Abstract
The hypothalamic melanocortin system, which controls appetite and energy expenditure, develops during the third trimester in primates. Thus, maternal nutrition and health may have a profound influence on the development of this system. To study the effects of chronic maternal high-fat diet (HFD) on the development of the melanocortin system in the fetal nonhuman primate, we placed adult female macaques on either a control (CTR) diet or a HFD for up to 4 yr. A subgroup of adult female HFD animals was also switched to CTR diet during the fifth year of the study (diet reversal). Third-trimester fetuses from mothers on HFD showed increases in proopiomelanocortin mRNA expression, whereas agouti-related protein mRNA and peptide levels were decreased in comparison with CTR fetuses. Proinflammatory cytokines, including IL-1β and IL-1 type 1 receptor, and markers of activated microglia were elevated in the hypothalamus, suggesting an activation of the local inflammatory response. Fetuses of diet-reversal mothers had normal melanocortin levels. These results raise the concern that chronic consumption of a HFD during pregnancy, independent of maternal obesity and diabetes, can lead to widespread activation of proinflammatory cytokines that may alter the development of the melanocortin system. The abnormalities in the fetal POMC system, if maintained into the postnatal period, could impact several systems, including body weight homeostasis, stress responses, and cardiovascular function. Indeed, the HFD offspring develop early-onset excess weight gain. These abnormalities may be prevented by healthful nutrient consumption during pregnancy even in obese and severely insulin-resistant individuals.
Consumption of a diet high in fats during pregnancy causes inflammation in the hypothalamus during a critical period of development for the melanocortin system.
The melanocortin system in the hypothalamus is pivotal in the regulation of energy balance and body-weight homeostasis. The primary agonist of the melanocortin system is the potent anorexic neuropeptide, α-MSH, a cleavage product of the proopiomelanocortin (POMC) gene. The POMC gene also can be cleaved into ACTH, β- and γ-MSH, and β-endorphin. The melanocortin system also has an endogenous inverse agonist, agouti-related peptide (AgRP). AgRP is a potent appetite stimulant and is exclusively expressed in the arcuate nucleus of the hypothalamus (ARH) where it is colocalized in neuropeptide Y (NPY) cells (1,2). The important melanocortin receptors are the MC3 and MC4 (3). The importance of this system in the regulation of energy homeostasis has been extensively studied in many species. Highlighting the importance of the melanocortin system, mutations in this system cause hyperphagia and obesity in mouse models as well as humans (3,4,5). Our group has demonstrated in the nonhuman primate (NHP) model that fasting is associated with decreased POMC gene expression and increased NPY and AgRP expression and that administration of α-MSH inhibits food intake and AgRP can stimulate food intake (6,7).
Although the effects of melanocortins on the regulation of energy homeostasis appears to be conserved in most species, the relative ontogeny of this system varies from species to species. In the developing rodent, the maturation of the melanocortin circuits occurs postnatally (8,9). More specifically, the ARH-NPY/melanocortin neurons are born around embryonic d 11.5–12.5 (10,11) (Dr. L. Zeltzer, personal communication); however, a function circuit doesn’t develop until the third postnatal week (9,12). Furthermore, leptin has been shown to be a critical neurotrophic factor for the development of ARH circuits (8,13). Increasing the quantity and quality of nutrition ingested postnatally during the critical period of the development of the melanocortin circuit, alters the sensitivity of ARH neurons to leptin and insulin, and induces an overweight phenotype that persists into adulthood (12,14,15). Moreover, maternal high-fat feeding specifically during pregnancy can also have long-term consequences on metabolic systems in rodent offspring (16,17), which is at least partially due to alterations in hypothalamic feeding circuitry.
In NHP, melanocortin neurocircuitry develops during the third trimester of pregnancy (18) and therefore is susceptible to perturbations from maternal nutrition and health (19). A variety of factors, such as overall fat and calorie load, glucose levels and insulin resistance, and hyperlipidemia and lipotoxicity, can affect the growing fetus. Pregnancies with poor glycemic control and/or gestational diabetes have a high risk of macrosomia at birth and obesity and insulin resistance later in life (20,21,22). Presently, greater than 50% of women of childbearing age are either overweight or obese (23). It is likely that the increased rate of childhood obesity in recent years is at least partly due to maternal diet and metabolic disease.
Previous studies from our group demonstrated that chronic maternal high-fat diet (HFD) consumption can cause lipotoxicity in the liver of the fetal and neonatal offspring (24). Furthermore, these animals display elevated levels of circulating inflammatory cytokines. Rodent studies have also shown that both HFD and cytokines can affect melanocortin neurons (25,26,27,28). In the present study, we tested the hypothesis that chronic consumption of HFD during pregnancy could alter the hypothalamic melanocortin system during this critical period of development, using a NHP model. We report alterations in both POMC and AgRP and activation of proinflammatory cytokines in the fetal hypothalamus exposed to maternal HFD (mHFD) in comparison with control (CTR) fetuses [maternal CTR diet (mCTR)]. We also demonstrate that developmental abnormalities in these fetuses can be prevented by reversing HFD animals to CTR diet [maternal diet reversal (mDR)] before pregnancy. These data suggest that maternal diet during fetal development may compromise the development of the melanocortin system.
Materials and Methods
All animal procedures were approved by the Oregon National Primate Research Center Institutional Animal Care and Use Committee and conformed to National Institutes of Health guidelines on the ethical use of animals. All animals were maintained in outdoor/indoor group harem housing (male to female ratio of 2:9) on a natural light schedule.
Part 1
In these studies, mCTR and mHFD tissues were harvested, processed, and analyzed first. Age- and weight-matched adult Macaca fuscata, Japanese macaques, were fed a CTR or HFD for up to 4 yr. The CTR diet (no. 5000; Purina Mills Co., St. Louis, MO) provided 13% calories from fat, and the HFD (test diet, 5A1F; Purina) supplied 35% of calories from fat and included calorically dense (peanut butter) treats. Pregnancies were detected by palpation and then later dated by ultrasound examination. Both groups consisted of primiparous and multiparous pregnant animals. All pregnancies were singleton. Fetuses were collected by cesarean section on gestational d 130 (G130) (early third trimester; full term is 175 d) from each pregnancy in CTR and HFD groups during yr 2, 3, and 4 on the diets. The fetuses were taken directly to necropsy and were deeply anesthetized with sodium pentobarbital (>30 mg/kg iv). The chest cavity was opened, and the brains flushed with 0.9% saline containing 5000 U/liter heparin via the carotids. Fetal brains were taken either fixative-perfused or fresh (mCTR, n = 4 fresh, n = 4 fixed; mHFD yr 3 and 4, n = 6 fixed; mHFD yr 2 and 4, n = 7 fresh). Both groups were balanced for sex, and the mHFD group was balanced for offspring from diet-sensitive and diet-resistant mothers. In the case of fixation, transcarotid perfusion of the head with 4% paraformaldehyde in buffered sodium phosphate (pH 7.4) was performed. The brain was removed, blocked, and postfixed overnight at 4 C in the same fixative and subsequently transferred for 24 h to 10% glycerol buffered with NaPO4 and for 48 h into 20% glycerol buffered with NaPO4. Tissue was frozen by submersion in −50 C or below methyl butane and then stored in −80 C until sectioning. When tissues were harvested fresh, the brain was removed and blocked. The hypothalamus was divided into three parts, anterior, dorsal, and ventral, and then stored in −80 C until RNA extraction.
Part 2
In yr 5 of the study, a subset of adults were switched to the CTR diet before the ensuing pregnancy (DR). Subsequently, mDR tissues were harvested and processed with fetal control (mCTR) tissues from that same year. All animals were fixative perfused as described above (mCTR, n = 4; mDR, n = 5). Both groups were balanced for sex, and the mDR group was balanced for offspring from diet-sensitive and diet-resistant mothers.
ARH NPY, POMC, and AgRP mRNA
Coronal hypothalamic sections (35 μm) were collected in 1:6 series using a freezing microtome, as previously described (18,29). Human NPY, POMC, and AgRP cRNA probes were transcribed from 500-, 1115-, and 400-bp cDNAs, respectively, and labeled with 100% [33P]UTP. In situ hybridization and imaging for NPY, POMC, and AgRP mRNA was performed using methods previously described (18,29). Integrated morphometry analysis was used to measure total density by multiplying total area by OD of an area approximating the ARH (reported in relative units). An average of six matched mid-ARH sections were analyzed for each animal.
Immunohistochemistry for neuropeptides and the IL-1 receptor
To characterize the development of AgRP- and NPY-immunoreactivity (-ir), a cocktail of goat anti-NPY (a kind gift of P. Larsen; 1:50,000), and guinea pig anti-AgRP (1:5000; Antibodies Australia, Melbourne, Australia) was used. To characterize the relative contribution of the POMC gene products, a cocktail of sheep anti-αMSH (no. AB5087, 1:3000; Chemicon, Temecula, CA) and rabbit anti-β-endorphin (no. AB5028, 1:3000; Chemicon) or rabbit anti-ACTH (no. 20070, 1:5000; Immunostar, Hudson, WI) antibodies were used. To characterize the expression of IL-1 receptor (IL-1R1), goat anti-mIL-1R1 (no. AF771, 1:2000; R&D Systems, Minneapolis, MN) antibody was used. IL-1R1, α-MSH, and AgRP specificity was tested using peptide blocking assays and found to be specific (see Supplemental Fig. 1 published on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org). Standard immunohistochemical methods were used as previously described (18). During immunostaining control experiments, antibodies for α-MSH, AgRP, and IL-1R1 was preabsorbed overnight with 50× excess of peptide and brought to a final volume of 50 μl with water (α-MSH, no. 043-01 from Phoenix Pharmaceuticals, Inc., Burlingame, CA; AgRP, kind gift of Neurocrine Biosciences Inc., San Diego, CA; IL-1R1, no. 771-MR from R&D Systems) and then used for standard immunohistochemistry. Primary or reabsorbed antibodies were diluted to their working concentrations in 2% donkey serum in 0.4% Triton X-100/potassium PBS and incubated with the tissue for 48 h at 4 C. Tissue was then washed in KPBS and fluorescent secondary antibodies applied for 1 h at room temperature at dilution of 1:200 (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA).
Confocal laser microscopy, as previously described (30), was used to capture the NPY, AgRP, and α-MSH immunofluorescent images. Four fields of view per section (upper/lower, right/left) in four equally spaced sections per animal were imaged and analyzed. Total immunoreactive fluorescent intensity was measured using the same conditions and same threshold for all images. Total gray value was measured for each image. Sections were blinded with respect to group. For qualitative illustration of α-MSH and IL-1R1, anatomically matched sections were chosen (n = 3 animals per group). Captured images are 10-μm-thick projections at either ×10 or ×25. A standardized adjustment of all the images was made for brightness and contrast for illustrative purposes using Photoshop (Adobe Systems Inc., San Jose, CA).
Immunohistochemistry for Iba1
Activated microglia were identified using chromogen immunohistochemistry for Iba1. Free-floating sections were rinsed in 0.05 m KPBS. Endogenous peroxidases were blocked using a 10-min incubation of 3% H2O2 in methanol and then preincubated in blocking buffer, consisting of 0.05 m KPBS with 0.4% Triton X-100 and 2% donkey serum, for 30 min. Sections were then incubated with rabbit anti-Iba1 antibody (1:1000; Wako Chemicals, Richmond, VA) for 48 h. After incubation, the tissue was again rinsed in 0.05 m KPBS, incubated in biotinylated donkey antirabbit IgG (1:600; Jackson ImmunoResearch) in KPBS with 0.4% Triton X-100 for 1 h, and then washed and incubated in avidin-biotin solution (Vectastain; Vector Laboratories, Burlingame, CA) for 1 h. Iba1-ir was visualized with 3,3′-diaminobenzidine enhanced with nickel chloride. Tissue sections were mounted on gelatin-coated glass slides and coverslipped. Iba1-ir was determined by measuring both immunoreactive area and integrated OD using Metamorph software. For these analyses, four images at ×10 magnification were captured from each section of three sections representing the left/right and medial/lateral aspects of the ARH. Anatomically matched sections were chosen for each animal from the rostral, medial, and caudal extents of the ARH. Thus, 12 images were captured from each brain, using identical camera settings. For the image analysis, identical threshold levels were used for each image to eliminate background. The values for all 12 readings were averaged to obtain a single value for each animal. Samples were analyzed for four animals from the mCTR, mHFD, and mDR groups. For publication, representative images were captured at ×4 and ×10 magnification, and contrast/brightness levels were adjusted (using identical settings) in Adobe Photoshop.
Cortisol assays
ACTH and cortisol assays were performed by the Oregon National Primate Research Center Endocrine Services Core. Briefly, hormones were measured in 10 μl monkey serum using an Immulite 2000 automated assay machine (Diagnostics Systems Laboratory, Inc., Los Angeles, CA). Inter- and intraassay coefficients of variation were 6.7 and 2.0%, respectively.
Proteome profiler cytokine array
Relative expression of plasma cytokines was quantified using the Proteome Profiler Human Cytokine Array, Panel A Array Kit (R&D Systems). The array was performed according to manufacturer’s exact specifications using 250 μl plasma (mCTR and mDR, n = 4 per group).
Real-time PCR of feeding-related targets
Standard quantitative real-time PCR (qPCR) protocol was followed as previously described (31). The following inventoried human primers/probe sets (Applied Biosystems, Inc., Foster City, CA) were validated for recognition of the NHP sequence: AgRP, no. Hs00361403_g1; brain-derived neurotrophic factor (BDNF), no. Hs00538277_m1; cocaine- and amphetamine-regulated transcript (CART), no. Hs00182861_m1; IGF-I, no. Hs00609566_m1; insulin receptor (INSR), no. Hs00169631_m1; insulin receptor substrate 1(IRS1), no. Hs00178563_m1; insulin receptor substrate 2 (IRS2), no. Hs00275843_s1; leptin receptor (LEPR), no. Hs00174497_m1; μ-opioid receptor (MOR), no. Hs0016850_m1; NPY, no. Hs00173470_m1; NPY1 receptor (NPY1R) no. Hs00197884_m1; suppressor of cytokine signaling-3 (SOCS3), no. Hs00269575_s1; and vascular endothelial growth factor (VEGF), no. Rh02621759_m1. All sequences had greater than 95% homology with the macaque genome according to published sequences. Macaque-specific probe and primer sets for MC4R and UCP2 were designed using Primer Express Software due to poor regional homology with human primers: MC4R, forward AGGCTTCACATTAAGAGGATTGCT, reverse ACGCCAATCAGGATGGTCAA, and probe CGCTCCCTTCATATTGGCGCCTTG, and UCP2, forward GGCAGAGTTCCTCTATCTCGTCTT, reverse CCCAACCATGATGCTGATTTC, and probe TGCCCCATCTCGGTTTTTCTCCATCT. All qPCR data are expressed as a ratio of target to 18S RNA expression. Beforehand, 18S RNA levels were determined to not be affected with respect to diet.
Inflammatory cytokines and receptors superarray
RNA isolation and reverse transcription was performed as previously described (24). qPCR was performed according to the manufacturer’s directions (RT2 Profiler PCR Array User Manual, version 1.5) using RT2 Profiler PCR Array Human Inflammatory Cytokines and Receptors array (catalog no. APH-011A) on an ABI 7300 Real-Time PCR System (Applied Biosystems). Auto cycle threshold (Ct) values were calculated using 7300 RQ Study Software version 1.3, verified, and adjusted as necessary. Gene expression values are expressed as ΔCt for each set of duplicates. The fold change for each gene was calculated as 2(−ΔΔCt), where ΔΔCt = ΔCt (mHFD group) − ΔCt (mCTR group). The wells from all significantly regulated genes were analyzed for specificity by demonstrating a single amplified band on an agarose gel and by DNA sequence analysis.
Statistical analysis
Comparisons were conducted using Student’s t test with Prism Biostatistical Software (version 4) (San Diego, CA). Differences were considered significant if P < 0.05. Data are presented as mean ± se.
Results
A complete characterization of our NHP maternal HFD model has been previously published (24). Briefly, adult pregnant monkeys on a HFD, as a group, have significantly increased body weight, adiposity, and fasting blood insulin and leptin levels during the third trimester. We previously reported that adult pregnant monkeys on a HFD segregate into diet-sensitive and diet-resistant phenotypes (24). The diet-sensitive animals have a 14% increase in body weight, with a near doubling of body fat, and a 3-fold increase in insulin secretion during iv glucose tolerance tests but have normal glucose clearance, indicating they are insulin resistant but not diabetic. In contrast, diet-resistant animals have no significant changes in any of these parameters, and their metabolic phenotype is the same as animals consuming a chow diet.
Previously, we demonstrated the mHFD offspring had slightly lower (but statistically significant) body weights at G130, but leptin and insulin levels were not different from mCTR (24). It should also be noted that leptin levels were low to undetectable in all offspring, despite some of the pregnant animals being very hyperleptinemic. This low fetal leptin indicates that leptin from the mother is not crossing the placental barrier in significant quantities at this early gestational age and that early third-trimester fetus has very little white adipose tissue. Furthermore, there were no differences in fetal circulating glucose or free fatty acids between the groups. Overall, the most notable phenotype of the mHFD, irrespective of maternal adiposity or insulin sensitivity, was evidence of nonalcoholic fatty liver disease, as indicated by increased liver triglycerides, oxidative stress/damage, and reprogramming of hepatic gluconeogenesis pathways. These offspring also have increased circulating levels of cytokines. In the data presented in this study, it should be noted that we observed no fetal segregation of effects dependent on maternal phenotype/response to the HFD; therefore, results from all mHFD were combined. Furthermore, both the mCTR and mHFD groups were balanced for the sex of the offspring, and no sex-dependent differences or abnormalities were observed in the fetuses, so results from males and females were combined.
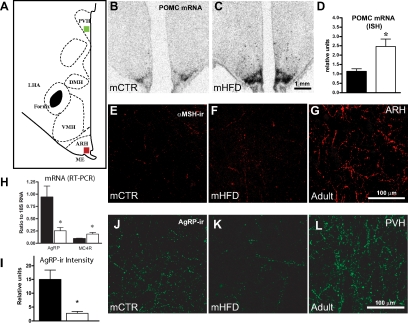
The present study focused on effects of fetal exposure to HFD on the development of the ARH melanocortin system. In the mCTR animals, POMC mRNA (Fig. 1B) was present throughout the rostral/caudal extent of the ARH; however, levels were low, consistent with this system being immature at this gestational age as previously shown (18). In mHFD animals, POMC expression was 2-fold up-regulated (P < 0.05) (Fig. 1, C and D). Consistent with previous studies (18), α-MSH-ir was low to undetectable in the mCTR group, and qualitatively, there was no apparent difference compared with the mHFD group (Fig. 1, E and F). In addition, levels of ACTH- and β-endorphin-ir were also low in all fetal animals (data not shown). In contrast, robust levels of α-MSH-ir (Fig. 1G) and ACTH- and β-endorphin-ir (data not shown) were present in the adult, validating these antibodies. Thus, the increased POMC expression does not appear to be translated into increased peptide levels.
Figure 1.
Effects of mHFD on the fetal melanocortin system. A, Schematic of hypothalamic area. B and C, Representative low-power autoradiographic images of POMC in situ hybridization signal in the ARH in mCTR (B) and mHFD (C) G130 hypothalamus. Images correspond to bregma −06.75 mm according to the brain atlas (75). Scale bar, 1 mm. D, Maternal consumption of HFD caused 2-fold up-regulation of POMC mRNA in fetal ARH (n = 4–5; *, P < 0.05) (black, mCTR; white, mHFD). E–G, Representative fluorescent confocal micrographs of αMSH-ir in ARH in approximate location of red square on schematic in A. α-MSH-ir is low to undetectable (mCTR, E; mHFD, F) in the ARH and not evident in other projection areas (data not shown). Note adult α-MSH-ir is robust in G. H, Maternal consumption of HFD caused a decrease in AgRP mRNA and increase in MC4R mRNA expression in G130 offspring by real-time PCR of medial basal hypothalamic blocks (n = 4–7; *, P < 0.05) (black, mCTR; white, mHFD). I, Overall AgRP-ir in PVH is 6-fold higher in mCTR than mHFD (n = 4–5; *, P < 0.05) (black, mCTR; white, mHFD). J–L, Representative fluorescent confocal micrographs of AgRP-ir in the PVH in the general area denoted by green square in A. PVH is a terminal projection field for AgRP fibers from ARH. mCTR AgRP-ir (J) is greater than mHFD (K). Adult immunoreactivity (L) is shown for comparison. Scale bar, 100 μm.
In the fetal hypothalamus, NPY mRNA, as determined by in situ hybridization, was present in the ARH and paraventricular nucleus of the hypothalamus (PVH), but there was no difference in the expression (reported in relative units) between the two groups (mCTR, n = 5, 3.78 ± 1.40; mHFD, n = 3, 3.29 ± 1.32) (data not shown). NPY mRNA was also quantified in separate hypothalamic samples by qPCR, and no difference was observed. In contrast, as determined by qPCR, mHFD exhibited a near 3-fold reduction in AgRP mRNA (mCTR, n = 4, 0.94 ± 0.22; mHFD, n = 7, 0.38 ± 0.11; P < 0.05) as well as a 2-fold up-regulation of MC4R mRNA (mCTR, n = 4, 0.93 ± 0.07; mHFD, n = 7, 1.80 ± 0.24; P < 0.05) (Fig. 1H). Expression of MC3R mRNA was also measured in these samples but was undetectable (not shown). A number of other relevant genes were also analyzed (Supplemental Table 1). Although BDNF, SOCS3, and UCP2 were elevated in F-HFD, none of these changes were statistically significant.
Because ARH AgRP/NPY projections to the PVH develop during the third trimester (18), NPY- and AgRP-ir were quantified in the PVH. No measurable difference in NPY-ir was detected in the PVH between mCTR and mHFD animals (mCTR, n = 4, 3.00 ± 0.62; mHFD, n = 7, 3.05 ± 0.56). In contrast, AgRP-ir fluorescence was decreased 6-fold in mHFD in the PVH (Fig. 1I). Although AgRP-ir fibers were readily detectable in mCTR (Fig. 1J), they were sparse in the F-HFD (Fig. 1K). AgRP-ir in the PVH in the adult is shown for comparison (Fig. 1L). Although not quantified, it is worth noting that the decrease in AgRP-ir was not limited to the PVH but was notably lower throughout the hypothalamus.
Cortisol is known to be elevated in response to HFD; it readily crosses the placenta and regulates hypothalamic neuropeptide expression (32,33). In our model, there was a small but significant increase in both maternal (CTR, 352.0 ± 49.8, n = 6; HFD, 461.1 ± 14.8, n = 8; P = 0.035) and fetal (mCTR, 103.1 ± 5.0, n = 7; mHFD, 128.1 ± 7.8, n = 8; P = 0.022) cortisol levels in the mHFD compared with mCTR, taken at the time of cesarean section. Circulating ACTH levels were also measured, but no differences were found (mCTR, n = 8, 81.2 ± 16.7 ng/ml; mHFD, n = 18, 94.7 ± 14.3 ng/ml).
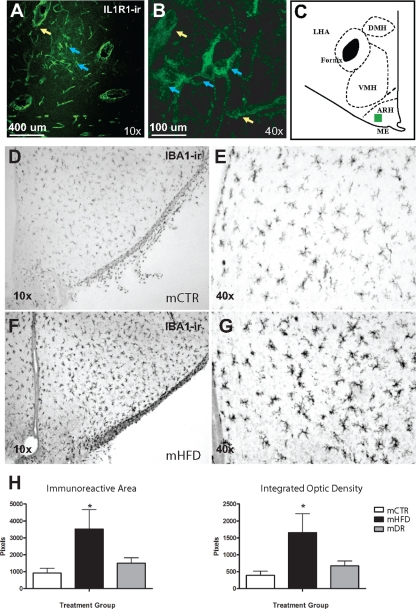
Because inflammatory cytokines are known to directly influence hypothalamic POMC and AgRP neurons (25,27,34), we used a qPCR-based cytokine-directed Superarray. Of 84 targets investigated, eight distinct inflammatory factors and receptors were up-regulated in F-HFD compared with F-CTR, including IL-1β and IL-1R1 (Table 1). To identify cellular localization of IL-1R1, immunohistochemistry was used. In both F-CTR and F-HFD, the vast majority of IL-1R1-ir was localized to endothelial cells of the microvasculature (Fig. 2, A and B), with some immunoreactivity localized to neurons (Fig. 2B) in the lateral aspects of the ARH (Fig. 2C). However, no qualitative difference could be determined. As an additional method to determine the impact of cytokines on the melanocortin system, we also stained sections with the marker of activated microglia Iba1. Iba1-ir, both immunoreactive area and density of staining, was significantly increased in ARH of the mHFD (Fig. 2, F–H) compared with mCTR group (Fig. 2, D and E).
Table 1.
Brain cytokines are up-regulated in mHFD compared with mCTR offspring
| Gene | Fold increase |
|---|---|
| IL-1 family | |
| IL-1β | 7.39a |
| IL-1R1 | 1.76a |
| IL-1F6 | 22.48b |
| IL-1F7 | 2.35b |
| BBB trafficking | |
| CCL26 (eotaxin) | 4.02b |
| CCR3 (eotaxin-R) | 7.53b |
| CCL19 (MIP-3) | 3.01a |
| CCL2 (MCP-1) | 3.09b |
The fold change for each gene was calculated as 2−ΔΔCt, where ΔΔCt = ΔCt (mHFD group) − ΔCt (mCTR group); n = 4–7. BBB, Blood-brain barrier.
P < 0.01.
P < 0.05.
Figure 2.
mHFD causes activation of inflammatory response in the fetal hypothalamus. A–C, IL-1R1-ir in the ventromedial hypothalamus. A, Representative ×10 confocal fluorescent micrograph of IL-1R1-ir shown in region demarcated by the green square in C (yellow arrows, vascular endothelial cells; blue arrows, lateral ARH neurons). B, Representative ×40 confocal fluorescent micrograph of IL-1R1-ir. D–H, Iba1-ir in the fetal hypothalamus: D and E, Iba1-ir in the ARH of a mCTR offspring; F and G, Iba1-ir in the ARH of a mHFD offspring. A and C represent ×4 magnification images of the ARH. B and D represent ×10 magnification images of the ventromedial region of the ARH. The third ventricle is on the left edge of the image. H, Quantitative analysis of Iba1-ir area (left) and integrated OD (right). n= 4 per group; *, P < 0.05.
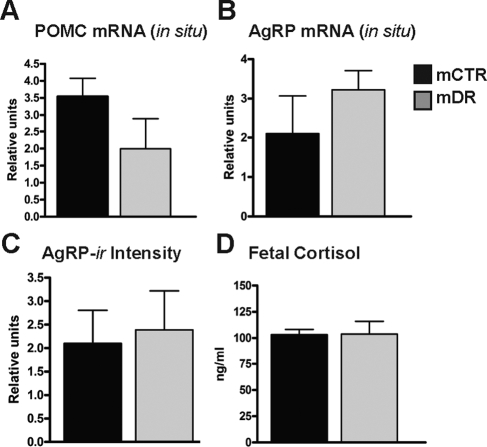
In part 2, we sought to determine the effect of switching chronic HFD animals (animals maintained on the HFD for 4 yr) to a CTR diet specifically during pregnancy. The fetal offspring of these animals (mDR) were compared with offspring of age-matched controls. Previously, our group had demonstrated that this DR prevented many of the abnormalities in the fetal liver (24). Compared with mCTR, the mDR offspring had normal POMC and AgRP mRNA expression (Fig. 3, A and B), as determined by in situ hybridization, and AgRP-ir (Fig. 3C). Furthermore, consistent with a normalization of the maternal cortisol in the HFD animals switched back to a control diet (not shown), the mDR offspring had normal cortisol (Fig. 3D) and circulating cytokine levels (Supplemental Table 2). Finally, we demonstrated that Iba1-ir was also normalized in the mDR animals compared with mCTR and mHFD groups (Fig. 2H).
Figure 3.
Effects of mDR on fetal POMC, AgRP, and cortisol. The mDR group is a cohort of animals that were maintained on the HFD for 4 yr and then switched back to the CTR just before the breeding season of the fifth year. A and B, Quantification of fetal in situ hybridization of POMC and AgRP mRNA; C, comparison of AgRP-ir fiber fluorescence; D, comparison of fetal cortisol measurements expressed in nanograms per milliliter (black, mCTR; gray, mDR).
Discussion
There is an abundance of population-based human studies demonstrating a strong association between maternal obesity and early-onset obesity and insulin resistance in the offspring (35,36,37,38,39). However, although these studies make suggestions toward intrauterine environment, including maternal diet, there are actually very few data directly investigating the link between maternal HFD consumption and metabolic disease in children. Furthermore, there is even less information pertaining to effects on brain development in humans. One of the major limitations to the human studies is the inability to accurately monitor dietary intake as well as a poor understanding in what is occurring early in development. In these studies, we use a NHP model of chronic maternal HFD consumption. A surprising result from our ongoing studies in this model is that the mHFD offspring have smaller body weights in the early third trimester but display catch-up growth and increased adiposity in the postnatal period. The cause of small for gestational age (SGA) is not fully understood, but the phenomenon of catch-up growth and the risk of obesity is well characterized in SGA babies, commonly referred to as Barker’s hypothesis or the thrifty-phenotype hypothesis (40,41,42). In rodents, which are born more developmentally immature compared with primates, early postnatal overnutrition and undernutrition results ultimately in obesity and insulin resistance, a result that has been directly linked to abnormalities in the development of hypothalamic circuits (9,43,44,45,46,47,48,49). However, the development of the circuits controlling body weight occurs in utero in the primate as opposed to postnatally in the rodent. This developmental difference in primates makes the fetus particularly dependent on maternal environment.
In the present study, we report that the hypothalamic melanocortin system of third-trimester offspring is altered by prolonged maternal exposure to HFD, exhibited by up-regulation of POMC and MC4R mRNA and down-regulation of AgRP mRNA in the ARH. In studies using adult rodents, there are many reports of altered melanocortin (POMC and AgRP) gene expression in response to HFD consumption (50,51,52,53,54,55,56,57). Therefore, the observed changes in POMC and AgRP mRNA may simply reflect an attempt to adapt to lipid excess. However, the effects of a HFD on the melanocortin system have never been reported in the NHP; thus, it is difficult to anticipate from the rodent studies what to expect in the fetal NHP brain in response to maternal HFD consumption. Studies involving maternal overfeeding (which results in fetal hyperglycemia) or direct fetal glucose infusion in the ovine model, which has a similar developmental profile for hypothalamic circuits, have reported similar increases in POMC gene expression in the fetal and neonatal offspring; however, changes in NPY and AgRP were not observed (58,59). The reason for the specific effects on POMC expression in the ovine model are not known but could be due to the difference in the effects of the fetal hyperglycemia, which was not observed in our studies, and the effects of the hyperlipidemia observed in our studies. In our model, the fetus experiences the constant passive influx of lipids over the course of 130 d gestation, before and leading up to the birth of POMC and AgRP neurons. Although the changes in POMC and AgRP mRNAs that we observed may be an appropriate adaptive response of the melanocortin neurons to high circulating nutrient levels under certain circumstances, it could have dire consequences on the development of these neurons and their projections. Interestingly, the effect of maternal HFD in the fetal hypothalamus was not widespread, because many neuropeptide systems were not different between the mCTR and mHFD offspring, including NPY, which is colocalized in AgRP neurons.
Based on the dramatic increased POMC mRNA observed, we initially hypothesized that the mHFD might display accelerated development and/or increased projections of POMC neurons to downstream hypothalamic targets. Surprisingly, αMSH-ir fibers in the PVH and ARH were sparse in both mCTR and mHFD offspring, similar to that previously described (18). This lack of peptide was also not due to differential production of other POMC cleavage productions, such as β-endorphin and ACTH, because they were similarly low in both the mCTR and mHFD offspring. Furthermore, prohormone convertases 1 and 2 mRNA expression was readily detectable in the hypothalamus (data not shown), indicating that the necessary enzymes are present for the production of α-MSH-ir. Therefore, it seems likely that the increased ARH POMC mRNA expression is not being translated to alter peptide levels. In contrast, AgRP-ir fiber projections to the PVH, which develop earlier than α-MSH projections, were significantly reduced, consistent with the reduction in AgRP mRNA. However, it is not possible to determine whether the decreased AgRP-ir was due to abnormalities in fiber projections or just a decrease in peptide levels. Nonetheless, these neurons are being abnormally driven during a critical period of development and could result in deficiencies in the development of the circuits, which could have broad physiological consequences later in life. Future studies will determine whether the abnormalities in the mHFD melanocortin system persist into the postnatal period; however, we have already reported that mHFD offspring display catch-up growth during the postnatal period, leading to early-onset obesity (24).
With this model, it is not possible to determine what is driving the changes in the melanocortins (i.e. POMC and AgRP), whether it is a direct effect of the lipids or indirect via activation of other systems. Melanocortin expression has been shown to be affected by central and peripheral proinflammatory cytokine administration and can function as an antiinflammatory agent in rodents and NHP. Specifically, in the rodent, IL-1β has been shown to directly activate POMC neurons, with increased neuron activation and peptide release, while directly inhibiting AgRP neurons, with increased mRNA expression and release (25,27,34). Conversely, α-MSH is known to antagonize many actions of IL-1 and other cytokines, including fever, anorexia, anxiety, and hypothalamic-pituitary-adrenal (HPA) axis activation (60,61,62,63,64,65). The neuroendocrine effects of cytokine exposure are known to be inhibited by α-MSH and augmented by AgRP infusion via melanocortin receptors in both the rodent and primate (66,67,68). These studies reflect an important relationship between the melanocortin and cytokine systems centrally. In our previous studies (24), we demonstrated that mHFD offspring have elevated circulating cytokine levels that may impact the central melanocortin system. In the current studies, we observed elevations in the basal hypothalamus in two main categories of cytokines: 1) the IL-1 family and 2) cytokines expressly involved with trafficking across the blood-brain barrier. Furthermore, we demonstrated that there is increased activation of microglia within the ARH and hypothalamus, supporting the concept of an overall inflammatory response. Taken as a whole, these data suggest that the maternal HFD is driving the proinflammatory pathway, which in turn may be driving POMC gene expression up and AgRP expression down in the fetus and may potentially impact the development of the blood-brain barrier.
High-fat feeding and proinflammatory cytokines are known to increase cortisol levels in adults (69). In the current study, we have observed significantly increased maternal cortisol levels in HFD offspring. It is unclear whether this fetal increase is due to a direct effect of the HFD or inflammatory cytokines on the fetal HPA axis or due to passive flux of maternal cortisol, which was also elevated. However, elevated cortisol levels during fetal development have been shown to prematurely activate the HPA axis and expedite the maturation of fetal organs (70). Excessive amounts of cortisol can cause intrauterine growth retardation, which in many models results in adult-onset obesity and diabetes (70). In our previous studies in these same animals, fetal weight was significantly reduced and may reflect intrauterine effects (24).
A crucial unanswered question remains as to whether these changes in the fetus persist into postnatal life and further on into adulthood and consequently impact long-term disease risk. Future studies in the primate are necessary to determine the persistence of fetal changes. Nevertheless, in the rodent, it is established that in utero exposure to impaired glucose tolerance and gestational diabetes results in long-term alterations in hypothalamic architecture and physiology (47,48,71). Furthermore, postnatal overfeeding in rodents also has long-lasting effects on hypothalamic function and hormone responsiveness (44,45,46,49). Finally, it has recently been demonstrated in an obesity-prone rat model, linked to polygenic inheritance, that the obese rats have disrupted development of the hypothalamic melanocortin system that is likely at least partially the cause of the susceptibility to diet-induced obesity (72,73). These observations highlight and support our assumption that the hypothalamic changes observed in mHFD fetal primate offspring contribute to long-term disease risks, including potential effects on body weight homeostasis, stress/anxiety, and cardiovascular disease. However, it is important to understand that the changes in the hypothalamic system, as well as our earlier observations in the fetal liver (24), occurred independently of maternal obesity or insulin resistance.
In humans, modest changes in body weight with diet intervention are known to improve metabolic parameters associated with obesity and diabetes. Thus, generalized clinical practice is to encourage limited weight gain (<15 lbs., normal 25–30 lbs.) for obese women during pregnancy. This is accomplished by a combination of limiting calorie quantity as well as altering the quality. Human studies have shown when weight gain during pregnancy is limited, there is a decreased incidence in macrosomia, cesarean delivery, and preeclampsia; however, SGA babies are more prevalent in these mothers (74). SGA is also associated with an obesity phenotype in children and adults. In this study, DR animals were placed on an ad libitum low-fat CTR diet specifically during pregnancy. Although this caused only a subtle change in the metabolic phenotype of these animals (see Ref. 24), the fetal hypothalamic melanocortin expression, activation of microglia, and circulating cortisol was normalized in this cohort. Thus, healthful, low-fat eating may be the best method to protect offspring of obese mothers from in utero-induced long-term body weight dysregulation.
Although only approximately 5% of the prevalent cases of obesity are due to single-locus gene mutations, a large majority of these are a result of MC4R and POMC mutations that result in varying levels of obesity, hyperphagia, hyperinsulinemia, and hyperglycemia, which often begin during childhood (5). This attests to the importance and susceptibility of the melanocortin system to body weight maintenance. Taken together, increased POMC and decreased AgRP expression implies targeted action of HFD on the melanocortin system. This action is in the absence of effects on other feeding-related pathways, notably NPY. Furthermore, there is a dramatic hypothalamic up-regulation of proinflammatory cytokines in offspring from mothers that consumed HFD during pregnancy. Although it is undetermined whether this environment will have lasting effects on the offspring, we hypothesize that this early challenge to the melanocortin pathway may be a critical factor in the development of early-onset obesity in juvenile offspring.
Supplementary Material
Footnotes
Disclosure Summary: None of the authors have disclosures related to this study.
First Published Online February 22, 2010
Abbreviations: AgRP, Agouti-related peptide; ARH, arcuate nucleus of the hypothalamus; Ct, cycle threshold; CTR, control; F-CTR, fetal control; G130, gestational d 130; HFD, high-fat diet; HPA, hypothalamic-pituitary-adrenal; ir, immunoreactivity; mCTR, maternal control diet; mDR, maternal diet reversal; mHFD, maternal high-fat diet; NPY, neuropeptide Y; NHP, nonhuman primate; POMC, proopiomelanocortin; PVH, paraventricular nucleus of the hypothalamus; qPCR, quantitative real-time PCR; SGA, small for gestational age.
References
- Ollmann MM, Wilson BD, Yang YK, Kerns JA, Chen Y, Gantz I, Barsh GS 1997 Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science 278:135–138 [DOI] [PubMed] [Google Scholar]
- Broberger C, Johansen J, Johansson C, Schalling M, Hökfelt T 1998 The neuropeptide Y/agouti gene-related protein (AGRP) brain circuitry in normal, anorectic, and monosodium glutamate-treated mice. Proc Natl Acad Sci USA 95:15043–15048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone RD 2005 Anatomy and regulation of the central melanocortin system. Nat Neurosci 8:571–578 [DOI] [PubMed] [Google Scholar]
- Farooqi IS, O'Rahilly S 2008 Mutations in ligands and receptors of the leptin-melanocortin pathway that lead to obesity. Nat Clin Pract Endocrinol Metab 4:569–577 [DOI] [PubMed] [Google Scholar]
- Farooqi IS, O'Rahilly S 2007 Genetic factors in human obesity. Obes Rev 8(Suppl 1):37–40 [DOI] [PubMed] [Google Scholar]
- Grove KL, Chen P, Koegler FH, Schiffmaker A, Smith MS, Cameron JL 2003 Fasting activates neuropeptide Y neurons in the arcuate nucleus and the paraventricular nucleus in the rhesus macaque. Brain Res Mol Brain Res 113:133–138 [DOI] [PubMed] [Google Scholar]
- Koegler FH, Grove KL, Schiffmacher A, Smith MS, Cameron JL 2001 Central melanocortin receptors mediate changes in food intake in the rhesus macaque. Endocrinology 142:2586–2592 [DOI] [PubMed] [Google Scholar]
- Bouret SG, Draper SJ, Simerly RB 2004 Formation of projection pathways from the arcuate nucleus of the hypothalamus to hypothalamic regions implicated in the neural control of feeding behavior in mice. J Neurosci 24:2797–2805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grove KL, Allen S, Grayson BE, Smith MS 2003 Postnatal development of the hypothalamic neuropeptide Y system. Neuroscience 116:393–406 [DOI] [PubMed] [Google Scholar]
- Ifft JD 1972 An autoradiographic study of the time of final division of neurons in rat hypothalamic nuclei. J Comp Neurol 144:193–204 [DOI] [PubMed] [Google Scholar]
- Khachaturian H, Lewis ME, Alessi NE, Watson SJ 1985 Time of origin of opioid peptide-containing neurons in the rat hypothalamus. J Comp Neurol 236:538–546 [DOI] [PubMed] [Google Scholar]
- Grove KL, Smith MS 2003 Ontogeny of the hypothalamic neuropeptide Y system. Physiol Behav 79:47–63 [DOI] [PubMed] [Google Scholar]
- Bouret SG, Draper SJ, Simerly RB 2004 Trophic action of leptin on hypothalamic neurons that regulate feeding. Science 304:108–110 [DOI] [PubMed] [Google Scholar]
- Plagemann A, Harder T, Rake A, Melchior K, Rohde W, Dörner G 1999 Increased number of galanin-neurons in the paraventricular hypothalamic nucleus of neonatally overfed weanling rats. Brain Res 818:160–163 [DOI] [PubMed] [Google Scholar]
- Grove KL, Grayson BE, Glavas MM, Xiao XQ, Smith MS 2005 Development of metabolic systems. Physiol Behav 86:646–660 [DOI] [PubMed] [Google Scholar]
- Guo F, Jen KL 1995 High-fat feeding during pregnancy and lactation affects offspring metabolism in rats. Physiol Behav 57:681–686 [DOI] [PubMed] [Google Scholar]
- Srinivasan M, Katewa SD, Palaniyappan A, Pandya JD, Patel MS 2006 Maternal high-fat diet consumption results in fetal malprogramming predisposing to the onset of metabolic syndrome-like phenotype in adulthood. Am J Physiol Endocrinol Metab 291:E792–E799 [DOI] [PubMed] [Google Scholar]
- Grayson BE, Allen SE, Billes SK, Williams SM, Smith MS, Grove KL 2006 Prenatal development of hypothalamic neuropeptide systems in the nonhuman primate. Neuroscience 143:975–986 [DOI] [PubMed] [Google Scholar]
- Dietz WH 1994 Critical periods in childhood for the development of obesity. Am J Clin Nutr 59:955–959 [DOI] [PubMed] [Google Scholar]
- Plagemann A, Harder T, Kohlhoff R, Rohde W, Dörner G 1997 Overweight and obesity in infants of mothers with long-term insulin-dependent diabetes or gestational diabetes. Int J Obes Relat Metab Disord 21:451–456 [DOI] [PubMed] [Google Scholar]
- Gillman MW, Rifas-Shiman S, Berkey CS, Field AE, Colditz GA 2003 Maternal gestational diabetes, birth weight, and adolescent obesity. Pediatrics 111:e221–e226 [DOI] [PubMed] [Google Scholar]
- Lindsay RS, Hanson RL, Bennett PH, Knowler WC 2000 Secular trends in birth weight, BMI, and diabetes in the offspring of diabetic mothers. Diabetes Care 23:1249–1254 [DOI] [PubMed] [Google Scholar]
- Baskin ML, Ard J, Franklin F, Allison DB 2005 Prevalence of obesity in the United States. Obes Rev 6:5–7 [DOI] [PubMed] [Google Scholar]
- McCurdy CE, Bishop JM, Williams SM, Grayson BE, Smith MS, Friedman JE, Grove KL 2009 Maternal high-fat diet triggers lipotoxicity in the fetal livers of nonhuman primates. J Clin Invest 119:323–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarlett JM, Jobst EE, Enriori PJ, Bowe DD, Batra AK, Grant WF, Cowley MA, Marks DL 2007 Regulation of central melanocortin signaling by interleukin-1β. Endocrinology 148:4217–4225 [DOI] [PubMed] [Google Scholar]
- Scarlett JM, Marks DL 2005 The use of melanocortin antagonists in cachexia of chronic disease. Expert Opin Investig Drugs 14:1233–1239 [DOI] [PubMed] [Google Scholar]
- Scarlett JM, Zhu X, Enriori PJ, Bowe DD, Batra AK, Levasseur PR, Grant WF, Meguid MM, Cowley MA, Marks DL 2008 Regulation of agouti-related protein messenger ribonucleic acid transcription and peptide secretion by acute and chronic inflammation. Endocrinology 149:4837–4845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enriori PJ, Evans AE, Sinnayah P, Jobst EE, Tonelli-Lemos L, Billes SK, Glavas MM, Grayson BE, Perello M, Nillni EA, Grove KL, Cowley MA 2007 Diet-induced obesity causes severe but reversible leptin resistance in arcuate melanocortin neurons. Cell Metab 5:181–194 [DOI] [PubMed] [Google Scholar]
- Grove KL, Sekhon HS, Brogan RS, Keller JA, Smith MS, Spindel ER 2001 Chronic maternal nicotine exposure alters neuronal systems in the arcuate nucleus that regulate feeding behavior in the newborn rhesus macaque. J Clin Endocrinol Metab 86:5420–5426 [DOI] [PubMed] [Google Scholar]
- Grove KL, Campbell RE, Ffrench-Mullen JM, Cowley MA, Smith MS 2000 Neuropeptide Y Y5 receptor protein in the cortical/limbic system and brainstem of the rat: expression on γ-aminobutyric acid and corticotropin-releasing hormone neurons. Neuroscience 100:731–740 [DOI] [PubMed] [Google Scholar]
- Xiao XQ, Grove KL, Lau SY, McWeeney S, Smith MS 2005 Deoxyribonucleic acid microarray analysis of gene expression pattern in the arcuate nucleus/ventromedial nucleus of hypothalamus during lactation. Endocrinology 146:4391–4398 [DOI] [PubMed] [Google Scholar]
- Power ML, Schulkin J 2006 Functions of corticotropin-releasing hormone in anthropoid primates: from brain to placenta. Am J Hum Biol 18:431–447 [DOI] [PubMed] [Google Scholar]
- Brooks AN, Currie IS, Gibson F, Thomas GB 1992 Neuroendocrine regulation of sheep fetuses. J Reprod Fertil Suppl 45:69–84 [PubMed] [Google Scholar]
- DeBoer MD, Scarlett JM, Levasseur PR, Grant WF, Marks DL 2009 Administration of IL-1β to the 4th ventricle causes anorexia that is blocked by agouti-related peptide and that coincides with activation of tyrosine-hydroxylase neurons in the nucleus of the solitary tract. Peptides 30:210–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM 2004 Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. JAMA 291:2847–2850 [DOI] [PubMed] [Google Scholar]
- Ong KK, Emmett PM, Noble S, Ness A, Dunger DB 2006 Dietary energy intake at the age of 4 months predicts postnatal weight gain and childhood body mass index. Pediatrics 117:e503–e508 [DOI] [PubMed] [Google Scholar]
- Schaefer-Graf UM, Pawliczak J, Passow D, Hartmann R, Rossi R, Bührer C, Harder T, Plagemann A, Vetter K, Kordonouri O 2005 Birth weight and parental BMI predict overweight in children from mothers with gestational diabetes. Diabetes Care 28:1745–1750 [DOI] [PubMed] [Google Scholar]
- Catalano PM, Presley L, Minium J, Hauguel-de Mouzon S 2009 Fetuses of obese mothers develop insulin resistance in utero. Diabetes Care 32:1076–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman DJ 21 October 2009 Effects of maternal obesity on fetal growth and body composition: implications for programming and future health. Semin Fetal Neonatal Med 10.1016/j.siny.2009.09.001 [DOI] [PubMed] [Google Scholar]
- Barker DJ, Eriksson JG, Forsén T, Osmond C 2002 Fetal origins of adult disease: strength of effects and biological basis. Int J Epidemiol 31:1235–1239 [DOI] [PubMed] [Google Scholar]
- Barker DJ 2007 The origins of the developmental origins theory. J Intern Med 261:412–417 [DOI] [PubMed] [Google Scholar]
- Barker DJ 1990 The fetal and infant origins of adult disease. BMJ 301:1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson BE, Kievit P, Smith MS, Grove KL 12 October 2009 Critical determinants of hypothalamic appetitive neuropeptide development and expression: Species considerations. Front Neuroendocrinol 10.1016/j.yfrne.2009.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidowa H, Plagemann A 2000 Different responses of ventromedial hypothalamic neurons to leptin in normal and early postnatally overfed rats. Neurosci Lett 293:21–24 [DOI] [PubMed] [Google Scholar]
- Davidowa H, Plagemann A 2000 Decreased inhibition by leptin of hypothalamic arcuate neurons in neonatally overfed young rats. Neuroreport 11:2795–2798 [DOI] [PubMed] [Google Scholar]
- Heidel E, Plagemann A, Davidowa H 1999 Increased response to NPY of hypothalamic VMN neurons in postnatally overfed juvenile rats. Neuroreport 10:1827–1831 [DOI] [PubMed] [Google Scholar]
- Plagemann A, Harder T, Melchior K, Rake A, Rohde W, Dörner G 1999 Elevation of hypothalamic neuropeptide Y-neurons in adult offspring of diabetic mother rats. Neuroreport 10:3211–3216 [DOI] [PubMed] [Google Scholar]
- Plagemann A, Harder T, Rake A, Janert U, Melchior K, Rohde W, Dörner G 1999 Morphological alterations of hypothalamic nuclei due to intrahypothalamic hyperinsulinism in newborn rats. Int J Dev Neurosci 17:37–44 [DOI] [PubMed] [Google Scholar]
- Plagemann A, Harder T, Rake A, Waas T, Melchior K, Ziska T, Rohde W, Dörner G 1999 Observations on the orexigenic hypothalamic neuropeptide Y-system in neonatally overfed weanling rats. J Neuroendocrinol 11:541–546 [DOI] [PubMed] [Google Scholar]
- Bergen HT, Mizuno T, Taylor J, Mobbs CV 1999 Resistance to diet-induced obesity is associated with increased proopiomelanocortin mRNA and decreased neuropeptide Y mRNA in the hypothalamus. Brain Res 851:198–203 [DOI] [PubMed] [Google Scholar]
- Torri C, Pedrazzi P, Leo G, Müller EE, Cocchi D, Agnati LF, Zoli M 2002 Diet-induced changes in hypothalamic pro-opio-melanocortin mRNA in the rat hypothalamus. Peptides 23:1063–1068 [DOI] [PubMed] [Google Scholar]
- Ziotopoulou M, Mantzoros CS, Hileman SM, Flier JS 2000 Differential expression of hypothalamic neuropeptides in the early phase of diet-induced obesity in mice. Am J Physiol Endocrinol Metab 279:E838–E845 [DOI] [PubMed] [Google Scholar]
- Huang XF, Han M, South T, Storlien L 2003 Altered levels of POMC, AgRP and MC4-R mRNA expression in the hypothalamus and other parts of the limbic system of mice prone or resistant to chronic high-energy diet-induced obesity. Brain Res 992:9–19 [DOI] [PubMed] [Google Scholar]
- Kim MS, Rossi M, Abusnana S, Sunter D, Morgan DG, Small CJ, Edwards CM, Heath MM, Stanley SA, Seal LJ, Bhatti JR, Smith DM, Ghatei MA, Bloom SR 2000 Hypothalamic localization of the feeding effect of agouti-related peptide and α-melanocyte-stimulating hormone. Diabetes 49:177–182 [DOI] [PubMed] [Google Scholar]
- Hansen MJ, Jovanovska V, Morris MJ 2004 Adaptive responses in hypothalamic neuropeptide Y in the face of prolonged high-fat feeding in the rat. J Neurochem 88:909–916 [DOI] [PubMed] [Google Scholar]
- Wang H, Storlien LH, Huang XF 2002 Effects of dietary fat types on body fatness, leptin, and ARC leptin receptor, NPY, and AgRP mRNA expression. Am J Physiol Endocrinol Metab 282:E1352–E1359 [DOI] [PubMed] [Google Scholar]
- Dunbar J, Lapanowski K, Barnes M, Rafols J 2005 Hypothalamic agouti-related protein immunoreactivity in food-restricted, obese, and insulin-treated animals: evidence for glia cell localization. Exp Neurol 191:184–192 [DOI] [PubMed] [Google Scholar]
- Muhlhausler BS, Adam CL, Findlay PA, Duffield JA, McMillen IC 2006 Increased maternal nutrition alters development of the appetite-regulating network in the brain. FASEB J 20:1257–1259 [DOI] [PubMed] [Google Scholar]
- Mühlhäusler BS, Adam CL, Marrocco EM, Findlay PA, Roberts CT, McFarlane JR, Kauter KG, McMillen IC 2005 Impact of glucose infusion on the structural and functional characteristics of adipose tissue and on hypothalamic gene expression for appetite regulatory neuropeptides in the sheep fetus during late gestation. J Physiol 565:185–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiltz ME, Lipton JM 1990 α-MSH peptides inhibit acute inflammation and contact sensitivity. Peptides 11:979–982 [DOI] [PubMed] [Google Scholar]
- Catania A, Airaghi L, Manfredi MG, Vivirito MC, Milazzo F, Lipton JM, Zanussi C 1993 Proopiomelanocortin-derived peptides and cytokines: relations in patients with acquired immunodeficiency syndrome. Clin Immunol Immunopathol 66:73–79 [DOI] [PubMed] [Google Scholar]
- Catania A, Manfredi MG, Airaghi L, Lipton JM 1992 The neuropeptide α-MSH in control of fever. Pharmacol Res 26(Suppl 2):72–73 [DOI] [PubMed] [Google Scholar]
- Macaluso A, McCoy D, Ceriani G, Watanabe T, Biltz J, Catania A, Lipton JM 1994 Antiinflammatory influences of α-MSH molecules: central neurogenic and peripheral actions. J Neurosci 14:2377–2382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vulliémoz NR, Xiao E, Xia-Zhang L, Ferin M, Wardlaw SL 2006 Melanocortin modulation of inflammatory cytokine and neuroendocrine responses to endotoxin in the monkey. Endocrinology 147:1878–1883 [DOI] [PubMed] [Google Scholar]
- Papadopoulos AD, Wardlaw SL 1999 Endogenous α-MSH modulates the hypothalamic-pituitary-adrenal response to the cytokine interleukin-1β. J Neuroendocrinol 11:315–319 [DOI] [PubMed] [Google Scholar]
- Xiao E, Xia-Zhang L, Vulliémoz NR, Ferin M, Wardlaw SL 2003 Agouti-related protein stimulates the hypothalamic-pituitary-adrenal (HPA) axis and enhances the HPA response to interleukin-1 in the primate. Endocrinology 144:1736–1741 [DOI] [PubMed] [Google Scholar]
- Huang QH, Hruby VJ, Tatro JB 1998 Systemic α-MSH suppresses LPS fever via central melanocortin receptors independently of its suppression of corticosterone and IL-6 release. Am J Physiol 275:R524–R530 [DOI] [PubMed] [Google Scholar]
- Weiss JM, Sundar SK, Cierpial MA, Ritchie JC 1991 Effects of interleukin-1 infused into brain are antagonized by α-MSH in a dose-dependent manner. Eur J Pharmacol 192:177–179 [DOI] [PubMed] [Google Scholar]
- Tannenbaum BM, Brindley DN, Tannenbaum GS, Dallman MF, McArthur MD, Meaney MJ 1997 High-fat feeding alters both basal and stress-induced hypothalamic-pituitary-adrenal activity in the rat. Am J Physiol 273:E1168–E1177 [DOI] [PubMed] [Google Scholar]
- Challis JR, Sloboda D, Matthews SG, Holloway A, Alfaidy N, Patel FA, Whittle W, Fraser M, Moss TJ, Newnham J 2001 The fetal placental hypothalamic-pituitary-adrenal (HPA) axis, parturition and post natal health. Mol Cell Endocrinol 185:135–144 [DOI] [PubMed] [Google Scholar]
- Franke K, Harder T, Aerts L, Melchior K, Fahrenkrog S, Rodekamp E, Ziska T, Van Assche FA, Dudenhausen JW, Plagemann A 2005 ‘Programming’ of orexigenic and anorexigenic hypothalamic neurons in offspring of treated and untreated diabetic mother rats. Brain Res 1031:276–283 [DOI] [PubMed] [Google Scholar]
- Bouret SG, Gorski JN, Patterson CM, Chen S, Levin BE, Simerly RB 2008 Hypothalamic neural projections are permanently disrupted in diet-induced obese rats. Cell Metab 7:179–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin BE 2000 Metabolic imprinting on genetically predisposed neural circuits perpetuates obesity. Nutrition 16:909–915 [DOI] [PubMed] [Google Scholar]
- Kiel DW, Dodson EA, Artal R, Boehmer TK, Leet TL 2007 Gestational weight gain and pregnancy outcomes in obese women: how much is enough? Obstet Gynecol 110:752–758 [DOI] [PubMed] [Google Scholar]
- Paxinos G, Huang XF, Toga AW 1999 The rhesus monkey brain in stereotaxic coordinates. New York: Academic Press [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.