Abstract
We have shown that in the perfused heart glucosamine improved functional recovery following ischemia and this appeared to be mediated via an increase in O-Linked N-acetylglucosamine (O-GlcNAc) levels on nucleocytoplasmic proteins. Several kinase pathways, specifically Akt and the mitogen activated protein kinases (MAPK) p38 and ERK1/2, which have been implicated in ischemic cardioprotection, have been reported to be modified in response to increased O-GlcNAc levels. Therefore, the goals of this study were to determine the effect of ischemia on O-GlcNAc levels and to evaluate whether the cardioprotection resulting from glucosamine treatment could be attributed to changes in ERK1/2, Akt and p38 phosphorylation. Isolated rat hearts were perfused with or without 5mM glucosamine and were subjected to 5, 10 or 30 min low flow ischemia (LFI) or 30 min LFI and 60min reperfusion. Glucosamine treatment attenuated ischemic contracture and improved functional recovery at the end of reperfusion. Glucosamine treatment increased flux through the hexosamine biosynthesis pathway and increased O-GlcNAc levels but had no effect on ATP levels. Glucosamine did not alter the response of either ERK1/2 or Akt to ischemia/reperfusion; however it significantly attenuated the ischemia-induced increase in p38 phosphorylation and paradoxically increased p38 phosphorylation at the end of reperfusion. These data support the notion that O-GlcNAc may play an important role as an internal stress response and that glucosamine-induced cardioprotection may be mediated via the p38 MAPK pathway.
Keywords: Glucosamine, hexosamine biosynthesis, MAPK, Akt/PKB, ischemia
Introduction
It was recently shown that in mammalian cells a variety of different stress stimuli increased the level of O-linked-N-acetylglucosamine (O-GlcNAc) on nuclear and cytoplasmic proteins. Inhibition of this response increased the sensitivity to stress whereas augmentation of the O-GlcNAc levels increased tolerance to the same stress stimuli and improved cell survival (63). Sohn et al. reported that in Chinese ovarian cancer cells inhibition of glutamine: fructose-6-phosphate amidotransferase (GFAT), which regulates the entry of glucose into the hexosamine biosynthesis pathway (HBP), decreased O-GlcNAc levels and reduced cell survival following heat stress (48). Taken together, these studies suggest that activation of metabolic pathways leading to increased levels of O-GlcNAc is an endogenous stress response of mammalian cells that could represent potential target for the treatment of ischemic injury.
O-GlcNAc transferase (OGT) is the enzyme catalyzing protein O-GlcNAc modifications and flux through this enzyme is very sensitive to the concentration of UDP-N-acetyl glucosamine (UDP-GlcNAc), which is an end product of the HBP (26, 57). Tissue levels of UDP-GlcNAc are very sensitive to circulating glucose levels and glutamine, the donor of the amine group, also increases HBP flux (57, 61). Glucosamine, which enters cells via the glucose transporter system and is phosphorylated to glucosamine-6-phosphate by hexokinase also increased HBP metabolites including UDP-GlcNAc (51). We have recently shown that in the heart, perfusion with glucosamine rapidly increased O-GlcNAc levels and decreased injury resulting from the calcium paradox and improved recovery of function following zero-flow ischemia and reperfusion (28). Inhibition of OGT with alloxan abrogated this protection; however, the mechanisms underlying the protection associated with glucosamine have yet to be elucidated.
The response of the heart to external stress stimuli, including ischemia is mediated, in part, by a number of signaling pathways, including extracellular-signal regulated kinase (ERK) and p38 mitogen activated protein kinase (MAPK) (36). The role of p38 in mediating the response of the heart to ischemic injury is somewhat controversial. In many studies ischemia increases p38 phosphorylation, and inhibition of p38 phosphorylation during sustained ischemia is reported to be cardioprotective (2, 3, 21, 31, 36, 39, 49). However, in contrast, brief activation of p38 prior to ischemia has been shown to contribute to the protection associated with ischemic preconditioning, although this protective effect is critically dependent on both timing and duration of activation (37). It is worth noting that p38 is frequently associated with activation of pro-apoptotic pathways such as Caspase-3, or p53 (22, 34, 64). However, αB-crystallin and HSP-27 are anti-apoptotic, have been shown to play a role in ischemic protection (12, 18, 32, 41) and are both downstream of p38 (27, 44). Recently several studies have demonstrated that ERK activation, especially during reperfusion is important in mediating protection associated with insulin and ischemic preconditioning (2, 3, 15, 37). A number of studies have also reported that Akt activation protects against ischemia/reperfusion injury in the heart (11, 14, 17). Recently Hausenloy and Yellon (15) described the reperfusion injury salvage kinase (RISK) pathway in which activation of pro-survival kinases Akt and ERK1/2 particularly at the time of reperfusion contribute to ischemic protection.
Interestingly activation of HBP and increased O-GlcNAc levels have been reported to modify p38 phosphorylation and activity (6, 13, 19). We have recently shown that increasing O-GlcNAc levels in human neutrophils, increased basal and agonist stimulated phosphorylation of both p38 and ERK1/2 (23). Other studies have also demonstrated that increased HBP and/or O-GlcNAc levels also affect Akt phosphorylation (40, 52). Taken together, these studies suggest that key kinases implicated in ischemic cardioprotection can be modulated by changes in HBP flux and O-GlcNAc levels. Therefore, in light of recent studies showing that increased O-GlcNAc levels were associated with increased tolerance to stress, we investigated the effect of ischemia on the flux through the hexosamine biosynthesis pathway and cardiac O-GlcNAc levels. We also examined the effect of glucosamine-mediated increase in protein O-GlcNAc levels on the response of p38, ERK1/2 and Akt phosphorylation to ischemia/reperfusion in the isolated perfused heart.
Methods
Animals
Animal experiments were approved by the University of Alabama Institutional Animal Care and Use Committee and conformed to the Guide for the Care and Usage of Laboratory Animals published by the National Institutes of Health (NIH Publication No. 85-23, 1996). Non-fasted, male Sprague-Dawley rats (Charles Rivers Laboratories) weighing 351±6 g were used.
Materials
All chemicals were purchased from Sigma-Aldrich unless otherwise stated.
Experimental groups
Hearts were divided into five perfusion groups: 1) Normoxia; 2) 5 minutes of LFI (0.3mls/min); 3) 10 minutes of LFI; 4) 30 minutes of LFI and 5) 30 minutes of LFI and 60 min reperfusion. In the normoxic group hearts were perfused for a total of 60 minutes. In the LFI groups hearts were allowed to stabilize for 30 minutes then subjected to LFI for the indicated time period. In each perfusion group hearts were perfused with or without glucosamine (5 mmol/L) for the duration of the experiment. There were 4–6 replicates in each group as indicated in the Table and Figure legends.
Isolated heart perfusions
Animals were anesthetized with intraperitoneal ketamine hydrochloride injection (100mg/kg, Lloyd Laboratories). Hearts were rapidly excised and perfused retrogradely, as previously described (9) and coronary flow was adjusted to maintain a constant perfusion pressure of 75 mmHg. The perfusion buffer consisted of a Krebs-Henseleit buffer containing in mmol/L: lactate 1.0, pyruvate 0.1, palmitate 0.32, glutamine 0.5 and 3% BSA (fatty acid free) (Serologicals Proteins Inc.) plus 50 μU/mL insulin (NovoNordisk). Cardiac function was monitored via a fluid-filled balloon placed into the left ventricle. End-diastolic pressure (EDP) was set to 5 mmHg by adjustingballoon volume. All hearts were paced at 320 beats/min rate during the whole experiment except the LFI period and the first five minutes of reperfusion. Ventricular fibrillation was defined as fast mechanical activity with minimal left ventricular developed pressure during the unpaced, first five minutes of reperfusion. At the end of the experiment hearts were freeze-clamped with liquid nitrogen cooled tongs.
Western blots
Hearts were ground to a fine powder under liquid nitrogen and homogenized in T-PER (Pierce) containing 5% protease inhibitor cocktail, 40 μmol/L PUGNAc (Carbogen) and 1 mmol/L sodium-orthovanadate and 20 mmol/L sodium-fluoride on ice and centrifuged for 10 min at 15000g. The protein concentration of the supernatant was measured using Bio-Rad Protein Assay Kit. Whole heart lysates were separated on 10% SDS-PAGE and transferred to PVDF membrane (Pall). Equal loading of protein was confirmed by Sypro Ruby staining (Bio-Rad) on the membranes. Blots were probed with the appropriate antibody in casein blocking buffer. Anti-O-GlcNAc antibody, CTD110.6 (Covance), total and phospho (Thr180/Tyr182) p38 (Santa Cruz), total and phospho (Thr202/Tyr204) ERK1/2 (Cell Signaling) and total and phospho (Ser473) Akt (Cell Signaling) antibodies were used. Blots were visualized with enhanced chemiluminescent assay (Pierce) and the signal was detected with UVP BioChemi System (UVP). Densitometry was quantified using Labworks analysis software (UVP).
HPLC
Approximately 50 mg of frozen tissue powder was homogenized in 1 mL ice-cold 0.3 mol/L perchloric acid (PCA) and centrifuged for 15 min at 15000g at 4°C. PCA was removed from the supernatant with 2 volumes of 1:4 trioctylamine:1,1,2-trichloro-trifluoroethan mixture. Samples were loaded on Partisil 10 SAX column (Beckman), nucleotide sugars were measured at 262 nm using 2 mL/min flow rate and linear salt and pH gradient from 5 mmol/L to 750 mmol/L (NH4)H2PO4 and from pH 2.8 to 3.7 (42). This method cannot separate UDP-GlcNAc from UDP-N-acetyl galactosamine (UDP-GalNAc) so the results are presented as the sum of UDP-GlcNAc and UDP-GalNAc (UDP-HexNAc); however in the heart the ratio of UDP-GlcNAc to UDP-GalNAc is approximately 3:1 (10).
Data analysis
Data are presented as means ± standard errors. Differences between experimental groups were evaluated with Student’s t-test for unpaired data, Two-way ANOVA or One-way ANOVA tests as appropriate. Statistical significant differences between groups were defined as p values < 0.05 and are indicated in the legends of figures.
Results
Glucosamine treatment improved functional recovery following ischemia
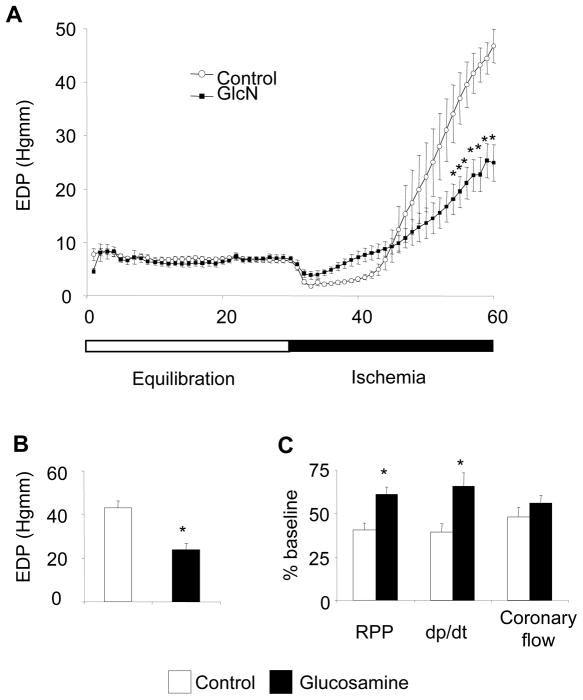
Before ischemia there was no significant difference in contractile function between control and glucosamine groups; however baseline coronary flow was significantly increased in the glucosamine group (Table 1). As previously reported in this model (54, 55), the onset of LFI resulted in a rapid decrease in contractile function and gradual increase in EDP (Fig. 1A). At the end of LFI, EDP was significantly attenuated in the glucosamine group (Control: 43.1 ± 3.1 vs. GlcN: 23.8 ± 3.0 mmHg; p<0.05) (Fig. 1B). After 30 minutes of LFI and 60 minutes reperfusion functional recovery was significantly higher in the glucosamine group compared to control (Fig. 1C); however, coronary flow was decreased by the same proportion in both groups compared to preischemic values (Fig. 1C). Interestingly, while four out of five control hearts (80%) fibrillated during early reperfusion none of the glucosamine treated hearts fibrillated (0%; p<0.05; chi-squared test).
Table 1.
| Control (n=17) | GlcN (n=17) | |
|---|---|---|
| LVDP (mmHg) | 100.6 ± 2.8 | 108.1 ± 3.4 |
| Heart rate (beats/min) | 322 ± 0.2 | 321 ± 0.3 |
| RPP (mmHg/min) | 32250 ± 830 | 34337 ± 1074 |
| +dp/dt (mmHg/s) | 4498 ± 172 | 4436 ± 209 |
| −dp/dt (mmHg/s) | 2436 ± 94 | 2457 ± 116 |
| EDP (mmHg) | 5.9 ± 0.2 | 6.0 ± 0.3 |
| Coronary flow (mL/min) | 13.6 ± 0.7 | 17.3 ± 0.9* |
p< 0,05 vs Control
Figure 1.
A: Trace of end diastolic pressure (n=11 in each group), *: p<0.05 vs. control (Two-Way ANOVA with Bonferroni posthoc test); B: End diastolic pressure at the end of 30 minutes ischemia (n=11 in each group), *: p<0.05 vs. control (Student’s t-test) C: Percentage of recovery of function compared to baseline values (n=5 in each group), * p<0.05 vs. control (Student’s t-test). (RPP: rate pressure product (heart rate x left ventricular developed pressure; dp/dt: maximal change in left ventricular pressure over time)
Effect of ischemia/reperfusion and glucosamine on UDP-HexNAc, O-GlcNAc and ATP
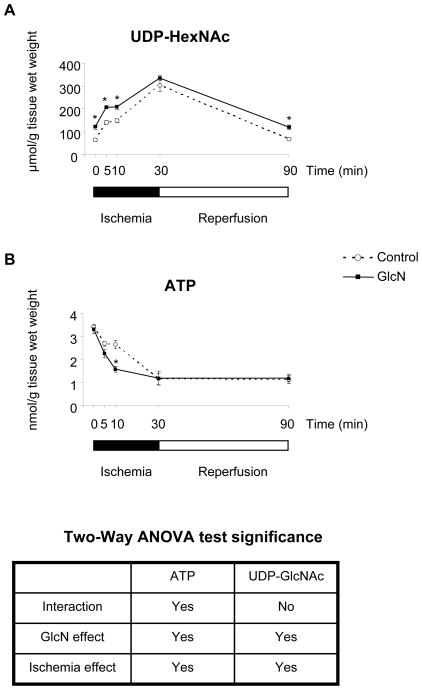
In the absence of glucosamine 5-min of ischemia significantly increased UDP-HexNAc concentrations, and longer periods of ischemia (10 and 30 minutes) increased levels further (Fig. 2A). After 60 minutes reperfusion UDP-HexNAc levels returned to the normoxic levels. Under normoxic perfusion conditions glucosamine increased UDP-HexNAc levels almost 2-fold (72 ± 7 Vs 126 ± 7 nmoles/g tissue wet weight, p<0.05; Fig. 2A). Similar to the control group there was a significant increase in UDP-HexNAc levels in response to ischemia; however after 30 minutes ischemia there was no significant difference in UDP-HexNAc levels between control and glucosamine treated groups (Fig. 2A). At the end of reperfusion UDP-HexNAc levels in the glucosamine treated group also decreased compared to end ischemia but were still elevated compared to control group.
Figure 2.
A: UDP-HexNAc levels B: ATP levels, (n=4 in each group), * p<0.05 vs. control (Two-Way ANOVA with Bonferroni posthoc test)
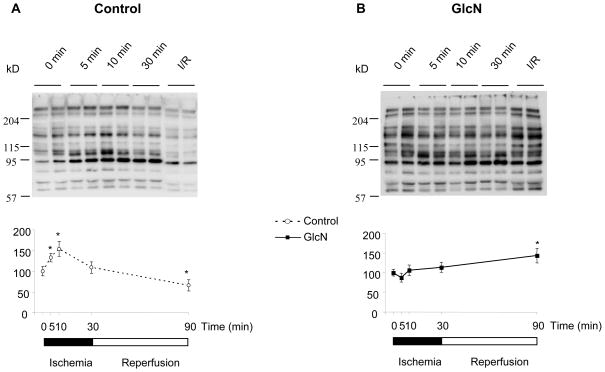
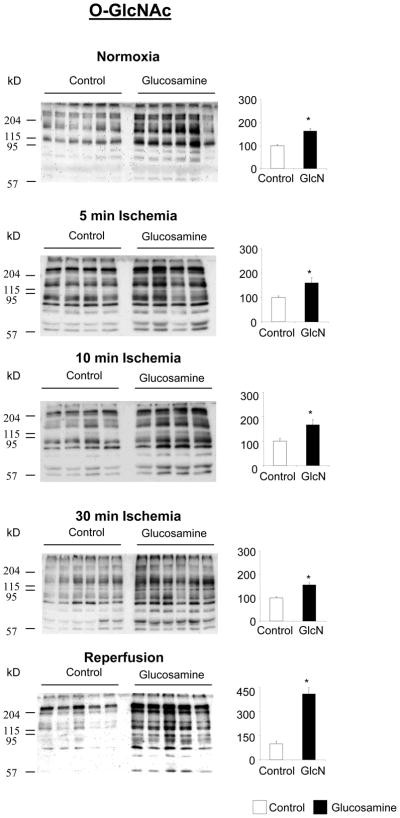
In the control group, protein O-GlcNAc levels increased in response to ischemia; after 10 minutes of ischemia O-GlcNAc levels were ~1.5-fold higher than normoxic levels. However, in contrast to UDP-HexNAc levels, O-GlcNAc levels declined between 10 and 30 min of ischemia and at the end of reperfusion they were significantly lower than normoxic levels (Fig. 3A). In contrast to the control group ischemia did not change O-GlcNAc levels in the glucosamine treated group; however at the end of reperfusion O-GlcNAc levels were significantly increased compared to normoxia (Fig. 3B). In Fig. 4 comparisons of O-GlcNAc levels between control and glucosamine treated groups are shown at each time point. It can be seen that glucosamine treatment significantly increased O-GlcNAc levels by at least 60% at all time points; the biggest difference in O-GlcNAc levels was at the end of reperfusion. This is presumably reflects the fact that in the control group, at the end of reperfusion, O-GlcNAc levels were lower compared to the normoxia, whereas in the glucosamine group they were higher (Fig. 3A, B).
Figure 3.
Effect of ischemia and reperfusion on O-GlcNAc. A: Top panel representative CTD110.6 immunoblots form control hearts, bottom panel: CTD110.6 area densities relative to 0 min ischemia (n=4 in each group) B: Top panel representative CTD110.6 immunoblots form glucosamine treated hearts, bottom panel: CTD110.6 area densities relative to 0 min ischemia (n=4 in each group); *: p<0.05 vs 0 min (One-Way ANOVA with Dunnet posthoc test)
Figure 4.
Effect of glucosamine on protein O-GlcNAc under normoxic conditions; after 5 minutes, 10 minutes and 30 minutes ischemia and following ischemia/reperfusion (I/R), left panels CTD110.6 immunoblots, right panels total area densities normalized to control group; * p<0.05 vs. control (Student’s t-test)
As expected ATP levels decreased in both control and glucosamine treated groups during ischemia and remained lower than normoxic perfusion at the end of ischemia/reperfusion (Fig. 2B). However, despite the attenuation of ischemic contracture and the improved functional recovery at the end of reperfusion, glucosamine treatment did not attenuate ATP loss during ischemia and did not increase ATP levels during reperfusion.
Effect of glucosamine on ERK, Akt and p38 phosphorylation
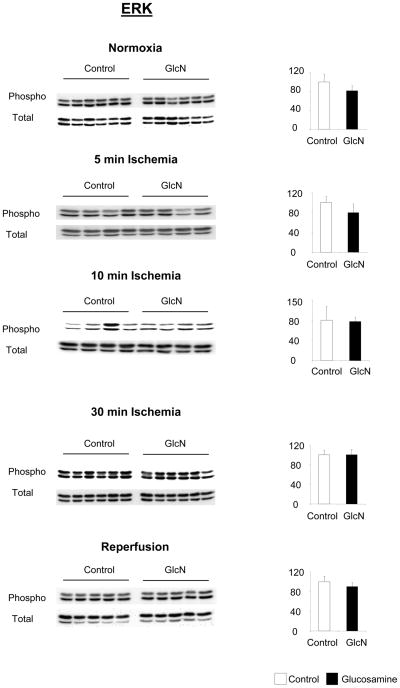
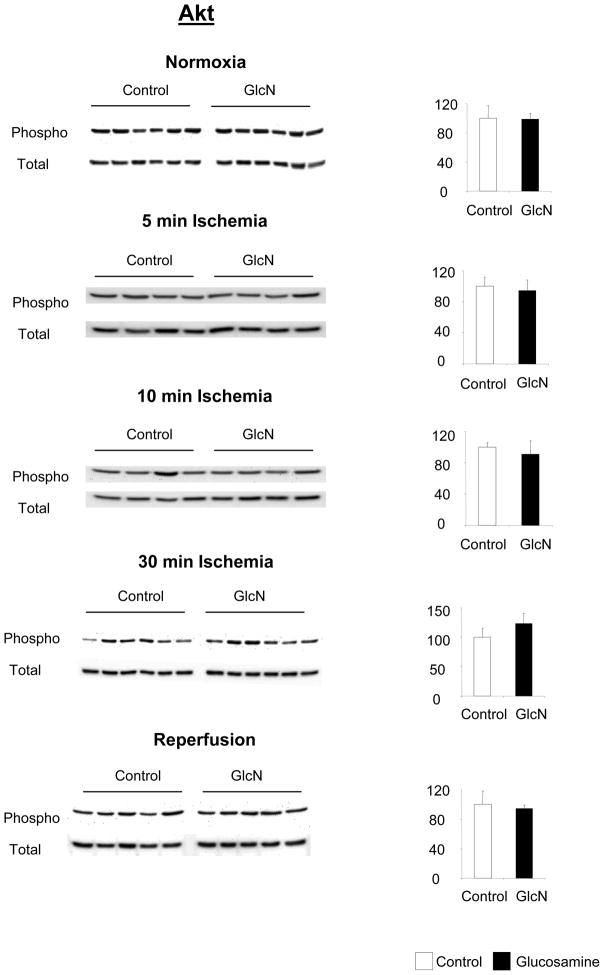
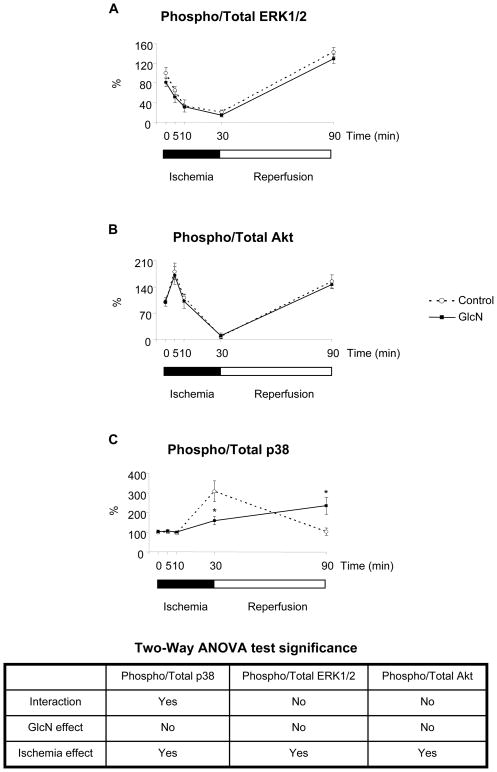
Comparisons of total and phosphorylation levels of ERK, Akt and p38 in control and glucosamine treated groups at each time point are shown in Figures 5, 6 and 7 respectively. The time course of changes in phosphorylation in the two groups normalized to normoxic conditions are summarized in Fig 8. Glucosamine treatment had no effect on ERK1/2 or Akt phosphorylation compared to the control under any conditions (Fig 5, 6). Consistent with other studies (15) there was a marked decrease in the ratio of phospho- to total-ERK1/2 during ischemia and a significant increase after reperfusion compared to normoxic conditions in both groups (Fig 8A). Akt phosphorylation increased following 5 minutes ischemia; however after longer period of ischemia Akt phosphorylation markedly decreased (Fig 8B) similar to that seen with ERK (Fig. 8A). At the end of reperfusion Akt phosphorylation returned to normoxic levels. The changes in Akt phosphorylation after 30 minutes ischemia and reperfusion are consistent with previous studies (46).
Figure 5.
Effect of glucosamine on ERK1/2 phosphorylation, under normoxic conditions, and following 5 minutes, 10 minutes and 30 minutes ischemia or ischemia/reperfusion (I/R), left panels phopho- and total-ERK1/2 immunoblots, right panels phosphor/total area densities normalized to control group; * p<0.05 vs. control (Student’s t-test)
Figure 6.
Effect of glucosamine on Akt phosphorylation, under normoxic conditions, and following 5 minutes, 10 minutes and 30 minutes ischemia or ischemia/reperfusion (I/R), left panels phopho- and total-Akt immunoblots, right panels phosphor/total area densities normalized to control group; * p<0.05 vs. control (Student’s t-test)
Figure 7.
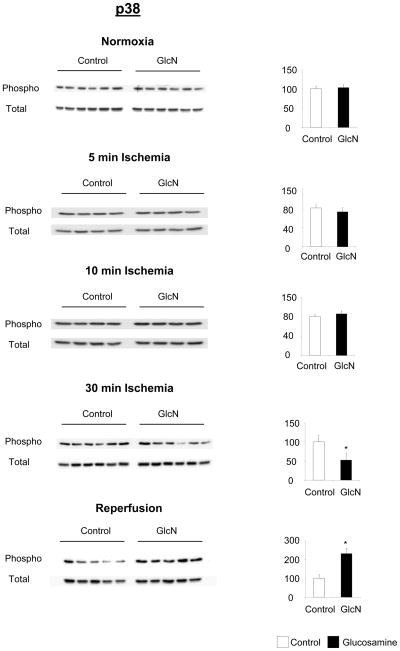
Effect of glucosamine on p38 phosphorylation, under normoxic conditions, and following 5 minutes, 10 minutes and 30 minutes ischemia or ischemia/reperfusion (I/R), left panels phopho- and total-p38 immunoblots, right panels phosphor/total area densities normalized to control group; * p<0.05 vs. control (Student’s t-test)
Figure 8.
Effect of ischemia/reperfusion and glucosamine on signaling pathways. A: ERK1/2 phosphorylation; B: Akt phosphorylation; C: p38 phosphorylation; *: p<0.05 (Two-Way ANOVA with Bonferroni posthoc test) (n=6 in 0 min, 30 groups, n=5 in 90 min group and n=4 in 5 min and 10 min groups)
Under normoxic conditions and after 5 and 10 min of ischemia p38 phosphorylation was unchanged by glucosamine treatment; however at the end of ischemia p38 phosphorylation was significantly attenuated in the glucosamine group (Fig. 7). In contrast at the end of reperfusion, p38 phosphorylation was increased in the glucosamine group (Fig. 7). In Fig. 8C it can be seen that consistent with previous reports ischemia increased phospho-p38 levels by more than 3-fold in control group compared to normoxic levels (5) (Fig. 8C). However, in the glucosamine group ischemia increased phospho-p38 by ~50%, consistent with the 30min data in Fig 7. At the end of reperfusion phospho-p38 levels returned to normoxic levels in the control group, whereas in the glucosamine group phospho-p38 levels were significantly elevated compared to both ischemic and normoxic conditions (Fig. 8C).
Interestingly we found that there was a significant linear correlation between O-GlcNAc levels and p38 phosphorylation in the glucosamine treated group (r2: 0.31, p<0.05, data not shown); however there was no significant correlation in the control hearts (r2: 0.01, p>0.7).
Discussion
In cell culture systems previous studies have demonstrated that O-GlcNAc levels are increased in response to stress and that augmentation of this response increased tolerance to stress (63). We show here for the first time that in the isolated perfused heart 5–10 min of ischemia significantly increased O-GlcNAc levels and this was associated with an increase in UDP-HexNAc levels consistent with an ischemia-induced increase in flux through the HBP. Surprisingly, however, after longer periods of ischemia, O-GlcNAc level decreased and after 60 min of reperfusion, they were significantly lower than normoxia. Thus, while the increase in O-GlcNAc with ischemia is consistent with the notion that this stress response pathway is active in the heart, the subsequent decrease during reperfusion, suggests that the regulation of this pathway may be more complex in the setting of ischemia/reperfusion in the intact heart, than previously reported in isolated cell systems (63). Nevertheless, the changes in O-GlcNAc levels in response to ischemia and reperfusion reinforce the notion that this is a highly dynamic post-translational modification that can be modulated in response to pathophysiological stimuli.
Consistent with our previous reports (28) glucosamine increased normoxic levels of both UDP-HexNAc and O-GlcNAc and improved functional recovery following ischemia reperfusion. Interestingly, while the addition of glucosamine increased O-GlcNAc levels at all time points relative to controls, ischemia did not result in a further increase; however, glucosamine did prevent the decrease in O-GlcNAc seen at the end of ischemia and during reperfusion. While the attachment of N-acetylgucosamine is important in the formation of proteoglycans in the Golgi and the endoplasmic reticulum, this is distinct from O-glycosylation, which is specific to the nucleus and in the cytosol (47). We have shown that that inhibition of nucleocytoplasmic O-glycosylation with alloxan, an OGT inhibitor, resulted in loss of protection of glucosamine treatment in isolated cardiomyocytes (8), which supports the notion that the cardioprotective effect of glucosamine is due to the increase in nucleocytoplasmic O-glycosylation. However, since glucosamine also increases the levels of UDP-GlcNAc, the substrate for N-glycosylation we cannot entirely rule out a possible effect associated with increased levels N-glycosylation. We also found that glucosamine markedly attenuated ischemic contracture and also reduced the incidence of arrhythmias on reperfusion. In addition glucosamine treatment significantly altered the response of p38 MAPK to ischemia reperfusion, without affecting the response of either ERK or Akt. These data provide further support for the idea that glucosamine cardioprotection is mediated via increased O-GlcNAc levels and show that this is associated with an altered response of p38 MAPK to ischemia/reperfusion.
It is increasingly apparent that protein O-GlcNAcylation is an important and widespread post-translational modification implicated in regulating a diverse range of cellular processes. The number of proteins identified as being capable of post-translational O-glycosylation are quickly growing and include NF-kB, Annexin, eNOS, αB-Crystallin, OGT, α-Tubulin, c-myc, HSP70 (53, 58). Increased levels of O-glycosylation have often been associated with adverse events such as insulin resistance (1); hyperglycemia induced apoptosis (29) and impaired excitation-contraction coupling (10). Paradoxically, recent studies have shown that stress increased levels of protein O-GlcNAc in several different mammalian cell lines and that increasing the levels of protein O-GlcNAc enhanced cell survival (48, 63). Sohn et al. also demonstrated that inhibition of the hexosamine biosynthesis pathway increased hyperthermal sensitivity of cells, suggesting that activation of this pathway maybe a component of endogenous cell survival pathway (48).
Zachara et al., (63) reported that O-GlcNAc levels increased in response to stress possibly as a consequence of an increase in OGT activity. Here in the intact heart we found that after 5–10 min of ischemia both UDP-HexNAc and O-GlcNAc level were significantly increased. Since flux through OGT is very sensitive to UDP-GlcNAc levels (26, 57), the increase in UDP-HexNAc could explain the increase in O-GlcNAc levels seen here; however, we cannot rule out an increase in OGT activity. The increase in UDP-HexNAc during ischemia could be due to either an increase in flux through the hexosamine pathway and/or a consequence of decreased utilization of UDP-GlcNAc via other pathways. Sohn et al., showed that inhibition of GFAT, which regulates the entry of glucose into the hexosamine biosynthesis pathway, prevented the stress induced increase in O-GlcNAc, and decreased hyperthermal tolerance. This would be consistent with increased flux through the hexosamine biosynthesis pathway also increasing UDP-GlcNAc. However, UDP-GlcNAc is also required for multiple N-glycosylation reactions that are involved in protein synthesis; since ischemia is known to inhibit protein synthesis (60) it is possible that this could also contribute to the increase in UDP-GlcNAc seen here.
Interestingly even though UDP-HexNAc continued to increase during ischemia, O-GlcNAc levels had decreased after 30 min ischemia. The dissociation between UDP-HexNAc and O-GlcNAc, suggests that OGT activity might be inhibited after prolonged ischemia. If so this could also contribute to the further decline in O-GlcNAc levels seen during reperfusion. Regulation of OGT activity is complex and poorly understood; however, OGT is known to be subject to both phosphorylation and O-GlcNAc modification and increased phosphorylation is believed to increase activity (25). Clearly more studies are needed to understand the regulation of the hexosamine biosynthesis pathway and O-GlcNAcylation in response to ischemia.
Given the wide range of proteins that are potential targets for O-GlcNAc modification the specific mechanisms underlying the protection associated with glucosamine are likely complex. Increasing energy production or slowing the rate of energy utilization during ischemia has repeatedly been shown to improve functional recovery on reperfusion. However, we found here that glucosamine did not attenuate ATP hydrolysis during ischemia or increase ATP levels during reperfusion, suggesting that the protection was not mediated by alterations in energy metabolism. It should be noted that maintenance of ATP levels either during ischemia or reperfusion, is not a prerequisite for cardioprotection. For example, although ischemic preconditioning significantly improves function recovery it is not necessarily associated with increased ATP levels during ischemia or reperfusion (7, 24), Furthermore, Champattanachai et al recently demonstrated that glucosamine treatment significantly decreased both necrosis and apoptosis in isolated cardiomyocytes in response to hypoxia/reoxygenation without altering ATP levels (8). Previous studies have provided strong evidence linking the protective effect of glucosamine to changes in O-GlcNAc levels (8, 28). However, glucosamine could potentially be metabolized via glycolysis; thus at this time we cannot rule out the possibility that mechanism of glucosamine cardioprotection could be due at least in part to the changes in substrate utilization. Clearly further studies are needed to examine the effect of glucosamine on the regulation of substrate metabolism in the heart.
Activation of the MAPK pathway, particularly p38 and ERK1/2 MAPK, has been implicated in ischemic cardioprotection (2, 3, 15, 21, 31, 36, 37, 39, 49). Since we have shown in another cell system that agonist stimulation of the MAPK pathway was enhanced in response to increased O-GlcNAc levels (23), we examined the effect of glucosamine on the response of p38 and ERK1/2 MAPK to ischemia/reperfusion. Here, we found that glucosamine significantly attenuated the ischemia-induced increase in p38 phosphorylation, which this is consistent with reports that inhibition of p38 during sustained ischemia is protective (2, 3, 21, 31, 36, 39, 49). Surprisingly, at the end of reperfusion p38 phosphorylation was increased in the glucosamine treated group. There is relatively little information regarding the impact of reperfusion on p38 phosphorylation or on whether modulation of p38 activation during reperfusion impacts recovery. However, it has been shown that brief activation of p38 may contribute to cardioprotection seen with ischemic preconditioning. Activation of p38 can be pro-apoptotic, mediated via Caspase-3 or p53 (21, 22, 34, 64); however, p38 has also been reported to activate pro-survival pathways. For example, αB-crystallin and HSP-27 are downstream of p38 and both have been shown not only to play a role in ischemic protection but also to subject to O-GlcNAc modification (43, 56, 58, 59). Furthermore, increased p38 phosphorylation has been associated with increased glucose transport (35), which might also be beneficial during reperfusion.
A number of studies have shown that increased ERK1/2 phosphorylation, especially during reperfusion is cardioprotective (2, 3, 15, 37). However, while we saw the decrease in ERK1/2 phosphorylation during ischemia and an increase on reperfusion as recently reported by Hausenloy (15), we found that glucosamine had no effect on ERK1/2 phosphorylation under any perfusion conditions. Akt has also been shown to have a protective effect in ischemia/reperfusion in the heart (14, 33). Recently, it has also been shown to mediate the effect of both ischemic pre- and postconditioning (15, 16, 50). We saw a brief increase in Akt phosphorylation after 5 minutes of ischemia; however, as described by Shao et al. (46) we saw a decrease in Akt phosphorylation at the end of ischemia and an increase during reperfusion. Despite the importance of Akt as a mediator of cardioprotection, we found no difference in the phosphorylation state of Akt between the control and the glucosamine group under any of the conditions. Thus, these data suggest that glucosamine cardioprotection cannot be attributed to activation of either ERK1/2 or Akt pro-survival pathways.
It remains to be determined whether the changes in p38 phosphorylation seen with glucosamine are a cause or an effect of the improved recovery. For example, the severity of ischemic contracture is determined in part by diastolic Ca2+ levels (45), thus since contracture was reduced the decrease in p38 phosphorylation during ischemia could be a consequence of lower cytosolic Ca2+. This is consistent with reports that glucosamine decreases Ca2+ entry into cardiomyocytes (20, 38) and increased cytosolic Ca2+ levels has been reported to activate p38 (30). Thus, a decrease in cytosolic Ca2+ during ischemia could in lead to a decrease in p38 phosphorylation; however, on reperfusion EDP is significantly reduced in the glucosamine group, suggesting lower diastolic Ca2+ levels, whereas p38 phosphorylation is increased. Consequently, the effects of glucosamine on p38 phosphorylation during ischemia and reperfusion are thus likely due to different mechanisms. It is also noteworthy that increased diastolic Ca2+ at the end of ischemia has been associated with increased incidence of ventricular fibrillation on reperfusion (45), and that here we found that glucosamine significantly reduced the incidence of ventricular fibrillation, from 80% to 0% on reperfusion. However, ventricular fibrillation was assessed only by qualtitive assessment of LVDP and heart rate; clearly a more detailed evaluation of the electrophysiological effects of glucosamine during reperfusion are needed.
Since these studies were performed in an ex vivo, isolated perfused heart model, it is premature to suggest that these results could be extrapolated to an in vivo environment. However, the substrates used here were designed to more closely mimic the in vivo metabolic milieu that in our earlier studies (28). It should also be noted that previously we demonstrated that glucosamine was protective against zero-flow ischemia; whereas here we used a model of very low flow ischemia, which may better reflect the conditions of myocardial infarction in vivo. Furthermore, in a rat model of trauma-hemorrhage and resuscitation we found that O-GlcNAc levels in the heart were markedly lower at the end of resuscitation compared to sham controls and that glucosamine treatment not only prevented the decrease in O-GlcNAc it was also associated with improved cardiac function (62). These data are supportive of the idea that similar strategies might protect against myocardial ischemic injury in vivo. It should also be noted that glucosamine is a widely nutritional supplement, used as a treatment of osteoarthritis; however the serum glucosamine concentrations with commonly used oral dose (1500 mg/day) (4) are well below the cytoprotective concentration (28, 62).
In conclusion we have shown that short term ischemia alone significantly increased UDP-GlcNAc levels in the heart and lead to increased cardiac O-glycosylation, which supports the notion that the hexosamine biosynthesis pathway and O-glycosylation is an endogenous stress activated pathway (63). We also show that glucosamine attenuated contracture development during low flow ischemia and improved functional recovery following reperfusion. This protection was associated with increased UDP-HexNAc levels and increased protein O-GlcNAc levels, but was not associated with increased ATP levels. We also found that glucosamine altered the response of p38 phosphorylation to ischemia and ischemia/reperfusion, and there was a significant correlation between phospho-p38 and O-GlcNAc levels in the glucosamine treated group, whereas it had no effect on ERK1/2 or Akt. Further studies are clearly warranted to better understand the specific mechanisms underlying the protection seen with glucosamine. Nevertheless, these data support the concept that increasing protein O-GlcNAc levels are cardioprotective and suggest that one possible mechanism may be via altered p38 MAPK activation.
Acknowledgments
We thank Clarence Forrest and Charlye Brocks for the technical assistance and Zachary T. Kneass and Tamas Nagy for their insightful input. This work was supported by grants from the NHLBI HL-076165 (RBM); HL-67464 (JCC) and SCCOR grant HL-077100.
References
- 1.Arias EB, Kim J, Cartee GD. Prolonged incubation in PUGNAc results in increased protein O-Linked glycosylation and insulin resistance in rat skeletal muscle. Diabetes. 2004;53:921–930. doi: 10.2337/diabetes.53.4.921. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong SC. Protein kinase activation and myocardial ischemia/reperfusion injury. Cardiovasc Res. 2004;61:427–436. doi: 10.1016/j.cardiores.2003.09.031. [DOI] [PubMed] [Google Scholar]
- 3.Baines CP, Molkentin JD. STRESS signaling pathways that modulate cardiac myocyte apoptosis. J Mol Cell Cardiol. 2005;38:47–62. doi: 10.1016/j.yjmcc.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 4.Biggee BA, Blinn CM, McAlindon TE, Nuite M, Silbert JE. Low Levels of Human Serum Glucosamine After Ingestion of Glucosamine Sulphate Relative to Capability for Peripheral Effectiveness. Ann Rheum Dis. 2005 doi: 10.1136/ard.2005.036368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bogoyevitch MA, Gillespie-Brown J, Ketterman AJ, Fuller SJ, Ben-Levy R, Ashworth A, Marshall CJ, Sugden PH. Stimulation of the stress-activated mitogen-activated protein kinase subfamilies in perfused heart. p38/RK mitogen-activated protein kinases and c-Jun N-terminal kinases are activated by ischemia/reperfusion. Circ Res. 1996;79:162–173. doi: 10.1161/01.res.79.2.162. [DOI] [PubMed] [Google Scholar]
- 6.Burt DJ, Gruden G, Thomas SM, Tutt P, Dell’Anna C, Viberti GC, Gnudi L. P38 mitogen-activated protein kinase mediates hexosamine-induced TGFbeta1 mRNA expression in human mesangial cells. Diabetologia. 2003;46:531–537. doi: 10.1007/s00125-003-1075-y. [DOI] [PubMed] [Google Scholar]
- 7.Cave AC, Garlick PB. Ischemic preconditioning and intracellular pH: a 31P NMR study in the isolated rat heart. Am J Physiol. 1997;272:H544–H552. doi: 10.1152/ajpheart.1997.272.1.H544. [DOI] [PubMed] [Google Scholar]
- 8.Champattanachai V, Marchase RB, Chatham JC. Glucosamine protects neonatal cardiomyocytes from ischemia-reperfusion injury via increased protein-associated O-GlcNAc. Am J Physiol Cell Physiol. 2006 doi: 10.1152/ajpcell.00162.2006. [DOI] [PubMed] [Google Scholar]
- 9.Chatham JC, Gao Z-P, Forder JR. The impact of 1 wk of diabetes on the regulation of myocardial carbohydrate and fatty acid oxidation. Am J Physiol Endocrinol Metab. 1999;277:E342–E351. doi: 10.1152/ajpendo.1999.277.2.E342. [DOI] [PubMed] [Google Scholar]
- 10.Clark RJ, McDonough PM, Swanson E, Trost SU, Suzuki M, Fukuda M, Dillmann WH. Diabetes and the accompanying hyperglycemia impairs cardiomyocyte cycling through increased nuclear O-GlcNAcylation. J Biol Chem. 2003;278:44230–44237. doi: 10.1074/jbc.M303810200. [DOI] [PubMed] [Google Scholar]
- 11.Cross TG, Scheel-Toellner D, Henriquez NV, Deacon E, Salmon M, Lord JM. Serine/threonine protein kinases and apoptosis. Exp Cell Res. 2000;256:34–41. doi: 10.1006/excr.2000.4836. [DOI] [PubMed] [Google Scholar]
- 12.Efthymiou CA, Mocanu MM, de Belleroche J, Wells DJ, Latchmann DS, Yellon DM. Heat shock protein 27 protects the heart against myocardial infarction. Basic Res Cardiol. 2004;99:392–394. doi: 10.1007/s00395-004-0483-6. [DOI] [PubMed] [Google Scholar]
- 13.Federici M, Menghini R, Mauriello A, Hribal ML, Ferrelli F, Lauro D, Sbraccia P, Spagnoli LG, Sesti G, Lauro R. Insulin-dependent activation of endothelial nitric oxide synthase is impaired by O-linked glycosylation modification of signaling proteins in human coronary endothelial cells. Circulation. 2002;106:466–472. doi: 10.1161/01.cir.0000023043.02648.51. [DOI] [PubMed] [Google Scholar]
- 14.Fujio Y, Nguyen T, Wencker D, Kitsis RN, Walsh K. Akt promotes survival of cardiomyocytes in vitro and protects against ischemia-reperfusion injury in mouse heart. Circulation. 2000;101:660–667. doi: 10.1161/01.cir.101.6.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hausenloy DJ, Tsang A, Mocanu MM, Yellon DM. Ischemic preconditioning protects by activating prosurvival kinases at reperfusion. Am J Physiol Heart Circ Physiol. 2005;288:H971–976. doi: 10.1152/ajpheart.00374.2004. [DOI] [PubMed] [Google Scholar]
- 16.Hausenloy DJ, Yellon DM. New directions for protecting the heart against ischaemia-reperfusion injury: targeting the Reperfusion Injury Salvage Kinase (RISK)-pathway. Cardiovasc Res. 2004;61:448–460. doi: 10.1016/j.cardiores.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 17.Hayashi K, Okumura K, Matsui H, Murase K, Kamiya H, Saburi Y, Numaguchi Y, Toki Y, Hayakawa T. Involvement of 1,2-diacylglycerol in improvement of heart function by etomoxir in diabetic rats. Life Sci. 2001;68:1515–1526. doi: 10.1016/s0024-3205(01)00953-5. [DOI] [PubMed] [Google Scholar]
- 18.Hollander JM, Martin JL, Belke DD, Scott BT, Swanson E, Krishnamoorthy V, Dillmann WH. Overexpression of wild-type heat shock protein 27 and a nonphosphorylatable heat shock protein 27 mutant protects against ischemia/reperfusion injury in a transgenic mouse model. Circulation. 2004;110:3544–3552. doi: 10.1161/01.CIR.0000148825.99184.50. [DOI] [PubMed] [Google Scholar]
- 19.Hsieh TJ, Fustier P, Zhang SL, Filep JG, Tang SS, Ingelfinger JR, Fantus IG, Hamet P, Chan JS. High glucose stimulates angiotensinogen gene expression and cell hypertrophy via activation of the hexosamine biosynthesis pathway in rat kidney proximal tubular cells. Endocrinology. 2003;144:4338–4349. doi: 10.1210/en.2003-0220. [DOI] [PubMed] [Google Scholar]
- 20.Hunton DL, Zou LY, Pang Y, Marchase RB. Adult Rat Cardiomyocytes Exhibit Capacitative Calcium Entry. Am J Physiol Heart Circ Physiol. 2004;286:H1124–H1132. doi: 10.1152/ajpheart.00162.2003. [DOI] [PubMed] [Google Scholar]
- 21.Kaiser RA, Bueno OF, Lips DJ, Doevendans PA, Jones F, Kimball TF, Molkentin JD. Targeted inhibition of p38 mitogen-activated protein kinase antagonizes cardiac injury and cell death following ischemia-reperfusion in vivo. J Biol Chem. 2004;279:15524–15530. doi: 10.1074/jbc.M313717200. [DOI] [PubMed] [Google Scholar]
- 22.Kim SJ, Hwang SG, Shin DY, Kang SS, Chun JS. p38 kinase regulates nitric oxide-induced apoptosis of articular chondrocytes by accumulating p53 via NFkappa B-dependent transcription and stabilization by serine 15 phosphorylation. J Biol Chem. 2002;277:33501–33508. doi: 10.1074/jbc.M202862200. [DOI] [PubMed] [Google Scholar]
- 23.Kneass ZT, Marchase RB. Protein O-GlcNAc modulates motiligy-associated signaling intermediates in neutrophils. J Biol Chem. 2005 doi: 10.1074/jbc.M414066200. [DOI] [PubMed] [Google Scholar]
- 24.Kolocassides KG, Galinanes M, Hearse DJ. Ischemic preconditioning, cardioplegia or both? Differing approaches to myocardial and vascular protection. J Mol Cell Cardiol. 1996;28:623–634. doi: 10.1006/jmcc.1996.0058. [DOI] [PubMed] [Google Scholar]
- 25.Kreppel LK, Blomberg MA, Hart GW. Dynamic glycosylation of nuclear and cytosolic proteins. Cloning and characterization of a unique O-GlcNAc transferase with multiple tetratricopeptide repeats. J Biol Chem. 1997;272:9308–9315. doi: 10.1074/jbc.272.14.9308. [DOI] [PubMed] [Google Scholar]
- 26.Kreppel LK, Hart GW. Regulation of a cytosolic and nuclear O-GlcNAc transferase. Role of the tetratricopeptide repeats. J Biol Chem. 1999;274:32015–32022. doi: 10.1074/jbc.274.45.32015. [DOI] [PubMed] [Google Scholar]
- 27.Larsen JK, Yamboliev IA, Weber LA, Gerthoffer WT. Phosphorylation of the 27-kDa heat shock protein via p38 MAP kinase and MAPKAP kinase in smooth muscle. Am J Physiol. 1997;273:L930–940. doi: 10.1152/ajplung.1997.273.5.L930. [DOI] [PubMed] [Google Scholar]
- 28.Liu J, Pang Y, Chang T, Bounelis P, Chatham JC, Marchase RB. Increased hexosamine biosynthesis and protein O-GlcNAc levels associated with myocardial protection against calcium paradox and ischemia. J Mol Cell Cardiol. 2006;40:303–312. doi: 10.1016/j.yjmcc.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 29.Liu K, Paterson AJ, Chin E, Kudlow JE. Glucose stimulates protein modification by O-linked GlcNAc in pancreatic beta cells: linkage of O-linked GlcNAc to beta cell death. Proc Natl Acad Sci U S A. 2000;97:2820–2825. doi: 10.1073/pnas.97.6.2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lotem J, Kama R, Sachs L. Suppression or induction of apoptosis by opposing pathways downstream from calcium-activated calcineurin. Proc Natl Acad Sci U S A. 1999;96:12016–12020. doi: 10.1073/pnas.96.21.12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mackay K, Mochly-Rosen D. Involvement of a p38 mitogen-activated protein kinase phosphatase in protecting neonatal rat cardiac myocytes from ischemia. J Mol Cell Cardiol. 2000;32:1585–1588. doi: 10.1006/jmcc.2000.1194. [DOI] [PubMed] [Google Scholar]
- 32.Martindale JJ, Wall JA, Martinez-Longoria DM, Aryal P, Rockman HA, Guo Y, Bolli R, Glembotski CC. Over-expression of MKK6 in the heart improves functional recovery from ischemia in vitro and protects against myocardial infarction in vivo. J Biol Chem. :2004. doi: 10.1074/jbc.M406690200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matsui T, Tao J, del Monte F, Lee KH, Li L, Picard M, Force TL, Franke TF, Hajjar RJ, Rosenzweig A. Akt activation preserves cardiac function and prevents injury after transient cardiac ischemia in vivo. Circulation. 2001;104:330–335. doi: 10.1161/01.cir.104.3.330. [DOI] [PubMed] [Google Scholar]
- 34.Mayr M, Hu Y, Hainaut H, Xu Q. Mechanical stress-induced DNA damage and rac-p38MAPK signal pathways mediate p53-dependent apoptosis in vascular smooth muscle cells. Faseb J. 2002;16:1423–1425. doi: 10.1096/fj.02-0042fje. [DOI] [PubMed] [Google Scholar]
- 35.McFalls EO, Hou M, Bache RJ, Best A, Marx D, Sikora J, Ward HB. Activation of p38 MAPK and increased glucose transport in chronic hibernating swine myocardium. Am J Physiol Heart Circ Physiol. 2004;287:H1328–1334. doi: 10.1152/ajpheart.01188.2003. [DOI] [PubMed] [Google Scholar]
- 36.Michel MC, Li Y, Heusch G. Mitogen-activated protein kinases in the heart. Naunyn Schmiedebergs Arch Pharmacol. 2001;363:245–266. doi: 10.1007/s002100000363. [DOI] [PubMed] [Google Scholar]
- 37.Mocanu MM, Baxter GF, Yue Y, Critz SD, Yellon DM. The p38 MAPK inhibitor, SB203580, abrogates ischaemic preconditioning in rat heart but timing of administration is critical. Basic Res Cardiol. 2000;95:472–478. doi: 10.1007/s003950070023. [DOI] [PubMed] [Google Scholar]
- 38.Nagy T, Champattanachai V, Marchase RB, Chatham JC. GLUCOSAMINE INHIBITS ANGIOTENSIN II INDUCED CYTOPLASMIC Ca2+ ELEVATION IN NEONATAL CARDIOMYOCYTES VIA PROTEIN-ASSOCIATED O-GlcNAc. Am J Physiol Cell Physiol. 2005 doi: 10.1152/ajpcell.00263.2005. [DOI] [PubMed] [Google Scholar]
- 39.Otsu K, Yamashita N, Nishida K, Hirotani S, Yamaguchi O, Watanabe T, Hikoso S, Higuchi Y, Matsumura Y, Maruyama M, Sudo T, Osada H, Hori M. Disruption of a single copy of the p38alpha MAP kinase gene leads to cardioprotection against ischemia-reperfusion. Biochem Biophys Res Commun. 2003;302:56–60. doi: 10.1016/s0006-291x(03)00096-2. [DOI] [PubMed] [Google Scholar]
- 40.Park SY, Ryu J, Lee W. O-GlcNAc modification on IRS-1 and Akt2 by PUGNAc inhibits their phosphorylation and induces insulin resistance in rat primary adipocytes. Exp Mol Med. 2005;37:220–229. doi: 10.1038/emm.2005.30. [DOI] [PubMed] [Google Scholar]
- 41.Ray PS, Martin JL, Swanson EA, Otani H, Dillmann WH, Das DK. Transgene overexpression of alphaB crystallin confers simultaneous protection against cardiomyocyte apoptosis and necrosis during myocardial ischemia and reperfusion. Faseb J. 2001;15:393–402. doi: 10.1096/fj.00-0199com. [DOI] [PubMed] [Google Scholar]
- 42.Robinson KA, Weinstein ML, Lindenmayer GE, Buse MG. Effects of diabetes and hyperglycemia on the hexosamine synthesis pathway in rat muscle and liver. Diabetes. 1995;44:1438–1446. doi: 10.2337/diab.44.12.1438. [DOI] [PubMed] [Google Scholar]
- 43.Roquemore EP, Chevrier MR, Cotter RJ, Hart GW. Dynamic O-GlcNAcylation of the small heat shock protein alpha B-crystallin. Biochemistry. 1996;35:3578–3586. doi: 10.1021/bi951918j. [DOI] [PubMed] [Google Scholar]
- 44.Schoolwerth AC, LaNoue KF, Hoover WJ. Glutamate transport in rat kidney mitochondria. J Biol Chem. 1983;258:1735–1739. [PubMed] [Google Scholar]
- 45.Seki S, Horikoshi K, Takeda H, Izumi T, Nagata A, Okumura H, Taniguchi M, Mochizuki S. Effects of sustained low-flow ischemia and reperfusion on Ca2+ transients and contractility in perfused rat hearts. Mol Cell Biochem. 2001;216:111–119. doi: 10.1023/a:1011067529272. [DOI] [PubMed] [Google Scholar]
- 46.Shao Z, Bhattacharya K, Hsich E, Park L, Walters B, Germann U, Wang YM, Kyriakis J, Mohanlal R, Kuida K, Namchuk M, Salituro F, Yao YM, Hou WM, Chen X, Aronovitz M, Tsichlis PN, Bhattacharya S, Force T, Kilter H. c-Jun N-terminal kinases mediate reactivation of Akt and cardiomyocyte survival after hypoxic injury in vitro and in vivo. Circ Res. 2006;98:111–118. doi: 10.1161/01.RES.0000197781.20524.b9. [DOI] [PubMed] [Google Scholar]
- 47.Slawson C, Housley MP, Hart GW. O-GlcNAc cycling: How a single sugar post-translational modification is changing the Way We think about signaling networks. J Cell Biochem. 2006;97:71–83. doi: 10.1002/jcb.20676. [DOI] [PubMed] [Google Scholar]
- 48.Sohn KC, Lee KY, Park JE, Do SI. OGT functions as a catalytic chaperone under heat stress response: a unique defense role of OGT in hyperthermia. Biochem Biophys Res Commun. 2004;322:1045–1051. doi: 10.1016/j.bbrc.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 49.Steenbergen C. The role of p38 mitogen-activated protein kinase in myocardial ischemia/reperfusion injury; relationship to ischemic preconditioning. Basic Res Cardiol. 2002;97:276–285. doi: 10.1007/s00395-002-0364-9. [DOI] [PubMed] [Google Scholar]
- 50.Tsang A, Hausenloy DJ, Mocanu MM, Yellon DM. Postconditioning: a form of “modified reperfusion” protects the myocardium by activating the phosphatidylinositol 3-kinase-Akt pathway. Circ Res. 2004;95:230–232. doi: 10.1161/01.RES.0000138303.76488.fe. [DOI] [PubMed] [Google Scholar]
- 51.Uldry M, Ibberson M, Hosokawa M, Thorens B. GLUT2 is a high affinity glucosamine transporter. FEBS Lett. 2002;524:199–203. doi: 10.1016/s0014-5793(02)03058-2. [DOI] [PubMed] [Google Scholar]
- 52.Vosseller K, Wells L, Lane MD, Hart GW. Elevated nucleocytoplasmic glycosylation by O-GlcNAc results in insulin resistance associated with defects in Akt activation in 3T3-L1 adipocytes. Proc Natl Acad Sci U S A. 2002;99:5313–5318. doi: 10.1073/pnas.072072399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Walgren JL, Vincent TS, Schey KL, Buse MG. High glucose and insulin promote O-GlcNAc modification of proteins, including alpha-tubulin. Am J Physiol Endocrinol Metab. 2003;284:E424–E434. doi: 10.1152/ajpendo.00382.2002. [DOI] [PubMed] [Google Scholar]
- 54.Wang P, Chatham JC. The onset of diabetes in Zucker diabetic fatty (ZDF) rats leads to improved recovery of function following ischemia in the isolated perfused heart. Am J Physiol Endocrin Met. 2004;286:E725–E736. doi: 10.1152/ajpendo.00295.2003. [DOI] [PubMed] [Google Scholar]
- 55.Wang P, Lloyd SG, Chatham JC. The impact of high glucose/high insulin and dichloroacetate treatment on carbohydrate oxidation and functional recovery following low flow ischemia. Circulation. 2005;111:2066–2072. doi: 10.1161/01.CIR.0000162466.06150.D4. [DOI] [PubMed] [Google Scholar]
- 56.Webster KA. Serine phosphorylation and suppression of apoptosis by the small heat shock protein alphaB-crystallin. Circ Res. 2003;92:130–132. doi: 10.1161/01.res.0000056967.51841.21. [DOI] [PubMed] [Google Scholar]
- 57.Wells L, Vosseller K, Hart GW. Glycosylation of nucleocytoplasmic proteins: signal transduction and O-GlcNAc. Science. 2001;291:2376–2378. doi: 10.1126/science.1058714. [DOI] [PubMed] [Google Scholar]
- 58.Wells L, Whalen SA, Hart GW. O-GlcNAc: a regulatory post-translational modification. Biochem Biophys Res Commun. 2003;302:435–441. doi: 10.1016/s0006-291x(03)00175-x. [DOI] [PubMed] [Google Scholar]
- 59.Whelan SA, Hart GW. Proteomic approaches to analyze the dynamic relationships between nucleocytoplasmic protein glycosylation and phosphorylation. Circ Res. 2003;93:1047–1058. doi: 10.1161/01.RES.0000103190.20260.37. [DOI] [PubMed] [Google Scholar]
- 60.Williams EH, Kao RL, Morgan HE. Protein degradation and synthesis during recovery from myocardial ischemia. Am J Physiol. 1981;240:E268–273. doi: 10.1152/ajpendo.1981.240.3.E268. [DOI] [PubMed] [Google Scholar]
- 61.Wu G, Haynes TE, Li H, Yan W, Meininger CJ. Glutamine metabolism to glucosamine is necessary for glutamine inhibition of endothelial nitric oxide synthesis. Biochem J. 2001;353:245–252. doi: 10.1042/0264-6021:3530245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang S, Zou LY, Bounelis P, Chaudry I, Chatham JC, Marchase RB. Glucosamine administration during resuscitation improves organ function after trauma hemorrhage. Shock. 2006;25:600–607. doi: 10.1097/01.shk.0000209563.07693.db. [DOI] [PubMed] [Google Scholar]
- 63.Zachara NE, O’Donnell N, Cheung WD, Mercer JJ, Marth JD, Hart GW. Dynamic O-GlcNAc modification of nucleocytoplasmic proteins in response to stress. A survival response of mammalian cells. J Biol Chem. 2004;279:30133–30142. doi: 10.1074/jbc.M403773200. [DOI] [PubMed] [Google Scholar]
- 64.Zhuang S, Demirs JT, Kochevar IE. p38 mitogen-activated protein kinase mediates bid cleavage, mitochondrial dysfunction, and caspase-3 activation during apoptosis induced by singlet oxygen but not by hydrogen peroxide. J Biol Chem. 2000;275:25939–25948. doi: 10.1074/jbc.M001185200. [DOI] [PubMed] [Google Scholar]