Abstract
Although removal of dopamine inhibition is established as a major factor in prolactin (PRL) release, a large body of evidence suggests that hypothalamic oxytocin (OT) may serve as a PRL-releasing hormone in the rat. PRL release is modulated by estradiol (E2), which rises between diestrus and proestrus of the estrous cycle, causing a PRL surge in the afternoon of proestrus. Given that E2 strongly modulates OT actions in both central and peripheral tissues, OT action on lactotrophs might also be modulated by the stage of the estrous cycle. To test this hypothesis, we have monitored PRL release and intracellular calcium levels ([Ca2+]i) induced by OT in pituitary lactotrophs obtained from female rats in either diestrus 1 or proestrus. We found that both secretory and [Ca2+]i responses to OT are significantly increased in lactotrophs obtained on proestrus. Moreover, we show that these differences are due to an increase in both the number of OT-responding lactotrophs and the magnitude of their individual [Ca2+]i responses. Both secretory and [Ca2+]i responses were abolished by a specific OT antagonist. Finally, dose-dependent studies show that the increased PRL-releasing effect of OT on proestrus is significant over a wide range of concentrations, particularly those observed in hypophyseal portal plasma. These results suggest that the rising E2 titers that culminate on proestrus facilitate the stimulatory action of OT on lactotrophs and support the notion that OT is a PRL-releasing hormone with an important role in the production of the proestrous surge of PRL.
Oxytocin triggers calcium entry and prolactin secretion in lactotrophs; these responses are larger in cells obtained from proestrous rats compared to diestrous 1 rats.
Removal from the inhibitory influence of dopamine (DA) is well established as a major event inducing the release of prolactin (PRL) by anterior pituitary lactotrophs under various physiological conditions, including the proestrous surge of PRL (1,2,3,4). In addition, stimulation of lactotrophs by one or more hypothalamic PRL releasing factors such as the nonapeptide oxytocin (OT) seems to be necessary to generate PRL surges (5,6).
A well-described anatomic and pharmacological framework supports a physiological role for OT in the physiological regulation of PRL secretion. First, terminals from hypothalamic oxytocinergic neurons are found both in the posterior pituitary (7) and the external zone of the median eminence (8,9). OT is released at these terminals and is transported to the anterior pituitary through portal vessels (10,11). Second, there is a temporal correlation between OT release and PRL secretion under a variety of experimental conditions and physiological paradigms (12,13,14). The concentration of OT in pituitary portal blood reaches a peak in the afternoon of proestrus, at the onset of the proestrous surge of PRL (15). Third, a subpopulation of lactotrophs possesses OT receptors (16,17,18,19,20). Fourth, peripheral administration of OT results in PRL release (21,22), an effect mediated by pituitary lactotrophs because OT can stimulate PRL secretion from dissociated pituitary cells (12,21,23,24,25) and increase the intracellular calcium concentration ([Ca2+]i) of identified lactotrophs (23). Finally, passive immunoneutralization of endogenous OT and peripheral administration of OT antagonists inhibit PRL surges observed in various physiological conditions (12,26,27), including the proestrous PRL surge (28,29). These results support the idea that hypothalamic OT participates in the PRL surge of proestrus by directly stimulating pituitary lactotrophs.
The rise in estradiol (E2) concentration during the estrous cycle decreases the inhibitory influence of DA on lactotrophs and facilitates PRL release (6,30). Although these effects are essential for the generation of the proestrous PRL surge (31), the multiple mechanisms of the E2-induced facilitation of PRL release are not completely characterized. Because OT is needed for the proestrous PRL surge, E2 might sensitize lactotrophs to the stimulatory effect of OT. Here, we ask whether the responsiveness of the lactotrophs to OT is increased after exposure to rising levels of E2 between diestrus 1 and proestrus.
To answer this question, we compared in vitro OT-induced responses in PRL secretion and [Ca2+]i between lactotrophs obtained from rats on the morning of diestrus 1 and lactotrophs from the afternoon of proestrus.
Materials and Methods
Chemicals
OT was obtained from Bachem Bioscience Inc. (King of Prussia, PA). The selective OT antagonist desGly-NH2-d(CH2)5[d-Tyr2,Thr4]ornithine vasotocin was obtained from GenScript Corp. (Scotch Plains, NJ) (32). All other compounds were from Sigma Chemical Co. (St. Louis, MO) if not otherwise specified.
Animals
Adult female Sprague-Dawley rats (>60 d of age) weighing 250–300 g (Charles River Laboratories, Raleigh, NC) were kept in standard rat cages under a 12-h light, 12-h dark cycle (lights on at 0600 h) with water and rat chow available ad libitum. The stage of the estrous cycle was determined by daily vaginal smears, taken between 0800 and 1000 h. Based on these, rats were assigned to either proestrus, estrus, diestrus 1, or diestrus 2. Only rats showing at least two regular 4- to 5-d cycles were used for the study. Experiments were performed on diestrus 1 and proestrus. Rats in diestrus 1 were euthanized under CO2 by decapitation in the morning (before 1000 h), whereas those in proestrus were killed in the afternoon by the time of the preovulatory surge (1700 h). All animal procedures were approved by the Florida State University Animal Care and Use Committee.
Cell dispersion and culture
Pituitary cell dispersion was adapted from a tissue dissociation method of postnatal cortical neurons (33). Briefly, pituitaries were removed on ice, separated from the neurointermediate lobe, and placed in chambers containing freshly prepared Hanks’ balanced salt solution (HBSS) with 1.26 mm CaCl2 and 0.7 mm Mg2+ supplemented with 25 mm HEPES, 1% BSA, 100 U/ml penicillin, and 100 μg/ml streptomycin and adjusted to pH 7.35–7.40. Buffer was filtered through a Nalgene membrane (Fisher, Rochester, NY) with a pore diameter of 0.22 μm. Hypophyses were washed three times with supplemented HBSS and then cut in 1-mm pieces. Fragments were then placed into a glass vial containing 5 mg/ml papain (Worthington, Lakewood, NJ) and 5 μl/pituitary of 1 mg/ml deoxyribonuclease I (Worthington) in HBSS. Pituitary explants were digested for 45 min at 37 C with a shaking rate of 30 rpm. Afterward, fragments were dispersed into individual cells by gentle trituration through Pasteur pipettes. The resulting suspension was filtered through a 40-μm nylon gauze and centrifuged 10 min at 600 × g and 4 C in HBSS without calcium. Supernatant was discarded and the cell pellet immediately resuspended in HBSS containing 1 mg/ml ovomucoid protease inhibitor and BSA (Worthington). A discontinuous density gradient was prepared with a 10 mg/ml albumin-inhibitor solution, and the pituitary cell suspension was carefully laid on top. After centrifugation, the cell pellet was resuspended in medium 199 (M199) (Invitrogen, Carlsbad, CA) containing Earle’s salts, 0.7 mm glutamine, 2.2 g/l sodium bicarbonate, 10% fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin. An aliquot of cellular suspension was taken to quantify hypophyseal cell yield, using a Neubauer chamber. Viability of cells, determined by trypan blue exclusion, was 95% or higher. Anterior pituitary cells were cultured as mixed cells or enriched lactotrophs in M199. A Percoll discontinuous density gradient procedure was used to obtain enriched lactotrophs (see below), and their further identification in single-cell studies was achieved through the addition of TRH (34). Experiments were performed 1 d after harvesting. Pituitary cells retain their in vivo-imprinted physiological status when cultured in vitro (35,36,37,38,39).
Lactotroph enrichment
We used the purification protocol originally described in Ref. 40. Briefly, 2-ml layers of Percoll at densities of 70, 60, 50, and 35% (from bottom to top) were sequentially added to a 15-ml Falcon tube. Freshly dispersed pituitary cells (up to 8 million in 2 ml) were then placed on top of this gradient. After 20 min, centrifugation at 600 × g, the cells at the interface between the 50% and the 35% layers were washed in M199 and centrifuged for 10 min at 600 × g, resuspended, counted, plated on 1.5 glass coverslips (0.4 million per coverslip) in 35 mm Petri dishes and cultured for one day in M199 with 10% fetal bovine serum. The percentage of lactotrophs obtained after enrichment was estimated using immunofluorescence labeling of lactotrophs with a goat anti-PRL antibody (C-17, dilution 1:500; Santa Cruz Biotechnology, Santa Cruz, CA) followed by a mouse antigoat antibody conjugated with cy-3 (dilution 1:1000; Jackson ImmunoResearch Laboratories, West Grove, PA). About 70–85% of the cells in lactotroph-enriched cultures were PRL positive. By comparison, cultures from cells obtained at the interface between the 70 and 60% density layers (enriched in somatotrophs) (40) contained less than 10% of PRL-positive cells.
Measurements of PRL release from perifused pituitary cells
Hormone secretion was monitored using cell column perifusion experiments. Briefly, 4 × 106 anterior pituitary cells were incubated with preswollen Cytodex-1 beads in a 35-mm petri dishes for 18 h. The beads were then transferred to 0.5-ml chambers and perifused with HEPES-buffered saline solution (HBS) containing 25 mm HEPES, 138 mm NaCl, 5 mm KCl, 10 mm α-d-glucose, 0.7 mm sodium phosphate, 1 mm MgCl2, 2 mm CaCl2, 0.1% BSA, 100 U/ml penicillin, and 100 μg/ml streptomycin for 2.5 h at a flow rate of 0.5 ml/min and at 37 C to establish stable basal secretion. Fractions were collected in 1-min intervals, stored at −20 C, and later assayed for PRL content by RIA.
Rat PRL RIA
Concentrations of PRL were measured with a rat PRL RIA kit as previously described (41). Rat PRL standards and antiserum were supplied by Dr. Albert Parlow through the National Hormone and Pituitary Program (Torrance, CA). 125I was purchased from PerkinElmer Life Sciences (Shelton, CT) and used to prepare PRL tracer by the chloramine T method. Results are expressed in terms of PRL reference preparation RP-3. The lower limit of detection for PRL was 0.10 ng/ml. The intraassay coefficient of variation was 5%.
Measurements of [Ca2+]i in individual lactotrophs
After 1 d in culture, enriched lactotrophs were rinsed once with HBS (without BSA) and then incubated in HBS containing 2 μm fura-2-AM (Molecular Probes, Carlsbad, CA) for 40 min at 37 C. The cells were then rinsed three times with HBS, and the coverslip was transferred into a recording chamber (RC-40; Warner Instruments, Hamden, CT) (volume ∼0.4 ml) on the stage of an inverted microscope and continuously perfused with HBS at room temperature. Drugs (OT, 10−7 m; TRH, 10−7 m) were bath applied for periods of 2 min.
Recordings were started 15 min after loading with fura-2. Cells were illuminated every 2 sec with 340- and 380-nm light beams (15–50 msec exposure each) from a 175-W xenon light source (DG4; Sutter Instrument, Novato, CA). Light intensity was decreased by 90% before reaching the cells. Light focusing and imaging was through a ×40, 0.9 NA objective (Nikon Instruments, Melville, NY). The light passed through an emission filter centered around 510 nm (Chroma Technology, Rockingham, VT), and images were acquired with a 12-bit CCD camera set to 8 × 8 binning, controlled by TI Workbench software developed by T. Inoue. Regions of interest (ROI) were drawn around selected cells (healthy cells that were not superimposed on other cells), and one background ROI was drawn in an empty area. For each ROI, a ratio r was calculated by averaging pixel values within each ROI for each excitation wavelength and dividing the values obtained after background subtraction: r = (ROI340 − ROI-background340)/(ROI380 − ROI-background380).
Lactotrophs were identified as the TRH-responsive cells (42,43). To check the accuracy of this criterion, we identified the PRL-positive cells with immunofluorescence, as described above, after two experiments (141 cells). Fewer than 5% of TRH-responsive cells were not PRL positive. Moreover, only 2% of PRL-positive cells failed to respond to TRH. TRH was also used as a control response because of the well defined lactotroph response to TRH.
Calculations and statistical analyses
Hormone secretion data were plotted as representative traces from at least six independent experiments. Absolute PRL concentrations (in nanograms per milliliter) were plotted as a function of time (minutes). For the dose-response curve and comparative analysis, secretion data are expressed as the area under the curve (AUC) means ± sem. The AUC measured the first 6 min of the secretion response, and is defined as follows:
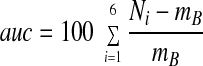 |
where Ni is the PRL concentration in fraction i and mB is the mean basal value during the six fractions that preceded the time of stimulation.
AUC values for each group (diestrus 1 and proestrus) were analyzed by the nonparametric Kruskal-Wallis test to evaluate significance of the dose-dependent effect within each group. All concentrations were independently tested at least six times, except for 10−2 and 10−3 m OT (n = 4), which were pooled together to obtain the saturation level. Concentration-dependent effects of OT on PRL release were further analyzed by nonlinear regression analysis, and dose-response curves were fitted to four-parameter sigmoid curves with ALLFIT 2.6 and GraphPad Prism 4 software, each producing comparable results. The curve that gave the highest R2 (regression coefficient) with the lowest possible Syx (sd of the residuals) was selected. The concentration at which the agonist displays half-maximal effect (EC50) is computed with its 95% confidence interval (CI).
Two-tailed comparisons of secretion responses for each agonist concentration and basal values between diestrous 1 and proestrous groups were performed by the nonparametric Wilcoxon test. Likewise, comparisons of the percentage of responding cells and amplitude of intracellular calcium responses between diestrus 1 and proestrus were assessed using the same test. Data are thus presented as box plots showing median, interquartile range, and full range. For all statistical comparisons, P < 0.05 was considered significant; however, exact P values are provided when appropriate.
Results
The PRL-secretory and Ca2+ response to OT differs in lactotrophs obtained from diestrous and proestrous rats
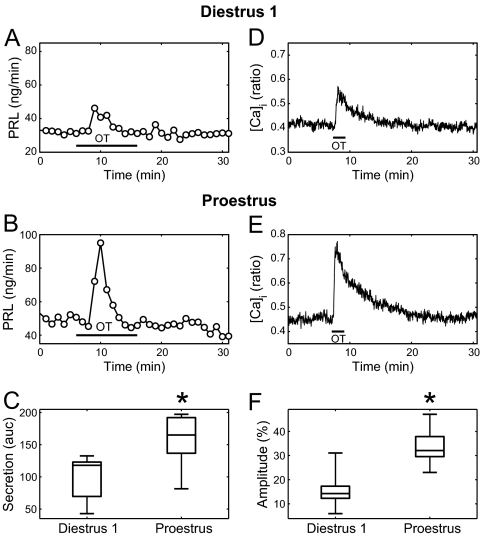
OT (100 nm) transiently stimulated PRL secretion in perifused anterior pituitary cells from both diestrus 1 (Fig. 1A) and proestrus animals (Fig. 1B); however, the PRL-releasing effect of OT in cells obtained from the latter group was much stronger. This was quantified by calculating the AUC (see Materials and Methods) of the response for 6 min after agonist application. We observed that the responses obtained with cells from proestrus animals were significantly larger than the responses from diestrus 1 animals (Fig. 1C, P < 0.01). Similarly, OT (100 nm) evoked increases of intracellular Ca2+ in lactotroph cells (identified as TRH-responding cells) obtained from both diestrous 1 (Fig. 1D) and proestrous (Fig. 1E) animals. The responses shown are averaged over all lactotrophs in a field of view. Consistent with our results in PRL secretion, the size of the Ca2+ response over the first 6 min was greater at proestrus (Fig. 1F, P < 0.0005).
Figure 1.
Comparison of the responses of lactotroph populations to OT at diestrus 1 and proestrus. A, OT application (100 nm for 10 min, horizontal bar) evokes a modest PRL increase in diestrus 1. Samples were collected every minute. B, OT application evokes a stronger PRL increase in proestrus. C, Box plots of the size of the PRL release in diestrus 1 (n = 7 experiments) and proestrus (n = 10). PRL release was normalized relative to the basal level and summed over 6 min (AUC). There is a significant difference between diestrus 1 and proestrus (*, P < 0.01). D, OT application (100 nm for 2 min, horizontal bar) evokes a modest [Ca2+]i increase on diestrus 1. Ratios were collected every 2 sec. E, OT application evokes a stronger [Ca2+]i increase on proestrus. F, Box plots of the size of the [Ca2+]i increase in diestrus 1 (n = 9) and proestrus (n = 9). Ratios were averaged over all TRH-responsive cells in a given experiment. The mean increase relative to baseline, over the first 6 min of this averaged response, was then computed and expressed as a percentage. Box plots show the median (middle bar), interquartile range (box), and range (whiskers). The difference between diestrus 1 and proestrus is significant (*, P < 0.0005).
Specificity of the PRL-secretory and Ca2+ response to OT
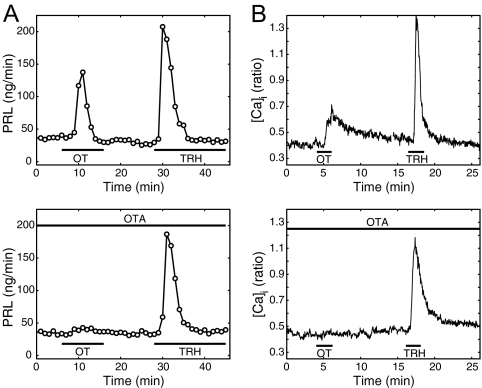
To ensure the specificity of the PRL and Ca2+ responses to OT, perifused anterior pituitary cells were stimulated with OT in the presence of desGly-NH2-d(CH2)5[d-Tyr2,Thr4]ornithine vasotocin, a selective OT receptor antagonist (OTA) (32), at a concentration of 100 μm. This concentration of OTA in blood circulation was sufficient to block PRL surges in ovariectomized (OVX) animals induced by either cervical stimulation (27) or by E2 injection (26). The presence of OTA abolished both release of PRL (Fig. 2A) and intracellular Ca2+ increase (Fig. 2B) in response to bath application of OT (100–1000 nm) but did not significantly affect the responses to TRH (Fig. 2). In additional studies, we observed that 1 μm OTA could still abolish the PRL-releasing effect of 100 nm OT (not shown). As additional evidence of the specificity of OT actions on lactotrophs, sub-micromolar concentrations of the related peptide arginine vasopressin did not evoke PRL release (not shown).
Figure 2.
Specificity of the response to OT. A, In control conditions (upper panel), both OT (1 μm) and TRH (100 nm) evoke an increase in the rate of PRL release. In the presence of the OTA (lower panel), there is no increase in PRL release after OT application (significantly different from the control response to OT, P < 0.03; n = 4). Samples were collected every minute. B, In control conditions (upper panel), both OT (100 nm) and TRH (100 nm) evoke an increase in intracellular free calcium ([Ca2+]i), measured as the fura-2 ratio. In the presence of OTA (lower panel), the calcium response to OT is abolished (average over 39 cells, response is significantly lower than in control, P < 0.0004). Cells were obtained from proestrus animals.
The physiological basis for the estrous cycle modulation of OT responses in lactotrophs
The increased response to OT on proestrus could be due to an increased fraction of lactotrophs responsive to the nonapeptide or an increase in their individual responses. To test these two possibilities, we compared the fraction of OT-responding lactotrophs and the size of their individual Ca2+ responses between diestrus 1 and proestrus.
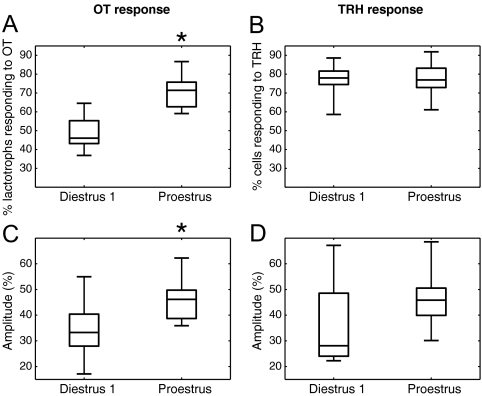
Figure 3A shows that the fraction of lactotrophs responding to OT was significantly greater on proestrus (P < 0.0005). Typically, just under 50% of the lactotrophs responded to OT on diestrus 1, but on proestrus, more than 70% were stimulated by the nonapeptide. The fraction of TRH-responsive cells in our lactotroph-enriched population was similar on diestrus 1 and proestrus (Fig. 3B, P > 0.85). Figure 3C shows that the OT-induced responses were greater in lactotrophs obtained on proestrus than diestrus 1. That is, the median response was larger in proestrous cells, as were the responses of the middle 50% of cells in the distribution (P < 0.015). By comparison, the individual responses to TRH were not significantly larger on proestrus (Fig. 3D, P > 0.13). Thus, both the fraction of lactotrophs responding to OT and the size of their individual responses is increased on proestrus.
Figure 3.
Comparison of the intracellular calcium responses of individual cells at diestrus 1 and proestrus. A, Fraction of lactotrophs (i.e. TRH-responding cells) responding to OT (100 nm) in lactotroph-enriched preparations. There is a significant difference between diestrus 1 and proestrus (*, P < 0.0005). B, Fractions of TRH-responding cells in lactotroph-enriched preparations. There is no significant difference between diestrus 1 and proestrus (P > 0.85). C, Response amplitude to OT (100 nm) relative to baseline, computed over the first 6 min for each individual OT-responding lactotroph and then averaged over the cells. The difference between diestrus 1 and proestrus is significant (*, P < 0.015). D, The response amplitude to TRH (100 nm) shows no significant difference between diestrus 1 and proestrus (P > 0.13). All panels were obtained from the same data set (diestrus 1, n = 9; proestrus, n = 9 experiments).
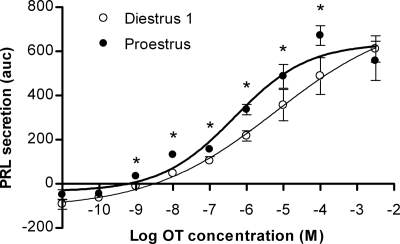
The findings above suggest that the PRL-releasing effect of OT in pituitary lactotrophs is modulated by the stage of the estrous cycle. To further address this hypothesis, we conducted dose-response experiments. Figure 4 shows the dose-response relationship for the PRL-releasing effects of OT in lactotrophs obtained at diestrus 1 and proestrus. OT stimulated PRL release from perifused cells in a dose-dependent manner (P < 0.0001) in both experimental groups, as previously reported for lactotrophs obtained from animals in different physiological states (12,25,28).
Figure 4.
Dose-response curves of the PRL-releasing effect of OT in perifused anterior pituitary cells from female rats during diestrus 1 (○) and proestrus (•). Results are shown as normalized AUC means ± sem from at least six independent experiments, except for 10−2 and 10−3 m OT (n = 4), which were pooled together to obtain the saturation level. *, Statistically significant differences (P < 0.05) between the two experimental groups for the same concentration of agonist. P values were as follows: OT 10−9 m, 0.012; 10−8 m, <0.0001; 10−7 m, 0.021; 10−6 m, 0.0031; 10−5 m, 0.027; 10−4 m, 0.022. Continuous lines represent the best fit curve for each experimental group. Regression curves were calculated and EC50 values determined as described in Materials and Methods: for diestrus 1, EC50 4.24 μm (95% CI = 2.79–6.44 μm), for proestrus, EC50 0.57 μm (95% CI = 0.16–2.03 μm). Mean basal values were 38.8 ± 1.3 and 51.1 ± 1.8 ng/min for diestrus 1 and proestrus, respectively (P < 0.0001).
When the PRL-releasing effect at each concentration of the nonapeptide was compared between the diestrous 1 and proestrous groups, it was found that the latter group evoked the greatest responses, and the difference was significant (two-tailed Wilcoxon test) over a wide range of OT concentrations, 1 nm through 100 μm (Fig. 4). At the lowest OT concentration in this range (1 nm), the PRL response was above the basal level only in cells from proestrus animals. This threshold OT concentration is close to estimates of the affinity of pituitary OT receptors from estrogen-treated female rats (19). For OT concentrations at saturation levels (above 100 μm), responses were no longer statistically different between the groups.
Nonlinear regression analysis further characterized the differential secretory response of lactotrophs to OT. Analysis showed that the OT-induced PRL release in lactotrophs obtained on diestrus 1 and proestrus could be best fit by two different (P < 0.0201) sigmoid curves (Fig. 4), with an estimated EC50 of 4.24 μm for diestrus 1 and 0.57 μm for proestrus. In the same conditions, TRH stimulated the secretion of PRL with an EC50 of 6.05 nm from the same cell cultures (not shown), consistent with previous reports (45).
Discussion
It has previously been shown that OT stimulates PRL secretion in vitro in cultures of rat anterior pituitary cells from males (21,24,28), randomly cycling and estrogen-primed females (25), and lactating and E2-primed OVX females (12). More recently, our lab has shown that bath application of OT to purified lactotrophs obtained from OVX female rats elevates intracellular Ca2+ levels and stimulates PRL secretion (23). The present work shows that the effects of OT on PRL secretion and intracellular Ca2+ are significantly greater in lactotrophs obtained from rats in proestrus than in diestrus 1. This is the first reported demonstration of a physiological modulation of the lactotroph sensitivity to the action of OT.
The anterior pituitary also expresses vasopressin V1b receptors (19), which can bind OT (46). However, arginine vasopressin at sub-micromolar concentrations failed to elicit a PRL response, as previously reported (21,47). Also, [Ca2+]i and secretion responses of lactotrophs to the nonapeptide were abolished by a highly specific OT antagonist. Although the relatively high concentration of OTA used (micromolar) could have blocked vasopressin receptors, the antagonist has been reported to have a very low affinity for these receptors (18). This is consistent with the findings that vasopressin receptors are confined to corticotrophs and possibly thyrotrophs, whereas lactotrophs express OT receptors (16,18,20). Thus, the lactotroph responses reported here are specifically mediated by OT receptors.
Data from Ca2+ imaging experiments also indicated that the lactotroph’s average response to OT was significantly up-regulated in cells obtained on proestrus, compared with diestrus 1. An analysis of individual cell responses revealed that such a difference was due to an increase of both the fraction of lactotrophs responsive to OT and the size (amplitude) of the individual calcium responses. This could be due to an increase in the number of OT receptors expressed per lactotroph in addition to an increase in the number of OT receptor-positive lactotrophs. Both are consistent with data showing that E2 up-regulates the expression of OT receptors in the anterior pituitary (16,18,19,26,48).
The enhanced intracellular Ca2+ response of proestrous lactotrophs to OT was reflected in the increased PRL-releasing effect of the nonapeptide that was observed in this stage. This was shown by a left shift of the dose-response curve of the PRL-releasing effect of OT in cells obtained on proestrus, resulting in a decrease of almost one order of magnitude of the concentration at which OT displayed half-maximal effect. Importantly, the augmented stimulatory effect of OT on PRL secretion in proestrus was observed at a wide range of agonist concentrations (1 nm to 100 μm) that includes those reported in rat portal blood (1–10 nm) (10,11). This suggests that the up-regulation of OT action on lactotrophs is physiologically significant.
The observed left shift of the dose-response curve in cells obtained on proestrus suggests that the ovarian steroids do more than modulate the number of OT receptors expressed in lactotrophs, which by itself would leave the EC50 unaltered. E2 has been reported to regulate the desensitization of OT receptors (49) as well as their coupling to downstream signaling pathways (50,51). Gonadal steroids are well-established modulators of the sensitivity of anterior pituitary cells to hypothalamic neurohormones (36), often targeting postreceptor events (52,53) and mechanisms controlling intracellular Ca2+ concentration (54,55,56,57). The nature of steroid-induced modulations is not only cell specific but also receptor specific, because E2 affects the response of lactotrophs to TRH and DA by changing the number of surface receptors, while leaving their EC50 unaltered (45).
The withdrawal of DA due to E2 could also have potentiated the PRL-releasing action of OT. This seems unlikely, however, because DA withdrawal has been shown to potentiate the PRL-releasing activity of TRH (3). If a decrease in DA concentration had been an important factor in the sensitization of the lactotrophs in proestrus, we should have also observed significantly enhanced responses to TRH.
Actions of OT are generally preceded by an increase in the responsiveness of the target tissue. In the uterus, myometrial cells are sensitized to the contractile effect of OT just before parturition (58,59,60). In the mammary gland, OT binding sites gradually increase throughout gestation and remain up-regulated during the ensuing lactation period (61). We now provide direct in vitro evidence that pituitary lactotroph cells are sensitized to the stimulatory effect of OT through specific OT receptors in the afternoon of proestrus. This OT-specific sensitization of lactotrophs in proestrus occurs in synchrony with the rise of OT concentrations in portal plasma and the decrease of dopaminergic input and action at the anterior pituitary, which are key events for the generation of the proestrous PRL surge. The coincidence of these events ensures the proper timing of the PRL surge. In addition, because OT plays additional roles in the regulation of ACTH (62), LH (63), and possibly GH release (44), the decreased responsiveness of lactotrophs to OT on diestrus may allow the nonapeptide to perform other actions without evoking a large PRL release. Taken together, the present results provide a strong foundation for the role of OT as a physiologically relevant PRL-releasing hormone. They call for additional experiments characterizing the intracellular pathways activated by OT in lactotrophs and how these might be modulated by ovarian steroids.
Acknowledgments
We thank Dr. Takafumi Inoue for providing and supporting the software TI Workbench for acquisition and analysis of intracellular calcium signals. We also thank Drs. Wei Wu and Maurizio Tomaiuolo for advice on statistical methods as well as Mrs. Ruth Cristancho-Gordo for her excellent technical assistance.
Footnotes
This work was supported by National Institutes of Health Grants DK-43200 and DA-19356.
Disclosure Summary: The authors have nothing to disclose.
First Published Online February 22, 2010
Abbreviations: AUC, Area under the curve; [Ca2+]i, intracellular calcium concentration; CI, confidence interval; DA, dopamine; E2, estradiol; HBS, HEPES-buffered saline solution; HBSS, Hanks’ balanced salt solution; M199, medium 199; OT, oxytocin; OTA, OT receptor antagonist; OVX, ovariectomized; PRL, prolactin; ROI, regions of interest.
References
- Ben-Jonathan N, Oliver C, Weiner HJ, Mical RS, Porter JC 1977 Dopamine in hypophysial portal plasma of the rat during the estrous cycle and throughout pregnancy. Endocrinology 100:452–458 [DOI] [PubMed] [Google Scholar]
- Ben-Jonathan N, Arbogast LA, Hyde JF 1989 Neuroendocrine Regulation of prolactin-release. Prog Neurobiol 33:399–447 [DOI] [PubMed] [Google Scholar]
- Martinez de la Escalera G, Weiner RI 1992 Dissociation of dopamine from its receptor as a signal in the pleiotropic hypothalamic regulation of prolactin secretion. Endocr Rev 13:241–255 [DOI] [PubMed] [Google Scholar]
- DeMaria JE, Livingstone JD, Freeman ME 1998 Characterization of the dopaminergic input to the pituitary gland throughout the estrous cycle of the rat. Neuroendocrinology 67:377–383 [DOI] [PubMed] [Google Scholar]
- McCann SM, Lumpkin MD, Mizunuma H, Khorram O, Ottlecz A, Samson WK 1984 Peptidergic and dopaminergic control of prolactin release. Trends Neurosci 7:127–131 [Google Scholar]
- Freeman ME, Kanyicska B, Lerant A, Nagy G 2000 Prolactin: structure, function, and regulation of secretion. Physiol Rev 80:1523–1631 [DOI] [PubMed] [Google Scholar]
- Vandesande F, Dierickx K 1975 Identification of the vasopressin producing and of the oxytocin producing neurons in the hypothalamic magnocellular neurosecretory system of the rat. Cell Tissue Res 164:153–162 [DOI] [PubMed] [Google Scholar]
- Sarkar DK, Frautschy SA, Mitsugi N 1992 Pituitary portal plasma levels of oxytocin during the estrous cycle, lactation, and hyperprolactinemia. Ann NY Acad Sci 652:397–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palkovits M 1984 Neuropeptides in the hypothalamo-hypophyseal system: lateral retrochiasmatic area as a common gate for neuronal fibers towards the median eminence. Peptides 5(Suppl 1):35–39 [DOI] [PubMed] [Google Scholar]
- Gibbs DM 1984 High concentrations of oxytocin in hypophysial portal plasma. Endocrinology 114:1216–1218 [DOI] [PubMed] [Google Scholar]
- Fink G, Robinson IC, Tannahill LA 1988 Effects of adrenalectomy and glucocorticoids on the peptides CRF-41, AVP and oxytocin in rat hypophysial portal blood. J Physiol 401:329–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson WK, Lumpkin MD, McCann SM 1986 Evidence for a physiological role for oxytocin in the control of prolactin secretion. Endocrinology 119:554–560 [DOI] [PubMed] [Google Scholar]
- Dawood MY, Khan-Dawood FS, Wahi RS, Fuchs F 1981 Oxytocin release and plasma anterior pituitary and gonadal hormones in women during lactation. J Clin Endocrinol Metab 52:678–683 [DOI] [PubMed] [Google Scholar]
- Lippert TH, Mueck AO, Seeger H, Pfaff A 2003 Effects of oxytocin outside pregnancy. Horm Res 60:262–271 [DOI] [PubMed] [Google Scholar]
- Sarkar DK, Gibbs DM 1984 Cyclic variation of oxytocin in the blood of pituitary portal vessels of rats. Neuroendocrinology 39:481–483 [DOI] [PubMed] [Google Scholar]
- Breton C, Pechoux C, Morel G, Zingg HH 1995 Oxytocin receptor messenger ribonucleic acid: characterization, regulation, and cellular localization in the rat pituitary gland. Endocrinology 136:2928–2936 [DOI] [PubMed] [Google Scholar]
- Samson WK, Schell DA 1995 Oxytocin and the anterior pituitary gland. Adv Exp Med Biol 395:355–364 [PubMed] [Google Scholar]
- Chadio SE, Antoni FA 1989 Characterization of oxytocin receptors in rat adenohypophysis using a radioiodinated receptor antagonist peptide. J Endocrinol 122:465–470 [DOI] [PubMed] [Google Scholar]
- Antoni FA 1986 Oxytocin receptors in rat adenohypophysis: evidence from radioligand binding studies. Endocrinology 119:2393–2395 [DOI] [PubMed] [Google Scholar]
- Samson WK, Alexander BD, Skala KD, Huang FL, Fulton RJ 1992 Ricin-cytotoxin conjugate administration reveals a physiologically relevant role for oxytocin in the control of gonadotropin secretion. Ann NY Acad Sci 652:411–422 [DOI] [PubMed] [Google Scholar]
- Lumpkin MD, Samson WK, McCann SM 1983 Hypothalamic and pituitary sites of action of oxytocin to alter prolactin secretion in the rat. Endocrinology 112:1711–1717 [DOI] [PubMed] [Google Scholar]
- Egli M, Bertram R, Toporikova N, Sellix MT, Blanco W, Freeman ME 2006 Prolactin secretory rhythm of mated rats induced by a single injection of oxytocin. Am J Physiol Endocrinol Metab 290:E566–E572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egli M, Bertram R, Sellix MT, Freeman ME 2004 Rhythmic secretion of prolactin in rats: action of oxytocin coordinated by vasoactive intestinal polypeptide of suprachiasmatic nucleus origin. Endocrinology 145:3386–3394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JW, Ben-Jonathan N 1994 Prolactin-releasing activity of neurohypophysial hormones: structure-function relationship. Endocrinology 134:114–118 [DOI] [PubMed] [Google Scholar]
- Chadio SE, Antoni FA 1993 Specific oxytocin agonist stimulates prolactin release but has no effect on inositol phosphate accumulation in isolated rat anterior pituitary cells. J Mol Endocrinol 10:107–114 [DOI] [PubMed] [Google Scholar]
- Kennett JE, Poletini MO, Fitch CA, Freeman ME 2009 Antagonism of oxytocin prevents suckling- and estradiol-induced, but not progesterone-induced, secretion of prolactin. Endocrinology 150:2292–2299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee DT, Poletini MO, Bertram R, Freeman ME 2007 Oxytocin action at the lactotroph is required for prolactin surges in cervically stimulated ovariectomized rats. Endocrinology 148:4649–4657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston CA, Negro-Vilar A 1988 Role of oxytocin on prolactin secretion during proestrus and in different physiological or pharmacological paradigms. Endocrinology 122:341–350 [DOI] [PubMed] [Google Scholar]
- Sarkar DK 1988 Immunoneutralization of oxytocin attenuates preovulatory prolactin secretion during proestrus in the rat. Neuroendocrinology 48:214–216 [DOI] [PubMed] [Google Scholar]
- Raymond V, Beaulieu M, Labrie F, Boissier J 1978 Potent antidopaminergic activity of estradiol at the pituitary level on prolactin release. Science 200:1173–1175 [DOI] [PubMed] [Google Scholar]
- Neill JD, Freeman ME, Tillson SA 1971 Control of proestrus surge of prolactin and luteinizing hormone secretion by estrogens in rat. Endocrinology 89:1448–1453 [DOI] [PubMed] [Google Scholar]
- Manning M, Miteva K, Pancheva S, Stoev S, Wo NC, Chan WY 1995 Design and synthesis of highly selective in vitro and in vivo uterine receptor antagonists of oxytocin: comparisons with atosiban. Int J Pept Protein Res 46:244–252 [DOI] [PubMed] [Google Scholar]
- Huettner JE, Baughman RW 1986 Primary culture of identified neurons from the visual cortex of postnatal rats. J Neurosci 6:3044–3060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshimizu T, Tomic M, Wong AOL, Zivadinovic D, Stojilkovic SS 2000 Characterization of purinergic receptors and receptor-channels expressed in anterior pituitary cells. Endocrinology 141:4091–4099 [DOI] [PubMed] [Google Scholar]
- Tomic M, Cesnajaj M, Catt KJ, Stojilkovic SS 1994 Developmental and physiological aspects of Ca2+ signaling in agonist-stimulated pituitary gonadotrophs. Endocrinology 135:1762–1771 [DOI] [PubMed] [Google Scholar]
- Lacau-Mengido IM, González Iglesias A, Lux-Lantos V, Libertun C, Becú-Villalobos D 1998 Ontogenic and sexual differences in pituitary GnRH receptors and intracellular Ca2+ mobilization induced by GnRH. Endocrine 8:177–183 [DOI] [PubMed] [Google Scholar]
- Tobin VA, Canny BJ 1996 Testosterone regulates gonadotropin-releasing hormone-induced calcium signals in male rat gonadotrophs. Endocrinology 137:1299–1305 [DOI] [PubMed] [Google Scholar]
- Close FT, Freeman ME 1997 Effects of ovarian steroid hormones on dopamine-controlled prolactin secretory responses in vitro. Neuroendocrinology 65:430–435 [DOI] [PubMed] [Google Scholar]
- Fitch CA, Freeman ME 1996 Effects of the estrous cycle stage on the prolactin secretory response to dopamine in vitro. Endocrine 4:59–63 [DOI] [PubMed] [Google Scholar]
- Velkeniers B, Hooghe-Peters EL, Hooghe R, Belayew A, Smets G, Claeys A, Robberecht P, Vanhaelst L 1988 Prolactin cell subpopulations separated on discontinuous Percoll gradient: an immunocytochemical, biochemical, and physiological characterization. Endocrinology 123:1619–1630 [DOI] [PubMed] [Google Scholar]
- Freeman ME, Sterman JR 1978 Ovarian steroid modulation of prolactin surges in cervically stimulated ovariectomized rats. Endocrinology 102:1915–1920 [DOI] [PubMed] [Google Scholar]
- Van Goor F, Zivadinovic D, Stojilkovic SS 2001 Differential expression of ionic channels in rat anterior pituitary cells. Mol Endocrinol 15:1222–1236 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Iglesias AE, Murano T, Li S, Tomic M, Stojilkovic SS 2008 Dopamine inhibits basal prolactin release in pituitary lactotrophs through pertussis toxin-sensitive and -insensitive signaling pathways. Endocrinology 149:1470–1479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björkstrand E, Hulting AL, Uvnäs-Moberg K 1997 Evidence for a dual function of oxytocin in the control of growth hormone secretion in rats. Regul Pept 69:1–5 [DOI] [PubMed] [Google Scholar]
- Zhang J, Chen C, Kukstas LA, Verrier D, Vincent JD, Israel JM 1990 In vitro effects of 17β-estradiol on thyrotropin-releasing hormone-induced and dopamine-inhibited prolactin release from adult male rat lactotrophs in primary culture. J Neuroendocrinol 2:277–284 [DOI] [PubMed] [Google Scholar]
- Schlosser SF, Almeida OF, Patchev VK, Yassouridis A, Elands J 1994 Oxytocin-stimulated release of adrenocorticotropin from the rat pituitary is mediated by arginine vasopressin receptors of the V1b type. Endocrinology 135:2058–2063 [DOI] [PubMed] [Google Scholar]
- Vaughan MK, Blask DE, Johnson LY, Reiter RJ 1975 Prolactin-releasing activity of arginine vasotocin in vitro. Horm Res 6:342–350 [DOI] [PubMed] [Google Scholar]
- Quiñones-Jenab V, Jenab S, Ogawa S, Adan RA, Burbach JP, Pfaff DW 1997 Effects of estrogen on oxytocin receptor messenger ribonucleic acid expression in the uterus, pituitary, and forebrain of the female rat. Neuroendocrinology 65:9–17 [DOI] [PubMed] [Google Scholar]
- Adachi S, Oku M 1995 The regulation of oxytocin receptor expression in human myometrial monolayer culture. J Smooth Muscle Res 31:175–187 [DOI] [PubMed] [Google Scholar]
- Zingg HH, Grazzini E, Breton C, Larcher A, Rozen F, Russo C, Guillon G, Mouillac B 1998 Genomic and non-genomic mechanisms of oxytocin receptor regulation. Adv Exp Med Biol 449:287–295 [DOI] [PubMed] [Google Scholar]
- Ludwig TE, Sun BC, Carnahan KG, Uzumcu M, Yelich JV, Geisert RD, Mirando MA 1998 Endometrial responsiveness to oxytocin during diestrus and early pregnancy in pigs is not controlled solely by changes in oxytocin receptor population density. Biol Reprod 58:769–777 [DOI] [PubMed] [Google Scholar]
- Platia MP, Catt KJ, Aguilera G 1986 Effects of 17β-estradiol on angiotensin II receptors and prolactin release in cultured pituitary cells. Endocrinology 119:2768–2772 [DOI] [PubMed] [Google Scholar]
- Livingstone JD, Lerant A, Freeman ME 1998 Ovarian steroids modulate responsiveness to dopamine and expression of G-proteins in lactotropes. Neuroendocrinology 68:172–179 [DOI] [PubMed] [Google Scholar]
- Stojilkovic SS 1998 Calcium signaling systems. In: Conn PM, Goodman HM, eds. Handbook of physiology, section 7, the endocrine system: cellular endocrinology. New York: Oxford Press; 177–224 [Google Scholar]
- Ortmann O, Stojilkovic SS, Cesnjaj M, Emons G, Catt KJ 1992 Modulation of cytoplasmic calcium signaling in rat pituitary gonadotrophs by estradiol and progesterone. Endocrinology 131:1565–1567 [DOI] [PubMed] [Google Scholar]
- Tobin VA, Millar RP, Canny BJ 1997 Testosterone acts directly at the pituitary to regulate gonadotropin-releasing hormone-induced calcium signals in male rat gonadotropes. Endocrinology 138:3314–3319 [DOI] [PubMed] [Google Scholar]
- Qiu J, Bosch MA, Jamali K, Xue C, Kelly MJ, Rønnekleiv OK 2006 Estrogen upregulates T-type calcium channels in the hypothalamus and pituitary. J Neurosci 26:11072–11082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larcher A, Neculcea J, Breton C, Arslan A, Rozen F, Russo C, Zingg HH 1995 Oxytocin receptor gene expression in the rat uterus during pregnancy and the estrous cycle and in response to gonadal steroid treatment. Endocrinology 136:5350–5356 [DOI] [PubMed] [Google Scholar]
- Gimpl G, Fahrenholz F 2001 The oxytocin receptor system: structure, function, and regulation. Physiol Rev 81:629–683 [DOI] [PubMed] [Google Scholar]
- Murata T, Narita K, Honda K, Higuchi T 2003 Changes of receptor mRNAs for oxytocin and estrogen during the estrous cycle in rat uterus. J Vet Med Sci 65:707–712 [DOI] [PubMed] [Google Scholar]
- Breton C, Di Scala-Guenot D, Zingg HH 2001 Oxytocin receptor gene expression in rat mammary gland: structural characterization and regulation. J Mol Endocrinol 27:175–189 [DOI] [PubMed] [Google Scholar]
- Link H, Dayanithi G, Föhr KJ, Gratzl M 1992 Oxytocin at physiological concentrations evokes adrenocorticotropin (ACTH) release from corticotrophs by increasing intracellular free calcium mobilized mainly from intracellular stores. Oxytocin displays synergistic or additive effects on ACTH-releasing factor or arginine vasopressin-induced ACTH secretion, respectively. Endocrinology 130:2183–2191 [DOI] [PubMed] [Google Scholar]
- Evans JJ 1996 Oxytocin and the control of LH. J Endocrinol 151:169–174 [DOI] [PubMed] [Google Scholar]