Abstract
In recent years, the glycoconjugates of many parasitic nematodes have attracted interest due to their immunogenic and immunomodulatory nature. Previous studies with the porcine roundworm parasite Ascaris suum have focussed on its glycosphingolipids which were found, in part, to be modified by phosphorylcholine. Using mass spectrometry and Western blotting, we have now analysed the PNGase A-released N-glycans of adults of this species. The presence of hybrid, bi- and triantennary N-glycans, some modified by core α1,6-fucose and peripheral phosphorylcholine, was demonstrated by LC/ESI-Q-TOF-MS/MS, as was the presence of paucimannosidic N-glycans, some of which carry core α1,3-fucose, and oligomannosidic oligosaccharides. Western blotting verified the presence of protein-bound phosphorylcholine and core α1,3-fucose, whereas glycosyltransferase assays showed the presence of core α1,6-fucosyltransferase and Lewis-type α1,3-fucosyltransferase activities. Although, the unusual tri- and tetrafucosylated glycans found in the model nematode Caenorhabditis elegans were not found, the vast majority of the N-glycans found in A. suum represent a subset of those found in C. elegans; thus, our data demonstrate that the latter is an interesting glycobiological model for parasitic nematodes.
Keywords: Ascaris, nematode, parasite, N-glycan, fucose, phosphorylcholine
Introduction
Ascaris suum is one of a number of nematode parasites which affects pigs resulting in a loss of productivity. Whereas the large adult roundworms reside in the gut, the larvae hatching from ingested eggs travel from the stomach or small intestine via the liver to the lungs, before the juvenile worms are coughed up and return to the gastrointestinal tract. The human parasite Ascaris lumbricoides completes a similar life cycle and infects a large proportion of the world's population; associated health problems include lung haemorrhage and inflammation, pneumonia, intestinal blockage and IgE-induced hypersensitivity. Helminths in general often have a major impact on the host's immune system and affect the balance of Th1 and Th2 responses [1]; some nematode proteins have immunomodulatory functions and, recently, non-infective nematodes (Trichuris suis) have been used successfully as a novel therapeutic for inflammatory bowel disease [2, 3]. Furthermore, A. lumbricoides infection has been suggested to be associated with protection from cerebral malaria [4] and natural immunity to this roundworm is associated with both increased IgE and inflammation [5]. Indeed, the mutual evolutionary interaction of nematodes with their hosts, the balance between pathogenicity, protection against other diseases and nematode survival and the apparent association of reduced nematode infections in developed countries with increased prevalance of allergies indicate the necessity to study the macromolecules (both proteins and carbohydrates) of these organisms.
The carbohydrates linked to proteins and lipids of nematodes have attracted significant attention in recent years due to their immunogenic and immunomodulatory nature [6]. For instance, phosphorylcholine-modified (PC) carbohydrates seem to have an important role in the immunomodulatory properties of parasites such as A. suum [7, 8] and the rodent parasite Acanthocheilonema viteae [9, 10], whereas their immunogenicity is shown by the production of antibodies recognising PC by rats infected with the intracellular muscle parasite Trichinella spiralis [11]. The relevant nematode PC-substituted oligosaccharides occur in two different groups [12]: on one hand, as PC-modified glycosphingolipids such as those found in A. suum and A. lumbricoides [13-16], in the human ‘river blindness’ parasite Onchocerca volvulus [17] and in Caenorhabditis elegans [18]. In these organisms the glycolipid-bound PC is linked to an N-acetylglucosamine residue; additionally, in the case of Ascaris glycolipids, phosphoethanolamine was also detected. On the other hand, PC-containing protein-linked N-glycans have been found in C. elegans [19-22], Ac. viteae [23], T. spiralis [24] and O. volvulus [25]. These N-glycans contain the typical trimannosyl core, with and without core fucosylation, and carry between one and four additional N-acetylglucosamine residues. In these PC-modified glycans, the core fucose is α1,6-linked as in mammals. Other N-glycans from nematodes also carry α1,3-fucose on the proximal [21, 26] and, uniquely, distal GlcNAc residues of the core [27, 28]. Fucose residues may be associated with the Th2-bias of the immune response to some nematodes [29] and core α1,3-fucose in particular is known to be immunogenic [30].
In initial studies we found that proteins in A. suum extracts strongly bound the phosphorylcholine-specific monoclonal IgA known as TEPC15, which also reacts with C. elegans glycolipids and glycoproteins [18] as well as lipopolysaccharides from a number of bacterial species [31, 32]. Also, we detected reactivity towards anti-horseradish peroxidase which recognises core α1,3-fucose residues [33]. However, to date no study has described the N-glycans from this organism; thus, structural explanation for these findings was absent. Therefore, we have adopted LC-ESI-MS-MS techniques to elucidate the structures of this parasite and indeed show the presence of PC-containing, as well as core α1,3-fucosylated, N-glycans.
Results
Western blotting
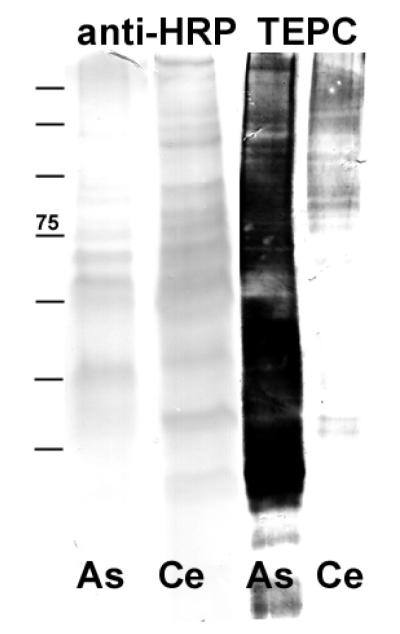
In an initial screen for glycan epitopes in Ascaris suum, a crude extract of an adult worm and, for comparative purposes, an extract of Caenorhabditis elegans were subject to SDS-PAGE and Western blotting with anti-horseradish peroxidase to test for the presence of core α1,3-fucose and TEPC15 to detect any phosphorylcholine-modified proteins (Figure 1). With TEPC15, the result was a much more intense staining of the A. suum extract compared with the protein extract of the nematode C. elegans, whereas for anti-horseradish peroxidase the opposite was observed.
Figure 1. Western blotting of Ascaris and Caenorhabditis extracts.
Equal amounts, in terms of protein, of nematode extracts were subject to blotting using either anti-horseradish peroxidase (recognising, e.g., core D1,3-fucose) or anti-phosphorylcholine (TEPC15) antibodies.
HPLC of pyridylaminated glycans
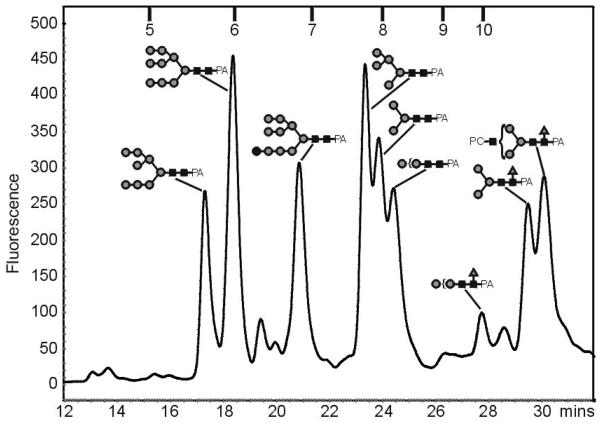
To examine the PC containing structures in A. suum more closely, the PNGase A-released N-glycans were, for further HPLC analysis and for better sensitivity with ESI-MS [34], derivatised at the reducing end with 2-aminopyridine. The RP-HPLC chromatogram of the glycan pool (Figure 2) revealed a number of peaks, which were collected and further analysed by ESI-MS. According to their masses, the major fractions were concluded to be typical oligomannosidic and core fucosylated glycans; also complex, difucosylated and PC-containing glycans were found (see Table 1). Selected fractions containing fucosylated N-glycans were then subject to a further round of purification, in order to remove co-eluting glycans prior to further analyses, by normal-phase HPLC (e.g., as used to purify the HexNAc3Hex3Fuc1PC1 glycan described below). The low amounts of the complex N-glycans, however, precluded a more exact investigation of their structures.
Figure 2. Fluorescence RP-HPLC chromatogram of PA-labelled N-glycans from Ascaris suum.
The peak assignment was performed with ESI-MS; the compositions of selected N-glycans are shown using the nomenclature of the Consortium for Functional Glycomics (www.functionalglycomics.org) with black squares indicating GlcNAc, grey circles mannose and grey triangles fucose; most annotated peaks also contain further structures (see Table 1). The retention times of external isomaltose oligomer standards (5 - 10 glucose units) are also shown.
Table 1. Summary of RP-HPLC data for 2-aminopyridylaminated glycans from Ascaris suum.
Fractions collected from the RP-HPLC run shown in Figure 2 were analysed by ESI-MS (m/z values are given for [M+H]+ forms; retention times are expressed in both minutes and glucose units (g.u.).
| Retention time | putative N-glycan | m/z |
|---|---|---|
| 17.13 (5.8 g.u.) | HexNAc2Hex8 | 1799.7772 |
| 18.18 (6.0 g.u.) | HexNAc2Hex9 | 1961.8134 |
| HexNAc2Hex7 | 1637.7037 | |
| 19.23 (6.3 g.u.) | HexNAc2Hex8 | 1799.7994 |
| 19.78 (6.5 g.u.) | HexNAc2Hex7 | 1637.7499 |
| HexNAc2Hex6 | 1475.9825 | |
| 20.68 (6.9 g.u.) | HexNAc2Hex11 | 2285.8366 |
| HexNAc2Hex10 | 2123.9421 | |
| HexNAc2Hex6 | 1475.6858 | |
| 23.18 (7.8 g.u.) | HexNAc2Hex5 | 1313.6149 |
| HexNAc3Hex3 | 1192.5357 | |
| 23.69 (8.0 g.u.) | HexNAc2Hex4 | 1151.5483 |
| HexNAc2Hex3 | 989.4521 | |
| 24.25 (8.2 g.u.) | HexNAc3Hex5PC1 | 1681.6625 |
| HexNAc3Hex3Fuc2 | 1484.7269 | |
| HexNAc4Hex3 | 1395.5761 | |
| HexNAc2Hex3Fuc2 | 1281.5733 | |
| HexNAc2Hex2Fuc2 | 1119.4913 | |
| HexNAc2Hex2Fuc1 | 973.4512 | |
| HexNAc2Hex2 | 827.4333 | |
| 26.21 (9.0 g.u.) | HexNAc4Hex5Fuc1 | 1865.7863 |
| HexNAc4Hex3Fuc1 | 1744.7407 | |
| HexNAc4Hex5 | 1719.6941 | |
| HexNAc4Hex4Fuc1 | 1703.7253 | |
| HexNAc4Hex3Fuc2 | 1687.7095 | |
| HexNAc3Hex5Fuc1 | 1662.6786 | |
| HexNAc3Hex4Fuc2 | 1646.6624 | |
| HexNAc5Hex3 | 1598.6602 | |
| HexNAc4Hex4 | 1557.6779 | |
| HexNAc4Hex3Fuc1 | 1541.6602 | |
| 27.58 (10.0 g.u.) | HexNAc2Hex2Fuc1 | 973.4674 |
| 28.45 | HexNAc3Hex3PC1 | 1357.6570 |
| HexNAc2Hex3 | 1338.5874 | |
| HexNAc2Hex4Fuc1 | 1297.5573 | |
| HexNAc2Hex1Fuc1 | 811.3865 | |
| 29.33 | HexNAc3Hex3PC1 | 1357.6575 |
| HexNAc2Hex3Fuc1 | 1135.4809 | |
| 29.93 | HexNAc5Hex3PC1 | 1763.7541 |
| HexNAc4Hex3PC2 | 1725.8147 | |
| HexNAc4Hex3Fuc1PC1 | 1706.7106 | |
| HexNAc4Hex3PC1 | 1560.6861 | |
| HexNAc3Hex3Fuc1PC1 | 1503.6277 | |
| HexNAc2Hex2Fuc1 | 973.4294 | |
| 31.60 | HexNAc5Hex4Fuc1PC1 | 1925.7566 |
| HexNAc5Hex3Fuc1PC1 | 1909.7701 |
Although the slightly-different RP-HPLC elution conditions used seemingly led to some shifts in the retention times in terms of glucose units (g.u.) as compared to an earlier study with C. elegans N-glycans [22], the general trend in the order of elution was the same, i.e., first the oligomannose were eluted, then difucosylated, PC-containing non-fucosylated, α1,6-fucosylated and PC-containing α1,6-fucosylated glycans. Specifically, fractions in the region from 5.8-8.0 g.u. were judged to primarily contain Glc0-1Man3-9GlcNAc2, whereas core α1,3/α1,6-difucosylated glycans (e.g., putative Man3GlcNAc2Fuc2) were found to elute in the region of 8.2-9.0 g.u. Putatively unmodified complex glycans (i.e., those with more than three HexNAc residues, but lacking PC and fucose) eluted at around 9 g.u., as expected from other studies [35]. The paucimannosidic and complex species putatively containing core α1,6-fucose were expected to be found in the region beyond 10 g.u., whereas modification by phosphorylcholine appears to lead to a slight increase in retention time as compared to the corresponding non-modified forms.
LC-ESI-MS of pyridylaminated glycans
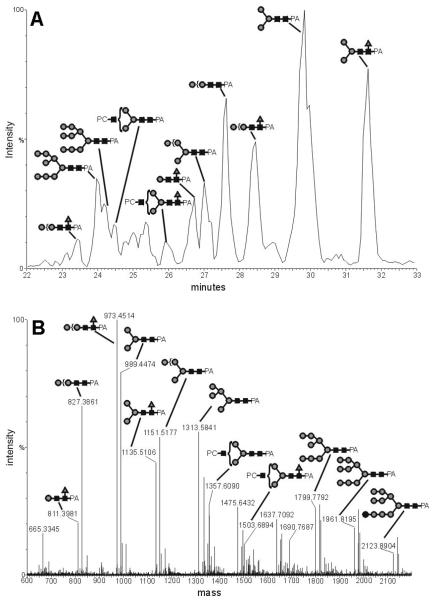
For a more detailed analysis, the derivatised glycans were examined using an LC-ESI-MS system. This approach showed two major advantages: first of all, the derivatised glycans were desalted on a pre-column, thus removing compounds which could suppress the ionisation. Secondly, the glycans were separated on a graphitised carbon column; thus not all glycans reached the electrospray needle simultaneously, thereby minimising ionisation suppression effects. The analysis of the whole PA-labelled glycan pool from A. suum (Figure 3) indicated that the major proportion of the N-glycans consists of structures with 2 HexNAc and between 3 to 11 hexose residues (i.e., paucimannosidic and oligomannosidic structures). More interestingly, a common glycan type, at least as judged by the ESI-MS signal intensity, is represented by PC-containing N-glycans, specifically hybrid and complex N-glycans with one or two PCs. Fucosylated forms of PC-modified and paucimannosidic glycans were also detected in this analysis.
Figure 3. LC-ESI-MS of 2-aminopyridine-derivatised N-glycans from Ascaris suum.
N-glycans were analysed by ESI-MS following graphitised carbon chromatography: A) the chromatogram in terms of ion intensity; B) the accumulated MS spectra from 23 to 32 minutes. The [M+H]+ Ions have been calculated by use of the Masslynx-MaxEnt3 software from the raw multiply-charged ion data. Selected peaks are annotated with black squares indicating GlcNAc, grey circles mannose and grey triangles fucose.
Glycosidase treatment of the whole glycan pool
In order to gain a global view of the modifications on A. suum N-glycans, the whole pyridylaminated-glycan pool was subject to a combined fucosidase and β1,3/β1,4-galactosidase digest prior to reanalysis by ESI-MS. These three glycosidases were employed since we hypothesised that, not only some structures were modified by fucose, but that extra hexose residues were present on some of the putatively complex and hybrid N-glycans. As summarised in Table 2, a subset of structures was indeed sensitive to this treatment, suggesting that some A. suum glycans are modified by α-linked fucose and β-linked galactose residues, with the assumption that the fucose residues removed are core α1,6-linked.
Table 2. Summary of ESI-MS data for 2-aminopyridylaminated glycans from Ascaris suum.
Proposed compositions, the predominant charged species, theoretical and observed m/z as well as sensitivity to combined fucosidase and galactosidase (‘glycosidase’) digestion, galactosidase digestion alone and the results of the HF treatment are shown. Due to in-source fragmentation, there is an inherent bias towards smaller species, which in part will not be naturally present on Ascaris glycoproteins.
| Glycan composition | [M+H]+ calc. | Predominant ion | m/z theoretical | m/z found | Glycosidase sensitive |
Galactosidase sensitive |
HF sensitive |
|---|---|---|---|---|---|---|---|
| Oligomannosidic and paucimannosidic structures | |||||||
| HexNAc2Hex1 | 665.2846 | [M+H]+ | 665.2846 | 665.3309 | |||
| HexNAc2Hex1Fuc1 | 811.3424 | [M+H]+ | 811.3424 | 811.3931 | |||
| HexNAc2Hex2 | 827.3373 | [M+H]+ | 827.3373 | 827.3730 | |||
| HexNAc2Hex2Fuc1 | 973.3952 | [M+H]+ | 973.3952 | 973.4291 | |||
| HexNAc2Hex3 | 989.3901 | [M+H]+ | 989.3901 | 989.4585 | * | ||
| HexNAc2Hex2Fuc2 | 1119.4531 | [M+H]+ | 1119.4531 | 1119.5190 | Yes | Yes | |
| HexNAc2Hex3Fuc1 | 1135.4481 | [M+H]+ | 1135.4481 | 1135.4785 | * | ||
| HexNAc2Hex4 | 1151.4429 | [M+H]+ | 1151.4429 | 1151.4956 | |||
| HexNAc2Hex3Fuc2 | 1281.5059 | [M+H]+ | 1281.5017 | 1281.5914 | Yes | Yes | |
| HexNAc2Hex4Fuc1 | 1297.5008 | [M+H]+ | 1297.5008 | 1297.5881 | Yes | ||
| HexNAc2Hex5 | 1313.4957 | [M+H]+ | 1313.4957 | 1313.5510 | |||
| HexNAc2Hex6 | 1475.5485 | [M+2H]2+ | 738.2779 | 738.3330 | |||
| HexNAc2Hex7 | 1637.6014 | [M+2H]2+ | 819.3044 | 819.3519 | |||
| HexNAc2Hex8 | 1799.6541 | [M+2H]2+ | 900.3307 | 900.3765 | |||
| HexNAc2Hex9 | 1961.7070 | [M+2H]2+ | 981.3572 | 981.4147 | |||
| HexNAc2Hex10 | 2123.7597 | [M+2H]2+ | 1062.3835 | 1062.4513 | |||
| HexNAc2Hex11 | 2285.8125 | [M+2H]2+ | 1143.4099 | 1143.5077 | |||
| Complex and hybrid structures | |||||||
| HexNAc3Hex3 | 1192.4696 | [M+H]+ | 1192.4696 | 1192.5438 | |||
| HexNAc3Hex3Fuc1 | 1338.5274 | [M+2H]2+ | 669.7673 | 669.8187 | Yes | ||
| HexNAc4Hex3 | 1395.5489 | [M+2H]2+ | 698.2781 | 698.2921 | |||
| HexNAc3Hex3Fuc2 | 1484.5853 | [M+2H]2+ | 742.7963 | 742.8596 | Yes | Yes | |
| HexNAc4Hex3Fuc1 | 1541.6069 | [M+2H]2+ | 771.3071 | 771.3533 | Yes | ||
| HexNAc4Hex4 | 1557.6018 | [M+2H]2+ | 779.3045 | 779.3304 | Yes | Yes | |
| HexNAc4Hex4Fuc1 | 1703.6596 | [M+2H]2+ | 852.3334 | 852.3865 | Yes | Yes | |
| HexNAc5Hex3 | 1598.6284 | [M+2H]2+ | 799.8178 | 799.8515 | |||
| HexNAc3Hex4Fuc2 | 1646.6381 | [M+2H]2+ | 823.8227 | 823.8736 | Yes | Yes | |
| HexNAc3Hex5Fuc1 | 1662.6330 | [M+2H]2+ | 831.8202 | 831.8696 | Yes | ||
| HexNAc4Hex3Fuc2 | 1687.6647 | [M+2H]2+ | 844.3360 | 844.3635 | Yes | Yes | |
| HexNAc4Hex5 | 1719.6545 | [M+2H]2+ | 860.3309 | 860.3995 | Yes | Yes | |
| HexNAc5Hex3Fuc1 | 1744.6862 | [M+2H]2+ | 872.8467 | 872.9117 | Yes | ||
| HexNAc4Hex5Fuc1 | 1865.7125 | [M+2H]2+ | 933.3599 | 933.4164 | Yes | Yes | |
| PC-containing structures | |||||||
| HexNAc3Hex3PC1 | 1357.5251 | [M+2H]2+ | 679.2662 | 679.3078 | Yes | ||
| HexNAc3Hex3Fuc1PC1 | 1503.5829 | [M+2H]2+ | 752.2951 | 752.3353 | Yes | Yes | |
| HexNAc4Hex3PC1 | 1560.6044 | [M+2H]2+ | 780.8058 | 780.8600 | Yes | ||
| HexNAc3Hex5PC1 | 1681.6306 | [M+2H]2 | 841.3189 | 841.3498 | Yes | ||
| HexNAc4Hex3Fuc1PC1 | 1706.6624 | [M+2H]2+ | 853.8365 | 853.8905 | Yes | Yes | |
| HexNAc4Hex3PC2 | 1725.6599 | [M+2H]2+ | 863.3336 | 863.3870 | Yes | ||
| HexNAc5Hex3PC1 | 1763.6839 | [M+2H]2+ | 882.3456 | 882.4037 | Yes | ||
| HexNAc4Hex4Fuc1PC1 | 1868.7151 | [M+2H]2+ | 934.8612 | 934.9277 | Yes | Yes | Yes |
| HexNAc5Hex3Fuc1PC1 | 1909.7417 | [M+2H]2+ | 955.3745 | 955.4325 | Yes | Yes | |
| HexNAc5Hex4PC1 | 1925.7366 | [M+2H]2+ | 963.3737 | 963.4075 | Yes | Yes | Yes |
| HexNAc4Hex5Fuc1PC1 | 2030.768 | [M+2H]2+ | 1015.8876 | 1015.9613 | Yes | Yes | Yes |
The intensity of the HexNAc2Hex3Fuc1 peak was reduced, but not abolished, after combined galactosidase/fucosidase digestion, because HexNAc2Hex3Fuc2 is digested to HexNAc2Hex3Fuc1, whereas the HexNAc2Hex3Fuc1 is in turn digested to HexNAc2Hex3, the intensity of which is concomitantly increased.
Repeating the analysis with β1,4-galactosidase alone indicated that the galactose residues are β1,4-linked and that only glycans with at least three N-acetylhexosamine residues (i.e., presumed hybrid and complex structures) contain this type of residue; based on previous experience with the Aspergillus galactosidase and on the resistance of in vitro Lewis-type fucosyltransferase reaction products to this enzyme (see below), the accessibility of the galactose residues of A. suum N-glycans to this treatment suggests that they do not form part of Lewis-type moieties. However, the low amounts of the galactosylated glycans, as well as of the complex structures in general, precluded a more thorough analysis. Thus, the focus of later experiments was on phosphorylcholine- and fucose-substituted N-glycans.
Hydrofluoric acid treatment
After the treatment with HF none of the PC containing N-glycans could be detected by MS analysis (see Table 2 for a summary). This is caused by the cleavage of the phosphodiester linkage, between the terminal sugar residue and the PC group [23]. Other than the PC–sugar linkage, also the fucose linked α1-3 to the inner GlcNAc is HF-sensitive [36]. Whereas in the untreated glycan pool double fucosylation was detected, all glycans containing two fucoses were absent after this chemical cleavage. This leads to the conclusion that in A. suum also core α1,3-linked fucose is present, a finding also suggested by the reactivity with anti-horseradish peroxidase (see above); these same difucosylated glycans were also fucosidase-sensitive, which suggests that the second fucose may be core α1,6-linked. The presence of such core difucosylated glycans is also suggested by their RP-HPLC retention time and the MSMS experiments discussed below. On the other hand, fucosidase digestion and MSMS experiments showed that the PC-containing N-glycans only carry one fucose which is α1,6-linked to the inner GlcNAc (see also below).
Analysis of PC-containing structures
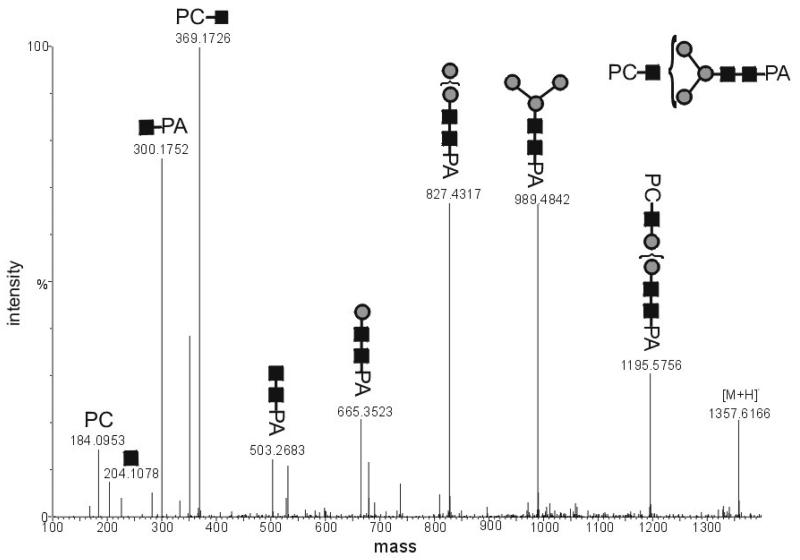
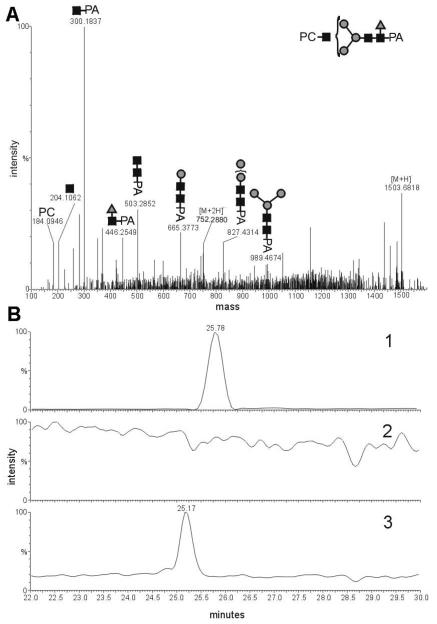
To gain more information about the position of the PC on the glycans, collision-induced dissociation tandem MS (CID-MSMS) experiments with a selected ion, whose m/z is in accordance with a putative HexNAc3Hex3PC1 structure, were performed (Figure 4). Particularly characteristic is the occurrence of an oxonium ion with m/z 369.2; this corresponds to a PC-residue linked to an N-acetylhexosamine. The high intensity of this fragment ion was interpreted as being compatible with the PC being linked to a non-reducing terminal N-acetylhexosamine, because only the breakage of one bound is necessary to obtain this ion. Overall, in MSMS experiments, no PC-containing fragment containing the pyridylamino moiety was detected which possessed less than three N-acetylhexosamine residues. These results agree well with the ESI-MS analysis in which the detected PC-modified structures contain at least three N-acetylhexosamine residues when modified by one PC and at least four N-acetylhexosamines when modified by a second PC. A hybrid structure, putatively of the form Man5GlcNAc3PC1 was also detected, which had an RP-HPLC elution time of 8.2 g.u. (Table 1); in C. elegans a glycan with a similar RP-HPLC retention time and the same mass has only been observed in a Golgi α-mannosidase II mutant [22]. Based on the linkages found in PC-substituted glycolipids in A. suum [15], it is presumed, but not proven, that the PC in all cases is linked through the 6-hydroxyl of GlcNAc.
Figure 4. CID-ESI-MS-MS analysis of a phosphorylcholine-modified Ascaris suum Nglycan.
The selected ion HexNAc3Hex3PC1-PA was in its [M+2H]2+ form (m/z 679.2679).
Some PC-containing structures were also putatively modified by fucose; thus, the linkage and the position of the fucose in these PC-containing N-glycans were also investigated. In CID-MS-MS experiments with the structure HexNAc3Hex3Fuc1PC1,it could be shown that the fucose was linked to the proximal N-acetylglucosamine residue at the reducing terminus, because a fragment of m/z 446.3 was detected (Figure 5A); this corresponds to a 2-aminopyridine-linked N-acetylhexosamine substituted by a fucose residue. In order to determine the linkage of the fucose, a 2α-HPLC purified HexNAc3Hex3Fuc1PC1 glycan was digested with α-fucosidase from bovine kidney, which should specifically remove only α1,6-bound fucose residues, whereas the core α1,3-fucose linkage is resistant to this enzyme. The fucosidase removed the fucose quantitatively, thus indicating that the fucose is indeed core α1,6-linked (Figure 5B). This result is compatible with the late retention time (beyond 10 g.u.) of this glycan.
Figure 5. Analysis of an Ascaris suum N-glycan modified by phosphorylcholine and fucose.
A) CID-MS-MS analysis of the presumed HexNAc3Hex3Fuc1PC1-PA in its [M+2H]2+ form (m/z 752.2880); B) LC-ESI-MS ion trace of 2-aminopyridine labeled Ascaris suum N-glycans. Chromatogram 1 shows the trace of m/z 752.30 (HexNAc3Hex3Fuc1PC1) of a 2-aminopyridine N-glycan fraction, purified by the “two-dimensional” mapping technique, before treatment with α-fucosidase. Chromatogram 2 shows the trace m/z 752.30 after incubation with α-fucosidase, showing that structures with this m/z were completely digested by this treatment. Chromatogram 3 shows the ion trace of m/z 679.27 (HexNAc3Hex3PC1) of the same fraction as in chromatogram 1, but after treatment with α-fucosidase and indicates a shift to lower retention time.
Analysis of core difucosylated glycans
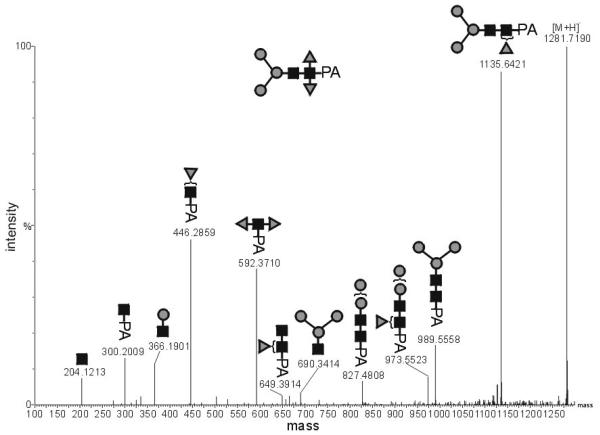
The weak staining in the western blot of an A. suum protein extract with anti-horseradish peroxidase was hypothesised to be due to species observed with the putative compositions HexNAc2Hex2Fuc2 and HexNAc2Hex3Fuc2 (see Tables 1 and 2). In CID-MSMS experiments with the HexNAc2Hex2Fuc2 species, a fragment of m/z 592.4 ([M+H]+ form) was detected, which corresponds to a 2-aminopyridine-linked N-acetylglucosamine substituted by two fucose residues (Figure 6). This suggests that these N-glycan structures indeed contain a core α1,3-linked fucose, as found in other invertebrates [37]; in this and other studies [22, 38], the RP-HPLC retention times of these difucosylated structures are approximately the same as that of HexNAc2Hex3 (putatively Man3GlcNAc3 or MM).
Figure 6. CID-ESI-MSMS analysis of a core difucosylated Ascaris suum N-glycan.
Fragments of the species HexNAc2Hex3Fuc2-PA in its [M+H]+ form (m/z 1281.7190) verify the presence of a disubstituted proximal HexNAc residue.
Fucosyltransferase activities in Ascaris suum
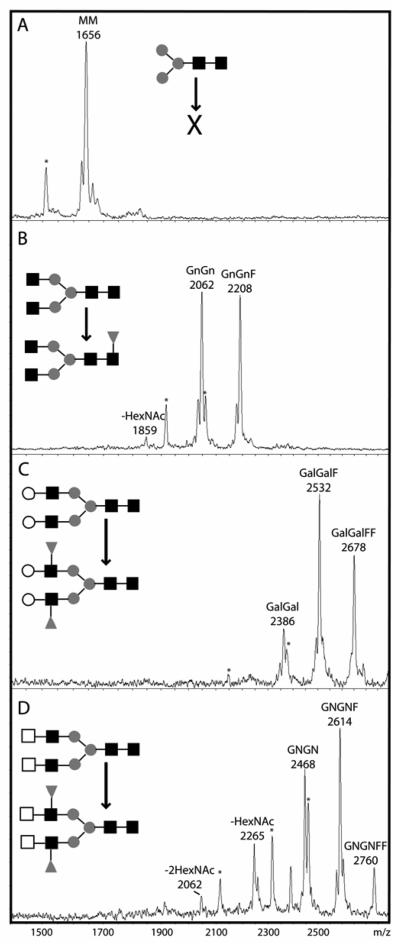
Considering the presence of core fucose residues on A. suum N-glycans, we performed fucosyltransferase assays using N-glycan acceptors previously used in studies on Caenorhabditis and Schistosoma [39]. Fucose transfer was detected towards dabsylated GnGn, GalGal and βGNβGN glycopeptides (Figure 7), but not towards MM even when repeated in the presence of Mg(II) instead of Mn(II). This latter result was somewhat unexpected because previously the only core α1,3-fucosyltransferase characterised from a nematode to date (i.e., FUT-1 from C. elegans which prefers Mg(II) as the activating cation) transfers fucose to MM [21]; this activity was found for both the native enzyme in extracts and the recombinant enzyme expressed in Pichia. Perhaps the undetectable levels of core α1,3-fucosylation with this substrate in vitro is compatible with the lower level of anti-horseradish peroxidase reactivity of A. suum proteins or that the enzyme is particularly unstable. It is interesting to note that the putative peptide encoded by a partial fucosyltransferase gene reconstructed from A. suum genome survey sequences displays its highest homology to C. elegans FUT-1 with 50% identity (data not shown); thus, it is possible that the A. suum core α]1,3-fucosyltransferase does indeed have a substrate specificity similar to that of C. elegans FUT-1.
Figure 7. Fucosyltransferase activities in an Ascaris suum extract.
Nematode extract was incubated with dabsyl-N-glycopeptides (A) MM, (B) GnGn, (C) GalGal or (D) EGNEGN (nomenclature based on that of Schachter) in the presence of GDP-Fuc for 5 hours (controls without GDP-Fuc were also performed, data not shown). The MM glycopeptide was apparently not modified, the GnGn substrate is the acceptor for a single fucose residue, the GalGal and βGNβGN for two fucose residues. Laser-induced degradation results, in part, in a decrease of m/z 132 (peaks marked by an asterisk). Hexosaminidase digestion products are indicated with −1HexNAc or −2HexNAc. Structures of substrates and products shown in the diagrammatic form of the Consortium for Functional Glycomics with black squares indicating GlcNAc, grey circles mannose, white squares GalNAc, white circles galactose and grey triangles fucose.
The transfer of only a seemingly single fucose to GnGn is, though, in keeping with previous data with C. elegans extracts and we assume this activity is due to a core α1,6-fucosyltransferase and is in accordance with the presence of core α1,6-fucose on glycans substituted by non-reducing terminal PC-GlcNAc moieties; the transfer of the second fucose to this substrate was not observed, suggesting that any core α1,3-fucosyltransferase in A. suum is not using the same substrate as that in, e.g., Schistosoma [39]. The GnGnF product was successfully digested with β-hexosaminidase and with PNGase F (data not shown) indicating that the fucose transferred was on the core pentasaccharide and not on the non-reducing termini; the PNGase F sensitivity confirms that the transferred core fucose was α1,6-linked and not α1,3-linked.
Interestingly, unlike C. elegans [40], both GalGal and βGNβGN could accept up to two fucose residues; this would suggest that Ascaris has the capability to generate Lewis-type structures in vitro and indeed, as shown above, Ascaris appears to be able to form potential acceptors for Lewis-type enzymes by transfer galactose to its N-glycans (although we could not detect the galactosylation reaction to N-glycans in vitro; data not shown). Considering the strict substrate specificity of previously-characterised invertebrate core α1,6-fucosyltransferases for GnGn [39], it was assumed that both fucoses are transferred to the antennae of GalGal and αGNαGN and indeed digestion of the GalGalF and GalGalFF products with β-galactosidase showed that, respectively, one or both galactose residues were resistant to digestion, compatible with the presence of Lewis groups on the enzymatic products, whereas unmodified GalGal was digested to GnGn. The possibility that one fucose transferred to GalGal was α1,3-linked to the core was ruled out by the complete digestion of the fucosylation products with PNGase F to a species with m/z 763, which corresponds to the non-glycosylated peptide (data not shown). However, as with C. elegans [41], no reactivity towards anti-Lewis antibodies was found in A. suum extracts and no mass spectrometric data suggested the presence of such structures on N-glycans. It is also noteworthy that, similar to C. elegans extract [39], A. suum extract apparently contains a hexosaminidase capable of removing HexNAc residues from αGNαGN. However, the ‘classical’ invertebrate hexosaminidase, removing a single GlcNAc from GnGn, only shows minor activity in this extract of A. suum. Thus, substrates for phosphorylcholinyltransferase and galactosyltransferase are retained in the parasite.
Discussion
Glycoconjugates either on the surfaces of cells or in secretions are of importance in cell-cell and host-parasite interactions; thus, it is to be expected that the glycosylation of parasites has a role in their biology and pathogenicity. Nematode parasites are remarkable, due to the relatively low mortality, but high morbidity, associated with them as well as their long survival in the host. Furthermore, in recent years the ‘hygiene hypothesis’ has been invoked to address the apparent inverse relationship between Western living styles and allergy [42]. Various nematodes [1] and trematodes [43] display a mixture of immunosupression, immunogenicity and molecular mimickry; these phenomena being often associated with glycans. Thus, it is interesting to compare the glycans of non-parasitic and parasitic nematodes for two reasons: first, the differences may yield clues as to the types of glycans which may aid the survival of the parasite in an appropriate host and, secondly, the similarities may enable relevant studies to be performed on genetically-tractable model organisms.
With the results of the present study, we can now compare the N-glycans of Ascaris with those of Caenorhabditis. The most obvious difference appears to be the relative simplicity of the A. suum N-glycome in comparison to that of the model organism; in particular, the tri- and tetrafucosylated N-glycans found in C. elegans, whose structures still remain to be entirely elucidated, are absent. On the other hand, difucosylated paucimannosidic structures are present and the typical MMF6 and oligomannosidic glycans are dominant. Indeed, based on the N-glycan cores detected, we estimate that, as judged by either ESI-MS or fluorescence intensity, 80-90% of A. suum N-glycans are either pauci- or oligomannosidic. However, due to the potential that the ionisation of each glycan type is not equal, an exact quantitation of the glycans is problematic.
Compatible with the high TEPC15 reactivity as judged by, e.g., previous immunohistochemical studies [14] and our Western blot data (Figure 1), a range of phosphorylcholine-modified glycans, some being multiantennary, are present; such glycans are also a feature of C. elegans [19, 20] and of filarial nematodes [25]. One PC-containing glycan (HexNAc3Hex5PC1) is also hybrid; thus, one can assume that the A. suum PC-transferase transfers not just to multiantennary glycans, but also to hybrid glycans containing a free non-reducing terminal N-acetylglucosamine residue; this finding is compatible with the inability of swainsonine, a mannosidase II inhibitor, to inhibit transfer of phosphorylcholine in a filarial nematode [44] as well as with the presence of hybrid PC-containing N-glycans in the C. elegans mannosidase II mutant [22]. Some PC-containing glycans also appeared to contain a terminal galactose residue; this, though, is a feature of the parasite and seemingly not of the model ‘worm’. Similar glycans, lacking PC, are also found in the parasitic cestode species Echinococcus and Taenia [45-47]. Unlike Trichinella [24, 48] or Onchocerca [25], however, there is no obvious evidence for non-reducing terminal modification by either LacdiNAc (GalNAcβ1,4GlcNAc) or chito-oligomers (GlcNAcβ1,4GlcNAc) in either Ascaris or Caenorhabditis. On the other hand, Galα1,3Galβ1,4GlcNAc units are present on the N-glycans of Parelaphostrongylus tenuis, a nematode parasite of deer [49], indicating that other nematodes do possess galactosyltransferases.
Many glycans of A. suum contain fucose, but this appears to be restricted to the core; Lewis-type structures, as found in the cattle parasite Dictyocaulus viviparus [36], were not detected. This is in keeping with the apparent lack of Lex as judged by Western blotting. Indeed, those complex and PC-containing structures found to be modified by fucose appear predominantly to contain solely α1,6-linked fucose, since treatment with α-fucosidase resulted in removal of fucose from all such structures. On the other hand, some paucimannosidic structures were found to be mono- and difucosylated; some of these are the typical MUF6 and MMF6 structures dominant in C. elegans, whereas modification of the proximal, pyridylaminated GlcNAc by both α1,3- and α1,6-fucose is found in many invertebrates, including the ruminant parasite Haemochus contortus [27], the aforementioned Parelaphostrongylus tenuis [49] and Drosophila melanogaster [38]. Unlike Schistosoma mansoni [50], no xylose was detected on the N-glycans, confirming that trematodes and nematodes have different glycosylation potentials. Thus, as in C. elegans, the cross-reactivity with anti-horseradish peroxidase is due to core α1,3-fucosylation [21]; this modification is an epitope for IgE from, amongst others, Haemonchus-infected sheep [51], some bee venom allergic subjects [52] and some food allergy patients [53]. However, perhaps due to low activity in A. suum, we did not detect an MM-modifying fucosyltransferase similar to the C. elegans FUT-1. We did, though, find both a GnGn-modifying fucosyltransferase (probably forming core α1,6-linkages) and a Lewis-epitope synthesising activity. It is possible that this latter type of enzyme has substrates which are not N-glycans in vivo, since fucose linked to LacdiNAc of A. suum glycolipids has been previously found [15]. A Lewis-type fucosyltransferase activity has also been found in H. contortus [54], but in this case a fucosylated LacdiNAc structure can be detected by Western blotting of a host-protective protein antigen [55], although it is unknown whether the epitope is on N- or O-linked glycans.
The accumulated structural and enzymatic data generate hints as to the glycosylation potential of A. suum. Thus, it appears that this organism must have a range of N-acetylglucosaminyltransferases required for N-glycan branching; indeed, in comparison, C. elegans possesses GlcNAc-TI, GlcNAc-TII and GlcNAc-TV genes [56-58]. The genome of Ascaris must, in addition to Golgi mannosidases and the ‘usual’ dolichol-linked oligosaccharide pathway enzymes, also encode homologues of known core α1,3- and α1,6-fucosyltransferases and galactosyltransferase(s). However, the identity of eukaryotic glycan-modifying PC-transferases remains elusive. Considering the glycomic similarities as well as results showing that antibodies raised against C. elegans strongly react with A. suum proteins (manuscript in preparation), there is potential to exploit C. elegans as a model to investigate the molecular nature and biological relevance of Ascaris glycosylation.
Experimental Procedures
Western blotting
Extracts of Ascaris suum and Caenorhabditis elegans were prepared as previously described [21]. Proteins were separated by SDS-PAGE on 12.5% gels and transferred to nitrocellulose using a semi-dry blotting apparatus. After blocking with 0.5% (w/v) BSA, membranes were incubated with either rabbit anti-horseradish peroxidase (1:12500) or TEPC15 (1:300). After washing, either an alkaline phosphatase conjugate of goat anti-rabbit (1:2000) or peroxidase-coupled goat anti-mouse IgA (1:1000) were used, with subsequent colour detection with respectively 5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium or 4-chloro-1-naphthol. Except for the phosphatase-conjugated goat anti-rabbit antibody (Vector Laboratories), all antibodies and detection reagents were purchased from Sigma.
Preparation of the N-glycans
Approximately 2 g of worm material were boiled in 10 ml water for 5 minutes prior to grinding. The extract was made up to 5% (v/v) with aqueous formic acid and incubated overnight with 9 mg pepsin (Sigma) at 37°C. After centrifugation at 39,000 × g for 30 min, the supernatant was applied to 15 ml Dowex AG50W × 2 equilibrated with 2% (v/v) acetic acid. The column was washed with 20 ml of 2% acetic acid and the (glyco)peptides were eluted with 0.6 M ammonium acetate pH 6. Orcinol-positive fractions were pooled and the volume was reduced by rotary evaporation. The (glyco)peptides were then applied to a Sephadex G25 column, which was then washed with 1% acetic acid. The orcinol-positive fractions were again pooled and subject to rotary evaporation. To reduce the free sugars in the A. suum peptide extract, which in preliminary trials otherwise interfered with the subsequent analyses, the dried sample was dissolved in 50 μl 5% ammonia in water (v/v) and 50 μl of a 1% sodium borohydride solution (w/v) was added. After incubation for 2 hours at room temperature, 2.5 μl acetic acid were added and the solution was dried under a stream of nitrogen prior to being dissolved in 200 μl 0.1 M citrate-phosphate, pH 5.0. After heat treatment at 95°C for 6 min to inactivate any residual pepsin, the sample was cooled and centrifuged prior to addition of 0.45 mU PNGase A and incubation at 37°C overnight. The sample was then acidified with 150 μl of 30% acetic acid (v/v) and applied to a 3 ml Dowex AG50W × 2 column. The PNGase released glycans were eluted with 2% acetic acid; orcinol-positive fractions were pooled and the volume was reduced by vacuum evaporation. The released glycans were then taken up in 100 μl 1% acetic acid and applied onto a Zorbax SPE C18 25mg cartridge previously washed with 65% (v/v) aqueous acetonitrile and equilibrated with 1% acetic acid; the glycans were then collected by washing with 1% acetic acid and dried.
Reversed phase HPLC analysis of pyridylaminated N-glycans
Fluorescent labelling of the N-glycans was performed basically as previous described [59]. The subsequent reversed phase HPLC experiments were performed on a Shimadzu HPLC System equipped with a fluorescence detector (excitation/emission at 320/400 nm) and a ODS Hypersil, 250 × 4 mm, 5 μm particle size column. Glycans were eluted using a gradient from 0 to 9% methanol in 50 mM ammonium acetate buffer, pH 4.4 over 30 minutes at a flow rate of 1.5 ml/min, with a final wash step from 30 - 33 minutes with 24% methanol.
LC-ESI MS Analysis
The 2-aminopyridine labelled N-glycans were subject to the above mentioned RPHPLC method and the fractions from 5 - 32 minutes were pooled, lyophilised and dissolved in water. The LC-ESI-MS experiments were carried out using a Q-TOF Ultima Global mass spectrometer (Micromass, Manchester, U.K.) equipped with an atmospheric pressure ionization electrospray interface and an upstream Micromass CapLC using a Thermo Aquastar 30 × 0.32 mm guard column and a Thermo Hypercarb 100 × 0.32 mm separation column. The flow rate was 4 μl/min, starting with 95% solvent A (aqueous 0.1% formic acid) and 5% solvent B (acetonitrile containing 0.1% formic acid); a separating gradient from 5 - 40% B was applied from 5 - 40 minutes. The MS instrument was calibrated with [Glu1]-Fibrinopeptide B in the mass range of 72-1285 amu. The sampling cone potential was 80 V, the capillary voltage 3.0 kV, the electrospray source temperature was 60 °C and the desolvation temperature 120 °C. Mass spectra were scanned over the range m/z 100 - 1900.
Exoglycosidase digestion of the pyridylaminated glycan pool
The complete pool of pyridylaminated glycans was dried and dissolved in 20 μl 0.1 M Citrate pH 5 prior to incubation at 37°C in the presence of 55 mU β1,4-specific galactosidase from Aspergillus oryzae, 0.25 mU β1,3-galactosidase from bovine testes and 3 mU α-fucosidase from bovine kidney. After 24 hours, another 0.25 mU of bovine testes β1,3-galactosidase were added and the incubation was continued for a further 24 hours prior to analysis by LC-ESI-MS.
Fucosidase digestion of selected glycans
Pyridylaminated oligosaccharides were fractionated by a “two-dimensional” mapping technique starting with the aforementioned RP-HPLC method. Peaks were collected, dried and fractionated in the second dimension by NP-HPLC. The normal phase HPLC experiments were performed on a Shimadzu HPLC System equipped with a fluorescence detector (excitation/emission 310/380 nm) and a TOSOH Biosep TSK gel Amide-80 column (250 × 4.6 mm). Solvent A was 10% acetonitrile, 3% acetic acid in water, pH 7.3 adjusted with triethylamine and B consisted of 95% acetonitrile and 5% water (v/v). A linear gradient from 73.5% to 47% B from 5 - 45 minutes was applied using a flow rate of 1 ml/min. Selected fractions were collected, dried and analysed with the LC-ESI-MS method described above; the structure of interest (HexNAc3Hex3Fuc1PC1) was subjected to a α-fucosidase digest. For this purpose the dried PA derivatised N-glycans were incubated in 20 μl 0.1 M Citrate pH 5 solution and 3 mU α-fucosidase from bovine kidney overnight at 37°C; subsequent analysis was again done by LC-ESI-MS.
Hydrofluoric acid treatment of glycans
Glycans were treated with hydrofluoric acid (HF) as described by Schneider and Ferguson [60]. The dried PA labelled glycans were placed on ice and incubated with 50 μl 48% HF in water (v/v) for 48h hours. The reagent was removed under a stream of nitrogen. The glycans were analysed afterwards with LC-ESI-MS.
Native fucosyltransferase assays
As previously described for C. elegans extracts [39], dabsylated glycopeptides (MM, GnGn, GalGal, EGNEGN; 0.1 mM; see Figure 7 for structures) were incubated in PCR tubes for 5 hours at 37 °C with 2 μl of A. suum extract, 40 mM MES, pH 6.5, 10 mM MnCl2 in the absence or presence of 10 mM GDP-Fucose (final volume 5 μl). Thereafter 0.2 μl were diluted with 0.8 μl water and mixed with 1 μl 1% (w/v) α-cyano-4-hydroxycinnamic acid in 70% acetonitrile on a MALDI-TOF MS plate prior to analysis with a Thermo Bioanalysis Dynamo instrument. Subsequent digestion of fucosylation products with Aspergillus β-galactosidase, jack bean β-hexosaminidase and PNGase F were performed as previously described prior to re-analysis by MALDI-TOF MS [39].
Acknowledgements
The authors thank Dr. Günter Lochnit, Universität Gießen, for the kind gift of A. suum material and Josef Voglmeir for assistance with glycan preparation and labelling. The authors also thank Dr. Friedrich Altmann for access to the Micromass Global ESI-Q-TOF MS funded by a grant from the Austrian Rat für Forschung und Technologieentwicklung to this department. This work was funded by a grant from the Austrian Fonds zur Förderung der wissenschaftlichen Forschung (P18447 to IBHW).
Abbreviations
- CID
collision-induced dissociation
- ESI
electrospray-ionisation
- g.u.
glucose units
- LC
liquid chromatography
- MALDI
matrix-assisted laser desorptionionisation
- MS
mass spectrometry
- NP
normal phase
- PC
phosphorylcholine
- PNGase
peptide N-glycosidase
- RP
reversed phase
- TOF
time-of-flight
The following N-glycan abbreviations are used in the text and the corresponding pictorical forms are shown in Figure 7:
- MM
Manα1-6(Manα1-3)Manβ1-4GlcNAcβ1-4-Asn
- GalGal
Galβ1-4GlcNAcβ1-2Manα1-6(Galβ1-4GlcNAcβ1-2Manα1-3)Manβ1-4GlcNAcβ1-4-Asn
- βGNβGN
GalNAcβ1-4GlcNAcβ1-2Manα1-6(GalNAcβ1-4GlcNAcβ1-2Manα1-3)Manβ1-4GlcNAcβ1-4-Asn
- GnGn
GlcNAcβ1-2Manα1-6(GlcNAcβ1-2Manα1-3)Manβ1-4GlcNAcβ1-4-Asn
References
- 1.Maizels RM, Balic A, Gomez-Escobar N, Nair M, Taylor MD, Allen JE. Helminth parasites--masters of regulation. Immunol Rev. 2004;201:89–116. doi: 10.1111/j.0105-2896.2004.00191.x. [DOI] [PubMed] [Google Scholar]
- 2.Summers RW, Elliott DE, Qadir K, Urban JF, Jr., Thompson R, Weinstock JV. Trichuris suis seems to be safe and possibly effective in the treatment of inflammatory bowel disease. Am J Gastroenterol. 2003;98:2034–2041. doi: 10.1111/j.1572-0241.2003.07660.x. [DOI] [PubMed] [Google Scholar]
- 3.Summers RW, Elliott DE, Urban JF, Jr., Thompson R, Weinstock JV. Trichuris suis therapy in Crohn's disease. Gut. 2005;54:87–90. doi: 10.1136/gut.2004.041749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nacher M, Gay F, Singhasivanon P, Krudsood S, Treeprasertsuk S, Mazier D, Vouldoukis I, Looareesuwan S. Ascaris lumbricoides infection is associated with protection from cerebral malaria. Parasite Immunol. 2000;22:107–113. doi: 10.1046/j.1365-3024.2000.00284.x. [DOI] [PubMed] [Google Scholar]
- 5.McSharry C, Xia Y, Holland CV, Kennedy MW. Natural immunity to Ascaris lumbricoides associated with immunoglobulin E antibody to ABA-1 allergen and inflammation indicators in children. Infect Immun. 1999;67:484–489. doi: 10.1128/iai.67.2.484-489.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dell A, Haslam SM, Morris HR, Khoo K-H. Immunogenic glycoconjugates implicated in parasitic nematode diseases. Biochim. Biophys. Acta. 1999;1455:353–362. doi: 10.1016/s0925-4439(99)00064-2. [DOI] [PubMed] [Google Scholar]
- 7.Harnett W, Harnett MM. Phosphorylcholine: friend or foe of the immune system? Immunol Today. 1999;20:125–129. doi: 10.1016/s0167-5699(98)01419-4. [DOI] [PubMed] [Google Scholar]
- 8.McInnes IB, Leung BP, Harnett M, Gracie JA, Liew FY, Harnett W. A novel therapeutic approach targeting articular inflammation using the filarial nematode-derived phosphorylcholine-containing glycoprotein ES-62. J Immunol. 2003;171:2127–2133. doi: 10.4049/jimmunol.171.4.2127. [DOI] [PubMed] [Google Scholar]
- 9.Deehan MR, Goodridge HS, Blair D, Lochnit G, Dennis RD, Geyer R, Harnett MM, Harnett W. Immunomodulatory properties of Ascaris suum glycosphingolipids - phosphorylcholine and non-phosphorylcholine-dependent effects. Parasite Immunol. 2002;24:463–469. doi: 10.1046/j.1365-3024.2002.00489.x. [DOI] [PubMed] [Google Scholar]
- 10.Allen JE, MacDonald AS. Profound suppression of cellular proliferation mediated by the secretions of nematodes. Parasite Immunol. 1998;20:241–247. doi: 10.1046/j.1365-3024.1998.00151.x. [DOI] [PubMed] [Google Scholar]
- 11.Peters PJ, Gagliardo LF, Sabin EA, Betchen AB, Ghosh K, Oblak JB, Appleton JA. Dominance of immunoglobulin G2c in the antiphosphorylcholine response of rats infected with Trichinella spiralis. Infect Immun. 1999;67:4661–4667. doi: 10.1128/iai.67.9.4661-4667.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lochnit G, Dennis RD, Geyer R. Phosphorylcholine substituents in nematodes: structures, occurrence and biological implications. Biol Chem. 2000;381:839–847. doi: 10.1515/BC.2000.106. [DOI] [PubMed] [Google Scholar]
- 13.Lochnit G, Dennis RD, Ulmer AJ, Geyer R. Structural elucidation and monokine-inducing activity of two biologically active zwitterionic glycosphingolipids derived from the porcine parasitic nematode Ascaris suum. J Biol Chem. 1998;273:466–474. doi: 10.1074/jbc.273.1.466. [DOI] [PubMed] [Google Scholar]
- 14.Lochnit G, Dennis RD, Müntefehr H, Nispel S, Geyer R. Immunohistochemical localization and differentiation of phosphocholine-containing antigens of the porcine, parasitic nematode, Ascaris suum. Parasitology. 2001;122:359–370. doi: 10.1017/s0031182001007326. [DOI] [PubMed] [Google Scholar]
- 15.Friedl CH, Lochnit G, Zähringer U, Bahr U, Geyer R. Structural elucidation of zwitterionic carbohydrates derived from glycosphingolipids of the porcine parasitc nematode Ascaris suum. Biochem. J. 2003;369:89–102. doi: 10.1042/BJ20021074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Riet E, Wuhrer M, Wahyuni S, Retra K, Deelder AM, Tielens AG, D VDK, Yazdanbakhsh M. Antibody responses to Ascaris-derived proteins and glycolipids: the role of phosphorylcholine. Parasite Immunol. 2006;28:363–371. doi: 10.1111/j.1365-3024.2006.00844.x. [DOI] [PubMed] [Google Scholar]
- 17.Wuhrer M, Rickhoff S, Dennis RD, Lochnit G, Soboslay PT, Baumeister S, Geyer R. Phosphocholine-containing, zwitterionic glycosphingolipids of adult Onchocerca volvulus as highly conserved antigenic structures of parasitic nematodes. Biochem. J. 2000;348:417–423. [PMC free article] [PubMed] [Google Scholar]
- 18.Gerdt S, Dennis RD, Borgonie G, Schnabel R, Geyer R. Isolation, characterization and immunolocalization of phosphorylcholine-substituted glycolipids in developmental stages of Caenorhabditis elegans. Eur J Biochem. 1999;266:952–963. doi: 10.1046/j.1432-1327.1999.00937.x. [DOI] [PubMed] [Google Scholar]
- 19.Haslam SM, Dell A. Hallmarks of Caenorhabditis elegans N-glycosylation: complexity and controversy. Biochimie. 2003;85:25–32. doi: 10.1016/s0300-9084(03)00041-5. [DOI] [PubMed] [Google Scholar]
- 20.Cipollo JF, Awad A, Costello CE, Robbins PW, Hirschberg CB. Biosynthesis in vitro of Caenorhabditis elegans phosphorylcholine oligosaccharides. Proc Natl Acad Sci U S A. 2004;101:3404–3408. doi: 10.1073/pnas.0400384101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paschinger K, Rendić D, Lochnit G, Jantsch V, Wilson IBH. Molecular basis of anti-horseradish peroxidase staining in Caenorhabditis elegans. J. Biol. Chem. 2004;279:49588–49598. doi: 10.1074/jbc.M408978200. [DOI] [PubMed] [Google Scholar]
- 22.Paschinger K, Hackl M, Gutternigg M, Kretschmer-Lubich D, Stemmer U, Jantsch V, Lochnit G, Wilson IBH. A deletion in the Golgi α-mannosidase II gene of Caenorhabditis elegans results in unexpected non-wild type N-glycan structures. J. Biol. Chem. 2006;281:28625–28277. doi: 10.1074/jbc.M602878200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haslam SM, Khoo KH, Houston KM, Harnett W, Morris HR, Dell A. Characterisation of the phosphorylcholine-containing N-linked oligosaccharides in the excretory-secretory 62 kDa glycoprotein of Acanthocheilonema viteae. Mol Biochem Parasitol. 1997;85:53–66. doi: 10.1016/s0166-6851(96)02807-1. [DOI] [PubMed] [Google Scholar]
- 24.Morelle W, Haslam SM, Olivier V, Appleton JA, Morris HR, Dell A. Phosphorylcholine-containing N-glycans of Trichinella spiralis: identification of multiantennary lacdiNAc structures. Glycobiology. 2000;10:941–950. doi: 10.1093/glycob/10.9.941. [DOI] [PubMed] [Google Scholar]
- 25.Haslam SM, Houston KM, Harnett W, Reason AJ, Morris HR, Dell A. Structural studies of N-glycans of filarial parasites. Conservation of phosphorylcholine-substituted glycans among species and discovery of novel chitooligomers. J Biol Chem. 1999;274:20953–20960. doi: 10.1074/jbc.274.30.20953. [DOI] [PubMed] [Google Scholar]
- 26.Haslam SM, Gems D, Morris HR, Dell A. The glycomes of Caenorhabditis elegans and other model organisms. Biochem. Soc. Symp. 2002;69:117–134. [PubMed] [Google Scholar]
- 27.Haslam SM, Coles GC, Munn EA, Smith TS, Smith HF, Morris HR, Dell A. Haemonchus contortus glycoproteins contain N-linked oligosaccharides with novel highly fucosylated core structures. J. Biol. Chem. 1996;271:30561–30570. doi: 10.1074/jbc.271.48.30561. [DOI] [PubMed] [Google Scholar]
- 28.Haslam SM, Coles GC, Reason AJ, Morris HR, Dell A. The novel core fucosylation of Haemonchus contortus N-glycans is stage specific. Mol. Biochem. Parasitol. 1998;93:143–147. doi: 10.1016/s0166-6851(98)00020-6. [DOI] [PubMed] [Google Scholar]
- 29.Tawill S, Le Goff L, Ali F, Blaxter M, Allen JE. Both free-living and parasitic nematodes induce a characteristic Th2 response that is dependent on the presence of intact glycans. Infect. Immun. 2004;72:398–407. doi: 10.1128/IAI.72.1.398-407.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bardor M, Faveeuw C, Fitchette AC, Gilbert D, Galas L, Trottein F, Faye L, Lerouge P. Immunoreactivity in mammals of two typical plant glycoepitopes, core α(1,3)-fucose and core xylose. Glycobiology. 2003;13:427–434. doi: 10.1093/glycob/cwg024. [DOI] [PubMed] [Google Scholar]
- 31.Leon MA, Young NM. Specificity for phosphorylcholine of six murine myeloma proteins reactive with Pneumococcus C polysaccharide and β-lipoprotein. Biochemistry. 1971;10:1424–1429. doi: 10.1021/bi00784a024. [DOI] [PubMed] [Google Scholar]
- 32.Weiser JN, Shchepetov M, Chong ST. Decoration of lipopolysaccharide with phosphorylcholine: a phase-variable characteristic of Haemophilus influenzae. Infect. Immun. 1997;65:943–950. doi: 10.1128/iai.65.3.943-950.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilson IBH, Harthill JE, Mullin NP, Ashford DA, Altmann F. Core α1,3-fucose is a key part of the epitope recognized by antibodies reacting against plant N-linked oligosaccharides and is present in a wide variety of plant extracts. Glycobiology. 1998;8:651–661. doi: 10.1093/glycob/8.7.651. [DOI] [PubMed] [Google Scholar]
- 34.Mo W, Sakamoto H, Nishikawa A, Kagi N, Langridge JI, Shimonishi Y, Takao T. Structural characterization of chemically derivatized oligosaccharides by nanoflow electrospray ionization mass spectrometry. Anal Chem. 1999;71:4100–4106. doi: 10.1021/ac990247i. [DOI] [PubMed] [Google Scholar]
- 35.Tomiya N, Awaya J, Kurono M, Endo S, Arata Y, Takahashi N. Analyses of N-linked oligosaccharides using a two-dimensional mapping technique. Anal Biochem. 1988;171:73–90. doi: 10.1016/0003-2697(88)90126-1. [DOI] [PubMed] [Google Scholar]
- 36.Haslam SM, Coles GC, Morris HR, Dell A. Structural characterisation of the N-glycans of Dictyocaulus viviparus: discovery of the Lewisx structure in a nematode. Glycobiology. 2000;10:223–229. doi: 10.1093/glycob/10.2.223. [DOI] [PubMed] [Google Scholar]
- 37.Wilson IBH. Glycosylation of proteins in plants and invertebrates. Curr. Opin. Struc. Biol. 2002;12:569–577. doi: 10.1016/s0959-440x(02)00367-6. [DOI] [PubMed] [Google Scholar]
- 38.Fabini G, Freilinger A, Altmann F, Wilson IBH. Identification of core α1,3-fucosylated glycans and the requisite fucosyltransferase in Drosophila melanogaster. Potential basis of the neural anti-horseradish peroxidase epitope. J. Biol. Chem. 2001;276:28058–28067. doi: 10.1074/jbc.M100573200. [DOI] [PubMed] [Google Scholar]
- 39.Paschinger K, Staudacher E, Stemmer U, Fabini G, Wilson IBH. Fucosyltransferase substrate specificity and the order of fucosylation in invertebrates. Glycobiology. 2005;15:463–474. doi: 10.1093/glycob/cwi028. [DOI] [PubMed] [Google Scholar]
- 40.Paschinger K, Fabini G, Schuster D, Rendić D, Wilson IBH. Definition of immunogenic carbohydrate epitopes. Acta Biochim. Pol. 2005;52:629–632. [PubMed] [Google Scholar]
- 41.Nyame AK, DeBose-Boyd R, Long TD, Tsang VCW, Cummings RD. Expression of Lex antigen in Schistosoma japonicum and S- haematobium and immune responses to Lex in infected animals: lack of Lex expression in other trematodes and nematodes. Glycobiology. 1998;8:615–624. doi: 10.1093/glycob/8.6.615. [DOI] [PubMed] [Google Scholar]
- 42.Yazdanbakhsh M, Kremsner PG, van Ree R. Allergy, parasites, and the hygiene hypothesis. Science. 2002;296:490–494. doi: 10.1126/science.296.5567.490. [DOI] [PubMed] [Google Scholar]
- 43.Van Die I, Cummings RD. Glycans modulate immune responses in helminth infections and allergy. Chem Immunol Allergy. 2006;90:91–112. doi: 10.1159/000088883. [DOI] [PubMed] [Google Scholar]
- 44.Houston KM, Cushley W, Harnett W. Studies on the Site and Mechanism of Attachment of Phosphorylcholine to a Filarial Nematode Secreted Glycoprotein. J. Biol. Chem. 1997;272:1527–1533. doi: 10.1074/jbc.272.3.1527. [DOI] [PubMed] [Google Scholar]
- 45.Khoo K-H, Nieto A, Morris HR, Dell A. Structural characterisation of the N-glycans from Echinococcus granulosus hydatid cyst membrane and protoscholeces. Mol Biochem Parasitol. 1997;86:237–248. doi: 10.1016/s0166-6851(97)00036-4. [DOI] [PubMed] [Google Scholar]
- 46.Haslam SM, Restrepo BI, Obregon-Henao A, Teale JM, Morris HR, Dell A. Structural characterization of the N-linked glycans from Taenia solium metacestodes. Mol Biochem Parasitol. 2003;126:103–107. doi: 10.1016/s0166-6851(02)00250-5. [DOI] [PubMed] [Google Scholar]
- 47.Lee JJ, Dissanayake S, Panico M, Morris HR, Dell A, Haslam SM. Mass spectrometric characterisation of Taenia crassiceps metacestode N-glycans. Mol Biochem Parasitol. 2005;143:245–249. doi: 10.1016/j.molbiopara.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 48.Morelle W, Haslam SM, Morris HR, Dell A. Characterization of the N-linked glycans of adult Trichinella spiralis. Mol Biochem Parasitol. 2000;109:171–177. doi: 10.1016/s0166-6851(00)00241-3. [DOI] [PubMed] [Google Scholar]
- 49.Duffy MS, Morris HR, Dell A, Appleton JA, Haslam SM. Protein glycosylation in Parelaphostrongylus tenuis -First description of the Galα1-3Gal sequence in a nematode. Glycobiology. 2006;16:854–862. doi: 10.1093/glycob/cwl001. [DOI] [PubMed] [Google Scholar]
- 50.Khoo K-H, Huang H-H, Lee K-M. Characteristic structural features of schistosome cercarial N-glycans: expression of Lewis X and core xylosylation. Glycobiology. 2001;11:149–163. doi: 10.1093/glycob/11.2.149. [DOI] [PubMed] [Google Scholar]
- 51.van Die I, Gomord V, Kooyman FNJ, van der Berg TK, Cummings RD, Vervelde L. Core α1→3-fucose is a common modifcation of N-glycans in parasitic helminths and constitutes an important epitope for IgE from Haemonchus contortus infected sheep. FEBS Lett. 1999;463:189–193. doi: 10.1016/s0014-5793(99)01508-2. [DOI] [PubMed] [Google Scholar]
- 52.Tretter V, Altmann F, Kubelka V, März L, Becker WM. Fucose α1,3-linked to the core region of glycoprotein N- glycans creates an important epitope for IgE from honeybee venom allergic individuals. Int Arch Allergy Immunol. 1993;102:259–266. doi: 10.1159/000236534. [DOI] [PubMed] [Google Scholar]
- 53.Bublin M, Radauer C, Wilson IBH, Kraft D, Scheiner O, Breiteneder H, Hoffmann-Sommergruber K. Cross-reactive N-glycans of Api g 5, a high molecular weight glycoprotein allergen from celery, are required for immunoglobulin E binding and activation of effector cells from allergic patients. FASEB J. 2003;17:1697–1699. doi: 10.1096/fj.02-0872fje. [DOI] [PubMed] [Google Scholar]
- 54.DeBose-Boyd RA, Nyame AK, Jasmer DP, Cummings RD. The ruminant parasite Haemonchus contortus expresses an α1,3- fucosyltransferase capable of synthesizing the Lewis x and sialyl Lewis x antigens. Glycoconjugate J. 1998;15:789–798. doi: 10.1023/a:1006912032273. [DOI] [PubMed] [Google Scholar]
- 55.Geldhof P, Newlands GF, Nyame K, Cummings R, Smith WD, Knox DP. Presence of the LDNF glycan on the host-protective H-gal-GP fraction from Haemonchus contortus. Parasite Immunol. 2005;27:55–60. doi: 10.1111/j.1365-3024.2005.00744.x. [DOI] [PubMed] [Google Scholar]
- 56.Chen S, Tan J, Reinhold VN, Spence AM, Schachter H. UDP-Nacetylglucosamine:α-3-D-mannoside β-1,2-N-acetylglucosaminyltransferase I and UDP-N-acetylglucosamine:α-6-D-mannoside β-1,2-N-acetylglucosaminyltransferase II in Caenorhabditis elegans. Biochim Biophys Acta. 2002;1573:271–279. doi: 10.1016/s0304-4165(02)00393-8. [DOI] [PubMed] [Google Scholar]
- 57.Warren CE, Krizius A, Roy PJ, Culotti JG, Dennis JW. The C. elegans gene, gly-2, can rescue the N-acetylglucosaminyltransferase V mutation of Lec4 cells. J. Biol. Chem. 2002;277:22829–22838. doi: 10.1074/jbc.M201390200. [DOI] [PubMed] [Google Scholar]
- 58.Schachter H. Protein glycosylation lessons from Caenorhabditis elegans. Curr Opin Struct Biol. 2004;14:607–616. doi: 10.1016/j.sbi.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 59.Hase S, Ibuki T, Ikenaka T. Reexamination of the pyridylamination used for fluorescence labelling of oligosaccharides and its application to glycoproteins. J Biochem (Tokyo) 1984;95:197–203. doi: 10.1093/oxfordjournals.jbchem.a134585. [DOI] [PubMed] [Google Scholar]
- 60.Schneider P, Ferguson MAJ. Microscale analysis of glycosylphosphatidylinositol structures. Methods Enzymol. 1995;250:614–630. doi: 10.1016/0076-6879(95)50100-2. [DOI] [PubMed] [Google Scholar]