Abstract
Excessive alcohol (ethanol) consumption is the hallmark of alcohol use disorders. The F1 hybrid cross between the C57BL/6J (B6) and FVB/NJ (FVB) inbred mouse strains, consumes more ethanol than either progenitor strain. The purpose of this study was to utilize ethanol drinking data and genetic information to map genes that result in over-dominant (or heterotic) ethanol drinking. About 600 B6 × FVB F2 mice, half of each sex, were tested for ethanol intake and preference in a 24-h, two-bottle water versus ethanol choice procedure, with ascending ethanol concentrations. They were then tested for ethanol intake in a drinking in the dark (DID) procedure, first, when there was no water choice and then when ethanol was offered versus water. DNA samples were obtained and genome-wide QTL analyses were performed to search for single QTLs (both additive and dominance effects) and interactions between pairs of QTLs, or epistasis. On average, F2 mice consumed excessive amounts of ethanol in the 24-h choice procedure, consistent with high levels of consumption seen in the F1 cross. Consumption in the DID procedure was similar or higher than amounts reported previously for the B6 progenitor. QTLs resulting in heightened consumption in heterozygous compared to homozygous animals were found on Chr 11, 15 and 16 for 24-h choice 30% ethanol consumption, and on Chr 11 for DID. No evidence was found for epistasis between any pair of significant or suggestive QTLs. This indicates that the hybrid over-dominance is due to intralocus interactions at the level of individual QTL.
Keywords: Heterosis, addiction, gene mapping, epistasis, alcoholism
Excessive alcohol (ethanol; EtOH) use disorders appear to have multiple etiologies. For example, they may develop as a consequence of poor impulse control Crews and Boettiger, 2009), genetic risk (Bohman et al. 1987; Schuckit 2009), or the interaction of heritable factors with environmental factors, such as parental divorce (Thompson et al. 2008). Behavioral and expression quantitative trait locus (QTL) mapping studies in rodents and humans have identified chromosomal regions, and nominated candidate genes, that may influence EtOH drinking (Belknap and Atkins 2001; Mulligan et al. 2006; Tabakoff et al. 2008; Ducci and Goldman, 2008). The majority of studies have focused on providing evidence for the locations of genes that independently influence EtOH intake; in combination, these genes account for a larger proportion of variation in intake than does each gene when considered alone. Technological advances are leading to more sophisticated gene network analyses designed to identify important signaling pathways (e.g., Song et al. 2009). However, epistatic (gene × gene) interactions influence complex traits (Godoy-Herrera, 1994; Chesler et al. 2005), including EtOH-related traits (Bergeson et al. 2003; Hood et al. 2001; Kuo et al. 2008; Palmer et al. 2003), and there is a need for the further development of methods for identifying these genetic interactions.
Mice from the first filial (F1) cross of the C57BL/6J (B6) and FVB/NJ (FVB) inbred mouse strains have been documented to display excessive EtOH intake, when measured in a 24-h two-bottle choice procedure (Blednov et al. 2005). The almost absolute preference of B6 mice for 10% EtOH over water has been recognized as a stable trait in this strain, not surpassed by any other strain (Belknap et al. 1993; Wahlsten et al. 2006; Yoneyama et al. 2008). Thus, the finding that B6FVBF1 cross mice consumed as much as twice the amount of EtOH consumed by B6 mice was a remarkable finding. The heightened EtOH consumption of the F1 hybrid above that of both the B6 and FVB progenitor strains defines an over-dominant trait, also known as heterosis (Bruell 1967).
Over-dominance could arise as a result of interactions between loci (gene × gene; aka, epistatic). Thus, excessive drinking in the heterozygote mice could be the result of epistatic interactions specifically between alleles that are unique to each of the progenitor strains. However, another possible source of over-dominance is interaction between unique alleles within a locus, rather than between loci (as is the case for epistasis). In this case, the expectation would be that individuals possessing both of the possible alleles at an influential locus, the heterozygotes, would exhibit heightened EtOH consumption compared to individuals homozygous at that locus. Of course, because EtOH consumption is influenced by multiple loci, both mechanisms could underlie the excessive drinking.
In the current work, we focused on the hypothesis that interactions, either within or between loci, play a role in the excessive drinking of the B6 × FVB intercross mice. We tested this hypothesis by carrying out QTL mapping based on both of these sources of non-additive genetic variation underlying EtOH drinking in B6FVBF2 intercross mice. In particular, dominance genetic variance in the F2 was the target of the scan rather than the more usual combined additive and dominance variance, and two-dimensional searches capable of detecting epistasis were also carried out for those chromosomal regions showing suggestive or significant effects on this phenotype. This paper describes the methods and results associated with our initial search for B6FVB heterozygous loci that increase EtOH consumption.
Methods
Subjects
Male and female, 6-week old B6 and FVB mice were purchased from The Jackson Laboratory for the purpose of establishing reciprocal F1 (B6 × FVB and FVB × B6) breeding pairs. Offspring of these pairs were bred at ~50 d of age to generate the F2 mice that were tested for EtOH consumption and used for QTL mapping. F2 mice of all reciprocal types were generated (i.e., B6FVBF1 × B6FVBF1; B6FVBF1 × FVBB6F1; FVBB6F1 × B6FVBF1; FVBB6F1 × FVBB6F1). All breeding pairs and offspring were maintained in polycarbonate cages lined with Bed-o’ cobs bedding (The Andersons, Inc., Maumee, OH) on a reverse 12h:12h light:dark cycle with lights off at 0900 h and room temperature at 21 +/− 2 C. Lights were set on a reverse light:dark cycle to accommodate the drinking in the dark (DID) procedure described below. Unlimited supplies of mouse block food (Purina 5001; St. Louis, MI) and water were freely accessible through wire cage tops or an automatic watering system. Breeders and pre-weanling mice were maintained on Thoren racks with a cage top filtering system. Weaned mice (21 +/− 1 d of age) were group housed with same-sex littermates in groups of 2–5, and maintained on free racks with filter tops to isolate cages from each other. Male and female mice weighed 20.4 ± 0.2 and 26.2 ± 0.2 g, respectively at the beginning of testing (Day -1, Fig 1) and 21.7 ± 0.1 and 26.1 ± 0.1 g, respectively, when weight was last measured (Day 24, Fig 1).
Figure 1.

Time course of EtOH drinking procedures. Mice were first acclimated to drinking from graduated drinking tubes, then offered a series of EtOH concentrations in tap water versus tap water for 24-h per day, and then tested in a single-bottle followed by a two-bottle choice drinking in the dark (DID) procedure. Note: Length of bars is not entirely to scale.
EtOH Drinking
The complete sequence of testing is shown in Figure 1.
Twenty-four hour, two-bottle choice EtOH drinking
Six-hundred and nineteen F2 mice (310 female and 309 male; mean ± SEM = 60 ± 0.4 d old on Day 1) were tested for EtOH consumption and preference using the same method used by Blednov et al. (2005) in which the over-dominant drinking phenotype of B6FVBF1 mice was first identified. They were tested in four passes of 164, 160, 158 and 137 mice, with all reciprocal crosses represented in each pass in approximately equal numbers. Mice were offered EtOH in tap water versus plain tap water across a twenty day period. They had first been acclimated to drinking from 25-ml glass graduated cylinders fitted with sipper tubes for two prior days. EtOH was offered in an ordered series of concentrations of 3, 10, 20, 30, and 20% (v/v) for 4 consecutive days at each concentration, and consumption of EtOH and of water were determined by reading tube volumes immediately after and 24-h after placement on the cages. To account for side preferences, the relative positions of the EtOH and water tubes were switched every two days (e.g., if the water tube was to the left of the EtOH tube on days 1 and 2, then it was switched to the right of the EtOH tube on days 3 and 4), and mice were weighed on the first day of each concentration change. Consumption (g/kg) and preference (EtOH ml/total ml) data from days 2 and 4 at each concentration were used to summarize drinking behavior for each individual animal. This average included data from both possible EtOH tube positions, and allowed the animals ample time to identify the new location of the EtOH tube after a position switch. Data were lost for 14 mice on some days due to misreading of tubes (indicated by increased rather than decreased volumes) or obvious tube leakage (indicated by near total loss of fluid from the tube and excessively wet underlying bedding), leaving complete data available for 303 female and 302 male mice. Evaporation and leakage due to animal rack disturbance were accounted for each day by correcting recorded volumes for lost volumes from fluid filled tubes placed on control cages not containing animals.
Drinking in the Dark (DID)
The same F2 mice were subsequently tested for limited access EtOH drinking using a DID procedure (Rhodes et al. 2005). Data were complete for 303 female and 300 male mice. After the last reading for 24-h, two-bottle choice drinking, a single 25-ml water-filled tube was placed on all cages. Twenty-four hours later, 3 h into the dark phase of the light:dark cycle (at 1200 h), this tube was replaced with a 10 ml tube containing 20% EtOH (v/v). The EtOH tube remained in place for 2 h, and was then replaced with a 25-ml water tube. This sequence was repeated for 2 additional days, and then on the fourth access day, animals were offered EtOH for 4 h, with measurements taken after 2 and 4 h. On the next day, mice were offered two 10-ml tubes for a 4-h period, one tube containing water and the other containing 20% EtOH, to determine if the water option would alter amount of EtOH consumed. The EtOH tube was placed in the same location as during the single-bottle test. Tube volumes were read at 2 and 4 h. All volumes were adjusted for leakage from tubes on cages not containing mice. Tail blood samples (20 μl) were obtained immediately after each 4-h drinking sessions (Days 25 and 26) to obtain blood EtOH concentration (BEC) data. BEC was measured by gas chromatography using standard methods in our laboratory (Boehm et al. 2000).
DNA Sampling and Extraction
Tail snips were obtained from all F2 mice when they were 42 ± 0.3 d old on average. This avoided temporal contiguity of possible stress axis activation and behavioral assessment. Samples were immediately frozen on dry ice and stored at −80°C until DNA was extracted, using reagents and a modified protocol from Gentra Systems (Minneapolis, MN). Briefly, tail tips were placed in cell lysis solution, proteinase K solution was added and tubes were incubated at 55–60°C overnight. Samples were cooled to room temperature, treated with RNase (DNase free), protein was precipitated to form a tight pellet, and the supernatant containing DNA was poured into fresh microcentrifuge tubes containing isopropanol. Centrifugation promoted DNA adherence to the tube bottom, supernatant was removed and replaced with 70% EtOH, centrifugation was repeated and the tubes were drained, inverted and allowed to dry. Remaining DNA was hydrated in TE buffer and stored at 2–8°C.
Genotyping
Mice were genotyped using a custom single nucleotide polymorphism (SNP) array and the Illumina Golden Gate Assay (San Diego, CA). Ninety-six informative SNPs were spaced at ~ 23.4 Mb intervals throughout the genome. The SNPs were validated using DNA from the B6 and FVB parental strains. The samples were analyzed locally using the Illumina BeadStation 500 X (Illumina, Inc., San Diego, CA) genotyping platform, and procedures were performed exactly as recommended by the manufacturer.
Data Analysis
To characterize the EtOH drinking of the F2 mice, drinking data were analyzed by repeated measures ANOVA using Statistica software (V 6.1; StatSoft, Inc., Tulsa, OK) with sex as a between groups factor and EtOH concentration or days as the repeated measure. Data from the 24-h, two-bottle choice phase and DID phase were analyzed separately. For significant two-way interactions, simple effects analysis was used (Keppel and Wickens, 2004) to identify the effect of one variable at specific levels of another (e.g., the effect of sex at particular concentrations of EtOH), and the Tukey HSD test was used for post-hoc mean comparisons. Pearson’s product-moment correlations were calculated between EtOH drinking, preference, and BEC measures to examine phenotypic relationships. Due to the large number of correlations performed, α was set at 0.01 to reduce Type I error.
QTL Mapping
Genome scans for QTLs were carried out by R/qtl software version 1.09 (Broman et al. 2003) running in the R programming environment, v.2.2, using the EM interval mapping option and the ConvertX option to search for X-linked QTLs (Broman et al. 2006). The Mainscan subprogram was used to search for single QTLs; Pairscan was used to search for interactions between pairs of QTLs, or epistasis. For each marker or interval between markers genome-wide, the value of a, the additive effect, or the average effect of an allele substitution (Falconer and Mackay 1996), was calculated as one-half the phenotypic difference between the means of the two homozygous genotypes, B6/B6 and FVB/FVB. In this case, the difference was always calculated as B6/B6 – FVB/FVB, which yields positive values of a when the B6/B6 genotype shows higher phenotypic values, and negative if lower. Also, d, the dominance deviation (Falconer and Mackay 1996; Kearsey and Pooni 1996), was calculated as the difference between the phenotypic mean of the heterozygotes and the average (midpoint) of the two homozygote classes. These two variables were then standardized by dividing each one by the pooled within genotype standard deviation generated by R/qtl. The sign of d was positive if the heterozygote mean trait values scored above the mean of the two homozygote classes (midpoint), and was negative if below. The ratio d/a was then determined (Bruell 1967; Kearsey and Pooni 1996); this value is 0 with no dominance, 1.0 with complete B6 allele dominance, and >1.0 with B6 allele over-dominance. On the other hand, negative d/a ratios reflects FVB dominance or over-dominance, respectively. The sign of d/a was positive if the B6 allele showed any degree of dominance (FVB allele is recessive), but was negative if the FVB allele showed any degree of dominance (B6 allele is recessive). A custom R script was used to pull out these data from the output matrix, and is available upon request from the last author.
The statistical significance of the observed standardized values of a and d at each QTL were tested against the null hypothesis that each value was zero. Because a and d were expressed in pooled within-genotype standard deviation units, the following equations were used to calculate Student’s t for a two-group, two-tailed comparison: t = |a| dfa1/2 and t =(|d| dfd1/2 )/2, where dfa = N homozygotes − 2, dfd = N − 2 (Rosenthal 1994), and N is the total number of mice. The significance threshold for a genome-wide search was determined empirically by permutation testing for a and d, and was estimated as p = 0.0004 (LOD 3.4) to yield a 5% risk of even one false positive result genome-wide. The resulting thresholds for significance from the above two equations were thus 0.204 for a and 0.287 for d. The test for over-dominance at a QTL was a test that |d| was significantly greater than |a| using the equation t = ((|d| − |a|) dfd 1/2)/2. Because the test for over-dominance was restricted to only the three QTLs where d was significantly greater than zero, p = 0.017 was used as the significance threshold for the choice experiment. For the DID experiment, this threshold was p = 0.01.
Results
Twenty-four Hour, Two-bottle Choice EtOH Drinking
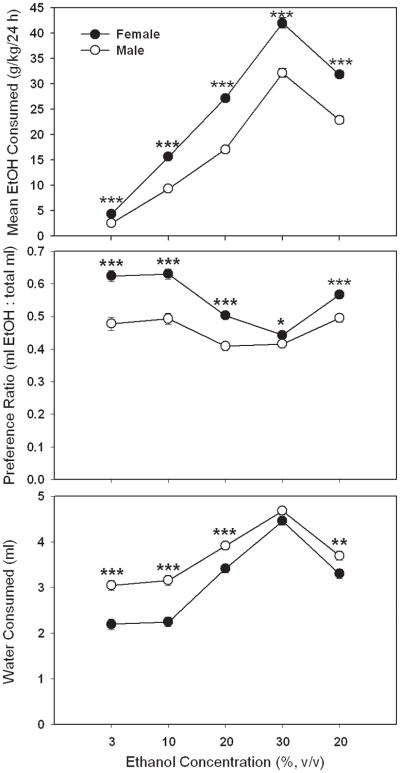
Two-bottle choice, continuous access EtOH consumption, preference and total volume consumption data are summarized in Figure 2 for male and female B6FVBF2 mice. Shown are mean consumption (Fig 2A) and preference (Fig 2B) values for each EtOH concentration, using the second day following each tube position switch (e.g., average of days 2 and 4 for 3%, days 6 and 8 for 6%, etc.). EtOH consumption increased with increasing concentration of EtOH in a sex-dependent manner (F[4,2412] = 21.6, p <.001 for the concentration × sex interaction). Female mice consumed more EtOH than male mice. Simple effect analyses indicated that the sex difference was significant for all concentrations of EtOH, including 3%, although the pattern of results shown in Fig 2A indicates a larger mean difference for higher EtOH concentrations. Female mice consumed 42 ± 0.9 g EtOH/kg/24 h on average, when EtOH was offered as a 30% concentration, whereas male mice consumed 32 ± 0.9 g EtOH/kg/24 h of the 30% solution (see Fig 2A). EtOH preference (Fig 2B) also varied with concentration and sex (F[4,2412] = 9.2, p <.001 for the concentration × sex interaction). Female mice showed a significantly higher preference for EtOH at all concentrations; however, this difference was smallest at the highest EtOH concentration. When total volume of fluid consumed was examined, a significant effect of EtOH concentration was found (F[4,2412] = 327, p <.001), but no effect of sex or interaction with sex was found (means ± SEM for the sexes combined were 5.78 ± 0.05, 6.13 ± 0.05, 6.75 ± 0.06, 8.03 ± 0.09, 7.44 ± 0.08 ml for the 3, 10, 20, 30 and 20% concentrations, respectively). For water consumption on the days when EtOH consumption was examined (Fig 2C), there was a significant effect of sex that varied with the concentration of EtOH offered versus water (F[4,2412] = 7.2, p < 0.001). Generally, males consumed more water compared to females, except when the 30% concentration of EtOH was the alternative solution.
Figure 2.
EtOH consumption and preference in a 24-h two-bottle choice procedure are greater in female than male F2 mice from the reciprocal cross of the C57BL/6J (B6) and FVB/NJ (FVB) inbred strains. Shown are means ± SEM for (A) EtOH consumption, (B) EtOH preference and (C) water consumption, when multiple concentrations of EtOH were offered versus water for 4 consecutive days at each EtOH concentration in the order listed along the x-axis. Means are the average of days 2 and 4, the last days after an EtOH tube position change for each concentration. Some error bars are hidden by the symbols. n = 303 female and 302 male; * p < 0.05, ** p < 0.01, *** p < 0.001 for the comparison of female and male mice at the indicated concentration.
Frequency distributions in Figure 3 show the range of EtOH consumption values in this population. The female distributions tend to be skewed toward higher levels of consumption. There are significant departures from normality in all except one case (see results in Figure 3); however, the departures from normality appear to be somewhat greater for the lower than higher EtOH concentrations.
Figure 3.

Frequency distributions for consumption of EtOH offered versus water. Data are expanded from those summarized in Fig 2. The black curves show the predicted normal distributions. Actual female (left panels) and male (right panels) data are shown as histograms. Listed in each panel are Chi-Square test results for the comparison of predicted normal and actual distributions.
Drinking in the Dark (DID)
Data from the DID phase of the study are shown in Figure 4. Data from days on which EtOH was offered for 2 h (Days 22–24) were analyzed using a repeated measures ANOVA, with sex as a grouping factor. Consumption of EtOH for each 2-h period during this single-bottle DID procedure was consistent across days (p = 0.72 for the main effect of Day), but female mice consumed more EtOH than male mice (F[1,606] = 21.1, p <.001). The 4-h drinking data collected on Days 25 and 26 were then examined in a separate repeated measures analysis. EtOH consumption was significantly reduced when water was offered as an alternative drinking solution (F[1,608] = 203.6, p <.001 for the main effect of day), and females continued to consume more EtOH than males (F[1,608] = 34.4, p <.001) regardless of whether EtOH was offered alone or versus water.
Figure 4.

EtOH consumption in single-bottle and two-bottle choice drinking in the dark (DID) procedures is greater in female than male B6FVBF2 mice, and EtOH intake is reduced when water is offered as an alternative source of fluid. Shown are means ± SEM for EtOH consumption during 2-h periods on five consecutive days (days 22–26 in Fig 1). EtOH was offered for 4 total hours on days 25 and 26. *** p < 0.001 for the comparison of data for the sexes combined on day 26 versus all means for the sexes combined on days 22–25 (Note: because there was no interaction of sex and day, effects for each sex were not analyzed across days).
Phenotypic Relationships
EtOH consumption and preference data from the 24-h and DID procedures were examined for relationships. Shown in Table 1 are Pearson Product-Moment correlations among these variables. Within the 24-h procedure, g/kg and preference values within particular concentrations of EtOH were significantly correlated at r ≥ 0.78 except for the 30% concentration for which r = 0.60, still highly significant. For two-bottle choice DID, consumption and preference were significantly, but not as strongly, correlated (r = 0.38 to 0.43). Considering intake for 24-h choice and g/kg EtOH consumed during DID (total 4 h) testing for the common 20% EtOH concentration, correlations ranged from r = 0.17 – 0.31. Interestingly, the correlations were generally larger between the 24-h choice and two-bottle choice data than between 24-h choice and single-bottle DID, suggesting that at least at this phenotypic level of investigation, the presence of a water bottle influences consistency of EtOH intake for individual animals. BEC data are most relevant to the EtOH intake data on the day on which BECs were measured. It is surprising that BEC correlated more strongly with EtOH consumption data when water was available (r=0.40) than when not available (r=0.18).
Table 1.
Phenotypic correlations (Pearson’s Product-Moment) between pairs of the 24 variables shown in Figure 5. The analysis was set for pairwise deletion of missing data. Group size was 583–617 B6FVBF2 mice for each correlation. Bold and italicized = p < 0.01. This more conservative α level was used to reduce the probability of concluding that two variables were associated when they were not, due to the large number of correlations examined in this experiment.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 - PR 3% | 1.00 | |||||||||||||||||
| 2 - g/kg 3% | 0.89 | 1.00 | ||||||||||||||||
| 3 - PR 10% | 0.40 | 0.38 | 1.00 | |||||||||||||||
| 4 - g/kg 10% | 0.39 | 0.51 | 0.88 | 1.00 | ||||||||||||||
| 5 - PR 20%-1 | 0.20 | 0.21 | 0.56 | 0.51 | 1.00 | |||||||||||||
| 6 - g/kg 20%-1 | 0.22 | 0.32 | 0.49 | 0.58 | 0.80 | 1.00 | ||||||||||||
| 7 - PR 30% | 0.09 | 0.09 | 0.34 | 0.31 | 0.41 | 0.35 | 1.00 | |||||||||||
| 8 - g/kg 30% | 0.09 | 0.23 | 0.16 | 0.30 | 0.20 | 0.36 | 0.60 | 1.00 | ||||||||||
| 9 - PR 20%-2 | 0.19 | 0.19 | 0.53 | 0.50 | 0.53 | 0.48 | 0.46 | 0.20 | 1.00 | |||||||||
| 10 - g/kg 20%-2 | 0.25 | 0.32 | 0.45 | 0.55 | 0.43 | 0.53 | 0.37 | 0.38 | 0.78 | 1.00 | ||||||||
| 11 - DID g/kg d22 | 0.04 | 0.12 | 0.10 | 0.19 | 0.16 | 0.28 | 0.09 | 0.25 | 0.12 | 0.21 | 1.00 | |||||||
| 12 - DID g/kg d23 | 0.03 | 0.08 | 0.08 | 0.14 | 0.15 | 0.20 | 0.10 | 0.25 | 0.12 | 0.16 | 0.56 | 1.00 | ||||||
| 13 - DID g/kg d24 | 0.04 | 0.07 | 0.06 | 0.09 | 0.09 | 0.19 | 0.06 | 0.18 | 0.08 | 0.15 | 0.57 | 0.63 | 1.00 | |||||
| 14 - DID g/kg d25 | 0.01 | 0.06 | 0.08 | 0.14 | 0.12 | 0.23 | 0.07 | 0.21 | 0.10 | 0.17 | 0.56 | 0.63 | 0.69 | 1.00 | ||||
| 15 - DID g/kg d26 | 0.12 | 0.17 | 0.17 | 0.23 | 0.19 | 0.31 | 0.13 | 0.24 | 0.24 | 0.30 | 0.46 | 0.48 | 0.55 | 0.55 | 1.00 | |||
| 16 - BEC d25 | 0.12 | 0.21 | 0.32 | 0.36 | 0.31 | 0.33 | 0.25 | 0.26 | 0.41 | 0.38 | 0.15 | 0.16 | 0.14 | 0.18 | 0.20 | 1.00 | ||
| 17 - BEC d26 | 0.15 | 0.19 | 0.30 | 0.32 | 0.27 | 0.26 | 0.24 | 0.19 | 0.38 | 0.34 | 0.13 | 0.13 | 0.06 | 0.05 | 0.40 | 0.38 | 1.00 | |
| 18 - PR d26-tot | 0.16 | 0.18 | 0.22 | 0.22 | 0.17 | 0.18 | 0.14 | 0.06 | 0.29 | 0.22 | 0.15 | 0.13 | 0.09 | 0.10 | 0.43 | 0.23 | 0.48 | 1.00 |
QTL scans
Consistent with the findings of Blednov et al. (2005), our most significant findings for over-dominant (or heterotic) effects for 24-h, two-bottle choice EtOH consumption were for consumption (g/kg/d) of the 30% concentration, which was offered on days 14 and 16. Figure 5 shows the QTL scan results expressed as traditional LOD scores distributed genome-wide. As can be seen, two QTLs emerged as significant, one on mid Chr 9 and the other on mid Chr 11. These LOD scores reflect both additive (a) and dominance (d) variation taken together, although a tends to have a greater influence on the LOD score than does d in an F2 (Falconer and Mackay, 1996). For Chr 9, there were multiple suggestive or significant results for markers spanning a 49–109 Mb region (~29–61 cM). For Chr 11, there were suggestive or significant results for markers spanning a 59 – 79 Mb region (~34–47 cM).
Figure 5.
Consumption of 30% EtOH in a two-bottle 24-h choice procedure is associated with QTL on chromosomes 9 and 11. Shown are LOD scores from the genome-wide QTL analysis indicating suggestive (bottom dashed line) and significant (top dashed line) associations of genetic markers with the average amount of 30% EtOH consumed on days 14 and 16. Chromosome number is listed along the x-axis.
More to the point concerning the effects of dominance are the results shown in Figure 6, which shows the corresponding values of a and d plotted separately for the same markers and intervals as the LOD score. Based on the magnitude of a alone, QTLs emerged as significant on mid Chr 9 and distal Chr 12, with 2-LOD support intervals of 42 – 110 Mb and 31 – 84 Mb, respectively. Based on the magnitude of d alone, QTLs emerged as significant on mid Chr 11, mid Chr 15, and mid to distal Chr 16 with 2-LOD support intervals of 61 – 88 Mb, 48 – 92 Mb, and 53 – 91 Mb, respectively. Over-dominance was also statistically significant for these same three dominance QTLs based on the magnitude of d being significantly greater than a for all three of these QTL regions. EtOH drinking values were considered for each of these dominance QTLs independently by separating mice into groups based on their genotype at the most significant marker in each of the dominance QTL regions. These results are shown in Figure 7. In each case, EtOH consumption was significantly greater in mice that were heterozygous for the marker, compared to mice that were homozygous B6 or FVB, which parallels the findings of Blednov et al. (2005) at the level of progenitor strain versus F1 means, rather than at the QTL level. In contrast, we found no evidence of epistasis between any pair of significant or suggestive QTLs based on either a or d or both combined (all ps >.01). Finally, Figure 8 shows the Illumina SNPs used for genotyping on the three relevant Chr, and shows the 2 LOD confidence intervals for these QTLs. To assess repeatability (aka, reliability), we used a split-half analysis where the QTL results of the odd numbered mice were compared to the even numbered mice. The vector of LOD scores genome-wide correlated 0.92 between the half samples using the Spearman-Brown method; thus, the reliability of this study was quite high.
Figure 6.
Additive (a; shown in grey) and dominance (d; shown in black) values plotted separately for the same markers and intervals as the LOD scores shown in Fig 6 for two-bottle choice 24-h 30% EtOH consumption. Significant QTLs were those for which a or d exceeded the absolute value of 0.204 and 0.287, respectively. Based on the magnitude of a alone, QTLs emerged as significant on mid Chr 9 and distal Chr 12. Based on the magnitude of d alone, QTLs emerged as significant on mid Chr 11, mid Chr 15, and mid to distal Chr 16.
Figure 7.

Results of dominance deviation QTL mapping in B6FVBF2 mice for 24-h, two-bottle choice 30% EtOH consumption (g/kg/d). Shown are mean ± SEM EtOH consumption values for mice homozygous B6, heterozygous B6FVB or homozygous FVB for markers significantly associated with excessive EtOH consumption in the heterozygous animals. Overdominance was evaluated for statistical significance by testing whether d was significantly greater than a as described in Methods. This comparison was significant for all three regions shown. Please note that the genotypes shown are at a specific marker, not between markers (interval analysis) as shown in Fig 6.
Figure 8.
Locations of genetic markers and 2 LOD confidence intervals for QTL associated with heterozygote overdominant 30% EtOH consumption on chromosomes 11, 15 and 16. Shown along the lengths of the chromosomes are the SNPs used for genotyping and their locations in b.
For the DID data, five variables were of interest and were subjected to QTL scans. First were g/kg 20% EtOH consumption on Days 25 and 26, when EtOH was offered alone and then vs. water. For these variables, no significant LOD scores from the traditional Mainscan output were seen, although two suggestive QTLs emerged as shown in Table 2. However, when a and d were analyzed separately, no significant results emerged for a, but for d, a significant over-dominant QTL was seen on Chr 11 (proximal to mid) for each of these two days as shown in Fig. 9. Next, the corresponding BEC measures taken on those same two days were examined. Mainscan detected two significant QTLs on Chr. 13 (mid) and 17 (proximal to mid). When a and d were analyzed separately, a was significant on Chr 13 (mid) and 17 (proximal to mid) and d was significant for Chr 11 (proximal to mid) for each of these two days as shown in Fig 9. Finally, for the EtOH preference measure derived from the two-bottle choice presentation on Day 26, Mainscan detected significant QTLs on Chr 13 (mid) and 17 (proximal to mid) as shown in Table 2, and when a and d were analyzed, a was significantly greater than zero only on Chr 17 (proximal to mid) and d was significant only on Chr 11 (proximal to mid) as shown in Fig 9. Thus, all of the five DID measures and the primary 24-h, two-bottle choice variable showed significant over-dominance on proximal to mid Chr. 11. For all six measures, the heterozygotes drank more EtOH than did either of the two homozygote classes. Pairscan did not reveal any evidence of epistasis among the significant or suggestive QTLs for any of the DID measures.
Table 2.
QTL scan results as LOD scores for the key variables for the drinking in the dark (DID) experiment. These variables are the Day 25 and 26 EtOH consumption in g/kg (gkg d25 and g/kg d26), BECs for those two days (BEC d25 and BEC d26) and the preference ratio for the 4-hr two-bottle choice presentation on Day 26 (PR d26). Only those chromosomal regions showing at least a suggestive (LOD > 1.9) or significant QTL (LOD > 3.4) are shown (significant are in bold and italics). Markers beginning with mm are SNP markers; the others are intervals between markers from EM interval mapping.
| Marker | Chr | Mb | gkg d25 | gkg d26 | BEC d25 | BEC d26 | PR d26 |
|---|---|---|---|---|---|---|---|
| c1.loc40 | 1 | 79.3202 | 1.3075 | 0.6523 | 0.1816 | 3.2001 | 0.7243 |
| mm13480953 | 11 | 33.8977 | 1.7837 | 2.6875 | 2.7031 | 0.9929 | 1.7631 |
| c11.loc10 | 11 | 43.8977 | 0.6858 | 1.5920 | 2.1994 | 0.4628 | 1.9294 |
| c11.loc20 | 11 | 53.8977 | 1.7923 | 1.6596 | 2.6250 | 2.0444 | 2.1245 |
| mm6313602 | 11 | 58.61899 | 2.9636 | 2.1592 | 2.3913 | 2.5865 | 2.0963 |
| c11.loc30 | 11 | 63.8977 | 3.1567 | 2.3744 | 2.2485 | 2.6755 | 1.7291 |
| c11.loc40 | 11 | 73.8977 | 3.2253 | 2.1313 | 1.3280 | 1.9698 | 0.7020 |
| mm13481117 | 11 | 78.86479 | 3.0617 | 1.7977 | 0.8917 | 1.4338 | 0.3304 |
| mm3657836 | 13 | 45.53282 | 0.9944 | 0.8713 | 3.4748 | 4.1910 | 0.9826 |
| c13.loc10 | 13 | 55.53282 | 0.2404 | 0.1407 | 1.2751 | 1.2192 | 3.5838 |
| c13.loc20 | 13 | 65.53282 | 0.2548 | 0.4092 | 2.7376 | 1.3430 | 1.1357 |
| mm13481870 | 13 | 67.44601 | 0.3059 | 0.5194 | 2.8693 | 1.5724 | 1.1894 |
| mm6384940 | 17 | 27.64924 | 0.2180 | 1.0826 | 3.3795 | 3.5889 | 5.1313 |
| c17.loc10 | 17 | 37.64924 | 1.6474 | 2.1415 | 3.4235 | 4.8628 | 4.8703 |
| mm3705130 | 17 | 46.93326 | 2.4804 | 2.0370 | 2.9118 | 4.9101 | 3.6691 |
| c17.loc20 | 17 | 47.64924 | 2.4472 | 2.0274 | 2.8248 | 4.8288 | 3.6045 |
| c17.loc30 | 17 | 57.64924 | 1.8867 | 1.6072 | 1.4029 | 3.0325 | 2.3536 |
Figure 9.
The magnitude of d (dominance deviation) on Chr 11 for all five of the DID measures and the 24-h, two-bottle choice measure (30% EtOH consumption; g/kg/d). For all six measures, a significant overdominant QTL was seen that conferred higher EtOH consumption, preference or blood EtOH concentration in the heterozygotes compared to either homozygote class.
Discussion
The current paper describes a method for mapping intralocus interactions that result in greater ethanol consumption amounts for heterozyogote than homozygote class mice. Large individual differences among mice from the F2 cross of the B6 and FVB inbred mouse strains in the amount of 10–30% EtOH consumed in a 24-h, two-bottle choice procedure were found. The high consumption values found in some mice are consistent with those seen in the F1 cross of these strains (Beldnov et al. 2005), when compared to the B6 and FVB progenitor inbred strains. The heterosis (Bruell 1967) or higher EtOH intake seen in the F1 compared to the intake of each progenitor strain, led to our interest in attempting to map loci that result in levels of consumption in heterozygotes that exceed those found in the B6 mouse. The distribution of EtOH consumption values tended to depart from normality to a somewhat greater extent for lower than for higher EtOH concentrations. B6FVBF2 mice also exhibited high levels of EtOH consumption using the typical single-bottle DID procedure, as well as a two-bottle DID procedure. Their consumption values for single-bottle DID were within a range comparable to those seen for B6 mice (e.g., Moore and Boehm 2009; Rhodes et al. 2005), and somewhat lower than achieved in a selective breeding project that was initiated from an 8-way cross of inbred mouse strains (Crabbe et al. 2009).
Genome scan results for QTLs were expressed as traditional LOD scores and examined for evidence of additive and dominance effects. For 24-h choice, 30% EtOH consumption, evidence for significant QTLs based on the traditional (Mainscan, or additive and dominance combined) analysis was found on Chr 9 and 11. Evidence for additive effects was found on Chr 9 and 12, whereas evidence for three significant QTLs influencing over-dominant EtOH intake was found on Chr 11, 15 and 16. For the five DID measures, only one over-dominant QTL was found, and for all five this was on Chr 11. Thus, the 24-h and DID measures all showed an over-dominant QTL on Chr 11. In contrast, we found no evidence of epistasis between any pair of significant or suggestive QTLs. This indicates that the over-dominance seen in the F1 versus the B6 and FVB progenitors (Blednov et al. 2005) was most likely due to intralocus interactions characteristic of dominance variation exceeding that due to additive variation, or in other words, over-dominance at the level of individual QTL.
These are the first data addressing additive vs dominance QTLs; however, evidence for EtOH consumption/preference QTLs, using traditional analyses, has been previously found for regions of chromosome 9 and 12 (Belknap and Atkins 2001; Gill and Boyle 2005; Vadasz et al. 2007). The current results suggest that the same genes or linked genes in the same regions influence 30% EtOH consumption in mice possessing B6 and FVB alleles. Gene expression analysis has nominated several cis-regulated candidate genes in the Chr 9 region as EtOH consumption quantitative trait genes (QTG) (Mulligan et al., 2006). To the best of our knowledge, none of these has yet been shown to play a functional role of its own or in a gene network that influences EtOH consumption. Data were obtained for multiple phenotypes in the same individual F2 animals so that phenotypic correlations could be examined. In general, the results suggest relatively weak relationships between EtOH consumption measures collected in a 24-h, two-bottle choice procedure and a single- or two-bottle DID procedure (r = 0.17 – 0.31). This may suggest that EtOH drinking behavior over a longer time-span is genetically divergent from that measured during the more restricted DID period. However, phenotypic correlations include genetic and environmental sources of variation. The 24-h choice data were obtained first, and these drinking data had the possibility of influencing subsequent DID results, an environment or genotype × environment source of variation. The DID behavior of mice of different genotypes within this heterogeneous population could have been differentially affected by drinking history. In addition, differential effects could be partly associated with the different amounts of EtOH consumed. We measured 24-h choice drinking first because phenotypes from this procedure were those that we wished to utilize for over-dominant QTL mapping, the overarching goal of this research. Rhodes et al. (2007) found that amount of EtOH consumed in a single-bottle DID procedure was genetically correlated with 24-h intake data for the 7 strains shared in common from the Mouse Phenome Project database (r = 0.70). The B6 strain was the highest EtOH consuming strain for both measures, but the FVB strain was not included in this analysis. It is not clear whether the disparity in correlational results is associated with a difference in the genotypes included in the analysis or with more significant environment or genotype × environment influences on DID behavior in the current study.
DID EtOH consumption amounts in the F2 mice did not appear to be excessive compared to those previously reported for B6 mice, yet evidence for an over-dominant QTL was identified. When this trait was examined in a direct comparison of B6, FVB and their F1 hybrid, unlike 24-h choice, EtOH consumption of the hybrid in the DID procedure closely resembled that of the B6 progenitor strain (Blednov et al. 2005). The absence of phenotypic over-dominance does not indicate that all genetic loci are acting additively with regard to a complex trait – one that is influence by several genes. In fact, heterozygosity at some locations could result in significantly reduced EtOH intake, while at others resulting in enhanced intake. Others could act purely additively or show complete dominance. The net results might be the appearance of an additive or dominant phenotype, when over-dominance could be present at one or more loci. F1 data indicate the absence of phenotypic heterosis, but the F2 mapping results suggest the presence of at least one over-dominant QTL.
Whereas the differences between procedures for measuring 24-h choice and DID are many, the differences between procedures for measuring single- vs two-bottle DID are few. The only significant difference is the introduction of a water-containing bottle during the limited access drinking period. Therefore, it might be expected that correspondence between the single- and two-bottle DID measures would be higher than between 24-h choice and DID, and his was the case. In addition, two-bottle DID was more highly correlated with two-bottle choice EtOH consumption than was single bottle DID EtOH consumption. Our correlations are phenotypic; however, to estimate genetic correlations, Rhodes et al. (2007) tested 8–12 inbred mouse strains, including B6 and FVB. They reported that g/kg intake for two 4-h, single-bottle tests, and BEC at the end of the two tests were highly genetically correlated (r = 0.89 and 0.91, respectively). In addition, the genetic correlation for EtOH intake in the single- and two-bottle tests was highly significant (r = 0.92). Thus, the genetic relationships between these traits appear to be stronger than reflected in the phenotypic correlations from the current study. However, the contribution to the results of alleles from multiple strains in the Rhodes et al. (2007) study versus only B6 and FVB alleles in the current investigation must be considered.
There has been little investigation of EtOH drinking levels in hybrid mice in which they have been compared to the progenitors used to derive them. However, F1 crosses involving, B6, D2, A/J, BALB/cJ, and CD-1 have shown largely intermediate EtOH consumption amounts in 24-h choice procedures, and in no case was over-dominance seen (Goodrick 1978; Short et al. 2006; Gabriel and Cunningham 2008). The QTL we have identified would be expected to be unique to populations carrying the same alleles as B6 and FVB mice, making intralocus interaction of the two allele types possible. The next step in our analysis of these d QTLs will be to examine the nature of the gene networks involved. We plan to accomplish this through gene expression analysis in advanced filial crosses of B6 and FVB mice (F3 and beyond), comparing individual mice that are heterozygous at a d QTL to those that are homozygous B6 or FVB. Expression differences within the QTL regions as well as those that may be coordinated with these differences may explain at least some portion of the over-dominant drinking seen in these hybrid mice. The over-dominant QTLs detected and described here provide an important screen to identify the strongest candidate genes for our future endeavors. Over-dominance should also be seen for the genes responsible for the QTLs at multiple levels of analysis from gene expression to protein function.
Acknowledgments
This work was supported by the Department of Veterans Affairs, and by NIAAA grants U01 AA016655, U01 AA013484 and P60 AA010760.
References
- Belknap JK, Atkins AL. The replicability of QTLs for murine alcohol preference drinking behavior across eight independent studies. Mamm Genome. 2001;12:893–899. doi: 10.1007/s00335-001-2074-2. [DOI] [PubMed] [Google Scholar]
- Belknap JK, Crabbe JC, Young ER. Voluntary consumption of ethanol in 15 inbred mouse strains. Psychopharmacology. 1993;112:503–510. doi: 10.1007/BF02244901. [DOI] [PubMed] [Google Scholar]
- Bergeson SE, Kyle Warren R, Crabbe JC, Metten P, Erwin VG, Belknap JK. Chromosomal loci influencing chronic alcohol withdrawal severity. Mamm Genome. 2003;14:454–463. doi: 10.1007/s00335-002-2254-4. [DOI] [PubMed] [Google Scholar]
- Blednov YA, Metten P, Finn DA, Rhodes JS, Bergeson SE, Harris RA, Crabbe JC. Hybrid C57BL/6J × FVB/NJ mice drink more alcohol that do C57BL/6J mice. Alcohol Clin Exp Res. 2005;29:1949–1958. doi: 10.1097/01.alc.0000187605.91468.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm SL, II, Schafer GL, Phillips TJ, Browman KE, Crabbe JC. Sensitivity to ethanol-induced motor incoordination in 5-HT(1B) receptor null mutant mice is task-dependent: implications for behavioral assessment of genetically altered mice. Behav Neurosci. 2000;114:401–409. [PubMed] [Google Scholar]
- Bohman M, Cloninger R, Sigvardsson S, von Knorring AL. The genetics of alcoholisms and related disorders. J Psychiatr Res. 1987;21:447–452. doi: 10.1016/0022-3956(87)90092-6. [DOI] [PubMed] [Google Scholar]
- Broman KW, Wu H, Sen S, Churchill GA. R/qtl: QTL mapping in experimental crosses. Bioinformatics. 2003;19:889–890. doi: 10.1093/bioinformatics/btg112. [DOI] [PubMed] [Google Scholar]
- Broman KW, Sen S, Owens SE, Manichaikul A, Southard-Smith EM, Churchill GA. The X chromosome in quantitative trait locus mapping. Genetics. 2006;174:2151–2158. doi: 10.1534/genetics.106.061176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruell JH. Behavioral heterosis. In: Hirsch J, editor. Behavior-Genetic Analysis. McGraw-Hill; New York: 1967. pp. 270–286. [Google Scholar]
- Chesler EJ, Lu L, Shou S, Qu Y, Gu J, Wang J, Hsu HC, Mountz JD, Baldwin NE, Langston MA, Threadgill DW, Manly KF, Williams RW. Complex trait analysis of gene expression uncovers polygenic and pleiotropic networks that modulate nervous system function. Nat Genet. 2005;37:233–242. doi: 10.1038/ng1518. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Metten P, Rhodes JS, Yu CH, Brown LL, Phillips TJ, Finn DA. A line of mice selected for high blood ethanol concentrations shows drinking in the dark to intoxication. Biol Psychiatry. 2009;65:662–670. doi: 10.1016/j.biopsych.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Boettiger CA. Impulsivity, frontal lobes and risk for addiction. Pharmacol Biochem Behav. 2009;93:237–247. doi: 10.1016/j.pbb.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducci F, Goldman D. Genetic approaches to addiction: genes and alcohol. Addiction. 2008;103:1414–1428. doi: 10.1111/j.1360-0443.2008.02203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falconer DS, Mackay TFC. Introduction to quantitative genetics. 4. Longman Group Ltd; Essex, England: 1996. [Google Scholar]
- Gabriel KI, Cunningham CL. Effects of maternal strain on ethanol responses in reciprocal F1 C57BL/6J and DBA/2J hybrid mice. Genes Brain Behav. 2008;7:276–287. doi: 10.1111/j.1601-183X.2007.00349.x. [DOI] [PubMed] [Google Scholar]
- Gill K, Boyle AE. Genetic analysis of alcohol intake in recombinant inbred and congenic strains derived from A/J and C57BL/6J progenitors. Mamm Genome. 2005;16:319–331. doi: 10.1007/s00335-004-2239-x. [DOI] [PubMed] [Google Scholar]
- Godoy-Herrera R. Biometrical analysis of larval digging in Drosophila melanogaster. Behav Genet. 1994;24:427–432. doi: 10.1007/BF01076178. [DOI] [PubMed] [Google Scholar]
- Goodrick CL. Ethanol selection by inbred mice. Mode of inheritance and the effect of age on the genetic system. J Stud Alcohol. 1978;39:19–38. doi: 10.15288/jsa.1978.39.19. [DOI] [PubMed] [Google Scholar]
- Hood HM, Belknap JK, Crabbe JC, Buck KJ. Genomewide search for epistasis in a complex trait: pentobarbital withdrawal convulsions in mice. Behav Genet. 2001;31:93–100. doi: 10.1023/a:1010214026692. [DOI] [PubMed] [Google Scholar]
- Kearsey, Pooni . The Genetical Analysis of Quantitative Traits. London: Chapman & Hall; 1996. [Google Scholar]
- Kuo PH, Kalsi G, Prescott CA, Hodgkinson CA, Goldman D, van den Oord EJ, Alexander J, Jiang C, Sullivan PF, Patterson DG, Walsh D, Kendler KS, Riley BP. Association of ADH and ALDH genes with alcohol dependence in the Irish affected sib pair study of alcohol dependence (IASPSAD) sample. Alcohol Clin Exp Res. 2008;32:785–795. doi: 10.1111/j.1530-0277.2008.00642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore EM, Boehm SL., II Site-specific microinjection of baclofen into the anterior ventral tegmental area reduces binge-like ethanol intake in male C57BL/6J mice. Behav Neurosci. 2009;123:555–563. doi: 10.1037/a0015345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan MK, Ponomarev I, Hitzemann RJ, Belknap JK, Tabakoff B, Harris RA, Crabbe JC, Blednov YA, Grahame NJ, Phillips TJ, Finn DA, Hoffman PL, Iyer VR, Koob GF, Bergeson SE. Toward understanding the genetics of alcohol drinking through transcriptome meta-analysis. Proc Natl Acad Sci USA. 2006;103:6368–6373. doi: 10.1073/pnas.0510188103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer AA, Low MJ, Grandy DK, Phillips TJ. Effects of a Drd2 deletion mutation on ethanol-induced locomotor stimulation and sensitization suggest a role for epistasis. Behav Genet. 2003;33:311–324. doi: 10.1023/a:1023450625826. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav. 2005;84:53–63. doi: 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Ford MM, Yu CH, Brown LL, Finn DA, Garland T, Jr, Crabbe JC. Mouse inbred strain differences in ethanol drinking to intoxication. Genes Brain Behav. 2007;6:1–18. doi: 10.1111/j.1601-183X.2006.00210.x. [DOI] [PubMed] [Google Scholar]
- Rosenthal . Parametric measures of effect size. In: Cooper H, Hedges LV, editors. The Handbook of Research Synthesis. New York: Russell Sage Fnd; 1994. pp. 232–244. [Google Scholar]
- Schuckit MA. An overview of genetic influences in alcoholism. J Subst Abuse Treat. 2009;36:S5–15. [PubMed] [Google Scholar]
- Short JL, Drago J, Lawrence AJ. Comparison of ethanol preference and neurochemical measures of mesolimbic dopamine and adenosine systems across different strains of mice. Alcohol Clin Exp Res. 2006;30:606–620. doi: 10.1111/j.1530-0277.2006.00071.x. [DOI] [PubMed] [Google Scholar]
- Song MJ, Lewis CK, Lance ER, Chesler EJ, Yordanova RK, Langston MA, Lodowski KH, Bergeson SE. Reconstructing generalized logical networks of transcriptional regulation in mouse brain from temporal gene expression data. EURASIP J Bioinform Syst Biol, article ID 545176. 2009:1–13. doi: 10.1155/2009/545176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabakoff B, Saba L, Kechris K, Hu W, Finn DA, Grahame NJ, Hoffman PL. The genomic determinants of alcohol preference in mice. Mann Genome. 2008;19:352–365. doi: 10.1007/s00335-008-9115-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RG, Jr, Lizardi D, Keyes KM, Hasin DS. Childhood or adolescent parental divorce/separation, parental history of alcohl problems, and offspring lifetime alcohol dependence. Drug Alcohol Depend. 2008;98:264–269. doi: 10.1016/j.drugalcdep.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadasz C, Saito M, Gyetvai BM, Oros M, Szakall I, Kovacs KM, Prasad VV, Morahan G, Toth R. Mapping of QTLs for oral alcohol self-administration in B6.C and B6.I quasi-congenic RQI strains. Neurochem Res. 2007;32:1099–1112. doi: 10.1007/s11064-006-9234-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlsten D, Bachmanov A, Finn DA, Crabbe JC. Stability of inbred mouse strain differences in behavior and brain size between laboratories and across decades. Proc Natl Acad Sci U S A. 2006;103:16364–16369. doi: 10.1073/pnas.0605342103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneyama N, Crabbe JC, Ford MM, Murillo A, Finn DA. Voluntary ethanol consumption in 22 inbred mouse strains. Alcohol. 2008;42:149–160. doi: 10.1016/j.alcohol.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]