ABSTRACT
Colonoscopy, the preferred procedure for colon cancer screening, has well-documented limitations. To improve colonoscopy's effectiveness, augmented endoscopy techniques, such as chromoendoscopy and narrow band imaging (NBI) have been evaluated. Both techniques are inexpensive, safe, and relatively easy to perform. Chromoendoscopy has an increasingly important role in surveillance of IBD, but significant work is needed to determine the optimal staining techniques and mucosal surface pattern analysis before this technique can be incorporated into routine clinical practice. NBI is a much newer technology with far less data. Well-designed prospective randomized controlled trials have failed to identify a benefit of NBI in screening colonoscopy, in surveillance for high-risk populations or as an adjunct for therapeutic procedures.
Keywords: Chromoendoscopy, narrow band imaging, colonoscopy, endoscopy
In the United States, colorectal cancer is currently the fifth leading cancer, with an estimated 146,970 new cases annually. It is the second largest cause of cancer death: Approximately 49,920 died of the disease in 2009.1 As dedicated colonoscopists, we must focus on our ability to meticulously detect and remove colorectal neoplasia with the ultimate goal being colorectal cancer prevention.
The practice of removing neoplastic colonic lesions during colonoscopy to reduce the incidence of colorectal cancer has been justified by the results of the National Polyp Study Workgroup cohort study, which showed that colonoscopy with removal of identified polyps reduced the incidence of colorectal cancer 76 to 90%.2
Currently, white light colonoscopy (colonoscopy), albeit imperfect, is the preferred colorectal cancer screening test.3 It is the best screening test to both detect and remove colonic neoplasms. However, substantial miss rates for adenoma and cancers have been reported with colonoscopy. The colonoscopy-miss rate for adenomas is not completely understood, but has been well documented by several studies showing a significant discordance in adenoma detection rate (ADR) during screening colonoscopy among practicing colonoscopists.
ADENOMA DETECTION RATE
Published ADRs during colonoscopy have ranged from 7% to 44%, whereas the mean number of adenomas detected per subject screen has ranged from 0.09 to 1.05.4,5,6,7,8 To study more closely the missed adenoma rate of colonoscopy, same-day tandem (back-to-back) colonoscopies have shown an overall adenoma-miss rate as high as 24%. Miss rates for adenomas ≤5 mm was 27%; adenomas 6–9 mm, 13%, adenomas ≥10 mm, 6%; and advanced adenomas, 11%.9,10,11 In individuals with Lynch syndrome (hereditary nonpolyposis colorectal cancer), miss rates as high as 55% have been reported.12 Colonoscopy-miss rates for colorectal cancer has been reported at ∼4%.13 Answers to the question of how meticulous a colonoscopist must be to prevent colorectal cancer are unknown, but evolving.
Computed Tomographic Colonography
In 2008, the American College of Radiology Imaging Network published one of the largest prospective, multicenter, head-to-head comparisons of computed tomographic colonography (CTC or virtual colonoscopy) to white light colonoscopy for adenoma detection in 2,531 asymptomatic adults ≥50 years. CTC's sensitivity for adenomas or cancers ≥10 mm was 90%, but the sensitivity for adenomas or cancers <5 mm was only 65%.14 These results were not as promising as Pickhardt's groundbreaking study reporting a 93.8% sensitivity of CTC for polyps ≥10 mm, which no subsequent multicenter study has ever achieved.15 In May 2009, the Centers for Medicare and Medicaid Services concluded “the evidence is inadequate to conclude that CT colonography is an appropriate colorectal cancer screening test… CT colonography for colorectal cancer screening remains noncovered.” However, the American College of Gastroenterology added, for the first time, CTC every 5 years as an alternate cancer detection test in their updated 2009 colorectal cancer screening guideline, removing double-contrast barium enema as an approved colorectal cancer detection test.3
QUALITY METRICS
Quality indicators for colonoscopy have focused mainly on the cecal intubation rate, adequacy of colonic cleansing, and colonoscopists' ADR as the most salient quality metrics for practicing colonoscopists.
Withdrawal Time
Several prospective studies have shown significant positive correlation between ADR and longer mean withdrawal time during colonoscopy in which no polyps are found or removed. Detection rates of adenomas and advanced adenomas (adenomas ≥10 mm, adenomas of any size with any villous features, and any polyp with high-grade dysplasia) were both significantly greater among endoscopists whose mean withdrawal time was ≥6 minutes. The advanced ADR was 2.46 times higher for colonoscopists whose average withdrawal times on a no-polyp-find colonoscopy were ≥6 minutes when compared with colonoscopists whose average withdrawal times were <6 minutes.16
More recent studies have shown that a minimum withdrawal time of at least 7 minutes provided significantly greater ADRs.17,18 The mean withdrawal time to virtually prevent colorectal cancer has not yet been defined.
Bowel Preparation
High-quality bowel preparation for colonoscopy is paramount to facilitate maximum mucosal visibility for colonoscopists. Several recently published prospective, randomized, blinded, controlled trials have compared whole-dose polyethylene glycol electrolyte (PEG-E) versus split-dose PEG-E, demonstrating that patients who received split-dose colonic preparation have a significantly better colon cleansing than those receiving whole-dose colonic preparation.19,20,21,22
High-Resolution Colonoscopy
A comparative study of ADR between standard-resolution (SR) and high-resolution (HR) colonoscopy with optimized withdrawal technique of at least 6 minutes and wide-angle field of view colonoscopy versus standard field of view showed no significant difference in ADR.23,24
Timing of Colonoscopy
Two studies investigating colonoscopists' fatigue on ADR have shown that time of the day that the colonoscopy is performed appears to be an independent predictor of adenoma detection, irrespective of adequate bowel preparation or completeness of the colonoscopy.25,26 Sanaka and colleagues' study of 3,619 asymptomatic outpatients with adequate bowel preparation and complete colonoscopy—in which 48.3% were performed in the morning and 51.7% in the afternoon—reported an ADR of 29.3% in the morning group versus 25.3% in the afternoon group (p = 0.008). In addition, there was a trend toward declining ADR for each subsequent hour of the day (p = 0.01).
Nonpolypoid Colonic Neoplasm
Flat adenomas have recently gained much attention and are a possible cause of missed adenomas or interval colorectal cancers because they may go unrecognized or may be dismissed by colonoscopists; their visualization at colonoscopy mandates an adequate bowel preparation and a meticulous examination of the colonic mucosa. The incidence of flat colonic neoplasia has been elucidated by Gorgun et al, whose evaluation of 3,115 adenomas removed during colonoscopy found 83% to be sessile, 10% to be flat, 6% to be pedunculated, and 1% to be depressed.27 This prevalence of flat and depressed adenomas was validated by a report of 1,819 adults Veterans Affairs Administration colorectal cancer screening patients.28
With the known limitations of colonoscopy addressed, we will review the ability of chromoendoscopy and narrow band imaging (NBI) to enhance mucosal detection of neoplastic tissue in both screening and surveillance colonoscopy.
CHROMOENDOSCOPY
Background
Chromoendoscopy, the technique of endoscopically applying contrast dyes to improve the detection of mucosal abnormalities, first appeared in the literature in the 1970s.29,30 Initially, this procedure was described as an adjunct in gastric cancer screening, where it facilitated the identification of dysplastic tissue. Tada31 first adapted the technique for colonoscopy in 1974. Over the next 20 years, the majority of the experience with this technique appeared in Japanese publications; however, in the 1990s, as interest grew in improving colonic adenoma detection, more trials of chromoendoscopy were conducted worldwide.
Chromoendoscopy techniques have been employed for the study of several conditions, including Barrett's esophagus, esophageal cancer, gastric cancer, celiac disease, and colon cancer. Although several inexpensive staining agents have been investigated, none to date have been approved by the U.S. Food and Drug Administration (FDA) for chromoendoscopy.
Types of Stains and Dyes
Chromoendoscopy staining agents can be broadly organized into the categories based on their mode of action (Table 1). Contrast dyes, including indigo carmine, pool in the crevices and irregularities of surface mucosa and highlight potentially abnormal tissue.
Table 1.
Staining Agents for Chromoendoscopy
| Stains | Mechanism of Action | Main Application |
|---|---|---|
| EMR, endoscopic mucosal resection. | ||
| ABSORPTIVE STAINS | ||
| Lugol's solution | Stains glycogen-containing epithelium | Esophageal squamous cell cancer |
| Barrett's esophagus | ||
| Methylene blue | Absorbed by epithelial cells, dysplasia, and cancer stain poorly | Barrett's esophagus |
| Gastric cancer | ||
| Chronic ulcerative colitis | ||
| Colonic neoplasms | ||
| Toluidine blue | Stains nuclei | Oral and esophageal squamous cell cancer |
| Crystal violet | Stains nuclei | Colonic neoplasms |
| Define margins of EMR | ||
| CONTRAST STAINS | ||
| Indigo carmine | Highlights mucosal topography | Colonic neoplasms |
| REACTIVE STAINS | ||
| Phenol red | pH sensitive color change | H. pylori infection |
| Congo red | pH sensitive color change | Ectopic gastric mucosa |
| Gastric cancer | ||
| Adequacy of vagotomy | ||
| H. pylori infection | ||
Vital dyes, such as methylene blue and crystal violet are actively absorbed by the gastrointestinal mucosa. Metaplastic or neoplastic mucosa often absorbs vital stains differently than normal mucosa, and these differences serve to highlight abnormal structures.
Finally, reactive dyes such as Congo red and phenol red respond to specific host conditions. Phenol red is pH sensitive, and when used in the stomach, it aids in the identification of Helicobacter pylori infection by changing color in lower acid concentrations. In chromoendoscopy of the colon, the majority of clinical trials have been conducted using two staining agents: methylene blue and indigo carmine.
Staining Techniques
A multitude of techniques have been described for the application of surface stains during chromoendoscopy. Some methods deliver the contrast dye prior to endoscopy by mixing it in the colonic lavage solution or administering it by mouth immediately after the colon preparation is completed.32 Although convenient, these methods suffer from inconsistent mucosal staining and are not routinely used.
The most widely accepted technique for applying surface stains employs a spray catheter inserted through the working channel of the colonoscope. This technique, while time consuming, allows for adequate surface preparation and targeted application of surface dyes. Spray catheter chromoendoscopy typically begins with cecal intubation using standard white light. Next, the colonic mucosa is prepared and stained in a segmental fashion starting with the cecum and progressing anatomically through the ascending, transverse, descending colon, sigmoid colon and rectum.
Most commonly, the mucosa is washed free of debris under white light. Some protocols call for the use of atropine or glucagon to minimize gut contractions and prevent uneven spraying. Once the mucosa is adequately prepared, a spray catheter (Table 2) is advanced ∼1 to 2 cm from the surface mucosa and dye is delivered until the entire mucosal surface is covered. Excess dye is removed prior to mucosal examination. The additional time required for chromoendoscopy varies dramatically depending on the technique used and operator experience.
Table 2.
Spray Catheters for Chromoendoscopy
| Manufacturer | Name | Length | Use | U.S. Price (11/09) |
|---|---|---|---|---|
| Olympus, Inc., Melville, NY | PW-6P-1 | 190 cm | Reusable | $225 |
| PW-5L-1 | 165 cm | Reusable | $225 | |
| PW-5V-1 | 240 cm | Reusable | $225 | |
| PW-6C-1.A | 105 cm | Reusable | $245 | |
| PW-6C-1.B | 105 cm | Reusable | $215 | |
| Wilson-Cook Medical, Inc., Winston Salem, NC | GT-7-SPRAY | 240 cm | Single use | $81 |
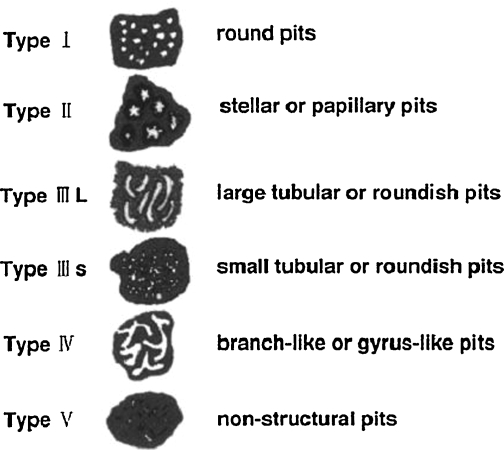
Successful chromoendoscopy depends not only on adequate mucosal staining, but also on observer identification of abnormal mucosa. Interpretation of stained mucosa is subject to observer variation.33,34,35 Most commonly, this is accomplished by the classification of the mucosal appearance according to a system described by Kudo et al (Fig. 1).36 Organizing lesions into groups based on mucosal surface patterns, this system allows endoscopists to estimate a lesion's malignant potential based on its endoscopic appearance. Kudo and colleagues' original work used high-magnification endoscopes capable of 70× to 150× magnification to assist in surface pattern analysis; not all chromoendoscopy trials have been conducted with high-magnification endoscopy.
Figure 1.
Pit pattern classification. (Reprinted from Miyamoto, Muto, Hamamoto, et al. Diagnosis of colorectal tumorous lesions by magnifying endoscopy. Gastrointest Endosc 2006;44:9, with permission from Elsevier.)
Several researchers have adapted the technique for use with high-resolution endoscopy: a technology that boasts enhanced surface detail from improved image resolution, but only minimal (1.5×&) magnification. Both high-magnification and high-resolution endoscopy have been described as adjuncts to chromoendoscopy; however, neither is central to the technique.
Safety Concerns
The use of surface dyes during chromoendoscopy has, in some cases, generated safety concerns. Prior in vitro biochemical studies have established that in the presence of light methylene blue can generate single-strand breaks in DNA.37,38,39 Around the time the first large prospective trials using methylene blue chromoendoscopy in the colon were published, Olliver et al40 released an in vivo evaluation of methylene blue chromoendoscopy for surveillance of Barrett's esophagus. Using DNA electrophoresis assays to assess levels of DNA damage, the authors evaluated tissue samples taken before and after staining. Higher levels of DNA damage appeared in the samples exposed to methylene blue and white light.
Kiesslich41 reported 20-month safety data on patients who had been subjects of chromoendoscopy. The subjects all had long-standing ulcerative colitis and had been enrolled in a prior chromoendoscopy investigation using methylene blue. At follow-up colonoscopy, significantly fewer lesions were found in the chromoendoscopy group when compared with controls. Citing these findings, he endorsed the use of methylene blue and concluded that prior observations regarding DNA damage were unlikely to have biologic consequences. In another endorsement, Dinis-Ribeiro and colleagues reported 3-year safety data on patients under methylene blue chromoendoscopy surveillance for chronic atrophic gastritis.42 Here again, the authors noted a lower incidence of neoplasia in the study population when compared with surveillance studies using white light.
As an alternative to methylene blue, indigo carmine is generally regarded as a safe dye because it is not absorbed by the colonic mucosa. In a direct comparison of the toxicity of these two dyes, Davies43 published an in vitro study using adenocarcinoma cell cultures that were exposed to white light for 2 minutes in the presence of either 0.1% indigo carmine or 0.1% methylene blue. Assays were performed on each group to quantify the DNA strand breaks. No significant increase in DNA damage was detected in the indigo carmine group when compared with the control group. The methylene blue group, on the other hand, had a significant increase in DNA damage. Drawing on these findings, Davies suggested that, assuming equivalent efficacy of the chromoendoscopic dyes, indigo carmine should be preferentially considered. To date, however, no long-term data exist that capture adverse clinical outcomes, and some groups continue to use methylene blue chromoendoscopy.
Adenoma Detection in Chromoendoscopy
Several trials have evaluated the ability of chromoendoscopy to increase detection of adenomas during screening colonoscopy. Kiesslich et al44 performed one of the first large prospective trials of this technique. The study included 100 patients and implemented selective mucosal staining. Abnormal mucosa seen on white light exam was further stained with 0.4% indigo carmine dye and characterized according to the Kudo classification system. In the subset of patients where no lesions were identified, the entire sigmoid colon was stained with 0.4% indigo carmine and reexamined. Using this technique the authors found 178 additional lesions in the sigmoid colon. This study failed to control for withdrawal time, which may have explained the increased detection with indigo carmine.
As larger and more carefully controlled studies have been published, some of the early promise of chromoendoscopy has faded. Booker et al45 conducted one of the first large randomized controlled trials of pan-colonic chromoendoscopy versus standard colonoscopy. This study included 259 patients and reported a statistically significant increase in detection rate for diminutive adenomas (89 vs. 36), as well as number of patients with ≥3 adenomas (15 vs. 3), in the chromoendoscopy group. Despite these findings, there was no significant overall increase in the detection of adenomas. In addition, this study failed to control for withdrawal time. Withdrawal times in the chromoendoscopy group were significantly longer than the control group (9 minutes, 5 seconds vs. 4 minutes, 52 seconds). Thus, the increased detection reported in the chromoendoscopy group may have been a result of a longer examination time and not from the contrast staining.
Hurlstone46 designed a second large randomized trial of pan-chromoendoscopy that controlled for scope withdrawal time and mucosal lavage volume. This study included 260 patients and compared pan-chromoendoscopy using 0.4% indigo carmine dye to a control group of targeted chromoendoscopy. Hurlstone reported a higher number of adenomas in the pan-chromoendoscopy group (112 vs. 57). The reported flat lesions (54 vs. 82), right-sided lesions (31 vs. 79), and patients with ≥3 adenomas (4 vs. 13) were significantly higher in the pan-chromoendoscopy group
Unfortunately, Hurlstone's findings were not replicated in a third randomized controlled trial investigating chromoendoscopy conducted by Le Rhun et al.47 This large prospective trial included 203 patients and compared adenoma detection of SR video colonoscopy against HR pan-colonic chromoendoscopy using indigo carmine 0.4%. The authors found that HR colonoscopy detected more polyps than SR colonoscopy. They also noted that the HR group found a higher number of flat adenomas <5 mm. Unlike Booker's or Hurlstone's studies, this trial did not find a higher number of patients with ≥3 adenomas in the HR group. In fact, the total number of adenomas detected was not statistically different between the HR and SR groups.
Chromoendoscopy in High-Risk Populations
Although the weight of evidence appears to not support the use of chromoendoscopy for adenoma detection in routine screening colonoscopy, several trials have investigated the benefit of chromoendoscopy in enhancing detection of dysplasia in high-risk populations.
In 2003, Matsumoto et al48 published the results of a chromoendoscopy surveillance program for patients with ulcerative colitis. The trial lasted 7 years; it followed 57 patients who had been diagnosed with pan-colitis at least 5 years earlier, and included 117 pancolonic chromoendoscopies using indigo carmine 0.2%. A higher diagnostic accuracy for dysplasia in biopsies targeted by chromoendoscopy versus random biopsies from standard colonoscopy was found (sensitivity of 38.1% vs. 85.7%, respectively).
In a similar study, Kiesslich et al49 performed a randomized controlled trial of chromoendoscopy to evaluate the early detection of intraepithelial neoplasia and colitis-associated colon carcinomas in patients with ulcerative colitis. In this study, 263 patients were randomized to receive either white light colonoscopy with random biopsies or pancolonic chromoendoscopy with targeted biopsies using 0.1% methylene blue. The trial found a stronger correlation between the endoscopic assessment of colonic inflammation and histopathologic findings in the chromoendoscopy group. Further, statistically higher numbers of intraepithelial neoplasias were detected in the chromoendoscopy group (32 vs. 10; p = 0.003). Chromoendoscopy allowed for the differentiation between nonneoplastic and neoplastic lesions with a sensitivity and specificity of 93%.
Rutter et al50 investigated pancolonic surveillance chromoendoscopy in patients with long-standing ulcerative colitis. The study featured 100 patients examined using a back-to-back design, beginning with white light colonoscopy using random mucosal biopsies followed by pancolonic chromoendoscopy using mucosal biopsies targeted by 0.1% indigo carmine staining. The authors failed to show a significant difference in detection of dysplasia between the two groups (7 vs. 2), but noted a strong trend toward significance (p = 0.06). Further, they found that biopsies targeted by dye spraying yielded significantly more dysplastic lesions than random biopsies did (5 vs. 0; p = 0.02).
Marion et al51 subsequently published a prospective trial comparing chromoendoscopy with methylene blue 0.01% to white light colonoscopy in 115 patients with inflammatory bowel disease (IBD). The authors detected significantly more dysplasia in the chromoendoscopy group (17 vs. 9; p = 0.001).
Although the majority of studies of chromoendoscopy in high-risk populations have examined patients with long-standing IBD, other high-risk groups have been evaluated. Hurlstone et al52 conducted a prospective study of 25 patients who fulfilled the modified Amsterdam criteria for hereditary nonpolyposis colon cancer (HNPCC). This study assessed the efficacy of high-magnification chromoendoscopy in the detection of neoplastic lesions in HNPCC. During their study, the authors employed a phased withdrawal protocol whereby the endoscope was withdrawn in segments. The colon was examined first with white light, then with targeted chromoendoscopy, and finally with complete segmental staining. Pancolonic chromoendoscopy using 0.5% indigo carmine as a contrast dye yielded significantly more adenomas than conventional colonoscopy: 35 versus 17 (p = 0.001). Additionally, significantly more flat adenomas were identified with chromoendoscopy: 4 versus 35 (p = 0.004).
The role for chromoendoscopy in surveillance colonoscopy for patients with IBD remains unclear. The Consensus Conference of Colorectal Cancer Screening and Surveillance in IBD recommendations only obliquely reference chromoendoscopy53 This report endorsed the incorporation of chromoendoscopy into surveillance colonoscopy given its ability to increase detection of dysplastic lesions.
Chromoendoscopy for Familial Adenomatous Polyposis
Finally, Matsumoto54 et al published a study comparing chromoendoscopy to NBI, white light colonoscopy, and autofluorescence in patients with familial adenomatous polyposis (FAP). Autofluorescence is a developing technology that detects differences in light-emitted tissues when observed under blue light. This very small, prospective study of 13 patients with known FAP showed that chromoendoscopy detected the greatest number of adenomas when compared with the other techniques. In addition, researchers noted that chromoendoscopy detected significantly more left-sided lesions (p < 0.05).
Chromoendoscopy to Predict Histology
The gold standard for the diagnosis of colorectal polyps is mucosal resection and histologic evaluation. However, as experience with chromoendoscopy and mucosal pit pattern analysis grew, some researchers set out to determine whether chromoendoscopy could provide an accurate prediction of the underlying histology of suspected lesions. Tung and colleagues55 conducted a trial of chromoendoscopy in 141 patients that compared endoscopic prediction of lesions to biopsy results; they found that the endoscopic prediction had a sensitivity and specificity of 93.8% and 64.6%, respectively, for neoplastic lesions. Not long after this study, Eisen et al56 published a multicenter study including 299 patients and 520 polyps, targeting suspected lesions during standard colonoscopy with indigo carmine 0.8% as a contrast, predicting the histology endoscopically, and finally obtaining mucosal biopsies. The endoscopic prediction of adenomatous polyps using chromoendoscopy had a sensitivity and specificity of 82%, but an accuracy of only 68%. In a single-examiner, large, prospective trial including 1,850 patients, Hurlstone et al57 performed a similar evaluation of the ability of chromoendoscopy to predict a lesions' final pathology; the sensitivity and specificity of chromoendoscopy were 98% and 92%, respectively.
Although not yet a suitable replacement for tissue biopsy, endoscopic prediction of histology using chromoendoscopy may yet prove to be an important tool in therapeutic endoscopy.
Chromoendoscopy for Evaluation of Mucosal Resection Margins
Hurlstone et al58 have described a technique using chromoendoscopy to assess adequacy endoscopic mucosal resection. In a large study of 602 patients, the authors reported a sensitivity of chromoendoscopy for predicting remnant tissue in the lateral and deep margins after mucosal resection of 79% and 80%, respectively. This technique may prove useful in the prediction of remnant tissue after endoscopic mucosal resection.
Narrow Band and Multiband Imaging
NBI is a technology designed to increase contrast on endoscopic examination without the time-consuming application of a surface dye. Olympus (Tokyo, Japan) has trademarked NBI technology: two main variations exist. In the United States, the system incorporates filters to limit the endoscopic light source to two wavelengths: 415 nm and 540 nm. A second system commercially available in Europe and Japan incorporates three discrete wavelengths: 415 nm, 445 nm, and 500 nm. The differences in these systems are largely related to differences in the image processors used in these geographic regions. Sometimes referred to as optical chromoendoscopy, NBI improves mucosal detail in two ways. First, it increases the relative contribution of surface structures to the final endoscopic image, as the shorter wavelength light does not penetrate deeply into the mucosa. Second, it highlights vascular structures by incorporating the specific wavelength that corresponds to the peak absorption spectrum for hemoglobin: 415 nm.59 Fuji Corporation (Saitama, Japan) has developed a multiband imaging technology to compete with NBI. Multiband imaging relies on a digital imaging processing technique that augments images taken during white light endoscopy, generating a virtual image that accentuates the contributions of specific wavelengths of light. To date, no published trials exist that compare the efficacy of NBI and multiband imaging.60
Narrow Band Increased Detection
Although the great majority of research in NBI has focused on Barrett's esophagus, several trials have studied the use of NBI in the colon. Three randomized controlled prospective clinical trials have examined the effect of pancolonic NBI on ADRs during screening colonoscopy. The first, conducted by Inoue et al, included 243 patients and reported a significantly higher number of adenomas detected, as well as a higher number of diminutive (<5 mm) adenomas detected in the patients examined with pancolonic NBI versus white light endoscopy.61 The results of this study fueled speculation that perhaps NBI colonography would provide a simpler, dyeless alternative to chromoendoscopy. Critics of the study noted that the number of NBI examinations was not distributed equally among endoscopists, raising the concern that endoscopy technique rather than NBI augmentation could explain the higher detection rates. A second trial by Adler et al included 401 patients and failed to show a higher detection rate.62 In the early phases of Adler's trial, preliminary data indicated a higher ADR with NBI. Over the course of the study, however, the ADR in the NBI group remained constant whereas the detection rate in the white light group improved. In the end, the difference in adenoma detection was not statistically significant between the two groups. The researchers further speculated that the increasing trend in white light detection rates may have been the result of a training effect that performing NBI colonography has on endoscopists. A third randomized controlled trial by Rex et al63 failed to show a significant difference in sensitivity between NBI and white light endoscopy. This trial included more than 434 patients and controlled for interobserver variations by using only one endoscopist. The authors found no difference between the NBI or white light examination groups in either the prevalence of adenomas or the total number of adenomas detected.
NBI Adenoma Detection in High-Risk Populations
Only one randomized controlled trial has evaluated adenoma detection using NBI colonoscopy in patients with IBD. Dekker et al64 conducted a prospective randomized crossover study comparing the ADR of white light colonoscopy to NBI colonoscopy in 42 patients with long-standing ulcerative colitis. The examinations were conducted at least 3 weeks apart. During the procedures, targeted biopsies were taken of suspicious tissue. In addition, random four-quadrant biopsies were taken during the conventional endoscopy. This trial found no significant difference in adenoma detection between the two examinations. The authors did qualify their results by noting that the NBI system used in the study was a prototype system and suffered from low light intensity.
NBI for Improved Prediction of Underlying Histology
Interest in perfecting optical biopsy technology has generated several trials aimed at determining the accuracy of NBI colonoscopy in predicting the histology of observed lesions.65,66,67,68,69,70 Several systems have been described, categorizing both mucosal pit patterns and vascular features of surface mucosa during narrow band endoscopy. However, no single system has been adopted in NBI colonoscopy the way that Kudo's pit pattern classification system has been in chromoendoscopy. In 2009, Van de Broek et al71 published a review of narrow-band colonoscopy studies. Using a random-effects pooled data analysis, they concluded that the overall sensitivity and specificity of NBI colonoscopy in correctly predicting neoplasia were 92% and 86%, respectively. In a separate analysis of trials of NBI in patients with long-standing ulcerative colitis, Van de Broek and colleagues concluded that the sensitivities and specificities were not as good, ranging from 75 to 80% and 81 to 84.2%, respectively.72,73
CONCLUSION
Colonoscopy continues to be the preferred procedure for colon cancer screening and has robust data to support its use in cancer surveillance. Despite this, several large, well-defined trials have identified its limitations. Significant strides have been made in developing ways to improve colonoscopy's effectiveness. Augmented endoscopy techniques, including chromoendoscopy and NBI, represent two of the best studied new techniques designed to improve the effectiveness of colonoscopy.
Both techniques are inexpensive, safe, and relatively easy to perform. According to the 2005 recommendations of the Consensus Conference of Colorectal Cancer Screening and Surveillance in IBD, chromoendoscopy should take an increasingly important role in surveillance of IBD. However, before this technique can be incorporated into routine clinical practice, work is needed to build consensus on optimal staining techniques and mucosal surface pattern analysis.
Several large, randomized controlled trials suggest that chromoendoscopy does not confer advantages to screening colonoscopy. The data are mixed with respect to surveillance examinations in high-risk populations; yet, several studies involving patients with IBD suggest that this technique could significantly improve disease surveillance. Chromoendoscopy may also hold promise as an adjunct in therapeutic endoscopy, though the experience of using chromoendoscopy to assess the adequacy of complete tissue resection is very limited.
NBI, by comparison, is a much newer technology, and available data evaluating NBI are far less extensive. Despite this, several clinical trials have examined a broad range of potential uses. Well-designed prospective randomized controlled trials have failed to identify a benefit of NBI in screening colonoscopy. Though fewer studies have examined NBI use in surveillance for high-risk populations or as an adjunct for therapeutic procedures, the available data do not demonstrate an advantage of this technique over standard colonoscopy.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun M J. Cancer statistics, 2009. CA Cancer J Clin. 2009;59(4):225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Winawer S J, Zauber A G, Ho M N, et al. The National Polyp Study Workgroup Prevention of colorectal cancer by colonoscopic polypectomy. N Engl J Med. 1993;329(27):1977–1981. doi: 10.1056/NEJM199312303292701. [DOI] [PubMed] [Google Scholar]
- 3.Rex D K, Johnson D A, Anderson J C, Schoenfeld P S, Burke C A, Inadomi J M, American College of Gastroenterology American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected] Am J Gastroenterol. 2009;104(3):739–750. doi: 10.1038/ajg.2009.104. Erratum in: Am J Gastroenterol 2009;104:1613. [DOI] [PubMed] [Google Scholar]
- 4.Chen S C, Rex D K. Endoscopist can be more powerful than age and male gender in predicting adenoma detection at colonoscopy. Am J Gastroenterol. 2007;102(4):856–861. doi: 10.1111/j.1572-0241.2006.01054.x. [DOI] [PubMed] [Google Scholar]
- 5.Barclay R L, Vicari J J, Doughty A S, Johanson J F, Greenlaw R L. Colonoscopic withdrawal times and adenoma detection during screening colonoscopy. N Engl J Med. 2006;355(24):2533–2541. doi: 10.1056/NEJMoa055498. [DOI] [PubMed] [Google Scholar]
- 6.Chen S C, Rex D K. Variable detection of nonadenomatous polyps by individual endoscopists at colonoscopy and correlation with adenoma detection. J Clin Gastroenterol. 2008;42(6):704–707. doi: 10.1097/MCG.0b013e31817236e5. [DOI] [PubMed] [Google Scholar]
- 7.Imperiale T F, Glowinski E A, Juliar B E, Azzouz F, Ransohoff D F. Variation in polyp detection rates at screening colonoscopy. Gastrointest Endosc. 2009;69(7):1288–1295. doi: 10.1016/j.gie.2007.11.043. [DOI] [PubMed] [Google Scholar]
- 8.Benson M E, Reichelderfer M, Said A, Gaumnitz E A, Pfau P R. Variation in colonoscopic technique and adenoma detection rates at an academic gastroenterology unit. Dig Dis Sci. 2010;55(1):166–171. doi: 10.1007/s10620-008-0703-2. [DOI] [PubMed] [Google Scholar]
- 9.Rex D K, Cutler C S, Lemmel G T, et al. Colonoscopic miss rates of adenomas determined by back-to-back colonoscopies. Gastroenterology. 1997;112(1):24–28. doi: 10.1016/s0016-5085(97)70214-2. [DOI] [PubMed] [Google Scholar]
- 10.Heresbach D, Barrioz T, Lapalus M G, et al. Miss rate for colorectal neoplastic polyps: a prospective multicenter study of back-to-back video colonoscopies. Endoscopy. 2008;40(4):284–290. doi: 10.1055/s-2007-995618. [DOI] [PubMed] [Google Scholar]
- 11.Rijn J C van, Reitsma J B, Stoker J, Bossuyt P M, Deventer S J van, Dekker E. Polyp miss rate determined by tandem colonoscopy: a systematic review. Am J Gastroenterol. 2006;101(2):343–350. doi: 10.1111/j.1572-0241.2006.00390.x. [DOI] [PubMed] [Google Scholar]
- 12.Stoffel E M, Turgeon D K, Stockwell D H, et al. Great Lakes-New England Clinical Epidemiology and Validation Center of the Early Detection Research Network. Missed adenomas during colonoscopic surveillance in individuals with Lynch syndrome (hereditary nonpolyposis colorectal cancer) Cancer Prev Res. 2008;1(6):470–475. doi: 10.1158/1940-6207.CAPR-08-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bressler B, Paszat L F, Vinden C, Li C, He J, Rabeneck L. Colonoscopic miss rates for right-sided colon cancer: a population-based analysis. Gastroenterology. 2004;127(2):452–456. doi: 10.1053/j.gastro.2004.05.032. [DOI] [PubMed] [Google Scholar]
- 14.Johnson C D, Chen M H, Toledano A Y, et al. Accuracy of CT colonography for detection of large adenomas and cancers. N Engl J Med. 2008;359(12):1207–1217. doi: 10.1056/NEJMoa0800996. Erratum in: N Engl J Med 2008;359:2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pickhardt P J, Choi J R, Hwang I, et al. Computed tomographic virtual colonoscopy to screen for colorectal neoplasia in asymptomatic adults. N Engl J Med. 2003;349(23):2191–2200. doi: 10.1056/NEJMoa031618. [DOI] [PubMed] [Google Scholar]
- 16.Barclay R L, Vicari J J, Doughty A S, Johanson J F, Greenlaw R L. Colonoscopic withdrawal times and adenoma detection during screening colonoscopy. N Engl J Med. 2006;355(24):2533–2541. doi: 10.1056/NEJMoa055498. [DOI] [PubMed] [Google Scholar]
- 17.Simmons D T, Harewood G C, Baron T H, et al. Impact of endoscopist withdrawal speed on polyp yield: implications for optimal colonoscopy withdrawal time. Aliment Pharmacol Ther. 2006;24(6):965–971. doi: 10.1111/j.1365-2036.2006.03080.x. [DOI] [PubMed] [Google Scholar]
- 18.Barclay R L, Vicari J J, Greenlaw R L. Effect of a time-dependent colonoscopic withdrawal protocol on adenoma detection during screening colonoscopy. Clin Gastroenterol Hepatol. 2008;6(10):1091–1098. doi: 10.1016/j.cgh.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 19.Abdul-Baki H, Hashash J G, Elhajj I I, et al. A randomized, controlled, double-blind trial of the adjunct use of tegaserod in whole-dose or split-dose polyethylene glycol electrolyte solution for colonoscopy preparation. Gastrointest Endosc. 2008;68(2):294–300, quiz 334, 336. doi: 10.1016/j.gie.2008.01.044. [DOI] [PubMed] [Google Scholar]
- 20.Park J S, Sohn C I, Hwang S J, et al. Quality and effect of single dose versus split dose of polyethylene glycol bowel preparation for early-morning colonoscopy. Endoscopy. 2007;39(7):616–619. doi: 10.1055/s-2007-966434. [DOI] [PubMed] [Google Scholar]
- 21.Aoun E, Abdul-Baki H, Azar C, et al. A randomized single-blind trial of split-dose PEG-electrolyte solution without dietary restriction compared with whole dose PEG-electrolyte solution with dietary restriction for colonoscopy preparation. Gastrointest Endosc. 2005;62(2):213–218. doi: 10.1016/s0016-5107(05)00371-8. [DOI] [PubMed] [Google Scholar]
- 22.El Sayed A M, Kanafani Z A, Mourad F H, et al. A randomized single-blind trial of whole versus split-dose polyethylene glycol-electrolyte solution for colonoscopy preparation. Gastrointest Endosc. 2003;58(1):36–40. doi: 10.1067/mge.2003.318. [DOI] [PubMed] [Google Scholar]
- 23.East J E, Stavrindis M, Thomas-Gibson S, Guenther T, Tekkis P P, Saunders B P. A comparative study of standard vs. high definition colonoscopy for adenoma and hyperplastic polyp detection with optimized withdrawal technique. Aliment Pharmacol Ther. 2008;28(6):768–776. doi: 10.1111/j.1365-2036.2008.03789.x. [DOI] [PubMed] [Google Scholar]
- 24.Rex D K, Chadalawada V, Helper D J. Wide angle colonoscopy with a prototype instrument: impact on miss rates and efficiency as determined by back-to-back colonoscopies. Am J Gastroenterol. 2003;98(9):2000–2005. doi: 10.1111/j.1572-0241.2003.07662.x. [DOI] [PubMed] [Google Scholar]
- 25.Sanaka M R, Deepinder F, Thota P N, Lopez R, Burke C A. Adenomas are detected more often in morning than in afternoon colonoscopy. Am J Gastroenterol. 2009;104(7):1659–1664, quiz 1665. doi: 10.1038/ajg.2009.249. [DOI] [PubMed] [Google Scholar]
- 26.Chan M Y, Cohen H, Spiegel B M. Fewer polyps detected by colonoscopy as the day progresses at a Veteran's Administration teaching hospital. Clin Gastroenterol Hepatol. 2009;7(1):1217–1223, quiz 1143. doi: 10.1016/j.cgh.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 27.Gorgun E, Church J. Flat adenomas of the large bowel: a single endoscopist study. Dis Colon Rectum. 2009;52(5):972–977. doi: 10.1007/DCR.0b013e3181a0aab4. [DOI] [PubMed] [Google Scholar]
- 28.Soetikno R M, Kaltenbach T, Rouse R V, et al. Prevalence of nonpolypoid (flat and depressed) colorectal neoplasms in asymptomatic and symptomatic adults. JAMA. 2008;299(9):1027–1035. doi: 10.1001/jama.299.9.1027. [DOI] [PubMed] [Google Scholar]
- 29.Kawai K, Ida K, Kohli Y. In: Maratka Z, Setka J, editor. Urgent Endoscopy of Digestive and Abdominal Diseases. Prague and Carlsbad: International Symposium of International Society of Gastrointestinal Endoscopy: Society of Gastroenterology and Nutrition 1971; Karger; 1971. On the dye scattering method for endoscopy, new field in gastrointestinal endoscopy.
- 30.Ida K, Kohli K, Shimamoto Y, Hashimoto Y, Kawai K. Endoscopical findings of fundic and pyloric gland area using dye scattering method. Endoscopy. 1973;5:21–26. [Google Scholar]
- 31.Tada M, Katoh S, Kohli Y, Kawai K. On the dye spraying method in colonofiberscopy. Endoscopy. 1977;8(2):70–74. doi: 10.1055/s-0028-1098379. [DOI] [PubMed] [Google Scholar]
- 32.Mitooka H, Fujimori T, Ohno S, et al. Chromoscopy of the colon using indigo carmine dye with electrolyte lavage solution. Gastrointest Endosc. 1992;38(3):373–374. doi: 10.1016/s0016-5107(92)70436-2. [DOI] [PubMed] [Google Scholar]
- 33.Dinis-Ribeiro M, da Costa-Pereira A, Lopes C, et al. Magnification chromoendoscopy for the diagnosis of gastric intestinal metaplasia and dysplasia. Gastrointest Endosc. 2003;57(4):498–504. doi: 10.1067/mge.2003.145. [DOI] [PubMed] [Google Scholar]
- 34.Meining A, Rösch T, Kiesslich R, Muders M, Sax F, Heldwein W. Inter- and intra-observer variability of magnification chromoendoscopy for detecting specialized intestinal metaplasia at the gastroesophageal junction. Endoscopy. 2004;36(2):160–164. doi: 10.1055/s-2004-814183. [DOI] [PubMed] [Google Scholar]
- 35.Huang Q, Fukami N, Kashida H, et al. Interobserver and intra-observer consistency in the endoscopic assessment of colonic pit patterns. Gastrointest Endosc. 2004;60(4):520–526. doi: 10.1016/s0016-5107(04)01880-2. [DOI] [PubMed] [Google Scholar]
- 36.Kudo S, Tamura S, Nakajima T, Yamano H, Kusaka H, Watanabe H. Diagnosis of colorectal tumorous lesions by magnifying endoscopy. Gastrointest Endosc. 1996;44(1):8–14. doi: 10.1016/s0016-5107(96)70222-5. [DOI] [PubMed] [Google Scholar]
- 37.Kumar S S, Ghosh A, Devasagayam T P, Chauhan P S. Effect of vanillin on methylene blue plus light-induced single-strand breaks in plasmid pBR322 DNA. Mutat Res. 2000;469(2):207–214. doi: 10.1016/s1383-5718(00)00074-7. [DOI] [PubMed] [Google Scholar]
- 38.Epe B, Hegler J, Wild D. Singlet oxygen as an ultimately reactive species in Salmonella typhimurium DNA damage induced by methylene blue/visible light. Carcinogenesis. 1989;10(11):2019–2024. doi: 10.1093/carcin/10.11.2019. [DOI] [PubMed] [Google Scholar]
- 39.Boiteux S, Gajewski E, Laval J, Dizdaroglu M. Substrate specificity of the Escherichia coli Fpg protein (formamidopyrimidine-DNA glycosylase): excision of purine lesions in DNA produced by ionizing radiation or photosensitization. Biochemistry. 1992;31(1):106–110. doi: 10.1021/bi00116a016. [DOI] [PubMed] [Google Scholar]
- 40.Olliver J R, Wild C P, Sahay P, Dexter S, Hardie L J. Chromoendoscopy with methylene blue and associated DNA damage in Barrett's oesophagus. Lancet. 2003;362(9381):373–374. doi: 10.1016/s0140-6736(03)14026-3. [DOI] [PubMed] [Google Scholar]
- 41.Kiesslich R, Burg J, Kaina B, Galle P R, Neurath M F. Safety and efficacy of methylene blue-aided chromoendoscopy in ulcerative colitis: a prospective pilot study upon previous chromoendoscopies. (abstract) Gastrointest Endosc. 2004;59:AB97. [Google Scholar]
- 42.Dinis-Ribeiro M, Moreira-Dias L. There is no clinical evidence of consequences after methylene blue chromoendoscopy. Gastrointest Endosc. 2008;67(7):1209. doi: 10.1016/j.gie.2007.12.043. [DOI] [PubMed] [Google Scholar]
- 43.Davies J, Burke D, Olliver J R, Hardie L J, Wild C P, Routledge M N. Methylene blue but not indigo carmine causes DNA damage to colonocytes in vitro and in vivo at concentrations used in clinical chromoendoscopy. Gut. 2007;56(1):155–156. doi: 10.1136/gut.2006.107300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kiesslich R, von Bergh M, Hahn M, Hermann G, Jung M. Chromoendoscopy with indigocarmine improves the detection of adenomatous and nonadenomatous lesions in the colon. Endoscopy. 2001;33(12):1001–1006. doi: 10.1055/s-2001-18932. [DOI] [PubMed] [Google Scholar]
- 45.Brooker J C, Saunders B P, Shah S G, et al. Total colonic dye-spray increases the detection of diminutive adenomas during routine colonoscopy: a randomized controlled trial. Gastrointest Endosc. 2002;56(3):333–338. doi: 10.1016/s0016-5107(02)70034-5. [DOI] [PubMed] [Google Scholar]
- 46.Hurlstone D P, Cross S S, Slater R, Sanders D S, Brown S. Detecting diminutive colorectal lesions at colonoscopy: a randomised controlled trial of pan-colonic versus targeted chromoscopy. Gut. 2004;53(3):376–380. doi: 10.1136/gut.2003.029868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Le Rhun M, Coron E, Parlier D, et al. High resolution colonoscopy with chromoscopy versus standard colonoscopy for the detection of colonic neoplasia: a randomized study. Clin Gastroenterol Hepatol. 2006;4(3):349–354. doi: 10.1016/j.cgh.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 48.Matsumoto T, Nakamura S, Jo Y, Yao T, Iida M. Chromoscopy might improve diagnostic accuracy in cancer surveillance for ulcerative colitis. Am J Gastroenterol. 2003;98(8):1827–1833. doi: 10.1111/j.1572-0241.2003.07580.x. [DOI] [PubMed] [Google Scholar]
- 49.Kiesslich R, Fritsch J, Holtmann M, et al. Methylene blue-aided chromoendoscopy for the detection of intraepithelial neoplasia and colon cancer in ulcerative colitis. Gastroenterology. 2003;124(4):880–888. doi: 10.1053/gast.2003.50146. [DOI] [PubMed] [Google Scholar]
- 50.Rutter M D, Saunders B P, Schofield G, Forbes A, Price A B, Talbot I C. Pancolonic indigo carmine dye spraying for the detection of dysplasia in ulcerative colitis. Gut. 2004;53(2):256–260. doi: 10.1136/gut.2003.016386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marion J F, Waye J D, Present D H, et al. Chromoendoscopy Study Group at Mount Sinai School of Medicine Chromoendoscopy-targeted biopsies are superior to standard colonoscopic surveillance for detecting dysplasia in inflammatory bowel disease patients: a prospective endoscopic trial. Am J Gastroenterol. 2008;103(9):2342–2349. doi: 10.1111/j.1572-0241.2008.01934.x. [DOI] [PubMed] [Google Scholar]
- 52.Hurlstone D P, Karajeh M, Cross S S, et al. The role of high-magnification-chromoscopic colonoscopy in hereditary nonpolyposis colorectal cancer screening: a prospective “back-to-back” endoscopic study. Am J Gastroenterol. 2005;100(10):2167–2173. doi: 10.1111/j.1572-0241.2005.41481.x. [DOI] [PubMed] [Google Scholar]
- 53.Itzkowitz S H, Present D H, Crohn's and Colitis Foundation of America Colon Cancer in IBD Study Group Consensus conference: colorectal cancer screening and surveillance in inflammatory bowel disease. Inflamm Bowel Dis. 2005;11(3):314–321. doi: 10.1097/01.mib.0000160811.76729.d5. [DOI] [PubMed] [Google Scholar]
- 54.Matsumoto T, Esaki M, Fujisawa R, Nakamura S, Yao T, Iida M. Chromoendoscopy, narrow-band imaging colonoscopy, and autofluorescence colonoscopy for detection of diminutive colorectal neoplasia in familial adenomatous polyposis. Dis Colon Rectum. 2009;52(6):1160–1165. doi: 10.1007/DCR.0b013e31819ef6fe. [DOI] [PubMed] [Google Scholar]
- 55.Tung S Y, Wu C S, Su M Y. Magnifying colonoscopy in differentiating neoplastic from nonneoplastic colorectal lesions. Am J Gastroenterol. 2001;96(9):2628–2632. doi: 10.1111/j.1572-0241.2001.04120.x. [DOI] [PubMed] [Google Scholar]
- 56.Eisen G M, Kim C Y, Fleischer D E, et al. High-resolution chromoendoscopy for classifying colonic polyps: a multicenter study. Gastrointest Endosc. 2002;55(6):687–694. doi: 10.1067/mge.2002.123619. [DOI] [PubMed] [Google Scholar]
- 57.Hurlstone D P, Cross S S, Adam I, et al. Efficacy of high magnification chromoscopic colonoscopy for the diagnosis of neoplasia in flat and depressed lesions of the colorectum: a prospective analysis. Gut. 2004;53(2):284–290. doi: 10.1136/gut.2003.027623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hurlstone D P, Cross S S, Brown S, Sanders D S, Lobo A J. A prospective evaluation of high-magnification chromoscopic colonoscopy in predicting completeness of EMR. Gastrointest Endosc. 2004;59(6):642–650. doi: 10.1016/s0016-5107(04)00156-7. [DOI] [PubMed] [Google Scholar]
- 59.Gono K, Obi T, Yamaguchi M, et al. Appearance of enhanced tissue features in narrow-band endoscopic imaging. J Biomed Opt. 2004;9(3):568–577. doi: 10.1117/1.1695563. [DOI] [PubMed] [Google Scholar]
- 60.Song L M, Adler D G, Conway J D, et al. ASGE TECHNOLOGY COMMITTEE Narrow band imaging and multiband imaging. Gastrointest Endosc. 2008;67(4):581–589. doi: 10.1016/j.gie.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 61.Inoue T, Murano M, Murano N, et al. Comparative study of conventional colonoscopy and pan-colonic narrow-band imaging system in the detection of neoplastic colonic polyps: a randomized, controlled trial. J Gastroenterol. 2008;43(1):45–50. doi: 10.1007/s00535-007-2125-x. [DOI] [PubMed] [Google Scholar]
- 62.Adler A, Pohl H, Papanikolaou I S, et al. A prospective randomised study on narrow-band imaging versus conventional colonoscopy for adenoma detection: does narrow-band imaging induce a learning effect? Gut. 2008;57(1):59–64. doi: 10.1136/gut.2007.123539. [DOI] [PubMed] [Google Scholar]
- 63.Rex D K, Helbig C C. High yields of small and flat adenomas with high-definition colonoscopes using either white light or narrow band imaging. Gastroenterology. 2007;133(1):42–47. doi: 10.1053/j.gastro.2007.04.029. [DOI] [PubMed] [Google Scholar]
- 64.Dekker E, den Broek F J van, Reitsma J B, et al. Narrow-band imaging compared with conventional colonoscopy for the detection of dysplasia in patients with longstanding ulcerative colitis. Endoscopy. 2007;39(3):216–221. doi: 10.1055/s-2007-966214. [DOI] [PubMed] [Google Scholar]
- 65.Su M Y, Hsu C M, Ho Y P, Chen P C, Lin C J, Chiu C T. Comparative study of conventional colonoscopy, chromoendoscopy, and narrow-band imaging systems in differential diagnosis of neoplastic and nonneoplastic colonic polyps. Am J Gastroenterol. 2006;101(12):2711–2716. doi: 10.1111/j.1572-0241.2006.00932.x. [DOI] [PubMed] [Google Scholar]
- 66.Chiu H M, Chang C Y, Chen C C, et al. A prospective comparative study of narrow-band imaging, chromoendoscopy, and conventional colonoscopy in the diagnosis of colorectal neoplasia. Gut. 2007;56(3):373–379. doi: 10.1136/gut.2006.099614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.East J E, Suzuki N, Saunders B P. Comparison of magnified pit pattern interpretation with narrow band imaging versus chromoendoscopy for diminutive colonic polyps: a pilot study. Gastrointest Endosc. 2007;66(2):310–316. doi: 10.1016/j.gie.2007.02.026. [DOI] [PubMed] [Google Scholar]
- 68.Rastogi A, Bansal A, Wani S, et al. Narrow-band imaging colonoscopy—a pilot feasibility study for the detection of polyps and correlation of surface patterns with polyp histologic diagnosis. Gastrointest Endosc. 2008;67(2):280–286. doi: 10.1016/j.gie.2007.07.036. [DOI] [PubMed] [Google Scholar]
- 69.Hirata M, Tanaka S, Oka S, et al. Evaluation of microvessels in colorectal tumors by narrow band imaging magnification. Gastrointest Endosc. 2007;66(5):945–952. doi: 10.1016/j.gie.2007.05.053. [DOI] [PubMed] [Google Scholar]
- 70.Machida H, Sano Y, Hamamoto Y, et al. Narrow-band imaging in the diagnosis of colorectal mucosal lesions: a pilot study. Endoscopy. 2004;36(12):1094–1098. doi: 10.1055/s-2004-826040. [DOI] [PubMed] [Google Scholar]
- 71.den Broek F J van, Reitsma J B, Curvers W L, Fockens P, Dekker E. Systematic review of narrow-band imaging for the detection and differentiation of neoplastic and nonneoplastic lesions in the colon (with videos) Gastrointest Endosc. 2009;69(1):124–135. doi: 10.1016/j.gie.2008.09.040. [DOI] [PubMed] [Google Scholar]
- 72.den Broek F J van, Fockens P, Eeden S van, et al. Endoscopic tri-modal imaging for surveillance in ulcerative colitis: randomised comparison of high-resolution endoscopy and autofluorescence imaging for neoplasia detection; and evaluation of narrow-band imaging for classification of lesions. Gut. 2008;57(8):1083–1089. doi: 10.1136/gut.2007.144097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Matsumoto T, Kudo T, Jo Y, Esaki M, Yao T, Iida M. Magnifying colonoscopy with narrow band imaging system for the diagnosis of dysplasia in ulcerative colitis: a pilot study. Gastrointest Endosc. 2007;66(5):957–965. doi: 10.1016/j.gie.2007.04.014. [DOI] [PubMed] [Google Scholar]