Abstract
Roux-en-Y gastric bypass (RYGB) surgery is the most effective treatment for morbid obesity and remission of associated type 2 diabetes, but the mechanisms involved are poorly understood. The aim of the present study was to develop and validate a rat model for RYGB surgery that allows repeated measurement of meal-induced changes in gut and pancreatic hormones via chronic venous catheters. Male Sprague Dawley rats made obese on a palatable high-fat diet were subjected to RYGB or sham surgery and compared with chow-fed, lean controls. Hormonal responses to a mixed-liquid test meal were examined by frequent blood sampling through chronically implanted jugular catheters in freely behaving rats, 3–4 months after surgery, when RYGB rats had significantly reduced body weight and fat mass compared with sham-operated rats. Hyperleptinemia, basal hyperinsulinemia, and hyperglycemia as well as postprandial glucose intolerance seen in sham-operated, obese rats were completely reversed by RYGB and no longer different from lean controls. Postprandial increases in glucagon-like peptide-1, peptide YY, and amylin as well as suppression of ghrelin levels were all significantly augmented in RYGB rats compared with both sham-operated obese and lean control rats. Thus, our rat model replicates most of the salient hormonal and glycemic changes reported in obese patients after RYGB, with the addition of amylin to the list of potential candidate hormones involved in hypophagia, weight loss, and remission of diabetes. The model will be useful for elucidating the specific peripheral and central mechanisms involved in the suppression of appetite, loss of body weight, and remission of type 2 diabetes.
A rat model for gastric bypass surgery shows all the clinically relevant beneficial effects seen in successfully treated patients and will thus be useful for elucidating the underlying mechanisms.
The prevalence of overweight and obesity continues to rise, with few effective treatments available. Bariatric surgery is increasingly carried out in morbidly obese patients and is now considered for cases of less severe obesity and nonobese diabetic patients because it produces significant sustained weight loss and because surgery including a gastroduodenal bypass rapidly restores insulin sensitivity and glucose homeostasis, even before significant weight loss (1,2,3). Despite a growing number of clinical and animal studies, the specific mechanisms leading to these beneficial effects remain largely unknown.
Changes in gut hormone secretion and action are suspected to play a major role. A number of clinical studies found exaggerated plasma levels of glucagon-like peptide (GLP)-1 and peptide YY (PYY) after Roux-en-Y gastric bypass (RYGB) surgery (4,5,6,7,8,9,10), gut hormones known to be involved in the control of satiety and glucose homeostasis. In one study, postprandial GLP-1 and PYY responses were correlated to the patients weight loss, and acute inhibition of GLP-1 and PYY secretion by somatostatin infusion resulted in larger meals taken by RYGB patients (6).
RYGB-induced decreases in ghrelin secretion may also contribute to reduced food intake and weight loss (11,12,13), but disparate findings make this explanation more controversial. In several studies, fasted levels of ghrelin were not significantly lower in RYGB compared with obese controls (4,5,7,10), but ingestion of a meal produced a greater suppression of ghrelin levels (5,10).
In rat models, various surgical procedures changing the normal flow of nutrients through the bowel, including RYGB, jejuno-ileal bypass, and ileal transposition generally resulted in similar effects on circulating hormone levels (7,14,15,16,17) but often based on single blood samples taken in the fasted or fed state relatively early after surgery, providing little dynamic information.
The aim of our study was to establish a rat model for RYGB that can be used for more invasive testing of specific mechanistic hypotheses not possible in patient populations. We earlier reported the efficacy of our model to reduce food intake and reverse obesity, and we identified behavioral changes of meal patterning, satiation, and food preference (18). Here we demonstrate the validity of the model regarding changes in basal and meal-induced plasma hormone levels by taking advantage of chronic vascular catheterization and multiplex hormone assays. This allowed simultaneous detection of several hormones in small volumes of plasma, sampled at multiple time points over the course of a mixed meal. In addition to GLP-1, PYY, ghrelin, gastric inhibitory peptide (GIP), insulin, and leptin, we measured plasma amylin levels because this hormone has recently been shown to synergize leptin and PYY signaling, leading to increased satiation, reduced food intake, and body weight and fat loss in obese rat models and humans (20,21,22).
Materials and Methods
Animals
Male Sprague Dawley rats weighing 200 g were purchased from Harlan Industries (Indianapolis, IN) and housed individually in wire-mesh cages at a constant temperature of 21–23 C with a 12-h light, 12-h dark cycle (lights on 0700, off at 1900 h). To render them obese, rats were given access for 14–16 wk to a three-choice cafeteria diet consisting of normal laboratory chow (kilocalorie percent: carbohydrate, 58; fat, 13.5; protein, 28.5, no. 5001; Purina LabDiet, Richmond, IN), high-fat chow (kilocalorie percent: carbohydrate, 20; fat, 60; protein, 20, D12492; Research Diets, New Brunswick, NJ), and chocolate-flavored liquid Ensure (kilocalorie percent: carbohydrate, 64; fat, 21.6; protein, 14.4; Abbott Laboratories, Columbus, OH). After surgery, only Ensure was available for the first 10 d, and a two-choice diet with regular and high-fat chow was available thereafter, except for brief periods when intake of single diets was measured.
All protocols involved in this study were approved by the Institutional Animal Care and Use Committee at the Pennington Biomedical Research Center in accordance with guidelines established by the National Institutes of Health.
Gastric bypass surgery and postoperative care
At the end of the fattening period, rats were randomly assigned to either RYGB or sham surgery. After overnight food deprivation, they were administered an antibiotic (Cefazolin, 50 mg/kg, sc), deeply anesthetized with isoflurane, and the abdominal organs exposed by laparotomy. First, a gastric pouch of approximately 20% of the total stomach volume was divided using a cutting stapler (ATW35; Ethicon Endo-Surgery Inc., Cincinnati, OH) with triple titanium staple lines on either side of the cut. Care was taken not to damage the neural and vascular supply to the esophagogastric junction and the pouch. Second, the jejunum was transected about 40 cm from the ileocecal valve (∼40 cm from the ligament of Treitz), and the distal cut end was anastomosed to the gastric pouch. Third, the proximal cut end of the jejunum was anastomosed to the side of the lower jejunum (∼25 cm from the ileocecal valve). This procedure resulted in an approximately 15-cm-long Roux limb, a 25-cm-long common limb, and a roughly 40-cm-long biliopancreatic limb. All nerves crossing the gastric cut line are obviously transected, leading to partial denervation of the pyloric sphincter, proximal duodenum, and pancreas. In addition, the continuity of the enteric nervous system is interrupted by the jejunal transection. However, the vagal and sympathetic supply to the gastric pouch (gastric branches) and most of the intestines, pancreas, and liver (vagal celiac and hepatic branches) remain intact.
Sham surgery consisted of the same procedure, except that the stapler was laid over only the stomach but not fired, the divided jejunum was sewn back together, and the small stab wound in the gastric fundus was closed with sutures. Rats were given a sc injection of 10 ml saline for replenishment of fluid.
On the day after surgery, rats were given access to a limited amount of chocolate-flavored Ensure, and ad libitum intake of Ensure as the only food source was monitored for the first 10 d after surgery. Ten days after surgery, rats were slowly adapted to eating normal chow and high-fat chow provided in increasing amounts on the cage floor.
Additionally, rats of similar age fed regular chow only and without any surgery served as lean controls. Body weight was monitored daily for the first month, and weekly body weight was recorded afterward. Body composition of animals (percent fat mass, fluid, and lean mass) was also measured before and 9–12 wk after surgery by using a Minispec LF 90 NMR analyzer (Bruker BioSpin Corp., The Woodlands, TX). This method uses whole-body magnetic resonance relaxometry in unanesthetized rodents with excellent linearity and reproducibility (23).
Rats used in this study were operated in different cohorts, and because the jugular catheterization was carried out 3 months after surgery, all RYGB rats were stable and healthy at that time. Of a total of 40 rats with RYGB surgery, eight rats (20%) died of complications, mostly during the operation or within the first 24 h and fewer when switched from Ensure to solid diets. Mortality was higher in the earlier surgeries and there was no mortality in the last 10 rats operated.
Jugular vein catheterization
Three to four months after surgery, rats of all three groups (RYGB, sham obese, and lean controls) were anesthetized with ketamine/xylazine/acepromazine cocktail (80/4.0/1.6 mg/kg, sc), and atropine (1 mg/kg, sc) was administered. Custom-made catheters made from silicone tubing (0.02 in. inner diameter, 0.037 in. outer diameter; Baxter, Deerfield, IL) were inserted into the jugular vein as previously reported (24). The catheter was tunneled under the skin to the top of the head, at which it was connected with a piece of stainless steel tubing and secured to the cranium with three screws and dental cement. In some rats, intraoral fistulas for delivery of taste stimuli directly into the oral cavity (25) were fitted as described earlier, with the external port joining the head piece.
Patency of catheters was checked during surgery by infusing and withdrawing through a 1-ml syringe filled with heparinized sterile saline. Catheters were then filled with heparinized glycerol solution, and the cranial port was capped. Patency of catheters was checked two to three times a week, and heparinized glycerol solution was replaced. Of a total of 19 jugular catheters implanted, two catheters lost patency before blood sampling tests could be performed. For the remaining catheters, the mean duration of patency was at least 2 wk. Jugular catheter implantation was followed by weight loss that was typically recovered within a few days. Tests were conducted when body weight had recovered to within about 5 g of preimplantation body weight.
Meal challenge and blood sampling
After recovery from jugular vein surgery (usually within 1 wk), overnight food-deprived rats were prepared for blood sampling by aspirating the catheter contents, infusing 0.5 ml heparinized saline, and connecting the exterior port with plastic tubing and a 1-ml syringe containing heparinized saline. After a rest period of 30 min, the remote blood sampling and meal-delivery protocol started with the first baseline blood sample. Immediately after the second baseline sample, a mixed meal consisting of 5 ml of chocolate-flavored Ensure was delivered through either the intraoral fistula or a spout in the test cage wall connected to an infusion pump. In either case, Ensure was delivered at a rate of 1 ml/min for 5 min. Additional blood samples (200 μl) were taken at 10, 20, 35, 60, and 90 min after the start of the meal.
Whole blood was transferred from the withdrawal syringe into centrifuge tubes containing a cocktail of serine protease inhibitor (Pefabloc) and protease inhibitor cocktail, both from Sigma (St. Louis. MO), and dipeptidyl-peptidase-IV inhibitor (Millipore, St. Charles, MO), to prevent degradation of hormone peptides, including deacylation of ghrelin. Tubes were spun at 2000 rpm for 10 min and plasma was collected and stored at −80 C.
Plasma hormone and glucose assays
Plasma insulin, leptin, acylated ghrelin, GIP, active amylin, and total PYY were measured on duplicate samples using the Milliplex rat gut hormone panel with Luminex xMAP technology (Millipore; Luminex Corp., Austin, TX). This assay allows simultaneous measurement of up to seven hormones with the same 25 μl plasma sample. Sensitivity for each hormone as indicated by the manufacturer was as follows: leptin (27 pg/ml), insulin (28 pg/ml); GIP (1 pg/ml); acylated ghrelin (2 pg/ml); PYY (16 pg/ml); and active amylin (20 pg/ml). Intraassay coefficient of variation was less than 7% and interassay variability was less than 24%.
Active GLP-1 was initially determined in the multiplex assay, but the baseline samples were below detection threshold. Therefore, GLP-1 was subsequently detected in a separate fluorescent ELISA (Millipore). Samples (100 μl) were diluted 1:2 with assay buffer as recommended, and the diluted samples were run in duplicates along with quality controls provided by the manufacturer. Sensitivity was 6.6 pg/ml, intraassay coefficient of variation was 6.7%, and interassay coefficient of variation was 4.0%. According to the manufacturer, all antisera are highly specific and display insignificant cross-reactivity to the other analytes within the panel.
Plasma glucose was analyzed in duplicate by commercially available Accu-Chek glucometer (Roche, Madison, WI), with an intraassay coefficient of variation of 3.4%.
Statistical analysis
Body composition data were analyzed by one-way ANOVA followed by Fisher’s least significant differences (LSD) post hoc test. Two-way repeated-measures ANOVA followed by Fisher’s LSD post hoc test was used to evaluate mean differences in body weight between groups across time and also changes in glucose and hormone responses between groups across time before and after a liquid meal challenge. Data are expressed as mean ± sem. Values for the area under the curve for glucose and hormones were calculated using the trapezoidal method, and one-way ANOVA followed by Fisher’s LSD post hoc test was used to evaluate the mean differences between groups. Homeostasis model assessment was used as an index of insulin sensitivity (26), and the mean difference between groups was analyzed by one-way ANOVA followed by Fisher’s LSD post hoc test. Simple regression analysis was performed to assess correlations between different hormones and body weight.
Results
RYGB leads to sustained body weight loss and reversal of obesity
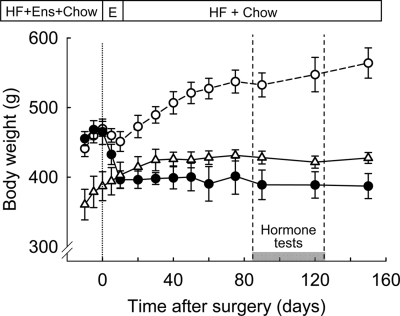
As previously reported, our rat model is characterized by a sustained effect on body weight that lasts at least 5 months (18) and contrasts with significant body weight gain during this period in sham-operated rats exposed to the same high-fat diet (Fig. 1). Body weight of our lean control rats was similar to the RYGB rats during the hormone challenge test period.
Figure 1.
Body weight of high-fat exposed RYGB (filled circles, n = 5) and sham-operated (open circles, n = 6) as well as chow-fed control rats (open triangles, n = 6). Availability of high-fat diet (HF), Ensure (E), and regular chow (chow) is indicated on top. The period of blood sampling and hormone tests is indicated by the gray bar.
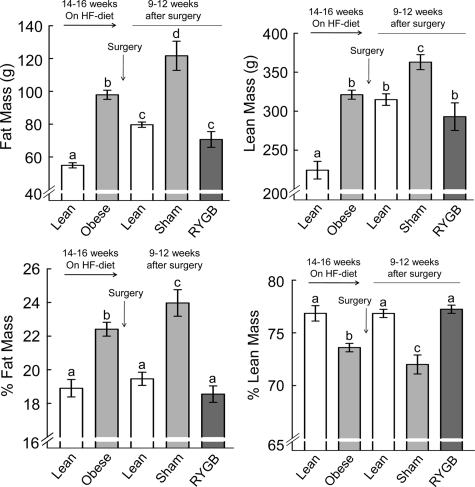
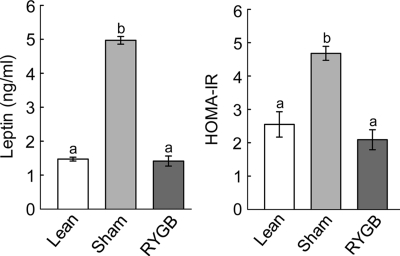
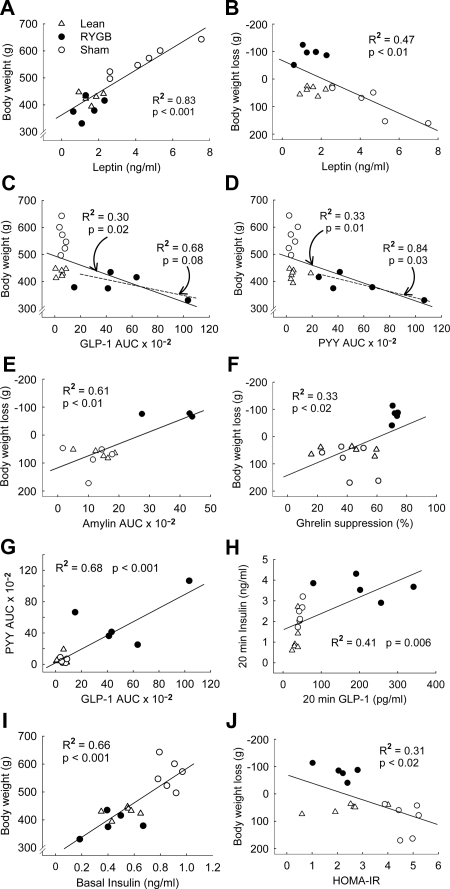
Measurement of body composition before surgery showed that the high-fat diet significantly and substantially increased fat mass and adiposity and decreased percent lean mass (P < 0.05; Fig. 2). Fat mass, adiposity, lean mass, and percent lean mass were matched and therefore identical for animals designated as RYGB or sham surgery (Fig. 2). Measurement of body composition 3 months after surgery, just before implantation of the chronic catheters, indicated that most of excess fat mass was lost after RYGB but that sham-operated rats continued to accumulate fat (Fig. 2). Fat mass of RYGB rats at 3 months after surgery was similar to weight-matched lean controls, and adiposity (percent fat mass) was not different from the level before exposure to the diet (Fig. 2). Fat mass at the time of testing was reflected in plasma leptin levels, with sham-operated/obese rats showing a 4-fold, significantly higher level compared with both RYGB and lean controls (P < 0.01; Fig. 3). If expressed per unit fat mass, leptin levels were still about 50% lower in RYGB and lean control rats, compared with sham-operated, obese rats (see Figs. 2 and 3). Furthermore, leptin levels showed significant positive correlations with both body weight (R2 = 0.83, P < 0.001) and body weight change (R2 = 0.47; P < 0.01) if all treatment groups were considered (see Fig. 7, A and B). There were no significant correlations if only the five RYGB rats were considered.
Figure 2.
Body composition as assessed by whole-body magnetic resonance relaxometry, showing absolute (upper panels) and relative (lower panels) fat mass (left panels) and lean mass (right panels). The left two bars in each panel show values before exposure to high-fat diet (white bars) and before surgery (light gray bars). Obese rats were then matched for body weight and adiposity and assigned for RYGB or sham surgery. The right three bars in each panel show values 9–12 wk after surgery for RYGB rats (dark gray bars), sham-operated (obese) rats (light gray bars), and nonoperated, age-matched, chow-fed lean rats (white bars). Bars that do not share a common letter are significantly different from each other (P < 0.05; based on ANOVA followed by Fisher’s LSD post hoc test).
Figure 3.
Left panel, Fasting plasma leptin levels in RYGB (dark gray bars), sham-operated obese rats (light gray bars), and age-matched chow-fed, lean controls (white bars) at 3 months after surgery. Right panel, HOMA-IR calculated from fasting insulin and glucose levels in Fig. 5. Bars that do not share a common letter are significantly (P < 0.05) different from each other (based on ANOVA followed by Fisher’s LSD post hoc test).
Figure 7.
Linear regression analysis of relationships between body weight or weight loss with various circulating hormones or postprandial hormone responses. Correlations included either all treatment groups (lean controls, open triangles; sham-operated obese, open circles; RYGB, filled circles; full regression lines) or only RYGB rats (dotted regression lines), and only significant correlations are shown. A and B, Leptin levels are positively correlated to body weight and negatively to body weight loss using all treatment groups. C and D, Areas under the curve (AUC) for GLP-1 and PYY are negatively correlated to body weight across all treatment groups and for RYGB rats only. E and F, AUC for amylin and percent suppression of acylated ghrelin are positively correlated with body weight loss. G, GLP-1 and PYY AUCs are positively correlated. H, Twenty-minute peaks of GLP-1 and insulin are positively correlated. I, Basal insulin is positively correlated with body weight. J, HOMA-IR is negatively correlated with body weight loss.
Lean mass of RYGB rats was significantly lower compared with sham-operated rats (P < 0.05), but the effect was mainly due to an increase of lean mass in sham-operated rats after surgery. Both absolute and relative lean mass of RYGB rats at the time of testing was not significantly different from presurgical levels and from chow-fed lean control rats (Fig. 2).
Effects of RYGB on food intake, food choice, and energy expenditure were reported in an earlier study (18) that included some of the same rats used in the present study. The initially greatly suppressed food intake recovered after the third postoperative week to a more moderate suppression of about 15–25% compared with sham-operated controls (18). Furthermore, RYGB rats gradually decreased preference for high fat and consumed almost 50% of energy from regular chow, whereas sham-operated rats continued to highly prefer a high-fat diet (18).
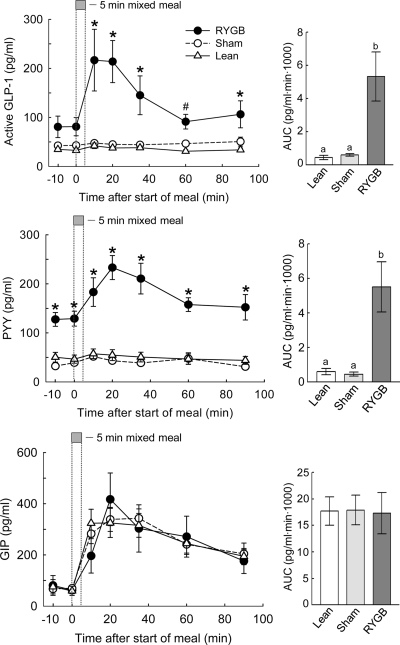
RYGB leads to increased basal and/or meal-stimulated GLP-1, PYY, and amylin plasma concentrations but does not affect GIP levels
In the overnight fasted state, PYY plasma levels were almost 3-fold higher in RYGB compared with both sham-operated/obese and lean controls (P < 0.05; Fig. 4). Basal GLP-1 levels were almost twice as high in RYGB compared with both sham/obese and lean controls, but this difference just missed statistical significance vs. lean rats (P = 0.07) and was not significant vs. sham/obese rats. Basal GIP (Fig. 4) and amylin (Fig. 5) levels were identical for the three groups.
Figure 4.
GLP-1, PYY, and GIP responses to a mixed meal in RYGB (filled circles), sham-operated, obese rats (open circles), and chow-fed lean controls (open triangles). Overnight food-deprived rats consumed 5 ml (∼5 kcal) of Ensure delivered at 1 ml/min, and jugular vein blood was sampled remotely at the times indicated. *, RYGB significantly (P < 0.05) different compared with both sham-operated/obese and lean controls; #, RYGB significantly (P < 0.05) different compared with lean controls. Areas under the curve (AUC) are shown in the bar graphs at the right, with bars that do not share a common letter significantly (P < 0.05) different from each other.
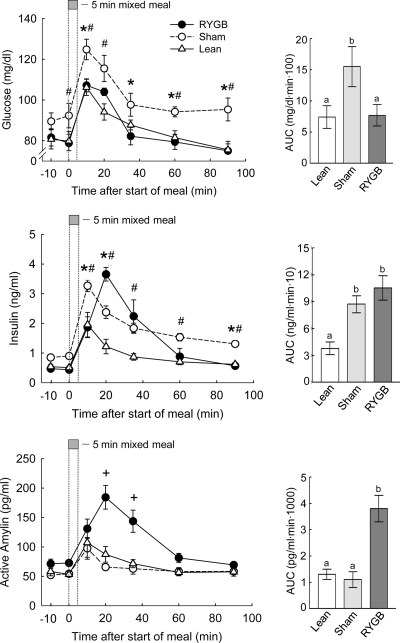
Figure 5.
Insulin, glucose, and amylin responses to a mixed meal in RYGB (filled circles), sham-operated/obese rats (open circles), and chow-fed, lean controls (open triangles). Overnight food-deprived rats consumed 5 ml (∼5 kcal) of Ensure delivered at 1 ml/min, and jugular vein blood was sampled remotely at the times indicated. *, P < 0.05 between sham and RYGB rats; #, P < 0.05 between sham and lean control rats; +, P < 0.05 between RYGB and both other groups. Areas under the curve (AUC) are shown in the bar graphs at the right, with bars that do not share a common letter significantly (P < 0.05) different from each other.
In response to a mixed meal of palatable Ensure, chow-fed lean controls and sham-operated obese rats showed modest but significant peak postprandial increases at 10 min in GLP-1 (+34% and +22%; P < 0.05) and PYY (+25% and +32%; P < 0.05; Fig. 4). Amylin was also significantly increased in lean (+100%; P < 0.05) and obese (+80%; P < 0.05) rats at 10 min (Fig. 5). In contrast, RYGB rats had much higher and persistent postprandial GLP-1, PYY, and amylin responses compared with both sham/obese and lean controls (see Figs. 4 and 5). Ninety minutes after the meal, all three hormones returned to their respective baseline, significantly higher for GLP-1 and PYY than in the control groups (P < 0.05). Areas under the curve for both hormones were about 6-fold higher in RYGB compared with both control groups (P < 0.05). The GIP response to the mixed meal did not differ among the three groups.
Regression analysis using all treatment groups (n = 17) further revealed that areas under the curve for GLP-1, PYY, and amylin showed significant negative correlations with body weight or body weight change (all Ps < 0.05; see Fig. 7, C–E) and significant positive correlations with body weight loss (all Ps < 0.05). If regression analysis was limited to RYGB animals (n = 5), only the area under the curve for PYY correlation with body weight was significant (P < 0.05), and the one for GLP-1 was marginally significant (P = 0.08; see Fig. 7, C and D). GIP (area under the curve) did not correlate with either body weight or body weight change and whether all or only RYGB animals were considered.
Furthermore, the area under the curve for GLP-1 was significantly positively correlated with the area under the curve for PYY (P < 0.05; see Fig. 7G), and peak GLP-1 levels were significantly positively correlated with peak insulin levels at 20 min when considering all treatment groups (P < 0.05; see Fig. 7H).
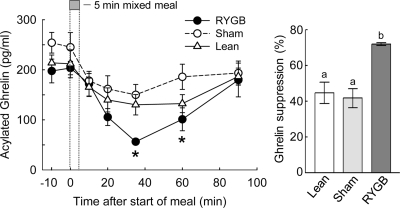
RYGB leads to greater meal-induced suppression of plasma ghrelin levels
Basal ghrelin levels tended to be higher in sham-operated, obese rats compared with RYGB rats, but the difference did not reach statistical significance (P = 0.15; Fig. 6). Upon meal ingestion, ghrelin levels declined for approximately 40 min, with the relative suppression being significantly greater in RYGB compared with both control groups (P < 0.05). Ninety minutes after the meal, ghrelin levels had almost returned to baseline levels. Regression analysis using all treatment groups (n = 17) revealed that maximal percent ghrelin suppression was significantly negatively correlated with body weight change (P < 0.05; see Fig. 7F) but not with absolute body weight. None of these correlations was significant when only the five RYGB animals were considered.
Figure 6.
Ghrelin responses to a mixed meal in RYGB (filled circles), sham-operated (obese) rats (open circles), and nonoperated, chow-fed lean controls (open triangles). Overnight food-deprived rats consumed 5 ml (∼5 kcal) of Ensure delivered at 1 ml/min, and jugular vein blood was sampled remotely at the times indicated. *, RYGB significantly (P < 0.05) different compared with sham-operated rats. Areas under the curve (AUC) are shown in the bar graph at the right, with bars that do not share a common letter significantly (P < 0.05) different from each other.
RYGB reverses glucose intolerance seen in sham/obese rats and improves insulin sensitivity
Basal plasma glucose levels were slightly higher in sham-operated, obese rats compared with both RYGB and lean controls, but the difference was statistically significant only compared with lean controls (P < 0.05; Fig. 5). Meal-stimulated glucose levels were generally higher in obese rats compared with both RYGB and lean rats. Compared with RYGB rats, this difference was significant at 10, 35, 60, and 90 min after the meal (P < 0.05). The area under the curve for plasma glucose was 2-fold, significantly higher in obese compared with RYGB and lean rats, which did not differ from each other (P < 0.05).
Similar to glucose, basal plasma insulin levels tended to be higher in obese compared with RYGB and lean rats, but the difference was not statistically significant (Fig. 5). Meal-stimulated insulin levels were lowest in lean controls. Whereas the early insulin response at 10 and 20 min was similar in RYGB and sham-operated obese rats, insulin levels at 60 and 90 min were significantly lower in RYGB rats (P < 0.05). The area under the curve for plasma insulin was about 2.5-fold higher in RYGB and obese compared with lean rats (P < 0.05). Basal insulin levels correlated significantly with body weight (P < 0.001; see Fig. 7I).
Computation of the homeostasis model assessment index for insulin resistance (HOMA-IR) showed that sham-operated, obese rats were significantly more insulin resistant than both RYGB and lean controls (P < 0.05; Fig. 3). If analyzed across all treatment groups, HOMA-IR significantly correlated with body weight change (R2 = 0.31; P < 0.02; Fig. 7J) and absolute body weight (R2 = 0.55; P < 0.001). However, none of the correlations were significant if only the five RYGB rats were considered.
Discussion
This is the first rat model of RYGB providing a comprehensive analysis of basal and meal stimulated hormone levels. Earlier studies in rats were limited by looking at single time points and/or by the breadth of hormone measurements or by stressful blood sampling and meal delivery techniques (7,14,15,16,17). Using remote blood sampling in undisturbed rats through chronic jugular catheters and multiplex hormone assays, we were able to determine both basal levels and the time course of meal-induced multiple hormonal responses with the same voluntarily ingested test meal delivered at a constant rate and duration.
Approximately 3 months after surgery, when RYGB rats had lost excess fat mass and leptin levels returned to lean control levels, mixed meal-induced plasma GLP-1 and PYY responses were greatly enhanced. Similarly exaggerated GLP-1 (7,9,27,28,29) and PYY (5,7,9,28,29) responses to a mixed meal or oral glucose (10) ingestion have been reported in RYGB patients 6 wk to 48 months after surgery. These two hormones, secreted from intestinal L cells, have been the leading candidates for mediation of the beneficial effects of RYGB on food intake, body weight, and glucose homeostasis.
Considerable evidence suggests that GLP-1 released from intestinal L cells suppresses food intake. Peripheral administration of GLP-1 or its stable analog exendin-4 decreases hunger and suppresses food intake in humans (30) and rodents (31), and administration of the GLP-1 receptor antagonist exendin 9–39 stimulates food intake in rats (32,33). However, mechanistic analysis of GLP’s effects on food intake is complicated by the fact that GLP-1 is also produced in the brain in which it acts as a neurotransmitter/modulator. This makes proof of origin of the natural ligand for central GLP-1-induced signaling difficult. In addition to suppressing food intake, GLP-1 also improves glucose homeostasis by controlling gastric emptying, hepatic glucose production, and insulin secretion (for a review see Ref. 34).
In a mouse model of duodeno-jejunal bypass surgery with an intact stomach, peripheral infusion of exendin 9–39 prevented the increased insulin secretion during an oral glucose tolerance test, but not insulin sensitivity, and only minimally rescued surgery-induced hypophagia, suggesting that increased GLP-1 levels might account for the enhancement of insulin secretion but play a minor role in hypophagia after the bypass procedure (35).
PYY is released from the same intestinal L cells and rapidly truncated to PYY (3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36) by dipeptidyl peptidase IV. Peripheral administration of PYY(3–36) suppresses food intake in humans and rodents (36,37), and PYY-deficient mice have an obese phenotype (38). Thus, increased meal-stimulated levels of PYY may be partly responsible for reduced food intake after RYGB.
In our rat model, basal and postprandial PYY levels were not different between obese and lean rats but were significantly elevated almost 3-fold 4 months after RYGB. The literature is controversial regarding fasting and meal-stimulated PYY levels in obese subjects. Some studies find that obese children (39) and adults (40) have significantly lower basal and meal-stimulated circulating PYY levels. Children with the lowest fasting levels of PYY were the most likely to succeed in a 1-yr weight loss program, after which PYY levels were restored to higher levels found in lean children (39). Together with reduced satiety in the obese and the fact that sensitivity to exogenous PYY remained intact (40), these findings suggest that low PYY levels might be a major factor contributing to the development of obesity. However, similar to our results in rats, other clinical studies did not report differences in fasted and meal-stimulated PYY levels between obese and lean subjects (5,27,41). Some of the discrepant outcomes may be explained by assays detecting either total PYY (39) or selectively PYY(3–36) (5,41). Nevertheless, our finding of greatly increased fasting and meal-stimulated PYY levels after RYGB agree with clinical studies and confirm PYY as a major candidate for the beneficial effects of RYGB.
Amylin, a hormone cosecreted with insulin from pancreatic β-cells and strongly implicated in the control of food intake, was significantly increased postprandially in our RYGB rats. Systemic administration of amylin suppresses food intake and chronic infusion attenuates the development of diet-induced obesity in rodents [(42) and see Ref. 43 for a recent review]. Furthermore, preclinical and clinical studies suggest that amylin agonism restores leptin responsiveness in diet-induced obesity (22) and enhances the anorexic actions of PYY(3–36) (21). Thus, amylin could play an important role in RYGB-induced anorexia and weight loss by its own effects on satiation by synergizing with elevated PYY levels and restoring leptin sensitivity in the face of rapidly decreasing circulating leptin levels.
To date, ghrelin is the only orexigenic gut hormone identified, and decreased ghrelin signaling after RYGB is another potential candidate mechanism for hypophagia and weight loss. Although we did not detect decreases in fasting ghrelin levels compared with both sham-operated obese and lean control rats, the postprandial suppression of plasma ghrelin levels was significantly greater after RYGB. Furthermore, the expected counterregulatory increase of ghrelin levels caused by hypophagia and weight loss after RYGB was not observed. Decreased ghrelin signaling was the first hormonal mechanism implicated in the beneficial weight-loss effects of RYGB because it was initially observed that RYGB patients had substantially reduced plasma ghrelin levels throughout the diurnal cycle despite weight loss compared with diet-induced increases of ghrelin levels in control subjects (11). This interpretation was supported by other clinical studies (4,44,45) and the observation that postoperative weight loss is correlated with the magnitude of the decrease in circulating ghrelin levels in a rat model for RYGB (46). Several other clinical studies did not find significantly decreased fasting ghrelin levels after RYGB compared with untreated obese subjects (5,10,13,47), but in some of these studies, meal-induced suppression of ghrelin was enhanced (5,10). In a recent prospective study, fasting and postprandial ghrelin did show small decreases at 26 and 52 wk after RYGB, but these changes were not statistically significant (48). Thus, although the effects of RYGB on ghrelin are highly variable, a relative ghrelin deficiency compared with the expected rise due to hypophagia and weight loss appears to be a common observation, keeping ghrelin on the list of major potential candidate mechanisms for the beneficial effects on weight loss after RYGB.
One of the hallmarks of RYGB is the rapid resolution of diabetes, which appears to be at least partially independent of weight loss (1,2,3), but the mechanisms involved have been elusive (for an in-depth discussion, see Ref. 49). Rubino and colleagues (50,51) demonstrated the usefulness of rat models by providing strong evidence for the involvement of an antiincretin factor released from the upper intestine on luminal contact with nutrients. The upper intestinal hypothesis suggests that after RYGB less of this hypothetical antiincretin is released because nutrients are no longer in contact with the upper intestine, resulting in improved insulin secretion. In contrast, the lower intestinal hypothesis suggests that the accelerated delivery of food to the Roux limb leads to exaggerated secretion of GLP-1, which strongly stimulates insulin secretion (19). It seems clear that the improvement of glycemic control depends on not only increased insulin secretion but also equally on heightened insulin sensitivity. However, the responsible mechanisms are not well understood.
Although our diet-induced obese rats were not diabetic, their fasting glucose levels were slightly but significantly elevated and postprandial glucose and insulin areas under the curve as well as HOMA-IR were about 2-fold higher compared with lean rats. This prediabetic state was fully prevented or reversed 4 months after RYGB. The increased early insulin response to a meal is consistent with a role for elevated GLP-1 after RYGB, but additional experiments with measurements of meal-induced hormone profiles at earlier time points after surgery will be necessary to sort out contributions of weight loss-dependent and -independent effects on insulin secretion and insulin sensitivity.
Our study has several limitations. Hormone responses were measured only at one postsurgical time point and with a specific type and amount of food. Longitudinal studies with measurements before and at several time points after surgery as well as different meal size and composition will be necessary to obtain a more complete picture of RYGB-induced changes in circulating hormones. The study relied on relatively small numbers of animals, preventing meaningful conclusions regarding correlations between RYGB-induced weight loss and basal hormone levels and postprandial responses. Furthermore, although we have characterized food intake, food choice, and energy expenditure during the first 3 months after surgery in a similar cohort of animals (18), we have not measured food intake and energy expenditure during the hormone measurement period.
In conclusion, we have demonstrated the validity of a rat model of RYGB that should be useful for further testing-specific mechanistic hypotheses explaining hypophagia, weight loss, and remission of obesity comorbidities.
Acknowledgments
The technical support of Laurie Wallace and Elaine de Heuval is gratefully acknowledged.
Footnotes
This work was supported by National Institutes of Health Grants DK47348 and DK071082 (to H.-R.B.) and the Alberta Children’s Hospital Professorship in Pediatric Surgical Research (to D.L.S.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online February 23, 2010
Abbreviations: GIP, Gastric inhibitory peptide; GLP, glucagon-like peptide; HOMA-IR, homeostasis model assessment index for insulin resistance; LSD, least significant differences; PYY, peptide YY; RYGB, Roux-en-Y gastric bypass.
References
- Schauer PR, Burguera B, Ikramuddin S, Cottam D, Gourash W, Hamad G, Eid GM, Mattar S, Ramanathan R, Barinas-Mitchel E, Rao RH, Kuller L, Kelley D 2003 Effect of laparoscopic Roux-en Y gastric bypass on type 2 diabetes mellitus. Ann Surg 238:467–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickremesekera K, Miller G, Naotunne TD, Knowles G, Stubbs RS 2005 Loss of insulin resistance after Roux-en-Y gastric bypass surgery: a time course study. Obes Surg 15:474–481 [DOI] [PubMed] [Google Scholar]
- Ali MR, Fuller WD, Rasmussen J 2009 Detailed description of early response of metabolic syndrome after laparoscopic Roux-en-Y gastric bypass. Surg Obes Relat Dis 5:346–351 [DOI] [PubMed] [Google Scholar]
- Korner J, Bessler M, Cirilo LJ, Conwell IM, Daud A, Restuccia NL, Wardlaw SL 2005 Effects of Roux-en-Y gastric bypass surgery on fasting and postprandial concentrations of plasma ghrelin, peptide YY, and insulin. J Clin Endocrinol Metab 90:359–365 [DOI] [PubMed] [Google Scholar]
- Korner J, Inabnet W, Conwell IM, Taveras C, Daud A, Olivero-Rivera L, Restuccia NL, Bessler M 2006 Differential effects of gastric bypass and banding on circulating gut hormone and leptin levels. Obesity (Silver Spring) 14:1553–1561 [DOI] [PubMed] [Google Scholar]
- le Roux CW, Welbourn R, Werling M, Osborne A, Kokkinos A, Laurenius A, Lönroth H, Fändriks L, Ghatei MA, Bloom SR, Olbers T 2007 Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Ann Surg 246:780–785 [DOI] [PubMed] [Google Scholar]
- le Roux CW, Aylwin SJ, Batterham RL, Borg CM, Coyle F, Prasad V, Shurey S, Ghatei MA, Patel AG, Bloom SR 2006 Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Ann Surg 243:108–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg CM, le Roux CW, Ghatei MA, Bloom SR, Patel AG, Aylwin SJ 2006 Progressive rise in gut hormone levels after Roux-en-Y gastric bypass suggests gut adaptation and explains altered satiety. Br J Surg 93:210–215 [DOI] [PubMed] [Google Scholar]
- Morínigo R, Moizé V, Musri M, Lacy AM, Navarro S, Marín JL, Delgado S, Casamitjana R, Vidal J 2006 Glucagon-like peptide-1, peptide YY, hunger, and satiety after gastric bypass surgery in morbidly obese subjects. J Clin Endocrinol Metab 91:1735–1740 [DOI] [PubMed] [Google Scholar]
- Rodieux F, Giusti V, D'Alessio DA, Suter M, Tappy L 2008 Effects of gastric bypass and gastric banding on glucose kinetics and gut hormone release. Obesity (Silver Spring) 16:298–305 [DOI] [PubMed] [Google Scholar]
- Cummings DE, Weigle DS, Frayo RS, Breen PA, Ma MK, Dellinger EP, Purnell JQ 2002 Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med 346:1623–1630 [DOI] [PubMed] [Google Scholar]
- Frühbeck G, Diez-Caballero A, Gil MJ, Montero I, Gómez-Ambrosi J, Salvador J, Cienfuegos JA 2004 The decrease in plasma ghrelin concentrations following bariatric surgery depends on the functional integrity of the fundus. Obes Surg 14:606–612 [DOI] [PubMed] [Google Scholar]
- Stoeckli R, Chanda R, Langer I, Keller U 2004 Changes of body weight and plasma ghrelin levels after gastric banding and gastric bypass. Obes Res 12:346–350 [DOI] [PubMed] [Google Scholar]
- Meirelles K, Ahmed T, Culnan DM, Lynch CJ, Lang CH, Cooney RN 2009 Mechanisms of glucose homeostasis after Roux-en-Y gastric bypass surgery in the obese, insulin-resistant Zucker rat. Ann Surg 249:277–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strader AD, Vahl TP, Jandacek RJ, Woods SC, D'Alessio DA, Seeley RJ 2005 Weight loss through ileal transposition is accompanied by increased ileal hormone secretion and synthesis in rats. Am J Physiol Endocrinol Metab 288:E447–E453 [DOI] [PubMed] [Google Scholar]
- Suzuki S, Ramos EJ, Goncalves CG, Chen C, Meguid MM 2005 Changes in GI hormones and their effect on gastric emptying and transit times after Roux-en-Y gastric bypass in rat model. Surgery 138:283–290 [DOI] [PubMed] [Google Scholar]
- Guijarro A, Osei-Hyiaman D, Harvey-White J, Kunos G, Suzuki S, Nadtochiy S, Brookes PS, Meguid MM 2008 Sustained weight loss after Roux-en-Y gastric bypass is characterized by down regulation of endocannabinoids and mitochondrial function. Ann Surg 247:779–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Shin AC, Lenard NR, Townsend RL, Patterson LM, Sigalet DL, Berthoud HR 2009 Meal patterns, satiety, and food choice in a rat model of Roux-en-Y gastric bypass surgery. Am J Physiol Regul Integr Comp Physiol 297:R1273–R1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holst JJ 1994 Glucagonlike peptide 1: a newly discovered gastrointestinal hormone. Gastroenterology 107:1848–1855 [DOI] [PubMed] [Google Scholar]
- Trevaskis JL, Coffey T, Cole R, Lei C, Wittmer C, Walsh B, Weyer C, Koda J, Baron AD, Parkes DG, Roth JD 2008 Amylin-mediated restoration of leptin responsiveness in diet-induced obesity: magnitude and mechanisms. Endocrinology 149:5679–5687 [DOI] [PubMed] [Google Scholar]
- Roth JD, Coffey T, Jodka CM, Maier H, Athanacio JR, Mack CM, Weyer C, Parkes DG 2007 Combination therapy with amylin and peptide YY[3–36] in obese rodents: anorexigenic synergy and weight loss additivity. Endocrinology 148:6054–6061 [DOI] [PubMed] [Google Scholar]
- Roth JD, Roland BL, Cole RL, Trevaskis JL, Weyer C, Koda JE, Anderson CM, Parkes DG, Baron AD 2008 Leptin responsiveness restored by amylin agonism in diet-induced obesity: evidence from nonclinical and clinical studies. Proc Natl Acad Sci USA 105:7257–7262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Künnecke B, Verry P, Bénardeau A, von Kienlin M 2004 Quantitative body composition analysis in awake mice and rats by magnetic resonance relaxometry. Obes Res 12:1604–1615 [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Trimble ER, Siegel EG, Bereiter DA, Jeanrenaud B 1980 Cephalic-phase insulin secretion in normal and pancreatic islet-transplanted rats. Am J Physiol 238:E336–E340 [DOI] [PubMed] [Google Scholar]
- Wyvell CL, Berridge KC 2000 Intra-accumbens amphetamine increases the conditioned incentive salience of sucrose reward: enhancement of reward “wanting” without enhanced “liking” or response reinforcement. J Neurosci 20:8122–8130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuki A, Sumida Y, Gabazza EC, Murashima S, Furuta M, Araki-Sasaki R, Hori Y, Yano Y, Adachi Y 2001 Homeostasis model assessment is a reliable indicator of insulin resistance during follow-up of patients with type 2 diabetes. Diabetes Care 24:362–365 [DOI] [PubMed] [Google Scholar]
- Korner J, Bessler M, Inabnet W, Taveras C, Holst JJ 2007 Exaggerated glucagon-like peptide-1 and blunted glucose-dependent insulinotropic peptide secretion are associated with Roux-en-Y gastric bypass but not adjustable gastric banding. Surg Obes Relat Dis 3:597–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laferrère B, Teixeira J, McGinty J, Tran H, Egger JR, Colarusso A, Kovack B, Bawa B, Koshy N, Lee H, Yapp K, Olivan B 2008 Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. J Clin Endocrinol Metab 93:2479–2485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laferrère B, Heshka S, Wang K, Khan Y, McGinty J, Teixeira J, Hart AB, Olivan B 2007 Incretin levels and effect are markedly enhanced 1 month after Roux-en-Y gastric bypass surgery in obese patients with type 2 diabetes. Diabetes Care 30:1709–1716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toft-Nielsen MB, Madsbad S, Holst JJ 1999 Continuous subcutaneous infusion of glucagon-like peptide 1 lowers plasma glucose and reduces appetite in type 2 diabetic patients. Diabetes Care 22:1137–1143 [DOI] [PubMed] [Google Scholar]
- Chelikani PK, Haver AC, Reidelberger RD 2005 Intravenous infusion of glucagon-like peptide-1 potently inhibits food intake, sham feeding, and gastric emptying in rats. Am J Physiol Regul Integr Comp Physiol 288:R1695–R1706 [DOI] [PubMed] [Google Scholar]
- Rüttimann EB, Arnold M, Hillebrand JJ, Geary N, Langhans W 2009 Intrameal hepatic portal and intraperitoneal infusions of glucagon-like peptide-1 reduce spontaneous meal size in the rat via different mechanisms. Endocrinology 150:1174–1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott CR, Monteiro M, Small CJ, Sajedi A, Smith KL, Parkinson JR, Ghatei MA, Bloom SR 2005 The inhibitory effects of peripheral administration of peptide YY(3–36) and glucagon-like peptide-1 on food intake are attenuated by ablation of the vagal-brainstem-hypothalamic pathway. Brain Res 1044:127–131 [DOI] [PubMed] [Google Scholar]
- Holst JJ 2007 The physiology of glucagon-like peptide 1. Physiol Rev 87:1409–1439 [DOI] [PubMed] [Google Scholar]
- Troy S, Soty M, Ribeiro L, Laval L, Migrenne S, Fioramonti X, Pillot B, Fauveau V, Aubert R, Viollet B, Foretz M, Leclerc J, Duchampt A, Zitoun C, Thorens B, Magnan C, Mithieux G, Andreelli F 2008 Intestinal gluconeogenesis is a key factor for early metabolic changes after gastric bypass but not after gastric lap-band in mice. Cell Metab 8:201–211 [DOI] [PubMed] [Google Scholar]
- Chelikani PK, Haver AC, Reidelberger RD 2007 Intermittent intraperitoneal infusion of peptide YY(3–36) reduces daily food intake and adiposity in obese rats. Am J Physiol Regul Integr Comp Physiol 293:R39–R46 [DOI] [PubMed] [Google Scholar]
- Talsania T, Anini Y, Siu S, Drucker DJ, Brubaker PL 2005 Peripheral exendin-4 and peptide YY(3–36) synergistically reduce food intake through different mechanisms in mice. Endocrinology 146:3748–3756 [DOI] [PubMed] [Google Scholar]
- Batterham RL, Heffron H, Kapoor S, Chivers JE, Chandarana K, Herzog H, Le Roux CW, Thomas EL, Bell JD, Withers DJ 2006 Critical role for peptide YY in protein-mediated satiation and body-weight regulation. Cell Metab 4:223–233 [DOI] [PubMed] [Google Scholar]
- Roth CL, Enriori PJ, Harz K, Woelfle J, Cowley MA, Reinehr T 2005 Peptide YY is a regulator of energy homeostasis in obese children before and after weight loss. J Clin Endocrinol Metab 90:6386–6391 [DOI] [PubMed] [Google Scholar]
- le Roux CW, Batterham RL, Aylwin SJ, Patterson M, Borg CM, Wynne KJ, Kent A, Vincent RP, Gardiner J, Ghatei MA, Bloom SR 2006 Attenuated peptide YY release in obese subjects is associated with reduced satiety. Endocrinology 147:3–8 [DOI] [PubMed] [Google Scholar]
- Holdstock C, Zethelius B, Sundbom M, Karlsson FA, Eden Engstrom B 2008 Postprandial changes in gut regulatory peptides in gastric bypass patients. Int J Obes (Lond) 32:1640–1646 [DOI] [PubMed] [Google Scholar]
- Mack C, Wilson J, Athanacio J, Reynolds J, Laugero K, Guss S, Vu C, Roth J, Parkes D 2007 Pharmacological actions of the peptide hormone amylin in the long-term regulation of food intake, food preference, and body weight. Am J Physiol Regul Integr Comp Physiol 293:R1855–R1863 [DOI] [PubMed] [Google Scholar]
- Lutz TA 2009 Control of food intake and energy expenditure by amylin-therapeutic implications. Int J Obes (Lond) 33(Suppl 1):S24–S27 [DOI] [PubMed] [Google Scholar]
- Frühbeck G, Diez Caballero A, Gil MJ 2004 Fundus functionality and ghrelin concentrations after bariatric surgery. N Engl J Med 350:308–309 [DOI] [PubMed] [Google Scholar]
- Tritos NA, Mun E, Bertkau A, Grayson R, Maratos-Flier E, Goldfine A 2003 Serum ghrelin levels in response to glucose load in obese subjects post-gastric bypass surgery. Obes Res 11:919–924 [DOI] [PubMed] [Google Scholar]
- Stylopoulos N, Davis P, Pettit JD, Rattner DW, Kaplan LM 2005 Changes in serum ghrelin predict weight loss after Roux-en-Y gastric bypass in rats. Surg Endosc 19:942–946 [DOI] [PubMed] [Google Scholar]
- Holdstock C, Engström BE, Ohrvall M, Lind L, Sundbom M, Karlsson FA 2003 Ghrelin and adipose tissue regulatory peptides: effect of gastric bypass surgery in obese humans. J Clin Endocrinol Metab 88:3177–3183 [DOI] [PubMed] [Google Scholar]
- Korner J, Inabnet W, Febres G, Conwell IM, McMahon DJ, Salas R, Taveras C, Schrope B, Bessler M 2009 Prospective study of gut hormone and metabolic changes after adjustable gastric banding and Roux-en-Y gastric bypass. Int J Obes (Lond) 33:786–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings DE 2009 Endocrine mechanisms mediating remission of diabetes after gastric bypass surgery. Int J Obes (Lond) 33(Suppl 1):S33–S40 [DOI] [PubMed] [Google Scholar]
- Moo TA, Rubino F 2008 Gastrointestinal surgery as treatment for type 2 diabetes. Curr Opin Endocrinol Diabetes Obes 15:153–158 [DOI] [PubMed] [Google Scholar]
- Rubino F 2008 Is type 2 diabetes an operable intestinal disease? A provocative yet reasonable hypothesis. Diabetes Care 31(Suppl 2):S290–S296 [DOI] [PubMed] [Google Scholar]