Abstract
Transcranial direct current stimulation (tDCS) is a novel intervention that can modulate brain excitability in health and disease; however, little is known about its effects on bilaterally innervated systems such as pharyngeal motor cortex. Here, we assess the effects of differing doses of tDCS on the physiology of healthy human pharyngeal motor cortex as a prelude to designing a therapeutic intervention in dysphagic patients. Healthy subjects (n = 17) underwent seven regimens of tDCS (anodal 10 min 1 mA, cathodal 10 min 1 mA, anodal 10 min 1.5 mA, cathodal 10 min 1.5 mA, anodal 20 min 1 mA, cathodal 20 min 1 mA, Sham) on separate days, in a double blind randomized order. Bihemispheric motor evoked potential (MEP) responses to single-pulse transcranial magnetic stimulation (TMS) as well as intracortical facilitation (ICF) and inhibition (ICI) were recorded using a swallowed pharyngeal catheter before and up to 60 min following the tDCS. Compared with sham, both 10 min 1.5 mA and 20 min 1 mA anodal stimulation induced increases in cortical excitability in the stimulated hemisphere (+44 ± 17% and +59 ± 16%, respectively; P < 0.005) whereas only 10 min 1.5 mA cathodal stimulation induced inhibition (−26 ± 4%, P = 0.02). There were neither contralateral hemisphere changes nor any evidence for ICI or ICF in driving the ipsilateral effects. In conclusion, anodal tDCS can alter pharyngeal motor cortex excitability in an intensity-dependent manner, with little evidence for transcallosal spread. Anodal stimulation may therefore provide a useful means of stimulating pharyngeal cortex and promoting recovery in dysphagic patients.
Keywords: swallowing, pharynx, plasticity
TRANSLATIONAL HIGHLIGHTS Dysphagia or swallowing problems are common after neurological damage such as stroke, being both distressing and difficult to treat. This study tested whether transcranial direct current stimulation (tDCS), a simple, noninvasive method of modulating excitability within the cortex, would affect pharyngeal motor cortex in healthy individuals. tDCS is able to both excite and inhibit pharyngeal motor cortex depending on the placement of the electrodes and specific stimulation parameters, suggesting potential therapeutic benefit in dysphagia after brain damage.
Transcranial direct current stimulation (tDCS) is a novel, noninvasive brain stimulation technique that delivers a small electric current continuously across the cerebral cortex. It appears to be both safe and well tolerated (10, 34) and is able to directly alter excitability within the brain for periods outlasting the duration of stimulation (20, 33). When used to modulate excitability within the hand motor cortex, tDCS has been shown to have physiological and functional effects on both the stimulated (4, 18, 23, 29) and unstimulated (contralateral) hemispheres (24, 41) and is also able to improve motor function following stroke (3, 9, 16, 25).
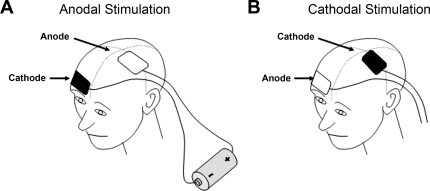
Of importance, the direction of change of excitability (excitation vs. inhibition) within the cortex is determined by the direction of current flow between the electrodes. Excitation typically occurs when the anode is placed over the motor cortex and the cathode over the supraorbital ridge (“anodal” tDCS), whereas inhibition is more pronounced when the current flow is reversed (“cathodal” tDCS) (Fig. 1) (30). The size and duration of these modulatory effects also appear to depend on a combination of current strength and duration (2, 29, 30, 32). An additional advantage of tDCS is that it provides a useful tool for placebo-controlled studies, since subjects are unable to easily distinguish between real and sham stimulation, the latter applied as 30 s of tDCS, which is enough to produce the initial sensation but no lasting change in cortical excitability (10).
Fig. 1.
Schematic demonstrating the principles of transcranial direct current stimulation (tDCS) including the placement of electrodes for anodal tDCS (A) and cathodal tDCS (B) of left hemisphere motor cortex.
Most studies with tDCS have focused on peripheral somatic motor systems, including the upper limb and paresis after stroke. By contrast, swallowing is a complex process incorporating many different muscles and cranial nerves, with oral, pharyngeal, and esophageal stages. Moreover, dysphagia is a common and distressing problem following neurological injury, and patients with swallowing problems following a stroke have a significantly higher morbidity and mortality compared with their nondysphagic peers (38). However, there is little evidence for the efficacy of current treatments and therefore any new therapy that has the potential to make a significant difference to the quality of life for these patients would be welcomed. Of relevance, the numerous pharyngeal muscles involved in swallowing are under bilateral, albeit asymmetric, cortical control (14). It has therefore been postulated that a lesion, for example stroke, to the hemisphere exerting the greatest control over swallowing produces dysphagia, whereas a lesion to the nondominant hemisphere appears to have no functional effect on swallowing (12). In addition, those patients whose swallowing improves poststroke show plasticity within the unaffected hemisphere concomitant with functional recovery (13). We have previously demonstrated that it is possible to modulate both excitability within the pharyngeal motor cortex and swallowing behavior, using other forms of stimulation including pharyngeal stimulation, repetitive transcranial magnetic stimulation (rTMS), and paired associative stimulation (8, 11, 26, 37), and more recently rTMS to pharyngeal motor cortex has been shown to improve dysphagia following stroke (21, 40). However, unlike with limb muscles, the effects of tDCS on areas of cortex controlling midline structures such as the pharynx have not yet been investigated.
Our aim was therefore to examine the effects of differing doses of tDCS to determine the optimal stimulation parameters for excitation and inhibition, as a prelude to studying the therapeutic effects of tDCS in dysphagic stroke patients.
METHODS
Subjects.
Seventeen healthy volunteers were recruited (10 female, 7 male, mean age 37.6 yr, range 22–60 yr). Sixteen were right handed. Inclusion criteria were: age over 18 yr, being in good health, and able to give written, informed consent. Exclusion criteria included a history of epilepsy, cardiac pacemaker, previous brain surgery, previous swallowing problems, pregnancy, metal in the head or eyes, or use of medication that acts on the central nervous system. An information sheet was given to the subjects prior to obtaining consent. Ethical approval was obtained from Salford and Trafford Local Research Ethics Committee and the study was in compliance with the Declaration of Helsinki.
Pharyngeal and thenar motor evoked potential measurements.
Pharyngeal motor evoked potentials (MEPs) were recorded through a swallowed 3.2-mm-diameter intraluminal catheter (Gaeltec, Dunvegan, Isle of Skye, UK) passed either transnasally or transorally depending on the subject's preference. The catheter houses a pair of bipolar platinum ring electrodes that were positioned in the pharynx to record the MEPs by intraluminal contact. The catheter was connected via a preamplifier and interface to a personal computer that recorded the traces using Signal Application Program 2.13 (Cambridge Electronic Design, Cambridge, UK). Analysis of the amplitude of the MEPs was also conducted using Signal Application Program. For thenar MEP recordings, surface electrodes were placed over the abductor digiti minimi (ADM) muscle of the hand contralateral to the hemisphere evoking the greatest pharyngeal MEPs.
TMS.
To assess cortical physiology, single-pulse transcranial magnetic stimulation (TMS) was employed and delivered through a Magstim 200 stimulator (Magstim, Whitland, Wales) connected to a 7-cm-diameter figure of eight coil. The cranial vertex was marked on the scalp, and the magnetic stimulator was initially discharged over both cortices to identify the site evoking the greatest pharyngeal response in each hemisphere. These sites were then also marked on the scalp. The pharyngeal motor threshold for each hemisphere was identified by using single pulses of stimulation to achieve evoked potentials of at least 20 μv on 50% of occasions. The scalp site evoking the greatest ADM response was then determined and marked as above. The TMS intensity capable of evoking MEP responses in ADM of ∼1 mV on 50% of occasions was then determined by using single pulses of TMS. Single pulse TMS at 10% of stimulator output above motor threshold was used at each time point to record 10 MEPs for each site (stimulated hemisphere, unstimulated hemisphere, and ADM). Threshold + 10% was chosen because it produces pharyngeal traces of consistent amplitude and, since pharyngeal thresholds are generally high, stimulating much higher than this can become uncomfortable, which may interfere with changes in cortical excitability.
In addition to corticocortical excitability, intracortical inhibition (ICI) and intracortical facilitation (ICF) were also measured by using paired pulses of TMS over the stimulated pharyngeal motor cortex to assess cortical properties to stimulation. Briefly, shorter intervals (3–5 ms) between stimuli produce ICI, which is thought to involve GABAergic mechanisms, whereas longer intervals (10–20 ms) tend to produce ICF, thought to involve glutamatergic mechanisms. ICI and ICF thus give a measure of whether any cortical change is being driven by increases or decreases in intracortical properties of inhibition or excitation. This employed a conditioning pulse given at 80% of pharyngeal motor threshold and a test pulse at 120% of pharyngeal motor threshold. The pairs were delivered at interstimulus intervals of 3, 5, 10, 15, and 20 ms with three pulses at each interstimulus interval, and the sizes of the resulting MEPs were recorded, averaged, and compared with the MEPs evoked by test pulses alone.
tDCS.
tDCS produces a weak electric current that, when applied to the scalp of humans, penetrates the brain to modify neuronal transmembrane potentials, thereby influencing the level of excitability and modulating firing (Fig. 1). This was delivered through the application of a custom-made device (Department of Medical Physics, Salford Royal NHS Foundation Trust) through two 25-cm2 rectangular surface electrodes (current density 0.04 mA/cm2 at 1 mA and 0.06 mA/cm2 at 1.5 mA), one placed over the “pharyngeal” area of the motor cortex producing the largest MEPs and the other overlying the contralateral supraorbital ridge. A water-soaked sponge was placed beneath the electrodes to optimize contact with the scalp, and the electrodes were held in place by adjustable rubber straps placed around the head. For the active interventions, the current was slowly ramped up to either 1 or 1.5 mA over 10 s, eliciting a transient tingling sensation, and remained on for either 10 or 20 min (depending on the protocol), before being slowly turned off over 10 s. For the sham intervention, the current was left on for 30 s, then switched off [this regimen evokes the same initial tingling sensation as active stimulation, without generating any significant cortical stimulation (10)], with the electrodes being left in place for a further 10 min.
Experimental protocol 1: low-intensity tDCS.
Since initial studies with tDCS in the hand motor system utilized a low-intensity paradigm (1 mA for 10 min) to modify motor cortex, our initial investigation duplicated these parameters in the pharyngeal motor system. Thus, for this protocol, subjects (n = 11) were seated in a comfortable reclining chair, the pharyngeal catheter was sited, and motor hot spots and thresholds for pharynx and hand were determined per the TMS methods outlined above. The hemisphere evoking the largest pharyngeal MEPs was then defined as the stimulated hemisphere. Baseline responses were recorded in response to single-pulse TMS over each hemisphere for pharynx and over the stimulated hemisphere for ADM.
Over three different sessions, the volunteer received the tDCS intervention in each of anodal (anode electrode placed over motor cortex), cathodal (cathode electrode placed over motor cortex), or sham (anode over motor cortex) positions. The studies took place on separate days with at least 4 days between each, and the order of the studies was randomly assigned for each participant. The interventions were given by an independent researcher and double blinded. In both the anodal and cathodal positions the stimulation was given for 10 min at 1 mA; sham stimulation was given as described in the methods above. Changes in TMS-evoked pharyngeal and thenar MEP amplitudes following tDCS were assessed immediately and then at 15, 30, 60, and 90 min.
Experimental protocol 2: higher intensity tDCS.
Because low-intensity tDCS was likely to be subthreshold for modulation of pharyngeal motor cortex, we also investigated a range of higher intensities and durations of tDCS. Thus subjects (n = 13) were studied as described in experimental protocol 1, but with increases to either the duration or intensity of tDCS as follows: anodal stimulation for 10 min at 1.5 mA; anodal stimulation for 20 min at 1 mA; cathodal stimulation for 10 min at 1.5 mA; cathodal stimulation for 20 min at 1 mA; and sham stimulation (see above).
The interventions were again given in a randomized order and double blinded. Changes in MEP amplitudes induced by the stimulation were assessed immediately following the intervention and then at 15, 30, and 60 min. In addition, ICI and ICF measurements were recorded from the stimulated hemisphere at baseline, immediately following tDCS and at 15, 30, and 60 min poststimulation to assess mechanisms of brain excitability in the pharyngeal system.
Data analysis.
The amplitude was defined as the maximum peak-to-peak voltage of the MEP. The amplitudes of individual MEPs in each group of 10 traces were determined and then averaged, to give a measure of cortical excitability. To minimize the interindividual variability in the amplitude of pharyngeal MEP responses, these data were normalized to baseline and are expressed in the results as a percentage change from baseline. For ICI and ICF three MEPs were recorded and averaged at each interstimulus interval (3, 5, 10, 15, and 20 ms) and compared with the averaged single test pulses. For each interstimulus interval the amplitude was then expressed as a proportion of the test pulse. Values for ICI were calculated by averaging the combined results for 3- and 5-ms interstimulus intervals, whereas ICF values were an average of the combined results from 10, 15, and 20 ms. Data are shown as means ± SE unless stated otherwise.
Statistical methods.
The statistical calculations were performed using SPSS (SPSS, Chicago, IL). Changes in excitability over time between the different groups and sham were compared by using a General Linear Models repeated-measures ANOVA. Where comparisons were made between single data points, a paired t-test was used. Statistical significance was taken as P < 0.05.
RESULTS
All subjects tolerated the TMS and tDCS procedures well. Average pharyngeal motor threshold to TMS was 62% (± 2%) of stimulator output over the dominant hemisphere (range 44–79%), 47% (± 3%) for the adjacent ADM (range 32–78%), and 69% (± 4%) for the nondominant hemisphere (range 45–91%). The average distance from the vertex to the site of maximal pharyngeal response (at the anterior bifurcation of the coil) for the right hemisphere was 3.9 cm lateral and 2.3 cm anterior, for the left hemisphere 4.1 cm lateral and 2.0 cm anterior, and for ADM responses 4.3 cm lateral and 2.4 cm anterior. The average impedance during tDCS was 10 kΩ (± 1 kΩ, range 7–14 kΩ).
Experiment 1: low-intensity tDCS.
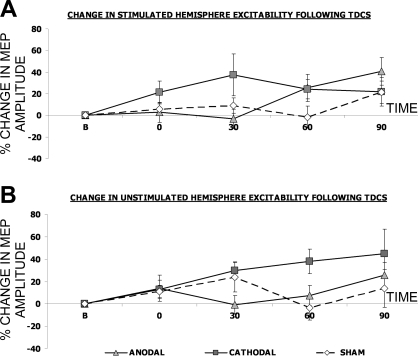
Compared with sham, anodal tDCS at 1 mA for 10 min had no effects on either the stimulated or the unstimulated pharyngeal motor cortex [F(1,10) = 0.8, P = 0.39 and F(1,10) < 0.01, P = 0.99, respectively] (Fig. 2 and Table 1). Although cathodal tDCS appeared to change both the stimulated and unstimulated hemispheres (Fig. 2) neither effect was significant [F(1,10) = 2.6, P = 0.14 and F(1,10) = 2.3, P = 0.12, respectively] and the appearances were largely due to variability. Furthermore, compared with sham, neither anodal nor cathodal tDCS for 10 min at 1 mA evoked any significant changes in cortical excitability for the control (ADM) muscle [F(1,10) = 1.2, P = 0.30 and F(1,10) = 1.2, P = 0.31, respectively; Table 2].
Fig. 2.
Percentage change (means ± SE) in pharyngeal motor evoked potential (MEP) amplitudes in response to single-pulse transcranial magnetic stimulation (TMS) over the stimulated hemisphere (A) and the unstimulated hemisphere (B), following either anodal (triangles), cathodal (squares), or sham (diamonds) tDCS given for 10 min at 1 mA tDCS. There were no significant changes from baseline.
Table 1.
Change in pharyngeal corticobulbar excitability of the unstimulated hemisphere after tDCS
| Immediate | 15 min | 30 min | 60 min | |
|---|---|---|---|---|
| Anodal 10 min 1 mA | 9±12 | −2±11 | −4±9 | 2±10 |
| Cathodal 10 min 1 mA | 13±8 | 47±14 | 30±8 | 38±11 |
| Anodal 10 min 1.5 mA | 7±14 | −13±9 | 14±10 | 4±10 |
| Cathodal 10 min 1.5 mA | −3±6 | −14±6 | −4±5 | 16±8 |
| Anodal 20 min 1 mA | 17±9 | 18±10 | −1±8 | 20±12 |
| Cathodal 20 min 1 mA | 2±9 | −18±6 | −4±5 | 0±8 |
| Sham | 1±6 | −14±6 | 10±5 | −1±5 |
Values show percent change from baseline (means ± SE), with no significant changes seen. tDCS, transcranial direct-current stimulation.
Table 2.
Change in corticospinal excitability of contralateral ADM after tDCS to pharyngeal motor cortex
| Immediate | 15 min | 30 min | 60 min | |
|---|---|---|---|---|
| Anodal 10 min 1 mA | 5±15 | −7±12 | 8±17 | 28±21 |
| Cathodal 10 min 1 mA | −3±12 | −5±10 | 11±13 | 13±12 |
| Anodal 10 min 1.5 mA | 12±8 | 14±11 | 30±14 | 23±8 |
| Cathodal 10 min 1.5 mA | 2±11 | 11±9 | −2±8 | 11±9 |
| Anodal 20 min 1 mA | −8±11 | 13±11 | 15±12 | 13±12 |
| Cathodal 20 min 1 mA | 4±9 | 8±13 | 9±21 | −6±6 |
| Sham | 1±7 | −3±6 | 20±14 | 14±14 |
Values show percent change from baseline (means ± SE), with no significant changes seen. ADM, abductor digiti minimi.
Experiment 2: higher intensity tDCS.
As with experiment 1, sham tDCS elicited no change in the amplitude of MEPs from either the pharyngeal or ADM sites (Figs. 3 and 4, and Table 2).
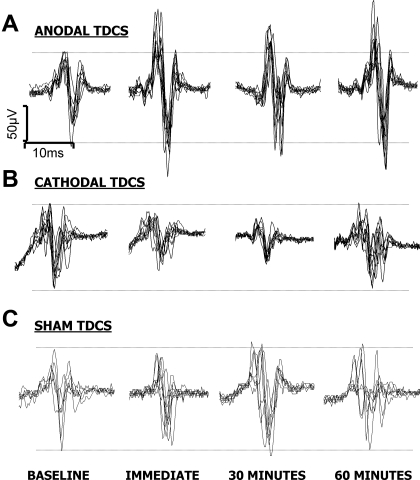
Fig. 3.
Overlaid traces of pharyngeal MEPs from the stimulated hemisphere of 1 subject, showing typical responses to 3 different tDCS paradigms [sham (C), anodal (A), and cathodal (B)]. Note the changes in amplitude following anodal and cathodal stimulation, which are not seen with sham stimulation alone.
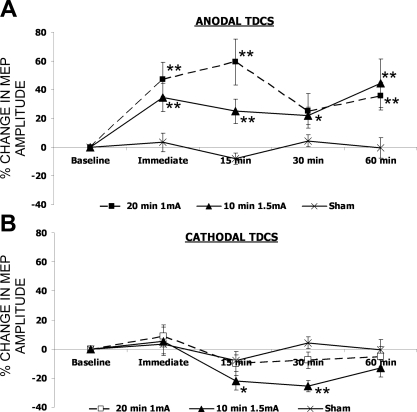
Fig. 4.
Percentage (means ± SE) change from baseline of stimulated hemisphere pharyngeal motor cortex excitability following anodal (A) or cathodal (B) tDCS for 20 min at 1 mA (squares), 10 min at 1.5 mA (triangles), or sham stimulation (cross). Significant changes in excitability are seen following 10 min 1.5 mA, 20 min 1 mA anodal, and 10 min 1.5 mA cathodal stimulation with repeated-measures ANOVA; *t-test P < 0.05 compared with baseline, **t-tests P < 0.05 compared with both baseline and sham.
Increasing either the duration of anodal tDCS to 20 min or the intensity to 1.5 mA produced an increase in corticobulbar excitability to the pharynx in the stimulated hemisphere compared with sham [F(1,12) = 18, P = 0.001 and F(1,12) = 16, P = 0.002, respectively] (Fig. 4). By contrast, pharyngeal excitability of the unstimulated hemisphere and excitability in ADM was unchanged [F(4,48) = 1.4, P = 0.25 and F(4,48) = 0.6, P = 0.65, respectively (Tables 1 and 2) ]. Following both anodal tDCS paradigms, excitability remained significantly elevated at 60 min poststimulation compared with both baseline and sham stimulation (P = 0.02 and P = 0.03, respectively; Fig. 4).
In comparison, only cathodal tDCS given at 1.5 mA for 10 min produced any significant decrease in pharyngeal excitability compared with sham [F(1,12) = 7, P = 0.02] (Figs. 3 and 4), with significant decreases in excitability compared with baseline seen at 15 and 30 min following tDCS (P = 0.006 and P < 0.001) and significant difference to sham stimulation at 30 min (P < 0.001, Fig. 4). There was no change in MEP amplitude for ADM (Table 2).
Changes in ICI and ICF.
There were no consistent changes for either ICI [anodal 1.5 mA 10 min F(1,12) = 2, P = 0.20; anodal 1 mA 20 min F(1,12) = 0.3, P = 0.60; cathodal 1.5 mA 10 min F(1,12) = 0.6, P = 0.46; cathodal 1 mA 20 min F(1,12) = 0.6, P = 0.46] or ICF [anodal 1.5 mA 10 min F(1,12) = 0.3, P = 0.58; anodal 1 mA 20 min F(1,12) = 0.04, P = 0.85; cathodal 1.5 mA 10 min F(1,12) = 2, P = 0.14; cathodal 1 mA 20 min F(1,12) = 2, P = 0.24].
DISCUSSION
These studies demonstrate that, in contrast to hand motor cortex, tDCS at 1 mA for 10 min is unable to excite or inhibit the pharyngeal motor cortex. However, increasing either the duration or intensity of tDCS above this level did induce changes in pharyngeal excitability and thus these observations merit discussion.
Although we recognized from previous studies using other neurostimulation techniques that higher intensities or durations of stimulation are often required to generate the same magnitude of response in pharyngeal motor cortex as seen in the hand motor cortex (11, 19, 26), we originally hypothesized that 10 min of tDCS, given at 1 mA, would modulate corticobulbar excitability as it does in the hand motor cortex (33), especially since we were stimulating with a slightly higher current density [0.04 mA/cm2 compared with the previously reported and effective level of 0.03 mA/cm2 (33)]. However, only when the duration of anodal stimulation reached 20 min or when a higher intensity was applied were we able to increase corticobulbar excitability. We were also aware, from previous work in our department, that it is particularly difficult to suppress excitability within the pharyngeal motor cortex (26), perhaps because of its strong interhemispheric connections and bilateral innervation pattern. It is therefore unsurprising that the lower levels of cathodal stimulation were ineffective, and although we could suppress excitability when the cathodal stimulation was increased to 1.5 mA, the changes were less pronounced and of shorter duration than with anodal stimulation. Other groups have looked at nonhand regions of motor cortex. For example, the excitatory and inhibitory effects of tDCS on leg motor cortex have been described, and although excitation could be provoked with anodal stimulation, even at 2 mA for 10 min they were unable to produce inhibition with cathodal stimulation (20). This supports the idea that motor cortical areas other than the hand area may respond differently to tDCS or require greater stimulation. Consequently, we might propose that further increasing both the intensity and duration of stimulation would lead to greater changes of excitability within the pharyngeal motor cortex; however, this then leads to potential safety issues (27).
Although functional imaging studies suggest that tDCS over the motor cortex can have widespread effects (22, 24) as well as modulating excitability within the primary motor cortex itself (18), we found no change in excitability of the contralateral pharyngeal motor cortex. This is in contrast to rTMS and paired associative stimulation of pharyngeal motor cortex, which do appear to have transcallosal effects (11, 37), although the degree of excitability is always greater in the stimulated hemisphere. Indeed, other groups looking at the hand motor areas have also failed to demonstrate bilateral effects of tDCS to primary motor cortex (23), implying that transcallosal pathways may not be as susceptible to the neural excitation properties of tDCS compared with TMS. Moreover, the mechanisms by which TMS and tDCS excite the brain have been explored and it has been suggested that the short-term effects of tDCS are driven by polarity-specific shifts of the resting membrane potential; in contrast, TMS excites the myelinated axonal membrane, thus generating transsynaptic effects (1, 32, 35). It is therefore conceivable that transcallosal excitation is more dependent on the latter mechanism, making cross-hemisphere effects less responsive to tDCS.
Despite a trend toward both increasing ICF following anodal stimulation and decreasing ICF and increasing ICI following cathodal tDCS, these changes were not statistically robust. Only a few studies have measured ICI and ICF following tDCS; Nitsche et al. (32) showed variation in both ICI and ICF following tDCS (although only with certain interstimulus intervals), whereas Siebner et al. were unable to demonstrate any alteration in ICI and ICF (36). To minimize potential confounding effects of multiple TMS pulses we used the same conditioning and test pulse intensities before and after stimulation with tDCS. We also limited measurement of ICI and ICF to three pairs of stimuli at each interstimulus interval and then averaged the inhibitory (3, 5 ms) and excitatory (10, 15, 20 ms) results. Given the inherent variability of MEPs there is a risk that this may have diluted the effects and we may have missed small changes at specific interstimulus intervals; however, the same methodology has been used previously in pharyngeal cortex and did demonstrate changes in ICI and ICF following rTMS (19). Magnetic resonance spectroscopy studies examining the effects of tDCS on hand motor cortex have shown that anodal stimulation causes a decrease in GABA, whereas cathodal stimulation decreases the levels of both glutamate and GABA (39). In addition, when a GABAA agonist is administered, anodal tDCS produces a delayed but enhanced increase in excitability, leading Nitsche et al. (28) to propose that this late excitability may therefore originate from remote cortical or subcortical areas. As such, there are likely to be complex interactions in considering the mechanisms by which tDCS alters excitability in motor cortex; given the important and extensive connections within pharyngeal motor cortex to multiple brain regions, this is likely to also be the case for this region of cortex. Perhaps future studies utilizing magnetic resonance spectroscopy may help further elucidate the mechanisms by which tDCS is acting on the pharyngeal motor cortex (37).
tDCS has also been used in combination with other neurostimulation techniques to modulate motor cortical excitability of the hand; the precise degree and direction of the effects appear to depend on the type of costimulation (repetitive transcranial magnetic stimulation vs. paired associative stimulation), timing of stimulation (simultaneous vs. preconditioning), and brain function (healthy vs. stroke) (5, 31, 36). In paretic stroke patients, both anodal tDCS to the affected hemisphere and cathodal tDCS to the unaffected hemisphere are able to improve hand function (3, 16, 17). Moreover, combining tDCS with peripheral stimulation or motor training further enhances the functional improvement (5, 6, 15). Now that we have demonstrated the significant effects of tDCS alone on pharyngeal motor cortex, we are drawn to hypothesize that, in combination with rTMS, paired associative stimulation or pharyngeal electrical stimulation, these effects could be greatly enhanced. Pharyngeal motor cortex plays an integral role in the control of normal swallowing function, and other neurostimulation techniques have shown that stimulation of pharyngeal cortex is able to improve dysphagia following stroke (7, 21, 40). Thus tDCS may also prove to be a valuable therapeutic tool for the treatment of poststroke dysphagia. However, the physiological differences, and recovery patterns of bilaterally innervated musculature should be taken into account. Hence our work supports the view that anodal stimulation of the unaffected hemisphere may be of greater benefit in treating dysphagia after unilateral hemispheric stroke than trying to inhibit maladaptive changes in the undamaged hemisphere with cathodal stimulation or through the direct application of anodal stimulation to the affected hemisphere.
In conclusion, we have been able to modulate plasticity within the areas of primary motor cortex controlling the pharynx in a manner dependent on the polarity, intensity, and duration of TDCS. These effects are similar in magnitude to those elicited by pharyngeal electrical stimulation, paired associative stimulation, and rTMS (8, 11, 19, 37). Moreover, the stimulation technique appears to be both safe and well tolerated (34), in addition to needing only small and easily transportable equipment, making it ideal for bedside use in patients. Therefore anodal tDCS, possibly in conjunction with other forms of neurostimulation, may provide a useful means of stimulating pharyngeal cortex and promoting recovery in dysphagic patients in the wider clinical setting.
GRANTS
This research was supported by Action Medical Research and The Wellcome Trust.
DISCLOSURES
No conflicts of interest are declared by the author(s).
ACKNOWLEDGMENTS
We thank the Medical Physics Department, Salford Royal Hospital, Salford, UK.
REFERENCES
- 1.Amassian VE, Maccabee PJ. Transcranial magnetic stimulation. Conf Proc IEEE Eng Med Biol Soc, p. 1620–1623, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Antal A, Kincses TZ, Nitsche MA, Bartfai O, Paulus W. Excitability changes induced in the human primary visual cortex by transcranial direct current stimulation: direct electrophysiological evidence. Invest Ophthalmol Vis Sci 45: 702–707, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Boggio PS, Nunes A, Rigonatti SP, Nitsche MA, Pascual-Leone A, Fregni F. Repeated sessions of noninvasive brain DC stimulation are associated with motor function improvement in stroke patients. Restor Neurol Neurosci 25: 123–129, 2007 [PubMed] [Google Scholar]
- 4.Boros K, Poreisz C, Munchau A, Paulus W, Nitsche MA. Premotor transcranial direct current stimulation (tDCS) affects primary motor excitability in humans. Eur J Neurosci 27: 1292–1300, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Celnik P, Paik NJ, Vandermeeren Y, Dimyan M, Cohen LG. Effects of combined peripheral nerve stimulation and brain polarization on performance of a motor sequence task after chronic stroke. Stroke 40: 1764–1771, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edwards DJ, Krebs HI, Rykman A, Zipse J, Thickbroom GW, Mastaglia FL, Pascual-Leone A, Volpe BT. Raised corticomotor excitability of M1 forearm area following anodal tDCS is sustained during robotic wrist therapy in chronic stroke. Restor Neurol Neurosci 27: 199–207, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fraser C, Power M, Hamdy S, Rothwell J, Hobday D, Hollander I, Tyrell P, Hobson A, Williams S, Thompson D. Driving plasticity in human adult motor cortex is associated with improved motor function after brain injury. Neuron 34: 831–840, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Fraser C, Rothwell J, Power M, Hobson A, Thompson D, Hamdy S. Differential changes in human pharyngoesophageal motor excitability induced by swallowing, pharyngeal stimulation, and anesthesia. Am J Physiol Gastrointest Liver Physiol 285: G137–G144, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Fregni F, Boggio P, Mansur C, Wagner T, Ferreira M, Lima M, Rigonatti S, Marcolin M, Freedman S, Nitsche M, Pascual-Leone A. Transcranial direct current stimulation of the unaffected hemisphere in stroke patients. Neuroreport 16: 1551–1555, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Gandiga PC, Hummel FC, Cohen LG. Transcranial DC stimulation (tDCS): a tool for double-blind sham-controlled clinical studies in brain stimulation. Clin Neurophysiol 117: 845–850, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Gow D, Rothwell J, Hobson A, Thompson D, Hamdy S. Induction of long-term plasticity in human swallowing motor cortex following repetitive cortical stimulation. Clin Neurophysiol 115: 1044–1051, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Hamdy S, Aziz Q, Rothwell JC, Crone R, Hughes D, Tallis RC, Thompson DG. Explaining oropharyngeal dysphagia after unilateral hemispheric stroke. Lancet 350: 686–692, 1997 [DOI] [PubMed] [Google Scholar]
- 13.Hamdy S, Aziz Q, Rothwell JC, Power M, Singh KD, Nicholson DA, Tallis RC, Thompson DG. Recovery of swallowing after dysphagic stroke relates to functional reorganization in the intact motor cortex. Gastroenterology 115: 1104–1112, 1998 [DOI] [PubMed] [Google Scholar]
- 14.Hamdy S, Rothwell JC, Aziz Q, Thompson DG. Organization and reorganization of human swallowing motor cortex: implications for recovery after stroke. Clin Sci (Lond) 99: 151–157, 2000 [PubMed] [Google Scholar]
- 15.Hesse S, Werner C, Schonhardt EM, Bardeleben A, Jenrich W, Kirker SG. Combined transcranial direct current stimulation and robot-assisted arm training in subacute stroke patients: a pilot study. Restor Neurol Neurosci 25: 9–15, 2007 [PubMed] [Google Scholar]
- 16.Hummel F, Celnik P, Giraux P, Floel A, Wu WH, Gerloff C, Cohen LG. Effects of non-invasive cortical stimulation on skilled motor function in chronic stroke. Brain 128: 490–499, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Hummel FC, Voller B, Celnik P, Floel A, Giraux P, Gerloff C, Cohen LG. Effects of brain polarization on reaction times and pinch force in chronic stroke. BMC Neurosci 7: 73, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jang SH, Ahn SH, Byun WM, Kim CS, Lee MY, Kwon YH. The effect of transcranial direct current stimulation on the cortical activation by motor task in the human brain: an fMRI study. Neurosci Lett 460: 117–120, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Jefferson S, Mistry S, Michou E, Singh S, Rothwell JC, Hamdy S. Reversal of a virtual lesion in human pharyngeal motor cortex by high frequency contra-lesional brain stimulation. Gastroenterology 137: 841–849, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Jeffery D, Norton J, Roy F, Gorassini M. Effects of transcranial direct current stimulation on the excitability of the leg motor cortex. Exp Brain Res 182: 281–287, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Khedr EM, Abo-Elfetoh N, Rothwell JC. Treatment of post-stroke dysphagia with repetitive transcranial magnetic stimulation. Acta Neurol Scand 119: 155–161, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Kwon YH, Ko MH, Ahn SH, Kim YH, Song JC, Lee CH, Chang MC, Jang SH. Primary motor cortex activation by transcranial direct current stimulation in the human brain. Neurosci Lett 435: 56–59, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Lang N, Nitsche MA, Paulus W, Rothwell JC, Lemon RN. Effects of transcranial direct current stimulation over the human motor cortex on corticospinal and transcallosal excitability. Exp Brain Res 156: 439–443, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Lang N, Siebner HR, Ward NS, Lee L, Nitsche MA, Paulus W, Rothwell JC, Lemon RN, Frackowiak RS. How does transcranial DC stimulation of the primary motor cortex alter regional neuronal activity in the human brain? Eur J Neurosci 22: 495–504, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mansur CG, Fregni F, Boggio P, Riberto M, Gallucci-Neto J, Santos CM, Wagner T, Rigonatti SP, Marcolin MA, Pascual-Leone A. A sham stimulation-controlled trial of rTMS of the unaffected hemisphere in stroke patients. Neurology 64: 1802–1804, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Mistry S, Verin E, Singh S, Jefferson S, Rothwell JC, Thompson DG, Hamdy S. Unilateral suppression of pharyngeal motor cortex to repetitive transcranial magnetic stimulation reveals functional asymmetry in the hemispheric projections to human swallowing. J Physiol 585: 525–538, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nitsche MA, Liebetanz D, Lang N, Antal A, Tergau F, Paulus W. Safety criteria for transcranial direct current stimulation (tDCS) in humans. Clin Neurophysiol 114: 2220–2222; author reply 2222–2223, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Nitsche MA, Liebetanz D, Schlitterlau A, Henschke U, Fricke K, Frommann K, Lang N, Henning S, Paulus W, Tergau F. GABAergic modulation of DC stimulation-induced motor cortex excitability shifts in humans. Eur J Neurosci 19: 2720–2726, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Nitsche MA, Nitsche MS, Klein CC, Tergau F, Rothwell JC, Paulus W. Level of action of cathodal DC polarisation induced inhibition of the human motor cortex. Clin Neurophysiol 114: 600–604, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol 527: 633–639, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nitsche MA, Roth A, Kuo MF, Fischer AK, Liebetanz D, Lang N, Tergau F, Paulus W. Timing-dependent modulation of associative plasticity by general network excitability in the human motor cortex. J Neurosci 27: 3807–3812, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nitsche MA, Seeber A, Frommann K, Klein CC, Rochford C, Nitsche MS, Fricke K, Liebetanz D, Lang N, Antal A, Paulus W, Tergau F. Modulating parameters of excitability during and after transcranial direct current stimulation of the human motor cortex. J Physiol 568: 291–303, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nitsche MAMD, Paulus WMD. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology 57: 1899–1901, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Poreisz C, Boros K, Antal A, Paulus W. Safety aspects of transcranial direct current stimulation concerning healthy subjects and patients. Brain Res Bull 72: 208–214, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Rothwell JC. Techniques and mechanisms of action of transcranial stimulation of the human motor cortex. J Neurosci Methods 74: 113–122, 1997 [DOI] [PubMed] [Google Scholar]
- 36.Siebner HR, Lang N, Rizzo V, Nitsche MA, Paulus W, Lemon RN, Rothwell JC. Preconditioning of low-frequency repetitive transcranial magnetic stimulation with transcranial direct current stimulation: evidence for homeostatic plasticity in the human motor cortex. J Neurosci 24: 3379–3385, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singh S, Mistry S, Jefferson S, Davies K, Rothwell J, Williams S, Hamdy S. A magnetic resonance spectroscopy study of brain glutamate in a model of plasticity in human pharyngeal motor cortex. Gastroenterology 136: 417–424, 2009 [DOI] [PubMed] [Google Scholar]
- 38.Smithard DG, O'Neill PA, Park C, Morris J, Wyatt R, England R, Martin DF. Complications and outcome after acute stroke: does dysphagia matter? Stroke 27: 1200–1204, 1996 [DOI] [PubMed] [Google Scholar]
- 39.Stagg CJ, Best JG, Stephenson MC, O'Shea J, Wylezinska M, Kincses ZT, Morris PG, Matthews PM, Johansen-Berg H. Polarity-sensitive modulation of cortical neurotransmitters by transcranial stimulation. J Neurosci 29: 5202–5206, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Verin E, Leroi AM. Poststroke dysphagia rehabilitation by repetitive transcranial magnetic stimulation: a noncontrolled pilot study. Dysphagia 24: 204–210, 2009 [DOI] [PubMed] [Google Scholar]
- 41.Vines BW, Nair DG, Schlaug G. Contralateral and ipsilateral motor effects after transcranial direct current stimulation. Neuroreport 17: 671–674, 2006 [DOI] [PubMed] [Google Scholar]