Abstract
Following traumatic brain injury, mitochondria sustain structural and functional impairment, which contributes to secondary damage that can continue for days after the initial injury. The present study investigated mitochondrial bioenergetic changes in the rat neocortex at 1 and 3 h after mild, moderate, and severe injuries. Brains from young adult Sprague-Dawley rats were harvested from the injured and contralateral cortex to assess possible changes in mitochondrial respiration abilities following a unilateral cortical contusion injury. Differential centrifugation was used to isolate synaptic and extrasynaptic mitochondria from cortical tissue. Bioenergetics was assessed using a Clark-type electrode and results were graphed as a function of injury severity and time post-injury. Respiration was significantly affected by all injury severity levels compared to uninjured tissue. Complex 1- and complex 2-driven respirations were affected proportionally to the severity of the injury, indicating that damage to mitochondria may occur on a gradient. Total oxygen utilization, respiratory control ratio, ATP production, and maximal respiration capabilities were all significantly decreased in the injured cortex at both 1 and 3 h post-trauma. Although mitochondria displayed bioenergetic deficits at 1 h following injury, damage was not exacerbated by 3 h. This study stresses the importance of early therapeutic intervention and suggests a window of approximately 1–3 h before greater dysfunction occurs.
Key words: Clark-type electrode, cortical contusion injury, mitochondrial bioenergetics, total mitochondria, traumatic brain injury
Introduction
Experimental traumatic brain injury (TBI) results in a rapid and significant loss of neurons in the tissue immediately below the site of the impact. TBI consists of a primary insult resulting from the biomechanical forces directly damaging neuronal tissue. The depth of the insult is directly proportional to the extent of neuronal tissue loss that ensues. The primary insult is then followed by secondary cascades that evolve over a period of hours to weeks after the trauma (Teasdale and Graham, 1998), and may offer opportunities for intervention (Robertson, 2004). Secondary damage is believed to be much more detrimental than the initial insult itself, which drives research to focus on what causes these impairments and how they can be minimized. In particular, mitochondria sustain considerable structural and functional damage following TBI (Xiong et al., 1997; Sullivan et al., 1998; Verweij et al., 2000; Lifshitz et al., 2003; Singh et al., 2006; Robertson et al., 2007). Neuronal survival is intimately linked to mitochondrial homeostasis. Mitochondria supply the central nervous system with energy (ATP), as well as regulating calcium within the cell (Khodorov et al., 1996). Several therapeutic interventions that target stabilizing mitochondria have shown promising results by reducing overall neuronal tissue damage as well as enhancing neurological outcome following TBI (Verweij et al., 1997; Sullivan et al., 1999; Sullivan et al., 2004; Hino et al., 2005; Xiong et al., 2005; Clark et al., 2007). Finsterer (2008) reviewed several drugs that could be utilized to improve aspects of mitochondrial bioenergetics in many respiratory chain diseases (e.g., epilepsy and dementia), some of which may be effective in TBI as well.
Experimental models of TBI have shown that mitochondrial damage occurs rapidly. Significant respiration changes are seen as early as 15–30 min post-injury (Sullivan et al., 1998; Singh et al., 2006), with peak mitochondrial dysfunction at 12–24 h (Xiong et al., 1997; Singh et al., 2007). Changes in mitochondrial bioenergetics can persist for up to 14 days (Xiong et al., 1997). Some of the very early changes in mitochondrial bioenergetics may represent part of the primary injury cascade and not be amenable to pharmacologic intervention. There is limited information concerning the interaction between injury severity and early mitochondrial dysfunction. Two relatively recent studies using transgenic mice reported no significant alterations in mitochondrial respiration at 24 h following a moderate insult, while a severe injury produced a significant functional deficit (Xiong et al., 2004; Xiong et al., 2005). The present study explored possible early mitochondrial bioenergetic changes (at 1 h and 3 h) at three different injury severities (mild, moderate, and severe).
Methods
Surgeries
This study used young adult male Sprague-Dawley (SD) rats (n = 63, 275–300 g; Harlan Labs, Indianapolis, IN), which were housed in group cages (2 per cage) on a 12-h light/dark cycle with free access to food and water.
All experimental protocols involving animals were approved by the University of Kentucky Animal Use and Care Committee. The animals were randomly assigned to one of nine groups: (I) Naïve: no anesthesia or any type of surgical intervention (n = 7); (II and III) Sham: rats subjected to a craniotomy alone without injury and assessed at 1 (n = 7) and 3 h (n = 6); (IV and V) Mild injury: (0.5 mm cortical displacement) assessed at 1 (n = 7) and 3 h (n = 6) post-injury; (VI and VII) Moderate injury: (1.0 mm cortical displacement) assessed at 1 (n = 6) and 3 h (n = 7) post-injury; (VIII and IX) Severe injury: (1.5 mm cortical displacement) assessed at 1 (n = 8) and 3 h (n = 6) post-injury.
Cortical contusions were carried out under isoflurane anesthesia (2%) as previously described (Sullivan et al., 2002). All injuries were produced using a pneumatic controlled cortical impact device (TBI 0310; Precision Systems and Instrumentation, Fairfax Station, VA) with a soft-stop Bimba cylinder (Bimba Manufacturing, Monee, IL). The depth of the impact was altered to result in different injury severities, while the impactor tip size (5 mm beveled), dwell time (400 ms), and velocity (3.5 m/sec) were held constant. The animals were maintained at 37°C and allowed to survive for 1 or 3 h, depending on their experimental group.
Total mitochondria isolation
The mitochondrial isolation protocol and all procedures were performed on ice. The animals were briefly gassed with CO2 until flaccid, decapitated, and the brains rapidly removed. The cortices were dissected and an 8-mm-diameter circular core of the ipsilateral and contralateral cortex at the site of the impact was extracted. The cortices were placed in separate glass dounce homogenizers with 4 mL of isolation buffer with 1 mM EGTA (215 mM mannitol, 75 mM sucrose, 0.1% BSA, 20 mM HEPES, and 1 mM EGTA; pH 7.2). The tissue cores were homogenized and mitochondria isolated by differential centrifugation. The homogenate was centrifuged twice at 1300 × g for 3 min in a microcentrifuge at 4°C. The resulting supernatant was diluted with isolation buffer containing EGTA and centrifuged at 13,000 × g for 10 min. The resulting pellet was resuspended in 500 μL of isolation buffer with EGTA and subjected to a pressure of 1200 psi for 10 min inside a nitrogen cell disruption bomb (Parr Instrument Company, Moline, IL) at 4°C. After rupturing the synaptosomes, the samples were brought up to a final volume of 2 mL with isolation buffer containing EGTA, and centrifuged at 13,000 × g for 10 min. The pellet was resuspended in isolation buffer without EGTA and centrifuged at 10,000 × g for 10 min. The final mitochondrial pellet was resuspended in isolation buffer without EGTA to yield a concentration of 10 mg/mL or higher. The protein concentration was determined using a bicinchoninic acid protein assay kit (Pierce, Rockford, IL).
Mitochondria respiration measurements
Mitochondrial functionality was assessed using an Oxytherm Clark-type oxygen electrode (OXYT1/ED; Hansatech Instruments, Norfolk, UK). Mitochondrial protein (∼180 μg taken from uninjured tissue and ∼200 μg from injured tissue) were placed in the sealed Oxytherm chamber containing respiration buffer (125 mM KCl, 0.1% BSA, 20 mM HEPES, 2 mM MgCl2, and 2.5 mM KH2PO4; pH 7.2) and was continuously stirred at a constant temperature of 37°C. The total volume of respiration buffer and mitochondrial protein placed in the chamber was 250 μL.
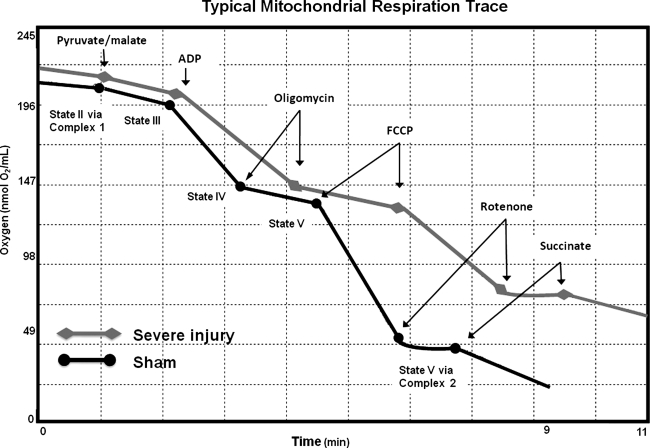
Rates of oxygen utilization were defined as the amount of oxygen (nmol) utilized over time (min) on consecutive administrations of oxidative substrates, similar to those described previously (Singh et al., 2007). The oxidative substrates were injected into the Oxytherm chamber sequentially as follows: state I: mitochondria with respiration buffer and no substrates added so respiration was minimal; state II (P/M): addition of 2.5 μL of 2.5 mM pyruvate and 5 mM malate (P/M) and allowed to reach a steady, constant rate; state III (ADP): addition of 1.25 μL of 150 μM ADP allowed to reach maximum rate; state IV (oligomycin): addition of 1.0 μL of 1 μM oligomycin allowed to reach minimum rate; state V (FCCP): addition of 1.0 μL of 1 μM FCCP allowed to reach maximum rate (0.2 μL of rotenone was added to shut down complex 1 activity, resulting in cessation of oxygen utilization); and finally state V (succinate): addition of 2.5 μL of 10 mM succinate and allowed to reach maximum rate. State V (succinate) represents maximum complex 2 activity, since FCCP is still present in the assay system. Figure 1 is a schematic drawing of a typical mitochondria respiration trace, depicting the sequence of substrate additions and subsequent oxygen utilization rates. The overall oxygen utilization rate was determined by measuring the amount of oxygen consumed throughout all states of respiration, divided by the time elapsed and amount of mitochondrial protein present in the assay. This measurement can detect alterations in the rate of oxygen utilized by mitochondria in response to substrate additions, which serves as an index of their overall respiration capacities. The respiratory control ratio (RCR) was determined by dividing the rate of oxygen utilization for state III (ADP) by state IV (oligomycin). RCRs tell how coupled the electron transport chain (ETC) is to ATP production. The states of respiration (III–V), overall oxygen utilization rates, and RCRs were all collected to further define respiration capabilities.
FIG. 1.
Typical oxygen utilization trace of healthy mitochondria taken from a sham animal. State I: no substrates for respiration have been added; no respiration is apparent. State II: addition of P/M, with basal rate of respiration. State III: addition of ADP completes the necessary substrates needed for operation of the electron transport chain (ETC); the high level of oxygen utilization indicates that ADP is getting converted into ATP. State IV: addition of oligomycin; return to basal rate of respiration since the ATP synthase is shut down and no electrons are allowed to return to the matrix. The ETC continues only to maintain mitochondrial membrane potential due to loss of protons back into the matrix. State V: addition of FCCP; this represents the maximum rate of respiration, causing uncoupling of the ETC to ATP synthesis, and allows protons to rush back into the matrix. Rotenone is then added to shut down complex 1–driven respiration. State V (succinate): addition of succinate; this is the maximum rate of respiration via complex 2, since FCCP is present in the system.
To ensure the quality of the isolation procedure, mitochondria isolated from the contralateral cortex had to produce RCRs of ≥5 to be included in the study. Respiration capabilities of mitochondria isolated from the contralateral cortex served as an internal control for extent of dysfunction as a result of the injury to the ipsilateral cortex. Previous studies, as well as our own, have shown no early changes in mitochondria respiration in the contralateral hemisphere following TBI (Sullivan et al., 2005; Opii et al., 2007; Pandya et al., 2007), or focal cerebral ischemia (Kuroda et al., 1996), supporting the use of the contralateral cortex as an appropriate “internal” control.
Statistical analyses
All data are reported as group means ± SD. Overall oxygen utilization and rates (states II through VI) are plotted as percentage of the contralateral rate to show the extent of damage dependent on injury severity and time post-injury. Actual rates of all states of respiration are also reported to show relative respiration capabilities across groups, consistency, and extent of biological variance. Respiration states (I–V) and state V (succinate) via complex 2 were calculated as nmol of oxygen/min/mg of mitochondria in 250 μL of respiration buffer, as described previously (Singh et al., 2006). RCRs are an index of the overall health of the mitochondria, and are reported for both ipsilateral and contralateral sides separately.
A paired t-test was utilized to detect possible hemispheric differences in naïve animals. An unpaired t-test was used to compare the ipsilateral sides of 1- and 3-h post-surgery sham animals to investigate the possible suppression of mitochondrial function due to the surgery (anesthesia and craniotomy). To ensure that sham animals were no different than naïve rats, 3-h post-surgery sham animals were compared to the respiration rates from naïve rats using an unpaired t-test. To show that the contralateral hemisphere could be used as an internal control for typical respiration and to reduce biological variance, an unpaired t-test was used to compare sham animals to contralateral tissue. Two separate t-tests were performed to compare mitochondrial respiration of sham animals to contralateral tissue from severely injured animals at the same time point post-injury (1-h contralateral to 1-h shams, and then 3-h contralateral to 3-h shams). A two-way ANOVA was used to determine the extent of mitochondrial dysfunction due to injury severity and time post-trauma. All ANOVAs were followed by a Student-Newman-Keuls post-hoc analysis. The alpha level was set at 0.05 for significance.
Results
NaÏve animals
Statistical analysis using a paired comparison t-test failed to reveal any significant hemispheric differences among naïve animals for any of the dependent measures of mitochondrial bioenergetics: RCR (t6 = 0.13, p > 0.1), overall oxygen utilization (t6 = 0.96, p > 0.1), P/M (t6 = 0.23, p > 0.1), ADP (t6 = 0.93, p > 0.1), oligomycin (t6 = 0.86, p > 0.1), FCCP (t6 = 1.6, p > 0.1), and succinate (t6 = 1.6, p > 0.1). These results support the idea that both hemispheres have the same mitochondrial respiration capacities.
Sham surgery animals
Animals were subjected to both anesthesia and craniotomy, and then killed at 1 or 3 h post-surgery. For each bioenergetic parameter (e.g., total oxygen utilization), t-tests were carried out to probe for alterations in mitochondrial function due to the general surgical procedure and no significant differences were found. A paired comparison statistical analysis failed to identify any significant differences in mitochondrial bioenergetics between the two hemispheres due to the surgical process (p > 0.1). These results indicate that the craniotomy and surgical procedures do not alter mitochondrial bioenergetics.
NaÏve versus sham comparison
The cortex immediately beneath the sham craniotomy site was also compared to values obtained from naïve animals. An unpaired t-test failed to identify any significant differences in respiration capabilities between naive and sham animals at either 1 or 3 h post-surgery (p > 0.1). These results support the use of sham animals as an appropriate control.
Comparison of sham versus contralateral tissue taken from injured animals
Two separate ANOVAs were used to compare the contralateral side of injured animals to sham-operated animals at 1 and 3 h post-injury. No significant differences (p > 0.1) in respiration capabilities were observed, indicating that the contralateral hemisphere of injured animals reflects that observed in the sham animals and can be used as an internal control for each subject. Since mitochondria from the contralateral hemisphere are unaffected by the insult, we compared ipsilateral respiration rates as a percent of the contralateral side to reduce biological variance.
Injury-induced mitochondria bioenergetics
Mitochondrial amounts used for analysis
Equal amounts of mitochondrial protein isolated from naïve, sham, or contralateral tissue of injured animals could produce similar, consistent respiration traces. The average amount of mitochondrial protein taken from uninjured cortical tissue used to test mitochondrial functionality was 180.1 μg ± 25 (n = 39), compared to 205.5 ± 27 μg from the site of injury. An unpaired t-test showed that these amounts of mitochondrial protein used for respiration analysis were significantly greater for the injured hemisphere (t39 = 5.2, p < 0.0001).
Overall oxygen utilization
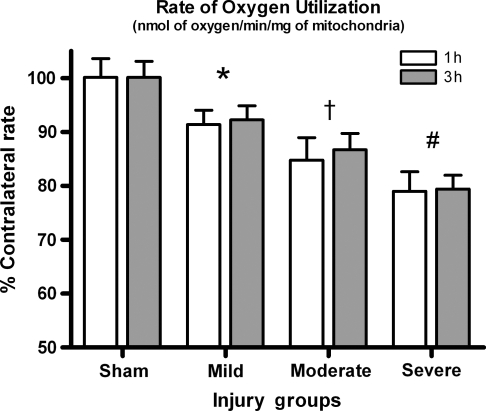
The overall rate of oxygen utilization demonstrated a significant effect for injury severity [F (3, 45) = 102.54, p < 0.0001], but not for time post-injury [F (3, 45) = 0.196, p > 0.1] (Fig. 2). Post-hoc analysis showed that all injured groups were significantly lower than sham animals (p < 0.05), and that as the injury severity increased, oxygen utilization declined significantly (p < 0.05).
FIG. 2.
Overall oxygen utilization rate. As injury severity increases the overall oxygen utilization significantly drops, indicating that the mitochondria are not responding to the additions of substrates as robustly following injury. Bars represent group means ± SD (*p < 0.01 compared to sham; †p < 0.05 compared to mild, #p < 0.01 compared to moderate).
Pyruvate/malate (state II respiration via complex 1)
A two-way ANOVA (time × injury severity) revealed no significant effect for time post-injury [F (1, 45) = 1.065, p > 0.1] or for the severity of injury [F (3, 45) = 0.114, p > 0.1]. All preparations contained equal amounts of respiring mitochondria and the state II respiration via complex 1 was used as baseline. This gave all groups equal ability to produce similar respiration traces, regardless of the amount of mitochondrial protein added, unless damage to components of the ETC existed as a result of the insult.
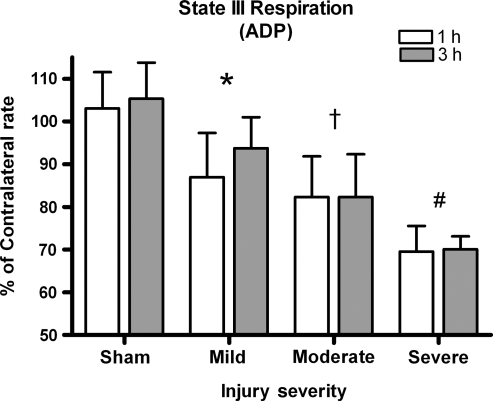
ADP (state III respiration)
Figure 3 depicts how efficiently ADP is utilized to make ATP. A two-way ANOVA was significant for effect of injury severity [F (3, 45) = 38.982, p < 0.0001], but not for time post-injury [F (1, 45) = 1.145, p > 0.1]. The magnitude of the deficit was directly proportional to mitochondria's ability to utilize ADP. No significant interaction between injury severity and time post-injury existed [F (3, 45) = 0.495, p > 0.1]. Post-hoc testing revealed that all injured groups were significantly lower than sham-operated controls (p < 0.05), and that as the injury severity increased the ability to utilize the ADP significantly decreased (p < 0.05).
FIG. 3.
ADP rates. The ability of mitochondria to phosphorylate ADP significantly drops with injury severity. Bars represent group means ± SD (*p < 0.05 compared to sham; †p < 0.05 compared to mild; #p < 0.05 compared to moderate).
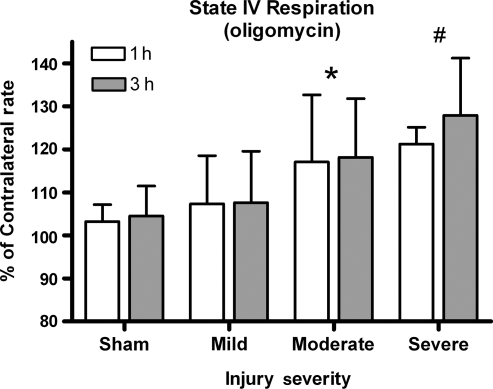
Oligomycin (state IV respiration)
Oligomycin was added to assess the possible changes in state IV respiration. A two-way ANOVA revealed a significant increase in state IV effect for injury severity [F (3, 45) = 10.46, p < 0.0001], but not for time post-injury [F (1, 45) = 0.627, p > 0.1] (Fig. 4). Post-hoc analysis revealed that the severely and moderately injured groups were significantly different from both the sham and mildly injured groups, but not from animals with moderate injuries (p < 0.05).
FIG. 4.
Oligomycin rates. The inner membrane becomes increasingly damaged, causing more of the proton to be lost back into the matrix, resulting in less of the proton gradient being coupled with ATP production. Bars represent group means ± SD (*p < 0.05 compared sham/mild; #p < 0.01 compared to sham/mild).
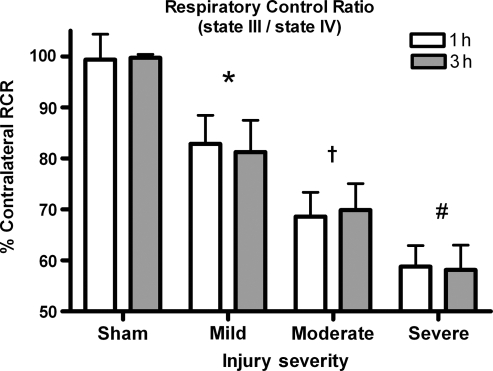
RCR (state III/state IV respiration)
Changes in the overall condition of the mitochondria and how coupled their ETC is to ATP production were assessed by comparing RCRs across groups. A two-way ANOVA revealed a significant effect for injury severity [F (3, 45) = 77.006, p < 0.0001], but not for time post-injury [F (3, 45) = 2.022, p > 0.1]. There was no significant interaction (p > 0.1) (Fig. 5). Post-hoc analysis revealed that all injured groups had significantly lower RCRs than sham-operated controls (p < 0.05) and, that as the injury severity increased, the RCRs significantly decreased (p < 0.05). The RCRs at 1 h were not significantly lower from those observed at 3 h post-trauma (p > 0.05).
FIG. 5.
Respiratory control ratio (RCR). RCR is an index of how coupled respiration is to phosphorylating ADP. A RCR of 5 indicates well-coupled mitochondria. RCRs significantly drop with increasing injury severity. The data suggest that mitochondria are displaying an uncoupling of respiration from ATP production, meaning a decreased ability of mitochondria to produce ATP. Bars represent group means ± SD (*p < 0.05 compared to sham; †p < 0.05 compared to mild injury; #p < 0.05 compared to moderate injury).
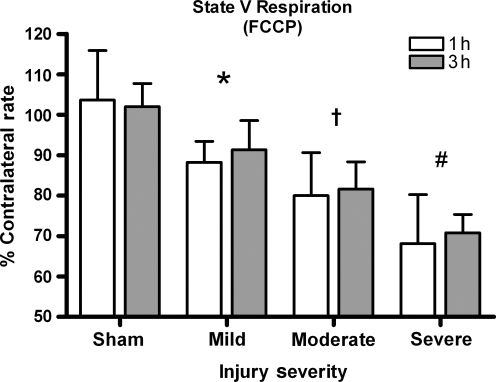
FCCP (state V respiration)
The metabolic poison FCCP was introduced into the Oxytherm chamber to monitor state V respiration. A two-way ANOVA revealed a significant effect for injury severity [F (3, 45) = 34.492, p < 0.0001], but not for time post-injury effect [F (3, 45) = 0.349, p > 0.1] (Fig. 6). Post-hoc testing revealed that all injury severity groups significantly differed from sham controls (p < 0.05) and, that as the injury severity increased, the FCCP rate significantly declined (p < 0.05).
FIG. 6.
FCCP rates. Maximal respiration capability drops with increasing injury severity. Bars represent group means ± SD (*p < 0.05 compared to sham; †p < 0.05 compared to mild injury; #p < 0.05 compared to moderate injury).
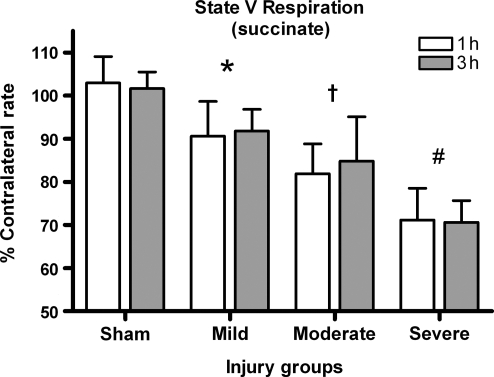
Succinate (state V respiration via complex 2)
Succinate was added to the chamber to study complex 2 (state V) respiration. A two-way ANOVA was not significant for time post-injury effect [F (1, 45) = 0.195, p > 0.1], but was significant for effect of injury severity [F (3, 45) = 51.416, p < 0.001] (Fig. 7). Post-hoc analysis revealed that all injured groups were significantly lower than sham-operated controls (p < 0.05) and, that as the injury severity increased, the ability to utilize succinate was significantly decreased (p < 0.05).
FIG. 7.
Succinate rates. Not only is Complex 1-driven respiration affected by injury, but Complex 2 as well. Bars are group means ± SD (*p < 0.05 compared to sham; †p < 0.05 compared to mild injury; #p < 0.05 compared to moderate injury).
Discussion
In the present study we performed a detailed analysis of mitochondrial bioenergetics following a mild, moderate, and severe unilateral cortical contusion injury (CCI). The results from this study are the first to demonstrate alterations in mitochondrial respiration with a mild injury. Animals subjected to a moderate and severe insult show even greater mitochondrial dysfunction. Changes were observed as early as 1 h post-trauma and remained constant for up to 3 h. We chose 1 and 3 h because these are times in the literature that conform to times when pharmacologic interventions are often carried out. These results support the idea that mitochondria are affected very early and precede many of the TBI injury cascades, including oxidative damage (Ansari et al., 2008). The fact that we can demonstrate functional mitochondrial respiration at early times post-trauma suggests that therapeutic interventions aimed at assisting mitochondria need to be initiated early for possible neuroprotection.
Our results differ from those of previous studies that have reported a lack of severity-dependent changes in mitochondrial respiration. Xiong and colleagues reported no significant alterations in mitochondrial bioenergetics 24 h following a moderate CCI in mice (Xiong et al., 2004, 2005), when deficits have been reported to be the greatest following a severe CCI in rats (Xiong et al., 1997). Discrepancies may be a result of species differences, mitochondrial purification procedures, or whether or not pooling tissue from multiple animals was used. Previous studies have shown that significant TBI-related behavioral dysfunction can occur without obvious tissue destruction and cavity formation (Hamm et al., 1992; Scheff et al., 1997), suggesting that a mild injury disrupts normal brain biochemical and physiological function. A mild injury has also been shown to activate cortical astrocytes despite the lack of any obvious tissue destruction (Baldwin and Scheff, 1996).
In the present study, bioenergetic measurements are reported as a percentage of the subject's uninjured contralateral hemisphere. Multiple experiments were undertaken to demonstrate that the contralateral uninjured cortex represents conditions normally observed in naïve animals. This provides a common baseline to compare results from multiple different experiments. As a further indicator of mitochondrial quality, only preparations in which the RCR value for the contralateral hemisphere was above 6.0 were used, a value equivalent to that observed in the sham-operated groups (Table 1). Previous reports have noted that an RCR above 5 indicates viable mitochondria (Xiong et al., 1997; Singh et al., 2006; Opii et al., 2007).
Table 1.
Respiratory Control Ratios (RCR) from the Contralateral Cortex
| |
1 h Post-injury |
3 h Post-injury |
||||
|---|---|---|---|---|---|---|
| Injury group | Mean ± SD | n | Mean ± SD | n | ||
| Sham | 6.7 | 0.7 | 7 | 6.7 | 0.4 | 6 |
| 0.5 mm | 6.4 | 0.6 | 7 | 6.9 | 0.6 | 6 |
| 1.0 mm | 6.4 | 0.5 | 6 | 6.7 | 0.7 | 7 |
| 1.5 mm | 6.4 | 0.5 | 8 | 6.8 | 0.6 | 6 |
RCRs (state III/state IV) are used as an overall index of the condition of isolated mitochondria. RCRs above 5 are indicative of healthy mitochondria that have a tight coupling of the electron transport chain with ATP production. There were no significant differences in RCRs of mitochondria taken from contralateral tissue of sham animals or injury groups. Data verify that mitochondria taken from the contralateral hemisphere could be used as an internal control. The use of an internal control helps reduce biological variance of making comparisons across animals.
In every mitochondrial preparation, including those from naïve animals, a portion of these organelles can be damaged or non-functional and still contribute to the overall protein concentration. Using the same amount of protein for each respiration assay assumes that the total protein content is primarily mitochondrial, and any alterations in bioenergetics are attributable to a failure of total functioning organelles. Following more severe injuries, a larger portion of the sample contains debris or non-functional mitochondria, making it difficult to accurately measure respiration rates. In efforts to try to maintain a consistent amount of functional mitochondrial protein, all groups were afforded the same starting potential to produce equivalent respiration traces. This was accomplished by adding an amount of mitochondrial protein that resulted in a standardized level of oxygen utilization during state II respiration (∼10–15 nmol O/min). Statistical analysis revealed no differences in state II respiration between any experimental groups (p > 0.1). In the present study, up to 14% more mitochondrial protein had to be used for injured cortex to maintain a standard level of state II respiration. This minor addition of sample for even the most severely injured animals suggests a high level of purity with our preparations, considering the magnitude of injury for some animals. Other studies using a similar mitochondrial isolation protocol report a relatively high variance (240%) in the amount of mitochondrial protein used for respiration analysis following a severe CCI in SD rats (Xiong et al., 1997) and mice (Xiong et al., 2004; Xiong et al., 2005). The necessity of using increased amounts of mitochondrial protein to obtain equivalent state II respiration rates indicates that some component(s) of the mitochondrial respiration chain sustained some type of structural and/or functional damage as a result of the insult.
The total oxygen utilization rate was monitored to determine if there were overt respiration changes occurring in tissue immediately below the area of impact. This parameter takes into account all states of the mitochondrial respiration combined. Figure 2 clearly shows an injury-dependent decline in the overall rate of oxygen utilization, indicative of a significant bioenergetic malfunction. Other researchers have reported an early decline in mitochondrial oxygen utilization following TBI (Verweij et al., 1997; Xiong et al., 1997; Clausen et al., 2001), but did not compare differences due to injury severity. In this study, a mild injury produced approximately a 10% decline in oxygen utilization compared to sham-operated controls, while a severe injury resulted in a 20% or greater deficit. The total oxygen utilization rate declined as a function of injury severity, but changes between 1 and 3 h were not found. While measurement of the overall oxygen utilization rate signifies a general overview of mitochondrial function, it cannot determine which particular complex(s) are significantly affected following injury. The robust nature of the injury-dependent decline in total oxygen utilization suggests that multiple respiration states are involved. It is necessary to investigate which complexes and states of respiration are most involved.
State III respiration is demonstrated by adding ADP following a stabilization of P/M oxygen utilization. As protons cross the inner membrane, the ETC increases and consequently more oxygen is consumed as ATP is synthesized. In this study, state III respiration significantly declined as a function of injury severity (Fig. 3). More severely injured animals showed a 30% reduction in state III respiration, while animals with a mild injury showed a 10% reduction. Since the amount of ADP available was constant, a decline would signify a decline in ATP production, a situation that would be deleterious for cells attempting to regain homeostasis following injury. There were no significant differences between 1 and 3 h post-trauma for state III respiration.
In a previous study researchers reported as much as a 43% drop in cortical ATP levels 15 min following a mild CCI, and a 51% drop in severely injured SD rats (Marklund et al., 2006). ATP levels were restored by 40 min post-trauma in animals with mild injury, but remained significantly reduced in animals receiving a severe insult. Sullivan and associates (1998) reported an approximately 30% drop in ATP levels 30 min following a moderate CCI in cortical synaptosomes that was not restored until 6 h post-trauma. More severe injuries place higher energy demands on neuronal tissue, which are compounded by greater mitochondrial respiration deficits. A lack of ATP production could be partially responsible for the progressive tissue loss that quickly begins in the cortex following TBI.
One direct cellular consequence of a significant drop in ATP would be an inability to acquire calcium homeostasis following TBI. Excitatory amino acids (EAAs) play an important role in the mechanisms of secondary injury following TBI (Nicholls and Budd, 1998; Sullivan et al., 1998; Jiang et al., 2001; Brustovetsky et al., 2002). Elevated EAAs increase intracellular calcium concentrations by mechanisms involving activation of NMDA-receptor/ion channels, the AMPA receptor, and voltage-operated calcium channels. In order to maintain calcium homeostasis and neuronal survival, calcium must be removed from the cytosolic compartment. This extrusion of calcium is primarily mediated through the sodium-calcium exchanger, which requires large amounts of ATP. If the deficits in ATP production are large enough, cell death is inevitable.
Calcium influx can also cause significant alterations in membrane potential that reflect an intermediate unstable state of mitochondria, which may lead to or reflect mitochondrial dysfunction (Vergun et al., 2003). The adenine nucleotide translocator (ANT) typically exchanges ATP in the matrix for cytosolic ADP across the inner mitochondrial membrane, but its function can be altered by oxidative stress or a large influx of calcium. Damage occurs to ANT following TBI (Opii et al., 2007), and may be responsible for the decline in ATP production, as well as enhanced formation of the devastating mitochondrial permeability transition pore (Halestrap et al., 2002; Vyssokikh and Brdiczka, 2003).
Intact healthy mitochondria maintain a proton gradient, primarily for synthesis of ATP, which is the dominant pathway for re-entry of protons into the mitochondrial matrix (Nicholls and Budd, 2000). Oligomycin is a metabolic poison that binds ATP synthase and inhibits ADP-stimulated respiration; following its addition mitochondria are locked into state IV respiration, where the extent of proton leakage into the matrix can be determined. State IV indicates the extent that the proton motive force is coupled with ATP production versus maintaining the basal metabolic rate. With an isolated mitochondrial preparation, an increase in oxygen utilization during state IV indicates an uncoupling of the ETC to ATP production, as well as the magnitude of damage to the inner mitochondrial membrane.
Figure 4 demonstrates an injury-related increase in state IV respiration that was most pronounced at the most severe levels (∼20%). A mild injury produced oligomycin respiration rates that were equivalent to sham-operated controls, while a moderate injury produced evidence that changes were occurring within the mitochondria. Others have reported state IV respiration being twice as high following a severe TBI in mice (Singh et al., 2006). A short (12–20 min) ischemic/reperfusion event has also been shown to result in an increase in state IV respiration, by ∼25% (Sciamanna et al., 1992). The lack of any time-dependent changes is indicative of a very rapid injury to the mitochondria within the first hour, which may have important implications in formulating therapeutic interventions.
The overall functionality of the mitochondria is often stated in terms of the RCR. This ratio is a measure of how coupled mitochondrial respiration is to ATP production. A high RCR (5–10) indicates fully functional organelles, while low ratios (<5) indicate that more of the proton motive force is being lost back into the matrix, or is being used for processes other than ATP production. As shown in Figure 5, even a mild injury significantly reduced the RCR, with further reductions observed with increased injury severity. Low RCRs indicate that the ability to produce ATP efficiently is significantly compromised, which would have consequences for injured neurons trying to regain cellular homeostasis. Several other studies support a reduction in RCRs at 1 h (Xiong et al., 1997; Robertson et al., 2006), as well as 3 h following TBI (Singh et al., 2006; Opii et al., 2007). The extended mitochondrial dysfunction that persists for at least 3 days after the initial insult may be amenable to pharmacological intervention.
FCCP is a pure uncoupler that acts as a protonophore, which allows the protons to freely pass back into the matrix, bypassing the ATP synthase at an extremely rapid rate. FCCP is added to determine maximum respiration capabilities of the ETC in its attempts to restore the dissipated proton gradient. We found a 10% reduction in maximum respiration abilities in animals receiving mild injuries, while animals subjected to a severe insult dropped 30% (Fig. 6). State V (FCCP) is important to assess, especially following an injury, because neurons and supporting cells depend heavily on mitochondria to produce large amounts of ATP and sequester the large influx of calcium. Due to the decline in cellular homeostasis following injury and higher energy demands, the mitochondria are probably operating near capacity. Other researchers report reductions of state V (FCCP) by more than 50% by 3 h post-trauma in rats (Opii et al., 2007), and similar reductions were seen in mice (Singh et al., 2006).
Following the addition of rotenone, which acts as a competitive inhibitor to block complex 1–driven respiration, succinate was added to determine if complex 2–driven respiration is affected by TBI. State V (succinate) was affected proportionally as a function of injury severity (Fig. 7). A mild injury produced approximately a reduction of 7% in state V (succinate) respiration, 15–20% in a moderate injury, and 30% following a severe injury. Data indicate that both complex 1– and complex 2–driven respiration are significantly affected by TBI, and that this can quickly evolve into a metabolic crisis because neurons require ATP for survival.
Several studies support complex 2 dysfunction following TBI. Xiong and associates (1997) showed that impairments of state III respiration by complex 1–driven respiration were more severe (∼45% drop from sham rates), but complex 2–driven respiration was still affected by the injury (∼25% drop from sham rates). Similarly, maximal increases in state IV respiration via complex 1 were 155% of sham values and 125% of sham values for complex 2, demonstrating that complex 2 is not impervious to damage. Verweij and colleagues (1997) reported a significant decrease in state III respiratory rates, RCRs, and P/O ratios with either succinate or NAD-linked substrates in SD rats following a severe unilateral CCI. Both complex 1 and 2 maximum respiration have been shown to drop by approximately 50% following a moderate unilateral CCI in SD rats (Opii et al., 2007). Significant changes in both NAD-linked and succinate-driven respiration have also been reported following ischemic/reperfusion injury (Sciamanna et al., 1992; Sciamanna and Lee, 1993), as well as spinal cord contusion injury (Jin et al., 2004).
When analyzing changes in mitochondrial bioenergetics following trauma, we realized that damage to mitochondria could occur at any number of sites in the molecular machinery, which could be responsible for declines in respiration. A few of the possible sites of malfunction are transporter proteins responsible for importing substrates inside mitochondria (Sugden and Holness, 2003); enzymes needed to initiate the tricarboxylic acid cycle, such as pyruvate dehydrogenase (Long et al., 2006); various complexes of the ETC used for production of proton motive force (Singh et al., 2006); or the ANT that exchanges ADP for ATP across the inner mitochondrial membrane (Opii et al., 2007). Future studies will have to probe the molecular and biochemical mechanisms for both the functional and structural changes that occur within the mitochondria following TBI.
Conclusion
In the present study we demonstrated that administration of a mild, moderate, or severe CCI results in mitochondrial dysfunction as early as 1 h post-trauma. The extent of bioenergetic dysfunction appears to be contingent on injury severity. The damage seen at 1 h remains constant for at least 3 h following the insult. Therapies should target stabilization of mitochondrial homeostasis and be initiated as early as possible, with the understanding that treatment may help prevent subsequent waves of mitochondrial dysfunction and secondary cascade events. Our data suggest that mitochondria experience a combination of damage to ETC components as well as the inner membrane. Decreased oxygen utilization at states III (ADP), V (FCCP), and V (succinate) all indicate that the mitochondria have sustained some type of damage to components of their ETC, resulting in lowered bioenergetic capabilities. State IV respiration increased across levels of injury severity, but was significantly higher only in the severely-injured groups, suggesting a high degree of inner membrane damage. Following an injury, mitochondria experience metabolic dysfunction (due to influx of high levels of Ca2+), and as a result are not able to produce the needed high levels of ATP. Immediately following injury, the affected neuronal tissue becomes necrotic, whereas the surviving brain parenchyma in the area of impact may be susceptible to apoptosis as various secondary cascades play out over the next several days (Kovesdi et al., 2007).
Acknowledgments
We thank Paula Thomason for carefully reading the manuscript. This work was supported by NIH grant AG21981 and training grant T32 DA022738-01.
Author Disclosure Statement
No conflicting financial interests exist.
References
- Ansari M.A. Roberts K.N. Scheff S.W. Oxidative stress and modification of synaptic proteins in hippocampus after traumatic brain injury. Free Radic. Biol. Med. 2008;45:443–452. doi: 10.1016/j.freeradbiomed.2008.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin S.A. Scheff S.W. Intermediate filament change in astrocytes following mild cortical contusion. Glia. 1996;16:266–275. doi: 10.1002/(SICI)1098-1136(199603)16:3<266::AID-GLIA9>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Brustovetsky N. Brustovetsky T. Jemmerson R. Dubinsky J.M. Calcium-induced cytochrome c release from CNS mitochondria is associated with the permeability transition and rupture of the outer membrane. J. Neurochem. 2002;80:207–218. doi: 10.1046/j.0022-3042.2001.00671.x. [DOI] [PubMed] [Google Scholar]
- Clark R.S. Nathaniel P.D. Zhang X. Dixon C.E. Alber S.M. Watkins S.C. Melick J.A. Kochanek P.M. Graham S.H. Boc-aspartyl(ome)-fluoromethylketone attenuates mitochondrial release of cytochrome C and delays brain tissue loss after traumatic brain injury in rats. J. Cereb. Blood Flow Metab. 2007;27:316–326. doi: 10.1038/sj.jcbfm.9600338. [DOI] [PubMed] [Google Scholar]
- Clausen T. Zauner A. Levasseur J.E. Rice A.C. Bullock R. Induced mitochondrial failure in the feline brain: Implications for understanding acute post-traumatic metabolic events. Brain Res. 2001;908:35–48. doi: 10.1016/s0006-8993(01)02566-5. [DOI] [PubMed] [Google Scholar]
- Finsterer J. Cognitive decline as a manifestation of mitochondrial disorders (mitochondrial dementia) J. Neurol. Sci. 2008;272:20–33. doi: 10.1016/j.jns.2008.05.011. [DOI] [PubMed] [Google Scholar]
- Halestrap A.P. McStay G.P. Clarke S.J. The permeability transition pore complex: Another view. Biochimie. 2002;84:153–166. doi: 10.1016/s0300-9084(02)01375-5. [DOI] [PubMed] [Google Scholar]
- Hamm R.J. Dixon C.E. Gbadebo D.M. Singha A.K. Jenkins L.W. Lyeth B.G. Hayes R.L. Cognitive deficits following traumatic brain injury produced by controlled cortical impact. J. Neurotrauma. 1992;9:11–20. doi: 10.1089/neu.1992.9.11. [DOI] [PubMed] [Google Scholar]
- Hino K. Nishikawa M. Sato E. Inoue M. L-carnitine inhibits hypoglycemia-induced brain damage in the rat. Brain Res. 2005;1053:77–87. doi: 10.1016/j.brainres.2005.06.062. [DOI] [PubMed] [Google Scholar]
- Jiang D. Sullivan P.G. Sensi S.L. Steward O. Weiss J.H. Zn(2+) induces permeability transition pore opening and release of pro-apoptotic peptides from neuronal mitochondria. J. Biol. Chem. 2001;276:47524–47529. doi: 10.1074/jbc.M108834200. [DOI] [PubMed] [Google Scholar]
- Jin Y. McEwen M.L. Nottingham S.A. Maragos W.F. Dragicevic N.B. Sullivan P.G. Springer J.E. The mitochondrial uncoupling agent 2,4-dinitrophenol improves mitochondrial function, attenuates oxidative damage, and increases white matter sparing in the contused spinal cord. J. Neurotrauma. 2004;21:1396–1404. doi: 10.1089/neu.2004.21.1396. [DOI] [PubMed] [Google Scholar]
- Khodorov B. Pinelis V. Storozhevykh T. Vergun O. Vinskaya N. Dominant role of mitochondria in protection against a delayed neuronal Ca2+ overload induced by endogenous excitatory amino acids following a glutamate pulse. F.E.B.S. Lett. 1996;393:135–138. doi: 10.1016/0014-5793(96)00873-3. [DOI] [PubMed] [Google Scholar]
- Kovesdi E. Czeiter E. Tamas A. Reglodi D. Szellar D. Pal J. Bukovics P. Doczi T. Buki A. Rescuing neurons and glia: Is inhibition of apoptosis useful? Prog. Brain Res. 2007;161:81–95. doi: 10.1016/S0079-6123(06)61006-6. [DOI] [PubMed] [Google Scholar]
- Kuroda S. Katsura K. Hillered L. Bates T.E. Siesjo B.K. Delayed treatment with alpha-phenyl-n-tert-butyl nitrone (PBN) attenuates secondary mitochondrial dysfunction after transient focal cerebral ischemia in the rat. Neurobiol. Dis. 1996;3:149–157. doi: 10.1006/nbdi.1996.0015. [DOI] [PubMed] [Google Scholar]
- Lifshitz J. Friberg H. Neumar R.W. Raghupathi R. Welsh F.A. Janmey P. Saatman K.E. Wieloch T. Grady M.S. McIntosh T.K. Structural and functional damage sustained by mitochondria after traumatic brain injury in the rat: Evidence for differentially sensitive populations in the cortex and hippocampus. J. Cereb. Blood Flow Metab. 2003;23:219–231. doi: 10.1097/01.WCB.0000040581.43808.03. [DOI] [PubMed] [Google Scholar]
- Long J. Wang X. Gao H. Liu Z. Liu C. Miao M. Liu J. Malonaldehyde acts as a mitochondrial toxin: Inhibitory effects on respiratory function and enzyme activities in isolated rat liver mitochondria. Life Sci. 2006;79:1466–1472. doi: 10.1016/j.lfs.2006.04.024. [DOI] [PubMed] [Google Scholar]
- Marklund N. Salci K. Ronquist G. Hillered L. Energy metabolic changes in the early post-injury period following traumatic brain injury in rats. Neurochem. Res. 2006;31:1085–1093. doi: 10.1007/s11064-006-9120-0. [DOI] [PubMed] [Google Scholar]
- Nicholls D.G. Budd S.L. Mitochondria and neuronal survival. Physiol. Rev. 2000;80:315–360. doi: 10.1152/physrev.2000.80.1.315. [DOI] [PubMed] [Google Scholar]
- Nicholls D.G. Budd S.L. Neuronal excitotoxicity: The role of mitochondria. Biofactors. 1998;8:287–299. doi: 10.1002/biof.5520080317. [DOI] [PubMed] [Google Scholar]
- Opii W.O. Nukala V.N. Sultana R. Pandya J.D. Day K.M. Merchant M.L. Klein J.B. Sullivan P.G. Butterfield D.A. Proteomic identification of oxidized mitochondrial proteins following experimental traumatic brain injury. J. Neurotrauma. 2007;24:772–789. doi: 10.1089/neu.2006.0229. [DOI] [PubMed] [Google Scholar]
- Pandya J.D. Pauly J.R. Nukala V.N. Sebastian A.H. Day K.M. Korde A.S. Maragos W.F. Hall E.D. Sullivan P.G. Post-injury administration of mitochondrial uncouplers increases tissue sparing and improves behavioral outcome following traumatic brain injury in rodents. J. Neurotrauma. 2007;24:798–811. doi: 10.1089/neu.2006.3673. [DOI] [PubMed] [Google Scholar]
- Robertson C.L. Mitochondrial dysfunction contributes to cell death following traumatic brain injury in adult and immature animals. J. Bioenerg. Biomembr. 2004;36:363–368. doi: 10.1023/B:JOBB.0000041769.06954.e4. [DOI] [PubMed] [Google Scholar]
- Robertson C.L. Puskar A. Hoffman G.E. Murphy A.Z. Saraswati M. Fiskum G. Physiologic progesterone reduces mitochondrial dysfunction and hippocampal cell loss after traumatic brain injury in female rats. Exp. Neurol. 2006;197:235–243. doi: 10.1016/j.expneurol.2005.09.014. [DOI] [PubMed] [Google Scholar]
- Robertson C.L. Saraswati M. Fiskum G. Mitochondrial dysfunction early after traumatic brain injury in immature rats. J. Neurochem. 2007;101:1248–1257. doi: 10.1111/j.1471-4159.2007.04489.x. [DOI] [PubMed] [Google Scholar]
- Scheff S.W. Baldwin S.A. Brown R.W. Kraemer P.J. Morris water maze deficits in rats following traumatic brain injury: Lateral controlled cortical impact. J. Neurotrauma. 1997;14:615–627. doi: 10.1089/neu.1997.14.615. [DOI] [PubMed] [Google Scholar]
- Sciamanna M.A. Lee C.P. Ischemia reperfusion-induced injury of forebrain mitochondria and protection by ascorbate. Arch. Biochem. Biophys. 1993;305:215–224. doi: 10.1006/abbi.1993.1414. [DOI] [PubMed] [Google Scholar]
- Sciamanna M.A. Zinkel J. Fabi A.Y. Lee C.P. Ischemic injury to rat forebrain mitochondria and cellular calcium homeostasis. Biochim. Biophys. Acta. 1992;1134:223–232. doi: 10.1016/0167-4889(92)90180-j. [DOI] [PubMed] [Google Scholar]
- Singh I.N. Sullivan P.G. Hall E.D. Peroxynitrite-mediated oxidative damage to brain mitochondria: Protective effects of peroxynitrite scavengers. J. Neurosci. Res. 2007;85:2216–2223. doi: 10.1002/jnr.21360. [DOI] [PubMed] [Google Scholar]
- Singh I.N. Sullivan P.G. Deng Y. Mbye L.H. Hall E.D. Time course of post-traumatic mitochondrial oxidative damage and dysfunction in a mouse model of focal traumatic brain injury: Implications for neuroprotective therapy. J. Cereb. Blood Flow Metab. 2006;26:1407–1418. doi: 10.1038/sj.jcbfm.9600297. [DOI] [PubMed] [Google Scholar]
- Sugden M.C. Holness M.J. Trials, tribulations and finally, a transporter: The identification of the mitochondrial pyruvate transporter. Biochem. J. 2003;374:E1–E2. doi: 10.1042/bj20031105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan P.G. Keller J.N. Bussen W.L. Scheff S.W. Cytochrome C release and caspase activation after traumatic brain injury. Brain Res. 2002;949:88–96. doi: 10.1016/s0006-8993(02)02968-2. [DOI] [PubMed] [Google Scholar]
- Sullivan P.G. Keller J.N. Mattson M.P. Scheff S.W. Traumatic brain injury alters synaptic homeostasis: Implications for Impaired mitochondrial and transport function. J. Neurotrauma. 1998;15:789–798. doi: 10.1089/neu.1998.15.789. [DOI] [PubMed] [Google Scholar]
- Sullivan P.G. Rabchevsky A.G. Waldmeier P.C. Springer J.E. Mitochondrial permeability transition in CNS trauma: Cause or effect of neuronal cell death? J. Neurosci. Res. 2005;79:231–239. doi: 10.1002/jnr.20292. [DOI] [PubMed] [Google Scholar]
- Sullivan P.G. Springer J.E. Hall E.D. Scheff S.W. Mitochondrial uncoupling as a therapeutic target following neuronal injury. J. Bioenerg. Biomembr. 2004;36:353–356. doi: 10.1023/B:JOBB.0000041767.30992.19. [DOI] [PubMed] [Google Scholar]
- Sullivan P.G. Thompson M.B. Scheff S.W. Cyclosporin A attenuates acute mitochondrial dysfunction following traumatic brain injury. Exp. Neurol. 1999;160:226–234. doi: 10.1006/exnr.1999.7197. [DOI] [PubMed] [Google Scholar]
- Teasdale G.M. Graham D.I. Craniocerebral trauma: Protection and retrieval of the neuronal population after injury. Neurosurgery. 1998;43:723–738. doi: 10.1097/00006123-199810000-00001. [DOI] [PubMed] [Google Scholar]
- Vergun O. Votyakova T.V. Reynolds I.J. Spontaneous changes in mitochondrial membrane potential in single isolated brain mitochondria. Biophys. J. 2003;85:3358–3366. doi: 10.1016/S0006-3495(03)74755-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verweij B.H. Muizelaar J.P. Vinas F.C. Peterson P.L. Xiong Y. Lee C.P. Impaired cerebral mitochondrial function after traumatic brain injury in humans. J. Neurosurg. 2000;93:815–820. doi: 10.3171/jns.2000.93.5.0815. [DOI] [PubMed] [Google Scholar]
- Verweij B.H. Muizelaar J.P. Vinas F.C. Peterson P.L. Xiong Y. Lee C.P. Mitochondrial dysfunction after experimental and human brain injury and its possible reversal with a selective N-type calcium channel antagonist (Snx-111) Neurol. Res. 1997;19:334–339. doi: 10.1080/01616412.1997.11740821. [DOI] [PubMed] [Google Scholar]
- Vyssokikh M.Y. Brdiczka D. The function of complexes between the outer mitochondrial membrane pore (Vdac) and the adenine nucleotide translocase in regulation of energy metabolism and apoptosis. Acta Biochim. Pol. 2003;50:389–404. [PubMed] [Google Scholar]
- Xiong Y. Gu Q. Peterson P.L. Muizelaar J.P. Lee C.P. Mitochondrial dysfunction and calcium perturbation induced by traumatic brain injury. J. Neurotrauma. 1997;14:23–34. doi: 10.1089/neu.1997.14.23. [DOI] [PubMed] [Google Scholar]
- Xiong Y. Shie F.S. Zhang J. Lee C.P. Ho Y.S. Prevention of mitochondrial dysfunction in post-traumatic mouse brain by superoxide dismutase. J. Neurochem. 2005;95:732–744. doi: 10.1111/j.1471-4159.2005.03412.x. [DOI] [PubMed] [Google Scholar]
- Xiong Y. Shie F.S. Zhang J. Lee C.P. Ho Y.S. The protective role of cellular glutathione peroxidase against trauma-induced mitochondrial dysfunction in the mouse brain. J. Stroke Cerebrovasc. Dis. 2004;13:129–137. doi: 10.1016/j.jstrokecerebrovasdis.2004.05.001. [DOI] [PubMed] [Google Scholar]