Abstract
In the present study we used electrophysiological techniques in an in vitro preparation of the rat dentate gyrus to examine the effect of muscarinic acetylcholine receptor activation on the intrinsic excitability of hilar neurons. We found that bath application of muscarine caused a direct depolarization in approximately eighty percent of mossy cells tested, and also produced a clear afterdepolarization (ADP) in nearly one hundred percent of trials. The ADP observed in hilar mossy cells is produced by opening of a Na+ permeant and yet largely TTX insensitive ion channel. It requires an increase in postsynaptic calcium for activation, and is blocked by flufenamic acid, an antagonist of a previously identified calcium activated non-selective cation channel (ICAN). Further, we demonstrate that induction of an ADP in current clamp causes release of cannabinoids, and subsequent depression of GABAergic transmission that is comparable to that produced in the same cells by a more conventional five second depolarization in voltage clamp. By contrast, other types of hilar neurons were less strongly depolarized by bath application of muscarinic agonists, and uniformly lacked a similar muscarinic ADP. Overall, the data presented here extend our understanding of the specific mechanisms through which muscarinic agonists are likely to modulate neuronal excitability in the hilar network, and further reveal a mechanism that could plausibly promote endocannabinoid mediated signaling in vivo.
Keywords: hippocampus, dentate, dentate gyrus, electrophysiology, acetylcholine, muscarine, mAChR
1. Introduction
The septo-hippocampal cholinergic system has been widely implicated in hippocampal dependent learning and memory tasks (Frotscher and Leranth, 1985; Gold, 2003; Hasselmo, 2006). Although extensive effort has been devoted to understanding how cholinergic compounds modulate both cells and circuits in area CA1 and CA3 of the hippocampus (Cobb and Davies, 2005; Lawrence, 2008), comparatively little is known about cholinergics in the hilus. This area of the dentate gyrus is home to an unusual class of local circuit neurons known as hilar mossy cells. Mossy cells are the only hippocampal local circuit neurons that have a glutamatergic phenotype. They are also unusual in having an axonal projection that runs primarily along the septotemporal axis of the hippocampus, where they likely provide excitatory input to both hilar interneurons and dentate granule cells (Amaral and Witter, 1989; Buckmaster et al., 1992; Jackson and Scharfman, 1996; Larimer and Strowbridge, 2008; Scharfman, 1995). These unusual features leave mossy cells well positioned to play a powerful role in modulation of information transfer from the dentate to CA3 (Henze and Buzsaki, 2007), and have also promoted significant interest in understanding their specific role in both memory formation and epileptogenesis. However, it is important to note that the hilar region of the dentate gyrus is also home to a much more heterogeneous group of more traditional GABAergic local circuit neurons. Attempts have been made to categorize GABAergic neurons in the hilus based on general axonal arborization, specific postsynaptic targets, and immunohistochemical markers, among other things, yet significant work in this area remains to be done (for recent review see Houser, 2007). Recent evidence has indicated that within the hilar region of the dentate gyrus, monosynaptic connections between GABAergic interneurons and mossy cells are more common than any other pairwise combination (Larimer and Strowbridge, 2008), and further that many GABAergic contacts to mossy cells originate from cholecystokinin rather than parvalbumin positive terminals (Acsady et al., 2000).
Our lab has recently shown that bath application of a muscarinic acetylcholine receptor (mAChR) agonist in the hilus can raise ambient GABA and selectively modulate glutamatergic signaling through activation of presynaptic GABAB receptors (Nahir et al., 2007). However, we have also noted that identical treatment with cholinergic agonists enhances depolarization induced suppression of inhibition as produced by direct depolarization of mossy cells (Hofmann et al., 2006). These types of findings suggest multiple sites of action and an overall powerful role for muscarinic systems in the modulation of hilar networks, and yet direct effects of muscarinic agonists on the intrinsic properties of hilar neurons have yet to be carefully examined.
In the present study we report that bath application of 5 μM muscarine typically depolarizes hilar mossy cells and also activates a muscarinic afterdepolarization (ADP). The ADP observed in mossy cells is likely carried by a calcium sensitive non-selective cation channel (ICAN), and can lead to endogenous cannabinoid (eCB) release and a subsequent inhibition of GABAergic transmission that resembles depolarization induced suppression of inhibition. By contrast, 50% of non-mossy cells tested were depolarized by muscarine, but none exhibited a similar muscarinic ADP.
2. Results
2.1. mAChR activation produces an afterdepolarization in hilar mossy cells but not in other hilar neurons
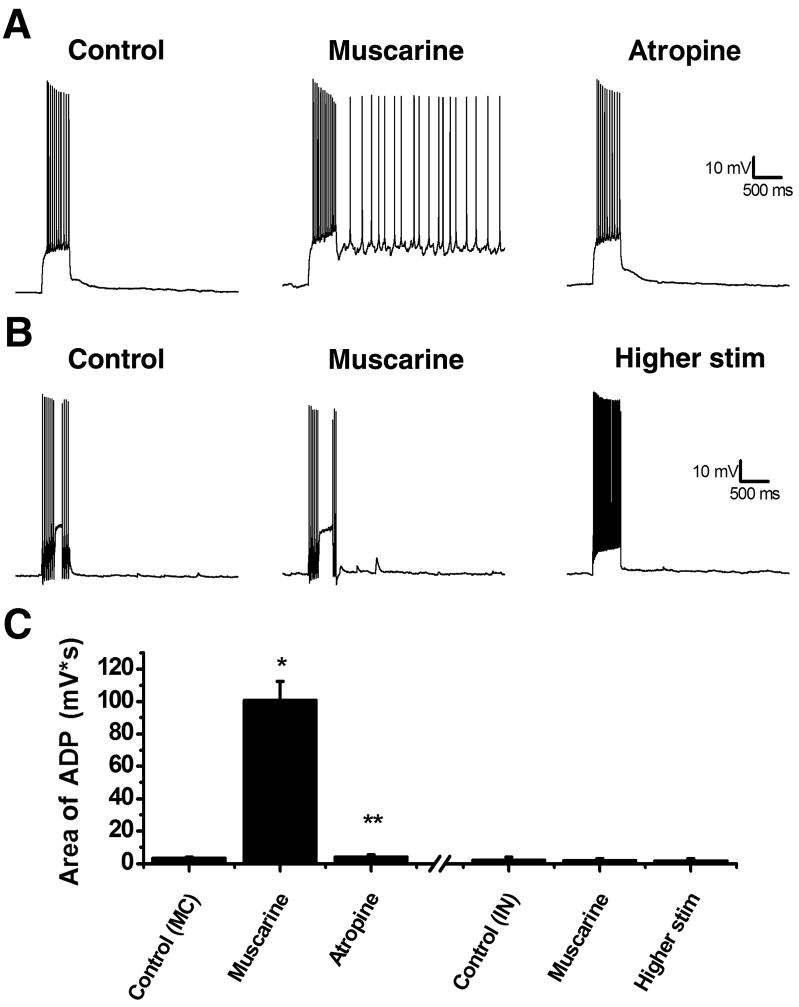
In this study we investigated the effect of mAChR activation on the intrinsic excitability of neurons located in the hilar region of the rat dentate gyrus. Mossy cells were patch clamped (see methods) in voltage clamp mode using a K-gluconate based internal solution. After briefly recording spontaneous EPSCs, ionotropic glutamate receptor antagonists (20 μM DNQX and 40 μM APV) were bath applied, the recording mode was switched to current clamp, and holding current was adjusted to obtain a resting membrane potential of −60 mV (average IHold: 4.05 ± 5.57 pA, n=40). Under these conditions we applied a 500 msec depolarizing pulse calibrated to produce a continuous train of action potentials (279 ± 18.8 pA on average, n=40). Following the depolarization, the membrane voltage quickly returned to −60 mV showing no evidence of an ADP (Fig. 1A, control). In order to examine the effect of mAChR activation, muscarine (5 μM) was bath applied while membrane potential was continuously monitored in current clamp. In 78% of mossy cells tested, bath application of muscarine caused a direct depolarization of ≥ 5 mV (average ΔV: 15.6 ± 0.86 mV), and in the majority of those cases (25 of 31) this depolarization was sufficient to induce firing of action potentials. We believe this direct depolarization is likely to depend on muscarinic modulation of a potassium conductance, as comparable bath application of a muscarinic agonist (3 μM CCh) had minimal effect on holding current or input resistance in 34 mossy cells voltage clamped with a CsMeSO3 based internal solution (change from baseline: 4.34 ± 3.52% and 2.60 ± 8.43%, respectively, n=34, p>0.05, data not shown). Nevertheless, in virtually all mossy cells tested (regardless of the magnitude of the direct depolarization) bath application of muscarine also resulted in the expression of a robust ADP observed following a 500 msec depolarizing pulse (Fig. 1A, muscarine). Over 40 mossy cells tested the average area of the ADP was 101 ± 11.6 mV*s in the presence of muscarine compared to 3.57 ± 0.45 mV*s before muscarine application (p < 0.05, Fig. 1C). Although (as noted above) the holding current was adjusted to maintain the membrane potential at −60 mV both before and after application of muscarine, the absolute size of the depolarizing step was the same in both conditions. Further, in a subset of cells tested, we noted that bath application of the general mAChR antagonist atropine (5 μM) completely blocked the muscarinic ADP (89.0 ± 27.3 mV*s in muscarine vs. 4.32 ± 1.13 mV*s in muscarine + atropine; n=9, p<0.05).
Figure 1. Muscarine can induce an ADP in mossy cells but not interneurons.
A, left: A mossy cell was held at a membrane potential of −60 mV and a 500 ms depolarizing pulse was applied to evoke a train of action potentials. After the depolarization the cell immediately returned to −60 mV. Middle: Following application of 5 μM muscarine an ADP was induced following the depolarizing current which could last for several seconds. Right: The ADP could be blocked by 5 μM atropine, a mAChR antagonist. B, left: A hilar interneuron was depolarized resulting in an immediate return to −60 mV. Middle: After application of muscarine, unlike mossy cells, there was no ADP. Right: An ADP could still not be produced with a larger somatic current injection. C: Summary plot showing the average area of ADP following the depolarizing pulse. The three bars left of the hash marks summarize the results of experiments in hilar mossy cells, while the three bars to the right of the hash marks are from hilar interneurons. *p<0.05 compared to baseline. **p<0.05 compared to muscarine. Higher stim was tested in 7 of 10 non-mossy hilar neurons.
By contrast, across 10 non-mossy hilar neurons examined in an identical fashion, 50% were depolarized by ≥ 5 mV in response to muscarine (average ΔV: 10.2 ± 1.7 mV), while the other 50 % were unaffected (average ΔV: 0.6 ± 0.6 mV). Interestingly, however, all 10 non-mossy hilar neurons examined lacked a muscarinic ADP in response to both moderate (192 ± 28.4 pA) and large (429 ± 42.1 pA) depolarizing pulses (Fig. 1B, control: 2.23 ± 1.70 mV*s; muscarine: 1.93 ± 0.86 mV*s; n=10, p > 0.05, and higher stim: 1.83 ± 1.02 mV*s, n=7, p > 0.05). There was no difference between non-mossy hilar neurons that were depolarized by muscarine and those that were not in terms of whole cell capacitance (109.88 ± 20.64 pF vs. 137.63 ± 22.63 pF), input resistance (213.97 ± 37.10 MΩ vs. 156.08 ± 14.75 MΩ), or holding current at −60 mV (−34.00 ± 5.10 pA vs. −30 ± 25.30 pA). Similarly, while there was some variability among non-mossy hilar neurons in firing pattern in response to depolarization, in degree of afterhyperpolarization observed after an action potential, and in sag currents observed in response to a hyperpolarizing pulse, none of these features were clearly related to the propensity to be depolarized by bath application of muscarine. Thus significant additional anatomical and immunohistochemical work would be necessary to further sub-divide non-mossy hilar cells based on susceptibility to mAChR mediated depolarization. Instead, for the remainder of this manuscript, we focused our attention on further characterizing the robust muscarinic ADP that was uniquely observed in hilar mossy cells.
2.2. ADP depends on a calcium activated non-selective cation channel
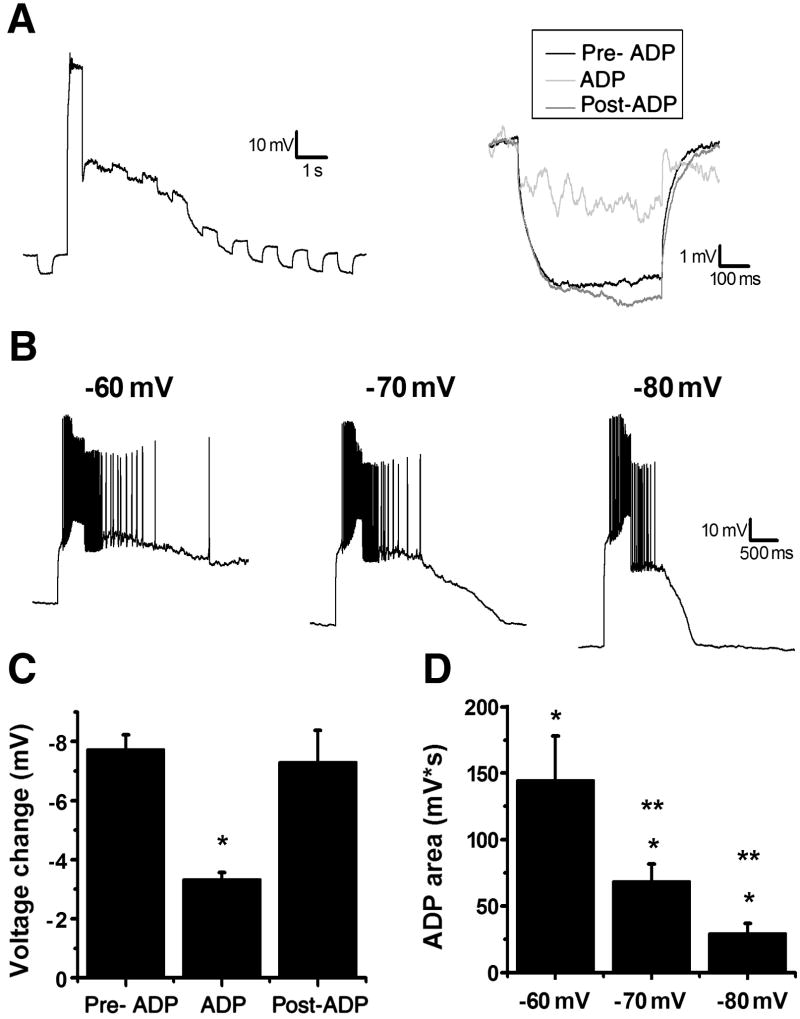
As a first step, we asked whether induction of a muscarinic ADP in hilar mossy cells was consistent with opening (as opposed to closing) of an ionic conductance. Hyperpolarizing steps of −40 pA for 500 ms were applied before, during, and after the ADP in the presence of 1 μM TTX (see below for TTX experiments) to prevent action potentials from contaminating the measurements (Fig. 2A). During the ADP there was a significant reduction in the change in voltage produced by the hyperpolarizing step, which returned to baseline levels after the ADP (Fig. 2C; pre-ADP: −7.72 ± 0.49 mV; ADP: −3.33 ± 0.24 mV; post-ADP: −7.30 ± 1.07 mV, n=4, p<0.05). Although other voltage dependent conductances may contribute to this observation, these data are consistent with the hypothesis that activation of the ADP is associated with a decrease in the input resistance, and thus likely the opening of an ion channel. To further characterize the current involved in the ADP we next investigated its voltage sensitivity in the absence of TTX. After observing an ADP at −60 mV we adjusted the holding current to obtain membrane potentials of −70 mV and −80 mV and compared the area of the ADP at each membrane potential (current injected for depolarization at each membrane potential was adjusted to evoke a similar number of action potentials). We found the ADP was present but reduced at both −70 mV and −80 mV when compared to −60 mV (Fig. 2C,E; −60 mV: 145 ± 33.2 mV*s; −70 mV: 68.6 ± 12.9 mV*s; −80 mV: 29.4 ± 7.70 mV*s; n=8, p<0.05). To verify this was not due to rundown we again examined the ADP at −60 mV, after having tested the hyperpolarized voltages, and noted no difference from the initial measurements (145 ± 33.2 mV*s vs. 147.5275 ± 31.9006 mV*s, n=8, p>0.05). The reduction in the area of the ADP at progressively more hyperpolarized membrane potentials suggests that there may be a voltage sensitive component to the muscarinic ADP observed in hilar mossy cells. However, we cannot explicitly rule out the possibility that more negative holding and plateau potentials hasten the ADP through alteration of intracellular calcium dynamics (see below).
Figure 2. ADP depends on opening a voltage dependent channel.
A, left: A 500 ms, −40 pA hyperpolarizing pulse was applied before and after the depolarization in the presence of 1 μM TTX to investigate changes in input resistance. Right: An inset of three hyperpolarizing pulses from the representative cell to the left: black represents the pulse before the depolarization, light gray is the first pulse after depolarization, and dark gray is a pulse after the membrane potential returned to −60 mV. B: A single cell representation of the effect of voltage on the ADP. The ADP is smaller at more hyperpolarized potentials (middle, −70 mV and right, −80 mV) compared to control (left, −60 mV). C: Summary plot of the Δvoltage to a hyperpolarizing pulse before, during, and after an ADP. *p<0.05 compared to pre-ADP. D: Summary plot of the area of the ADP at different membrane potentials. *p<0.05 compared to control (before muscarine). **p<0.05 compared to muscarine at −60 mV.
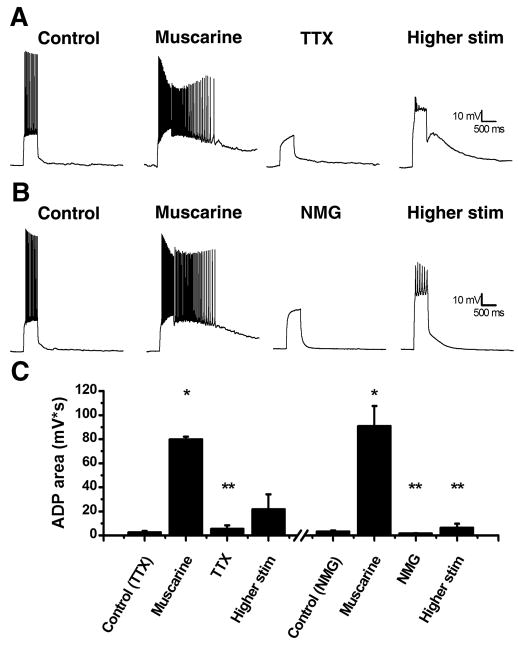
Previous research has shown several different currents involved in the production of ADPs throughout the CNS including a TTX-sensitive Na+ current, an mGluR activated inward current, and a calcium activated non-selective current, ICAN (Fraser and MacVicar, 1996; Young et al., 2004; Yue et al., 2005). As was apparent above, we found the muscarinic ADP as observed in hilar mossy cells does not require TTX sensitive Na+ channels. For these experiments we first obtained an ADP in the presence of muscarine and then examined the effect of 1 μM TTX on the ADP. We found that bath application of TTX eliminated the ADP (Fig. 3A,C; muscarine: 79.8567 ± 20.9213 mV*s; muscarine + TTX: 5.5456 ± 2.7981 mV*s; n=5; p<0.05), however, this could be due to the reduced depolarization (or possibly reduced calcium influx) produced by the current step in the absence of action potentials. Consequently, we increased the amount of current injected in the presence of TTX (by an average of 426 ± 32 pA) and were ultimately able to induce an ADP that was not significantly different in area than that observed in the absence of TTX (Fig. 3A,C; higher stim: 21.8351 ± 12.1584 mV*s; n=5; p>0.05). This observation suggests that while TTX sensitive Na+ channels may contribute indirectly to the induction of the ADP, they are not required to carry the current. However, as the ADP is clearly depolarizing, we next asked whether the current carried by the ADP is heavily dependent on sodium ions. To test this, we applied ACSF with NMG substituted for NaCl to create a nominally Na+ free solution. We found application of this solution eliminated the ADP (Fig. 3B,C; muscarine: 90.9087 ± 16.6110 mV*s; muscarine + NMG: 1.6265 ± 0.2536 mV*s; n=5, p<0.05). However, in contrast to our observations with TTX, in NMG containing solutions the ADP was not rescued with larger current injections (Fig. 3B,C; higher stim: 6.4868 ± 3.2309 mV*s; n=5, p<0.05). This result implies that a large portion of the inward current observed during an ADP is carried by sodium ions. While closing a K+ selective channel may produce robust depolarization, opening of a Na+ permeant channel is required to produce an inward current. As such, these data also reinforce the results of the input resistance experiments in suggesting the current that carries the ADP is most likely to depend on opening, rather than closing, of an ion channel.
Figure 3. Induction of the ADP does not depend on voltage gated sodium channels but does require sodium ions.
A: A representative cell before (left trace) and after (middle left trace) application of muscarine resulting in an ADP. When 1 μM TTX is applied the ADP is blocked (middle right trace) until the depolarizing current is increased resulting in a smaller ADP (right trace). B: A representative cell before (left trace) and after (middle left trace) application of muscarine resulting in an ADP. When the cell is perfused with a nominally sodium free ACSF containing NMG substituted for NaCl the ADP is blocked (middle right trace) even after increased depolarization (right trace). C: Summary plot of the ADP area under different conditions. *p<0.05 compared to control. **p<0.05 compared to muscarine.
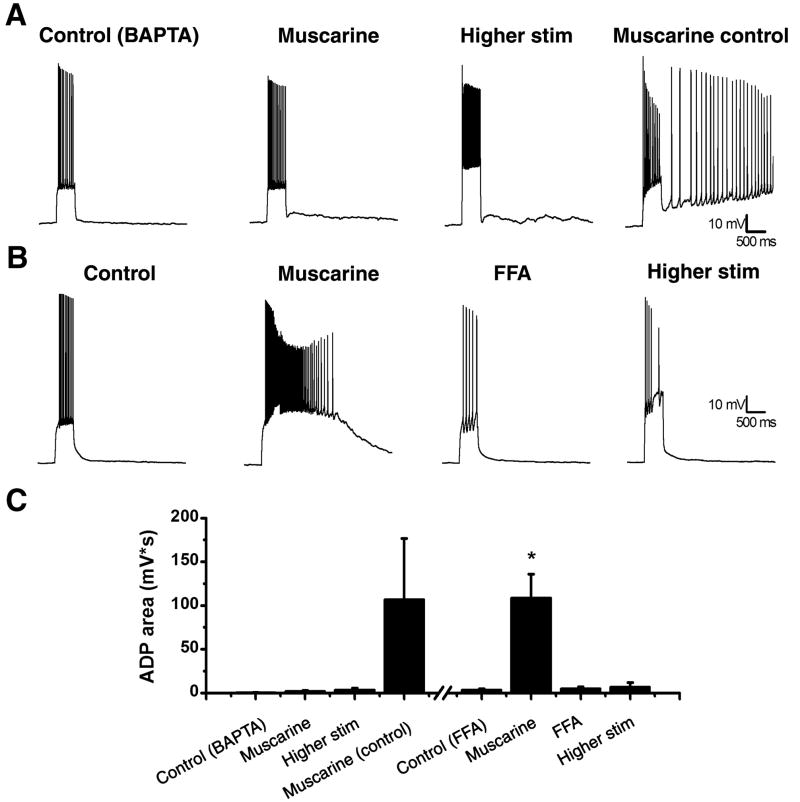
Next we sought to examine the calcium dependence of the ADP as observed in hilar mossy cells. Towards that end, we placed 10 mM BAPTA, a calcium chelator, into our internal solution. In the presence of BAPTA, we were unable to produce an ADP following muscarine application (Fig. 4A,C; control: 0.60 ± 0.16 mV*s; muscarine: 2.12 ± 0.78 mV*s; higher stim: 3.71 ± 1.75 mV*s; n=5, p>0.05). For same day controls we were able to produce an ADP with muscarine absent BAPTA suggesting the agonist was functionally active (Fig. 4A,C). Interestingly, although BAPTA internal completely eliminated the muscarinic ADP, it failed to significantly reduce the direct depolarization caused by bath application of 5 μM muscarine (change in membrane potential: 15.6 ± 8.61 vs. 11.6 ± 2.59 mV, n=31,5, p>0.05).
Figure 4. ADPs in mossy cells depend on calcium and depend on ICAN.
A: The internal solution was filled with 10 mM BAPTA, a calcium chelator, which blocked the ability to induce an ADP even with a higher stimulation (left, middle left, and middle right trace). A same day control showed an ADP could be produced with the muscarine (right trace). B: A representative cell before (left trace) and after (middle left trace) application of muscarine. The ICAN blocker, FFA (100 μM), blocked the ADP (middle right trace) and could not be recovered with higher stimulation (right trace). C: Summary plot of the ADP area under different conditions. For the BAPTA data set either 5 μM muscarine or 3 μM carbachol was used. *p<0.05 compared to control. **p<0.05 compared to muscarine.
Collectively, all the results presented above are consistent with the hypothesis that the muscarinic ADP as observed in hilar mossy cells is mediated by activation of a calcium activated and non-selective cation channel (ICAN). To test that hypothesis directly, we bath applied the relatively nonselective ICAN antagonist flufenamic acid (FFA, 100 μM) (Ghamari-Langroudi and Bourque, 2002; Partridge and Valenzuela, 2000; Pressler et al., 2007; Pressler and Strowbridge, 2006), and found that mossy cells are unable to produce an ADP in the presence of both FFA and 5 μM muscarine (Fig. 4B,C; muscarine: 109 ± 27.3 mV*s; FFA: 5.12 ± 2.03 mV*s; higher stim: 6.86 ± 4.95 mV*s; n=6).
2.3. ADP induction can result in the release of eCBs
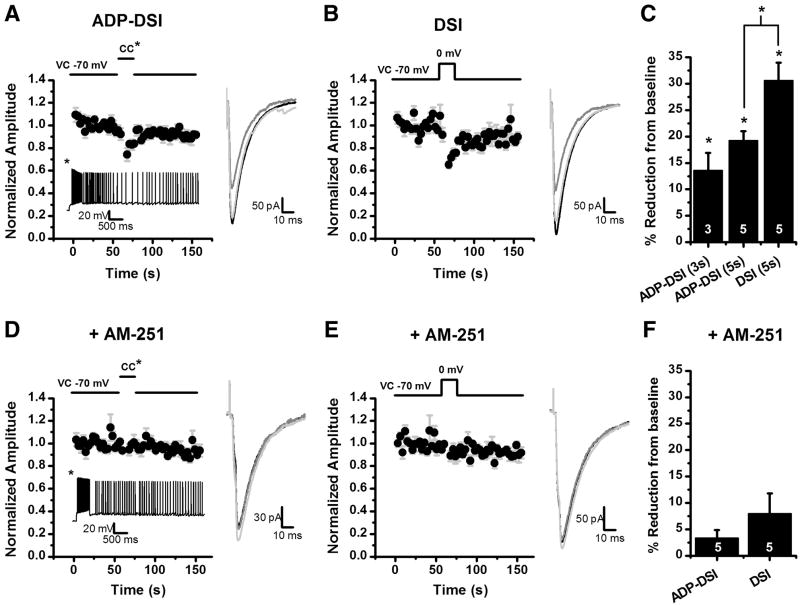
After characterizing the ADP, we sought to investigate a possible functional significance for this prolonged depolarization. Previous results have indicated that mossy cells can release eCBs in a calcium dependent manner to transiently reduce inhibitory transmission in a phenomenon known as depolarization induced suppression of inhibition (Hofmann et al., 2006; Howard et al., 2007). However, failed attempts to elicit DSI at in vivo firing rates in CA1 have brought into question whether the five second depolarizations typically used to induce eCB release are physiologically relevant (Hampson et al., 2003). We reasoned that since the ADP results in a prolonged depolarization, then it could possibly be a physiological mechanism for the release of eCBs from mossy cells. To test this hypothesis we switched to a high chloride internal solution so we could observe evoked IPSCs (eIPSCs) at −70 mV in voltage clamp. After observing an ADP in current clamp we switched to voltage clamp, and used a concentric bipolar stimulator to evoke IPSCs. Cells were stimulated at 0.33 Hz for a one minute baseline, followed by a mode switch to current clamp. Once in current clamp we applied a depolarizing pulse (as in previous experiments) to induce an ADP. Three to five seconds after ADP onset the mode was switched back to voltage clamp and IPSCs were evoked for an additional 1.5 minutes. Using this approach we found that ADPs terminated after three seconds transiently reduced eIPSC amplitude by 13.5 ± 3.3% (n=3, p=0.05). Further, in a separate group of cells where the ADP was terminated after five seconds evoked IPSC amplitude was reduced by 19.2 ± 1.86% (p<0.01, Fig. 5A). By comparison, in the same cells a conventional 5 second depolarization from −70 to 0 mV in voltage clamp reduced eIPSC amplitude by 30.6 ± 3.42% (n=5, p<0.01, Fig. 5B). These data suggest that ADP induction may lead to cannabinoid release and CB1 receptor dependent inhibition of evoked GABAergic transmission. In order to further validate that hypothesis, we noted that both ADP induced suppression of inhibition and conventional DSI are absent in slices pretreated the CB1 receptor antagonist AM-251 (ADP induced suppression of inhibition: 3.30 ± 1.55%, n=5, p>0.05, Fig. 5D; DSI: 7.93 ± 3.87%, n=5, p>0.05, Fig. 5E). Importantly, the average area of the ADP observed in the presence of AM-251 (66.6 ± 23.1 mV*s) was not significantly different than that which produced transient suppression of evoked IPSCs in the absence of AM-251 (p>0.05).
Figure 5. ADP can cause a release of eCBs resulting in DSI.
eIPSCs were evoked with a concentric bipolar stimulator placed in the hilus at 0.33 Hz in the presence of 5 μM muscarine or 3 μM carbachol. A: For ADP-DSI a mixed mode protocol was used. Following a baseline the cell was switched to current clamp and a 500 ms depolarizing pulse was applied evoking a train of action potentials and resulting in an ADP. Five seconds after the depolarization the cell was switched back to voltage clamp and eIPSCs were evoked again at 0.33 Hz. The ADP resulted in a transient decrease in the eIPSC amplitude (DSI). The inset below the graph is an ADP from a representative cell in current clamp. B: For the DSI protocol, instead of switching to current clamp the cell was depolarized in voltage clamp from −70 mV to 0 mV for 5 seconds resulting in a transient decrease in the eIPSC amplitude (DSI). A–B: The insets to the right of the graphs are the average of 8 sweeps for the baseline (black), 2 sweeps for DSI (dark gray), and 8 sweeps for recovery (light gray). C: Summary plot of the amount of DSI for 3 second and 5 second ADPs and conventional DSI (5 second depolarization). *p<0.05. D: ADP-DSI was attempted after a 20 minute preincubation in 5 μM AM-251, a CB1 receptor antagonist. Induction of a similar ADP no longer resulted in a transient decrease in eIPSCs. Inset below the graph is an ADP from a representative cell in current clamp. E: A depolarization from −70 to 0 mV for 5 seconds also did not result in a transient decrease in eIPSCs in the presence of AM-251. D–E: The insets to the right of the graphs are the average of 8 sweeps for the baseline (black), 2 sweeps for DSI (dark gray), and 8 sweeps for recovery (light gray). F: Summary plot of the amount of DSI under each induction protocol in the presence of AM-251.
3. Discussion
In the present study we have shown evidence that activation of mAChRs differentially affects the intrinsic excitability of mossy cells and other hilar interneurons. Application of muscarine often resulted in a direct depolarization of mossy cells, likely through modulation of a Cs+ sensitive conductance, and further activated a robust ADP. The ADP observed in hilar mossy cells is carried by a calcium activated and Na+ permeant ion channel that that is blocked by the ICAN antagonist FFA. Further work revealed that activation of an ADP in hilar mossy cells produces transient CB1R dependent suppression of inhibition similar to that produced by a five second depolarization in voltage clamp (DSI). By contrast, only fifty percent of other hilar neurons tested were directly depolarized by muscarine, and only mossy cells had a muscarinic ADP.
ADPs have been described in many different areas of the CNS including the hippocampus (Fraser and MacVicar, 1996; Jensen et al., 1996; Lawrence et al., 2006b; McQuiston and Madison, 1999; Young et al., 2004), olfactory bulb (Pressler et al., 2007; Pressler and Strowbridge, 2006), and prefrontal cortex (Haj-Dahmane and Andrade, 1996). Research has shown the mechanism responsible for induction of these ADPs can vary. For example, prior studies have implicated both mGluR activated inward currents (Young et al., 2004) and TTX sensitive Na+ channels (Yue et al., 2005) in the induction of an ADP. However, one of the most common mechanisms is the opening of a calcium activated non-selective cation current (ICAN), especially for muscarinic induced ADPs (Fraser and MacVicar, 1996; Haj-Dahmane and Andrade, 1998; Lawrence et al., 2006b; Pressler et al., 2007). Similar to other ICAN mediated ADPs, we find that the ADP in hilar mossy cells is sodium and calcium dependent, and is blocked by the ICAN antagonist FFA. In fact, several properties of ICAN could account for the cell to cell variability in size and duration of the ADP we observed. Previous research has shown that the open probability for ICAN currents depends upon the cytoplasmic calcium concentration which is regulated in large part by mitochondria (Partridge, 1994; Razani-Boroujerdi and Partridge, 1993). Thus, cell to cell variability in calcium uptake or modulators of mitochondrial function could allow for the variability in the area of the ADP (Mattson, 2007). It has also been suggested that phosphorylation by PKA can decrease the amount of time ICAN is in the open state (Partridge, 1994), which could also be responsible for the cell to cell variability.
In the present study we also implicated the ADP as a possible physiological mechanism for the production of eCBs and subsequent inhibition of GABAergic transmission. In our lab we have previously shown that a depolarization in mossy cells can produce eCBs in a calcium sensitive manner resulting in DSI; however, to induce eCB production we used a non-physiological depolarization (−70 to 0 mV for 5 s) consistent with previously published reports of DSI (Hofmann et al., 2006; Kreitzer and Regehr, 2001; Ohno-Shosaku et al., 2001; Wilson and Nicoll, 2001). Previous research in the hippocampus was unable to reproduce DSI using in vivo firing rates bringing into question whether DSI could be induced under physiological conditions (Hampson et al., 2003). However, our data implicate an ADP induced depolarization as a possible physiological mechanism capable of producing sustained calcium influx and production of endocannabinoids. Potentially consistent with these data, our lab and others have previously demonstrated enhanced DSI following activation of mAChRs (Hashimotodani et al., 2005; Hofmann et al., 2006; Kim et al., 2002; Ohno-Shosaku et al., 2003). A recent study in cerebellar stellate basket cells showed that brief trains of somatic action potentials can result in increases in dendritic calcium causing the release of eCBs and a transient inhibition of glutamatergic transmission; however, to obtain eCB release at in vivo firing rates low level activation of mGluR1/5 receptors was required to lower the calcium requirement of eCB release (Myoga et al., 2009). This report provides a possible precedent for the pairing of action potentials with the activation of mAChRs to obtain DSI in mossy cells. In fact, mossy cells are well situated for this scenario because they have cholinergic synaptic connections to their soma and proximal dendrites (Deller et al., 1999). Future studies should investigate the in vivo activation of the ADP to further characterize this possible physiological mechanism for induction of eCB dependent signaling. These experiments could be attempted with either local application or uncaging of acetylcholine to mimic endogenous release. An additional possibility would be to test the hilus for intact cholinergic medial septal inputs using a previously described septo-hippocampal parasagittal slicing technique used in both CA1 and CA3 (Toth et al., 1997; Widmer et al., 2006).
It will also be interesting to investigate other functional consequences of mAChR activation in hilar mossy cells. Previous research in the olfactory bulb and hippocampus has shown application of muscarine results in decreases in action potential latency and jitter to sinusoidal waves (Lawrence et al., 2006a; Pressler et al., 2007). In addition, mAChR activation increases the firing reliability at theta frequencies in hippocampal interneurons (Lawrence et al., 2006a) and increases the number of action potentials fired in response to multiple stimuli resulting in increased output of GABAergic transmission in olfactory granule cells (Pressler et al., 2007). Finally, previous research has implicated activation of ICAN in the rising phase of excitotoxicity due to its lack of inactivation (Partridge et al., 1994; Partridge and Valenzuela, 1999; Tatsumi and Katayama, 1994). Because mossy cells are susceptible to ischemic and excitotoxic death (Freund and Magloczky, 1993; Hsu and Buzsaki, 1993; Magloczky and Freund, 1993) and they have been implicated in theories of epilepsy (Houser, 1999; Lothman et al., 1996; Ratzliff et al., 2002; Santhakumar et al., 2000; Sloviter et al., 2003), prolonged activation of an ADP, and consequent activation of ICAN, could provide a possible mechanism for mossy cell death or the initiation, progression, or maintenance of seizures. Perhaps mAChR activation could push mossy cells past the threshold of excitotoxicity by pairing the activation of the ADP and a reduction in the GABAergic input to mossy cells (due to the production of eCBs). Indeed, previous research has implicated ICAN in sustaining cortical seizures (Schiller, 2004), and antiepileptic drugs have been shown to reduce CCh induced depolarizing plateau potentials in the subiculum (D’Antuono et al., 2007).
4. Experimental Procedures
Male Sprague-Dawley rats between 18–25 days old were given an intraperitoneal injection of ketamine (80–100 mg/kg) and were decapitated using a small animal guillotine. The brain was rapidly removed and a vibratome was used to cut 300 μm thick horizontal slices which were then submerged in a chamber filled with artificial cerebral spinal fluid (ACSF) consisting of (in mM): 124 NaCl, 2.5 KCl, 1.2 NaH2PO4, 2.5 MgSO4, 10 D-glucose, 1 CaCl2, and 25.9 NaHCO3. The slices were incubated at 30–35 °C for 30 minutes and then allowed to equilibrate to room temperature for an additional 30 minutes before the start of experiments. All animal procedures were approved by the IACUC at the University of Florida and conformed to animal welfare guidelines issued by the NIH.
Slices were transferred to a recording chamber perfused at a rate of 2 mL/min with ACSF saturated with 95% O2-5% CO2 and heated to 30 °C for whole-cell patch clamp recordings. The ACSF consisted of (in mM): 126 NaCl, 3 KCl, 1.2 NaHPO4, 1.5 MgSO, 11 D-glucose, 2.4 CaCl2, and 25.9 NaHCO3. For experiments in Fig. 3B N-methyl-D-glucamine (NMG) was substituted for NaCl to create a nominally Na+ free ACSF. Cells were visualized with infrared differential interference contrast microscopy using an Olympus BX51WI microscope. Recording pipettes were pulled for whole cell patch clamp recordings on a Flaming/Brown electrode puller (Sutter P-97, Sutter Instruments, Novato, CA) and filled with an internal patch solution consisting of (in mM): 140 K-gluconate, 8 KCl, 0.1 CaCl2, 2 MgCl2, 1 EGTA, 2 Na2ATP, 0.3 NaGTP, 10 HEPES, 0.063 sulforhodamine 101, and the pH was adjusted to 7.3 using KOH. For experiments in Fig. 4A, 10 mM 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA) was added to the internal solution to chelate calcium. For experiments in Fig. 5 a high chloride internal patch solution was used consisting of (in mM): 90 K-gluconate, 55 KCl, 0.1 CaCl2, 2 MgCl2, 1 EGTA, 2 Na2ATP, 0.3 NaGTP, 10 HEPES, 0.063 sulforhodamine 101, and the pH was adjusted to 7.3 using KOH. Current clamp and voltage clamp experiments were performed using an Axon Multiclamp 700A or 700B amplifier. The data were recorded at 20 kHz, filtered at 2 kHz, and recorded using Clampex version 9 or 10 (Molecular Devices, Sunnyvale, CA). Access resistance was typically between 10 and 30 MΩ and uncompensated.
All experiments were performed in the presence of the glutamate antagonists DNQX (20 μM) and APV (40 μM) to block ionotropic glutamate receptors. For each cell, holding current was adjusted in current clamp to achieve a resting membrane potential of −60 mV. This adjustment was made once after application of ionotropic glutamate receptor antagonists, and one additional time (as needed) after application of muscarine. ADPs were induced by injecting a depolarizing current for 500 ms calibrated to induce a continuous train of action potentials. Any long lasting ADPs were terminated by a hyperpolarizing pulse of −100 pA for 1 s at the end of each sweep (sweep duration limited ADP length to 25 s). One set of ADP measurements consisted of two sweeps with depolarizing pulses and two interleaved sweeps with −100 pA hyperpolarizing pulses with an intersweep interval of 30 s. ADPs were measured by taking the area underneath the signal immediately after the depolarizing step until the resting membrane potential came within 2 mV of −60 mV. Values reported for ADPs are the average of that measurement taken from two depolarizing sweeps. This analysis was automated using software written in OriginPro 8 (OriginLab, Northampton, MA) by Ben Nahir.
For experiments investigating changes in input resistance during the ADP (Fig. 2) a 500 ms, −40 pA hyperpolarizing pulse was applied before the depolarizing pulse, and immediately following the depolarization it was applied once every second until the end of the sweep. These experiments were done in the presence of 1 μM TTX to prevent action potentials from contaminating our measurements. For experiments in Fig. 5, evoked responses were generated at 0.33 Hz using a concentric bipolar stimulator placed in the hilus and connected to a constant current stimulus isolator (World Precision Instruments, Sarasota, FL). Current intensity varied between 30–100 μA and lasted for 0.1 ms. Due to the high internal [Cl-] used in these experiments (see above), evoked responses were observed as inward currents in voltage clamp mode. A mixed mode (voltage clamp/current clamp) protocol was used to determine whether a muscarinic ADP could produce functionally relevant release of eCBs. Specifically, a one minute baseline of eIPSCs was recorded in voltage clamp. Immediately following this baseline period an ADP was induced in current clamp (via 500 msec depolarization) and allowed to continue for up to five seconds. At the end of this five second period, voltage clamp was restored and eIPSCs were recorded for an additional 1.5 minutes. This constituted one set, and 3–5 sets of this ADP-induced suppression of inhibition were tested for each cell. Following completion, conventional DSI was evaluated purely in voltage clamp using a 5 s depolarization from −70 mV to 0 mV in the same cell. Depolarization induced (and ADP-induced) suppression of inhibition was calculated as previously reported (Hofmann et al., 2006). Briefly, the mean amplitude of the initial 2 eIPSCs (observed over 6 s) following the ADP or depolarization were divided by the mean amplitude of the 8 eIPSCs (observed over 24 s) prior to the ADP or depolarization. This value was then converted to be expressed as a percent change from baseline.
Mossy cells were distinguished from other hilar neurons using physiological and anatomical criteria as previously described (Frazier et al., 2003; Hofmann et al., 2006; Nahir et al., 2007). In brief, typical mossy cells appear larger than other hilar neurons when observed under IR DIC, and have multiple differences apparent after patching. Anatomical features including multiple primary dendritic branches and the presence of multiple large thorny excrescences on the proximal dendrites have been described in a number of our prior publications (Frazier et al., 2003; Hofmann et al., 2006; Nahir et al., 2007). In the present manuscript we noted that compared to non-mossy hilar neurons, mossy cells displayed significantly larger whole cell capacitance (241.43 ± 5.76 pF vs. 123.76 ± 15.16 pF, n=40, 10, p<0.05) and significantly smaller whole cell input resistance (102.67 ± 4.75 MΩ vs. 185.03 ± 21.15 MΩ, n=40, 10, p<0.05). Further, mossy cells received higher frequency and larger amplitude spontaneous EPSCs than non-mossy cells (17.88 ± 1.51 Hz vs. 9.93 ± 2.36 Hz, and 52.59 ± 2.17 pA vs. 34.62 ± 4.92 pA, respectively, n=40, 10, and p<0.05, in both cases). Similarly, large amplitude spontaneous events (≥ 200 pA), which were noted to occur in mossy cells with a frequency of 0.47 ± 0.10 Hz, n=40, were virtually absent in non-mossy cells (0.08 ± 0.05 Hz, n=10, p=0.05). Finally, holding current required to clamp mossy cells at −60 mV in current clamp (after application of NBQX and APV, but prior to application of muscarinic agonists) was significantly smaller for mossy cells than for non-mossy hilar neurons (4.05 ± 5.57 pA vs. −32.0 ± 12.18 pA, n=40, 10, p<0.05). Statistical significance was determined by using the Student’s t-test. For all figures the error bars represent the SE. All drugs were purchased from either Sigma (St. Louis, MO) or Tocris (Ellisville, MO).
Acknowledgments
This work was supported by NIDA R01 DA019576. We thank Dr. Roger Papke for helpful comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acsady L, Katona I, Martinez-Guijarro FJ, Buzsaki G, Freund TF. Unusual Target Selectivity of Perisomatic Inhibitory Cells in the Hilar Region of the Rat Hippocampus. Journal of Neuroscience. 2000;20:6907–6919. doi: 10.1523/JNEUROSCI.20-18-06907.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral DG, Witter MP. The three-dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience. 1989;31:571–591. doi: 10.1016/0306-4522(89)90424-7. [DOI] [PubMed] [Google Scholar]
- Buckmaster PS, Strowbridge BW, Kunkel DD, Schmiege DL, Schwartzkroin PA. Mossy cell axonal projections to the dentate gyrus molecular layer in the rat hippocampal slice. Hippocampus. 1992;2:349–362. doi: 10.1002/hipo.450020403. [DOI] [PubMed] [Google Scholar]
- Cobb SR, Davies CH. Cholinergic modulation of hippocampal cells and circuits. J Physiol. 2005;562:81–88. doi: 10.1113/jphysiol.2004.076539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Antuono M, Kawasaki H, Palmieri C, Curia G, Biagini G, Avoli M. Antiepileptic drugs and muscarinic receptor-dependent excitation in the rat subiculum. Neuropharmacology. 2007;52:1291–1302. doi: 10.1016/j.neuropharm.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Deller T, Katona I, Cozzari C, Frotscher M, Freund TF. Cholinergic innervation of mossy cells in the rat fascia dentata. Hippocampus. 1999;9:314–320. doi: 10.1002/(SICI)1098-1063(1999)9:3<314::AID-HIPO10>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Fraser DD, MacVicar BA. Cholinergic-dependent plateau potential in hippocampal CA1 pyramidal neurons. J Neurosci. 1996;16:4113–4128. doi: 10.1523/JNEUROSCI.16-13-04113.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier CJ, Strowbridge BW, Papke RL. Nicotinic receptors on local circuit neurons in dentate gyrus: a potential role in regulation of granule cell excitability. J Neurophysiol. 2003;89:3018–3028. doi: 10.1152/jn.01036.2002. [DOI] [PubMed] [Google Scholar]
- Freund TF, Magloczky Z. Early degeneration of calretinin-containing neurons in the rat hippocampus after ischemia. Neuroscience. 1993;56:581–596. doi: 10.1016/0306-4522(93)90358-m. [DOI] [PubMed] [Google Scholar]
- Frotscher M, Leranth C. Cholinergic innervation of the rat hippocampus as revealed by choline acetyltransferase immunocytochemistry: a combined light and electron microscopic study. J Comp Neurol. 1985;239:237–246. doi: 10.1002/cne.902390210. [DOI] [PubMed] [Google Scholar]
- Ghamari-Langroudi M, Bourque CW. Flufenamic acid blocks depolarizing afterpotentials and phasic firing in rat supraoptic neurones. J Physiol. 2002;545:537–542. doi: 10.1113/jphysiol.2002.033589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold PE. Acetylcholine modulation of neural systems involved in learning and memory. Neurobiol Learn Mem. 2003;80:194–210. doi: 10.1016/j.nlm.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Haj-Dahmane S, Andrade R. Muscarinic activation of a voltage-dependent cation nonselective current in rat association cortex. J Neurosci. 1996;16:3848–3861. doi: 10.1523/JNEUROSCI.16-12-03848.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haj-Dahmane S, Andrade R. Ionic mechanism of the slow afterdepolarization induced by muscarinic receptor activation in rat prefrontal cortex. J Neurophysiol. 1998;80:1197–1210. doi: 10.1152/jn.1998.80.3.1197. [DOI] [PubMed] [Google Scholar]
- Hampson RE, Zhuang SY, Weiner JL, Deadwyler SA. Functional significance of cannabinoid-mediated, depolarization-induced suppression of inhibition (DSI) in the hippocampus. J Neurophysiol. 2003;90:55–64. doi: 10.1152/jn.01161.2002. [DOI] [PubMed] [Google Scholar]
- Hashimotodani Y, Ohno-Shosaku T, Tsubokawa H, Ogata H, Emoto K, Maejima T, Araishi K, Shin HS, Kano M. Phospholipase Cbeta serves as a coincidence detector through its Ca2+ dependency for triggering retrograde endocannabinoid signal. Neuron. 2005;45:257–268. doi: 10.1016/j.neuron.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME. The role of acetylcholine in learning and memory. Curr Opin Neurobiol. 2006;16:710–715. doi: 10.1016/j.conb.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henze DA, Buzsaki G. Hilar mossy cells: functional identification and activity in vivo. Prog Brain Res. 2007;163:199–216. doi: 10.1016/S0079-6123(07)63012-X. [DOI] [PubMed] [Google Scholar]
- Hofmann ME, Nahir B, Frazier CJ. Endocannabinoid-mediated depolarization-induced suppression of inhibition in hilar mossy cells of the rat dentate gyrus. J Neurophysiol. 2006;96:2501–2512. doi: 10.1152/jn.00310.2006. [DOI] [PubMed] [Google Scholar]
- Houser CR. Neuronal loss and synaptic reorganization in temporal lobe epilepsy. Adv Neurol. 1999;79:743–761. [PubMed] [Google Scholar]
- Houser CR. Interneurons of the dentate gyrus: an overview of cell types, terminal fields and neurochemical identity. Prog Brain Res. 2007;163:217–232. doi: 10.1016/S0079-6123(07)63013-1. [DOI] [PubMed] [Google Scholar]
- Howard AL, Neu A, Morgan RJ, Echegoyen JC, Soltesz I. Opposing modifications in intrinsic currents and synaptic inputs in post-traumatic mossy cells: evidence for single-cell homeostasis in a hyperexcitable network. J Neurophysiol. 2007;97:2394–2409. doi: 10.1152/jn.00509.2006. [DOI] [PubMed] [Google Scholar]
- Hsu M, Buzsaki G. Vulnerability of mossy fiber targets in the rat hippocampus to forebrain ischemia. J Neurosci. 1993;13:3964–3979. doi: 10.1523/JNEUROSCI.13-09-03964.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson MB, Scharfman HE. Positive feedback from hilar mossy cells to granule cells in the dentate gyrus revealed by voltage-sensitive dye and microelectrode recording. J Neurophysiol. 1996;76:601–616. doi: 10.1152/jn.1996.76.1.601. [DOI] [PubMed] [Google Scholar]
- Jensen MS, Azouz R, Yaari Y. Spike after-depolarization and burst generation in adult rat hippocampal CA1 pyramidal cells. J Physiol. 1996;492 (Pt 1):199–210. doi: 10.1113/jphysiol.1996.sp021301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Isokawa M, Ledent C, Alger BE. Activation of muscarinic acetylcholine receptors enhances the release of endogenous cannabinoids in the hippocampus. J Neurosci. 2002;22:10182–10191. doi: 10.1523/JNEUROSCI.22-23-10182.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Regehr WG. Cerebellar depolarization-induced suppression of inhibition is mediated by endogenous cannabinoids. J Neurosci. 2001;21:RC174. doi: 10.1523/JNEUROSCI.21-20-j0005.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larimer P, Strowbridge BW. Nonrandom local circuits in the dentate gyrus. J Neurosci. 2008;28:12212–12223. doi: 10.1523/JNEUROSCI.3612-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence JJ. Cholinergic control of GABA release: emerging parallels between neocortex and hippocampus. Trends Neurosci. 2008;31:317–327. doi: 10.1016/j.tins.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Lawrence JJ, Grinspan ZM, Statland JM, McBain CJ. Muscarinic receptor activation tunes mouse stratum oriens interneurones to amplify spike reliability. J Physiol. 2006a;571:555–562. doi: 10.1113/jphysiol.2005.103218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence JJ, Statland JM, Grinspan ZM, McBain CJ. Cell type-specific dependence of muscarinic signalling in mouse hippocampal stratum oriens interneurones. J Physiol. 2006b;570:595–610. doi: 10.1113/jphysiol.2005.100875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lothman EW, Bertram EH, 3rd, Kapur J, Bekenstein JW. Temporal lobe epilepsy: studies in a rat model showing dormancy of GABAergic inhibitory interneurons. Epilepsy Res Suppl. 1996;12:145–156. [PubMed] [Google Scholar]
- Magloczky Z, Freund TF. Selective neuronal death in the contralateral hippocampus following unilateral kainate injections into the CA3 subfield. Neuroscience. 1993;56:317–335. doi: 10.1016/0306-4522(93)90334-c. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Mitochondrial regulation of neuronal plasticity. Neurochem Res. 2007;32:707–715. doi: 10.1007/s11064-006-9170-3. [DOI] [PubMed] [Google Scholar]
- McQuiston AR, Madison DV. Muscarinic receptor activity induces an afterdepolarization in a subpopulation of hippocampal CA1 interneurons. J Neurosci. 1999;19:5703–5710. doi: 10.1523/JNEUROSCI.19-14-05703.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myoga MH, Beierlein M, Regehr WG. Somatic spikes regulate dendritic signaling in small neurons in the absence of backpropagating action potentials. J Neurosci. 2009;29:7803–7814. doi: 10.1523/JNEUROSCI.0030-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahir B, Bhatia C, Frazier CJ. Presynaptic inhibition of excitatory afferents to hilar mossy cells. J Neurophysiol. 2007;97:4036–4047. doi: 10.1152/jn.00069.2007. [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Maejima T, Kano M. Endogenous cannabinoids mediate retrograde signals from depolarized postsynaptic neurons to presynaptic terminals. Neuron. 2001;29:729–738. doi: 10.1016/s0896-6273(01)00247-1. [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Matsui M, Fukudome Y, Shosaku J, Tsubokawa H, Taketo MM, Manabe T, Kano M. Postsynaptic M1 and M3 receptors are responsible for the muscarinic enhancement of retrograde endocannabinoid signalling in the hippocampus. Eur J Neurosci. 2003;18:109–116. doi: 10.1046/j.1460-9568.2003.02732.x. [DOI] [PubMed] [Google Scholar]
- Partridge LD. Cytoplasmic Ca2+ activity regulation as measured by a calcium-activated current. Brain Res. 1994;647:76–82. doi: 10.1016/0006-8993(94)91400-1. [DOI] [PubMed] [Google Scholar]
- Partridge LD, Muller TH, Swandulla D. Calcium-activated non-selective channels in the nervous system. Brain Res Brain Res Rev. 1994;19:319–325. doi: 10.1016/0165-0173(94)90017-5. [DOI] [PubMed] [Google Scholar]
- Partridge LD, Valenzuela CF. Ca2+ store-dependent potentiation of Ca2+-activated non-selective cation channels in rat hippocampal neurones in vitro. J Physiol. 1999;521(Pt 3):617–627. doi: 10.1111/j.1469-7793.1999.00617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge LD, Valenzuela CF. Block of hippocampal CAN channels by flufenamate. Brain Res. 2000;867:143–148. doi: 10.1016/s0006-8993(00)02275-7. [DOI] [PubMed] [Google Scholar]
- Pressler RT, Inoue T, Strowbridge BW. Muscarinic receptor activation modulates granule cell excitability and potentiates inhibition onto mitral cells in the rat olfactory bulb. J Neurosci. 2007;27:10969–10981. doi: 10.1523/JNEUROSCI.2961-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressler RT, Strowbridge BW. Blanes cells mediate persistent feedforward inhibition onto granule cells in the olfactory bulb. Neuron. 2006;49:889–904. doi: 10.1016/j.neuron.2006.02.019. [DOI] [PubMed] [Google Scholar]
- Ratzliff AH, Santhakumar V, Howard A, Soltesz I. Mossy cells in epilepsy: rigor mortis or vigor mortis? Trends Neurosci. 2002;25:140–144. doi: 10.1016/s0166-2236(00)02122-6. [DOI] [PubMed] [Google Scholar]
- Razani-Boroujerdi S, Partridge LD. Activation and modulation of calcium-activated non-selective cation channels from embryonic chick sensory neurons. Brain Res. 1993;623:195–200. doi: 10.1016/0006-8993(93)91427-t. [DOI] [PubMed] [Google Scholar]
- Santhakumar V, Bender R, Frotscher M, Ross ST, Hollrigel GS, Toth Z, Soltesz I. Granule cell hyperexcitability in the early post-traumatic rat dentate gyrus: the ‘irritable mossy cell’ hypothesis. J Physiol. 2000;524(Pt 1):117–134. doi: 10.1111/j.1469-7793.2000.00117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE. Electrophysiological evidence that dentate hilar mossy cells are excitatory and innervate both granule cells and interneurons. J Neurophysiol. 1995;74:179–194. doi: 10.1152/jn.1995.74.1.179. [DOI] [PubMed] [Google Scholar]
- Schiller Y. Activation of a calcium-activated cation current during epileptiform discharges and its possible role in sustaining seizure-like events in neocortical slices. J Neurophysiol. 2004;92:862–872. doi: 10.1152/jn.00972.2003. [DOI] [PubMed] [Google Scholar]
- Sloviter RS, Zappone CA, Harvey BD, Bumanglag AV, Bender RA, Frotscher M. “Dormant basket cell” hypothesis revisited: relative vulnerabilities of dentate gyrus mossy cells and inhibitory interneurons after hippocampal status epilepticus in the rat. J Comp Neurol. 2003;459:44–76. doi: 10.1002/cne.10630. [DOI] [PubMed] [Google Scholar]
- Tatsumi H, Katayama Y. Brief increases in intracellular Ca2+ activate K+ current and non-selective cation current in rat nucleus basalis neurons. Neuroscience. 1994;58:553–561. doi: 10.1016/0306-4522(94)90080-9. [DOI] [PubMed] [Google Scholar]
- Toth K, Freund TF, Miles R. Disinhibition of rat hippocampal pyramidal cells by GABAergic afferents from the septum. J Physiol. 1997;500 (Pt 2):463–474. doi: 10.1113/jphysiol.1997.sp022033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widmer H, Ferrigan L, Davies CH, Cobb SR. Evoked slow muscarinic acetylcholinergic synaptic potentials in rat hippocampal interneurons. Hippocampus. 2006;16:617–628. doi: 10.1002/hipo.20191. [DOI] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature. 2001;410:588–592. doi: 10.1038/35069076. [DOI] [PubMed] [Google Scholar]
- Young SR, Chuang SC, Wong RK. Modulation of afterpotentials and firing pattern in guinea pig CA3 neurones by group I metabotropic glutamate receptors. J Physiol. 2004;554:371–385. doi: 10.1113/jphysiol.2003.051847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue C, Remy S, Su H, Beck H, Yaari Y. Proximal persistent Na+ channels drive spike afterdepolarizations and associated bursting in adult CA1 pyramidal cells. J Neurosci. 2005;25:9704–9720. doi: 10.1523/JNEUROSCI.1621-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]