Abstract
We tested the novel hypothesis that endogenous adenosine (eADO) activates low-affinity A3 receptors in a model of neurogenic diarrhea in the guinea pig colon. Dimaprit activation of H2 receptors was used to trigger a cyclic coordinated response of contraction and Cl− secretion. Contraction-relaxation was monitored by sonomicrometry (via intracrystal distance) simultaneously with short-circuit current (Isc, Cl− secretion). The short interplexus reflex coordinated response was attenuated or abolished by antagonists at H2 (cimetidine), 5-hydroxytryptamine 4 receptor (RS39604), neurokinin-1 receptor (GR82334), or nicotinic (mecamylamine) receptors. The A1 agonist 2-chloro-N6-cyclopentyladenosine (CCPA) abolished coordinated responses, and A1 antagonists could restore normal responses. A1-selective antagonists alone [8-cyclopentyltheophylline (CPT), 1,3-dipropyl-8-(2-amino-4-chlorophenyl)xanthine (PACPX), or 8-cyclopentyl-N3-[3-(4-(fluorosulfonyl)benzoyloxy)propyl]-xanthine (FSCPX)] caused a concentration-dependent augmentation of crypt cell secretion or contraction and acted at nanomolar concentrations. The A3 agonist N6-(3-iodobenzyl)-adenosine-5′-N-methyluronamide (IB-MECA) abolished coordinated responses and the A3 antagonist 3-ethyl-5-benzyl-2-methyl-4-phenylethynyl-6-phenyl-1,4-(±)-dihydropyridine-3,5-dicarboxylate (MRS1191) could restore and further augment responses. The IB-MECA effect was resistant to knockdown of adenosine A1 receptor with the irreversible antagonist FSCPX; the IC50 for IB-MECA was 0.8 μM. MRS1191 alone could augment or unmask coordinated responses to dimaprit, and IB-MECA suppressed them. MRS1191 augmented distension-evoked reflex Isc responses. Adenosine deaminase mimicked actions of adenosine receptor antagonists. A3 receptor immunoreactivity was differentially expressed in enteric neurons of different parts of colon. After tetrodotoxin, IB-MECA caused circular muscle relaxation. The data support the novel concept that eADO acts at low-affinity A3 receptors in addition to high-affinity A1 receptors to suppress coordinated responses triggered by immune-histamine H2 receptor activation. The short interplexus circuit activated by histamine involves adenosine, acetylcholine, substance P, and serotonin. We postulate that A3 receptor modulation may occur in gut inflammatory diseases or allergic responses involving mast cell and histamine release.
Keywords: adenosine A3 receptor, adenosine A1 receptor, neurogenic diarrhea, sonomicrometry, motility, Cl− secretion, coordination of motility and secretion, endogenous adenosine
adenosine (ADO) A1, A2, and A3 receptor (R) proteins or mRNA exist in rodent (30) and human intestinal tract (14). Functional evidence for A1 inhibitory, non-A1 (putative A3), and A2 excitatory receptors exists on enteric neurons (15). A preliminary report suggested that an inhibitory limb of the musculomotor reflex may be activated by putative A3 receptors in the rodent distal colon (24). One study to date provided pharmacological evidence for inhibitory A3 receptors in synaptic transmission in neurons of the intact submucosal plexus of the human jejunum (65). Endogenous adenosine (eADO) provides ongoing inhibitory effects in enterochromaffin (EC) cells (17) and the enteric nervous system (ENS) in rodents (13, 15, 16, 18) and humans (65) and in hypoxic conditions (29) by activating high-affinity A1 and putative low-affinity A3 receptors. This has been deduced from pharmacological studies with selective agonists or antagonists at these receptors. In human EC cells or ENS, functional A3 receptors can be revealed by selective A3 agonists [i.e., N6-(3-iodobenzyl)-adenosine-5′-N-methyluronamide (IB-MECA)] or antagonists [3-ethyl-5-benzyl-2-methyl-4-phenylethynyl-6-phenyl-1,4-(±)-dihydropyridine-3,5-dicarboxylate (MRS1191) or MRS1220] (17, 65).
It has been hypothesized that high-affinity inhibitory A1 receptors are involved in physiological regulation whereas low-affinity inhibitory A3 receptors are more likely to be activated by eADO in pathophysiological states because they require high levels of eADO to activate them that are more easily achieved in disease states (3, 40, 44, 48, 57, 58). Ongoing studies are beginning to unravel the potential protective and therapeutic role of selectively targeting eADO (26, 53), A3 (37, 43), A2A (2, 8, 45, 46), and A2B receptors (41) in inflamed gut and models of inflammatory bowel diseases (IBD) with drug agonists or antagonists depending on the receptor. Endogenous adenosine is a metabolite of ATP that acts at A1, A2A, and A2B receptors to influence mucosal secretion and motor reflexes (18). Increasing the concentration of eADO is one mechanism by which several drugs may reduce gut inflammation (25, 26, 54). An adenosine kinase inhibitor (26, 54) or the A3 agonist IB-MECA (43) may be beneficial in murine models of colitis or a rat 2,4,6-trinitrobenzenesulfonic acid-induced colitis model (37), but the mechanisms involved remain poorly understood. IB-MECA is well tolerated orally by healthy human volunteers (63) and has been in Phase II clinical trials for another chronic inflammatory disease, rheumatoid arthritis, and is apparently without toxicity (www.canfite.com/develop.html).
Our recent bioinformatics analysis provided evidence for differential expression and dysregulation of adenosine A3 receptor and 11 other purinergic receptors or targets in purinergic signaling pathways in mucosal biopsies or peripheral blood mononuclear cells in Crohn's disease and ulcerative colitis; Crohn's disease and ulcerative colitis could be distinguished on the basis of unique profiles of purine gene expression and dysregulation (50). Expression of A1 or A3 receptors or mRNA has been shown to be sensitive to intestinal inflammation in a rabbit ileitis-Crohn's disease model (61) or human IBD (50) and adenosine A1 receptor (ADOA1R) downregulation leads to disruption of neurotransmission (28).
Complex neural circuits in the ENS coordinate motility, secretion, vascular tone, epithelial transport, and immune responses in the gut to ensure effective digestion, absorption, transit of luminal contents, and elimination of waste products. Immune-neural communication is involved in response to pathogens, allergens in the diet, mucosal irritants, or gut inflammation and to host-defense mechanisms (24). The role of A3 receptors in the ENS or such integrated responses or reflexes in normal or disease states of the gut remains unknown.
Mast cells are emerging as an important cell type in the immunopathobiology of IBD, microscopic colitis, neurogenic secretory diarrhea, and irritable bowel syndrome (19, 32, 33, 34, 35, 52, 64). Histamine release from mast cells (among other immune mediators) may be relevant in human allergy and luminal responses to food allergens and gut inflammatory diseases, and excitatory effects of a mast cell mediator cocktail were recently reported to occur on human submucous neurons (52). Neurons from sensitized or infected animals had an exaggerated response to histamine, and it has led to the hypothesis by Wood and colleagues (21, 32–35) that its release from mast cells during gut inflammation and actions in the enteric nerve plexuses contribute to the observed pathobiology. Dimaprit mimics the action of the mast cell mediator histamine in intact neural circuits of the gut; it activates submucosal neurons and triggers a short interplexus reflex of coordinated muscle contraction and Cl− secretion (19–21, 23, 24). Our goal was to determine whether activation of low-affinity A3 receptors leads to suppression of immune-neural communication and the short interplexus reflex triggered by dimaprit. Our findings indicate that adenosine A3 receptors (ADOA3Rs) in addition to ADOA1Rs are involved in suppression of the stereotypic coordinated reflex response to dimaprit. It is postulated that endogenous ADO, A3, and A1 receptors may provide potential novel therapeutic targets for immune-neural modulation in disease states of the gut.
METHODS
The method of euthanasia and the entire study have been approved by The Ohio State University Institutional Laboratory Animal Care and Use Committee.
Tissue preparation.
Male albino Hartley guinea pigs (Harlan-Sprague-Dawley, Indianapolis, IN) weighing 300–400 g were stunned by a blow to the head and exsanguinated. Segments of distal colon ∼8 cm proximal to the anus were excised and placed in a petri dish with ice-cold Krebs-Ringer buffer and gassed with 95% O2-5% CO2 mixture, buffered at pH 7.2–7.4. The composition of the Krebs-Ringer buffer (in mM) was 120 NaCl, 6 KCl, 1.2 MgCl2·6 H2O, 1.3 NaH2PO4·H2O, 14.4 NaHCO3, 2.5 CaCl2·H2O, and 10 glucose. The segments were opened along the mesenteric border, the luminal contents were removed, whole-thickness segments were pinned serosal side up onto one-half of a flux (Ussing) chamber, and the chamber was reconnected (Fig. 1). The mucosal and serosal compartments of the Ussing chamber were bathed on both sides with 5–10 ml gassed Krebs-Ringer buffer maintained at 37°C. The crystals were affixed parallel to the circumferential axis of the gut segment, so that segmentation-type contractions and relaxations of the circular muscle could be recorded and measured in response to the H2 receptor agonist dimaprit.
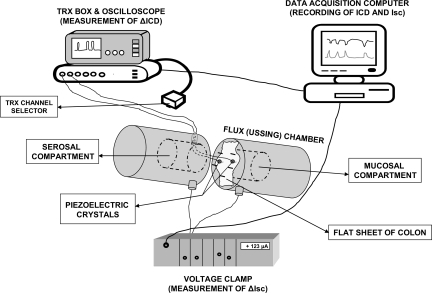
Fig. 1.
Illustration of the experimental setup for simultaneous monitoring of muscle contraction/relaxation by sonomicrometry [as change (Δ) in intracrystal distance (ICD)] and Cl− secretion in Ussing flux chambers [as change in short-circuit current (Isc)]. The traces were recorded and stored on the data-acquisition computer, and 1-mm piezoelectric crystals were glued in the circular direction of the whole-thickness flat sheets of colon at a distance of 4–5 mm apart. ΔICD was measured in μm distance between the piezoelectric crystals. A shorter distance between crystals indicates contraction and a larger distance relaxation. Drugs can be added to either serosal or mucosal compartments. Aperture area of the Ussing chamber = 0.785 cm2. ΔIsc was measured in μA/0.785 cm2 and then normalized to an area of 1 cm2. Details are described in methods.
For motility measurements we used miniature strain-gauge transducers sutured to the serosal surface in the circular muscle direction (CM) according to our previous study (19), or sonomicrometry with two piezoelectric crystals, 1 mm in diameter (Sonometrics, London, ON, Canada), attached to the serosa of the tissue in the Ussing chamber and connected to the TRX (ultrasound transceiver unit) box through a side opening. They were glued 4–5 mm apart to the serosal surface in the CM, with a small drop of cyanoacrylate tissue adhesive (Vetbond; 3M Animal Care Products, St. Paul, MN).
Electrical measurements.
For Cl− secretion measurements gut segments with a serosal surface area of 0.785 cm2 were bathed on both sides with Krebs-Ringer solution and bubbled with 95% O2-5% CO2 mixture, buffered at pH ∼7.2–7.4. Flux chambers were equipped with Ringer-agar bridges and calomel half-cells for measurement of the transmural potential difference (PD). A short-circuit current (Isc) is passed from a voltage-clamp apparatus (Physiological Instruments, VC-600, Houston, TX) via silver-silver chloride electrodes to abolish the PD. The Isc represents the algebraic sum of net fluxes of actively transported ions. Changes in Isc to dimaprit or carbachol were previously shown to represent chloride secretion (19, 21–23). The difference between peak responses and baseline Isc before drug addition was used to calculate changes in Isc.
Ultrasonomicrometry.
To study motility we adopted a novel ultrasonomicrometry technique for in vitro measurements in whole-thickness tissues of colon (illustrated in Fig. 1); this is a digital ultrasonic measurement system that utilizes ultrasound energy. Sonomicrometry estimates motion with high spatial and temporal resolution and is suitable for quantifying gastrointestinal contractile activity (1). The sonomicrometer (model TRX-6; Sonometrics, London, ON, Canada) has two main components: an external six-channel ultrasound transceiver unit (TRX box), connected to up to six omnidirectional piezocrystals, and a data-acquisition Pentium computer, running SonoLAB acquisition software and SonoVIEW postprocessing software. Ultrasonic signals are produced by a network of piezoelectric crystals that are implanted into the tissue. To transmit sound waves, a piezocrystal needs to be electrically stimulated. The potential applied to the crystal causes it to oscillate at its characteristic frequency, which in turn results in generation of a short ultrasound burst with a resonant frequency of ∼1.5 MHz. The velocity of the ultrasound in aqueous media is ∼1.59 mm/μs. Each implanted receiving crystal then begins to vibrate upon reception of the sonic signal and generates a weak electrical signal amplified in the receiving circuit. The time between the transmitting and the receiving signals (called transit time) is measured by use of high-frequency digital counters and is converted to distance. Since the velocity of the ultrasound is known, exact distance measurement between a given crystal pair is calculated by the SonoLAB software by the equation Distance = Velocity × Time. During an experiment the computer rapidly switches crystal modes from transmitting to receiving, thus allowing the user to obtain measurements between any of the implanted crystals. In the protocol that we designed, we used one crystal pair, and readings were acquired at a sampling rate of 90 Hz, with a duration of the transmit pulse of 312.5 ns. The arrival of the ultrasound wavefront to the receiving crystal was monitored on an oscilloscope as the first reliably marked peak of the receiving signal. The sensitivity of the circuits was adjusted on the TRX box during each experiment, so that small amplitude noise signals would not interfere with the trace record. The intercrystal distance (ICD) and Isc were recorded on the data-acquisition computer, as well as on a chart recorder (BD 101 Kipp & Zonen), connected to the analog output of the computer and to the voltage-clamp current output.
Strain-gauge recordings.
The strain-gauge amplifier combination was precalibrated by clamping the transducer at the suture holes on one end while hanging weights on the other end. To measure contractility, the leads from the transducer were passed through a hole in the flux chamber and sealed with bone wax. The transducer was connected to a strain-gauge amplifier for continuous monitoring of contractile activity in the CM direction. Outputs of the strain-gauge and voltage-clamp amplifiers were digitized by use of an RC Electronics/IBM PC-based waveform analysis system. A minimum of 45 min was allowed before measurement of baseline activity. For experiments, Isc and tension were recorded for a 10- to 15-min period before addition of dimaprit to the serosal side. Responses to dimaprit were usually recorded for 1–3 h (19).
Experimental protocols to study neural ADOA1R or ADOA3R and dimaprit responses.
Selective ADO receptor antagonists [8-cyclopentyltheophylline (CPT); 1,3-dipropyl-8-(2-amino-4-chlorophenyl)xanthine (PACPX); 8-cyclopentyl-N3-[3-(4-(fluorosulfonyl)benzoyloxy)propyl]-xanthine (FSCPX); MRS1191] were tested alone to unmask the ongoing influence of eADO on A1 or A3 receptors on coordinated responses. Selective ADO receptor agonists [2-chloro-N6-cyclopentyladenosine (CCPA); IB-MECA] were used to activate putative A1 or A3 receptors. Receptor antagonists were also used to confirm effects at A1 or A3 receptors. Pharmacological blockade and protection of A1 or knockdown experiments of A1 using an irreversible A1 antagonist FSCPX were used to functionally isolate A3 receptors and study them in the absence of A1 with an A3 agonist or antagonist. Direct effects of adenosine to influence smooth muscle contraction or crypt cell secretion were studied in tetrodotoxin (TTX)-poisoned preparations. Immunochemical identification and localization of A3 receptors was done in the rodent colon to identify A3 receptor immunoreactivity (ir) in enteric ganglia. Effects of A3 receptor antagonist on distension-evoked Isc responses were also studied in the guinea pig distal colon. To further characterize the interplexus reflex, various antagonists or inhibitors were tested on the coordinated responses including mecamylamine (nicotinic receptor antagonist), RS39604 (5HT4 receptor antagonist), GR82334 (neurokinin-1 receptor antagonist), cimetidine (histamine H2 receptor antagonist), a Na+Cl− cotransport inhibitor bumetanide or TTX.
Immunohistochemistry.
Preparations of microdissected submucosa-submucous plexus, longitudinal muscle-myenteric plexus (LMMP), or paraffin sections (5–10 μm) of guinea pig, mouse, or rat distal colon were used in immunohistochemical identification of A3 receptor-immunoreactivity (ir) in neurons of the ENS (14). A rabbit anti-rat A3 receptor antibody from Alpha Diagnostic (cat. no. A3R31-A, San Antonio, TX) was used at a dilution of 1:100–1:200 for immunochemical detection of A3-ir. Immunoreactivity is visualized by using a secondary antibody, bio-SP donkey anti-rabbit-IgG (Jackson Laboratories), at a dilution of 1:200. The antigen is a 15-aa peptide of rat A3R/AA3R (gene accession no. P28647). The specificity of anti-A3 antibody was tested by preabsorption of the rabbit anti-rat A3 receptor antiserum with the 15-aa immunogenic peptide (according to recommendations from Alpha Diagnostics).
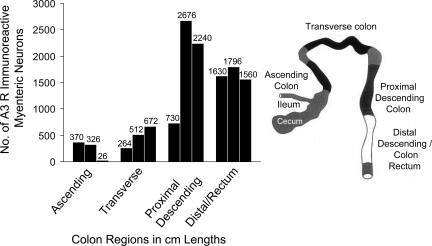
A3 receptor-ir neurons were counted in the rat colon myenteric plexus from the entire colon of two animals. Microdissected LMMP preparations were processed and stained for A3 receptor-ir, and then the number of A3 receptor-ir cell bodies of neurons was counted in the entire colon under transmitted illumination by use of an Olympus IMT-2 inverted scope and a ×20 objective (×200 magnification). The distribution of A3 receptor-ir neurons from oral to distal colon was quantified by arbitrarily dividing the colon into 1-cm segments; data are illustrated in the form of a histogram (Fig. 14).
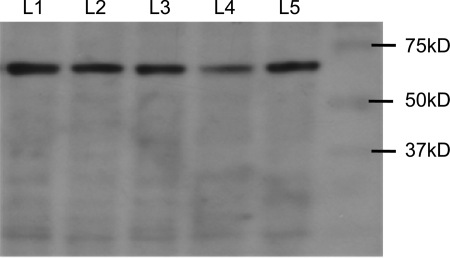
Fig. 14.
Western blot analysis for A3 receptor expression revealed an immunogenic band at ∼66 kDa. Data are for 5 different animals denoted by L1–L5; 100 μg protein lysate was used for each preparation. The rabbit anti-rat A3 receptor antiserum was used for Western blots as described in methods.
Western blots.
Two polyclonal antisera were used for Western blots: 1) rabbit anti-rat A3 receptor antibody (1:100 dilution, Alpha Diagnostic) and a secondary antibody goat anti-rabbit IgG-horseradish peroxidase (HRP; sc-2004, 1:500 dilution, Santa Cruz Biotechnology, Santa Cruz, CA) and 2) goat anti-human A3 receptor antibody (1:500 dilution) and secondary antibody donkey anti-goat IgG-HRP (1:2000 dilution, sc-2020). The proteins were isolated from colons of C57Bl/6 mice. A 100-μg sample of protein lysate was separated by SDS-PAGE and then transferred to polyvinylidene difluoride membranes (Bio-Rad, Richmond, CA). Membranes were incubated at 4°C overnight with the rabbit primary antibody for A3 receptors followed by incubation with HRP-conjugated goat anti-rabbit IgG for 2 h at room temperature. The immunogenic band for A3 receptors was revealed with enhanced chemiluminescence Western blot analysis detection reagent (Amersham Biosciences, Pittsburgh, PA). Immunoabsorption with immunogenic peptide was used to confirm specificity of antisera for A3 receptors.
Statistics.
Mean values ± SE were reported. Statistical significance was calculated by paired or unpaired Student's t-test, depending on the experimental design. ANOVA was used to evaluate significant differences between data points on concentration-response curves. For multiple comparisons, ANOVA followed by post hoc (Dunnett's or Newman-Keuls) tests was used. EC50 and IC50 values are extrapolated from sigmoid cumulative concentration-response curves fitted by a nonlinear curve fitting program (GraphPad Prism 3 applied to Figs. 5–8). Statistical analysis was done by using Stat-View 54.51 (Abacus Concepts, Calabasas, CA). Data was analyzed from a minimum of four to six animals for most experiments unless otherwise indicated. Differences are considered statistically significant at P < 0.05.
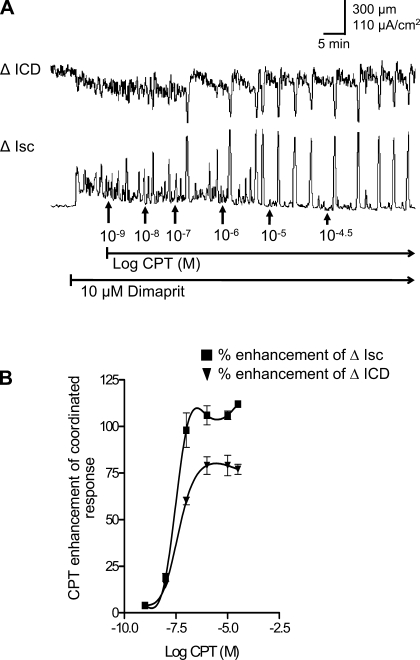
Fig. 5.
Effects of the A1 antagonist CPT on coordinated responses to dimaprit in whole-thickness preparations of guinea pig distal colon. A: CPT caused a concentration-dependent augmentation of the peak ICD and Isc responses. B: concentration-response curves for CPT on coordinated responses (n = 6).
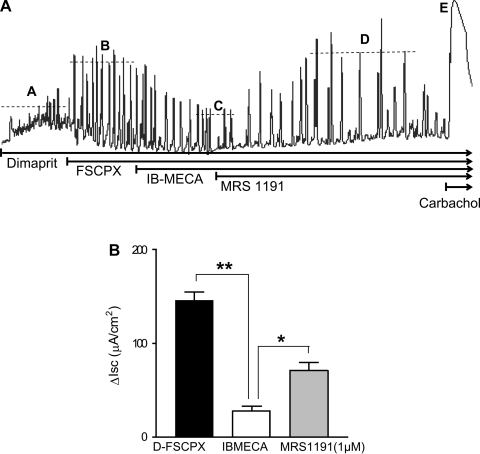
Fig. 8.
Effects of adenosine A3 receptor-selective compounds on Isc responses to dimaprit in guinea pig distal colon. A: representative tracing of a response to dimaprit that is enhanced by the irreversible A1 antagonist FSCPX. In the presence of FSCPX to eliminate A1 effects, the A3 agonist IB-MECA (2 μM) can suppress the recurrent Isc responses. The A3 antagonist 3-ethyl-5-benzyl-2-methyl-4-phenylethynyl-6-phenyl-1,4-(±)-dihydropyridine-3,5-dicarboxylate (MRS1191; 5 μM) can reverse the inhibitory effect. B: IB-MECA caused a significant reduction in Isc in the presence of A1 knockdown, and MRS1191 significantly reversed the effect (**P < 0.01; *P < 0.05, n = 5). Dashed lines A–E represent average peak responses with each intervention.
RESULTS
Sonomicrometry in combination with chloride secretion.
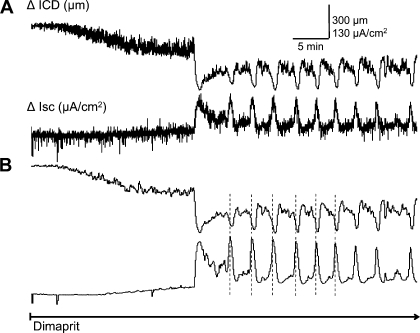
The histamine H2 receptor agonist dimaprit was used to elicit a stereotypic cyclical increase in Isc in coordination with motility. Figure 1 illustrates the experimental setup for simultaneous monitoring of muscle contraction and relaxation by sonomicrometry (as a change in intercrystal distance, ΔICD) and Cl− secretion in Ussing Flux chambers (as a change in short-circuit current, ΔIsc). A typical response to dimaprit is shown in Fig. 2. The coordinated response is triggered by dimaprit (10–100 μM) within 5–25 min and lasts up to 3–4 h (18, 20, 22); the stereotypic cyclical response does not occur in the absence of dimaprit in 10 preparations recorded for 3–4 h without any drugs. In more than 80% of preparations, the response is initiated in 5–10 min with dimaprit. Signal processing of the recorded responses is done by a smoothing software application to remove transient noise as shown in Fig. 2B. These curves are used for quantitative analysis.
Fig. 2.
Signal processing sequence of a coordinated response to H2 receptor activation by dimaprit in whole-thickness preparations of guinea pig colon. Simultaneous recording of circular muscle contraction (ΔICD) and ion transport (Isc) reveals a coordinated motility and secretory response to dimaprit. A: raw data trace of coordinated changes in ICD and Isc in response to dimaprit. B: smoothing is applied to all traces to remove transient noise.
Dimaprit (10 μM) caused cyclical increases in Isc of 170 ± 23 μA/cm2 at frequencies of 1.2 cycles/5 min. Cyclical changes in Isc were coordinated with phasic large-amplitude CM contractions (LACs) measuring an ICD of 260 ± 22.5 μm (n = 6); this is equivalent to 66% of the peak carbachol response of 335 ± 56 μm (n = 4). We could confirm that blockade on nerve conduction by 0.5 μM TTX abolishes coordinated Isc responses and circular muscle contractions (see Fig. 11) as shown previously in strain-gauge recordings (19); severing myenteric-submucous plexus connections abolished contractions but not Isc responses confirming that the effect of dimaprit is triggered in the submucous plexus (data not shown). Initiation of the coordinated responses is time locked but peak responses are out of phase; there is a time lag between Isc and ICD peaks = 15 ± 1 s (note: time lag is not apparent in the slow time-scale of tracings in figures).
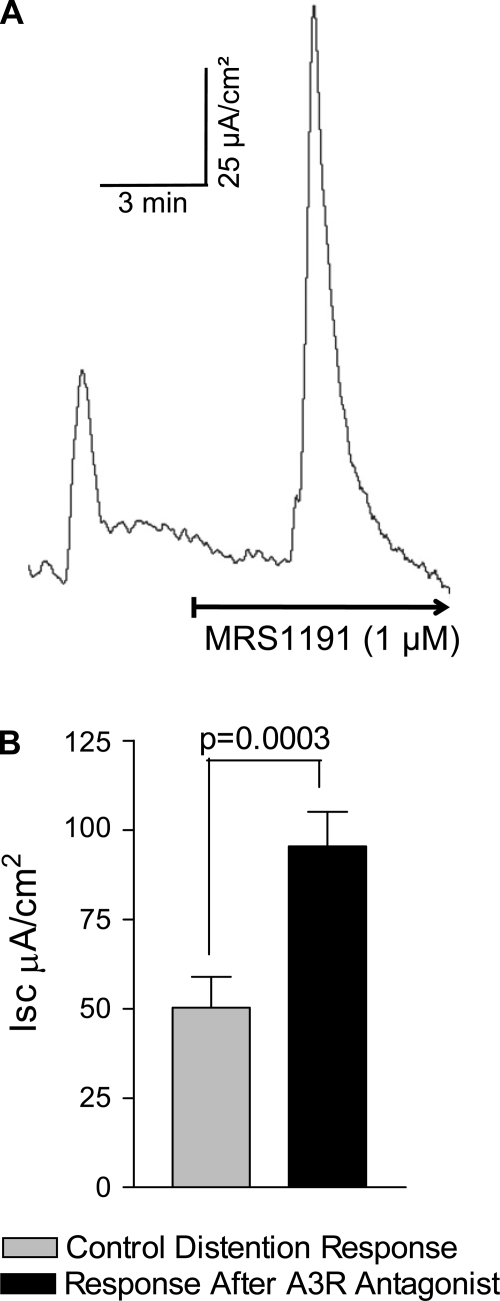
Fig. 11.
The A3 receptor antagonist MRS1191 augments Isc/chloride secretion evoked by distention. Distention was evoked by removal of 10 μl of fluid for 10 s from the serosal compartment. A: effect of MRS1191 on the distention reflex response in a mucosa-submucosa preparation. B: a significant increase in distention-evoked Isc response occurs after MRS1191 (n = 4 tissues from 4 animals).
ADO A1 receptor effects on muscle length in combination with chloride secretion.
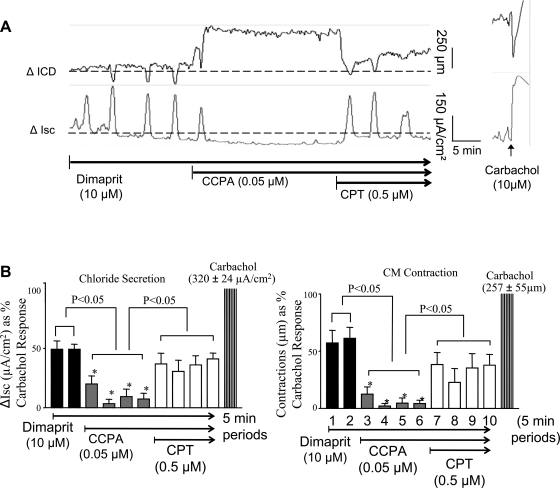
The A1 receptor agonist CCPA reduced or abolished cyclical Isc and LACs (monitored by sonomicrometry). An example of the inhibitory effect of CCPA on coordinated responses is shown in Fig. 3A. CCPA could abolish coordinated responses and cause further muscle relaxation and reduce baseline Isc. The ADOA1R antagonist CPT (0.5–2.5 μM) could reverse the effects of CCPA on Isc and ICD. The pooled data are summarized in Fig. 3B.
Fig. 3.
Pharmacological actions of an adenosine A1 agonist 2-chloro-N6-cyclopentyladenosine (CCPA) and an A1 antagonist 8-cyclopentyltheophylline (CPT) on dimaprit-evoked coordinated responses. A: CCPA abolishes the coordinated responses and further influences baseline secretion (Isc) and resting tone (ICD baseline). Further application of CPT can reverse the inhibitory effect of CCPA. B: pooled data indicating that chloride secretion and circular muscle direction (CM) contraction are nearly abolished by CCPA and significantly reversed by the A1 antagonist CPT; each bar is a value calculated at 5-min intervals (n = 6).
ADO A1 receptor effects on muscle tension in combination with chloride secretion.
In some experiments, we also investigated the role of ADOA1Rs on coordination of motility and secretion comparing muscle responses with sonomicrometry or strain-gauge recordings. A1 receptors were suitable targets because adenosine was shown to inhibit gut neuroeffector transmission to muscle and epithelia (15, 18, 22). In strain-gauge coordination studies, dimaprit caused LACs of 1.4 ± 0.4 g tension in association with large increases in Isc of 116 ± 51 μA/cm2 (n = 5).
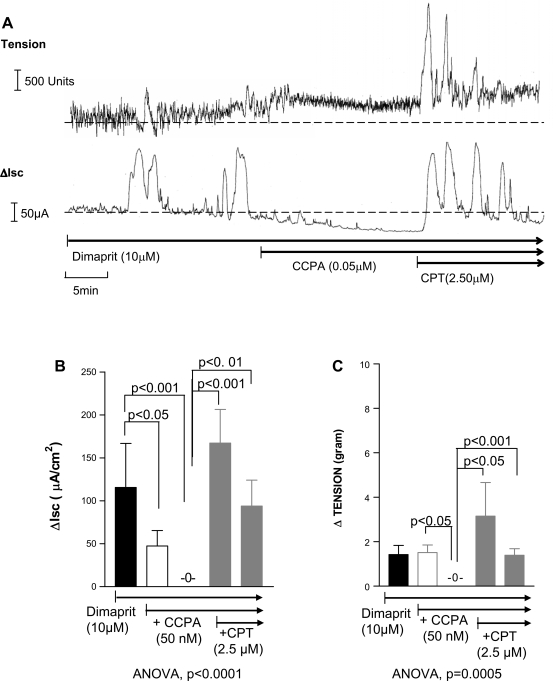
The A1 receptor agonist CCPA (50 nM) could reduce or abolish cyclical Isc and LACs or tension responses. CCPA caused further relaxation of the muscle and reduced baseline Isc responses. Coordination of Isc and LACs was lost after CCPA. The ADOA1R antagonist CPT (0.5–2.5 μM) reversed the effects of the A1 agonist on Isc and tension (Fig. 4) and further enhanced coordinated responses.
Fig. 4.
Simultaneous recording of muscle contraction (tension) and Isc in whole-thickness preparations of guinea pig distal colon. A: the dimaprit-evoked response is abolished by the A1 agonist CCPA and reversed and enhanced by the A1 antagonist CPT. B and C: pooled data showing that CCPA can abolish Isc and tension responses and CPT can significantly reverse inhibition; n = 4. Each bar represents consecutive peak responses at 5- and 10-min incubations of A1 agonist CCPA alone and then 5 and 10 min of CCPA + A1 antagonist CPT.
ADO A1 receptor antagonists augment coordinated responses.
The selective A1 receptor antagonist CPT alone caused a concentration dependent augmentation of coordinated responses (Fig. 5). The apparent ED50 concentration of changes in ICD (reductions indicative of contraction) is 60 nM CPT. The apparent ED50 concentration for facilitation of secretion is 30 nM CPT. At 0.1–1 μM CPT, maximum effect is observed on coordinated responses (Fig. 5B); Isc was enhanced by >100% (P = 0.0001, n = 6) and ICD by 75% (P = 0.0006, n = 6). In two additional experiments, PACPX alone could also augment the coordinated responses in a similar manner by >100% (also see Fig. 11).
Effects of the adenosine A3 selective agonist IB-MECA.
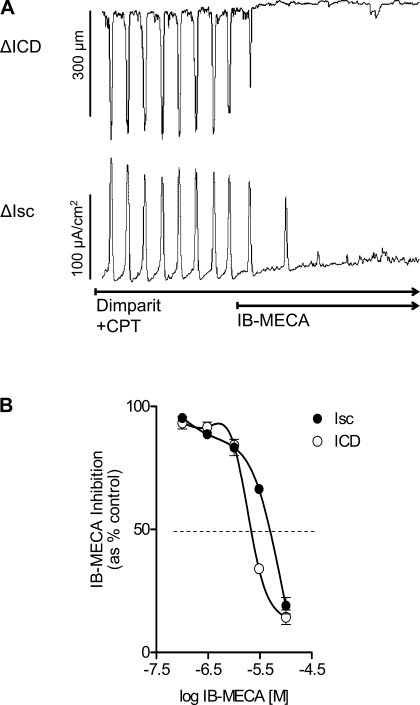
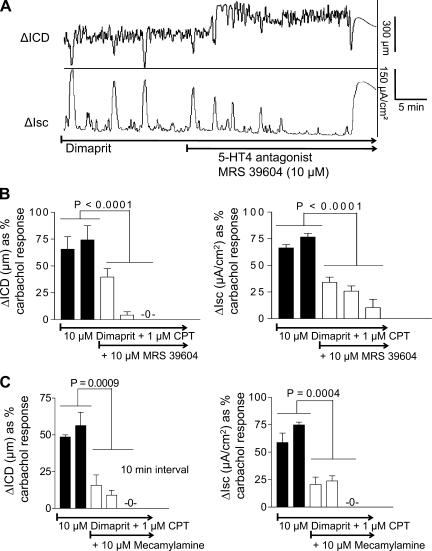
On the basis of experiments with the A1 antagonist CPT, 0.5–2.5 μM CPT should be sufficient to protect and block effects at the high-affinity A1 receptors. As shown in Fig. 6, in the presence of pharmacological blockade of A1 receptors with CPT, the selective A3 agonist IB-MECA (10 μM) is able to abolish the coordinated responses to dimaprit in the colon (Fig. 6). IB-MECA abolished coordinated responses in the continued presence of CPT blockade with an IC50 for Isc = 2.1 μM and IC50 for contraction (ICD) = 7.1 μM (n = 6 each). In the presence of the high-affinity lipid-soluble antagonist PACPX, 5 μM IB-MECA could abolish the Isc response that was reversed by the adenosine A3 receptor antagonist MRS1191 (5 μM-10 μM, P < 0.001, n = 5).
Fig. 6.
Effect of the adenosine A3 selective agonist IB-MECA on dimaprit-evoked coordinated responses in whole-thickness preparations of guinea pig distal colon. A: N6-(3-iodobenzyl)-adenosine-5′-N-methyluronamide (IB-MECA; 10 μM) could abolish the response to dimaprit in the presence of 1 μM CPT. B: concentration-dependent inhibition of coordinated responses by IB-MECA (n = 6, ANOVA, P < 0.001 for each curve for Isc or ΔICD).
Knockdown with FSCPX and IB-MECA responses.
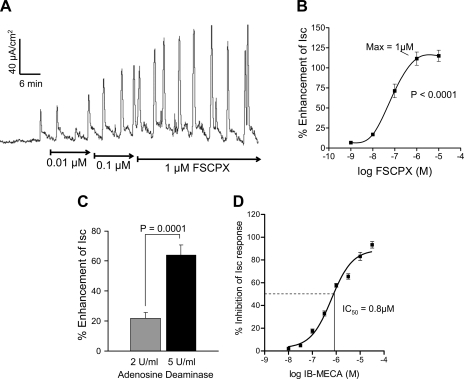
The irreversible A1 receptor antagonist was used to provide pharmacological “knockdown” of the high-affinity ADOA1R to further study putative A3 low-affinity inhibitory receptors. As shown in Fig. 7, FSCPX alone caused a concentration-dependent augmentation of the Isc secretory response in the colon. The ED50 concentration is ∼80 nM for FSCPX and maximum response is observed at 1 μM FSCPX (Fig. 7B). In addition, the enzyme adenosine deaminase that breaks down eADO also augmented the Isc response in a concentration-dependent manner at 1 and 5 U/ml (Fig. 7C). In the continuous presence of 1 μM FSCPX to “knock down the A1 receptor” by irreversibly binding to the A1 receptor, the selective A3 agonist IB-MECA is still able to inhibit or abolish the secretory response to dimaprit (Fig. 7D). The IC50 concentration of IB-MECA for inhibition of the Isc responses is 0.8 μM.
Fig. 7.
Effect of the irreversible adenosine A1 receptor antagonist 8-cyclopentyl-N3-[3-(4-(fluorosulfonyl)benzoyloxy)propyl]-xanthine (FSCPX) or adenosine deaminase on Isc/chloride secretion in the guinea pig distal colon. A: FSCPX caused a concentration-dependent augmentation of the Isc response. B: concentration-response curve to FSCPX for enhancement of the Isc response (n = 8, ANOVA, P < 0.001). The maximum effect of FSCPX was observed at a 1 μM concentration. C: adenosine deaminase augmented the Isc response at 1 or 5 U/ml concentration. D: concentration-effect curve for the A3 agonist IB-MECA for inhibition of Isc/chloride secretion in guinea pig distal colon. The IC50 concentration of IB-MECA is 0.8 μM. FSCPX (1 μM) knockdown was used to eliminate possible interactions of IB-MECA with inhibitory A1 receptors; n = 8 (ANOVA, P < 0.0001).
After FSCPX knockdown, the selective A3 receptor antagonist MRS1191 can reverse the inhibitory effect of IB-MECA (Fig. 8A). Pooled data for the inhibitory effect of IB-MECA and pharmacological antagonism with MRS1191 are shown in Fig. 8B.
Enhancing effects of the A3 receptor antagonist MRS1191 alone.
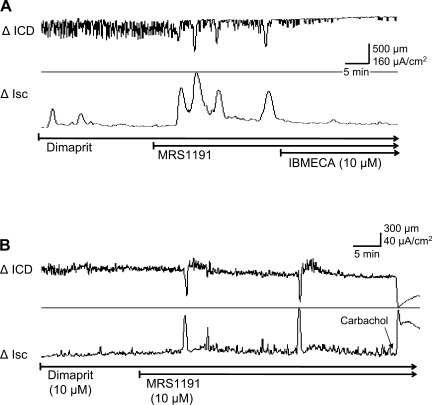
Application of the selective A3 receptor antagonist MRS1191 (1–10 μM) alone can augment the coordinated cyclical responses to dimaprit on secretion and motility. Two separate examples are illustrated in Fig. 9. MRS1191 can unmask a cyclical pattern of motility and secretion in some preparations or cause a remarkable augmentation of the peak responses. MRS1191 was shown to enhance or unmask (Fig. 9, A and B) coordinated responses; enhancement of the peak responses varied from 100 to 400%. Application of the A3 receptor agonist IB-MECA could overcome the effect of 1 μM MRS1191 and abolish coordinated responses and baseline fluctuations in muscle length (n = 10 preparations from 6 animals).
Fig. 9.
Effect of the adenosine A3 receptor antagonist MRS1191 alone on the dimaprit-evoked coordination responses in whole-thickness guinea pig colon. A: MRS1191 caused a remarkable augmentation in recurrent elevations in Isc/chloride secretory responses and unmasked a recurrent circular muscle contractile response (ΔICD). The A3 agonist IB-MECA could overcome the effect of MRS1191. B: MRS1191 unmasks coordination responses to dimaprit in another preparation.
Direct effects of the A3 receptor agonist IB-MECA on muscle.
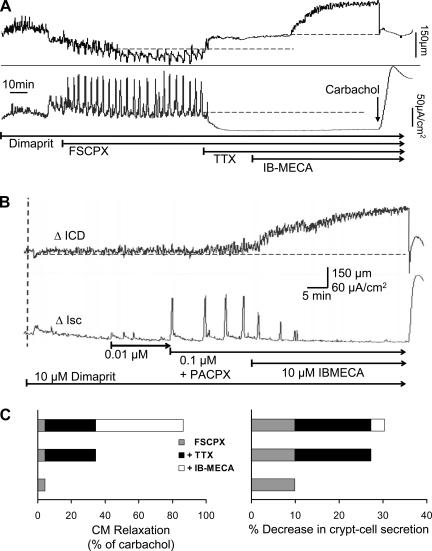
In the presence of FSCPX the coordinated response to dimaprit is enhanced, although in some cases Isc recurrent responses are more sensitive than ICD responses (Fig. 10A). In some cases, A1 knockdown as shown in Fig. 10B with PACPX can selectively unmask or augment the recurrent Isc response without any apparent effect on contractions or muscle tone (n = 3). FSCPX did not cause a significant ΔICD of the resting muscle tone; the ΔICD was a 4.4 ± 2.4% increase over baseline (normalized to % of carbachol response) that represents a change or relaxation of 18 ± 14.7 μm (P > 0.05, n = 6). Baseline Isc-crypt cell secretion was significantly decreased by 9.9% ± 0.6% (n = 7, P < 0.05), which represents a reduction of 17.3 ± 5.9 μA/cm2. Further application of TTX (1 μM) in the presence of FSCPX blocked coordinated responses to dimaprit and caused an increase in ICD, reflecting a reduction in muscle tone equivalent to 29.9 ± 12.9% of the carbachol response (n = 3). TTX also caused a reduction in Isc equivalent to 17.4 ± 3.9% of the carbachol response (P < 0.05, n = 3). In the continued presence of FSCPX and TTX, the A3 agonist IB-MECA caused significant relaxation of the circular muscle represented by a 52 ± 12.9% increase in ICD (P < 0.05, n = 3, Fig. 10A); ΔICD = 197 ± 67 μm. IB-MECA had no effect on Isc-crypt cell secretion after treatment with FSCPX and TTX; ΔIsc = 3 ± 1.5% of carbachol response (P > 0.05, n = 3). In some cases, where 0.1 μM TTX was used to block neural coordinated responses to dimaprit and after FSCPX knockdown, emergent or unmasked myogenic activity (not shown, ΔICD = 254.4 μm ± 24, n = 3) could also be reduced by 48.4% with IB-MECA (n = 3, P < 0.0001, i.e., relaxation).
Fig. 10.
Direct effects of IB-MECA on coordination responses to dimaprit in whole-thickness preparations of guinea pig distal colon. A: FSCPX unmasks a robust recurrent Isc response in coordination with CM contractions. Application of tetrodotoxin (TTX; 0.5 μM) to eliminate any neural contribution could abolish coordinated responses and cause further relaxation of the muscle and basal Isc/secretion. IB-MECA caused a further reduction in muscle tone but no effect on Isc in TTX-poisoned preparations. B: the selective lipid-soluble A1 receptor antagonist 1,3-dipropyl-8-(2-amino-4-chlorophenyl)xanthine (PACPX; 0.1 μM) unmasks a robust recurrent Isc response to dimaprit but did not cause a similar effect on CM contractions. IB-MECA could abolish the effect on Isc and cause further muscle relaxation. C: in nerve-poisoned preparations with FSCPX/A1 knockdown, IB-MECA was able to cause direct CM relaxation (P < 0.05) and had no effect on crypt cell secretion (see results for details).
Distension evoked Isc responses.
MRS1191 (1 μM) enhanced the TTX-sensitive distension-evoked Isc response by 70–150%. The distension response increased from 50 ± 8.6 to 95 ± 9.6 μA/cm2 (P = .003, n = 4) (Fig. 11).
Expression of A3 receptors.
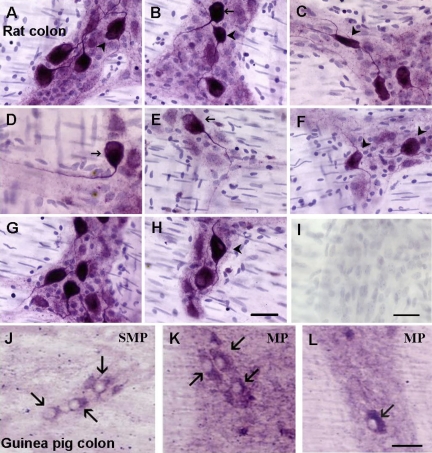
A3 receptor-ir occurs in myenteric and submucous neurons of guinea pig, mouse, and rat colon (n = 4 each species). Neurons with A3 receptor-ir are shown in Fig. 12 for rat and guinea pig; mouse evaluation was performed but was not included in Fig. 12. Neurons with Dogiel type I and Dogiel type II morphology are visualized with the anti-rat A3 receptor antiserum in rat colon. Omitting primary antibody or preabsorption of the anti-A3 receptor antiserum with the antigenic peptide eliminated positive A3 receptor-ir on enteric neurons and staining in the ganglia.
Fig. 12.
A3 immunoreactivity in enteric neurons of the rodent distal colon. Visualization of A3-immunoreactive neurons in rat distal colon myenteric plexus (A–H). Neurons with Dogiel type II morphology and multiple long processes as well as uniaxonal neurons express A3-immunoreactivity. I: negative control. Arrowheads, Dogiel type II neurons; arrows, Dogiel type I neurons. J–L: A3-immunoreactivity is expressed in guinea pig myenteric and submucous neurons. Arrows, positive neurons; SMP, submucosa; MP, myenteric plexus; calibration bar, 40 μm (see methods for details).
Rat colon was selected for further quantification of A3 receptor-ir expression because the antiserum was raised against a rat antigen and works best in rat tissue. In the rat colon, the distribution profile of ADOA3R-ir revealed a region-selective expression of the receptor. A3 receptors are expressed in the highest numbers in the proximal descending and sigmoid/rectal area of the colon (Fig. 13). Western blot analysis identified an immunogenic band between 60 and 70 kDa for the A3 receptor in rodent colon; a 66-kDa band is reported for the A3 receptor (Fig. 14). Both the antibody from Santa Cruz and Alpha Diagnostics revealed an A3 immunogenic band, but the Alpha Diagnostic antiserum also detects other nonspecific bands outside the range of 50–75 kDa.
Fig. 13.
Distribution of A3-immunoreactive neurons in the myenteric plexus of the rat colon. A3-immunoreactive neuronal cell bodies were quantitated in 1-cm lengths of longitudinal muscle myenteric plexus preparations from ascending colon to distal rectum for the whole length of the colon. Ascending and transverse colon have the lowest expression of A3-immunoreactive neurons. Descending colon and rectum have a 3- to 4-fold greater expression of A3-immunoreactive neurons than proximal colon. Similar results were obtained in 1 other animal (not shown).
Effects of nonadenosinergic receptor antagonists on coordinated responses.
Table 1 is a descriptive summary of actions of agonists, antagonists, and inhibitors of the coordinated responses to dimaprit. The H2 receptor antagonist cimetidine could abolish the dimaprit-evoked coordinated responses. In contrast, the Na+Cl− cotransport inhibitor bumetanide abolished the Isc response but had no effect on cyclic contractions and ICD responses in the muscle. The 5HT4 antagonist 1-[4-amino-5-chloro-2-(3,5-dimethoxyphenyl)methyloxy]-3-[1-[2-methylsulfonylamino]ethyl] piperidin-4-yl]propan-1-one hydrochloride (RS 39604) could abolish the coordinated responses elicited by dimaprit (Fig. 15, A and B). Blockade of nicotinic receptors by mecamylamine could abolish responses (Fig. 15C). The neurokinin-1 antagonist GR 82334 (1 μM) could also block or abolish the coordinated responses to dimaprit (in n = 3 preparations, ΔICD = 60–95% inhibition of dimaprit responses).
Table 1.
Descriptive summary of pharmacological actions and relative effects of receptor agonists or antagonists (of A1, A3, nicotinic, 5HT4, NK1, H2 receptors) or inhibitors on the dimaprit-induced stereotype cyclical coordination response in guinea pig distal colon
| Effect |
|||
|---|---|---|---|
| Drug | Receptor | Cyclic Crypt Cell Secretion (via ΔIsc) | Cyclic Contraction (via ΔICD) |
| Antagonist | |||
| CPT, 0.5 μM | A1 | 133% ↑ | 64% ↑ |
| PACPX, 0.1 μM | A1 | 160% ↑ | |
| FSCPX, 1.0 μM | A1 | 120% ↑ | |
| MRS1191, 1–10 μM | A3 | 130% ↑ | 110% ↑ |
| RS 39604, 10 μM | 5HT4 | 100% ↓ | 100% ↓ |
| Cimetidine, 10 μM | H2 | 100% ↓ | 100% ↓ |
| Mecamylamine, 10 μM | Nic-R | 100% ↓ | 100% ↓ |
| GR 82334, 1 μM | NK1-R | 60–95% ↓ | 100% ↓ |
| Inhibitors | |||
| Bumetenide | Na+Cl− cotransport | 100% ↓ | 0% ↓ |
| Basal secretion | Circular muscle tone | ||
| TTX, 0.1–0.25 μM | Na+ channels | 10% ↓ | 33% ↓ (relaxation) |
| TTX + IB-MECA | No effect | 52% ↓ (relaxation) | |
| Agonists | |||
| CCPA, 0.05 μM | A1 | 100% ↓ | 100% ↓ |
| IB-MECA, 1–10 μM (+FSCPX knockdown) | A3 | 100% ↓ | 100% ↓ |
ΔIsc, change in short-circuit current; ΔICD, change in intracrystal distance; CPT; 8-cyclopentyltheophylline; PACPX; 1,3-dipropyl-8-(2-amino-4-chlorophenyl)xanthine; FSCPX; 8-cyclopentyl-N3-[3-(4-(fluorosulfonyl)benzoyloxy)propyl]-N1-propylxanthine; MRS1191, 3-ethyl-5-benzyl-2-methyl-4-phenylethynyl-6-phenyl-1,4-(±)-dihydropyridine-3,5-dicarboxylate; RS 39604, 1-[4-amino-5-chloro-2-(3,5-dimethoxyphenyl)methyloxy]-3-[1-[2-methylsulfonylamino]ethyl]piperidin-4-yl]propan-1-one hydrochloride; Nic-R, nicotinic receptor; NK1, neurokinin-1; TTX, tetrodotoxin; IB-MECA, N6-(3-iodobenzyl)-adenosine-5′-N-methyluronamide; CCPA, 2-chloro-N6-cyclopentyladenosine.
Fig. 15.
Effects of cholinergic and 5-HT antagonists on dimaprit evoked coordination responses in whole-thickness guinea pig colon. A: representative tracing showing that the 5HT4 antagonist 1-[4-amino-5-chloro-2-(3,5-dimethoxyphenyl)methyloxy]-3-[1-[2-methylsulfonylamino] ethyl]piperidin-4-yl]propan-1-one hydrochloride (RS 39604) could abolish the recurrent responses to CM contraction (ΔICD) and secretion (ΔIsc); the experiment was performed in the presence of the A1 receptor antagonist CPT to enhance and optimize control responses. B: averaged data for RS 39604 showing that over time it reduced and eventually abolished recurrent coordinated responses to dimaprit; bars are at 5-min intervals (n = 6). D: the nicotinic receptor antagonist mecamylamine (10 μM) could also abolish the coordinated responses to dimaprit.
DISCUSSION
Overall, the functional role of adenosine A3 receptors in physiological or pathophysiological circumstances was largely unknown in the gastrointestinal tract. We provide evidence in support of the hypothesis that activation of A3 receptors leads to suppression of gut-motor reflexes and immune-neural communication in the guinea pig colon. To test this hypothesis we adopted the dimaprit-H2 receptor model of neurogenic secretory diarrhea previously established in our laboratories. The H2 receptor agonist dimaprit elicits a stereotypic cyclical motor response characterized by coordinated secretion and contraction in the guinea pig distal colon. Motor responses are triggered by activation of H2 receptors that are sensitive to blockade by cimetidine (19, 22, 23); once triggered they continue for hours without desensitization, and this is consistent with the underlying recurrent excitation of enteric neurons that occurs with continuous exposure to histamine. Wood and colleagues (21, 32–35) hypothesized that release of histamine from mast cells during gut inflammation and its actions in the ENS contribute to the observed pathobiology. Therefore, inhibitory effects of eADO observed in our study may be relevant in such pathological situations.
Histamine H2 receptor-induced cyclical secretion involves cholinergic and VIP-ergic pathways in the submucous plexus (23). An H2 receptor-activated chloride conductance is involved in the excitability of myenteric neurons (60), and these receptors could contribute to the short interplexus reflex but do not trigger it; since severing the interplexus connections prevents the coordinated response and results exclusively in a neurosecretomotor response, it is evident that the response is triggered at submucous neurons and then spreads to the myenteric plexus via the short interplexus neural circuit.
We developed a new approach to study coordinated responses in whole-thickness colon preparations by combining two techniques to simultaneously record muscle contractions by in vitro sonomicrometry and chloride secretion by Isc in Ussing flux chambers. This made it possible to carry out detailed pharmacological analysis of A3 receptors that is most difficult with strain-gauge recordings. Data from sonomicrometry (ΔICD) or the more established technique strain-gauge recordings (grams of tension) showed that eADO can activate A1 receptors to suppress coordination responses. In previous studies, sonomicrometry was used to track transit and motility throughout the gastrointestinal tract (1).
The reflex coordinated response involves a short interplexus reflex contained within the 0.7-cm2 whole-thickness tissue used in our experiments. Afferent Dogiel type II neurons with AH cell behavior send one process to myenteric plexus (36), and these are likely to be involved in coordination responses to histamine. Sensitivity of coordinated dimaprit responses to the nonpurinergic antagonists mecamylamine, RS 39604, and GR 82334 indicated that 5-HT, acetylcholine, and substance P (or a tachykinin) are excitatory mediators in the short interplexus reflex.
Endogenous ADO provides ongoing inhibitory effects in human EC cells (17) and the ENS in rodents (12, 13, 15, 16, 18, 22) or humans (65) or hypoxic conditions (29) by activating high-affinity A1 and putative low-affinity A3 receptors. Ongoing activation of A1 receptors by eADO is able to attenuate neuronal excitability and excitatory synaptic transmission in the ENS (16) and inhibit mucosal reflex chloride secretion (22) and H2 receptor-evoked excitation in myenteric AH neurons (60). Our data suggests that eADO modulates the coordinated response triggered by H2 receptor activation of submucous neurons via A1 receptors in both nerve plexuses (15, 22, 60). Our data also suggest that eADO is high enough to activate low-affinity A3 receptors in this model of neurogenic diarrhea. Low micromolar concentrations of eADO (1–2 μM) are needed to activate A3 receptors, and previous studies in our laboratory indicated that baseline extracellular concentrations were ∼200 nM eADO, but when ADO deaminase and uptake of eADO were blocked by inhibitors, then the extracellular concentration was elevated to 2.2 μM eADO, sufficient to activate A3 sites. The interstitial levels of eADO are likely to be much higher than perfusion levels of eADO that we calculated (17). Endogenous ADO release is sufficient to activate A3 receptors and inhibit mechanically evoked Ca2+ responses or 5-HT release from EC cells (17). Miniature eADO biosensors could measure even higher 5–10 μM eADO in neuronal tissue in the brain (27), but they too underestimate eADO (27). The eADO achieved at the receptor near sites of release or in response to dimaprit activation is therefore likely to be in the micromolar range. In our study, adenosine deaminase augments secretomotor responses by inactivating eADO release and attenuation of its inhibitory effects on enteric neurons. Mast cells, neurons, neutrophils, and endothelial cells can release even higher levels of eADO at sites of inflammation, infection, and metabolic stress. In unstressed tissues, eADP concentrations are ≤1 μM whereas in inflamed or ischemic tissues they increase to as high as 10–100 μM (3, 40, 43, 48, 57, 58); eADP is a precursor of extracellular eADO in tissues.
Activation of A3 receptors in rodents may cause mast cell degranulation and release of histamine (and other mediators) (39, 51, 56). IB-MECA alone does not evoke any excitatory agonist responses or mimic the cyclic neuromotor behavior triggered by dimaprit that would be expected to occur if IB-MECA caused significant mast cell degranulation and histaminergic responses. This argues against the possibility that adenosine can activate mucosal tissue mast cells to release histamine and modulate coordinated secretion and contraction responses in normal gut. This is plausible since it has been shown that A3 receptors are localized to certain mast cells and not others (39). Therefore, in normal colon, the effect of ADOA3R activation is suppression of immune-histaminergic neuromodulation triggered by dimaprit in our model.
Selective A1 (CPDPX, FSCPX, CPT) and A3 antagonists (MRS1191) alone could augment or unmask a cyclic pattern of contraction and secretion in response to dimaprit H2 receptor activation. Antagonists were able to block or restore inhibitory effects of selective agonists indicating that they were acting at the receptors to prevent the effect of eADO at A1 or A3 receptors respectively. Antagonists alone had variable effects in different preparations suggestive of differential eADO release; although antagonists could still prevent agonist inhibitory effects in tissues they had little or no dimaprit enhancing effects (data not shown), further supporting an A1 and A3 receptor interaction.
The low-affinity A3 receptor provides inhibitory modulation of coordinated motor responses. MRS1191 is reported to be a high-affinity A3 antagonist at the human receptors with >1,300-fold selectivity over A1 or A2a receptors (47). In rodents, the Ki at A3 sites is in the low micromolar range at 1.4 μM compared with 39.8 μM for A1 sites. Therefore, it is a useful A3 antagonist across species. Bearing also in mind that affinity is not equivalent to efficacy and functional response in tissues, at the concentrations used (1–10 μM), MRS1191 is relatively selective for A3 over A1 or other receptors. In addition, after FSCPX knockdown of A1 receptors, MRS1191 alone is still able to augment or unmask coordinated responses or to reverse the inhibition produced by the A3 agonist IB-MECA. FSCPX has been shown to be an irreversible antagonist at high-affinity A1 receptors and has been used in knockdown studies to eliminate the influence of A1 receptors (59). The concentration of 1 μM FSCPX used is the same as reported to fully and irreversibly occupy and block A1 receptors. Furthermore, we determined the optimal concentration of FSCPX in our coordination studies by determining the apparent concentration-response curve to FSCPX, i.e., 1 μM FSCPX caused a maximum augmentation on gut responses, indicating that at this concentration, the interaction of eADO at A1 receptors is fully blocked, enabling us to study putative inhibitory effects via the low-affinity A3 receptors more reliably using A3 agonists or antagonists. We did not test whether our agonists or antagonists also interact with A2 receptors and relied on their selectivity for A1 or A3 receptors as described earlier; their involvement in the inhibitory effects of A3 compounds on dimaprit-induced secretory responses is unlikely since activation of A2 receptors is known to stimulate Cl− secretion (14, 18, 55) and elicit slow membrane depolarization and excitation in enteric neurons (4, 12, 15). Some contribution of A2b-effects with A3 agonists and antagonists cannot be entirely ruled out (10). Studies in A3AR−/− knockout mice are planned (9).
The actions of eADO at ADOA3Rs in the guinea pig colon are not restricted to the short interplexus reflex. MRS1191 augmented the distension-evoked Isc response, suggesting that eADO release can suppress distension reflexes via A3 receptors. The local distension reflex involves a different neural circuit than the interplexus circuit triggered by histamine. Both are abolished by TTX but differ in that nicotinic cholinergic transmission is not involved in the distension reflex (32).
Histamine acts at excitatory H1 and H2 as well as inhibitory H3 receptors on guinea pig enteric neurons (64). Dimaprit is a selective H2 receptor agonist with more than 1,000-fold separation of activity between H2 and H1 receptors. A potential caveat is that it has antagonistic effects at H3 receptors with moderate affinity but no stimulation of H3 receptors (62a). H3 receptors are presynaptic inhibitory receptors in the guinea pig enteric nervous system, but it is unlikely that any interaction at these receptors contributes to the net excitatory effect of dimaprit on coordinated responses because all effects of dimaprit (e.g., coordinated responses) could be blocked by the H2 receptor antagonist cimetidine. Dimaprit also interacts with H4 receptors but these are not present in the guinea pig ENS unlike the human (7, 21, 62). The specific cells and sites of action of eADO in suppressing the short interplexus reflex response at H2 or other receptors in rodents or humans remain unclear. There is abundant expression of A3-ir in both nerve plexuses in mouse, rat, and guinea pig colon. In rat, a unique profile of A3 receptor expression and distribution was identified with differential expression of A3-ir in different segments; both uniaxonal and multipolar neurons can be visualized by their A3-ir. These receptors likely play a role in the short interplexus reflex. Our earlier study identified A3-ir in human ENS including VIP-immunoreactive neurons that may represent secretomotor neurons (14, 18). A recent Ca imaging study of synaptic Ca transients indicated that eADO modulates synaptic transmission in the human submucosal plexus via putative A3 receptors (65), but its effects on histamine-evoked excitatory responses in the human ENS are unknown.
In TTX-poisoned preparations, it is clear that functional ADOA3R also exist on muscle that can mediate circular muscle relaxation and reduction in basal tone; this does not appear to be the case on crypt cells, since IB-MECA could not influence basal Isc responses in TTX-poisoned preparations. The effect of IB-MECA to cause muscle relaxation is likely via A3 receptors since it occurred after A1 knockdown with FSCPX or in tissues exposed to 100 nM PACPX, a high-affinity lipid-soluble ADOA1R selective antagonist. TTX unmasked a dramatic direct effect of IB-MECA on tone and circular muscle relaxation not observed in tissues not exposed to TTX; this is an interesting finding suggesting that activity in intact neural circuits prevents smooth muscle relaxation or reduction in tone in response to ADOA3R activation. One could speculate that such direct effects could become important in abnormal or disease states in which neural activity in the ENS is compromised such as in postoperative ileus or abnormal motility due to mesenteric thrombosis or gut infarct and ischemia, pseudoobstruction, gut spasm, or aganglionosis, in which inhibitory motor tone and general neural-motor behavior is altered.
In conclusion, endogenous adenosine is involved in immunomodulation of histamine responses in a short interplexus reflex that coordinates motility and secretion in the guinea pig colon. Ongoing release of eADO also activates low-affinity A3 receptors to suppress actions of histamine. It also acts on A3 receptors to suppress a distention-evoked neurosecretory reflex. The short interplexus reflex involves nicotinic cholinergic, serotonergic, and peptidergic transmission. It is proposed that the short interplexus reflex may be an important local gut reflex in the integrated circuits that may play an important role in immunomodulation of the ENS and motor behaviors. It will be of considerable interest and clinical relevance to ascertain whether eADO can also suppress short-reflex responses to other mediators released from mast cells such as cytokines, prostaglandins, proteases, etc. (11, 64). Mast cells are emerging as an important immune cell type in the pathogenesis of IBD, microscopic colitis, neurogenic secretory diarrhea, luminal responses to food allergens, and irritable bowel syndrome (19, 32–35, 52, 64). In inflamed tissues, cells other than mast cells can release or secrete even higher levels of eADO at sites of inflammation, infection, and metabolic stress (3, 40, 44, 48, 57, 58). Our study provides a basis for postulating a novel mechanism of gut neuroprotection via eADO against immune-neuromodulation and motor disturbances via dual activation of A1 and A3 receptors.
GRANTS
This research was supported by National Institutes of Health (NIH) Grants RO1 DK44179-14 to F. L. Christofi, NCRR 1S10RR11434 to F. L. Christofi, and DK57016 to H. J. Cooke.
DISCLOSURES
No conflicts of interest are declared by the author(s).
REFERENCES
- 1.Adelson DW, Million M. Tracking the moveable feast: sonomicrometry and gastrointestinal motility. News Physiol Sci 19: 27–32, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Antonioli L, Fornai M, Colucci R, Ghisu N, Tuccori M, Del Tacca M, Blandizzi C. Pharmacological modulation of adenosine system: novel options for treatment of inflammatory bowel diseases. Inflamm Bowel Dis 14: 566–574, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Baggott JE, Morgan SL, Ha TS, Alarcon GS, Koopman WJ, Krumdieck CL. Antifolates in rheumatoid arthritis: a hypothetical mechanism of action. Clin Exp Rheumatol 11: S101–S105, 1993 [PubMed] [Google Scholar]
- 4.Barajas-Lopez C, Surprenant A, North RA. Adenosine A1 and A2 receptors mediate presynaptic inhibition and postsynaptic excitation in guinea pig submucosal neurons. J Pharmacol Exp Ther 258: 490–495, 1991 [PubMed] [Google Scholar]
- 5.Bozarov A, Wang YZ, Javed N, Christofi FL, Cooke HJ. Comparison of adenosine A1 receptor activation on repetitive reflex Cl secretion coordinated with repetitive muscle activity measured by sonomicrometry and force transducer tension (Abstract). Gastroenterology 128, Suppl 2: A–608, 2005 [Google Scholar]
- 6.Bozarov A, Wang YZ, Yu JG, Cooke HJ, Christofi FL. Endogenous adenosine acts at A1 and A3 inhibitory receptors to modulate the stereotype motor behavior of the gut triggered by histamine or distension (Abstract). Gastroenterology 130, Suppl 2: A29, 2006 [Google Scholar]
- 7.Breunig E, Michel K, Zeller F, Seidl S, Weyhern CW, Schemann M. Histamine excites neurons in the human submucous plexus through activation of H1, H2, H3 and H4 receptors. J Physiol 583: 731–742, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cavalcante IC, Castro MV, Barreto AR, Sullivan GW, Vale M, Almeida PR, Linden J, Rieger JM, Cunha FQ, Guerrant RL, Ribeiro RA, Brito GA. Effect of novel A2A adenosine receptor agonist ATL 313 on Clostridium difficile toxin A-induced murine ileal enteritis. Infect Immun 74: 2606–2612, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cerniway RJ, Yang Z, Jacobson MA, Linden J, Mathern GP. Targeted deletion of A3 adenosine receptors improves tolerance to ischemia-reperfusion injury in mouse myocardium. Am J Physiol Heart Circ Physiol 281: H1751–H1758, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Chandrasekharan BP, Kolachala VL, Dalmasso G, Merlin D, Ravid K, Sitaraman SV, Srinivasan S. Adenosine 2B receptors (A2bAR) on enteric neurons regulate murine distal colonic motility. FASEB J 23: 2727–2734, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Z, Suntres Z, Palmer J, Guzman J, Javed A, Xue J, Yu JG, Cooke H, Awad H, Hassanain HH, Cardounel AJ, Christofi FL. Cyclic AMP signaling contributes to neural plasticity and hyperexcitability in AH sensory neurons following intestinal Trichinella spiralis-induced inflammation. Int J Parasitol 37: 743–761, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Christofi FL, Baidan LV, Fertel RH, Wood JD. Adenosine A2 receptor-mediated excitation of a subset of AH/Type 2 neurons and elevation of cAMP levels in myenteric ganglia of guinea pig ileum. Neurogastroenterol Motil 6: 67–78, 1994 [Google Scholar]
- 13.Christofi FL, Wood JD. Electrophysiological subtypes of inhibitory P1 purinoceptors on myenteric neurones of guinea pig small bowel. Br J Pharmacol 113: 703–710, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christofi FL, Zhang H, Yu JG, Guzman J, Xue J, Kim M, Wang YZ, Cooke HJ. Differential gene expression of adenosine A1, A2a, A2b, and A3 receptors in the human enteric nervous system. J Comp Neurol 439: 46–64, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Christofi FL. Unlocking mysteries of gut sensory transmission: is adenosine the key? News Physiol Sci 16: 201–207, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Christofi FL, Wood JD. Presynaptic inhibition by adenosine A1 receptors on guinea pig small intestinal myenteric neurons. Gastroenterology 104: 1420–1429, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Christofi FL, Kim M, Wunderlich JE, Xue J, Suntres Z, Cardounel AJ, Javed NH, Yu JG, Grants I, Cooke HJ. Endogenous adenosine differentially modulates 5-hydroxytryptamine from a human enterochromaffin cell model. Gastroenterology 127: 188–202, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Christofi FL. Purinergic receptors and gastrointestinal secretomotor function. Purinergic Signal 4: 213–236, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cooke HJ, Wang YZ, Rogers R. Coordination of Cl− secretion and contraction by a histamine H2-receptor agonist in guinea pig distal colon. Am J Physiol Gastrointest Liver Physiol 265: G973–G978, 1993 [DOI] [PubMed] [Google Scholar]
- 20.Cooke HJ. Neuroimmune signaling in regulation of intestinal ion transport. Am J Physiol Gastrointest Liver Physiol 266: G167–G178, 1994 [DOI] [PubMed] [Google Scholar]
- 21.Cooke HJ, Wang YZ. H3 receptors: modulation of histamine-stimulated neural pathways influencing electrogenic ion transport in the guinea pig colon. J Auton Nerv Syst 50: 201–207, 1994 [DOI] [PubMed] [Google Scholar]
- 22.Cooke HJ, Wang Y, Liu CY, Zhang H, Christofi FL. Activation of neuronal adenosine A1 receptors suppresses secretory reflexes in the guinea pig colon. Am J Physiol Gastrointest Liver Physiol 276: G451–G462, 1999 [DOI] [PubMed] [Google Scholar]
- 23.Cooke HJ, Wang YZ, Reddix R, Javed NH. Cholinergic and VIP-ergic pathways mediate histamine H2 receptor-induced cyclical secretion in the guinea pig colon. Am J Physiol Gastrointest Liver Physiol 268: G465–G470, 1995 [DOI] [PubMed] [Google Scholar]
- 24.Cooke HJ, Javed N, Christofi FL. Enteric neural reflexes and secretion. In: Autonomic Nervous System, Autonomic Neuroimmunology, edited by Goetzl EJ, Blennerhassett MG, Bienestock J. Reading, UK: Hardwood Academic, 2003, vol. 16, p. 35–59 [Google Scholar]
- 25.Cronstein BN, Naime D, Ostad E. The anti-inflammatory mechanism of methotrexate: increased adenosine release at inflamed sites diminished leukocyte accumulation in an in vivo model of inflammation. J Clin Invest 92: 2675–2682, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cronstein B, Naime D, Firestein G. The anti-inflammatory effects of an adenosine kinase inhibitor are mediated by adenosine. Arthritis Rheum 38: 1040–1045, 1995 [DOI] [PubMed] [Google Scholar]
- 27.Dale N, Gourine AV, Llaudet E, Bulmer D, Thomas T, Spyer KM. Rapid adenosine release in the nucleus tractus solitarii during defense response in rats: real-time measurement in vivo. J Physiol 544: 149–160, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Man JG, Seerden TC, De Winter BY, Van Marck EA, Herman AG, Pelckmans PA. Alteration of the purinergic modulation of enteric neurotransmission in the mouse ileum during chronic intestinal inflammation. Br J Pharmacol 139: 172–184, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deshpande NA, McDonald TJ, Cook MA. Endogenous interstitial adenosine in isolated myenteric neural networks varies inversely with prevailing Po2. Am J Physiol Gastrointest Liver Physiol 276: G875–G885, 1999 [DOI] [PubMed] [Google Scholar]
- 30.Dixon AK, Gubitz AK, Sirinathsinghji DJ, Richardson PJ, Freeman TC. Tissue distribution of adenosine receptor mRNAs in the rat. Br J Pharmacol 118: 1461–1468, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frieling T, Wood JD, Cooke HJ. Submucosal reflexes: distension-evoked ion transport in the guinea pig distal colon. Am J Physiol Gastrointest Liver Physiol 263: G91–G96, 1992 [DOI] [PubMed] [Google Scholar]
- 32.Frieling T. Different types of stimuli evoke spike discharge in submucous neurons of the large intestine driving cyclical chloride secretion. In: Advances in the Innervation of the Gastrointestinal Tract, edited by Holle GE, Wood JD. Amsterdam: Excerpta Medica, 1992, p. 425–431 [Google Scholar]
- 33.Frieling T, Cooke HJ, Wood JD. Histamine receptors on submucous neurons in guinea pig colon. Am J Physiol Gastrointest Liver Physiol 264: G74–G80, 1993 [DOI] [PubMed] [Google Scholar]
- 34.Frieling T, Cooke HJ, Wood JD. Neuroimmune communication in the submucous plexus of guinea pig colon after sensitization to milk antigen. Am J Physiol Gastrointest Liver Physiol 267: G1087–G1093, 1994 [DOI] [PubMed] [Google Scholar]
- 35.Frieling T, Palmer JM, Cooke HJ, Wood JD. Neuroimmune communication in the submucous plexus of guinea pig colon after infection with Trichinella spiralis. Gastroenterology 107: 1602–1609, 1994 [DOI] [PubMed] [Google Scholar]
- 36.Furness JB, Jones C, Nurgali K, Clerc N. Intrinsic primary afferent neurons and nerve circuits within the intestine. Prog Neurobiol 72: 143–164, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Guzman J, Yu JG, Suntres Z, Bozarov A, Cooke H, Javed N, Auer H, Palatini J, Hassanain HH, Cardounel AJ, Javed A, Grants I, Wunderlich JE, Christofi FL. ADOA3R as a therapeutic target in experimental colitis: proof by validated high-density oligonucleotide microarray analysis. Inflamm Bowel Dis 12: 766–789, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Hannon JP, Tigani B, Schuurman HJ, Fozard JR. Suppression of adenosine A3 receptor-mediated hypotension and mast cell degranulation in the rat by dexamethasone. J Pharmacol Exp Ther 302: 725–730, 2002 [DOI] [PubMed] [Google Scholar]
- 40.Hasko G, Cronstein BN. Adenosine: an endogenous regulator of innate immunity. Trends Immunol 25: 33–39, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Kolachala VL, Vijay-Kumar M, Dalmasso G, Yang D, Linden J, Wang L, Gewirtz A, Ravid K, Merlin D, Sitaraman SV. A2B adenosine receptor gene deletion attenuates murine colitis. Gastroenterology 135: 861–870, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lomax AE, Fernández E, Sharkey KA. Plasticity of the enteric nervous system during intestinal inflammation. Neurogastroenterol Motil 17: 4–15, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Mabley J, Soriano F, Pacher P, Hasko G, Marton A, Wallace R, Salzman A, Szabo C. The adenosine A3 receptor agonist, N6-(3-iodobenyl)-adenosine-5′-N-methyluronamide, is protective in two murine models of colitis. Eur J Pharmacol 466: 323–329, 2003 [DOI] [PubMed] [Google Scholar]
- 44.Martin C, Leone M, Viviand X, Ayem ML, Guieu R. High adenosine plasma concentration as a prognostic index for outcome in patients with septic shock. Crit Care Med 28: 3198–3202, 2000 [DOI] [PubMed] [Google Scholar]
- 45.Naganuma M, Wiznerowicz EB, Lappas CM, Linden J, Worthington MT, Ernst PB. Cutting edge: critical role for A2A adenosine receptors in the T-cell-mediated regulation of colitis. J Immunol 177: 2765–2769, 2006 [DOI] [PubMed] [Google Scholar]
- 46.Odashima M, Bamias G, Rivera-Nieves J, Linden J, Nast CC, Moskaluk CA, Marini M, Sugawara K, Kozaiwa K, Otaka M, Watanabe S, Cominelli F. Activation of A2A adenosine receptor attenuates intestinal inflammation in animal models of inflammatory bowel disease. Gastroenterology 129: 26–33, 2005 [DOI] [PubMed] [Google Scholar]
- 47.Pal S, Choi WJ, Choe SA, Heller CL, Gao ZG, Chinn M, Jacobson KA, Hou X, Lee SK, Kim HO, Jeong LS. Structure-activity relationships of truncated adenosine derivatives as highly potent and selective human A3 adenosine receptor antagonists. Bioorg Med Chem 17: 3733–3738, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev 50: 413–492, 1998 [PubMed] [Google Scholar]
- 49.Ramkumar V, Stiles GL, Beaven MA, Ali H. The A3 adenosine receptor is the unique adenosine receptor which facilitates release of allergic mediators in mast cells. J Biol Chem 268: 16887–16890, 1993 [PubMed] [Google Scholar]
- 50.Rybaczyk L, Rozmiarek A, Circle K, Grants I, Needleman BJ, Wunderlich JE, Huang K, Christofi FL. A new bioinformatics approach to analyze gene expressions and signaling pathways reveals unique purine gene dysregulation profiles that distinguish between CD and UC. Inflamm Bowel Dis 15: 971–984, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Salvatore CA, Tilley SL, Latour AM, Fletcher DS, Koller BH, Jacobson MA. Disruption of the A(3) adenosine receptor gene in mice and its effect on stimulated inflammatory cells. J Biol Chem 275: 4429–4434, 2000 [DOI] [PubMed] [Google Scholar]
- 52.Schemann M, Michel K, Ceregrzyn M, Zeller F, Seidl S, Bischoff SC. Human mast cell mediator cocktail excites neurons in human and guinea pig enteric nervous system. Neurogastroenterol Motil 17: 281–289, 2005 [DOI] [PubMed] [Google Scholar]
- 53.Sharkey KA, Kroese A. Consequences of intestinal inflammation on the enteric nervous system: neuronal activation induced by inflammatory mediators. Anat Rec 262: 79–90, 2001 [DOI] [PubMed] [Google Scholar]
- 54.Siegmund B, Rieder F, Albrich S, Wolf K, Bidlingmaier C, Firestein G, Boyle D, Lehr H, Loher F, Hatmann G, Entres S, Eigler A. Adenosine kinase inhibitor GP515 improves experimental colitis in mice. J Pharmacol Exp Ther 296: 99–105, 2001 [PubMed] [Google Scholar]
- 55.Sitaraman SV, Merlin D, Wang L, Wong M, Gewirtz AT, Si-Tahar M, Madara JL. Neutrophil-epithelial crosstalk at the intestinal luminal surface mediated by reciprocal secretion of adenosine and IL-6. J Clin Invest 107: 861–869, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smith SR, Denhardt G, Terminelli C. A role for histamine in cytokine modulation by the adenosine A(3) receptor agonist, 2-Cl−IB-MECA. Eur J Pharmacol 457: 57–69, 2002 [DOI] [PubMed] [Google Scholar]
- 57.Sottofattori E, Anzaldi M, Ottonello L. HPLC determination of adenosine in human synovial fluid. J Pharm Biomed Anal 24: 1143–1146, 2001 [DOI] [PubMed] [Google Scholar]
- 58.Sperlagh B, Doda M, Baranyi M, Hasko G. Ischemic-like condition releases norepinephrine and purines from different sources in superfused rat spleen strips. J Neuroimmunol 111: 45–54, 2000 [DOI] [PubMed] [Google Scholar]
- 59.Srinivas M, Shryock JC, Scammells PJ, Ruble J, Baker SP, Belardinelli L. A novel irreversible antagonist of the A1-adenosine receptor. Mol Pharmacol 50: 196–205, 1996 [PubMed] [Google Scholar]
- 60.Starodub AM, Wood JD. Histamine H2 receptor activated chloride conductance in myenteric neurons from guinea pig small intestine. J Neurophysiol 83: 1809–1816, 2000 [DOI] [PubMed] [Google Scholar]
- 61.Sundaram U, Hassanain H, Suntres Z, Yu JG, Cooke HJ, Guzman J, Christofi FL. Rabbit chronic ileitis leads to up-regulation of adenosine A1/A3 gene products, oxidative stress, and immune modulation. Biochem Pharmacol 65: 1529–1538, 2003 [DOI] [PubMed] [Google Scholar]
- 62.Takagaki K, Osawa S, Horio Y. Cytokine responses of intraepithelial lymphocytes are regulated by histamine H2 receptor. J Gastroenterol 44: 285–296, 2009 [DOI] [PubMed] [Google Scholar]
- 62a.Van der Goot H, Timmerman H. Selective ligands as tools to study histamine receptors. Eur J Med Chem 35: 5–20, 2000 [DOI] [PubMed] [Google Scholar]
- 63.Van Troostenburg AR, Clark EV, Carey WD, Warrington SJ, Kerns WD, Cohn I, Silverman MH, Bar-Yehuday S, Fong KL, Fishman P. Tolerability, pharmacokinetics and concentration-dependent hemodynamic effects of oral CF101, an A3 adenosine receptor agonist, in healthy young men. Int J Clin Pharmacol Ther 42: 434–442, 2004 [DOI] [PubMed] [Google Scholar]
- 64.Wood JD. Histamine, mast cells, and the enteric nervous system in the irritable bowel syndrome, enteritis and food allergies. Gut 55: 445–447, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wunderlich JE, Needleman BJ, Chen Z, Yu JG, Wang YZ, Grants I, Mikami D, Melvin WS, Cooke HJ, Christofi FL. Dual purinergic synaptic transmission in the human enteric nervous system. Am J Physiol Gastrointest Liver Physiol 294: G554–G566, 2008 [DOI] [PubMed] [Google Scholar]