Abstract
Differences in cardiovascular disease outcomes between men and women have long been recognized and attributed, in part, to gender and sex steroids. Gender dimorphisms also exist with respect to the roles of progenitor and stem cells in post-ischemic myocardial and endothelial repair and regeneration. Understanding how these cells are influenced by donor gender and the recipient hormonal milieu may enable researchers to further account for the gender-related disparities in clinical outcomes as well as utilize the beneficial effects of these hormones to optimize transplanted cell function and survival. This review discusses (1) the cardiovascular effects of sex steroids (specifically estradiol and testosterone); (2) the therapeutic potentials of endothelial progenitor cells, mesenchymal stem cells, and embryonic stem cells; and (3) the direct effect of sex steroids on these cell types.
Keywords: Progenitor Cells, Stem Cell Therapy, Sex Steroids, Gender Differences, Cardiovascular Disease
Introduction
Gender differences exist in numerous types of injuries and disease states including renal ischemia/reperfusion (I/R) injury [1], trauma/hemorrhage [2], sepsis [3], intestinal ischemia-induced organ injury, cardiovascular disease [4], and myocardial inflammation [5]. With regard to cardiovascular disease, premenopausal women are at lower risk for atherosclerosis, coronary artery disease, and myocardial infarction compared to postmenopausal women and age-matched men [4, 6]. Such gender dimorphisms have been linked at least in part to differences in sex steroid expression, specifically estrogen.
Within the last decade, stem cell and progenitor cell-based therapies have been applied to the injured heart with promising initial results. Clinical trials have utilized bone marrow cell populations that include endothelial progenitor cells (EPCs) and mesenchymal stem cells (MSCs). As a circulating population, EPCs may home to areas of tissue injury and augment endothelial protection and repair [7]. MSCs in addition to other bone marrow-derived mononuclear cells may also be mobilized from bone marrow and traffic to injured myocardium or may be therapeutically applied in a more directed fashion [8]. In vitro and animal models have also investigated the similar application of embryonic stem cells (ESCs) [9].
Therefore, it is not surprising that as estrogen has been observed in multiple models to confer direct protective on the vasculature, its effect on the function of progenitor and stem cells has also drawn increasing interest. It is becoming increasingly important to understand how these cells are affected by endogenous and exogenous influences including sex steroids as well as how their function may be optimized prior to their therapeutic application. To this purpose, we review the cardiovascular effects of sex steroids; the therapeutic potentials of EPCs, MSCs, and ESCs; and the direct effect of sex steroids on these cell types.
Roles of Sex Steroids in Cardiovascular Disease
Estrogen, specifically 17β-estradiol (E2), has been demonstrated to exert multiple cardiovascular protective effects in animal models [10]. Many of these effects are exerted directly on the vasculature and involve modulation of atherogenic and vasoreactive mechanisms (Table 1). The further recognition of the role of endothelial dysfunction in atherosclerosis and cardiovascular disease has also guided investigation into how estrogen protects and repairs damaged endothelium.
Table 1.
Protective effects of estrogen (17β-estradiol) on the vasculature
| Site of action | Effect | Reference |
|---|---|---|
| Vascular smooth muscle | ↓ Vasoreactivity | [118] |
| ↓ LDL oxidation | ||
| ↓ Proliferation | ||
| Tunica intima | ↓ Atherosclerosis | [119, 120] |
| ↑ NO synthesis | ||
| Vascular endothelium | ↓ Macrophage and platelet adhesion | [121–123] |
| ↓ Reactive oxygen species | ||
| ↑ Post-injury re-endothelialization | ||
| Pulmonary vasculature | ↓ Hypoxia-induced vasoreactivity | [124] |
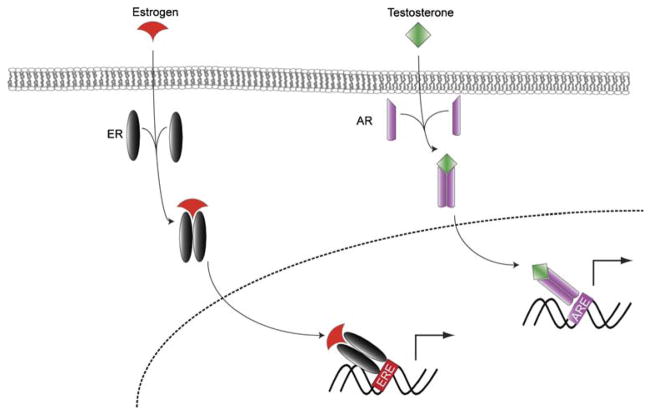
Estrogen functions primarily by signaling via estrogen receptors (ER)α and β which belong to the steroid/thyroid superfamily of nuclear receptors [11, 12]. These receptors are expressed by a wide variety of cells including vascular smooth muscle cells, endothelial cells, EPCs, MSCs, and other progenitor and stem cells. Following ligand binding, ERs mediate their effects through either genomic or non-genomic mechanisms. Genomic mechanisms include regulation of gene transcription through the direct binding of the nuclear estrogen receptor to estrogen response elements or other transcriptional regulator sequences (Fig. 1) [13]. Consequently, estrogen may suppress pro-atherogenic genes and induce athero-protective genes, downregulate interleukin (IL)-6 expression [14], and increase production of protective growth factors including vascular endothelial growth factor (VEGF) and insulin-like growth factor-1 (IGF-1) [15, 16]. E2 has also been shown to upregulate suppressor of cytokine signaling (SOCS) protein expression with resultant resistance to deleterious tumor necrosis factor-α (TNF-α) signaling in females [17, 18]. Non-genomic effects involve the direct action of estrogen on the vasculature including the rapid activation of endothelial nitric oxide synthetase (eNOS) and vasodilation which may augment tissue perfusion [19, 20].
Fig. 1.
After entering the cytoplasmic space, estrogen and androgen bind to and induce dimerization of their respective receptors. The dimers then translocate to the nucleus where they engage either estrogen response elements (ERE) or androgen response elements (ARE) to regulate gene transcription. ER estrogen receptor, AR androgen receptor
Evidence supporting the protective role of E2 in the setting of vascular injury includes the observations that E2 increased re-endothelialization, increased endothelial functional recovery (increased nitric oxide production), and decreased neointimal formation in a dose-dependent fashion in ovariectomized (OVX) mice following carotid artery injury [21]. This E2-induced re-endothelialization appears to be mediated by ERα [22, 23]. ERβ, on the other hand, has separately been shown to mediate vasculoprotective effects in reproductive organs [24] and myocardial protection during ischemia/reperfusion injury via upregulation of PI3K/Akt and decreased cardiomyocyte apoptosis [25]. Interestingly, E2 may also protect the vasculature in the absence of ERα or ERβ as shown in mouse knockout models [26, 27]. Specifically, early atheroprotection has recently been shown to occur independently of ERα in OVX ERα −/− mice treated with exogenous E2 [12]. Thus, while ERα and ERβ are important mediators of E2-induced vasculoprotection, other receptors or signaling pathways are likely involved.
The promising results of these early animal studies have not been fully realized in clinical trials, however. In the Heart and Estrogen/Progestin Replacement Study that included menopausal women with documented coronary artery disease, there was no reduction in cardiovascular events with exogenous hormone therapy [28, 29]. In addition, hormonal therapy was associated with an increased risk of early coronary events and venous thrombo-embolic events. The Women’s Health Initiative Estrogen/Progesterone Study was also stopped early due to increased risks of breast cancer, coronary events, and stroke [30]. Similarly, the unopposed estrogen arm of this study was stopped due to an increased risk of stroke without any change in heart disease risk [31]. Further research is warranted to explain these discrepancies between the results of the animal studies and the clinical outcomes following estrogen therapy.
The evidence that men have a greater incidence of coronary artery disease (CAD) and myocardial infarction (MI) than age-matched women also raised the hypothesis that testosterone (T) negatively affects the cardiovascular system. As demonstrated in a rat model of I/R injury, T exhibits deleterious effects on the myocardium specifically by downregulating signal transducer and activator of transcription 3 (STAT3) and suppressor of cytokine signaling 3 (SOCS3) expression during acute I/R [32]. However, other evidence suggests that T may actually possess vasculoprotective properties as well. Exogenous T was shown to inhibit aortic atherosclerosis in castrated male rabbits [33]. In addition, reduced plasma T was associated with increased arterial stiffness in men [34, 35], and the oral administration of T in men with CAD improved brachial artery vasoreactivity [36]. Furthermore, the acute administration of T in men with CAD had beneficial effects on exercise-induced myocardial ischemia [37].
Endothelial Progenitor Cells
Bone marrow-derived endothelial progenitor cells are self-renewing and capable of differentiating into mature endothelial cells. As a circulating pool, EPCs offer an immediate mechanism of repair of endothelial damage [7], and it was this ability to home to ischemic sites and differentiate into mature endothelial cells that led to their discovery [38]. Given the protective endothelial effects of E2 as well as the eventually recognized role of EPCs in maintaining endothelial integrity, it was further hypothesized that E2 may confer some of its vasculoprotective effects through regulation of EPC function.
Ischemic/injured tissue recruits EPCs through the local release of growth factors and cytokines. Both endogenous and exogenous granulocyte colony-stimulating factor and granulocyte colony-stimulating factor have been directly shown to enhance EPC mobilization and migration [39, 40]. Hypoxia-inducible factors, which are activated under low oxygen levels, induce expression of stromal cell-derived factor-1 (SDF-1) in endothelial cells with subsequent increase in the adhesion, migration, and homing of circulating EPC to ischemic areas via the CXCR4 receptor [41]. In a rat model of MI, skeletal myoblasts over-expressing SDF-1 produced an endogenous gradient that facilitated stem and progenitor cell home migration to areas of infarction and increased angiogenesis [42]. EPC migration has also been shown to be mediated by VEGF receptor 1 (flt1) and 2 (flk1) in an eNOS-dependent fashion [43, 44]. These receptors may also facilitate the increased levels of circulating EPCs following acute MI and vascular trauma [45, 46] and in relation to plasma VEGF levels [47].
EPCs have demonstrated therapeutic efficacy for ischemic disease in early animal and human studies. Mice hindlimb ischemia models have shown that endogenously mobilized and exogenously delivered EPCs improve tissue perfusion and limb recovery [40, 48]. Post-ischemic injection of EPCs resulted in improved myocardial capillary density and function 28 days later in a rat coronary artery ligation model [49]. Clinical trials investigating the use of EPC mobilization as well as exogenous intracoronary infusion or direct intramyocardial injection in patients with acute and chronic myocardial ischemia have shown promising preliminary results with improvements in left ventricular (LV) function and myocardial neovascularization following these directed therapies (reviewed in [50]). While one study ended early due to a greater-than-expected rate of in-stent restenosis in patients who received cell therapy [51], phase I and II trials are on-going.
In addition to their therapeutic potential, EPCs may also serve as predictors of cardiovascular disease outcomes. Hill et al. [52] found EPCs to be more predictive of vascular reactivity compared to conventional cardiac risk factors. In the same study, EPCs from high-risk subjects exhibited greater in vitro senescence compared to low-risk subjects. Werner et al. [53] observed that increased levels of EPCs were associated with a reduced risk of adverse cardiovascular disease outcomes but were not predictive of MI or death from all causes. Similarly, levels of circulating EPCs and function have also inversely correlated with age-related decreases in VEGF [54], diabetes [55, 56], cardiac allograft vasculopathy [57], unstable angina [58], chronic renal failure [59], essential hypertension [60], and acute stroke patients [61].
Sex Steroids and Endothelial Progenitor Cells
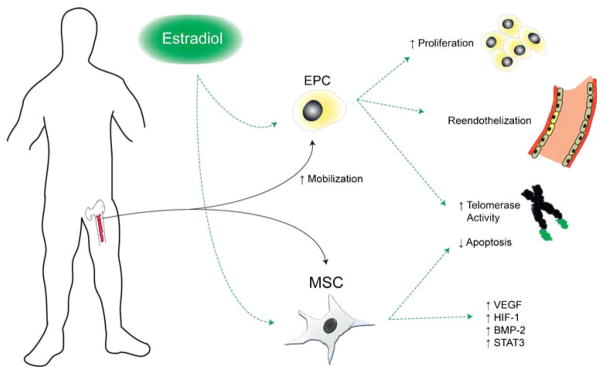
Investigations into the effect of E2 on EPCs have focused primarily on EPC mobilization, survival, and promotion of re-endothelialization. Numerous studies have demonstrated either a positive association between levels of circulating EPCs and E2 or an ability of E2 to directly stimulate mobilization of these cells (Fig. 2) [62, 63]. E2 mediates this action via ERα and ERβ; however, ERα may play a more significant role [15]. Following ER ligand binding, activation of the PI3K pathway, eNOS induction, and fibroblast growth factor (FGF)-2 production appear to be critical steps in bone marrow EPC mobilization [62, 64–66]. E2-induced EPC mobilization is also associated with accelerated re-endothelialization following vascular injury [62, 63] and direct incorporation into areas of myocardial ischemia with subsequently reduced LV scarring and increased LV function [65].
Fig. 2.
Effects of estradiol on endothelial progenitor cell and mesenchymal stem cell function. EPC endothelial progenitor cell, MSC mesenchymal stem cell, VEGF vascular endothelial growth factor, HIF-1 hypoxia-inducible factor-1, BMP-2 bone morphogenetic protein-2
E2 also affects EPC survival and growth kinetics. Specifically, E2 inhibits apoptosis via attenuation of caspase 8 activity [62]. It also inhibits EPC senescence onset in culture, increases telomerase activity, increases mitogenic activity, and activates the Akt survival pathway [60].
Hormonal status may be an important determinant of in vivo EPC function. Levels of circulating EPCs are greater in premenopausal women compared to postmenopausal women [67]. EPC levels also correlate with the level of circulating E2 during the menstrual cycle [68, 69] and are higher in fertile women than in young men or postmenopausal women. However, there is no difference in levels of EPCs between postmenopausal women and age-matched men [68]. In addition, EPCs from middle-aged women exhibit greater colony-forming capacity and migratory activity in vitro compared to those from age-matched men, providing further evidence of the effect of endogenous E2 on EPC function [70]. Finally, linear regression analysis indicates that gender and age correlate with EPC levels independent of other cardiovascular parameters.
While EPCs express androgen receptors (AR), there are more limited data regarding the effect of androgens on EPCs [71]. In one study, hypogonadal males had decreased basal circulating EPCs which increased following T treatment [71]. However, in this study, both T and estrogen levels increased following T treatment, and the peripheral conversion of T to estrogen could not be excluded as a cause of the increased EPC levels. As a follow-up study, EPCs were treated with a synthetic nonaromatizable androgen in vitro and demonstrated increased migration and proliferation by an AR-mediated mechanism [72].
The effects of androgens on EPC mobilization have recently been challenged. By separately isolating early and late EPCs, Fadini et al. [73] found that androgen stimulation had no effect on late EPC expansion and adhesion in vitro. Castration decreased levels of circulating EPCs, but this effect was not reversed with exogenous T administration. In a sample of healthy middle-aged men, the level of circulating EPCs more closely correlated with E2 than with T. Pro-survival and growth-stimulatory effects of T may also be restricted to more mature progenitor cells [74]. Furthermore, in an analysis of plasma steroid levels in males with inflammatory bowel disease, EPC levels did not correlate with T levels [75]. Thus, while the data are conflicting regarding the full role of androgens in EPC proliferation and function, T appears to be less influential than E2 in this capacity
Mesenchymal Stem Cells
MSCs derived from bone marrow are self-renewing cells capable of differentiating into multiple cell types including osteoblasts, adipocytes, chondrocytes, endothelial cells, and potentially cardiomyocytes [76, 77]. In addition to their potential for differentiation, MSCs may protect ischemic tissue via the paracrine release of growth factors and anti-inflammatory cytokines that mitigate the ischemia-induced local inflammatory response [78]. MSCs also exhibit unique immunologic characteristics in that allogeneic MSCs may be able to evade the host immune system and potentially even suppress local activation of host T lymphocytes [79]. These latter properties in particular have spurred interest in developing the cells as a readily available source of cells for the treatment of various tissue injuries.
Several randomized and nonrandomized clinical trials have evaluated the use of autologous bone marrow-derived mononuclear cells for the treatment of acute and chronic myocardial ischemia [80, 81]. While this population of cells represents both hematopoietic and mesenchymal progenitor and stem cells, the cumulative beneficial effects of transplantation of these cells has promoted further investigation into the use of the individual cell types for myocardial and peripheral ischemia.
Sex Steroids and Mesenchymal Stem Cells
MSCs exhibit gender-related differences in paracrine function [82]. In vitro, female MSCs as compared to male MSCs produced more VEGF and less TNF-α in response to stress stimuli such as lipopolysaccharide and hypoxia. Since VEGF is an important paracrine factor in MSC-mediated myocardial protection following ischemia [83], this suggested that source gender may be an important determinant of MSC function and potential cardioprotection. In addition, the decreased production of TNF-α, a pro-inflammatory mediator of I/R-related myocardial dysfunction and apoptosis, suggests that female MSCs are more resistant to certain injurious stimuli [84]. Using an ex vivo model of isolated heart perfusion, intra-coronary infusion of female MSCs was associated with greater post-ischemic myocardial functional recovery compared to male MSCs [85]. Given that these cells were removed during the in vivo estrous cycle, these observed responses may reflect the inherent chronic effects of estrogen or estrogen-independent functions. Exogenous administration E2 may also play a role on MSC function as E2 stimulates male MSC production of VEGF in vitro [86]. Moreover, hearts infused with E2-treated MSCs exhibited greater functional recovery after I/R compared to those infused with untreated MSCs [86]. Cumulatively, these data suggest that exposure to estrogen may augment MSC function and potentially MSC-mediated cardioprotection.
The roles of ERα and ERβ in MSC function have also been evaluated. Our lab observed that E2 as well as ERα but not ERβ agonism can stimulate production of VEGF and hypoxia-inducible factor-1α (HIF-1α) as well as activate STAT3 in vitro (Fig. 2) [87]. These effects were abolished in ERα and STAT3 but not ERβ-KO MSCs. The HIF-1 family of cytokines is upregulated in response to reduced tissue oxygenation and may mediate growth factor production including VEGF [88]. Following induction by cellular stress and growth factors, STAT3 participates in several cell functions including cell survival/apoptosis, proliferation, inflammation, and angiogenesis [89, 90]. STAT3 functions as a direct transcriptional activator of VEGF in MSCs and other cell types [89, 91], a process that is in part mediated by HIF-1α [92]. In addition, STAT3 is a direct target gene of E2 as evidenced by its upregulation in response to E2 or ERα agonism [93]. E2-stimulated MSC production of HIF-1α and VEGF occurs also via protein kinase C, PI3K, Akt, SAPK/JNK, and ERK-mediated mechanisms [94]. E2 also stimulates MSC production of bone morphogenetic protein-2 (BMP-2) via both ERα and ERβ, although with greater reliance on ERα [95].
Following the demonstration that TNFR1 plays a detrimental role in the myocardium during I/R injury [96], attention was given to the potential roles of TNFR1 and TNFR2 in MSC function. MSCs from male TNFR1-KO mice produce more VEGF and less TNF-α and IL-6. In vitro, we observed that male TNFR1KO MSCs underwent less apoptosis in vitro compared to wild-type MSCs and were equal to that of wild-type female MSCs [97]. Ablation of TNFR1 in female MSCs also resulted in improved paracrine function. Similar investigation into the role of TNFR2 signaling in gender-related MSC functional differences demonstrated that TNFR2 is a more significant regulator of VEGF and IGF-1 production in male but not female MSCs [98].
In addition to enhancing MSC paracrine functions, E2 has been shown to increase MSC proliferation [94, 99] and differentiation [100]. The osteogenic differentiation of murine MSCs appears to be promoted by ERα and possibly inhibited by ERβ in response [101]. Lastly, E2 may induce MSC telomerase activity via ERα and delay the onset of senescence [102].
The role of androgens in MSC function is less clear. In vitro, MSCs from castrated male rats showed increased VEGF production compared to MSCs from normal rats, and exogenous T decreased VEGF production by female MSCs, thereby suggesting that T may have an inhibitory effect [103]. The full effect of T on MSCs including its mechanisms of action remains to be elucidated.
Embryonic Stem Cells
ESCs are undifferentiated, totipotent cells obtained from the inner cell mass of blastocysts that exhibit an extensive capacity for differentiation. This potential has led to the investigation of their use in the regenerative therapy for a wide range of pathologies. Early animal studies in which undifferentiated murine ESCs were transplanted into ischemic myocardium resulted in cardiac recovery following ischemia primarily via the paracrine release of growth factors [9, 104]. However, undifferentiated ESCs also possess potential for disorganized growth and induction of intramyocardial immune reactions [105, 106], and the former may be due to a lesser capability of the native myocardial environment to drive cardiogenic transformation of ESCs compared to the embryo itself [107]. One strategy for overcoming this limitation involves preprogramming ESCs to cardiac-specific differentiation through treatment with TNF-α [108]. Transplantation of these preprogrammed cells resulted in no teratoma formation at any cell load in contrast to the observed 70% rate of teratoma formation following delivery of equivalent ESC loads. In addition, these preprogrammed stem cells also promoted recovery of cardiac function following LAD ligation. Alternatively, more differentiated, ESC-derived cardiomyocytes obtained from postnatal tissue exhibit similar functional and molecular characteristics as mature cardiomyocytes but lack the adverse growth characteristics of undifferentiated ESCs [109, 110]. Similarly, Nelson et al. [111] recently demonstrated that fibroblasts can be reprogrammed with the human stemness factors OCT3/4, SOX2, KLF4, and c-MYC and exhibit ESC characteristics. Importantly, these induced pleuripotent stem cells demonstrated the ability to engraft into the native myocardium in an organized fashion and to restore post-ischemic myocardial function.
ESCs express ERα and ERβ during early embryonic development [112]. E2 has been shown to stimulate ESC expression of ERα and ERβ expression; increase mRNA expression of the proto-oncogenes c-fos, c-jun, and c-myc; and increase proliferation in part via ERK [113]. Little is known regarding the effect of E2 on the paracrine properties of ESCs, however.
ESCs also express androgen receptors [114], and treatment with nilutamide, a nonsteroidal antiandrogen, stimulates their growth and Akt expression. However, ESC proliferation is unaffected by androgen treatment. Treatment of murine ESCs with T in vitro resulted in the development of a cardiomyocyte-like phenotype with spontaneous contractility and expression of cardiac markers [115]. This effect was inhibited with the addition of flutamide, providing further evidence for the role of the AR-mediated signaling in this cell population. Interestingly, these ESCs were also found to produce T at levels similar to unstimulated Leydig cells, suggesting a possible autocrine role of T.
The recent development of proteomics is enabling investigators to conduct large-scale analyses of paracrine factors involved in cell differentiation as well as characterization of intracellular secretory processes (secretome) [116]. In addition, transcriptome investigation including systems expression profiling with bioinformatic network analysis is facilitating the investigation of spatiotemporal expression patterns of surface biomarkers and cardiogenic genes which may allow for the selection of specific progenitor and stem cell subpopulations that possess the greatest potential for organized cardiac differentiation [117]. These techniques will undoubtedly play important roles in the investigation of the role of sex hormones in stem cell differentiation and function as well as the development of strategies for optimizing the therapeutic efficacy of these cells.
Conclusion
Source gender may be a significant determinant of progenitor and stem cell function via direct actions of sex steroids on these cells. Understanding these mechanisms may enable us to further understand the apparent discrepancy in cardiovascular disease outcomes between men and women. In addition, by utilizing the beneficial effects of E2 on stem and progenitor cell function, these cells may be optimized during ex vivo expansion prior to their therapeutic use in order to improve post-transplantation function and survival.
Acknowledgments
This work was supported by the following NIH grants: R01 GM070628, R01 HL085595, F32 HL092718, F32 HL092719, and F32 HL093987.
Abbreviations
- AR
Androgen receptor
- BMP-2
Bone morphogenetic protein-2
- CAD
Coronary artery disease
- E2
17β-estradiol
- eNOS
Endothelial nitric oxide synthase
- ER
Estrogen receptor
- EPC
Endothelial progenitor cell
- ESC
Embryonic stem cell
- FGF
Fibroblast growth factor
- HIF
Hypoxia-inducible factor
- IGF-1
Insulin-like growth factor-1
- IL
Interleukin
- I/R
Ischemia/reperfusion
- MI
Myocardial infarction
- MSC
Mesenchymal stem cell
- OVX
Ovariectomized
- SDF-1
Stromal cell-derived factor-1
- SOCS/3
Suppressor of cytokine signaling
- STAT3
Signal transducer and activator of transcription
- T
Testosterone
- TNF
Tumor necrosis factor
- TNFR
Tumor necrosis factor receptor
- VEGF
Vascular endothelial growth factor
- VEGR
Vascular endothelial growth factor receptor
References
- 1.Kher A, Meldrum KK, Wang M, Tsai BM, Pitcher JM, Meldrum DR. Cellular and molecular mechanisms of sex differences in renal ischemia–reperfusion injury. Cardiovascular Research. 2005;67:594–603. doi: 10.1016/j.cardiores.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 2.Choudhry MA, Schwacha MG, Hubbard WJ, Kerby JD, Rue LW, Bland KI, et al. Gender differences in acute response to trauma–hemorrhage. Shock. 2005;24(Suppl 1):101–106. doi: 10.1097/01.shk.0000191341.31530.5e. [DOI] [PubMed] [Google Scholar]
- 3.Choudhry MA, Bland KI, Chaudry IH. Gender and susceptibility to sepsis following trauma. Endocr Metab Immune Disord Drug Targets. 2006;6:127–135. doi: 10.2174/187153006777442422. [DOI] [PubMed] [Google Scholar]
- 4.Stampfer MJ, Colditz GA, Willett WC, Manson JE, Rosner B, Speizer FE, et al. Postmenopausal estrogen therapy and cardiovascular disease. Ten-year follow-up from the nurses’ health study. New England Journal of Medicine. 1991;325:756–762. doi: 10.1056/NEJM199109123251102. [DOI] [PubMed] [Google Scholar]
- 5.Kher A, Wang M, Tsai BM, Pitcher JM, Greenbaum ES, Nagy RD, et al. Sex differences in the myocardial inflammatory response to acute injury. Shock. 2005;23:1–10. doi: 10.1097/01.shk.0000148055.12387.15. [DOI] [PubMed] [Google Scholar]
- 6.Grady D, Rubin SM, Petitti DB, Fox CS, Black D, Ettinger B, et al. Hormone therapy to prevent disease and prolong life in postmenopausal women. Annals of Internal Medicine. 1992;117:1016–1037. doi: 10.7326/0003-4819-117-12-1016. [DOI] [PubMed] [Google Scholar]
- 7.Urbich C, Dimmeler S. Endothelial progenitor cells: Characterization and role in vascular biology. Circulation Research. 2004;95:343–353. doi: 10.1161/01.RES.0000137877.89448.78. [DOI] [PubMed] [Google Scholar]
- 8.Crisostomo PR, Wang M, Markel TA, Lahm T, Abarbanell AM, Herrmann JL, et al. Stem cell mechanisms and paracrine effects: Potential in cardiac surgery. Shock. 2007;28:375–383. doi: 10.1097/shk.0b013e318058a817. [DOI] [PubMed] [Google Scholar]
- 9.Crisostomo PR, Abarbanell AM, Wang M, Lahm T, Wang Y, Meldrum DR. Embryonic stem cells attenuate myocardial dysfunction and inflammation after surgical global ischemia via paracrine actions. American Journal of Physiology. Heart and Circulatory Physiology. 2008;295:H1726–H1735. doi: 10.1152/ajpheart.00236.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farhat MY, Lavigne MC, Ramwell PW. The vascular protective effects of estrogen. FASEB Journal. 1996;10:615–624. [PubMed] [Google Scholar]
- 11.Mosselman S, Polman J, Dijkema R. ER beta: Identification and characterization of a novel human estrogen receptor. FEBS Letters. 1996;392:49–53. doi: 10.1016/0014-5793(96)00782-x. [DOI] [PubMed] [Google Scholar]
- 12.Villablanca AC, Tenwolde A, Lee M, Huck M, Mumenthaler S, Rutledge JC. 17beta-estradiol prevents early-stage atherosclerosis in estrogen receptor-alpha deficient female mice. J Cardiovasc Transl Res. 2009;2:289–299. doi: 10.1007/s12265-009-9103-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nilsson S, Makela S, Treuter E, Tujague M, Thomsen J, Andersson G, et al. Mechanisms of estrogen action. Physiological Reviews. 2001;81:1535–1565. doi: 10.1152/physrev.2001.81.4.1535. [DOI] [PubMed] [Google Scholar]
- 14.Liu H, Liu K, Bodenner DL. Estrogen receptor inhibits interleukin-6 gene expression by disruption of nuclear factor kappaB transactivation. Cytokine. 2005;31:251–257. doi: 10.1016/j.cyto.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 15.Hamada H, Kim MK, Iwakura A, Ii M, Thorne T, Qin G, et al. Estrogen receptors alpha and beta mediate contribution of bone marrow-derived endothelial progenitor cells to functional recovery after myocardial infarction. Circulation. 2006;114:2261–2270. doi: 10.1161/CIRCULATIONAHA.106.631465. [DOI] [PubMed] [Google Scholar]
- 16.Shao R, Egecioglu E, Weijdegard B, Kopchick JJ, Fernandez-Rodriguez J, Andersson N, et al. Dynamic regulation of estrogen receptor-alpha isoform expression in the mouse fallopian tube: Mechanistic insight into estrogen-dependent production and secretion of insulin-like growth factors. Am J Physiol Endocrinol Metab. 2007;293:E1430–E1442. doi: 10.1152/ajpendo.00384.2007. [DOI] [PubMed] [Google Scholar]
- 17.Matthews J, Almlof T, Kietz S, Leers J, Gustafsson JA. Estrogen receptor-alpha regulates SOCS-3 expression in human breast cancer cells. Biochemical and Biophysical Research Communications. 2005;335:168–174. doi: 10.1016/j.bbrc.2005.07.057. [DOI] [PubMed] [Google Scholar]
- 18.Roggia C, Gao Y, Cenci S, Weitzmann MN, Toraldo G, Isaia G, et al. Up-regulation of TNF-producing T cells in the bone marrow: A key mechanism by which estrogen deficiency induces bone loss in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:13960–13965. doi: 10.1073/pnas.251534698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leung SW, Teoh H, Keung W, Man RY. Non-genomic vascular actions of female sex hormones: Physiological implications and signalling pathways. Clinical and Experimental Pharmacology and Physiology. 2007;34:822–826. doi: 10.1111/j.1440-1681.2007.04686.x. [DOI] [PubMed] [Google Scholar]
- 20.Kim KH, Moriarty K, Bender JR. Vascular cell signaling by membrane estrogen receptors. Steroids. 2008;73:864–869. doi: 10.1016/j.steroids.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krasinski K, Spyridopoulos I, Asahara T, van der Zee R, Isner JM, Losordo DW. Estradiol accelerates functional endothelial recovery after arterial injury. Circulation. 1997;95:1768–1772. doi: 10.1161/01.cir.95.7.1768. [DOI] [PubMed] [Google Scholar]
- 22.Brouchet L, Krust A, Dupont S, Chambon P, Bayard F, Arnal JF. Estradiol accelerates reendothelialization in mouse carotid artery through estrogen receptor-alpha but not estrogen receptor-beta. Circulation. 2001;103:423–428. doi: 10.1161/01.cir.103.3.423. [DOI] [PubMed] [Google Scholar]
- 23.Toutain CE, Filipe C, Billon A, Fontaine C, Brouchet L, Guery JC, et al. Estrogen receptor alpha expression in both endothelium and hematopoietic cells is required for the accelerative effect of estradiol on reendothelialization. Arteriosclerosis, Thrombosis and Vascular Biology. 2009;29:1543–1550. doi: 10.1161/ATVBAHA.109.192849. [DOI] [PubMed] [Google Scholar]
- 24.Makela S, Savolainen H, Aavik E, Myllarniemi M, Strauss L, Taskinen E, et al. Differentiation between vasculo-protective and uterotrophic effects of ligands with different binding affinities to estrogen receptors alpha and beta. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:7077–7082. doi: 10.1073/pnas.96.12.7077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang M, Wang Y, Weil B, Abarbanell A, Herrmann J, Tan J, et al. Estrogen receptor beta mediates increased activation of PI3K/Akt signaling and improved myocardial function in female hearts following acute ischemia. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology. 2009;296:R972–R978. doi: 10.1152/ajpregu.00045.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iafrati MD, Karas RH, Aronovitz M, Kim S, Sullivan TR, Jr, Lubahn DB, et al. Estrogen inhibits the vascular injury response in estrogen receptor alpha-deficient mice. Nature Medicine. 1997;3:545–548. doi: 10.1038/nm0597-545. [DOI] [PubMed] [Google Scholar]
- 27.Karas RH, Hodgin JB, Kwoun M, Krege JH, Aronovitz M, Mackey W, et al. Estrogen inhibits the vascular injury response in estrogen receptor beta-deficient female mice. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:15133–15136. doi: 10.1073/pnas.96.26.15133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/Progestin Replacement Study (HERS) Research Group. JAMA. 1998;280:605–613. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- 29.Grady D, Herrington D, Bittner V, Blumenthal R, Davidson M, Hlatky M, et al. Cardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/Progestin Replacement Study follow-up (HERS II) JAMA. 2002;288:49–57. doi: 10.1001/jama.288.1.49. [DOI] [PubMed] [Google Scholar]
- 30.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 31.Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: The Women’s Health Initiative randomized controlled trial. JAMA. 2004;291:1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 32.Wang M, Wang Y, Abarbanell A, Tan J, Weil B, Herrmann J, et al. Both endogenous and exogenous testosterone decrease myocardial STAT3 activation and SOCS3 expression after acute ischemia and reperfusion. Surgery. 2009;146:138–144. doi: 10.1016/j.surg.2009.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alexandersen P, Haarbo J, Byrjalsen I, Lawaetz H, Christiansen C. Natural androgens inhibit male atherosclerosis: A study in castrated, cholesterol-fed rabbits. Circulation Research. 1999;84:813–819. doi: 10.1161/01.res.84.7.813. [DOI] [PubMed] [Google Scholar]
- 34.Dockery F, Bulpitt CJ, Donaldson M, Fernandez S, Rajkumar C. The relationship between androgens and arterial stiffness in older men. Journal of the American Geriatrics Society. 2003;51:1627–1632. doi: 10.1046/j.1532-5415.2003.51515.x. [DOI] [PubMed] [Google Scholar]
- 35.Hougaku H, Fleg JL, Najjar SS, Lakatta EG, Harman SM, Blackman MR, et al. Relationship between androgenic hormones and arterial stiffness, based on longitudinal hormone measurements. Am J Physiol Endocrinol Metab. 2006;290:E234–E242. doi: 10.1152/ajpendo.00059.2005. [DOI] [PubMed] [Google Scholar]
- 36.Kang SM, Jang Y, Kim JY, Chung N, Cho SY, Chae JS, et al. Effect of oral administration of testosterone on brachial arterial vasoreactivity in men with coronary artery disease. American Journal of Cardiology. 2002;89:862–864. doi: 10.1016/s0002-9149(02)02202-6. [DOI] [PubMed] [Google Scholar]
- 37.Rosano GM, Leonardo F, Pagnotta P, Pelliccia F, Panina G, Cerquetani E, et al. Acute anti-ischemic effect of testosterone in men with coronary artery disease. Circulation. 1999;99:1666–1670. doi: 10.1161/01.cir.99.13.1666. [DOI] [PubMed] [Google Scholar]
- 38.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 39.Kocher AA, Schuster MD, Szabolcs MJ, Takuma S, Burkhoff D, Wang J, et al. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nature Medicine. 2001;7:430–436. doi: 10.1038/86498. [DOI] [PubMed] [Google Scholar]
- 40.Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, et al. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nature Medicine. 1999;5:434–438. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- 41.Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nature Medicine. 2004;10:858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 42.Elmadbouh I, Haider H, Jiang S, Idris NM, Lu G, Ashraf M. Ex vivo delivered stromal cell-derived factor-1alpha promotes stem cell homing and induces angiomyogenesis in the infarcted myocardium. Journal of Molecular and Cellular Cardiology. 2007;42:792–803. doi: 10.1016/j.yjmcc.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li B, Sharpe EE, Maupin AB, Teleron AA, Pyle AL, Carmeliet P, et al. VEGF and PlGF promote adult vasculogenesis by enhancing EPC recruitment and vessel formation at the site of tumor neovascularization. FASEB Journal. 2006;20:1495–1497. doi: 10.1096/fj.05-5137fje. [DOI] [PubMed] [Google Scholar]
- 44.Aicher A, Heeschen C, Mildner-Rihm C, Urbich C, Ihling C, Technau-Ihling K, et al. Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells. Nature Medicine. 2003;9:1370–1376. doi: 10.1038/nm948. [DOI] [PubMed] [Google Scholar]
- 45.Shintani S, Murohara T, Ikeda H, Ueno T, Honma T, Katoh A, et al. Mobilization of endothelial progenitor cells in patients with acute myocardial infarction. Circulation. 2001;103:2776–2779. doi: 10.1161/hc2301.092122. [DOI] [PubMed] [Google Scholar]
- 46.Gill M, Dias S, Hattori K, Rivera ML, Hicklin D, Witte L, et al. Vascular trauma induces rapid but transient mobilization of VEGFR2(+)AC133(+) endothelial precursor cells. Circulation Research. 2001;88:167–174. doi: 10.1161/01.res.88.2.167. [DOI] [PubMed] [Google Scholar]
- 47.Asahara T, Takahashi T, Masuda H, Kalka C, Chen D, Iwaguro H, et al. VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO Journal. 1999;18:3964–3972. doi: 10.1093/emboj/18.14.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kalka C, Masuda H, Takahashi T, Kalka-Moll WM, Silver M, Kearney M, et al. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:3422–3427. doi: 10.1073/pnas.070046397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kawamoto A, Gwon HC, Iwaguro H, Yamaguchi JI, Uchida S, Masuda H, et al. Therapeutic potential of ex vivo expanded endothelial progenitor cells for myocardial ischemia. Circulation. 2001;103:634–637. doi: 10.1161/01.cir.103.5.634. [DOI] [PubMed] [Google Scholar]
- 50.Jujo K, Ii M, Losordo DW. Endothelial progenitor cells in neovascularization of infarcted myocardium. Journal of Molecular and Cellular Cardiology. 2008;45:530–544. doi: 10.1016/j.yjmcc.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kang HJ, Kim HS, Zhang SY, Park KW, Cho HJ, Koo BK, et al. Effects of intracoronary infusion of peripheral blood stem-cells mobilised with granulocyte-colony stimulating factor on left ventricular systolic function and restenosis after coronary stenting in myocardial infarction: The MAGIC cell randomised clinical trial. Lancet. 2004;363:751–756. doi: 10.1016/S0140-6736(04)15689-4. [DOI] [PubMed] [Google Scholar]
- 52.Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. New England Journal of Medicine. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 53.Werner N, Kosiol S, Schiegl T, Ahlers P, Walenta K, Link A, et al. Circulating endothelial progenitor cells and cardiovascular outcomes. New England Journal of Medicine. 2005;353:999–1007. doi: 10.1056/NEJMoa043814. [DOI] [PubMed] [Google Scholar]
- 54.Scheubel RJ, Zorn H, Silber RE, Kuss O, Morawietz H, Holtz J, et al. Age-dependent depression in circulating endothelial progenitor cells in patients undergoing coronary artery bypass grafting. Journal of the American College of Cardiology. 2003;42:2073–2080. doi: 10.1016/j.jacc.2003.07.025. [DOI] [PubMed] [Google Scholar]
- 55.Tepper OM, Galiano RD, Capla JM, Kalka C, Gagne PJ, Jacobowitz GR, et al. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation. 2002;106:2781–2786. doi: 10.1161/01.cir.0000039526.42991.93. [DOI] [PubMed] [Google Scholar]
- 56.Loomans CJ, de Koning EJ, Staal FJ, Rookmaaker MB, Verseyden C, de Boer HC, et al. Endothelial progenitor cell dysfunction: A novel concept in the pathogenesis of vascular complications of type 1 diabetes. Diabetes. 2004;53:195–199. doi: 10.2337/diabetes.53.1.195. [DOI] [PubMed] [Google Scholar]
- 57.Simper D, Wang S, Deb A, Holmes D, McGregor C, Frantz R, et al. Endothelial progenitor cells are decreased in blood of cardiac allograft patients with vasculopathy and endothelial cells of noncardiac origin are enriched in transplant atherosclerosis. Circulation. 2003;108:143–149. doi: 10.1161/01.CIR.0000081703.34526.5D. [DOI] [PubMed] [Google Scholar]
- 58.George J, Goldstein E, Abashidze S, Deutsch V, Shmilovich H, Finkelstein A, et al. Circulating endothelial progenitor cells in patients with unstable angina: Association with systemic inflammation. European Heart Journal. 2004;25:1003–1008. doi: 10.1016/j.ehj.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 59.Choi JH, Kim KL, Huh W, Kim B, Byun J, Suh W, et al. Decreased number and impaired angiogenic function of endothelial progenitor cells in patients with chronic renal failure. Arteriosclerosis, Thrombosis, and Vascular Biology. 2004;24:1246–1252. doi: 10.1161/01.ATV.0000133488.56221.4a. [DOI] [PubMed] [Google Scholar]
- 60.Imanishi T, Moriwaki C, Hano T, Nishio I. Endothelial progenitor cell senescence is accelerated in both experimental hypertensive rats and patients with essential hypertension. Journal of Hypertension. 2005;23:1831–1837. doi: 10.1097/01.hjh.0000183524.73746.1b. [DOI] [PubMed] [Google Scholar]
- 61.Ghani U, Shuaib A, Salam A, Nasir A, Shuaib U, Jeerakathil T, et al. Endothelial progenitor cells during cerebrovascular disease. Stroke. 2005;36:151–153. doi: 10.1161/01.STR.0000149944.15406.16. [DOI] [PubMed] [Google Scholar]
- 62.Iwakura A, Luedemann C, Shastry S, Hanley A, Kearney M, Aikawa R, et al. Estrogen-mediated, endothelial nitric oxide synthase-dependent mobilization of bone marrow-derived endothelial progenitor cells contributes to reendothelialization after arterial injury. Circulation. 2003;108:3115–3121. doi: 10.1161/01.CIR.0000106906.56972.83. [DOI] [PubMed] [Google Scholar]
- 63.Strehlow K, Werner N, Berweiler J, Link A, Dirnagl U, Priller J, et al. Estrogen increases bone marrow-derived endothelial progenitor cell production and diminishes neointima formation. Circulation. 2003;107:3059–3065. doi: 10.1161/01.CIR.0000077911.81151.30. [DOI] [PubMed] [Google Scholar]
- 64.Zhao X, Huang L, Yin Y, Fang Y, Zhao J, Chen J. Estrogen induces endothelial progenitor cells proliferation and migration by estrogen receptors and PI3K-dependent pathways. Microvascular Research. 2008;75:45–52. doi: 10.1016/j.mvr.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 65.Iwakura A, Shastry S, Luedemann C, Hamada H, Kawamoto A, Kishore R, et al. Estradiol enhances recovery after myocardial infarction by augmenting incorporation of bone marrow-derived endothelial progenitor cells into sites of ischemia-induced neovascularization via endothelial nitric oxide synthase-mediated activation of matrix metalloproteinase-9. Circulation. 2006;113:1605–1614. doi: 10.1161/CIRCULATIONAHA.105.553925. [DOI] [PubMed] [Google Scholar]
- 66.Fontaine V, Filipe C, Werner N, Gourdy P, Billon A, Garmy-Susini B, et al. Essential role of bone marrow fibroblast growth factor-2 in the effect of estradiol on reendothelialization and endothelial progenitor cell mobilization. American Journal of Pathology. 2006;169:1855–1862. doi: 10.2353/ajpath.2006.060260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rousseau A, Ayoubi F, Deveaux C, Charbit B, Delmau C, Christin-Maitre S, et al. Impact of age and gender interaction on circulating endothelial progenitor cells in healthy subjects. Fertility and Sterility. 2009 doi: 10.1016/j.fertnstert.2008.10.062. (in press) [DOI] [PubMed] [Google Scholar]
- 68.Lemieux C, Cloutier I, Tanguay JF. Menstrual cycle influences endothelial progenitor cell regulation: A link to gender differences in vascular protection? International Journal of Cardiology. 2009;136:200–210. doi: 10.1016/j.ijcard.2008.04.054. [DOI] [PubMed] [Google Scholar]
- 69.Fadini GP, de Kreutzenberg S, Albiero M, Coracina A, Pagnin E, Baesso I, et al. Gender differences in endothelial progenitor cells and cardiovascular risk profile: The role of female estrogens. Arteriosclerosis, Thrombosis, and Vascular Biology. 2008;28:997–1004. doi: 10.1161/ATVBAHA.107.159558. [DOI] [PubMed] [Google Scholar]
- 70.Hoetzer GL, MacEneaney OJ, Irmiger HM, Keith R, Van Guilder GP, Stauffer BL, et al. Gender differences in circulating endothelial progenitor cell colony-forming capacity and migratory activity in middle-aged adults. American Journal of Cardiology. 2007;99:46–48. doi: 10.1016/j.amjcard.2006.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Foresta C, Caretta N, Lana A, De Toni L, Biagioli A, Ferlin A, et al. Reduced number of circulating endothelial progenitor cells in hypogonadal men. Journal of Clinical Endocrinology and Metabolism. 2006;91:4599–4602. doi: 10.1210/jc.2006-0763. [DOI] [PubMed] [Google Scholar]
- 72.Foresta C, Zuccarello D, De Toni L, Garolla A, Caretta N, Ferlin A. Androgens stimulate endothelial progenitor cells through an androgen receptor-mediated pathway. Clinical Endocrinology (Oxford) 2008;68:284–289. doi: 10.1111/j.1365-2265.2007.03036.x. [DOI] [PubMed] [Google Scholar]
- 73.Fadini GP, Albiero M, Cignarella A, Bolego C, Pinna C, Boscaro E, et al. Effects of androgens on endothelial progenitor cells in vitro and in vivo. Clinical Science (London) 2009;117:355–364. doi: 10.1042/CS20090077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim SW, Hwang JH, Cheon JM, Park NS, Park SE, Park SJ, et al. Direct and indirect effects of androgens on survival of hematopoietic progenitor cells in vitro. Journal of Korean Medical Science. 2005;20:409–416. doi: 10.3346/jkms.2005.20.3.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Garolla A, D’Inca R, Checchin D, Biagioli A, De Toni L, Nicoletti V, et al. Reduced endothelial progenitor cell number and function in inflammatory bowel disease: A possible link to the pathogenesis. American Journal of Gastroenterology. 2009;104:2500–2507. doi: 10.1038/ajg.2009.332. [DOI] [PubMed] [Google Scholar]
- 76.Conget PA, Minguell JJ. Phenotypical and functional properties of human bone marrow mesenchymal progenitor cells. Journal of Cellular Physiology. 1999;181:67–73. doi: 10.1002/(SICI)1097-4652(199910)181:1<67::AID-JCP7>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 77.Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105:93–98. doi: 10.1161/hc0102.101442. [DOI] [PubMed] [Google Scholar]
- 78.Crisostomo PR, Markel TA, Wang Y, Meldrum DR. Surgically relevant aspects of stem cell paracrine effects. Surgery. 2008;143:577–581. doi: 10.1016/j.surg.2007.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838–3843. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- 80.Abdel-Latif A, Bolli R, Tleyjeh IM, Montori VM, Perin EC, Hornung CA, et al. Adult bone marrow-derived cells for cardiac repair: A systematic review and meta-analysis. Archives of Internal Medicine. 2007;167:989–997. doi: 10.1001/archinte.167.10.989. [DOI] [PubMed] [Google Scholar]
- 81.Lipinski MJ, Biondi-Zoccai GG, Abbate A, Khianey R, Sheiban I, Bartunek J, et al. Impact of intracoronary cell therapy on left ventricular function in the setting of acute myocardial infarction: A collaborative systematic review and meta-analysis of controlled clinical trials. Journal of the American College of Cardiology. 2007;50:1761–1767. doi: 10.1016/j.jacc.2007.07.041. [DOI] [PubMed] [Google Scholar]
- 82.Crisostomo PR, Wang M, Herring CM, Morrell ED, Seshadri P, Meldrum KK, et al. Sex dimorphisms in activated mesenchymal stem cell function. Shock. 2006;26:571–574. doi: 10.1097/01.shk.0000233195.63859.ef. [DOI] [PubMed] [Google Scholar]
- 83.Hiasa K, Egashira K, Kitamoto S, Ishibashi M, Inoue S, Ni W, et al. Bone marrow mononuclear cell therapy limits myocardial infarct size through vascular endothelial growth factor. Basic Research in Cardiology. 2004;99:165–172. doi: 10.1007/s00395-004-0456-9. [DOI] [PubMed] [Google Scholar]
- 84.Meldrum DR. Tumor necrosis factor in the heart. American Journal of Physiology. 1998;274:R577–R595. doi: 10.1152/ajpregu.1998.274.3.R577. [DOI] [PubMed] [Google Scholar]
- 85.Crisostomo PR, Markel TA, Wang M, Lahm T, Lillemoe KD, Meldrum DR. In the adult mesenchymal stem cell population, source gender is a biologically relevant aspect of protective power. Surgery. 2007;142:215–221. doi: 10.1016/j.surg.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 86.Erwin GS, Crisostomo PR, Wang Y, Wang M, Markel TA, Guzman M, et al. Estradiol-treated mesenchymal stem cells improve myocardial recovery after ischemia. Journal of Surgical Research. 2009;152:319–324. doi: 10.1016/j.jss.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang M, Tan J, Coffey A, Fehrenbacher J, Weil BR, Meldrum DR. Signal transducer and activator of transcription 3-stimulated hypoxia inducible factor-1alpha mediates estrogen receptor-alpha-induced mesenchymal stem cell vascular endothelial growth factor production. Journal of Thoracic and Cardiovascular Surgery. 2009;138:163–171. 171, e161. doi: 10.1016/j.jtcvs.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, et al. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Molecular and Cellular Biology. 1996;16:4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang M, Zhang W, Crisostomo P, Markel T, Meldrum KK, Fu XY, et al. STAT3 mediates bone marrow mesenchymal stem cell VEGF production. Journal of Molecular and Cellular Cardiology. 2007;42:1009–1015. doi: 10.1016/j.yjmcc.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Niu G, Wright KL, Huang M, Song L, Haura E, Turkson J, et al. Constitutive Stat3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene. 2002;21:2000–2008. doi: 10.1038/sj.onc.1205260. [DOI] [PubMed] [Google Scholar]
- 91.Platt DH, Bartoli M, El-Remessy AB, Al-Shabrawey M, Lemtalsi T, Fulton D, et al. Peroxynitrite increases VEGF expression in vascular endothelial cells via STAT3. Free Radical Biology and Medicine. 2005;39:1353–1361. doi: 10.1016/j.freeradbiomed.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 92.Xu Q, Briggs J, Park S, Niu G, Kortylewski M, Zhang S, et al. Targeting Stat3 blocks both HIF-1 and VEGF expression induced by multiple oncogenic growth signaling pathways. Oncogene. 2005;24:5552–5560. doi: 10.1038/sj.onc.1208719. [DOI] [PubMed] [Google Scholar]
- 93.Gao H, Bryzgalova G, Hedman E, Khan A, Efendic S, Gustafsson JA, et al. Long-term administration of estradiol decreases expression of hepatic lipogenic genes and improves insulin sensitivity in ob/ob mice: A possible mechanism is through direct regulation of signal transducer and activator of transcription 3. Molecular Endocrinology. 2006;20:1287–1299. doi: 10.1210/me.2006-0012. [DOI] [PubMed] [Google Scholar]
- 94.Yun SP, Lee MY, Ryu JM, Song CH, Han HJ. Role of HIF-1alpha and VEGF in human mesenchymal stem cell proliferation by 17beta-estradiol: Involvement of PKC, PI3K/Akt, and MAPKs. American Journal of Physiology. Cell Physiology. 2009;296:C317–C326. doi: 10.1152/ajpcell.00415.2008. [DOI] [PubMed] [Google Scholar]
- 95.Zhou S, Turgeman G, Harris SE, Leitman DC, Komm BS, Bodine PV, et al. Estrogens activate bone morphogenetic protein-2 gene transcription in mouse mesenchymal stem cells. Molecular Endocrinology. 2003;17:56–66. doi: 10.1210/me.2002-0210. [DOI] [PubMed] [Google Scholar]
- 96.Wang M, Tsai BM, Crisostomo PR, Meldrum DR. Tumor necrosis factor receptor 1 signaling resistance in the female myocardium during ischemia. Circulation. 2006;114:I282–I289. doi: 10.1161/CIRCULATIONAHA.105.001164. [DOI] [PubMed] [Google Scholar]
- 97.Crisostomo PR, Wang M, Herring CM, Markel TA, Meldrum KK, Lillemoe KD, et al. Gender differences in injury induced mesenchymal stem cell apoptosis and VEGF, TNF, IL-6 expression: Role of the 55 kDa TNF receptor (TNFR1) Journal of Molecular and Cellular Cardiology. 2007;42:142–149. doi: 10.1016/j.yjmcc.2006.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Markel TA, Crisostomo PR, Wang M, Wang Y, Lahm T, Novotny NM, et al. TNFR1 signaling resistance associated with female stem cell cytokine production is independent of TNFR2-mediated pathways. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology. 2008;295:R1124–R1130. doi: 10.1152/ajpregu.90508.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.DiSilvio L, Jameson J, Gamie Z, Giannoudis PV, Tsiridis E. In vitro evaluation of the direct effect of estradiol on human osteoblasts (HOB) and human mesenchymal stem cells (h-MSCs) Injury. 2006;37(Suppl 3):S33–S42. doi: 10.1016/j.injury.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 100.Hong L, Colpan A, Peptan IA. Modulations of 17-beta estradiol on osteogenic and adipogenic differentiations of human mesenchymal stem cells. Tissue Engineering. 2006;12:2747–2753. doi: 10.1089/ten.2006.12.2747. [DOI] [PubMed] [Google Scholar]
- 101.Zhou S, Zilberman Y, Wassermann K, Bain SD, Sadovsky Y, Gazit D. Estrogen modulates estrogen receptor alpha and beta expression, osteogenic activity, and apoptosis in mesenchymal stem cells (MSCs) of osteoporotic mice. Journal of Cellular Biochemistry. Supplement, Suppl. 2001;36:144–155. doi: 10.1002/jcb.1096. [DOI] [PubMed] [Google Scholar]
- 102.Cha Y, Kwon SJ, Seol W, Park KS. Estrogen receptor-alpha mediates the effects of estradiol on telomerase activity in human mesenchymal stem cells. Molecules and Cells. 2008;26:454–458. [PubMed] [Google Scholar]
- 103.Ray R, Herring CM, Markel TA, Crisostomo PR, Wang M, Weil B, et al. Deleterious effects of endogenous and exogenous testosterone on mesenchymal stem cell VEGF production. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology. 2008;294:R1498–R1503. doi: 10.1152/ajpregu.00897.2007. [DOI] [PubMed] [Google Scholar]
- 104.Min JY, Yang Y, Sullivan MF, Ke Q, Converso KL, Chen Y, et al. Long-term improvement of cardiac function in rats after infarction by transplantation of embryonic stem cells. Journal of Thoracic and Cardiovascular Surgery. 2003;125:361–369. doi: 10.1067/mtc.2003.101. [DOI] [PubMed] [Google Scholar]
- 105.Leor J, Gerecht S, Cohen S, Miller L, Holbova R, Ziskind A, et al. Human embryonic stem cell transplantation to repair the infarcted myocardium. Heart. 2007;93:1278–1284. doi: 10.1136/hrt.2006.093161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nussbaum J, Minami E, Laflamme MA, Virag JA, Ware CB, Masino A, et al. Transplantation of undifferentiated murine embryonic stem cells in the heart: Teratoma formation and immune response. FASEB Journal. 2007;21:1345–1357. doi: 10.1096/fj.06-6769com. [DOI] [PubMed] [Google Scholar]
- 107.Behfar A, Zingman LV, Hodgson DM, Rauzier JM, Kane GC, Terzic A, et al. Stem cell differentiation requires a paracrine pathway in the heart. FASEB Journal. 2002;16:1558–1566. doi: 10.1096/fj.02-0072com. [DOI] [PubMed] [Google Scholar]
- 108.Behfar A, Perez-Terzic C, Faustino RS, Arrell DK, Hodgson DM, Yamada S, et al. Cardiopoietic programming of embryonic stem cells for tumor-free heart repair. Journal of Experimental Medicine. 2007;204:405–420. doi: 10.1084/jem.20061916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nature Biotechnology. 2007;25:1015–1024. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 110.Caspi O, Huber I, Kehat I, Habib M, Arbel G, Gepstein A, et al. Transplantation of human embryonic stem cell-derived cardiomyocytes improves myocardial performance in infarcted rat hearts. Journal of the American College of Cardiology. 2007;50:1884–1893. doi: 10.1016/j.jacc.2007.07.054. [DOI] [PubMed] [Google Scholar]
- 111.Nelson TJ, Martinez-Fernandez A, Yamada S, Perez-Terzic C, Ikeda Y, Terzic A. Repair of acute myocardial infarction by human stemness factors induced pluripotent stem cells. Circulation. 2009;120:408–416. doi: 10.1161/CIRCULATIONAHA.109.865154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hong SH, Nah HY, Lee YJ, Lee JW, Park JH, Kim SJ, et al. Expression of estrogen receptor-alpha and -beta, glucocorticoid receptor, and progesterone receptor genes in human embryonic stem cells and embryoid bodies. Molecules and Cells. 2004;18:320–325. [PubMed] [Google Scholar]
- 113.Han HJ, Heo JS, Lee YJ. Estradiol-17beta stimulates proliferation of mouse embryonic stem cells: Involvement of MAPKs and CDKs as well as protooncogenes. American Journal of Physiology. Cell Physiology. 2006;290:C1067–C1075. doi: 10.1152/ajpcell.00222.2005. [DOI] [PubMed] [Google Scholar]
- 114.Chang CY, Hsuuw YD, Huang FJ, Shyr CR, Chang SY, Huang CK, et al. Androgenic and antiandrogenic effects and expression of androgen receptor in mouse embryonic stem cells. Fertility and Sterility. 2006;85(Suppl 1):1195–1203. doi: 10.1016/j.fertnstert.2005.11.031. [DOI] [PubMed] [Google Scholar]
- 115.Goldman-Johnson DR, de Kretser DM, Morrison JR. Evidence that androgens regulate early developmental events, prior to sexual differentiation. Endocrinology. 2008;149:5–14. doi: 10.1210/en.2007-1123. [DOI] [PubMed] [Google Scholar]
- 116.Arrell DK, Niederlander NJ, Faustino RS, Behfar A, Terzic A. Cardioinductive network guiding stem cell differentiation revealed by proteomic cartography of tumor necrosis factor alpha-primed endodermal secretome. Stem Cells. 2008;26:387–400. doi: 10.1634/stemcells.2007-0599. [DOI] [PubMed] [Google Scholar]
- 117.Nelson TJ, Faustino RS, Chiriac A, Crespo-Diaz R, Behfar A, Terzic A. CXCR4+/FLK-1+ biomarkers select a cardiopoietic lineage from embryonic stem cells. Stem Cells. 2008;26:1464–1473. doi: 10.1634/stemcells.2007-0808. [DOI] [PubMed] [Google Scholar]
- 118.Keaney JF, Jr, Shwaery GT, Xu A, Nicolosi RJ, Loscalzo J, Foxall TL, et al. 17beta-estradiol preserves endothelial vasodilator function and limits low-density lipoprotein oxidation in hypercholesterolemic swine. Circulation. 1994;89:2251–2259. doi: 10.1161/01.cir.89.5.2251. [DOI] [PubMed] [Google Scholar]
- 119.Moskowitz MS, Moskowitz AA, Bradford WL, Jr, Wissler RW. Changes in serum lipids and coronary arteries of the rat in response to estrogens. AMA Archives of Pathology. 1956;61:245–263. [PubMed] [Google Scholar]
- 120.Adams MR, Kaplan JR, Manuck SB, Koritnik DR, Parks JS, Wolfe MS, et al. Inhibition of coronary artery atherosclerosis by 17-beta estradiol in ovariectomized monkeys: Lack of an effect of added progesterone. Arteriosclerosis. 1990;10:1051–1057. doi: 10.1161/01.atv.10.6.1051. [DOI] [PubMed] [Google Scholar]
- 121.Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- 122.Bath PM, Hassall DG, Gladwin AM, Palmer RM, Martin JF. Nitric oxide and prostacyclin. Divergence of inhibitory effects on monocyte chemotaxis and adhesion to endothelium in vitro. Arteriosclerosis and Thrombosis. 1991;11:254–260. doi: 10.1161/01.atv.11.2.254. [DOI] [PubMed] [Google Scholar]
- 123.Radomski MW, Palmer RM, Moncada S. Endogenous nitric oxide inhibits human platelet adhesion to vascular endothelium. Lancet. 1987;2:1057–1058. doi: 10.1016/s0140-6736(87)91481-4. [DOI] [PubMed] [Google Scholar]
- 124.Lahm T, Patel KM, Crisostomo PR, Markel TA, Wang M, Herring C, et al. Endogenous estrogen attenuates pulmonary artery vasoreactivity and acute hypoxic pulmonary vasoconstriction: The effects of sex and menstrual cycle. Am J Physiol Endocrinol Metab. 2007;293:E865–E871. doi: 10.1152/ajpendo.00201.2007. [DOI] [PubMed] [Google Scholar]