Abstract
The cholestatic bile acid taurolithocholate (TLC) inhibits biliary secretion of organic anions and hepatic uptake of taurocholate (TC). TLC has been suggested to induce retrieval of Mrp2 from the canalicular membrane via the phosphoinositide-3-kinase (PI3K)/PKB-dependent activation of novel protein kinase Cε (nPKCε) in rat hepatocytes. The aim of the present study was to determine whether TLC-induced inhibition of TC uptake may also involve PI3K-dependent activation of PKCε in HuH7 cells stably transfected with human Na+-dependent TC-cotransporting polypeptide (NTCP) (HuH-NTCP cells). To avoid direct competition for uptake, cells were pretreated with TLC, washed, and then incubated with 3H-TC to determine TC uptake. TLC produced time- and dose-dependent inhibition of TC uptake. TLC inhibited TC uptake competitively without affecting NTCP membrane translocation. A PI3K inhibitor failed to reverse TLC-induced TC uptake inhibition and TLC-inhibited PKB phosphorylation. TLC did activate nPKCε as evidenced by increased membrane translocation and nPKCε-Ser729 phosphorylation. Overexpression of dominant negative-nPKCε reversed TLC-induced inhibition of PKB phosphorylation but not of TC uptake. Finally, cAMP prevented TLC-induced inhibition of TC uptake via the PI3K pathway, and the prevention is due to the sum of cAMP-induced stimulation and TLC-induced inhibition of TC uptake. Taken together, these results suggest that TLC-induced inhibition of PKB, but not of TC uptake, is mediated via nPKCε. Activation of nPKCε and inhibition of TC uptake by TLC are not mediated via the PI3K/PKB pathway.
Keywords: dominant negative-novel protein kinase Cε, inhibition kinetics, PKB/Akt, Na+-dependent taurocholate-cotransporting polypeptide, phosphoinositide-3-kinase
transhepatic transport of organic solutes plays an important role in hepatic bile formation, and cholestasis results when hepatic solute transport is inhibited (1). Taurolithocholate (TLC), a secondary bile salt, has been known to produce acute cholestasis by inhibiting biliary secretion of organic anions (18) and has been shown to inhibit taurocholate (TC) uptake in rat hepatocytes (29). The inhibition of biliary organic anion secretion by TLC involves retrieval of Bsep (9) and Mrp2 (2) from the canalicular membrane and may be exacerbated by the inhibition of bile acid uptake. However, the cellular mechanism by which TLC inhibits TC uptake is not known.
Hepatic uptake of TC is primarily mediated via Na+-dependent TC-cotransporting polypeptide (NTCP). Cyclic AMP has been shown to stimulate TC uptake by translocating Ntcp to the plasma membrane via phosphoinositide-3-kinase (PI3K) signaling pathway (36, 39). Because inhibition of TC uptake by TLC is noncompetitive in rat hepatocytes (29), it is likely that TLC inhibits TC uptake by retrieving NTCP from the sinusoidal membrane. Interestingly, a recent study showed that inhibition of TC uptake by bosentan, a dual antagonist of endothelin receptors A and B, is noncompetitive for rat Ntcp and competitive for human NTCP (19). Whether TLC also inhibits TC uptake by Ntcp and NTCP differently is not known. Such a difference would indicate a difference in the regulation of Ntcp/NTCP in rats/humans.
The retrieval of Mrp2 and inhibition of biliary excretion of Mrp2 substrate by TLC has been suggested to be mediated via the PI3K-dependent activation of the novel protein kinase Cε (nPKCε) in rat hepatocytes (3). Thus it is possible that a similar mechanism (i.e., PI3K/nPKCε pathway) may also be involved in TLC-induced inhibition of TC uptake by Ntcp. However, stimulation of TC uptake and translocation of Ntcp by cAMP has been shown to be mediated via PI3K signaling pathway in rat hepatocytes as well as in HuH7 cells transfected with Ntcp (36, 39). Thus TLC may inhibit TC uptake by a PI3K-independent mechanism. However, the role of PI3K/nPKCε in TLC-induced inhibition of TC uptake has not been studied.
The aim of the present study was to determine the mechanism by which TLC inhibits TC uptake mediated via human NTCP using HuH7 cells stably transfected with NTCP (HuH-NTCP cells). More specifically, we determined the kinetics of inhibition and the role of PI3K/PKCε signaling pathway. Our studies showed that TLC inhibits TC uptake by NTCP competitively and activates PKCε without activating PI3K. In addition, the inhibition of TC uptake by TLC is not mediated via PKCε, and cAMP can reverse the effect of TLC.
MATERIALS AND METHODS
Materials.
Lipofectamine reagent kit was purchased from Life Technologies/GIBCO (Carlsbad, CA). 8-Chlorophenylthio (CPT)-cAMP, TC (Na-salt), LY294002, wortmannin, aprotinin, leupeptin, okadaic acid, and collagenase were obtained from Sigma Chemical (St. Louis, MO). [3H]TC (56 mCi/mmol) was purchased from Perkin-Elmer (Boston, MA). The dominant negative nPKCε plasmid (pHACE-DN-PKCε) was produced by point mutation of K437R (32) and was generously provided by Dr. Jae-Won Soh (Inha University, South Korea). Human NTCP antibody (31) was generously provided by Dr. Shneider (Children's Hospital, Pittsburg, PA). HuH-NTCP cells are HuH7 cells, a human hepatocellular carcinoma cell line, stably transfected with human NTCP (16) and were generously provided by Dr. Gores (Rochester, MN). Anti-phospho PKCε-S729 antibody was obtained from Abcam (Cambridge, MA), and antibodies for nPKCε and PKB were from Cell Signaling Technology (Danvers, MA).
Rat hepatocyte preparation.
Rat hepatocytes were isolated from male Wistar rats (200–250 g), cultured as previously described (37), and used to confirm PKB activation by TLC and to determine Ntcp translocation by TLC. The protocol for harvesting livers was approved by the Institutional Animal Care and Use Committee.
Cell culture and transfections.
HuH-NTCP cells were cultured in Eagle's minimum essential medium supplemented with 10% fetal bovine serum, 100,000 U/l penicillin, 100 mg/l streptomycin, 25 μg/ml amphotericin B, and 1.2 g/l G418 at 37°C in a 5% CO2-95% O2 air incubator. The cells were transiently transfected with empty vector or DN-PKCε using Lipofectamine as described previously (36). The transfection efficiency ranged from 25–42% on the basis of cells transfected with green fluorescence protein under similar experimental conditions.
TC uptake.
TC uptake in HuH-NTCP cells was determined as previously described (36). Briefly, following pretreatment with TLC, cells were washed twice with 0.5 ml HEPES buffer and then incubated with 20 μM TC containing 3H-TC (100 dpm/pmol) for 2 min. For TC uptake kinetic studies, cells were treated with different concentrations of TLC and TC as described in the figure legends. Cells were then washed twice with 0.5 ml ice-cold HEPES buffer and lysed in 0.25 ml of a lysis buffer (20 mM Tris, 150 mM NaCl, 1% Triton X-100, 1 mM PMSF, 1 mM EDTA, 1 mM EGTA, 2.5 mM Na4P2O7, 1 mM β-glycerophosphate, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 500 nM okadaic acid, and 1 mM orthovanadate, pH 7.5). Aliquots of cell lysate were counted for radioactivity, and TC uptake rate was expressed in pmol/min per mg protein.
NTCP translocation.
A cell surface protein biotinylation method was used to quantitate NTCP translocation in HuH-NTCP cells (36). Briefly, following various treatments, cell surface proteins were biotinylated by exposing HuH-Ntcp cells to sulfo-NHS-LC-Biotin (0.5 mg/ml; Pierce, Rockford, IL) followed by preparation of cell lysate used to determine biotinylated and total NTCP mass. Biotinylated proteins were isolated using streptavidin-agarose beads and then subjected to immunoblot analysis to determine plasma membrane NTCP. The absence of biotinylated actin was routinely checked to assure that intracellular proteins were not biotinylated (38, 39). Proteins (10–50 μg) from whole cell lysate and streptavidin-agarose-containing biotinylated proteins were subjected to immunoblot analysis as previously described (25).
nPKCε translocation and phosphorylation.
The effect of TLC and PMA on nPKCε activity was assessed from membrane translocation as described by us and others (2, 5, 28) and nPKCε-Ser729 phosphorylation (6, 7, 26, 40). Briefly, HuH-NTCP cells treated with TLC or PMA were homogenized followed by centrifugation at 400 g. The homogenate supernatant was centrifuged at 148,000 g for 1 h. The resultant supernatant was saved as the cytosolic fraction. The pellet was washed three times at 12,000 g and then dissolved in the lysis buffer. The dissolved pellet was centrifuged at 12,000 g for 15 min, and the resulting supernatant was saved as the membrane fraction. The cytosolic and membrane fractions were subjected to immunoblot analysis for nPKCε. The phosphorylation of nPKCε at Ser729 was evaluated by immunoblot analysis of cell lysates using phospho-specific antibodies. The blots were first probed for phospho-nPKCε-Ser729 followed by total nPKCε. To correct for loading variations, the result was expressed as a ratio of phospho/total nPKCε with the control ratio set at 1.0.
Other methods.
Phosphorylation of PKB (also known as Akt) was determined using phospho-PKB-S473 antibody as previously described (39). The Lowry method was used to determine cell protein (22). The blots were scanned in grayscale using Adobe Photoshop (Adobe System, San Jose, CA), and the relative band densities were quantitated using Signal Gel (Jandel Scientific Software, San Rafael, CA). All values are expressed as means ± SE. Analysis of variance or Student's t-test was used to statistically analyze data with P < 0.05 considered significant.
RESULTS
TLC-induced inhibition of TC uptake in HuH-NTCP cells.
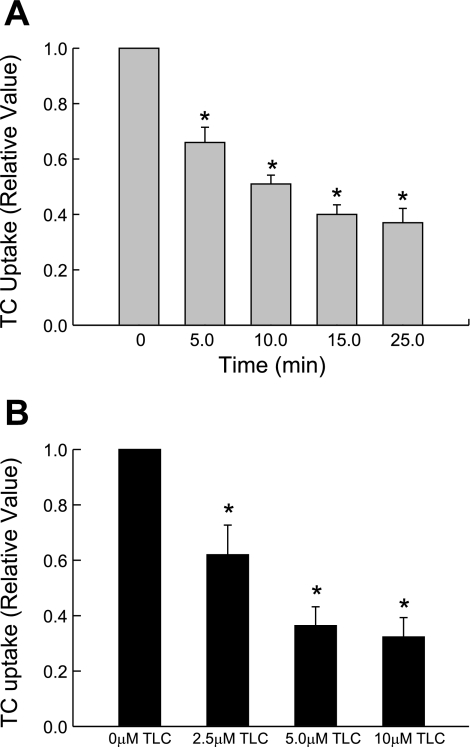
Our initial time-dependent study showed that 10 μM TLC inhibited TC uptake by 35% as early as 5 min and by 60% by 25 min (Fig. 1A). Cell viability was not affected by 10 μM TLC. We did not extend the studies beyond 25 min because the inhibition of bile formation by TLC stabilizes by 15–20 min (2). Previous studies showed that TLC (2.5, 5, and 10 μM) can inhibit bile formation in isolated perfused rat livers (2) and TC uptake in rat hepatocytes (29). Thus we used these concentrations to further study the effect of TLC. The inhibition of TC uptake by TLC was concentration dependent and ranged from 40% at 2.5 μM to 70% at 10 μM in 25 min (Fig. 1B). These results suggest that TLC inhibits TC uptake by human NTCP. We next determined whether TLC inhibits TC uptake by decreasing NTCP translocation.
Fig. 1.
Time- and concentration-dependent effect of taurolithocholate (TLC) on taurocholate (TC) uptake. HuH7 cells stably transfected with human Na+-dependent TC-cotransporting polypeptide (NTCP) (HuH-NTCP cells) were incubated with 10 μM TLC for indicated time (A) or with indicated TLC concentrations for 25 min (B) before determining TC uptake. Values are means ± SE (n = 4). *Significantly different (P < 0.05) from 0 min (A) or 0 μM TLC (B) values.
TLC does not inhibit NTCP translocation and competitively inhibits TC uptake in HuH-NTCP cells.
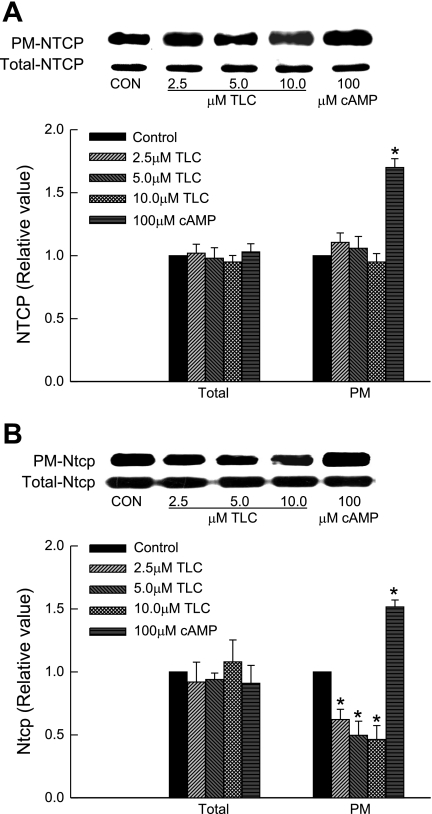
A previous study in rat hepatocytes showed that TLC inhibits TC uptake noncompetitively, i.e., by decreasing Vmax (29). A decrease in Vmax may be due to a decrease in plasma membrane NTCP induced by TLC. Thus we determined the concentration-dependent effect of TLC on NTCP translocation. Unexpectedly, we observed that TLC at concentrations found to inhibit TC uptake did not affect plasma membrane NTCP level (Fig. 2A). As reported before for rat Ntcp (25, 38), cAMP increased plasma membrane NTCP. Neither TLC nor cAMP affected total NTCP. Thus TLC does not affect membrane translocation of NTCP. We then determined whether TLC affects plasma membrane distribution of rat Ntcp because this has not been studied before and would be expected from noncompetitive inhibition. The result (Fig. 2B) shows that TLC decreased plasma membrane Ntcp in rat hepatocytes by 38, 50, and 54% at 2.5, 5, and 10 μM TLC, respectively, without affecting total Ntcp. As shown before (25, 38), cAMP increased plasma membrane Ntcp by 45%. Thus TLC differentially affects plasma membrane distribution of Ntcp and NTCP.
Fig. 2.
Effect of TLC on plasma membrane (PM) NTCP. HuH-NTCP cells (A) or rat hepatocytes (B) were incubated with TLC for 25 min or 8-chlorophenylthio (CPT)-CAMP for 15 min followed by biotinylation of cell surface proteins and immunoblot analysis of biotinylated (PM-NTCP/Ntcp) and total cell lysate NTCP/Ntcp (Total-NTCP/Ntcp). Top: typical NTCP/Ntcp immunoblots. Bottom: results of densitometric analysis (means ± SE, n = 4). *Significantly different (P < 0.05) from respective control values. Con, control.
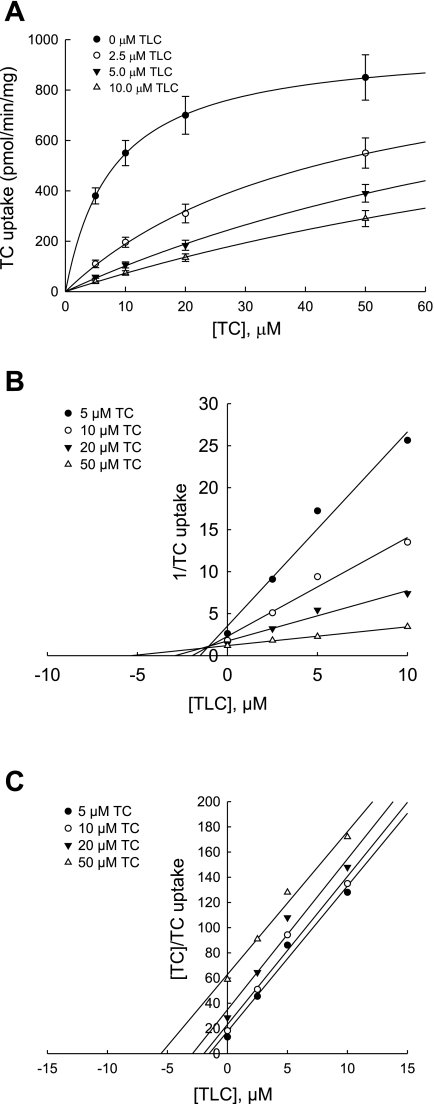
The above results raised the possibility that TLC inhibits TC uptake by rat Ntcp differently than by human NTCP. Thus we determined the type of inhibition of TC uptake by TLC in HuH-NTCP cells. Inhibition of TC uptake at different TC concentrations by different concentrations of TLC was determined (Fig. 3A), and the Dixon plot (1/TC uptake vs. [TLC]) was used to determine the type of inhibition (Fig. 3B). TLC increased estimated Km values for TC (7, 45, 118, and 147 μM at 0, 2.5, 5.0, and 10 μM TLC, respectively) without significantly affecting the estimated Vmax values. In addition, the lines in the Dixon plot intersected above the x-axis, indicating competitive inhibition. Because the Dixon plot cannot distinguish between competitive and mixed inhibition, we subjected the data to Cornish-Bowden plot, [TC]/TC uptake vs. [TLC] (8). This plot resulted in parallel lines (Fig. 3C) indicative of competitive inhibition. The slopes of the regression lines (11.54 ± 1.22, 11.76 ± 1.39, 11.96 ± 1.54, and 11.33 ± 0.94 for 5, 10, 20, and 50 μM TC, respectively) were not significantly different from each other. This result confirmed that TLC inhibits TC uptake in HuH-NTCP cell competitively. A competitive inhibition is consistent with the result that TLC does not affect plasma membrane NTCP.
Fig. 3.
Competitive inhibition of TC uptake by TLC. HuH-NTCP cells were incubated with 0, 2.5, 5.0, or 10.0 μM TLC for 15 min followed by determination of TC (5.0, 10.0, 20.0, and 50.0 μM) uptake. Concentration-dependent effect of TLC on TC uptake (means ± SE, n = 3) is shown in A, and Dixon and Cornish-Bowden plots of data in A showing competitive inhibition are presented in B and C, respectively. The solid lines represent the regression lines through the data points. The estimated Km value for TC and the estimated inhibitor constant (Ki) for TLC were 7.9 ± 0.13 μM and 1.1 ± 0.03 μM, respectively.
The estimated inhibitor constant (Ki) for TLC was 1.05 ± 0.03 μM (means ± SE, n = 3), a value sixfold lower than the reported value of 7 μM for the inhibition of rat Ntcp by TLC (29). Thus TLC is a more potent inhibitor of human NTCP than rat Ntcp. The estimated Km value for TC was 7.94 ± 0.13 μM (means ± SE, n = 3), and this value was comparable to those (2–8 μM) previously reported for human NTCP (15, 17, 19, 30).
TLC-induced inhibition of TC uptake is not mediated via PI3K.
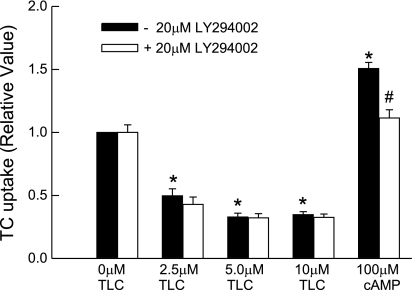
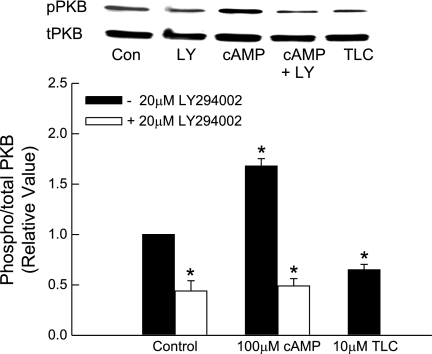
TLC has been suggested to induce cholestasis via PI3K-dependent activation of PKCε (3). If a similar mechanism is involved, PI3K inhibitor should reverse TLC-induced inhibition of TC uptake. Thus we studied the effect of LY294002, an inhibitor of PI3K. Interestingly, LY294002 failed to reverse TLC-induced inhibition of TC uptake (Fig. 4). Similar results were obtained with wortmannin (data not shown). In the same study, cAMP-induced increases in TC uptake were inhibited by LY294002 (Fig. 4); cAMP-induced stimulation of TC uptake by rat hepatocytes is PI3K dependent (38). This result raised the question whether TLC activates PI3K in HuH-NTCP cells.
Fig. 4.
Phosphoinositide-3-kinase (PI3K)-independent inhibition of TC uptake by TLC. HuH-Ntcp cells were pretreated with or without 20 μM LY294002 for 20 min and then with different concentrations of TLC or 100 μM CPT-cAMP before determining TC uptake. Values are means ± SE (n = 3); *P < 0.05 compared with 0 μM TLC and #P < 0.05 compared with cAMP value in the absence of LY294002.
TLC does not activate PI3K in HuH-NTCP cells.
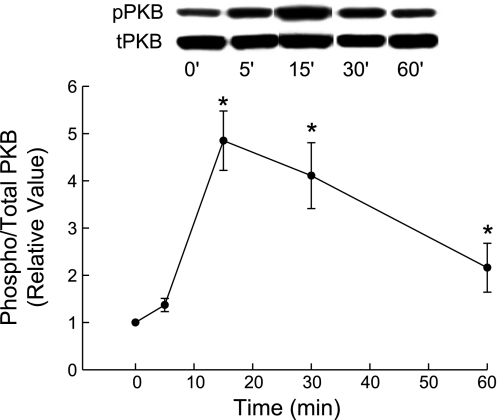
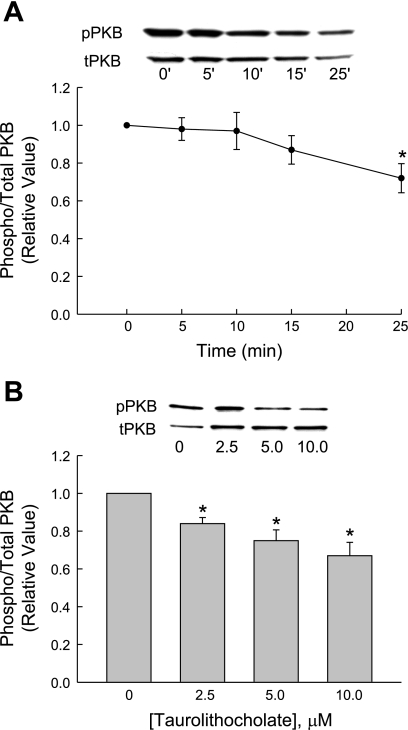
TLC has been shown to stimulate PI3K, as evidenced by TLC-induced, PI3K-dependent phosphorylation of PKB in rat hepatocytes (3). We have also observed a transient increase in PKB phosphorylation with 25 μM TLC by 15 min in rat hepatocytes (Fig. 5). Qualitatively, similar results were also obtained with 5 μM TLC (data not shown). Thus we determined whether TLC activates PI3K in HuH-NTCP cells by studying the effect on PKB phosphorylation, a PI3K-dependent process (33, 34). First we determined the time-dependent effect of 10 μM TLC on PKB phosphorylation. TLC did not have any effect on PKB phosphorylation until 25 min when it inhibited PKB phosphorylation (Fig. 6A). Further studies showed that incubation of HuH-NTCP cells with TLC for 25 min resulted in a concentration-dependent inhibition of PKB phosphorylation (Fig. 6B). It may be noted that TLC-sulfate also inhibits PKB phosphorylation in rat hepatocytes (14). To confirm that PI3K-dependent PKB phosphorylation occurs in HuH-NTCP cells, we determined the effect of LY294002 on cAMP-induced PKB phosphorylation. We have previously shown that cAMP stimulates PKB phosphorylation in HuH-Ntcp cells (23). In this study (Fig. 7), cAMP stimulated and TLC inhibited PKB phosphorylation, and LY294002 did inhibit cAMP-induced phosphorylation of PKB, indicating inhibition of PI3K. These results suggest that TLC does not activate PI3K/PKB pathway in HuH-NTCP cells, and hence its effect on TC uptake is unlikely to be mediated via PI3K kinase. These results also suggest that TLC-induced activation of PKCε may not be PI3K dependent in HuH-NTCP. This, however, does not rule out the possibility of PI3K-independent activation of nPKCε by TLC in HuH-NTCP cells. Thus we determined the effect of TLC on nPKCε.
Fig. 5.
Time-dependent effect of TLC on PKB phosphorylation in rat hepatocytes. Cells were treated with 25 μM TLC for the indicated time followed by determination of phospho (pPKB) and total PKB (tPKB, loading control). Values are means ± SE (n = 4); *significantly different from 0-min values (P < 0.05).
Fig. 6.
Time- and concentration-dependent effect of TLC on PKB phosphorylation. HuH-NTCP cells were treated with 10 μM TLC for the indicated time (A) or with indicated TLC concentrations for 25 min (B) followed by determination of pPKB and tPKB. Values are means ± SE (n = 4); *significantly different from 0-min (A) or 0 μM TLC (B) values (P < 0.05).
Fig. 7.
Inhibition of cAMP-induced activation of PKB by LY294002 (LY). HuH-NTCP cells were pretreated with or without 20 μM LY294002 for 20 min followed by incubation with 100 μM CPT-cAMP for 15 min. Cells were also treated with 10 μM TLC for 25 min (negative control). Top: typical immunoblot analysis of pPKB and tPKB. Bottom: result of densitometric analysis (means ± SE; n = 3). *Significantly (P < 0.05) different from control values in the absence of LY294002.
TLC activates PKCε in HuH-NTCP cells.
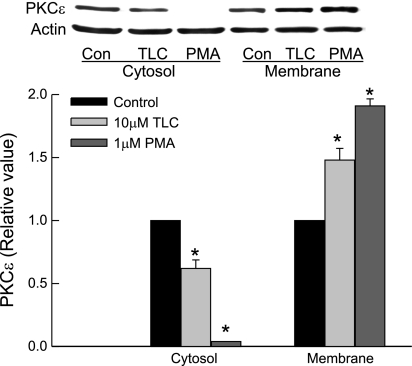
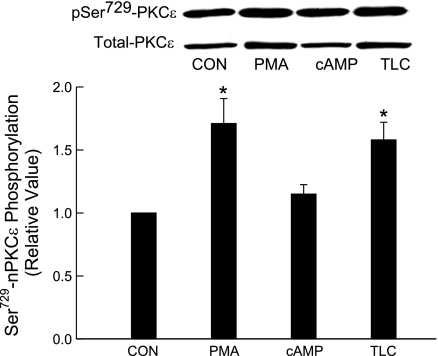
Two methods were used to study the effect of TLC on PKCε: 1) membrane translocation of PKCε and 2) phosphorylation of PKCε at Ser729. On the basis of membrane translocation studies, TLC has been shown to activate PKCε in rat hepatocytes (3, 4). Phosphorylation at Ser729 has been suggested to be involved in translocation and activation of nPKCε (6, 7, 26, 40). Membrane translocation studies in HuH-NTCP cells showed that 10 μM TLC increased and decreased PKCε in membrane and cytosol, respectively (Fig. 8). PMA (1 μM) used as a positive control also produced similar effects. TLC and PMA, but not cAMP, induced PKCε phosphorylation at Ser729 (Fig. 9). Cyclic AMP has previously been shown not to activate nPKCε (translocation studies) in rat hepatocytes (28). Taken together, these results suggest that TLC activates nPKCε in HuH-NTCP cells. Thus TLC-induced inhibition of TC uptake may be mediated via nPKCε, and hence we studied the role of nPKCε.
Fig. 8.
Membrane translocation of novel protein kinase Cε (nPKCε) by TLC and PMA. HuH-NTCP cells were treated with 1 μM PMA or 10 μM TLC for 15 min followed by preparation of crude membranes and cytosol and immunoblot analysis of nPKCε (actin for loading control). Top: typical immunoblot analysis of nPKCε and actin. Bottom: result of densitometric analysis (means ± SE; n = 4); *significantly different from respective control values (P < 0.05).
Fig. 9.
Effects of TLC, PMA, and cAMP on nPKCε-Ser729 phosphorylation. HuH-NTCP cells were treated with 1 μM PMA, 100 μM CPT-CAMP, or 10 μM TLC for 15 min followed by determination of phospho PKCε and total PKCε. Top: typical immunoblot analysis of phospho (pSer729-PKCε) and total PKCε (loading control). Bottom: result of densitometric analysis (means ± SE; n = 4). *Significantly different from control values (P < 0.05).
Role of nPKCε on TLC-induced inhibition of TC uptake and PKB.
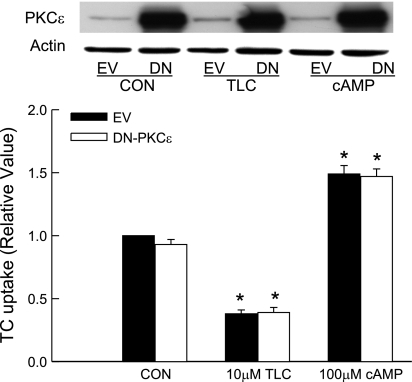
We studied the effect of a DN-PKCε (HA tagged) to define the role of PKCε. This mutant has been used previously to study the role of nPKCε in other cell types (6, 7, 21, 32). Overexpression of DN-PKCε resulted in several fold increases in PKCε compared with cells transfected with the empty vector (Fig. 10); the expression of DN-PKCε was also confirmed by expression of HA tag (Fig. 11). Despite this high expression of DN-PKCε, neither basal TC uptake nor inhibition of TC uptake by TLC was affected. This result would suggest that TLC-induced inhibition of TC uptake by NTCP is not mediated via PKCε. Also as expected, increases in TC uptake induced by cAMP were not affected by DN-PKCε (Fig. 10). Studies with cAMP were included as a negative control because cAMP does not activate PKCε in rat hepatocytes (28).
Fig. 10.
Effect of dominant negative-PKCε (DN-PKCε) on TC uptake by TLC and cAMP. HuH-NTCP cells were transfected with 1 μg DN-PKCε (DN) or empty vector (EV) followed by incubation with 10 μM TLC or 100 μM CPT-cAMP (cAMP) for 15 min and determination of TC uptake and nPKCε immunoblot analysis. Top: typical immunoblot analysis of nPKCε and actin. Bottom: result of TC uptake (means ± SE; n = 4). *Significantly different from control EV value (P < 0.05).
Fig. 11.
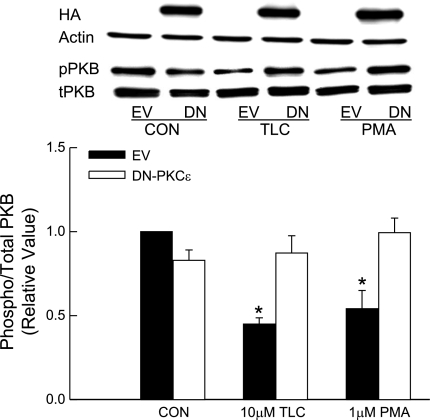
Effect of DN-PKCε on PKB phosphorylation by TLC and PMA. HuH-NTCP cells were transfected with 1 μg DN-PKCε or empty vector followed by incubation with 10 μM TLC or 1 μM PMA for 15 min. Cell lysates were subjected to immunoblot analysis for HA with actin for loading control, pPKB, and tPKB. Top: typical immunoblot analysis. Bottom: result of densitometric analysis (means ± SE; n = 3). *Significantly different from control EV value (P < 0.05).
nPKCε has been shown to inhibit PKB in mouse keratinocytes (20) and myeloid 32D cells (21). Thus we determined whether TLC induced inhibition of PKB in HuH-NTCP cells is mediated via PKCε. Overexpression of DN-PKCε decreased basal PKB phosphorylation by 20% (Fig. 11). Like TLC, PMA also inhibited PKB phosphorylation. Both PMA and TLC-induced decreases in PKB phosphorylation were reversed by DN-PKCε (Fig. 11), indicating that nPKCε mediated inhibition of PKB by TLC and PMA in HuH-NTCP cells.
Effect of cAMP on TLC-induced inhibition of TC uptake.
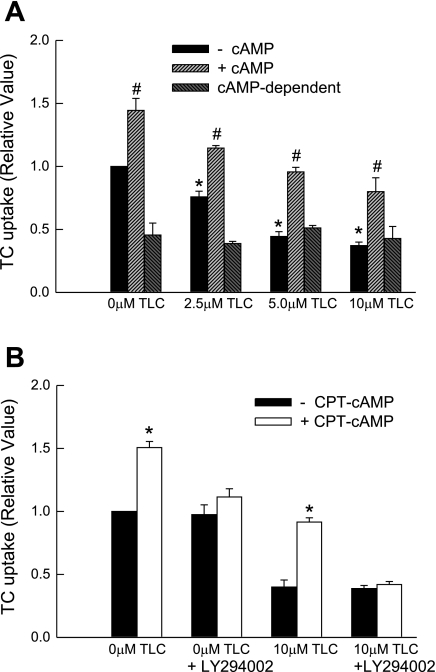
Cyclic AMP has been shown to reverse TLC-induced retrieval of Bsep (9). We next determined whether cAMP can reverse the effect of TLC by preincubating HUH-NTCP cells with or without 100 μM CPT-cAMP. In these studies, TLC inhibited TC uptake by 25, 55, and 63% at 2.5, 5.0, and 10.0 μM, respectively, in the absence of CPT-cAMP (Fig. 12A). As previously shown for rat Ntcp (36), CPT-CAMP increased TC uptake by 50% in the absence of TLC in HuH-NTCP cells. When preincubated with CPT-cAMP, the ability of TLC to inhibit TC uptake decreased at all concentrations studied (Fig. 12A). For example, at 10 μM TLC, TC uptake increased from 37% to 80% of control in the presence of cAMP. In addition, the ability to prevent TLC-induced inhibition of TC uptake by cAMP was abolished in the presence of PI3K inhibitor (Fig. 12B). These results would suggest that cAMP prevents TLC-induced inhibition of TC uptake by NTCP via a PI3K-dependent mechanism. However, it does not appear that cAMP actually reverses the effect of TLC. Rather, the stimulatory effect of cAMP is additive to the inhibitory effect of TLC. This is evident from the result that cAMP-dependent stimulation of TC uptake, as determined from the difference in TC uptake in the presence and absence of cAMP at different concentrations of TLC, remained unchanged (Fig. 12A). In other words, TC uptake in the presence of cAMP and TLC represents a sum of the stimulatory and inhibitory effects of cAMP and TLC, respectively. This explanation is consistent with the findings that cAMP stimulates TC uptake by increasing NTCP translocation to the plasma membrane, whereas TLC does not affect translocation.
Fig. 12.
PI3K-dependent prevention of TLC-induced TC uptake inhibition by cAMP. A: HuH-NTCP cells were pretreated 100 μM CPT-cAMP for 15 min and then with the indicated concentrations of TLC before determining TC uptake. cAMP-dependent values represent the difference between TC uptake in the presence and absence of CPT-cAMP. *Significantly different (P < 0.05) from 0 μM TLC values in the absence of CPT-cAMP and #significantly different from respective values in the absence of CPT-cAMP. B: HuH-NTCP cells were pretreated with or without 100 μM cAMP for 15 min and 20 μM LY294002 for 20 min and then with TLC for 25 min before determining TC uptake. Values are means ± SE (n = 3–4); *significantly different (P < 0.05) from respective values in the absence of CPT-cAMP.
DISCUSSION
The aim of the present study was to determine the role of nPKCε in TLC-induced inhibition of TC uptake. Our studies in HuH-NTCP cells show that TLC inhibits TC uptake via NTCP competitively and activates PKCε in a PI3K-independent manner. TLC-induced inhibition of TC uptake is not mediated via nPKCε, and cAMP can reverse the effect of TLC. In addition, TLC-induced inhibition of PKB is mediated via nPKCε.
The present study showed that TLC inhibits TC uptake by NTCP competitively. This is in contrast to noncompetitive inhibition of TC uptake by TLC in rat hepatocytes (29). Thus different mechanisms are involved in TLC-induced inhibition of TC uptake by rat Ntcp and human NTCP. A similar difference in the mechanism of inhibition of TC uptake by bosentan has been reported for rat Ntcp and human NTCP (19). The reason for this difference is unclear, and initial studies with NTCP/Ntcp chimeras failed to identify a sequence in Ntcp and NTCP that may be responsible for this difference (19). The Ki value (1 μM) for the inhibition of NTCP (present study) is sevenfold lower than that reported (7 μM) for the inhibition of rat Ntcp (29). Thus TLC is a more potent inhibitor of TC uptake by NTCP than Ntcp and by extension is likely to be more toxic for human liver. In contrast to TLC, cAMP stimulates translocation of both Ntcp (25) and NTCP to the plasma membrane (Fig. 2), and cAMP-induced stimulation of TC uptake is PI3K dependent in both rat hepatocytes (36, 38) and HuH-NTCP cells (Fig. 4). Thus cAMP-mediated regulation of rat Ntcp and human NTCP appears to be similar.
TLC activates nPKCε in rat hepatocytes (3, 4) and in HuH-NTCP cells (Figs. 8 and 9). Studies in rat hepatocytes suggested that TLC-induced activation of nPKCε is mediated via PI3K (3). PI3K products have been shown to activate nPKCε in vitro (35), and IFNγ-induced activation of nPKCε in mesangial cells is inhibited by PI3K inhibitor (7). However, nPKCε and PMA have been shown to inhibit granulocyte colony-stimulating factor-induced activation of PKB in myeloid 32D cells (21), and activation of nPKCδ and nPKCε results in inhibition of PKB in mouse keratinocytes (20). Thus whether nPKCε regulates PKB activity or PI3K regulates nPKCε activity appears to be dependent on cell type and stimulus. In the present study, TLC did inhibit and did not stimulate PKB phosphorylation (Fig. 6A), a PI3K-dependent process (33, 34), indicating that TLC-induced activation of nPKCε is not mediated via PI3K/PKB in HuH-NTCP cells. Furthermore, TLC-induced inhibition of PKB is mediated via nPKCε (Fig. 11). These results suggest that, whereas the PI3K/PKB signaling pathway regulates nPKCε in rat hepatocytes (3), nPKCε negatively regulates the PKB signaling pathway in human-derived hepatic cells. In a recent study, TLC has been suggested to induce apoptosis by upregulating activator protein-1 in HepG2-Ntcp hepatoma cells (27). Considering the antiapoptotic role of PI3K/PKB in different cell types (10–12), it is likely that TLC may produce apoptosis via nPKCε-mediated inhibition of PKB in human hepatoma cells.
Cyclic AMP has been shown to translocate Bsep to canalicular membrane (24) and to reverse TLC-induced inhibition of bile acid secretion and retrieval of Bsep (9). The present study showed that cAMP can reverse TLC-induced inhibition of TC uptake (Fig. 12A) via the PI3K signaling pathway (Fig. 12B). Thus the reversal of TLC-induced cholestasis by cAMP appears to involve both sinusoidal uptake and canalicular secretion of bile acids. The present study also suggests that the prevention of TLC-induced TC uptake inhibition by cAMP is attributable to an additive effect and not to reversal of TLC effect. It is possible that similar mechanism may also be involved in the reversal of TLC-induced Bsep retrieval. In that case, cAMP may reverse TLC-induced cholestasis by stimulating transhepatic transport of bile acids and not by reversing the effect of TLC on bile acid transport.
The cellular mechanism by which TLC inhibits TC uptake competitively in HuH-NTCP cells is unclear. The present study showed that TLC-induced inhibition of TC uptake is not mediated via nPKCε (Fig. 10). In a previous study, we reported that cAMP-induced TC uptake is mediated via the PI3K/PKB signaling pathway (36). Because TLC inhibited PI3K/PKB signaling pathway, it is possible that the inhibition of TC uptake may be attributable to inhibition of PI3K. However, this is unlikely for two reasons. TLC inhibits TC uptake as early as 5 min (Fig. 1A), but it does not inhibit PI3K until 25 min (Fig. 6A). Moreover, DN-PKB does not inhibit basal TC uptake in HuH-Ntcp cells (36). TLC-sulfate and glycochenodeoxycholate has been shown to activate ERK1/2, p38 MAPK, and JNK1/2 in rat hepatocytes (13, 14). Our preliminary study showed that TLC activated p38 MAPK and JNK1/2 in HuH-NTCP cells (C. Schanhoff, C. Webster, M. Anwer, unpublished observations). However, little is known about the role of these kinases in the regulation of hepatic TC uptake. We have previously reported that cAMP activates p38 MAPK, but cAMP-induced stimulation of TC uptake is not mediated via p38 MAPK in rat hepatocytes (36). Thus it is unlikely that p38 MAPK is involved in TLC-induced inhibition of TC uptake. Whether the effect of TLC is mediated via ERK1/2 and/or JNK1/2 is presently under investigation.
It is also possible that the inhibition of TC uptake by TLC may be attributable to binding to NTCP from the cytoplasmic side (i.e., transinhibition); the present study design would preclude an interaction from the outside. Similarly, whether the inhibition is attributable to nonspecific binding to plasma membrane cannot be ruled out. However, the observed time-dependent effect would not be consistent with this hypothesis because binding to plasma membrane would be expected to be rapid compared with changes in uptake.
In summary, the present study showed that TLC-induced inhibition of PKB, but not TC uptake, is mediated via nPKCε in HuH-NTCP cells. In addition, the prevention of TLC-induced inhibition of TC uptake by cAMP is mediated via the PI3K pathway.
DISCLOSURES
No conflicts of interest are declared by the author(s).
GRANTS
This study was supported in part by National Institutes of Health Grants DK-33436 (M. Anwer), DK-65975 (C. Webster), and DK-56108 (B. Bouscarel).
ACKNOWLEDGMENTS
We thank Holly Jameson and Ariel Hobson for excellent technical assistance.
REFERENCES
- 1.Anwer MS. Cellular regulation of hepatic bile acid transport in health and cholestasis. Hepatology 39: 581–589, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Beuers U, Bilzer M, Chittattu A, Kullak-Ublick GA, Keppler D, Paumgartner G, Dombrowski F. Tauroursodeoxycholic acid inserts the apical conjugate export pump, Mrp2, into canalicular membranes and stimulates organic anion secretion by protein kinase C-dependent mechanisms in cholestatic rat liver. Hepatology 33: 1206–1216, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Beuers U, Denk GU, Soroka CJ, Wimmer R, Rust C, Paumgartner G, Boyer JL. Taurolithocholic acid exerts cholestatic effects via phosphatidylinositol-3 kinase-dependent mechanisms in perfused rat livers and rat hepatocyte couplets. J Biol Chem 278: 17810–17818, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Beuers U, Probst I, Soroka C, Boyer JL, Kullak-Ublick GA, Paumgartner G. Modulation of protein kinase C by taurolithocholic acid in isolated rat hepatocytes. Hepatology 29: 477–482, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Braiman L, Alt A, Kuroki T, Ohba M, Bak A, Tennenbaum T, Sampson SR. Protein kinase Cdelta mediates insulin-induced glucose transport in primary cultures of rat skeletal muscle. Mol Endocrinol 13: 2002–2012, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Cenni V, Doppler H, Sonnenburg ED, Maraldi N, Newton AC, Toker A. Regulation of novel protein kinase C epsilon by phosphorylation. Biochem J 363: 537–545, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choudhury GG. A linear signal transduction pathway involving phosphatidylinositol 3-kinase, protein kinase Cepsilon, and MAPK in mesangial cells regulates interferon-gamma-induced STAT1alpha transcriptional activation. J Biol Chem 279: 27399–27409, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Cornish-Bowden A. A simple graphical method for determining the inhibition constants of mixed, uncompetitive and non-competitive inhibitors. Biochem J 137: 143–144, 1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crocenzi FA, Mottino AD, Sanchez Pozzi EJ, Pellegrino JM, Rodriguez Garay EA, Milkiewicz P, Vore M, Coleman R, Roma MG. Impaired localisation and transport function of canalicular Bsep in taurolithocholate induced cholestasis in the rat. Gut 52: 1170–1177, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev 13: 2905–2927, 1999 [DOI] [PubMed] [Google Scholar]
- 11.Franke TF, Hornik CP, Segev L, Shostak GA, Sugimoto C. PI3K/Akt and apoptosis: size matters. Oncogene 22: 8983–8998, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Franke TF, Kaplan DR, Cantley LC. PI3K: downstream AKTion blocks apoptosis. Cell 88: 435–437, 1997 [DOI] [PubMed] [Google Scholar]
- 13.Graf D, Kurz AK, Reinehr R, Fischer R, Kircheis G, Haussinger D. Prevention of bile acid-induced apoptosis by betaine in rat liver. Hepatology 36: 829–839, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Graf D, Reinehr R, Kurz AK, Fischer R, Haussinger D. Inhibition of taurolithocholate 3-sulfate-induced apoptosis by cyclic AMP in rat hepatocytes involves protein kinase A-dependent and -independent mechanisms. Arch Biochem Biophys 415: 34–42, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Hagenbuch B, Meier PJ. Molecular cloning, chromosomal localization, and functional characterization of a human liver Na+/bile acid cotransporter. J Clin Invest 93: 1326–1331, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higuchi H, Bronk SF, Takikawa Y, Werneburg N, Takimoto R, El Deiry W, Gores GJ. The bile acid glycochenodeoxycholate induces trail-receptor 2/DR5 expression and apoptosis. J Biol Chem 276: 38610–38618, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Ho RH, Leake BF, Roberts RL, Lee W, Kim RB. Ethnicity-dependent polymorphism in Na+-taurocholate cotransporting polypeptide (SLC10A1) reveals a domain critical for bile acid substrate recognition. J Biol Chem 279: 7213–7222, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Javitt NB, Emerman S. Effect of sodium taurolithocholate on bile flow and bile acid excretion. J Clin Invest 47: 1002–1014, 1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leslie EM, Watkins PB, Kim RB, Brouwer KL. Differential inhibition of rat and human Na+-dependent taurocholate cotransporting polypeptide (NTCP/SLC10A1) by bosentan: a mechanism for species differences in hepatotoxicity. J Pharmacol Exp Ther 321: 1170–1178, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Li L, Sampat K, Hu N, Zakari J, Yuspa SH. Protein kinase C negatively regulates Akt activity and modifies UVC-induced apoptosis in mouse keratinocytes. J Biol Chem 281: 3237–3243, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Liu H, Qiu Y, Xiao L, Dong F. Involvement of protein kinase Cepsilon in the negative regulation of Akt activation stimulated by granulocyte colony-stimulating factor. J Immunol 176: 2407–2413, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Lowry DH, Rosenberg NJ, Farr AL, Randall RJ. Protein measurement with folin phenol reagent. J Biol Chem 193: 265–275, 1951 [PubMed] [Google Scholar]
- 23.McConkey M, Gillin H, Webster CR, Anwer MS. Cross-talk between protein kinases Czeta and B in cyclic AMP-mediated sodium taurocholate co-transporting polypeptide translocation in hepatocytes. J Biol Chem 279: 20882–20888, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Misra S, Varticovski L, Arias IM. Mechanisms by which cAMP increases bile acid secretion in rat liver and canalicular membrane vesicles. Am J Physiol Gastrointest Liver Physiol 285: G316–G324, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Mukhopadhayay S, Ananthanarayanan M, Stieger B, Meier PJ, Suchy FJ, Anwer MS. cAMP increases liver Na+-taurocholate cotransport by translocating transporter to plasma membranes. Am J Physiol Gastrointest Liver Physiol 273: G842–G848, 1997 [DOI] [PubMed] [Google Scholar]
- 26.Parekh D, Ziegler W, Yonezawa K, Hara K, Parker PJ. Mammalian TOR controls one of two kinase pathways acting upon nPKCdelta and nPKCepsilon. J Biol Chem 274: 34758–34764, 1999 [DOI] [PubMed] [Google Scholar]
- 27.Pusl T, Vennegeerts T, Wimmer R, Denk GU, Beuers U, Rust C. Tauroursodeoxycholic acid reduces bile acid-induced apoptosis by modulation of AP-1. Biochem Biophys Res Commun 367: 208–212, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Schonhoff CM, Gillin H, Webster CR, Anwer MS. Protein kinase Cdelta mediates cyclic adenosine monophosphate-stimulated translocation of sodium taurocholate cotransporting polypeptide and multidrug resistant associated protein 2 in rat hepatocytes. Hepatology 47: 1309–1316, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Schwenk M, Schwarz LR, Greim H. Taurolithocholate inhibits taurocholate uptake by isolated hepatocytes at low concentrations. Naunyn Schmiedebergs Arch Pharmacol 298: 175–179, 1977 [DOI] [PubMed] [Google Scholar]
- 30.Shitara Y, Li AP, Kato Y, Lu C, Ito K, Itoh T, Sugiyama Y. Function of uptake transporters for taurocholate and estradiol 17beta-D-glucuronide in cryopreserved human hepatocytes. Drug Metab Pharmacokinet 18: 33–41, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Shneider BL, Fox VL, Schwarz KB, Watson CL, Ananthanarayanan M, Thevananther S, Christie DM, Hardikar W, Setchell KDR, Meili-Vergani G, Suchy FJ, Mowat AP. Hepatic basolateral sodium-dependent-bile acid transporter expression in two unusual cases of hypercholanemia and in extrahepatic biliary atresia. Hepatology 25: 1176–1183, 1997 [DOI] [PubMed] [Google Scholar]
- 32.Soh JW, Lee EH, Prywes R, Weinstein IB. Novel roles of specific isoforms of protein kinase C in activation of the c-fos serum response element. Mol Cell Biol 19: 1313–1324, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stephens L, Anderson K, Stokoe D, Erdjument-Bromage H, Painter GF, Holmes AB, Gaffney PRJ, Reese CB, McCormick F, Tempst P, Coadwell J, Hawkins PT. Protein kinase B kinases that mediate phosphatidylinositol 3,4,5-trisphosphate-dependent activations on protein kinase B. Science 279: 710–714, 1998 [DOI] [PubMed] [Google Scholar]
- 34.Stokoe D, Stephens LR, Copeland T, Gaffney PRJ, Reese CB, Painter GF, Holmes AB, McCormick F, Hawkins PT. Dual role of phosphatidylinositol-3,4,5-trisphosphate in the activation of protein kinase B. Science 277: 567–570, 1997 [DOI] [PubMed] [Google Scholar]
- 35.Toker A, Meyer M, Reddy KK, Falck JR, Aneja R, Aneja S, Parra A, Burns DJ, Ballas LM, Cantley LC. Activation of protein kinase C family members by the novel polyphosphoinositides PtdIns-3,4-P2 and PtdIns-3,4,5-P3. J Biol Chem 269: 32358–32367, 1994 [PubMed] [Google Scholar]
- 36.Webster CR, Srinivasulu U, Ananthanarayanan M, Suchy FJ, Anwer MS. Protein kinase B/Akt mediates cAMP- and cell swelling-stimulated Na+/taurocholate cotransport and Ntcp translocation. J Biol Chem 277: 28578–28583, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Webster CR, Usechak P, Anwer MS. cAMP inhibits bile acid-induced apoptosis by blocking caspase activation and cytochrome c release. Am J Physiol Gastrointest Liver Physiol 283: G727–G738, 2002 [DOI] [PubMed] [Google Scholar]
- 38.Webster CRL, Anwer MS. Role of the PI3K/PKB signaling pathway in cAMP-mediated translocation of rat liver Ntcp. Am J Physiol Gastrointest Liver Physiol 277: G1165–G1172, 1999 [DOI] [PubMed] [Google Scholar]
- 39.Webster CRL, Blanch CJ, Philips J, Anwer MS. Cell swelling-induced translocation of rat liver Na+/taurocholate cotransport polypeptide is mediated via the phosphoinositide 3-kinase signaling pathway. J Biol Chem 275: 29754–29760, 2000 [DOI] [PubMed] [Google Scholar]
- 40.Xu TR, He G, Dobson K, England K, Rumsby M. Phosphorylation at Ser729 specifies a Golgi localisation for protein kinase C epsilon (PKCepsilon) in 3T3 fibroblasts. Cell Signal 19: 1986–1995, 2007 [DOI] [PubMed] [Google Scholar]