Abstract
Background
Many recent studies have employed repetitive transcranial magnetic stimulation (rTMS) to study brain-behavior relationships. However, the pulse-to-pulse neural effects of rapid delivery of multiple TMS pulses are unknown largely because of TMS-evoked electrical artifacts limiting recording of brain activity.
Objective
In this study, TMS-related artifacts were removed with independent component analysis (ICA), which allowed for the investigation of the neurophysiological effects of rTMS with simultaneous electroencephalographic (EEG) recordings.
Methods
10-Hz, 3-sec rTMS trains (110% of motor threshold) were delivered to the postcentral gyrus and superior parietal lobule in 16 young adults. Simultaneous EEG recordings were made with a TMS-compatible system. The stereotypical pattern of TMS-related electrical artifacts was identified by ICA.
Results
Removal of artifacts allowed for identification of a series of five evoked brain potentials occurring within 100 ms of each TMS pulse. With the exception of the first potential, for both areas targeted, there was a quadratic relationship between potential peak amplitude and pulse number within the TMS train. This was characterized by a decrease followed by a rise in amplitude.
Conclusions
ICA is an effective method for removal of TMS-evoked electrical artifacts in EEG data. With the use of this procedure we found that the physiological responses to TMS pulses delivered in a high-frequency train of pulses are not independent. The sensitivity of the magnitude of these responses to recent stimulation history suggests a complex recruitment of multiple neuronal events with different temporal dynamics.
Keywords: transcranial magnetic stimulation, rTMS, electroencephalography, EEG, ERP, independent component analysis
Introduction
The ability to noninvasively alter neuronal processing with transcranial magnetic stimulation (TMS) has provided an important tool with which to study mind-brain relationships (1, 2). Repetitive TMS (rTMS) has generally been thought to produce a “virtual lesion”, thereby impairing task performance (3, 4). As such, rTMS provides a powerful method to examine whether or not a given brain area plays a causal role in task performance (e.g., 5, 6). However, some studies have produced seemingly paradoxical rTMS-related improvements in performance, with, for example, rTMS to the parietal cortex producing improved performance on tests of mental rotation (7) and working memory (8, 9). These findings indicate that rTMS may have more complex effects on brain function than initially thought (3, 4, 10)
Indeed, recent neuroimaging and electrophysiological studies have shown that the physiological effects of TMS are neither short-lived nor limited to the brain area that is targeted (11-14). For example, previous electroencephalogram (EEG) studies have shown that delivery of a single pulse of TMS elicits a sequence of event-related potential (ERP) components (15, 16). In order to understand how rTMS modulates brain activity, and hence task performance, it is thus important to combine it with neuroimaging methods such as EEG (17-20). The combination of rTMS and EEG is particularly promising given its high temporal resolution, as illustrated by recent combined rTMS-EEG studies that have revealed changes in neuronal oscillations post-stimulation (19, 20). However, cortical activity changes during rTMS stimulation have not been previously investigated. This is likely related to the fact that each TMS pulse produces artifacts in the recorded data arising from the large current produced by the magnetic discharge of each pulse. Although recent TMS-compatible EEG systems have minimized such artifacts and allowed for analysis of neural activity to within 10 ms of the TMS pulse (21), at higher intensities of stimulation the artifact is more prominent (e.g., 22).
From in vitro and in vivo studies of microstimulation of neurons, it is known that multiple stimuli delivered close in time can lead either to a potentiated (e.g., paired-pulse facilitation and synaptic augmentation, 23, 24) or depressed (e.g., 24, 25 (paired-pulse depression), 26 (synaptic depression)) response, depending on the cell type and details of the stimulation protocol. Several recent studies suggest that rTMS may enhance cortical excitability. For example, studies of TMS of the motor cortex have shown evidence of potentiation of the motor evoked potential (MEP; 27 (paired-pulse TMS), 28 (5 Hz rTMS)), and of the TMS-evoked ERP following a 5-Hz rTMS train (29). Other studies, however, using rTMS-evoked MEPs (30, 31) and long-interval paired-pulse stimulation (32, 33), have described evidence for TMS-induced cortical inhibition. Some of this variability across studies may be due to individual differences in baseline cortical activity (34), as well as to complex, cumulative effects of rTMS that may depend on the number of pulses delivered (20). Thus, rTMS effects are likely to include both decreases and increases in cortical excitability.
The current study used simultaneous rTMS and EEG to examine whether rTMS-evoked brain responses change over the course of a train of pulses during a cognitive task. To this end, rTMS was applied at 10 Hz to the superior parietal lobule (SPL) and post-central gyrus (PCG) during the delay period of a visual working memory task, while EEG was recorded (cf. 9). Several studies have used similar rTMS parameters to investigate brain-behavior relations, and we have used this precise stimulation protocol to produce both impairment (35) and improvement (9) of behavioral performance. Furthermore, the two target areas were chosen because our previous studies have shown that SPL and PCG rTMS produce differential behavioral effects. The relationship between the effects of rTMS and task performance will be presented elsewhere. Here, we use these data to examine the possibility of pulse-to-pulse interactions or cumulative effects of multiple successive pulses with rTMS (20) by measuring the strength of TMS-induced brain responses.
Recently, there have been several attempts to remove rTMS-related EEG artifacts post-hoc, in order to be able to study the neurophysiological effects of rTMS. Some groups have avoided the artifact by limiting their analysis to the late effects of rTMS (e.g., 36). Another group sought to remove the artifact by subtracting the rTMS response in the EEG during a rest condition from that measured during task performance (37). However, both these methods preclude the study of the immediate effects of TMS on brain activity. An alternative class of approaches employs offline digital signal processing to remove TMS-related artifacts from the EEG signal without affecting underlying neural activity (38, 39). In the present study, we used independent component analysis (ICA) as a method of removing the TMS-related artifact. Because the electrical artifact is temporally and spatially predictable, ICA provides a relatively simple method with which to remove it without affecting the underlying neurophysiological activity.
Methods
Subjects
16 young adults (12 male, mean age = 22.5 [S.D.=3.8]) participated in the study. Subjects reported no neurological, psychiatric or other excluding medical conditions as determined by a psychiatrist or clinical psychologist who administered a structured psychiatric diagnostic interview (MINI, 40) and mood assessment (HAM-D, 41). The study was approved by the local ethics committee. All subjects provided informed consent and were compensated monetarily.
Behavioral Task
Subjects performed a delayed-recognition working memory task. Half the trials, randomly distributed, required memory for spatial locations, while the other half of trials required memory for shapes. In half the trials, randomly distributed and orthogonal to the factor of trial type, a 3-sec, 10-Hz rTMS train was applied to the target brain area time-locked to the onset of the 3-sec delay period. Because the behavioral context is being treated as incidental to the effects of rTMS, the data presented here are collapsed across tasks.
For safety reasons, the number of TMS pulses delivered to each subject must be limited (42). In order to obtain data from an adequate number of trials of each condition, the experiment therefore consisted of two sessions that took place on separate days. In each session, a different brain area was targeted. The order of the brain area stimulated was counterbalanced across subjects. In each session, subjects performed 192 memory trials (96 per trial type), divided into 8 task blocks. During half of these trials (n=96), randomly distributed, rTMS was applied.
rTMS
rTMS was applied to two different brain areas: the SPL and the PCG. The SPL was chosen based on a previous finding from our group that rTMS of SPL leads to an improvement in task performance (9). The area representing the leg in the somatosensory cortex of the PCG served as a control region for the behavioral study (9, 35). Both areas were identified based on individual anatomy from whole-brain anatomical MRIs that were obtained for each subject prior to the study (GE Signa VH/I, 256 sagittal slices, 0.5 mm × 0.5 mm × 0.8 mm). An infrared-based frameless stereotaxy system was used to accurately target each brain area with the TMS coil (eXimia NBS, Nexstim, Helsinki, Finland). For all subjects rTMS was applied to the left hemisphere.
In rTMSpresent trials, a 10-Hz rTMS train (110% of resting motor threshold1) was applied during the entire 3-sec delay period (30 pulses). For each brain area targeted (and, hence, for each session), a total of 2,880 pulses were delivered. TMS was delivered with a Magstim Standard Rapid magnetic stimulator fit with a 70-mm figure-8 stimulating coil (Magstim, Whitland, UK). Because rTMSpresent and rTMSabsent trials were randomly intermixed, the intertrain interval varied. A minimum of 17.1 seconds separated each train. White noise was played in the background during all trials.
EEG recordings
EEG was recorded with a 60-channel carbon cap and TMS-compatible amplifier (Nexstim, Helsinki, Finland). This amplifier is designed to avoid saturation by the TMS pulse by employing a sample-and-hold circuit that keeps the output of the amplifier constant from 100 μs pre- to 2 ms poststimulus (21). To reduce residual TMS-related artifacts, the impedance at each electrode was kept below 3 kΩ. The right mastoid was used as the reference and eye movements were recorded using two additional electrodes. Data were acquired at 1450 Hz sampling rate with 16-bit resolution.
Pre-analysis
Data were processed offline using the EEGLAB toolbox (44) running in a MATLAB environment (Mathworks, Natick, MA). The data were first down-sampled to 500 Hz and then bandpass filtered between 0.1 and 500 Hz. Following this, the data were cleaned of large movement-related artifacts and channels with excessive noise were reinterpolated using spherical spline interpolation (45). Prior to analysis, the data were rereferenced to an average of all 60 channels.
Removing TMS artifacts with ICA
Because of smearing of the electrical signals by the skull, physiological EEG signals, even if from a very localized source, are detected by many electrodes on the scalp. TMS-related electrical artifacts, on the other hand, originate from outside the skull and, with TMS-compatible EEG systems, localize to only the few electrodes surrounding the TMS coil (e.g., 16). TMS-related artifacts are also temporally predictable. They reliably occur with delivery of each pulse and typically last a few milliseconds (21, 46). Thus, they are readily distinguishable from physiological signals.
ICA, a method that separates statistically independent sources from a mixed signal, is ideally suited to separate the electrical artifacts from physiological data in the EEG recordings. It is a technique that has already been successfully applied to removal of other non-neurophysiological EEG artifacts (e.g., 47, 48). In this study, the residual TMS artifacts, as well as other stereotypical artifacts, such as eye-blinks and some muscle contractions were identified and removed using ICA as implemented in EEGLAB (48). Two rounds of ICA were performed on the data. This was to ensure that less prominent TMS artifacts would also be detected. The first ICA was performed on continuous EEG data, and the second ICA was performed on data from only the epoch during which rTMS was applied. TMS artifact components were identified using three characteristics. First, as described above, the artifact should be relatively spatially localized (Fig. 1a). Second, the power spectrum of an ICA artifact component should have a strong peak at 10 Hz (accompanied by peaks at every harmonic of 10 Hz) because rTMS was delivered at 10 Hz. Although physiological EEG signals often also have a peak at around 10 Hz (the α-band peak), this peak typically covers a wider frequency range, does not have strong harmonics, and has a general 1/frequency pattern in the power spectrum. In contrast, the TMS artifact typically consists of a sharp peak at the frequency of stimulation (and corresponding harmonics), which is superimposed on a flat power spectrum (Fig. 1b). A third criterion concerned the component activity. With the TMS compatible EEG system, the artifact, if present, is limited to the first 10-15 ms after the pulse (21, 46). Because the timing of the TMS train was known, a component reflecting the ICA artifact therefore had to peak within a few milliseconds of the start of each TMS pulse in the train (Figs. 1c-d).
Figure 1.
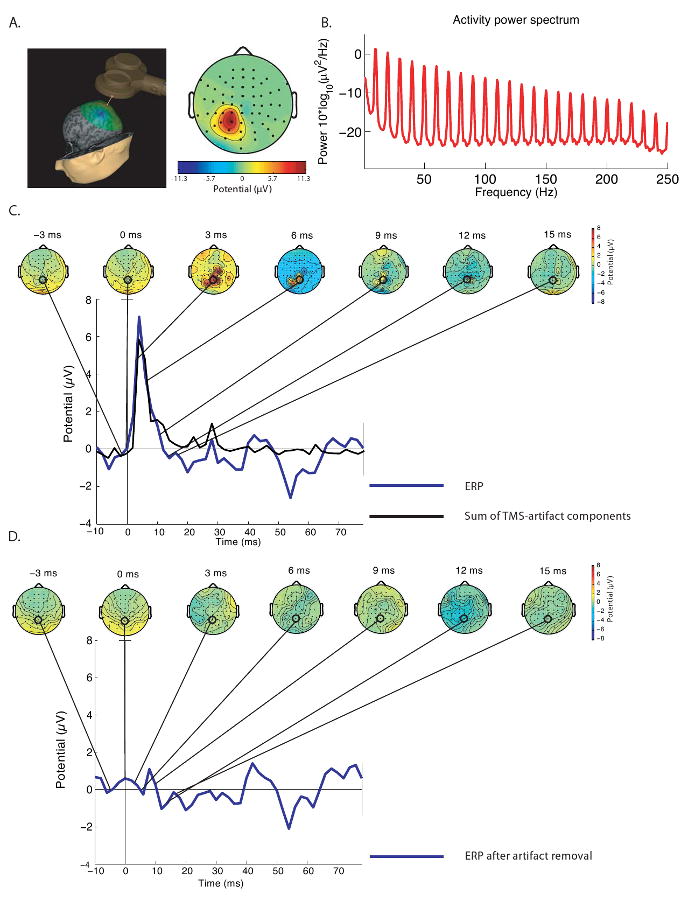
Representative TMS-artifact component identified by ICA. A) This panel illustrates that a typical artifact was limited to the electrodes immediately below or next to the location of stimulation. In this example, the SPL was targeted (near electrode P1). B) Power spectrum of the artifact reveals a strong, sharp peak at 10 Hz and corresponding harmonics. C and D illustrate the effectiveness of ICA in removing TMS-related EEG artifacts for a representative subject. Shown are scalp topographies time-locked to the onset of the TMS pulse before (C) and after (D) removal of 7 ICA components representing the TMS-related artifact. Also shown is the summed time course of activity of these 7 artifact components (black line, C), as well as the TMS-induced evoked responses after artifact removal (blue line, D). As can be seen in this figure, the artifact was most prominent in the first 10 ms after the TMS pulse.
For several subjects, ICA also produced one or more components that seemed to contain elements of both neurophysiological data and TMS artifact. If such a component was identified after the first ICA, it was kept for further analysis, with the idea that the second ICA may separate the two. If such components were still present after the second ICA step, they were then discarded. Although removal of physiologically relevant data with removal of ICA components is concerning, it should be noted that relatively few components that included both TMS artifact and physiological activity were removed. Of further importance, these components typically explained relatively little variance in the data (see Results).
ERP Analysis
To examine whether TMS-evoked brain responses changed as a function of pulse position in the train, we used the global field power (GFP; 49). GFP indexes the overall strength of the electric field of evoked potentials and is computed as the square root of the sum of squares of the average-referenced activity over all channels.
As a first analysis, we collapsed the data across time by averaging the GFP across the 100-ms time window after each pulse (GFPmean). This allowed us to assess changes in the amplitude of rTMS-evoked ERPs without making any assumptions about their temporal or topographic distribution. GFPmean values were calculated both for the ICA artifact-corrected data and for data for which ICA components (including those representing TMS artifacts) were not removed. In this way, we could examine whether the ICA procedure removed physiologically important information that may have affected our results. Subsequent analyses looked at the temporal pattern of the GFP after each pulse and more specifically determined the timing of rTMS-related changes in brain activity. GFPmean and GFP values were submitted to separate repeated measures analyses of variance (ANOVAs) with pulse number as a within-subject variable. Whenever a significant main effect of pulse number was found, polynomial tests were run to assess whether evoked potential amplitudes changed as a function of pulse number. Additionally, to determine if there are differences in the scalp distribution of the responses to SPL versus PCG rTMS, we performed a 2-way (Electrode × rTMS target) ANOVA on vector normalized measured potentials (50).
Results
TMS artifact removal
Prior to ICA artifact removal, of the 192 trials collected per brain area, an average (±S.D.) of 12.3±8.0% was removed due to large movement-related artifacts. Overall, of a mean of 55.0±4.0 components produced by ICA, an average of 7.4±3.4 TMS artifact-related components were identified and removed after the first round of ICA. The second round of ICA led to the removal of an additional 5.8±2.2 TMS artifact components. An average of 1.6±1.4 components that contained both physiological data and TMS artifact were removed. These components explained 13.9±16.7% of the variance of the epoched dataset.
Response during rTMS
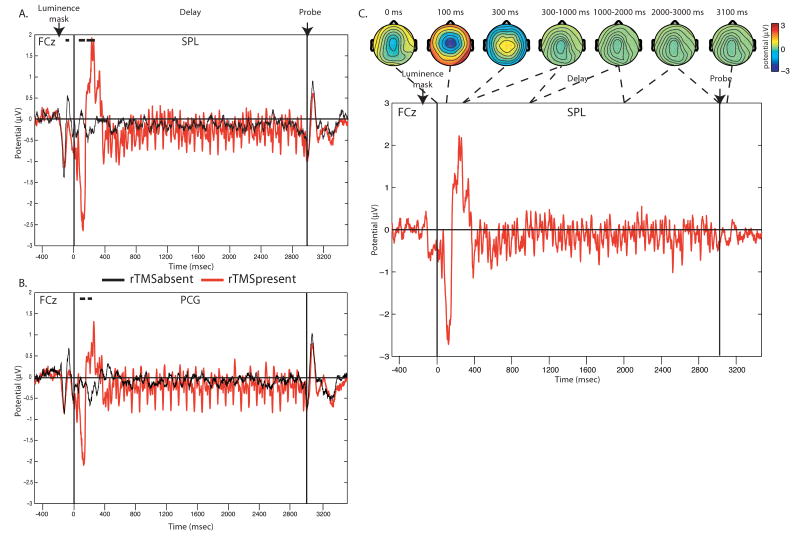
Figure 2 shows the effects of rTMS on brain activity during the delay period of the task. As can be seen in this figure, both when SPL and PCG were targeted, the onset of the rTMS train was associated with an early negativity around 100 ms, followed by a broader positivity around 200-400 ms that was not present in rTMSabsent trials. These responses were strongest over midline frontal scalp regions (Fig. 2c). The fact that this initial volley of responses was maximal over sites that were not directly under the TMS coil and did not vary as a function of stimulation site indicates that it may reflect processes related to a general orienting response to the onset of the rTMS train. Following this initial sequence of responses, the delivery of each pulse in the rTMS train was associated with a specific pattern of evoked potentials, which depended on the site of stimulation (described in detail below). We chose to only focus on brain responses elicited after the 4th pulse, to avoid contamination of our analysis by the initial orienting response. In addition, the 30th pulse was excluded from our analyses, because the brain response to this last pulse overlapped with the brain response elicited by the onset of the probe stimulus that was presented immediately after the delay period ended.
Figure 2.
Delay-period neuronal activity, averaged across all subjects, at electrode FCz in the absence (black line) and presence (red line) of 10 Hz rTMS, shown separately for SPL (A) and PCG (B) stimulation. As can be seen, the onset of the rTMS train was associated with a strong negativity around 100 ms, followed by a positivity between 200 and 400 ms. Lines above the graph indicate time period for which the rTMSpresent – rTMSabsent contrast revealed a significant difference in neuronal activity (paired t-test, p<0.05) for at least 20 consecutive samples (40 ms). (C) The difference in delay-period activity in rTMSpresent vs. rTMSabsent trials with scalp topographies (shown for SPL rTMS).
ERP as a function of pulse number
Collapsed across time
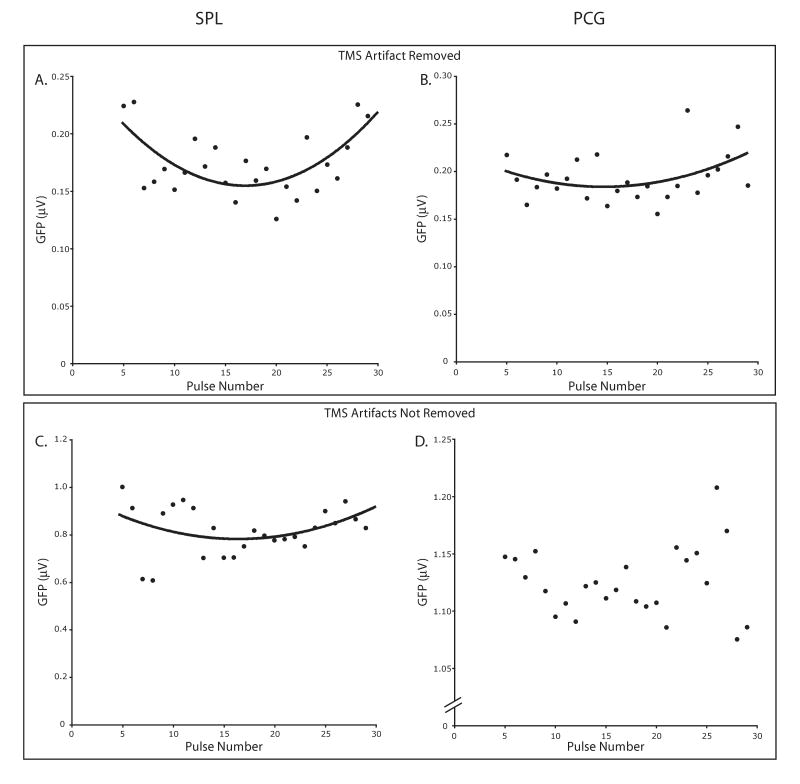
We first examined changes in the amplitude of rTMS-evoked ERPs without making any assumptions about the temporal or topographic distribution of the response using GFPmean. When rTMS was applied to the SPL, the GFPmean elicited by each pulse showed a significant effect of pulse number [F(24)=4.09; p<0.0001]. This was driven by a significant quadratic trend [F(1,15)=20.11; p<0.0005], reflecting a dip in amplitude halfway through the train (Fig. 3a). When the PCG was targeted, a significant effect of pulse number on GFPmean was observed [F(24)=6.28; p<0.0001]. Here, too, the data showed a dip in response amplitude halfway through the rTMS train as indexed by a significant quadratic trend [F(1,15)=7.75; p<0.05; Fig. 3b].
Figure 3.
Mean changes in the amplitude of TMS pulse-evoked brain responses across the rTMS train with and without removal of ICA components. Shown are GFPmean values averaged across the 100-ms time window after each pulse for pulses 5-29. A and B show GFPmean values after removal of ICA components representing TMS-related artifacts for SPL and PCG rTMS, respectively. C and D represent the same data without the removal of these components. Significant quadratic relationships between pulse number and GFPmean amplitude are displayed.
It is possible that the removal of TMS-artifacts using ICA may have affected our measurement of the rTMS-evoked physiological responses. Therefore, we repeated the above analysis on data for which ICA components were not removed. Because the amplitude of the TMS-related artifact should be identical for each pulse, any changes in GFPmean as a function of pulse number should be due to differences in physiological response. The effects of rTMS on GFPmean values calculated for the uncorrected data, though more noisy, were very similar to those found after artifact correction. When the SPL was targeted, the uncorrected data also showed a significant effect of pulse number [F(24)=3.72; p<0.0001], driven by a quadratic relationship between pulse number and GFPmean [F(1,15)=18.46; p<0.001; Fig. 3c]. With PCG rTMS, we again found a significant main effect of pulse number [F(24)=2.16; p<0.005]. This was driven by a marginally significant tertiary relationship between GFPmean and pulse number [F(1,15)=3.67; p=0.07; Fig. 3d].
In addition to these relatively fast effects of rTMS on evoked brain activity, which were measured within the 3-second delay period, it was also possible that rTMS had more slowly developing physiological effects. To test for this possibility, we looked at changes in the GFPmean across the 8 task blocks into which the experimental session was divided. Specifically, GFPmean values were averaged across the 360 pulses present in each task block. These values were entered as dependent variables in repeated measure ANOVAs that assessed effects of task block on rTMS-evoked brain activity, separately for SPL and PCG rTMS. For both SPL and PCG rTMS, we found no significant effect of task block (both Fs<1.34). We repeated this analysis with the same data, but without removal of ICA components and once again found no significant effect of task block on GFPmean amplitude for either SPL or PCG (both Fs<1.12). These findings are not indicative of more slowly developing effects of rTMS on neural function.
Temporal pattern of the response
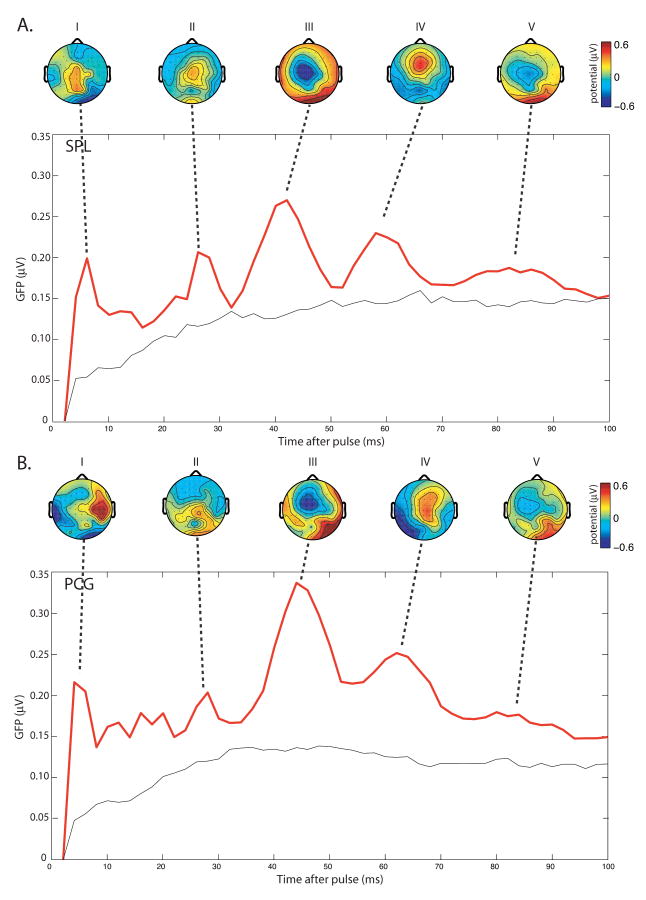
As described above, we found that the GFPmean changed quadratically with pulse number. To more precisely determine the timing of these rTMS-related effects, we next looked at the temporal pattern of the TMS-evoked response after each pulse. As can be seen in Fig. 4, both SPL and PCG rTMS elicited a sequence of brain responses as reflected by the different peaks in the GFP function. These peaks are indicative of high variance between channels and reflect a maximum of the total underlying brain activity that contributes to the surface potential field (49). For both areas targeted, five prominent GFP peaks (Peaks I-V) were identified. The five peaks occurred at approximately 4, 26, 42, 60, and 84 ms post-pulse. The scalp topographies of the ERPs corresponding to each peak are also displayed in Fig. 4. With the SPL targeted, Peak I was maximal at approximately the site of stimulation (near electrode Pz). With PCG targeted, Peak I was maximal at electrode C6, contralateral to the site of stimulation. The scalp topographies corresponding to each subsequent peak appeared similar for SPL and PCG rTMS, with maximal activity over central scalp regions. However, analyses directly comparing the scalp topographies of the different potential peaks between SPL and PCG rTMS revealed significant differences in evoked responses. Specifically, the spatial distribution of the first and fourth potential evoked by SPL and PCG rTMS were significantly different [Peak I: F(59,885)=2.11; p<0.0001; Peak IV: F(59,885)=1.65; p<0.005].
Figure 4.
Mean spatio-temporal pattern of brain potentials evoked by rTMS. GFP waveforms computed over 100 ms, averaged across subjects, are shown separately for rTMS to the SPL (A) and PCG (B), averaged across pulses 5-29. For each GFP peak, the corresponding scalp topography is plotted.
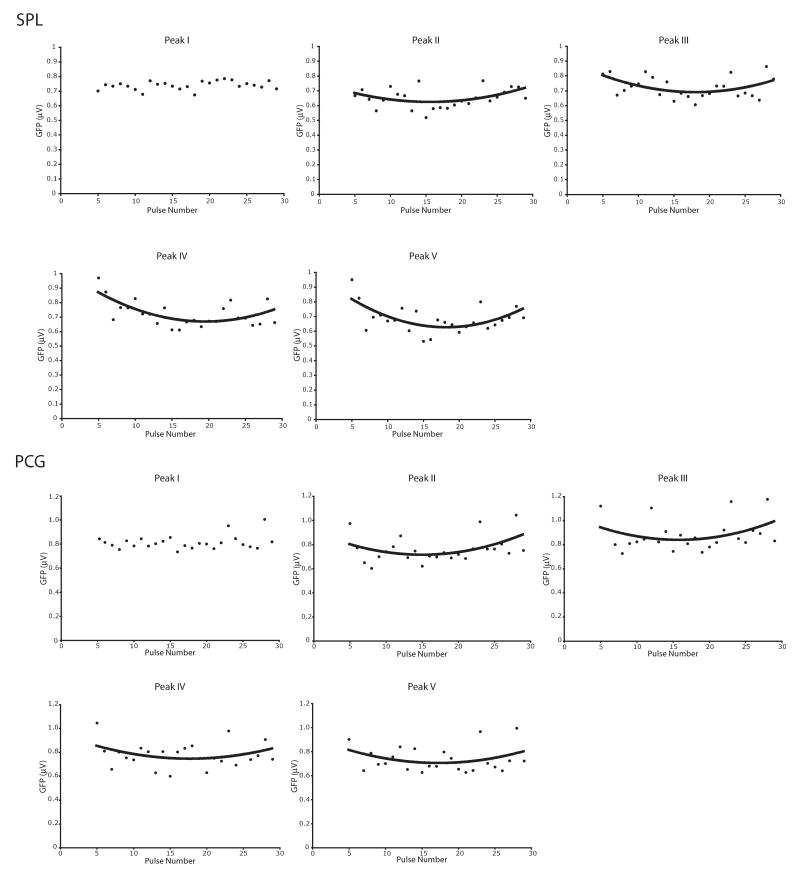
To determine whether there is a relationship between pulse number and TMS-evoked brain responses, for each subject and peak separately, we first calculated mean GFP using a 3-sample (6-ms) window around the peak. These values were entered as dependent variables into a repeated-measures ANOVA with pulse number as a within-subject variable, separately for each GFP peak and target site. Fig. 5 plots the mean amplitude of each GFP peak for each pulse, peak and target site separately. As can be seen from this figure, except for peak I, GFP amplitudes generally changed across the pulse train in a quadratic manner, similar to GFPmean amplitudes. These rTMS effects are described in more detail in Table 1.
Figure 5.
Effects of pulse number in the rTMS train on TMS-evoked brain response amplitudes. Shown are GFP peak amplitudes for pulses 5 to 29 in the rTMS train, separately for SPL (top panel) and PCG (bottom panel) rTMS. With the exception of Peak I, for both SPL and PCG rTMS a quadratic relationship between pulse number and peak amplitude is evident for all peaks.
Table 1.
Relationship between GFP amplitude and pulse number reported separately for each peak in the TMS-evoked response.
| Brain Area Targeted | Peak Number | Effect of pulse number F(24) | Polynomial relationship between peak amplitude and pulse number |
|---|---|---|---|
| SPL | I | 0.66; n.s. | None |
| II | 1.56; p<0.05 | Quadratic; F(1,15) = 10.93; p<0.005 | |
| III | 1.52; p=0.06 | Quadratic; F(1,15) = 6.26; p<0.05 | |
| IV | 2.56; p<0.0001 | Linear; F(1,15) = 4.71; p=0.05 Quadratic; F(1,15) = 12.50; p<0.005 |
|
| V | 2.37; p<0.0005 | Quadratic; F(1,15) = 8.97; p<0.01 | |
| PCG | I | 2.11; p<0.005 | None |
| II | 3.31; p<0.0001 | Quadratic; F(1,15) = 7.90; p<0.05 | |
| III | 3.65; p<0.0001 | Quadratic; F(1,15) = 4.21; p=0.06 | |
| IV | 2.91; p<0.0001 | Quadratic; F(1,15) = 3.00; p=0.10 | |
| V | 3.15; p<0.0001 | Quadratic; F(1,15) = 3.46; p=0.08 |
Discussion
The current study examined the neurophysiological effects of high-frequency rTMS using simultaneous EEG recordings. Our data show that ICA can provide an effective method for removal of TMS-related artifact from EEG data with minimal effect on the measurement of neuronal responses. We found that TMS-evoked brain responses were affected by high-frequency repetitive stimulation, with most evoked responses decreasing in amplitude across the first few pulses, then increasing for the remaining pulses of the rTMS train. This novel finding suggests that rTMS evokes a series of neuronal events that evolves as a function of recent stimulation history.
TMS artifact removal
The current data illustrate the usefulness of ICA in removing TMS-related artifacts from EEG data. ICA successfully identified TMS artifacts, which generally occurred directly below the coil, and within 10-15 ms of pulse delivery. After artifact removal we observed the stereotypical sequence of brain potentials after each TMS pulse in the train that has been described in previous, single-pulse TMS studies (14, 15, 22). Nevertheless, it is possible that our method for artifact removal also removed some brain activity. ICA is a statistical algorithm that is useful for removing artifacts that are linearly and independently mixed with signals of interest. Thus, because the TMS artifact and the immediate brain response induced by TMS are highly temporally correlated, ICA may not be able to separate the two sources. Although we did identify and remove components that seemed to contain both artifact and neuronal activity, relatively few such components were identified, and these components explained little variance in the data. Furthermore, analysis of the data without artifact removal revealed, though less clearly, the same general pattern of responses as when the artifact was removed with ICA. We thus believe that ICA can remove TMS-related artifacts with only minimal effects on the measurement of neuronal responses. It is important to note, however, that this method is not likely to remove TMS-related neuronal activity that is less well defined in terms of timing and scalp topography and that, in some contexts, may also be considered artifactual (such as the brain response associated with TMS-related auditory stimuli). Inclusion of a control, such as a controlled auditory stimulation condition during rTMSabsent trials, may be useful in this respect. Nonetheless, the ICA method allows for detection of subtle effects of rTMS on evoked EEG response, as well as performance of more complex analyses (i.e. spectral analysis) without contamination by the artifact. It should be noted that it may be necessary to run ICA twice, to ensure that ICA will also identify relatively weak TMS artifacts.
Effect of repetitive pulses on TMS-evoked ERPs
After an initial orienting response, each pulse in the rTMS train elicited a sequence of ERPs that were very similar both in time course and scalp topography across the rTMS train. However, for both brain areas targeted (SPL and PCG), we observed a quadratic relationship between the amplitude of evoked response and pulse number, characterized by a dip in the response halfway through the rTMS train. Except for the initial peak after the TMS pulse, this pattern was observed for each peak of the TMS-related ERP. These data extend findings of several previous studies that have reported enhanced MEPs immediately after the delivery of a sequence of pulses (e.g., 28, 51), by showing that rTMS may modulate pulse-to-pulse neural excitability during the stimulation train. Studies with paired-pulse delivery of TMS have shown that the physiological response to a pulse is affected by the delivery of a prior pulse, even with an inter-pulse interval of several hundred milliseconds (33, 52). The pattern of results observed in the present study corroborates these findings and shows that the physiological response to a pulse delivered in a high-frequency train of pulses also depends on the number of pulses that have previously been delivered. Together, these data suggest that the physiological effects of rTMS are more complex than simple linear summation of the neural response. Furthermore, they provide additional support for the notion that rTMS has more complex effects than production of a “virtual lesion” with induction of neural noise (10).
The current findings also demonstrate that, as with single-pulse TMS (15, 16), the effects of rTMS are not limited to the time period immediately after the pulse or localized to a circumscribed brain area. Thus, rTMS-related improvements in behavior (7-9) may be better explained by more electrophysiological changes involving multiple brain areas, rather than simple inhibition or potentiation of localized neural activity.
The neurophysiological processes underlying TMS-evoked brain responses are not well understood (53), although there is some evidence that these responses reflect the immediate change in current induced by the magnetic pulse (16). One can therefore only speculate about the cellular basis of the observed quadratic pattern in the TMS-evoked response across our train of 30 pulses. There are at least two possible explanations for the observed effects. One is that rTMS leads to both depression and potentiation. This is conceivable given prior work with repeated stimulation showing dynamic patterns of synaptic response due to an interaction between synaptic depression and potentiation (54). Notably, Mongillo et al. (55) described a role for residual calcium levels in transient synaptic potentiation. Their model proposes an interaction between the decrease of available resources with each spike (leading to depression of response) and an increase in synaptic efficacy associated with an increase in residual synaptic calcium levels with each spike. Thus, the pattern of the amplitude of the synaptic response with each spike is determined by the time constants relating to these two opposing effects. There has been at least one experimental demonstration that the time constant for the depressing effect is lower than that of the potentiating effect (which was found to be around 1 sec.; 54), a pattern that, at least qualitatively, fits the timing of our findings of initial depression followed by subsequent enhancement of the TMS-evoked response.
A second explanation for the pattern of findings described in this study is that because TMS affects a large population of neurons, it is possible that the initial decrease and subsequent increase in response amplitudes reflect stimulation of two different groups of cell types. Specifically, it is possible that the initial depression of ERP amplitudes was due to a depression of response in excitatory synapses, whereas the subsequent enhancement of ERP amplitudes was due to a delayed depression of the response in inhibitory synapses (or similarly an initial potentiation of inhibition followed by later potentiation of excitation). In line with this possibility, Quartarone et al. (56) suggest that rTMS may affect specific subpopulations of neurons differently, an effect that cannot be distinguished by EEG due to its relatively low spatial resolution.
A question that remains for future studies is whether the pulse-to-pulse changes in rTMS response depend on the frequency of stimulation. Is there something specific about the timing of 10-Hz rTMS that leads to the observed changes in amplitude with subsequent TMS pulses? Based on previous paired-pulse TMS studies showing that neuronal excitability is highly dependent on the inter-pulse interval, one may predict that the timing between pulses (and hence frequency of rTMS) will affect cortical excitability from one pulse to the next. Future studies are necessary to examine the effects of stimulation frequency and other parameters, such as intensity or number of stimuli delivered, on brain activity, and may allow for a further characterization of the effects of rTMS on brain function.
Conclusions
We found that TMS-evoked brain responses were affected by repetitive stimulation, with an initial depression of the TMS ERP followed by a late potentiation. This finding suggests that 10-Hz rTMS may evoke multiple cellular mechanisms. Furthermore, this study showed that ICA provides a relatively simple and effective method for TMS-related artifact removal from EEG data.
Acknowledgments
We would like to thank Lawrence Greischar, Alex Shackman and Michael Kruepke for their expert help. This work also benefited from discussions with Eva Feredoes and Marcello Massimini, and was supported by NIH grants MH078705 (M.H.) and MH064498 (B.R.P.) and by NARSAD (G.T.).
Footnotes
As measured with an electromyogram at the first dorsal interosseus muscle. Furthermore, minor adjustments to the stimulation intensity were made to correct for scalp-to-cortex distance differences between each target brain area and the motor cortex (43).
Financial Disclosures: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Terao Y, Ugawa Y. Studying higher cerebral functions by transcranial magnetic stimulation. Suppl Clin Neurophysiol. 2006;59:9–17. doi: 10.1016/s1567-424x(09)70006-6. [DOI] [PubMed] [Google Scholar]
- 2.Cowey A, Walsh V. Tickling the brain: studying visual sensation, perception and cognition by transcranial magnetic stimulation. Prog Brain Res. 2001;134:411–25. doi: 10.1016/s0079-6123(01)34027-x. [DOI] [PubMed] [Google Scholar]
- 3.Pascual-Leone A, Walsh V, Rothwell J. Transcranial magnetic stimulation in cognitive neuroscience--virtual lesion, chronometry, and functional connectivity. Curr Opin Neurobiol. 2000;10(2):232–7. doi: 10.1016/s0959-4388(00)00081-7. [DOI] [PubMed] [Google Scholar]
- 4.Walsh V, Rushworth M. A primer of magnetic stimulation as a tool for neuropsychology. Neuropsychologia. 1999;37(2):125–35. [PubMed] [Google Scholar]
- 5.Pascual-Leone A, Bartres-Faz D, Keenan JP. Transcranial magnetic stimulation: studying the brain--behaviour relationship by induction of ‘virtual lesions’. Philos Trans R Soc Lond B Biol Sci. 1999;354(1387):1229–38. doi: 10.1098/rstb.1999.0476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sack AT, Linden DEJ. Combining transcranial magnetic stimulation and functional imaging in cognitive brain research: possibilities and limitations. Brain Res Rev. 2003;43(1):41–56. doi: 10.1016/s0165-0173(03)00191-7. [DOI] [PubMed] [Google Scholar]
- 7.Klimesch W, Sauseng P, Gerloff C. Enhancing cognitive performance with repetitive transcranial magnetic stimulation at human individual alpha frequency. Eur J Neurosci. 2003;17(5):1129–33. doi: 10.1046/j.1460-9568.2003.02517.x. [DOI] [PubMed] [Google Scholar]
- 8.Luber B, Kinnunen LH, Rakitin BC, Ellsasser R, Stern Y, Lisanby SH. Facilitation of performance in a working memory task with rTMS stimulation of the precuneus: Frequency- and time-dependent effects. Brain Res. 2007;1128(1):120–9. doi: 10.1016/j.brainres.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 9.Hamidi M, Tononi G, Postle BR. Evaluating frontal and parietal contributions to spatial working memory with repetitive transcranial magnetic stimulation. Brain Res. 2008;1230:202–10. doi: 10.1016/j.brainres.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silvanto J, Muggleton NG. New light through old windows: Moving beyond the “virtual lesion” approach to transcranial magnetic stimulation. NeuroImage. 2008;39(2):549–52. doi: 10.1016/j.neuroimage.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 11.Ferrarelli F, Haraldsson HM, Barnhart TE, Roberts AD, Oakes TR, Massimini M, et al. A [17F]-fluoromethane PET/TMS study of effective connectivity. Brain Res Bull. 2004;64(2):103–13. doi: 10.1016/j.brainresbull.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 12.Sack AT, Kohler A, Bestmann S, Linden DEJ, Dechent P, Goebel R, et al. Imaging the Brain Activity Changes Underlying Impaired Visuospatial Judgments: Simultaneous fMRI, TMS, and Behavioral Studies. Cereb Cortex. 2007;17(12):2841–52. doi: 10.1093/cercor/bhm013. [DOI] [PubMed] [Google Scholar]
- 13.Bestmann S, Baudewig J, Siebner HR, Rothwell JC, Frahm J. BOLD MRI responses to repetitive TMS over human dorsal premotor cortex. NeuroImage. 2005;28(1):22–9. doi: 10.1016/j.neuroimage.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 14.Massimini M, Ferrarelli F, Huber R, Esser SK, Singh H, Tononi G. Breakdown of Cortical Effective Connectivity During Sleep. Science. 2005;309(5744):2228–32. doi: 10.1126/science.1117256. [DOI] [PubMed] [Google Scholar]
- 15.Bonato C, Miniussi C, Rossini PM. Transcranial magnetic stimulation and cortical evoked potentials: A TMS/EEG co-registration study. Clin Neurophysiol. 2006;117(8):1699–707. doi: 10.1016/j.clinph.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 16.Komssi S, Kahkonen S, Ilmoniemi RJ. The effect of stimulus intensity on brain responses evoked by transcranial magnetic stimulation. Human Brain Mapping. 2004;21(3):154–64. doi: 10.1002/hbm.10159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mottaghy FM, Krause BJ, Kemna LJ, Topper R, Tellmann L, Beu M, et al. Modulation of the neuronal circuitry subserving working memory in healthy human subjects by repetitive transcranial magnetic stimulation. Neurosci Lett. 2000;280(3):167–70. doi: 10.1016/s0304-3940(00)00798-9. [DOI] [PubMed] [Google Scholar]
- 18.Siebner HR, Peller M, Willoch F, Minoshima S, Boecker H, Auer C, et al. Lasting cortical activation after repetitive TMS of the motor cortex: A glucose metabolic study. Neurology. 2000;54(4):956–63. doi: 10.1212/wnl.54.4.956. [DOI] [PubMed] [Google Scholar]
- 19.Fuggetta G, Pavone EF, Fiaschi A, Manganotti P. Acute modulation of cortical oscillatory activities during short trains of high-frequency repetitive transcranial magnetic stimulation of the human motor cortex: A combined EEG and TMS study. Human Brain Mapping. 2008;29(1):1–13. doi: 10.1002/hbm.20371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brignani D, Manganotti P, Rossini PM, Miniussi C. Modulation of cortical oscillatory activity during transcranial magnetic stimulation. Human Brain Mapping. 2008;29(5):603–12. doi: 10.1002/hbm.20423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Virtanen J, Ruohonen J, Naatanen R, Ilmoniemi RJ. Instrumentation for the measurement of electric brain responses to transcranial magnetic stimulation. Med Biol Eng Comput. 1999;37(3):322–6. doi: 10.1007/BF02513307. [DOI] [PubMed] [Google Scholar]
- 22.Kahkonen S, Komssi S, Wilenius J, Ilmoniemi RJ. Prefrontal transcranial magnetic stimulation produces intensity-dependent EEG responses in humans. NeuroImage. 2005;24(4):955–60. doi: 10.1016/j.neuroimage.2004.09.048. [DOI] [PubMed] [Google Scholar]
- 23.Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64(1):355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]
- 24.Hempel CM, Hartman KH, Wang XJ, Turrigiano GG, Nelson SB. Multiple Forms of Short-Term Plasticity at Excitatory Synapses in Rat Medial Prefrontal Cortex. J Neurophysiol. 2000;83(5):3031–41. doi: 10.1152/jn.2000.83.5.3031. [DOI] [PubMed] [Google Scholar]
- 25.Saviane C, Savtchenko LP, Raffaelli G, Voronin LL, Cherubini E. Frequency-dependent shift from paired-pulse facilitation to paired-pulse depression at unitary CA3-CA3 synapses in the rat hippocampus. J Physiol. 2002;544(2):469–76. doi: 10.1113/jphysiol.2002.026609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abbott LF, Varela JA, Sen K, Nelson SB. Synaptic Depression and Cortical Gain Control. Science. 1997;275(5297):221–4. doi: 10.1126/science.275.5297.221. [DOI] [PubMed] [Google Scholar]
- 27.Hamada M, Hanajima R, Terao Y, Arai N, Furubayashi T, Inomata-Terada S, et al. Origin of facilitation in repetitive, 1.5-ms interval, paired pulse transcranial magnetic stimulation (rPPS) of the human motor cortex. Clin Neurophysiol. 2007;118(7):1596–601. doi: 10.1016/j.clinph.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 28.Di Lazzaro V, Oliviero A, Berardelli A, Mazzone P, Insola A, Pilato F, et al. Direct demonstration of the effects of repetitive transcranial magnetic stimulation on the excitability of the human motor cortex. Exp Brain Res. 2002;144:549–53. doi: 10.1007/s00221-002-1106-9. [DOI] [PubMed] [Google Scholar]
- 29.Esser SK, Huber R, Massimini M, Peterson MJ, Ferrarelli F, Tononi G. A direct demonstration of cortical LTP in humans: A combined TMS/EEG study. Brain Res Bull. 2006;69(1):86–94. doi: 10.1016/j.brainresbull.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 30.Fitzgerald PB, Fountain S, Daskalakis ZJ. A comprehensive review of the effects of rTMS on motor cortical excitability and inhibition. Clin Neurophysiol. 2006;117:2584–96. doi: 10.1016/j.clinph.2006.06.712. [DOI] [PubMed] [Google Scholar]
- 31.Romeo S, Gilio F, Pedace F, Ozkaynak S, Inghilleri M, Manfredi M, et al. Changes in the cortical silent period after repetitive magnetic stimulation of cortical motor areas. Exp Brain Res. 2000;135(4):504–10. doi: 10.1007/s002210000541. [DOI] [PubMed] [Google Scholar]
- 32.Berardelli A, Rona S, Inghilleri M, Manfredi M. Cortical inhibition in Parkinson's disease: A study with paired magnetic stimulation. Brain. 1996;119(1):71–7. doi: 10.1093/brain/119.1.71. [DOI] [PubMed] [Google Scholar]
- 33.Daskalakis ZJ, Farzan F, Barr MS, Maller JJ, Chen R, Fitzgerald PB. Long-Interval Cortical Inhibition from the Dorsolateral Prefrontal Cortex: A TMS-EEG Study. Neuropsychopharmacology. 2008;33:2860–9. doi: 10.1038/npp.2008.22. [DOI] [PubMed] [Google Scholar]
- 34.Daskalakis ZJ, Moller B, Christensen BK, Fitzgerald PB, Gunraj C, Chen R. The effects of repetitive transcranial magnetic stimulation on cortical inhibition in healthy human subjects. Exp Brain Res. 2006;174(3):403–12. doi: 10.1007/s00221-006-0472-0. [DOI] [PubMed] [Google Scholar]
- 35.Postle BR, Ferrarelli F, Hamidi M, Feredoes E, Massimini M, Peterson M, et al. Repetitive Transcranial Magnetic Stimulation Dissociates Working Memory Manipulation from Retention Functions in the Prefrontal, but not Posterior Parietal, Cortex. J Cogn Neurosci. 2006;18(10):1712–22. doi: 10.1162/jocn.2006.18.10.1712. [DOI] [PubMed] [Google Scholar]
- 36.Fuggetta G, Pavone EF, Walsh V, Kiss M, Eimer M. Cortico-cortical interactions in spatial attention: A combined ERP/TMS study. J Neurophysiol. 2006;95:3277–80. doi: 10.1152/jn.01273.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thut G, Ives JR, Kampmann F, Pastor MA, Pascual-Leone A. A new device and protocol for combining TMS and online recordings of EEG and evoked potentials. J Neurosci Meth. 2005;141(2):207–17. doi: 10.1016/j.jneumeth.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 38.Litvak V, Komssi S, Scherg M, Hoechstetter K, Classen J, Zaaroor M, et al. Artifact correction and source analysis of early electroencephalographic responses evoked by transcranial magnetic stimulation over primary motor cortex. NeuroImage. 2007;37(1):56–70. doi: 10.1016/j.neuroimage.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 39.Morbidi F, Garulli A, Prattichizzo D, Rizzo C, Manganotti P, Rossi S. Off-line removal of TMS-induced artifacts on human electroencephalography by Kalman filter. J Neurosci Meth. 2007;162(1-2):293–302. doi: 10.1016/j.jneumeth.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 40.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(S20):22–33. [PubMed] [Google Scholar]
- 41.Hamilton M. A rating scale for depression. J Neurol, Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wasserman E. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5-7, 1996. Electroencephalogr Clin Neurophysiol. 1998;108:1–16. doi: 10.1016/s0168-5597(97)00096-8. [DOI] [PubMed] [Google Scholar]
- 43.Stokes MG, Chambers CD, Gould IC, Henderson TR, Janko NE, Allen NB, et al. A simple metric for scaling motor threshold based on scalp-cortex distance: Application to studies using transcranial magnetic stimulation. J Neurophysiol. 2005;94(6):4520–7. doi: 10.1152/jn.00067.2005. [DOI] [PubMed] [Google Scholar]
- 44.Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 45.Perrin F, Pernier J, Bertrand O, Echallier JF. Spherical splines for scalp potential and current density mapping. Electroencephalogr Clin Neurophysiol. 1989;72:184–7. doi: 10.1016/0013-4694(89)90180-6. [DOI] [PubMed] [Google Scholar]
- 46.Ilmoniemi RJ, Virtanen J, Ruohonen J, Karhu J, Aronen HJ, Naatanen R, et al. Neuronal responses to magnetic stimulation reveal cortical reactivity and connectivity. Neuroreport. 1997;8:3537–40. doi: 10.1097/00001756-199711100-00024. [DOI] [PubMed] [Google Scholar]
- 47.Crespo-Garcia M, Atienza M, Cantero J. Muscle Artifact Removal from Human Sleep EEG by Using Independent Component Analysis. Annals of Biomedical Engineering. 2008;36(3):467–75. doi: 10.1007/s10439-008-9442-y. [DOI] [PubMed] [Google Scholar]
- 48.Jung T, Makeig S, Humphries C, Lee T, McKeown MJ, Iragui V, et al. Removing electroencephalographic artifacts by blind source separation. Psychophysiology. 2000;37:163–78. [PubMed] [Google Scholar]
- 49.Lehmann D, Skrandies W. Spatial analysis of evoked potentials in man - a review. Prog Neurobiol. 1984;23(3):227–50. doi: 10.1016/0301-0082(84)90003-0. [DOI] [PubMed] [Google Scholar]
- 50.McCarthy G, Wood CC. Scalp distributions of event-related potentials: An ambiguity associated with analysis of variance models. Electroencephalogr Clin Neurophysiol. 1985;62:203–8. doi: 10.1016/0168-5597(85)90015-2. [DOI] [PubMed] [Google Scholar]
- 51.Takano B, Drzezga A, Peller M, Sax I, Schwaiger M, Lee L, et al. Short-term modulation of regional excitability and blood flow in human motor cortex following rapid-rate transcranial magnetic stimulation. NeuroImage. 2004;23(3):849–59. doi: 10.1016/j.neuroimage.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 52.Boroojerdi B, Kopylev L, Battaglia F, Facchini S, Ziemann U, Muellbacher W, et al. Reproducibility of intracortical inhibition and facilitation using the paired-pulse paradigm. Muscle Nerve. 2000;23:1594–7. doi: 10.1002/1097-4598(200010)23:10<1594::aid-mus19>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 53.Lioumis P, Kicic D, Savolainen P, Makela JP, Kahkonen S. Reproducibility of TMS-Evoked EEG responses. Human Brain Mapping. doi: 10.1002/hbm.20608. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang Y, Markram H, Goodman PH, Berger TK, Ma J, Goldman-Rakic PS. Heterogeneity in the pyramidal network of the medial prefrontal cortex. Nat Neurosci. 2006;9(4):534–42. doi: 10.1038/nn1670. [DOI] [PubMed] [Google Scholar]
- 55.Mongillo G, Barak O, Tsodyks M. Synaptic theory of working memory. Science. 2008;319:1543–6. doi: 10.1126/science.1150769. [DOI] [PubMed] [Google Scholar]
- 56.Quartarone A, Bagnato S, Rizzo V, Morgante F, Sant'Angelo A, Battaglia F, et al. Distinct changes in cortical and spinal excitability following high-frequency repetitive TMS to the human motor cortex. Exp Brain Res. 2005;161(1):114–24. doi: 10.1007/s00221-004-2052-5. [DOI] [PubMed] [Google Scholar]