Abstract
The gastrointestinal hormone CCK exists in various molecular forms, with differences in bioactivity between the well-characterized CCK-8 and larger CCK-58 previously reported. We have compared the effects of these peptides on cytosolic calcium concentration ([Ca2+]c), mitochondrial metabolism, enzyme secretion, and cell fate in murine isolated pancreatic acinar cells using fluorescence confocal microscopy and patch-clamp electrophysiology. CCK-58 (1–10 pM) induced transient, oscillatory increases of [Ca2+]c, which showed apical to basolateral progression and were associated with a rise of mitochondrial NAD(P)H. CCK-58 (10 pM) induced zymogen exocytosis in isolated cells and amylase secretion from isolated cells and whole tissues. Hyperstimulation with supraphysiological CCK-58 (5 nM) induced a single large increase of [Ca2+]c that declined to a plateau, which remained above the basal level 20 min after application and was dependent on external Ca2+ entry. In cells dispersed from the same tissues, CCK-8 induced similar patterns of responses to those of CCK-58, with oscillatory increases of [Ca2+]c at lower (pM) concentrations and sustained responses at 5 nM. CCK-58 and CCK-8 exhibited similar profiles of action on cell death, with increases in necrosis at high CCK-58 and CCK-8 (10 nM) that were not significantly different between peptides. The present experiments indicate that CCK-8 and CCK-58 have essentially identical actions on the acinar cell at high and low agonist concentrations, suggesting an action via the same receptor and that the differences observed in an intact rat model may result from indirect effects of the peptides. Our data strengthen the argument that CCK-58 is an important physiological form of this gastrointestinal hormone.
Keywords: mitochondria, necrosis, apoptosis
the pancreatic secretagogue hormone CCK occurs naturally in several molecular forms. Although the shorter octapeptide CCK-8 has been most extensively studied to date, evidence has accumulated to suggest that CCK-58 may be the more physiologically relevant peptide. It has been demonstrated that CCK-58 is the principal circulating type of CCK in humans (8) and dogs (9) and is the only detectable form of the hormone in the rat (26). Although CCK-58 and CCK-8 share an identical primary sequence at the COOH terminus, CCK-58 may possess regions outside that influence its bioactivity (27), and qualitative in vivo differences between the action of these two forms have been previously reported (29, 36).
At physiological concentrations, CCK-8 stimulates pancreatic digestive enzyme secretion via the generation of discrete apical cytosolic calcium concentration ([Ca2+]c) elevations coupled to mitochondrial energy production (24). However, at supraphysiological concentrations, stimulation with CCK-8 leads to development of pancreatitis (35), a widely employed experimental rodent model (31). Interestingly, for both physiological and supraphysiological concentrations of agonist, CCK-58 stimulates more fluid than CCK-8 (36), which may explain why CCK-58 does not cause severe pancreatitis (35).
For many years this gastrointestinal hormone had been thought to act only indirectly to elicit digestive enzyme secretion from the human pancreas. It was proposed that the secretagogue action of CCK occurs exclusively via stimulation of parasympathetic vagal nerves releasing acetylcholine, which in turn activates muscarinic (M3) receptors on pancreatic acinar cells to induce exocrine secretion (7, 18), rather than CCK acting directly to stimulate the acinar cell via its own (CCK1) receptors, as is widely documented in rodent models (24). This view of an indirect action of CCK on human pancreas had been based largely on an inability to detect Ca2+]c responses to CCK-8 stimulation of isolated human pancreatic acinar cells (14). However, this negative result may reflect the difficulty of obtaining high quality cells from resection specimens because supramaximal concentrations of carbachol were required to elicit [Ca2+]c responses in this study (14, 30).
Recent evidence has shown that fully functional CCK receptors are present in human isolated pancreatic acinar cells, the direct activation of which by physiological (pM) CCK-8 induced increases of [Ca2+]c, stimulation of mitochondrial metabolism, and exocytosis of digestive enzymes (17), similar to changes previously characterized in rodent cells (24). Furthermore, CCK-58 also induced elevation of [Ca2+]c in human pancreatic acinar cells (17), and these new findings strongly suggest important common themes within human and animal secretory physiology, delineating the necessity for elucidation of the actions of the different molecular forms of CCK in animal models.
Therefore, in the present study, we have evaluated the in vitro effects of physiological (pM) and supramaximal (nM) concentrations of CCK-58 on [Ca2+]c, mitochondrial function, digestive enzyme secretion, and cell damage in murine isolated pancreatic acinar cells, comparing its actions with those of CCK-8. Our results indicate that the two peptides induce remarkably similar effects, providing fresh evidence for the ability of CCK-58 to act as the principal, physiological form of this gastrointestinal hormone.
MATERIALS AND METHODS
Cell preparation and solutions.
Freshly isolated pancreatic acinar cells and acinar clusters of two or three cells were prepared from the pancreases of adult CD1 mice using collagenase (Worthington Biochemical, Lakewood, NJ) as in our previous work (6). The mice were euthanized by stunning and cervical dislocation. In accordance with the Animals (Scientific Procedures) Act of 1986 (UK), training and overseeing of procedures were conducted by competent personnel from the University of Liverpool (in compliance with national requirements). All experiments were performed at room temperature (23–25°C), and cells were used within 4 h of isolation. The extracellular solution contained (in mM): 140 NaCl, 4.7 KCl, 1.13 MgCl2, 1 CaCl2, 10 D-glucose, and 10 HEPES (adjusted to pH 7.35 using NaOH) (all chemicals highest grade available from Sigma, Gillingham, UK).
Measurements of [Ca2+]C, NAD(P)H, and exocytosis.
Confocal imaging of cells loaded with fluorescent dyes was performed using a Zeiss LSM510 system (Carl Zeiss, Jena, Germany). Freshly dispersed cells were loaded with Fluo 4-AM (3 μM) for 30 min at room temperature to measure [Ca2+]C. The fluorescence of Fluo 4 was excited by an argon 488-nm laser line and emitted light collected from 505–550 nm. Images were composed of 256 × 256 pixels, and optical sections were 5–6 μm. A C-Apochromat ×63 objective with a numerical aperture of 1.2 was used, and image analysis was completed with LSM510 software.
Mitochondrial metabolism was assessed contemporaneously by monitoring changes of reduced NAD(P)H autofluorescence in the perigranular region of the acinar cells using the fast-switching multi-channel mode of the Zeiss LSM510 confocal microscope system (excitation 363 nm, emission 390–450 nm).
Exocytotic responses were measured using quinacrine (10 μM), loaded at 37°C for 10 min, of which selectively stained granules have been used to quantify exocytosis from a variety of cell types including rodent pancreatic acinar cells in response to acetylcholine (excitation 488 nm; emission >505 nm) (2, 20).
Fluorescence measurements are expressed either as absolute values, where appropriate, or as normalized changes from basal fluorescence (F/F0 ratio), where F0 represents the initial fluorescence recorded at the start of the experiment, and F represents the fluorescence recorded at specific time points (n represents the number of cells studied for each experimental protocol).
Measurements of amylase secretion.
Amylase measurements were performed in two ways. For analysis of concentration-dependent CCK secretion, isolated acini were incubated with CCK (1 pM-10 nM) or a control solution for 30 min at 37°C, and the media were reserved. After being washed, cells were permeabilized with Triton X-100 (0.1%) (Calbiochem, San Diego, CA), and the lysate was reserved. Both the secreted fraction and the lysate were tested in 96-well plate format according to manufacturer's instructions (EnzChek Ultra Amylase Assay Kit; Molecular Probes, Eugene, OR) using a Beckman Coulter Biomek dtx880 fluorometer (Beckman Coulter, High Wycombe, UK), and amylase secretion was expressed as a percentage of total amylase. Additionally, online fluorometric measurements were carried out as previously described (17, 22). Briefly, small segments of mouse pancreas (total weight 90 mg) were placed in a Perspex flow chamber (1 ml volume) and superfused with physiological saline solution (1 ml/min) at 37°C. After a period of equilibration, tissues were stimulated with CCK (10 pM) and amylase secretion was measured fluorometrically (excitation 485 nm; emission 520 nm) using amylase substrate [DQ starch from corn, BODIPY FL conjugate (100 μg/ml); EnzChek Ultra Amylase Assay Kit, Molecular Probes], and secretion was expressed as units per minute per milligram of tissue. In the present study, α-amylase was used as a standard for calibration.
Patch-clamp current recording.
The whole cell configuration of the patch-clamp technique was used to record calcium-activated chloride currents (IClCa) from single pancreatic acinar cells as previously described (5). Patch pipettes were pulled from borosilicate glass capillaries (Harvard Apparatus, Edenbridge, Kent, UK) with a resistance of 2–3 MΩ when filled with an intracellular solution of composition (in mM): 140 KCl, 1.5 MgCl2, 2 MgATP, 10 HEPES, and 0.1 EGTA, pH 7.2. Whole-cell currents were sampled at 10 kHz using an EPC8 amplifier and Pulse software (HEKA; Lambrecht, Pfalz, Germany) with the membrane voltage clamped at −30 mV. Cells were stimulated with either CCK-8 or CCK-58 (5 pM–5 nM).
Measurements of cell damage and death.
Detection of early apoptosis and necrosis was performed contemporaneously using dual loading of freshly isolated pancreatic acinar cells. For measurement of apoptosis, cells were loaded with R110-aspartic acid amide (20 μM), a fluorescent indicator-linked general caspase substrate, at room temperature for 30 min, as described previously (4). Propidium iodide (1 μM) staining was employed to detect necrosis; early apoptotic and nonapoptotic cells do not show staining with propidium iodide. After loading, cells were washed and resuspended in calcium-free buffer solution. A portion of cells were then treated with either 10 nM CCK-58 or 10 nM CCK-8 for 30 min at room temperature before fluorescence of R110-aspartic acid amide (excitation 488 nm, emission >505 nm) and propidium iodide (excitation 488 nm and 630–693 nm emission) was recorded using a Zeiss LSM510 confocal microscope system (Carl Zeiss) with a ×63 C-Apochromat water immersion objective, aperture set at 1.2. In separate experiments, freshly dispersed cells were continuously perfused with a high concentration (20 nM) of CCK-58 or CCK-8 on the microscope stage at 37°C for 20 min, and membrane damage (blebbing) was evaluated by the light-transmitted image. At the end of the period of exposure to CCK-58 or CCK-8, rhodamine 110, bis-(CBZ-L-isoleucyl-L-prolyl-L-arginine amide) dihydrochloride (BZiPAR), a cell-permeant substrate for trypsin that becomes fluorescent after cleavage by the protease (excitation 488 nm, emission >505 nm), was applied to the perfusion chamber bath to evaluate intracellular activation of trypsinogen.
Statistical analysis.
Statistical analysis (one-way ANOVA) was performed using the statistical software package Origin 7. A value of P < 0.05 was considered to be significant.
Chemicals.
Human synthetic sulfated CCK-58 was obtained from UCLA Peptide Synthesis Facility (Dr. J. R. Reeve, Jr., Director). The human CCK-58 was synthesized using an Applied Biosystems Peptide Synthesizer (Foster City, CA), unblocked, and purified to >90% as described for the synthesis of rat CCK-58 (29). After confocal imaging, the CCK-58 solution was collected, concentrated, and compared with the starting peptide by reverse-phase HPLC. After the experiment, the CCK-58 eluted in the same position as the CCK-58 starting material, indicating that CCK-8 had not been produced during the exposure to acinar cells.
Fluo 4-AM, R110-aspartic acid amide, and BZiPAR were purchased from Molecular Probes. All other chemicals were from Sigma-Aldrich of the highest grade available.
RESULTS
Effects of CCK-58 and CCK-8 on [Ca2+]c and mitochondrial metabolism.
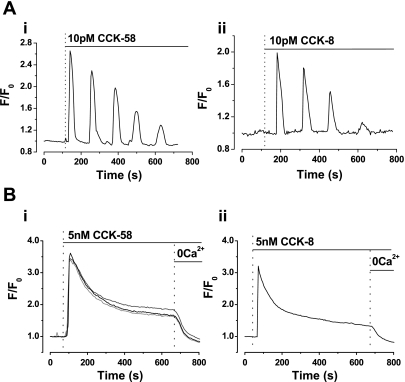
Application of CCK-58 (1–10 pM) caused global, oscillatory increases of [Ca2+]c in isolated acinar cells (34 of 37 cells; Fig. 1A). In contrast, a supraphysiological concentration of CCK-58 (5 nM) induced a single large “spike” increase of [Ca2+]c that declined to a steady plateau and remained above the basal level at 20 min after application. Withdrawal of extracellular Ca2+ from the bathing solution (and addition of 1 mM EGTA) during continued exposure to 5 nM CCK-58 resulted in a return of [Ca2+]c to the prestimulation baseline level, indicating that the plateau was dependent on Ca2+ entry (Fig. 1B; 18 of 18 cells).
Fig. 1.
Comparative effects of low (pM) and high (nM) CCK-58 and CCK-8 on cytosolic calcium concentration ([Ca2+]c) in isolated murine pancreatic acinar cells. Representative traces show that application of 10 pM (A) CCK-58 (i) and CCK-8 (ii) induced oscillatory rises of [Ca2+]c, whereas application of 5 nM (B) CCK-58 (i) (recordings from 3 cells shown) and CCK-8 (ii) evoked single “spike” increases in [Ca2+]c, followed by plateau sustained elevations above the basal level. When extracellular Ca2+ (with EGTA) was removed from the perfusion solution, the plateau increase in [Ca2+]c was reversed to basal levels, indicating the dependence on Ca2+ influx into the cell. F0 represents the initial fluorescence recorded at the start of the experiment, and F represents the fluorescence recorded at specific time points.
In cells dispersed from the same tissues, CCK-8 induced similar patterns of responses to those of CCK-58, with oscillatory increases of cytosolic [Ca2+] at low concentrations (1–10 pM) (12 of 15 cells; Fig. 1B) and sustained elevations at a higher concentration (5 nM) (10 of 10 cells; Fig. 1B).
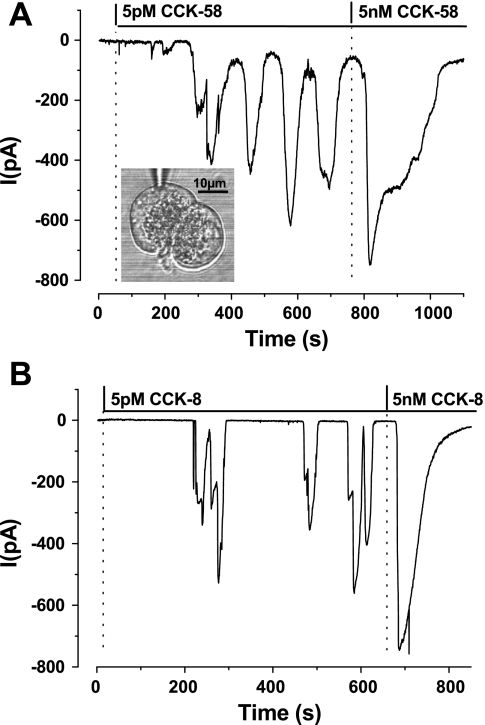
In whole cell patch-clamp experiments, the elevations of [Ca2+]c could also be detected as Ca2+-dependent chloride currents (IClCa), as characterized previously for both CCK-8 and acetylcholine (21). CCK-58 and CCK-8 produced similar oscillatory inward currents at low (pM) concentrations, whereas a single, large and more sustained current was observed in response to higher (nM) concentrations (Fig. 2, A and B).
Fig. 2.
Typical recordings obtained from whole cell patch-clamp experiments, contrasting the effects of CCK-58 (A) (5 pM-5 nM, n = 5) with CCK-8 (B) (5 pM-5 nM, n = 6) to induce Ca2+-dependent chloride currents (IClCa) in isolated pancreatic acinar cells. Cells were held at −30 mV, and CCK was applied once the membrane had been ruptured mechanically by suction. At the lower (5 pM) concentration both CCK-58 and CCK-8 induced oscillatory inward IClCa currents, denoting rises of [Ca2+]c, whereas at 5 nM a larger, broader single current was observed. The inset in A shows a light-transmitted image of a doublet of pancreatic acinar cells with the patch-pipette recording from the upper cell.
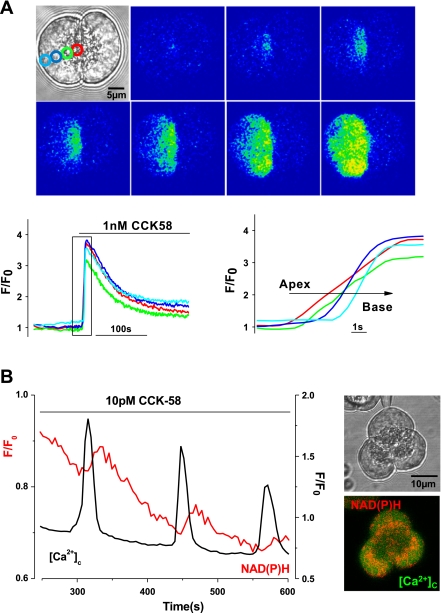
The elevations of [Ca2+]c induced by physiological concentrations of CCK-58 followed patterns characteristic of CCK-8 in this cell type. First, the transient rises of [Ca2+]c exhibited apical to basolateral progression (Fig. 3A). In addition, each rise of [Ca2+]c was associated with a rise of mitochondrial NAD(P)H autofluorescence in the perigranular region (Fig. 3B), denoting the process of stimulus-metabolism coupling that generates ATP necessary for digestive enzyme secretion (24).
Fig. 3.
Characteristics of CCK-58-induced elevations of [Ca2+]c in isolated pancreatic acinar cells. A: typical fluorescent images and traces showing the apical to basal progression of the rise in [Ca2+]c recorded in 1 of a doublet of acinar cells stimulated by CCK-58 (1 nM, n = 6). Regions of interest for measurement of the apical (red) to basal (blue) [Ca2+]c signals are shown in the inset light-transmitted image. B: typical trace showing the stimulus-metabolism coupling induced by 10 pM CCK-58. After each elevation of [Ca2+]c induced by CCK-58, a slower rise of NAD(P)H (red) was observed. The perigranular distribution of the mitochondrial belt can be seen clearly by the NAD(P)H (red) in the inset fluorescent image of an acinar cell triplet. Similar responses to 10 pM CCK-8 were observed in separate experiments (n = 6).
Effects of CCK-58 and CCK-8 on digestive enzyme secretion.
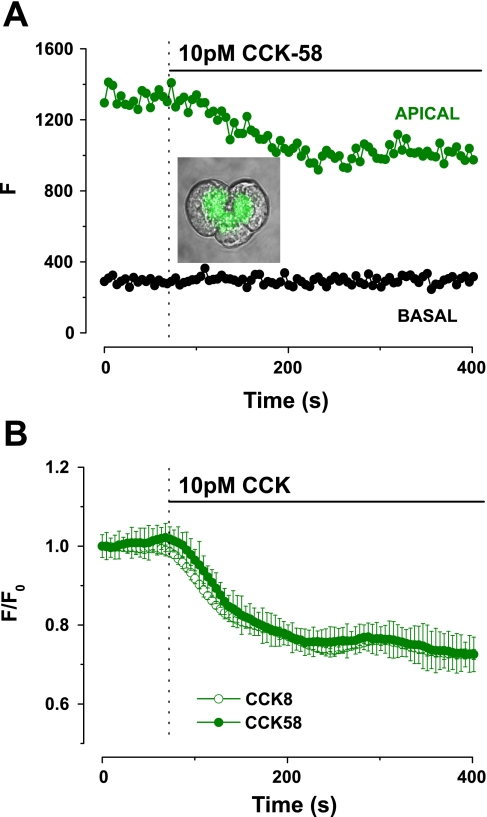
The effects of CCK-58 were compared with those of CCK-8 on digestive enzyme secretion using three established methods. First, quinacrine fluorescence was used to measure exocytosis. Quinacrine is an acidophilic fluorescent dye that is preferentially taken up by secretory granules and has been used to detect and quantify exocytosis in a variety of isolated cell types, including murine (2, 20) and human (17) pancreatic acinar cells. The typical quinacrine (green) staining of the granules can be seen in the inset image of Fig. 4A. A decrease in apical quinacrine fluorescence in response to 10 pM CCK-58, denoting exocytosis from the granules, was observed with no change in the basal area (Fig. 4A). No difference was detected between the actions of CCK-58 (n = 16) and CCK-8 (n = 11), such that the rate of onset and maximal decrease in quinacrine fluorescence were not significantly different between the two peptides (Fig. 4, A and B).
Fig. 4.
Comparative effects of CCK-58 and CCK-8 on digestive enzyme secretion in murine pancreatic acinar cells. A: typical trace showing exocytosis that occurred in response to a physiological concentration (10 pM) of CCK-58. Typical quinacrine staining (green) of zymogen granules is shown within the inset light-transmitted image. On stimulation with CCK-58 apical quinacrine fluorescence (F) fell from a steady baseline to a plateau level, with no change in the basal area. B: exocytotic responses induced by CCK-58 (n = 16) and CCK-8 (n = 11) were not significantly different. All fluorescence changes were normalized from basal values (F/F0).
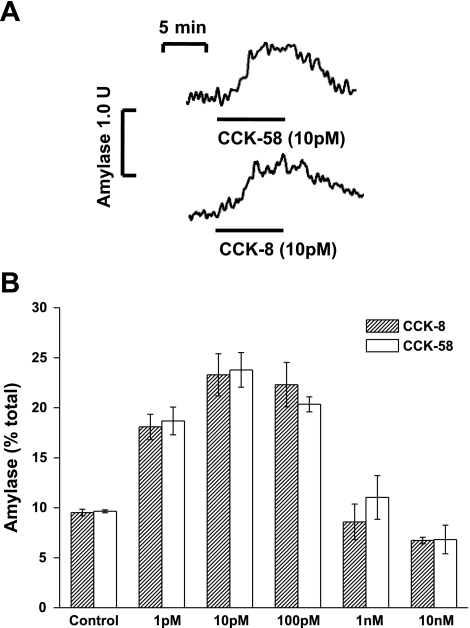
Direct measurement of amylase secretion was achieved in two ways: an online fluorometric assay using segments of murine pancreatic tissue, as reported previously (22), and a plate-format assay using isolated acinar cells. In the online format, application of 10 pM CCK-58 induced a sustained and reversible increase in amylase secretion from a basal value of 0.55 U/min per 100 mg of tissue to 0.98 U/min per 100 mg of tissue, an approximate doubling of the basal secretion rate (Fig. 5A). Similar responses were seen when CCK-8 was applied (Fig. 5A), causing a rise of secretion rate to 0.80 U/min per 100 mg of tissue from a basal rate of 0.49 U/min per 100 mg of tissue. In plate format, CCK-58 induced concentration-dependent effects on amylase secretion from freshly isolated cells, such that basal levels were increased by 114.7% and 146.6% in response to 1 and 10 pM CCK-58, respectively, whereas 10 nM CCK-58 inhibited secretion by 29.25% (Fig. 5B). No significant differences between the effects of CCK-58 (n = 3) and CCK-8 (n = 3) on amylase secretion were detected at any concentration (P > 0.05).
Fig. 5.
A: typical traces showing the reversible amylase secretion in response to CCK-58 and CCK-8 from superfused segments of murine pancreatic tissue, monitored fluorometrically in the flow cell effluent (n = 5). CCK-58 and CCK-8 increased amylase secretion to 0.98 and 0.80 U/min per 100 mg of tissue from basal levels of 0.55 and 0.49 U/min per 100 mg of tissue, respectively. B: concentration-dependent effects of CCK-58 (n = 3) and CCK-8 (n = 3) (1 pM-10 nM) on amylase secretion from isolated murine pancreatic cells, expressed as a percentage of total amylase. No significant difference between CCK-58 and CCK-8 responses was detected at all concentrations tested (P > 0.05).
Effects of CCK-58 and CCK-8 on cell damage and fate.
Because it has been shown that CCK-58 hyperstimulation does not cause pancreatitis in the rat, unlike similar treatment with CCK-8 (35), the effects of CCK-58 and CCK-8 were compared on several parameters of cellular damage and fate in murine isolated pancreatic acinar cells.
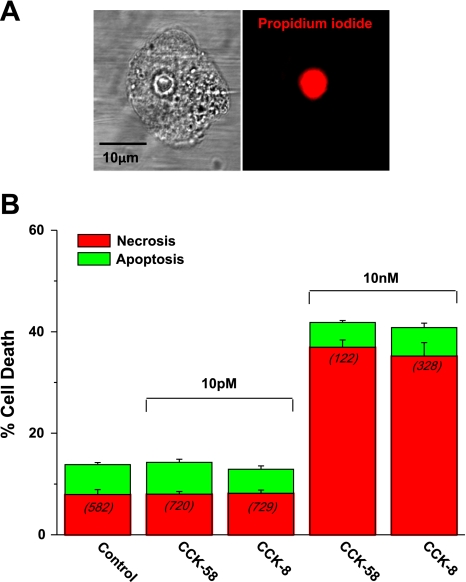
First, a cell death assay was employed to detect early apoptosis (general caspase substrate) and necrosis (propidium iodide). Physiological (10 pM) concentrations of CCK-58 and CCK-8 produced no significant increase in total cell death compared with controls (Fig. 6B). In contrast, hyperstimulation with 10 nM CCK-58 and CCK-8 produced significant 3.4- and 3.3-fold increases in total cell death, respectively, from time-matched, non-stimulated controls. This was largely attributable to an increase in necrosis from 7.9% (control) to 36.9% and 35.2%, with CCK-58 and CCK-8 treatments, respectively. A typical necrotic cell following CCK-58 hyperstimulation is shown in Fig. 6A, with bright nuclear propidium iodide staining clearly evident.
Fig. 6.
Comparative effects of CCK-58 and CCK-8 on cell death. A: light-transmitted and propidium iodide fluorescent images showing bright staining of the nucleus of a necrotic cell after exposure to 10 nM CCK-58 for 30 min at 37°C. B: mean data from such experiments comparing the effects of CCK-58 and CCK-8 on apoptosis (green) and necrosis (red). No significant increase in total cell death was observed with physiological (10 pM) CCK-58 or CCK-8 stimulation. However, total cell death was increased by hyperstimulation with 10 nM of either peptide. Necrosis significantly increased in response to 10 nM CCK-58 or CCK-8, whereas apoptosis remained at control levels. No significant difference between CCK-58 and CCK-8 was detected in these experiments. (Bars show mean percentage of cell death ± SE. The number of cells analyzed in each group are in parentheses.)
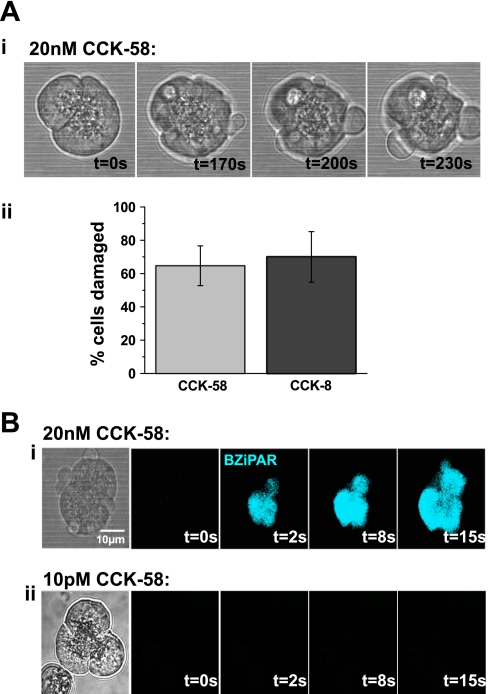
In addition, in separate experiments in which cells were continuously perfused with a high concentration (20 nM) of CCK-58 or CCK-8 on the microscope stage at 37°C for 20 min, membrane damage, seen as blebbing, was noticeable in a majority of cells (Fig. 7A), an effect that developed over the period of exposure to supramaximal CCK. There was no significant difference between the detrimental effects of CCK-58 and CCK-8, with 65% and 70% of cells exhibiting membrane damage, respectively (Fig. 7A). After this period of exposure to either CCK-58 or CCK-8 (20 nM), bath application of the trypsin probe BZiPAR to the perfusion chamber caused an increase of fluorescence in the damaged cells that originated in the apical (granular) area of the cell and quickly progressed to the basolateral region as the activated probe diffused away from the site of activation. This did not occur when the cells had been perfused with CCK at a lower concentration of 10 pM (Fig. 7B).
Fig. 7.
Comparative effects of CCK-58 and CCK-8 on cell membrane damage and intracellular trypsin activation. A: i: light-transmitted images showing blebbing of the plasmalemmal membrane induced by stimulation with 20 nM CCK-58 for 20 min at 37°C, which increased with exposure time. ii: Mean data comparing the effects of CCK-58 (n = 17) and CCK-8 (n = 10) in this experimental protocol show that there was no significant difference detected between the detrimental actions of these peptides. (Bars show mean levels of cell death ± SE. The number of cells analyzed in each group are in parentheses.) B: light-transmitted image and rhodamine 110, bis-(CBZ-L-isoleucyl-L-prolyl-L-arginine amide) dihydrochloride (BZiPAR) fluorescent images of a doublet of acinar cells damaged by exposure to 20 nM CCK-58 for 20 min at 7°C (i). Trypsin activity (blue) can be seen at various time points, following bath application of BZiPAR (10 nM) at t = 0 s, commencing in the granular, apical pole of the cell (t = 2 s), and spreading to the basolateral region. Similar trypsin activity was detected in response to both hyperstimulation with CCK-58 (6 of 6 cells) and CCK-8 (6 of 6 cells) but not by stimulation with 10 pM CCK-58 (ii) for the same period (9 of 9 cells).
DISCUSSION
The present study has shown that CCK-58 exhibits a strikingly similar profile of action to that of CCK-8 in murine isolated pancreatic acinar cells. Thus at low, picomolar concentrations, CCK-58 induced transient oscillatory rises of [Ca2+]c, which were generated at the apical, granular pole of the cell and spread to the basolateral region. Such a pattern of response is characteristic of CCK-8 in rodent isolated pancreatic cells (16, 24), and in the present study physiological CCK-58 and CCK-8 exhibited similar effects. In accord with these observations, a recent study in cultured Chinese hamster ovary cells has shown that CCK-58 and CCK-8 stimulate the CCK1 receptor with a similar efficacy although a fivefold lower potency was observed with CCK-58 (34).
Pancreatic acinar cells are highly polarized cells that exhibit a distinct morphology and whose [Ca2+]c signals are tightly regulated to facilitate secretory function (23). One particular feature is a perigranular mitochondrial belt that constitutes a “buffer barrier” to restrict transmission of [Ca2+]c signals from the granular region (32), to protect the nucleus from excessive localized elevations of [Ca2+]c in the basolateral region, and to function as a source of ATP necessary to fuel secretion. Thus, when [Ca2+]c is elevated close to the mitochondria in response to physiological stimuli, these organelles temporarily uptake Ca2+, which in turn stimulates Ca2+-dependent dehydrogenase enzymes to generate NADH via the Krebs cycle, thus fueling the electron transport chain and production of ATP (11). Such stimulation of mitochondrial metabolism was visualized by an increase in NAD(P)H autofluorescence in the perigranular region of the isolated cell in response to CCK-58-induced oscillatory rises of [Ca2+]c and was comparable to that seen with CCK-8 (present study) (33).
The functional consequence of these oscillatory rises of [Ca2+]c and coupling to mitochondrial metabolism is digestive enzyme secretion, which was demonstrated using different techniques. First, quinacrine loading into the zymogen granules provided real-time measurement of exocytosis (2, 17, 20), and, in the present investigation, CCK-58 and CCK-8 produced identical exocytotic responses. In a standard isolated cell assay, both CCK-58 and CCK-8 (1 pM-10 nM) exhibited a similar bell-shaped response, with increases of amylase secretion at pM concentrations and inhibition at higher nM concentrations. Furthermore, no differences between the profile of action of CCK-58 and CCK-8 on amylase secretion from intact tissues were detected, suggesting that both peptides employ a common transduction pathway to activate the secretory machinery. Recently we have demonstrated that 10 pM CCK-8 induced an approximate doubling of the basal amylase secretion from human pancreatic segments (17). In agreement with this, the present study in murine pancreatic tissue has shown that the same concentration of CCK-8 and CCK-58 (10 pM) caused similar amylase secretion rates, approximate doublings of basal secretion.
Hyperstimulation of the rodent pancreas with the CCK analog cerulein is a well-characterized model of experimental acute pancreatitis (19, 31), and intriguing recent in vivo data have suggested that, in contrast to CCK-8, stimulation with supramaximal CCK-58 does not induce pancreatitis in the rat (35). When isolated acinar cells were hyperstimulated with nanomolar CCK-58 or CCK-8, the characteristics of the induced [Ca2+]c signals changed from being oscillatory in nature, observed with picomolar stimulation, to a single, broad peak followed by a sustained rise of [Ca2+]c, a response observed with both peptides. This sustained elevation of [Ca2+]c was dependent on influx of Ca2+ into the cell from the external medium, and accordingly was reversed under Ca2+-free conditions. This type of [Ca2+]c elevation induced by high (nM) CCK-8 causes activation of trypsinogen within the acinar cell and vacuolization, features indicative of acute pancreatitis (15, 25). In the present study, trypsin activation was detected after hyperstimulation with CCK-58, using the fluorescent probe BZiPAR, consistent with its action on [Ca2+]c, and was a feature shared by CCK-8. Additionally, damage to the membrane of isolated acinar cells, manifested as basolateral blebs, was also observed during perfusion with both high CCK-58 and CCK-8, an effect that developed over the period of stimulation. Morphological changes, including basolateral blebbing and actin redistribution, in response to high concentrations of CCK-8 have previously been documented in mouse pancreatic acini (1). In the present experiments, no significant difference in membrane damage induced by the two peptides was detected. Furthermore, in a separate assay designed to assess cell fate (apoptosis and necrosis) using fluorescent probes, hyperstimulation of pancreatic acinar cells with 10 nM CCK-58 or CCK-8 for a 30-min period caused approximate threefold increases in total cell death, whereas no significant changes were induced by physiological (pM) concentrations of either peptide. Such detrimental effects of hyperstimulation were attributable to cellular necrosis rather than apoptosis, which remained at control levels, most likely via the generation of large, sustained elevations of [Ca2+]c and consequent inhibition of mitochondrial function (3). No differences between the actions of CCK-58 and CCK-8 on cell fate were detected.
The differences between the in vitro and in vivo experiments on stimulation of pancreatic function by CCK-8 and CCK-58 may result from CCK-58 being able to reach sites not attainable by CCK-8. This could arise from the shorter peptide being digested by endopeptidases in the vicinity of the CCK1 receptor while CCK-58 is not degraded, a suggestion supported by the fact that CCK-58 is much more stable to endopeptidase 24:11 than CCK-8 (28). Therefore, CCK-58 may reach sites that stimulate enzyme secretion by another mediator such as gastrin-releasing peptide (GRP), which is present in pancreatic nerves (12). GRP is a candidate because its amphibian counterpart, bombesin, stimulates enzyme release (13) but does not cause pancreatitis when incubated with acinar cells at high concentrations (10). Thus both direct and indirect stimulation might cause enzyme release in the whole animal, whereas there is no indirect stimulation with purified acinar cells. This model may be tested in several future experiments, including evaluation of whether GRP might ameliorate pancreatitis caused by supraphysiological concentrations of CCK-8 in vitro or in vivo, whether vagotomy could enable CCK-58 to cause pancreatitis in vivo, and whether GRP antibodies might enable CCK-58 to cause pancreatitis in vivo or GRP might stimulate pancreatic fluid in the in vivo model. Furthermore, species differences may be important and contribute to the reported experimental differences between CCK-8 and CCK-58.
In conclusion, our results indicate that CCK-58 and CCK-8 peptides induce remarkably similar effects on [Ca2+]c, mitochondrial function, digestive enzyme secretion, and cell damage, providing clear evidence that CCK-58 is capable of activating the full spectrum of stimulus-secretion coupling in pancreatic acinar cells, compatible with its role as the principal, physiological form of this gastrointestinal hormone. Furthermore, our present data suggest that the reported in vivo differences between the actions of CCK-58 and CCK-8 are unlikely to be accounted for by their abilities to stimulate the CCK1 receptor on the pancreatic acinar cell, but that additional prereceptor factors may contribute. Recent evidence has suggested that pancreatic chloride and water secretion is better maintained by CCK-58 than by CCK-8 in an in vivo rodent model, which may constitute a basis for its inability to induce pancreatitis (35), and further studies with CCK-58 are clearly necessary to elucidate the mechanism(s) involved.
GRANTS
This work was supported by Programme, Cooperative, and Component Grants from the Medical Research Council (UK) and by National Institutes of Health Grants DK 33850 (to J. Reeve, Jr.), DK 37482 (to G. green), by the Veterans Administration Research Service, and by the NIH CURE: Digestive Diseases Research Center Grant DK41301.
DISCLOSURES
No conflicts of interest are declared by the author(s).
ACKNOWLEDGMENTS
O. Petersen is a Medical Research Council Professor; R. Mukherjee is supported by an Amelie Waring Clinical Research Fellowship from CORE; E. McLaughlin was supported by a Clinical Training Fellowship from the Royal College of Surgeons of England.
REFERENCES
- 1.Bi Y, Page SL, Williams JA. Rho and Rac promote acinar morphological changes, actin reorganization, and amylase secretion. Am J Physiol Gastrointest Liver Physiol 289: G561–G570, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Campos-Toimil M, Edwardson JM, Thomas P. Real-time studies of zymogen granule exocytosis in intact rat pancreatic acinar cells. J Physiol 528: 317–326, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Criddle DN, Gerasimenko JV, Baumgartner HK, Jaffar M, Voronina S, Sutton R, Petersen OH, Gerasimenko OV. Calcium signalling and pancreatic cell death: apoptosis or necrosis? Cell Death Differ 14: 1285–1294, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Criddle DN, Gillies S, Baumgartner-Wilson HK, Jaffar M, Chinje EC, Passmore S, Chvanov M, Barrow S, Gerasimenko OV, Tepikin AV, Sutton R, Petersen OH. Menadione-induced reactive oxygen species generation via redox cycling promotes apoptosis of murine pancreatic acinar cells. J Biol Chem 281: 40485–40492, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Criddle DN, Murphy J, Fistetto G, Barrow S, Tepikin AV, Neoptolemos JP, Sutton R, Petersen OH. Fatty acid ethyl esters cause pancreatic calcium toxicity via inositol trisphosphate receptors and loss of ATP synthesis. Gastroenterology 130: 781–793, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Criddle DN, Raraty MG, Neoptolemos JP, Tepikin AV, Petersen OH, Sutton R. Ethanol toxicity in pancreatic acinar cells: mediation by nonoxidative fatty acid metabolites. Proc Natl Acad Sci USA 101: 10738–10743, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dufresne M, Seva C, Fourmy D. Cholecystokinin and gastrin receptors. Physiol Rev 86: 805–847, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Eberlein GA, Eysselein VE, Hesse WH, Goebell H, Schaefer M, Reeve JR., Jr Detection of cholecystokinin-58 in human blood by inhibition of degradation. Am J Physiol Gastrointest Liver Physiol 253: G477–G482, 1987 [DOI] [PubMed] [Google Scholar]
- 9.Eysselein VE, Eberlein GA, Hesse WH, Singer MV, Goebell H, Reeve JR., Jr Cholecystokinin-58 is the major circulating form of cholecystokinin in canine blood. J Biol Chem 262: 214–217, 1987 [PubMed] [Google Scholar]
- 10.Grady T, Mah'Moud M, Otani T, Rhee S, Lerch MM, Gorelick FS. Zymogen proteolysis within the pancreatic acinar cell is associated with cellular injury. Am J Physiol Gastrointest Liver Physiol 275: G1010–G1017, 1998 [DOI] [PubMed] [Google Scholar]
- 11.Hajnoczky G, Robb-Gaspers LD, Seitz MB, Thomas AP. Decoding of cytosolic calcium oscillations in the mitochondria. Cell 82: 415–424, 1995 [DOI] [PubMed] [Google Scholar]
- 12.Holst JJ, Knuhtsen S, Jensen SL, Fahrenkrug J, Larsson LI, Nielsen OV. Interrelation of nerves and hormones in stomach and pancreas. Scand J Gastroenterol Suppl 82: 85–99, 1983 [PubMed] [Google Scholar]
- 13.Iwatsuki N, Petersen OH. In vitro action of bombesin on amylase secretion, membrane potential, and membrane resistance in rat and mouse pancreatic acinar cells. A comparison with other secretagogues. J Clin Invest 61: 41–46, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ji B, Bi Y, Simeone D, Mortensen RM, Logsdon CD. Human pancreatic acinar cells lack functional responses to cholecystokinin and gastrin. Gastroenterology 121: 1380–1390, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Kruger B, Albrecht E, Lerch MM. The role of intracellular calcium signaling in premature protease activation and the onset of pancreatitis. Am J Pathol 157: 43–50, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maruyama Y, Inooka G, Li YX, Miyashita Y, Kasai H. Agonist-induced localized Ca2+ spikes directly triggering exocytotic secretion in exocrine pancreas. EMBO J 12: 3017–3022, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murphy JA, Criddle DN, Sherwood M, Chvanov M, Mukherjee R, McLaughlin E, Booth D, Gerasimenko JV, Raraty MG, Ghaneh P, Neoptolemos JP, Gerasimenko OV, Tepikin AV, Green GM, Reeve JR, Jr, Petersen OH, Sutton R. Direct activation of cytosolic Ca2+ signaling and enzyme secretion by cholecystokinin in human pancreatic acinar cells. Gastroenterology 135: 632–641, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Owyang C, Logsdon CD. New insights into neurohormonal regulation of pancreatic secretion. Gastroenterology 127: 957–969, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Pandol SJ, Gukovsky I, Satoh A, Lugea A, Gukovskaya AS. Animal and in vitro models of alcoholic pancreatitis: role of cholecystokinin. Pancreas 27: 297–300, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Park MK, Lee M, Petersen OH. Morphological and functional changes of dissociated single pancreatic acinar cells: testing the suitability of the single cell as a model for exocytosis and calcium signaling. Cell Calcium 35: 367–379, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Petersen OH. Ca2+ signalling and Ca2+-activated ion channels in exocrine acinar cells. Cell Calcium 38: 171–200, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Petersen OH, Findlay I, Iwatsuki N, Singh J, Gallacher DV, Fuller CM, Pearson GT, Dunne MJ, Morris AP. Human pancreatic acinar cells: studies of stimulus-secretion coupling. Gastroenterology 89: 109–117, 1985 [DOI] [PubMed] [Google Scholar]
- 23.Petersen OH, Sutton R, Criddle DN. Failure of calcium microdomain generation and pathological consequences. Cell Calcium 40: 593–600, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Petersen OH, Tepikin AV. Polarized calcium signaling in exocrine gland cells. Annu Rev Physiol 70: 273–299, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Raraty M, Ward J, Erdemli G, Vaillant C, Neoptolemos JP, Sutton R, Petersen OH. Calcium-dependent enzyme activation and vacuole formation in the apical granular region of pancreatic acinar cells. Proc Natl Acad Sci USA 97: 13126–13131, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reeve JR, Jr, Green GM, Chew P, Eysselein VE, Keire DA. CCK-58 is the only detectable endocrine form of cholecystokinin in rat. Am J Physiol Gastrointest Liver Physiol 285: G255–G265, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Reeve JR, Jr, Liddle RA, Shively JE, Lee TD, Keire DA, Chew P, Vigna SR. Sequence variation outside the “active” region of dog and rabbit cholecystokinin-58 results in bioactivity differences. Pancreas 32: 306–313, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Reeve JR, Jr, McVey DC, Bunnett NW, Solomon TE, Keire DA, Ho FJ, Davis MT, Lee TD, Shively JE, Vigna SR. Differences in receptor binding and stability to enzymatic digestion between CCK-8 and CCK-58. Pancreas 25: e50–e55, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Reeve JR, Jr, Wu SV, Keire DA, Faull K, Chew P, Solomon TE, Green GM, Coskun T. Differential bile-pancreatic secretory effects of CCK-58 and CCK-8. Am J Physiol Gastrointest Liver Physiol 286: G395–G402, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Saluja A, Logsdon C, Garg P. Direct versus indirect action of cholecystokinin on human pancreatic acinar cells: is it time for a judgment after a century of trial? Gastroenterology 135: 357–360, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Saluja AK, Lerch MM, Phillips PA, Dudeja V. Why does pancreatic overstimulation cause pancreatitis? Annu Rev Physiol 69: 249–269, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Tinel H, Cancela JM, Mogami H, Gerasimenko JV, Gerasimenko OV, Tepikin AV, Petersen OH. Active mitochondria surrounding the pancreatic acinar granule region prevent spreading of inositol trisphosphate-evoked local cytosolic Ca(2+) signals. EMBO J 18: 4999–5008, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Voronina S, Sukhomlin T, Johnson PR, Erdemli G, Petersen OH, Tepikin A. Correlation of NADH and Ca2+ signals in mouse pancreatic acinar cells. J Physiol 539: 41–52, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu SV, Harikumar KG, Burgess RJ, Reeve J, Jr, Miller LJ. Effects of cholecystokinin-58 on Type 1 cholecystokinin receptor function and regulation. Receptor binding, activation, internalization and oligomerization. Am J Physiol Gastrointest Liver Physiol 295: G641–G647, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamamoto M, Reeve JR, Jr, Green GM. Supramaximal CCK-58 does not induce pancreatitis in the rat: role of pancreatic water secretion. Am J Physiol Gastrointest Liver Physiol 292: G964–G974, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Yamamoto M, Reeve JR, Jr, Keire DA, Green GM. Water and enzyme secretion are tightly coupled in pancreatic secretion stimulated by food or CCK-58 but not by CCK-8. Am J Physiol Gastrointest Liver Physiol 288: G866–G879, 2005 [DOI] [PubMed] [Google Scholar]