Abstract
Sensory systems must be able to extract features of environmental cues within the context of the different physiological states of the organism and often temper their activity in a state-dependent manner via the process of neuromodulation. We examined the effects of the neuromodulator serotonin on a well-characterized sensory circuit, the antennal lobe of Drosophila melanogaster, using two-photon microscopy and the genetically expressed calcium indicator, G-CaMP. Serotonin enhances sensitivity of the antennal lobe output projection neurons in an odor-specific manner. For odorants that sparsely activate the antennal lobe, serotonin enhances projection neuron responses and causes an offset of the projection neuron tuning curve, most likely by increasing projection neuron sensitivity. However, for an odorant that evokes a broad activation pattern, serotonin enhances projection neuron responses in some, but not all, glomeruli. Further, serotonin enhances the responses of inhibitory local interneurons, resulting in a reduction of neurotransmitter release from the olfactory sensory neurons via GABAB receptor-dependent presynaptic inhibition, which may be a mechanism underlying the odorant-specific modulation of projection neuron responses. Our data suggest that the complexity of serotonin modulation in the antennal lobe accommodates coding stability in a glomerular pattern and flexible projection neuron sensitivity under different physiological conditions.
Keywords: olfaction, neuromodulation, serotonin, antennal lobes
INTRODUCTION
The response to a sensory stimulus changes with the physiological state of an organism. The nervous system must alter the acuity and resolution of different sensory systems so that processing of certain stimuli is enhanced, while less relevant stimuli can be neglected. This is accomplished, in part, by the local release of neuromodulators, substances often released within a specific context (e.g., fear, hunger, and reproduction), causing alteration of the response properties and synaptic efficiency of individual neurons without directly causing excitation or inhibition (Kupfermann, 1979; Katz, 1999). This results in large-scale changes in the response properties of a neural network and affects how information is processed.
Neuromodulation is usually mediated by G-protein–couple receptors (GPCRs). The Drosophila genome contains at least 44 genes coding for neuropeptide GPCRs, three genes for protein hormone GPCRs, and 21 genes for biogenic amine GPCRs (Hauser et al., 2006). One limit to the study of neuromodulation is the inherent complexity of most neural circuits. To this end, we studied the neuromodulatory actions of serotonin (5-hydroxytryptamine; 5HT) on olfactory processing in the antennal lobe (AL) of Drosophila melanogaster, which is well characterized, of reduced complexity, compared to the vertebrate olfactory bulb, and allows for the use of many genetic experimental tools.
The AL is the first center of olfactory processing in Drosophila. Each olfactory receptor neuron (ORN) expresses one or a few olfactory receptor genes (ORs) (Clyne et al., 1999; Vosshall et al., 1999; Gao et al., 2000; Vosshall et al., 2000; van der Goldman et al., 2005; Couto et al., 2005; Fishilevich & Vosshall, 2005) and responds to a particular set of odorants (de Bruyne et al., 2001; Ng et al., 2002; Stensmyr et al., 2003; Pelz et al., 2006). Axon terminals of ORNs expressing the same ORs innervate stereotyped glomeruli (Gao et al., 2000, Vosshall et al., 2000; Couto et al., 2005; Fishilevich & Vosshall, 2005). Glomeruli also contain the neurites of projection neurons (PNs) that output to downstream areas of processing and intrinsic local interneurons (LNs) that exert lateral inhibitory (Ng et al., 2002; Wilson & Laurent, 2005; Silbering & Galizia 2007; Olsen & Wilson, 2008; Root et al., 2008; Silbering et al., 2008) and excitatory (Olsen et al., 2007; Root et al., 2007; Shang et al., 2007; Silbering et al., 2008) influences on the odor-evoked responses of PNs. Investigating neuromodulation in specific populations of AL neurons is crucial to understanding the role of neuromodulation in olfactory processing.
There is evidence in insects to suggest that serotonin plays a role in olfactory processing. In Drosophila, glomeruli receive innervation from two 5HT-immunoreactive neurons (Dacks et al., 2006; Roy et al., 2007). Moths possess morphologically similar 5HT-ir neurons (Kent et al., 1987; Dacks et al., 2006), and 5HT enhances the excitability and responses of AL neurons (Kloppenburg & Hildebrand, 1995; Kloppenburg et al., 1999; Hill et al., 2003; Dacks et al., 2008). The increased excitability is caused by a reduction in two K+ conductances (Mercer et al., 1995). The levels of 5HT in the ALs vary throughout the day, peaking when moths are most active (Kloppenburg et al., 1999), and 5HT increases the behavioral sensitivity of males to sex pheromone (Linn & Roelofs, 1986; Gatellier et al., 2004). In this study, we examined the effects of 5HT on specific populations of PNs and the interactions between glomeruli in Drosophila. Optical imaging coupled with the specific expression of G-CaMP, a genetically expressed calcium indicator, provides a system to measure neuromodulation in select neurons. We found that 5HT enhances the response magnitude and sensitivity of PNs in an odor-dependent manner (indicating a modulation of the intrinsic local circuitry), and 5HT increases the responsiveness of inhibitory local interneurons. Modulation of multiple neuron types in the AL is a potential mechanism to alter olfactory sensitivity while maintaining feature stability. This study lays out the foundation for future genetic dissection of neuromodulation and its contribution to olfactory information processing.
METHODS
Flies
Flies were raised on standard medium at 22–25°C and adult females (except for the cis-11-vaccenyl acetate experiments) were used at 2–5 days posteclosion. Several transgenic flies were used: 1) UAS-GCaMP56 (Wang et al., 2003), 2) GH146-Gal4 (Stocker et al., 1997), 3) GAD1-Gal4 (Ng et al., 2002), 4) UAS-spH (Ng et al., 2002) with the transgene mobilized onto the third chromosome (Root et al., 2008), 5) Or83b-Gal4 (Wang et al., 2003), 6) GABABR2-Gal4 (Root et al., 2008), and 7) UAS-CD8GFP. The heads of flies were removed and pinned down by the proboscis in a Sylgard (Dow Corning; Midland, MI, USA) dish, using a fine tungsten wire in calcium-free adult hemolymph-like (AHL) saline [108 mM NaCl, 5 mM KCL, 8.2 mM MgCl2, 4mM NaHCO3, 1 mM NaH2PO4, 5 mM trehalose, 10 mM sucrose, 5 mM HEPES (pH 7.5, 265 mOsm)]. The cuticle surrounding the brain was removed, except for the proboscis and antennae, with only the antennal nerves intact. The calcium-free AHL saline was then replaced with 2 mL of AHL saline containing 2 mM of CaCl2 (Wang et al., 2003).
Physiological Preparations
Three different types of preparations were used to examine the effects of 5HT on AL responses, with the following experimental goals: 1) test the responses of the PNs to an olfactory stimulus before and after 5HT application; 2) test the responses of PNs to multiple olfactory cues either in the presence or absence of 5HT; and 3) test the responses of AL cell types to antennal nerve shock. First, to test the responses of PNs to olfactory stimuli before and after 5HT treatment, liquid odor was applied onto the submerged preparation, as described previously (Root et al., 2007). Three 3-second stimulations, each separated by 2 minutes, were delivered for each treatment. If the preparation moved out of position during the experiment, it was discarded and only one odorant was tested for each preparation. cis-11-vaccenyl acetate (a generous gift from Dr. Dean Smith MD, Phd), ethyl hexanoate (Sigma, St. Louis, MO, USA), and isoamyl acetate (Sigma, St. Louis, MO, USA) were presented at dilutions of 1:50–100, 1:2,500,000, and 1:20,000, respectively, in AHL. For submerged preparations, two-tailed Student's t-tests for dependent samples were used to test before and after pharmacological treatments in the same animals.
Second, to test the responses of PNs to multiple olfactory stimuli, the brain-antennae preparation was embedded in 2% agarose (type VII-A; Sigma, St. Louis, MO, USA) and stimulated, as described previously (Wang et al., 2003; Root et al., 2008). Preparations were embedded in either agarose or agarose in which 5HT had been added. A constant flow of 1 L/min was applied onto the antennae via a 12-mm tube, and solenoids were used to divert a specific proportion of odor-laden air into the common air flow from 100-mL bottles containing 20 uL of pure odor on filter paper. All olfactory stimuli were tested at least twice, and if there was a shift of 25% or more in the integral of ΔF/F (ΔF/F dT; see data analysis), the preparation was discarded to ensure that the recordings were stable. The odorants used in this preparation were isoamyl acetate, 3-hexanol (Sigma, St. Louis, MO, USA), 3-heptanol (Sigma, St. Louis, MO, USA), 3-octanol (Fluka, St. Louis, MO, USA), 3-nonanol (Fluka, St. Louis, MO, USA), and 3-decanol (Tokyo Chemical Industry, Tokyo, Japan), and concentrations of odorant delivered were estimated based on vapor pressure (Lide, 2008). For the embedded preparations, two-tailed Student's t-tests for independent samples were used to test for significant differences between populations of flies that were given different pharmacological treatment.
Last, to test the effects of 5HT on the precise activation of AL cellular elements, electrical stimulation of the antennal nerve before and after pharmacological manipulation was applied, as described previously (Root et al., 2008). Briefly, a borosilicate capillary tube was pulled to a fine tip, which was broken and then fire-polished to 1.5 times the width of the antennal nerve. The nerve was sucked into the electrode and 1-ms electric shocks at 10 V were applied, using a Grass stimulator (Grass Technologies; West Warwich, RI, USA) at 100 Hz. Thus, for every 10 ms of stimulus, one electrical “spike” was delivered to the preparation. The duration of electric stimulation was increased, delivering 1, 2, 4, 8, 16, 32, and 45 spikes. The middle value of 8 spikes was retested at the end of each treatment to ensure that the antennal nerve had not slipped in the suction electrode. If there was a shift of 15% for the retested values, the preparation was discarded. The GAD1-Gal4 flies were stimulated twice at a single intensity of 8 spikes with 2 minutes between each stimulation before and after either saline or 5HT application.
Pharmacology
Because 5HT is light sensitive, we took measures (dissecting under dim light conditions, covering preparations, etc.) to decrease the light exposure to all preparations (whether exposed to 5HT or saline). In addition, 5HT was never used if it had been prepared more than 3 hours previously. Serotonin (Sigma) was applied to the bath or agarose at a final concentration of 10–4 M to remain comparable with previous studies on the effects of 5HT on the AL (Kloppenburg & Hildebrand, 1995; Mercer et al., 1995; Kloppenburg et al., 1999; Dacks et al., 2008) and was also tested at 10–6 and 10–8 M. Methysergide (Tocris Bioscience, Ellisville, MO, USA) was similarly applied at a final dilution of 50 μM in 0.1% dimethyl sulfoxide (DMSO). CGP54626 (Tocris Bioscience, Ellisville, MO, USA) was dissolved as 2,000X stocks in DMSO and applied at a final dilution of 25 μM. In the Results section, “control” describes pretreatment responses. All pharmacological agents were applied for 10 minutes before taking measurements.
Two-Photon Microscopy and Data Analysis
All imaging experiments were conducted on a custom-built two-photon microscope with a 40X water-immersion lens, as described previously (Wang et al., 2003). Images were acquired at 4 Hz and 128×128 pixels for live experiments. At the end of each experiment, a 512×512 pixel z-stack was acquired to verify the identity of glomeruli. The imaging data were analyzed by using Igor Pro 6.0 (Wavemetrics, Lake Oswego, OR, USA) and a custom macro. Tests for normality (Kolmogorov-Smirnov) were performed in SigmaStat (Systat Software Inc., Ashburn, Virginia, USA). If data were fit with a normal distribution, two-tailed Student's t-tests for dependent (submerged preparations) and independent samples (embedded preparations) were performed in Statistica (Statsoft, Tulsa, Oklahoma, USA). If data were not normally distributed, data were analyzed with a Wilcoxon matched-pairs test (Statistica; Statsoft). For quantification of GABAB receptors, data obtained by Root et al. (2008) were reanalyzed to compare the levels of GABAB receptors expressed by ORNs innervating the VM2 and DM2 glomeruli. This analysis was performed as described in Root et al. (2008).
RESULTS
5HT Enhances the Olfactory Responses of Projection Neurons
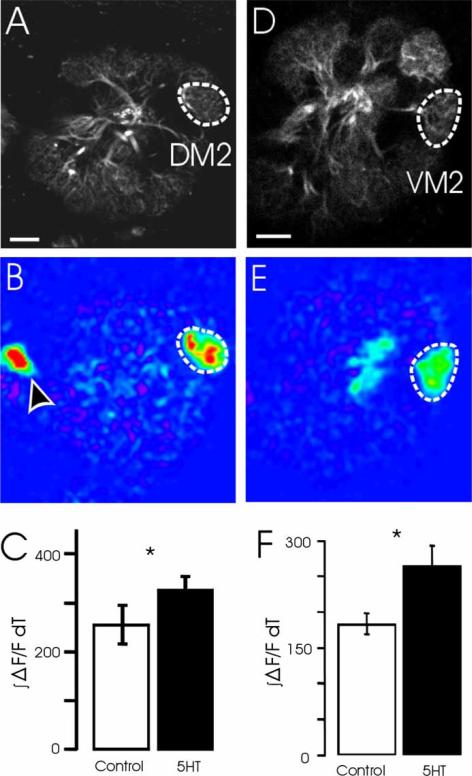
We first investigated the effects of 5HT on PN activity. Coupling two-photon microscopy with the expression of the calcium sensor, G-CaMP (Nakai et al., 2001), in only PNs, we measured the effect of 5HT in PNs of a specific glomerulus. Flies bearing the GH146-Gal4 and UAS-GCaMP transgenes express G-CaMP in most AL PNs (Stocker et al., 1997), which allowed for the observation of calcium influx, most likely via nicotinic acetylcholine receptors, into the dendrites of PNs (Goldberg et al., 1999; Oertner et al., 2001). Using this imaging system, we confirmed that PNs of the DA1 glomerulus respond to the male pheromone, cis-11-vacenyl acetate (Ha & Smith, 2006; Ejima et al., 2007; Kurtovic et al., 2007; Schlief & Wilson, 2007; Root et al., 2008). Serotonin application significantly increased the response of DA1 PNs to cVA (Figure 1D and 1E) (n=11, P <0.05, t-test), and this increase was eliminated by methysergide (Figure 1E) (n=8, P =0.21, t-test), an antagonist of all four Drosophila 5HT receptors (Saudou et al., 1992; Colas et al., 1995). Ethyl hexanoate (Figure 2A and 2B) and 3-heptanol (Figure 2D and 2E) at low concentrations excited only the DM2 and VM2 glomeruli, respectively. Similarly, the responses of DM2 PNs to ethyl hexanoate were significantly enhanced by 5HT (Figure 2C; n=5, P <0.05, t-test), as were the responses of VM2 PNs to 3-heptanol (Figure 2F; P <0.05, t-test). Thus, 5HT appears to enhance PN responses.
Figure 1.
Serotonin (5HT) enhances the responses of PNs to cis-11-vaccenyl acetate (cVA). (A) Single optical plane through the AL reveals the DA1 glomerulus (hatched white outline). DA1 PN responses to the pheromone, cVA, before (B) and after (C) 5HT application. (D) Time course of fluorescent change (ΔF/F) in response to repeated pulses of cVA before (control) and after exposure to 5HT. (E) 5HT enhanced the responses to cVA (n=11 flies. *P <0.05), and this enhancement was blocked by the 5HT antagonist, methysergide (MS) (n=8; n.s., not significant). Data in D and E are represented as mean±SEM. ∫ΔF/F dt, integral of ΔF/F during the total response period. Scale bar=10 μm.
Figure 2.
Serotonin enhances the responses of PNs to odorants eliciting sparse AL activation. (A) Single optical plane through the AL reveals the DM2 glomerulus (hatched white outline). (B) Response of DM2 PNs to ethyl hexanoate (EH). Arrowhead indicates a PN cell body. (C) 5HT significantly enhanced the DM2 responses to EH (n=5 flies; *P <0.05). (D) Single optical plane through the AL reveals the VM2 glomerulus (hatched white outline). (E) Response of VM2 PNs to 3-heptanol. (F) 5HT significantly enhanced the VM2 responses to 3-heptanol (n=5 flies; *P <0.05). Data in C and F are represented as mean±SEM. Scale bar=10 μm.
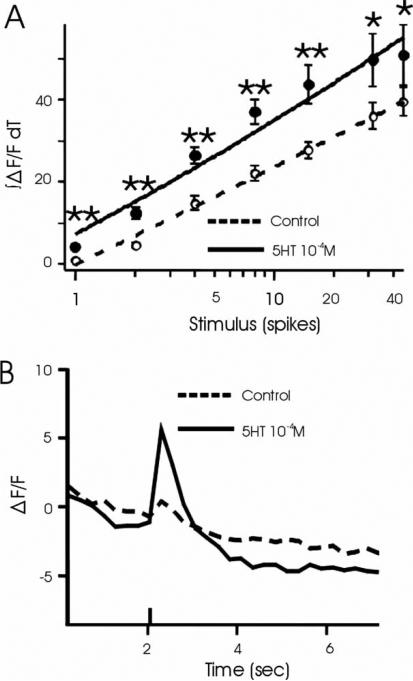
In the moth Manduca sexta, 5HT causes an increase in membrane resistance via a reduction in two K+ channel conductances (Mercer et al., 1995), leading to the prediction that 5HT should enhance PN sensitivity (Kloppenburg & Hildebrand, 1995; Kloppenburg et al., 1999; Dacks et al., 2008). Therefore, we investigated the effect of 5HT on PN sensitivity in Drosophila PNs by plotting the input-output function of PNs in response to electrical stimulations of the antennal nerve before and after 5HT application. Serotonin enhanced the responses of PNs across the AL at all stimulus intensities (Figure 3A; n=9, P <0.05–0.01, t-test), thus shifting the input-output curve to lower intensities, although the application of 5HT did not alter the slope of the input-output curve (n=9, P =.79, t-test). The relative time course of the responses measured across the AL were unaffected by 5HT (Supplemental Figure 1A) and saline application did not affect PN responses (Supplemental Figure 1B; n=9, P=0.14–0.85, t-test). In addition, the application of 5HT at 10–6 and 10–8M resulted in a relatively weak enhancement of PN responses (Supplemental Figure 1C and D, respectively). Although only one of nine preparations responded to the weakest level of nerve stimulation before 5HT exposure, responses to this stimulus intensity were elicited in all preparations after 5HT exposure (Figure 3B), suggesting that 5HT increases PN sensitivity as PNs respond to previously subthreshold stimuli after 5HT exposure.
Figure 3.
Serotonin lowers the response threshold of PNs. (A) PN responses to stimulation of the antennal nerve measured across the entire AL are enhanced at all stimulus intensities by 5HT at 10–4 M (n=9; *P <0.05; **P <0.005). (B) Serotonin application results in subthreshold stimuli to evoke responses. Average time course of responses to the lowest intensity stimulation (1 spike), as measured across the entire AL before (hatched line) and after (solid line) 5HT application. Stimulus onset at time 2 seconds, as indicated by black bar. Data in A are represented as mean±SEM.
The finding that 5HT shifts the sensitivity of PNs predicts that the odor-tuning curve should be offset by 5HT. To test this prediction, we measured the effects of 5HT on the responses of PNs in the DM2 and VM2 glomeruli to five structurally similar alcohols of increasing carbon chain length (3-hexanol through 3-decanol). Each alcohol odorant was presented at a moderate concentration of 5 ppm (Figure 4A). The responses to 3-octanol and 3-nonanol were significantly enhanced for DM2 PNs of flies exposed to 5HT, compared to control flies (n=11 and 9, respectively, P <0.01, t-test) (Figure 4B), while the responses of VM2 PNs were significantly enhanced (P <0.01, t-test) for 3-heptanol (although not 3-hexanol, due to the inconsistency of VM2 PN responses to this odor) (Figure 4C). In these experiments, when olfactory stimuli that sparsely activated the AL were tested, a 5HT-induced modulation of transmitter release from ORNs or sensitivity of PNs may have been an underlying mechanism of enhancement of PN responses. Nonetheless, these results provide further evidence that 5HT enhances the sensitivity of PNs to odor stimulation, which causes an offset of the odor-tuning curve for each glomerulus.
Figure 4.
Serotonin causes an offset in the responses of PNs. (A) The DM2 and VM2 glomeruli (hatched white outline) and responses of these glomeruli to alcohols of increasing carbon chain length; C-6=3-hexanol, C-7=3-heptanol, C-8=3-octanol, C-9=3-nonanol, and C-10=3-decanol. (B) 5HT causes an offset of the odor-tuning curve of DM2 PNs. Responses to C-8 and C-9 were significantly higher in flies exposed to 5HT, compared to control flies (n=11 and 9, respectively; *P <0.05; **P <0.01). (C) Responses measured from the VM2 to C-7 were significantly higher in flies exposed to 5HT, compared to control flies (n=11 and 9, respectively; *P <0.05). Data in B and C are represented as mean±SEM. Scale bar=10 μm.
Response Enhancement by 5HT is Odorant Specific
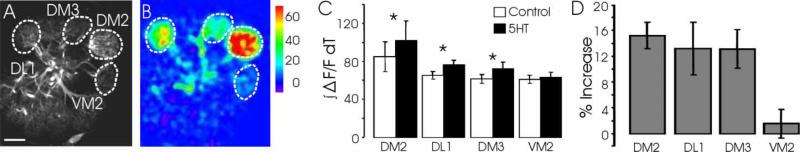
In the above experiments, the AL was sparsely activated by odors at low concentration, and lateral interactions were likely minimally evoked. To further investigate how 5HT affects interglomerular interactions, the effects of 5HT on PN responses to isoamyl acetate (IAA), an odorant that excites multiple glomeruli, were tested. At a moderate concentration, IAA excites four different glomeruli in one optical plane (Figure 5A and 5B). The responses of PNs in three of the glomeruli (the DM2, DL1, and DM3) were significantly enhanced by 5HT (Figure 5C and 5D; ~ 15% increase, n=8 flies; P <0.05, t-test for DM3, DL1, and a Wilcoxon matched pairs test was used for DM2 due to nonparametric distribution of data for this glomerulus), whereas the response of PNs in the VM2 glomerulus to IAA was not enhanced by 5HT (Figure 5C and 5D; P=0.33, t-test). This effect could not have been due to differential expression levels of 5HT receptors on VM2 PNs or the ORNs that project to the VM2 glomerulus, as the responses of VM2 PNs to 3-heptanol were enhanced by 5HT application (Figure 2D–2F). Further, the properties of the differential enhancement were not limited to just one concentration of IAA. In a concentration range of 2.8 to 45 ppm (Figure 6A), the response of the DM2 glomerulus was always enhanced for flies exposed to 5HT, compared to control flies (Figure 6B; n=9 and 13, respectively, P <0.05 and 0.01, t-test), while VM2 PN responses were not significantly enhanced (Figure 6C; P=0.27–0.63, t-test), demonstrating that the lack of enhancement of VM2 responses was not due to response saturation.
Figure 5.
Differential enhancement of PN responses by 5HT is odor dependent. (A) Single optical plane through the AL reveals the DL1, DM3, DM2, and VM2 glomeruli (hatched white outlines). (B) Responses of PNs in the four glomeruli depicted in A to iso-amyl acetate (IAA). (C) 5HT significantly enhanced the responses in the DM2, DL1, and DM3 PNs to IAA (n=8 flies; *P <0.05), but did not enhance the response of VM2 PNs to IAA (n=8 flies; P=0.326). (D) Percent increase in responses of DM2, DM3, DL1, and VM2 PNs after 5HT application. Data in C and D are represented as mean±SEM. Scale bar=10 μm.
Figure 6.
Differential enhancement by 5HT persists across odor concentration. (A) The DM2 and VM2 glomeruli (hatched white outline) and their responses to increasing concentrations of IAA, from 2.8 to 44.8 ppm. (B) The responses of DM2 PNs to all concentrations of IAA were significantly higher for flies exposed to 5HT, compared to control flies (n=9 and 13, respectively; *P <0.05; **P <0.01). (C) VM2 PNs responses were not significantly different for 5HT-treated or control flies at any concentration of IAA tested (n=9 and 13, respectively; P=0.27–0.63). (D) Proportional increase after 5HT application for the DM2 (white) and VM2 (black) at all stimulus intensities. Comparisons were only significant at the 4-spike stimulus intensity (n=6 and 4, respectively; *P <0.05; **P <0.01). Scale bar=10 μm.
We next asked if the responses of DM2 and VM2 PNs are modulated by 5HT in the presence of strong lateral activity. Electrical stimulation of the antennal nerve generates uniform activation of ORNs and 5HT application resulted in equal proportional enhancement of PN responses in the VM2 and DM2 glomeruli (Figure 6D; for those preparations in which they were visible; n=4 and 6, respectively). Thus, the responses of VM2 PNs can be modulated by 5HT even in the presence of strong lateral influences, suggesting that the odorant-specific 5HT-modulation is likely due to heterogeneous lateral interactions that are revealed with the application of specific odorants (such as IAA). The equal proportional enhancement of the responses of VM2 and DM2 PNs to electrical stimulation, in combination with the enhancement of VM2 PN responses to 3-heptanol, eliminate the possibility that the differential enhancement of responses of VM2 and DM2 PNs to IAA is due to differences in the expression levels of 5HT receptors.
To address the possibility that the differential enhancement of PN responses by 5HT is due to a differential increase in the responses of ORNs (thus increasing the amount of input to the PNs), we examined the effects of serotonin on ORN neurotransmitter release in flies bearing the Or83b-Gal4 and UAS-spH transgenes (Figure 7). These flies express the activity reporter, synaptopHluorin (Ng et al., 2002), in all ORNs that express the relatively ubiquitous Or83b protein (Larsson et al., 2004). Surprisingly, 5HT significantly decreased the strength of ORN responses to electrical stimulation of the antennal nerve (Figure 7A; t-test, P <0.05, n=6). This could either have been due to a direct decrease in the excitability of ORNs or an enhancement of the presynaptic inhibition impinging upon ORNs, which in Drosophila is mediated by GABAB receptor expression on ORNs (Olson & Wilson, 2008; Root et al., 2008). To test the possibility that 5HT enhances presynaptic inhibition, we applied the GABAB receptor antagonist, CGP54626 (as described in Root et al., 2008), to block any presynaptic inhibition experienced by the ORNs and then applied 5HT. When 5HT was applied to preparations that had been pretreated with CGP54626, there was no longer any effect of 5HT on ORN response (Figure 7B; t-test, P=0.15–0.80, n=5), suggesting that the attenuation of ORN responses by 5HT was due to an enhancement of the influence of GABAergic LNs.
Figure 7.
Serotonin suppresses ORN transmission via GABAB receptor signaling. (A) Serotonin attenuates ORN transmission elicited by antennal nerve stimulation (t-test, *P <0.05, n=6). Upper panel: representative traces of synaptopHluorin (spH) fluorescence change over time before (hatched line) and after (solid line) application of 5HT at 10–4 M; traces are the average of three trials in response to 80 spikes. Lower panel: responses to increasing intensity of antennal nerve stimulation before (hatched line) and after (solid line) 5HT application. (B) The 5HT-dependent attenuation of ORN responses is eliminated by the application of the GABAB receptor antagonist, CGP 54626 (t-test, P=0.15–0.8, n=5). Upper panel: representative traces of spH fluorescence change over time for CGP54626 alone (hatched line) and after (solid line) CGP54626 and 5HT at 10–4 M; traces are the average of three trials in response to 80 spikes. Lower panel: responses to increasing intensity of antennal nerve stimulation for CGP54626 alone (hatched line) and after (solid line) CGP54626 and 5HT at 10–4 M. Data in lower panels are represented as mean±SEM.
To further confirm that 5HT modulates the activity of GABAergic LNs, we examined the effects of 5HT on the responses of flies bearing the UAS-GCaMP and GAD1-Gal4 transgenes, which label most of the GABAergic LNs within the AL (Ng et al., 2002). Serotonin application enhanced the responses of these LNs (Figure 8B and 8C) to antennal nerve stimulation (Figure 8D and Figure 8E) (n=9, P <0.005, t-test), while saline application did not (Figure 8F) (n=7, P=0.91, t-test). Further, there was no statistically significant difference in the magnitude of response enhancement for LNs in the VM2 glomerulus, compared to the DM2 glomerulus (Figure 8G) (n=7, P=0.29, t-test). This indicated that the differential enhancement of PN responses to IAA was not likely due to a greater enhancement of those LNs innervating the VM2 glomerulus. To determine if there was a difference in the level of expression of GABAB receptors expressed by ORNs innervating the VM2 and DM2 glomeruli, we measured the reporter expression levels in flies bearing the GABABR2-Gal4 and UAS-CD8GFP transgenes, which had been analyzed in Root et al. (2008). There was a statistically significant difference in the levels of GABAB receptors expressed by ORNs innervating the VM2 glomerulus, compared to those innervating the DM2 glomerulus (Figure 8H) (n=4, P <0.05, t-test). These results suggest that while 5HT enhances the responses of PNs and GABAergic LNs, the resultant increase in presynaptic inhibition impinging upon the ORNs may have balanced the enhancement of PN responses in those glomeruli in which the ORNs express high levels of the GABAB receptor.
Figure 8.
Serotonin enhances the responses of GABAergic LNs to antennal nerve stimulation. (A) Single optical plane through the AL reveals the arborization patterns of GAD1-Gal4 LNs. LN responses to antennal nerve stimulation before (B) and after (C) 5HT application. (D) Average time course of LN responses across all preparations to middle-intensity stimulation (8 spikes), as measured across the entire AL before (hatched line) and after (solid line) 5HT application. (E) Responses of GABAergic LNs to antennal nerve stimulation of 8 spikes in stimulus duration were enhanced by 5HT (n=9, *P <0.005). (F) Responses of GAD1-Gal4 neurons to antennal nerve stimulation of 8 spikes in stimulus duration were unaffected by saline (n=7, P=0.60). (G) Responses of GAD1-Gal4 neurons in the DM2 and VM2 glomeruli to electrical stimulation of the antennal nerve were equally enhanced by 5HT application (n=7, P=0.29, t-test). (H) Reporter intensity for ORNs innervating the VM2 glomerulus are significantly higher, compared to the DM2 glomerulus (n=4, *P <0.05, t-test). Data in B–H are represented as mean±SEM. Scale bar=10 μm.
DISCUSSION
In this study, we have investigated modulation of different neuronal populations in the Drosophila AL by 5HT, with two-photon imaging of neural activity in response to odor and electrical stimulation. Serotonin appears to have two primary effects: an enhancement of PN and LN responses and a concomitant increase in lateral interactions mediated by LNs. The sex pheromone, cVA, and the odorants, ethyl hexanoate and 3-heptanol, selectively activate the DA1, DM2, and VM2 glomeruli, respectively, and the response strength of PNs in these glomeruli is enhanced by 5HT. Studies of the AL in moths have established that the excitability of AL neurons is enhanced by 5HT (Kloppenburg & Hildebrand, 1995; Kloppenburg et al., 1999; Hill et al., 2003; Dacks et al., 2008), which causes a reduction of two K+ conductances (Mercer et al., 1995; Kloppenburg et al., 1999). The resultant increase in membrane resistance should, therefore, decrease the amount of input current required to elicit a response, thus increasing the sensitivity of AL neurons. Our results are consistent with these effects of 5HT observed in the ALs of moth. Serotonin causes Drosophila PNs to respond to intensities of antennal nerve stimulation that were previously subthreshold (Figure 3B). It is possible that 5HT causes a similar reduction in K+ conductances in Drosophila AL neurons; however, further biophysical studies are necessary to determine if this is the case.
When we examined the effects of 5HT on the responses of PNs to odors that activated large portions of the AL, we found evidence that 5HT modulated lateral interactions within the AL. For the odor, IAA, 5HT enhanced the responses of PNs in many (including the DM2), but not all, glomeruli (in particular, the VM2). The responses of the VM2 PNs to 3-heptanol (Figure 2F) and antennal nerve stimulation (Figure 6D) were enhanced by 5HT, indicating that the differential effects of 5HT on IAA responses were due to modulation of lateral interactions within the AL and not due to the VM2 PNs being immune to the effects of 5HT. Serotonin attenuated the responses of ORNs in a GABAB-receptor–dependent manner (Figure 7) and enhanced the responses of GABAergic LNs (Figure 8D and Figure 8E), indicating that 5HT enhanced the presynaptic inhibition impinging upon ORNs. Root et al. (2008) reported that there is heterogeneous expression of GABAB receptors by the ORNs innervating different glomeruli, and using previously unpublished data from that study, we observed a greater expression of GABAB receptors by ORNs innervating the VM2 glomerulus, compared to the DM2 glomerulus (Figure 8H), which likely explains the differences in the effects of 5HT on the responses of PNs in these glomeruli for IAA. This does not, however, preclude the possibility that 5HT modulates lateral inhibition or excitation impinging directly upon PNs. There is a rich diversity of LNs interconnecting the glomeruli of the AL (Homberg et al., 1990; Christensen et al., 1993; Fonta et al., 1993; Shang et al., 2007; Seki & Kanzaki, 2008). In Drosophila, different glomeruli receive variable innervation from LNs (Wilson & Laurent, 2005), and the lateral interactions between glomeruli are nonuniform (Ng et al., 2002; Silbering & Galizia, 2007; Olsen et al., 2007; Shang et al., 2007). Heterogeneous inhibitory interactions within the AL have been demonstrated in other insect species (Sachse & Galizia, 2002; Linster et al., 2005; Reisenman et al., 2008), and so, modulation of LN activity by 5HT likely has nonuniform effects across glomeruli.
Comparing what is known already from moths with what we have found in Drosophila, several underlying principles begin to emerge. In this study, we show that, as in moths (Kloppenburg & Hildebrand, 1995; Kloppenburg et al., 1999; Hill et al., 2003; Dacks et al., 2008), 5HT enhances the responses of both PNs and LNs in the AL of Drosophila. We, further, went on to show that this did not simply result in a simple increase in activity across the entirety of the AL, but rather that the modulation of the lateral network resulted in an enhancement of presynaptic inhibition, likely resulting, at least in part, in an odor-dependant enhancement of responses, as in the case for the odor IAA. It should be noted that the VM2 glomerulus produced weaker responses to IAA, compared to the other glomeruli that were enhanced. This suggests that 5HT may serve to enhance strongly activated glomeruli within the pattern of AL activation and enhance suppression of more weakly activated glomeruli. Serotonin may enhance only certain features of the AL representation that are key for the behavioral sensitivity of flies or moths to a given odor, and the effects of 5HT on the local network, at least in part, result in a dampening mechanism to prevent a nonspecific enhancement of all activity (potentially via an enhancement of presynaptic inhibition; Figure 7).
Both flies and moths possess a single morphologically similar neuron (the contralaterally projecting, serotonin-immunoreactive, deutocerebral, or CSD neuron) that innervates the antennal lobes (Kent et al., 1987; Dacks et al., 2006), and electron microscopy studies in the moth revealed serotonergic synaptic release sites within the AL (Sun et al., 1993). The levels of 5HT in the AL of moths vary throughout the day, reaching their peak when moths are most active and reaching their trough when moths are least active. Recordings in moths have demonstrated that this neuron responds to mechanosensory stimulation of the antennae by wind (Hill et al., 2002), suggesting that this neuron releases 5HT in the AL when the moths are actively tracking odor sources. Most, if not all, behaviors exhibited by moths are, at least in part, mediated by olfactory stimuli, and so, it appears that the CSD neuron affects olfactory processing only when the moths (and, potentially, flies) are most likely to be using the olfactory system. This could, therefore, subserve a function to modulate the acuity and sensitivity of the antennal lobe, based on the arousal state of the individual animal.
A sensory system must temper its function based on the current physiological state. Neuromodulation accommodates state-dependent adjustment of neural function, whereby synaptic strength is altered by affecting presynaptic release of primary neurotransmitters or the effective synaptic current in postsynaptic cells (Kupfermann, 1979; Katz, 1999). By adjusting the excitability of the cellular elements within a network, neuromodulators can alter network properties (Destexhe & Marder 2004). In moths, the levels of 5HT in the AL cycle throughout the day, peaking when moths are most active (Kloppenburg et al., 1999), and 5HT injection increases olfactory behavioral sensitivity (Linn & Roelofs, 1986; Gatellier et al., 2004), suggesting that 5HT may be a mechanism by which arousal modifies the performance of the AL to best suit the physiological state. Elevating 5HT levels promotes sleep in Drosophila via the 5HT1A receptors (Yuan et al., 2006). However, the 5HT1A receptor is not required for the enhancement by 5HT of PN responses to cVA in the AL of Drosophila (data not shown). Circadian entrainment in Drosophila requires the 5HT1B and 5HT2 receptors (Yuan et al., 2005). Four 5HT receptor genes, 5HT1A, 5HT1B, 5HT2, and 5HT7, have been identified in the Drosophila genome (Saudou et al. 1992; Colas et al., 1995). Therefore, it is possible that different 5HT receptors participate in different neural circuits to regulate complex behavioral traits in response to the release of 5HT.
Findings from this study suggest that in addition to similarity in the anatomical organization of the olfactory system, neuromodulation is similar in different animal species as well. In the AL of Manduca, 5HT enhances the responses of both PNs and LNs (Kloppenburg & Hildebrand, 1995; Kloppenburg et al., 1999; Dacks et al., 2008) and causes odorant-dependent modulation of olfactory responses (Dacks et al., 2008). In the rat, 5HT causes a depolarization of the membrane potential of some mitral cells in the olfactory bulb and a hyperpolarization of other mitral cells that is blocked by GABAA receptor antagonists (Hardy et al., 2005). Further, a recent study in mice found, similar to Drosophila, that the responses of ORNs were attenuated by 5HT, an effect mediated by GABAB-receptor–dependent presynaptic inhibition (Petzhold et al., 2009). This suggests that across diverse phyla, 5HT enhances the responses of output neurons from the primary olfactory neuropil, yet concurrently modulates the activity of the local circuitry. The activation of specific sets of local interneurons by a given odor results in patterns of lateral excitation and inhibition, which are likely enhanced by 5HT. The overall effect of this form of modulation is an enhancement of certain response features over others, although future experiments with genetic manipulations of 5HT receptors in specific populations of AL neurons should shed light on the behavioral significance of the apparent selective modulation of specific olfactory response features by 5HT.
Supplementary Material
Supplemental Figure 1. (A) Time course of PN responses are unaffected by 5HT. Stimulus onset at time 2 seconds, as indicated by black bar. (Ai) Average time course of responses to 8 spikes in stimulation, as measured across the entire AL before (hatched line) and after (solid line) 5HT application. (Aii) Time course of responses to 8 spikes in stimulation, as measured across the entire AL before and after 5HT application normalized to the maximum value for each treatment. (B) PN responses are unaffected by saline addition (n=9, P=0.14–0.85). (C) PN responses are enhanced at lower stimulus intensities by 5HT at 10–6 M (n=9, *P <0.05; **P <0.005). (D) PN responses are enhanced at lower stimulus intensities by 5HT at 10–8 M (n=9; *P <0.01; **P <0.005). Data in B–D are represented as mean±SEM.
ACKNOWLEDGEMENTS
The authors thank Drs. Carolina Reisenman PhD, Lynne Oland PhD, Angelique Paulk PhD, and Jason Worrell PhD, as well as Josh Martin, Penny Dacks, and Aaron Beyerlein for comments on the manuscript for this article. The authors would also like to thank Dr. Marco Gallio PhD for helpful discussions. The cis-11-vaccenyl acetate was a generous gift from Dr. Dean Smith MD, PhD. This work was partially supported by a research grant from the Whitehall Foundation (to JWW) and a grant from the National Institute of Deafness and other Communication Disorders (R01DC-009597, to JWW; R01DC-05652, to AJN). JWW is a Beckman investigator, a Hellman Faculty scholar, and a Searle scholar. AMD was funded by the Center for Insect Science through NIH Training Grant #1 K12 Gm00708. CMR was funded by an NRSA training grant from NIH-NIDCD (1F31DC009511).
Footnotes
Declaration of interest: The authors report no financial conflicts of interest. The authors alone are responsible for the content and writing of this paper.
REFERENCES
- Christensen TA, Waldrop BR, Harrow ID, Hildebrand JG. Local interneurons and information processing in the olfactory glomeruli of the moth Manduca sexta. J Comp Physiol A. 1993;173(4):385–399. doi: 10.1007/BF00193512. [DOI] [PubMed] [Google Scholar]
- Clyne PJ, Certel SJ, de Bruyne M, Zaslavsky L, Johnson WA, Carlson JR. The odor specificities of a subset of olfactory receptor neurons are governed by Acj6, a POU-domain transcription factor. Neuron. 1999;22:339–347. doi: 10.1016/s0896-6273(00)81094-6. [DOI] [PubMed] [Google Scholar]
- Colas JF, Launay JM, Kellermann O, Rosay P, Maroteaux L. Drosophila 5-HT2 serotonin receptor: coexpression with fushi-tarazu during segmentation. Proc Natl Acad Sci USA. 1995;92(12):5441–5445. doi: 10.1073/pnas.92.12.5441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couto A, Alenius M, Dickson BJ. Molecular, anatomical, and functional organization of the Drosophila olfactory system. Curr Biol. 2005;15:1535–1547. doi: 10.1016/j.cub.2005.07.034. [DOI] [PubMed] [Google Scholar]
- Dacks AM, Christensen TA, Hildebrand JG. Phylogeny of a serotonin-immunoreactive neuron in the primary olfactory center of the insect brain. J Comp Neurol. 2006;498:727–746. doi: 10.1002/cne.21076. [DOI] [PubMed] [Google Scholar]
- Dacks AM, Christensen TA, Hildebrand JG. Modulation of olfactory processing in the antennal lobe of Manduca sexta by serotonin. J Neurophysiol. 2008;99(5):2077–2085. doi: 10.1152/jn.01372.2007. [DOI] [PubMed] [Google Scholar]
- de Bruyne M, Foster K, Carlson JR. Odor coding in the Drosophila antenna. Neuron. 2001;30:537–552. doi: 10.1016/s0896-6273(01)00289-6. [DOI] [PubMed] [Google Scholar]
- Destexhe A, Marder E. Plasticity in single neuron and circuit computations. Nature. 2004;431(7010):789–795. doi: 10.1038/nature03011. [DOI] [PubMed] [Google Scholar]
- Ejima A, Smith BP, Lucas C, van der Goes van Naters W, Miller CJ, Carlson JR, et al. Generalization of courtship learning in Drosophila is mediated by cis-vaccenyl acetate. Curr Biol. 2007;17(7):599–605. doi: 10.1016/j.cub.2007.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishilevich E, Vosshall LB. Genetic and functional subdivision of the Drosophila antennal lobe. Curr Biol. 2005;15:1548–1553. doi: 10.1016/j.cub.2005.07.066. [DOI] [PubMed] [Google Scholar]
- Fonta C, Sun X, Masson C. Morphology and spatial distribution of bee antennal lobe interneurones responsive to odours. Chem Senses. 1993;18:101–119. [Google Scholar]
- Gao Q, Yuan B, Chess A. Convergent projections of Drosophila olfactory neurons to specific glomeruli in the antennal lobe. Nat Neurosci. 2000;3:780–785. doi: 10.1038/77680. [DOI] [PubMed] [Google Scholar]
- Gatellier L, Nagao T, Kanzaki R. Serotonin modifies the sensitivity of the male silkmoth to pheromone. J Exp Biol. 2004;207:2487–2496. doi: 10.1242/jeb.01035. [DOI] [PubMed] [Google Scholar]
- Goldberg F, Grunewald B, Rosenboom H, Menzel R. Nicotinic acetylcholine currents of cultured Kenyon cells from the mushroom bodies of the honey bee Apis mellifera. J Physiol. 1999;514:759–768. doi: 10.1111/j.1469-7793.1999.759ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha TS, Smith DP. A pheromone receptor mediates 11-cis-vaccenyl acetate-induced responses in Drosophila. J Neurosci. 2006;26(34):8727–8733. doi: 10.1523/JNEUROSCI.0876-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy A, Palouzier-Paulignan B, Duchamp A, Royet JP, Duchamp-Viret P. 5-hydroxytryptamine action in the rat olfactory bulb: in vitro electrophysiological patch-clamp recordings of juxtaglomerular and mitral cells. Neuroscience. 2005;131:717–731. doi: 10.1016/j.neuroscience.2004.10.034. [DOI] [PubMed] [Google Scholar]
- Hauser F, Cazzamali G, Williamson M, Blenau W, Grimmelikhuijzen CJ. A review of neurohormone GPCRs present in the fruitfly Drosophila melanogaster and the honey bee Apis mellifera. Prog Neurobiol. 2006;80:1–19. doi: 10.1016/j.pneurobio.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Hill ES, Iwano M, Gatellier L, Kanzaki R. Morphology and physiology of the serotonin-immunoreactive putative antennal lobe feedback neuron in the male silkmoth Bombyx mori. Chem Senses. 2002;27:475–483. doi: 10.1093/chemse/27.5.475. [DOI] [PubMed] [Google Scholar]
- Hill ES, Okada K, Kanzaki R. Visualization of modulatory effects of serotonin in the silkmoth antennal lobe. J Exp Biol. 2003;206(2):345–352. doi: 10.1242/jeb.00080. [DOI] [PubMed] [Google Scholar]
- Homberg U, Kingan TG, Hildebrand JG. Distribution of FMRFamide-like immunoreactivity in the brain and suboesophageal ganglion of the sphinx moth Manduca sexta and colocalization with SCPB-, BPP-, and GABA-like immunoreactivity. Cell Tissue Res. 1990;259(3):401–419. doi: 10.1007/BF01740767. [DOI] [PubMed] [Google Scholar]
- Katz PS. Beyond Neurotransmission; Neuromodulation and Its Importance for Information Processing. Oxford University Press; New York: 1999. What are we talking about? [Google Scholar]
- Kent KS, Hoskins SG, Hildebrand JG. A novel serotonin-immunoreactive neuron in the antennal lobe of the sphinx moth Manduca sexta persists throughout postembryonic life. J Neurobiol. 1987;18:451–465. doi: 10.1002/neu.480180506. [DOI] [PubMed] [Google Scholar]
- Kloppenburg P, Ferns D, Mercer AR. Serotonin enhances central olfactory neuron responses to female sex pheromone in the male sphinx moth, Manduca sexta. J Neurosci. 1999;19:8172–8181. doi: 10.1523/JNEUROSCI.19-19-08172.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloppenburg P, Hildebrand JG. Neuromodulation by 5-hydroxytryptamine in the antennal lobe of the sphinx moth Manduca sexta. J Exp Biol. 1995;198:603–611. doi: 10.1242/jeb.198.3.603. [DOI] [PubMed] [Google Scholar]
- Kupfermann I. Modulatory actions of neurotransmitters. Ann Rev Neurosci. 1979;2:447–465. doi: 10.1146/annurev.ne.02.030179.002311. [DOI] [PubMed] [Google Scholar]
- Kurtovic A, Widmer A, Dickson BJ. A single class of olfactory neurons mediates behavioural responses to a Drosophila sex pheromone. Nature. 2007;446(7135):542–546. doi: 10.1038/nature05672. [DOI] [PubMed] [Google Scholar]
- Larsson MC, Domingos AI, Jones WD, Chiappe ME, Amrein H, Vosshall LB. Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron. 2004;43(5):703–714. doi: 10.1016/j.neuron.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Lide D, editor. CRC Handbook of Chemistry and Physics. 88th ed. CRC Press; Boca Raton, Florida, USA: 2008. Vapor pressure. [Google Scholar]
- Linn CE, Roelofs WL. Modulatory effects of octopamine and serotonin on male sensitivity and periodicity of response to sex pheromone in the cabbage looper moth, Trichoplusia ni. Arch Insect Bioch Mol Biol. 1986;3:161–172. [Google Scholar]
- Linster C, Sachse S, Galizia CG. Computational modeling suggests that response properties rather than spatial position determine connectivity between olfactory glomeruli. J Neurophysiol. 2005;93(6):3410–3417. doi: 10.1152/jn.01285.2004. [DOI] [PubMed] [Google Scholar]
- Mercer A, Hayashi J, Hildebrand JG. Modulatory effects of 5-hydroxytryptamine on voltage-activated currents in cultured antennal lobe neurons of the sphinx moth, Manduca sexta. J Exp Biol. 1995;198:613–627. doi: 10.1242/jeb.198.3.613. [DOI] [PubMed] [Google Scholar]
- Nakai J, Ohkura M, Imoto K. A high signal-to-noise Ca(2+) probe composed of a single green fluorescent protein. Nat Biotechnol. 2001;19:137–141. doi: 10.1038/84397. [DOI] [PubMed] [Google Scholar]
- Ng M, Roorda RD, Lima SQ, Zemelman BV, Morcillo P, Miesenbock G. Transmission of olfactory information between three populations of neurons in the antennal lobe of the fly. Neuron. 2002;36:463–474. doi: 10.1016/s0896-6273(02)00975-3. [DOI] [PubMed] [Google Scholar]
- Oertner TG, Brotz TM, Borst A. Mechanisms of dendritic calcium signaling in fly neurons. J Neurophysiol. 2001;85:439–447. doi: 10.1152/jn.2001.85.1.439. [DOI] [PubMed] [Google Scholar]
- Olsen SR, Bhandawat V, Wilson RI. Excitatory interactions between olfactory processing channels in the Drosophila antennal lobe. Neuron. 2007;54(1):89–103. doi: 10.1016/j.neuron.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen SR, Wilson RI. Lateral presynaptic inhibition mediates gain control in an olfactory circuit. Nature. 2008;452(7190):956–960. doi: 10.1038/nature06864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelz D, Roeske T, Syed Z, de Bruyne M, Galizia CG. The molecular receptive range of an olfactory receptor in vivo (Drosophila melanogaster Or22a). J Neurobiol. 2006;66:1544–1563. doi: 10.1002/neu.20333. [DOI] [PubMed] [Google Scholar]
- Petzold GC, Hagiwara A, Murthy VN. Serotonergic modulation of odor input to the mammalian olfactory bulb. Nat Neurosci. 2009 doi: 10.1038/nn.2335. published online May 10, 2009, doi:10.1038/nn.2335. [DOI] [PubMed] [Google Scholar]
- Reisenman CE, Heinbockel T, Hildebrand JG. Inhibitory interactions among olfactory glomeruli do not necessarily reflect spatial proximity. J Neurophysiol. 2008;100(2):554–564. doi: 10.1152/jn.90231.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root CM, Masuyama K, Green DS, Enell LE, Nässel DR, Lee CH, et al. A presynaptic gain control mechanism fine-tunes olfactory behavior. Neuron. 2008;59(2):311–321. doi: 10.1016/j.neuron.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root CM, Semmelhack JL, Wong AM, Flores J, Wang JW. Propagation of olfactory information in Drosophila. Proc Natl Acad Sci U S A. 2007;104(28):11826–11831. doi: 10.1073/pnas.0704523104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy B, Singh AP, Shetty C, Chaudhary V, North A, Landgraf M, et al. Metamorphosis of an identified serotonergic neuron in the Drosophila olfactory system. Neural Develop. 2007;2:ARTN 20. doi: 10.1186/1749-8104-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachse S, Galizia CG. Role of inhibition for temporal and spatial odor representation in olfactory output neurons: a calcium imaging study. J Neurophysiol. 2002;87(2):1106–1117. doi: 10.1152/jn.00325.2001. [DOI] [PubMed] [Google Scholar]
- Saudou F, Boschert U, Amlaiky N, Plassat JL, Hen R. A family of Drosophila serotonin receptors with distinct intracellular signaling properties and expression patterns. EMBO J. 1992;11(1):7–17. doi: 10.1002/j.1460-2075.1992.tb05021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlief ML, Wilson RI. Olfactory processing and behavior downstream from highly selective receptor neurons. Nat Neurosci. 2007;10(5):623–630. doi: 10.1038/nn1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki Y, Kanzaki R. Comprehensive morphological identification and GABA immunocytochemistry of antennal lobe local interneurons in Bombyx mori. J Comp Neurol. 2008;506(1):93–107. doi: 10.1002/cne.21528. [DOI] [PubMed] [Google Scholar]
- Shang Y, Claridge-Chang A, Sjulson L, Pypaert M, Miesenböck G. Excitatory local circuits and their implications for olfactory processing in the fly antennal lobe. Cell. 2007;128(3):601–612. doi: 10.1016/j.cell.2006.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silbering AF, Galizia CG. Processing of odor mixtures in the Drosophila antennal lobe reveals both global inhibition and glomerulus-specific interactions. J Neurosci. 2007;27(44):11966–11977. doi: 10.1523/JNEUROSCI.3099-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silbering AF, Okada R, Ito K, Galizia CG. Olfactory information processing in the Drosophila antennal lobe: anything goes? J Neurosci. 2008;28(49):13075–13087. doi: 10.1523/JNEUROSCI.2973-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker RF, Heimbeck G, Gendre N, de Belle JS. Neuroblast ablation in Drosophila P[GAL4] lines reveals origins of olfactory interneurons. J Neurobiol. 1997;32:443–456. doi: 10.1002/(sici)1097-4695(199705)32:5<443::aid-neu1>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Stensmyr MC, Giodano E, Balloi A, Angioy AM, Hansson BS. Novel natural ligands for Drosophila olfactory receptor neurones. J Exp Biol. 2003;206:715–724. doi: 10.1242/jeb.00143. [DOI] [PubMed] [Google Scholar]
- Sun XJ, Tolbert LP, Hildebrand JG. Ramification pattern and ultrastructural characteristics of the serotonin-immunoreactive neuron in the antennal lobe of the moth Manduca sexta: a laser scanning confocal and electron microscopic study. J Comp Neurol. 1993;338:5–16. doi: 10.1002/cne.903380103. [DOI] [PubMed] [Google Scholar]
- van der Goldman AL, van der Goes van Naters W, Lessing D, Warr CG, Carlson JR. Coexpression of two functional odor receptors in one neuron. Neuron. 2005;45:661–666. doi: 10.1016/j.neuron.2005.01.025. [DOI] [PubMed] [Google Scholar]
- Wang JW, Wong AM, Flores J, Vosshall LB, Axel R. Two-photon calcium imaging reveals an odor-evoked map of activity in the fly brain. Cell. 2003;112:271–282. doi: 10.1016/s0092-8674(03)00004-7. [DOI] [PubMed] [Google Scholar]
- Vosshall LB, Amrein H, Morozov PS, Rzhetsky A, Axel R. A spatial map of olfactory receptor expression in the Drosophila antenna. Cell. 1999;96:725–736. doi: 10.1016/s0092-8674(00)80582-6. [DOI] [PubMed] [Google Scholar]
- Vosshall LB, Wong AM, Axel R. An olfactory sensory map in the fly brain. Cell. 2000;102:147–159. doi: 10.1016/s0092-8674(00)00021-0. [DOI] [PubMed] [Google Scholar]
- Wilson RI, Laurent G. Role of GABAergic inhibition in shaping odor-evoked spatiotemporal patterns in the Drosophila antennal lobe. J Neurosci. 2005;25(40):9069–9079. doi: 10.1523/JNEUROSCI.2070-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Q, Joiner WJ, Sehgal A. A sleep-promoting role for the Drosophila serotonin receptor 1A. Curr Biol. 2006;16:1051–1062. doi: 10.1016/j.cub.2006.04.032. [DOI] [PubMed] [Google Scholar]
- Yuan Q, Lin F, Zheng X, Sehgal A. Serotonin modulates circadian entrainment in Drosophila. Neuron. 2005;47:115–127. doi: 10.1016/j.neuron.2005.05.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. (A) Time course of PN responses are unaffected by 5HT. Stimulus onset at time 2 seconds, as indicated by black bar. (Ai) Average time course of responses to 8 spikes in stimulation, as measured across the entire AL before (hatched line) and after (solid line) 5HT application. (Aii) Time course of responses to 8 spikes in stimulation, as measured across the entire AL before and after 5HT application normalized to the maximum value for each treatment. (B) PN responses are unaffected by saline addition (n=9, P=0.14–0.85). (C) PN responses are enhanced at lower stimulus intensities by 5HT at 10–6 M (n=9, *P <0.05; **P <0.005). (D) PN responses are enhanced at lower stimulus intensities by 5HT at 10–8 M (n=9; *P <0.01; **P <0.005). Data in B–D are represented as mean±SEM.