Abstract
Converging evidence suggests that the motivation to seek cocaine during the postpartum period is significantly impacted by the competing incentives of offspring, a stimulus unique to this life stage. In the present study, the functional role of the medial preoptic area (mPOA), a critical site involved in maternal responsiveness, on processing incentive value of pup-associated cues and influencing response allocation for pup- over cocaine-associated environments was investigated using a concurrent pup/cocaine choice conditioned place preference (CPP) paradigm. Early postpartum females with bilateral guide cannulae aimed into the mPOA or into anatomical control sites were conditioned, from postpartum days (PPD) 4 to 7, to associate different uniquely featured environments with pups or cocaine. CPP was tested on PPD8 following intra-mPOA infusions of either 2% bupivacaine or saline vehicle. In two additional experiments, the effects of intra-mPOA infusions of bupivacaine on expression of conditioned responding induced by environments associated with either pups or cocaine were examined separately. Transient inactivation of the mPOA selectively blocked the conditioned preferences for pup-associated environments, significantly contrasting the robust pup-CPP found in non-surgical and intra-mPOA vehicle-treated females. In contrast, mPOA inactivation failed to alter cocaine-CPP in postpartum females. When given a choice between environments associated with pups or cocaine, transient functional inactivation of the mPOA altered choice behavior, biasing the preference of females toward cocaine-associated environments, such that almost all preferred cocaine- and none the pup-associated option. The anatomical specificity was revealed when inactivation of adjacent regions to the mPOA did not affect CPP responses for pups. The findings support a critical role for the mPOA in mediating pup-seeking behavior, and further suggest that the competing properties of pups over alternative incentives, including drugs of abuse, rely on mPOA integrity to provide relevant pup-related information to the circuitry underlying the choice behavior between pups and alternative stimuli.
Keywords: bupivacaine neuronal inactivation, cocaine, conditioned place preference, maternal motivation, mPOA, postpartum period
Cocaine use by women during the postpartum period remains a pressing public health problem, often with a detrimental effect on mother-infant interactions and infant development, and lifelong-negative consequences for both the mother and child (Roland and Volpe, 1989; Dembo et al., 1990; Burns et al., 1991; Kelly et al., 1991; Murphy et al., 1991; Barabach et al., 1992; Frank et al., 1993; Ball et al., 1997; Burns et al., 1997; D’Apolito, 1998; Johnson and Leff, 1999; Sterk et al., 2000; Walton-Moss and Becker, 2000; Minnes et al., 2005). On the other hand, studies examining temporal cocaine use patterns have generally reported a decrease in cocaine use during late pregnancy and lactation (Richardson and Day, 1991; Cornelius et al., 1994), together with an increased willingness to seek help and undergo addiction recovery treatment, suggesting that this period is one in which competitive maternal motivation (to care for their child) might influence drug use (Gottwald and Thurman, 1994; Barnet et al., 1995).
Preclinical research using maternal rodents provide remarkable parallels to these human reports, demonstrating a cocaine impact on maternal care-giving (Sobrian et al., 1990; Heyser et al., 1992; Zimmerberg and Gray, 1992; Johns et al., 1994; Kinsley et al., 1994; Peeke et al., 1994; Vernotica et al., 1996, 1999) and suggesting a complex motivational picture for cocaine and pup stimuli. For instance, there is a notable reduction of cocaine self-administration around parturition and early postpartum in chronically cocaine-exposed (for a period starting prior to breeding and during gestation and lactation) female rats relative to their levels prior to pregnancy (Hecht et al., 1999). This voluntary reduction in cocaine self-administration appeared to be the result of postpartum females spending considerable amounts of the test time performing maternal caregiving behaviors toward the pups (Hecht et al., 1999). This suggests that the pups, a powerful incentive to postpartum females (Wilsoncroft, 1969; Hauser and Gandelman, 1985; Fleming et al., 1989, 1994; Lee et al., 1999; Wansaw et al., 2008), may be a competing motivation during this period.
To compare the relative incentive value of pups versus cocaine in the postpartum period, prior studies in our laboratory used a concurrent pup/cocaine choice conditioned place preference (CPP) procedure that offered postpartum maternal rats a choice between an environment associated with maternal interaction with pups versus an environment associated with cocaine administration. These experiments demonstrated that the incentive salience of pups can exceed that of cocaine during the early postpartum period (Mattson et al., 2001; Seip and Morrell, 2007; Pereira et al., 2008). While cocaine remains highly reinforcing throughout the postpartum period, readily inducing robust place preference responses across a wide range of doses, the conditioned preferences of postpartum females for environments associated with cocaine are substantially reduced when pup-associated environments became the alternative option (Mattson et al., 2001; Seip and Morrell, 2007; Pereira et al., 2008; Seip et al., 2008). Collectively, these converging lines of evidence strongly suggest that, regardless of previous history of cocaine exposure, during the unique period of early postpartum the reinforcing efficacy of cocaine might be influenced by competition from the maternal motivation to seek and interact with the pups, resulting in relatively decreased cocaine-seeking behaviors.
How the incentive properties of such diverse stimuli, as pups and cocaine, are integrated by the neurobiological substrate that mediates incentive motivational processes is not completely understood. Certainly, however, the prevailing view in the literature is that subregional and subcircuit specialization across particular brain structures selectively processes stimulus-specific information, incentive comparisons, and ultimately mediates the choice among qualitatively different stimuli including natural versus pharmacological stimuli (and conditioned stimuli associated with them) (Brown et al., 1992; Carelli et al., 2000; Carelli and Ijames, 2001; Carelli and Wondolowski, 2003; Carelli and Wightman, 2004; Di Chiara et al., 1993; Neisewander et al., 2000; Schroeder et al., 2000; Schroeder et al., 2001; Grigson, 2002; Kelley and Berridge, 2002; Mattson and Morrell, 2005). One of the best studied set of CNS components mediating motivated responses to stimuli is the mesocorticolimbic dopaminergic system, including projections from the ventral tegmental area (VTA) to the nucleus accumbens (NA) and medial prefrontal cortex (mPFC) which are particularly important in attributing incentive value to stimuli (Robinson and Berridge, 1993; Robbins and Everitt, 1996; Ikemoto and Panksepp, 1999; Salamone and Correa, 2002), as are projections from the mPFC, hippocampus, and amygdala (BLA) to the NA (Groenewegen et al., 1996, 1999; Groenewegen and Uylings, 2000). In the case of a natural stimulus it is likely that additional specific brain regions contribute information to this circuit so that motivated responses appropriate for the natural stimulus can be generated.
In the particular state of motherhood, we posit that the medial preoptic area (mPOA), a site that integrates the hormonal and sensory inputs related to the maternal state (Numan and Insel, 2003), likely contributes pup-specific information to this motivational circuitry to influence the behavioral allocation toward offspring and related stimuli. Support for this hypothesis is provided by our previous work examining expression of Fos showing that subsets of neurons within the mPFC, NA, BLA, and mPOA were differentially activated depending on whether postpartum maternal rats expressed a conditioned place preference for pup- or cocaine-associated environments in a concurrent pup/cocaine choice CPP procedure (Mattson and Morrell, 2005). Notably, the mPOA was the only region that showed substantially greater activation (c-Fos and CART-IR) of neurons when postpartum females chose pup- over cocaine-associated environments. Data from additional studies shows that the mPOA mediates motivational processing of reproductive behaviors, including sexual (Band and Hull, 1990; Ågmo and Gómez, 1993; Paredes et al., 1993; Hoshina et al., 1994; Paredes and Baum, 1995; van Furth et al., 1995; Kindon et al., 1996; Paredes et al., 1998; Xiao et al., 2005; García-Horsman et al., 2008; Hurtazo et al., 2008; Sakuma, 2008) and maternal behavior (Lee et al., 1999; Numan and Insel, 2003; Pereira and Morrell, 2009). Furthermore, the mPOA has connections with VTA, NA, and mPFC (Numan and Callahan, 1980; Numan and Smith, 1984; Numan and Numan, 1997; Stack et al., 2002; Numan et al., 2005; Numan, 2006) that potentially allow pup-responsive mPOA neurons to interact with the motivational circuitry to influence the choice behavior for pups over alternative stimuli.
In the present study, we used the CPP procedure to investigate the hypothesis that the mPOA is a critical site mediating motivational processing of pup reinforcement, influencing maternal choice for pup- over cocaine-associated cues (interpreted as a measure of motivation, i.e. stimulus-seeking). Specifically, intra-mPOA microinfusion of bupivacaine was employed to test the hypothesis that transient neuronal inactivation of the mPOA would selectively block the conditioned preferences of postpartum females for pup-associated environments, shifting the response allocation toward cocaine-associated environment preference. To this aim, in Experiments 1 and 2, the effects of intra-mPOA administration of bupivacaine on expression of conditioned responding induced by environments associated with either pups or cocaine were examined separately. Experiment 3 examined the effects of transient inactivation of the mPOA during expression of place conditioning on the choice of postpartum females for pups- versus cocaine-associated environments in a concurrent pup/cocaine choice CPP procedure.
A methodological approach using transient site-specific neuronal inactivation by infusion of bupivacaine hydrochloride (an amide local anesthetic which blocks voltage-dependent sodium channels) was chosen to allow the analysis of the function of the mPOA in a temporally specific manner (Lomber, 1999). Specifically, this method allows the timing of the regional inactivation to be optimally chosen to be only at the time of the expression of a previously learned and established CPP, without interfering with the prior associative learning of the subject (i.e. acquisition phase), or with the memory consolidation of these learned associations, both needed to establish a CPP. Furthermore, this method preserves entirely the maternal caregiving in the conditioned environment, and in the homecage, so that interpretation of the inactivation of the mPOA is not confounded by effects other than at CPP test.
Experimental Procedures
Animals
All subjects were primiparous postpartum Sprague-Dawley female rats (original stock from Charles River Laboratories, Kingston, NY) bred in our colony at the Rutgers University Laboratory Animal Facility (accredited by the American Association for Accreditation of Laboratory Animal Care). Before giving birth, pregnant females were housed in individual cages (25.5 cm wide × 47 cm long × 23 cm high) lined with fresh woodchip bedding (Beta chip, Northeastern Products Corp., Warrensburg, NY) and containing shredded paper towels as nest-building material. Postpartum females remained with their pups for 24h after parturition, without interruption. On postpartum day 1 (birth=day 0), litters were culled to four male and four female pups per dam. All females were kept on a 12-hr light/dark cycle (light on at 0700AM) at 22 ± 1 °C, with ad libitum access to water, rat chow (Lab Diet 5008, PMI Nutrition International, LLC, Brentwood, MO) and sunflowers seeds.
All animal care and experimental procedures performed in this study were in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23, revised 1996), and were reviewed and approved by the Rutgers University Animal Care and Facilities Committee. All efforts were made to minimize the number of animals used and their suffering.
Stereotaxic Surgery
Females were randomly assigned to and prepared for one of the following sets of cannula placements: (1) females stereotaxically implanted with bilateral guide cannulae aimed at the mPOA, or (2) location control groups aimed immediately adjacent to but outside the mPOA and (3) females not subjected to surgery (non-surgical control group).
On day 1 postpartum, females were anesthetized with 1mL/kg of a solution that contained ketamine HCl (75.0 mg/mL), xylazine (7.5 mg/mL) and acepromazine maleate (1.5 mg/mL). Following anesthesia, females received a subcutaneous injection of 0.5% Marcaine along the intended incision area and were placed in a Kopf stereotaxic instrument. The incisor bar on the stereotaxic apparatus was set to 3.2 mm below the interaural line such that a flat skull position was achieved (with Bregma and Lambda at the same vertical coordinate). All females received bilateral implantations of 22-gauge stainless steel guide cannulae (Plastics One, Roanoke, VA, USA). For the mPOA site, guide cannulae were implanted 2.0 mm dorsal to target at the following coordinates: AP-0.5 (from bregma), ML±0.5 (from midline), and DV-6.5 (from the skull surface). For the dorsal control site, guide cannulae were implanted at the following coordinates: AP-0.5, ML±0.5, and DV-5.0. The guide cannulae were secured to the skull with stainless steel screws and cranioplastic cement. To maintain patency of the cannulae prior to injection, dummy stylets were inserted. Immediately after surgery, females were reunited with their pups in their home cages. Overall condition and health were evaluated daily, particularly after surgery and during CPP conditioning and testing procedures. All females remained healthy throughout the experiment, fully exhibiting typical maternal behaviors, and their pups gained weight and developed normally.
Conditioned Place Preference Apparatus
The place preference apparatus consisted of three equal-sized chambers (21.0 cm wide × 27.5 cm long × 20.5 cm high) made of clear Plexiglas, connected by manually operated guillotine doors. Each side chamber contained unique contextual cues (that remained present throughout all conditioning and testing sessions) of wallpaper (vertical or horizontal black-and-white stripes) and tactile flooring, either small paper squares (Alpha-Dri, W. F. Fisher & Sons) or corncob pieces (Bed-o-Cobs, The Andersons, Maumee, OH) scattered on solid grey floors, becoming a unique environment for the association learning and testing of the CPP procedure. The center chamber had white wallpaper and solid grey floor. Luminance (measured with a Konica Minolta Luminance Meter LS-100, Japan) was equal in all three chambers. Subject location within the CPP apparatus was monitored by means of infrared beam breaks, providing data on the time spent and locomotor activity in each chamber via an automated computer based interface (Med Associates Inc., St. Albans, VT, USA/MED-PC® Version IV Research Control and Data Acquisition System).
Experimental Design and Procedure
Separate groups of postpartum female rats were used for each of the CPP experiments of this study. In all three experiments, the behavioral procedure consisted of a 5-day schedule with three distinct phases, pre-conditioning, conditioning, and post-conditioning testing.
At the pre-conditioning session, females were exposed to the CPP apparatus for a single baseline session before conditioning, i.e. prior to any exposure to unconditioned stimuli in the apparatus, to identify preconditioning chamber preferences, on day 4 postpartum. Each female was placed into the center chamber and allowed free access to the entire apparatus for 60 min. The amount of time spent in each chamber was used to assess individual unconditioned chamber preferences. The majority (68%) of females did not exhibit an unconditioned preference for either conditioning side chamber, and chamber assignment was counterbalanced (on a group basis). Of those females that did exhibit a side chamber preference, relevant unconditioned stimulus was assigned to the least-preferred chamber. In the particular case of Experiment 3, females exhibiting an unconditioned side chamber preference were counterbalanced for which chamber was to be paired with either unconditioned stimulus (i.e. pups or cocaine).
Experimental groups of early postpartum females were exposed to unconditioned stimuli (pups and/or cocaine) during conditioning between postpartum days (PPD) 4–7, and then tested for their conditioned preferences on PPD8 (~12:00 PM). All parametric aspects of conditioning (i.e. number of conditioning sessions, session duration, cocaine doses and route of administration, etc) used in the present study were selected based on our previous research demonstrating that it reliably produces maximal CPP effect sizes in postpartum females for both pups and cocaine (Mattson et al., 2001; Seip and Morrell, 2007; Pereira et al., 2008; Seip et al., 2008; Wansaw et al., 2008). Specifically, the present study uses intraperitoneal (IP) administration of cocaine and hence 30-min cocaine-conditioning sessions to maximize chamber exposure during rising and peak plasma concentrations of the drug (Lau et al. 1991; Festa et al. 2004), which is important for establishing a positive incentive salience for the drug experience. On the other hand, at least 1-h pup-conditioning sessions are needed to allow postpartum females’ full expression of maternal behavior, in order to elicit robust pup-associated CPP (Fleming et al. 1994; Mattson et al. 2001, 2003).
Intracranial Injection Procedure Females were gently handled once daily for approximately 5 min for 2 days before CPP testing, to habituate them to the experimenter handling associated with the intracranial drug administration as well as having their stylets removed and reinserted. On postpartum day 8, just before CPP testing, females were gently restrained while their dummy stylets were removed and 28-gauge stainless steel injectors were inserted into the guide cannulae, extending 2.0 mm beyond the tip of the guide cannulae. Injectors were connected by PE-10 tubing to 10μL Hamilton syringes, and bilateral infusions were driven simultaneously by a two-syringe infusion pump (Harvard 22 syringe pump; Harvard Apparatus, Holliston, MA). Infusions of bupivacaine (2% solution; Sigma, St. Louis, MO) or saline vehicle were delivered at a rate of 0.5μL/min. Each side received 1.0 μL total volume, and the injectors were left in place for an additional 1 min to allow for diffusion of the drug. Immediately after, stylets were replaced. Assignment to bupivacaine or vehicle treatment was counterbalanced, so that half of the females received site-specific bilateral infusions of bupivacaine, and the other half received site-specific bilateral infusions of saline. Five min later, postpartum females were placed in the CPP apparatus and their conditioned place preference for stimulus-associated environment was evaluated during a 60-min test. Behavioral control females did not undergo surgery but were similarly handled and tested as the cannulated groups.
Bupivacaine hydrochloride in a dose of 20mg/mL dissolved in 0.9% saline solution was used to suppress neural activity of the mPOA throughout the entire CPP test session. Bupivacaine, like the structurally similar amide-linked anesthetic lidocaine, produces an immediate neuronal inactivation by blocking voltage-dependent sodium channels, and hence action potential conductance both in neuronal cell bodies and axons (Hille, 1966 and 1977). We chose bupivacaine due to its longer duration of action over lidocaine (lidocaine approximately 10–30 min range, bupivacaine approximately 30–75 min range, Caterall and Mackie, 1986). An infusion of 1 μL volume of 2% bupivacaine was selected on the basis of previous studies of estimates of the effective spread (i.e. the distance over which there is a physiological effect) and time course of neural inactivation caused by intracranial lidocaine infusion (Boehnke and Rasmusson, 2001; Tehovnik and Summer, 1997). Furthermore, using an infusion protocol, including infusion rate and volume, and coordinates identical to those utilized in the present study, we have previously established functional site specificity of the mPOA inactivation with respect to adjacent dorsal, rostral, lateral, and caudal anatomical control sites (Pereira and Morrell, 2009), demonstrating that bupivacaine administration resulted in selective inactivation of the mPOA only.
Specific Experiments
Experiment 1 - Effect of transient mPOA Inactivation on the expression of pup-induced CPP
This experiment examined the effect of transient inactivation of the mPOA during expression of place conditioning on the preference of postpartum females for pup-associated environments. Postpartum females were separately conditioned to associate different uniquely featured environments with their own 8-pup litter or with no specific unconditioned stimulus (empty chamber), once a day from PPD4-7. During each exposure session interval for either unconditioned stimulus, the females were confined for 1 h to the respective side chamber by closing the guillotine doors. Conditioning sessions started at 11:00 AM for the empty chamber condition. Litters were removed from their maternal cages and housed in same-litter groups in small cages. Females were returned to their home cage after the morning conditioning session for 1 h without their pups. Pup-conditioning sessions started at 13:00 PM. Immediately prior to the session, each female’s 8-pup litter was placed in the corresponding conditioning chamber. Thus, postpartum females underwent the pup-conditioning session after being deprived of pups for 2h, and their pups, used for conditioning, were also deprived of maternal care for the same duration.
Conditioned place preference for the pup-associated environment was tested on PPD8, the day after the last conditioning session, by allowing females to explore all three chambers for 60 min, with no unconditioned stimuli present. Before CPP testing, females received site-specific bilateral infusions (1.0μL/side) of either 2% bupivacaine or saline vehicle.
Experiment 2 - Effect of transient mPOA inactivation on the expression of cocaine-induced CPP
This experiment examined the effect of transient inactivation of the mPOA during expression of place conditioning on the preference of postpartum females for cocaine-associated environments. A 5.0 mg/kg dose of cocaine was selected on the basis of previous research showing that it produces maximal CPP responses in postpartum females (Seip et al., 2008). Postpartum females received separate conditioning sessions with each one of two unconditioned stimuli, cocaine or saline, daily from PPD4-7; each stimulus for 30 min at a time. Immediately before each conditioning session, females were intraperitoneally injected with 5mg/mL/kg cocaine or same volume of saline, and confined to their respective chamber. Cocaine hydrochloride (National Institute of Drug Abuse, Research Triangle Park, NC, USA) was freshly dissolved daily in 0.9% saline solution. The cocaine-conditioning session was always last each day, ensuring at least 12 h for residual cocaine to clear from females’ circulation before subsequent conditioning sessions (Lau et al., 1991; Wansaw et al., 2005). No postpartum females developed any skin lesions or other pathology in the abdominal cavity or the mammary glands due to IP cocaine injections.
The conditioned place preferences of postpartum females for the cocaine-associated environment was tested on PPD8 following intracranial infusion (1.0μL/side) of either 2% bupivacaine or saline vehicle. Five minutes later, females were placed in the center chamber of the CPP apparatus and allowed free access to the entire CPP apparatus for 60 min, with no unconditioned stimuli present. Because cocaine-CPP is retained in a single retest for up to a week after initial chamber preferences (Seip et al., 2008), postpartum females were retested the following day with the alternate treatment (within-subject design).
Experiment 3 - Effect of transient mPOA inactivation on motivated choice of pup- versus cocaine-associated environments in a concurrent pup/cocaine choice CPP
This experiment examined the effect of transient inactivation of the mPOA during expression of place conditioning on the choice of postpartum females for pup- versus cocaine-associated environments in a concurrent pup/cocaine choice CPP procedure. To allow for the analysis of female’s behavioral choice following transient mPOA inactivation, it was important to choose a design that would provide preference categories represented relatively equally, and with a sufficient number of females with either a pup- or cocaine-associated CPP, so that the effect of a transient inactivation of the mPOA could be detected in relation to this baseline preference. As shown in Figures 1A and 1B, both 1.0 and 5.0 mg/kg IP cocaine similarly induced robust cocaine-CPP in postpartum females. In a concurrent pup/cocaine choice CPP procedure, the 5.0 mg/kg cocaine dose continues to establish a place preference in the majority of the females (67%), with only 10% preferring the pup-associated environment (Figure 1C and 1D). When given a choice between environments associated with pups versus 1.0 mg/kg IP cocaine, however, the desired bimodal distribution of preferences emerges, with 40% of the females preferring the cocaine- and 40% preferring the pup-associated environment (Figure 1C and 1D). On the basis of this preliminary data, a dosage of 1.0 mg/kg of intraperitoneally-administered cocaine was chosen for the concurrent pup/cocaine choice CPP study to enable us to detect statistically significant effects of transient mPOA inactivation on the choice behavior of early postpartum females.
Figure 1.
Comparison of the conditioned response of postpartum females to an environment associated with either 1.0 or 5.0 mg/kg IP cocaine versus one associated with saline or pups. (A) Conditioned chamber preferences and (B) mean time spent in each chamber of the apparatus during the cocaine-CPP test session, after conditioning with IP injections of either 1.0 or 5.0 mg/kg cocaine and saline. (C) Conditioned chamber preferences and (D) mean time spent in each chamber of the apparatus during the concurrent pup/cocaine-CPP test session, after conditioning with IP cocaine injections (either 1.0 or 5.0 mg/kg) and maternal interaction with pups.
In the current experiment, postpartum females were separately conditioned to associate different uniquely featured environments with each one of two unconditioned stimuli: their own 8-pup litter or 1mg/mL/kg IP injection of cocaine. Pup-conditioning sessions started at 11:00 AM, after females were pup deprived for 2 hours. Postpartum females were confined to the assigned pup-conditioning chamber for 1 hour with their pups. Females and pups were then returned to their home cage and left undisturbed for 2 hours before receiving the cocaine-conditioning session. Females were intraperitoneally injected with cocaine and immediately placed in the alternate conditioning chamber for 30 minutes.
On PPD8, following intracranial infusions (1.0μL/side) of either 2% bupivacaine or saline vehicle, CPP was assessed by allowing females free access to the entire apparatus for 60 min.
Histology and Analysis of infusion Sites
Upon completion of CPP testing, postpartum females were deeply anesthetized with Nembutal and perfused intracardially with saline followed by a 4.0% formaldehyde solution. Brains were removed and stored in 4.0% formaldehyde for 48h. The brains were frozen using dry ice and serial cross sections of 30μm were cut around the implantation site on a cryostat. The sections were mounted on slides, stained with Cresyl violet, and cover slipped. The location of injection sites were documented with the aide of a Zeiss Axioplan microscope equipped with a Neurolucida imaging system, and the neuroanatomical atlas of Paxinos and Watson (1997).
Statistical Analysis
The time spent in each chamber during pre- and postconditioning sessions was used to identify subjects with preference for a particular apparatus chamber. A subject was categorized as having a preference for a particular apparatus chamber if spent at least 50% of the total session time in one chamber and that was at least 25% greater than either time in any other chamber (Mattson et al., 2001). Subjects that did not meet the preference criterion were categorized as having no preference.
CPP results are expressed as proportion of individual subjects exhibiting an environment preference and as mean ± SEM time the group spent in each apparatus environment. Preconditioning environment preferences and times were compared to those of postconditioning, to provide evidence of conditioning. CPP was operationally defined as a statistically significant increase in conditioned preferences of individuals and group time spent in the stimulus- (pup, cocaine) associated environment postconditioning relative to preconditioning baseline. Chi-square goodness-of-fit test was used to analyze categorical data from pre- and postconditioning environment preferences of the individual females. Between-group chamber preference comparisons were examined using Chi-square test of independence and Fisher’s exact test. Environment times were analyzed using two-way repeated measures ANOVA, with group as the between-subject variable and chamber (side) and session (preconditioning vs. postconditioning) as within-subject factors. Locomotor scores are expressed as mean ± SEM and were analyzed using a one-way ANOVA. Significant main effects and interactions were further analyzed using Tukey’s HSD tests. A significance level of p<0.05 was used for all data.
Results
Location of the Cannulae Implantation Site
Figures 2A, 3A, and 4A represent schematic cross sections of the rat brain showing the placement of the injection cannulae tips based on the microscopic analysis of cresyl violet-stained sections. Figure 2A shows the placement of the injection cannula tips for the mPOA (nBUP=10 and nVEH=9) groups tested for pup-CPP, and Figure 3A shows those for the groups of postpartum females tested for cocaine-CPP (n=12). Figure 4A shows the location of the mPOA infusion sites for the postpartum groups tested in the concurrent pup/cocaine choice CPP procedure (nBUP=9 and nVEH=8). Histological analysis demonstrated that all mPOA groups had sites distributed in a similar overall pattern, with most of the bilateral injections sites located between −0.26 and −0.88 mm from bregma.
Figure 2.
Effect of transient inactivation of the mPOA on expression of pup-induced CPP. (A) Schematic representation, based on the microscopic analysis of cresyl violet-stained sections, of mPOA and dorsal control sites for all rats receiving infusion treatments. Circles represent the most ventral extent of the injector tracks in the brain. Plates were taken from the atlas of Paxinos and Watson (1997). Numbers beside each plate indicate the distance caudal to bregma in millimeters. (B) Pup-associated chamber preferences and times during the pre- and postconditioning sessions. (C) Conditioned chamber preferences and (D) mean time spent in each chamber of the CPP apparatus during the test session by non-surgical control (n=10), dorsal bupivacaine-treated (n=7), mPOA bupivacaine-treated (n=10), and mPOA vehicle-treated (n=9) postpartum females. * denotes significant differences between groups, and # indicates significant within-group differences in pre- vs. postconditioning responding; all P<0.05.
Figure 3.
Effect of transient inactivation of the mPOA on expression of cocaine-induced CPP. (A) Schematic representation, based on the microscopic analysis of cresyl violet-stained sections, of injection sites for all rats receiving infusion treatments. (B) Cocaine-associated chamber preferences and times during the pre- and postconditioning sessions. (C) Conditioned chamber preferences and (D) mean time spent in each chamber of the CPP apparatus by non-surgical control females (n=12), and mPOA cannulated females (n=12) following either vehicle or inactivation treatment with 2% bupivacaine. * denotes significant differences between groups, and # indicates significant within-group differences in pre- vs. postconditioning responding; all P<0.05.
Figure 4.
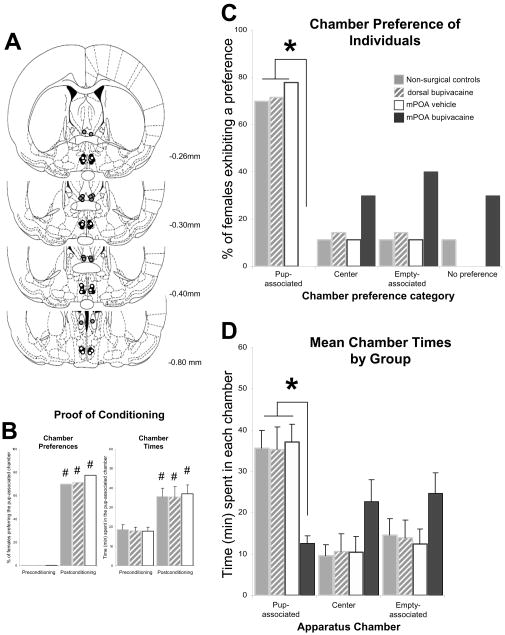
Effect of transient mPOA inactivation on motivated choice of pup- versus cocaine-associated environments in a concurrent pup/cocaine-CPP. (A) Schematic representation, based on the microscopic analysis of cresyl violet-stained sections, of injection sites for all rats receiving infusion treatments. (B) Chamber preferences and times during the pre- and postconditioning sessions for the subset of females that exhibited a conditioned preference for either pup- or cocaine-associated environments. (C) Conditioned chamber preferences and (D) mean time spent in each chamber of the CPP apparatus during the test session by non-surgical control (n=10), mPOA bupivacaine-treated (n=9), and mPOA vehicle-treated (n=8) postpartum females. *denotes significant differences between groups, and # indicates significant within-group differences in pre- vs. postconditioning responding; all P<0.05.
Experiment 1 - Effect of Transient mPOA Inactivation on Expression of Pup-induced CPP
In the preconditioning test, the majority of postpartum females showed no preference for either side compartments, and, on average, spent similar amounts of time in each chamber of the CPP apparatus. Importantly, unconditioned chamber preferences [χ2=5.137, df=6, P=ns] and times were similar and did not differ among groups [chamber × group interaction: F(3,32)=0.1649, P=ns], indicating that differences in baseline responding cannot account for any selective effect of the bupivacaine-induced changes in conditioned preferences.
After conditioning, non-surgical control females exhibited a robust CPP for the pup-associated environment as demonstrated by comparison analysis of pre- and postconditioning environment preferences [χ2 (3, N=10)=40.533, P<0.05]. Similarly, postpartum females receiving infusion of saline vehicle into the mPOA prior to CPP testing exhibited substantial conditioned preference for the pup-associated environment [preconditioning versus postconditioning environment preferences: χ2 (3, N=9)=40.083, P<0.05]. Thus, the majority of females receiving vehicle infusion into the mPOA preferred the environment associated with the pups (78%), and this proportion was not different from the non-surgical control group (70%) (Fisher’s Exact Test, P=ns), demonstrating that surgery, handling and intracranial drug administration procedures neither affected the acquisition nor the expression of pup-CPP of postpartum females (Figure 2C). Furthermore, the time spent in the pup-associated environment significantly increased in both the non-surgical control group and in the group receiving microinfusion of saline vehicle into the mPOA following conditioning [session × group interaction: F(3,32)=4.36, P<0.05; chamber × session interaction: F(1,32)=35.4, P<0.001; chamber × group × session interaction: F(3,32)=5.7, P<0.01]. In addition, both groups similarly spent more test time in the pup-associated environment than in the alternative one associated with no unconditioned stimuli (Tukey’s HSD test: both Ps<0.05; Figure 2D). Thus, both environment preferences and times changed significantly as a function of conditioning, indicating a robust CPP associated with pups (see Figure 2B).
In contrast, intra-mPOA infusions of bupivacaine prior to CPP testing completely blocked the expression of the conditioned preferences for the pup-associated environment. Thus, none of the mPOA bupivacaine-treated females preferred the environment associated with pups (0%), whereas the majority of control females (both groups) preferred the pup-associated option (70–80%, Fisher’s Exact Test, both Ps<0.05; Figure 2C), indicating that neuronal inactivation, not the mechanical or osmotic effects of drug infusion, affected expression of pup-CPP in postpartum females. Furthermore, the time spent in the pup-associated environment was significantly reduced in mPOA-inactivated females compared to notable time spent in the pup-associated environment by both control groups (Tukey’s HSD test: both Ps<0.001; Figure 2D).
Proof of Anatomical Specificity
Regional specificity limited to mPOA of the CPP effects was demonstrated since inactivation of anatomical sites adjacent to the mPOA had no effect on CPP for pup-associated environments. Figure 2A shows the implant locations for the group of postpartum females tested for pup-associated CPP following infusion of bupivacaine into adjacent dorsal sites to the mPOA (n=7). In addition to this deliberately chosen dorsal control site, as a result of the normal variation in placements found with stereotaxic surgery targeting a small brain region, nine additional postpartum females treated with bupivacaine before CPP testing had infusion sites that bilaterally targeted control structures immediately outside the mPOA. Three females had an asymmetrical placement that resulted in a unilateral infusion into the lateral mPOA and a unilateral infusion into the third ventricle, 2 females had their cannulae rostral to the mPOA into the diagonal band of Broca; and 4 females had their cannulae caudal to the mPOA into the anterior hypothalamus.
Most of theses control females receiving pre-testing bupivacaine infusion into dorsal sites preferred the environment associated with pups (71%). Thus, the conditioned environment preferences and times of dorsal control females were not different from those of non-surgical and vehicle-treated control females [χ2=1.778, df=6, P=ns; session × chamber × group interaction: F(2, 23)=0.001, P=ns; Figures 2C and 2D]. Similarly, of those postpartum females with cannulae sites that bilaterally targeted control structures either rostral or caudal to the mPOA, 78% showed robust pup-associated CPP after bupivacaine infusions (caudal to the mPOA, 3 of 4; rostral, 2 of 2; unilateral 2 of 3 preferred the environment associated with pups). Thus, the disruptive effect on pup-associated CPP responses produced by pre-testing bupivacaine infusion into the mPOA appear unlikely to have been mediated by diffusion to these other dorsal, anterior, or posterior structures.
Experiment 2 - Effect of Transient mPOA Inactivation on Expression of Cocaine-induced CPP
Prior to conditioning, unconditioned chamber preferences [χ2=3.152, df=3, P=ns] and times [chamber × group interaction: F(1,22)=0.078, P=ns] were similar and did not differ among groups. Following conditioning, a significant CPP to 5.0 mg/kg IP cocaine occurred in both the non-surgical and the vehicle-treated control groups, with chamber preferences [preconditioning versus postconditioning environment preferences: χ2 (3, N=12)=8.643, P<0.05 and χ2 (3, N=12)=8.637, P<0.05, respectively] and times [session × chamber interaction: F(1,22)=57.2, P<0.01] differing statistically between the pre- and postconditioning sessions (see Figure 3B).
There was no significant difference in chamber preference [χ2=, df=3, P=ns] nor times [session × chamber × group interaction: F(1,22)=0.61, P=ns] between the non-surgical control and saline microinjection groups during the CPP test (Figure 3C–D).
Transient mPOA inactivation had no impact on the motivational responsivity of postpartum females to cocaine-related stimuli (Figure 3C–D). Thus, intra-mPOA bupivacaine infusion prior to CPP testing affected neither the number of early postpartum females preferring the cocaine-associated environment (Fisher’s Exact Test, both Ps=ns), nor the time spent by the group in the cocaine-associated environment (Tukey’s HSD test, both Ps=ns) compared to the non-surgical control group. Consequently, postconditioning environment preferences did not differ within and between groups, with the vast majority of postpartum females, regardless of treatment, preferring the cocaine-associated environment. All groups also similarly spent more time in the cocaine-associated environment than in the saline-associated environment (Tukey’s HSD test, all Ps=ns; Figure 3D).
Experiment 3 - Effect of transient mPOA inactivation on motivated choice of pup- versus cocaine-associated environments in a concurrent pup/cocaine choice CPP
Prior to conditioning (at preconditioning test) the majority of postpartum females showed no preference for either side compartment, and, on average, spent similar amounts of time in each chamber of the CPP apparatus. Furthermore, there were no differences in the unconditioned chamber preferences between groups [χ2=1.893, df=6 P=ns], nor were there significant group [F(2,24)=0.047, P=ns], chamber [F(1,24)=0.0001, P=ns] and group × chamber interaction [F(2,24)=0.055, P=ns] effects. A robust CPP effect was demonstrated, as environment preferences [behavioral control group: χ2(3, N=10)=22.67, P<0.05 and vehicle-treated group: χ2(3, N=8)=11.2, P<0.05] and times changed significantly between the pre- and postconditioning sessions (see Figure 4B).
After conditioning, most females from the two control groups developed a preference for environments associated with one of the two unconditioned stimuli, either cocaine or pups (Figure 4C). There was no significant difference in environment preferences between the non-surgical and saline microinjection control groups [χ2=0.064, df=3, P=ns; Figure 4C]. Thus, 40% of the non-surgical control females showed a preference for the cocaine-associated environment, and 40% preferred the pup-associated environment. Similarly, the vehicle-treated group showed a bimodal distribution in which postpartum females preferred either the pup- or the cocaine-associated environment after conditioning.
In contrast, intra-mPOA infusions of bupivacaine just prior to conditioned place preference testing selectively and entirely blocked the conditioned preference of postpartum females for the pup-associated environment (Figure 4C). Furthermore, a substantial and statistically significant majority of the postpartum females infused with bupivacaine into the mPOA preferred the cocaine-associated environment (78%). Thus, the majority of early postpartum females preferred the cocaine- over the pup-associated environment following pre-testing bupivacaine infusion into the mPOA, highly contrasting the bimodal distribution of conditioned preferences of both control groups for pup- and cocaine-associated environments (Figure 4C).
As shown in Figure 4D, the analysis of environment times during the CPP test revealed a significant chamber × group interaction effect [F(2,24)=3.74, P<0.05], with overall time spent in the cocaine-associated environment significantly longer in the bupivacaine-treated group than in either non-surgical or vehicle-treated control groups (Tukey’s HSD test, both Ps<0.05). Pup- and cocaine-associated environment times during the expression test were similar and did not differ between the two control groups (Tukey’s HSD test, all Ps=ns). However, the time spent in the cocaine-associated environment by bupivacaine-treated females was significantly longer than the time spent in the pup-associated environment (Tukey’s HSD test, P<0.05; Figure 4D).
Five additional postpartum females treated with bupivacaine before testing had infusion sites that bilaterally targeted structures immediately rostral or caudal to the mPOA. Of these females, 3/5 preferred the pup-associated environment and 2/5 the preferred the cocaine-associated environment, resulting in a postconditioning environmental preference pattern similar to that of the control groups. This further supports the anatomical specificity of the data, showing that bilateral infusions of bupivacaine into the mPOA are needed to block the conditioned preferences of postpartum females for the pup-associated environment.
It is important to note that the CPP responses of mPOA bupivacaine-treated females for cocaine-associated environments in the concurrent pup/cocaine choice procedure are comparable in magnitude to those of postpartum females presented with a CPP choice between an environment associated with 1.0mg/kg IP cocaine and one associated with saline (78% and 75%, respectively; Figures 1 and 4). This together with the fact that transient inactivation of the mPOA completely and selectively blocked the conditioned preference of postpartum females for the pup-associated environment, regardless of which alternative option was presented (Experiments 1 and 3), suggest that the shift in the choice preference toward the cocaine-associated environments induced by mPOA inactivation in the concurrent choice procedure is most likely due to the result of a reduction in pup-associated environment preferences.
Transient inactivation of mPOA did not affect locomotor activity during CPP test
Locomotor activity during the expression test did not differ between the groups, suggesting that exploratory behaviors remained intact during CPP testing. Across mPOA-cannulated groups, locomotor scores during the 60-min CPP test were similar regardless of whether females received bupivacaine or saline treatment, and were not different from non-surgical controls [treatment × group interaction: Experiment 1: F(2,24)=0.06, P=ns; Experiment 2: F(1,22)=0.0137, P=ns; Experiment 3: F(2,26)=1.17, P=ns].
Discussion
The present results indicate that the functional integrity of the mPOA is necessary for the expression of context-induced pup- but not cocaine-seeking behavior. Thus, transient inactivation of the mPOA, produced by infusion of bupivacaine, completely eliminated the ability of pup-associated environmental cues to promote pup-seeking behavior, while conditioned behavioral responses to cocaine-associated stimuli were left intact. When given a choice between environments associated with pups or cocaine, functional inactivation of the mPOA substantially shifted the choice preference of early postpartum females toward cocaine-associated environments, demonstrating that the mPOA is necessary to promote the choice of pup- over cocaine-associated environments. Collectively, the present findings provide strong evidence that the functional integrity of the mPOA is necessary for the expression of context-induced pup-seeking behavior, suggesting that the mPOA encodes information about goal-directed behaviors for pups. Furthermore, these results demonstrate that the mPOA influences response allocation processes by providing relevant pup-related signal into the circuitry processing the choice behavior between pups and alternative stimuli.
Bupivacaine inactivation has been successfully used in behavioral studies in our laboratory and the laboratories of others to induce effective temporary neural inhibition of discrete brain areas (Waraczynski and Perkins, 2000; Ragozzino et al., 2002; Evans et al., 2003; Holahan and White, 2004; Broadbent et al., 2006; Perrin et al., 2007; Floresco et al., 2008; Hsu and Packard, 2008; Hurtazo et al., 2008; Pereira and Morrell, 2009). The effective spread of neural inactivation of the structurally similar amide-linked anesthetic lidocaine has been well characterized both in cortical and subcortical structures using electrophysiological and other indices of neural activity (Malpeli and Schiller 1979; Sandkühler and Gebhart, 1984; Sandkühler et al., 1987; Tehovnik and Sommer, 1997; Boehnke and Rasmusson, 2001; Pereira de Vasconcelos et al., 2006). Accordingly, the functional spread of intra-cerebral bupivacaine infusion for the present parameters (1μL, 2%) would be approximately spherical with a radius of 500–620 μm from the tip of the injector (Tehovnik and Sommer, 1997; Boehnke and Rasmusson, 2001). Since most of the infusion sites in the present study were centrally located within the mPOA, and taking into account the approximate extent of the mPOA and the limited diffusion of bupivacaine, the vast majority of the physiological effects of bupivacaine infusions would be expected to be limited to tissue inclusive of the mPOA. Consistent with this is the fact that the disruptive effect of intra-mPOA infusion of bupivacaine on conditioned preferences for pup-associated environments was anatomically specific. Thus, infusions of bupivacaine into neighboring adjacent sites dorsal, rostral, or caudal to the mPOA were ineffective at blocking the expression of pup-associated environment preferences. As a complete blockade of the pup-CPP was observed following mPOA inactivation, the present results demonstrate that the expression of pup-seeking behavior motivated by pup-associated environmental stimuli depends upon the functional integrity of the mPOA.
Given that intra-mPOA bupivacaine infusion impaired conditioned responding in a stimulus-specific manner (i.e., selectively blocked the expression of a pup-CPP and failed to alter cocaine-CPP) and did not alter locomotor activity of females, it is unlikely that intra-mPOA bupivacaine-induced impairments in pup-seeking behavior were due to: (1) nonspecific performance deficits (i.e., sensory, motor, attentional processing and/or general memory impairment), (2) bupivacaine-induced novelty (i.e., a novel state produced by mPOA inactivation could have nonspecifically and unconditionally disrupted the expression of the CPP), (3) a state-dependent effect, such that differing states between acquisition (without bupivacaine treatment) and expression (with bupivacaine treatment) may have interfered with the retrieval of that association during the test session; Tzschenthe and Schmidt, 1997), or (4) induction of an aversive state. In other words, if any of the above-mentioned possibilities accounted for why bupivacaine infusion into the mPOA blocked pup-CPP, then it is unclear why any of these effects would have only selectively affected the expression of a pup-CPP and not of cocaine-CPP in postpartum females.
It is therefore reasonable to argue that transient mPOA inactivation impaired the ability of pup-associated stimuli to elicit pup-seeking behaviors by selectively disrupting pup-related motivational processes. Possibly the mPOA is involved in determining the incentive salience of pup-associated (environmental) conditioned stimuli and/or pups themselves. Specifically, mPOA inactivation might have blocked the expression of a previously learned pup-induced CPP by reducing the salience of the conditioned effects of pups and/or the incentive efficacy of the pups. In this sense, conditioned preference has been shown to be sensitive to postconditioning changes in incentive value, induced by stimulus devaluation manipulation such as sensory-specific satiety or experimentally induced aversion (Adams, 1982; Yin and Knowlton, 2002). Several lines of research support the notion that the mPOA is involved in incentive motivational processes, particularly in relation to social incentives, including maternal and sexual-motivated behaviors. For instance, using an operant bar-pressing procedure, Lee and colleagues (1999) demonstrated that bar-pressing for access to pups in postpartum females is substantially reduced following permanent mPOA lesions, while food-reinforced bar-pressing was unimpaired. Similarly, several reports using both permanent mPOA lesions and pharmacological manipulations have demonstrated involvement of the mPOA in sexual incentive motivation in both male (Band and Hull, 1990; Hughes et al., 1990; Ågmo and Gómez, 1993; Paredes et al., 1993; Paredes and Baum, 1995; van Furth et al., 1995; Kindon et al., 1996; Paredes et al., 1998; García-Horsman et al., 2008; Hurtazo et al., 2008) and female rats (Hoshina et al., 1994; Xiao et al., 2005; Coria-Avila et al., 2008; Sakuma, 2008).
An alternative, although not exclusive, possibility that is somewhat more speculative, concerns the potential role of the mPOA in the storage of pup-related associations. That is, mPOA inactivation might have disrupted the ability of pup-associated stimuli to elicit pup-seeking behaviors due to unsuccessful retrieval of the association between environmental cues and the affective state produced by the unconditioned stimulus. Certainly, the role of mPOA in integrating converging pup-related sensory information coupled with previous findings indicating that plastic changes consistent with the storage of information within this structure can occur (Morgan et al., 1999; Numan and Insel, 2003) provide a basis for the speculation that the mPOA may serve as a locus for the storage of pup-context related associations. Our recent finding that transient mPOA inactivation selectively impaired the expression of active components of maternal behavior, including retrieval, licking and nest building, without affecting nursing behaviors (Pereira and Morrell, 2009), however argues against this idea. This finding demonstrates that bupivacaine-treated postpartum females do not forget about pups nor find pups aversive, as shown by their willingness to make and maintain physical contact with them, but they are just less motivated to actively interact with them (Pereira and Morrell, 2009). Thus, rather than acting as a location for storage of information, we posit that the mPOA is more likely key in the incentive motivational effects of the pup-associated environment that underlie approach behaviors during CPP testing.
The present findings make very clear that the mPOA is not necessary for the expression of cocaine-seeking behavior elicited by environmental cues repeatedly paired with cocaine. Thus we posit that the mPOA is not a neural site involved in processing the incentive motivational value of cocaine-associated stimuli.
This differential involvement of the mPOA in mediating conditioned responding to pup- but not cocaine-associated stimuli is consistent with enhanced activation of c-Fos and CART-IR neurons in the mPOA exclusively when the preference choice of pup- over cocaine-associated environments is made by postpartum females (Mattson and Morrell, 2005). The fact that the mPOA is not necessary for the expression of cocaine-CPP, but is critically involved in mediating both the unconditioned behavioral activation produced by pups (Pereira and Morrell, 2009) as well as the expression of the conditioned responses to pup-related stimuli (present results, Lee et al., 1999), raises the interesting possibility that the blunting of cocaine-seeking behaviors observed during postpartum (Hecht et al., 1999) may result from maternal state-induced mPOA activating effects on the circuitry that mediate and modulate response allocation.
Certainly, important functional changes within the mPOA occur around parturition to prepare the female rat for her maternal role and promote maternal responsiveness to pup-related stimuli (Numan and Insel, 2003). An intriguing corollary of the previous assumption is that, if indeed mPOA is responsible for the competing effects of pups on cocaine, then this capacity of the mPOA remains relatively intact, even following extensive cocaine exposure before, and across gestation and the postpartum period, suggesting that regardless of previous history of exposure, cocaine does not abolish the overall ability of the mPOA in mediating maternal incentive motivation (Hecht et al., 1999). In support of this, female rats that were treated with cocaine daily for 30 days (20 days of pregnancy and 10 days of the postpartum period) showed impaired maternal behaviors only while cocaine was in their circulatory system, a behavioral effect that was remarkably similar to that of a single acute systemic cocaine injection on maternal responsiveness (Sobrian et al., 1990; Heyser et al., 1992; Zimmerberg and Gray, 1992; Johns et al., 1994; Kinsley et al., 1994; Peeke et al., 1994; Vernotica et al., 1996). These same cocaine-exposed females, when drug free exhibited typical maternal behaviors, regardless of the number of days they had been previously exposed to cocaine (Vernotica et al., 1996).
Collectively, the present results suggest that the mPOA, a site that integrates the hormonal and sensory inputs related to the maternal state, is a key component in motivational processing of pup-related incentive stimuli. These data further suggest that the competing effects of pups over alternative incentives, including drugs of abuse, rely on mPOA integrity to provide important pup-related information to the choice circuitry and hence influence the process through which goal-directed action-outcome associations influence behavior. Thus, functional inactivation via bupivacaine effectively eliminated mPOA-mediated input to the circuitry, silencing the influence of pup-related cues and thereby allowing the conditioned effects of cocaine, which are mediated by other areas, to exert relatively greater influence on the choice process. We posit that the mPOA influences the integration of the stimulus value of pup-related information at several points within the motivational circuitry. Much data shows that the salience and the activational effects of pups and related stimuli depends on mPOA interactions with two maior components of the mesolimbic dopamine (DA) system, the VTA and NA (Numan and Callahan 1980; Numan and Smith, 1984; Hansen et al., 1991a,b; Numan and Numan, 1997; Numan and Insel, 2003; Champagne et al., 2004; Numan et al., 2005; Numan, 2006; Pereira and Ferreira, 2006; Pereira and Morrell, 2009), allowing pup-responsive mPOA neurons to influence, via NA, the motor systems required for goal-directed behavior. Accordingly, transient inactivation of the VTA has been shown to block the expression of pup-CPP (Seip and Morrell, 2009). The nucleus accumbens also receives convergent synaptic inputs from cortical and limbic structures such as the mPFC, the hippocampus and amygdala (Groenewegen et al., 1996; Groenewegen et al., 1999; Groenewegen and Uylings, 2000; Saper, 2000; Eichenbaum and Cohen, 2001). It has been suggested that the NA mediates goal-directed behavior by integrating hippocampus-dependent contextual information and amygdala-dependent affective information with mPFC executive functions, including attentional processes and response allocation to select appropriate behavioral responses (Mogenson et al., 1980; Groenewegen et al., 1999). We posit that during the postpartum period, the NA also integrates mPOA-dependent pup-related information. The mPOA might also influence the response allocation process through direct reciprocal interactions with the mPFC (Groenewegen and Uylings, 2000; Saper, 2000). Specifically, the mPFC is thought to modulate the salience and motivational significance of stimuli (Tzschentke, 2000; Dalley et al., 2004) and regulate goal-directed behaviors by regulating attention to the sensory input that enters the BLA and hippocampus (Kolb, 1984; Rosenkranz and Grace, 2001) and by mediating BLA input to the NA (Jackson and Moghaddam, 2001), respectively. Ongoing research is examining the functional role of these other brain areas to further refine the understanding of the neural circuitry underlying response selection among the competing alternatives: pup- and cocaine-associated environments, during the postpartum period.
In conclusion, the present study has demonstrated that motivation for pups and cocaine can be differentially modulated. A better understanding of the neural substrate underlying response selection among competing alternatives might importantly contribute to developing strategies for combating maternal drug abuse during the postpartum period. The present results strongly suggest that interventions that build upon promoting and/or restoring early mother-infant bonding might be more successful in terms of facilitating maintenance of maternal abstinence. In this sense, several studies have shown a more positive outcome in those practice-based and home-based intervention programs for postpartum substance using women that had special emphasis on enhancing parenting effectiveness (i.e., maternal-infant bonding), particularly in terms of reducing parenting stress and maternal depression, as well as maintaining greater levels of abstinence from illicit drug use (Ashley et al., 2003; McComish et al., 2003; Nair et al., 2003; Mullins et al., 2004; Porowski et al., 2004; Porter and Porter, 2004).
Acknowledgments
This research was supported by a NARSAD Young Investigator Award and NIH DA027945 awarded to MP and NIH DA014025 awarded to JIM. The authors thank Dr. Andrew M. Farrar and María José Zuluaga, M.Sc. for their support and critical comments on the manuscript, and the Laboratory Animal Facility staff at Rutgers University, Newark Campus for animal breeding and care.
List of Abbreviations
- BLA
Basolateral Amygdala
- CPP
Conditioned Place Preference
- mPFC
Medial Prefrontal Cortex
- mPOA
Medial Preoptic Area
- NA
Nucleus Accumbens
- PPD
Postpartum Day
- VTA
Ventral Tegmental Area
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams CD. Variations in the sensitivity of instrumental responding to reinforcer devaluation. Q J Exp Psychol B. 1982;34:77–98. [Google Scholar]
- Ågmo A, Gómez M. Sexual reinforcement is blocked by infusion of naloxone into the medial preoptic area. Behav Neurosci. 1993;107(5):812–818. doi: 10.1037//0735-7044.107.5.812. [DOI] [PubMed] [Google Scholar]
- Ashley OS, Marsden ME, Brady TM. Effectiveness of substance abuse treatment programming for women: a review. Am J Drug Alcohol Abuse. 2003;29(1):19–53. doi: 10.1081/ada-120018838. [DOI] [PubMed] [Google Scholar]
- Ball SA, Maves LC, DeTeso JA, Schottenfeld RS. Maternal attentiveness of cocaine abusers during child-based assessments. Am J Addict. 1997;6(2):135–143. [PubMed] [Google Scholar]
- Band LC, Hull EM. Morphine and dynorphin(1-13) microinjected into the medial preoptic area and nucleus accumbens: effects on sexual behavior in male rats. Brain Res. 1990;524(1):77–84. doi: 10.1016/0006-8993(90)90494-v. [DOI] [PubMed] [Google Scholar]
- Barabach LM, Glazer G, Norris SC. Maternal perception and parent-infant interaction of vulnerable cocaine-exposed couplets. J Perinat Neonatal Nurs. 1992;6(3):76–84. doi: 10.1097/00005237-199212000-00010. [DOI] [PubMed] [Google Scholar]
- Barnet B, Duggan AK, Wilson MD, Joffe A. Association between postpartum substance use and depressive symptoms, stress, and social support in adolescent mothers. Pediatrics. 1995;96(4 Pt 1):659–666. [PubMed] [Google Scholar]
- Boehnke SE, Rasmusson DD. Time course and effective spread of lidocaine and tetrodotoxin delivered via microdialysis: an electrophysiological study in cerebral cortex. J Neurosci Methods. 2001;105(2):133–141. doi: 10.1016/s0165-0270(00)00348-4. [DOI] [PubMed] [Google Scholar]
- Broadbent NJ, Squire LR, Clark RE. Reversible hippocampal lesions disrupt watermaze performance during both recent and remote memory tests. Learn Mem. 2006;13(2):187–191. doi: 10.1101/lm.134706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EE, Robertson GS, Fibiger HC. Evidence for conditional neuronal activation following exposure to a cocaine-paired environment: role of forebrain limbic structures. J Neurosci. 1992;12(10):4112–4121. doi: 10.1523/JNEUROSCI.12-10-04112.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns K, Chethik L, Burns WJ, Clark R. Dyadic disturbances in cocaine-abusing mothers and their infants. J Clin Psychol. 1991;47(2):316–319. doi: 10.1002/1097-4679(199103)47:2<316::aid-jclp2270470220>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Burns K, Chethik L, Burns WJ, Clark R. The early relationship of drug abusing mothers and their infants: an assessment at eight to twelve months of age. J Clin Psychol. 1997;53(3):279–287. doi: 10.1002/(sici)1097-4679(199704)53:3<279::aid-jclp11>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Carelli RM, Ijames SG. Selective activation of accumbens neurons by cocaine-associated stimuli during a water/cocaine multiple schedule. Brain Res. 2001;907(1–2):156–161. doi: 10.1016/s0006-8993(01)02604-x. [DOI] [PubMed] [Google Scholar]
- Carelli RM, Ijames SG, Crumling AJ. Evidence that separate neural circuits in the nucleus accumbens encode cocaine versus “natural” (water and food) reward. J Neurosci. 2000;20(11):4255–4266. doi: 10.1523/JNEUROSCI.20-11-04255.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carelli RM, Wightman RM. Functional microcircuitry in the accumbens underlying drug addiction: insights from real-time signaling during behavior. Curr Opin Neurobiol. 2004;14(6):763–768. doi: 10.1016/j.conb.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Carelli RM, Wondolowski J. Selective encoding of cocaine versus natural rewards by nucleus accumbens neurons is not related to chronic drug exposure. J Neurosci. 2003;23(35):11214–11223. doi: 10.1523/JNEUROSCI.23-35-11214.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall WA, Mackie K. Local anesthetics. In: Hardman JG, Limbard LE, Molinoff PB, Ruddon RW, Gilman A, editors. Goodman and Gilman’s the pharmacological basis of therapeutics. 9. New York: McGraw-Hill; 1996. pp. 321–347. [Google Scholar]
- Champagne FA, Chretien P, Stevenson CW, Zhang TY, Gratton A, Meaney MJ. Variations in nucleus accumbens dopamine associated with individual differences in maternal behavior in the rat. J Neurosci. 2004;24(17):4113–4123. doi: 10.1523/JNEUROSCI.5322-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coria-Avila GA, Hernández-Aguilar ME, Toledo-Cárdenas R, García-Hernández LI, Manzo J, Pacheco P, Miquel M, Pfaus JG. Biological and neural bases of partner preferences in rodents: models to understand human pair bonds. Rev Neurol. 2008;47(4):209–214. [PubMed] [Google Scholar]
- Cornelius MD, Richardson GA, Day NL, Cornelius JR, Geva D, Taylor PM. A comparison of prenatal drinking in two recent samples of adolescents and adults. J Stud Alcohol. 1994;55(4):412–419. doi: 10.15288/jsa.1994.55.412. [DOI] [PubMed] [Google Scholar]
- D’Apolito K. Substance abuse: infant and childhood outcomes. J Pediatr Nurs. 1998;13(5):307–316. doi: 10.1016/S0882-5963(98)80017-1. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Cardinal RN, Robbins TW. Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci Biobehav Rev. 2004;28(7):771–784. doi: 10.1016/j.neubiorev.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Dembo R, Williams L, La Voie L, Getreu A, Berry E, Genung L, Schmeidler J, Wish ED, Kern J. A longitudinal study of the relationships among alcohol use, marijuana/hashish use, cocaine use, and emotional/psychological functioning problems in a cohort of high-risk youths. Int J Addict. 1990;25(11):1341–1382. doi: 10.3109/10826089009056224. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Acquas E, Tanda G, Cadoni C. Drugs of abuse: biochemical surrogates of specific aspects of natural reward? Biochem Soc Symp. 1993;59:65–81. [PubMed] [Google Scholar]
- Eichenbaum H, Cohen NJ. From Conditioning to Conscious Recollection: Memory Systems of the Brain. New York: Oxford University Press; 2001. [Google Scholar]
- Evans SB, Wilkinson CW, Gronbeck P, Bennett JL, Taborsky GJ, Jr, Figlewicz DP. Inactivation of the PVN during hypoglycemia partially simulates hypoglycemia-associated autonomic failure. Am J Physiol Regul Integr Comp Physiol. 2003;284(1):R57–65. doi: 10.1152/ajpregu.00439.2002. [DOI] [PubMed] [Google Scholar]
- Festa ED, Russo SJ, Gazi FM, Niyomcha T, Kemen LM, Lin SN, Foltz R, Jenab S, Quinones-Jenab V. Sex differences in cocaine-induced behavioral responses, pharmacokinetics, and monoamine levels. Neuropharmacology. 2004;46(5):672–687. doi: 10.1016/j.neuropharm.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Cheung U, Myhal N, Kessler Z. Effects of maternal hormones on ‘timidity’ and attraction to pup-related odors in female rats. Physiol Behav. 1989;46(3):449–453. doi: 10.1016/0031-9384(89)90019-x. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Korsmit M, Deller M. Rat pups are potent reinforcers to the maternal animal: effects of experience parity, hormones, and dopamine function. Psychobiology. 1994;22:44–53. [Google Scholar]
- Floresco SB, Block AE, Tse MT. Inactivation of the medial prefrontal cortex of the rat impairs strategy set-shifting, but not reversal learning, using a novel, automated procedure. Behav Brain Res. 2008;190(1):85–96. doi: 10.1016/j.bbr.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Frank DA, Bresnahan K, Zuckerman BS. Maternal cocaine use: impact on child health and development. Adv Pediatr. 1993;40:65–99. [PubMed] [Google Scholar]
- García-Horsman SP, Agmo A, Paredes RG. Infusions of naloxone into the medial preoptic area, ventromedial nucleus of the hypothalamus, and amygdala block conditioned place preference induced by paced mating behavior. Horm Behav. 2008;54(5):709–716. doi: 10.1016/j.yhbeh.2008.07.011. [DOI] [PubMed] [Google Scholar]
- Gottwald SR, Thurman SK. The effects of prenatal cocaine exposure on mother-infant interaction and infant arousal in the newborn period. Topics in early Childhood Special Education. 1994;14:217–231. [Google Scholar]
- Grigson PS. Like drugs for chocolate: separate rewards modulated by common mechanisms? Physiol Behav. 2002;76(3):389–395. doi: 10.1016/s0031-9384(02)00758-8. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Uylings HB. The prefrontal cortex and the integration of sensory, limbic and autonomic information. Prog Brain Res. 2000;126:3–28. doi: 10.1016/S0079-6123(00)26003-2. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Wright CI, Beijer AV. The nucleus accumbens: gateway for limbic structures to reach the motor system? Prog Brain Res. 1996;107:485–511. doi: 10.1016/s0079-6123(08)61883-x. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Wright CI, Beijer AV, Voorn P. Convergence and segregation of ventral striatal inputs and outputs. Ann N Y Acad Sci. 1999;877:49–63. doi: 10.1111/j.1749-6632.1999.tb09260.x. [DOI] [PubMed] [Google Scholar]
- Hansen S, Harthon C, Wallin E, Löfberg L, Svensson K. Mesotelencephalic dopamine system and reproductive behavior in the female rat: effects of ventral tegmental 6-hydroxydopamine lesions on maternal and sexual responsiveness. Behav Neurosci. 1991a;105:588–598. doi: 10.1037//0735-7044.105.4.588. [DOI] [PubMed] [Google Scholar]
- Hansen S, Harthon C, Wallin E, Löfberg L, Svensson K. The effects of 6-OHDA-induced dopamine depletions in the ventral or dorsal striatum on maternal and sexual behavior in the female rat. Pharmacol Biochem Behav. 1991b;39:71–77. doi: 10.1016/0091-3057(91)90399-m. [DOI] [PubMed] [Google Scholar]
- Hauser H, Gandelman R. Lever pressing for pups: evidence for hormonal influence upon maternal behavior of mice. Horm Behav. 1985;19(4):454–468. doi: 10.1016/0018-506x(85)90041-8. [DOI] [PubMed] [Google Scholar]
- Hecht GS, Spear NE, Spear LP. Changes in progressive ratio responding for intravenous cocaine throughout the reproductive process in female rats. Dev Psychobiol. 1999;35(2):136–145. [PubMed] [Google Scholar]
- Heyser CJ, Molina VA, Spear LP. A fostering study of the effects of prenatal cocaine exposure: I. Maternal behaviors. Neurotoxicol Teratol. 1992;14(6):415–421. doi: 10.1016/0892-0362(92)90052-c. [DOI] [PubMed] [Google Scholar]
- Hille B. Common mode of action of three agents that decrease the transient change in sodium permeability in nerves. Nature. 1966;210(5042):1220–1222. doi: 10.1038/2101220a0. [DOI] [PubMed] [Google Scholar]
- Hille B. The pH-dependent rate of action of local anesthetics on the node of Ranvier. J Gen Physiol. 1977;69(4):475–496. doi: 10.1085/jgp.69.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holahan MR, White NM. Intra-amygdala muscimol injections impair freezing and place avoidance in aversive contextual conditioning. Learn Mem. 2004;11(4):436–46. doi: 10.1101/lm.64704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshina Y, Takeo T, Nakano K, Sato T, Sakuma Y. Axon-sparing lesion of the preoptic area enhances receptivity and diminishes proceptivity among components of female rat sexual behavior. Behav Brain Res. 1994;61(2):197–204. doi: 10.1016/0166-4328(94)90160-0. [DOI] [PubMed] [Google Scholar]
- Hsu E, Packard MG. Medial prefrontal cortex infusions of bupivacaine or AP-5 block extinction of amphetamine conditioned place preference. Neurobiol Learn Mem. 2008;89(4):504–512. doi: 10.1016/j.nlm.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Hughes AM, Everitt BJ, Herbert J. Comparative effects of preoptic area infusions of opioid peptides, lesions and castration on sexual behaviour in male rats: studies of instrumental behaviour, conditioned place preference and partner preference. Psychopharmacology (Berl) 1990;102(2):243–256. doi: 10.1007/BF02245929. [DOI] [PubMed] [Google Scholar]
- Hurtazo HA, Paredes RG, Agmo A. Inactivation of the medial preoptic area/anterior hypothalamus by lidocaine reduces male sexual behavior and sexual incentive motivation in male rats. Neuroscience. 2008;152(2):331–337. doi: 10.1016/j.neuroscience.2007.10.063. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Panksepp J. The role of nucleus accumbens dopamine in motivated behavior: a unifying interpretation with special reference to reward-seeking. Brain Res Rev. 1999;31:6–341. doi: 10.1016/s0165-0173(99)00023-5. [DOI] [PubMed] [Google Scholar]
- Jackson ME, Moghaddam B. Amygdala regulation of nucleus accumbens dopamine output is governed by the prefrontal cortex. J Neurosci. 2001;21(2):676–681. doi: 10.1523/JNEUROSCI.21-02-00676.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns JM, Noonan LR, Zimmerman LI, Li L, Pedersen CA. Effects of chronic and acute cocaine treatment on the onset of maternal behavior and aggression in Sprague-Dawley rats. Behav Neurosci. 1994;108(1):107–112. doi: 10.1037//0735-7044.108.1.107. Erratum in: Behav Neurosci 109(2):257, 1995. [DOI] [PubMed] [Google Scholar]
- Johnson JL, Leff M. Children of substance abusers: overview of research findings. Pediatrics. 1999;103:1085–1099. [PubMed] [Google Scholar]
- Kelley AE, Berridge KC. The neuroscience of natural rewards: relevance to addictive drugs. J Neurosci. 2002;22(9):3306–3311. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly SJ, Walsh JH, Thompson K. Birth outcomes, health problems, and neglect with prenatal exposure to cocaine. Pediatr Nurs. 1991;17:130–136. [PubMed] [Google Scholar]
- Kindon HA, Baum MJ, Paredes RJ. Medial preoptic/anterior hypothalamic lesions induce a female-typical profile of sexual partner preference in male ferrets. Horm Behav. 1996;30(4):514–527. doi: 10.1006/hbeh.1996.0055. [DOI] [PubMed] [Google Scholar]
- Kinsley CH, Turco D, Bauer A, Beverly M, Wellman J, Graham AL. Cocaine alters the onset and maintenance of maternal behavior in lactating rats. Pharmacol Biochem Behav. 1994;47(4):857–864. doi: 10.1016/0091-3057(94)90288-7. [DOI] [PubMed] [Google Scholar]
- Kolb B. Functions of the frontal cortex of the rat: a comparative review. Brain Res. 1984;320(1):65–98. doi: 10.1016/0165-0173(84)90018-3. [DOI] [PubMed] [Google Scholar]
- Lau CE, Imam A, Ma F, Falk JL. Acute effects of cocaine on spontaneous and discriminative motor functions: relation to route of administration and pharmacokinetics. J Pharmacol Exp Ther. 1991;257(1):444–456. [PubMed] [Google Scholar]
- Lee A, Clancy S, Fleming AS. Mother rats bar-press for pups: effects of lesions of the mpoa and limbic sites on maternal behavior and operant responding for pup-reinforcement. Behav Brain Res. 1999;100(1–2):15–31. doi: 10.1016/s0166-4328(98)00109-0. Corrected and republished in: Behav Brain Res. 2000;108(2):215-213. [DOI] [PubMed] [Google Scholar]
- Lomber SG. The advantages and limitations of permanent or reversible deactivation techniques in the assessment of neural function. J Neurosci Methods. 1999;86(2):109–117. doi: 10.1016/s0165-0270(98)00160-5. [DOI] [PubMed] [Google Scholar]
- Malpeli JG, Schiller PH. A method of reversible inactivation of small regions of brain tissue. J Neurosci Methods. 1979;1(2):143–151. doi: 10.1016/0165-0270(79)90011-6. [DOI] [PubMed] [Google Scholar]
- Mattson BJ, Morrell JI. Preference for cocaine- versus pup-associated cues differentially activates neurons expressing either Fos or cocaine- and amphetamine-regulated transcript in lactating, maternal rodents. Neuroscience. 2005;135(2):315–328. doi: 10.1016/j.neuroscience.2005.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson BJ, Williams S, Rosenblatt JS, Morrell JI. Comparison of two positive reinforcing stimuli: pups and cocaine throughout the postpartum period. Behav Neurosci. 2001;115(3):683–694. doi: 10.1037//0735-7044.115.3.683. [DOI] [PubMed] [Google Scholar]
- McComish JF, Greenberg R, Ager J, Essenmacher L, Orgain LS, Bacik WJ., Jr Family-focused substance abuse treatment: a program evaluation. J Psychoactive Drugs. 2003;35(3):321–331. doi: 10.1080/02791072.2003.10400015. [DOI] [PubMed] [Google Scholar]
- Minnes S, Singer LT, Arendt R, Satayathum S. Effects of prenatal cocaine/polydrug use on maternal-infant feeding interactions during the first year of life. J Dev Behav Pediatr. 2005;26(3):194–200. doi: 10.1097/00004703-200506000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogenson GJ, Jones DL, Yim CY. From motivation to action: functional interface between the limbic system and the motor system. Prog Neurobiol. 1980;14(2–3):69–97. doi: 10.1016/0301-0082(80)90018-0. [DOI] [PubMed] [Google Scholar]
- Morgan HD, Watchus JA, Milgram NW, Fleming AS. The long lasting effects of electrical simulation of the medial preoptic area and medial amygdala on maternal behavior in female rats. Behav Brain Res. 1999;99(1):61–73. doi: 10.1016/s0166-4328(98)00070-9. [DOI] [PubMed] [Google Scholar]
- Mullins SM, Suarez M, Ondersma SJ, Page MC. The impact of motivational interviewing on substance abuse treatment retention: a randomized control trial of women involved with child welfare. J Subst Abuse Treat. 2004;27(1):51–58. doi: 10.1016/j.jsat.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Murphy JM, Jellinek M, Quinn D, Smith G, Poitrast FG, Goshko M. Substance abuse and serious child mistreatment: prevalence, risk, and outcome in a court sample. Child Abuse Negl. 1991;15(3):197–211. doi: 10.1016/0145-2134(91)90065-l. [DOI] [PubMed] [Google Scholar]
- Nair P, Schuler ME, Black MM, Kettinger L, Harrington D. Cumulative environmental risk in substance abusing women: early intervention, parenting stress, child abuse potential and child development. Child Abuse Negl. 2003;27(9):997–1017. doi: 10.1016/s0145-2134(03)00169-8. [DOI] [PubMed] [Google Scholar]
- Neisewander JL, Baker DA, Fuchs RA, Tran-Nguyen LT, Palmer A, Marshall JF. Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine self-administration environment. J Neurosci. 2000;20(2):798–805. doi: 10.1523/JNEUROSCI.20-02-00798.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numan M. Hypothalamic neural circuits regulating maternal responsiveness toward infants. Behav Cogn Neurosci Rev. 2006;5(4):163–190. doi: 10.1177/1534582306288790. [DOI] [PubMed] [Google Scholar]
- Numan M, Callahan EC. The connections of the medial preoptic region and maternal behavior in the rat. Physiol Behav. 1980;25(5):653–665. doi: 10.1016/0031-9384(80)90367-4. [DOI] [PubMed] [Google Scholar]
- Numan M, Insel TR. The neurobiology of parental behavior. New York: Springer-Verlag; 2003. [Google Scholar]
- Numan M, Numan MJ. Projection sites of medial preoptic and ventral bed nucleus of the stria terminalis neurons that express Fos during maternal behavior in female rats. J Neuroendocrinol. 1997;9:369–384. doi: 10.1046/j.1365-2826.1997.t01-1-00597.x. [DOI] [PubMed] [Google Scholar]
- Numan M, Numan MJ, Schwarz JM, Neuner CM, Flood TF, Smith CD. Medial preoptic area interactions with the nucleus accumbens-ventral pallidum circuit and maternal behavior in rats. Behav Brain Res. 2005;158:53–68. doi: 10.1016/j.bbr.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Numan M, Smith HG. Maternal behavior in rats: evidence for the involvement of preoptic projections to the ventral tegmental area. Behav Neurosci. 1984;98:712–727. doi: 10.1037//0735-7044.98.4.712. [DOI] [PubMed] [Google Scholar]
- Paredes RG, Baum MJ. Altered sexual partner preference in male ferrets given excitotoxic lesions of the preoptic area/anterior hypothalamus. J Neurosci. 1995;15(10):6619–6630. doi: 10.1523/JNEUROSCI.15-10-06619.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredes RG, Highland L, Karam P. Socio-sexual behavior in male rats after lesions of the medial preoptic area: evidence for reduced sexual motivation. Brain Res. 1993;618(2):271–276. doi: 10.1016/0006-8993(93)91275-w. [DOI] [PubMed] [Google Scholar]
- Paredes RG, Tzschentke T, Nakach N. Lesions of the medial preoptic area/anterior hypothalamus (MPOA/AH) modify partner preference in male rats. Brain Res. 1998;813(1):1–8. doi: 10.1016/s0006-8993(98)00914-7. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 3. New York: Academic Press; 1997. [DOI] [PubMed] [Google Scholar]
- Peeke HV, Dark KA, Salamy A, Salfi M, Shah SN. Cocaine exposure prebreeding to weaning: maternal and offspring effects. Pharmacol Biochem Behav. 1994;48(2):403–410. doi: 10.1016/0091-3057(94)90544-4. [DOI] [PubMed] [Google Scholar]
- Pereira M, Ferreira A. Demanding pups improve maternal behavioral impairments in sensitized and haloperidol-treated lactating female rats. Behav Brain Res. 2006;175(1):139–148. doi: 10.1016/j.bbr.2006.08.013. [DOI] [PubMed] [Google Scholar]
- Pereira M, Morrell JI. The changing role of the medial preoptic area in the regulation of maternal behavior across the postpartum period: Facilitation followed by inhibition. Behav Brain Res. 2009;205:238–248. doi: 10.1016/j.bbr.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira M, Seip KM, Morrell JI. Maternal Motivation and Its Neural Substrate Across the Postpartum Period. In: Bridges RS, editor. The Neurobiology of the Parental Mind. Ch 3. Elsevier; San Diego, CA: 2008. [Google Scholar]
- Pereira de Vasconcelos A, Klur S, Muller C, Cosquer B, Lopez J, Certa U, Cassel JC. Reversible inactivation of the dorsal hippocampus by tetrodotoxin or lidocaine: a comparative study on cerebral functional activity and motor coordination in the rat. Neuroscience. 2006;141(4):1649–1663. doi: 10.1016/j.neuroscience.2006.05.023. [DOI] [PubMed] [Google Scholar]
- Perrin G, Meurisse M, Lévy F. Inactivation of the medial preoptic area or the bed nucleus of the stria terminalis differentially disrupts maternal behavior in sheep. Horm Behav. 2007;52(4):461–473. doi: 10.1016/j.yhbeh.2007.06.010. [DOI] [PubMed] [Google Scholar]
- Porowski A, Burgdorf K, Herrell J. Effectiveness and sustainability of residential substance abuse treatment programs for pregnant and parenting women. Eval Program Plann. 2004;27:191–198. [Google Scholar]
- Porter LS, Porter BO. A blended infant massage--parenting enhancement program for recovering substance-abusing mothers. Pediatr Nurs. 2004;30(5):363–72. [PubMed] [Google Scholar]
- Ragozzino ME, Jih J, Tzavos A. Involvement of the dorsomedial striatum in behavioral flexibility: role of muscarinic cholinergic receptors. Brain Res. 2002;953(1–2):205–214. doi: 10.1016/s0006-8993(02)03287-0. [DOI] [PubMed] [Google Scholar]
- Richardson GA, Day NL. Maternal and neonatal effects of moderate cocaine use during pregnancy. Neurotoxicol Teratol. 1991;13(4):455–460. doi: 10.1016/0892-0362(91)90095-e. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ. Neurobehavioural mechanisms of reward and motivation. Curr Opin Neurobiol. 1996;6:228–236. doi: 10.1016/s0959-4388(96)80077-8. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Roland EH, Volpe JJ. Effect of maternal cocaine use on the fetus and newborn: review of the literature. Pediatr Neurosci. 1989;15(2):88–94. doi: 10.1159/000120449. [DOI] [PubMed] [Google Scholar]
- Rosenkranz JA, Grace AA. Dopamine attenuates prefrontal cortical suppression of sensory inputs to the basolateral amygdala of rats. J Neurosci. 2001;21(11):4090–4103. doi: 10.1523/JNEUROSCI.21-11-04090.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuma Y. Neural substrates for sexual preference and motivation in the female and male rat. Ann N Y Acad Sci. 2008;1129:55–60. doi: 10.1196/annals.1417.009. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M. Motivational views of reinforcement: implications for understanding the behavioral functions of nucleus accumbens dopamine. Behav Brain Res. 2002;137:3–25. doi: 10.1016/s0166-4328(02)00282-6. [DOI] [PubMed] [Google Scholar]
- Sandkühler J, Gebhart GF. Relative contributions of the nucleus raphe magnus and adjacent medullary reticular formation to the inhibition by stimulation in the periaqueductal gray of a spinal nociceptive reflex in the pentobarbital anesthetized rat. Brain Res. 1984;305(1):77–87. doi: 10.1016/0006-8993(84)91121-1. [DOI] [PubMed] [Google Scholar]
- Sandkühler J, Maisch B, Zimmermann M. The use of local anaesthetic microinjections to identify central pathways: a quantitative evaluation of the time course and extent of the neuronal block. Exp Brain Res. 1987;68(1):168–78. doi: 10.1007/BF00255242. [DOI] [PubMed] [Google Scholar]
- Saper CB. Hypothalamic connections with the cerebral cortex. Prog Brain Res. 2000;126:39–48. doi: 10.1016/S0079-6123(00)26005-6. [DOI] [PubMed] [Google Scholar]
- Schroeder BE, Binzak JM, Kelley AE. A common profile of prefrontal cortical activation following exposure to nicotine- or chocolate-associated contextual cues. Neuroscience. 2001;105(3):535–545. doi: 10.1016/s0306-4522(01)00221-4. [DOI] [PubMed] [Google Scholar]
- Schroeder BE, Holahan MR, Landry CF, Kelley AE. Morphine-associated environmental cues elicit conditioned gene expression. Synapse. 2000;37(2):146–158. doi: 10.1002/1098-2396(200008)37:2<146::AID-SYN8>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Seip KM, Morrell JI. Transient inactivation of the ventral tegmental area selectively disrupts the expression of conditioned place preference for pup- but not cocaine-paired contexts. Behav Neurosci. 2009;123(6):1325–1338. doi: 10.1037/a0017666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seip KM, Morrell JI. Increasing the incentive salience of cocaine challenges preference for pup- over cocaine-associated stimuli during early postpartum: place preference and locomotor analyses in the lactating female rat. Psychopharmacology (Berl) 2007;194(3):309–319. doi: 10.1007/s00213-007-0841-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seip KM, Pereira M, Wansaw MP, Reiss JI, Dziopa EI, Morrell JI. Incentive salience of cocaine across the postpartum period of the female rat. Psychopharmacology (Berl) 2008;199(1):119–130. doi: 10.1007/s00213-008-1140-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobrian SK, Burton LE, Robinson NL, Ashe WK, James H, Stokes DL, Turner LM. Neurobehavioral and immunological effects of prenatal cocaine exposure in rat. Pharmacol Biochem Behav. 1990;35(3):617–629. doi: 10.1016/0091-3057(90)90299-w. [DOI] [PubMed] [Google Scholar]
- Stack EC, Balakrishnan R, Numan MJ, Numan M. A functional neuroanatomical investigation of the role of the medial preoptic area in neural circuits regulating maternal behavior. Behav Brain Res. 2002;131(1–2):17–36. doi: 10.1016/s0166-4328(01)00370-9. [DOI] [PubMed] [Google Scholar]
- Sterk C, Elifson K, Theall K. Women and drug treatment experiences: A generational comparison of mothers and daughters. Journal of Drug Issues. 2000;30(4):839–862. [Google Scholar]
- Tehovnik EJ, Sommer MA. Effective spread and timecourse of neural inactivation caused by lidocaine injection in monkey cerebral cortex. J Neurosci Methods. 1997;74(1):17–26. doi: 10.1016/s0165-0270(97)02229-2. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM. The medial prefrontal cortex as a part of the brain reward system. Amino Acids. 2000;19(1):211–219. doi: 10.1007/s007260070051. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM, Schmidt WJ. Interactions of MK-801 and GYKI 52466 with morphine and amphetamine in place preference conditioning and behavioural sensitization. Behav Brain Res. 1997;84(1–2):99–107. doi: 10.1016/s0166-4328(97)83329-3. [DOI] [PubMed] [Google Scholar]
- van Furth WR, Wolterink G, van Ree JM. Regulation of masculine sexual behavior: involvement of brain opioids and dopamine. Brain Res Rev. 1995;21(2):162–184. doi: 10.1016/0165-0173(96)82985-7. [DOI] [PubMed] [Google Scholar]
- Vernotica EM, Lisciotto CA, Rosenblatt JS, Morrell JI. Cocaine transiently impairs maternal behavior in the rat. Behav Neurosci. 1996;110(2):315–323. doi: 10.1037//0735-7044.110.2.315. [DOI] [PubMed] [Google Scholar]
- Vernotica EM, Rosenblatt JS, Morrell JI. Microinfusion of cocaine into the medial preoptic area or nucleus accumbens transiently impairs maternal behavior in the rat. Behav Neurosci. 1999;113(2):377–390. doi: 10.1037//0735-7044.113.2.377. [DOI] [PubMed] [Google Scholar]
- Walton-Moss BJ, Becker KL. Women and substance use disorders. Lippincotts Prim Care Pract. 2000;4(3):290–301. [PubMed] [Google Scholar]
- Wang S, Redgrave P. Microinjections of muscimol into lateral superior colliculus disrupt orienting and oral movements in the formalin model of pain. Neuroscience. 1997;81(4):967–88. doi: 10.1016/s0306-4522(97)00191-7. [DOI] [PubMed] [Google Scholar]
- Wansaw MP, Lin SN, Morrell JI. Plasma cocaine levels, metabolites, and locomotor activity after subcutaneous cocaine injection are stable across the postpartum period in rats. Pharmacol Biochem Behav. 2005;82(1):55–66. doi: 10.1016/j.pbb.2005.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wansaw MP, Pereira M, Morrell JI. Characterization of maternal motivation in the lactating rat: Contrasts between early and late postpartum responses. Horm Behav. 2008;54(2):294–301. doi: 10.1016/j.yhbeh.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waraczynski M, Perkins M. Temporary inactivation of the retrorubral fields decreases the rewarding effect of medial forebrain bundle stimulation. Brain Res. 2000;885(2):154–65. doi: 10.1016/s0006-8993(00)02908-5. [DOI] [PubMed] [Google Scholar]
- Wilsoncroft WE. Babies by bar-press: Maternal behavior in the rat. Behav Res Meth Instrum. 1969;1:229–230. [Google Scholar]
- Xiao K, Kondo Y, Sakuma Y. Differential regulation of female rat olfactory preference and copulatory pacing by the lateral septum and medial preoptic area. Neuroendocrinology. 2005;81(1):56–62. doi: 10.1159/000084893. [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ. Reinforcer devaluation abolishes conditioned cue preference: evidence for stimulus-stimulus associations. Behav Neurosci. 2002;116(1):174–177. doi: 10.1037//0735-7044.116.1.174. [DOI] [PubMed] [Google Scholar]
- Zimmerberg B, Gray MS. The effects of cocaine on maternal behaviors in the rat. Physiol Behav. 1992;52(2):379–384. doi: 10.1016/0031-9384(92)90287-c. [DOI] [PubMed] [Google Scholar]