Abstract
Cytochrome P450c17 (P450c17) is the single microsomal enzyme that catalyzes steroid 17α-hydroxylase and 17,20 lyase activities. The ratio of lyase to hydroxylase activity of human P450c17 determines whether steroidogenesis leads to the synthesis of cortisol or sex steroids. This ratio is regulated posttranslationally by factors that influence the efficiency of electron transfer from P450 oxidoreductase to P450c17. One factor favoring more efficient electron transfer and 17,20 lyase activity is cAMP-dependent serine/threonine phosphorylation of P450c17. Identifying the responsible kinase(s) and the P450c17 residues that undergo phosphorylation has been challenging, partly because of difficulties in preparing biochemically useful amounts of pure, catalytically active P450c17. We describe a modified strategy for preparing P450c17 in which the traditional carboxy-terminal 4×His tag is replaced by 3×Gly6×His. This construct permits more rotational freedom of the protein when bound to the nickel affinity column, reducing steric associations between the protein and the column, and permitting a single-step chromatographic purification to apparent homogeneity. Using this vector, we explored P450c17 phosphorylation by mutagenesis of Ser and/or Thr residues to Asp or Glu to mimic the approximate size and charge of phospho-Ser or phospho-Thr. This strategy did not identify Ser and/or Thr site(s) that increase the ratio of lyase to hydroxylase activity, suggesting that the regulatory phosphorylation strategy of human P450c17 is very complicated. Although previous work has excluded protein kinase A (PKA) as the responsible kinase, the cAMP-inducible nature of the phosphorylation-associated increase in lyase activity suggests that PKA may play a role, possibly as a priming kinase. Using our novel vector and a series of mutations, we identified the P450c17 site phosphorylated by PKA as Ser258.
The cAMP-inducible nature of the phosphorylation-associated increase in lyase activity suggests that protein kinase A may play a role possibly as a priming kinase.
Cytochrome P450c17 (P450c17) is the single microsomal steroidogenic cytochrome P450 enzyme that catalyzes both the 17α-hydroxylase activity needed to produce cortisol and the 17,20 lyase activity needed to produce sex steroids (1,2,3). Only a single form of P450c17, encoded by a single gene, is expressed in both the adrenals and gonads (4,5). Uniquely among mammals, rodents fail to express P450c17 in their adrenals and hence must use corticosterone instead of cortisol as their glucocorticoid (6). Phosphorylation of human P450c17 on serine and threonine residues increases 17,20 lyase activity without altering 17α-hydroxylase activity (7,8,9). The kinase(s) responsible for this phosphorylation and the sites of phosphorylation that contribute to the augmented 17,20 lyase activity have been unclear. Initial studies implicated a cAMP-dependent process, possibly involving protein kinase A (PKA) (7), but a subsequent study found that PKA was not the responsible kinase (10). To address this question further, we screened potential phosphorylation sites on P450c17 by site-directed mutagenesis.
Our previous efforts were hampered by the difficulty in preparing large amounts of pure, catalytically active human P450c17. Dramatic improvements in the levels of bacterial expression of P450c17 were achieved by Imai et al. by altering the N-terminal sequence of P450c17 from MWELVALL to MALLLAVF and adding a carboxy-terminal 4×His tag for affinity purification (11). However, the purification of a membrane-bound protein such as P450c17 can require multiple steps, even when using a C-terminal His tag. We constructed a new P450c17 bacterial expression plasmid, which engineered a Gly-Gly-Gly spacer preceding a 6×His tag in the C terminus. This C terminally modified P450c17 was readily purified by a single pass over Ni-NTA (nickel nitrilotriacetic acid) agarose.
Using the N-terminal modifications of Imai et al. (11) and our C-terminal modifications, we produced a series of purified, catalytically active Ser to Asp or Thr to Glu P450c17 mutants, but none of those mimicked the effect of phosphorylation on activating 17,20 lyase activity. Because PKA is one of the kinases that can phosphorylate P450c17, we mapped its preferred site. PKA phosphorylated wild-type P450c17 but did not phosphorylate Ser258Ala P450c17. Optimizing the PKA recognition motif to 255Arg-Arg-Asp-Ser258 and then to Ser256Arg/Asp257Ala improved phosphorylation by PKA to a greater extent than the wild-type P450c17, whereas the Ser256Arg/Ser258Ala mutant could not be phosphorylated by PKA at all. These results show that Ser258 is the phosphorylation site of PKA.
Materials and Methods
Construction of human P450c17 expression plasmid pCWH17mod(G3H6)
The human P450c17 cDNA (4) and its N-terminal derivitization to optimize bacterial expression as pCWH17mod(His)4 (11) have been described. A fragment of the coding region spanning an internal Xba1 site was amplified using the primer pair, 5′-CAAATGG CAACTCTAGACATCG-3′ (forward) and 5′-CCCAAGCTT CAGTG ATG GTG ATG GTG ATG ACC GCC ACC GGT GCT ACC CTC AGC CTG GGC T-3′ (reverse). The reverse primer encodes three Gly codons followed by six His codons and a stop codon with an overlapping HindIII site. This PCR product was digested with Xba1 and HindIII and then ligated into pCWH17mod(His)4 which had previously been digested with Xba1 and HindIII and dephosphorylated with Antarctic Phosphatase (New England Biolabs, Beverly, MA). After transformation into Escherichia coli, the transformants of this ligation were screened by PCR with the primer pair 5′-TCACACAGGAAACAGGATCCA-3′ (forward) and 5′-GTG ATG GTG ATG ACC GCC ACC-3′ (reverse). A positive clone was picked, sequenced, and designated pCWH17mod(G3H6).
Expression and purification of P450c17
A single colony of E. coli strain JM109 transformed with pCWH17mod(G3H6) was grown to saturation in 10 ml Luria- Bertani medium containing 100 μg/ml ampicillin at 37 C in an incubator shaking at 220 rpm. This culture was seeded into 1 liter terrific broth containing 100 μg/ml carbenicillin, supplemented with 40 μm FeCl3, 4 μm ZnCl2, 2 μm CoCl2, 2 μm Na2MoO4, 2 μm CaCl2, 2 μm CuCl2, 2 μm H3BO3 (12), and 1 mm thiamine and grown at 37 C in an incubator shaking at 220 rpm to OD600 = 0.2; then δ-aminolevulinic acid was added to 0.5 mm. Then culture temperature was lowered to 28 C, and when the OD600 reached 0.4, isopropyl-1-thio-β-d-galactopyranoside was added to 0.5 mm to induce P450c17 expression. Induction continued at 28 C with reduced shaking (120–150rpm) for 2 d. All subsequent steps were done at 4 C. The bacteria were harvested at 5000 × g for 10 min and resuspended in 20 ml of 0.1 m Tris-acetate, pH 7.8; 0.5 mm EDTA; and 0.5 m sucrose. Lysozyme was added to 0.2 mg/ml for 2 h or more to digest the bacterial cell walls, and spheroplasts were collected by centrifugation at 12,000 × g for 10 min and homogenized in buffer A [50 mm potassium phosphate, pH 7.4; 10 mm MgCl2; 0.1 mm EDTA; 20% glycerol; 1 mm dithiothreitol (DTT); 40 μm progesterone; 0.2 mm phenylmethylsulfonyl fluoride (PMSF); 1 μg/ml deoxyribonuclease 1] with a Wheaton homogenizer. The homogenized spheroplasts were then sonicated at 4 C with eight to nine cycles of 25 sec on, 30 sec off using a 550 Sonic dismembranator (Fisher Scientific) at 30% power.
The lysate was cleared by centrifugation at 12,000 × g for 10 min, and then the supernatant was centrifuged at 265,000 × g for 45 min to pellet the membranes. The membrane-associated human P450c17 in the pellet was solublized in buffer B (50 mm potassium phosphate, pH 7.4; 20% glycerol; 0.5% Triton X-114; 0.2% sodium cholate; 10 mm imidazole; 40 μm progesterone; 0.1 mm PMSF), and the resulting solution was cleared by centrifugation at 27,000 × g for 25 min. The supernatant (20 ml) was loaded slowly onto Ni-NTA agarose column (1.6 cm × 2.5 cm), which had been preequilibrated with buffer C (25 mm potassium phosphate, pH 7.4; 10% glycerol; 0.1% Triton X-100; 0.1% sodium cholate; 10 mm imidazole; 40 μm progesterone; 0.1 mm PMSF). The column was consecutively washed with buffer C, buffer D (buffer C plus 300 mm NaCl), and buffer E (25 mm potassium phosphate, pH 7.4; 10% glycerol; 0.1% Triton X-100; 0.1% sodium cholate; 50 mm imidazole; 40 μm progesterone; 0.1 mm PMSF). Finally, P450c17 was eluted with buffer F (25 mm potassium phosphate, pH 7.4; 10% glycerol; 0.2% sodium cholate; 300 mm imidazole; 0.1 mm PMSF). Purified P450c17 was then desalted into buffer G (25 mm potassium phosphate, pH 7.4; 10% glycerol; 0.2 mm DTT; 0.1% sodium cholate) by passage through a Sephadex G-25 column (1.6 cm × 10 cm) or by dialysis against buffer G. All purification steps were performed at about 0–4 C. Protein concentration was determined by the Bradford method, and the purity was examined by SDS-PAGE and Coomassie brilliant blue staining. P450 content was determined using a molar extinction difference of 91 cm−1/mm−1 between 450 and 490 nm. The baseline spectrum from 400–500 nm of 5 mg protein was measured after the addition of 20 mg sodium dithionite, and the reduced CO spectrum was measured after the sample was bubbled gently with carbon monoxide for 1 min.
Generation, expression, and purification of P450c17 mutants
Human P450c17 mutants were built in expression plasmid pCWH17mod(G3H6) by PCR-mediated site-directed mutagenesis essentially as described (10) and confirmed by DNA sequencing. Mutant human P450c17 proteins were expressed and purified essentially as described for wild-type human P450c17.
Assay of P450c17 activities
P450c17 activities were assayed as described (10,12) with recombinant human P450 oxidoreductase (POR) as the electron donor. Each 200-μl reaction contained 10 pmol purified human P450c17, 20 pmol purified human POR, 20 μg phosphatidylcholine, 50 mm potassium phosphate (pH 7.4), 20% glycerol, 6 mm potassium acetate, 10 mm MgCl2, 1 mm reduced glutathione, 2 U glucose-6-phosphate dehydrogenase, 1.5 mm glucose-6-phosphate, 0.5 mm of reduced NADPH (nicotinamide adenine dinucleotide phosphate), and a radiolabeled substrate. For 17α-hydroxylase activity, 1 μm 14C-progesterone (50 nCi; American Radiolabeled Chemicals, St. Louis, MO) was added; for the 17,20 lyase activity, 1 μm 3H-17α-hydroxypregnenolone (17OH-preg; 50 nCi; American Radiolabeled Chemicals) was added. Wherever applicable, 10 pmol of cytochrome b5 was added to facilitate 17,20 lyase activity (9,13). Assays were conducted at 37 C for 2 h and terminated by addition of 550 μl of 1:1 ethyl acetate-isooctane for steroid isolation. Steroids were extracted from assay mixtures by vigorously mixing. After centrifugation, the upper organic phase was removed and dried through evaporation under gentle nitrogen stream. Steroids from each assay were dissolved in 30 μl dichloromethane and analyzed by thin-layer chromatography on the silica gel plates (PE SIL G/UV; Whatman, Middlesex, UK) with mixture of 3:1 chloroform-ethyl acetate as developer. Radiolabeled steroids were visualized by exposing the thin-layer chromatography plate to a phosphorimager screen (GE Healthcare, Piscataway, NJ), quantitated with software ImageQuant (IQMac, version 1.2 Alias; GE Health) for the determination of enzymatic activities in femtomoles of steroid converted per picomoles of P450c17 per minute.
In vitro phosphorylation of P450c17 by PKA
The catalytic subunit of PKA (Sigma, St. Louis, MO) and purified recombinant human wild-type or mutant P450c17 was incubated with 20 μm ATP (1 μCi γ-32P-ATP), 12 mm MgCl2, and 50 mm potassium phosphate (pH 7.5), 20% glycerol, 1 mm DTT, protease inhibitor cocktail (catalog no. 539134; Calbiochem, La Jolla, CA), phosphatase inhibitor cocktail set 1 (catalog no. 524624; Calbiochem,) and 100 nm okadaic acid (catalog no. 459620; Calbiochem) at 30 C for 10 min (unless otherwise indicated). After incubation, the reaction was terminated by addition of sodium dodecyl sulfate sample buffer and analyzed by SDS-PAGE. After staining with Coomassie brilliant blue and destaining, the gels were exposed to a phosphorimager screen or film (BioMax MS film; Kodak, Rochester, NY). To quantitate 32P incorporation into P450c17, the P450c17 bands were excised and subjected to scintillation counting. The efficiency of the incorporation of 32P was determined as picomoles of 32P incorporated per picomole of P450c17. In some cases, phosphorylated human P450c17 was assayed for activities as described above.
Statistical analyses
Statistical analyses were performed using two-tailed unpaired t tests, and significance was accepted for tests where P < 0.05.
Results
Construction, expression, and purification of human P450c17
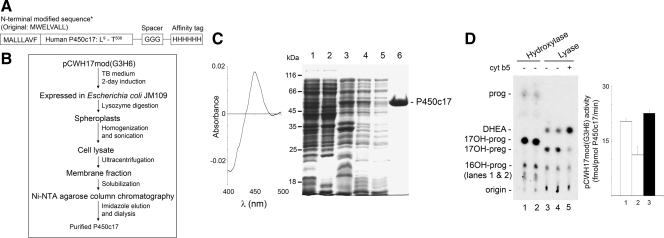
The addition of a carboxy-terminal polyhistidine or other tag has become a universally used tactic to prepare bacterially expressed recombinant proteins, and we and others have used it in the preparation of membrane-bound cytochrome P450 enzymes (8,9,10,11,14,15,16,17,18,19,20,21). However, in contrast to soluble, cytoplasmic proteins, the use of a His tag or FLAG tag has resulted in poor yields and contaminated preparations of many P450 enzymes, apparently because both the hydrophobic enzyme protein and the tag interact with the column material. Using the conventional N-terminally modified P450c17 expression vector pCWH17mod(His)4 engineered by the Waterman group, purification of the bacterially expressed P450c17 requires three steps of column chromatography on Ni-NTA agarose, followed by two consecutive passes on hydroxylapatite columns (11). Because this protocol gave poor yields and was inconvenient for preparing multiple P450c17 mutants, we designed a new expression plasmid, pCWH17mod(G3H6), in which the 4×His tag in pCWH17mod(His)4 is replaced by a 6×His tag downstream from a 3×Gly spacer (Fig. 1A). The 3×Gly was inserted to improve flexibility between the P450c17 moiety and the histidine affinity tag, which was lengthened to permit more stringent washing conditions. This construct permitted the purification of P450c17 with a single pass on the Ni-NTA agarose column rather than three steps of column chromatography in the conventional protocol (Fig. 1, B and C). The purified protein appears to be homogeneous after this single step (Fig. 1C) and retains full 17α-hydroxylase and 17,20 lyase activities (Fig. 1D). This P450c17 protein with its N- and C-terminal modifications is referred to as wild type to distinguish it from the 27 site-directed mutants described in Table 1.
Figure 1.
Construction, expression, and purification of recombinant human P450c17. A, Diagram of the modified form of human P450c17 expressed by pCWH17mod(G3H6); single letter amino acid codes are used to indicate the relevant sequences. The eight N-terminal residues are modified as described (11), the residues from Leu9 to Thr508 are those of wild-type P450c17 (4,5), and the Gly3His6 tag precedes the stop codon. B, Flow chart of purification of recombinant human P450c17 from bacteria. C, Purification of recombinant human P450c17 from bacteria. Dithionite-reduced carbon monoxide-difference spectra of pCWH17mod(G3H6) is shown in the left panel. The specific content is 9.9 pmol/μg protein. In the right panel, samples from various purification steps were analyzed by 12% SDS-PAGE and stained with Coomassie brilliant blue. Lane 1, Cell lysate (∼50 μg protein); lane 2, supernatant from ultracentrifugation (∼50 μg); lane 3, membrane fraction (pellet from ultracentrifugation) (∼25 μg); lane 4, solubilized membrane fraction (∼10 μg); lane 5, flow-through from the Ni-NTA agarose column (∼10 μg); lane 6, purified recombinant human P450c17 (10 μg). The migration of molecular weight markers (kilodaltons) is shown on the left. D, 17α-Hydroxylase and 17,20 lyase activities of the bacterially expressed recombinant human P450c17. Left panel, Radiolabeled progesterone (prog) was used as the substrate for the hydroxylase assays (lanes 1 and 2), and 17OH-preg was used as the substrate for the lyase assays (both at 1 μm). The hydroxylase assay yields 17OHP (17OH-prog) and a small amount of 16OH-progesterone (16OH-prog); the lyase reaction yields dehydroepiandrosterone (DHEA). Some minor contaminant in the substrate steroids are not metabolized. The assays were done in duplicates in lanes 1 and 2 for the hydroxylase reaction and lanes 3 and 4 for the lyase reaction. In lane 5, 10 pmol cytochrome b5 were added to the lyase reaction. Right panel, The activities of pCWH17mod(G3H6) were quantitated for the hydroxylase assay (lane 1), lyase assay in the absence of cytochrome b5 (lane 2), and 17,20 lyase activity in the presence of 10 pmol cytochrome b5 (lane 3); data are mean ± sd.
Table 1.
Effects of mutations of candidate sites for phosphorylation of P450c17
| Mutation | Hydroxylase activity of wt (%) | Lyase activity of wt without b5 (%) | Lyase activity of wt with b5 (%) |
|---|---|---|---|
| WT | 100 (20 ± 0.73) | 100 (8.9 ± 2.3) | 100 (22 ± 7.9) |
| S65D | 9 | 49 | 18 |
| T70E | 88 | 114 | 67 |
| T72E | 53 | 98 | 12 |
| S94D | 43 | 18 | 6 |
| S168D | 8 | 0 | 0 |
| S187D | 2 | 22 | 4 |
| S256D | 62 | 31 | 33 |
| S258D | 75 | 17 | 23 |
| S288D | 10 | 77 | 10 |
| T343E | 87 | 39 | 48 |
| S380D | 17 | 13 | 16 |
| S427D | 21 | 4 | 0 |
| S431D | 4 | 6 | 0 |
| S441D | 20 ± 8 (P < 0.0001)a | 0 (P = 0.0003)a | 4 |
| S94D/S234D | 66 ± 15 (P = 0.0116)a | 10 ± 2 (P = 0.0005)a | 13 |
| S94D/T265E | 31 ± 11 (P < 0.0001)a | 14 ± 4 (P = 0.0008)a | 3 |
| S94D/S309D | 0 | 27 | 3 |
| S94D/T314E | 43 ± 8 (P < 0.0001)a | 14 ± 4 (P = 0.0008)a | 2 |
| S94D/T343E | 110 | 0 | 0 |
| S94D/T356E | 6 | 21 | 12 |
| S94D/S379D | 1 ± 2 (P < 0.0001)a | 7 ± 2 (P = 0.0004)a | 1 |
| S94D/S380D | 0 | 19 | 7 |
| S94D/S427D | 5 | 0 | 0 |
| S94D/S429D | 6 ± 5 (P < 0.0001)a | 15 ± 5 (P = 0.0010)a | 2 |
| T341E/T343E | 67 | 9 | 44 |
| S256D/S258D/T260D | 8 | 0 | 0 |
| S94D/T341E/T343E | 5 | 6 | 2 |
The data are mean ± sd of quadruplicate experiments of hydroxylase and 17,20 lyase assays for constructs S441D, S94D/S234D, S94D/T265E, S94D/T314E, S94D/S379D, and S94D/S429D and duplicate experiments for the remaining constructs. The hydroxylase and lyase activities for the WT construct are given in parentheses as femtomoles per picomole P450c17 per minute (mean ± sd). WT, Wild type.
Significant differences in hydroxylase and lyase activities from WT for constructs S441D, S94D/S234D, S94D/T265E, S94D/T314E, S94D/S379D, and S94D/S429D.
Phosphomimetic screening of P450c17 regulatory phosphorylation site(s)
Phosphorylation of serine and/or threonine residues of P450c17 in response to cAMP increases 17,20-lyase activity without influencing 17α-hydroxylase activity (7,8,9). Of the 508 amino acid residues in the human P450c17 protein, 57 are serine and threonine residues (4). Based on amino acid sequence alignments of mammalian P450c17, computational phosphorylation site predictions and evaluations of surface accessibility of P450c17 Ser/Thr residues, we identified about a dozen serine and threonine residues as candidate sites of regulatory phosphorylation (Table 1). To test the potential role(s) of candidate Ser/Thr residues, we built P450c17 mutants changing Ser to Asp and/or Thr to Glu or Asp because these changes approximate the size and charge of phosphoserine and phosphothreonine, respectively (phosphomimetic screening) (Table 1). Because Ser/Thr phosphorylation increases the 17,20 lyase activity of P450c17 (7,8,9), we expected to see a similar increase in lyase activity when an Asp or Glu is substituted for the relevant Ser/Thr. We chose to change Ser to Asp and Thr to Glu according to the volumes of the amino acids and generated 14 mutants: S65D, T70E, T72E, S94D, S168D, S187D, S256D, S258D, S288D, T343E, S380D, S427D, S431D, and S441D (Table 1). However, none of these mutants had a significant increase in lyase activity, and, somewhat surprisingly, all except T70E and T343E lost substantial 17α-hydroxylase activity (Table 1).
We previously found that mutation S94A virtually abolished lyase activity but maintained about half of the 17α-hydroxylase activity (10). Other mutations, such as R347H and R356Q (22), have also selectively reduced lyase activity of P450c17 by affecting the P450c17 domain that interacts with POR (23), and E305G selectively reduced lyase activity directly in the catalytic active site (24), but the mechanism by which mutation of S94 would selectively affect 17,20 lyase activity is unclear. One possibility is that it affects binding of cytochrome b5. The residues of b5 that interact with P450c17 to promote lyase activity have been mapped by mutagenesis to E48 and E49 (25), but the corresponding interacting sites on P450c17 have not been reported, although detailed mapping of CYP2B4 identified seven sites that interact with b5, six of which are on the proximal face between residues 122 and 139 (26). Furthermore, it is not known whether phosphorylation of a single Ser or Thr residue of P450c17 increases 17,20 lyase activity or whether several residues must be phosphorylated. To test the potential role of S94, we constructed and tested a series of double mutants (S94D/S234D, S94D/T265E, S94D/S309D, S94D/T314E, S94D/T343E, S94D/T356E, S94D/S379D, S94D/S380D, S94D/S427D, and S94D/S429D), but none of these had significantly increased lyase activity (Table 1). Similarly, we generated the mutants T341E/T343E, S256D/S258D/T260D, and S94D/T341E/T343E to examine the possibility that neighboring serine or threonine residues might participate in regulatory phosphorylation sites, but none of these mutants had significantly increased lyase activity (Table 1).
Identification of Ser258 as the site phosphorylated by PKA
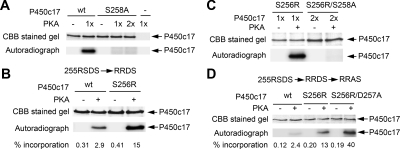
cAMP and cAMP-dependent PKA appear to participate in the phosphorylation of human P450c17 in vivo (7) and in vitro (10). However, the site(s) phosphorylated by PKA have not been mapped. The preferred sequence that undergoes phosphorylation by PKA is R-R-X-S/T (27). By linear sequence analysis, P450c17 has a favorable-appearing sequence 255Arg-Ser-Asp-Ser258, and based on the computational model of P450c17 (28), this region is predicted to be surface exposed and hence available to interact with PKA. To test the potential role of Ser 258 as a PKA phosphorylation site, we constructed, expressed, purified, and tested a series of P450c17 mutants (Fig. 2). PKA phosphorylated wild-type P450c17 but not the S258A mutant; even when the amount of PKA in the phosphorylation reaction was doubled, S258A did not yield a detectable phosphorylation signal (Fig. 2A). Ser258 lies in the sequence 255Arg-Ser-Asp-Ser258, which is a recognizable, but not optimal, recognition/phosphorylation sequence for PKA; we reasoned that this site should be improved by changing Ser 256 to Arg to match the optimal PKA recognition motif, R-R-X-S/T (27). Indeed, the S256R mutant was phosphorylated to about 5-fold higher level than wild type (Fig. 2B). However, the S256R/S258A double mutant was not phosphorylated at all, consistent with PKA phosphorylation occurring at Ser258 (Fig. 2C). The mutant S256R/D257A was phosphorylated by PKA to about a 3-fold higher level than the S256R mutant (Fig. 2D), suggesting that elimination of the negatively charged Asp257 residue in this motif further facilitates the PKA phosphorylation. This result is consistent with the general understanding that a negative charge within the PKA recognition motif may affect the charge-charge interaction between the negatively charged PKA and its positively charged substrate for recognition (29). Thus, Ser258 is the site on human P450c17 phosphorylated by PKA.
Figure 2.
PKA phosphorylates Ser258. In each panel, the amount of P450c17 is indicated by staining with Coomassie brilliant blue (CBB), and phosphorylation is assessed by autoradiography of incorporated 32P. A, S258A is not phosphorylated. Wild-type (wt) and S258A human P450c17 (3 μg each) were treated without (−) and with (+) 0.5 U PKA catalytic subunit (one time) or with 1.0 U PKA catalytic subunit (two times) and 20 μm ATP (1μCi γ-32P-ATP) for 30 min at 30 C. B, S256R increases phosphorylation. S256R and wild-type (wt) P450c17 (5 μg each) were incubated with 0.5U PKA catalytic subunit for 10 min at 30 C. The percentages of 32P incorporated (picomoles 32P per picomole P450c17) of each sample are indicated below the figure. C, S256R does not promote phosphorylation of S258A. S256R mutant (1 μg) or S256R/S258A double mutant (2 μg) was treated with 0.5 U PKA catalytic subunit for 30 min at 30 C. D, D257A promotes phosphorylation. Wild-type human P450c17, S256R mutant, and S256R/D257A double mutant (0.75 μg each) were treated with 0.5 U PKA catalytic subunit for 10 min at 30 C. The percentages of 32P incorporation are indicated below the figure, as in B.
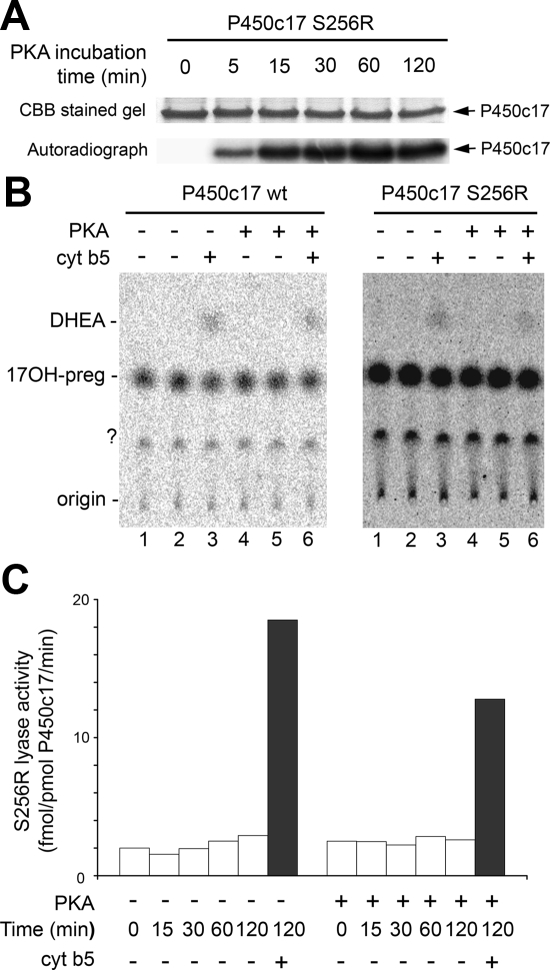
To assess the effect of Ser258 phosphorylation on 17,20 lyase activity, we prepared S256R P450c17 and phosphorylated Ser258 with PKA at 30 C for 0–120 min (Fig. 3A). However, the phosphorylated wild-type and S256R mutant displayed no increase in 17,20 lyase activity (Fig. 3, B and C). Thus, phosphorylation of Ser258 alone is not sufficient to constitute the phosphorylation-induced increase in the 17,20 lyase activity of P450c17. This result is consistent with the S258D mutant having decreased lyase activity, contrary to anticipated activation (Table 1) (10,30). Whether the Ser258 PKA phosphorylation site functions in concert with other phosphorylation site(s) of unknown protein serine/threonine kinase(s) to regulate P450c17 lyase activity remains unknown.
Figure 3.
Effect of S258 phosphorylation on catalysis by P450c17. A, The S256R mutant (4 μg) was phosphorylated by 0.5 U PKA catalytic subunit with 20 μm ATP (1 μCi γ-32P-ATP) for the indicated times and analyzed by SDS-PAGE. Staining with Coomassie brilliant blue (CBB) and autoradiography of incorporated 32P are shown. B, Unphosphorylated and PKA-phosphorylated P450c17 wild-type (left panel) and S256R mutant (right panel) (10 pmol each) were assayed for 17,20 lyase activity with 1 μm 3H-17OH-preg. The lane numbers identify corresponding experiments in each panel. Each form of P450c17 was preincubated in the presence or absence of PKA for 30 min at 30 C followed by lyase assay for 2 h in the absence or presence of 10 pmol cytochrome b5 (cyt b5) in lanes 3 and 6. The experiment was done in duplicates for lanes 1–2 and 4–5 for the unphosphorylated and PKA-phosphorylated forms of P450c17, respectively. DHEA, Dehydroepiandrosterone. C, The S256R mutant was preincubated in the absence or presence of PKA for the indicated times followed by 2 h incubation with steroids for the 17,20 lyase assay in the absence (open bars) or presence (closed bars) of cytochrome b5. The data are means from duplicate experiments.
Discussion
P450c17 is the only enzyme that catalyzes 17α-hydroxylase or 17,20 lyase activity; in its absence, no 17-hydroxysteroids and no sex steroids are produced (31,32). Understanding the 17,20 lyase activity of P450c17 is central to studies of sex steroid biosynthesis and its disorders, especially premature, exaggerated adrenarche (33,34,35,36) and the polycystic ovary syndrome (37,38). There are important differences in the biochemistry of P450c17 from different species, especially with respect to the 17,20 lyase reaction: porcine and hamster P450c17 catalyze 17,20 lyase activity with both the Δ4 steroid substrate, 17OH-progesterone (17OHP), and the Δ5 substrate, 17OH-preg (3,39). Rat (40), trout (41), and guinea pig (42) P450c17 function primarily with 17OHP, and human P450c17 exhibits about a 50-fold preference for 17OH-preg (13,43). The Michaelis constants for the three main reactions catalyzed by human P450c17 (Δ5 17-hydroxylase, Δ4 17-hydroxylase, and Δ5 17,20 lyase) are essentially the same (0.73–0.83 μm), but the maximum velocities differ substantially, depending on additional factors that regulate the availability of electrons (13). These include the molar ratio of P450 oxidoreductase to P450c17 (44,45), the allosteric action of cytochrome b5 (13,23), and the Ser/Thr phosphorylation of P450c17 (7,8,9) (for review see Ref. 46).
The phosphorylation of P450c17 has been difficult to study. The initial report showed that P450c17 phosphorylation was responsive to cAMP and suggested a role for PKA (7). Subsequent work showed counterbalancing dephosphorylation by protein phosphatase 2A, which, in turn, was regulated by phosphoprotein SET (8). Many kinases and potential phosphorylation sites have been studied. Microarray studies showed that human adrenal NCI-H295A cells, which phosphorylate P450c17 in response to cAMP, yielding increased 17,20 lyase activity, do not express many serine/threonine kinases, including mammalian target of rapamycin, MAPK3/ERK1, MAPK1/ERK2, MAP2K1/MAPK kinase 1, and MAP2K2/MAPK kinase 2 (10). Six other serine/threonine kinases were induced greater than 2-fold by cAMP, but RNA interference studies showed no effect of their knockdown on 17,20 lyase activity (10). Pharmacological inhibitor studies suggested a role for rho-associated coiled-coil containing kinase (ROCK)-1, and both ROCK1 and PKA could phosphorylate bacterially expressed P450c17 in vitro, but neither directly influenced 17,20 lyase activity, even though overexpression of ROCK1 in cells induced 17,20 lyase activity and its knockdown decreased 17,20 lyase activity (10). Thus, prior work identified kinases (PKA, ROCK1) that influence 17,20 lyase activity in a whole-cell milieu, suggesting they participate in a signal transduction pathway, but to date no kinase has phosphorylated P450c17 in vitro with a consequent direct increase in the ratio of 17,20 lyase to 17α-hydroxylase activity.
Similarly, the P450c17 phosphorylation site(s) that promote lyase activity have been elusive. Because it is not possible to insert Ser-PO4 or Thr-PO4 into a protein by site-directed mutagenesis, most studies of serine/threonine phosphorylation have substituted either Asp or Glu for Ser or Thr to approximate the size and charge of a Ser-PO4 or Thr-PO4 side groups; Asp is often superior (47). The side chain of Ser-PO4 is −CH2PO3− and that of Thr-PO4 is −CH(CH3)PO3. Thus, Asp and Glu are imperfect models−, with pI (isoelectric point) values of 2.7 and 2.9, respectively, whereas the side chain of Asp is −CH2CO2− with a pI of 3.0 and the side chain of Glu is −CH2CH2CO2− with a pI of 3.2. Thus, Asp and Glu are imperfect models of Ser-PO4 or Thr-PO4, and hence, one cannot draw clear conclusions from the failure of the Ser/Thr mutagenesis to yield constitutively active 17,20 lyase activity. The serine phosphorylation that affects 17,20 lyase activity does not affect 17α-hydroxylase activity (7,8,9); hence, we expected that the mutations S65D, S168D, S187D, S288D, S380D, S427D, S431D, and S441D would have retained wild-type 17α-hydroxylase activity. However, all of these mutants had diminished 17α-hydroxylase activity, suggesting that the structure of P450c17 is highly intolerant to amino acid replacements. Thus, the tactic of modeling Ser or Thr phosphorylation by substituting Asp or Glu, which has been successful with many but not all proteins, does not appear to be effective with human P450c17.
Acknowledgments
We thank Izabella Damm for excellent technical assistance.
Footnotes
This work was supported by National Institutes of Health Grant DK37922 and a University of California, San Francisco, School of Medicine bridge grant (to W.L.M.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online February 16, 2010
Abbreviations: DTT, Dithiothreitol; 17OHP, 17OH-progesterone; 17OH-preg, 17α-hydroxypregnenolone; P450c17, cytochrome P450c17; PKA, protein kinase A; PMSF, phenylmethylsulfonyl fluoride; POR, P450 oxidoreductase; ROCK, rho-associated coiled-coil containing kinase.
References
- Nakajin S, Shively JE, Yuan PM, Hall PF 1981 Microsomal cytochrome P450 from neonatal pig testis: two enzymatic activities (17α-hydroxylase and C17,20-lyase) associated with one protein. Biochemistry 20:4037–4042 [DOI] [PubMed] [Google Scholar]
- Nakajin S, Hall PF 1981 Microsomal cytochrome P450 from neonatal pig testis. Purification and properties of a C21 steroid side-chain cleavage system (17α-hydroxylase-C17,20 lyase). J Biol Chem 256:3871–3876 [PubMed] [Google Scholar]
- Nakajin S, Shinoda M, Haniu M, Shively JE, Hall PF 1984 C21 steroid side-chain cleavage enzyme from porcine adrenal microsomes. Purification and characterization of the 17α-hydroxylase/C17,20 lyase cytochrome P450. J Biol Chem 259:3971–3976 [PubMed] [Google Scholar]
- Chung BC, Picado-Leonard J, Haniu M, Bienkowski M, Hall PF, Shively JE, Miller WL 1987 Cytochrome P450c17 (steroid 17α-hydroxylase/17,20 lyase): cloning of human adrenal and testis cDNAs indicates the same gene is expressed in both tissues. Proc Natl Acad Sci USA 84:407–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picado-Leonard J, Miller WL 1987 Cloning and sequence of the human gene encoding P450c17 (steroid 17α-hydroxylase/17,20 lyase): similarity to the gene for P450c21. DNA 6:439–448 [DOI] [PubMed] [Google Scholar]
- Voutilainen R, Tapanainen J, Chung BC, Matteson KJ, Miller WL 1986 Hormonal regulation of P450scc (20,22-desmolase) and P450c17 (17α-hydroxylase/17,20-lyase) in cultured human granulosa cells. J Clin Endocrinol Metab 63:202–207 [DOI] [PubMed] [Google Scholar]
- Zhang LH, Rodriguez H, Ohno S, Miller WL 1995 Serine phosphorylation of human P450c17 increases 17,20 lyase activity: Implications for adrenarche and for the polycystic ovary syndrome. Proc Natl Acad Sci USA 92:10619–10623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey AV, Mellon SH, Miller WL 2003 Protein phosphatase 2A and phosphoprotein SET regulate androgen production by P450c17. J Biol Chem 278:2837–2844 [DOI] [PubMed] [Google Scholar]
- Pandey AV, Miller WL 2005 Regulation of 17,20 lyase activity by cytochrome b5 and by serine phosphorylation of P450c17. J Biol Chem 280:13265–13271 [DOI] [PubMed] [Google Scholar]
- Tee MK, Dong Q, Miller WL 2008 Pathways leading to the phosphorylation of P450c17 and to the post-translational regulation of androgen biosynthesis. Endocrinology 149:2667–2677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai T, Globerman H, Gertner JM, Kagawa N, Waterman MR 1993 Expression and purification of functional human 17α-hydroxylase/17,20 lyase (P450c17) in Escherichia coli. J Biol Chem 268:19681–19689 [PubMed] [Google Scholar]
- Huang N, Pandey AV, Agrawal V, Reardon W, Lapunzina PD, Mowat D, Jabs EW, Van Vliet G, Sack J, Flück CE, Miller WL 2005 Diversity and function of mutations in P450 oxidoreductase in patients with Antley-Bixler syndrome and disordered steroidogenesis. Am J Hum Genet 76:729–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auchus RJ, Lee TC, Miller WL 1998 Cytochrome b5 augments the 17,20 lyase activity of human P450c17 without direct electron transfer. J Biol Chem 273:3158–3165 [DOI] [PubMed] [Google Scholar]
- Doray B, Chen CD, Kemper B 2001 N-terminal deletions and His-tag fusions dramatically affect expression of cytochrome P450 2C2 in bacteria. Arch Biochem Biophys 393:143–153 [DOI] [PubMed] [Google Scholar]
- Owaki A, Takamasa A, Yamazaki T, Kominami S 2002 Membrane reconstitution of recombinant guinea pig cytochrome P45017α and the effects of site-directed mutagenesis on androgen formation. J Steroid Biochem Mol Biol 81:255–262 [DOI] [PubMed] [Google Scholar]
- Chernogolov A, Behlke J, Schunck WH, Roots I, Schwarz D 2003 Human CYP1A1 allelic variants: baculovirus expression and purification, hydrodynamic, spectral, and catalytical properties and their potency in the formation of all-trans-retinoic acid. Protein Expr Purif 28:259–269 [DOI] [PubMed] [Google Scholar]
- Gilep AA, Estabrook RW, Usanov SA 2003 Molecular cloning and heterologous expression in E. coli of cytochrome P45017α. Comparison of structural and functional properties of substrate-specific cytochromes P450 from different species. Biochemistry (Mosc) 68:86–98 [DOI] [PubMed] [Google Scholar]
- Mast N, Andersson U, Nakayama K, Bjorkhem I, Pikuleva IA 2004 Expression of human cytochrome P450 46A1 in Escherichia coli: effects of N- and C-terminal modifications. Arch Biochem Biophys 428:99–108 [DOI] [PubMed] [Google Scholar]
- Guengerich FP, Martin MV 2006 Purification of cytochromes P450: products of bacterial recombinant expression systems. Methods Mol Biol 320:31–37 [DOI] [PubMed] [Google Scholar]
- Hamann T, Laursen T, Møller BL 2009 Functional expression of N-terminally tagged membrane bound cytochrome P450. Protein Expr Purif 68:18–21 [DOI] [PubMed] [Google Scholar]
- Emoto C, Murayama N, Wakiya S, Yamazaki H 2009 Effects of histidine-tag on recombinant human cytochrome P450 3A5 catalytic activity in reconstitution systems. Drug Metab Lett 3:207–211 [DOI] [PubMed] [Google Scholar]
- Geller DH, Auchus RJ, Mendonça BB, Miller WL 1997 The genetic and functional basis of isolated 17,20 lyase deficiency. Nat Genet 17:201–205 [DOI] [PubMed] [Google Scholar]
- Geller DH, Auchus RJ, Miller WL 1999 P450c17 mutations R347H and R358Q selectively disrupt 17,20-lyase activity by disrupting interactions with P450 oxidoreductase and cytochrome b5. Mol Endocrinol 13:167–175 [DOI] [PubMed] [Google Scholar]
- Sherbet DP, Tiosano D, Kwist KM, Hochberg Z, Auchus RJ 2003 CYP17 mutation E305G causes isolated 17,20 lyase deficiency by selectively altering substrate binding. J Biol Chem 278:48563–48569 [DOI] [PubMed] [Google Scholar]
- Naffin-Olivos JL, Auchus RJ 2006 Human cytochrome b5 requires residues E48 and E49 to stimulate the 17,20-lyase activity of cytochrome P450c17. Biochemistry 45:755–762 [DOI] [PubMed] [Google Scholar]
- Bridges A, Gruenke L, Chang YT, Vakser IA, Loew G, Waskell L 1998 Identification of the binding site on cytochrome P450 2B4 for cytochrome b5 and cytochrome P450 reductase. J Biol Chem 273:17036–17049 [DOI] [PubMed] [Google Scholar]
- Montminy MR 1997 Transcriptional regulation by cyclic AMP. Annu Rev Biochem 66:807–822 [DOI] [PubMed] [Google Scholar]
- Auchus RJ, Miller WL 1999 Molecular modeling of human P450c17 (17α-hydroxylase/17,20-lyase): Insights into reaction mechanisms and effects of mutations. Mol Endocrinol 13:1169–1182 [DOI] [PubMed] [Google Scholar]
- Taylor SS, Kim C, Vigil D, Haste NM, Yang J, Wu J, Anand GS 2005 Dynamics of signaling by PKA. Biochim Biophys Acta 1754:25–37 [DOI] [PubMed] [Google Scholar]
- Souter I, Munir I, Mallick P, Weitsman SR, Geller DH, Magoffin DA 2006 Mutagenesis of putative serine-threonine phosphorylation sites proximal to Arg255 of human cytochrome P450c17 does not selectively promote its 17,20 lyase activity. Ferti Steril 85(Suppl 1):1290–1299 [DOI] [PubMed] [Google Scholar]
- Biglieri EG, Herron MA, Brust N 1966 17α-Hydroxylation deficiency in man. J Clin Invest 15:1945–1954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auchus RJ 2001 The genetics, pathophysiology, and management of human deficiencies of P450c17. Endocrinol Metab Clin North Am 30:101–119, vii [DOI] [PubMed] [Google Scholar]
- Schiebinger RJ, Albertson BD, Cassorla FG, Bowyer DW, Geelhoed GW, Cutler Jr GB, Loriaux DL 1981 The developmental changes in plasma adrenal androgens during infancy and adrenarche are associated with changing activities of adrenal microsomal 17-hydroxylase and 17,20 desmolase. J Clin Invest 67:1177–1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couch RM, Muller J, Winter JSD 1986 Regulation of the activities of 17α-hydroxylase and 17,20-desmolase in the human adrenal cortex: kinetic analysis and inhibition by endogenous steroids. J Clin Endocrinol Metab 63:613–618 [DOI] [PubMed] [Google Scholar]
- Arlt W, Martens JW, Song M, Wang JT, Auchus RJ, Miller WL 2002 Molecular evolution of adrenarche: structural and functional analysis of P450c17 from four primate species. Endocrinology 143:4665–4672 [DOI] [PubMed] [Google Scholar]
- Nguyen AD, Corbin CJ, Pattison JC, Bird IM, Conley AJ 2009 The developmental increase in adrenocortical 17,20-lyase activity (biochemical adrenarche) is driven primarily by increasing cytochrome b5 in neonatal rhesus macaques. Endocrinology 150:1748–1756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickenheisser JK, Nelson-DeGrave VL, Quinn PG, McAllister JM 2004 Increased cytochrome P450 17α-hydroxylase promoter function in theca cells isolated from patients with polycystic ovary syndrome involves nuclear factor-1. Mol Endocrinol 18:588–605 [DOI] [PubMed] [Google Scholar]
- Wickenheisser JK, Nelson-Degrave VL, McAllister JM 2005 Dysregulation of cytochrome P450 17α-hydroxylase messenger ribonucleic acid stability in theca cells isolated from women with polycystic ovary syndrome. J Clin Endocrinol Metab 90:1720–1727 [DOI] [PubMed] [Google Scholar]
- Cloutier M, Fleury A, Courtemanche J, Ducharme L, Mason JI, LeHoux JG 1997 Characterization of the adrenal cytochrome P450c17 in the hamster, a small animal model for the study of adrenal dehydroepiandrosterone biosynthesis. DNA Cell Biol 16:357–368 [DOI] [PubMed] [Google Scholar]
- Brock BJ, Waterman MR 1999 Biochemical differences between rat and human cytochrome P450c17 support the different steroidogenic needs of these two species. Biochemistry 38:1598–1606 [DOI] [PubMed] [Google Scholar]
- Sakai N, Tanaka M, Adachi S, Miller WL, Nagahama Y 1992 Rainbow trout cytochrome P450c17 (17α-hydroxylase/17,20 lyase) cDNA cloning, enzymatic properties and temporal pattern of ovarian P450c17 mRNA expression during oogenesis. FEBS Lett 301:60–64 [DOI] [PubMed] [Google Scholar]
- Tremblay Y, Fleury A, Beaudoin C, Valée M, Bélanger A 1994 Molecular cloning and expression of guinea pig cytochrome P450c17 cDNA (steroid 17α-hydroxylase/17,20 lyase): tissue distribution, regulation, and substrate specificity of the expressed enzyme. DNA Cell Biol 13:1199–1212 [DOI] [PubMed] [Google Scholar]
- Lin D, Harikrishna JA, Moore CC, Jones KL, Miller WL 1991 Missense mutation Ser106→ Pro causes 17α-hydroxylase deficiency. J Biol Chem 266:15992–15998 [PubMed] [Google Scholar]
- Yanagibashi K, Hall PF 1986 Role of electron transport in the regulation of the lyase activity of C-21 side-chain cleavage P450 from porcine adrenal and testicular microsomes. J Biol Chem 261:8429–8433 [PubMed] [Google Scholar]
- Lin D, Black SM, Nagahama Y, Miller WL 1993 Steroid 17α-hydroxylase and 17,20 lyase activities of P450c17: contributions of serine106 and P450 reductase. Endocrinology 132:2498–2506 [DOI] [PubMed] [Google Scholar]
- Miller WL 2005 Regulation of steroidogenesis by electron transfer. Endocrinology 146:2544–2550 [DOI] [PubMed] [Google Scholar]
- Huang W, Erickson RL 1994 Constitutive activation of Mek1 by mutation of serine phosphorylation sites. Proc Natl Acad Sci USA 91:8960–8963 [DOI] [PMC free article] [PubMed] [Google Scholar]