Abstract
Appropriate interactions between serotonin (5-HT) and stress pathways are critical for maintaining homeostasis. Dysregulation of hypothalamic-pituitary-adrenal (HPA) stress axis is a common feature in affective disorders in which an involvement of 5-HT neurocircuitry has been implicated in disease vulnerability and treatment responsiveness. Because there is a greater prevalence of affective disorders in women, sex differences in the 5-HTergic influence on stress pathways may contribute to disease disparity. Therefore, our studies compared stress or citalopram-induced corticosterone levels in male and female mice. To determine whether sex-dependent HPA axis responsiveness was mediated by the difference in testosterone levels, testosterone-treated females were also examined. Gene expression patterns in 5-HTergic and stress neurocircuitry were analyzed to determine sites of potential sex differences and mechanisms of testosterone action. As expected, restraint stress corticosterone levels were higher in intact females and were masculinized by testosterone. Interestingly, citalopram administration independent of stress resulted in a greater corticosterone response in females, which was also masculinized by testosterone. Analyses along the 5-HT-HPA axis revealed sex differences including greater pituitary 5-HT receptors and adrenal weights in females. Moreover, in stress-regulatory regions, we found sex differences in glucocorticoid receptor and glutamic acid decarboxylase expression supportive of greater inhibitory modulation and feedback potential in males. Taken together, these data suggest that multiple sites related to 5-HTergic stimulation, corticosterone production, and negative feedback of HPA neurocircuitry combine to produce higher female stress responsiveness. These studies support a potential for sex-specific involvement of 5-HT and stress pathways in the etiology of affective disorders.
The higher prevalence of affective disorders in women may partially involve a greater contribution of serotonin in the stimulation of stress responses.
The onset of affective disorders is highly associated with stressful life events, and patients with major depression frequently exhibit hyperactivity of the hypothalamic-pituitary-adrenal (HPA) stress axis (1,2). The prevalence of affective disorders is 2 times greater in females than males (3), which may relate to sex differences in stress sensitivity and serotonin (5-HT) neurocircuitry, in which 5-HT dysfunction has been implicated in the etiology of depression (4,5,6,7). Rodent and human studies have shown that females exhibit higher levels of glucocorticoids (corticosterone in rodents and cortisol in humans) in response to various stressors (8,9,10,11,12). Gonadal hormones are at least partly responsible for these sex differences because androgen administration decreases many indices of HPA axis activity including secretion of ACTH and glucocorticoids and stress-induced c-fos mRNA expression in the paraventricular nucleus (PVN) (10,13,14,15,16). Conversely, treatment with estrogens increases these measures, leading to greater stress responsivity (13,17,18).
The mechanisms by which testosterone blunts the HPA stress axis are not well understood. In addition to direct effects on the PVN, anterior pituitary, and adrenal gland, gonadal hormones may act on multiple upstream regulatory pathways outside the hypothalamus. Gonadal hormones also affect HPA axis responses by acting on negative feedback pathways, involving diverse regions including the hippocampus, lateral septum, and bed nucleus of the stria terminalis (19,20). Females demonstrate decreased glucocorticoid-mediated negative feedback ability, likely due to effects of estrogen or absence of testosterone (17,21,22). Glucocorticoid receptor activation and the inhibitory neurotransmitter γ-aminobutyric acid (GABA) play pivotal roles in mediating the return to homeostasis after stress exposure. Sex differences and the influence of gonadal hormones on these parameters in stress regulatory regions may be important in termination of HPA axis responses.
The 5-HTergic projection from the midbrain raphe nuclei to the PVN is one important signal involved in HPA axis activation (23,24). Pharmacological stimulation of this system by acute administration of a selective serotonin reuptake inhibitor (SSRI) or 5-HT agonist increases plasma ACTH and corticosterone in which 5-HT receptor subtypes, including 5-HT1A, -2A, and -2C, are thought to mediate these effects (25,26,27,28,29,30). Sex differences in the 5-HT system, such as decreased serotonin transporter (SERT) binding in female rodents and humans or a greater stress-induced increase in amygdalar 5-HT levels in females (6,26,31), may influence the HPA stress axis response and/or feedback mechanisms essential in homeostatic maintenance. However, little is known regarding such potential sex differences in the 5-HTergic influence and markers of negative feedback that may contribute to greater female HPA axis activity. Therefore, we compared corticosterone responses to a restraint stress or after an acute SSRI injection in male and female mice. Ovariectomized and testosterone-treated females have been included to determine whether sex differences in testosterone levels can account for stress outcomes in females. Mechanistically, gene expression patterns along the HPA axis were also investigated to ascertain potential targets of testosterone. We hypothesized that the greater female corticosterone stress response may involve a heightened 5-HTergic stimulation and include a reduced glucocorticoid and GABA-mediated negative feedback capacity.
Materials and Methods
Animals
Six-week-old male (n = 12) and female (n = 36) C57BL/6J mice were purchased from The Jackson Laboratory (Bar Harbor, ME). After 1 wk of habituation, females were assigned to one of three groups: 1) gonadally intact, 2) ovariectomized (OVX) plus vehicle (V), or 3) OVX plus testosterone propionate (TP; Sigma-Aldrich, St. Louis, MO). Bilateral ovariectomy surgery was performed under isoflurane anesthesia followed by implantation of a SILASTIC brand capsule (Dow Corning, Midland, MI; inner diameter 1.98 mm, outer diameter 3.18 mm) that contained 3 mm of TP. Vehicle capsules were empty. Implants were inserted sc, caudal to the scapula. Males remained gonadally intact. Cycle stage was determined in intact females on the days of HPA axis testing and day the animals were killed for quantitative RT-PCR (QRT-PCR) analyses using methods described previously (32). All mice were group housed under controlled conditions of a 12-h light, 12-h dark cycle with access to food and water ad libitum. All procedures were approved by the Institutional Animal Care and Use Committee at the University of Pennsylvania.
Corticosterone analysis
At 9 wk, all groups of mice (n = 6) were exposed to a 15 min restraint in a 50-ml conical tube (33). Testing occurred between 0900 and 1200 h. Blood samples were collected from a tail nick at four time points: time 0, 15, 30, and 120 min. After 4 wk of rest, citalopram activation of the HPA axis (in the absence of any additional stressor) was assessed in all groups (n = 6). Citalopram (Sigma-Aldrich) was dissolved in water and 0.9% saline (1:4 mixture) and injected ip at a dose of 15 mg/kg in 100 μl vol. Vehicle injections were a 1:4 mixture of water and 0.9% saline. Blood samples were collected from a tail nick immediately on removal from the cage before the injection (time 0). The ip injection followed and additional blood samples were taken at 15, 30, and 120 min. Samples from both restraint and citalopram experiments were collected into EDTA-treated tubes, immediately centrifuged, and stored at −80 C. Corticosterone concentrations in plasma samples were measured using an 125I RIA kit (MP Biomedicals, Orangeburg, NY). The minimum detection limit of the assay was 7.7 ng/ml and intraassay coefficient of variation was 7.3%. Area under the curve (AUC) was also analyzed. Corticosterone rise was calculated by subtracting the time 0 value from the time 30 value for each mouse. Rate of recovery was calculated by subtracting the time 120 value from the time 30 value for each mouse.
QRT-PCR
To identify stress-relevant genes in the brain, pituitary gland, and adrenal gland involved in mediating sex differences in HPA axis responses, a separate cohort of intact male, intact female, OVX+V female, and OVX+TP female mice was used (n = 6). Approximately 2 wk after OVX surgeries, mice were decapitated under isoflurane anesthesia. Trunk blood was collected into EDTA-treated tubes for plasma testosterone analysis, described below. Whole brains were removed and frozen on dry ice. Whole pituitary glands and left and right adrenal glands were collected and frozen in liquid nitrogen. Adrenal glands were weighed before RNA isolation. Whole brains were sectioned at 300 μm. Using a hollow needle (Ted Pella, Redding, CA), brains regions were micropunched based on outlines in the mouse brain atlas by Paxinos and Franklin (34) as follows: lateral septum (LS) −0.75-m diameter punches taken on left hemisphere on sections corresponding to Figs. 27–29 of the mouse atlas; bed nucleus of stria terminalis (BNST) −0.75-mm diameter bilateral punches corresponding to Figs. 31 and 32; PVN, −1.25-mm diameter punches on the midline corresponding to Figs. 36–40; central and basolateral nuclei of the amygdala, −1.25-mm diameter bilateral punches corresponding to Figs. 38–42; dorsal hippocampus, −0.75-mm diameter bilateral punches corresponding to Figs. 43–45; ventral hippocampus, −1.25-mm diameter bilateral punches corresponding to Figs. 54–56. RNA was isolated from pituitary, left adrenal, and brain regions as previously described (35). Expression of target genes was determined by QRT-PCR using TaqMan gene expression assays (Applied Biosystems, Foster City, CA). Samples were run in duplicate for the target gene and for β-actin as the endogenous control on the same 96-well plate. PCRs were conducted on the Applied Biosystems 7500 fast real-time PCR system as previously described (35). For each sample, the cycle threshold (CT) value for β-actin was subtracted from the target threshold value, defined as ΔCT. Raw ΔCT values were converted to fold change by calculating 2(−ΔCT) for each sample and normalizing each value to the average for the male group.
Tryptophan hydroxylase-2 (TPH2) in situ hybridization
Due to the functional diversity within the dorsal raphe nucleus, we examined TPH2 by in situ hybridization. The caudal portion of the brains were coronally sectioned at 20 μm and mounted on Colorfrost/Plus slides (Fisher Scientific, Waltham, MA). Sections corresponding to Figs. 65–72 of the mouse atlas (34) were postfixed in 4% paraformaldehyde and hybridized as previously described (36). Tissue was incubated overnight with a 35S-uridine 5-triphosphate-labeled antisense RNA probe generated from TPH2 DNA (generously provided by Dr. Michael Bader, Max-Delbruch-Center for Molecular Medicine, Berlin, Germany). For silver grain analysis, slides were dipped in NTB salt liquid nuclear emulsion (Eastman Kodak, Rochester, NY) and exposed for 5 d. Slides were developed and counterstained with hematoxylin. TPH2 expression was quantified using IPLab imaging software (BD Biosciences, San Jose, CA). Brain sections were anatomically matched for each mouse. Under bright-field illumination, a region of interest was drawn and silver grains present on cells within the region were counted in dark-field. Total number of grains and grain density (grains per cell) were analyzed (n = 6–7).
Testosterone analysis
Plasma samples collected from trunk blood (n = 6–7) were assayed using a commercial 125I RIA kit for total testosterone (Diagnostic Products Corp., Los Angeles, CA). The minimum detection limit of the assay was 4 ng/dl and intraassay coefficient of variation was 17%. Data were converted to nanograms per milliliter.
Statistical analysis
Corticosterone data were analyzed using repeated-measures multivariate ANOVA (time × group for restraint experiment; time × group × drug for citalopram experiment). AUC, corticosterone rise, and recovery rate were analyzed by one-way ANOVA for restraint experiment and two-way ANOVA (group × drug) for the citalopram experiment. All other data were analyzed by one-way ANOVA for effect of group. Main effects and interactions were further explored with Tukey honestly significant difference post hoc test. Significant differences were identified at P < 0.05. Statistical analyses were performed with JMP software (SAS Institute, Cary, NC). All data are reported as mean ± sem.
Results
HPA axis responses to restraint stress and citalopram
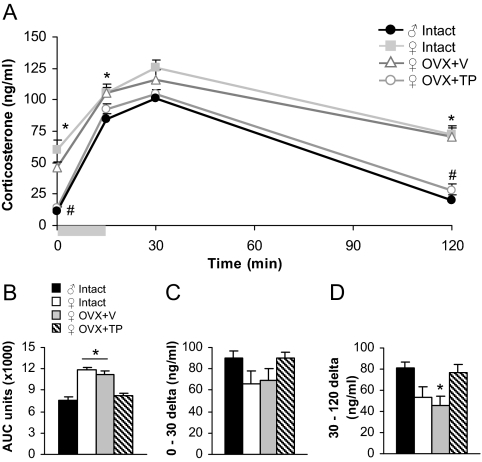
To examine sex differences and the role of testosterone in stress-induced HPA axis activity, corticosterone was assessed in mice exposed to a 15-min restraint stress (Fig. 1A). A repeated-measures ANOVA revealed main effects of group [F(3,20) = 47.53; P < 0.0001], time [F(3,18) = 21.42; P < 0.0001], and a group × time interaction [F(9,44) = 2.26; P < 0.05]. Analysis of individual times showed that basally (at time 0), intact, and OVX+V females exhibited greater corticosterone than males [F(3,20) = 23.64; P < 0.0001, main effect of group followed by post hoc tests]. This basal sex difference was masculinized by TP treatment. At time 15, at the termination of restraint, intact and OVX+V females continued to show elevated corticosterone compared with males [F(3,20) = 4.38; P < 0.05, main effect of group followed by post hoc tests]. At the peak response at time 30, there were no differences among groups. However, a sex difference reemerged at time 120, and TP treatment in females reduced corticosterone to male levels [F(3,20) = 23.54; P < 0.0001, main effect of group followed by post hoc tests].
Figure 1.
Sex differences in corticosterone levels after restraint stress were masculinized by testosterone. A, Intact females exhibited greater corticosterone basally (at time 0) as well as after restraint (at 15 min) and during recovery (at 120 min) compared with males. *, P < 0.05. OVX+V females were not different from intact females, but corticosterone in OVX+TP females was significantly reduced to male levels at times 0 and 120. #, P < 0.05. Gray bar represents period of restraint stress. B, AUC analysis showed an overall sex difference in restraint-induced corticosterone that was masculinized by TP exposure. *, P < 0.05, different from males and OVX+TP females. C, The rise in corticosterone from 0 to 30 min due to restraint stress was not different among groups. D, The rate of recovery from 30 to 120 min was lower in OVX+V females compared with males. *, P < 0.05.
Differences in overall corticosterone release and in the rise and recovery of corticosterone were analyzed using AUC (Fig. 1B), the Δ from time 0 to 30 (Fig. 1C), and the Δ from time 30 to 120 (Fig. 1D), respectively. There was a main effect of group for AUC [F(3,20) = 26.58; P < 0.0001], indicating that intact females overall release greater levels of corticosterone than males (P < 0.05 by post hoc tests). TP treatment masculinized the response. The restraint-induced rise in corticosterone from time 0 to 30 was not different among groups. The rate of recovery from time 30 to 120, however, was significantly lower in OVX+V females compared with males [F(3,20) = 4.25; P < 0.05, main effect of group followed by post hoc tests]. All intact females were in the estrus phase of the cycle during testing, and thus, variability in corticosterone in this group is unlikely to be attributed to the cycling hormone levels.
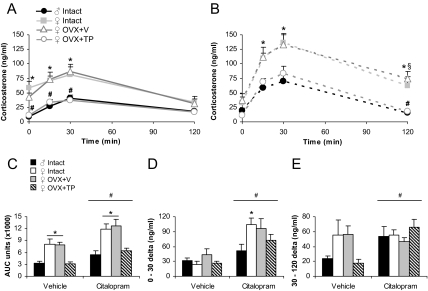
To examine whether sex differences in HPA axis activity relate to the serotonergic influence on stress pathways, we administered citalopram (15 mg/kg) or vehicle and analyzed corticosterone (Fig. 2, A and B). Overall, there were main effects of group [F(3,36) = 10.67; P < 0.0001], time [F(3,34) = 22.59; P < 0.0001], and a time × drug interaction [F(3,34) = 7.67; P < 0.001] by repeated-measures ANOVA. Basally, at time 0, there was a trend for intact and OVX+V females to display higher corticosterone than males and OVX+TP females [F(3,44) = 2.55; P = 0.07, effect of group]. We found a sex difference in corticosterone at all postinjection times that was ameliorated by TP [F(3,44) = 7.33, 6.98, and 12.31 for times 15, 30, and 120; P < 0.001, main effect of group followed by post hoc tests]. Additionally, during recovery at time 120, a group × drug interaction [F(3,44) = 2.98; P < 0.05] indicated that citalopram increased corticosterone in the OVX+V female group compared with vehicle-injected controls (P < 0.05 by post hoc tests).
Figure 2.
Sex differences in corticosterone levels in response to citalopram (15 mg/kg) were masculinized by testosterone. Intact females exhibited greater corticosterone than males after ip injection of vehicle (A) and citalopram (B). *, P < 0.05. Corticosterone was not affected by OVX+V but was masculinized by TP. #, P < 0.05, compared with intact and OVX+V females. Citalopram delayed recovery at time 120 in OVX+V females. §, P < 0.05, compared with vehicle. The ip injection occurred immediately after time 0. C, Analysis of AUC revealed overall sex difference in corticosterone that was masculinized by TP in response to vehicle or citalopram injections. *, P < 0.05, different from males and OVX+TP females. Citalopram significantly increased overall levels of corticosterone. #, P < 0.05, main effect of drug. D, The rise of corticosterone from 0 to 30 min in response to vehicle or citalopram injections was greater in intact females than males. *, P < 0.05. Citalopram caused a greater rise than vehicle. #, P < 0.0001. E, The rate of recovery from 30 to 120 min was higher with citalopram treatment than vehicle. #, P < 0.05.
Analysis of AUC (Fig. 2C) confirmed greater overall levels of corticosterone in intact females compared with males after vehicle and citalopram injections [F(3,44) = 13.02; P < 0.0001, main effect of group followed by post hoc tests]. TP treatment masculinized these effects. Furthermore, citalopram increased AUC in all groups relative to vehicle [F(1,46) = 6.42; P < 0.05]. The rise in corticosterone from time 0 to 30 (Fig. 2D) was significantly increased by citalopram [F(1,46) = 22.98; P < 0.0001] and was greater in intact females than males [F(3,44) = 4.04; P < 0.05, main effect of group followed by post hoc tests]. The recovery rate of corticosterone from time 30 to 120 (Fig. 2E) was faster with citalopram treatment compared with vehicle [F(1,40) = 4.76; P < 0.05]. A significant group × drug interaction [F(3,38) = 2.96; P < 0.05] suggests that recovery rate in vehicle-treated males and OVX+TP females is particularly shallow due to the minimal rise of corticosterone in these groups. Intact females were split between the proestrus and estrus phases of the cycle during testing, and there was no correlation between cycle stage and corticosterone [F(1,9) = 0.03; P = 0.86 by repeated measures ANOVA].
Gene expression by QRT-PCR in primary tissues of the HPA axis
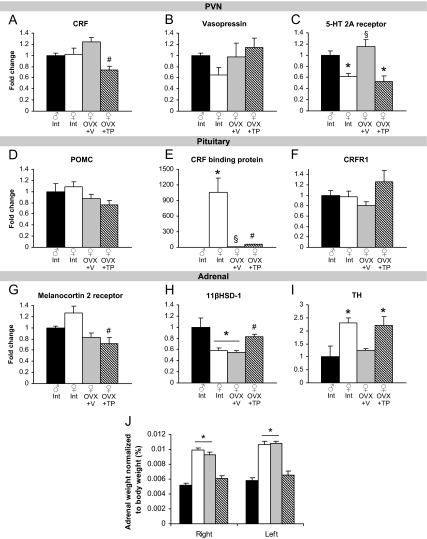
We examined resting mRNA levels of stress-relevant genes in the brain, pituitary gland, and adrenal gland to identify the targets of testosterone’s action in blunting stress responsivity. In the PVN, we did not observe sex differences in the expression of corticotropin-releasing factor (CRF) (Fig. 3A) or vasopressin (Fig. 3B). TP exposure in females reduced CRF mRNA [F(3,20) = 6.71; P < 0.01, main effect of group followed by post hoc tests]. Intact females had lower 5-HT2A receptor mRNA in the PVN than males, which was increased by ovariectomy [F(3,18) = 15.79; P < 0.0001, main effect of group followed by post hoc tests; Fig. 3C]. In the pituitary, there were no sex differences in proopiomelanocortin (POMC) (Fig. 3D) or CRF receptor-1 (Fig. 3F). A greater than 1000-fold elevation in CRF binding protein mRNA was found in intact females relative to males [F(3,20) = 135.1; P < 0.0001, main effect of group followed by post hoc tests; Fig. 3E]. Melanocortin-2 receptor in the adrenal gland was greater in intact females than males and was reduced by TP exposure [F(3,17) = 6.06; P < 0.01, Fig. 3G]. 11β-Hydroxysteroid dehydrogenase (11βHSD)-1 was reduced in intact and OVX+V females compared with males and increased by TP treatment [F(3,18) = 7.49; P < 0.01, main effect of group followed by post hoc tests; Fig. 3H]. We found sex differences in adrenal TH in which intact females had greater expression than males [F(3,18) = 5.13; P < 0.01, main effect of group followed by post hoc tests; Fig. 3I]. Tyrosine hydroxylase (TH) in OVX+TP females was also elevated above male levels. Intact females were split between the diestrus (three mice) and estrus (3) phases of the cycle when brains were removed for QRT-PCR analysis.
Figure 3.
Effects of sex and gonadal hormones on stress-related gene expression in primary tissues of the HPA axis. A, TP exposure in females reduced CRF mRNA in the PVN. #, P < 0.05, different from OVX+V. B, There were no differences in expression of vasopressin in the PVN. C, Intact and TP-treated females displayed lower 5-HT 2A receptor than males in the PVN. *, P < 0.05, compared with males. Expression was increased in OVX+V females. §, P < 0.05, different from intact and TP-treated females. D and F, There were no differences in POMC or CRF receptor-1 in the pituitary. E, There is a dramatic sex difference in CRF binding protein. *, P < 0.05, compared with males. OVX+V females also displayed greater expression than males but reduced from intact females. §, P < 0.05, different from all other groups. TP exposure in females increased CRF binding protein mRNA. #, P < 0.05, different from all other groups. G, TP treatment in females reduced melanocortin 2 receptor mRNA in the adrenal gland. #, P < 0.05, different from intact females. H, 11βHSD-1 mRNA was reduced in intact and OVX+V females (*, P < 0.05, different from males) and was increased by TP treatment. #, P < 0.05, different from OVX+V females. I, Intact and OVX+TP females displayed greater expression of TH than males. *, P < 0.05, different from males. J, Sex differences in adrenal weight were masculinized by TP treatment. Both the right and left adrenal glands were heavier in intact and OVX+V females compared with males and OVX+TP females. *, P < 0.05.
Adrenal weight
To investigate potential sex differences in corticosterone production, we weighed the adrenal glands. We found that intact and OVX+V females had heavier right and left adrenals glands than males [F(3,24) = 12.73 and 18.48 for left and right; P < 0.0001, main effect of group followed by post hoc tests; Fig. 3J]. This sex difference was masculinized by TP administration in females.
TPH2 mRNA expression by in situ hybridization
We hypothesized that greater responses to citalopram in intact females may be due to differences in serotonin synthesis. Thus, we examined TPH2 mRNA expression in regions of the dorsal raphe nucleus (Fig. 4A). In the middle ventromedial subregion, there was a trend for group differences in TPH2 mRNA (P = 0.08; Fig. 4B). There were no differences in the caudal dorsomedial subregion (Fig. 4C).
Figure 4.
There were no sex differences in TPH2 mRNA in the dorsal raphe (DR) nucleus. A, Representative images of TPH2 silver grains in the middle vmDR. B, There was a trend for group differences in TPH2 in the middle vmDR. C, There were no differences in the caudal dmDR. Diagram of middle DR adapted from mouse atlas (34). aq, Aqueduct; dmDR, dorsomedial DR; vmDR, ventromedial DR; xscp, decussation of the superior cerebellar peduncle.
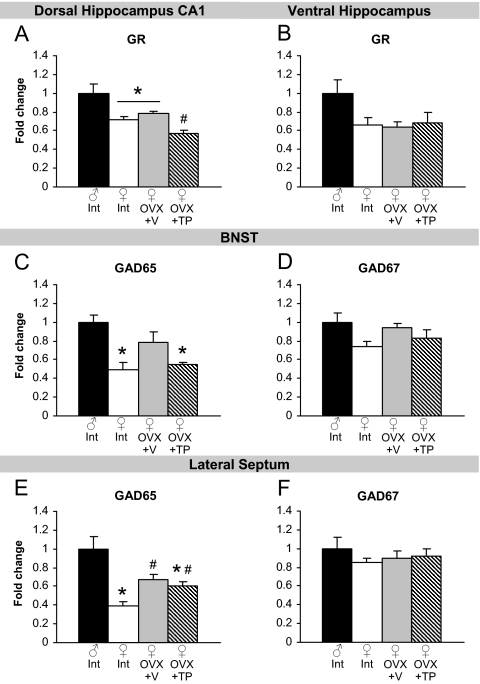
Gene expression by QRT-PCR in feedback regions of the HPA axis
Genes in brain regions that can inhibit HPA axis responsivity may also contribute to sex differences in stress. We began by measuring glucocorticoid receptor (GR) mRNA in subregions of the hippocampus, a major site for negative feedback of the HPA axis. Intact and OVX+V females showed lower levels of GR mRNA than males in the CA1 subfield of the dorsal hippocampus [F(3,20) = 14.50; P < 0.0001, main effect of group followed by post hoc tests; Fig. 5A]. The OVX+TP group showed further reductions in GR expression. In the ventral hippocampus, females showed reduced GR compared with males, but the effect was not significant (P = 0.11, effect of group; Fig. 5B). We examined glutamic acid decarboxylase (GAD) isoforms 65 and 67 (GAD65 and GAD67), enzymes involved in GABA synthesis, in the BNST and LS. We found that intact females express reduced GAD65 mRNA compared with males in the BNST [F(3,20) = 7.39; P < 0.01, main effect of group followed by post hoc tests; Fig. 5C]. In addition, GAD65 in OVX+TP females was also lower than males. There was a strong trend for lower GAD67 expression in intact females compared with males in the BNST (P = 0.06, effect of group; Fig. 5D). Females also had higher expression of GAD65 in the LS than males [F(3,19) = 12.19; P < 0.0001, Fig. 5E]. Ovariectomy increased GAD65 from intact females but remained lower than males. There were no differences in GAD67 expression in the LS (Fig. 5F). Sex differences in additional genes throughout the stress circuitry are listed in Table 1.
Figure 5.
Effects of sex and gonadal hormones on stress-related gene expression in feedback regions of the HPA axis. A, Intact and OVX+V females had lower GR mRNA expression in the dorsal hippocampus CA1 region than males. *, P < 0.05. TP treatment decreased GR further. #, P < 0.05, compared with OVX+V females. B, There were no group differences in GR mRNA in the ventral hippocampus. C and E, Intact and OVX+TP females had lower expression of GAD65 mRNA in the BNST and lateral septum than males. *, P < 0.05. Both OVX groups had higher expression than intact females in the lateral septum. #, P < 0.05. D and F, There was a trend for group differences in GAD67 in the BNST and no differences in the lateral septum.
Table 1.
Stress-related gene expression by QRT-PCR
| Fold change relative to ♂ Intact
|
F value | P value | ||||
|---|---|---|---|---|---|---|
| ? Intact | ♀ Intact | ♀ OVX+V | ♀ OVX+TP | |||
| Pituitary | ||||||
| Serotonin 1A receptor | 1.00 | 6.97a | 0.79 | 1.26 | F(3,19) = 5.27 | <0.01 |
| Serotonin 2A receptor | 1.00 | 1.68 | 0.97 | 0.72b | F(3,20) = 5.83 | <0.01 |
| PVN | ||||||
| Adrenergic-α1B receptor | 1.00 | 0.88 | 1.05 | 0.88 | F(3,20) = 1.37 | 0.28 |
| Estrogen receptor-β | 1.00 | 0.73 | 0.96 | 0.81 | F(3,19) = 2.15 | 0.13 |
| GR | 1.00 | 0.92 | 0.82 | 0.79 | F(3,19) = 2.48 | 0.09 |
| Metabotropic glutamate receptor 1 | 1.00 | 0.75 | 0.85 | 0.88 | F(3,19) = 0.87 | 0.47 |
| Serotonin 2C receptor | 1.00 | 0.99 | 0.74 | 0.82 | F(3,20) = 2.46 | 0.09 |
| Spinophilin | 1.00 | 0.96 | 0.94 | 1.00 | F(3,20) = 0.08 | 0.97 |
| Dorsal hippocampus CA1 subfield | ||||||
| Mineralocorticoid receptor | 1.00 | 0.95 | 1.00 | 0.88 | F(3,20) = 0.70 | 0.56 |
| Serotonin 2A receptor | 1.00 | 1.11 | 0.99 | 1.20 | F(3,20) = 2.21 | 0.12 |
| Dorsal hippocampus CA3 subfield | ||||||
| GR | 1.00 | 1.49 | 1.34 | 1.12 | F(3,20) = 1.31 | 0.30 |
| Ventral hippocampus | ||||||
| GAD65 | 1.00 | 0.90 | 0.66 | 0.62 | F(3,18) = 2.32 | 0.11 |
| GAD67 | 1.00 | 1.32 | 1.00 | 0.96# | F(3,18) = 3.20 | <0.05 |
| Metabotropic glutamate receptor 1 | 1.00 | 1.42a | 1.05 | 1.09 | F(3,19) = 4.37 | <0.05 |
| Mineralocorticoid receptor | 1.00 | 1.10 | 0.91 | 0.94 | F(3,18) = 0.61 | 0.61 |
| BNST | ||||||
| CRF | 1.00 | 0.77 | 1.04 | 0.76 | F(3,20) = 0.79 | 0.51 |
| Amygdala | ||||||
| CRF | 1.00 | 0.76 | 0.77 | 1.09 | F(3,19) = 0.98 | 0.42 |
P < 0.05, post hoc test showing difference from males.
P < 0.05, post hoc test showing difference from intact females.
Plasma testosterone
Testosterone levels were measured in plasma samples collected 2 wk after OVX surgeries and TP pellet implantation, corresponding to the timing of restraint stress testing and tissue harvesting for QRT-PCR and in situ hybridization. Plasma testosterone in males was 0.41 ± 0.09 and 17.31 ± 0.96 ng/ml in OVX+TP females.
Discussion
Appropriate interactions between 5-HT and stress pathways are critical for maintaining homeostasis (26,27). Heightened HPA stress axis activity is a common feature in affective disorders in which an involvement of 5-HT neurocircuitry has been implicated in disease vulnerability and treatment responsiveness. A greater prevalence of affective disorders in women suggests that sex differences in the 5-HTergic influence on stress pathways may potentially contribute to disease disparity. Therefore, our studies compared citalopram-induced corticosterone levels with restraint stress levels in male and female mice. Females treated with TP were also examined to determine whether higher stress responses in females is attributed to normally low testosterone levels. To identify targets of testosterone’s modulation of HPA axis activity, we measured expression of stress-related genes in brain regions and peripheral tissues involved in stress circuitry.
As expected, intact females exhibited higher overall corticosterone compared with males in response to a restraint stress, with higher basal levels and at poststress time points including stress recovery at 120 min. There was no sex difference in the rate of rise for corticosterone from time 0 to 30 min, indicating that intact females respond similarly to restraint but have overall higher basal circulating corticosterone compared with males. Furthermore, the stress recovery time for females was greater than for males in which the decline in corticosterone from 30 to 120 min was faster in males. Because testosterone is a known modulator of the HPA stress axis (11), we found that activational TP was able to ameliorate sex differences in corticosterone after restraint with TP-treated females looking identical with males for overall corticosterone, rate of rise after restraint, and rate of recovery.
Sex differences in HPA axis stress responsivity may be due to a greater 5-HTergic contribution in females. Therefore, we examined corticosterone levels in response to an acute administration of the SSRI, citalopram, in the absence of restraint. Citalopram increased corticosterone in all groups relative to vehicle treatment, but the increase between time 0 and 30 was 2-fold greater in intact females than in males. In addition, females showed a delayed rate of recovery after citalopram. Interestingly, the overall corticosterone response, measured by AUC, to the stress of an ip injection (vehicle) was also higher in females. In contrast to the rise of citalopram-induced corticosterone, the Δ between time 0 and 30 due to a vehicle injection was similar in males and females. This Δ in vehicle females would have been greater if the time 0 level had been lower and more typical of basal values. Due to the stimulatory effect of acute 5-HT on corticosterone (25,27,29), the higher basal values in females relative to males and the greater rise after citalopram administration suggest that there may be a greater endogenous 5-HTergic contribution to the HPA stress axis in females. However, the effect of citalopram augmented an existing sex difference, indicating that additional mechanisms underlie the higher corticosterone levels in females. Future studies using a specific 5-HT receptor antagonist would be informative as to the contribution of 5-HT in these sex differences. Additionally, similar to findings with restraint stress, TP administration in females ameliorated sex differences in response to citalopram by reducing the maximal rise of corticosterone as well as increasing the rate of recovery. ACTH levels were not measured but may be informative as to additional points of 5-HTergic actions. Comparison of restraint vs. SSRI-mediated corticosterone levels here suggests that 5-HT may possibly play a greater role in the female stress response than in males.
To determine the potential mechanism by which testosterone acts to reduce stress and 5-Htergic-mediated corticosterone levels, we examined gene expression patterns across the 5-HT-HPA axis. In the PVN, we did not find a sex difference in postsynaptic 5-HT2C receptor expression and we found greater levels of 5-HT2A mRNA in males compared with intact females. Because these 5-HT receptors have been localized to CRF neurons in the PVN and have been shown to activate the HPA axis, these results were surprising (28,37). In addition, ovariectomy led to increased 5-HT2A mRNA, suggesting that ovarian hormones suppress expression of this gene. TP treatment normalized expression to intact female levels, which may be due to testosterone aromatization to estradiol. No significant effects were found between groups for CRF or vasopressin expression in the PVN. It remains possible that alterations in presynaptic SERT levels could account for a greater impact of citalopram in females. Although this has not been examined, female mice have been reported to have reduced SERT binding in the hippocampus and amygdala compared with males (26). However, overall SERT levels in the PVN are extremely low and difficult to detect using standard autoradiographical techniques.
We also looked for potential differences in upstream serotonergic input to the PVN by measuring dorsal raphe TPH2 expression, the rate-limiting enzyme in 5-HT synthesis. Because the raphe is a complex heterogeneous nucleus receiving distinct CRF inputs, we analyzed TPH2 expression by in situ hybridization to have greater detailed anatomical specificity. Surprisingly, there were no significant sex differences in TPH2 in the middle ventromedial or caudal dorsomedial subregions of the dorsal raphe, regions known to be responsive to gonadal hormones and receiving CRF input. Whereas estradiol has been shown to increase TPH2 in ovariectomized female rats (38), we did not find a difference between intact and ovariectomized females or an effect of TP on TPH2 expression in mice.
At the level of the pituitary, greater expression of 5-HT1A receptors in intact females than in males and a similar trend for 5-HT2A receptors was detected. 5-HT has been shown to directly promote ACTH release from pituitary cells (39), suggesting that 5-HT stimulation could lead to greater HPA axis activation in females due to the enhanced sensitivity at the level of the pituitary. We did not detect a sex difference for POMC or CRF receptor-1 expression here. However, all female groups independent of hormonal status had significantly higher levels of pituitary CRF binding protein than males, suggesting that there may be an additional impact of sex chromosomes on gene expression (40). As previously shown, ovarian hormones also play a large role in CRF binding protein regulation (41), indicated by the steep decrease in levels in OVX females compared with the intact group. TP treatment in OVX females increased expression, which may be caused by testosterone aromatization to estradiol. CRF binding protein is thought to inhibit CRF activity by binding CRF and preventing its action on CRF receptors. However, the role of CRF binding protein in the pituitary is not known and may not be involved in ACTH production. Certainly, the direction of the effect detected in our data does not support a function in blocking HPA axis responses.
In the adrenal gland, there were no sex differences in expression of the melanocortin-2 receptor for ACTH. We detected a reduction in female 11βHSD-1, the enzyme responsible for the conversion of dehydrocorticosterone to active corticosterone, which was reversed with TP treatment. This effect appears to be in opposition to what would be expected with higher female corticosterone production. Because previous studies have reported an inhibitory effect of testosterone metabolites on 11βHSD-1, measures of 11βHSD-1 enzymatic activity may be informative for influences of testosterone outside of transcriptional regulation examined here, suggesting that testosterone could reduce cortisone conversion, thus promoting feedback to increase 11βHSD-1 gene expression (42). Adrenal TH was also measured in these mice as a potential marker of sympathetic output. Increased adrenal TH has been associated with heightened corticosterone secretion related to a proposed mechanism of increased cholesterol transfer and glucocorticoid synthesis (43). TH levels were significantly higher in intact and TP-treated females compared with males and OVX females, supporting a role for estrogen regulation of this gene in which effects of TP in females are likely via aromatization to estradiol in the adrenal medulla. Previous studies have shown a sex-specific outcome of estrogen to increase TH expression in females but reduce TH in males in the mouse brain (44).
The clearest mechanistic result in our studies that was supportive of a hypothesized pattern in which a basal sex difference would be reversed by testosterone and would fit with a heightened corticosterone production in females was surprisingly not at the level of gene expression. Instead, we found that females had 2-fold greater relative adrenal weights compared with males. Similar to the corticosterone pattern after restraint or SSRI treatment, female adrenal weights were unchanged by ovariectomy but were masculinized by TP treatment. Previous studies have similarly shown a sex difference in adrenal weight and an effect of testosterone in gonadectomized males to reduce weight (45,46). Specifically, testosterone replacement led to atrophy of the cells in the zona fasciculata region of the adrenal cortex (46). Our results suggest that with minimal testosterone in intact females, the adrenal gland cortex is hypertrophied, increasing the capacity to synthesize corticosterone in response to ACTH stimulation. This may account for aspects of the sex differences in overall HPA axis activity after restraint or serotonergic stimulation.
In addition to the activation of the HPA stress axis, many brain regions that innervate the PVN modulate its inhibition. We hypothesized that genes involved in negative regulation of the PVN may be more highly expressed in males than intact females and be regulated by testosterone. Inhibition of the HPA axis can occur through glucocorticoid-mediated negative feedback or GABA innervation from multiple regions outside the PVN (19). The hippocampus is one of the primary mediators of GR-mediated negative feedback (47). Fitting with our hypothesis, females regardless of hormonal state showed reduced GR in the dorsal and ventral hippocampus, which may support a role of sex chromosomes to regulate this gene independent of gonadal hormone modulation. Additionally, administration of a 5-HT precursor has been shown to reduced hippocampal GR (48), suggesting a potential mechanism by which greater endogenous 5-HT activity in females may lead to reduced negative feedback. Furthermore, the presence or absence of androgen receptors within the hippocampus could also influence the ability of testosterone to function in female GR expression (49,50). Our data suggest that greater corticosterone negative feedback in females given testosterone occurs via a GR-independent mechanism.
We further investigated negative regulation of the HPA axis by examining gene expression for indicators of GABA synthesis, GAD isoforms 65 and 67. A growing body of evidence has implicated the BNST, and to a lesser degree the LS, as major relay centers for limbic inhibitory control of the PVN (19,20,51). Thus, sex differences in GABAergic projections from the BNST and LS may be critical regulators of HPA axis control. Ovarian hormones are known to regulate GAD expression in various brain regions (52), but sex differences have not been investigated in the BNST and septum. Overall, our results were supportive of a reduced basal inhibitory tone from both of these brain regions in females with reduced expression of GAD65 and GAD67 compared with males, although we cannot confirm that these GABA neurons are PVN projecting. Whereas androgen receptor and GAD distribution overlap in the BNST and LS as reported in males (53,54,55), testosterone administration in females here did not ameliorate sex differences. 5-HT appears to increase or decrease GAD activity, depending on the tissue (56,57), and thus, it is unclear whether sex differences in basal 5-HT transmission are responsible for our GAD results. Whereas these data are only indirect measures of potential GABA production, GAD mRNA levels have been positively correlated with firing rates of GABA neurons (58,59). GAD65 is preferentially involved in the synthesis of GABA for vesicular synaptic release after acute demand (60). This suggests that during an acute stress response, females may have a reduced capacity to rapidly synthesize an immediately usable pool of GABA and that this ability may be important in blunting a stress response.
In summary, our studies demonstrated robust sex differences in HPA axis activity in which females exhibited higher stress and citalopram-induced corticosterone levels that may partially involve a greater and more prolonged contribution of 5-HT stimulation. Gonadal hormones are at least partly responsible for these sex differences because testosterone administration in females reduces these effects to be identical with that of males. Female sensitivity to serotonergic stimulation may result from sex differences at multiple levels in HPA axis regulation including greater expression of 5-HT receptors in the pituitary, increased adrenal gland weights, and the potential for reduced GR-mediated negative feedback and GABAergic inhibition. This may lead to a decreased ability to maintain homeostasis in females and an increased susceptibility to stress-related psychiatric disease. Furthermore, the heightened HPA axis response to acute SSRI treatment in females may be important in consideration of sex-specific outcomes in antidepressant therapy.
Acknowledgments
The authors acknowledge the tremendous contributions and founding studies of Dr. Elizabeth A. Young (University of Michigan) in her research on sex differences in stress and depression. She will be greatly missed.
Footnotes
This work was supported by National Institutes of Health Grant MH073030.
Disclosure Summary: The authors have nothing to disclose.
First Published Online February 25, 2010
Abbreviations: AUC, Area under the curve; BNST, bed nucleus of stria terminalis; CRF, corticotropin-releasing factor; CT, cycle threshold; GABA,γ-aminobutyric acid; GAD, glutamic acid decarboxylase; GR, glucocorticoid receptor; HPA, hypothalamic -pituitary-adrenal; 11βHSD, 11β-hydroxysteroid dehydrogenase; 5-HT, serotonin; LS, lateral septum; OVX, ovariectomized; POMC, proopiomelanocortin; PVN, paraventricular nucleus; QRT-PCR, quantitative RT-PCR; SERT, serotonin transporter; SSRI, selective serotonin reuptake inhibitor; TH, tyrosine hydroxylase; TP, testosterone propionate; TPH2, tryptophan hydroxylase-2; V, vehicle.
References
- Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB 1999 The role of corticotropin-releasing factor in depression and anxiety disorders. J Endocrinol 160:1–12 [DOI] [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, Prescott CA 1999 Causal relationship between stressful life events and the onset of major depression. Am J Psychiatry 156:837–841 [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, Wittchen HU, Kendler KS 1994 Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry 51:8–19 [DOI] [PubMed] [Google Scholar]
- Cowen PJ, Parry-Billings M, Newsholme EA 1989 Decreased plasma tryptophan levels in major depression. J Affect Disord 16:27–31 [DOI] [PubMed] [Google Scholar]
- Owens MJ, Nemeroff CB 1994 Role of serotonin in the pathophysiology of depression: focus on the serotonin transporter. Clin Chem 40:288–295 [PubMed] [Google Scholar]
- Mann JJ, Huang YY, Underwood MD, Kassir SA, Oppenheim S, Kelly TM, Dwork AJ, Arango V 2000 A serotonin transporter gene promoter polymorphism (5-HTTLPR) and prefrontal cortical binding in major depression and suicide. Arch Gen Psychiatry 57:729–738 [DOI] [PubMed] [Google Scholar]
- Boldrini M, Underwood MD, Mann JJ, Arango V 2005 More tryptophan hydroxylase in the brainstem dorsal raphe nucleus in depressed suicides. Brain Res 1041:19–28 [DOI] [PubMed] [Google Scholar]
- Stroud LR, Salovey P, Epel ES 2002 Sex differences in stress responses: social rejection versus achievement stress. Biol Psychiatry 52:318–327 [DOI] [PubMed] [Google Scholar]
- Heuser IJ, Gotthardt U, Schweiger U, Schmider J, Lammers CH, Dettling M, Holsboer F 1994 Age-associated changes of pituitary-adrenocortical hormone regulation in humans: importance of gender. Neurobiol Aging 15:227–231 [DOI] [PubMed] [Google Scholar]
- Goel N, Bale TL 2008 Organizational and activational effects of testosterone on masculinization of female physiological and behavioral stress responses. Endocrinology 149:6399–6405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa RJ, Burgess LH, Kerr JE, O'Keefe JA 1994 Gonadal steroid hormone receptors and sex differences in the hypothalamo-pituitary-adrenal axis. Horm Behav 28:464–476 [DOI] [PubMed] [Google Scholar]
- Viau V, Bingham B, Davis J, Lee P, Wong M 2005 Gender and puberty interact on the stress-induced activation of parvocellular neurosecretory neurons and corticotropin-releasing hormone messenger ribonucleic acid expression in the rat. Endocrinology 146:137–146 [DOI] [PubMed] [Google Scholar]
- Lund TD, Munson DJ, Haldy ME, Handa RJ 2004 Androgen inhibits, while oestrogen enhances, restraint-induced activation of neuropeptide neurones in the paraventricular nucleus of the hypothalamus. J Neuroendocrinol 16:272–278 [DOI] [PubMed] [Google Scholar]
- Rubinow DR, Roca CA, Schmidt PJ, Danaceau MA, Putnam K, Cizza G, Chrousos G, Nieman L 2005 Testosterone suppression of CRH-stimulated cortisol in men. Neuropsychopharmacology 30:1906–1912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale JV, Wood SA, Atkinson HC, Harbuz MS, Lightman SL 2004 Gonadal steroid replacement reverses gonadectomy-induced changes in the corticosterone pulse profile and stress-induced hypothalamic-pituitary-adrenal axis activity of male and female rats. J Neuroendocrinol 16:989–998 [DOI] [PubMed] [Google Scholar]
- Bingaman EW, Magnuson DJ, Gray TS, Handa RJ 1994 Androgen inhibits the increases in hypothalamic corticotropin-releasing hormone (CRH) and CRH-immunoreactivity following gonadectomy. Neuroendocrinology 59:228–234 [DOI] [PubMed] [Google Scholar]
- Burgess LH, Handa RJ 1992 Chronic estrogen-induced alterations in adrenocorticotropin and corticosterone secretion, and glucocorticoid receptor-mediated functions in female rats. Endocrinology 131:1261–1269 [DOI] [PubMed] [Google Scholar]
- Viau V, Meaney MJ 1991 Variations in the hypothalamic-pituitary-adrenal response to stress during the estrous cycle in the rat. Endocrinology 129:2503–2511 [DOI] [PubMed] [Google Scholar]
- Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, Cullinan WE 2003 Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol 24:151–180 [DOI] [PubMed] [Google Scholar]
- Silverman AJ, Hoffman DL, Zimmerman EA 1981 The descending afferent connections of the paraventricular nucleus of the hypothalamus (PVN). Brain Res Bull 6:47–61 [DOI] [PubMed] [Google Scholar]
- Young EA 1996 Sex differences in response to exogenous corticosterone: a rat model of hypercortisolemia. Mol Psychiatry 1:313–319 [PubMed] [Google Scholar]
- Daneva T, Spinedi E, Hadid R, Jacquier MC, Giacomini M, Gaillard RC 1993 Transient sex-related changes in the mice hypothalamo-pituitary-adrenal (HPA) axis during the acute phase of the inflammatory process. Mediators Inflamm 2:123–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen PJ, Hay-Schmidt A, Vrang N, Mikkelsen JD 1996 Origin of projections from the midbrain raphe nuclei to the hypothalamic paraventricular nucleus in the rat: a combined retrograde and anterograde tracing study. Neuroscience 70:963–988 [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Swanson LW, Steinbusch HW, Verhofstad AA 1983 The distribution and cells of origin of serotonergic inputs to the paraventricular and supraoptic nuclei of the rat. Brain Res 277:355–360 [DOI] [PubMed] [Google Scholar]
- Fuller RW, Snoddy HD, Clemens JA 1978 The effect of quipazine, a serotonin receptor agonist, on serum corticosterone concentration in rats. Endocr Res Commun 5:161–171 [DOI] [PubMed] [Google Scholar]
- McEuen JG, Semsar KA, Lim MA, Bale TL 2009 Influence of sex and CRF pathways as determinants in 5-HT sensitivity. Endocrinology 150:3709–3716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen JB, Jessop DS, Harbuz MS, Mørk A, Sánchez C, Mikkelsen JD 1999 Acute and long-term treatments with the selective serotonin reuptake inhibitor citalopram modulate the HPA axis activity at different levels in male rats. J Neuroendocrinol 11:465–471 [DOI] [PubMed] [Google Scholar]
- Heisler LK, Pronchuk N, Nonogaki K, Zhou L, Raber J, Tung L, Yeo GS, O'Rahilly S, Colmers WF, Elmquist JK, Tecott LH 2007 Serotonin activates the hypothalamic-pituitary-adrenal axis via serotonin 2C receptor stimulation. J Neurosci 27:6956–6964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen H, Kjaer A, Warberg J, Knigge U 2001 Differential effect of serotonin 5-HT(1A) receptor antagonists on the secretion of corticotropin and prolactin. Neuroendocrinology 73:322–333 [DOI] [PubMed] [Google Scholar]
- Hemrick-Luecke SK, Evans DC 2002 Comparison of the potency of MDL 100,907 and SB 242084 in blocking the serotonin (5-HT)(2) receptor agonist-induced increases in rat serum corticosterone concentrations: evidence for 5-HT(2A) receptor mediation of the HPA axis. Neuropharmacology 42:162–169 [DOI] [PubMed] [Google Scholar]
- Mitsushima D, Yamada K, Takase K, Funabashi T, Kimura F 2006 Sex differences in the basolateral amygdala: the extracellular levels of serotonin and dopamine, and their responses to restraint stress in rats. Eur J Neurosci 24:3245–3254 [DOI] [PubMed] [Google Scholar]
- Nelson JF, Felicio LS, Randall PK, Sims C, Finch CE 1982 A longitudinal study of estrous cyclicity in aging C57BL/6J mice: I. Cycle frequency, length and vaginal cytology. Biol Reprod 27:327–339 [DOI] [PubMed] [Google Scholar]
- Goel N, Bale TL 2007 Identifying early behavioral and molecular markers of future stress sensitivity. Endocrinology 148:4585–4591 [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ 2001 The mouse brain atlas in stereotaxic coordinates. 2nd ed. San Diego: Academic Press [Google Scholar]
- Teegarden SL, Nestler EJ, Bale TL 2008 ΔFosB-mediated alterations in dopamine signaling are normalized by a palatable high-fat diet. Biol Psychiatry 64:941–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL, Dorsa DM 1995 Sex differences in and effects of estrogen on oxytocin receptor messenger ribonucleic acid expression in the ventromedial hypothalamus. Endocrinology 136:27–32 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Gray TS, D'Souza DN, Carrasco GA, Damjanoska KJ, Dudas B, Garcia F, Zainelli GM, Hanley NR, Battaglia G, Muma NA, Van de Kar LD 2004 Desensitization of 5-HT1A receptors by 5-HT2A receptors in neuroendocrine neurons in vivo. J Pharmacol Exp Ther 310:59–66 [DOI] [PubMed] [Google Scholar]
- Hiroi R, McDevitt RA, Neumaier JF 2006 Estrogen selectively increases tryptophan hydroxylase-2 mRNA expression in distinct subregions of rat midbrain raphe nucleus: association between gene expression and anxiety behavior in the open field. Biol Psychiatry 60:288–295 [DOI] [PubMed] [Google Scholar]
- Calogero AE, Bagdy G, Moncada ML, D'Agata R 1993 Effect of selective serotonin agonists on basal, corticotrophin-releasing hormone- and vasopressin-induced ACTH release in vitro from rat pituitary cells. J Endocrinol 136:381–387 [DOI] [PubMed] [Google Scholar]
- Carruth LL, Reisert I, Arnold AP 2002 Sex chromosome genes directly affect brain sexual differentiation. Nat Neurosci 5:933–934 [DOI] [PubMed] [Google Scholar]
- Speert DB, McClennen SJ, Seasholtz AF 2002 Sexually dimorphic expression of corticotropin-releasing hormone-binding protein in the mouse pituitary. Endocrinology 143:4730–4741 [DOI] [PubMed] [Google Scholar]
- Latif SA, Pardo HA, Hardy MP, Morris DJ 2005 Endogenous selective inhibitors of 11β-hydroxysteroid dehydrogenase isoforms 1 and 2 of adrenal origin. Mol Cell Endocrinol 243:43–50 [DOI] [PubMed] [Google Scholar]
- Otawa M, Arai H, Atomi Y 2007 Molecular aspects of adrenal regulation for circadian glucocorticoid synthesis by chronic voluntary exercise. Life Sci 80:725–731 [DOI] [PubMed] [Google Scholar]
- Thanky NR, Son JH, Herbison AE 2002 Sex differences in the regulation of tyrosine hydroxylase gene transcription by estrogen in the locus coeruleus of TH9-LacZ transgenic mice. Brain Res Mol Brain Res 104:220–226 [DOI] [PubMed] [Google Scholar]
- Critchlow V, Liebelt RA, Bar-Sela M, Mountcastle W, Lipscomb HS 1963 Sex difference in resting pituitary-adrenal function in the rat. Am J Physiol 205:807–815 [DOI] [PubMed] [Google Scholar]
- Malendowicz LK 1979 Sex differences in adrenocortical structure and function. V. The effects of postpubertal gonadectomy and gonadal hormone replacement on nuclear-cytoplasmic ratio, morphology and histochemistry of rat adrenal cortex. Folia Histochem Cytochem (Krakow) 17:195–214 [PubMed] [Google Scholar]
- Jacobson L, Sapolsky R 1991 The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenocortical axis. Endocr Rev 12:118–134 [DOI] [PubMed] [Google Scholar]
- Sémont A, Fache M, Héry F, Faudon M, Youssouf F, Héry M 2000 Regulation of central corticosteroid receptors following short-term activation of serotonin transmission by 5-hydroxy-l-tryptophan or fluoxetine. J Neuroendocrinol 12:736–744 [DOI] [PubMed] [Google Scholar]
- Ahima RS, Harlan RE 1992 Regulation of glucocorticoid receptor immunoreactivity in the rat hippocampus by androgenic-anabolic steroids. Brain Res 585:311–314 [DOI] [PubMed] [Google Scholar]
- Kerr JE, Beck SG, Handa RJ 1996 Androgens modulate glucocorticoid receptor mRNA, but not mineralocorticoid receptor mRNA levels, in the rat hippocampus. J Neuroendocrinol 8:439–447 [DOI] [PubMed] [Google Scholar]
- Radley JJ, Gosselink KL, Sawchenko PE 2009 A discrete GABAergic relay mediates prefrontal cortical inhibition of the neuroendocrine stress response. J Neurosci 29:7330–7340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, Kaufman LC, Brooks PJ, Pfaff DW, Schwartz- Giblin S 1995 Estrogen modulation of mRNA levels for the two forms of glutamic acid decarboxylase (GAD) in female rat brain. J Comp Neurol 360:685–697 [DOI] [PubMed] [Google Scholar]
- Castañeda MT, Sanabria ER, Hernandez S, Ayala A, Reyna TA, Wu JY, Colom LV 2005 Glutamic acid decarboxylase isoforms are differentially distributed in the septal region of the rat. Neurosci Res 52:107–119 [DOI] [PubMed] [Google Scholar]
- Choi DC, Nguyen MM, Tamashiro KL, Ma LY, Sakai RR, Herman JP 2006 Chronic social stress in the visible burrow system modulates stress-related gene expression in the bed nucleus of the stria terminalis. Physiol Behav 89:301–310 [DOI] [PubMed] [Google Scholar]
- Williamson M, Viau V 2007 Androgen receptor expressing neurons that project to the paraventricular nucleus of the hypothalamus in the male rat. J Comp Neurol 503:717–740 [DOI] [PubMed] [Google Scholar]
- Di Cara B, Samuel D, Salin P, Kerkerian-Le Goff L, Daszuta A 2003 Serotonergic regulation of the GABAergic transmission in the rat basal ganglia. Synapse 50:144–150 [DOI] [PubMed] [Google Scholar]
- Wang YY, Legendre P, Huang J, Wang W, Wu SX, Li YQ 2008 The effect of serotonin on GABA synthesis in cultured rat spinal dorsal horn neurons. J Chem Neuroanat 36:150–159 [DOI] [PubMed] [Google Scholar]
- Hendry SH, Jones EG 1986 Reduction in number of immunostained GABAergic neurones in deprived-eye dominance columns of monkey area 17. Nature 320:750–753 [DOI] [PubMed] [Google Scholar]
- Litwak J, Mercugliano M, Chesselet MF, Oltmans GA 1990 Increased glutamic acid decarboxylase (GAD) mRNA and GAD activity in cerebellar Purkinje cells following lesion-induced increases in cell firing. Neurosci Lett 116:179–183 [DOI] [PubMed] [Google Scholar]
- Soghomonian JJ, Martin DL 1998 Two isoforms of glutamate decarboxylase: why? Trends Pharmacol Sci 19:500–505 [DOI] [PubMed] [Google Scholar]