Abstract
Previous research using α-fetoprotein knockout and aromatase knockout (ArKO) female mice suggested that the developing hypothalamic mechanisms that later control feminine sexual behavior are protected prenatally from estradiol, whereas shortly after birth, they may be stimulated by this same sex hormone. In the present study, we found that the amount of progesterone receptor immunoreactivity (PR-ir) in the anteroventral periventricular nucleus and medial part of the medial preoptic nucleus was significantly lower in ArKO female mice than in wild-type (WT) females at several prepubertal ages including postnatal d 15 (P15), P15, P20, and P25 but not neonatally at P0, P5, or P10. Likewise, PR-ir in the lateral subdivision of the ventromedial hypothalamic nucleus was significantly lower at P25 in ArKO vs. WT female mice but not at earlier postnatal ages. PR-ir was consistently higher in male than in female WT mice in the anteroventral periventricular nucleus and medial preoptic nucleus over P0–P10 and in the ventromedial hypothalamic nucleus over P0–P20. In these brain regions across these latter ages, PR-ir in male ArKO mice was significantly lower than in WT males and resembled the values seen in WT females, confirming previous reports that estradiol formed in the developing male hypothalamus from testicular testosterone is responsible for male-typical levels of neural PR expression. Thus, estradiol induces both female- and male-typical expression of PR postnatally in the mouse hypothalamus. Future experiments will determine whether this estradiol-induced PR expression contributes to either female- or male-typical brain and behavioral differentiation.
Estradiol induces female-typical expression of progesterone receptor prepubertally in the mouse hypothalamus, perhaps thereby contributing to female brain sexual differentiation.
A central tenet of contemporary theories of mammalian brain and behavioral sexual differentiation is that an organizational action of testosterone, secreted perinatally by the male’s testes, controls male-typical facets of brain and behavioral development, whereas no active perinatal sex hormone signaling is required for female-typical differentiation. In rodents (1) and carnivores (2), much evidence suggests that many, although not all, of the perinatal organizational actions of testosterone on male nervous system development actually result from the cellular effects of estradiol formed via the local, neural aromatization of circulating testosterone. It was proposed many years ago (3) that the brain of the developing female rodent fetus is protected from the actions of maternal estradiol and/or from aromatized products of testosterone derived from adjacent male fetuses by the relatively high-affinity binding of this sex hormone by circulating α-fetoprotein (AFP). Indeed, Bakker et al. (4) found that the capacity of AFP-null mutant [AFP-knockout (KO)] female mice to display feminine receptive sexual behavior (lordosis) after adult ovariectomy and treatment with estradiol plus progesterone was severely attenuated. Likewise, AFP-KO females had a male-like number of tyrosine hydroxylase immunoreactive (ir) cells in the sexually differentiated anteroventral periventricular nucleus (AVPv) of the hypothalamus. Both of these behavioral and morphological consequences of the AFP-null mutation in female mice were reversed by transplacental treatment with an aromatase-inhibiting drug, 1,4,-androstatriene-3,17-dione (ATD). These results provide evidence that circulating AFP binds circulating estradiol in female fetuses so as to protect their brains from the potential defeminizing actions of this hormone that otherwise normally occur in the male nervous system in response to estradiol synthesized from testosterone after it is taken up from the circulation.
Toran-Allerand (5) first proposed that estradiol may actively contribute to the differentiation of female-typical aspects of brain and behavioral sexual differentiation. Early studies (6,7) reported that removing the ovaries neonatally from female rats reduced their later lordotic responsiveness to ovarian hormones and that treatment with estradiol over peripubertal ages reversed the behavioral deficits induced by neonatal ovariectomy. More recently (8,9), it was proposed that different doses of genes expressed off the X chromosome in XY (male) vs. XX (female) mice influence aspects of brain and behavioral sexual differentiation, perhaps reflecting a nonhormonal, genetic signaling mechanism that actively promotes female-typical brain sexual differentiation. Additional evidence of a possible contribution of estradiol to female-typical behavioral development involved the report (10) that aromatase-KO (ArKO) female mice showed significantly lower levels of lordosis behavior than wild-type (WT) controls after adult ovariectomy and treatment with ovarian hormones. ArKO females also showed significantly lower levels of olfactory investigation of urinary odors from male and female conspecifics and significantly less male-like mounting behavior toward a stimulus female than WT females. Finally, ArKO females had significantly fewer kisspeptin neurons than WT females in the AVPv (11). These results have resurrected the question of whether estradiol, acting during the first several weeks of life, actively contributes to female-typical brain and behavioral sexual differentiation (12), perhaps beginning after postnatal d 7 (P7) when the rat ovary first produces estradiol (13).
One approach to the question of whether estradiol actively contributes to female-typical brain sexual differentiation would be to compare the postnatal neural expression of an estradiol-dependent gene in ArKO female mice, which produce no estradiol in any tissue, and in WT control females. The expression of progesterone receptor (PR) in the hypothalamus of adult female rodents is well known to be dramatically up-regulated by estradiol (14,15). Likewise, Wagner and co-workers have conducted an extensive series of experiments showing that the number of PR-ir cells in the medial preoptic area (MPOA), the lateral part of the ventromedial hypothalamic nucleus (VMH-L), and AVPv of both rat (16) and mouse (17,18) is significantly greater in neonatal males than in females. Additional work from this group (18,19) showed that estradiol, formed via the neural aromatization of testosterone in male neonates, is responsible for the higher, male-typical levels of PR expression in the MPOA. Finally, another study from this group (20) found that transplacental administration of the aromatase blocker ATD significantly reduced PR-ir in the MPOA of female rats killed at the end of gestation. Based on these results, we sought to use the expression of PR in three different hypothalamic regions, AVPv, MPOA, and VMH-L, as an index of estradiol’s action in the female mouse brain across the first 25 d of postnatal development. We predicted that if estradiol normally acts in the developing female mouse hypothalamus, we would see significantly less PR-ir in some or all of these three brain regions from ArKO females (which cannot synthesize estradiol) vs. WT females at one or several of the postnatal ages studied. Finally, to confirm that our method of monitoring PR-ir in postnatal hypothalamic regions was reliable, we analyzed PR-ir in the same hypothalamic regions and at the same postnatal ages in WT males in an attempt to replicate the previous reports of Wagner et al. (21) of a sex difference in hypothalamic expression of PR shortly after birth. We also added groups of ArKO males to further test the hypothesis (15) that estrogenic metabolites of testosterone, formed in the male hypothalamus, stimulate the high, male-typical levels of neonatal PR expression.
Materials and Methods
Animals and tissue preparation
ArKO mice were generated by targeted disruption of exons 1 and 2 of the Cyp 19 gene and consequently could not convert androgens into estrogens (22). Heterozygous males and females of the C57BL/6J strain were bred to generate WT, heterozygous, and homozygous-null (ArKO) offspring at the Center for Cellular and Molecular Neurobiology (now named GIGA Neurosciences), University of Liège, Liège, Belgium. Food (phytoestrogen-free mouse chow D10001 AIN-76A; Brogaarden, Lynge, Denmark) and water were available to mice ad libitum. To obtain brain tissues at postnatal ages, pregnant females were checked daily for parturition toward the end of pregnancy. Because all breeding females were housed under a 12-h light, 12-h dark cycle (lights on between 0800 and 2000 h), parturition usually occurred in the morning, 1–2 h after lights on. The day of birth was designated as P0. Brain tissues were collected on P0, P5, P10, P15, P20, and P25. We collected brains at these 5-d intervals because neonatal estradiol has been shown to induce hypothalamic PR expression in fewer than 5 days (15). Postnatal mice were decapitated whereupon brains were rapidly removed from the skull and immediately immersion fixed in 5% acrolein in 0.1 m PBS (pH 7.6) for 2.5 h, rinsed twice in PBS for 30 min, and then cryoprotected in 30% sucrose and when sunken, frozen on dry ice and stored at −80 C until being processed for PR immunocytochemistry. The sex of each pup was determined by visual inspection of the ano-genital distance, and the presence of ovaries or testes was confirmed by laparotomy. To determine the genotype of the pup, the tail was kept and stored at −20 C for later PCR analysis of DNA. The number of animals used for each group was as follows: female WT, P0 = 8, P5 = 8, P10 = 8, P15 = 8, P20 = 8, and P25 = 8; female ArKO, P0 = 7, P5 = 7, P10 = 8, P15 = 8, P20 = 8, and P25 = 7; male WT, P0 = 6, P5 = 7, P10 = 7, P15 = 8, P20 = 7, and P25 = 7; and male ArKO, P0 = 7, P5 = 9, P10 = 7, P15 = 8, P20 = 8, and P25 = 8.
Brain sections (30 μm thick) were cut on a Leica CM3050S cryostat. Forebrains were cut coronally from the level of the end of the olfactory bulb. Sections were saved in three different series, placed in antifreeze solution, and stored at −20 C for later immunocytochemistry.
All experiments were conducted in accordance with the guidelines set forth by the National Institutes of Health Guiding Principles for the Care and Use of Research Animals and were approved by the Committees for Ethical Animal Use at the University of Liège and at Boston University.
Immunocytochemistry
Every effort was made to include an equal number of brains from all 24 groups in each immunocytochemistry run. PR immunocytochemistry was carried out on free-floating sections. All incubations were carried out at room temperature (22 C), and all washes of brain tissue sections were performed using Tris-buffered saline (TBS, 0.05 m) or TBS containing 0.2% Triton X-100 (TBST). Briefly, sections were rinsed and incubated in 10% sodium borohydride for 15 min. Endogenous peroxidase activity was quenched by incubating the sections for 30 min with 3% hydrogen peroxide. Aspecific binding sites were then blocked by incubating sections for 30 min with 5% normal goat serum (Dako Cytomation, Glostrup, Denmark). Sections were then incubated with a rabbit polyclonal antibody (1/1000 in TBST-normal goat serum 2%; Dako Cytomation) against the DNA-binding domain of the human PR for 72 h at 4 C. This antibody detects both the A and B isoforms of PR (23) and has been validated for specific staining of PR in mouse brain by Wagner et al. (21) and in the Bakker laboratory (unpublished results). Sections were then incubated for 1 h in a goat antirabbit biotinylated antibody (1:800 in TBST; Dako Cytomation). Sections were then incubated for 1 h in avidin-biotin complex (Vector Laboratories, Burlingame, CA) and then reacted for 7 min with 3,3′-diaminobenzidine tetrahydrochloride (DAB kit; Vector). Sections were then washed, mounted onto gelatin-coated slices, dried overnight, and coverslipped with a gelatin-based medium.
Image analysis and statistics
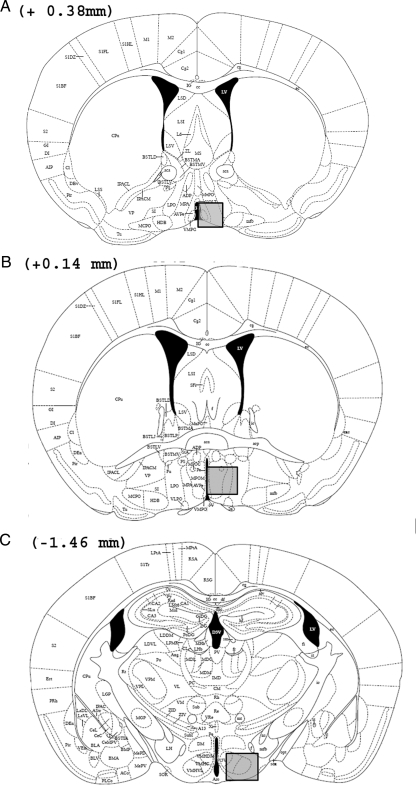
The relative amount of PR-ir was measured in three brain areas (Fig. 1): the AVPv (Fig. 28 of the mouse atlas: interaural, 4.18 mm; bregma, 0.38 mm), the medial part of the MPOA (Fig. 30 of the mouse atlas: interaural, 3.94 mm; bregma, 0.14 mm), and the VMH-L (Fig. 43 of the mouse atlas: interaural, 2.34 mm; bregma, −1.46 mm) (24). PR-ir was quantified by an experimenter, who was blind to the sex/genotype of the mice, using computer-assisted image analysis. Sections were digitized through a video camera (CCD camera, XC-77CE, Sony) attached to a microscope (Olympus MTV-3 with ×20 objective), and PR-ir was quantified with a PC-based image analysis system using the particle-counting protocol of the NIH Image program (version 1.37; Wayne Rasband, National Institutes of Health, Bethesda, MD). Digital images were made binary, and a manual threshold was used for discriminating the labeled material from the background. With a ×20 objective, exclusion thresholds were set at five (low threshold) and 200 (high threshold) pixels to remove from the quantification dark objects that were not the same size as a cell nucleus. The relative amount of PR-ir in the AVPv, MPOA, and VMH-L was measured in one entire field (720 × 480 pixels; area = 328,769 μm2) placed in a standardized manner based on predefined anatomical landmarks in the sections (e.g. edge of the third ventricle or prominent fiber tracts) and determined by measuring the area (square micrometers) covered by thresholded pixels [those pixels with a gray level higher than a defined threshold density (specific immunoreactive staining)]. Threshold was determined as a constant function of the background OD defined as the mean OD three to five times the sd higher than the mean background density. The mean background density was measured in a region devoid of PR-ir, immediately lateral to the analyzed region containing PR-ir. We measured the relative amount of PR-ir in sections from the right hemisphere of each animal that included the three selected brain regions.
Figure 1.
Drawings taken from the mouse brain atlas of Paxinos and Franklin (24) showing the location of forebrain regions in which PR-ir cells were quantified (shaded areas in each panel) in the AVPv (A), MPOA (B). and VMH-L (C). The distance of each coronal brain slice in front of (+) or behind (−) bregma is given for each panel.
Statistical analysis was performed using a three-way ANOVA [P < 0.05; factors included age (six) × genotype (four) × sex (two)], followed by planned pairwise comparisons using Fisher least significant difference post hoc tests (P < 0.05).
Results
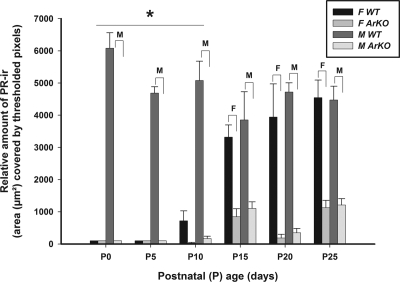
Representative examples of PR-ir cells in the MPOA of WT vs. ArKO female and male mice killed at P10 are shown in Fig. 2. PR-ir first appeared in the MPOA of WT females at P10, and the levels of PR expression progressively increased in WT females over P10–P25 (Fig. 3). Over this prepubertal period, PR-ir was consistently lower in ArKO females than in WT females. ANOVA revealed a significant effect of genotype [F(1,154) = 367.7; P < 0.0001], and post hoc comparisons showed that relative amount of PR-ir was significantly lower (P < 0.05) in ArKO female than in WT female mice at each of the prepubertal ages P15–P25. WT male mice consistently had more PR-ir in the MPOA than WT females across the neonatal ages P0–P10. ANOVA revealed a significant effect of sex [F(1,154) = 80.4; P < 0.0001], and post hoc comparisons revealed significant (P < 0.05) differences between WT male and WT female relative amount of PR-ir at P0–P10 but not at later ages. The relative amount of PR-ir in the MPOA was consistently significantly (P < 0.05) lower in ArKO males than in WT males at all six postnatal ages studied.
Figure 2.
Representative photomicrographs show coronal sections of the MPOA with PR-ir cells in a WT female (A), an ArKO female (B), a WT male (C), and an ArKO male (D) at P10. V, Third ventricle. Scale bar, 40 μm.
Figure 3.
The relative amount (mean ± sem) of PR-ir in the MPOA on P0, P5, P10, P15, P20, and P25 in male (M) and female (F) mice that were either WT or ArKO. *, Post hoc comparisons indicated a significant (P < 0.05) sex difference in WT mice at the ages indicated. Post hoc comparisons also indicated significant (P < 0.05) differences between WT vs. ArKO females (F over brackets) and between WT and ArKO males (M over brackets) at the ages shown.
PR-ir first appeared in the AVPv of WT females at P10, and the levels of PR expression progressively increased in WT females over P10–P25 (Fig. 4). Over this prepubertal period, PR-ir was consistently lower in ArKO females than in WT females. ANOVA revealed a significant effect of genotype [F(1,150) = 148.1; P < 0.0001], and post hoc comparisons showed that relative amount of PR-ir was significantly lower (P < 0.05) in ArKO female than in WT female mice at each of the ages P15–P25. WT male mice consistently had more PR-ir in the AVPv than WT females across the neonatal ages P0–P10. ANOVA revealed a significant effect of sex [F(1,150) = 42.7; P < 0.0001], and post hoc comparisons revealed significant (P < 0.05) differences between WT male and WT female relative amount of PR-ir at P0–P10 but not at later ages. The relative amount of PR-ir in the AVPv was consistently significantly (P < 0.05) lower in ArKO males than in WT males at all six postnatal ages studied.
Figure 4.
The relative amount (mean ± sem) of PR-ir in the AVPv on P0, P5, P10, P15, P20, and P25 in male (M) and female (F) mice that were either WT or ArKO. *, Post hoc comparisons indicated a significant (P < 0.05) sex difference in WT mice at the ages indicated. Post hoc comparisons also indicated significant (P < 0.05) differences in WT vs. ArKO females (F over brackets) and between WT and ArKO males (M over brackets) at the ages shown.
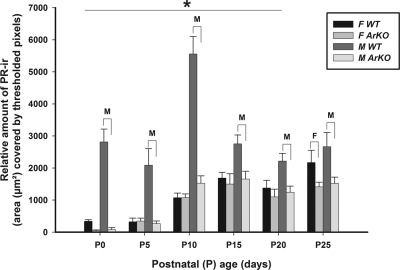
There was a progressive increase in the relative amount of PR-ir in the VMH-L of both WT and ArKO female mice across the postnatal ages sampled (Fig. 5), with the values in ArKO females being lower than in WT females only at P25. ANOVA revealed a significant effect of genotype [F(1,156) = 101.4; P < 0.0001], and post hoc comparisons showed that relative amount of PR-ir was significantly (P < 0.05) lower in ArKO females than in WT females only at P25. There was much more PR-ir in the VMH-L of WT males than in WT females across the postnatal ages P0–P20. ANOVA revealed a significant effect of sex [F(1,156) = 81.8; P < 0.0001], and post hoc comparisons showed that the relative amount of PR-ir was significantly higher (P < 0.05) in the VMH-L of WT males than WT females over P0–P20. Again, there was significantly (P < 0.05) less PR-ir in the VMH-L of ArKO males than WT males across all six postnatal ages sampled. Thus, the pattern of PR-ir in the VMH-L was clearly different from that observed for the MPOA and AVPv.
Figure 5.
The relative amount (mean ± sem) of PR-ir in the VMH-L on P0, P5, P10, P15, P20, and P25 in male (M) and female (F) mice that were either WT or ArKO. *, Post hoc comparisons indicated a significant (P < 0.05) sex difference in WT mice at the ages indicated. Post hoc comparisons also indicated significant (P < 0.05) differences in WT vs. ArKO females (F over brackets) and between WT and ArKO males (M over brackets) at the ages shown.
Discussion
The present results support the view (12) that estradiol contributes prepubertally to at least one female-typical aspect of development in both the MPOA and the AVPv, the expression of PR. Thus, in WT female mice, we first detected PR-ir cells in both of these brain regions at P10, and the levels of PR expression steadily increased in WT females killed over the ages P15–P25. Our observation that PR-ir values in both the MPOA and the AVPv were significantly lower in ArKO females than in WT females across the ages P15–P25 suggests that estradiol normally stimulates PR expression at these prepubertal ages in WT female mice. Wagner et al. (18) also first detected PR-ir cells in the MPOA of WT female mice killed at P8 (older ages were not studied), and this level of PR expression was not different from that of estradiol receptor (ER)-α KO females. It is not known whether PR expression would be reduced in ER-α KO female mice killed at later postnatal ages (e.g. P10–P25). The results of several studies using female rats further support our conclusion that estradiol normally stimulates female-typical levels of prepubertal PR expression in the mouse MPOA, although the exact timing of estradiol’s postnatal action may differ between the two species. Thus, ovariectomy shortly after birth significantly reduced PR-ir in the MPOA of female rats killed at P13 (14) or at P14 (16). In the latter study (16), administration of estradiol to ovariectomized female rats significantly augmented PR-ir in the MPOA across a range (P7–P67) of postnatal ages. Also, neonatal administration of an ER-α, but not an ER-β agonist, reliably stimulated PR expression in the MPOA of female rats killed on P7 (25). Finally (20), transplacental administration of the aromatase-blocking drug ATD significantly reduced PR-ir present in the female rat MPOA at the end of gestation.
The traditional view is that female-typical aspects of hypothalamic sexual differentiation in mammals occur without the benefit of any active sex hormone signaling (12). The present data from mice along with the rat data cited above suggest, however, that the female-typical, prepubertal profile of PR expression in the MPOA as well as the AVPv depends on a stimulatory action of estradiol. More research is needed to identify the sources of this estradiol, which may include the females’ ovaries and/or de novo synthesis in the female hypothalamus (20). Additional studies are also needed to see whether the prepubertal increase in neural PR expression seen in WT females is simply a passive, inconsequential response to increased ovarian production of estradiol or, alternatively, whether long-lasting morphological and/or behavioral neuroendocrine consequences of this prepubertal estrogenic stimulation of PR expression can be identified in the female rodent MPOA and AVPv. A feminizing role for estradiol has been reported in the development of the sexually dimorphic expression of kisspeptin (females more than males), an important modulator of GnRH secretion (11,26). Both studies reported decreased numbers of kisspeptin neurons in the rostral periventricular area of the third ventricle, which includes the AVPv, in adult female ArKO mice, even after treatment with estradiol in adulthood (11). Clarkson et al. (26) also showed that ovariectomy of female mice at P15 resulted in a 70–90% reduction in kisspeptin expression within the rostral periventricular area of the third ventricle analyzed at either P30 or P60, whereas administration of 17β-estradiol to P15-ovariectomized mice from P15–P30 or P22–P30 completely restored kisspeptin expression in this brain region. These results suggest that the sex difference in kisspeptin neuronal number in WT mice reflects a prepubertal, organizational action of estradiol in females. Additional evidence of an organizational role for prepubertal ovarian hormones is the observation (27) that ovariectomy on P20 reduced the later incorporation of newly born neurons into the female rat AVPv. More research is needed to determine whether any of these prepubertal, organizational actions of estradiol on the developing female nervous system are mediated by the induction of PR and the concurrent effects of progesterone.
Compared with the timing of estradiol action in the MPOA and AVPv, our results suggest that there is a delay in the postnatal age at which the female-typical profile of VMH-L PR expression in mice depends on the stimulatory action of estradiol. Thus, at ages P0–P20, there were no differences in PR-ir cell number between WT and ArKO females; only at P25 was PR-ir cell number significantly lower in ArKO than in WT females. Results obtained in rats (16) point to a similar, albeit shorter, delay in the postnatal age when estradiol first stimulates PR expression in the female VMH-L. Thus, ovariectomy on P0 failed to reduce PR-ir cell number in the VMH-L of female rats killed on P4, and administering estradiol to neonatally ovariectomized females failed to stimulate PR expression at P7, whereas significant estradiol-induced PR expression was seen over ages P14–P67. In another study (25), administration of a specific ER-α agonist on P5 and P6 was even more effective than estradiol in stimulating PR-ir cell number in the VMH-L of female rats killed at P7. Furthermore, concurrent administration of an ER-β agonist together with the ER-α agonist reduced the stimulation of PR expression on P7 otherwise obtained by administering the ER-α agonist alone, suggesting that an early neonatal period exists when ER-β activation inhibits ER-α-induced transcriptional activity. After an initial neonatal delay in both female rats and mice, estradiol, acting via ER-α, reliably stimulates PR expression in the VMH-L across the life span. At the age of puberty, this estradiol-stimulated expression of PR in the VMH contributes importantly to the induction of proceptive and receptive aspects of feminine mating behaviors shown in response to progesterone (28). Ovarian steroids are thought to stimulate sexual receptivity (lordosis) in female rodents by acting in the VMH-L (28). The observation (10) that ArKO female mice showed significantly less lordosis behavior than WT control females after ovariectomy, and treatment with estradiol and progesterone in adulthood is correlated with the present observation that PR expression was significantly reduced at least at one prepubertal age (P25). Again, more research is needed to determine whether a causal link exists between the absence of an estradiol-induced stimulation of PR expression (with a resultant loss of responsiveness to progesterone) in the VMH-L on or after P25 in ArKO females and their later behavioral refractoriness to ovarian hormones.
The present results confirm and extend to P10 a previous report (18) that PR-ir cell number was significantly greater in both the MPOA and VMH-L of WT male than WT female mice killed at P0, P4, and P8. A similar sex difference in PR expression in the MPOA across perinatal ages was also reported in the rat (15,29). Another report from that group (21) showed that the organizational effects of testicular hormone (presumably testosterone), as opposed to genes expressed directly off the sex chromosomes, stimulate increased expression of PR neonatally in the MPOA and VMH-L of male vs. female mice. Indeed, castration of male rats on the day of birth significantly reduced PR expression in the MPOA over the next several days (19). Many of the perinatal actions of testosterone in the male rodent hypothalamus depend on the neural aromatization of this androgen into estradiol and the subsequent activation of ER-α and/or -β (1). Our observation that PR-ir in the MPOA, AVPv, and VMH-L was significantly lower in ArKO males than in WT males across a range of postnatal ages (P0–P25) suggests that estrogenic metabolites of testosterone play an essential role in establishing the male-typical profile of hypothalamic PR expression in neonatal mice. A similar conclusion can be drawn from the report (18) that PR-ir values in both the MPOA and VMH-L were significantly lower in male mice with a null mutation of ER-α than in WT males killed at P0, P4, and P8. Likewise, transplacental administration of the aromatase blocker ATD significantly attenuated PR expression in the MPOA and AVPv of male rats killed at the end of gestation (15). Taken together, these results for mice and rats strongly suggest that the male-typical profile of PR expression in both MPOA and VMH-L depends on the stimulatory action of estradiol formed via the neural aromatization of testosterone secreted perinatally by the testes.
Just as it is unknown whether prepubertal estradiol-induced expression of hypothalamic PR expression contributes in the long term to female-typical brain and behavioral differentiation, it is uncertain whether the increased neonatal expression of PR in the male rodent MPOA, AVPv, and/or VMH-L contributes to the testosterone-dependent, male-typical organization of brain and behavior. It has been reported (14) that neonatal treatment of female rats with the PR antagonist RU486 significantly attenuated the masculinizing effect of neonatal testosterone on the volume of the sexually dimorphic nucleus of the POA, whereas in another study (30), neonatal RU486 significantly reduced the proportion of male rats that showed mounting behavior in adult tests with an estrous female rat. Interpretation of these results is complicated by the fact the RU486 antagonizes glucocorticoid receptors in addition to PR (31). Also, other studies (32,33) revealed either no significant disruption or an enhancement of masculine sexual behavior in PRKO male mice. Likewise, there was no disruption of normal male-male aggressive behavior in PRKO males (34). More research is needed to determine whether other male-typical as well as female-typical aspects of brain, behavioral, and/or reproductive neuroendocrine sexual differentiation are disrupted in PRKO mice of either sex.
Footnotes
This work was supported by National Institutes of Health Grant HD044897 and Fonds National de la Recherche Scientifique (FNRS) MIS F.4502.07. J.B. is a research associate of the Belgian FNRS.
Disclosure Summary: The authors have nothing to disclose.
First Published Online February 24, 2010
Abbreviations: AFP, α-Fetoprotein; ArKO, aromatase-KO; ATD, 1,4,-androstatriene-3,17-dione; AVPv, anteroventral periventricular nucleus; ER, estradiol receptor; ir, immunoreactive; KO, knockout; MPOA, medial preoptic area; P7, postnatal d 7; PR, progesterone receptor; TBS, Tris-buffered saline; TBST, TBS containing 0.2% Triton X-100; VMH-L, lateral part of the ventromedial hypothalamic nucleus; WT, wild type.
References
- De Vries GJ, Simerly RB 2002 Anatomy, development, and function of sexually dimorphic neural circuits in the mammalian brain. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, eds. Hormones, brain and behavior. Amsterdam: Academic Press; 137–191 [Google Scholar]
- Baum MJ, Carroll RS, Cherrv JA, Tobet SA 1990 Steroidal control of behavioural, neuroendocrine and brain sexual differentiation: studies in a carnivore, the ferret. J Neuroendocrinol 2:401–418 [DOI] [PubMed] [Google Scholar]
- McEwen BS, Plapinger L, Chaptal C, Gerlach J, Wallach G 1975 Role of fetoneonatal estrogen binding proteins in the associations of estrogen with neonatal brain cell nuclear receptors. Brain Res 96:400–406 [DOI] [PubMed] [Google Scholar]
- Bakker J, De Mees C, Douhard Q, Balthazart J, Gabant P, Szpirer J, Szpirer C 2006 Alpha-fetoprotein protects the developing female mouse brain from masculinization and defeminization by estrogens. Nat Neurosci 9:220–226 [DOI] [PubMed] [Google Scholar]
- Toran-Allerand CD 1976 Sex steroids and the development of the newborn mouse hypothalamus and preoptic area in vitro: implications for sexual differentiation. Brain Res 106:407–412 [DOI] [PubMed] [Google Scholar]
- Gerall AA, Dunlap JL, Hendricks SE 1973 Effect of ovarian secretions on female behavioral potentiality in the rat. J Comp Physiol Psychol 82:449–465 [DOI] [PubMed] [Google Scholar]
- Steward J, Cygan D 1980 Ovarian hormones act early in development to feminize adult open-field behavior in the rat. Horm Behav 14:20–32 [DOI] [PubMed] [Google Scholar]
- Arnold AP, Xu J, Grisham W, Chen X, Kim YH, Itoh Y 2004 Sex chromosomes and brain sexual differentiation. Endocrinology 145:1057–1062 [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Rissman EF, Simerly RB, Yang LY, Scordalakes EM, Auger CJ, Swain A, Lovell-Badge R, Burgoyne PS, Arnold AP 2002 A model system for study of sex chromosome effects on sexually dimorphic neural and behavioral traits. J Neurosci 22:9005–9014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker J, Honda S, Harada N, Balthazart J 2002 The aromatase knock-out mouse provides new evidence that estradiol is required during development in the female for the expression of sociosexual behaviors in adulthood. J Neurosci 22:9104–9112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker J, Pierman S, Gonzalez-Martinez D 27 November 2009 Effects of aromatase mutation (ArKO) on the sexual differentiation of kisspeptin neuronal numbers and their activation by same versus opposite sex urinary pheromones. Horm Behav 10.1016/j.yhbeh.2009.11.005 [DOI] [PubMed] [Google Scholar]
- Bakker J, Baum MJ 2008 Role for estradiol in female-typical brain and behavioral sexual differentiation. Front Neuroendocrinol 29:1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weniger JP, Zeis A, Chouraqui J 1993 Estrogen production by fetal and infantile rat ovaries. Reprod Nutr Dev 33:129–136 [DOI] [PubMed] [Google Scholar]
- Quadros PS, Lopez V, De Vries GJ, Chung WC, Wagner CK 2002 Progesterone receptors and the sexual differentiation of the medial preoptic nucleus. J Neurobiol 51:24–32 [DOI] [PubMed] [Google Scholar]
- Quadros PS, Pfau JL, Goldstein AY, De Vries GJ, Wagner CK 2002 Sex differences in progesterone receptor expression: a potential mechanism for estradiol-mediated sexual differentiation. Endocrinology 143:3727–3739 [DOI] [PubMed] [Google Scholar]
- Quadros PS, Wagner CK 2008 Regulation of progesterone receptor expression by estradiol is dependent on age, sex and region in the rat brain. Endocrinology 149:3054–3061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudwa AE, Harada N, Honda SI, Rissman EF 2009 Regulation of progestin receptors in medial amygdala: estradiol, phytoestrogens and sex. Physiol Behav 97:146–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner CK, Pfau JL, De Vries GJ, Merchenthaler IJ 2001 Sex differences in progesterone receptor immunoreactivity in neonatal mouse brain depend on estrogen receptor α expression. J Neurobiol 47:176–182 [DOI] [PubMed] [Google Scholar]
- Quadros PS, Goldstein AY, De Vries GJ, Wagner CK 2002 Regulation of sex differences in progesterone receptor expression in the medial preoptic nucleus of postnatal rats. J Neuroendocrinol 14:761–767 [DOI] [PubMed] [Google Scholar]
- Jahagirdar V, Quadros PS, Wagner CK 2008 Endogenous oestradiol regulates progesterone receptor expression in the brain of female rat fetuses: what is the source of oestradiol? J Neuroendocrinol 20:359–365 [DOI] [PubMed] [Google Scholar]
- Wagner CK, Xu J, Pfau JL, Quadros PS, De Vries GJ, Arnold AP 2004 Neonatal mice possessing an Sry transgene show a masculinized pattern of progesterone receptor expression in the brain independent of sex chromosome status. Endocrinology 145:1046–1049 [DOI] [PubMed] [Google Scholar]
- Honda S, Harada N, Ito S, Takagi Y, Maeda S 1998 Disruption of sexual behavior in male aromatase-deficient mice lacking exons 1 and 2 of the cyp19 gene. Biochem Biophys Res Commun 252:445–449 [DOI] [PubMed] [Google Scholar]
- Traish AM, Wotiz HH 1990 Monoclonal and polyclonal antibodies to human progesterone receptor peptide-(533–547) recognize a specific site in unactivated (8S) and activated (4S) progesterone receptor and distinguish between intact and proteolyzed receptors. Endocrinology 127:1167–1175 [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ 2001 The mouse brain in stereotaxic coordinates. San Diego: Academic Press [Google Scholar]
- Gonzales KL, Tetel MJ, Wagner CK 2008 Estrogen receptor (ER)β modulates ERα responses to estrogens in the developing rat ventromedial nucleus of the hypothalamus. Endocrinology 149:4615–4621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson J, Boon WC, Simpson ER, Herbison AE 2009 Postnatal development of an estradiol-kisspeptin positive feedback mechanism implicated in puberty onset. Endocrinology 150:3214–3220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed EI, Zehr JL, Schulz KM, Lorenz BH, DonCarlos LL, Sisk CLT 2008 Pubertal hormones modulate the addition of new cells to sexually dimorphic brain regions. Nat Neurosci 11:995–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein JD, Erskine MS 2002 Feminine sexual behavior: cellular integration of hormonal and afferent information in the rodent forebrain. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, eds. Hormones, brain and behavior. Amsterdam: Academic Press; 139–214 [Google Scholar]
- Wagner CK, Nakayama AY, De Vries GJ 1998 Potential role of maternal progesterone in the sexual differentiation of the brain. Endocrinology 139:3658–3661 [DOI] [PubMed] [Google Scholar]
- Lonstein JS, Quadros PS, Wagner CK 2001 Effects of neonatal RU486 on adult sexual, parental, and fearful behaviors in rats. Behav Neurosci 115:58–70 [DOI] [PubMed] [Google Scholar]
- Munden PM, Schmidt TJ 1992 Mifepristone blocks specific glucocorticoid receptor binding in rabbit iris-ciliary body. Arch Ophthalmol 110:703–705 [DOI] [PubMed] [Google Scholar]
- Phelps SM, Lydon JP, O'Malley BW, Crews D 1998 Regulation of male sexual behavior by progesterone receptor, sexual experience, and androgen. Horm Behav 34:294–302 [DOI] [PubMed] [Google Scholar]
- Schneider JS, Burgess C, Sleiter NC, DonCarlos LL, Lydon JP, O'Malley B, Levine JE 2005 Enhanced sexual behaviors and androgen receptor immunoreactivity in the male progesterone receptor knockout mouse. Endocrinology 146:4340–4348 [DOI] [PubMed] [Google Scholar]
- Schneider JS, Stone MK, Wynne-Edwards KE, Horton TH, Lydon J, O'Malley B, Levine JE 2003 Progesterone receptors mediate male aggression toward infants. Proc Natl Acad Sci USA 100:2951–2956 [DOI] [PMC free article] [PubMed] [Google Scholar]