Abstract
Myofibroblastic hepatic stellate cells (MF-HSC) are derived from quiescent hepatic stellate cells (Q-HSC). Q-HSC express certain epithelial cell markers and have been reported to form junctional complexes similar to epithelial cells. We have shown that Hedgehog (Hh) signaling plays a key role in HSC growth. Because Hh ligands regulate epithelial-to-mesenchymal transition (EMT), we determined whether Q-HSC express EMT markers and then assessed whether these markers change as Q-HSC transition into MF-HSC and whether the process is modulated by Hh signaling. Q-HSC were isolated from healthy livers and cultured to promote myofibroblastic transition. Changes in mRNA and protein expression of epithelial and mesenchymal markers, Hh ligands, and target genes were monitored in HSC treated with and without cyclopamine (an Hh inhibitor). Studies were repeated in primary human HSC and clonally derived HSC from a cirrhotic rat. Q-HSC activation in vitro (culture) and in vivo (CCl4-induced cirrhosis) resulted in decreased expression of Hh-interacting protein (Hhip, an Hh antagonist), the EMT inhibitors bone morphogenic protein (BMP-7) and inhibitor of differentiation (Id2), the adherens junction component E-cadherin, and epithelial keratins 7 and 19 and increased expression of Gli2 (an Hh target gene) and mesenchymal markers, including the mesenchyme-associated transcription factors Lhx2 and Msx2, the myofibroblast marker α-smooth muscle actin, and matrix molecules such as collagen. Cyclopamine reverted myofibroblastic transition, reducing mesenchymal gene expression while increasing epithelial markers in rodent and human HSC. We conclude that Hh signaling plays a key role in transition of Q-HSC into MF-HSC. Our findings suggest that Q-HSC are capable of transitioning between epithelial and mesenchymal fates.
Keywords: bone morphogenetic protein-7, cyclopamine, fibrosis, proliferation, regeneration
hepatic stellate cells (HSC) have important roles in health and disease. In healthy livers, quiescent HSC (Q-HSC) reside in the space of Disse, store retinoids, and produce molecules that are trophic for neighboring hepatocytes. Various factors that are released during liver injury stimulate a complex, incompletely understood process that causes Q-HSC to transition into myofibroblastic HSC (MF-HSC) and proliferate. Accumulation of MF-HSC plays an important role in liver repair. On the other hand, because MF-HSC are fibrogenic, overgrowth of MF-HSC populations contributes to progression of liver fibrosis. Conversely, regression of liver fibrosis occurs when MF-HSC are induced to undergo apoptosis. The endogenous mechanisms that promote apoptosis of MF-HSC during fibrosis regression, as well as the origin of replacement Q-HSC, are also somewhat unclear (7).
Published data suggest that HSC are multipotent cells. For example, heterogeneity of HSC populations has been well documented in healthy adult livers (7). In vitro, primary HSC have been shown to express markers of neural cells, mature and immature liver epithelial cells, endothelial cells, and myofibroblasts, depending on the culture conditions. Although there is little argument that Q-HSC are capable of transitioning into myofibroblastic cells in vivo and in vitro, it is not widely accepted that this process might be reversible or that HSC in intact livers might be capable of transitioning into cells other than myofibroblasts. The latter dogma is challenged, however, by double-immunofluorescence staining and confocal microscopy studies of Q-HSC in normal rat livers that demonstrated membranous expression of the adherens junction protein E-cadherin in cells that coexpressed the HSC marker glial fibrillary acidic protein (GFAP) (26). Other groups also reported that HSC might be capable of acquiring epithelial characteristics. Higashi et al. (15) showed that the human stellate cell line L190 expresses several adherens junction proteins and forms intercellular adhesive structures with typical ultrastructural features of adherens junctions when grown to confluence. These authors subsequently demonstrated colocalization of pan-cadherin and β-catenin staining at the contact regions between cultured human stellate cells and confirmed expression of several adherens junction proteins (N-cadherin, α-catenin, and β-catenin) in rat HSC by Western blotting of protein extracts from primary rat HSC (15). Others showed that HSC express connexin-43 and form functional gap junctions with each other in culture, resulting in cell-to-cell transfer of Lucifer yellow dye and propagation of intracellular calcium signals (6, 10). HSC also form functional gap junctions with hepatocytes in coculture (40). Thus, similar to many types of typical epithelial cells, HSC have the ability to adhere to other cells and form cellular sheets under certain circumstances but to exist as single cells in tissue mesenchyme in other situations. The mechanisms that regulate such complex cell fate decisions remain obscure, however.
Epithelial-to-mesenchymal transition (EMT) is the process by which epithelial cells disassociate from their neighbors, gradually acquire a motile phenotype, and eventually migrate out of epithelial sheets and into adjacent mesenchyme (18). Orchestration of the multiple events that are required for such global phenotypic changes involves the collaboration of various factors. Among these, transforming growth factor-β (TGF-β) plays a particularly prominent role (55). TGF-β is produced by many types of cells, including HSC, and increases in TGF-β promote the accumulation of MF-HSC during liver fibrosis (7). Another HSC-derived factor that has been shown to promote myofibroblast accumulation during liver injury and to stimulate EMT is Sonic Hh (Shh) (34, 36). Cross talk between the TGF-β and Hedgehog (Hh) signaling pathways appears to occur, because TGF-β has been shown to induce expression of Hh ligands and to stabilize expression of Gli proteins (Hh-regulated transcription factors) (16, 38). Conversely, Gli proteins help control the expression of Snail, a factor that is required for TGF-β-mediated induction of EMT (25). Thus EMT might provide a unifying mechanism for the events that transpire as Q-HSC become MF-HSC. As such, it is an important process to investigate in animal and cell culture models of HSC activation. Also, because reversal of phenotypic changes that are caused by EMT can sometimes be accomplished by mesenchymal-to-epithelial transition (MET) (1), MET might provide a novel mechanism to reduce fibrosis and replenish Q-HSC populations once liver injury resolves. Hence, our goals were to determine whether Hh signaling regulates the transition of Q-HSC into MF-HSC, to assess whether expression of factors that control EMT or mark its occurrence vary during this process, and to evaluate the reversibility of EMT in HSC. Our results prove that Hh pathway activation plays a key role in the transition of Q-HSC into MF-HSC and demonstrate, for the first time, that inhibition of Hh signaling permits MF-HSC to undergo an MET-like process that permits them to reacquire a more quiescent phenotype. These findings suggest that HSC represent a pool of multifunctional liver cells that are capable of transitioning between epithelial and mesenchymal fates and identify mechanisms that control this process. Such knowledge has important diagnostic and therapeutic implications for individuals with liver fibrosis.
MATERIALS AND METHODS
Animals and experimental design.
Adult, male Sprague-Dawley rats were purchased from Charles River Laboratories (Wilmington, MA). Age- and sex-matched (n = 6 each) C57BL/6 mice (Jackson Laboratories, Bar Harbor, ME) received intraperitoneal injections of CCl4 (0.5 mg/kg, Sigma-Aldrich, St. Louis, MO) or corn oil vehicle, as previously described (4). Mice were killed after 8 wk of treatment. Animal experiments fulfilled National Institutes of Health and Duke University Institutional Animal Care and Use Committee requirements for humane animal care.
Cell isolation and culture.
HSC were isolated from normal Sprague-Dawley rats as described previously (53). Briefly, after in situ perfusion of the liver with pronase (Roche, Indianapolis, IN) and collagenase (Roche), dispersed cell suspensions were layered on a discontinuous density gradient of 5.8% Larcoll (Sigma-Aldrich) and 15.6% Histodenz (Sigma-Aldrich). The resulting upper layer consisted of >98% HSC. Purity and viability were verified by phase-contrast microscopy examining autofluorescence and propidium iodide exclusion (50 μg/ml; Roche). HSC were cultured in 10% serum-supplemented DMEM (Invitrogen, Carlsbad, CA) with streptomycin-penicillin.
Pronase/collagenase digestion and density gradient centrifugation were also used to isolate primary stellate cells from a residual segment of healthy human liver that was used for split-liver transplantation. After a portion of human liver was minced and digested in pronase and collagenase, dispersed cell suspensions were layered on a discontinuous density gradient as described above. Viability and purity of the isolated cells were validated as described above. Human tissue was collected under Duke University Institutional Review Board approval.
The human HSC line LX-2 (provided by S. L. Friedman, Mount Sinai School of Medicine, New York, NY) was cultured in serum-supplemented DMEM (51).
The clonally derived rat HSC lines 8B and 5H were cultured in serum-supplemented RPMI 1640 medium (Invitrogen) (11).
The normal rat cholangiocyte (NRC) line (provided by N. F. LaRusso, Mayo Clinic, Rochester, MN) was cultured on collagen-coated dishes in serum-supplemented medium as described elsewhere (48).
Pharmacological inhibition of Hh signaling.
Primary HSC were treated with cyclopamine (3 μM; Calbiochem, San Diego, CA), an inhibitor of Hh signaling, or its catalytically inactive analog tomatidine (3 μM) for 4 or 7 days as previously described (42). LX-2 and 8B cells were treated with cyclopamine (3 μM) or tomatidine (3 μM) for 4 days.
mRNA quantification by real-time RT-PCR.
Total RNA was extracted using TRIzol (Invitrogen), reverse-transcribed to cDNA templates, and amplified using a SYBR Green PCR Master Mix (Bio-Rad Laboratories, Hercules, CA) as previously described (4, 33). Quantitative RT-PCR (qRT-PCR) was performed using iQ-SYBR Green Supermix (Bio-Rad) as previously described (36, 53). Specificity for all primers (Table 1) was confirmed by cloning and sequencing of PCR products. Samples were analyzed in triplicate. Target gene levels are presented as a ratio of levels in treated cells or tissues to levels detected in corresponding control cells or tissues, according to the ΔΔCt method (27).
Table 1.
RT-PCR primers for analysis
| Gene | GenBank Accession No. | Sequence |
|---|---|---|
| α−SMA | X06801 | |
| Forward | GTGGATCACCAAGCAGGAGGAGT | |
| Reverse | CATAGCACGATGGTCGATTG | |
| Col1α1 | XM_213440 | |
| Forward | CTGCATACACAATGGCCTAA | |
| Reverse | GGGTCCCTCGACTCCTA | |
| Fibronectin | NM_019143 | |
| Forward | GTGGCTGCCTTCAACTTCTC | |
| Reverse | GTGGGTTGCAAACCTTCAAT | |
| TGF-β | NM_021578 | |
| Forward | TTGCCCTCTACAACCAACACAA | |
| Reverse | GGCTTGCGACCCACGTAGTA | |
| PPARγ | NM_013124 | |
| Forward | CCCTGGCAAAGCATTTGTAT | |
| Reverse | ACTGGCACCCTTGAAAAATG | |
| GFAP | NM_017009 | |
| Forward | GGGAGTCGGCCAGTTACCAG | |
| Reverse | CCCGCATCTCCACCGTCT | |
| K7 | NM_033073 | |
| Forward | TAGAGTCCAGCATCGCAGAG | |
| Reverse | CACAGGTCCCATTCCGTC | |
| K19 | NM_199498 | |
| Forward | GTCCACACTACGCAGATCCA | |
| Reverse | CAAGCAGGCTTCGGTAGGT | |
| S100A4 | NM_012618 | |
| Forward | ATACTCAGGCAACGAGGGTG | |
| Reverse | CTTCCGGGGCTCCTTATC | |
| BMP-7 | XM_342591 | |
| Forward | GTGGTCAACCCTCGGCACA | |
| Reverse | GGCGTCTTGGAGCGATTCTG | |
| Desmoplakin | XM_001058477 | |
| Forward | GGAAGTCAGCCAAGCAAAAC | |
| Reverse | GGCTCTCCTTTTCACACTGC | |
| E-cadherin | NM_009864 | |
| Forward | ACCTCTGGGCTGGACCGA | |
| Reverse | CCTGATACGTGCTTGGGTTGAA | |
| Shh | NM_017221 | |
| Forward | ACAAGAAACTCCGAACGATT | |
| Reverse | GCCCTCAGTCACTCGAAG | |
| Hhip | XM_238042 | |
| Forward | TGTGCCGTGGATCGAC | |
| Reverse | GATCTCCGAACACGTAGCTT | |
| Gli2 | XM_222557 | |
| Forward | ATAAGCGGAGCAAGGTCAAG | |
| Reverse | CAGTGGCAGTTGGTCTCGTA | |
| S9 | NM_029767 | |
| Forward | GACTCCGGAACAAACGTGAGGT | |
| Reverse | CTTCATCTTGCCCTCGTCCA | |
| Col1α1 | NM_000088 | |
| Forward | TGTGAGGCCACGCATGAG | |
| Reverse | CAGATCACGTCATCGCACAA | |
| PAI-1 | NM_000602 | |
| Forward | CTCTCTCTGCCCTCACCAAC | |
| Reverse | GTGGAGAGGCTCTTGGTCTG | |
| PPARγ | NM_138712 | |
| Forward | CGTGGCCGCAGATTTGAA | |
| Reverse | CTTCCATTACGGAGAGATCCAC | |
| β-Actin | NM_001101 | |
| Forward | TGGCATCCACGAAACTACCT | |
| Reverse | ACGGAGTACTTGCGCTCAG | |
| Snail | NM_053805 | |
| Forward | CATCCCTCCTGCGTCC | |
| Reverse | CCTAACAAGTGACGGCCATT |
α-SMA, α-smooth muscle actin; Col1α1, collagenase 1α1; TGF-β, transforming growth factor-β; PPARγ, peroxisome proliferator -activated receptor-γ; GFAP, glial fibrillary acidic protein; K7 and K19, ketatins 7 and 19; BMP-7, bone morphogenic protein-7; Shh, Sonic Hedgehog; Hhip, Hedgehog-interacting protein; PAI-1, plasminogen activator inhibitor-1.
Western blotting.
Whole cell proteins (20 μg) were separated by PAGE and transferred to nylon membranes. Primary antibodies were as follows: anti-α-smooth muscle actin (α-SMA, 1:2,000 dilution; Dako, Carpentaria, CA), anti-keratin 7 (K7, 1:2,000 dilution; Santa Cruz Biotechnology, Santa Cruz, CA), anti-keratin 19 (K19, 1:2,000 dilution; Dako), anti-Gli2 (1:2,000 dilution; Santa Cruz Biotechnology), anti-BMP-7 (1:1,500 dilution; Cell Signaling Technology, Danvers, MA), anti-Shh (1:2,000 dilution; Santa Cruz Biotechnology), inhibitor of differentiation 2 (Id2, 1:1,500 dilution; BD Biosciences, San Jose, CA), and anti-β-actin (1:2,000 dilution; Sigma-Aldrich). Appropriate secondary antibodies were used with antigens demonstrated by enhanced chemiluminescence (Pierce Biotechnology, Rockford, IL).
Cytochemistry and immunocytochemistry.
Immediately after isolation, freshly isolated primary rat HSC were washed in PBS, centrifuged onto glass slides in a Cytospin (Shandon, Pittsburgh, PA) at 600 rpm for 5 min, and fixed in 4% paraformaldehyde (Sigma) at 4°C for 10 min. After permeabilization in 0.1% Triton X-100 for 10 min, specimens were washed in TBS-0.01% Tween 20 (TBST). Indirect immunocytochemistry was performed using anti-cytokeratin 19 (1:50 dilution; Dako) as the primary antibody for 45 min at room temperature. Secondary antibody detection was performed with goat anti-mouse IgG (1:100 dilution; Alexa Fluor 488, Invitrogen) for 30 min at room temperature. Omission of primary antibodies eliminated staining, demonstrating specificity. After the cell preparations were washed in TBST, they were rinsed in propanediol (Sigma) twice at room temperature for 5 min each before incubation in Oil Red O solution (Newcomer Supply, Middleton, WI) for 7 min at room temperature. Cell preparations were then rinsed in 85% propanediol and distilled water for 5 min, and coverslips were applied in fluorescent mounting medium containing 4,6-diamidino-2-phenylindole (Vector Laboratories, Burlingame, CA).
Statistical analysis.
Results are expressed as means ± SE, unless indicated otherwise. Comparisons between groups were performed using the nonparametric Wilcoxon's rank sums test. All analyses were performed using SAS version 9.1 statistical software (SAS Institute, Cary, NC). P values are two-tailed, and significance was accepted at the 5% level.
RESULTS
Expression of mesenchymal and epithelial markers changes during myofibroblastic transition of primary rat HSC in culture.
We used standard techniques to isolate HSC from the livers of healthy rats and cultured them for up to 7 days. RNA and protein were harvested at regular intervals. Changes in gene expression were analyzed by qRT-PCR. As expected, expression of myofibroblastic markers, such as α-SMA, collagen 1α1 (Col1α1), and fibronectin, was neglible in freshly plated cells but gradually increased, so that, by day 7, expression of each of these was ≥50-fold higher than baseline (Fig. 1A).
Fig. 1.
Changes in expression of epithelial and mesenchymal genes during culture-induced transition of primary rat quiescent hepatic stellate cells (Q-HSC) into myofibroblastic hepatic stellate cells (MF-HSC). Primary HSC were isolated from 6 healthy adult male rats per experiment, pooled, and cultured on plastic dishes in serum-containing medium. RNA was isolated at different time points, and changes in gene expression were evaluated by quantitative RT-PCR (qRT-PCR). A: myofibroblastic markers [α-smooth muscle actin (α-SMA), collagen 1α1 (Col1α1), and fibronectin]. B: markers of quiescent HSC [peroxisome proliferator-activated receptor-γ (PPARγ) and glial fibrillary acidic protein (GFAP)]. C: epithelial markers [keratin 7 (K7), keratin 19 (K19), and desmoplakin]. Values are means ± SE of triplicate experiments. *P < 0.05; **P < 0.01; †P < 0.005.
Q-HSC expressed typical markers of Q-HSC, including peroxisome proliferator-activated receptor-γ (PPARγ) and GFAP (Fig. 1B). Typical ductular cell markers, such as K7 and K19, and desmoplakin, a ubiquitous component of epithelial junctional complexes, were also easily demonstrated in our freshly plated HSC (Fig. 1C). Expression of Q-HSC markers, the epithelial keratins, and the epithelial junctional complex component was downregulated with time in culture, so that expression of these markers had virtually disappeared by day 7 of culture. Thus, in addition to their typical quiescence markers, freshly isolated primary HSC expressed markers of immature ductular-type epithelial cells, and expression of these genes decreased as the cells acquired a more mesenchymal phenotype, characterized by robust expression of several myofibroblastic markers (Fig. 1).
Although adult primary HSC can activate expression of hepatocyte and cholangiocyte genes when cultured under some conditions (7), HSC are thought to arise from septum transversum mesenchyme (30), not from endoderm, which provides the progenitors of mature liver epithelial cells during embryogenesis (22, 54). HSC precursors in fetal livers are desmin-positive and express the stem cell-related transcription factor Lhx2 (22), as well as the mesenchyme-enriched transcription factor Msx2 (2). Therefore, we examined expression of these HSC-related transcription factors in our isolates and correlated changes in expression of these factors, other HSC markers, and several ductular cell markers. Expression of these genes was also compared in HSC and NRC, which has been extensively investigated by cholangiocyte biologists and shown to exhibit the phenotype of large, mature cholangiocytes (48). Q-HSC expressed Lhx2, Msx2, and desmin, whereas none of these mRNAs were detected in NRC (Fig. 2A). Both cell types expressed PPARγ and GFAP (2 classical markers of Q-HSC; Fig. 2B), as well as K7 and K19 (2 typical cholangiocyte markers; Fig. 2C). Levels of the EMT inhibitors BMP-7, Id2, and desmoplakin were comparable in NRC and Q-HSC, and HSC expression of each of these factors decreased significantly by culture day 7 (Fig. 2D), when the HSC had become myofibroblastic (Fig. 1A).
Fig. 2.
Comparison of epithelial and mesenchymal gene expression in normal rat cholangiocytes (NRC) and primary rat stellate cells. Primary HSC were isolated from healthy adult male rats and cultured on plastic dishes in serum-containing medium. NRC were cultured on collagen-coated dishes in serum-supplemented medium. RNA was isolated from NRC and quiescent and culture-activated HSC (day 7), with changes in gene expression evaluated by qRT-PCR. A: HSC-associated markers (Lhx2, Msx2, and desmin). B: markers of quiescent HSC (PPARγ and GFAP). C: epithelial markers (K7 and K19). D: factors that inhibit EMT [bone morphogenetic protein-7 (BMP-7), inhibitor of differentiation (Id2), and desmoplakin]. Values are means ± SE of triplicate experiments. *P < 0.05; **P < 0.01; †P < 0.005.
The pattern of these variations in gene expression suggested that freshly isolated primary Q-HSC, similar to normal cholangiocytes, express epithelial genes as well as factors that reinforce their epithelial phenotype. During culture, expression of the epithelial maintenance factors seems to be repressed, permitting the HSC to acquire a less epithelial and more mesenchymal phenotype. Because this process is reminiscent of EMT, we examined the primary HSC at multiple time points during culture to determine whether other classical EMT markers were affected. In addition to downregulating expression of BMP-7 and its target gene Id2, culture-induced transition of HSC repressed expression of E-cadherin (an Id2-regulated component of epithelial junctional complexes that antagonizes EMT; Fig. 3A) (23) while upregulating expression of several factors that promote EMT, including Snail, TGF-β, and S100A4 (Fig. 3B) (56). Immunocytochemistry of freshly isolated Q-HSC confirmed that these lipocytic cells expressed K19 (Fig. 4A). Western blot analysis was used to track changes in cellular proteins during culture and confirmed that these changes in mRNA expression were accompanied by similar changes at the protein level (Fig. 4B). HSC content of α-SMA protein increased dramatically during culture, whereas expression of K7, K19, BMP-7, and Id2 proteins virtually disappeared. Thus the aggregate gene expression changes provide strong support for the concept that Q-HSC have epithelial features and undergo an EMT-like process to acquire a myofibroblastic phenotype during standard culture conditions.
Fig. 3.
Culture-related changes in HSC expression of genes that regulate epithelial-to-mesenchymal transitions (EMT) determined by qRT-PCR analysis of RNA obtained from the primary rat HSC described in Fig. 1. A: factors that inhibit EMT (BMP-7, Id2, and E-cadherin). B: factors that promote EMT [Snail, transforming growth factor-β (TGF-β), and S100A4]. C: Hedgehog (Hh) signaling factors known to regulate EMT [Hedgehog-interacting protein (Hhip), Sonic Hh (Shh), and Gli2]. Values are means ± SE of triplicate experiments. *P < 0.05; **P < 0.01; †P < 0.005.
Fig. 4.
Culture-induced changes in protein expression of EMT-related factors during transition of Q-HSC into MF-HSC. A: Oil Red O staining to detect neutral lipids and immunocytochemistry for K19, an epithelial keratin, in a representative, freshly isolated HSC from preparations described in Fig. 1. Immunocytochemistry in NRC confirms K19 positivity. Magnification ×40. B: Western blot analysis of protein harvested from these primary rat HSC. Results are representative of triplicate experiments.
Differential regulation of mesenchymal and epithelial markers is regulated by Hh signaling in rat primary HSC.
Given evidence that the Hh pathway promotes EMT (3, 34), culture-related changes in HSC expression of Hhip (a competitive antagonist of Hh ligands), Shh ligand, and Gli2 were evaluated (Fig. 3C). Freshly isolated HSC expressed Hhip, which was rapidly silenced during culture, falling by >90% within 1 day. Conversely, Shh and Gli2 were expressed at low levels in freshly isolated HSC but were strongly induced during culture. Expression of Shh and Gli2 was more than fivefold greater on day 7 than at baseline (Fig. 3C). We previously reported that cultured rat MF-HSC produce biologically active Shh protein (50, 53). Here, we performed additional Western blot analysis to confirm that increases in Shh and Gli2 mRNA were accompanied by accumulation of these proteins (Fig. 4B). Together with other data shown in Figs. 1–4, these results demonstrate that Hh pathway induction occurs during the transition from a pattern of epithelial gene expression to a mesenchymal pattern in rat primary HSC.
To evaluate potential cause-effect relationships between Hh pathway activation in HSC and the acquisition of a myofibroblastic phenotype, day 4 culture-activated MF-HSC were treated with cyclopamine, a potent and highly specific Hh pathway inhibitor (42), for 4 or 7 days. Results were compared with cultures that were comparably treated with tomatidine, a biologically inert cyclopamine analog. Tomatidine had no effect on HSC gene expression (data not shown), so results in cyclopamine-treated cells were normalized to findings in tomatidine-treated cultures. Compared with the latter, cyclopamine-treated cultures expressed significantly less α-SMA, Col1α1, and S100A4 (Fig. 5A). Conversely, cyclopamine-treated cultures expressed significantly greater levels of epithelial markers, including K7, K19, and desmoplakin (Fig. 5B), as well as the EMT-inhibitory factors E-cadherin and BMP-7, than tomatidine-treated MF-HSC (Fig. 5C).
Fig. 5.
Effects of inhibiting Hh signaling on mRNA expression of epithelial and mesenchymal markers in cultured primary rat HSC. Primary stellate cells were isolated from another 2 healthy adult male rats per experiment, pooled, and cultured on plastic in serum-containing medium for 4 days. Then cyclopamine (Cyc, a pharmacological inhibitor of Hh signaling) or tomatidine (Tom, an inert cyclopamine analog) was added, and cultures were harvested after an additional 4 or 7 days. RNA was isolated, and changes in gene expression were monitored by qRT-PCR. A: myofibroblastic markers (α-SMA, Col1α1, and S100A4). B: epithelial markers (K7, K19, and desmoplakin). C: EMT-inhibitory markers (E-cadherin and BMP-7). Values are means ± SE of triplicate experiments. *P < 0.05.
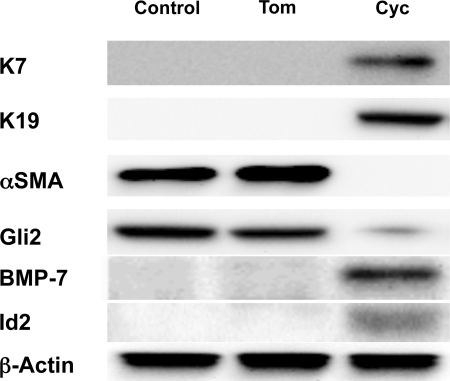
To verify that changes in mRNA expression were accompanied by changes in the cellular proteins, whole cell protein was harvested and expression of representative epithelial and mesenchymal markers was evaluated by Western blot. Treatment with cyclopamine, but not tomatidine, increased whole cell content of K7, K19, and BMP-7 protein and inhibited accumulation of α-SMA protein (Fig. 6). Hence, the aggregate results demonstrate that cyclopamine treatment of MF-HSC inhibited their expression of several mesenchymal markers while restoring their expression of several epithelial markers. Therefore, Hh pathway activation seems to promote EMT in primary rat HSC, as it does in rodent ductular-type cells (34).
Fig. 6.
Effects of inhibiting Hh signaling on protein expression of representative epithelial and mesenchymal markers in cultured primary rat HSC. Whole cell protein was isolated from HSC cultures described in Fig. 5. Changes in protein expression of K7, K19, α-SMA, and BMP-7 were assessed by Western blot analysis (40 μg whole cell protein/lane). β-Actin was used as a loading control.
Hh signaling regulates expression of EMT-related genes in human HSC.
Since gene expression changes in primary rat HSC preparations may have simply reflected species-specific phenomena or differential outgrowth of rare, contaminating cell types, the effects of Hh pathway modulation were assessed in primary human HSC and LX-2, a well-characterized human HSC line that was derived by clonal expansion of transformed MF-HSC (51). Primary HSC were harvested from a residual segment of healthy human liver tissue that was used for split-liver transplantation. Gene expression was compared in these Q-HSC and MF-HSC (Fig. 7). Primary human Q-HSC expressed the mesenchyme-associated transcription factors Lhx2 and Msx2 and the HSC marker desmin. Expression of each of these genes increased when the cells became myofibroblastic (Fig. 7A). Treatment of primary human MF-HSC with cyclopamine (but not tomatidine) repressed expression of all the mesenchymal markers. Similar responses were observed in the clonal human stellate cell line LX-2 (Fig. 7C).
Fig. 7.
Changes in mRNA expression of mesenchymal markers in cultured human HSC during myofibroblastic transition and effects of Hh pathway modulation. A and B: primary HSC were harvested from a residual ssegment of healthy human liver tissue that was used for split-liver transplantation. HSC were culture activated to MF-HSC on plastic dishes in serum-containing medium. qRT-PCR was done to compare gene expression in Q-HSC and MF-HSC (A) and after MF-HSC were treated with tomatidine or cyclopamine for 4 days (B). C: effects of similar treatment with tomatidine or cyclopamine on gene expression in the clonal human MF-HSC line LX-2. Values are means ± SE of triplicate experiments. *P < 0.05; †P < 0.005.
Further analysis of the primary human HSC confirmed that cyclopamine treatment inhibited Hh pathway activity in these cells, as evidenced by decreased expression of the Hh target genes Gli1 and Gli2 (Fig. 8A). Blocking Hh signaling in primary human HSC increased their expression of BMP-7 and Id2 (EMT inhibitors; Fig. 8A) and repressed expression of Snail, Slug, and S100A4 (factors that typically stimulate EMT; Fig. 8B) (56). These reciprocal changes in EMT inhibitors and enhancers were accompanied by increased expression of mRNAs that encode junctional complex components in epithelial cells, such as E-cadherin and desmoplakin, as well as typical markers of Q-HSC, such as PPARγ (Fig. 8C). Concomitantly, various myofibroblast genes (e.g., α-SMA, Col1α1, and plasminogen activator inhibitor-1) were downregulated (Fig. 8D). These findings further support the concept that Hh pathway activity modulates EMT in MF-HSC and demonstrate that such responses are conserved across species, occurring in rat and human HSC. Western blot analysis confirmed that changes in mRNA expression were accompanied by concomitant changes in protein expression (Fig. 9). Together with data shown in Figs. 7 and 8, these results confirm that Hh pathway induction occurs during the transition from a pattern of epithelial gene expression to a mesenchymal pattern in primary human HSC, as occurs in rodent HSC.
Fig. 8.
Effects of Hh pathway inhibition on expression of EMT-related genes in primary human MF-HSC. Primary human HSC described in Fig. 7 were treated with tomatidine or cyclopamine, and gene expression changes were compared by qRT-PCR. A: Hh signaling pathway factors (Gli1 and Gli2) and EMT inhibitors (BMP-7 and Id2). B: EMT promoters (Snail, Slug, and S100A4). C: epithelial markers (E-cadherin and desmoplakin) and the HSC quiescence marker PPARγ. D: myofibroblastic markers [α-SMA, Col1α1, and plasminogen activator inhibitor-1 (PAI-1)]. Values are means ± SE of triplicate experiments. *P < 0.05; **P < 0.01.
Fig. 9.
Effects of Hh signaling inhibition on protein expression of representative epithelial and mesenchymal markers in cultured primary human HSC. Whole cell protein was isolated from HSC cultures described in Fig. 7. Changes in protein expression of K7, K19, α-SMA, Gli2, BMP-7, and Id2 were assessed by Western blot (40 μg whole cell protein/lane). β-Actin was used as a loading control.
Hh pathway activation and changes in expression of genes that regulate EMT occur during CCl4-induced cirrhosis.
The previous data strongly support the concept that transition of Q-HSC into MF-HSC in culture involves an EMT-like process that is regulated by the Hh pathway. To assess whether similar mechanisms might be involved in the generation of myofibroblasts in vivo, liver RNA was isolated from mice chronically injured with CCl4 for 8 wk to induce cirrhosis. Expression of Hh ligands, Hhip, and Gli2 was evaluated by qRT-PCR. Results were compared with expression in vehicle-treated controls. Compared with controls, cirrhotic livers exhibited increased expression of Shh ligand and Gli2 and significantly reduced expression of Hhip, demonstrating that Hh pathway activation occurs during CCl4-induced cirrhosis (Fig. 10A). Increased Hh signaling was associated with altered expression of several factors that regulate EMT: mRNA levels of desmoplakin, BMP-7, and Id2 (genes that inhibit EMT) fell (Fig. 10B), whereas expression of pro-EMT factors, such as TGF-β and S100A4, increased (Fig. 10C). These findings support the concept that Hh pathway activation and EMT occur during the evolution of CCl4-induced cirrhosis and suggest that mechanisms that regulate HSC transdifferentiation in vitro may also operate during hepatic fibrogenesis in vivo.
Fig. 10.
Changes in expression of genes that regulate epithelial-to-mesenchymal transitions (EMT) and Hh pathway activation during CCl4-induced cirrhosis. Liver RNA was isolated from mice that had been treated with CCl4 for 8 wk to induce cirrhosis and compared with age- and sex-matched littermates treated with corn oil vehicle (n = 6 each). qRT-PCR was used to assess expression of Hh signaling factors known to regulate EMT. A: Shh, Gli2, and Hh-interacting protein (Hhip). B: epithelial genes that inhibit EMT (desmoplakin, BMP-7, and Id2). C: factors that promote EMT (TGF-β and S100A4). *P < 0.05.
Evidence for Hh pathway activation and EMT in clonal HSC from livers with CCl4-induced cirrhosis.
To assess whether myofibroblasts in cirrhotic livers might have been derived via an EMT-like process, we used qRT-PCR to analyze gene expression in two different clonal lines that were derived from primary HSC isolated from a single rat with CCl4-induced cirrhosis (11). Our results confirm earlier reports that such clonally derived cells, although heterogeneous, are generally fibroblastic. Compared with freshly isolated, rat primary Q-HSC, for example, both clonal lines significantly overexpressed several mesenchymal genes, including α-SMA, Col1α1, fibronectin, vimentin, and S100A4 (Fig. 11A). Both also expressed higher levels of Gli2 than were expressed by primary Q-HSC, and one of the lines (8B) expressed increased mRNA levels of Shh ligand (Fig. 11B). In contrast to primary Q-HSC (but similar to primary rat MF-HSC), neither of the MF-HSC lines expressed detectable levels of Hhip (data not shown). Several typical epithelial cell markers that were easily detected in primary Q-HSC, including K7, desmoplakin, and E-cadherin, were measurable (but much less abundant) in the clonal myofibroblastic lines, particularly 8B (Fig. 11C). Conversely, expression of two EMT-inhibitory factors, BMP-7 and Id2, was significantly repressed in both myofibroblastic clones compared with primary Q-HSC, being least expressed in the 8B cell line, which had the most myofibroblastic phenotype (Fig. 11D). These findings suggest that HSC populations in cirrhotic livers are heterogeneous and contain Hh-responsive fibroblastic cells that exhibit a pattern of gene expression that primary HSC acquire as they undergo an Hh-regulated EMT-like process and transition to become MF-HSC in vitro.
Fig. 11.
Evidence for Hh pathway activation and EMT in clonally derived MF-HSC from livers with CCl4-induced cirrhosis. Clonally derived HSC lines (8B and 5H) from a single rat with CCl4-induced cirrhosis were compared with freshly isolated primary rat Q-HSC to assess markers associated with EMT and Hh signaling. A: mesenchymal marker expression (α-SMA, Col1α1, fibronectin, vimentin, and S100A4). B: Hh signaling (Shh and Gli2). C: epithelial markers (K7, desmoplakin, and E-cadherin). D: EMT markers (BMP-7 and Id2). For each gene, expression was normalized to that of a housekeeping gene (S9) in the same RNA sample and then expressed relative to expression of the same gene in primary Q-HSC. 8B were then treated with tomatidine or cyclopamine, and effects on gene expression changes were assessed by qRT-PCR. E: EMT-inhibitory markers (BMP-7 and desmoplakin) and the Q-HSC marker PPARγ. F: myofibroblastic markers (α-SMA, Col1α1, and S100A4). Values are means ± SE of triplicate experiments. *P < 0.05; **P < 0.01.
We previously reported that mice with an overly active Hh pathway develop increased hepatic fibrosis during chronic liver injury (33, 45). The present studies link Hh pathway activation and hepatic fibrogenesis during CCl4-induced liver injury with repression of EMT inhibitors, such as BMP-7. Other investigators have demonstrated that CCl4-induced cirrhosis is reversed in mice treated with BMP-7 (21). Therefore, we treated the clonal 8B MF-HSC line with cyclopamine to determine whether blocking Hh signaling in myofibroblasts that were generated during CCl4-induced liver fibrosis enhanced their expression of BMP-7 and suppressed their myofibroblastic phenotype. Significantly more BMP-7 mRNA was expressed in cyclopamine- than in tomatidine-treated 8B cells (Fig. 11E). This was accompanied by increased expression of genes that encode epithelial junctional complexes, including desmoplakin, as well as upregulation of Q-HSC markers, such as PPARγ (Fig. 11E), but repression of various mesenchymal markers, such as α-SMA and Col1α1 (Fig. 11F). Thus a strategy that specifically increased BMP-7 in myofibroblasts from cirrhotic livers (i.e., direct inhibition of Hh pathway activity) tended to reverse their myofibroblastic phenotype and caused them to acquire a more quiescent and epithelial phenotype. Such data are consistent with the concept that Hh pathway activation provokes an EMT-like response that promotes the transdifferentiation of Q-HSC into MF-HSC in vivo, as it does in vitro. Moreover, this process appears to be somewhat reversible long after it was initiated, with restoration of a more quiescent HSC phenotype via an MET-like process when Hh activity declines and expression of EMT inhibitors, such as BMP-7, is restored.
DISCUSSION
We have shown that activation of the Hh pathway promotes the transition of Q-HSC into MF-HSC and that Hh signaling must remain active in MF-HSC in order for such cells to maintain their myofibroblastic phenotype. Our results demonstrate that Q-HSC strongly express Hhip. Hhip complexes with Hh ligands and prevents them from engaging cell surface receptors that initiate Hh signaling in Hh-responsive cells (46). This inhibits Hh pathway activity and helps explain why expression of Hh target genes, including the Hh-induced transcription factors Gli1 and Gli2, are neglible in Q-HSC. During fibrogenic liver injury, such as that evoked by chronic CCl4 exposure, we found that Hhip is downregulated and expression of Shh ligand and Gli2 increases. Thus CCl4-induced fibrogenic liver injury is characterized by repression of Hh pathway inhibitors, induction of factors that promote Hh signaling, and increased expression of Hh-stimulated target genes. A similar process occurs during fibrogenesis induced by bile duct ligation in rats (33) and during the progression of fatty liver-related fibrosis in patients (45).
Chronic CCl4 exposure is known to promote the transition of Q-HSC into MF-HSC, expand myofibroblastic populations, and cause progressive hepatic fibrosis (4, 5). A similar transdifferentiation process occurs when primary Q-HSC from healthy livers are cultured on plastic in serum-containing medium. As occurred during liver fibrosis in vivo, when primary rat or human Q-HSC transitioned to become collagen-producing MF-HSC in vitro, Hhip was downregulated and expression of Shh increased. The Hh pathway became activated in such MF-HSC, as evidenced by increased expression of Gli2. Conversely, blocking Hh signaling with cyclopamine increased Hhip expression and repressed expression of Gli2 and multiple myofibroblast-associated genes, including α-SMA and Col1α1. Our analysis of clonal myofibroblast cell lines from a liver with CCl4-induced fibrosis verified that the Hh pathway was also strongly activated in those cells. Similar to the culture-activated MF-HSC, cells that had become myofibroblastic in vivo during CCl4-related fibrogenesis upregulated Hhip and reverted to a more quiescent phenotype when they were treated with cyclopamine to repress Hh signaling. Therefore, Hh pathway activation promotes cell-autonomous transition of Q-HSC into MF-HSC, and the cells remain myofibroblastic as long as Hh signaling persists but revert to a more quiescent and epithelial phenotype when Hh signaling is inhibited.
Our work also identifies a novel Hh-regulated mechanism for transition of Q-HSC into MF-HSC, namely, EMT. The Hh pathway is a well-recognized regulator of EMT during development and neoplasia (3, 13). EMT is a complex process that is orchestrated by cross talk among several signaling pathways that collaborate to affect global, but gradual, changes in cell structure and function that permit individual cells to disrupt their connections to neighboring cells, disassociate, and migrate (18). A reverse process, i.e., MET, is apparently also possible (1). Detailed discussions of the mechanisms that regulate EMT/MET were published recently (1, 18, 19, 56) and are beyond the scope of this study. Suffice it to say that, other than Hh, one of the major factors that promotes EMT is TGF-β1. TGF-β interacts with certain TGF-β receptors to activate receptor-associated kinases, such as activin-linked kinase-5, downstream factors, such as Smads 2 and 3, and several TGF-β-regulated transcription factors, such as Snail and Slug (55). Cross talk between Hh and TGF-β signaling pathways is probably important in modulating EMT, because TGF-β has been shown to induce expression of certain Hh ligands (16) and to stabilize levels of Gli proteins (38), whereas Gli proteins are known to induce transcription of Snail (25). Among its other functions, Snail helps repress transcription of E-cadherin, leading to the disruption of junctional complexes that maintain epithelial integrity (20). BMP-7 is the most extensively characterized inhibitor of EMT. Epithelial cells constitutively express BMP-7, which maintains expression of E-cadherin by regulating activity of Id2 (23).
EMT has been demonstrated to occur in certain types of cultured adult liver epithelial cells, including hepatocytes and cholangiocytes (17, 34, 57). There is some evidence that these cells may also be capable of undergoing EMT during chronic liver injury (57), although the extent and significance of the process in damaged livers remain highly controversial (18). Recent work shows that endothelial cells in some tissues can also be induced to disassociate from each other, migrate into adjacent stroma, and acquire a migratory/mesenchymal phenotype (9). This process has been dubbed endothelial-to-mesenchymal transition. Whether endothelial-to-mesenchymal transition occurs during liver injury is unclear. The present study provides novel evidence that the transdifferentiation of adult liver Q-HSC into MF-HSC occurs via a process that resembles EMT. Moreover, such MF-HSC appear to be capable of undergoing an MET-like process that permits them to resume a less myofibroblastic, more epithelial, phenotype. When presented at various scientific meetings, these findings have been met with considerable skepticism, because Q-HSC are not generally considered to be epithelial cells. During liver development, HSC are thought to be derived from cardiac-associated mesenchymal cells that migrate into the nascent liver bud, mingle with infiltrating endodermal progenitors, and ultimately take up residence beneath the mesothelial lining of the primitive liver (2, 28). Such cells express mesenchymal markers, such as desmin and the mesenchyme-associated transcription factors Lhx2 and Msx2 (2). As in fetal livers, HSC in adult livers reside in the stroma and express Lhx2 and desmin. Thus they are also generally considered to be mesenchymal. However, results from the present study, as well as previously published data (6, 10, 15, 26), demonstrate that several genes that encode epithelium-associated keratins and components of junctional complexes, including E-cadherin, desmoplakin, and connexins, are actively expressed in freshly isolated Q-HSC. In addition, HSC have been shown to form intercellular junctions with other HSC and with hepatocytes in vitro (6, 10). Confocal microscopy evidence indicates that this process may also occur in intact liver tissue (26). The ability to form cell-to-cell connections and generate adherent cell layers is a key characteristic of epithelial cells (19). Our data demonstrate that as HSC become more myofibroblastic in vivo and in vitro, they downregulate these epithelial traits. However, expression of all the various epithelium-associated factors is restored when the mechanism that initially stimulated myofibroblastic transition (i.e., Hh pathway activation) is interrupted. Thus HSC appear to be capable of acquiring a somewhat epithelial phenotype under circumstances that constrain Hh signaling and transitioning to a less epithelial/more mesenchymal phenotype in other contexts that promote Hh pathway activity.
The biological significance of HSC EMT during adult liver injury and repair remains somewhat uncertain. Although MF-HSC are no longer believed to be the sole source of excess matrix during liver fibrogenesis, they remain an important contributor (7). Thus Hh-related activation of the EMT-like process that mediates the transition of Q-HSC into MF-HSC is likely to be important in the pathogenesis of cirrhosis. This may explain, in part, why treatments that increase BMP-7 are antifibrogenic in the liver (21), as in several other tissues (24, 31, 32, 47). Evidence that MF-HSC are capable of MET and that Q-HSC exhibit various epithelial characteristics also has intriguing implications. For example, MET of MF-HSC may play a role in the gradual dissipation of myofibroblasts, resolution of liver fibrosis, reaccumulation of Q-HSC, and reacquisition of normal liver architecture that occur when biliary obstruction is reversed by creating a biliary-enteric anastomosis in bile duct-ligated rats, because gradual silencing of Hh signaling occurs after Roux-en-Y gastric bypass (33). In addition, reduced Hh signaling would be expected to inhibit proliferation and potentiate apoptosis of any HSC that remain myofibroblastic after biliary obstruction is reversed, because MF-HSC require Hh pathway activity for optimal growth and viability (42, 53). Preventing the formation and accumulation of MF-HSC is also likely to exert other positive effects in livers that are recovering from cholestasis, because MF-HSC are an important source of Hh ligands that modulate the behavior of other Hh-responsive liver cells. For example, Shh released from MF-HSC has been shown to induce activation of liver sinusoidal endothelial cells (50) and promote EMT and upregulate expression of chemokines in neighboring ductular cells (35). Therefore, inhibiting EMT and stimulating MET in HSC would be predicted to inhibit hepatic microvascular abnormalities, block derivation of myofibroblasts from ductular cells, and reduce hepatic recruitment of various types of inflammatory cells.
Whether MET of MF-HSC plays other roles in the regenerative process is unknown, because although HSC can be induced to acquire features of hepatocytes and cholangiocytes when cultured (7), it is unclear whether they are capable of transitioning into cells other than myofibroblasts in vivo. Skepticism persists, because it remains debatable which germ layer(s) gives rise to HSC. As mentioned earlier, HSC are derived from cardiac-associated mesenchyme (22). Cardiac mesenchyme is composed of cells that originated from cardiac mesoderm, as well as cells that infiltrated the heart field from the neural crest (43). HSC in fetal livers clearly express Lhx2 and Msx2 (2). Msx2 is a mesoderm-enriched transcription factor (8), whereas Lhx2 is known to be expressed by neural and skin progenitors (29, 37) and, therefore, presumably marks ectodermal derivatives. Therefore, HSC populations in fetal livers appear to contain cells that were derived from mesoderm, ectoderm, and/or multipotent progenitors that retain the capacity to generate lineages for both germ layers. Adult HSC also express mesodermal markers (Msx2 and desmin), various ectodermal markers (GFAP), and neural cell adhesion molecule and can be induced to express endoderm-associated factors, particularly those that are found in ductular-type (K7 and K9) cells. Markers of multipotent progenitors (e.g., CD133, Gata6, and Oct4) have also been demonstrated in adult HSC under certain culture conditions (7).
These findings are intriguing, because HSC in fetal and adult livers express Lhx2 and Msx2, and these transcription factors are known to interact with the Hh pathway to regulate the fate of various types of multipotent progenitor cells in other tissues (12, 37, 39). For example, neuroepithelial cells in the developing brain (12, 29) and hair follicle stem cells in adult skin express Lhx2 (37), and in each of these tissues, Lhx2 functions as a selector gene to specify cell identity via cell-autonomous and non-cell-autonomous mechanisms. Hepatic morphogenesis is severely disturbed in Lhx2 knockout mice (49). By embryonic day 14.5, the livers of these animals have accumulated large numbers of ductular-appearing cells that express endodermal makers. Here, we report that Lhx2 mRNA was not detected in mature rat cholangiocytes but that Q-HSC from healthy rodent and human livers expressed Lhx2 and that Lhx2 mRNA levels increased further as the HSC became myofibroblastic. We also demonstrated that treating MF-HSC with cyclopamine reciprocally regulated expression of Lhx2 and ductular markers. Thus our data suggest that Lhx2 supports a more mesodermal (and less endodermal) phenotype in adult HSC populations. These findings are consistent with evidence that knocking down Lxh2 in mice resulted in reduced accumulation of desmin-expressing mesodermal cells while expanding the endodermal (ductular cell) compartment during liver morphogenesis (49) and suggest that Lhx2 may also help specify the identity of progenitors in liver, as it does in brain and skin.
More research is required to determine whether similar transitions occur in adult liver tissue and help reconstitute the pool of endodermal cells. Lineage tracing approaches are required to map the fates of various types of adult liver cells during liver injury. We are aware of only three studies in animal models of liver injury that have utilized this strategy (41, 52, 57). One of these used expression of the Q-HSC-associated gene GFAP to mark cells and demonstrated labeling of Q-HSC, myofibroblasts, ductular cells, and hepatocytes, suggesting that stellate cells, cholangiocytes, and hepatocytes may share a common lineage that is derived from multiprogenitor cells that express GFAP (52). After injury, transient coexpression of epithelial and mesenchymal markers was demonstrated in some of the labeled cells, supporting the possibility that liver injury triggered EMT/MET in these mice. If verified by further work, this observation would be very exciting, because it suggests that the relative sizes of the endodermal compartment (e.g., mature hepatocytes and cholangiocytes) and the mesectodermal compartment (which presumably includes mature HSC) (2) remains flexible in adults and depends on the balance between Hh-modulated mechanisms that regulate EMT and MET in multifunctional progenitors. This might help explain a report that administration of the EMT inhibitor BMP-7 promoted hepatocyte regeneration after partial hepatectomy (44). In any case, the aggregate results indicate that HSC belong on the list of adult liver cell types that are capable of undergoing EMT, suggest that EMT (and MET) probably play a larger role in remodeling injured adult livers than previously suspected, and identify the Hh pathway as a key regulator of EMT/MET in liver cells, including resident HSC. These findings, in turn, identify Hh ligands, BMP-7, and other EMT regulators as potential therapeutic targets that might be manipulated to optimize regeneration and prevent cirrhosis in patients with chronic liver injury.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant 1R01 DK-077794-01A2 (A. M. Diehl) and the Barton F. Haynes Award from the Department of Medicine, Duke University Medical Center (S. S. Choi).
DISCLOSURES
No conflicts of interest are declared by the author(s).
REFERENCES
- 1.Acloque H, Adams MS, Fishwick K, Bronner-Fraser M, Nieto MA. Epithelial-mesenchymal transitions: the importance of changing cell state in development and disease. J Clin Invest 119: 1438–1449, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asahina K, Tsai SY, Li P, Ishii M, Maxson RE, Jr, Sucov HM, Tsukamoto H. Mesenchymal origin of hepatic stellate cells, submesothelial cells, and perivascular mesenchymal cells during mouse liver development. Hepatology 49: 998–1011, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bailey JM, Singh PK, Hollingsworth MA. Cancer metastasis facilitated by developmental pathways: Sonic hedgehog, Notch, and bone morphogenic proteins. J Cell Biochem 102: 829–839, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Choi SS, Sicklick JK, Ma Q, Yang L, Huang J, Qi Y, Chen W, Li YX, Goldschmidt-Clairmont P, Diehl AM. Sustained activation of Rac-1 in hepatic stellate cells promotes liver injury and fibrosis in mice. Hepatology 44: 1267–1277, 2006 [DOI] [PubMed] [Google Scholar]
- 5.De Minicis S, Seki E, Uchinami H, Kluwe J, Zhang Y, Brenner DA, Schwabe RF. Gene expression profiles during hepatic stellate cell activation in culture and in vivo. Gastroenterology 132: 1937–1946, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Fischer R, Reinehr R, Lu TP, Schonicke A, Warskulat U, Dienes HP, Haussinger D. Intercellular communication via gap junctions in activated rat hepatic stellate cells. Gastroenterology 128: 433–448, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev 88: 125–172, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gong SG, Kiba A. The role of Xmsx-2 in the anterior-posterior patterning of the mesoderm in Xenopus laevis. Differentiation 65: 131–140, 1999 [DOI] [PubMed] [Google Scholar]
- 9.Goumans MJ, van Zonneveld AJ, ten Dijke P. Transforming growth factor-β-induced endothelial-to-mesenchymal transition: a switch to cardiac fibrosis? Trends Cardiovasc Med 18: 293–298, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Greenwel P, Rubin J, Schwartz M, Hertzberg EL, Rojkind M. Liver fat-storing cell clones obtained from a CCl4-cirrhotic rat are heterogeneous with regard to proliferation, expression of extracellular matrix components, interleukin-6, and connexin 43. Lab Invest 69: 210–216, 1993 [PubMed] [Google Scholar]
- 11.Greenwel P, Schwartz M, Rosas M, Peyrol S, Grimaud JA, Rojkind M. Characterization of fat-storing cell lines derived from normal and CCl4-cirrhotic livers. Lab Invest 65: 644–653, 1991 [PubMed] [Google Scholar]
- 12.Hebert JM, Fishell G. The genetics of early telencephalon patterning: some assembly required. Nat Rev Neurosci 9: 678–685, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heisenberg CP, Solnica-Krezel L. Back and forth between cell fate specification and movement during vertebrate gastrulation. Curr Opin Genet Dev 18: 311–316, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higashi N, Kojima N, Miura M, Imai K, Sato M, Senoo H. Cell-cell junctions between mammalian (human and rat) hepatic stellate cells. Cell Tissue Res 317: 35–43, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Jung Y, Brown KD, Witek RP, Omenetti A, Yang L, Vandongen HM, Milton RJ, Hines IN, Rippe RA, Spahr L, Rubbia-Brandt L, Diehl AM. Accumulation of hedgehog-responsive progenitors parallels alcoholic liver disease severity in mice and humans. Gastroenterology 134: 1532–1543, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaimori A, Potter J, Kaimori JY, Wang C, Mezey E, Koteish A. Transforming growth factor-β1 induces an epithelial-to-mesenchymal transition state in mouse hepatocytes in vitro. J Biol Chem 282: 22089–22101, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Kalluri R. EMT: when epithelial cells decide to become mesenchymal-like cells. J Clin Invest 119: 1417–1419, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest 119: 1420–1428, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katoh Y, Katoh M. Hedgehog signaling, epithelial-to-mesenchymal transition and miRNA. Int J Mol Med 22: 271–275, 2008 [PubMed] [Google Scholar]
- 21.Kinoshita K, Iimuro Y, Otogawa K, Saika S, Inagaki Y, Nakajima Y, Kawada N, Fujimoto J, Friedman SL, Ikeda K. Adenovirus-mediated expression of BMP-7 suppresses the development of liver fibrosis in rats. Gut 56: 706–714, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kolterud A, Wandzioch E, Carlsson L. Lhx2 is expressed in the septum transversum mesenchyme that becomes an integral part of the liver and the formation of these cells is independent of functional Lhx2. Gene Expr Patterns 4: 521–528, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Kondo M, Cubillo E, Tobiume K, Shirakihara T, Fukuda N, Suzuki H, Shimizu K, Takehara K, Cano A, Saitoh M, Miyazono K. A role for Id in the regulation of TGF-β-induced epithelial-mesenchymal transdifferentiation. Cell Death Differ 11: 1092–1101, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Kowanetz M, Valcourt U, Bergstrom R, Heldin CH, Moustakas A. Id2 and Id3 define the potency of cell proliferation and differentiation responses to transforming growth factor-β and bone morphogenetic protein. Mol Cell Biol 24: 4241–4254, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li X, Deng W, Nail CD, Bailey SK, Kraus MH, Ruppert JM, Lobo-Ruppert SM. Snail induction is an early response to Gli1 that determines the efficiency of epithelial transformation. Oncogene 25: 609–621, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lim YS, Lee HS. [The expression of E-cadherin in human and rat hepatic stellate cells: evidence of epithelial-mesenchymal transition]. Taehan Kan Hakhoe Chi 8: 90–99, 2002 [PubMed] [Google Scholar]
- 27.Livak KJ, Schmittgn TD. Analysis of relative gene expression data using real time quantitative PCR and the 2(Δ-Δ) CT method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Loo CK, Wu XJ. Origin of stellate cells from submesothelial cells in a developing human liver. Liver Int 28: 1437–1445, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Mangale VS, Hirokawa KE, Satyaki PR, Gokulchandran N, Chikbire S, Subramanian L, Shetty AS, Martynoga B, Paul J, Mai MV, Li Y, Flanagan LA, Tole S, Monuki ES. Lhx2 selector activity specifies cortical identity and suppresses hippocampal organizer fate. Science 319: 304–309, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Medlock ES, Haar JL. The liver hemopoietic environment. I. Developing hepatocytes and their role in fetal hemopoiesis. Anat Rec 207: 31–41, 1983 [DOI] [PubMed] [Google Scholar]
- 31.Myllarniemi M, Lindholm P, Ryynanen MJ, Kliment CR, Salmenkivi K, Keski-Oja J, Kinnula VL, Oury TD, Koli K. Gremlin-mediated decrease in bone morphogenetic protein signaling promotes pulmonary fibrosis. Am J Respir Crit Care Med 177: 321–329, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nguyen TQ, Goldschmeding R. Bone morphogenetic protein-7 and connective tissue growth factor: novel targets for treatment of renal fibrosis? Pharm Res 25: 2416–2426, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Omenetti A, Popov Y, Jung Y, Choi SS, Witek RP, Yang L, Brown KD, Schuppan D, Diehl AM. The hedgehog pathway regulates remodelling responses to biliary obstruction in rats. Gut 57: 1275–1282, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Omenetti A, Porrello A, Jung Y, Yang L, Popov Y, Choi SS, Witek RP, Alpini G, Venter J, Vandongen HM, Syn WK, Baroni GS, Benedetti A, Schuppan D, Diehl AM. Hedgehog signaling regulates epithelial-mesenchymal transition during biliary fibrosis in rodents and humans. J Clin Invest 118: 3331–3342, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Omenetti A, Syn WK, Jung Y, Francis H, Porrello A, Witek RP, Choi SS, Yang L, Mayo MJ, Gershwin ME, Alpini G, Diehl AM. Repair-related activation of hedgehog signaling promotes cholangiocyte chemokine production. Hepatology 50: 518–527, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Omenetti A, Yang L, Li YX, McCall SJ, Jung Y, Sicklick JK, Huang J, Choi S, Suzuki A, Diehl AM. Hedgehog-mediated mesenchymal-epithelial interactions modulate hepatic response to bile duct ligation. Lab Invest 87: 499–514, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Rhee H, Polak L, Fuchs E. Lhx2 maintains stem cell character in hair follicles. Science 312: 1946–1949, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Riobo NA, Lu K, Ai X, Haines GM, Emerson CP., Jr Phosphoinositide 3-kinase and Akt are essential for Sonic Hedgehog signaling. Proc Natl Acad Sci USA 103: 4505–4510, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rodriguez-Esteban C, Schwabe JW, Pena JD, Rincon-Limas DE, Magallon J, Botas J, Belmonte JC. Lhx2, a vertebrate homologue of apterous, regulates vertebrate limb outgrowth. Development 125: 3925–3934, 1998 [DOI] [PubMed] [Google Scholar]
- 40.Rojkind M, Novikoff PM, Greenwel P, Rubin J, Rojas-Valencia L, de Carvalho AC, Stockert R, Spray D, Hertzberg EL, Wolkoff AW. Characterization and functional studies on rat liver fat-storing cell line and freshly isolated hepatocyte coculture system. Am J Pathol 146: 1508–1520, 1995 [PMC free article] [PubMed] [Google Scholar]
- 41.Sackett SD, Li Z, Hurtt R, Gao Y, Wells RG, Brondell K, Kaestner KH, Greenbaum LE. Foxl1 is a marker of bipotential hepatic progenitor cells in mice. Hepatology 49: 920–929, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sicklick JK, Li YX, Choi SS, Qi Y, Chen W, Bustamante M, Huang J, Zdanowicz M, Camp T, Torbenson MS, Rojkind M, Diehl AM. Role for hedgehog signaling in hepatic stellate cell activation and viability. Lab Invest 85: 1368–1380, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Snarr BS, Kern CB, Wessels A. Origin and fate of cardiac mesenchyme. Dev Dyn 237: 2804–2819, 2008 [DOI] [PubMed] [Google Scholar]
- 44.Sugimoto H, Yang C, LeBleu VS, Soubasakos MA, Giraldo M, Zeisberg M, Kalluri R. BMP-7 functions as a novel hormone to facilitate liver regeneration. FASEB J 21: 256–264, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Syn WK, Omenetti A, Abdelmalek M, Guy CD, Yang L, Wang J, Witek RP, Fearing CM, Pereira TA, Teaberry V, Choi SS, Vancells JC, Karaca G, Diehl AM. Hedgehog-mediated epithelial-to-mesenchymal transition and fibrogenic repair in non-alcoholic fatty liver disease. Gastroenterology 137: 1478–1488, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tada M, Kanai F, Tanaka Y, Tateishi K, Ohta M, Asaoka Y, Seto M, Muroyama R, Fukai K, Imazeki F, Kawabe T, Yokosuka O, Omata M. Down-regulation of hedgehog-interacting protein through genetic and epigenetic alterations in human hepatocellular carcinoma. Clin Cancer Res 14: 3768–3776, 2008 [DOI] [PubMed] [Google Scholar]
- 47.Towbin JA. Scarring in the heart—a reversible phenomenon? N Engl J Med 357: 1767–1768, 2007 [DOI] [PubMed] [Google Scholar]
- 48.Vroman B, LaRusso NF. Development and characterization of polarized primary cultures of rat intrahepatic bile duct epithelial cells. Lab Invest 74: 303–313, 1996 [PubMed] [Google Scholar]
- 49.Wandzioch E, Kolterud A, Jacobsson M, Friedman SL, Carlsson L. Lhx2−/− mice develop liver fibrosis. Proc Natl Acad Sci USA 101: 16549–16554, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Witek RP, Yang L, Liu R, Jung Y, Omenetti A, Syn WK, Choi SS, Cheong Y, Fearing CM, Agboola KM, Chen W, Diehl AM. Liver cell-derived microparticles activate hedgehog signaling and alter gene expression in hepatic endothelial cells. Gastroenterology 136: 320–322, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu L, Hui AY, Albanis E, Arthur MJ, O'Byrne SM, Blaner WS, Mukherjee P, Friedman SL, Eng FJ. Human hepatic stellate cell lines, LX1 and LX2: new tools for analysis of hepatic fibrosis. Gut 54: 142–151, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang L, Jung Y, Omenetti A, Witek RP, Choi S, Vandongen HM, Huang J, Alpini GD, Diehl AM. Fate-mapping evidence that hepatic stellate cells are epithelial progenitors in adult mouse livers. Stem Cells 26: 2104–2113, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang L, Wang Y, Mao H, Fleig S, Omenetti A, Brown KD, Sicklick JK, Li YX, Diehl AM. Sonic hedgehog is an autocrine viability factor for myofibroblastic hepatic stellate cells. J Hepatol 48: 98–106, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zaret KS, Grompe M. Generation and regeneration of cells of the liver and pancreas. Science 322: 1490–1494, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zavadil J, Bottinger EP. TGF-β and epithelial-to-mesenchymal transitions. Oncogene 24: 5764–5774, 2005 [DOI] [PubMed] [Google Scholar]
- 56.Zeisberg M, Neilson EG. Biomarkers for epithelial-mesenchymal transitions. J Clin Invest 119: 1429–1437, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zeisberg M, Yang C, Martino M, Duncan MB, Fieder F, Tanjore H, Kalluri R. Fibroblasts derive from hepatocytes in liver fibrosis via epithelial to mesenchymal transition. J Biol Chem 282: 23337–23347, 2007 [DOI] [PubMed] [Google Scholar]