Abstract
The mechanisms linking intrauterine growth retardation (IUGR) with adulthood obesity and diabetes are unclear. These studies investigated energy homeostasis in 8- and 20-wk-old male and female mice subjected to protein deficiency in utero. Pregnant C57BL/6J female mice were fed a protein-deficient diet (6% protein). Undernourished offspring (UO) and controls (CO) were cross-fostered to lactating dams fed a 20% control diet. The 24-h profiles of energy expenditure, feeding behavior, physical activity, and whole-body substrate preference was assessed using 8-wk UO and CO weaned onto control diet. Blood chemistries, glucose tolerance, and expression of genes involved in hepatic lipid and glucose metabolism were analyzed in 8- and 20-wk-old CO and UO fed control or a high-fat diet. UO exhibited IUGR with catch-up growth at 8 wk of age and increased severity of diet-induced obesity and insulin resistance by 20 wk of age. Therefore, fetal malnutrition in the C57BL/6J mouse increases sensitivity to diet-induced obesity. Abnormal daily rhythms in food intake and metabolism, increased lipogenesis, and inflammation preceded obesity in the UO group. Arrhythmic expression of circadian oscillator genes was evident in brain, liver, and muscle of UO at 8 and 20 wk of age. Expression of the clock-associated nuclear receptor and transcription repressor Rev-erbα was reduced in liver and muscle of UO. Altered circadian physiology may be symptomatic of the metabolic dysregulation associated with IUGR, and altered feeding behavior and substrate metabolism may contribute to the obese phenotype.
Protein malnutrition during pregnancy results in offspring that have altered circadian rhythms in metabolism that may contribute to obesity and type II diabetes at adulthood.
A compromised environment encountered during fetal life and infancy is considered a risk factor for adult-onset diseases such as type 2 diabetes, hyperlipidemia, hypertension, and obesity (1). Many clinical problems can arise during gestation include metabolic, genetic, vascular, autoimmune, and infective disorders that may lead to reduced fetal birth weight (2). Early studies in humans found evidence that males of low birth weight were seven times more likely than normal birth weight infants to develop glucose intolerance, suggesting that low birth weight is correlated with a metabolic syndrome (3). Although the evidence supporting a link between fetal environment and propensity for the development of metabolic disease is growing, the mechanisms linking intrauterine growth retardation (IUGR) to adult disease are not clear.
Rodent models of IUGR have been developed to investigate the mechanisms linking fetal environment with adult disease. In rodent models, a strong correlation exists between fetal nutrition and insulin action after birth (4). In the rodent model, maternal undernutrition (through caloric malnutrition, protein restriction, or uterine ligation) during various stages of pregnancy results in hypoactivity, hyperphagia, hyperinsulinemia, hyperleptinemia, elevated blood pressure, and obesity in the offspring; these factors are exacerbated by hypercaloric nutrition and last throughout adulthood (5,6). Specifically, maternal protein restriction when administered to rodent dams reduces the birth weight of pups, suggesting IUGR. This is compensated by rapid catch-up growth, and at adulthood, rat offspring subject to IUGR related to protein malnutrition have a tendency for an exacerbated diet-induced obesity, hyperinsulinemia, and hyperleptinemia (7,8). Rat offspring are more insulin sensitive in early adulthood (3 months) but later become glucose intolerant and hyperinsulinemic associated with altered muscle and adipose tissue glucose uptake (9). Changes in hepatic expression of glucocorticoid receptors, peroxisome proliferator-activated receptor α (Pparα), and phosphoenolpyruvate carboxykinase (PEPCK or Pck1) in offspring of protein-deficient dams suggest altered glucose and lipid metabolism in liver (10,11,12). Several studies have used a candidate approach to investigate the function of known factors such as leptin in this process (13). These studies provided insight into the consequence of fetal malnutrition; however, the underlying mechanisms remain unclear.
The aim of this study was to investigate how a compromised fetal environment leads to changes in the 24-h metabolic profile in the C57BL/6J mouse subjected to a protein-restricted diet throughout gestation. The majority of metabolic studies in the literature associated with IUGR are performed in rats. The C57BL/6J mouse is the most commonly used model of diet-induced obesity and insulin resistance (14). We demonstrate that adult C57BL/6J mice subject to prenatal exposure to a low-protein diet develop many of the classic hallmarks of diabetes, including glucose intolerance and increased adiposity as well as displaying an altered circadian physiology with respect to physical activity and energy expenditure that precedes the onset of obesity. Protein-malnourished mice also exhibited altered expression of canonical clock oscillators (CCO) in a tissue-dependent fashion. CCO involve both a positive limb, comprised of circadian locomoter output cycles kaput (Clock), neuronal PAS domain protein 2 (Npas2), and aryl hydrocarbon receptor nuclear translocator-like (Arntl, also known as Bmal1). The transcription and translation of these clock oscillators is regulated through a feedback loop involving a negative limb that comprises the Cryptochrome (Cry) and Period (Per) genes (15,16). These data therefore indicate that the effects of the fetal environment on adult health may include alterations in the function of systems that govern circadian rhythms in behavior and metabolism.
Materials and Methods
Experimental animals
All studies were approved by the Pennington Biomedical Research Center’s Institutional Animal Care and Use Committee. C57BL/6J male and female mice purchased from The Jackson Laboratory (Bar Harbor, ME) were maintained on a 12-h light, 12-h dark cycle (lights on 0600–1800 h or circadian time CT0 and CT12). Breeding protocols were based on those used by Datta et al. (17). Two weeks before mating, nulliparous female and age-matched male mice were fed either a low-protein diet (6% protein, TD90016; Harlan Teklad, Madison, WI) or a control diet (20% protein TD91352; both diets are isocaloric but differ in the amount of protein in the form of casein). After acclimation to the chow, breeders were placed together. Females housed with males were checked daily for vaginal plugs. Offspring of protein-deficient and control (fed the 20% control diet during pregnancy) dams were cross-fostered to lactating females fed control diet within 24 h of birth and then weaned onto the 20% protein (TD91352) isocaloric control diet. All litters were culled to an equal number between protein-deficient and control/surrogate dams for all subsequent experiments (n = 6 per litter). Dams (both protein-deficient and control surrogates) were allowed to have only one litter before being euthanized to control for smaller litter sizes from subsequent pregnancies in protein-deficient dams.
For experiments examining the effect of diet on obesity and insulin resistance, male and female undernourished offspring (UO) and control offspring (CO) were fed either control diet or a high-fat diet (HFD, 60% kcal for fat, Research Diets D12492) from 8 wk of age for a period of 12 wk to address the effects of dietary challenge on metabolic stability.
Serum analysis
At 8 and 20 wk of age, serum glucose, cholesterol, and triglycerides (TG) were measured in UO and CO male and female mice after an overnight fast. Insulin, leptin, TNFα, and plasminogen activator inhibitor-1 (PAI-1) were measured using the Lincoplex biomarker immunoassay kit (Linco Research, St. Charles, MO) according to manufacturer’s recommendations.
Wheel-running activity
At 8 wk of age, wheel-running activity data (MiniMitter, Bend, OR) was recorded as previously described (18). Mice were allowed 14 d of acclimation in the wheel cages, and data were collected for 14 d after that.
Glucose tolerance test (GTT)
At 8 and 20 wk of age, male and female UO and CO were subjected to a GTT after an overnight fast (food was removed at 1900 h, and test was administered soon after lights on at 0800 h). A bolus of glucose was given ip (1 g/kg), and blood glucose levels were read using a glucometer at 15-min intervals beginning before the injection of glucose and continuing for 1 h. Direct comparisons at each time point between UO and CO was used for significance as well as area under the curve (AUC).
Analysis of energy expenditure (EE) and food intake
After 3 d acclimation, activity, food intake, and EE were measured using a 16-chamber comprehensive laboratory animal monitoring system (CLAMS; Columbus Instruments, Columbus, OH), as previously described (18). Food intake, activity, respiratory exchange ratio (RER) and EE recordings were collected over 72 h. Food intake in pregnant dams was assessed in wire mesh cages taking spillage into account.
Gene expression and data analysis
Total RNA from liver, muscle, and retroperitoneal white adipose tissue (rWAT) was obtained from snap-frozen specimens, and genes of interest were analyzed using RT-PCR and normalized using cyclophilinB as a control gene as described previously (19). For cortical gene analysis, whole brains were isolated and frozen in an isopentane bath. The hindbrain was removed and the forebrain transferred to a cryostat. One hundred-micrometer sections were shaved off of the forebrain until a rostral section, roughly −2.92 mm interaural/0.88 mm Bregma, was reached. Using a punch tool with a 0.5-mm interior diameter needle, all of the cortical forebrain was removed, being careful not to isolate tissue from hippocampal, thalamic, or hypothalamic regions. The medial basal hypothalamus (MBH) was excised at the time of necropsy and snap frozen for latter gene expression analysis. For gene expression analysis, graphs depict average gene expression ± sem (n = 6 per group). RNA was isolated at two time points, CT6 (1200 h) and CT18 (2400 h). Only comparisons of maternal nutrition groups (UO vs. CO) at each specific time point (circadian time CT6 or CT18) were analyzed.
Statistical analysis
For analysis, Student’s t test was used for body weight serum data between the two groups (UO and CO). Two-way ANOVA was used to analyze data from wheel running, CLAMS, or gene expression, respectively.
Results
C57BL/6J mice subject to low-protein diet in utero exhibit IUGR, catch-up growth, and increased sensitivity to HFD
We could not detect a significant increase in food intake during pregnancy (CO dams 3.9 ± 0.6 g vs. UO dams 4.2 ± 0.3 g, collected during the final week of pregnancy); however, during the first week of the acclimation phase, there did appear to be a slight increase in food intake. Average food intake was not statistically significant during acclimation; CO dams 3.3 ± 0.6 g vs. UO dams 4.1 ± 0.2 g of food eaten during a 24-h period (data not shown). UO from dams fed a low-protein diet weighed 40% less at birth compared with CO, suggesting IUGR (Table 1). However, catch-up growth during the neonatal period resulted in similar body weights for UO and CO pups at weaning and at 8 wk of age in mice fed control diet (Table 1). As predicted, the effect of HFD on obesity in male and female mice was exacerbated in the UO group relative to CO. Significant increases in body weight and adiposity (10% difference in adiposity) were observed in UO fed HFD compared with the CO group fed HFD irrespective of sex (Table 1). In contrast, in the control chow-fed groups, there was no significant difference in body weight or adiposity of UO compared with CO for either male or females (Table 1).
Table 1.
Effects of maternal nutrition and diet during adulthood on body weight of normal (CO) and fetal malnourished (UO) B6 mice
| CO
|
UO
|
Statistical analysis | |||
|---|---|---|---|---|---|
| Chow (n = 20) | HFD (n = 20) | Chow (n = 20) | HFD (n = 20) | ||
| Birth weight (g) | 1.22 ± 0.04 | ND | 0.75 ± 0.05 | ND | P < 0.05 (CO vs. UO) |
| Wean weight (g) | 8.60 ± 0.20 | ND | 7.80 ± 0.30 | ND | P < 0.05 (CO vs. UO) |
| Body weight (g) | |||||
| Males, 8 wk | 20.2 ± 1.8 | ND | 22.1 ± 1.5 | ND | Maternal diet NS, adult diet, P < 0.01, interaction P < 0.05 |
| Males, 20 wk | 25.2 ± 1.2 | 36.2 ± 3.8 | 28.6 ± 2.2 | 42.4 ± 1.8a | Maternal diet NS, adult diet P < 0.01, interaction P < 0.05 |
| Females, 8 wk | 19.8 ± 1.5 | ND | 23.2 ± 2.8 | ND | |
| Females, 20 wk | 21.5 ± 1.9 | 29.2 ± 4.3 | 24.9 ± 2.8 | 35.2 ± 4.0a | |
| Body composition: fat mass (% body weight) | |||||
| Males, 8 wk | 6.2 ± 1.5 | 5.8 ± 2.2 | |||
| Males, 20 wk | 11.1 ± 2.5 | 18.3 ± 2.5 | 13.8 ± 1.6 | 28.1 ± 3.4a | Maternal diet NS, adult diet P < 0.001, interaction P < 0.05 |
| Females, 8 wk | 5.4 ± 1.0 | ND | 4.2 ± 2.1 | ND | Maternal diet NS, adult diet P < 0.01, interaction P < 0.05 |
| Females, 20 wk | 8.2 ± 2.3 | 15.4 ± 2.1 | 10.4 ± 2.9 | 24.3 ± 5.5a | |
For statistical analysis, body weight data at birth through 8 wk of age were compared using Student’s t test. The interaction of diet in utero (maternal diet) and from 8–20 wk of age (adult diet) was assessed using two-way ANOVA. ND, No data; NS, not significant.
P < 0.05, statistically significant for interaction of adult diet, CO vs. UO.
Glucose clearance is altered in male and female UO at 8 and 20 wk of age
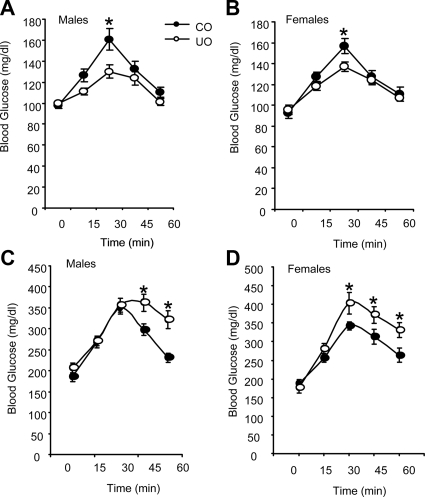
Based on initial observations suggesting abnormal rhythms in food intake and metabolism to be presented later in the manuscript, we examined serum chemistries at mid-light cycle (CT6, 1200 h) and mid-dark cycle (CT18, 2400 h). Rats subject to protein malnutrition in utero initially exhibit a heightened response to insulin but develop insulin resistance with age and exposure to a western diet (20,21). Here, we observed a similar phenomenon of improved glucose clearance preceding the development of insulin resistance. At 8 wk of age, male and female UO fed the control diet exhibited significantly lower serum glucose during the lights-on period (Table 2 and Supplemental Table 1, published on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org) and a significant improvement in glucose clearance after GTT for both male and female UO (Fig. 1, A and B; AUC demonstrated a significantly improved glucose clearance in CO and UO; P < 0.05 for both male and female mice). Exposure to HFD for 12 wk was associated with hyperinsulinemia, irrespective of fetal environment in male and female UO and CO; however, this was more severe in the UO group (Table 2 and Supplemental Table 1). Glucose intolerance associated with HFD was also more severe in the UO compared with CO for both male and female mice (Fig. 1, C and D; AUC demonstrated a significantly impaired glucose clearance in UO compared with CO; P < 0.01). UO offspring weaned onto the control diet were glucose intolerant at 20 wk of age, with only the 15-min time point being significant for both sexes compared with CO (data not shown; AUC did demonstrate a reduced glucose clearance for male and female UO; P = 0.05).
Table 2.
Effects of maternal nutrition on serum lipid, glucose, and markers of inflammation in male CO and UO
| Control diet
|
HFD
|
|||||||
|---|---|---|---|---|---|---|---|---|
| CO | UO | CO | UO | CO | UO | CO | UO | |
| Time | CT6 | CT6 | CT18 | CT18 | CT6 | CT6 | CT18 | CT18 |
| Age 8 wk | n = 15 | n = 15 | n = 20 | n = 20 | n = 15 | n = 15 | n = 20 | n = 20 |
| Glucose (mg/dl) | 110 ± 10 | 70 ± 15a | 126 ± 13 | 122 ± 12 | ND | ND | ND | ND |
| Insulin (ng/ml) | 1.2 ± 0.3 | 0.8 ± 0.5 | 1.0 ± 0.2 | 1.5 ± 0.4 | ND | ND | ND | ND |
| Blood lipids | ||||||||
| TG (mg/dl) | 18 ± 5 | 20 ± 8 | 24 ± 3 | 26 ± 5 | ND | ND | ND | ND |
| Cholesterol (mg/dl) | 35 ± 8 | 40 ± 10 | 42 ± 5 | 50 ± 7 | ND | ND | ND | ND |
| Age 20 wk | ||||||||
| Glucose (mg/dl) | 102 ± 15 | 135 ± 10a | 121 ± 12 | 158 ± 15a | 247 ± 20 | 290 ± 29 | 313 ± 10 | 380 ± 21a |
| Insulin (ng/ml) | 1.5 ± 0.3 | 1.7 ± 0.5 | 1.7 ± 0.2 | 1.8 ± 0.3 | 3.5 ± 0.8 | 6.2 ± 0.7a | 4.0 ± 0.6 | 6.5 ± 0.8a |
| Leptin (ng/ml) | 10 ± 3 | 11 ± 4 | ND | ND | 22 ± 5 | 35 ± 7a | ND | ND |
| Blood lipids | ||||||||
| TG (mg/dl) | 49 ± 12 | 84 ± 10a | 52 ± 8 | 94 ± 15a | 154 ± 12 | 175 ± 12a | 162 ± 15 | 192 ± 12a |
| Cholesterol (mg/dl) | 35 ± 10 | 54 ± 8a | 40 ± 8 | 59 ± 4a | 62 ± 15 | 102 ± 18a | 88 ± 12 | 122 ± 8a |
| Inflammatory markers | ||||||||
| TNFα (ng/ml) | 115 ± 25 | 106 ± 16 | 102 ± 5 | 93 ± 10 | 145 ± 20 | 159 ± 10 | 160 ± 30 | 210 ± 14a |
| PAI-1 (ng/ml) | 8 ± 8 | 12 ± 3 | 7 ± 2 | 10 ± 4 | 15.0 ± 8.0 | 22 ± 15 | 28 ± 10 | 38 ± 15 |
Serum samples were collected from mice in mid-light phase (CT6) or mid-dark phase (CT18). ND, No data.
P < 0.05 for effects of adult diet at either CT6 or CT18, CO vs. UO.
Figure 1.
UO have age-dependent differences in glucose clearance compared with age-matched controls. Age-matched UO and CO were fasted overnight and then administered a bolus of glucose (1 g/kg) by ip injection. Whole blood glucose levels were monitored every 15 min for 1 h. Graphs depict GTT for male (A) and female (B) UO and CO at 8 wk of age and fed a standard rodent diet. Twenty-week-old male (C) and female (D) UO and CO were fed a HFD for 12 wk and then subject to GTT as described. For all groups, n = 10 per group. Each point in the graph represents an average ± sem. *, P < 0.05 vs. CO. Differences for each time point measured was analyzed by Student’s t test.
Blood chemistry analysis for male and female UO and CO at 8 and 20 wk of age fed a chow or HFD
At 8 wk, there were no significant differences in serum TG or cholesterol at either CT6 or CT18 (Table 2). We did not observe any change in serum levels of leptin in UO or CO at 8 wk of age, nor did we observe significant changes in inflammatory markers at this age (data not shown). However, the effect of age and HFD to increase both parameters was exacerbated in the UO group. In male mice, at CT6 and CT18 time points, significant increases in serum TG and cholesterol were observed in HFD-fed UO compared with CO. Additionally, measurements of circulating serum levels of leptin correlated with adiposity data and were higher in male UO compared with CO at 20 wk of age and after HFD (Table 2). Serum profiles for female UO and CO were analyzed at 8 and 20 wk on control and HFD. The effects of fetal environment and diet on blood chemistries were similar to those observed in males (Supplemental Table 1).
Altered circadian rhythms in energy metabolism and feeding behavior precede obesity in male C57BL/6J mice subject to protein malnutrition in utero
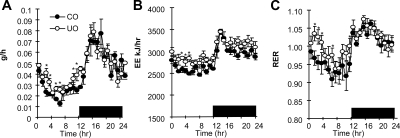
To begin to address the mechanisms by which fetal malnutrition exacerbate diet-induced obesity of the C57BL/6J mouse, we performed experiments analyzing energy metabolism in preobese UO mice. The 24-h profile of feeding behavior and energy metabolism was assessed using male preobese weight-matched UO and CO mice fed the control (20% protein) diet (n = 8 per group, age 8 wk). UO mice exhibited hyperphagia during the lights-on period compared with controls, with normal food intake during the lights-off period (Fig. 2A). EE was also significantly higher in UO mice compared with CO during the lights-on period (Fig. 2B). We did observe a statistical difference in total energy expenditure in UO compared with age- and weight-matched CO during the lights-off phase (average EE for the 12 h of lights off, CO 3011 ± 15 kj/h vs. UO 3177 ± 10 kj/hr, P < 0.05, data not graphed). The RER, an indicator of substrate preference (fat vs. carbohydrate), was higher in UO mice during the daytime, indicating reduced fatty acid oxidation (Fig. 2C). In both UO and controls, RER during the dark period was more than 1.0, coinciding with the peak period of ingestion of the control diet. The RER of UO mice during the first 4 h of daytime (CT0–CT4) remained higher than 1.0, a value that suggests lipogenesis (22). Home cage movement, measured by infrared beam breaks along the x- and z-axis, was reduced by 20% during the lights-off period from CT19–CT22 (P < 0.01); however, no significant difference was observed during the lights-on phase (data not shown). Therefore, altered EE and RER during the lights-on period cannot be explained by altered physical activity.
Figure 2.
UO mice have increased EE and RER during the daytime coupled with hyperphagia. Graphs depict 24-h food intake (A) and metabolic profile (B, TEE; C, RER) of male UO and CO mice as recorded by indirect calorimetry; UO and CO were of similar body weight (18 ± 0.3 vs. 18 ± 0.5 g) and percent fat mass (8.5 vs. 9 g) for UO and CO, respectively. Mice were 8 wk of age; n = 8 per group. Each point in the graph represents an average ± sem for 3 d of recordings. *, P < 0.05 vs. CO. Differences between UO and CO for 1-h bins for each parameter measured were analyzed using Student’s t test.
UO exhibit abnormal rhythms in wheel-running behavior at 8 wk of age
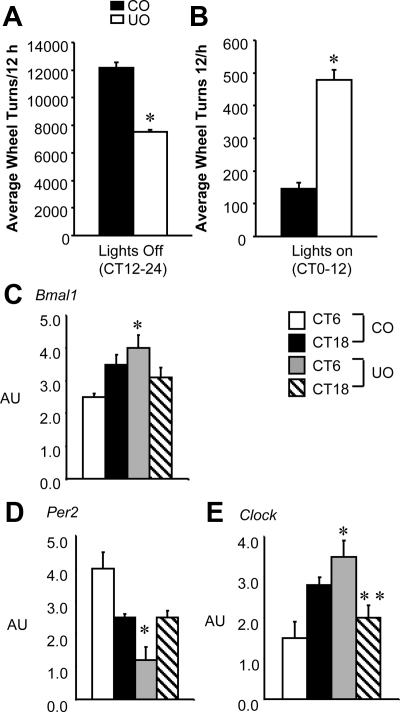
Rats subject to caloric malnutrition in utero exhibit hypoactive patterns of wheel running during the lights-off period (23,24). The CLAMS data presented here (Fig. 2A) suggested that UO mice exhibit abnormal feeding activity during the lights off, suggesting abnormal circadian function. To determine whether C57BL/6J mice subjected to a prenatal malnourished environment such as protein deficiency exhibit a similar wheel-running phenotype observed in the rat, male CO and UO (n = 10 per group) were placed in cages containing running wheels. UO were hypoactive during the lights-off period (Fig. 3A). UO also exhibited increased wheel running during the lights-on period (Fig. 3B). Female UO (n = 15) did not display an increased wheel-running behavior during the lights-on period; however, female mice did display a significant decrease in wheel running during the lights-off phase compared with CO females (Supplemental Fig. 1). For this reason, experiments pertaining to females were centered mainly on metabolic stability after exposure to protein deficiency in utero.
Figure 3.
UO mice have nocturnal hypoactivity coupled with daytime hyperactivity and have abnormal clock gene expression in brain. A, The cumulative average of wheel running ± sem over a 14-d period of UO and CO mice from CT12–24 or the lights-off period; B, average ± sem of lights-on wheel running (CT0–12). Both groups were of similar body weight (17.0 ± 0.5 vs. 17.5 ± 0.5 g for UO and CO, respectively) and were 8 wk of age (n = 20 per group). *, Significantly different from CO, P < 0.001 by Student’s t test. RNA was isolated from brain of CO and UO at two time points, CT6 and CT18 (n = 6 per group). AU, Arbitrary units. C, Bmal1; *, P < 0.01 for differences between maternal nutrition groups and time point CT6. D, Per2; *, P < 0.01 for differences between maternal nutrition groups and CT6. E, Clock; *, P < 0.01 for interaction between maternal nutrition and CT6; **, P < 0.05 for interaction between maternal nutrition and CT18.
Abnormal cortical circadian oscillator gene expression in the brain of UO mice
The expression of circadian rhythms of wakefulness and sleep homeostasis is governed by many factors, including CCO that maintain the normal daily rhythm of activity (25). We analyzed expression of core circadian oscillator (CCO) genes in the cortical and hypothalamic region of the central nervous system at CT6 (1200 h) and CT18 (2400 h) to ascertain whether UO expression of these genes was altered.
Bmal1, Per2, and Clock genes were successfully analyzed by RT-PCR in the cortex of UO and CO. An effect of maternal nutrition groups (CO vs. UO) as analyzed with time as a factor revealed a significant difference in expression of Bmal1, Per2, and Clock in the cortex of UO compared with CO at the CT6 time point (Fig. 3, C–E). Clock gene expression was also significantly different at CT18 in UO compared with CO. No difference in cortical expression of Npas2 was detected between groups at either time point (data not shown).
We next explored the possibility of altered clock genes in the MBH by RT-PCR because the master clock of the suprachiasmatic nucleus resides in this region. We did not observe any changes in MBH circadian genes at the two time points examined between UO and CO mice (data not shown).
To investigate whether the hyperphagia of UO is due to altered expression of orexigenic or anorectic neuropeptides, we analyzed MBH expression of neuropeptide Y (Npy) and proopiomelanocortin in this brain region. UO demonstrated a marked increase in Npy expression compared with CO mice at the CT6 time point in 8-wk-old mice (arbitrary units, 3.2 ± 0.8 vs. 6.8 ± 1.3, CO vs. UO at CT6, P < 0.05; n = 6). Npy expression was not significantly different between CO and UO at the CT18 time point (arbitrary units, 5.6 ± 0.4 vs. 4.9 ± 0.6, CO vs. UO at CT18, n = 6). In contrast, no change was observed in Pomc, an anorexigenic gene at either time point between CT6 and CT18 for CO vs. UO (arbitrary units, 9.3 ± 1.2 vs. 10.4 ± 0.8, CO vs. UO at CT6 n = 6; 3.8 ± 0.7 vs. 3.3 ± 0.5, CO vs. UO at CT18, n = 6).
Analysis of genes related to clock activity and lipid and glucose biosynthesis in the liver are deregulated in preobese UO mice
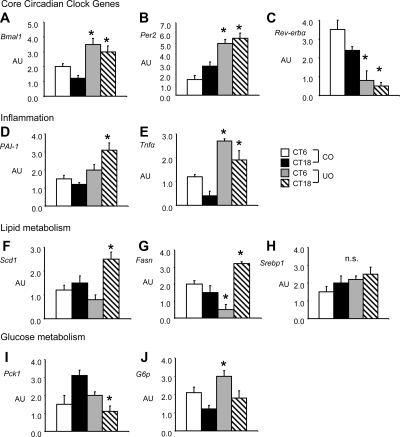
To further investigate abnormal circadian rhythms in energy metabolism of UO, the expression of genes involved in clock activity and energy metabolism was examined in liver at CT6 (1200 h) and CT18 (2400 h) in 8-wk control diet-fed male mice. Expression of Bmal1 was increased and did not exhibit evidence of oscillation in UO (Fig. 4A). Bmal1 activates transcription of Per2; a similar abnormal pattern of increased expression and loss of oscillation was observed for Per2 mRNA in UO (Fig. 4B). Transcription of Bmal1 is repressed by Rev-erbα; UO exhibited a marked reduction in Rev-erbα expression at CT6 and CT18 (Fig. 4C). Exposure in utero to a low-protein diet is thus associated with deregulation of circadian oscillators.
Figure 4.
Expression of genes involved in inflammation and lipid and glucose biosynthesis in the liver are deregulated in UO mice. AU, Arbitrary units. n = 6 per group for time points CT6 and CT18. A, Bmal1; *, P < 0.01 for differences between CO and UO at time point CT6 and CT18; B, Per2; *, P < 0.01 for differences between maternal nutrition groups at time point CT6 and CT18; C, Rev-erbα; *, P < 0.01 for interaction between maternal nutrition groups and CT6 and CT18; D, Pai-1; *, P < 0.01 for interaction between maternal nutrition groups and CT18; E, Tnfα; *, P < 0.01 for interaction between maternal nutrition groups and CT6 and for CT18; F, Scd1; *, P < 0.01 for interaction between maternal nutrition groups and CT18; G, Fasn; *, P < 0.01 for interaction between maternal nutrition groups and CT6 and for CT18; H, Srebf1, not significant (n.s.); I, Pck1; *, P < 0.01 for interaction between maternal nutrition groups and CT18; J, G6p; *, P < 0.01 for interaction between maternal nutrition groups and CT6.
These data suggest arrhythmic clock function in liver, perhaps driven by loss of Rev-erbα expression and reduced activity. Rev-erbα is also a transcriptional repressor of Pai-1, a regulator of fibrinolysis linked to inflammation and atherosclerosis through abnormal thrombosis (26). UO mice exhibited deregulated Pai-1 expression compared with control mice, and the expression was increased, suggesting loss of repression (Fig. 4D). Expression of another marker of inflammation that is increased by obesity, Tnf-α, was also increased in UO compared with control mice at both time points observed (Fig. 4E). There was also a significant increase in circulating levels of TNF-α; however, serum PAI-1 levels were not significantly different from CO in groups fed a HFD (Table 2).
Expression of genes involved in lipogenesis and triglyceride synthesis, stearoyl CoA-desaturase 1 (Scd1) (Fig. 4F), and fatty acid synthase (Fasn) (Fig. 4G), were also significantly increased in UO at CT18, which coincides with the period of maximum food consumption compared with CO. However, expression of sterol regulatory element binding protein-1 (Srebp-1a and -1c, two isoforms encoded by Srebf1), a gene involved in insulin-dependent regulation of lipogenic genes, was not significantly increased in UO (Fig. 4H). Interestingly, expression of phosphoenolpyruvate carboxykinase gene (Pck1), which functions as a gluconeogenic enzyme, was markedly reduced at CT18 in UO compared with CO (Fig. 4I). UO exhibited an increased expression of glucose-6 phosphatase (G6p), which converts glucose-6 phosphate to glucose and facilitates hepatic glucose secretion, at CT6 (Fig. 4J).
Altered clock gene regulation in UO at 20 wk of age exposed to control or a HFD
Analysis of clock gene expression was extended to the muscle and WAT of age- and weight-matched UO and CO male mice. Separate groups of CO and UO mice were either maintained on the 20% (Teklad TD91352) protein control diet from the age of weaning or given a HFD (60% kcal from fat) at 8 wk of age. At the age of 20 wk, male UO and CO were analyzed for clock gene expression in liver, muscle, and WAT. Figure 5A demonstrates that the apparent deregulation of Rev-erbα gene expression in liver initially observed at 8 wk of age is retained in 20-wk-old control and HFD-fed mice. Overall, exposure to HFD appeared to suppress the expression of Bmal1 and Per2 in liver, and we again observed evidence of constitutive elevation of expression at CT6 and CT18 (Fig. 6, A and B). Although we could reproduce the effect of the fetal environment, the expression pattern of Rev-erbα was not affected by the adult diet (Fig. 5A).
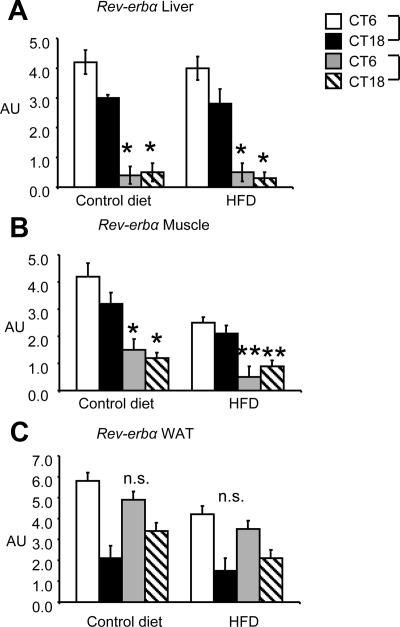
Figure 5.
Expression of Rev-erbα in liver, muscle, and rWAT in UO and CO at 20 wk of age. UO and CO at 8 wk were left on standard rodent chow or subject to HFD for 12 wk. Liver, muscle, and adipose tissue were removed (n = 10 per group for CT6 and CT18). AU, Arbitrary units. A, Liver Rev-erbα; *, P < 0.01 for interaction between maternal nutrition groups and CT6 and CT18, for control diet; *, P < 0.01 for interaction between maternal nutrition groups and CT6 and CT18 for HFD; B, muscle Rev-erbα; *, P < 0.01 for interaction between maternal nutrition groups and CT6 and CT18 for control diet; **, P < 0.05 for interaction between maternal nutrition groups and CT6 or CT18, for HFD; C, rWAT; no significant interaction was observed between maternal nutrition groups at either time point.
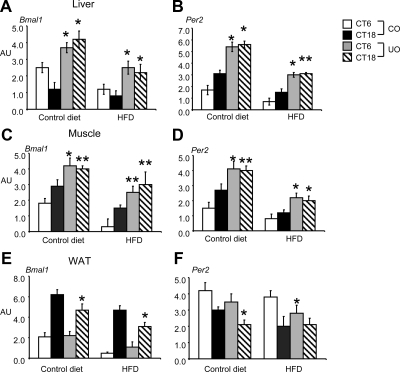
Figure 6.
Expression of Bmal1 and Per2 in liver, muscle, and rWAT at 20 wk of age. AU, Arbitrary units. n = 6 per group for time points CT6 and CT18. A, Liver Bmal1; *, P < 0.01 for differences maternal nutrition groups at CT6 and CT18, for control diet; *, P < 0.01 for differences between maternal nutrition groups at CT6 and CT18, for HFD; B, liver Per2; *, P < 0.01 for differences between maternal nutrition groups at CT6 and CT18, for control diet; *, P < 0.01 for differences between maternal nutrition groups at CT6; **, P < 0.05 for CT18, for HFD; C, muscle Bmal1; *, P < 0.01 for differences between maternal nutrition groups at CT6; **, P < 0.05 for CT18 for control diet; **, P < 0.05 for differences between maternal nutrition groups at CT6; **, P < 0.05 for CT18, for HFD; D, muscle Per2; *, P < 0.01 for differences between maternal nutrition groups at CT6; **, P < 0.05 for CT18 for control diet; *, P < 0.01 for differences between maternal nutrition groups at CT6 and CT18, for HFD; E, WAT Bmal1; **, P < 0.05 for maternal nutrition groups and CT18 for both control and HFD; F, WAT Per2; **, P < 0.05 for maternal nutrition groups and CT18 for control and HFD.
Importantly, we observed a similar asynchronous pattern of Rev-erbα, Bmal1, and Per2 expression in skeletal muscle from UO and CO fed either control or HFD (Figs. 5B and 6, C and D). Surprisingly, Rev-erbα and clock gene expression in rWAT isolated from UO or CO showed similar patterns of expression between either control or HFD fed mice (Figs. 5C and 6, E and F). There were significant differences between maternal nutrition groups in expression of Bmal1 and Per2 in adipose tissue at CT18 for Bmal1 and CT18 for Per2 (control diet) and CT6 (HFD) (Fig. 6, E and F).
Discussion
These studies further demonstrate the association of fetal environment and adult disease, showing that adult C57BL/6J mice subject to prenatal exposure to a low-protein diet develop many of the classic hallmarks of type 2 diabetes, including glucose intolerance and increased adiposity. Since the preparation of this manuscript, the C57BL/6J mouse model has recently been published as relevant to IUGR rat models. Protein-restricted male C57BL/6J offspring developed increased adiposity and glucose intolerance at 32 wk of age (27). In another study, microarray analysis of adipose tissue gene expression suggested a number of lipogenic genes were altered in offspring exposed to maternal protein restriction (28). Collectively, these results indicate that the C57BL/6J may be useful for studies investigating the link between fetal environment and the health span of the adult. The novel observation of these experiments is the altered circadian physiology that may be symptomatic of an altered fetal environment, and which is observable before the onset of obesity.
Interestingly, at 8 wk of age, the 24-h profile of energy metabolism in C57BL/6J mice subject to maternal protein deprivation in utero demonstrated stark differences in circadian physiology. As observed in rats subject to malnutrition in the form of calorie restriction (23), UO exhibited lights-off hypoactivity; however, an increase in physical activity was observed during the lights-on period. UO also exhibited hyperphagia restricted to the lights-on period, associated with increased EE and RER and fasting hypoglycemia at 8 wk of age. Exposure to a low-protein environment in utero also leads to improper ingestive behavior during a period normally dominated by rapid eye movement sleep in rodents, coupled with abnormal substrate selection in the preobese state. Collectively, these data may suggest a behavioral phenotype similar to the night-eating syndrome (29). Excess food consumption during the daytime is coupled with a prolonged period during which carbohydrates are converted to fatty acid (RER > 1). Further evidence for an increase in lipogenesis comes from the observation of increased Fas and Scd1 mRNA. These observations suggest a compromised ability to appropriately regulate substrate metabolism. Whether hypoglycemia coupled with reduced capacity to increase fat oxidation during the lights-on period contributes to the coincident hyperphagia is not clear but is an attractive hypothesis. One could speculate that the altered ingestive behavior observed in UO may be driven by abnormal hypothalamic expression during the lights-on phase in UO through Npy, which has been extensively shown to be primarily orexigenic, and neuropeptide Y injected into the paraventricular nucleus is the most potent central appetite stimulant known and also inhibits thermogenesis; repeated administration rapidly induces obesity (30). Altered peripheral and central cortical clock gene expression was observed in UO relative to CO in both lean and high-fat-fed mice, with expression also affected by age and tissue specificity. Importantly, these sequelae associated with UO were observed before the onset of obesity, suggesting a causative role in the metabolic phenotype.
In mouse models of severe insulin resistance, the retention of insulin stimulation of Srebf1 is thought to contribute to dyslipidemia (31). In the current study, we observed a pronounced increased expression in lipogenic genes at CT18, coinciding with reduced Pck1 expression. This could indicate increased insulin signaling acting to suppress glucose production while stimulating lipogenesis. Although we observed no increase in Srebf1 expression, it is nevertheless possible that posttranslational modification of the protein (cleavage and translocation of the N-terminal domain to the nucleus) is involved because this action coincides with peak nutrient ingestion (32,33). That this phenomenon was observed only during lights off may indicate abnormal clock function. Indeed, clock activity has been linked to regulation of circadian rhythms in expression of genes involved in lipogenesis and gluconeogenesis (34). Malnutrition studies performed in rats have demonstrated abnormal glucose production by the liver after stimulation with insulin and glucagon; however, mechanisms controlling the rate of glucose homeostasis by the liver of UO in rats or mice is unknown (35).
Male and female UO at an early age appeared to have a significantly better glucose tolerance than age-matched CO. Although the mechanism driving this response has not been studied in detail, rat offspring have been shown to respond in a similar physiological fashion. It has been suggested that this response stems from increased insulin sensitivity; however, in rats, total insulin concentration early in life is reduced (36). In our mouse model, this does not appear to be the case because we did not observe any changes in total insulin concentration at 8 wk of age in UO compared with CO. In vitro studies looking at basal glucose uptake from isolated muscle, liver, and fat of male rats exposed to protein restriction in utero revealed increased glucose uptake possibly due to increased expression of insulin receptor, phosphatidylinositol 3-kinase, and β-adrenergic receptors (20,35,37,38,39). Epidemiological studies have confirmed some of the findings observed in the animal studies mentioned, although there is considerable variability among age and populations tested (40,41). The mechanisms involved in the reversal of glucose and insulin sensitivity in adulthood are not currently known.
Our data suggest that altered circadian physiology may be symptomatic of exposure to a suboptimal environment during fetal development. Circadian rhythms involve circadian oscillators that maintain a cycle of transcriptional and translational activity with a tau (measure of circadian rhythm activity) of approximately 24 h (42). A heterodimer comprised of Bmal1 and either Clock or Npas2 forms the positive component, increasing transcription of genes containing the E-box motif in the promoter, including the Period (Per1-3) and Cryptochrome (Cry1-2), and Rev-erbα genes. Per/Cry heterodimers form one arm of the negative loop, inhibiting the transcriptional activity of Bmal1, whereas Rev-erbα represses Bmal1 transcription. Npas2 is required for normal regulation of Per2 expression in cultured cells and forebrain (15,16). Originally linked to circadian cycles driven by photic inputs, recent evidence has linked clock activity to the metabolic syndrome. Homozygous Clock (ClockΔ19) mutants on the C57BL/6J background exhibit increased propensity for diet-induced obesity, dyslipidemia, and hepatic steatosis (43). Aberrant clock function has also been linked to increased oxidative stress and an impaired ability to detoxify xenobiotics (44). How changes in clock oscillation alter metabolic outputs or vice versa is not clear. Mice fed a HFD have altered periods of locomotor activity as well as impaired oscillation of clock-controlled genes involved in metabolism in brain, fat, and liver (45). One potential link between clocks and metabolism may stem from tissue and cellular differences in redox state because this may provide a nutrient sensor that initiates or suppresses clocks depending on nutrient status (46). Although the mechanism is not clear at this time, our data suggest that aberrant regulation of the clock mechanism may be an underlying feature of the metabolic syndrome associated with compromised fetal development.
Dysregulation of metabolic pathways involved in lipid and carbohydrate metabolism coupled with inflammation are hallmark signals of ongoing metabolic disease (47). UO clearly exhibited arrhythmic clock oscillation in the liver and muscle, potentially as a result of a marked reduction in expression of Rev-erbα, although a direct relationship requires further investigation. Rev-erbα is known to regulate biological processes relevant to the metabolic syndrome. Rev-erbα promotes adipogenesis, whereas tissue-specific loss of expression of Rev-erbα in skeletal muscle increases aerobic lipid metabolism and mitochondrial content (48,49). Loss of Rev-erbα function in knockout mice alters clock mechanisms, resulting in increased Bmal1 and Clock expression (50). Hepatic Rev-erbα is also involved in regulating the expression of genes involved in lipid synthesis, and altered Rev-erbα expression is associated with dyslipidemia and inflammation (51,52,53).
One can hypothesize that exposure to stressors such as malnutrition alters the metabolic physiology of the dam, thus initiating metabolic instability in the offspring before birth. Factors such as glucocorticoids and leptin play an important role for proper fetal development (54). It has been shown that maternal malnutrition increases fetal glucocorticoid exposure (55,56), and circulating glucocorticoid levels are known to significantly reduce Rev-erbα expression (57). Whether early developmental exposure to glucocorticoids induces a functional alteration in clock oscillation in UO mice remains to be established.
Diets restricting the amount of protein during development affect the activity of promoter regions of genes involved in hepatic metabolism in rats (58,59). This mechanism is thought to result from impaired transcription when CpG islands (CG-dinucleotide-rich regions in the promoters of genes) become methylated, thus controlling facets of genetic turnover (60). This regulation of methyl group metabolism was found to be reversed by the addition of folate to the protein-restricted diet (61). Thus hypo- or hypermethlyation of a particular gene can affect turnover and expression of downstream effector genes in malnourished fetal metabolism. Further study is needed to determine whether either Rev-erbα or another component of the clock system in UO mice is warranted because this may play a key role in modification of gene expression after malnutrition.
Interestingly, an altered circadian profile in sleep state has been observed in rats subject to a protein-deficient environment in utero. Protein deficiency in utero has been shown to disrupt rapid eye movement sleep in rats, potentially stemming from significant changes in serotonergic regulation resulting in behavioral alterations and memory function (17). Loss of core clock oscillatory function in the central nervous system is also associated with aberrant circadian rhythms (50). Additionally, alterations in core clock oscillation due to altered expression in Per2 have been associated with inheritable sleep alterations in humans (62). Further study of circadian physiology in relation to metabolic instability as well as how clocks and clock-associated genes and protein function in peripheral tissues and central nervous system is clearly warranted in this model of maternal programming of the fetus.
In summary, these results demonstrate that fetal malnutrition is associated with a phenotype in young preobese adult mice consistent with increased propensity for metabolic diseases. The altered expression of genes involved in clock, glucose, and lipid metabolism observed at an early age coupled with altered circadian and metabolic profiles may be key to determining mechanisms that explain how a malnourished fetal environment contributes to adult disease.
Supplementary Material
Acknowledgments
We thank members of the Pennington Biomedical Research Center Animal Phenotyping Core Facility and the Department of Comparative Biology for their help during the course of these studies.
Footnotes
This work was supported by a P&F grant award (to G.M.S.) through the Clinical Nutrition Research Unit Center Grant 1P30 DK072476 entitled “Nutritional Programming: Environmental and Molecular Interactions” sponsored by the National Institute of Diabetes and Digestive and Kidney Diseases. G.M.S. is also supported by the American Diabetes Association (1-09-JF-51). A.A.B. is supported by DK073189 and the Pennington Biomedical Research Foundation.
Current address for A.A.B.: Department of Metabolism and Aging, The Scripps Research Institute, Jupiter, Florida 33458.
Disclosure Summary: The authors state they have nothing to disclose.
First Published Online February 16, 2010
For editorial see page 1385
Abbreviations: AUC, Area under the curve; CCO, canonical clock oscillators; CLAMS, comprehensive laboratory animal monitoring system; CO, control offspring; CT, circadian time; EE, energy expenditure; GTT, glucose tolerance test; HFD, high-fat diet; IUGR, intrauterine growth retardation; MBH, medial basal hypothalamus; PAI-1, plasminogen activator inhibitor-1; RER, respiratory exchange ratio; rWAT, retroperitoneal white adipose tissue; TG, triglycerides; UO, undernourished offspring.
References
- Victora CG, Adair L, Fall C, Hallal PC, Martorell R, Richter L, Sachdev HS 2008 Maternal and child undernutrition: consequences for adult health and human capital. Lancet 371:340–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cetin I, Foidart JM, Miozzo M, Raun T, Jansson T, Tsatsaris V, Reik W, Cross J, Hauguel-de-Mouzon S, Illsley N, Kingdom J, Huppertz B 2004 Fetal growth restriction: a workshop report. Placenta 25:753–757 [DOI] [PubMed] [Google Scholar]
- Hales CN, Barker DJ, Clark PM, Cox LJ, Fall C, Osmond C, Winter PD 1991 Fetal and infant growth and impaired glucose tolerance at age 64. BMJ 303:1019–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker CJ, Arch JR, Cawthorne MA 2005 Fetal origins of insulin resistance and obesity. Proc Nutr Soc 64:143–151 [DOI] [PubMed] [Google Scholar]
- Langley-Evans SC 2006 Developmental programming of health and disease. Proc Nutr Soc 65:97–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley-Evans SC, Bellinger L, McMullen S 2005 Animal models of programming: early life influences on appetite and feeding behaviour. Matern Child Nutr 1:142–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ, Gluckman PD, Godfrey KM, Harding JE, Owens JA, Robinson JS 1993 Fetal nutrition and cardiovascular disease in adult life. Lancet 341:938–941 [DOI] [PubMed] [Google Scholar]
- Hales CN, Barker DJ 2001 The thrifty phenotype hypothesis. Br Med Bull 60:5–20 [DOI] [PubMed] [Google Scholar]
- Hales CN, Ozanne SE 2003 For debate: Fetal and early postnatal growth restriction lead to diabetes, the metabolic syndrome and renal failure. Diabetologia 46:1013–1019 [DOI] [PubMed] [Google Scholar]
- Burns SP, Desai M, Cohen RD, Hales CN, Iles RA, Germain JP, Going TC, Bailey RA 1997 Gluconeogenesis, glucose handling, and structural changes in livers of the adult offspring of rats partially deprived of protein during pregnancy and lactation. J Clin Invest 100:1768–1774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluckman PD, Breier BH, Oliver M, Harding J, Bassett N 1990 Fetal growth in late gestation: a constrained pattern of growth. Acta Paediatr Scand Suppl 367:105–110 [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Cutfield W, Hofman P, Hanson MA 2005 The fetal, neonatal, and infant environments-the long-term consequences for disease risk. Early Hum Dev 81:51–59 [DOI] [PubMed] [Google Scholar]
- Vickers MH, Gluckman PD, Coveny AH, Hofman PL, Cutfield WS, Gertler A, Breier BH, Harris M 2008 The effect of neonatal leptin treatment on postnatal weight gain in male rats is dependent on maternal nutritional status during pregnancy. Endocrinology 149:1906–1913 [DOI] [PubMed] [Google Scholar]
- Collins S, Martin TL, Surwit RS, Robidoux J 2004 Genetic vulnerability to diet-induced obesity in the C57BL/6J mouse: physiological and molecular characteristics. Physiol Behav 81:243–248 [DOI] [PubMed] [Google Scholar]
- Dudley CA, Erbel-Sieler C, Estill SJ, Reick M, Franken P, Pitts S, McKnight SL 2003 Altered patterns of sleep and behavioral adaptability in NPAS2-deficient mice. Science 301:379–383 [DOI] [PubMed] [Google Scholar]
- Reick M, Garcia JA, Dudley C, McKnight SL 2001 NPAS2: an analog of clock operative in the mammalian forebrain. Science 293:506–509 [DOI] [PubMed] [Google Scholar]
- Datta S, Patterson EH, Vincitore M, Tonkiss J, Morgane PJ, Galler JR 2000 Prenatal protein malnourished rats show changes in sleep/wake behavior as adults. J Sleep Res 9:71–79 [DOI] [PubMed] [Google Scholar]
- Sutton GM, Perez-Tilve D, Nogueiras R, Fang J, Kim JK, Cone RD, Gimble JM, Tschöp MH, Butler AA 2008 The melanocortin-3 receptor is required for entrainment to meal intake. J Neurosci 28:12946–12955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvonic S, Ptitsyn AA, Conrad SA, Scott LK, Floyd ZE, Kilroy G, Wu X, Goh BC, Mynatt RL, Gimble JM 2006 Characterization of peripheral circadian clocks in adipose tissues. Diabetes 55:962–970 [DOI] [PubMed] [Google Scholar]
- Ozanne SE, Wang CL, Coleman N, Smith GD 1996 Altered muscle insulin sensitivity in the male offspring of protein-malnourished rats. Am J Physiol 271:E1128–E1134 [DOI] [PubMed] [Google Scholar]
- Ozanne SE 1999 Programming of hepatic and peripheral tissue insulin sensitivity by maternal protein restriction. Biochem Soc Trans 27:94–97 [DOI] [PubMed] [Google Scholar]
- Elia M, Livesey G 1988 Theory and validity of indirect calorimetry during net lipid synthesis. Am J Clin Nutr 47:591–607 [DOI] [PubMed] [Google Scholar]
- Vickers MH, Breier BH, McCarthy D, Gluckman PD 2003 Sedentary behavior during postnatal life is determined by the prenatal environment and exacerbated by postnatal hypercaloric nutrition. Am J Physiol Regul Integr Comp Physiol 285:R271–R273 [DOI] [PubMed] [Google Scholar]
- Vickers MH, Breier BH, Cutfield WS, Hofman PL, Gluckman PD 2000 Fetal origins of hyperphagia, obesity, and hypertension and postnatal amplification by hypercaloric nutrition. Am J Physiol Endocrinol Metab 279:E83–E87 [DOI] [PubMed] [Google Scholar]
- Takahashi JS, Hong HK, Ko CH, McDearmon EL 2008 The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat Rev Genet 9:764–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Yin L, Lazar MA 2006 The orphan nuclear receptor Rev-erbα regulates circadian expression of plasminogen activator inhibitor type 1. J Biol Chem 281:33842–33848 [DOI] [PubMed] [Google Scholar]
- Bhasin KK, van Nas A, Martin LJ, Davis RC, Devaskar SU, Lusis AJ 2009 Maternal low-protein diet or hypercholesterolemia reduces circulating essential amino acids and leads to intrauterine growth restriction. Diabetes 58:559–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bol VV, Delattre AI, Reusens B, Raes M, Remacle C 2009 Forced catch-up growth after fetal protein restriction alters the adipose tissue gene expression program leading to obesity in adult mice. Am J Physiol Regul Integr Comp Physiol 297:R291–R299 [DOI] [PubMed] [Google Scholar]
- Holmbäck U, Forslund A, Forslund J, Hambraeus L, Lennernäs M, Lowden A, Stridsberg M, Akerstedt T 2002 Metabolic responses to nocturnal eating in men are affected by sources of dietary energy. J Nutr 132:1892–1899 [DOI] [PubMed] [Google Scholar]
- Williams G, Harrold JA, Cutler DJ 2000 The hypothalamus and the regulation of energy homeostasis: lifting the lid on a black box. Proc Nutr Soc 59:385–396 [DOI] [PubMed] [Google Scholar]
- Shimano H 2002 Sterol regulatory element-binding protein family as global regulators of lipid synthetic genes in energy metabolism. Vitam Horm 65:167–194 [DOI] [PubMed] [Google Scholar]
- Yellaturu CR, Deng X, Park EA, Raghow R, Elam MB 2009 Insulin enhances the biogenesis of nuclear sterol regulatory element-binding protein (SREBP)-1c by posttranscriptional down-regulation of Insig-2A and its dissociation from SREBP cleavage-activating protein (SCAP).SREBP-1c complex. J Biol Chem 284:31726–31734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato R 2009 SREBPs: protein interaction and SREBPs. FEBS J 276:622–627 [DOI] [PubMed] [Google Scholar]
- Rudic RD, McNamara P, Curtis AM, Boston RC, Panda S, Hogenesch JB, Fitzgerald GA 2004 BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLoS Biol 2:e377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozanne SE, Smith GD, Tikerpae J, Hales CN 1996 Altered regulation of hepatic glucose output in the male offspring of protein-malnourished rat dams. Am J Physiol 270:E559–E564 [DOI] [PubMed] [Google Scholar]
- Shepherd PR, Crowther NJ, Desai M, Hales CN, Ozanne SE 1997 Altered adipocyte properties in the offspring of protein malnourished rats. Br J Nutr 78:121–129 [DOI] [PubMed] [Google Scholar]
- Ozanne SE, Hales CN 2002 Early programming of glucose-insulin metabolism. Trends Endocrinol Metab 13:368–373 [DOI] [PubMed] [Google Scholar]
- Ozanne SE, Jensen CB, Tingey KJ, Storgaard H, Madsbad S, Vaag AA 2005 Low birthweight is associated with specific changes in muscle insulin-signalling protein expression. Diabetologia 48:547–552 [DOI] [PubMed] [Google Scholar]
- Ozanne SE, Nave BT, Wang CL, Shepherd PR, Prins J, Smith GD 1997 Poor fetal nutrition causes long-term changes in expression of insulin signaling components in adipocytes. Am J Physiol 273:E46–E51 [DOI] [PubMed] [Google Scholar]
- Ravelli AC, van der Meulen JH, Michels RP, Osmond C, Barker DJ, Hales CN, Bleker OP 1998 Glucose tolerance in adults after prenatal exposure to famine. Lancet 351:173–177 [DOI] [PubMed] [Google Scholar]
- Ravelli AC, van Der Meulen JH, Osmond C, Barker DJ, Bleker OP 1999 Obesity at the age of 50 y in men and women exposed to famine prenatally. Am J Clin Nutr 70:811–816 [DOI] [PubMed] [Google Scholar]
- Allada R, Emery P, Takahashi JS, Rosbash M 2001 Stopping time: the genetics of fly and mouse circadian clocks. Annu Rev Neurosci 24:1091–1119 [DOI] [PubMed] [Google Scholar]
- Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, Laposky A, Losee-Olson S, Easton A, Jensen DR, Eckel RH, Takahashi JS, Bass J 2005 Obesity and metabolic syndrome in circadian Clock mutant mice. Science 308:1043–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gachon F, Olela FF, Schaad O, Descombes P, Schibler U 2006 The circadian PAR-domain basic leucine zipper transcription factors DBP, TEF, and HLF modulate basal and inducible xenobiotic detoxification. Cell Metab 4:25–36 [DOI] [PubMed] [Google Scholar]
- Kohsaka A, Laposky AD, Ramsey KM, Estrada C, Joshu C, Kobayashi Y, Turek FW, Bass J 2007 High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab 6:414–421 [DOI] [PubMed] [Google Scholar]
- Rutter J, Reick M, Wu LC, McKnight SL 2001 Regulation of clock and NPAS2 DNA binding by the redox state of NAD cofactors. Science 293:510–514 [DOI] [PubMed] [Google Scholar]
- Karalis KP, Giannogonas P, Kodela E, Koutmani Y, Zoumakis M, Teli T 2009 Mechanisms of obesity and related pathology: linking immune responses to metabolic stress. FEBS J 276:5747–5754 [DOI] [PubMed] [Google Scholar]
- Pircher P, Chomez P, Yu F, Vennström B, Larsson L 2005 Aberrant expression of myosin isoforms in skeletal muscles from mice lacking the rev-erbAα orphan receptor gene. Am J Physiol Regul Integr Comp Physiol 288:R482–R490 [DOI] [PubMed] [Google Scholar]
- Fontaine C, Dubois G, Duguay Y, Helledie T, Vu-Dac N, Gervois P, Soncin F, Mandrup S, Fruchart JC, Fruchart-Najib J, Staels B 2003 The orphan nuclear receptor Rev-Erbalpha is a peroxisome proliferator-activated receptor (PPAR)γ target gene and promotes PPARγ-induced adipocyte differentiation. J Biol Chem 278:37672–37680 [DOI] [PubMed] [Google Scholar]
- Preitner N, Damiola F, Lopez-Molina L, Zakany J, Duboule D, Albrecht U, Schibler U 2002 The orphan nuclear receptor REV-ERBα controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell 110:251–260 [DOI] [PubMed] [Google Scholar]
- Raspé E, Duez H, Mansén A, Fontaine C, Fiévet C, Fruchart JC, Vennström B, Staels B 2002 Identification of Rev-erbα as a physiological repressor of apoC-III gene transcription. J Lipid Res 43:2172–2179 [DOI] [PubMed] [Google Scholar]
- Duez H, Staels B 2008 Rev-erbα gives a time cue to metabolism. FEBS Lett 582:19–25 [DOI] [PubMed] [Google Scholar]
- Le Martelot G, Claudel T, Gatfield D, Schaad O, Kornmann B, Sasso GL, Moschetta A, Schibler U 2009 REV-ERBα participates in circadian SREBP signaling and bile acid homeostasis. PLoS Biol 7:e1000181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozanne SE 2001 Metabolic programming in animals. Br Med Bull 60:143–152 [DOI] [PubMed] [Google Scholar]
- Le Minh N, Damiola F, Tronche F, Schütz G, Schibler U 2001 Glucocorticoid hormones inhibit food-induced phase-shifting of peripheral circadian oscillators. EMBO J 20:7128–7136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram CE, Hanson MA 2002 Prenatal programming of postnatal endocrine responses by glucocorticoids. Reproduction 124:459–467 [DOI] [PubMed] [Google Scholar]
- Torra IP, Tsibulsky V, Delaunay F, Saladin R, Laudet V, Fruchart JC, Kosykh V, Staels B 2000 Circadian and glucocorticoid regulation of Rev-erbalpha expression in liver. Endocrinology 141:3799–3806 [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Lillycrop KA, Vickers MH, Pleasants AB, Phillips ES, Beedle AS, Burdge GC, Hanson MA 2007 Metabolic plasticity during mammalian development is directionally dependent on early nutritional status. Proc Natl Acad Sci USA 104:12796–12800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillycrop KA, Phillips ES, Torrens C, Hanson MA, Jackson AA, Burdge GC 2008 Feeding pregnant rats a protein-restricted diet persistently alters the methylation of specific cytosines in the hepatic PPARα promoter of the offspring. Br J Nutr 100:278–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin A 1998 CpG methylation, chromatin structure and gene silencing-a three-way connection. EMBO J 17:4905–4908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillycrop KA, Phillips ES, Jackson AA, Hanson MA, Burdge GC 2005 Dietary protein restriction of pregnant rats induces and folic acid supplementation prevents epigenetic modification of hepatic gene expression in the offspring. J Nutr 135:1382–1386 [DOI] [PubMed] [Google Scholar]
- Toh KL, Jones CR, He Y, Eide EJ, Hinz WA, Virshup DM, Ptácek LJ, Fu YH 2001 An hPer2 phosphorylation site mutation in familial advanced sleep phase syndrome. Science 291:1040–1043 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.