Abstract
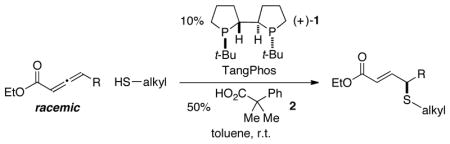
A method for the catalytic asymmetric γ sulfenylation of carbonyl compounds has been developed. In the presence of an appropriate catalyst, thiols not only add to the γ position of allenoates, overcoming their propensity to add to the β position in the absence of a catalyst, but do so with very good enantioselectivity. Sulfur nucleophiles are now added to the three families of nucleophiles (carbon, nitrogen, and oxygen) that had earlier been shown to participate in catalyzed γ additions. The phosphine catalyst of choice, TangPhos, had previously only been employed as a chiral ligand for transition metals, not as an efficient enantioselective nucleophilic catalyst.
Chiral sulfur-containing compounds have important applications in many areas of chemistry and biology, serving, for example, as antibiotics, as ligands for metal-based catalysts, as catalysts themselves, and as chiral auxiliaries.1 With respect to the catalytic enantioselective synthesis of sulfur-containing molecules, the conjugate addition of thiols to the β position of α,β-unsaturated carbonyl compounds has been the focus of intense interest.2 Furthermore, there has been recent progress in catalytic asymmetric sulfenylation α to a carbonyl group.3 In contrast, we are not aware of any methods for catalytic enantioselective sulfenylation of the γ position of carbonyl compounds.4
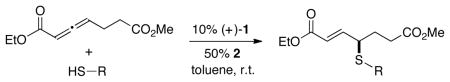
Trost and others have established that phosphines can catalyze certain γ additions of carbon, nitrogen, and oxygen nucleophiles to 2,3-allenoates and/or 2-alkynoates;5–8 on the other hand, the corresponding γ additions of sulfur nucleophiles have not been achieved. In this report, we describe a method that not only accomplishes γ functionalizations with this new family of nucleophiles, but also provides highly enantioenriched products (eq 1).9–12
 |
(1) |
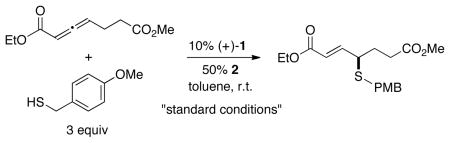
In the case of the carbon, nitrogen, and oxygen nucleophiles that have previously been employed in phosphine-catalyzed γ additions, there is generally no reaction between the nucleophile and an allenoate at room temperature in the absence of a catalyst. In contrast, thiols do react with allenoates, although not to afford the γ-addition product (Table 1, entry 1); instead, the uncatalyzed process leads to addition of the thiol to the β position.
Table 1.
Effect of Reaction Parameters on the Catalytic Asymmetric γ Addition of Thiols to Allenoates.
 | |||
|---|---|---|---|
| entry | change from the “standard conditions” | yield (%)a | ee (%) |
| 1 | no (+)-1 and no 2 | 0b | – |
| 2 | none | 89 | 92 |
| 3 | no 2 | <5 | – |
| 4 | PhOH instead of 2 | <5 | – |
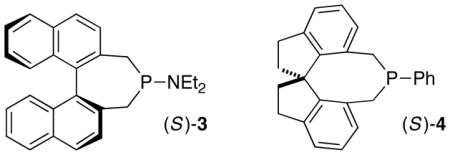
| 5 | (S)-3 instead of (+)-1 | <5 | – |
| 6 | (S)-4 instead of (+)-1 | 81 | 80 |
| 7 | 1.1, instead of 3, equiv of thiol | 80 | 92 |
All data are the average of two experiments.
The yield of the γ-addition product was determined by 1H NMR analysis with dibromomethane as an internal standard.
Addition occurs predominantly at the β position.
Nevertheless, through the use of an appropriate catalyst, the regioselectivity of the addition process can be altered such that the desired γ-addition product is generated not only in good yield, but also with very good enantioselectivity. In particular, chiral bisphosphine TangPhos (1), originally developed by Zhang as a ligand for rhodium-catalyzed asymmetric hydrogenations of olefins,13 along with a carboxylic acid additive,14 serves as a useful catalyst system, furnishing the γ-sulfenylated product in 89% yield and 92% ee (Table 1, entry 2). To the best of our knowledge, this is the first application of TangPhos as an effective chiral nucleophilic catalyst.15,16
In the absence of carboxylic acid 2, or if 2 is replaced by phenol,5d very little of the γ-addition product is observed (Table 1, entries 3 and 4). Other chiral phosphines (e.g., see entries 55d and 67d) furnish lower yield and/or ee. The use of 1.1 equivalents of thiol leads to a small loss in yield and no change in enantioselectivity (entry 7).
This phosphine-catalyzed asymmetric γ addition of thiols proceeds in good yield for an array of allenoates (Table 2).17 Thus, carbon–sulfur bond formation occurs with high ee in the presence of a variety of functional groups, including alkenes, alkynes, ethers, acetals, esters, and halides.
Table 2.
Catalytic Asymmetric γ Addition of Thiols to Allenoates: Scope with Respect to the Allenoate.
 | |||
|---|---|---|---|
| entry | R | yield (%)a | ee (%) |
| 1 | n-Pr | 80 | 91 |
| 2 | 78 | 92 | |
| 3 | 81 | 91 | |
| 4 | 87 | 85 | |
| 5 | (CH2)4OBn | 81 | 93 |
| 6 | 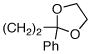 |
89 | 90 |
| 7 | (CH2)2CO2Me | 72 | 92 |
| 8 | (CH2)3Cl | 82 | 93 |
All data are the average of two experiments.
Yield of purified product.
This method for the catalytic asymmetric synthesis of sulfides is versatile not only with regard to the allenoate, but also the thiol (Table 3). A variety of substituted benzyl thiols, including hindered substrates, add to the γ position in good yield and ee (entries 1–5). Furthermore, heterocycles are compatible with the reaction conditions (entries 6 and 7). TangPhos also efficiently catalyzes the asymmetric γ addition of thiols that are not benzylic (entries 8–11); for the substrate illustrated in entry 11, exclusive γ addition by sulfur (none by oxygen7) is observed.
Table 3.
Catalytic Asymmetric γ Addition of Thiols to Allenoates: Scope with Respect to the Thiol.
 | |||
|---|---|---|---|
| entry | HS–R | yield (%)a | ee (%) |
| 1 | HS–Bn | 77 | 92 |
| 2 | 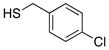 |
77 | 90 |
| 3 | 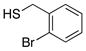 |
76 | 92 |
| 4 | 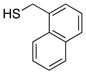 |
77 | 94 |
| 5 | 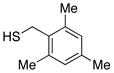 |
83 | 95 |
| 6 | 72 | 92 | |
| 7 | 67 | 89 | |
| 8 | 78 | 87 | |
| 9 | 80 | 93 | |
| 10 | 73 | 85 | |
| 11 | 79 | 88 | |
All data are the average of two experiments.
Yield of purified product.
The enantioenriched sulfides that are produced via phosphine-catalyzed γ additions to allenoates can be transformed into other useful compounds. For example, the sulfide can be converted into a thiol (eq 2), or highly stereoselective functionalizations of the olefin can be achieved (eq 3).
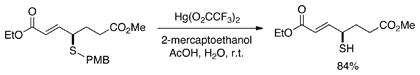 |
(2) |
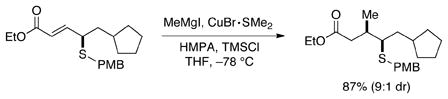 |
(3) |
In summary, the first method for the catalytic asymmetric γ sulfenylation of carbonyl compounds has been developed. Thus, in the presence of an appropriate catalyst, thiols not only add to the γ position of allenoates, overcoming their propensity to add to the β position in the absence of a catalyst, but do so with very good enantioselectivity. Sulfur nucleophiles are now added to the three families of nucleophiles (carbon, nitrogen, and oxygen) that had earlier been shown to participate in catalyzed γ additions. The phosphine catalyst of choice, TangPhos, had previously only been employed as a chiral ligand for transition metals, not as an efficient enantioselective nucleophilic catalyst. The development of additional phosphine-catalyzed asymmetric reactions is underway.
Supplementary Material
Acknowledgments
Support has been provided by the National Institutes of Health (National Institute of General Medical Sciences, grant R01-GM57034), Merck, and Novartis. We thank Dr. Ying Kit Chung for preliminary studies.
Footnotes
Supporting Information Available: Experimental procedures and compound characterization data. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.For some leading references, see: Damani LA, editor. Sulphur-Containing Drugs and Related Organic Compounds. John Wiley and Sons; New York: 1989. Toru T, Bolm C, editors. Organosulfur Chemistry in Asymmetric Synthesis. Wiley–VCH; New York: 2008. Pellissier H, editor. Chiral Sulfur Ligands: Asymmetric Catalysis. Royal Society of Chemistry; Cambridge, UK: 2009.
- 2.For a recent review, see: Enders D, Lüttgen K, Narine AA. Synthesis. 2007:959–980.For a recent example, see: Liu Y, Sun B, Wang B, Wakem M, Deng L. J Am Chem Soc. 2009;131:418–419. doi: 10.1021/ja8085092.
- 3.For a pioneering study, see: Marigo M, Wabnitz TC, Fielenbach D, Jørgensen KA. Angew Chem, Int Ed. 2005;44:794–797. doi: 10.1002/anie.200462101.
- 4.For a catalytic asymmetric method for the addition of nitrogen in the γ position of aldehydes, see: Bertelsen S, Marigo M, Brandes S, Diner P, Jørgensen KA. J Am Chem Soc. 2006;128:12973–12980. doi: 10.1021/ja064637f.
- 5.For some examples of the use of carbon nucleophiles, see: Trost BM, Li CJ. J Am Chem Soc. 1994;116:3167–3168.Zhang C, Lu X. Synlett. 1995:645–646.Chen Z, Zhu G, Jiang Q, Xiao D, Cao P, Zhang X. J Org Chem. 1998;63:5631–5635.Smith SW, Fu GC. J Am Chem Soc. 2009;131:14231–14233. doi: 10.1021/ja9061823.
- 6.For some examples of the use of nitrogen nucleophiles, see: Trost BM, Dake GR. J Org Chem. 1997;62:5670–5671.Liu B, Davis R, Joshi B, Reynolds DW. J Org Chem. 2002;67:4595–4598. doi: 10.1021/jo016154u.Lu C, Lu X. Org Lett. 2002;4:4677–4679. doi: 10.1021/ol0270733.
- 7.For some examples of the use of oxygen nucleophiles, see: Trost BM, Li CJ. J Am Chem Soc. 1994;116:10819–10820.(b) Reference 5b. Alvarez-Ibarra C, Csaky AG, de la Oliva CG. Tetrahedron Lett. 1999;40:8465–8467.Chung YK, Fu GC. Angew Chem, Int Ed. 2009;48:2225–2227. doi: 10.1002/anie.200805377.
- 8.For a review of catalytic enantioselective reactions of allenoates, see: Cowen BJ, Miller SJ. Chem Soc Rev. 2009;38:3102–3116. doi: 10.1039/b816700c.
- 9.For previous studies of catalytic enantioselective γ additions to allenoates/alkynoates wherein a γ stereocenter is produced, see References 5d and 7d.
- 10.For an investigation of catalytic enantioselective γ additions to allenoates/alkynoates wherein the stereochemistry of the δ carbon is controlled, see Reference 5c.
- 11.For a discussion of the difficulty in synthesizing this family of products, their utility, as well as an alternative route to their synthesis from triazolyated thiols, see: Armstrong A, Challinor L, Moir JH. Angew Chem, Int Ed. 2007;46:5369–5372. doi: 10.1002/anie.200701459.
- 12.Allenoates are readily synthesized by treatment of acid chlorides with Wittig reagents.
- 13.Tang W, Zhang X. Angew Chem, Int Ed. 2002;41:1612–1614. doi: 10.1002/1521-3773(20020503)41:9<1612::aid-anie1612>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 14.The potential benefit of additives such as carboxylic acids is described in the initial report by Trost (Reference 5a).
- 15.For reviews and leading references to nucleophilic catalysis by phosphines, see: Methot JL, Roush WR. Adv Synth Catal. 2004;346:1035–1050.Ye LW, Zhou J, Tang Y. Chem Soc Rev. 2008;37:1140–1152. doi: 10.1039/b717758e.Lu X, Zhang C, Xu Z. Acc Chem Res. 2001;34:535–544. doi: 10.1021/ar000253x.
- 16.For a review of enantioselective catalysis by chiral phosphines, see: Marinetti A, Voituriez A. Synlett. 2010:174–194.
- 17.Notes: (a) In all cases, the Z isomer of the product is not detected. (b) On a gram-scale, the reaction illustrated in entry 3 of Table 2 proceeds in 87% yield (purified product) and 91% ee. (c) At partial conversion, no kinetic resolution of the allenoate is observed. (d) By 31P NMR spectroscopy, we have determined that TangPhos is not protonated by acid 2 in toluene at room temperature. When TangPhos (10%), acid 2 (50%), and an allenoate are mixed, 31P NMR spectroscopy at −40 °C indicates that two compounds may be predominant (neither is TangPhos itself; compound 1: δ 79 (d) and 57 (d); compound 2: δ 64 (s)); upon addition of a thiol, both appear to be transformed into the γ-addition product, with liberation of TangPhos. Under the standard reaction conditions, the same resonances are observed by 31P NMR spectroscopy during the reaction (upon cooling to −40 °C; there is no resonance due to TangPhos), and TangPhos reappears when the reaction is complete. (e) TangPhos is susceptible to oxidation: After exposure to air for three days at room temperature, quantitative conversion to the bis(phosphine oxide) is observed (according to 31P NMR spectroscopy). The bis(phosphine oxide) is not an effective catalyst for γ additions of thiols to allenoates. (f) In an initial investigation, γ additions of ArSH proceed in low yields under our standard conditions. (g) Preliminary studies with truncated (monophosphine) relatives of TangPhos have furnished little of the γ-addition product.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


