Abstract
A prospective, randomized, placebo-controlled study was conducted in a baboon model to determine if a thiazolidinedione agonist of peroxisome proliferator-activated receptor-γ, pioglitazone, can impede the development of endometriosis. Endometriosis was induced using laparoscopic, intrapelvic injection of eutopic menstrual endometrium, previously incubated with placebo or pioglitazone for 30 min, in 12 female baboons with a normal pelvis that had undergone at least one menstrual cycle since the time of captivity. At this point, the 12 baboons were randomized into two groups and treated from the day of induction. They received either PBS tablets (n = 6, placebo control, placebo tablets once a day by mouth) or pioglitazone (n = 6, test drug, 7.5 mg by mouth each day). A second and final laparoscopy was performed in the baboons to record the extent of endometriotic lesions between 24 and 42 d after induction (no difference in length of treatment between the two groups, P = 0.38). A videolaparoscopy was performed to document the number and surface area of endometriotic lesions. The surface area and volume of endometriotic lesions were significantly lower in pioglitazone treated baboons than the placebo group (surface area, 48.6 vs. 159.0 mm2, respectively, P = 0.049; vol, 23.7 vs. 131.8 mm3, respectively, P = 0.041). The surface area (3.5 vs. 17.8 mm2, P = 0.017, pioglizatone vs. placebo) and overall number (1.5 vs. 9.5, P = 0.007, pioglizatone vs. placebo) of red lesions were lower in the pioglitazone group. A peroxisome proliferator-activated receptor-γ ligand, pioglitazone, effectively reduced the initiation of endometriotic disease in the baboon endometriosis model. Using this animal model, we have shown that thiazolidinedione is a promising drug for preventive treatment of endometriosis.
A peroxisome proliferator-activated receptor-γ ligand, pioglitazone, reduces the development of endometriosis in the baboon model.
Endometriosis is a common etiology for women who present with chronic pelvic pain while still desiring to conceive. At this time, effective treatment concurrently addressing both entities is lacking. An ideal treatment would eliminate endometriotic lesions, prevent recurrence, be affordable with few if any side effect, and not impede ovulation. Immune modulating drugs have been studied as candidate treatment options given the implicit role of an abnormal immune response seen with this disease (1,2,3,4,5).
Peroxisome proliferator-activated receptors (PPARs) are members of the nuclear hormone receptor superfamily that affect gene expression upon ligand binding (6). Such ligands encompass endogenous fatty acids and eicosanoids in addition to synthetic ligands, including nonsteroidal antiinflammatory drugs (7) and thiazolidinediones (TZDs) such as pioglitazone used in this study. Although the current medical utilization of TZDs is for type 2 diabetes as an insulin-sensitizing drug, complementary actions of this class of drug makes it an attractive medical option to target endometriotic disease. These favorable pleiotropic properties of TZDs involve antiinflammatory (8,9), antiangiogenesis (10), antiproliferative toward endometrial cells (11), and antiestrogenic (12,13,14) influences.
Endometriotic stromal cells express PPAR-γ (15) and a recent study noted a positive correlation between PPAR-γ protein expression and pelvic pain from endometriosis (16). We and others have shown a beneficial effect of a PPAR-γ agonist in the rat model of endometriosis (17,18,19). Decreased adhesions also have been shown after treatment with TZDs (20). Subsequently, the baboon model of endometriosis was used in a therapeutic model to demonstrate a reduction in the number of existing endometriotic lesions (21).
The baboon model of surgically transplanted endometrial tissue provides a primate model to prospectively study the effects of experimental drugs on preventing the establishment of ectopic endometrial tissue (4,22,23). This ideal model has enabled our current study wherein we proposed that an oral PPAR-γ agonist, pioglitazone, could suppress proliferation, impair the peritoneal immune response, as well as diminish estrogen production from endometriotic lesions and thereby prevent the development of endometriotic lesions compared with the untreated, control group. Therefore, the objective of this study, using a preventative baboon model of endometriosis, was to assess the ability of pioglitazone to impede the growth of recently inoculated ectopic endometrial tissue.
Materials and Methods
Animals and laparoscopy
Twelve female baboons (Papio anubis) of proven fertility (10–17 kg) were studied at the Institute of Primate Research. All animals that were tested and were negative for common pathogens (bacterial and viral infections and parasites) were used in this study. Before study initiation, each animal had undergone at least one menstrual cycle in captivity. Animals were housed in single cages. The baboons were randomly selected for treatment arm just before the induction laparoscopy (laparoscopy no. 1) until there were six baboons per treatment group, 12 overall. All animal procedures and care were conducted in accordance with the Institute of Primate Research standard operating procedures. The Institutional Scientific Evaluation and Review Committee and Animal Care and Use Committee of the Institute of Primate Research approved the study.
Induction of endometriosis
On the first or second day after onset of menses, endometrial tissue was extracted from each baboon by uterine curettage and fragmented through an 18-gauge needle. After such processesing, the endometrium had a paste-like consistency that was then incubated for 30 min before seeding with saline (controls, n = 6) or pioglitazone (cases, n = 6). The dose of pioglitazone used for this incubation was 15 mg pioglitazone for each gram of menstrual paste. During laparoscopy no. 1, the resulting paste (1000 ± 250 mg) was autologously seeded onto various peritoneal sites (uterosacral ligaments, uterovesical fold, pouch of Douglas, ovaries and ovarian fossae), as described previously (24). Oral drug treatment was initiated in all 12 baboons on this same day, i.e. laparoscopy no. 1 (see Fig. 1). A second and final laparoscopy was performed in all 12 baboons to record the extent of endometriotic lesions. The interval between induction and second laparoscopy was a mean of 31.3 ± 1.8 d (median 28.5, range 24–42 d) and was comparable (P = 0.38) in the pioglitazone group [mean of 29 ± 5, median 28.5 (range 24–38) d] and in the placebo group [mean of 33.7 ± 7.1, median 33 (range 27–42) d]. The slight variation in time interval between induction laparoscopy and screening laparoscopy can be explained entirely by logistic circumstances at the time of the study in the Institute of Primate Research. The final surgery was performed by one surgeon (Chai, D C) and in a blinded fashion. The number, surface area, and type of lesion (typical, red, white, and suspicious) were recorded. Lesions were measured using the tip of the laparoscopic suction irrigator as a 5-mm measuring caliper. The surface area (square millimeters) of an endometriotic lesion (and an endometriotic lesion-related adhesion) was determined by multiplying length (millimeters) × width (millimeters). Volume (cubic millimeters) of each lesion was assessed by multiplying length (millimeters) × width (millimeters) × height (millimeters). The total cumulative surface area and total cumulative number of lesions were calculated for each baboon. Adhesions involving the ovary, fallopian tube, cul-de-sac, and other adhesions in the abdominal cavity were recorded separately. At least one biopsy of an endometriotic lesion was taken from each baboon for pathologic confirmation of the disease.
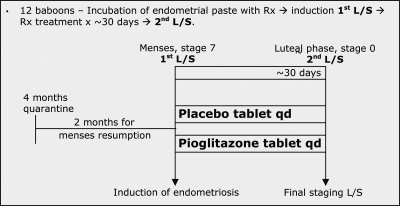
Figure 1.
Study protocol timeline. Summary figure depicting the time interval between baboon quarantine through resumption of menses and into the actual two laparoscopies performed for this project. L/S, Laparoscopy; qd, every day; Rx, drug; Tx, treatment. Stages refer to the appearance of the baboon perineum used for menstrual dating.
Timeline of study protocol
For each baboon, changes in the pattern of the menstrual and the peritoneal cycle were carefully monitored during the study. As noted in Fig. 1, the study baboons were observed for two normal menstrual cycles before the study was initiated with their subsequent third menses used as the date for the first laparoscopy (induction). The average interval between menses in baboons is 33 d (25). In baboons, perineal inflation and deflation correspond with follicular and luteal phase, respectively. Ovulation is known to occur approximately 3 d before perineal deflation, with a margin of error of 2 d (26). Daily perineal inspection in each baboon allowed determination of the onset of perineal inflation (start of the perineal cycle, corresponding to the initiation of the follicular phase) and perineal deflation during the total duration of the study period.
Histology
Single biopsy samples from each baboon were formalin fixed and embedded in paraffin blocks, sectioned at 5-μm thickness, stained with hematoxylin and eosin, and examined using a light microscope. Histological confirmation of the clinical diagnosis of endometriosis was defined as the presence of both endometrial glands and stroma in biopsies of suspected endometriotic lesions.
Drug treatment
The PPAR-γ agonist, pioglitazone used in this study was manufactured by Takeda Pharmaceuticals North America, Inc. (Actos; Deerfield, IL). A placebo tablet was provided by the University of Michigan Investigational Drug Services. The dose of pioglitazone was calculated based on the average female baboon weight being approximately one-fifth that of the average adult woman. This pioglitazone dose was equivalent to 37.5 mg/d in a 65-kg woman. Twelve baboons were randomly assigned to treatment with either placebo tablet (n = 6; tablet orally daily) or pioglitazone (n = 6; 7.5 mg tablet orally daily). Both placebo and pioglitazone pills were hidden in a banana, and each baboon was observed eating this banana by an Institute of Primate Research technician. Because of uncertain absorption from oral administration, the pioglitazone dose is at the upper range of the Food and Drug Administration-approved dose for a weight-matched woman.
Statistical analysis
Student’s t tests were conducted to compare the outcome measures between the placebo and pioglitazone groups. When variables were not normally distributed and hence did not meet the normality assumption for Student’s t tests, we used Student’s t tests on natural logarithm transformed measures if data could be transformed to be normally distributed. This was because parametric tests (such as Student’s t tests) have greater statistical power than nonparametric tests (such as Wilcoxon rank sum tests). When data could not be transformed to be normally distributed, Wilcoxon rank sum tests were used for comparisons between the two groups. P values less than 0.05 were considered as statistically significant. Data analysis was performed using SAS 9.1.3 (SAS Institute, Inc., Cary, NC) and SPSS 14.0.2 (SPSS, Inc., Chicago, IL). nQuery Advisor 6.01 (Statistical Solutions, Saugus, MA) was used for the post hoc power analysis.
Results
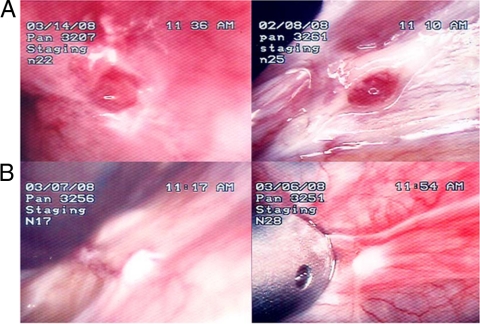
The presence of endometriosis was histologically confirmed by hematoxylin and eosin staining in the solitary endometriotic implant biopsies taken from each baboon. Table 1 lists the overall results for the total number of lesions, total surface area, total volume of endometriotic lesions after treatment, and the surface area of red lesions in both treatment groups. Table 2 reports the number of white, typical, and red lesions for the two treatment groups. Representative macroscopic appearances of the endometriotic lesions in the two treatment groups are shown in Fig. 2, A and B.
Table 1.
Overall results for each treatment group
| Placebo (n = 6) | Pioglitazone (n = 6) | P | Power | |
|---|---|---|---|---|
| Total number of lesions | 71, 60, 31, 63, 25, 125 [61.5 (25–125); 62.5 ± 35.7] | 37, 33, 23, 8, 43, 33 [33.0 (8–43); 29.5 ± 12.4] | 0.0751 | 0.44 |
| Total SA (mm2) | 117, 72, 242, 322, 48, 154 [135.3 (48–322); 159.0 ± 105.1] | 39, 60, 70, 72, 8, 43 [51.5 (8–72); 48.6 ± 24.0] | 0.0493 | 0.54 |
| Total volume (mm3) | 327, 248, 36, 66, 22, 92 [78.8 (22–327); 131.8 ± 125.6] | 26, 33, 13, 3, 39, 29 [27.4 (3–39); 23.7 ± 13.4] | 0.0411 | 0.58 |
| SA of red lesions (mm2) | 2, 12, 24, 35, 19, 15 [16.8 (2–35); 17.8 ± 11.4] | 9, 2, 0, 6, 0, 5 [3.3 (0–9); 3.5 ± 3.4] | 0.0173 | 0.68 |
All data are presented as raw individual values for each of the six baboons in each group [median (range); mean ± sd]. SA, surface area.
Table 2.
Number of specific lesion types for the two treatment groups
| Placebo (n = 6) | Pioglitazone (n = 6) | P | Power | |
|---|---|---|---|---|
| White lesions | 58, 23, 46, 48, 15, 119 [47 (15–119); 51.5 ± 36.8] | 20, 32, 42, 32, 8, 33 32 (8–42); 27.8 ± 12.0 | 0.1623 | 0.32 |
| Typical lesions | 3, 2, 1, 0, 3, 0 [1.5 (0–3); 1.5 ± 1.4] | 0, 0, 1, 0, 0, 0 [0 (0–1); 0.2 ± 0.4] | 0.1061 | 0.42 |
| Red lesions | 2, 6, 13, 23, 7, 6 [6.5 (2–23); 9.5 ± 7.5] | 3, 1, 0, 1, 0, 4 [1 (0–4); 1.5 ± 1.6] | 0.0065 | 0.76 |
| Typical and red lesions | 5, 8, 14, 23, 10, 6 [9.0 (5–23); 11.0 ± 6.7] | 3, 1, 1, 1, 0, 4 [1 (0–4); 1.7 ± 1.5] | 0.0022 | 0.85 |
All data are presented as raw individual values for each of the six baboons in each group [median (range); mean ± sd].
Figure 2.
Gross appearance of endometriotic lesions at the end of treatment. Laparoscopic appearance of endometriotic lesions in four representative baboons at the final laparoscopy (staging laparoscopy no. 2) at the end of treatment. A, Baboon PAN 3207 and 3261, placebo treatment. B, Baboon PAN 3256 and 3251 after pioglitazone treatment.
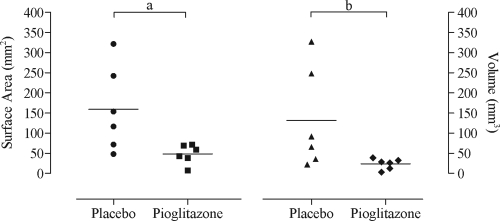
The surface area (mean: 48.6 vs. 159.0 mm2, P = 0.049, pioglizatone vs. placebo) and volume (mean: 23.7 vs. 131.8 mm3, P = 0.041, pioglizatone vs. placebo) of endometriotic lesions (Fig. 3) were significantly lower in pioglitazone treated baboons than the placebo group.
Figure 3.
Surface area and volume scatter plot. Scatter plot showing total surface area (square millimeters) and volume (cubic millimeters) after the respective treatment, placebo vs. pioglitazone (six baboons/group). Solid horizontal lines represent the mean for each group. a, Student’s t test, P = 0.0493. b, Wilcoxon rank sum test, P = 0.0411.
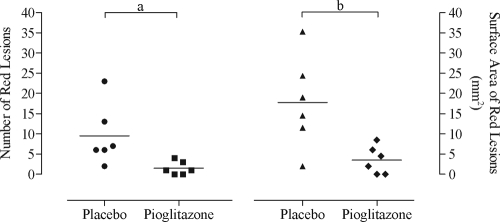
The number (mean: 1.5 vs. 9.5, P = 0.007, pioglizatone vs. placebo) and surface area (mean: 3.5 vs. 17.8 mm2, P = 0.017, pioglizatone vs. placebo) of red lesions were lower in the pioglitazone group (Fig. 4).
Figure 4.
Red lesion surface area and volume scatter plot. Scatter plot showing red lesion number and surface area (surface area, square millimeters) after the respective treatment, placebo vs. pioglitazone (six baboons/group). Solid horizontal lines represent the mean for each group. a, Wilcoxon rank sum test, P = 0.0065. b, Wilcoxon rank sum test, P = 0.0173.
Discussion
Retrograde menstruation is the leading pathophysiologic theory for the development of endometriotic lesions, and this phenomenon is known to lead to an inflammatory response (27,28,29). Controlling this aberrant immune response with antiinflammatory medicines could prove to be useful in the management of this debilitating disorder. PPAR-γ plays a fundamental role in abrogating the immune response by inhibiting the expression of inflammatory cytokines as well as modifying the immune cell phenotype into an antiinflammatory milieu. Moreover, because PPAR-γ ligands have been shown to regulate cell growth, apoptosis, angiogenesis in human endometrial cells, and estrogen biosynthesis, it was posited that this class of drug might offer an effective novel treatment for endometriosis. In particular, TZDs could provide a medical option that could at once diminish endometriotic disease burden and the resulting symptomatology, with respect to pain as well as subfertility.
To date, there have been three favorable studies using TZDs in the rat model of endometriosis and one in a therapeutic baboon model of endometriosis (17,18,20,21). Before this preventative study, a TZD, rosiglitazone, was used to assess whether it could significantly diminish endometriotic lesions that were documented before treatment (21). Rosiglitazone was able to reduce the surface area (∼50% decrease in relative change compared with placebo) of induced peritoneal endometriosis. In an in vitro model of the early endometriotic lesion, a PPAR-γ ligand, ciglitazone, significantly decreased adhesion of an endometrial epithelioid cell line to peritoneal mesothelial cells (30). Taken together, we designed this study as a proof-of-concept preventative study compared with the previously published therapeutic baboon model. As noted, the menstrual sample was incubated for 30 min before seeding and subsequent systemic pioglitazone administration. Prevention studies are planned to compare the effect of local in vitro treatment with systemic treatment in the manner that we looked at the effect of recombinant human TNFRSF1A and the development of endometriosis in the baboon (4). We observed a significant systemic effect of recombinant human TNFRSF1A in the prevention of endometriosis that was seen as independent from the in vitro exposure group. The results presented in this follow-up study provide the first subhuman primate evidence that treatment with a TZD can reduce the surface area and volume of lesions in a preventative baboon model. Furthermore, our results advocate that pioglitazone may block the development of the more active red lesions. A recent case report documented red vesicular lesions with focal hemorrhage at the time of laparoscopy timed during menstruation, thus further suggesting the active phenotype of such lesions (31).
Providing a more idyllic therapy option for treating endometriosis pain should possess a modicum of side effects, low-cost burden, proven efficacy at diminishing pelvic pain, and sparing fertility potential during treatment. Clinical data have exonerated pioglitazone from the increased hepatoxicity risk as seen with other TZDs (32). Pioglitazone has been studied in a prospective, randomized trial of cardiovascular outcomes. There was a trend toward benefit from pioglitazone (33,34,35). This makes physiologic sense given the fact that pioglitazone has a favorable effect on lipids (36). Secondary end-point data from clinical trials show increased fracture risk in diabetic women exposed to TZDs (37,38). These fractures were concentrated in the appendicular skeleton, suggesting a predilection for cortical rather than trabecular bone loss. When the manufacturer of pioglitazone, Takeda Pharmaceuticals North America, Inc., reviewed their clinical trial databases, they reported that the fracture rate for pioglitazone users was 1.9 per 100 person years compared with a rate of 1.1 per 100 person years in the control group. In vitro studies have shown that TZDs inhibit osteoblast differentiation (39) and promote osteoclastogenesis (40). It is not inconceivable to develop a selective TZD that may circumvent a detrimental effect such as bone-loss, whereas preserving the beneficial PPAR-γ target gene effect necessary to ameliorate endometriosis (41,42).
Pioglitazone is classified as a pregnancy category C drug due to animal evidence of nonmalforming embryotoxicity with 40 times the maximum human dose (no such effect was seen at a dose 17 times the maximum human dose). No evidence of teratogenicity exists in either preclinical or clinical trials. This would suggest that it may be safe to take the TZDs up until the time a pregnancy is confirmed. Human data on the utility of TZDs in treating endometriosis are limited to one small case series of women with endometriosis using rosiglitazone (43). Two of the three patients exhibited improvement in severity of symptoms and pain levels with a concurrent decrease in pain medication. The third patient experienced no change. There was no adverse effect to the rosiglitazone in these few participants.
Pioglitazone has been studied in women with polycystic ovary syndrome exhibiting chronic anovulation or oligoamenorrhea (44,45,46). A dose of 45 mg/d led to 54% of pioglitazone subjects exhibiting more than three regular menstrual cycles over a 6-month time period, in contrast to only 37% of the placebo subjects. Recent studies have found that cinnamon and extracts from the flowers of Echinacea purpurea can act as an activator of PPAR-γ (47,48). Simple compounds such as these thus have the potential to offer endometriosis pain treatment that is at once affordable, nontoxic, and noncontraceptive. Taken together with our previous findings using a therapeutic model, this study purports that PPAR-γ agonists such as pioglitazone may not only treat endometriotic lesions, but can also partially prevent the development of endometriosis in baboons. Moreover, pioglitazone seemed to inhibit the development of active red lesions compared with placebo. As such, pioglitazone, or another PPAR-γ agonist, offers a promising new therapeutic modality for not only addressing pelvic pain but preserving fertility in such women. In future research, we plan to do a randomized placebo-controlled trial to test the hypothesis that pioglitazone can reduce endometriosis-associated pain and endometriosis-associated infertility in women with active peritoneal endometriotic lesions.
Footnotes
This work was supported in part by the National Institutes of Health Grant 5K23HD043952-02 (to D.I.L.) and by the Bayer Droegemueller Award in Clinical Research. D.I.L. has received research grant support from Bayer Schering Pharma and the World Endometriosis Research Foundation through grants from Bayer Schering Pharma and Takeda.
Disclosure Summary: The authors have nothing to disclose.
First Published Online February 16, 2010
Abbreviations: PPAR, Peroxisome proliferator-activated receptor; TZD, thiazolidinedione.
References
- Agic A, Xu H, Finas D, Banz C, Diedrich K, Hornung D 2006 Is endometriosis associated with systemic subclinical inflammation? Gynecol Obstet Invest 62:139–147 [DOI] [PubMed] [Google Scholar]
- Lebovic DI, Mueller MD, Taylor RN 2001 Immunobiology of endometriosis. Fertil Steril 75:1–10 [DOI] [PubMed] [Google Scholar]
- Barrier BF, Bates GW, Leland MM, Leach DA, Robinson RD, Propst AM 2004 Efficacy of anti-tumor necrosis factor therapy in the treatment of spontaneous endometriosis in baboons. Fertil Steril 81(Suppl 1):775–779 [DOI] [PubMed] [Google Scholar]
- D'Hooghe TM, Nugent NP, Cuneo S, Chai DC, Deer F, Debrock S, Kyama CM, Mihalyi A, Mwenda JM 2006 Recombinant human TNFRSF1A (r-hTBP1) inhibits the development of endometriosis in baboons: a prospective, randomized, placebo- and drug-controlled study. Biol Reprod 74:131–136 [DOI] [PubMed] [Google Scholar]
- Falconer H, Mwenda JM, Chai DC, Wagner C, Song XY, Mihalyi A, Simsa P, Kyama C, Cornillie FJ, Bergqvist A, Fried G, D'Hooghe TM 2006 Treatment with anti-TNF monoclonal antibody (c5N) reduces the extent of induced endometriosis in the baboon. Hum Reprod 21:1856–1862 [DOI] [PubMed] [Google Scholar]
- Brown JD, Plutzky J 2007 Peroxisome proliferator-activated receptors as transcriptional nodal points and therapeutic targets. Circulation 115:518–533 [DOI] [PubMed] [Google Scholar]
- Willson TM, Brown PJ, Sternbach DD, Henke BR 2000 The PPARs: from orphan receptors to drug discovery. J Med Chem 43:527–550 [DOI] [PubMed] [Google Scholar]
- Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK 1998 The peroxisome proliferator-activated receptor-γ is a negative regulator of macrophage activation. Nature 391:79–82 [DOI] [PubMed] [Google Scholar]
- Jiang C, Ting AT, Seed B 1998 PPAR-γ agonists inhibit production of monocyte inflammatory cytokines. Nature 391:82–86 [DOI] [PubMed] [Google Scholar]
- Peeters LL, Vigne JL, Tee MK, Zhao D, Waite LL, Taylor RN 2005 PPAR γ represses VEGF expression in human endometrial cells: implications for uterine angiogenesis. Angiogenesis 8:373–379 [DOI] [PubMed] [Google Scholar]
- Wu Y, Guo SW 2009 Peroxisome proliferator-activated receptor-γ and retinoid X receptor agonists synergistically suppress proliferation of immortalized endometrial stromal cells. Fertil Steril 91:2142–2147 [DOI] [PubMed] [Google Scholar]
- Mu YM, Yanase T, Nishi Y, Takayanagi R, Goto K, Nawata H 2001 Combined treatment with specific ligands for PPARγ:RXR nuclear receptor system markedly inhibits the expression of cytochrome P450arom in human granulosa cancer cells. Mol Cell Endocrinol 181:239–248 [DOI] [PubMed] [Google Scholar]
- Yanase T, Mu YM, Nishi Y, Goto K, Nomura M, Okabe T, Takayanagi R, Nawata H 2001 Regulation of aromatase by nuclear receptors. J Steroid Biochem Mol Biol 79:187–192 [DOI] [PubMed] [Google Scholar]
- Fan W, Yanase T, Morinaga H, Mu YM, Nomura M, Okabe T, Goto K, Harada N, Nawata H 2005 Activation of peroxisome proliferator-activated receptor-γ and retinoid X receptor inhibits aromatase transcription via nuclear factor-κB. Endocrinology 146:85–92 [DOI] [PubMed] [Google Scholar]
- Ohama Y, Harada T, Iwabe T, Taniguchi F, Takenaka Y, Terakawa N 2008 Peroxisome proliferator-activated receptor-γ ligand reduced tumor necrosis factor-α-induced interleukin-8 production and growth in endometriotic stromal cells. Fertil Steril 89:311–317 [DOI] [PubMed] [Google Scholar]
- McKinnon B, Bersinger NA, Huber AW, Kuhn A, Mueller MD 2009 PPAR-γ expression in peritoneal endometriotic lesions correlates with pain experienced by patients. Fertil Steril 93:293–296 [DOI] [PubMed] [Google Scholar]
- Lebovic DI, Kir M, Casey CL 2004 Peroxisome proliferator-activated receptor-γ induces regression of endometrial explants in a rat model of endometriosis. Fertil Steril 82(Suppl 3):1008–1013 [DOI] [PubMed] [Google Scholar]
- Aytan H, Caliskan AC, Demirturk F, Aytan P, Koseoglu DR 2007 Peroxisome proliferator-activated receptor-γ agonist rosiglitazone reduces the size of experimental endometriosis in the rat model. Aust NZ J Obstet Gynaecol 47:321–325 [DOI] [PubMed] [Google Scholar]
- Demirturk F, Aytan H, Caliskan AC, Aytan P, Koseoglu DR 2006 Effect of peroxisome proliferator-activated receptor-γ agonist rosiglitazone on the induction of endometriosis in an experimental rat model. J Soc Gynecol Investig 13:58–62 [DOI] [PubMed] [Google Scholar]
- Demirturk F, Aytan H, Caliskan A, Aytan P, Yener T, Koseoglu D, Yenisehirli A 2006 The effect of rosiglitazone in the prevention of intra-abdominal adhesion formation in a rat uterine horn model. Hum Reprod 21:3008–3013 [DOI] [PubMed] [Google Scholar]
- Lebovic DI, Mwenda JM, Chai DC, Mueller MD, Santi A, Fisseha S, D'Hooghe T 2007 PPAR-γ receptor ligand induces regression of endometrial explants in baboons: a prospective, randomized, placebo- and drug-controlled study. Fertil Steril 88:1108–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Hooghe TM, Bambra CS, Raeymaekers BM, De Jonge I, Hill JA, Koninckx PR 1995 The effects of immunosuppression on development and progression of endometriosis in baboons (Papio anubis). Fertil Steril 64:172–178 [PubMed] [Google Scholar]
- Falconer H, Mwenda JM, Chai DC, Wagner C, Song XY, Mihalyi A, Simsa P, Kyama C, Cornillie FJ, Bergqvist A, Fried G, D'Hooghe TM 2006 Treatment with anti-TNF monoclonal antibody (c5N) reduces the extent of induced endometriosis in the baboon. Hum Reprod 21:1856–1862 [DOI] [PubMed] [Google Scholar]
- D'Hooghe TM, Bambra CS, Raeymaekers BM, De Jonge I, Lauweryns JM, Koninckx PR 1995 Intrapelvic injection of menstrual endometrium causes endometriosis in baboons (Papio cynocephalus and Papio anubis). Am J Obstet Gynecol 173:125–134 [DOI] [PubMed] [Google Scholar]
- Stevens VC 1997 Some reproductive studies in the baboon. Hum Reprod Update 3:533–540 [DOI] [PubMed] [Google Scholar]
- Kriewaldt FH, Hendrickx AG 1968 Reproductive parameters of the baboon. Lab Anim Care 18:361–370 [PubMed] [Google Scholar]
- Kyama CM, Overbergh L, Debrock S, Valckx D, Vander Perre S, Meuleman C, Mihalyi A, Mwenda JM, Mathieu C, D'Hooghe TM 2006 Increased peritoneal and endometrial gene expression of biologically relevant cytokines and growth factors during the menstrual phase in women with endometriosis. Fertil Steril 85:1667–1675 [DOI] [PubMed] [Google Scholar]
- D'Hooghe TM, Bambra CS, Xiao L, Peixe K, Hill JA 2001 Effect of menstruation and intrapelvic injection of endometrium on inflammatory parameters of peritoneal fluid in the baboon (Papio anubis and Papio cynocephalus). Am J Obstet Gynecol 184:917–925 [DOI] [PubMed] [Google Scholar]
- Orasanu G, Ziouzenkova O, Devchand PR, Nehra V, Hamdy O, Horton ES, Plutzky J 2008 The peroxisome proliferator-activated receptor-γ agonist pioglitazone represses inflammation in a peroxisome proliferator-activated receptor-α-dependent manner in vitro and in vivo in mice. J Am Coll Cardiol 52:869–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavoussi SK, Witz CA, Binkley PA, Nair AS, Lebovic DI 2009 Peroxisome-proliferator activator receptor-γ activation decreases attachment of endometrial cells to peritoneal mesothelial cells in an in vitro model of the early endometriotic lesion. Mol Hum Reprod 15:687–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burney RO, Lathi RB 2009 Menstrual bleeding from an endometriotic lesion. Fertil Steril 91:1926–1927 [DOI] [PubMed] [Google Scholar]
- Lebovitz HE, Kreider M, Freed MI 2002 Evaluation of liver function in type 2 diabetic patients during clinical trials: evidence that rosiglitazone does not cause hepatic dysfunction. Diabetes Care 25:815–821 [DOI] [PubMed] [Google Scholar]
- Dormandy JA, Charbonnel B, Eckland DJ, Erdmann E, Massi-Benedetti M, Moules IK, Skene AM, Tan MH, Lefèbvre PJ, Murray GD, Standl E, Wilcox RG, Wilhelmsen L, Betteridge J, Birkeland K, Golay A, Heine RJ, Korányi L, Laakso M, Mokán M, Norkus A, Pirags V, Podar T, Scheen A, Scherbaum W, Schernthaner G, Schmitz O, Skrha J, Smith U, Taton J 2005 Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive study (PROspective pioglitAzone clinical trial in macrovascular events): a randomised controlled trial. Lancet 366:1279–1289 [Google Scholar]
- Zinn A, Felson S, Fisher E, Schwartzbard A 2008 Reassessing the cardiovascular risks and benefits of thiazolidinediones. Clin Cardiol 31:397–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoff AM, Wolski K, Nicholls SJ, Nissen SE 2007 Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus: a meta-analysis of randomized trials. JAMA 298:1180–1188 [DOI] [PubMed] [Google Scholar]
- Deeg MA, Tan MH 2008 Pioglitazone versus rosiglitazone: effects on lipids, lipoproteins, and apolipoproteins in head-to-head randomized clinical studies. PPAR Res 2008:520465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, Kravitz BG, Lachin JM, O'Neill MC, Zinman B, Viberti G 2006 Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 355:2427–2443 [DOI] [PubMed] [Google Scholar]
- Kahn SE, Zinman B, Lachin JM, Haffner SM, Herman WH, Holman RR, Kravitz BG, Yu D, Heise MA, Aftring RP, Viberti G 2008 Rosiglitazone-associated fractures in type 2 diabetes: an analysis from a diabetes outcome progression trial (ADOPT). Diabetes Care 31:845–851 [DOI] [PubMed] [Google Scholar]
- Akune T, Ohba S, Kamekura S, Yamaguchi M, Chung UI, Kubota N, Terauchi Y, Harada Y, Azuma Y, Nakamura K, Kadowaki T, Kawaguchi H 2004 PPARγ insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J Clin Invest 113:846–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y, Chong LW, Evans RM 2007 PPAR-γ regulates osteoclastogenesis in mice. Nat Med 13:1496–1503 [DOI] [PubMed] [Google Scholar]
- Lazarenko OP, Rzonca SO, Suva LJ, Lecka-Czernik B 2006 Netoglitazone is a PPAR-γ ligand with selective effects on bone and fat. Bone 38:74–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motani A, Wang Z, Weiszmann J, McGee LR, Lee G, Liu Q, Staunton J, Fang Z, Fuentes H, Lindstrom M, Liu J, Biermann DH, Jaen J, Walker NP, Learned RM, Chen JL, Li Y 2009 INT131: a selective modulator of PPAR γ. J Mol Biol 386:1301–1311 [DOI] [PubMed] [Google Scholar]
- Moravek MB, Ward EA, Lebovic DI 2009 Thiazolidinediones as therapy for endometriosis: a case series. Gynecol Obstet Invest 68:167–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota H, Goto T, Yoshioka T, Ohyama N 2008 Successful pregnancies treated with pioglitazone in infertile patients with polycystic ovary syndrome. Fertil Steril 90:709–713 [DOI] [PubMed] [Google Scholar]
- Aroda VR, Ciaraldi TP, Burke P, Mudaliar S, Clopton P, Phillips S, Chang RJ, Henry RR 2009 Metabolic and hormonal changes induced by pioglitazone in polycystic ovary syndrome: a randomized, placebo-controlled clinical trial. J Clin Endocrinol Metab 94:469–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffler MS, Patel K, Dahan MH, Yoo RY, Malcom PJ, Chang RJ 2003 Enhanced granulosa cell responsiveness to follicle-stimulating hormone during insulin infusion in women with polycystic ovary syndrome treated with pioglitazone. J Clin Endocrinol Metab 88:5624–5631 [DOI] [PubMed] [Google Scholar]
- Sheng X, Zhang Y, Gong Z, Huang C, Zang YQ 2008 Improved insulin resistance and lipid metabolism by cinnamon extract through activation of peroxisome proliferator-activated receptors. PPAR Res 2008:581348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen KB, Petersen RK, Petersen S, Kristiansen K, Christensen LP 2009 Activation of PPARγ by metabolites from the flowers of purple coneflower (Echinacea purpurea). J Nat Prod 72:933–937 [DOI] [PubMed] [Google Scholar]