Abstract
Gene expression starts with transcription and is followed by multiple posttranscriptional processes that carry out the splicing, capping, polyadenylation, and export of each mRNA. Interest in posttranscriptional regulation has increased recently with explosive discoveries of large numbers of noncoding RNAs such as microRNAs, yet posttranscriptional processes depend largely on the functions of RNA-binding proteins as well. Glucocorticoid nuclear receptors are classical examples of environmentally reactive activators and repressors of transcription, but there has also been a significant increase in studies of the role of posttranscriptional regulation in endocrine responses, including insulin and insulin receptors, and parathyroid hormone as well as other hormonal responses, at the levels of RNA stability and translation. On the global level, the transcriptome is defined as the total RNA complement of the genome, and thereby, represents the accumulated levels of all expressed RNAs, because they are each being produced and eventually degraded in either the nucleus or the cytoplasm. In addition to RNA turnover, the many underlying posttranscriptional layers noted above that follow from the transcriptome function within a dynamic ribonucleoprotein (RNP) environment of global RNA-protein and RNA-RNA interactions. With the exception of the spliceosome and the ribosome, thousands of heterodispersed RNP complexes wherein RNAs are dynamically processed, trafficked, and exchanged are heterogeneous in size and composition, thus providing significant challenges to their investigation. Among the diverse RNPs that show dynamic features in the cytoplasm are processing bodies and stress granules as well as a large number of smaller heterogeneous RNPs distributed throughout the cell. Although the localization of functionally related RNAs within these RNPs are responsive to developmental and environmental signals, recent studies have begun to elucidate the global RNA components of RNPs that are dynamically coordinated in response to these signals. Among the factors that have been found to affect coordinated RNA regulation are developmental signals and treatments with small molecule drugs, hormones, and toxins, but this field is just beginning to understand the role of RNA dynamics in these responses.
Cellular responses to hormones and other small molecules can activate coordinated changes in the stability and translation of specific subpopulations of functionally related mRNAs.
Much of molecular biology and biochemistry over the past several decades has focused on transcription, in part, because genetics provided a practical means of elucidating gene and protein functions (1). RNA in eukaryotes, being more heterogeneous and unstable compared with DNA, was challenging to study (2). However, the decades of the 1970s and 1980s brought advancing progress in understanding RNA binding and regulation that followed from the sequencing of small RNAs, including 5s RNA, transfer RNAs, and small nuclear RNAs, and the advent of RT-PCR, the discovery of ribozymes, and the elucidation of numerous families of RNA-binding proteins (RBPs) (3,4). Sequencing of mammalian genomes revealed that cellular RNA processing factors, including RBPs, appear to outnumber transcription factors by as much as 2-fold (2,5). Moreover, RNA transcripts were found to be much more diverse, heterogeneous, and of low abundance individually than previously thought; therefore, increasing the challenge. The explosion of research on RNA per se proceeded in parallel with new understanding of RNA-protein interactions and RNA structure, whereas the discovery in Caenorhabditis elegans in 1993 of small regulatory RNAs by Victor Ambros’ Lab (6) and Ruvkun’s Lab (7), now called microRNAs, reemerged on the global level in the late 1990s. There is increasing evidence that microRNAs and other small noncoding RNAs are interdependent with RBPs, in some cases cooperating and in other cases competing for functional outcomes (8,9,10,11,12,13). The entire field of posttranscriptional gene regulation is undergoing a renaissance of discovery that is impacting on all fields of biology and medicine.
Posttranscriptional Gene Regulation: Coupling and Coordination
In bacterial cells, the transcriptional apparatus is directly coupled to the translational apparatus as transcripts begin to be translated by ribosomes before their transcription has terminated. On the contrary, eukaryotic transcription and translation cannot be directly coupled because of the nuclear membrane and the compartmentalization of RNA diversification and processing. Therefore, the final decision as to which genes will be expressed as proteins in eukaryotes is made independently in the cytoplasm, although early signals or tags such as the Exon-Junction-Complex placed on the pre-mRNA in the nucleus can influence translational outcomes (14). Many studies suggest that RBPs indirectly couple transcriptional and subsequent posttranscriptional steps by interacting with their target transcripts (15). Although progressive coupling of these processes is widely accepted, many studies have demonstrated that posttranscriptional events involving multiple mRNAs are highly coordinated as well (2,16). Both coupling and coordination are important in determining how, when, and where to translate functionally related subpopulations of mRNAs (17). Moreover, unless the posttranscriptional pathway functions with precision, transcriptional regulatory events will become discordant. Reports from many labs have demonstrated that RBPs, including export proteins, provide coordinating functions at all steps along the posttranscriptional regulatory chain (2,14,16,18,19,20,21). As depicted in Fig. 1, discrete subpopulations of mRNAs reside in ribonucleoprotein (RNP) complexes (Fig. 1, inset) and can be coordinately processed: spliced, transported, stabilized or degraded, localized, or translated into protein.
Figure 1.
Pathway of gene expression in eukaryotic cells showing intervening steps of mRNA processing (red) that are coupled together with RBPs coordinating multiple mRNAs from transcription to translation. These processes are dynamic with multiple copies of each mRNA changing within and among heterogeneous RNPs in time and space (inset). The approach of using microarrays or deep sequencing to identify RNP-associated subsets of functionally related RNAs (RIP-chip or RIP-seq) is depicted, as is the use of nuclear run-on array measurements to assess nascent transcripts before RNA processing. [Adapted with permission from J. D. Keene: Proc Natl Acad Sci USA 98:7018–7024, 2001 (2). ©National Academy of Sciences.]
All posttranscriptional steps require the proper functioning of RBPs as components of RNP complexes whether or not noncoding regulatory RNAs are involved. Several laboratories have used RNP-immunoprecipitation-microchip (RIP-chip) or RIP with high-throughput sequencing (RIP-seq) to demonstrate that RBPs can interact with distinct subsets of the global mRNA population (Fig. 1) (5,18,22,23,24,25,26,27). Thus, data from many laboratories indicate that eukaryotic gene expression is highly coordinated and that transcription factors, RBPs, and microRNAs function together to form coherent gene expression network modules (reviewed in Refs. 16,20 and 21). Regardless of the exact mechanisms, the final outcome of protein synthesis is a mRNP-driven process that responds dynamically to the environment and cellular growth conditions.
RBPs are universal in living cells, and in eukaryotes, are estimated to number approximately 650 in yeast and over 2500 in mammals (2,5,14,28). Although some RBPs are thought to bind RNA with little or no sequence specificity, many and possibly most RBPs are specific for binding to distinct subpopulations of RNAs (2,22). Because RBPs can bind to more than one mRNA with sequence specificity, such interactions play an important role in regulating RNA localization, coexpression, and overall coordination of functionally related groups of mRNAs.
Multitargeting of RNA by RBPs and MicroRNAs
Early indications that RBPs were able to target multiple mRNAs at specific sequence elements were demonstrated in vitro using the embryonic lethal abnormal vision (ELAV)/HuB RBP and the fragile-X-mental retardation protein (FMRP). HuB is a RNA-recognition motif-containing RBP, one of four members of the ELAV/Hu family [HuA (aka: HuR), HuB, HuC, HuD] that was shown to bind to AU-rich elements in the 3′ untranslated regions (UTRs) of proto-oncogene and cytokine encoding transcripts (29) and to approximately 100 AU-rich human brain mRNA targets (30). These findings were later expanded using cell extracts, and the in vitro studied AU-rich mRNA targets confirmed, using RIP-chip experiments with HuB, e1F4E, and HuR (22). Based upon binding of FMRP to its own mRNA in vitro and binding to total cell radioactive RNA to FMRP in vitro, Warren and co-workers (31) surmised that it could bind to as many as 4% of human mRNAs, but neither the RNA targets nor the RNA-binding sequences of FMRP were identified at that time. However, many potential mRNA targets of FMRP were later identified also using the RIP-chip procedure (32), and at least one putative RNA-binding element was identified (33,34). The RIP-chip and RIP-seq approach has since been used with nearly 100 RBPs from different species to examine RNA targets and specific binding sequences of RBPs (reviewed in Refs. 16,20 and 35). These demonstrations that RBPs could bind to multiple mRNAs on a global level led to the proposal that they could serve as a coordinating mechanism for posttranscriptional processes (2,17,22,30). In addition, methods were devised to assess changes in populations of mRNA during decay and during translational activation (Fig. 2). The results of many of these studies suggested that the coordinated events affect specific subsets of functionally related mRNAs (reviewed in Ref. 16).
Figure 2.
The complexity of transcriptomics can be reduced by probing its underlying layers. Transcriptomics has generated data with overwhelming complexity. Polysome-arrays and decay arrays dissect layers of gene expression and can also reveal coordinated posttranscriptional events. mRNAs identified using RIP-chip are functionally related as coordinated and dynamic RNP modules (posttranscriptional RNA operons and regulons). There are procedures used to globally quantify RNAs at the levels of RNA stability or translation that are not otherwise evident from complex transcriptomic analysis. Depiction of a microarray (middle) that displays transcriptomic data representing the accumulated levels of each mRNA depending upon both its rate of synthesis and its rate of degradation. Methods such as RIP-chip (and RIP-seq), RNA decay-array analysis, and polysome gradient-array analysis have been devised in recent years to assess these “under layers” of the transcriptome. RNA turnover due to differences in RNA stability among the RNAs is depicted as up and down arrows below the array.
The original discovery of microRNAs was based upon single antisense interactions between each of two small RNA transcripts (lin-4) and a single mRNA (lin-14) (6,7). Only later did the suggestion of multitargeting of microRNAs to multiple mRNAs emerge based upon computational predictions of complementary sequences between the microRNAs and many potential mRNA targets (36,37,38). These trans-acting small RNA regulators of mRNAs, stimulated by the discovery of RNA interference by Mello and co-workers (39), were believed, like the RBPs, to target multiple mRNAs, leading to profound phenotypic changes. Both experimental data and improved computational algorithms have overwhelmingly confirmed this prediction (40). The functional relationship among the multiple targets of microRNAs has remained unclear to date, although this could be a result of a lack of information regarding the combinatorial interactions between microRNAs and RBPs. Also, many articles compare microRNAs with transcription factors and leave out the analogy to RBPs entirely, whereas a few recent reports have linked the effects of these 3′UTR-interacting factors (8,9,10,13). The emergence of small noncoding RNA biology has been profound in recent years, and in some aspects overwhelming in its impact, because nearly every laboratory now uses RNA interference to study their respective biological systems and disease models.
RNA Dynamics
Along the complex pathway of gene expression from the nucleus to the cytoplasm, the final decisions that lead to protein production are posttranscriptional. Although transcription is necessary to originate each mRNA, the steps of splicing, nuclear export, RNA stability, localization, and eventually translation require precision in order for gene expression signals at transcription to achieve their intended expression outcome. Numerous recent studies have demonstrated that the flow of genetic information on these posttranscriptional levels is highly organized and combinatorial (Fig. 3). As noted, each step of posttranscriptional gene expression is intimately connected to the next step and the regulatory processes are coordinated by RBPs, and noncoding RNAs are believed to particulate in this regulation. This chain of interconnected RNA processing steps orchestrates the production of thousands of proteins in time and space in response to cellular signals in normal or diseased states (41,42,43,44,45,46,47; for review, see Ref. 35).
Figure 3.
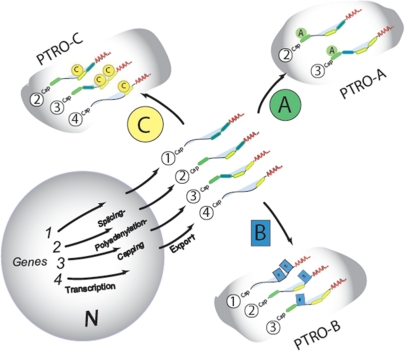
Multiple copies allow multiple combinations of mRNAs. Illustration of the multiple lives of each mRNA based on the posttranscriptional RNA operon/regulon model (16). Functionally related mRNAs are “clustered” in time and space such that the proteins encoded by them can be coordinately produced in concert. Each of the four mRNAs has the potential to be a member of more than one subset because the protein it encodes serves multiple functional roles in different cellular processes. The colored circles represent different RBPs or different posttranslationally modified isoforms of a given RBP that bind to the colored bars in the 3′ or 5′ UTRs. Noncoding RNAs that bind in combination with RBPs to sequences in these mRNAs can affect posttranscriptional outcomes such as RNA stability and translation. Posttranscriptional RNA Operons (PTRos) are coordinated subsets of functionally related mRNAs in association with regulatory RBPs and noncoding RNAs that are spliced, transported, stabilized, localized, or translated in a coherent manner. Multiple PTRos can share certain mRNAs to form overlapping posttranscriptional regulons that coordinate the production of several related subsets of functionally related proteins.
RNAs are highly dynamic, and multitargeting by RBPs plays an important role in generating as well as regulating dynamic RNA networks. Because RNA can be generated, used, and then destroyed over relatively short intervals, it can serve multiple transient functions and be replaced when needed. Whether being acted upon in cis, or acting as a trans-combinatorial regulator of other RNAs, the dynamic interactions can be exceedingly brief. Indeed, the multiple copies of each mRNA allow each individual species to have multiple lives in that each is capable of joining together with any functionally related group of mRNAs as long as it contains the appropriate RNA binding element that is also present in the other RNAs in the subset (14,17,18,20). For example, in the case of hormonal responses, one can imagine how rapidly responding changes at the level of RNA stability provide an advantage to multicellular systems (48).
An important feature of RNA networks is that a significant proportion of cellular proteins encode RBPs that in turn regulate the mRNAs encoding other RBPs. This property of the ribonome forms a “regulators of regulators” feature that can serve to coordinate upstream and downstream functions of gene expression. Indeed, there is massive feedback from translation to transcription and from translation to other posttranscriptional processes providing stability and resilience to global RNA networks by making them interdependent (49,50). Thus, at least two gene expression networks, one transcriptionally derived and one or more posttranscriptionally derived, are linked in a dynamic network of cross talk because every transcription factor must be translated and every translation factor must be transcribed. In any case, translation is the final decision along the chain of gene expression and it has recently been found to have its own overarching mode of modular coordination that we have termed posttranscriptional RNA operons and regulons (see Fig. 3) (reviewed in Refs. 16,20 and 35).
Most cellular events are dynamic in time and space as is readily apparent in time-lapse imaging of cells and embryos. Although all molecules and macromolecules are dynamic in their local shapes and allostery, traditional molecular biological and biochemical experiments do not generally consider events in both time and space; certainly not on a global basis. Moreover, molecular structural studies have only recently been designed to look beyond static crystallographic analysis and are beginning to use nuclear magnetic resonance and liquid imaging of increasingly larger peptides and DNA and RNA to understand molecular dynamics (51,52). The field that investigates transient molecular complexes is rapidly emerging and has broad implications for understanding the structural and functional dynamics of molecules at all levels of cell regulation. RNAs are known to be dynamic on multiple levels as detected, for example, by time-lapse experiments using florescent probes and molecular tags (51). However, global analyses that assess RNA dynamics have not been extensively used. Yet most developmental processes and chemical activation events are believed to alter the levels and locations of RNAs. Hormonal and inflammatory responses as well as genotoxic effects on cells are examples of cellular activation events that involve both transcriptional activation as well as posttranscriptional regulation of RNA stability and translation (28,53,54,55,56,57,58). Also, see accompanying minireviews in this issue (59,60). This is a field of active investigation because these important cellular responses underlie many normal physiological and pathological processes.
The accumulated global levels of mRNAs in cells, referred to as the transcriptome, represent the balance between on-going transcription and on-going changes in RNA stability (Figs. 1 and 2) (2). This important fact has generally been overlooked in gene expression profiling studies because the results obtained from total cellular or tumor RNA using microarrays is frequently assumed to represent RNA production. However, without a means to determine global changes in the stability of these RNAs as well as their levels of translation, we are left with a very incomplete understanding of the pathway of gene expression and the eventual outcomes. Thus, a variety of experimental approaches emerged, including nuclear run-on en masse (Fig. 1, inset), RNA decay-array analysis and polysome gradient-array analysis (Fig. 2). For example, a study that used nuclear run-on analysis of nascent transcripts globally during T-cell activation and compared the changes in RNA abundance demonstrated that more than one half of the changes in RNA were due to effects of RNA stability (61). Unfortunately, this very important aspect of transcriptomics analysis has been largely disregarded in many studies that seek to understand disease states using gene expression signatures. Moreover, most experiments that assess global transcription do so by measuring mRNA production, and those transcripts were very likely subjected to the effects of RNA stability. Therefore, interpretations of transcriptional outcomes need to consider this fact in determining mechanisms that coordinate global gene expression.
Coordinated Gene Expression and the Posttranscriptional Operon Model
As noted above, molecular events in gene expression from transcription to translation are closely interconnected to one another (Fig. 1). Jacob and Monod discovered prokaryotic DNA operons in which genes are expressed in functionally related units. The DNA operon model demonstrated that the initiation of transcription usually results in the production of long polycistronic mRNAs that contain multiple gene transcripts in tandem. This polycistronic architecture allows multiple genes to be expressed in concert and to be regulated sequentially as groups. Although a high percentage of bacterial genes are contained in operons, functionally related genes are not physically clustered on the genomes of eukaryotes. In fact, DNA operons have not been found in human cells, suggesting that other mechanisms of gene coordination may exist. In addition, with minor exceptions, polycistronic mRNAs are not common in mammalian cells, but instead, mRNA transcripts are monocistronic. Despite these facts, it is still generally assumed by most scientists that gene expression in mammalian cells is coordinated solely at the level of transcription.
Our laboratory demonstrated that during the induction of neuronal differentiation, Hu proteins bind to mRNA subpopulations within RNPs that change combinatorially in a dynamic pattern, indicating coordinated remodeling of mRNPs (17,22). As noted above, the demonstration that ELAV/Hu proteins are multitargeted to bind discrete subsets of mRNAs in neurons and other cells led to the theory of posttranscriptional RNA operons (or regulons) (Fig. 3) (17). This concept has been supported by data from many laboratories in many species by using RIP-chip and other experimental and bioinformatics procedures (summarized in Ref. 35). Recent studies with activated T cells have examined the dynamics of global mRNA populations using a probabilistic approach applying Gaussian mixture modeling and related statistical methods (27,47). One of the benefits of this approach is that one can assign a continuous metric to each mRNA in the population after cellular perturbation. Data representing these values allowed a database to be queried from the drug-gene-disease connectivity map (62) to identify small molecule drugs that affect posttranscriptional regulators and thereby generated a drug phenotype of HuR-mediated RNA stability during T-cell activation (47). Overall, the field of global RNA dynamics is at its beginnings and can provide insights into responses to developmental and environmental signals as well as hormone receptor functions and other endocrine systems in which rapid responses of the gene expression apparatus have provided an important physiological adaptation.
Future Perspectives
Novel approaches to understanding responses to hormones and other endocrine mediators at the posttranscriptional level of gene expression are developing at a rapid pace. Although many of these systems remain poorly understood, investigative methods to elucidate the underlying bases using quantitative cellular dynamics will be necessary (63). The advent of microarrays set forth an opportunity to analyze RNA regulatory factors on a global basis, and more recently, the introduction of high-throughput “deep” sequencing procedures has provided a powerful means to derive even more global information such as the roles of noncoding RNAs in posttranscriptional regulation. It will be important to exploit these strategies to better understand gene expression dynamics after hormone treatments and physiological responses.
Footnotes
Disclosure Summary: The author has a financial relationship with Ribonomics, Inc., and MBL, Inc., which hold licenses to RIP-chip technologies mentioned in this article.
Abbreviations: ELAV, Embryonic lethal abnormal vision; FMRP, fragile-X-mental retardation protein; RBP, RNA-binding protein; RIP-chip, RNP-immunoprecipitation-microchip; RIP-seq, RIP with high-throughput sequencing; RNP, ribonucleoprotein; UTR, untranslated region.
References
- Orphanides G, Reinberg D 2002 A unified theory of gene expression. Cell 108:439–451 [DOI] [PubMed] [Google Scholar]
- Keene JD 2001 Ribonucleoprotein infrastructure regulating the flow of genetic information between the genome and the proteome. Proc Natl Acad Sci USA 98:7018–7024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenan DJ, Query CC, Keene JD 1991 RNA recognition: towards identifying determinants of specificity. Trends Biochem Sci 16:214–220 [DOI] [PubMed] [Google Scholar]
- Burd CG, Dreyfuss G 1994 Conserved structures and diversity of functions of RNA-binding proteins. Science 265:615–621 [DOI] [PubMed] [Google Scholar]
- Hogan DJ, Riordan DP, Gerber AP, Herschlag D, Brown PO 2008 Diverse RNA-binding proteins interact with functionally related sets of RNAs, suggesting an extensive regulatory system. PLoS Biol 6:e255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RC, Feinbaum RL, Ambros V 1993 The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 75:843–854 [DOI] [PubMed] [Google Scholar]
- Wightman B, Ha I, Ruvkun G 1993 Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell 75:855–862 [DOI] [PubMed] [Google Scholar]
- Jing Q, Huang S, Guth S, Zarubin T, Motoyama A, Chen J, Di Padova F, Lin SC, Gram H, Han J 2005 Involvement of microRNA in AU-rich element-mediated mRNA instability. Cell 120:623–634 [DOI] [PubMed] [Google Scholar]
- Bhattacharyya SN, Habermacher R, Martine U, Closs EI, Filipowicz W 2006 Relief of microRNA-mediated translational repression in human cells subjected to stress. Cell 125:1111–1124 [DOI] [PubMed] [Google Scholar]
- Leung AK, Sharp PA 2007 microRNAs: a safeguard against turmoil? Cell 130:581–585 [DOI] [PubMed] [Google Scholar]
- Parker R, Sheth U 2007 P bodies and the control of mRNA translation and degradation. Mol Cell 25:635–646 [DOI] [PubMed] [Google Scholar]
- Kim HH, Kuwano Y, Srikantan S, Lee EK, Martindale JL, Gorospe M 2009 HuR recruits let-7/RISC to repress c-Myc expression. Genes Dev 23:1743–1748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipowicz W, Bhattacharyya SN, Sonenberg N 2008 Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat Rev Genet 9:102–114 [DOI] [PubMed] [Google Scholar]
- Moore MJ 2005 From birth to death: the complex lives of eukaryotic mRNAs. Science 309:1514–1518 [DOI] [PubMed] [Google Scholar]
- Maniatis T, Reed R 2002 An extensive network of coupling among gene expression machines. Nature 416:499–506 [DOI] [PubMed] [Google Scholar]
- Keene JD 2007 RNA regulons: coordination of posttranscriptional events. Nat Rev Genet 8:533–543 [DOI] [PubMed] [Google Scholar]
- Keene JD, Tenenbaum SA 2002 Eukaryotic mRNPs may represent posttranscriptional operons. Mol Cell 9:1161–1167 [DOI] [PubMed] [Google Scholar]
- Hieronymus H, Silver PA 2004 A systems view of mRNP biology. Genes Dev 18:2845–2860 [DOI] [PubMed] [Google Scholar]
- Yisraeli JK 2005 VICKZ proteins: a multi-talented family of regulatory RNA-binding proteins. Biol Cell 97:87–96 [DOI] [PubMed] [Google Scholar]
- Halbeisen RE, Galgano A, Scherrer T, Gerber AP 2008 Post-transcriptional gene regulation: from genome-wide studies to principles. Cell Mol Life Sci 65:798–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansfield KD, Keene JD 2009 The ribonome: a dominant force in gene expression. Biol Cell 101:169–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenenbaum SA, Carson CC, Lager PJ, Keene JD 2000 Identifying mRNA subsets in messenger ribonucleoprotein complexes by using cDNA arrays. Proc Natl Acad Sci USA 97:14085–14090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takizawa PA, DeRisi JL, Wilhelm JE, Vale RD 2000 Plasma membrane compartmentalization in yeast by messenger RNA transport and a septin diffusion barrier. Science 290:341–344 [DOI] [PubMed] [Google Scholar]
- Ule J, Jensen K, Mele A, Darnell RB 2005 CLIP: a method for identifying protein-RNA interaction sites in living cells. Methods 37:376–386 [DOI] [PubMed] [Google Scholar]
- López de Silanes I, Zhan M, Lal A, Yang X, Gorospe M 2004 Identification of a target RNA motif for RNA-binding protein HuR. Proc Natl Acad Sci USA 101:2987–2992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licatalosi DD, Mele A, Fak JJ, Ule J, Kayikci M, Chi SW, Clark TA, Schweitzer AC, Blume JE, Wang X, Darnell JC, Darnell RB 2008 HITS-CLIP yields genome-wide insights into brain alternative RNA processing. Nature 456:464–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris AR, Mukherjee N, Keene JD 2008 Ribonomic analysis of human Pum1 reveals cis-trans conservation across species despite evolution of diverse mRNA target sets. Mol Cell Biol 28:4093–4103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brivanlou AH, Darnell JE Jr 2002 Signal transduction and the control of gene expression. Science 295:813–818 [DOI] [PubMed] [Google Scholar]
- Levine TD, Gao F, King PH, Andrews LG, Keene JD 1993 Hel-N1: an autoimmune RNA-binding protein with specificity for 3′ uridylate-rich untranslated regions of growth factor mRNAs. Mol Cell Biol 13:3494–3504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao FB, Carson CC, Levine TD, Keene JD 1994 Selection of a subset of mRNAs from 3′UTR combinatorial libraries using neuronal RNA-binding protein, Hel-N1. Proc Natl Acad Sci USA 91:11207–11211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashley Jr CT, Wilkinson KD, Reines D, Warren ST 1993 FMR1 protein: conserved RNP family domains and selective RNA binding. Science 262:563–566 [DOI] [PubMed] [Google Scholar]
- Brown V, Jin P, Ceman S, Darnell JC, O'Donnell WT, Tenenbaum SA, Jin X, Feng Y, Wilkinson KD, Keene JD, Darnell RB, Warren ST 2001 Microarray identification of FMRP-associated brain mRNAs and altered mRNA translational profiles in fragile X syndrome. Cell 107:477–487 [DOI] [PubMed] [Google Scholar]
- Darnell JC, Jensen KB, Jin P, Brown V, Warren ST, Darnell RB 2001 Fragile X mental retardation protein targets G quartet mRNAs important for neuronal function. Cell 107:489–499 [DOI] [PubMed] [Google Scholar]
- Zanotti KJ, Lackey PE, Evans GL, Mihailescu MR 2006 Thermodynamics of the fragile X mental retardation protein RGG box interactions with G quartet forming RNA. Biochemistry 45:8319–8330 [DOI] [PubMed] [Google Scholar]
- Morris AD, Mukherjee N, Keene JD 14 August 2009 Systematic analysis of posttranscriptional gene expression. Syst Biol Med 10.1002/wsbm.54 [DOI] [PubMed] [Google Scholar]
- Lai EC 2002 microRNAs are complementary to 3′UTR sequence motifs that mediate negative post-transcriptional regulation. Nat Genet 30:363–364 [DOI] [PubMed] [Google Scholar]
- Rhoades MW, Reinhart BJ, Lim LP, Burge CB, Bartel B, Bartel DP 2002 Prediction of plant microRNA targets. Cell 110:513–520 [DOI] [PubMed] [Google Scholar]
- Lewis BP, Shih IH, Jones-Rhoades MW, Bartel DP, Burge CB 2003 Prediction of mammalian microRNA targets. Cell 115:787–798 [DOI] [PubMed] [Google Scholar]
- Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC 1998 Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391:806–811 [DOI] [PubMed] [Google Scholar]
- Bartel DP 2004 MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116:281–297 [DOI] [PubMed] [Google Scholar]
- Raghavan A, Dhalla M, Bakheet T, Ogilvie RL, Vlasova IA, Khabar KS, Williams BR, Bohjanen PR 2004 Patterns of coordinate down-regulation of ARE-containing transcripts following immune cell activation. Genomics 84:1002–1013 [DOI] [PubMed] [Google Scholar]
- Hao S, Baltimore D 2009 The stability of mRNA influences the temporal order of the induction of genes encoding inflammatory molecules. Nat Immunol 10:281–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson P 2009 Intrinsic mRNA stability helps compose the inflammatory symphony. Nat Immunol 10:233–234 [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay R, Ray PS, Arif A, Brady AK, Kinter M, Fox PL 2008 DAPK-ZIPK-L13a axis constitutes a negative-feedback module regulating inflammatory gene expression. Mol Cell 32:371–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas K, Chaudhuri S, Leaman DW, Komar AA, Musiyenko A, Barik S, Mazumder B 2009 Genome-wide polysome profiling reveals an inflammation-responsive posttranscriptional operon in γ interferon-activated monocytes. Mol Cell Biol 29:458–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazan-Mamczarz K, Hagner PR, Dai B, Wood WH, Zhang Y, Becker KG, Liu Z, Gartenhaus RB 2008 Identification of transformation-related pathways in a breast epithelial cell model using a ribonomics approach. Cancer Res 68:7730–7735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee N, Lager PJ, Friedersdorf MB, Thompson MA, Keene JD 2009 Coordinated post-transcriptional mRNA population dynamics during T-cell activation. Mol Syst Biol 5:288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhawan L, Liu B, Blaxall BC, Taubman MB 2007 A novel role for the glucocorticoid receptor in the regulation of monocyte chemoattractant protein-1 mRNA stability. J Biol Chem 282:10146–10152 [DOI] [PubMed] [Google Scholar]
- Penalva LO, Burdick MD, Lin SM, Sutterluety H, Keene JD 2004 RNA-binding proteins to assess gene expression states of co-cultivated cells in response to tumor cells. Mol Cancer 3:24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullmann Jr R, Kim HH, Abdelmohsen K, Lal A, Martindale JL, Yang X, Gorospe M 2007 Analysis of turnover and translation regulatory RNA-binding protein expression through binding to cognate mRNAs. Mol Cell Biol 27:6265–6278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shav-Tal Y, Darzacq X, Shenoy SM, Fusco D, Janicki SM, Spector DL, Singer RH 2004 Dynamics of single mRNPs in nuclei of living cells. Science 304:1797–1800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hura GL, Menon AL, Hammel M, Rambo RP, Poole 2nd FL, Tsutakawa SE, Jenney Jr FE, Classen S, Frankel KA, Hopkins RC, Yang SJ, Scott JW, Dillard BD, Adams MW, Tainer JA 2009 Robust, high-throughput solution structural analyses by small angle X-ray scattering (SAXS). Nat Methods 6:606–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson P, Phillips K, Stoecklin G, Kedersha N 2004 Post-transcriptional regulation of proinflammatory proteins. J Leukoc Biol 76:42–47 [DOI] [PubMed] [Google Scholar]
- Blackshear PJ 2002 Tristetraprolin and other CCCH tandem zinc-finger proteins in the regulation of mRNA turnover. Biochem Soc Trans 30:945–952 [DOI] [PubMed] [Google Scholar]
- Gorospe M 2003 HuR in the mammalian genotoxic response. Cell Cycle 2:412–414 [PubMed] [Google Scholar]
- Greenman IC, Gomez E, Moore CE, Herbert TP 2007 Distinct glucose-dependent stress responses revealed by translational profiling in pancreatic β-cells. J Endocrinol 192:179–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Heller NM, Gorospe M, Atasoy U, Stellato C 2005 The role of post-transcriptional regulation in chemokine gene expression in inflammation and allergy. Eur Respir J 26:933–947 [DOI] [PubMed] [Google Scholar]
- Pryzbylkowski P, Obajimi O, Keen JC 2008 Trichostatin A and 5 Aza-2′ deoxycytidine decrease estrogen receptor mRNA stability in ER positive MCF7 cells through modulation of HuR. Breast Cancer Res Treat 111:15–25 [DOI] [PubMed] [Google Scholar]
- Lee EK, Gorospe M 2010 Minireview: posttranscriptional regulation of the insulin and insulin-like growth factor systems. Endocrinology 151:1403–1408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naveh-Many T 2010 Minireview: the play of proteins on the parathyroid hormone messenger ribonucleic acid regulates its expression. Endocrinology 151:1398–1402 [DOI] [PubMed] [Google Scholar]
- Cheadle C, Fan J, Cho-Chung YS, Werner T, Ray J, Do L, Gorospe M, Becker KG 2005 Control of gene expression during T cell activation: alternate regulation of mRNA transcription and mRNA stability. BMC Genomics 6:75–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb J, Crawford ED, Peck D, Modell JW, Blat IC, Wrobel MJ, Lerner J, Brunet JP, Subramanian A, Ross KN, Reich M, Hieronymus H, Wei G, Armstrong SA, Haggarty SJ, Clemons PA, Wei R, Carr SA, Lander ES, Golub TR 2006 The Connectivity Map: using gene-expression signatures to connect small molecules, genes, and disease. Science 313:1929–1935 [DOI] [PubMed] [Google Scholar]
- Wist AD, Berger SI, Iyengar R 2009 Systems pharmacology and genome medicine: a future perspective. Genome Med 1:11 [DOI] [PMC free article] [PubMed] [Google Scholar]