Abstract
The phylogeny within the order Choreotrichida is reconstructed using (i) morphologic, ontogenetic, and ultrastructural evidence for the cladistic approach and (ii) the small subunit ribosomal RNA (SSrRNA) gene sequences, including the new sequence of Rimostrombidium lacustris. The morphologic cladograms and the gene trees converge rather well for the Choreotrichida, demonstrating that hyaline and agglutinated loricae do not characterize distinct lineages, i.e., both lorica types can be associated with the most highly developed ciliary pattern. The position of Rimostrombidium lacustris within the family Strobilidiidae is corroborated by the genealogical analyses. The diagnosis of the genus Tintinnidium is improved, adding cytological features, and the genus is divided into two subgenera based on the structure of the somatic kineties. The diagnosis of the family Lohmanniellidae and the genus Lohmanniella are improved, and Rimostrombidium glacicolum Petz, Song and Wilbert, 1995 is affiliated.
Keywords: Choreotrichida, Ciliary patterns, Morphology, Phylogeny, Taxonomy, SSrRNA
Introduction
Choreotrichid ciliates reveal a high diversity, especially in the marine microzooplankton, comprising four families with ~170 aloricate species and ~15 families with 1200 tintinnids. While the aloricate Choreotrichida are comparatively well-investigated, knowledge about the tintinnids is largely restricted to lorica features which, however, show a considerable infraspecific variability due to environmental conditions and the life cycle (Bernatzky et al. 1981; Biernacka 1952; Burkovsky 1973; Davis 1978, 1981; Gold and Morales 1975; Hofker 1931; Laval-Peuto 1981).
Kofoid and Campbell (1939) based a reconstruction of tintinnid evolution on lorica structures, concluding that species with an agglutinated lorica are ancestral and those with a hyaline lorica are derived. Likewise, Tappan and Loeblich (1968) regarded species with an agglutinated lorica and a ventral kinety and those with a hyaline lorica and without ventral kinety as distinct lineages (superfamilies). In contrast to these studies, Laval-Peuto and Brownlee (1986) concentrated on features of the ciliature, using data from the literature and Brownlee’s Master’s Thesis. They considered the density and length of the somatic kineties and the absence or presence of specialized kineties as well as the orientation of the adoral zone of membranelles and established four tintinnid categories without designating an evolutionary sequence. Similarly, Pierce (1997) included literature data and his own findings in a reconstruction of tintinnid evolution. He created three categories, employing the structure of the somatic kineties, ventral ciliary differentiations (ventral kinety, ventral organelles), and the orientation of the adoral zone of membranelles, but also did not suggest an evolutionary sequence. Pierce (1997) only concluded that the genera Tintinnopsis and Stenosemella are more derived than the genus Eutintinnus. In contrast to the preceding studies, our cladistic analyses comprise the whole order Choreotrichida (superclass Spirotricha, class Oligotrichea, subclass Oligotrichia). For that purpose, the descriptions of 57 protargol-impregnated species are utilized, including the ciliary patterns of four reinvestigated tintinnids, as well as ontogenetic and ultrastructural studies.
Molecular phylogenies inferred from the small subunit ribosomal RNA (SSrRNA) genes confirmed the monophyly of the order Choreotrichida and the suborder Tintinnina (Agatha et al. 2004, 2005; Kim et al. 2005; Modeo et al. 2003; Snoeyenbos-West et al. 2002; Strüder-Kypke and Lynn 2003). They also provided a first evolutionary model for the tintinnid taxa, contradicting earlier suggestions that species with hyaline loricae generally derived from species with agglutinated loricae. According to the molecular phylogenies, the genus Tintinnidium branches basally within the tintinnids, followed by Eutintinnus, Favella, and a dense group consisting of Metacylis, Tintinnopsis, Rhabdonella, and Codonellopsis. The aloricate choreotrichids do not form a monophyletic cluster since the genus Strombidinopsis branches basally to a clade comprising both the tintinnids and the remaining aloricate choreotrichids (Parastrombidinopsis, Strobilidium, and Pelagostrobilidium).
Our study adds the gene sequence of a further strobilidiid genus, viz., Rimostrombidium, and compares the phylogenetic trees inferred from morphological and molecular data.
Materials and methods
Acquisition of morphological data
In the present paper, “morphological” evidence refers to all observable characters in the whole organism, as distinct from molecular characters. The classification follows Agatha (2004a).
The current revisions of the suborder Strobilidiina by Maeda (1986) and the suborder Tintinnina by Kofoid and Campbell (1929, 1939) were exclusively based on live or preserved material and thus do not provide details of the ciliary pattern. Accordingly, only the 76 recent species descriptions and redescriptions involving the application of silver impregnation techniques were used for the reconstruction of evolution among the Choreotrichida (Table 1). Additionally, detailed live observations and nuclear stains, e.g., by Biernacka (1952), Brandt (1906, 1907), Campbell (1926, 1927), Coats and Heinbokel (1982), Daday (1887), Entz (1884, 1885, 1909a, b, 1929, 1937), Fauré-Fremiet (1908, 1924), Haeckel (1873), Hofker (1931), Jörgensen (1927),Laackmann (1906), Merkle (1909), Schweyer (1909), and Sterki (1879), were considered. On the other hand, the data on the tintinnid Parafavella denticulata were excluded, as Hedin (1976a) probably confused Strombidinopsis specimens with P. denticulata cells that had abandoned their loricae. This assumption is corroborated by the PhD Thesis of R.W. Pierce (1996, Univ. Rhode Island, USA): the ciliary pattern of the two Parafavella species he studied differs from that shown by Hedin (1976a).
Table 1.
Choreotrichid species studied after silver impregnation
| Species | Reference |
|---|---|
| Suborder Strobilidiina Jankowski, 1980 | |
| Family Leegaardiellidae Lynn and Montagnes, 1988 | |
| Leegaardiella Lynn and Montagnes, 1988 | |
| L. elbraechteri Petz et al., 1995 | [[27]] |
| L. ovalis Lynn and Montagnes, 1988 | [[21]] |
| L. sol Lynn and Montagnes, 1988 | [[21]] |
| Family Lohmanniellidae Montagnes and Lynn, 1991 | |
| Lohmanniella Leegaard, 1915 | |
| L. glacicola (Petz et al., 1995) nov. comb. | [[27]] |
| L. oviformis Leegaard, 1915 | [[1],[21]] |
| Family Strobilidiidae Kahl in Doflein and Reichenow, 1929 | |
| Pelagostrobilidium Petz et al., 1995 | |
| P. epacrum (Lynn and Montagnes, 1988) Agatha et al., 2005 | [[21]] |
| P. neptuni (Montagnes and Taylor, 1994) Petz et al., 1995 | [[5],[25],[27]] |
| P. simile Song and Bradbury, 1998 | [[31]] |
| P. spirale (Leegaard, 1915) Petz et al., 1995 | [[21]] |
| Rimostrombidium Jankowski, 1978 | |
| R. armeniensis (Zharikov, 1987) Foissner et al., 1999 | [[35]] |
| R. brachykinetum Krainer, 1995 | [[16],[19]] |
| R. caudatum (Kahl, 1932) Agatha and Riedel-Lorjé, 1998 | [[3],[20]] |
| R. conicum (Kahl, 1932) Petz and Foissner, 1992 | [[3]] |
| R. humile (Penard, 1922) Petz and Foissner, 1992 | [[15],[16],[19]] |
| R. hyalinum (Mirabdullaev, 1985) Petz and Foissner, 1992 | [[16],[24]] |
| R. lacustris (Foissner et al., 1988) Petz and Foissner, 1992 | [[14],[16],[19]] |
| R. multinucleatum (Lynn and Montagnes, 1988) Petz and Foissner, 1992 | [[21]] |
| R. orientale Song and Bradbury, 1998 | [[31]] |
| R. sphaericum (Lynn and Montagnes, 1988) Petz and Foissner, 1992 | [[21]] |
| R. undinum (Martin and Montagnes, 1993) Petz et al., 1995 | [[23]] |
| R. veniliae (Montagnes and Taylor, 1994) Petz et al., 1995 | [[3],[25]] |
| Strobilidium Schewiakoff, 1892 | |
| S. caudatum (Fromentel, 1876) Foissner, 1987 | [[11],[26]] |
| Family Strombidinopsidae Small and Lynn, 1985 | |
| Strombidinopsis Kent, 1881 | |
| S. acuminata Fauré-Fremiet, 1924 | [[10],[22],[30]] |
| S. azerbaijanica Alekperov and Asadullayeva, 1997 | [[6]] |
| S. batos Lynn et al., 1991 | [[22]] |
| S. cercionis Lynn et al., 1991 | [[22]] |
| S. chilorhax Lynn et al., 1991 | [[22]] |
| S. elegans Song and Bradbury, 1998 | [[31]] |
| S. elongata Song and Bradbury, 1998 | [[31]] |
| S. grandis Xu and Bai, 1998 | [[34]] |
| S. jeokjo Jeong et al., 2004 | [[17]] |
| S. minima (Gruber, 1884) Lynn et al., 1991 | [[2],[20],[31]] |
| S. multiauris Montagnes and Taylor, 1994 | [[25]] |
| S. sphaira Lynn et al., 1991 | [[22]] |
| S. spinifera (Leegaard, 1915) Lynn et al., 1991 | [[10],[22]] |
| Parastrombidinopsis Kim et al., 2005 | |
| P. shimi Kim et al., 2005 | [[18]] |
| Suborder Tintinnina Kofoid and Campbell, 1929 | |
| Family Tintinnidiidae Kofoid and Campbell, 1929 | |
| Tintinnidium Kent, 1881 | |
| T. fluviatile (Stein, 1863) Kent, 1881; (A) | [[13]] |
| T. pusillum Entz, 1909; (A) | [[13]] |
| T. semiciliatum (Sterki, 1879) Kent, 1881; (A) | [[7],[32]] |
| Membranicola Foissner et al., 1999 | |
| M. tamari Foissner et al., 1999; (B) | [[16]] |
| Family Nolaclusiliidae Sniezek et al., 1991 | |
| Nolaclusilis Snyder and Brownlee, 1991 | |
| N. bicornis Snyder and Brownlee, 1991; (E) | [[29]] |
| N. hudsonicus Sniezek et al., 1991; (E) | [[28]] |
| Family Codonellidae Kent, 1881 | |
| Tintinnopsis Stein, 1867 | |
| T. brasiliensis Kofoid and Campbell, 1929; (C) | [[8]] |
| T. campanula (Ehrenberg, 1840) Daday, 1887; (C) | [[1]] |
| T. cylindrata Kofoid and Campbell, 1929; (A) | [[13]] |
| T. cylindrica Daday, 1887; (C) | [[4]] |
| T. fimbriata Meunier, 1919; (C) | [[1]] |
| T. tubulosoides Meunier, 1910; (C) | [[1]] |
| Codonella Haeckel, 1873 | |
| C. cratera (Leidy, 1877) Imhof, 1885; (C) | [[13]] |
| Family Codonellopsidae Kofoid and Campbell, 1929 | |
| Codonellopsis Jörgensen, 1924 | |
| C. glacialis (Laackmann, 1907) Kofoid and Campbell, 1929; (D) | [[27]] |
| Stenosemella Jörgensen, 1924 | |
| S. lacustris Foissner and O’Donoghue, 1990; (C) | [[12]] |
| S. nivalis (Meunier, 1910) Kofoid and Campbell, 1929; (D) | [[1]] |
| Family Ptychocylididae Kofoid and Campbell, 1929 | |
| Cymatocylis Laackmann, 1910 | |
| C. calyciformis (Laackmann, 1907) Laackmann, 1910; (E) | [[27]] |
| C. affinis/convallaria Laackmann, 1910; (E) | [[27],[33]] |
| Family Tintinnidae Claparède and Lachmann, 1859 | |
| Eutintinnus Kofoid and Campbell, 1939 | |
| E. angustatus (Daday, 1887) Kofoid and Campbell, 1939; (E) | [[9]] |
| E. pectinis (Kofoid and Campbell, 1929) Kofoid and Campbell, 1939; (E) | [[9]] |
| E. tenuis (Kofoid and Campbell, 1929) Kofoid and Campbell, 1939; (E) | [[9]] |
Five lorica types are distinguished: (A) – agglutinated and flexible; (B) – agglutinated, flexible, and with subterminal membrane; (C) – agglutinated and stiff; (D) – with hyaline collar and agglutinated bowl; (E) – entirely hyaline.
observations by SA
Descriptions of choreotrichid ontogenesis were available for Codonella (Entz 1885, 1909b; Petz and Foissner 1993), Codonellopsis, Cymatocylis (Petz et al. 1995), Eutintinnus (Coats and Heinbokel 1982), Favella (Entz 1909b; Laval-Peuto 1981, 1994; Schweyer 1909), Leegaardiella (Petz et al. 1995), Lohmanniella (SA own observ.), Pelagostrobilidium (Agatha et al. 2005), Rimostrombidium (Agatha and Riedel-Lorjé 1998; Foissner et al. 1991, 1999; Krainer 1995; Song and Bradbury 1998), Stenosemella (SA own observ.; Hofker 1931), Strobilidium (Deroux 1974; Kormos and Kormos 1958; Petz and Foissner 1992), Strombidinopsis (Agatha 2003b; Dale and Lynn 1998), Tintinnidium (Entz 1885, 1909b; Petz and Foissner 1993), Tintinnopsis (Agatha and Riedel-Lorjé 2006; Biernacka 1952; Brownlee 1983; Entz 1909b; Hofker 1931; Petz and Foissner 1993), and Tintinnus (Fauré-Fremiet 1908).
Scanning and/or transmission electron microscopic studies on the cell morphology were conducted on Climacocylis (Laval-Peuto and Barria de Cao 1987), Codonaria (Laval 1971; Laval-Peuto 1994; Laval-Peuto and Barria de Cao 1987), Codonella (Foissner et al. 1999; Laval-Peuto and Barria de Cao 1987), Codonellopsis (Laval 1971; Laval-Peuto and Barria de Cao 1987), Cymatocylis (Wasik and Mikołajczyk 1992, 1994), Cyttarocylis (Laval 1971; Laval-Peuto 1975, 1976, 1994; Laval-Peuto and Barria de Cao 1987; Laval-Peuto and Brownlee 1986), Dictyocysta (Laval-Peuto and Barria de Cao 1987), Favella (Hedin 1975), Membranicola (Foissner et al. 1999), Parafavella (Hedin 1975; Sokolova and Gerassimova 1984), Pelagostrobilidium (Agatha et al. 2005; Montagnes and Taylor 1994), Petalotricha (Laval 1971, 1972; Laval-Peuto 1994), Proplectella (Laval 1971), Ptychocylis (Hedin 1976b), Rhabdonella (Laval-Peuto and Barria de Cao 1987), Rimostrombidium (Foissner et al. 1999; Grim 1987; Montagnes and Taylor 1994), Stenosemella (SA own observ.; Capriulo et al. 1986; Laval-Peuto and Barria de Cao 1987), Strobilidium (Foissner et al. 1991; Petz and Foissner 1992), Strombidinopsis (Agatha 2003b; Montagnes and Taylor 1994), Tintinnidium (Petz and Foissner 1993), Tintinnopsis (Capriulo et al. 1986; Foissner et al. 1999; Gold 1979; Laval-Peuto et al. 1979), Undella, and Xystonella (Laval 1971; Laval-Peuto 1994; Laval-Peuto and Barria de Cao 1987). It seemed reasonable to assign these scattered investigations of the ontogenesis and ultrastructure to the closest relatives in the cladistic analyses (see characters 21, 22, 24–30 and Table 3, below).
Table 3.
Distribution of character states among the taxa cladistically analysed with the computer programs (Fig. 5)
| 5 | 10 | 15 | 20 | 25 | 30 | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stichotrichida | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Halteria | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 |
| Pelagohalteria | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 |
| Meseres | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 |
| Oligotrichida | 1 | 1 | 0 | 2 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 2 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 |
| Strombidinopsis | 1 | 1 | 1 | 2 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 1 | 1 | 0 | 1 | 1 |
| Parastrombidinopsis | 1 | 1 | 2 | 2 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 1 | 1 | 0 | 1 | 1 |
|
Leegaardiella elbraechteri |
1 | 1 | 1 | 2 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 1 | 1 | 0 | 1 | 1 |
|
Leegaardiella ovalis |
1 | 1 | 1 | 2 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 2 | 1 | 1 | 0 | 1 | 1 |
|
Leegaardiella sol |
1 | 1 | 1 | 2 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 1 | 1 | 0 | 1 | 1 |
| Lohmanniella | 1 | 1 | 1 | 2 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 2 | 1 | 1 | 0 | 1 | 1 |
| Strobilidium | 1 | 1 | 1 | 2 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 4 | 1 | 2 | 1 | 1 | 0 | 2 | 0 | 0 | 0 | 1 | 2 | 1 | 1 | 0 | 1 | 1 |
| Pelagostrobilidium | 1 | 1 | 1 | 2 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 4 | 1 | 1 | 1 | 1 | 0 | 2 | 0 | 0 | 0 | 1 | 2 | 1 | 1 | 0 | 1 | 1 |
| Rimostrombidium | 1 | 1 | 1 | 2 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 4 | 1 | 0 | 1 | 1 | 0 | 2 | 0 | 0 | 0 | 1 | 2 | 1 | 1 | 0 | 1 | 1 |
|
Tintinnidium semiciliatum |
1 | 1 | 1 | 2 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 3 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 0 | 1 | 1 |
|
Tintinnidium fluviatile |
1 | 1 | 1 | 2 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 0 | 1 | 1 |
|
Tintinnidium pusillum |
1 | 1 | 1 | 2 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 0 | 1 | 1 |
|
Tintinnopsis cylindrata |
1 | 1 | 1 | 2 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 4 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 0 | 1 | 1 |
| Membranicola | 1 | 1 | 1 | 2 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 2 | 1 | 2 | 1 | 1 | 0 | 1 | 1 |
| Nolaclusilis | 1 | 1 | 1 | 2 | 0 | 0 | 1 | 1 | 1 | 2 | 0 | 0 | 0 | 3 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 5 | 1 | 2 | 1 | 1 | 0 | 1 | 1 |
| Eutintinnus | 1 | 1 | 1 | 2 | 0 | 0 | 1 | 1 | 1 | 2 | 1 | 0 | 0 | 3 | 0 | 0 | 1 | 0 | 0 | 3 | 1 | 1 | 5 | 1 | 2 | 1 | 1 | 0 | 1 | 1 |
|
Tintinnopsis brasiliensis |
1 | 1 | 1 | 2 | 0 | 0 | 1 | 1 | 1 | 2 | 2 | 0 | 1 | 3 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 3 | 1 | 2 | 1 | 1 | 0 | 1 | 1 |
| Codonella | 1 | 1 | 1 | 2 | 0 | 0 | 1 | 1 | 1 | 2 | 2 | 1 | 1 | 3 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 3 | 1 | 2 | 1 | 1 | 0 | 1 | 1 |
| Codonellopsis | 1 | 1 | 1 | 2 | 0 | 0 | 1 | 1 | 1 | 2 | 2 | 1 | 1 | 3 | 0 | 0 | 1 | 0 | 0 | 3 | 1 | 1 | 4 | 1 | 2 | 1 | 1 | 0 | 1 | 1 |
| Cymatocylis | 1 | 1 | 1 | 2 | 0 | 0 | 1 | 1 | 1 | 2 | 2 | 1 | 1 | 3 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 5 | 1 | 2 | 1 | 1 | 0 | 1 | 1 |
|
other Tintinnopsis species |
1 | 1 | 1 | 2 | 0 | 0 | 1 | 1 | 1 | 2 | 2 | 1 | 1 | 3 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 3 | 1 | 2 | 1 | 1 | 0 | 1 | 1 |
|
Stenosemella lacustris |
1 | 1 | 1 | 2 | 0 | 0 | 1 | 1 | 1 | 2 | 2 | 1 | 1 | 3 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 3 | 1 | 2 | 1 | 1 | 0 | 1 | 1 |
|
Stenosemella nivalis |
1 | 1 | 1 | 2 | 0 | 0 | 1 | 1 | 1 | 2 | 2 | 1 | 1 | 3 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 4 | 1 | 2 | 1 | 1 | 0 | 1 | 1 |
For the present study, the neotype slides of Lohmanniella oviformis kindly provided by D.H. Lynn (University of Guelph, Canada) were reinvestigated (Table 1). The specimens revealed a dikinetidal structure of the somatic kineties; therefore, the diagnoses of the family Lohmanniellidae and the genus Lohmanniella are emended and Rimostrombidium glacicolum Petz, Song and Wilbert (1995) is affiliated (see ‘Taxonomic Implications’, below). Additionally, the ciliary patterns of four tintinnid species were reinvestigated in this study (Table 1), employing the protargol impregnation method of Song and Wilbert (1995). The specimens were collected in the Irish Sea off the Isle of Man (Stenosemella nivalis), in the North Sea off the Island of Sylt (Tintinnopsis campanula, Tintinnopsis fimbriata), and in the mixo-polyhaline basins of the polder Speicherkoog Dithmarschen at the North Sea coast of Germany (Tintinnopsis tubulosoides). The somatic ciliature of all these species consists of a right, left, and lateral ciliary field as well as a ventral, dorsal, and posterior kinety.
The kinetal maps depict the morphostatic ciliary pattern of protargol-impregnated specimens in two dimensions, that is, the cortex is drawn as cut longitudinally on the dorsal side. Horizontal bars symbolize the collar membranelles (Choi et al. 1992; Foissner and Wilbert 1979). The terminology follows Agatha and Riedel-Lorjé (2006).
Cladistic analyses
Phylogenetic relationships among the Oligotrichea, with emphasis on the order Choreotrichida, were elucidated by applying Hennig’s argumentation method (Table 2, below; Hennig 1966) as well as the computer programs PAUP* ver. 4.0b10 (Swofford 2002) and Hennig86. The order Stichotrichida (superclass Spirotricha, class Hypotrichea) was used as outgroup. The computed parsimony trees were based on ordered (Wagner/Farris optimization) states in the characters 3, 4, 11, 14, and 25 (see Tables 2 and 3, below). With Hennig86, the most parsimonious tree was found by an exhaustive analysis of the equally weighted characters, and a strict consensus tree was calculated. Using the computer program PAUP*, the 50% majority-rule consensus tree was found by the heuristic analyses of equally weighted or successively weighted characters and optimized by the application of the accelerated transformation (ACCTRAN). The bootstrap method with heuristic search included 1000 replicates and used the establishment of the starting tree/s by stepwise addition, a random addition of further taxa, and the tree-bisection-reconnection (TBR) branch-swapping algorithm. One tree was held at each step during stepwise addition. The resulting tree was imported into TreeView (http://taxonomy.zoology.gla.ac.uk/rod/treeview.html).
Table 2.
Character states and coding used for the construction of the traditional cladogram shown in Fig. 4
| Character states | |
|---|---|
| Apomorphy | Plesiomorphy |
| 1 Cell shape usually globular to obconical (coded 1) | Cell shape usually distinctly dorsoventrally flattened (coded 0) |
| 2 Adoral zone of membranelles usually apical (coded 1) | Adoral zone of membranelles mainly ventral (coded 0) |
| 3# Adoral zone of membranelles closed (coded 1) or with minute ventral gap (coded 2) |
Adoral zone of membranelles C-shaped (coded 0) |
| 4# 30–50% (coded 1) or 0% (coded 2) of adoral polykinetids four-rowed |
> 90% of adoral polykinetids four-rowed (coded 0) |
| 5 Collar polykinetids bipartited (coded 1) | Collar polykinetids continuous (coded 0) |
| 6 Undulating membrane/es often diplo- or polystichomonad (coded 1) |
Undulating membrane/es invariably monostichomonad (coded 0) |
| 7 Paroral membrane absent (coded 1) | Endoral and paroral membrane present (coded 0) |
| 8 Cirri absent (coded 1) | Cirri present (coded 0) |
| 9 Somatic kineties arranged in a right and left ciliary field (coded 1) |
Somatic kineties more or less equidistantly arranged (coded 0) |
| 10 Two ventral organelles (coded 1) or one specialized ventral kinety (coded 2) |
Specialized ventral kinety/organelles absent (coded 0) |
| 11# Two dorsal kineties (coded 1) or one dorsal kinety (coded 2) | Specialized dorsal kinety/kineties absent (coded 0) |
| 12 Posterior kinety present (coded 1) | Specialized posterior kinety absent (coded 0) |
| 13 Lateral ciliary field present (coded 1) | Lateral ciliary field absent (coded 0) |
| 14# Unspecialized somatic kineties: dikinetidal, with posterior cilia at least in some dikinetids (coded 1); dikinetidal, without anterior cilia in at least some dikinetids (coded 2); partially (coded 3) or entirely (coded 4) monokinetidal |
Unspecialized somatic kineties dikinetidal, with cilia only at each anterior basal body (coded 0) |
| 15 Somatic kinetids condensed (coded 1) | Somatic kinetids distinctly separate (coded 0) |
| 16 Some unspecialized somatic kineties distinctly curved (coded 1) or forming posterior spiral (coded 2) |
Unspecialized somatic kineties meridional (coded 0) |
| 17 ≥40% of unspecialized somatic kineties shortened (coded 1) | Unspecialized somatic kineties extend from adoral zone of membranelles to posterior cell end (coded 0) |
| 18 Kinetal lips present (coded 1) | Kinetal lips absent (coded 0) |
| 19 Polysaccharidic cortical platelets present (coded 1) | Cortical platelets absent (coded 0) |
| 20 Majority of members with one ellipsoidal macronuclear nodule (coded 1), one C-shaped macronucleus (coded 2), or more than two macronuclear nodules (coded 3) |
Majority of members with two macronuclear nodules (coded 0) |
| 21 Cell contractility present (coded 1) | Cell contractility absent (coded 0) |
| 22 Capsules (coded 1) or oligotrichid extrusomes (coded 2) present |
Capsules and oligotrichid extrusomes absent (coded 0) |
| 23 Lorica: agglutinated and flexible (coded 1); agglutinated, flexible, and with subterminal membrane (coded 2); agglutinated and stiff (coded 3); posterior portion agglutinated, anterior hyaline (coded 4); entirely hyaline (coded 5) |
Majority of taxa without lorica (coded 0) |
| 24 Enantiotropy (coded 1) | Homeotropy (coded 0) |
| 25# Stomatogenesis hypoapokinetal in tube (coded 1) or in pouch (coded 2) |
Stomatogenesis epiapokinetal (coded 0) |
| 26 Posterior end of oral primordium curves rightwards (coded 1) |
Anterior portion of oral primordium curves rightwards (coded 0) |
| 27 Undulating membranes originate de novo (coded 1) | Undulating membranes originate from oral primordium or cirral anlagen (coded 0) |
| 28 Somatic kineties originate de novo (coded 1) | Somatic kineties originate at least partially by intrakinetal proliferation of basal bodies (coded 0) |
| 29 Reorganization of somatic kineties indistinct or absent (coded 1) |
Reorganization of somatic kineties (coded 0) |
| 30 Interlocking arrangement of conjugants (coded 1) | Parallel or oblique arrangement of conjugants (coded 0) |
The coding is mainly based on the out-group comparison with the order Stichotrichida.
Ordered characters (Wagner/Farris optimization).
Polymorphic characters (e.g., cell shape, position of adoral zone of membranelles, nuclear apparatus) were considered, as they contribute important phylogenetic information and thus consistently increase the accuracy of the analyses (Poe and Wiens 2000). Therefore, the “majority method” was applied which codes a polymorphic taxon as having the trait that is most common among the taxa considered (Wiens 2000).
Genera or orders represent the terminal taxa; only when part of the constituting species distinctly deviates in some respect, are species or subgenera the terminal taxa, viz., in Leegaardiella, Tintinnidium, and Tintinnopsis in which conspicuous differences in the somatic ciliary pattern occur or in Stenosemella in which the lorica structure varies considerably.
DNA extraction and amplification
Rimostrombidium lacustris was sampled from the pond close to the Department of Organismal Biology in Salzburg, Austria. The identification of the species was based on live observations and protargol impregnations. Its DNA was extracted from ethanol-fixed cells, following the modified Chelex extraction method (Strüder-Kypke and Lynn 2003). For the subsequent PCR reactions, 18 μl of the supernatant were used. The PCR amplification was performed in a Perkin-Elmer GeneAmp 2400 thermocycler (PE Applied Biosystems, Mississauga, ON, Canada), using the forward Primer A (5′-AACCTGGTTGATCCTGCCAGT-3′; Medlin et al. 1988) and the reverse Primer B (5′-TGATCCTTCTGCAGGTTCACCTAC-3′; Medlin et al. 1988). The PCR product was subsequently cloned (TOPO TA Cloning kit, Invitrogen, Carlsbad, CA, USA) and re-amplified with Primer A and Primer B. These PCR products were purified with the GeneClean II kit (Qbiogen, Carlsbad, CA, USA). Sequencing was performed in both directions in an ABI Prism 377 Automated DNA Sequencer (Applied Biosystems Inc., Foster City, CA, USA), using dye terminator and Taq FS with two forward and two reverse internal SSrRNA primers (Elwood et al. 1985) and the amplification primers.
Sequence availability and phylogenetic analyses
The nucleotide sequences used are available from the GenBank/EMBL databases and have the following accession numbers: Codonellopsis americana AY143571 (Strüder-Kypke and Lynn 2003), Eutintinnus pectinis AY143570 (Strüder-Kypke and Lynn 2003), Eutintinnus pectinis AF399171 (Snoeyenbos-West et al. 2002), Eutintinnus sp. (ENB-99) AY143569 (Strüder-Kypke and Lynn 2003), Favella ehrenbergii AF399164 (Snoeyenbos-West et al. 2002), Favella panamensis AY143572 (Strüder-Kypke and Lynn 2003), Gonostomum strenuum AJ310493 (Bernhard et al. 2001), Halteria grandinella AF194410 (Shin et al. 2000), Laboea strobila AF399151 (Snoeyenbos-West et al. 2002), Laboea strobila AY302563 (Agatha et al. 2004), Laurentiella strenua AJ310487 (Bernhard et al. 2001), Metacylis angulata AY143568 (Strüder-Kypke and Lynn 2003), Metacylis sp. (MNB-99) AY143567 (Strüder-Kypke and Lynn 2003), Novistrombidium testaceum AJ488910 (Modeo et al. 2003), Oxytricha granulifera X53486 (Schlegel et al. 1991), Parastrombidinopsis shimi AJ786648 (Kim et al. 2005), Pelagostrobilidium neptuni AY541683 (Agatha et al. 2005), Rhabdonella hebe AY143566 (Strüder-Kypke and Lynn 2003), Steinia sphagnicola AJ310494 (Bernhard et al. 2001), Strobilidium caudatum AY143573 (Strüder-Kypke and Lynn 2003), Strombidinopsis jeokjo AJ628250 (Jeong et al. 2004), Strombidinopsis sp. AF399132 (Snoeyenbos-West et al. 2002), Strombidium biarmatum AY541684 (Agatha et al. 2005), Strombidium inclinatum AJ488911 (Modeo et al. 2003), Strombidium purpureum U97112 (Hirt et al. unpubl.), Strombidium sp. 1 (SBB99-1) AY143565 (Strüder-Kypke and Lynn 2003), Strombidium sp. 2 (SNB99-2) AY143564 (Strüder-Kypke and Lynn 2003), Strombidium stylifer (submitted as S. styliferum) AY257125 (McManus et al. unpubl.), Tetmemena pustulata (submitted as Stylonychia pustulata) X03947, M14600 (Elwood et al. 1985), Tintinnidium mucicola AY143563 (Strüder-Kypke and Lynn 2003), Tintinnopsis dadayi AY143562 (Strüder-Kypke and Lynn 2003), Tintinnopsis fimbriata AY143560 (Strüder-Kypke and Lynn 2003), Tintinnopsis tocantinensis AY143561 (Strüder-Kypke and Lynn 2003), Tintinnopsis tubulosoides AF399110 (Snoeyenbos-West et al. 2002), and Urostyla grandis AF164129 (Hewitt et al. 2003).
The sequence fragments of Rimostrombidium lacustris were imported into Sequencher ver. 4.0.5 (Gene Codes Corp.), and ambiguous nucleotide positions at the beginning and end of the fragments were deleted. All fragments were assembled into a contig (set of overlapping DNA segments) and checked for sequencing errors. The new sequence was added to our existing DCSE (Dedicated Comparative Sequence Editor; De Rijk and De Wachter 1993) database and automatically aligned against already existing strobilidiid sequences. Considering secondary structural features of the SSrRNA molecule, the alignment was further refined. Hypervariable positions were excluded from the file prepared for the phylogenetic analyses, thus resulting in a data set that comprised 1756 nucleotide positions. The alignment is available from M.C. Strüder-Kypke upon request. Missing nucleotides at the beginning or the end of sequences were treated as missing by MrBayes and PAUP*, and gaps within the alignment were regarded as fifth character state.
As best model of nucleotide substitution for our data, MrModeltest ver. 2 (Nylander 2004; Posada and Crandall 1998) determined the general-time-reversible (GTR) model, considering invariable sites and gamma distributed substitution rates among sites. This model (n = 6, rates = invgamma) was implemented into MrBayes ver. 3.1.1, a phylogenetic program employing Bayesian inference (Huelsenbeck and Ronquist 2001; Ronquist and Huelsenbeck 2003), and a maximum likelihood tree (BI) was calculated. Two parallel runs were performed, and the posterior probability of a phylogeny out of 1,000,000 trees, approximating it with the Markov Chain Monte Carlo (MCMC) and sampling every 50th generation (tree), was computed, discarding the first 2000 trees as burn-in. A maximum parsimony (MP) analysis was performed with PAUP* ver. 4.0b10 (Swofford 2002). Three hundred and seventy-three parsimony-informative characters were analyzed with the TBR branch-swapping algorithm. Species were added randomly (n = 10) and the data were bootstrap resampled 1000 times. PHYLIP ver. 3.65 (Felsenstein 2004) was employed to construct a distance matrix tree. DNADIST was used to calculate the genetic distances with the Kimura-2-parameter model corrected for gamma-distributed substitution rates among sites (Kimura 1980). The distance tree was constructed, applying the neighbor joining (NJ) algorithm (Saitou and Nei 1987), and the data were bootstrap resampled 1000 times.
Results and discussion
Evolution of somatic ciliary patterns
Based on the available data, we hypothesize the following evolutionary sequence for the development of the somatic ciliary patterns in choreotrichid ciliates: The ancestor of the order Choreotrichida had some longitudinal somatic kineties composed of dikinetids, each with a distinct cilium only at the anterior basal body, as we find it in the dorsal kineties of the class Hypotrichea, the somatic kineties/bristle complexes of the subclass Halteriia, and the ventral kinety of the order Oligotrichida (Fig. 1a; Agatha 2004a, b). First, a cilium developed additionally at each posterior basal body of the dikinetids in the order Choreotrichida. The genus Leegaardiella represents all transition stages from the monociliated to the diciliated dikinetids: in Leegaardiella ovalis only the anterior basal body of each dikinetid is ciliated; Leegaardiella elbraechteri shows both monociliated and diciliated dikinetids; and Leegaardiella sol possesses exclusively diciliated dikinetids (Figs 1a and b). The diciliated state of the dikinetids was independently achieved in the family Strombidinopsidae, as the alternatives, i.e., a convergent development of bipartited collar membranelles among the Leegaardiella species or the occurrence of continuous membranelles as a retrogressive apomorphy in the remaining Choreotrichida, are less likely. The diciliated dikinetidal pattern then gave rise to two further ciliary patterns: (i) the Lohmanniella pattern (Fig. 1c) with dikinetids having bare anterior basal bodies and (ii) the pattern of the ancestor of the suborder Tintinnina with a right and left ciliary field separated by a blank ventral stripe and reduced anterior cilia in the posterior dikinetids (Figs 1e, 2a, and 3a). It is more parsimonious to assume a transformation of dikinetids into ciliated monokinetids by the loss of the unciliated basal bodies than by the loss of cilia plus basal bodies; during the ontogenesis of the halteriid Meseres corlissi, the bare basal bodies of the parental somatic dikinetids are also resorbed prior to the ciliated ones (Petz and Foissner 1992). Therefore, the pattern of the family Strobilidiidae (Fig. 1d) did not evolve from the Strombidinopsidae pattern (diciliated dikinetids) but rather from the Lohmanniella pattern by the degeneration of the bare anterior basal bodies and a condensation of the kinetids.
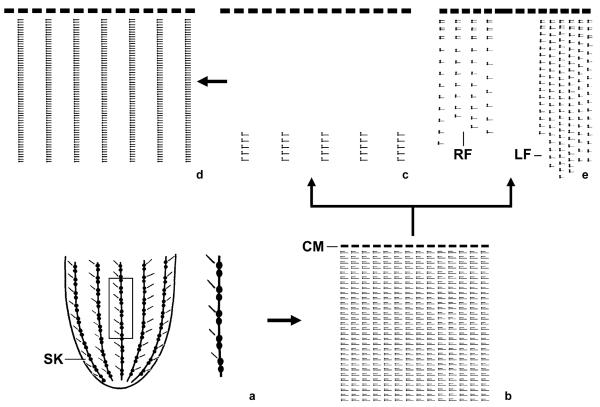
Fig. 1.
Kinetal maps showing the early evolution of the choreotrichid ciliary patterns. (a) The ancestor had many longitudinal somatic kineties consisting of dikinetids, each with a cilium only at the anterior basal body (see detail). (b) The ciliary pattern of Leegaardiella sol and the Strombidinopsidae reveals diciliated somatic dikinetids. (c, d) First, the anterior cilia of the dikinetids were reduced, producing the Lohmanniellidae pattern (c) which probably gave rise to the Strobilidiidae pattern (d) by the reduction of the bare anterior basal bodies of the dikinetids and a condensation of the resulting monokinetids. (e) In the hypothetical ancestor of the tintinnids, a right and left ciliary field developed and the anterior cilia of the dikinetids were reduced in the posterior kinety portions. CM, collar membranelles; LF, left ciliary field; RF, right ciliary field; SK, somatic kineties.
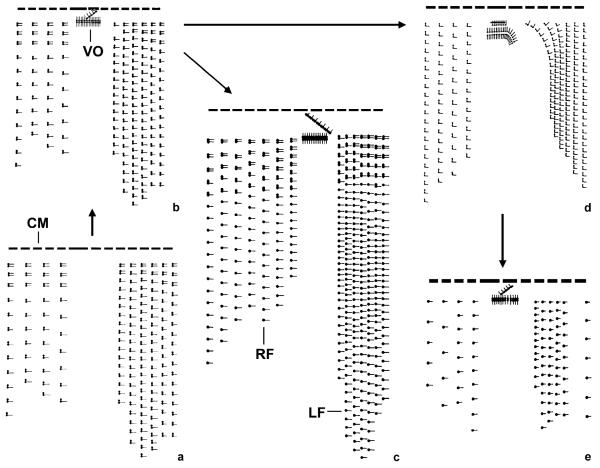
Fig. 2.
Kinetal maps showing the evolution of ciliary patterns in tintinnids with two ventral organelles. (a) The hypothetical ancestor of the tintinnids probably had a right and left ciliary field, and the anterior cilia of the dikinetids were reduced in the posterior portion of the kineties. (b) Two dikinetidal ventral organelles were introduced, resulting in the Tintinnidium (Tintinnidium) pattern (after Foissner and Wilbert 1979). (c) The bare anterior basal bodies of the dikinetids were partially lost in the posterior portion of the kineties, giving rise to the Tintinnidium (Semitintinnidium) pattern (after Blatterer and Foissner 1990). (d) The anterior cilia of the dikinetids were entirely lost, producing the Membranicola pattern (after Foissner et al. 1999). (e) The bare anterior basal bodies of the dikinetids were lost, creating the pattern of Tintinnopsis cylindrata (after Foissner and Wilbert 1979). CM, collar membranelles; LF, left ciliary field; RF, right ciliary field; VO, ventral organelles.
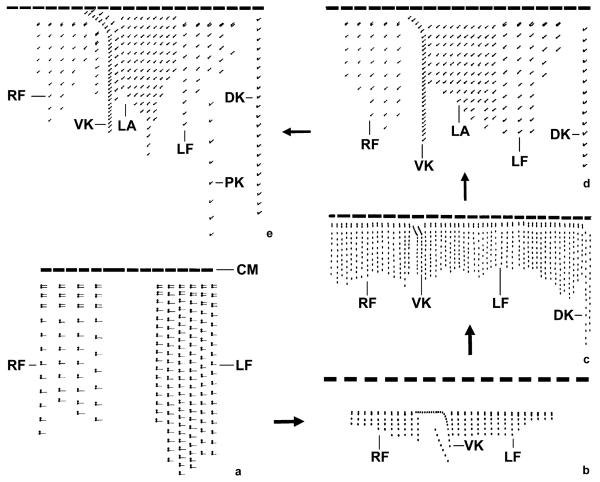
Fig. 3.
Kinetal maps showing the evolution of the ciliary patterns in tintinnids with a ventral kinety. (a) The hypothetical ancestor of the tintinnids probably had a right and left ciliary field, and the anterior cilia of the dikinetids were reduced in the posterior portion of the kineties. (b) A ventral kinety was created and the dikinetids, except for the anteriormost ones, transformed into monokinetids, generating the Nolaclusilis pattern (after Snyder and Brownlee 1991). (c) Dorsal kineties developed, producing the Eutintinnus pattern (after Choi et al. 1992). (d) The introduction of a lateral ciliary field and the loss of one dorsal kinety yielded the pattern of Tintinnopsis brasiliensis (after Cai et al. 2006). (e) The addition of a posterior kinety gave rise to the most complex ciliary pattern so far known. Such a pattern is found in the genera Codonella, Codonellopsis, Cymatocylis, Stenosemella, and Tintinnopsis (except for Tintinnopsis cylindrata and Tintinnopsis brasiliensis). CM, collar membranelles; DK, dorsal kinety/kineties; LA, lateral ciliary field; LF, left ciliary field; PK, posterior kinety; RF, right ciliary field; VK, ventral kinety.
Descending from the tintinnid ancestor, we hypothesize two evolutionary lineages based on (i) the development of two dikinetidal ventral organelles (Fig. 2) and (ii) the introduction of a specialized monokinetidal ventral kinety (Fig. 3). Accordingly, the following pattern in the first lineage comprised two de-novo-originating ventral organelles (Petz and Foissner 1993) as well as a right and left ciliary field; a similar pattern occurs in Tintinnidium fluviatile and Tintinnidium pusillum (Fig. 2b). The subsequent split was characterized by (i) the reduction of the bare anterior basal bodies of the posterior dikinetids, creating a pattern found in Tintinnidium semiciliatum (Fig. 2c), and (ii) the loss of all anterior cilia of the dikinetids, producing the Membranicola pattern (Fig. 2d). According to the more parsimonious assumption that dikinetids without anterior cilia were transformed into monokinetids (see Lohmanniellidae and Strobilidiidae), the Tintinnopsis cylindrata pattern (Fig. 2e) likely evolved from the Membranicola pattern.
In the second lineage of tintinnid evolution, a ventral kinety was introduced and the dikinetids of the right and left ciliary field, except for the anteriormost ones, transformed into ciliated monokinetids, producing a ciliary pattern known from Nolaclusilis (Fig. 3b). The two specialized monokinetidal kineties on the ventral side of Nolaclusilis are similar in structure and course to the ventral and second kinety of Codonella, Codonellopsis, Cymatocylis, Stenosemella, and Tintinnopsis (except for Tintinnopsis cylindrata); thus, we assume a homology between those structures, while Petz and Foissner (1993) suggested a homology with the dikinetidal ventral organelles mentioned above. Since the ventral organelles and the ventral kinety differ in their origin (de novo vs. intrakinetal proliferation of basal bodies), ontogenetic studies on Nolaclusilis are required to confirm our hypothesis and the proper familial affiliation. Next, two dorsal kineties evolved, creating the Eutintinnus pattern (Fig. 3c). Subsequently, the number of dorsal kineties was reduced to one and a lateral ciliary field developed, generating a pattern known from Tintinnopsis brasiliensis (Fig. 3d). Finally, a posterior kinety was introduced, creating the most complex tintinnid ciliary pattern so far known (Fig. 3e). This pattern is found with minute deviations in the families Codonellidae, Codonellopsidae, and Ptychocylididae. Thus, it is associated with different lorica structures, i.e., with agglutinated and stiff loricae, loricae composed of a hyaline collar and an agglutinated bowl, and entirely hyaline loricae (Table 1). All these species are marine, with the exception of the two freshwater species Codonella cratera and Stenosemella lacustris.
In contrast to the related class Hypotrichea and order Oligotrichida, the structure of the somatic kineties is less constant and conserved in the order Choreotrichida.
Morphological characters, character states, and convergences considered
The order Stichotrichida (class Hypotrichea) is chosen as outgroup for the phylogenetic reconstruction of the order Choreotrichida, since previous genetic genealogies invariably revealed a sister group relationship to the class Oligotrichea (Agatha et al. 2004, 2005; Kim et al. 2005; Modeo et al. 2003; Snoeyenbos-West et al. 2002; Strüder-Kypke and Lynn 2003). Indeed, ultrastructural evidence (the presence of a perilemma, absent or transient kinetodesmal fibrils in somatic kineties) corroborates these findings, and the resulting morphologic phylogeny is more parsimonious than the assumption of a sister group relationship between the orders Hypotrichida and Stichotrichida. The common ancestor of the order Stichotrichida and the class Oligotrichea presumably had (i) a benthic life style, (ii) a dorsoventrally flattened cell shape, (iii) some dorsal somatic kineties composed of dikinetids, each with a distinct cilium only at the anterior basal body, (iv) ventral cirri, (v) an adoral zone composed of paramembranelles, (vi) a monostichomonad endoral and paroral membrane, (vii) a perilemma, (viii) a layer of longitudinal microtubules underneath the alveoli, (ix) absent or transient kinetodesmal fibrils in the somatic kineties, (x) an intrakinetal proliferation of kinetids in the dorsal somatic kineties, (xi) a ventral and dorsal reorganization of the somatic ciliature, (xii) an apokinetal development of the oral ciliature, (xiii) gene-sized fragments in the macronucleus, and (xiv) a macronuclear replication band (convergence in some Cyrtophorida and Chonotrichida).
The cladistic inferences are based on nine groups of characters: the cell shape (character 1), the morphology of the oral apparatus (characters 2–7), the somatic ciliature (characters 8–17), features of the cell cortex (characters 18 and 19), the nuclear apparatus (character 20), special organelles and cell structures (characters 21 and 22), the lorica structure (character 23), ontogenetic particulars (characters 24–29), and the conjugation (character 30). The characters and their states are summarized in Table 2 and their distribution among the taxa is shown in Table 3.
Character 1 (cell shape)
The ancestor of the classes Hypotrichea (orders Stichotrichida and Hypotrichida) and Oligotrichea was probably a dorsoventrally flattened ciliate crawling on the substrate by means of ventral cirri. The “dorsalization” process suggested by Foissner et al. (2004) for the halteriid organization, i.e., an extension of the dorsal side with the longitudinal somatic kineties and a reduction of the ventral side, except for a rather narrow stomatogenic stripe (determinative region) posterior to the buccal vertex, might have generated the globular, ellipsoidal, or obconical cell shape of the Oligotrichea, which is advantageous for a planktonic life style. Simultaneously, the adoral zone of membranelles became apically located (see Character 2) and the ventrally arranged cirri were lost (see Character 8). Likely, this process also caused the enantiotropic division mode (see Character 24). A similar transformation is recognizable in the planktonic cyrtophorid Phascolodon vorticella (Foissner et al. 1999): compared with its benthic, ventrally flattened relatives, the species has apically arranged cilia, an involuted ventral side, a more extended dorsal side, and thus a more ellipsoidal cell shape.
The benthic or semibenthic Oligotrichida probably became secondarily dorsoventrally flattened because their flattening is less distinct as in the Stichotrichida and occurs together with specialized thigmotactic membranelles (Agatha 2004b).
Character 2 (position of membranellar zone)
The adoral zone of membranelles mainly extends on the ventral side of the Stichotrichida and is used for filter feeding, while the cirri perform the locomotion. The derived apical position in the Oligotrichea might be due to the “dorsalization” process (see Character 1), which simultaneously implies the loss of the cirri by the reduction of the ventral side. Thus, the adoral zone of membranelles is also responsible for the locomotion, as the somatic cilia (the formerly dorsal cilia) are usually minute and probably for thigmotactic purposes only (Fauré-Fremiet and Ganier 1970). Only in the Halteriia, the somatic ciliature may participate in the cell’s movement by the formation of long jumping bristles or cilia (Tamar 1967, 1979). In some tintinnids, e.g., Salpingacantha Kofoid and Campbell, 1929 and Amphorellopsis acuta (Schmidt, 1901) Kofoid and Campbell, 1929, the adoral zone of membranelles is obliquely arranged in the anterior cell portion (Laval-Peuto 1994; Laval-Peuto and Brownlee 1986; Lynn and Small 2002; Small and Lynn 1985), representing a retrogressive apomorphy (Laval-Peuto and Brownlee 1986; Wasik and Mikołajczyk 1992). Since the somatic ciliature of these species was not described and cannot be inferred from the illustrations of protargol-impregnated specimens (Laval-Peuto 1994; Laval-Peuto and Brownlee 1986; Lynn and Small 2002; Small and Lynn 1985), their phylogenetic position remains doubtful. According to Laval-Peuto and Brownlee (1986), a large number of membranelles in the buccal cavity indicates a high evolutionary state. Since this feature shows a considerable variability, especially in the aloricate Choreotrichida, it was not considered in the present reconstruction. Furthermore, the highest number of membranelles occurs in the buccal cavity of the naked taxa.
Character 3 (shape of membranellar zone)
The Stichotrichida, Halteriia, and Oligotrichida have a C-shaped adoral zone of membranelles. Hence, the closed zone of the Choreotrichida is considered the apomorphic state. Parastrombidinopsis is characterized by a slightly open adoral zone of membranelles, which is otherwise typical for the Choreotrichida, i.e., the collar polykinetids extend across the peristomial rim and the proximal ones are elongated, extending into the buccal cavity. Parastrombidinopsis probably represents a further derived stage, i.e., a secondarily slightly opened adoral zone of membranelles, especially as the Leegaardiellidae, which already have a closed zone, branch more basally in the Choreotrichida.
Character 4 (number of basal body rows in adoral polykinetids)
The majority of membranelles comprises four rows of basal bodies in most Stichotrichida (Berger 1999; Foissner and Al-Rasheid 2006; Foissner et al. 2002), while there are three rows in the Oligotrichida (literature mentioned in Agatha 2004b; Laval-Peuto et al. 1986; Wilbert and Song 2005; Xu and Song 2006; Xu et al. 2006) and Choreotrichida (literature mentioned in Table 1; Hedin 1976b; Laval 1972; Laval-Peuto 1975; Laval-Peuto et al. 1979; Petz and Foissner 1993; Wasik and Mikołajczyk 1992). The two-rowed structure of the collar membranelles found in several protargol-impregnated choreotrichid ciliates was interpreted by Dale and Lynn (1998) as a misobservation. The subclass Halteriia has both three-rowed collar and four-rowed buccal polykinetids in a ratio of 1–2.5:1 (Foissner et al. 1999; Grain 1972; Grim 1974; Krainer 1995; Petz and Foissner 1992), possibly representing a transition stage between the order Stichotrichida and subclass Oligotrichia.
Character 5 (bipartited collar polykinetids)
The adoral polykinetids are continuous in the orders Stichotrichida, Halteriida, Oligotrichida, and Choreotrichida, with the exception of the family Leegaardiellidae, in which the collar polykinetids are bipartited.
In some Strombidinopsis species, the collar membranelles are bipartited into an outer portion with long cilia and an inner portion with short cilia inserting in continuous polykinetids. Since most Strombidinopsidae have common membranelles, i.e., the length of the cilia gradually decreases from the distal to the proximal end of the organelles, the bipartited membranelles are apparently not an early developmental stage of the bipartited polykinetids.
Character 6 (structure of undulating membrane/es)
A monostichomonad structure of the endoral and paroral membrane is regarded as the plesiomorphic state. The diplo- or polystichomonad undulating membranes in most Stichotrichida thus represent the derived state, which probably developed convergently in several Hypotrichida.
Character 7 (number of undulating membranes)
The orders Stichotrichida and Halteriida have two undulating membranes: an endoral and a paroral membrane. Hence, the absence of the paroral in the subclass Oligotrichia is probably an apomorphy, especially as a tendency to reduce this organelles was observed during the ontogenesis in a halteriid species (Foissner pers. comm.).
Character 8 (cirri)
It is assumed that the cirri developed once, i.e., in the ancestor of the classes Hypotrichea (Hypotrichida and outgroup Stichotrichida) and Oligotrichea; when the ancestor of the Oligotrichea entered the pelagial and the “dorsalization” process took place (see Character 1), the cirri were lost. Although no transition stages are known, this assumption seems likely since otherwise the cirri and the formation of the longitudinal basal body streaks during ontogenesis must have developed convergently in the orders Hypotrichida and Stichotrichida.
Character 9 (right and left ciliary field)
The somatic kineties are more or less equidistantly arranged in the Stichotrichida, Halteriia, and most aloricate Choreotrichida; the Oligotrichida usually have only a longitudinal ventral kinety and a distinctly curved girdle kinety. Within the suborder Tintinnina, the kineties are distinctly more closely arranged in the left than in the right half of the cell, and the two ciliary fields are separated by a broad, blank ventral stripe. This new ciliary pattern is regarded as an autapomorphy of the suborder. Frequently, one or two specialized kineties also separate the fields dorsally (see Character 11).
Character 10 (ventral differentiations)
The somatic kineties usually have a uniform structure in the Stichotrichida, Halteriia, Oligotrichida, and aloricate Choreotrichida. Hence, the two dikinetidal and de-novo-originating ventral organelles in Membranicola, Tintinnidium, and Tintinnopsis cylindrata as well as the monokinetidal ventral kinety in Codonella, Codonellopsis, Cymatocylis, Eutintinnus, Nolaclusilis (see ‘Evolution of somatic ciliary patterns’), Stenosemella, and the other Tintinnopsis species likely represent derived states.
Character 11 (dorsal differentiations)
Similar to the ventral differentiations (see Character 10), the occurrence of specialized dorsal kineties is regarded as an apomorphy. Eutintinnus has two dorsal kineties, of which one was probably later reduced in the ancestor of Codonella, Codonellopsis, Cymatocylis, Stenosemella, and Tintinnopsis (except for Tintinnopsis cylindrata).
Character 12 (posterior kinety)
The introduction of a kinety posterior to the left or lateral ciliary field is considered the derived state, as the somatic kineties usually commence at the same level posterior to the adoral zone of membranelles in the Stichotrichida, Halteriia, and Choreotrichida; the Oligotrichida only have an anteriorly shortened ventral and a distinctly curved girdle kinety (Agatha 2004b). It is assumed that the short row of monokinetids in the posterior cell portion of Nolaclusilis hudsonicus represents a fragment of the ventral kinety and not a specialized posterior kinety, as such a structure is lacking in its congener N. bicornis. Likewise, the short monokinetidal kineties posterior to the left ciliary field in Eutintinnus pectinis are regarded as kinety fragments.
Character 13 (lateral ciliary field)
Apart from the specialized ciliary rows, the somatic kineties generally show an identical structure in Eutintinnus, Membranicola, Nolaclusilis, Tintinnidium, and Tintinnopsis cylindrata. The lateral ciliary field inserted between the ventral kinety and the left ciliary field, however, differs from the existing ciliary fields in its structure (entirely monokinetidal vs. monokinetidal with an anterior dikinetid) as well as in its very closely spaced kineties and kinetids. Therefore, this field is assumed to be a synapomorphy of Codonella, Codonellopsis, Cymatocylis, Stenosemella, and Tintinnopsis (except for Tintinnopsis cylindrata).
Character 14 (structure of somatic kinetids)
According to the evolution of the ciliary patterns hypothesized above, the ancestral dikinetids with a cilium only at each anterior basal body became diciliated, lost their anterior cilia, and finally the anterior basal bodies of the dikinetids. Hence, the character states are ordered. Some convergences are assumed, as the alternatives are less likely and parsimonious.
Character 15 (condensation of somatic kinetids)
The somatic kinetids are distinctly separate in the Stichotrichida, Halteriia, Oligotrichida, and the Choreotrichida, with the exception of the family Strobilidiidae and the genera Halteria and Pelagohalteria in which the kinetids are closely spaced. Since the kinetid condensation in the halteriids is probably due to the formation of jumping bristles, it is considered a convergence; the alternative would necessitate five convergences.
Character 16 (curvature of somatic kineties)
As suggested by the “dorsalization” process (see Character 1), the somatic kineties in the Oligotrichea correspond to the longitudinal dorsal kineties of the Stichotrichida. The plesiomorphic state with meridional ciliary rows is found in the Halteriia and Oligotrichia, with the exception of the Oligotrichida (Agatha 2004a, b) and some somatic ciliary rows of Pelagostrobilidium and Leegaardiella ovalis which are distinctly curved; the assumption of convergences is more parsimonious than the alternative. In Strobilidium, three kineties form a unique posterior spiral.
Character 17 (length of somatic kineties)
In the Stichotrichida, the somatic kineties extend between the adoral zone of membranelles and the posterior cell end. In the Oligotrichea, with the exception of the Strombidinopsidae, shortened kineties occur. An anterior and/or posterior shortening of more than 40% of the unspecialized somatic ciliary rows is regarded as the apomorphic state. For parsimonious reasons, the longitudinal kineties of the Strombidinopsidae are regarded as a retrogressive apomorphy because the alternative would require four convergent decreases in kinety length, viz., in the Halteriida, Oligotrichida, Leegaardiellidae, and the remaining Choreotrichida.
Character 18 (kinetal lips; cytoplasmic flaps)
An ultrastructural study showed that thin sheets of plasma membrane enclosing cytoplasm and a single layer of microtubules overlie the proximal portion of the somatic cilia, which are thus directed to the left and horizontally attached to the cell surface in the Strobilidiidae (Grim 1987). Since such a structure is unique in the superclass Spirotricha, it is regarded as the derived state.
Character 19 (cortical platelets)
Alveolate protists are characterized by cortical alveoli which occasionally contain platelets, e.g., in the hypotrichid Euplotes (Hausmann and Kaiser 1979) and the dinoflagellates (Taylor 1987). The polysaccharidic cortical platelets of the order Oligotrichida are, however, not within the alveoli but underneath the microtubular layer subjacent the alveoli (Fauré-Fremiet and Ganier 1970; Laval-Peuto and Febvre 1986; Modeo et al. 2003). A similar but convergent arrangement is only found in the clevelandellid family Sicuophoridae (Lynn and Small 2002).
Character 20 (macronucleus number and shape)
Two macronuclear nodules probably represent the ancestral state, occurring in most members of the Stichotrichida, Strombidinopsidae, and Tintinnina, as well as in Leegaardiella elbraechteri, Leegaardiella sol, and Membranicola. The two replication bands traversing the C-shaped macronucleus of Strobilidium caudatum indicate a fusion of the two nodules which probably developed convergently in some Hypotrichida, e.g., Euplotes and Aspidisca (Raikov 1982). In Lohmanniella, Leegaardiella ovalis, Tintinnidium, and Tintinnopsis cylindrata, the macronuclear nodule reveals only a single replication band, implying the reduction of the second nodule; likewise, there is only one nodule in the majority of the Oligotrichida, representing a convergent state. On the other hand, an increased number of macronuclear nodules is specific for some tintinnid genera and species, in which it probably developed independently from the oligotrichid family Tontoniidae.
Character 21 (contractility)
Within the Spirotricha, a distinct contractility of the posterior cell half is only known from the suborder Tintinnina and the oligotrichid family Tontoniidae. Since the contractile elements in the two taxa differ in their ultrastructure (Agatha 2004a, b; Greuet et al. 1986), they probably achieved the contractility independently.
Character 22 (extrusomes)
Ultrastructural studies on tintinnids revealed five types of capsules whose position and orientation indicate that they might be extrusive (Laval-Peuto and Barria de Cao 1987). Striae, accessory combs, and tentaculoids are assumed to be always associated with capsules. One or more of this organelle were found in Climacocylis, Codonaria (Laval 1971; Laval-Peuto and Barria de Cao 1987), Codonella (Daday 1887; Entz 1909a; Laval 1971; Laval-Peuto and Barria de Cao 1987), Codonellopsis (Laval 1971; Laval-Peuto and Barria de Cao 1987), Cymatocylis (Wasik and Mikołajczyk 1992), Cyttarocylis (Entz 1909b; Laval 1971; Laval-Peuto 1975, 1994; Laval-Peuto and Barria de Cao 1987), Dadayiella (Entz 1884), Dictyocysta (Laval 1971; Laval-Peuto and Barria de Cao 1987), Eutintinnus (Daday 1887; Entz 1909b; Fauré-Fremiet 1924; Schweyer 1909), Favella (Entz 1909b; Hedin 1975), Nolaclusilis (Sniezek et al. 1991; Snyder and Brownlee 1991), Parafavella (Hedin 1975; Sokolova and Gerassimova 1984), Petalotricha (Entz 1909b, 1929; Laval 1971, 1972; Laval-Peuto 1994), Proplectella (Laval 1971), Ptychocylis (Hedin 1976b; Schweyer 1909), Rhabdonella (Hofker 1931; Laval 1971; Laval-Peuto and Barria de Cao 1987), Stenosemella (SA own observ.; Campbell 1926; Capriulo et al. 1986; Hofker 1931; Laval-Peuto and Barria de Cao 1987), Tintinnidium (Entz 1885; Foissner and Wilbert 1979; Hofker 1931), Tintinnopsis (Capriulo et al. 1986; Entz 1909b; Fauré-Fremiet 1924; Gold 1979; Haeckel 1873; Hofker 1931; Laval-Peuto et al. 1979; Schweyer 1909), Tintinnus (Entz 1909b; Schweyer 1909), Undella (Daday 1887; Laval 1971; Laval-Peuto 1994; Laval-Peuto and Barria de Cao 1987), and Xystonella (Laval 1971; Laval-Peuto 1994; Laval-Peuto and Barria de Cao 1987). Genera with agglutinated loricae have only capsules of types I or II, while genera with hyaline loricae have only capsules of types III, IV, and V (Laval-Peuto and Barria de Cao 1987). The observations concerning the presence of capsules, striae, accessory combs, and tentaculoids are occasionally contradictory, possibly because their occurrence is correlated with food availability (Capriulo et al. 1986). It seems reasonable to assign these scattered observations to all tintinnids, especially as a convergent development of capsules in the different tintinnid families is less parsimonious.
Acicular extrusomes (trichites) characterize the Oligotrichida (Agatha 2004a), while argyrophilic granules of unknown nature are found in the oral region of the Stichotrichida (Foissner and Al-Rasheid 2006), the halteriids Halteria and Meseres (Foissner pers. comm.), the oligotrichids Strombidium rhynchum (SA pers. obs.; Lynn et al. 1988), Strombidium constrictum (SA pers. obs.), and Novistrombidium apsheronicum (Agatha 2003a), as well as in the strobilidiids Pelagostrobilidium neptuni, Pelagostrobilidium spirale, Rimostrombidium humile, Rimostrombidium lacustris, and Rimostrombidium veniliae. In the tintinnids, several types of mucocysts were also observed (Laval-Peuto and Barria de Cao 1987).
Character 23 (lorica)
The lorica is considered a strong tintinnid autapomorphy, although convergent structures developed in several ciliate groups, e.g., folliculinids, suctorians, and peritrichs. Comparable lorica-like structures are formed by close relatives, for instance, the loricae of some stichotrichid ciliates (e.g., Stichotricha, Chaetospira; Foissner et al. 1991), the mineral envelope of Strombidinopsis minima, and the hyaline, possibly mucilaginous cover of Leegaardiella elbraechteri. Even in these cases, homology with the tintinnid lorica is rejected as it is less parsimonious. Additionally, the covers of Strombidinopsis minima and Leegaardiella elbraechteri do not enclose the cell loosely like a lorica, but are directly attached to the cell surface. Although there is apparently a great diversity in lorica structures, only five distinct types are used in our analyses. It is assumed that the ancestral tintinnids had an agglutinated and flexible lorica similar to the mineral envelope of Strombidinopsis minima.
Character 24 (division mode)
The enantiotropic division mode is the most important autapomorphy of the Oligotrichea, although a modified, probably convergently developed type is found in the prostomatid Balanion (Agatha 2004a; Foissner et al. 1990; Petz and Foissner 1993). In the Oligotrichea, it very likely resulted from the “dorsalization” process (see Character 1) which reduced the ventral side to a small determinative region, in which the oral primordium develops. The generation of the voluminous adoral zone of membranelles, however, requires some space, which is only found at or in the posterior cell portion. The Choreotrichida show a less pronounced kind of enantiotropy compared to the Halteriia and Oligotrichida (Agatha 2004a, b), which might be correlated with the formation of the oral primordium within a pouch and the circular arrangement of almost all membranelles on the peristomial rim, a structure restricted to the Choreotrichida.
Character 25 (stomatogenic mode)
Stomatogenesis takes place on the cell surface, with the exception of the entodiniomorphids (Noirot-Timothée 1960), the Hypotrichida (Foissner 1996), and the Oligotrichia (Agatha 2004a). Although transitions to a subsurface development of the oral primordium are found in the Stichotrichida (Foissner 1983), the hypoapokinetal stomatogenesis is regarded as derived state which originated convergently in the taxa mentioned above, as other argumentations are less parsimonious (Agatha 2004a). The shape of the subsurface cave, in which the oral primordium develops, probably depends on the shape of the adoral zone of membranelles, i.e., a C-shaped zone necessitates a tube (convergence to the Hypotrichida), while a closed zone requires a pouch. Accordingly, a parallel development of the closed zone and the subsurface pouch (see Character 3) is assumed.
Character 26 (curvature of oral primordium)
Agatha (2004a, b) pointed out that the rightwards curvature of the oral primordium’s anterior end supports a closer affiliation of the Halteriia with the Stichotrichida, as it is lacking or less pronounced in the Oligotrichia. On the other hand, the posterior end of the oral primordium of the Oligotrichia performs a distinct rightwards curvature, which is absent or less conspicuous in the Stichotrichida and Halteriia and thus is considered an apomorphy.
Character 27 (origin of undulating membranes)
While the undulating membranes of the Stichotrichida are generated by the oral primordium or cirral anlagen as is common in ciliates (Foissner 1996), they originate de novo in the Oligotrichea (Agatha 2004a, c).
Character 28 (origin of somatic ciliature)
Considering the “dorsalization” process (see Character 1), the somatic kineties of the Oligotrichea correspond to the dorsal kineties of the Stichotrichida. Both types of kineties are usually generated by intrakinetal proliferation of kinetids (Agatha 2004a). Therefore, the de-novo-generation of the entire somatic ciliature in the Halteriia is regarded as an apomorphy, whereas the two ventral organelles in the Tintinnina (see Character 10) are not considered here because they constitute only a minor and specialized part of the somatic ciliature.
Character 29 (reorganization of somatic ciliature)
While the somatic ciliature is usually not noticeably reconstructed during ontogenesis, the reorganization typically embraces the somatic ciliature of the ventral and dorsal side in the Stichotrichida (Foissner 1996). Due to the “dorsalization” process (see Character 1), it is assumed that the somatic ciliature of the Oligotrichea represents the dorsal kineties, which are also entirely reorganized in the Halteriia (Agatha 2004a). In the Oligotrichia, such a process, however, is not recognizable as it is indistinct or secondarily absent; thus, this feature is a retrogressive apomorphy. The Hypotrichida also lack a dorsal reorganization.
Character 30 (conjugation)
Conjugating Stichotrichida (and Hypotrichida) fuse in their anterior portions, overlapping laterally or forming an oblique angle with their main cell axes. The choreotrichid ciliate Pelagostrobilidium sp. reveals a unique interlocking arrangement of the conjugants (Ota and Taniguchi 2003), which is also found in Halteria (SA and Foissner pers. obs.). In the tintinnids, the lorica might prevent such an intimate connection of the conjugants (Laval-Peuto 1983). These findings strongly corroborate the sister group relationship inferred from the morphologic evidence (Agatha 2004a; Foissner et al. 2005; Petz and Foissner 1992). In the analyses, an interlocking arrangement of the conjugants is also assumed for the Oligotrichida.
Characters not considered
Particle arrays on somatic cilia
Bardele (1981) performed freeze-fracture studies on the particle arrays in the membrane of somatic cilia. According to these data, Strombidium (order Oligotrichida) forms a sister group to Halteria (subclass Halteriia), and the Oligotrichea cluster with the Hypotrichea, supporting the cladograms presented here. However, more data are required for consideration of this character in phylogenetic studies.
Ultrastructure of lorica wall
Transmission electron microscopic studies revealed that the lorica wall is either unilaminar (Eutintinnus: Laval-Peuto 1980, 1994), trilaminar (Codonellopsis, Cymatocylis: Wasik et al. 1997; Cyttarocylis: Laval-Peuto 1980; Helicostomella, Laackmanniella: Wasik et al. 1997; Parafavella: Hedin 1975; Wasik et al. 1997; Petalotricha: Laval-Peuto 1980, 1994; Proplectella: Laval-Peuto 1980; Tintinnopsis: Wasik et al. 1997; Undella: Laval-Peuto 1980, 1994), or composed of irregular alveoli (Favella: Hedin 1975). The wall structure might vary within a genus, e.g., Cymatocylis drygalskii and C. vanhoeffeni have a trilaminar wall, but the number of layers decreases from three at the horn to one at the lorica opening in C. convallaria/affinis (Wasik et al. 1997). Differences in the agglutination properties are not reflected by the microstructure. The ability to agglutinate particles on the entire lorica or merely on its posterior portion is a taxonomic feature, but not the density and quality of the particles (Laval-Peuto 1980; Laval-Peuto and Brownlee 1986). Primary, secondary, and tertiary structures were described from light microscopy and frequently used for the classification of tintinnids (e.g., Brandt 1906, 1907; Kofoid and Campbell 1929, 1939); however, they are without ultrastructural foundation. On the other hand, differences in the lorica microstructure caused by the chemical composition and the polymerization process are potentially useful phylogenetic and taxonomic features (Laval-Peuto 1980; Laval-Peuto and Brownlee 1986).
Kinetal density index
Laval-Peuto and Brownlee (1986) postulated a decrease in the kinetal density index (number of kineties per 10 μm of circumference) during tintinnid evolution. However, this index apparently varies distinctly within a genus (e.g., Eutintinnus: 6.4–18.3 kineties per 10 μm) and thus is rather a species-specific feature. Assuming the “dorsalization” process (see Character 1), the ancestor of the Oligotrichea had probably few somatic (dorsal) kineties, as usually only five or six are found in the Stichotrichida, about seven in the Halteriia, two in the Oligotrichida, six in the Strobilidiidae, five in the Lohmanniellidae, and four in the Leegaardiellidae. In contrast to the suggestion of Laval-Peuto and Brownlee (1986), the somatic kineties thus apparently became numerous in the Strombidinopsidae and Tintinnina.
Minute deviations in somatic ciliary patterns
Besides the differences in the presence/absence of the main ciliary components (right, left and lateral ciliary field; ventral, dorsal, and posterior kinety), subtle deviations in the ciliary patterns were observed, concerning (i) the course of the ventral (first) kinety; (ii) the position and number of dikinetids at the anterior end of the second and third kineties; (iii) the course and structure of the last kinety; (iv) the structure of the dorsal kinety/kineties; (v) the structure, position, and course of the posterior kinety; and (vi) the occurrence of fragmented kineties. In the future, these features will probably contribute to a better understanding of tintinnid evolution and a revision of the tintinnid taxonomy. Since the majority of authors, however, did not mention these details, they could not be considered in the present analyses.
Length of cilia
In the Stichotrichida, the cilia of the somatic kineties have a uniform length as in the Oligotrichea, with the exception of (i) the oligotrichids Strombidium apolatum (Wilbert and Song 2005) and Strombidium rapulum (Xu et al. 2006), in which the cilia of the ventral kinety are distinctly longer than those of the girdle kinety, (ii) the choreotrichids Strombidinopsis minima and Strombidinopsis cheshiri, in which the posterior cilia of the dikinetids are slightly longer than the anterior ones, and (iii) some tintinnids, in which the cilia of the specialized kineties (ventral organelles as well as ventral, dorsal, and posterior kinety) are usually longer than those of the ordinary ciliary rows. Additionally, the anterior dikinetids in the right and left ciliary field of tintinnids often bear an elongated cilium at the anterior or both basal bodies (soies, Fauré-Fremiet 1924). These features were not included in the analyses as the length of the cilia was rarely measured.
Reorganization of oral apparatus
In the ontogenesis of the Stichotrichida, the reorganization of the oral apparatus is a variable feature (Foissner 1996). Accordingly, the apparent persistence of the parental oral structures in the Oligotrichea is not a useful cladistic character.
Resting cysts
The resting cyst of the halteriid ciliate Meseres corlissi is five-layered and includes a metacyst (Foissner et al. 2005). It is thus not only typical for the Stichotrichida, but also for the Hypotrichida and Phacodiniida Small and Lynn, 1985, indicating a plesiomorphic state. On the other hand, the ectocyst structure, the lepidosomes, and the cyst wall composition relate M. corlissi more closely to the Oligotrichia, especially as the spines of the Pelagostrombidium cyst (order Oligotrichida; Müller 2002) are interpreted as lepidosomes, a feature which is apparently absent in cysts of the Stichotrichida. Since data on Oligotrichea cysts are generally insufficient, the phylogenetic significance of the resting cyst characters remains unknown (Foissner 2005).
The studies on M. corlissi also revealed a complete resorption of the somatic and oral ciliature during encystment (Foissner 2005) as we find it in most Stichotrichida, whereas the somatic ciliature is retained in the Hypotrichida (Lynn and Small 2002; Martin-Gonzalez et al. 1992). None of the few studies concerning the encystment and excystment of the Oligotrichia mentioned the fate of the ciliature (Fauré-Fremiet 1948; Jonsson 1994; Kim and Taniguchi 1995; Montagnes et al. 2002; Müller 1996, 2002; Müller and Wünsch 1999; Reid 1987; Reid and John 1978). Additionally, the state of this character in the ancestor of the Hypotrichea and Oligotrichea is unknown.
Hennigian argumentation scheme
Apparently, some branches in the argumentation scheme lack apomorphies (Fig. 4). Actually, (i) the taxon-specific features were not considered for the clusters of Halteria and Pelagohalteria and Leegaardiella elbraechteri and Leegaardiella sol, respectively; (ii) the details of the somatic ciliature were not taken into account for the subgenus Tintinnidium (Tintinnidium fluviatile, Tintinnidium pusillum) and the most highly developed tintinnids comprising the genera Codonella, Cymatocylis, Stenosemella, and Tintinnopsis (except for Tintinnopsis brasiliensis and Tintinnopsis cylindrata); and (iii) further data on the lorica structure and shape were not included (Cymatocylis, Nolaclusilis). Only in Meseres, Strombidinopsis, Rimostrombidium, and the cluster of Lohmanniella and the Strobilidiidae, were no apomorphies found. This might be due to the scarce knowledge about these groups or to the persistence of the virtually/essentially unchanged parental lineages (Ax 1984; Mayr and Bock 2002).
Fig. 4.
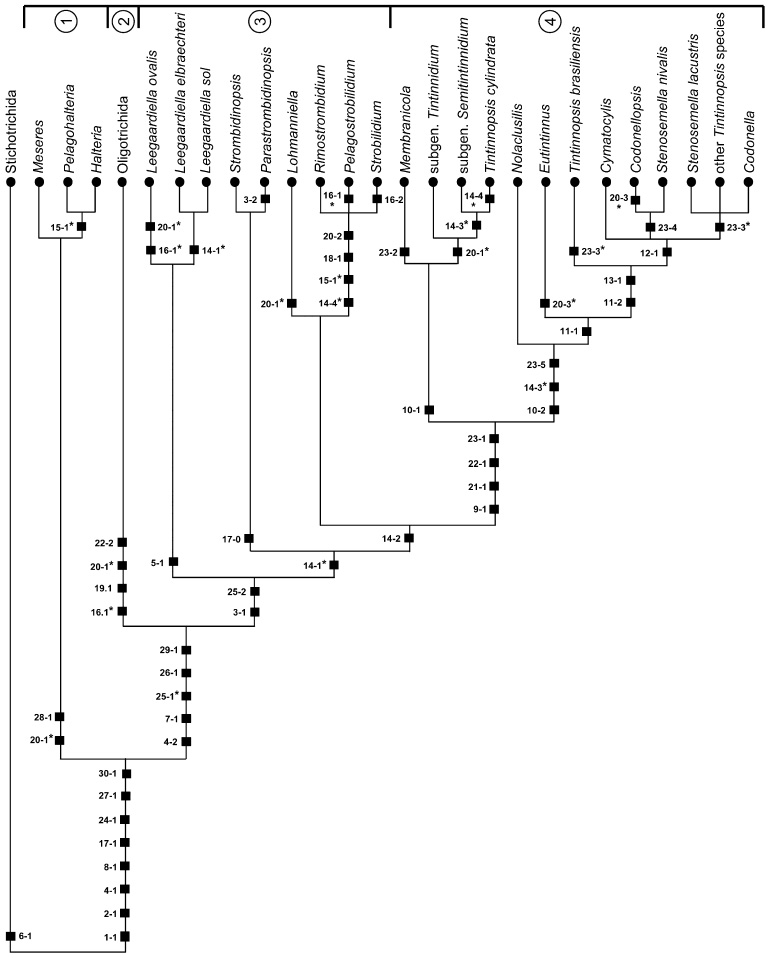
Cladistic scheme generated by the Hennigian argumentation scheme. For character coding, see Table 2 and section on character states. Black squares mark apomorphies. Asterisks denote convergences. 1, order Halteriida (class Oligotrichea, subclass Halteriia); 2, order Oligotrichida (class Oligotrichea, subclass Oligotrichia); 3, suborder Strobilidiina (class Oligotrichea, subclass Oligotrichia, order Choreotrichida); 4, suborder Tintinnina (class Oligotrichea, subclass Oligotrichia, order Choreotrichida).
The cladogram shows a paraphyly of the genus Tintinnidium due to a sister group relationship of Tintinnopsis cylindrata with the subgenus Semitintinnidium (Fig. 4). Since the generic affiliation of Tintinnopsis cylindrata is uncertain, these findings should not be overinterpreted. The genus Tintinnopsis is also paraphyletic, not only due to the position of Tintinnopsis cylindrata but also because Tintinnopsis brasiliensis lacks a posterior kinety and thus represents a sister group to the cluster of tintinnids with the most complex ciliary pattern including the remaining Tintinnopsis species.
According to illustrations of protargol-impregnated specimens, Tintinnidium mucicola has only a right and left ciliary field separated by a blank ventral stripe and composed of dikinetids with a cilium only at each posterior basal body (Laval-Peuto 1994; Laval-Peuto and Brownlee 1986; Lynn and Small 2002; Small and Lynn 1985). Thus, the species branches basal to the other tintinnids due to this simple ciliary pattern and its flexible and agglutinated lorica. The complete loss of the anterior cilia of the dikinetids probably represents an apomorphy of the species and a convergence to Membranicola.
Favella panamensis has a right, left, and lateral ciliary field as well as a ventral kinety, a posterior kinety, and two dorsal kineties as inferred from illustrations of a protargol-impregnated specimen (Lynn and Small 2002; Small and Lynn 1985). Accordingly, this species with a hyaline lorica clusters with the tintinnids possessing the most complex ciliary pattern (see above), but differs in the number of dorsal kineties (two vs. one). Three equally parsimonious trees with one convergence each can be established for tintinnids with dorsal kineties: (i) the ancestor had one dorsal kinety, and Eutintinnus and F. panamensis convergently produced a second one; (ii) the ancestor had two dorsal kineties which were reduced when the lateral ciliary field was introduced, and F. panamensis created again a second dorsal kinety; or (iii) the ancestor had two dorsal kineties which were convergently reduced to one in Tintinnopsis brasiliensis and the most complex tintinnids. Since a convergent introduction of a second kinety (case i) or a retrogressive introduction of a second dorsal kinety (case ii) seem less likely, the latter solution is favoured.
Computer analyses
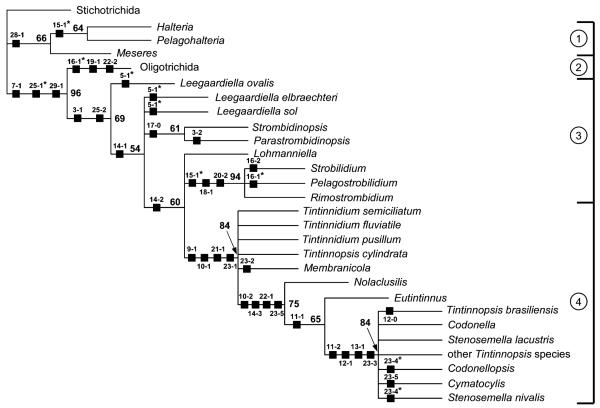
The analyses of the data matrix (Table 3), employing Hennig86 and PAUP* with equally weighted characters produced 700 trees each possibly due to the rather high rate of parsimony uninformative characters (eight out of 30 characters). Those created by Hennig86 are slightly longer (L = 59) and have slightly lower consistency (Ci = 77) and retention indices (Ri = 91) than those established with PAUP* (L = 58, Ci = 79, Ri = 93). The 126 trees found after successive approximations character weighting (SACW; Farris 1969) by PAUP* (24 characters with weight one and six characters with weight other than one) have a length of only 43, a consistency index of 89, and a retention index of 97. Nevertheless, both 50% majority-rule consensus PAUP* trees show an identical topology (Fig. 5), differing only in the bootstrap values which are usually somewhat higher after successive weighting. They are congruent with the strict consensus Hennig86 tree (not shown), except for the relationships of Leegaardiella elbraechteri and Leegaardiella sol (unresolved vs. forming a cluster with a sister group relationship to the Strombidinopsidae).
Fig. 5.
Maximum parsimony tree inferred from equally weighted morphologic data of the class Oligotrichea generated with the computer program PAUP* ver. 4.0b10 (Swofford 2002), using the Stichotrichida as outgroup. For character coding, see Table 3. The tree is the 50% majority-rule consent of 700 trees (length = 58, consistency index = 79, retention index = 93, rescaled consistency index = 73). Numbers on the branches are the bootstrap values (percentage out of 1000 replicates) for the internal nodes. Black squares mark main apomorphies. Asterisks denote convergences. 1, order Halteriida (class Oligotrichea, subclass Halteriia); 2, order Oligotrichida (class Oligotrichea, subclass Oligotrichia); 3, suborder Strobilidiina (class Oligotrichea, subclass Oligotrichia, order Choreotrichida); 4, suborder Tintinnina (class Oligotrichea, subclass Oligotrichia, order Choreotrichida).
Gene sequence analyses
The partial SSrRNA gene sequence of Rimostrombidium lacustris is 1756 nucleotides in length, has a GC content of 45%, and is available under the GenBank accession number DQ986131.
Phylogenetic analyses of gene sequences
The three phylogenetic methods (BI, MP, NJ) resulted in trees with congruent topologies; thus, only the maximum likelihood tree is shown, including all support values for the internal nodes (Fig. 6). Several signature positions can be defined in the SSrRNA gene sequence, but only a single short sequence is truly unique to the Oligotrichea (Strüder-Kypke and Lynn 2003). The newly sequenced species, Rimostrombidium lacustris, always clusters with rather high support (0.89 BI, 98% MP, 95% NJ) with Strobilidium caudatum; both species group with Pelagostrobilidium neptuni, forming a clade corresponding to the family Strobilidiidae. Strombidinopsis is the most ancestral choreotrichid genus, followed by Parastrombidinopsis which shows a sister group relationship to the Strobilidiidae; hence, the family Strombidinopsidae is paraphyletic. However, the support values are only low (0.69 BI, 30% MP, 18% NJ). The suborder Tintinnina is monophyletic and possesses several signature positions in the primary structure of the SSrRNA gene (Strüder-Kypke and Lynn 2003), although all analyses fail to give reliable support values for this clade (0.66 BI, 36% MP, 41% NJ). Tintinnidium mucicola branches basal to all other tintinnids, followed by Eutintinnus (1.0 BI, 100% MP, NJ) and Favella panamensis (0.99 BI, 97% MP, 99% NJ). The remaining topology reveals a paraphyly of the genera Tintinnopsis, Favella, and Metacylis. A more detailed discussion is given by Strüder-Kypke and Lynn (2003).
Fig. 6.
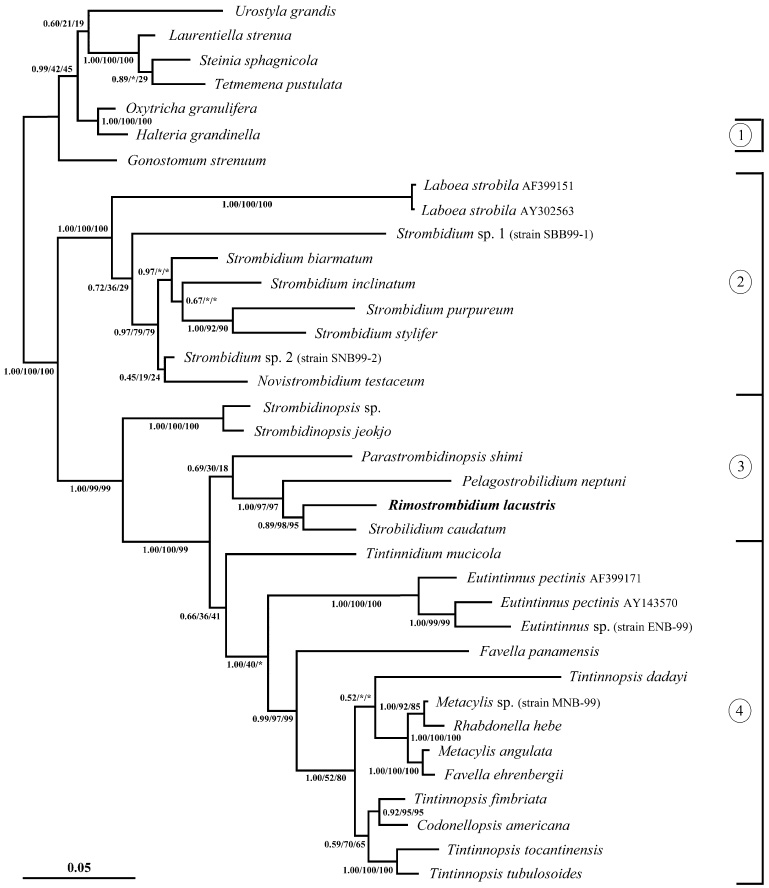
Maximum likelihood tree of SSrRNA gene sequences computed with MrBayes ver. 3.1.1 (Ronquist and Huelsenbeck 2003), based on the GTR model with gamma-distribution plus invariable sites, determined by MrModeltest (Nylander 2004). The first numbers at the nodes are the posterior probability values out of 1,000,000 trees from a maximum likelihood analysis, employing the Bayesian Inference; the second and third numbers are the bootstrap values (percent out of 1000 replicates) for the maximum parsimony (Swofford 2002) and neighbour-joining analysis (Saitou and Nei 1987), respectively. An asterisk indicates bootstrap values of <10%. The scale bar represents five expected changes per 100 positions. The new sequence appears in bold face: 1, order Halteriida (class Oligotrichea, subclass Halteriia); 2, order Oligotrichida (class Oligotrichea, subclass Oligotrichia); 3, suborder Strobilidiina (class Oligotrichea, subclass Oligotrichia, order Choreotrichida); and 4, suborder Tintinnina (class Oligotrichea, subclass Oligotrichia, order Choreotrichida).
Comparison of Hennigian scheme with computed trees
Compared with the computed genealogies, the argumentation tree provides a better resolution among the Tintinnina with ventral organelles or a lateral ciliary field (Figs. 4 and 5). Additionally, the Leegaardiellidae are monophyletic due to the bipartited collar polykinetids in the Hennigian scheme (Fig. 4), while the family is paraphyletic in the computed cladograms (Fig. 5). Likewise, the topology differs concerning the Lohmanniellidae (a sister group to the Strobilidiidae vs. an unresolved relationship to the Strobilidiidae and Tintinnina). Additionally, the Strombidinopsidae form a sister group to the cluster comprising the Lohmanniellidae, Strobilidiidae, and Tintinnina in the argumentation scheme, but represent in the computed trees a sister group to the cluster embracing Leegaardiella elbraechteri and Leegaardiella sol (Hennig86) or reveal an unresolved relationship to Leegaardiella elbraechteri, Leegaardiella sol, and a cluster composed of the Lohmanniellidae, Strobilidiidae, and Tintinnina (PAUP*; Fig. 5).
Comparison of morphologic and genetic trees
The species sets for the two approaches share eight terminal taxa, ten if Tintinnidium mucicola and Favella panamensis are included in the morphologic trees. Concerning these overlapping terminal taxa, the morphologic trees are congruent with the gene trees, except for the topology of the family Strombidinopsidae (monophyletic vs. paraphyletic; see ‘Phylogenetic analyses of gene sequences’). However, the low support values for the grouping of Parastrombidinopsis with the Strobilidiidae in the molecular phylogeny show that this cluster may be an artifact. The paraphyly of the genus Tintinnopsis is recognizable in both kinds of trees. Tintinnids with hyaline loricae are neither monophyletic nor do they represent the most highly developed group (Figs 4–6). On the contrary, the development of a hyaline lorica from an agglutinated and flexible one and followed by a partially or entirely agglutinated and stiff lorica is the most parsimonious assumption for the reconstruction of the phylogenetic trees (Figs 4–6).
Comparison with former studies
According to the interpretation of the line drawings from Brownlee’s Master’s Thesis provided by Small and Lynn (1985), Laval-Peuto and Brownlee (1986) and Laval-Peuto (1994), as well as Lynn and Small (2002), Tintinnidium mucicola has the simplest tintinnid ciliary pattern (see ‘Hennigian argumentation scheme’), while Codonella cratera, Climacocylis scalaroides, Favella panamensis (see ‘Hennigian argumentation scheme’),Protorhabdonella simplex, Stenosemella steini, Tintinnopsis baltica, and Tintinnopsis subacuta have the most complex pattern. Details of the somatic ciliature of Amphorellopsis acuta and Salpingacantha sp. are not recognizable, while the pattern of Eutintinnus pectinis roughly matches the findings of Choi et al. (1992).
In their phylogenetic considerations, Laval-Peuto and Brownlee (1986) established four tintinnid categories: (i) a plesiomorphic group with Parafavella denticulata (probably a Strombidinopsis species; see ‘Acquisition of morphological data’), Tintinnidium fluviatile, Tintinnidium mucicola, Tintinnidium pusillum, and Tintinnopsis cylindrata; (ii) a group with a high kinetal density index, high specialization of the somatic ciliature, and buccal membranelles, containing Climacocylis scalaroides, Codonella cratera, Favella panamensis, Stenosemella steini, Tintinnopsis baltica, and Tintinnopsis subacuta; (iii) a group with a low kinetal density index, short ciliary rows, and buccal membranelles, comprising Eutintinnus pectinis and Protorhabdonella simplex; and (iv) a group with a low kinetal density index, short ciliary rows, and an obliquely orientated adoral zone of membranelles, including Amphorellopsis acuta and Salpingacantha. While the first group is congruent with the phylogenetic trees in the early branching of the Strombidinopsidae and tintinnids with ventral organelles, an evolutionary sequence of the latter three categories was not provided; thus, a further comparison is impossible.
In an abstract, Pierce (1997) presented a reconstruction of tintinnid phylogeny, using Strombidinopsis acuminata as outgroup. Three groups of tintinnids were established, again without suggesting an evolutionary sequence: (i) tintinnids with ventral organelles and dikinetidal somatic kineties (Tintinnidium), (ii) tintinnids with an oblique adoral zone of membranelles, an undifferentiated ventral kinety, and dikinetidal somatic kineties (Salpingella), and (iii) tintinnids with a somatic ciliature comprising ventral kineties and ciliary rows composed of monokinetids and an anterior dikinetid (the remaining tintinnids). Pierce (1997) concluded that species with agglutinated loricae, e.g., Tintinnopsis and Stenosemella, are more derived than species with tube-like loricae, such as Eutintinnus. Concerning these three genera, the succession is congruent with the findings of the present cladistic inferences and the gene trees.
The observations that (i) hyaline and agglutinated loricae do not form distinct lineages and (ii) species with agglutinated loricae possess the most complex ciliary pattern (this study, Laval-Peuto and Brownlee 1986; Strüder-Kypke and Lynn 2003) are in conflict with the lorica-based phylogenetic models of Kofoid and Campbell (1939) and Tappan and Loeblich (1968).
Petz and Foissner (1993) regarded Codonella cratera as representative of the genus and affiliated Tintinnopsis subacuta and Tintinnopsis baltica due to a similar lorica shape and ciliary pattern, as inferred from Brownlee’s illustrations (see above). Since Tintinnopsis cylindrica also shares the most complex ciliary pattern, but has a tubular instead of an amphoriform lorica (Agatha and Riedel-Lorjé 2006), the similarity in the lorica shapes is the only feature that supports an assignment of Tintinnopsis subacuta and Tintinnopsis baltica to Codonella. Until we know much more about the variability of the cell and lorica features in tintinnids, we should thus refrain from such species transfers. Otherwise, the already large taxonomic confusion within the suborder Tintinnina will only be increased.
According to most genealogies inferred from SSrRNA and ITS genes (Agatha et al. 2004, 2005; Kim et al. 2005; Modeo et al. 2003; Snoeyenbos-West et al. 2002; Strüder-Kypke and Lynn 2003), the Choreotrichida, Strobilidiidae, and Tintinnina are monophyletic as in the findings presented here.
Taxonomic implications
Genus Tintinnidium Kent, 1881
Remarks
The genus Tintinnidium is paraphyletic in the cladistic reconstruction as Tintinnidium semiciliatum clusters with Tintinnopsis cylindrata (Fig. 4). The generic affiliation of the latter species, however, is uncertain because the ciliary pattern of the type species Tintinnopsis beroidea is unknown (Agatha and Riedel-Lorjé 2006; Laval-Peuto and Brownlee 1986; Petz and Foissner 1993). In every case, it seems reasonable to subdivide the tintinnids with ventral organelles, a flexible and agglutinated lorica without subterminal membrane and one macronuclear nodule, into two subgenera, mainly using the structure of the somatic kineties. When Tintinnopsis cylindrata will definitely be assigned to a genus, the establishment of a further subgenus for this species might also be justified.
Since the type species of the genus Tintinnidium as well as several congeners were studied after protargol impregnation (Table 1), the genus diagnosis is emended to include not only lorica characteristics but also cytological features.
Improved diagnosis
Lorica tubular, composed of gelatinous, flexible material, with embedded particles of biotic and abiotic origin. One macronucleus and one micronucleus. Somatic kineties arranged in a right and left ciliary field with dikinetids at least in the anterior portions. Two ventral organelles. Adoral zone of membranelles perpendicular to main cell axis.
Type species
Tintinnus fluviatilis Stein, 1863 (subsequent designation under the plenary powers by the International Commission on Zoological Nomenclature (ICZN) 1970, opinion 926).
Comparison with similar taxa
The occurrence of ventral organelles is apparently restricted to the freshwater genera Tintinnidium and Membranicola and the species Tintinnopsis cylindrata. Membranicola differs from Tintinnidium in a subterminal lorica membrane, and its sole species has two macronuclear nodules (Foissner et al. 1999). Tintinnopsis cylindrata has entirely monokinetidal somatic kineties, and Tintinnidium mucicola apparently lacks ventral organelles and a ventral kinety (although Laval-Peuto 1994 referred to an unspecialized dikinetidal kinety of the left ciliary field as such) and thus probably represents a distinct genus; however, the illustrations provided by Laval-Peuto (1994), Laval-Peuto and Brownlee (1986), Lynn and Small (2002), as well as Small and Lynn (1985) are insufficient for the establishment of a new genus.
Subgenus Tintinnidium Kent, 1881 nov. stat
Diagnosis
Somatic kineties entirely dikinetidal. Freely swimming.
Type species
Tintinnus fluviatilis Stein, 1863.
Remarks
Besides the type species Tintinnidium (Tintinnidium) fluviatile (Stein, 1863) Kent, 1881 (Fig. 2b), the subgenus also includes Tintinnidium (Tintinnidium) pusillum Entz, 1909.
Subgenus Semitintinnidium nov. subgen
Diagnosis
Somatic kineties dikinetidal in the anterior portion and monokinetidal in the posterior. Lorica attached to substrate.
Type species
Tintinnus semiciliatus Sterki, 1879.
Etymology
Composite of the Greek prefix semi (half) and Tintinnidium, referring to the monokinetidal posterior portion of the somatic kineties.
Comparison with related taxa
Semitintinnidium is distinguished from the subgenus Tintinnidium by the structure of the somatic kineties (dikinetidal anterior and monokinetidal posterior portion vs. entirely dikinetidal) and the life style (attached to the substrate by the lorica vs. freely swimming). Tintinnidium emarginatum redescribed by Song and Wilbert (1989) is regarded as a synonym of Tintinnidium (Semitintinnidium) semiciliatum (Sterki, 1879) Kent, 1881 (Fig. 2c; Blatterer and Foissner 1990; Foissner et al. 1991).
Family Lohmanniellidae Montagnes and Lynn, 1991
Remarks
The reinvestigation of the type species Lohmanniella oviformis necessitates an improvement of the family and genus diagnosis.
Improved diagnosis
Aloricate choreotrichids with somatic kineties restricted to the posterior cell portion and composed of dikinetids, each with a cilium only at the posterior basal body. Collar polykinetids continuous.
Type genus
Lohmanniella Leegaard, 1915.
Genus Lohmanniella Leegaard, 1915
Improved diagnosis
With characters of the family.
Type species
Lohmanniella oviformis Leegaard, 1915 (subsequent designation by Lynn and Montagnes 1988).
Comparison with similar taxa
According to the improved genus diagnosis, Rimostrombidium glacicolum Petz, Song and Wilbert, 1995 is assigned to Lohmanniella: Lohmanniella glacicola (Petz, Song and Wilbert, 1995) nov. comb. The authors already recognized that somatic kineties composed of distinctly separate dikinetids are uncommon for the genus Rimostrombidium which is characterized by kineties with condensed monokinetids (see Characters 14 and 15); additionally, the species lacks the kinetal lips typical for the Strobilidiidae (see Character 18).
Acknowledgments
The cladistic analyses were financed by the Austrian Science Foundation (FWF, Project P17752-B06), while the morphologic studies were supported by the Umweltbundesamt, Germany (Project OSHA 6.5), a grant by the Biologische Anstalt Helgoland, Germany, and the Austrian Science Foundation (FWF, Project T116-B06). Thanks go to J.C. Riedel-Lorjé (Institut für Frischwasserund Abwasserbiologie, Hamburg, Germany), M. Elbrächter (Biologische Anstalt Helgoland, Island of Sylt, Germany), and D.J.S. Montagnes (School of Biological Sciences, University of Liverpool, UK) for placing their laboratories at the senior author’s disposal. Special thanks are also given to W. Foissner, University of Salzburg, Austria, for his constructive criticism. We also want to thank D.H. Lynn in whose laboratory the molecular work was conducted and who also partly supported the molecular study with a grant from the Natural Sciences and Engineering Research Council (NSERC).
References
- Agatha S. Morphology and ontogenesis of Novistrombidium apsheronicum nov. comb. and Strombidium arenicola (Protozoa, Ciliophora): a comparative light microscopical and SEM study. Eur. J. Protistol. 2003a;39:245–266. [Google Scholar]
- Agatha S. Redescription of Strombidinopsis minima (Gruber, 1884) Lynn et al., 1991 (Protozoa, Ciliophora), with notes on its ontogenesis and distribution. Eur. J. Protistol. 2003b;39:233–244. [Google Scholar]
- Agatha S. A cladistic approach for the classification of oligotrichid ciliates (Ciliophora: Spirotricha) Acta Protozool. 2004a;43:201–217. [PMC free article] [PubMed] [Google Scholar]
- Agatha S. Evolution of ciliary patterns in the Oligotrichida (Ciliophora, Spirotricha) and its taxonomic implications. Zoology. 2004b;107:153–168. doi: 10.1016/j.zool.2004.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agatha S. New observations on the tontoniid ciliate Spirotontonia grandis (Suzuki and Han, 2000) Agatha, 2004 (Ciliophora, Oligotrichida, Tontoniidae); comparison with the similar Laboea strobila. Eur. J. Protistol. 2004c;40:295–301. [Google Scholar]
- Agatha S, Riedel-Lorjé JC. Morphology, infraciliature, and ecology of some strobilidiine ciliates (Ciliophora, Oligotrichea) from coastal brackish water basins of Germany. Eur. J. Protistol. 1998;34:10–17. [Google Scholar]
- Agatha S, Riedel-Lorjé JC. Redescription of Tintinnopsis cylindrica Daday, 1887 (Ciliophora, Spirotricha) and unification of tintinnid terminology. Acta Protozool. 2006;45:137–151. [PMC free article] [PubMed] [Google Scholar]
- Agatha S, Strüder-Kypke MC, Beran A. Morphologic and genetic variability in the marine planktonic ciliate Laboea strobila Lohmann, 1908 (Ciliophora, Oligotrichia), with notes on its ontogenesis. J. Eukaryot. Microbiol. 2004;51:267–281. doi: 10.1111/j.1550-7408.2004.tb00567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agatha S, Strüder-Kypke MC, Beran A, Lynn DH. Pelagostrobilidium neptuni (Montagnes and Taylor, 1994) and Strombidium biarmatum nov. spec. (Ciliophora, Oligotrichea): phylogenetic position inferred from morphology, ontogenesis, and gene sequence data. Eur. J. Protistol. 2005;41:65–83. doi: 10.1016/j.ejop.2004.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alekperov IK, Asadullayeva ES. New and little-known ciliates (orders Nassulida – Oligotrichida) from the Caspian Sea Apsheronian coast Communication 2. Zool. Zh. 1997;76:1411–1417. (in Russian with English summary) [Google Scholar]
- Ax P. Systematisierung der lebenden Natur aufgrund ihrer Phylogenese. G. Fischer Verlag; Stuttgart: 1984. Das phylogenetische System. [Google Scholar]
- Bardele CF. Functional and phylogenetic aspects of the ciliary membrane: a comparative freeze-fracture study. Biosystems. 1981;14:403–421. doi: 10.1016/0303-2647(81)90046-0. [DOI] [PubMed] [Google Scholar]
- Berger H. Monographiae biol. 78. Kluwer Academic Publishers; Dordrecht: 1999. Monograph of the Oxytrichidae (Ciliophora, Hypotrichia) [Google Scholar]
- Bernatzky G, Foissner W, Schubert G. Rasterelektronenmikroskopische und biometrische Untersuchungen über die Variabilität der Form, Struktur und Größe des Gehäuses einiger limnischer Tintinnina (Protozoa, Ciliophora) Zool. Scr. 1981;10:81–90. [Google Scholar]
- Bernhard D, Stechmann A, Foissner W, Ammermann D, Hehn M, Schlegel M. Phylogenetic relationships within the class Spirotrichea (Ciliophora) inferred from small subunit rRNA gene sequences. Mol. Phylogen. Evol. 2001;21:86–92. doi: 10.1006/mpev.2001.0997. [DOI] [PubMed] [Google Scholar]
- Biernacka I. Studia nad rozrodem nicktórych gatunków rodzaju Tintinnopsis Stein (Studies on the reproduction of some species of the genus Tintinnopsis Stein) Ann. Univ. Mariae Curie – Sklodowska. 1952;6:211–247. (with Russian and English summary) [Google Scholar]
- Blatterer H, Foissner W. Beiträge zur Ciliatenfauna (Protozoa: Ciliophora) der Amper (Bayern, Bundesrepublik Deutschland) Arch. Protistenkd. 1990;138:93–115. (with English summary) [Google Scholar]
- Brandt K. Die Tintinnodeen der Plankton-Expedition. Tafelerklärungen nebst kurzer Diagnose der neuen Arten. In: Hensen V, editor. Ergeb. Plankton-Exped. Humboldt-Stiftung. vol. 3. La. Lipsius and Tischer; Kiel, Leipzig: 1906. pp. 1–33. + plates 1-70. [Google Scholar]
- Brandt K. Die Tintinnodeen der Plankton-Expedition. Systematischer Teil. In: Hensen V, editor. Ergeb. Plankton-Exped. Humboldt-Stiftung. Vol. 3. La. Lipsius and Tischer; Kiel, Leipzig: 1907. pp. 1–488. [Google Scholar]
- Brownlee DC. Stomatogenesis in the tintinnine ciliates with notes on lorica formation. J. Protozool. 1983;30:1A. [Google Scholar]
- Burkovsky IV. Variability of Parafavella denticulata in the White Sea. Zool. Zh. 1973;52:1277–1285. (in Russian with English summary) [Google Scholar]
- Cai S, Song W, Xu D, Chiang K. Morphological studies on the infraciliature of a planktonic ciliate, Tintinnopsis brasiliensis (Ciliophora: Tintinina) J. Ocean Univ. China. 2006;5:55–57. [Google Scholar]
- Campbell AS. The cytology of Tintinnopsis nucula (Fol) Laackmann with an account of its neuromotor apparatus, division, and a new intranuclear parasite. Univ. Calif. Publ. Zool. 1926;29:179–237. [Google Scholar]
- Campbell AS. Studies on the marine ciliate Favella (Jörgensen), with special regard to the neuromotor apparatus and its rôle in the formation of the lorica. Univ. Calif. Publ. Zool. 1927;29:429–452. [Google Scholar]
- Capriulo GM, Taveras J, Gold K. Ciliate feeding: effect of food presence or absence on occurrence of striae in tintinnids. Mar. Ecol. Prog. Ser. 1986;30:145–158. [Google Scholar]
- Choi JK, Coats DW, Brownlee DC, Small EB. Morphology and infraciliature of three species of Eutintinnus (Ciliophora; Tintinnina) with guidelines for interpreting protargol-stained tintinnine ciliates. J. Protozool. 1992;39:80–92. [Google Scholar]
- Coats DW, Heinbokel JF. A study of reproduction and other life cycle phenomena in planktonic protists using an acridine orange fluorescence technique. Mar. Biol. 1982;67:71–79. [Google Scholar]
- von Daday E. Monographie der Familie der Tintinnodeen. Mitt. zool. Stn Neapel. 1887;7:473–591. +plates 18-21. [Google Scholar]
- Dale T, Lynn DH. Stomatogenesis of the ciliate genus Strombidinopsis with an improved description of S. spiniferum and S. acuminatum. J. Eukaryot. Microbiol. 1998;45:210–217. [Google Scholar]
- Davis CC. Variations of the lorica in the genus Parafavella (Protozoa: Tintinnida) in northern Norway waters. Can. J. Zool. 1978;56:1822–1827. [Google Scholar]
- Davis CC. Variations of lorica shape in the genus Ptychocylis (Protozoa: Tintinnina) in relation to species identification. J. Plankton Res. 1981;3:433–443. [Google Scholar]
- De Rijk P, De Wachter R. DCSE v2.54, an interactive tool for sequence alignment and secondary structure research. Comput. Applic. Biosci. 1993;9:735–740. doi: 10.1093/bioinformatics/9.6.735. [DOI] [PubMed] [Google Scholar]
- Deroux G. Quelques précisions sur Strobilidium gyrans Schewiakoff. Cah. Biol. Mar. 1974;15:571–588. [Google Scholar]
- Elwood HJ, Olsen GJ, Sogin ML. The small-subunit ribosomal RNA gene sequences from the hypotrichous ciliates Oxytricha nova and Stylonychia pustulata. Mol. Biol. Evol. 1985;2:399–410. doi: 10.1093/oxfordjournals.molbev.a040362. [DOI] [PubMed] [Google Scholar]
- Entz G., Sr. Über Infusorien des Golfes von Neapel. Mitt. zool. Stn Neapel. 1884;5:289–444. +plates 20-25. [Google Scholar]
- Entz G., Sr. Zur näheren Kenntnis der Tintinnoden. Mitt. zool. Stn Neapel. 1885;6:185–216. [Google Scholar]
- Entz G., Jr. Die Süsswasser-Tintinniden. Math. Naturw. Ber. Ung. 1909a;25:197–225. [Google Scholar]
- Entz G., Jr. Studien über Organisation und Biologie der Tintinniden. Arch. Protistenkd. 1909b;15:93–226. +plates 8-21. [Google Scholar]
- Entz G., Jr. Über Struktur und Funktion der Membranulae der Tintinniden, speziell von Petalotricha ampulla. Xth Congress of International Zoology; Evertébrés. 1929. pp. 887–895. Section V. [Google Scholar]
- Entz G., Jr. Fibrillen in “Favella Ehrenbergii” Jörgensen (“Ciliata, Oligotricha”). International Congress of Zoology; Lisboa. 1937.1937. pp. 1446–1448. [Google Scholar]
- Farris JS. A successive approximation approach to character weighting. Syst. Zool. 1969;18:374–385. [Google Scholar]
- Fauré-Fremiet E. Le Tintinnidium inquilinum. Arch. Protistenkd. 1908;11:225–251. +plate 12. [Google Scholar]
- Fauré-Fremiet E. Contribution a la connaissance des infusoires planktoniques. Bull. Biol. Fr. Belg. Suppl. 1924;6:1–171. [Google Scholar]
- Fauré-Fremiet E. Le rythme de marée du Strombidium oculatum Gruber. Bull. Biol. Fr. Belg. 1948;82:3–23. [PubMed] [Google Scholar]
- Fauré-Fremiet E, Ganier M-C. Structure fine du Strombidium sulcatum Cl. et L. (Ciliata Oligotrichida). Protistologica. 1970;6:207–223. (with English summary) [PubMed] [Google Scholar]
- Felsenstein J. PHYLIP (Phylogeny Interference Package) version 3.6a2. Department of Genetics, University of Washington; Seattle, Washington: 2004. Distributed by the author. [Google Scholar]
- Foissner W. Morphologie und Morphogenese von Psilotricha succisa (O. F. Müller, 1786) nov. comb. (Ciliophora, Hypotrichida) Protistologica. 1983;19:479–493. [Google Scholar]
- Foissner W. Ontogenesis in ciliated protozoa, with emphasis on stomatogenesis. In: Hausmann K, Bradbury PC, editors. Ciliates: Cells as Organisms. G. Fischer Verlag; Stuttgart: 1996. pp. 95–177. [Google Scholar]
- Foissner W. The unusual, lepidosome-coated resting cyst of Meseres corlissi (Ciliophora: Oligotrichea): transmission electron microscopy and phylogeny. Acta Protozool. 2005;44:217–230. [Google Scholar]
- Foissner W, Al-Rasheid K. A unified organization of the stichotrichine oral apparatus, including a description of the buccal seal (Ciliophora: Spirotrichea) Acta Protozool. 2006;45:1–16. [Google Scholar]
- Foissner W, O’Donoghue PJ. Morphology and infraciliature of some freshwater ciliates (Protozoa: Ciliophora) from Western and South Australia. Invertebr. Taxon. 1990;3:661–696. [Google Scholar]
- Foissner W, Wilbert N. Morphologie, Infraciliatur und Ökologie der limnischen Tintinnina: Tintinnidium fluviatile Stein, Tintinnidium pusillum Entz, Tintinnopsis cylindrata Daday und Codonella cratera (Leidy) (Ciliophora, Polyhymenophora) J. Protozool. 1979;26:90–103. [Google Scholar]
- Foissner W, Skogstad A, Pratt JR. Morphology and infraciliature of Trochiliopsis australis n. sp., Pelagohalteria viridis (Fromentel, 1876) n. g., n. comb., and Strobilidium lacustris n. sp. (Protozoa, Ciliophora) J. Protozool. 1988;35:489–497. [Google Scholar]
- Foissner W, Oleksiv I, Müller H. Morphologie und Infraciliatur einiger Ciliaten (Protozoa: Ciliophora) aus stagnierenden Gewässern. Arch. Protistenkd. 1990;138:191–206. [Google Scholar]
- Foissner W, Blatterer H, Berger H, Kohmann F. Band I: Cyrtophorida, Oligotrichida, Hypotrichia, Colpodea. Informationsberichte des Bayer. Landesamtes für Wasserwirtschaft; 1991. Taxonomische und ökologische Revision der Ciliaten des Saprobiensystems. 1/91. [Google Scholar]
- Foissner W, Berger H, Schaumburg J. Informationsberichte des Bayer. Landesamtes für Wasserwirtschaft; 1999. Identification and ecology of limnetic plankton ciliates. 3/99. [Google Scholar]
- Foissner W, Agatha S, Berger H. Soil Ciliates (Protozoa, Ciliophora) from Namibia (Southwest Africa), with Emphasis on Two Contrasting Environments, the Etosha Region and the Namib Desert. Part I: Text and Line Drawings. Part II: Photographs, Denisia. 2002;5 [Google Scholar]
- Foissner W, Moon-van der Staay SY, van der Staay GWM, Hackstein JHP, Krautgartner W-D, Berger H. Reconciling classical and molecular phylogenies in the stichotrichines (Ciliophora, Spirotrichea), including new sequences from some rare species. Eur. J. Protistol. 2004;40:265–281. [Google Scholar]
- Foissner W, Müller H, Weisse T. The unusual, lepidosome-coated resting cyst of Meseres corlissi (Ciliophora: Oligotrichea): light and scanning electron microscopy, cytochemistry. Acta Protozool. 2005;44:201–215. [Google Scholar]
- Gold K. Scanning electron microscopy of Tintinnopsis parva: studies on particle accumulation and the striae. J. Protozool. 1979;26:415–419. [Google Scholar]
- Gold K, Morales EA. Seasonal changes in lorica sizes and the species of Tintinnida in the New York Bight. J. Protozool. 1975;22:520–528. [Google Scholar]
- Grain J. Etude ultrastructurale d’Halteria grandinella O.F.M., (cilié oligotriche) et considérations phylogénétiques. Protistologica. 1972;8:179–197. [Google Scholar]
- Greuet C, Gayol P, Salvano P, Laval-Peuto M. Preliminary report on the ultrastructural organization of the contractile appendix of Tontonia appendiculariformis (Ciliophora Oligotrichina) Cell Motil. 1986;6:217–224. [Google Scholar]
- Grim JN. A protargol study of the fiber system of the ciliate Halteria. Trans. Am. microsc. Soc. 1974;93:421–425. [Google Scholar]
- Grim JN. The kinetid structures of the choreotrichous ciliate Strobilidium velox and an assessment of its evolutionary lineage. J. Protozool. 1987;34:117–123. [Google Scholar]
- Haeckel E. Ueber einige neue pelagische Infusorien. Jen. Z. Naturw. 1873;7:561–568. +plates 27, 28. [Google Scholar]
- Hausmann K, Kaiser J. Arrangement and structure of plates in the cortical alveoli of the hypotrich ciliate, Euplotes vannus. J. Ultrastruct. Res. 1979;67:15–22. doi: 10.1016/s0022-5320(79)80013-1. [DOI] [PubMed] [Google Scholar]
- Hedin H. On the ultrastructure of Favella ehrenbergii (Claparède & Lachmann) and Parafavella gigantea (Brandt), Protozoa, Ciliata, Tintinnida. Zoon. 1975;3:11–18. [Google Scholar]
- Hedin H. Examination of the tintinnid ciliate Parafavella denticulata (Ehrenberg) by scanning electron microscopy and the Bodian protargol technique. Acta Zool. 1976a;57:113–118. [Google Scholar]
- Hedin H. Microtubules and microfilaments in the tintinnid ciliate Ptychocylis minor Jörgensen. Zoon. 1976b;4:3–10. [Google Scholar]
- Hennig W. Phylogenetic Systematics. University of Illinois Press; Urbana, Chicago, London: 1966. [Google Scholar]
- Hewitt EA, Müller KM, Cannone J, Hogan DJ, Gutell R, Prescott DM. Phylogenetic relationships among 28 spirotrichous ciliates documented by rDNA. Mol. Phylogen. Evol. 2003;29:258–267. doi: 10.1016/s1055-7903(03)00097-6. [DOI] [PubMed] [Google Scholar]
- Hofker J. Studien über Tintinnoidea. Arch. Protistenkd. 1931;75:315–402. [Google Scholar]
- Huelsenbeck JP, Ronquist F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics. 2001;17:754–755. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- International Commission on Zoological Nomenclature (ICZN) Opinion 926. Tintinnidium Kent, 1881, and Leprotintinnus Jörgensen, 1900 (Ciliophora): designation of type-species under the plenary powers. Bull. Zool. Nom. 1970;27:89–90. [Google Scholar]
- Jeong HJ, Kim JS, Kim S, Song JY, Lee I, Lee G-H. Strombidinopsis jeokjo n. sp. (Ciliophora: Choreotrichida) from the coastal waters off western Korea: morphology and small subunit ribosomal DNA gene sequence. J. Eukaryot. Microbiol. 2004;51:451–455. doi: 10.1111/j.1550-7408.2004.tb00393.x. [DOI] [PubMed] [Google Scholar]
- Jonsson PR. Tidal rhythm of cyst formation in the rock pool ciliate Strombidium oculatum Gruber (Ciliophora, Oligotrichida): a description of the functional biology and an analysis of the tidal synchronization of encystment. J. Exp. Mar. Biol. Ecol. 1994;175:77–103. [Google Scholar]
- Jörgensen E. Ciliata. Tintinnidae. Tierwelt N.- und Ostsee. 1927;8:1–26. (Teil II. c1) [Google Scholar]
- Kim Y-O, Taniguchi A. Excystment of the oligotrich ciliate Strombidium conicum. Aquat. Microb. Ecol. 1995;9:149–156. [Google Scholar]
- Kim JS, Jeong HJ, Strüder-Kypke MC, Lynn DH, Kim S, Kim JH, Lee SH. Parastrombidinopsis shimi n. gen., n. sp. (Ciliophora: Choreotrichia) from the coastal waters of Korea: morphology and small subunit ribosomal DNA sequence. J. Eukaryot. Microbiol. 2005;52:514–522. doi: 10.1111/j.1550-7408.2005.00062.x. [DOI] [PubMed] [Google Scholar]
- Kimura M. A simple model for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980;16:111–120. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- Kofoid CA, Campbell AS. A conspectus of the marine and fresh-water Ciliata belonging to the suborder Tintinnoinea, with descriptions of new species principally from the Agassiz Expedition to the eastern tropical Pacific 1904-1905. Univ. Calif. Publ. Zool. 1929;34:1–403. [Google Scholar]
- Kofoid CA, Campbell AS. Reports on the scientific results of the expedition to the eastern tropical Pacific, in charge of Alexander Agassiz, by the US Fish Commission Steamer “Albatross,” from October, 1904, to March, 1905, Lieut.-Commander L.N. Garrett, U.S.N., Commanding. 37. The Ciliata: The Tintinnoinea. Bull. Mus. Comp. Zool., Harvard. 1939;84:1–473. +plates 1–36. [Google Scholar]
- Kormos J, Kormos K. Die Zellteilungstypen der Protozoen. Acta Biol. Hung. 1958;8:127–148. [Google Scholar]
- Krainer K-H. Taxonomische Untersuchungen an neuen und wenig bekannten planktischen Ciliaten (Protozoa: Ciliophora) aus Baggerseen in Österreich. Lauterbornia. 1995;21:39–68. [Google Scholar]
- Laackmann H. Ungeschlechtliche und geschlechtliche Fortpflanzung der Tintinnen. Wiss. Meeresunters., Abt. Kiel. 1906;10:13–38. +plates 1-3. [Google Scholar]
- Laval M. Mise en évidence par la microscopie électronique d’un organite d’un type nouveau chez les ciliés Tintinnides. C. r. hebd. Séanc. Acad. Sci., Paris, Série D. 1971;273:1383–1386. +plates 1, 2. [PubMed] [Google Scholar]
- Laval M. Ultrastructure de Petalotricha ampulla (Fol) Comparaison avec d’autres Tintinnides et avec les autres ordres de ciliés. Protistologica. 1972;8:369–386. (with English summary) [Google Scholar]
- Laval-Peuto M. Cortex, périlemme et réticulum vésiculeux de Cyttarocylis brandti (cilié Tintinnide). Les ciliés a périlemme. Protistologica. 1975;11:83–98. (with English summary) [Google Scholar]
- Laval-Peuto M. Ultrastructure de la ciliature et de l’infraciliature adorales de Cyttarocylis brandti (cilié) J. Protozool. 1976;23:13A. [Google Scholar]
- Laval-Peuto M. Ultrastructure of the lorica-wall of Tintinnina (Ciliata, Spirotricha) J. Protozool. 1980;27:83A. [Google Scholar]
- Laval-Peuto M. Construction of the lorica in Ciliata Tintinnina. In vivo study of Favella ehrenbergii: variability of the phenotypes during the cycle, biology, statistics, biometry. Protistologica. 1981;17:249–272. (with French summary) [Google Scholar]
- Laval-Peuto M. Sexual reproduction in Favella ehrenbergii (Ciliophora, Tintinnina) Taxonomical implications. Protistologica. 1983;19:503–512. (with French summary) [Google Scholar]
- Laval-Peuto M, Barria de Cao MS. Les capsules, extrusomes caracteristiques des Tintinnina (Ciliophora), permettent une classification evolutive des genres et des familles du sous-ordre. Ile Réun. Scientif. GRECO 88, Trav. C.R.M. 1987;8:53–59. [Google Scholar]
- Laval-Peuto M, Brownlee DC. Identification and systematics of the Tintinnina (Ciliophora): evaluation and suggestions for improvement. Ann. Inst. Océanogr., Paris. 1986;62:69–84. (with French summary) [Google Scholar]
- Laval-Peuto M, Febvre M. On plastid symbiosis in Tontonia appendiculariformis (Ciliophora, Oligotrichina) Biosystems. 1986;19:137–158. doi: 10.1016/0303-2647(86)90026-2. [DOI] [PubMed] [Google Scholar]
- Laval-Peuto M, Gold K, Storm ER. The ultrastructure of Tintinnopsis parva. Trans. Am. microsc. Soc. 1979;98:204–212. [Google Scholar]
- Laval-Peuto M, Salvano P, Gayol P, Greuet C. Mixotrophy in marine planktonic ciliates: ultrastructural study of Tontonia appendiculariformis (Ciliophora, Oligotrichina) Mar. Microb. Food Webs. 1986;1:81–104. [Google Scholar]
- Laval-Peuto M. Classe des Oligotrichea Bütschli, 1887. Ordre des Tintinnida Kofoid et Campbell, 1929. In: de Puytorac P, editor. Traité de Zoologie. Anatomie, systématique, biologie. II. Infusoires ciliés. 2. Systématique. Masson; Paris: 1994. pp. 181–219. [Google Scholar]
- Lei Y, Xu K, Song W. Free living ciliates from marine farming ponds. In: Song W, editor. Progress in Protozoology. Qingdao Ocean University Press; Qingdao: 1999. pp. 269–295. (in Chinese) [Google Scholar]
- Lynn DH, Montagnes DJS, Small EB. Taxonomic descriptions of some conspicuous species in the family Strombidiidae (Ciliophora: Oligotrichida) from the Isles of Shoals, Gulf of Maine. J. Mar. Biol. Ass. UK. 1988;68:259–276. [Google Scholar]
- Lynn DH, Montagnes DJS, Dale T, Gilron GL, Strom SL. A reassessment of the genus Strombidinopsis (Ciliophora, Choreotrichida) with descriptions of four new planktonic species and remarks on its taxonomy and phylogeny. J. Mar. Biol. Ass. UK. 1991;71:597–612. [Google Scholar]
- Lynn DH, Montagnes DJS. Taxonomic descriptions of some conspicuous species of strobilidiine ciliates (Ciliophora: Choreotrichida) from the Isles of Shoals, Gulf of Maine. J. Mar. Biol. Ass. UK. 1988;68:639–658. [Google Scholar]
- Lynn DH, Small EB. Phylum Ciliophora Doflein, 1901. In: Lee JJ, Leedale GF, Bradbury P, editors. An Illustrated Guide to the Protozoa. second ed. Vol. 2000. Allen Press; Lawrence: 2002. pp. 371–656. Organisms Traditionally Referred to as Protozoa, or Newly Discovered Groups. Society of Protozoologists. [Google Scholar]
- Maeda M. An illustrated guide to the species of the Families Halteriidae and Strobilidiidae (Oligotrichida, Ciliophora), free swimming protozoa common in the aquatic environment. Bull. Ocean Res. Inst., Univ. Tokyo. 1986;21:1–67. [Google Scholar]
- Martin AJ, Montagnes DJS. Winter ciliates in a British Columbian fjord: six new species and an analysis of ciliate putative prey. J. Eukaryot. Microbiol. 1993;40:535–549. [Google Scholar]
- Martin-Gonzalez A, Benitez L, Gutierrez JC. Ultrastructural analysis of resting cysts and encystment in Colpoda inflata. 2 Encystment process and a review of ciliate resting cyst classification. Cytobios. 1992;72:93–106. [Google Scholar]
- Mayr E, Bock WJ. Classifications and other ordering systems. J. Zool. Syst. Evol. Res. 2002;40:169–194. [Google Scholar]
- Medlin L, Elwood HJ, Stickel S, Sogin ML. The characterization of enzymatically amplified eukaryotic 16S-like rRNA-coding regions. Gene. 1988;71:491–499. doi: 10.1016/0378-1119(88)90066-2. [DOI] [PubMed] [Google Scholar]
- Merkle H. Untersuchung an Tintinnodeen der Ost- und Nordsee. Wiss. Meeresunters., Abt. Kiel. 1909;11:139–186. +plates 2, 3. [Google Scholar]
- Mirabdullaev IM. Two new species of the oligociliated infusorians (Ciliophora, Oligotrichida) from water reservoirs of Uzbekistan. Zool. Zh. 1985;64:1892–1893. (in Russian with English summary) [Google Scholar]
- Modeo L, Petroni G, Rosati G, Montagnes DJS. A multidisciplinary approach to describe protists: redescriptions of Novistrombidium testaceum Anigstein 1914 and Strombidium inclinatum Montagnes, Taylor, and Lynn 1990 (Ciliophora, Oligotrichia) J. Eukaryot. Microbiol. 2003;50:175–189. doi: 10.1111/j.1550-7408.2003.tb00114.x. [DOI] [PubMed] [Google Scholar]
- Montagnes DJS, Taylor FJR. The salient features of five marine ciliates in the class Spirotrichea (Oligotrichia), with notes on their culturing and behaviour. J. Eukaryot. Microbiol. 1994;41:569–586. [Google Scholar]
- Montagnes DJS, Lowe CD, Poulton A, Jonsson PR. Redescription of Strombidium oculatum Gruber 1884 (Ciliophora, Oligotrichia) J. Eukaryot. Microbiol. 2002;49:329–337. doi: 10.1111/j.1550-7408.2002.tb00379.x. [DOI] [PubMed] [Google Scholar]
- Müller H. Encystment of the freshwater ciliate Pelagostrombidium fallax (Ciliophora, Oligotrichida) in laboratory culture. Aquat. Microb. Ecol. 1996;11:289–295. [Google Scholar]
- Müller H. Laboratory study of the life cycle of a freshwater strombidiid ciliate. Aquat. Microb. Ecol. 2002;29:189–197. [Google Scholar]
- Müller H, Wünsch C. Seasonal dynamics of cyst formation of pelagic strombidiid ciliates in a deep prealpine lake. Aquat. Microb. Ecol. 1999;17:37–47. [Google Scholar]
- Noirot-Timothée C. Étude d’une famille de ciliés: les “Ophryoscolecidae”. Structures et ultrastructures. Ann. Sci. Nat. (Zool.) 1960;12:527–718. [Google Scholar]
- Nylander JAA. MrModeltest v2. Program distributed by the author. Evolutionary Biology Centre, Uppsala University; Uppsala, Sweden: 2004. [Google Scholar]
- Ota T, Taniguchi A. Conjugation in the marine aloricate oligotrich Pelagostrobilidium (Ciliophora: Oligotrichia) Eur. J. Protistol. 2003;39:149–160. [Google Scholar]
- Petz W, Foissner W. Morphology and morphogenesis of Strobilidium caudatum (Fromentel), Meseres corlissi n. sp., Halteria grandinella (Müller), and Strombidium rehwaldi n. sp., and a proposed phylogenetic system for oligotrich ciliates (Protozoa, Ciliophora) J. Protozool. 1992;39:159–176. [Google Scholar]
- Petz W, Foissner W. Morphogenesis in some freshwater tintinnids (Ciliophora, Oligotrichida) Eur. J. Protistol. 1993;29:106–120. doi: 10.1016/S0932-4739(11)80303-2. [DOI] [PubMed] [Google Scholar]
- Petz W, Song W, Wilbert N. Taxonomy and Ecology of the Ciliate Fauna (Protozoa, Ciliophora) in the Endopagial and Pelagial of the Weddell Sea, Antarctica. Stapfia. 1995;40 [Google Scholar]
- Pierce RW. Revised phylogeny and systematics of tintinnids based on infraciliature. J. Eukaryot. Microbiol. 1997;44(Suppl.):12A. [Google Scholar]
- Poe S, Wiens JJ. Character selection and the methodology of morphological phylogenetics. In: Wiens JJ, editor. Phylogenetic Analysis of Morphological Data. Smithsonian Institution Press; Washington, London: 2000. pp. 20–36. [Google Scholar]
- Posada D, Crandall KA. MODELTEST: testing the model of DNA substitution. Bioinformatics. 1998;14:817–818. doi: 10.1093/bioinformatics/14.9.817. [DOI] [PubMed] [Google Scholar]
- Raikov IB. Cell Biology Monographs 9. Springer; Wien: 1982. The Protozoan Nucleus, Morphology and Evolution. [Google Scholar]
- Reid PC. Mass encystment of a planktonic oligotrich ciliate. Mar. Biol. 1987;95:221–230. [Google Scholar]
- Reid PC, John AWG. Tintinnid cysts. J. Mar. Biol. Ass. UK. 1978;58:551–557. +plate 1. [Google Scholar]
- Ronquist F, Huelsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Schlegel M, Elwood HJ, Sogin ML. Molecular evolution in hypotrichous ciliates: sequence of the small subunit ribosomal RNA genes from Onychodromus quadricornutus and Oxytricha granulifera (Oxytrichidae, Hypotrichida, Ciliophora) J. Mol. Evol. 1991;32:64–69. doi: 10.1007/BF02099930. [DOI] [PubMed] [Google Scholar]
- Schweyer A. Zur Kenntnis des Tintinnodeenweichkörpers, nebst einleitenden Worten über die Hülsenstruktur und die Hülsenbildung. Arch. Protistenkd. 1909;18:134–189. +plates 10, 11. [Google Scholar]
- Shin MK, Hwang UW, Kim W, Wright A-DG, Krawczyk C, Lynn DH. Phylogenetic position of the ciliates Phacodinium (order Phacodiniida) and Protocruzia (subclass Protocruziidia) and systematics of the spirotrich ciliates examined by small subunit ribosomal RNA gene sequences. Eur. J. Protistol. 2000;36:293–302. [Google Scholar]
- Small EB, Lynn DH. Phylum Ciliophora Doflein, 1901. In: Lee JJ, Hutner SH, Bovee EC, editors. An Illustrated Guide to the Protozoa. Society of Protozoologists, Allen Press; Lawrence, Kansas: 1985. pp. 393–575. [Google Scholar]
- Sniezek JH, Capriulo GM, Small EB, Russo A. Nolaclusilis hudsonicus n. sp. (Nolaclusiliidae n. fam.) a bilaterally symmetrical tintinnine ciliate from the lower Hudson River estuary. J. Protozool. 1991;38:589–594. [Google Scholar]
- Snoeyenbos-West OLO, Salcedo T, McManus GB, Katz LA. Insights into the diversity of choreotrich and oligotrich ciliates (Class: Spirotrichea) based on genealogical analyses of multiple loci. Int. J. Syst. Evol. Microbiol. 2002;52:1901–1913. doi: 10.1099/00207713-52-5-1901. [DOI] [PubMed] [Google Scholar]
- Snyder RA, Brownlee DC. Nolaclusilis bicornis n. g., n. sp. (Tintinnina: Tintinnidiidae): a tintinnine ciliate with novel lorica and cell morphology from the Chesapeake Bay estuary. J. Protozool. 1991;38:583–589. [Google Scholar]
- Snyder RA, Ohman MD. Description of a new species of Strombidinopsidae (Ciliophora: Choreotrichida) from coastal waters of Southern California, USA. Trans. Am. microsc. Soc. 1991;110:237–243. [Google Scholar]
- Sokolova YY, Gerassimova ZP. The ultrastructure of the ciliates Parafavella denticulata Ehrenberg, 1840. Tsitologiya. 1984;26:1237–1245. +plates 1-3 (in Russian with English summary) [Google Scholar]
- Song W, Bradbury PC. Studies on some new and rare reported marine planktonic ciliates (Ciliophora: Oligotrichia) from coastal waters in north China. J. Mar. Biol. Ass. UK. 1998;78:767–794. [Google Scholar]
- Song W, Wilbert N. Taxonomische Untersuchungen an Aufwuchsciliaten (Protozoa, Ciliophora) im Poppelsdorfer Weiher, Bonn. Lauterbornia. 1989;3:1–221. [Google Scholar]
- Song W, Wilbert N. Benthische Ciliaten des Süßwassers. In: Röttger R, editor. Praktikum der Protozoologie. G. Fischer; Stuttgart: 1995. pp. 156–168. [Google Scholar]
- Sterki V. Tintinnus semiciliatus. Eine neue Infusorienart. Z. Wiss. Zool. 1879;32:460–465. +plate 28. [Google Scholar]
- Strüder-Kypke MC, Lynn DH. Sequence analyses of the small subunit rRNA gene confirm the paraphyly of oligotrich ciliates sensu lato and support the monophyly of the subclasses Oligotrichia and Choreotrichia (Ciliophora, Spirotrichea) J. Zool. Lond. 2003;260:87–97. [Google Scholar]
- Swofford DL. PAUP*. Phylogenetic analysis using parsimony (* and other methods) Version 4.0b10 Sinauer Assocation; Sunderland, MA: 2002. [Google Scholar]
- Tamar H. The movements and responses of Halteria grandinella. Acta Protozool. 1967;4:365–381. +plates 1-3 (with German summary) [Google Scholar]
- Tamar H. The movements of jumping ciliates. Arch. Protistenkd. 1979;122:290–327. [Google Scholar]
- Tappan H, Loeblich AR., Jr. Lorica composition of modern and fossil Tintinnida (ciliate Protozoa), systematics, geologic distribution, and some new tertiary taxa. J. Paleontol. 1968;42:1378–1394. [Google Scholar]
- Taylor FJR. Dinoflagellate morphology. In: Taylor FJR, editor. The Biology of Dinoflagellates. Botanical Monographs 21. Blackwell Science Publishers; Oxford: 1987. pp. 24–91. [Google Scholar]
- Wasik A, Mikołajczyk E. The morphology and ultrastructure of the Antarctic ciliate, Cymatocylis convallaria (Tintinnina) Acta Protozool. 1992;31:233–239. [Google Scholar]
- Wasik A, Mikołajczyk E. Infraciliature of Cymatocylis affinis/convallaria (Tintinnina) Acta Protozool. 1994;33:79–85. [Google Scholar]
- Wasik A, Mikołajczyk E, Gołȩbiowska M. Morphology and microstructure of selected Tintinnina loricae. Acta Protozool. 1997;36:31–38. [Google Scholar]
- Wiens JJ. Coding morphological variation within species and higher taxa for phylogenetic analysis. In: Wiens JJ, editor. Phylogenetic Analysis of Morphological Data. Smithsonian Institution Press; Washington, London: 2000. pp. 115–145. [Google Scholar]
- Wilbert N, Song W. New contributions to the marine benthic ciliates from the Antarctic area, including description of seven new species (Protozoa, Ciliophora) J. nat. Hist. 2005;39:935–973. [Google Scholar]
- Xu R, Bai Q. Strombidinopsis grandis: a new species of Strombidinopsis. Trop. Oceanol. 1998;17:40–43. (in Chinese with English summary) [Google Scholar]
- Xu D, Song W. Hapantotypification and morphological redescription of the marine planktonic ciliate, Spirostrombidium cinctum (Kahl, 1932) Petz, Song et Wilbert, 1995 (Ciliophora: Oligotrichida) Acta Protozool. 2006;45:17–25. [Google Scholar]
- Xu D, Song W, Sun P, Chen X. Morphology and infraciliature of the oligotrich ciliate Strombidium rapulum (Yagiu, 1933) Kahl, 1934 (Protozoa, Ciliophora, Oligotrichida) from the intestine of sea urchin Hemicentrotus pulcherrimus Agassiz. Zootaxa. 2006;1113:33–40. [Google Scholar]
- Zharikov VV. A new species of fresh-water infusorians (Oligotrichida) from waters of Armenia. Zool. Zh. 1987;66:930–932. (in Russian with English summary) [Google Scholar]