treatment. Application of neutral IS did not alter responses to stretch in either muscular (A) or muscular-mucosal (C) fibers. In contrast, application of acidic IS sensitized responses to stretch in both muscular (B: saline F = 12.57 and CYP F = 15.11, P < 0.002 for both) and muscular-mucosal fibers (D: saline F = 12.58 and CYP F = 21.46, P < 0.003 for both). The effect of IS on stretch sensitivity of muscular fibers, but not muscular-mucosal fibers, was more pronounced in CYP- than saline-treated mice (B: F = 4.314, *P = 0.043).
Fig. 3.
Response thresholds of muscular and muscular-mucosal colorectal fibers from CYP- and saline-treated mice before (pre) and after (post) application of acidic IS. Response thresholds to stretch were extrapolated from the ramp stretch stimulus (see Fig. 1) and are reported as means ± SE. *P < 0.05 vs. pre-IS.
Excitation of urinary bladder muscular afferents by IS.
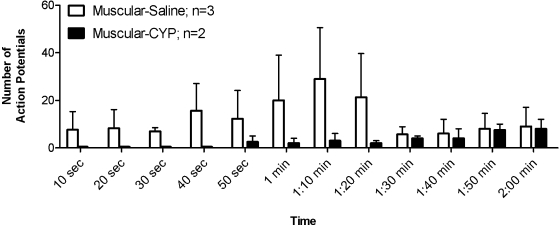
Only 13% (2 of 15) of urinary bladder muscular fibers tested in CYP-treated mice were excited during the application of acidic IS, compared with ∼60% (3 of 5) of muscular fibers from saline-treated mice (Fisher's exact test, P = 0.0726). Moreover, the group of IS-responsive fibers showed a tendency toward a reduced sensitivity in CYP-treated mice. The total number of APs was less (saline 80.0 ± 34.4 vs. CYP 32.5 ± 0.5; t-test, P > 0.3) and the latency (seconds) to effect longer (saline 15.5 ± 7.1 vs. CYP 60.5 ± 13.8; t-test, P < 0.05) in CYP-treated mice, supporting the impression of reduced sensitivity. Figure 4 summarizes the reduced responsiveness of urinary bladder muscular fibers in CYP- relative to saline-treated mice.
Fig. 4.
Responses of urinary bladder muscular afferents to a 60-s application of acidic IS (bin width = 10 s). CYP treatment reduced the chemosensitivity of muscular bladder fibers (F = 4.753, P = 0.036), reflected by an increased latency to effect and a tendency toward a reduced number of action potentials evoked by the IS application.
Responses of urinary bladder muscular afferents to stretch.
The basal response to stretch of muscular afferents in the urinary bladder did not differ between saline- and CYP-treated mice. Responses of the same afferents were significantly greater after exposure to acidic IS (saline: F = 5.021, P = 0.0447; CYP: F = 10.53, P = 0.0019). However, there was no significant difference in the magnitude of sensitization between saline- and CYP-treated mice.
Histology and MPO analysis.
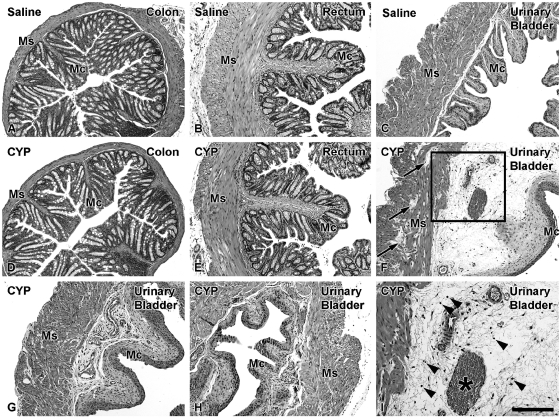
No signs of edema were observed in the colorectum of either saline (Fig. 5, A–C) - or CYP (Fig. 5, D and E)-treated mice, and neither were distinguishable from naive mice (data not shown). In contrast, urinary bladders from CYP-treated mice showed variable edema ranging from moderate (Fig. 5, F and I) to minor (Fig. 5G) and virtually absent (Fig. 5H). Edema scores of the urinary bladder from naive and saline- and CYP-treated mice were 0.04, 0.0, and 0.76, respectively (Kruskal-Wallis test, P = 0.0038). This was consistent with an increase in the percentage ratio of bladder to body weight from CYP-treated (0.15%) compared with naive (0.11%) and saline-treated (0.11%) mice (Kruskal-Wallis test, P = 0.0017).
Fig. 5.
Bright-field photomicrographs showing colon (A, D), rectum (B, E), and urinary bladder (C, F, G–I) histology from saline (A–C)- and CYP (D–I)-treated mice. Ms, muscular layers; Mc, mucosal layer; asterisk in I, blood vessel. Colon (D) and rectum (E) histology in CYP-treated mice was normal (compare with saline-treated mice in A, B). In contrast, a variable degree of edema from moderate (F) to mild (G) and minor (H) is observed in CYP-treated mice relative to saline-treated mice (C). Note the presence of edema in the muscular wall (arrows in F) in some CYP-treated animals. Boxed area in F is at a higher magnification in I, showing several cell profiles possibly corresponding to inflammatory infiltrate (arrowheads in I). Scale bar in I: 100 μm (A–H), 50 μm (I).
Only very low inflammatory cell infiltration and no signs of hemorrhage were detected in naive or saline-treated mice (data not shown). In CYP-treated mice, minor signs of inflammatory cell infiltration (Fig. 5I) and hemorrhage were observed. Thus infiltration + hemorrhage scores from naive and saline- and CYP-treated mice were 0.37, 0.33, and 0.58, respectively (Kruskal-Wallis test, P = 0.2721). MPO activity was low in all experimental groups (in all cases, below 0.012 units of MPO/g of colon).
DISCUSSION
This study documents that CYP-induced cystitis results in sensitization of colorectal afferents, supporting previous reports of CYP-produced hypersensitivity to colorectal distension in rats (49) and mice (8). The CYP treatment protocol we used produced only a mild bladder inflammation (not inconsistent with human interstitial cystitis/painful bladder syndrome) and no colorectal inflammation. Despite the modest bladder insult, mice treated with CYP exhibited 1) significant increases in response magnitude (i.e., sensitization) of both muscular and muscular-mucosal colorectal pelvic nerve afferents to stretch after exposure of their receptive endings to an acidic IS; 2) a significant decrease in response threshold of muscular afferents; and 3) recruitment of a greater proportion of muscular chemosensitive receptive endings.
We utilized a model of CYP-induced cystitis with the intention of developing a chronic, mild inflammation of the urinary bladder to study the potential occurrence of cross-organ sensitization of the colorectum. We observed that, indeed, CYP treatment induces sensitization of colorectal afferent fibers, supporting the occurrence of cross-organ sensitization between this organ and the urinary bladder. Because CYP is a prodrug that is metabolized by the liver to produce the bladder irritant acrolein, which is responsible for the occurrence of cystitis both in rodents and humans (18, 32), we interpret the present data as supporting cross-organ sensitization following bladder inflammation. Other reported adverse effects of CYP in mice are damage of hemopoietic and lymphoid organs (3, 21), alopecia (30), and, at high doses, lung and cardiac toxicity (21, 30). No further evidence for alterations in other organs has been reported thus far. However, it could be hypothesized that CYP directly affects colorectal tissue and/or afferents when injected into the intraperitoneal cavity. We consider this to be unlikely; we found no histological or inflammatory changes in the colorectum of CYP-treated mice, suggesting the lack of a direct effect (or effect of acrolein) in the colon. Interestingly, C57BL/6 mice, the strain used in the present study, appear to be more resistant to the adverse effects of CYP/acrolein than other mouse strains (10, 20). Collectively, the present and previous studies suggest that a direct effect of CYP on colorectal afferents is unlikely and that the sensitization of colorectal afferents likely derives from alterations induced by cystitis.
Under normal conditions, colon and bladder are functionally related (59). Importantly, in pathological circumstances this association may underlie the occurrence of shared painful symptoms. In fact, cross-organ sensitization is evident in patients with IBS, who often exhibit signs of urinary bladder hypersensitivity: nocturia, frequency and urgency, incomplete bladder emptying, back pain, and, in women, dyspareunia (62). Some of these clinical observations have been corroborated in rodents, where acute (49) or chronic (8, 35) colorectal irritation in mouse (49) and rat (35, 48) induces “colon-to-bladder” sensitization, with increased frequency of bladder contractions, reduced intercontraction intervals (49), and altered micturition reflexes (35).
Although human clinical and rodent behavioral evidence regarding cross-organ sensitization is abundant, the mechanisms by which colon and bladder sensory output interact in pathological situations remain unclear. Two basic hypotheses have been proposed: 1) sensitization of peripheral and/or central endings of sensory (afferent) neurons that may be associated with inflammatory changes in the organs themselves and 2) development of central sensitization of second- and higher-order neurons at different levels of the neuraxis.
The concept of peripherally initiated and maintained cross-organ sensitization between colorectum and urinary bladder has only recently been acknowledged. Thus Ustinova and colleagues (57) reported both increased spontaneous activity as well as augmented bladder afferent responses to distension and intravesical chemical stimulation in rats with acute colorectal inflammation. Conversely, in the present study we show that afferent fibers innervating the mouse colorectum become sensitized during cystitis, supporting the observation of hypersensitivity to colorectal distension in CYP-treated mice (8).
The fiber types involved in the bladder-to-colon sensitization presented here are stretch-sensitive colorectal muscular and muscular-mucosal fibers. These fibers were sensitized by acidic (but not neutral; Ref. 29) IS in both saline- and CYP-treated mice. However, only the muscular fibers exhibited greater sensitization in CYP- than in saline-treated mice, suggesting their prominent role in colorectal hypersensitivity evident during balloon distension (8). In addition to changes in mechanosensitivity, we also noted that the proportion of stretch-sensitive colorectal afferents (muscular and muscular-mucosal) that responded to application of IS to their receptive endings was significantly greater in CYP- than saline-treated mice. In contrast, the chemosensitivity of bladder afferents was decreased, consistent with previous reports showing that exposure of the urinary bladder to the toxic CYP metabolite acrolein affects afferents innervating the bladder, including capsaicin-sensitive afferents (1, 37, 60). Moreover, acrolein appears to contribute to the production of free radicals (6) and induces neurofilament-L aggregation (28).
As mentioned above, we found that only acidic IS was able to induce mechanical sensitization in muscular and muscular-mucosal fibers. Accordingly, in a previous study we found a synergistic interaction between low pH and the chemical components of IS because the use of neutral IS in wild-type mice, even though able to excite a proportion of colorectal afferent fibers, failed to induce mechanical sensitization of stretch-sensitive fibers (29). Interestingly, this occurrence suggests that protons, acting through receptors such as those belonging to the acid-sensing ion channel (ASIC) family, may be involved in cross-organ sensitization. In previous work from this laboratory, we have shown that the ASIC3 and TRPV1 channels have an important role in colon mechanosensation (29). Using ASIC3-knockout mice, we showed that, although the loss of the acid-sensing channel does not affect the ability of acidic IS to excite colorectal primary afferents, it impairs its sensitizing effect on the response to mechanical stimulation of muscular and muscular-mucosal fibers. In other words, ASIC3 knockout mice exhibit reduced acidic IS-induced mechanical sensitization of muscular and muscular-mucosal afferent fibers than do wild-type mice (29). The mechanism(s) by which ASIC3 channels, displaying the highest acid sensitivity (34), contribute to mechanical sensitization is not clearly understood (26, 61). Further research, along with the development of ASIC agonists and antagonists with improved selectivity, will be necessary to elucidate the role of ASIC channels in colorectal mechanical sensitization and, potentially, in cross-organ sensitization.
Independent of the intracellular mechanisms involved in the sensitization of primary afferents during cross-organ sensitization, the question remains: How does referral of visceral sensation and cross-organ sensitization occur in the periphery? The principal mechanism advanced so far is based on what have been called dichotomizing fibers (i.e., sensory endings of a single neuron innervating two different tissues). Recent anatomical studies have described dichotomizing neurons innervating the urinary bladder and the colon in rat (16, 31, 40) and mouse (16). In support, cultured lumbosacral bladder sensory neurons from rats with colitis exhibit increases in a capsaicin-produced inward current as well as in the peak amplitude of tetrodotoxin-resistant (TTX-R) Na+ currents (39). Furthermore, upregulation of CGRP was observed in rat bladder sensory neurons after colorectal inflammation (50). It is possible that similar mechanisms were involved in the bladder-to-colon cross talk, presumably leading to the sensitization of primary afferents reported here. However, despite their existence and supporting evidence reviewed above and elsewhere (13, 38), the small number of dichotomizing neurons within the relatively low proportion of visceral sensory neurons raises a cautionary note about their role in cross-organ sensitization.
Alternatively, sensory neurons innervating different organs could be cross-sensitized in a number of additional ways that do not require dichotomizing fibers. Thus electrical and/or chemical coupling have been proposed as potential mechanisms of cross-excitation between neurons within the dorsal root ganglia or at the level of peripheral nerves (for reviews, see Refs. 13, 14, 42). Similar mechanisms could contribute to cross talk between neighboring sensory neurons innervating different visceral organs.
It is also possible that organ inflammatory processes contribute to peripheral generation of cross-organ sensitization. For example, rats with chronic colitis exhibit an increase in urinary bladder mast cell density compared with control rats (58). Acute signs of inflammation such as plasma extravasation have also been described in the bladder after colorectal or uterine inflammation (64) or experimental endometriosis, associated in the latter case with decreased micturition thresholds (43). To the contrary, Foreman and colleagues (39, 52) found no detectable histological changes in the bladder wall of rats with chronic colitis. Likewise, we did not detect changes in histology or MPO activity, a marker for neutrophil infiltration and inflammation, in the colorectum of mice with cystitis. Interestingly, acute (but not chronic) colitis in rats was shown to alter the contractility of the detrusor in the absence of morphological changes or inflammatory infiltration of the bladder (47). Differences in the outcomes of these studies may relate to methodological issues. However, the absence of histological changes in a cross-sensitized organ does not preclude functional alterations.
Central mechanisms also have been proposed to explain the occurrence of referral and cross-organ sensitization. In fact, organ insult not only leads to sensitization of the primary afferent input but also increases the excitability of second (and higher)-order central neurons, providing a central nervous system mechanistic explanation for cross-organ and cross-tissue referral and sensitization (24, 45, 65). Accordingly, organ disease or experimental inflammation is viewed as increasing the excitability of spinal neurons, which is reflected by 1) increased responses to stimuli applied to non-diseased/inflamed tissues that provide convergent input onto the same spinal neuron and 2) an increase in the area of referred sensations by the same mechanism.
Because bladder and colon afferents project to the same spinal segments (thoracolumbar and lumbosacral) and often converge on the same spinal neurons (4, 44, 51), the role of second-order neurons in viscero-visceral hypersensitivity has dominated thinking about central mechanisms of cross-organ sensitization. For example, colitis sensitizes lumbosacral spinal convergent neurons to distension of the urinary bladder (52). Viscero-visceral convergence of bladder and colon inputs was also shown in Barrington's nucleus (the pontine micturition center), where neurons responding to distension of both organs were found (27, 53, 54). Whether these or other brain stem neurons become cross-sensitized and contribute to sensitization of colon or bladder during disease or experimental insult of the neighboring organ remains to be elucidated.
Finally, the generation of antidromically produced dorsal root reflexes has been proposed as another central mechanism of organ cross-sensitization (63).
In summary, we show that pelvic nerve colorectal and bladder afferents exhibit altered local sensory processing in mice with CYP-induced cystitis. Relative to saline-treated control mice, CYP-treated mice exhibit increases in mechano- and chemosensitivity of colorectal sensory afferents (i.e., sensitization). This sensitization arises in the absence of colorectal inflammation and only modest bladder inflammation and appears to contribute to the generation of cross-organ sensitization.
GRANTS
This study was supported by National Institute of Neurological Disorders and Stroke Grants NS-19912 and NS-35790 (G. F. Gebhart) and an International Association for the Study of Pain (IASP) Early Career Research Award (P. R. Brumovsky).
DISCLOSURES
No conflicts of interest are declared by the author(s).
ACKNOWLEDGMENTS
We thank Dr. Robert Garman for his help in the evaluation of the histological sections and technical advice.
Footnotes
The online version of this article contains supplemental material.
REFERENCES
- 1.Ahluwalia A, Maggi CA, Santicioli P, Lecci A, Giuliani S. Characterization of the capsaicin-sensitive component of cyclophosphamide-induced inflammation in the rat urinary bladder. Br J Pharmacol 111: 1017–1022, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alagiri M, Chottiner S, Ratner V, Slade D, Hanno PM. Interstitial cystitis: unexplained associations with other chronic disease and pain syndromes. Urology 49: 52–57, 1997 [DOI] [PubMed] [Google Scholar]
- 3.Anton E. Delayed toxicity of cyclophosphamide on the bladder of DBA/2 and C57BL/6 female mouse. Int J Exp Pathol 83: 47–53, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Applebaum AE, Vance WH, Coggeshall RE. Segmental localization of sensory cells that innervate the bladder. J Comp Neurol 192: 203–209, 1980 [DOI] [PubMed] [Google Scholar]
- 5.Arendt-Nielsen L, Schipper KP, Dimcevski G, Sumikura H, Krarup AL, Giamberardino MA, Drewes AM. Viscero-somatic reflexes in referred pain areas evoked by capsaicin stimulation of the human gut. Eur J Pain 12: 544–551, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Batista CK, Brito GA, Souza ML, Leitao BT, Cunha FQ, Ribeiro RA. A model of hemorrhagic cystitis induced with acrolein in mice. Braz J Med Biol Res 39: 1475–1481, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Berkley KJ. A life of pelvic pain. Physiol Behav 86: 272–280, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Bielefeldt K, Lamb K, Gebhart GF. Convergence of sensory pathways in the development of somatic and visceral hypersensitivity. Am J Physiol Gastrointest Liver Physiol 291: G658–G665, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Bjorling DE, Elkahwaji JE, Bushman W, Janda LM, Boldon K, Hopkins WJ, Wang ZY. Acute acrolein-induced cystitis in mice. BJU Int 99: 1523–1529, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Bon K, Lichtensteiger CA, Wilson SG, Mogil JS. Characterization of cyclophosphamide cystitis, a model of visceral and referred pain, in the mouse: species and strain differences. J Urol 170: 1008–1012, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Boucher M, Meen M, Codron JP, Coudore F, Kemeny JL, Eschalier A. Cyclophosphamide-induced cystitis in freely-moving conscious rats: behavioral approach to a new model of visceral pain. J Urol 164: 203–208, 2000 [PubMed] [Google Scholar]
- 12.Brierley SM, Jones RC, III, Gebhart GF, Blackshaw LA. Splanchnic and pelvic mechanosensory afferents signal different qualities of colonic stimuli in mice. Gastroenterology 127: 166–178, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Brumovsky P, Gebhart GF. Visceral organ cross-sensitization—an integrated perspective. Auton Neurosci (August11, 2009). doi:10.1016/j.autneu.2009.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brumovsky P, Shi TS, Landry M, Villar MJ, Hokfelt T. Neuropeptide tyrosine and pain. Trends Pharmacol Sci 28: 93–102, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Cervero F. Neurophysiology of gastrointestinal pain. Baillieres Clin Gastroenterol 2: 183–199, 1988 [DOI] [PubMed] [Google Scholar]
- 16.Christianson JA, Liang R, Ustinova EE, Davis BM, Fraser MO, Pezzone MA. Convergence of bladder and colon sensory innervation occurs at the primary afferent level. Pain 128: 235–243, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cox PJ. Cyclophosphamide cystitis—identification of acrolein as the causative agent. Biochem Pharmacol 28: 2045–2049, 1979 [DOI] [PubMed] [Google Scholar]
- 18.Dechant KL, Brogden RN, Pilkington T, Faulds D. Ifosfamide/mesna. A review of its antineoplastic activity, pharmacokinetic properties and therapeutic efficacy in cancer. Drugs 42: 428–467, 1991 [DOI] [PubMed] [Google Scholar]
- 19.Foreman RD. Neurological mechanisms of chest pain and cardiac disease. Cleve Clin J Med 74, Suppl 1: S30–S33, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Fraiser L, Kehrer JP. Murine strain differences in metabolism and bladder toxicity of cyclophosphamide. Toxicology 75: 257–272, 1992 [DOI] [PubMed] [Google Scholar]
- 21.Fraiser LH, Kanekal S, Kehrer JP. Cyclophosphamide toxicity. Characterising and avoiding the problem. Drugs 42: 781–795, 1991 [DOI] [PubMed] [Google Scholar]
- 22.Francis CY, Duffy JN, Whorwell PJ, Morris J. High prevalence of irritable bowel syndrome in patients attending urological outpatient departments. Dig Dis Sci 42: 404–407, 1997 [DOI] [PubMed] [Google Scholar]
- 23.Francis CY, Whorwell PJ. The irritable bowel syndrome. Postgrad Med J 73: 1–7, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gebhart GF, Ness TJ. Central mechanisms of visceral pain. Can J Physiol Pharmacol 69: 627–634, 1991 [DOI] [PubMed] [Google Scholar]
- 25.Giamberardino MA, Affaitati G, Costantini R. Chapter 24. Referred pain from internal organs. Handb Clin Neurol 81: 343–361, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Holzer P. Taste Receptors in the Gastrointestinal Tract. V. Acid sensing in the gastrointestinal tract. Am J Physiol Gastrointest Liver Physiol 292: G699–G705, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hubscher CH, Kaddumi EG, Johnson RD. Brain stem convergence of pelvic viscerosomatic inputs via spinal and vagal afferents. Neuroreport 15: 1299–1302, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Jeong MS, Kang JH. Acrolein, the toxic endogenous aldehyde, induces neurofilament-L aggregation. BMB Rep 41: 635–639, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Jones RC, III, Xu L, Gebhart GF. The mechanosensitivity of mouse colon afferent fibers and their sensitization by inflammatory mediators require transient receptor potential vanilloid 1 and acid-sensing ion channel 3. J Neurosci 25: 10981–10989, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kanekal S, Fraiser L, Kehrer JP. Pharmacokinetics, metabolic activation, and lung toxicity of cyclophosphamide in C57/B16 and ICR mice. Toxicol Appl Pharmacol 114: 1–8, 1992 [DOI] [PubMed] [Google Scholar]
- 31.Keast JR, de Groat WC. Segmental distribution and peptide content of primary afferent neurons innervating the urogenital organs and colon of male rats. J Comp Neurol 319: 615–623, 1992 [DOI] [PubMed] [Google Scholar]
- 32.Korkmaz A, Topal T, Oter S. Pathophysiological aspects of cyclophosphamide and ifosfamide induced hemorrhagic cystitis; implication of reactive oxygen and nitrogen species as well as PARP activation. Cell Biol Toxicol 23: 303–312, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Krawisz JE, Sharon P, Stenson WF. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology 87: 1344–1350, 1984 [PubMed] [Google Scholar]
- 34.Kress M, Waldmann R. Acid sensing ionic channels. Curr Top Membranes 57: 241–276, 2006 [Google Scholar]
- 35.Lamb K, Zhong F, Gebhart GF, Bielefeldt K. Experimental colitis in mice and sensitization of converging visceral and somatic afferent pathways. Am J Physiol Gastrointest Liver Physiol 290: G451–G457, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Lanteri-Minet M, Bon K, de PJ, Michiels JF, Menetrey D. Cyclophosphamide cystitis as a model of visceral pain in rats: model elaboration and spinal structures involved as revealed by the expression of c-Fos and Krox-24 proteins. Exp Brain Res 105: 220–232, 1995 [DOI] [PubMed] [Google Scholar]
- 37.Maggi CA, Lecci A, Santicioli P, Del BE, Giuliani S. Cyclophosphamide cystitis in rats: involvement of capsaicin-sensitive primary afferents. J Auton Nerv Syst 38: 201–208, 1992 [DOI] [PubMed] [Google Scholar]
- 38.Malykhina AP. Neural mechanisms of pelvic organ cross-sensitization. Neuroscience 149: 660–672, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Malykhina AP, Qin C, Foreman RD, Akbarali HI. Colonic inflammation increases Na+ currents in bladder sensory neurons. Neuroreport 15: 2601–2605, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Malykhina AP, Qin C, Greenwood-Van MB, Foreman RD, Lupu F, Akbarali HI. Hyperexcitability of convergent colon and bladder dorsal root ganglion neurons after colonic inflammation: mechanism for pelvic organ cross-talk. Neurogastroenterol Motil 18: 936–948, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Matheis A, Martens U, Kruse J, Enck P. Irritable bowel syndrome and chronic pelvic pain: a singular or two different clinical syndrome? World J Gastroenterol 13: 3446–3455, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meyer RA, Ringkamp M. A role for uninjured afferents in neuropathic pain. Sheng Li Xue Bao 60: 605–609, 2008 [PubMed] [Google Scholar]
- 43.Morrison TC, Dmitrieva N, Winnard KP, Berkley KJ. Opposing viscerovisceral effects of surgically induced endometriosis and a control abdominal surgery on the rat bladder. Fertil Steril 86: 1067–1073, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Ness TJ, Gebhart GF. Characterization of neurons responsive to noxious colorectal distension in the T13-L2 spinal cord of the rat. J Neurophysiol 60: 1419–1438, 1988 [DOI] [PubMed] [Google Scholar]
- 45.Ness TJ, Gebhart GF. Visceral pain: a review of experimental studies. Pain 41: 167–234, 1990 [DOI] [PubMed] [Google Scholar]
- 46.Neuhuber WL, Appelt M, Polak JM, Baier-Kustermann W, Abelli L, Ferri GL. Rectospinal neurons: cell bodies, pathways, immunocytochemistry and ultrastructure. Neuroscience 56: 367–378, 1993 [DOI] [PubMed] [Google Scholar]
- 47.Noronha R, Akbarali H, Malykhina A, Foreman RD, Greenwood-Van MB. Changes in urinary bladder smooth muscle function in response to colonic inflammation. Am J Physiol Renal Physiol 293: F1461–F1467, 2007 [DOI] [PubMed] [Google Scholar]
- 48.Peng HY, Chen GD, Tung KC, Lai CY, Hsien MC, Chiu CH, Lu HT, Liao JM, Lee SD, Lin TB. Colon mustard oil instillation induced cross-organ reflex sensitization on the pelvic-urethra reflex activity in rats. Pain 142: 75–88, 2009 [DOI] [PubMed] [Google Scholar]
- 49.Pezzone MA, Liang R, Fraser MO. A model of neural cross-talk and irritation in the pelvis: implications for the overlap of chronic pelvic pain disorders. Gastroenterology 128: 1953–1964, 2005 [DOI] [PubMed] [Google Scholar]
- 50.Qiao LY, Grider JR. Up-regulation of calcitonin gene-related peptide and receptor tyrosine kinase TrkB in rat bladder afferent neurons following TNBS colitis. Exp Neurol 204: 667–679, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Qin C, Foreman RD. Viscerovisceral convergence of urinary bladder and colorectal inputs to lumbosacral spinal neurons in rats. Neuroreport 15: 467–471, 2004 [DOI] [PubMed] [Google Scholar]
- 52.Qin C, Malykhina AP, Akbarali HI, Foreman RD. Cross-organ sensitization of lumbosacral spinal neurons receiving urinary bladder input in rats with inflamed colon. Gastroenterology 129: 1967–1978, 2005 [DOI] [PubMed] [Google Scholar]
- 53.Rouzade-Dominguez ML, Miselis R, Valentino RJ. Central representation of bladder and colon revealed by dual transsynaptic tracing in the rat: substrates for pelvic visceral coordination. Eur J Neurosci 18: 3311–3324, 2003 [DOI] [PubMed] [Google Scholar]
- 54.Rouzade-Dominguez ML, Pernar L, Beck S, Valentino RJ. Convergent responses of Barrington's nucleus neurons to pelvic visceral stimuli in the rat: a juxtacellular labelling study. Eur J Neurosci 18: 3325–3334, 2003 [DOI] [PubMed] [Google Scholar]
- 55.Rudick CN, Chen MC, Mongiu AK, Klumpp DJ. Organ cross talk modulates pelvic pain. Am J Physiol Regul Integr Comp Physiol 293: R1191–R1198, 2007 [DOI] [PubMed] [Google Scholar]
- 56.Suckow SK, Caudle RM. Identification and immunohistochemical characterization of colospinal afferent neurons in the rat. Neuroscience 153: 803–813, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ustinova EE, Fraser MO, Pezzone MA. Colonic irritation in the rat sensitizes urinary bladder afferents to mechanical and chemical stimuli: an afferent origin of pelvic organ cross-sensitization. Am J Physiol Renal Physiol 290: F1478–F1487, 2006 [DOI] [PubMed] [Google Scholar]
- 58.Ustinova EE, Gutkin DW, Pezzone MA. Sensitization of pelvic nerve afferents and mast cell infiltration in the urinary bladder following chronic colonic irritation is mediated by neuropeptides. Am J Physiol Renal Physiol 292: F123–F130, 2007 [DOI] [PubMed] [Google Scholar]
- 59.Vilensky JA, Bell DR, Gilman S. “On the physiology of micturition” by Denny-Brown and Robertson: a classic paper revisited. Urology 64: 182–186, 2004 [DOI] [PubMed] [Google Scholar]
- 60.Wang ZY, Wang P, Merriam FV, Bjorling DE. Lack of TRPV1 inhibits cystitis-induced increased mechanical sensitivity in mice. Pain 139: 158–167, 2008 [DOI] [PubMed] [Google Scholar]
- 61.Wemmie JA, Price MP, Welsh MJ. Acid-sensing ion channels: advances, questions and therapeutic opportunities. Trends Neurosci 29: 578–586, 2006 [DOI] [PubMed] [Google Scholar]
- 62.Whorwell PJ, McCallum M, Creed FH, Roberts CT. Non-colonic features of irritable bowel syndrome. Gut 27: 37–40, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Willis WD., Jr Dorsal root potentials and dorsal root reflexes: a double-edged sword. Exp Brain Res 124: 395–421, 1999 [DOI] [PubMed] [Google Scholar]
- 64.Winnard KP, Dmitrieva N, Berkley KJ. Cross-organ interactions between reproductive, gastrointestinal, and urinary tracts: modulation by estrous stage and involvement of the hypogastric nerve. Am J Physiol Regul Integr Comp Physiol 291: R1592–R1601, 2006 [DOI] [PubMed] [Google Scholar]
- 65.Woolf CJ. Central sensitization: uncovering the relation between pain and plasticity. Anesthesiology 106: 864–867, 2007 [DOI] [PubMed] [Google Scholar]
- 66.Xu L, Gebhart GF. Characterization of mouse lumbar splanchnic and pelvic nerve urinary bladder mechanosensory afferents. J Neurophysiol 99: 244–253, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]