Abstract
Subtyping of substance dependence disorders holds promise for a number of important research areas including phenotyping for genetic studies, characterizing clinical course, and matching treatment and prevention strategies. This study sought to investigate whether a dichotomous construct similar to Babor’s Types A/B and Cloninger’s Types I/II for alcohol dependence can be identified for cannabis dependence in a Native American sample. In addition, heritability of this construct and its behavior in a genetic linkage analyses were evaluated. Information on cannabis use and dependence symptoms and other psychiatric disorders was obtained using the Semi-Structured Assessment for the Genetics of Alcoholism (SSAGA) from a community sample of 606 American Indians. Hierarchical average linkage and K means cluster analysis was used, and a 3-cluster solution was found to generate the best separation of variables. Ninety-one percent of cannabis dependent participants fell into one of the two subtypes: Type A/I cluster (N=114, 56%) and Type B/II cluster (N=70, 35%). Heritability (estimated using SOLAR) was only significant for the Type B/II cluster (h2 =0.44, S.E.=0.18, p<0.01). Evidence for linkage was found for the Type B/II cluster (vs. no diagnosis) on chromosome 16 (@139 cM, LOD score= 4.4), and on chromosome 19 (@74 cM, LOD score= 6.4). Regions of interest for this phenotype (LOD > 1.5) were also located on chromosomes 14,21,22. These findings suggest that a Type B/II cannabis dependence phenotype can be identified in this population and that it is in part heritable and linked to areas of the genome identified previously for drug dependence phenotypes in this population as well as in other studies.
Keywords: American Indians, cannabis dependence, heritability
INTRODUCTION
Cannabis use and use disorders are prevalent in the industrialized world, including in Europe, the United States, Canada, and Australia (Anthony et al. 1994; Swift et al. 2001; Vega et al. 2002; SAMHSA 2003). In the U.S., cannabis use disorders (abuse and dependence) rank third, behind only tobacco and alcohol, in frequency of substance use disorders (Anthony et al. 1994; SAMHSA 2003). The 2003 National Survey on Drug Use and Health (SAMHSA 2003) reported that 40.6% of the U.S. population had used cannabis or hashish during their lifetime and that 4.2 million individuals age 12 years and older met criteria for past year cannabis abuse or dependence using DSM-IV (American Psychiatric Association 1994) criteria. The 2001–2002 National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) found that 8.45% of the population had a lifetime DSM-IV diagnosis of either cannabis dependence (1.30%) or abuse (7.16%) (Conway et al. 2006). Using DSM-III-R criteria, the National Co morbidity Survey (NCS) found that 46.3% of the population had a lifetime history of extra medical use of cannabis and 4.2 % of the population aged 18–54 years had a lifetime DSM-III-R (American Psychiatric Association 1987) history of cannabis dependence (Anthony et al. 1994).
Persistent cannabis use is associated with significant morbidity. Persistent use poses health problems similar to those of tobacco (Taylor et al. 2000; Mittleman et al. 2001; Fisher et al. 2005; Hashibe et al. 2005; Tashkin 2005), is implicated in syndrome characterized by apathy, loss of goal-directed behavior, and cognitive impairment termed the “amotivational syndrome” (Sharma 1975; Pope et al. 2001; Solowij et al. 2002; Schuckit 2006), and is associated with impaired educational and work performance (Kandel & Chen 2000; Lynskey & Hall 2000; Swift et al. 2001; Schuckit 2006). Cannabis use, particularly by adolescents and young adults, may also facilitate progression to other illicit drug use (the “gateway” drug hypothesis) (Fergusson & Horwood 2000; Lynskey et al. 2003). In the general U.S. population, cannabis dependence is significantly co-morbid, not only with alcohol and other drug dependence, but also with anxiety, depression, and personality disorders (Regier et al. 1990; Troisi et al. 1998; Agosti et al. 2002; Conway et al. 2006; Stinson et al. 2006) suggesting that cannabis dependence shares etiological relationships with other substance use and psychiatric disorders.
Work to date suggests that there are significant genetic as well as environmental risk factors for substance dependence disorders (Tsuang et al. 1996, 1998; Kendler & Prescott 1998; Maes et al. 1999; Kendler et al. 2000, 2003, 2007; Miles et al. 2001; Lynskey et al. 2002; Rhee et al. 2003; Wilhelmsen & Ehlers 2005; Ehlers et al. 2007; Hopfer et al. 2007; Agrawal et al. 2008; Kranzler et al. 2008). Multiple genetic and environmental risk factors for substance dependence disorders raise the possibility that a substance dependence diagnosis is a phenotypically similar but etiologically heterogeneous diagnosis. Etiologic heterogeneity also raises the possibility that subtyping of dependence disorders with more homogeneous environmental and genetic determinants might lead to more successful characterization of etiological risk and protective factors.
Most of the work on subtyping substance dependence disorders has focused on alcohol dependence. These efforts have produced several sub typing schemes (Hesselbrock & Hesselbrock 2006). The best known of these are Cloninger’s Types I and II (Cloninger et al. 1981) and Babor’s Types A and B (Babor et al. 1992; Brown et al. 1994). Cloninger’s Type II and Babor’s Type B are similar in having earlier onset of drinking problems, more antisocial and other psychiatric co-morbidity, a more severe course as opposed to Type I and Type A. A Type A/B dichotomy has been found for alcohol dependence in several ethnicities (Hesselbrock & Hesselbrock 2006). Evidence suggests that type II/B might be more heritable than type I/A (Cloninger et al. 1981; Foroud et al. 1998), raising the possibility that Type II/B is more genetically based and Type I/A more environmentally based (Hesselbrock & Hesselbrock 2006).
Recently, efforts have been made to subtype drug use disorders using cluster analysis. Ball and colleagues (1995) adduced evidence for a Type A/B in cocaine abusers. Feingold and colleagues (1996) adduced evidence for a Type A/B dichotomy from a group of alcohol, cocaine, marijuana, and opiate abusers approximately 60% of whom were recruited from treatment programs. Kranzler and colleagues (2008), in a study of cocaine dependence in 1393 subjects from 660 small nuclear families, identified 6 clusters, four of which showed rates of cocaine dependence of 78–100% with heritability ranging from 0.32 to 0.50. In those 4 clusters, rates of ASPD ranged from 9.3 to 19.9% and major depression episode from 6.7 to 20.1%. Gelernter and colleagues (2006) used cluster analytic methods to identify three clusters of moderate to heavy opioid users with heritability ranging from 0.40 to 0.65. These studies are producing specific genome wide scan information (Gelernter et al. 2005, 2006).
Efforts to subtype substance dependence disorders in select ethnic minority populations are important for several reasons. First, ethnic minority populations may be more homogeneous in terms of both genetic and environmental risk factors and so they may offer advantages for characterizing these risk and protective factors for substance use disorders over more heterogeneous samples taken from the general population. Second, some ethnic minority populations bear a higher burden from some substance use disorders than the general population. Characterizing the specific risk and protective factors in these groups likely to lead to more effective treatment and prevention strategies is an important public health objective.
Some Native American tribes have been reported to have high rates of substance use disorders, including cannabis dependence (Mitchell et al. 2003; Gilder et al. 2006, 2007). In previous work with a Native American community, Ehlers and colleagues have found high rates of lifetime DSM-III-R cannabis dependence (43% for men, 24% for women) (Gilder et al. 2006) and have characterized some of the risk factors associated with cannabis dependence. Early cannabis use was found to be significantly associated with cannabis dependence (Ehlers et al. 2007). Unlike the general population, independent anxiety and affective disorders were not comorbid with cannabis dependence (Gilder et al. 2006). The aims of the present study were to: 1) further characterize cannabis dependence in this sample by subtyping the participants with cannabis dependence using cluster analytic techniques; 2) determine if those subtypes have differential heritability; and 3) conduct a linkage analysis of heritable subtypes of cannabis dependence.
METHODS
Participants
Participants, known collectively as Mission Indians, were recruited from eight geographically contiguous reservations with a total population of about 3,000 individuals. Participants were recruited using a combination of a venue-based method for sampling hard-to-reach populations (Kalton & Anderson 1986; Muhib et al. 2001) as well as a respondent-driven procedure (Heckathorn 1997). The venues included: tribal halls, health clinics, tribal libraries, and stores on the reservations. Fliers advertising the study were placed in each venue with the telephone number of the tribal recruitment coordinator. The venues were also regularly visited by the tribal recruitment coordinator who approached potential participants to offer information about and enrollment in the study. Approximately half of the participants were recruited using each method. A 10–25% rate of refusal using the venue method occurred depending on venue. The refusal rate in the respondent-driven procedure is not known. Potential participants contacted the tribal recruitment coordinator or the study coordinator at the research facility. At that time, interested participants were given a brief description of the study, told transportation would be provided, and informed as to the amount they would be paid for participation. Individuals who elected to participate by the venue method were encouraged to inform other eligible participants about the study (respondent-driven procedure). Transportation from home to The Scripps Research Institute was provided by the study.
To be included in the study, participants had to be Mission Indian, at least 1/16th Native American Heritage (NAH), between the age of 18 and 70 years, and be mobile enough to be transported from his or her home to the General Clinical Research Center (GCRC) of The Scripps Research Institute (TSRI). The protocol for the study was approved by the Institutional Review Board (IRB) of TSRI, the Scientific Advisory Committee of the GCRC, and the Indian Health Council, a tribal review group overseeing health issues for the reservations where recruitment was undertaken.
Potential participants first met individually with research staff to have the study explained and give written informed consent. During a screening period, participants had blood pressure and pulse taken, took an alcohol breathalyzer test to assess blood alcohol concentration, and completed a questionnaire that was used to gather information on demographics, personal medical history, ethnicity, and substance use history (Schuckit 1985). No individuals with detectable blood alcohol levels were included in the study dataset. During the screening period, the study coordinator noted whether the participant was agitated, tremulous, or diaphoretic.
Psychiatric diagnoses and symptom history
Each participant also completed an interview with the SSAGA (Bucholz et al. 1994), which was used to collect demographic information, make lifetime substance dependence and psychiatric disorder diagnoses according to DSM-III-R criteria (American Psychiatric Association, 1987), and collect other information on cannabis use and use related symptomatology. The SSAGA is a fully structured, poly-diagnostic psychiatric interview that has undergone both reliability and validity testing (Bucholz et al. 1994; Hesselbrock et al. 1999). It has been used in another Native American sample (Hesselbrock et al. 2000; Hesselbrock et al. 2003). Interviewers were trained by personnel from COGA. All best final diagnoses were made by a research psychiatrist/addiction specialist (DAG).
Diagnostic criteria and lifetime rates of cannabis dependence, four anxiety disorders (panic disorder with or without agoraphobia, agoraphobia without panic, social phobia, and obsessive-compulsive disorder), three affective disorders (major depressive disorder, bipolar I disorder, and dysthymic disorder), childhood conduct disorder (onset of three or more conduct disorder symptoms before age 15), and adult antisocial personality disorder (ASPD) were evaluated. In the present study, diagnoses of anxiety and affective disorders were considered only if they were independent of alcohol and drug use. Criteria for diagnosing anxiety and affective disorders independent of, as opposed to induced by, cannabis, alcohol, or other substances followed those developed by Schuckit and colleagues (1997a, 1997b), Hesselbrock and colleagues (2000), and DSM-IV-TR (American Psychiatric Association 2000). These criteria have been described previously (Gilder et al. 2004, 2006). Severity of the most severe lifetime depression episode was calculated as the number of DSM-III-R symptoms in addition to depressed mood during that episode, whether or not that episode was deemed independent of alcohol or drug use and whether or not the depression episode met criteria for major depression episode. In addition, the number and kind of cannabis withdrawal symptoms, the severity of cannabis use and age of onset of cannabis use were determined.
Clinical Data Analysis
SPSS (version 11 for Mac, SPSS, Inc., Chicago, IL) was used to generate hierarchical average linkage and K means cluster analysis from clinical variables selected a priori as likely to contribute to the heritability of cannabis dependence. These variables were: age of first cannabis use, the number of childhood (<15 years) and adult (>=15 years) antisocial symptoms, the number of co-morbid lifetime anxiety and affective disorders, the number of DSM-III-R cannabis dependence criteria, the number of cannabis withdrawal symptoms, and severity of cannabis use. The number of cluster solutions was also specified a priori as two, three, and four. The number of cluster solutions was kept low in order to generate cluster solutions likely to be clinically meaningful.
Subsequently, comparisons of each clinical variable in each pair of clusters in each cluster solution were undertaken. For example, age of onset of use was compared in each pair of clusters in the two-cluster solution (1 vs. 2), the three-cluster solution (1 vs. 2, 1 vs. 3, and 2 vs. 3), and the four-cluster solution (1 vs. 2, 1 vs. 3, 1vs. 4, 2 vs. 3, 2 vs. 4, 3 vs. 4). The three-cluster solution generated the best separation of the data (see Results, below). Demographic variables, which had not been used to generate the clusters, were then compared in each pair of clusters in the three-cluster solution. Continuous variables, including all clinical variables, age, and years of education, were compared using analysis of variance (ANOVA). Dichotomous variables, including gender, current employment (yes vs. no), annual household income (<$20K vs. >=$20K), Native American Heritage (<50% vs. >=50%), and whether currently married (yes vs. no), were compared using chi-square and Fisher exact test. Significance was set at p<0.05.
Genetic data analyses
One hundred and seventy-two pedigrees containing 1548 individuals were used in the genetic analyses. Of these, 584 individuals were from 106 extended families where multiple family members were directly interviewed, data from these individuals were used to calculate heritability of the phenotypes. Sixty-six additional families have only a single individual with direct interview data. The individuals in these 66 families were not included in the linkage analyses but were included in the co-morbidity analyses and in the calculation of trait means and variance in order to determine the impact of covariates. Four hundred and sixteen individuals from 81 extended pedigree families have both genotype and phenotype data and were used in the linkage analyses. The family sizes for the 81 families ranged between 4 and 41 members (average 13.9±8.9) with between 2 and 15 individuals having both genotype and phenotype data (average 5.0±3.3). The 416 individuals within the 81 families that were genetically informative include: 142 parent-child, 260 sibling, 53 half-sibling, 11 grandparent-grandchild, 235 avuncular, and 240 cousin relative pairs. Only sib, half-sib, avuncular and cousin pairs were included as being potentially genetically informative. Family structure and genotype consistency were verified and described previously (Ehlers et al. 2004; Ehlers & Wilhelmsen 2005, 2006, 2007).
DNA was isolated from whole blood using an automated DNA extraction procedure, genotyping was done as previously described (Wilhelmsen et al. 2003). Genotypes were determined for a panel of 791 autosomal microsatellite polymorphisms (Weber & May 1989) using fluorescently labeled PCR primers under conditions recommended by the manufacturer (HD5 version 2.0; Applied Biosystems, Foster City, CA). The HD5 panel set has an average marker-to-marker distance of 4.6 cM, and an average heterozygosity of greater than 77% in a Caucasian population. Allele frequencies were estimated from the entire Mission Indian population with genotype data. Gender and age accounted for greater than 5% of the phenotypic variance for each of the phenotypes. Therefore, age and gender were included as covariates in the analyses.
Genotypes were ultimately determined for 416 participants. The total additive genetic variance (heritability, h2) and its standard error were estimated for the CD, ASPD, ASPD/CD phenotypes using SOLAR (http://solar.sfbrgenetics.org/). Two approaches to estimating heritability were used for the three traits. In the first approach the trait was modeled as a latent normally distributed variable with a threshold above which an individual is considered “affected”. Using a second approach, the same trait was modeled as if it was a normally distributed variable. In this case heritability is higher for the trait modeled as if it was a normally distributed variable. These methods can occasionally give very dissimilar results presumably because of factors related to convergence. In this analysis it was required that both methodologies provide support for heritability prior to linkage analyses and the lower estimate is presented. Variance component estimate methods were used to calculate LOD scores using SOLAR v2.0.4 (Almasy & Blangero, 1998). Simulation analysis was used to estimate empirical LOD scores and make appropriate genome wide adjustments for non-normality (Blangero et al. 2000).
RESULTS
Demographics
Six hundred and six individuals completed an interview with the SSAGA. Overall, the demographic characteristics of the sample, including mean age (30.4 ± 11.85 yrs), years of education (11.6 ± 1.6 yrs), economic status (47% of the sample had an annual household income < $20K), employment (44% of the sample had any current employment and 32% of the sample had current fulltime employment), and current marital status (single= 65%, married =19%, divorced=8%, separated=7%, widowed=2%), are similar to available information for the tribe from U.S. census data (United States Bureau of the Census 1990). Forty-four percent of men and 26% of women had lifetime DSM-III-R cannabis dependence. Demographic characteristics of participants with and without DSM-III-R cannabis dependence were compared. There were no significant differences between the groups for age, economic status, employment, marital status, and Native American Heritage. Compared to participants without cannabis dependence, participants with cannabis dependence were more likely to be male (p<0.001), younger (p<0.05), and have fewer years of education (p<0.01).
Cluster Analysis
Two hundred and two individuals with lifetime DSM-III-R cannabis dependence provided the information on variables associated with cannabis use and cannabis dependence used to generate clusters. Two, 3, and 4 cluster solutions were considered. The three-cluster solution showed the best separation of the data as evidenced by significant separation of a larger number of variables than was seen in the two and four cluster solutions. Therefore the three cluster solution was used in subsequent analyses including estimates of heritability and linkage.
In the three cluster solution, the most common cluster was a Type A/I cluster (N=114, 56%), and the second most common was a Type B/II (N=70, 35%). The Type B/II cluster, as compared to the Type A/I cluster, was characterized by a earlier age of onset of use (mean 11.5 compared to 13.9 years), more conduct and adult antisocial behaviors, and more cannabis dependence and withdrawal symptoms. The third cluster, called “Cluster 3” in this analysis, was the least common (N=18, 9%); it was not different from the Type A/I cluster on any variable except age of onset of use (mean 19.9 compared to 13.9 years). The Type B/II cluster, as compared to Cluster 3, was characterized by earlier age of onset of use (mean 11.5 compared to 19.4 years), more conduct and adult antisocial behaviors, and more cannabis dependence symptoms, but not more withdrawal symptoms. Numbers of independent anxiety and affective disorders did not differ in any pairwise cluster comparison.
Demographic characteristics of the three clusters and post hoc comparisons of pairs of clusters for each demographic variable are shown in Table 2. Pairs of clusters did not differ in any demographic variable except age and gender. Cluster 3 participants (mean age 38.3 years) were significantly older than participants in both the Type II/B (mean age 28.2 years, p<0.001) and Type I/A (mean age 26.8 years, p<0.001) clusters. The Type II/B and Type I/A clusters did not significantly differ on age. Both the Type I/A cluster (p<0.03) and the Type II/B (p<0.002)) differed in the proportion of women in the cluster based on the total number of women/men ascertained.
Table 2.
Means and counts of demographic variables in each cluster and comparison of variables in each pair.
| Variable | Cluster 1 |
Cluster 2 |
Cluster 3 |
1 vs 2 p-value |
1 vs 3 p-value |
2 vs 3 p-value |
|---|---|---|---|---|---|---|
| Mean; se | Mean; se | Mean; se | ||||
| Age | 26.8; 0.83 | 28.2; 1.16 | 38.3; 1.65 | 0.60 | 0.001 | 0.001 |
| Number of years of education |
11.5; 0.13 | 11.0; 0.18 | 11.3; 0.42 | 0.10 | 0.84 | 0.80 |
|
N, % of total population |
N, % of total population |
N, % of total population |
||||
| Male | 59, 23 | 42, 16 | 10, 04 | 0.03 | 0.002 | 0.229 |
| Female | 55, 16 | 28, 08 | 8, 02 | |||
| N, % | N, % | N, % | ||||
| Un-Employed | 62, 56 | 41, 63 | 8, 44 | 0.428 | 0.447 | 0.182 |
| Employed | 49, 44 | 24, 37 | 10, 56 | |||
| Income | 0.641 | 0.804 | 1.000 | |||
| < 20 K/year | 53, 48 | 29, 44 | 8, 44 | |||
| ≥ 20 K/year | 57, 52 | 37, 56 | 10, 56 | |||
| Native American Ancestry |
0.446 | 0.316 | 0.792 | |||
| < 50 | 66, 58 | 36, 51 | 8, 44 | |||
| ≥ 50 | 48, 42 | 34, 49 | 10, 56 | |||
| Not married |
98, 86 | 60, 87 | 12, 67 | 1.000 | 0.080 | 0.074 |
| Married | 16, 14 | 9, 13 | 6, 33 | |||
Heritability and Linkage
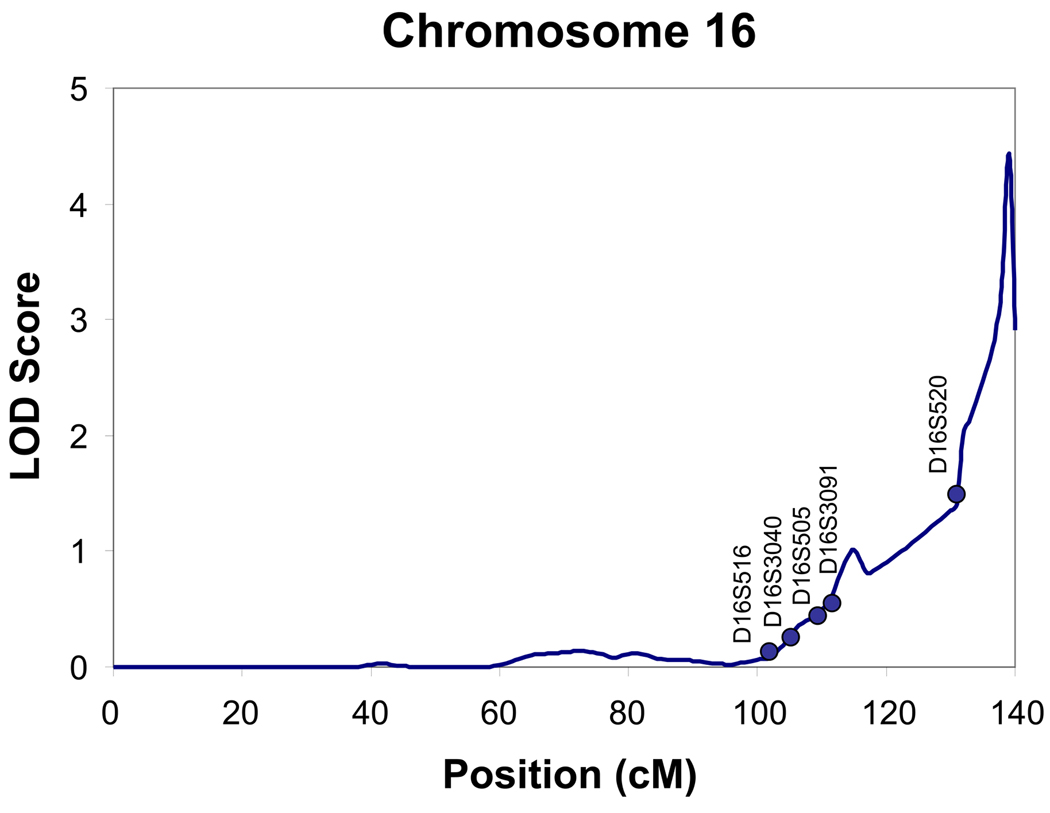
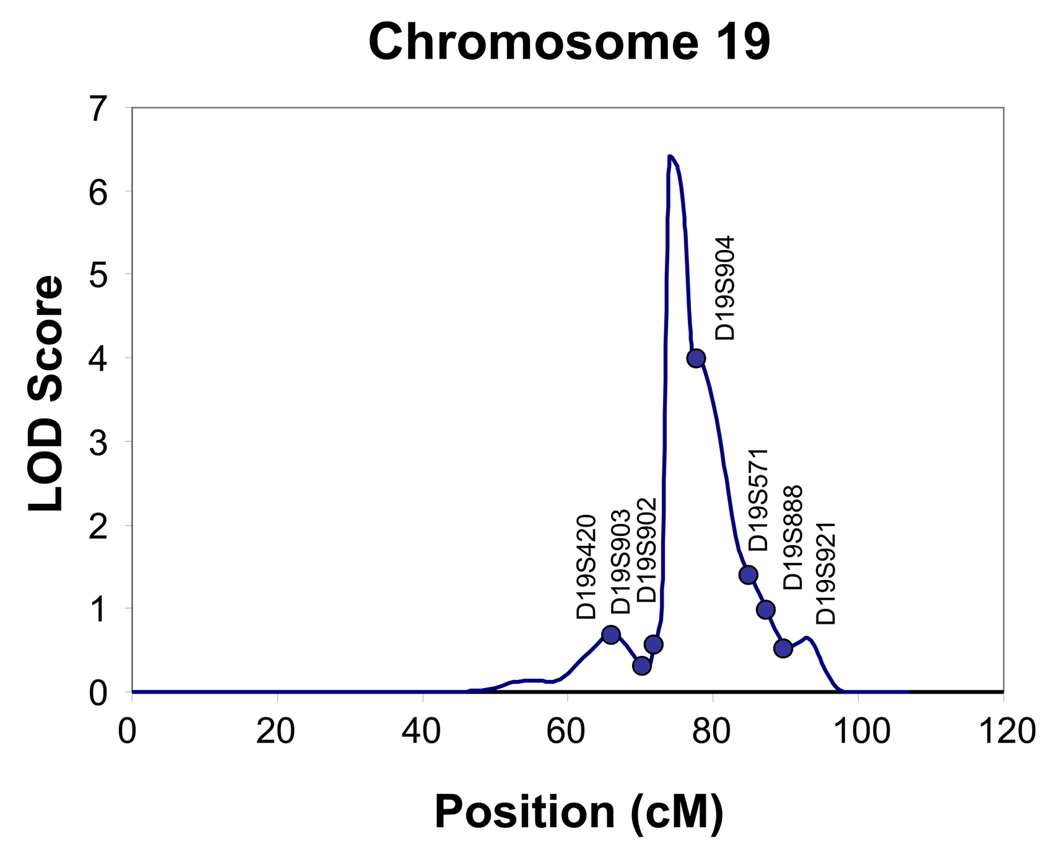
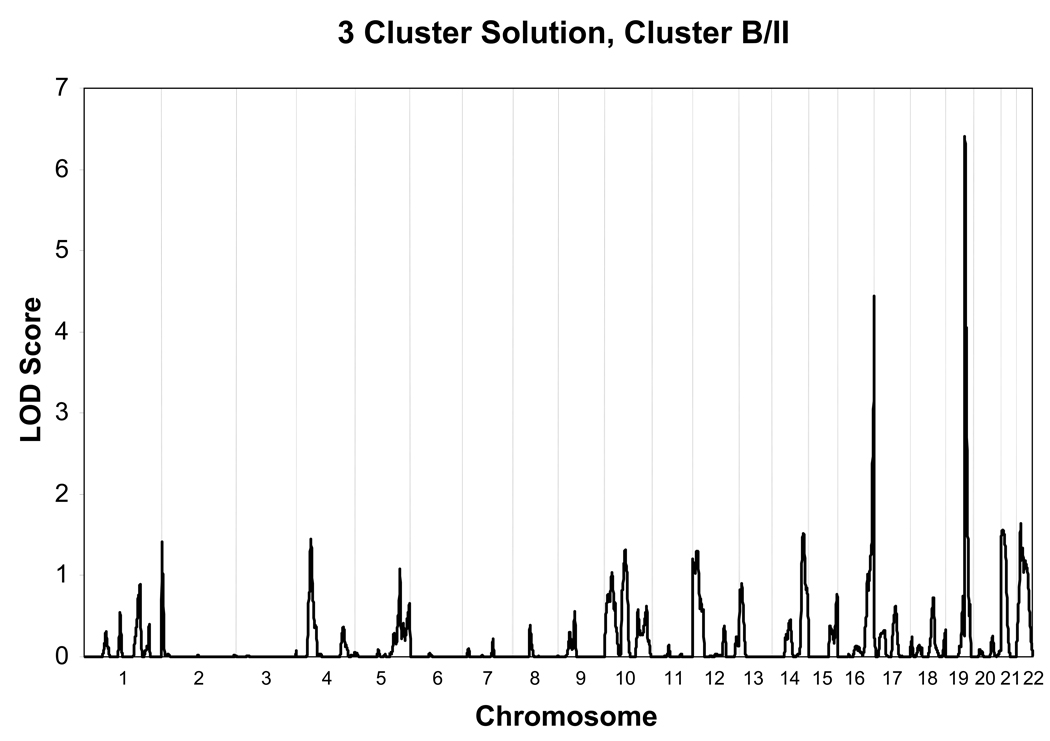
Heritability of each of the three clusters was tested and found to be significant only for the Type B/II cluster (h2 =.44, S.E. = 0.18, p<0.01). In the Type B/II cluster, age at interview and gender were significant covariates. Gender (p=0.000006) and age (p= 0.02) together accounted for 5% of the variance respectively. As seen in Fig. 1 and Fig. 2, evidence for linkage was found for the Type B/II cluster vs. no diagnosis, on chromosome 16 (@139 cM, LOD score= 4.4), and on chromosome 19 (@74 cM, LOD score= 6.4). Regions of interest for this phenotype (LOD > 1.5) were also located on chromosome 14, at 122 cM, on chromosome, 21 at 7 cM and on chromosome 22 at 17 cM as seen in Fig. 3 and listed in Table 3.
Figure 1.
Multipoint Linkage Analysis for Type II/B Cannabis cluster phenotype for chromosome 16. The analysis assumes a latent normally distributed variable with a threshold above which an individual is affected. Log of the Odds (LOD) score (Y-axis) is plotted for the chromosome location map (in centimorgans (cM), X-axis).
Figure 2.
Multipoint Linkage Analysis for the Type II/B Cannabis cluster phenotype for chromosome 19. The analysis assumes a latent normally distributed variable with a threshold above which an individual is affected. Log of the Odds (LOD) score (Y-axis) is plotted for the chromosome location map (in centimorgans (cM), X-axis).
Figure 3.
Multipoint Linkage Analysis for Type II/B Cannabis cluster phenotype for the entire genome. Results for each chromosome are aligned end to end with the p terminus on the left. Vertical lines indicate the boundaries between the chromosomes. The numbers above on the X-axis indicate the chromosome locations.
Table 3.
Chromosome locations for a type II/B cannabis cluster
| CHR | LOC (cm) |
LOD | Nearest marker |
Supporting references (phenotype) |
|---|---|---|---|---|
| 14 | 122 | 1.5 | D14S985 | Agrawal et al., 2008 (cannabis dep) |
| Ehlers et al., 2008 (ASPD/CD) | ||||
| 16 | 139 | 4.4 | D16S520 | Ehlers et al., 2007 (Any drug dep) |
| Hill et al., 2004 (Alc dep) | ||||
| Ma et al., 2003 (Drinking) | ||||
| Sheffield et al., 1999 (Alc Dep) | ||||
| 19 | 74 | 6.4 | D19S902 | Dick et al., 2004 (Conduct symptoms) |
| 21 | 7 | 1.6 | D21S1899/ D2151904 |
Ehlers et al., 2007 (Any drug dep) |
| Uhl et al., 2001 (Drug dep) | ||||
| 22 | 17 | 1.6 | D22S315 |
Ehlers & Wilhelmsen, 2006 (Reg. tobacco use) |
| Saccone et al., 2000 (Max drinks) | ||||
Abbreviations: CHR (chromosome number), LOC (location), dep (dependence), ASPD/CD (antisocial personality disorder/conduct disorder), Alc dep (Alcohol dependence), Max drinks (maximum number of drinks in a 24 hour period). Supporting references are best estimates of proximity within 35 cM considering the use of different maps and populations.
DISCUSSION
The results of this study suggest that, in general, a dichotomous typology similar to Babor’s Types A/B (Babor et al. 1992) and Cloninger’s Types I/II (Cloninger et al. 1981) for alcohol dependence characterizes cannabis dependence in this Indian community with the exception of an infrequent late onset cluster that may represent a temporal cohort effect. In this sample, the Type B/II cluster (in comparison to the Type A/I cluster) for cannabis dependence had an earlier age of onset of use, more childhood conduct and adult antisocial behaviors, and more cannabis dependence and withdrawal symptoms. Numbers of independent anxiety and affective disorders did not differ between the clusters. The Type B/II cluster was found to be heritable (h2 = 0.44, S.E. = 0.18, p<0.01), while the Type A/I and Cluster 3 were not found to be heritable.
These results are similar to the B/II and A/I typology established in previous studies of alcoholics (Cloninger et al. 1981; Babor et al. 1992; Schuckit et al. 1995; Feingold et al. 1996; Babor & Caetano 2006; Hesselbrock & Hesselbrock 2006) in a variety of studies using inpatient, outpatient, and community samples. Using cluster analytic techniques, a similar Type B/II vs. a Type A/I typology has been identified in substance use disorders, including cocaine, opiates, and cannabis (Ball et al. 1995; Feingold et al. 1996; Kranzler et al. 2008). To our knowledge, Feingold and colleagues (1996) have made the only other attempt to examine a sample of cannabis dependent participants for the Type B/II vs. A/I subtypes. Their sample consisted of predominantly treatment seeking substance users and psychiatric patients. Mean age in that sample was 35.7 years (S.D. = 11.5) and ethnicity reported as 32% African American, 6% Hispanic, and 63% White. Variables which were highly correlated with cluster membership in their study were withdrawal avoidance, dependence severity, polydrug use, higher scores on the Drug Abuse Screening Test and Michigan Alcoholism Screening Test, and an anxiety index from the Addiction Severity Index.
Feingold and colleagues (1996) also compared their Type B and Type A cannabis use disorder clusters using variables not used to establish the clusters. They found an approximately 40% – 60% ratio between Type B and Type A clusters in all four substance classes, a ratio similar to that found in this study. Feingold and colleagues (1996) also found no significant gender differences between Types B and A in any of their substance use disorder clusters. In the present study while the type I/A cluster had an almost equal distribution of men and women the type II/B cluster contained approximately 40% women and 60% men.
In addition to the Type II/B and Type I/A clusters, the cluster analysis used in the present study also generated a third cannabis dependence cluster. This third cluster was infrequent (n=18, 8.9% of the total sample) and differed from the Type I/A cluster only in having an age of onset of first cannabis intoxication of 19.4 years (as opposed to 13.9 years, p < 0.001) and a mean age at interview of 38.3 years (as opposed to 26.8 years, p < 0.001) (Table 2). We believe that the most parsimonious explanation for the third cluster is that it represents a temporal cohort effect, possibly marking a period of increased cannabis availability in the 1980s. Alternatively, or in addition, this small third cluster may represent individuals who had onset of cannabis use later in life due to other environmental variables.
This cluster analytic approach may also be useful in identifying etiological relationships between disorders. As an example in this study, the numbers of DSM-III-R anxiety and affective disorders did not differ between heritable and non-heritable subtypes of cannabis dependence. This finding is consistent with a previous finding in this sample that cannabis dependence was not co-morbid with anxiety and affective disorders (Gilder et al. 2006). Taken together, these findings suggest that anxiety and affective disorders may not be as etiologically related to cannabis dependence in this population as they may be in other populations where co-morbidity is more pronounced (Regier et al. 1990; Grant 1995; Grant & Pickering 1998; Agosti et al. 2002).
Several studies suggest that there is a moderate genetic influence on cannabis dependence and recently there have been several reports that have identified regions in the genome that may be linked to cannabis dependence (Kendler & Prescott 1998; Tsuang et al. 1998; Maes et al. 1999; Miles et al. 2001; Lynskey et al. 2002; Kendler et al. 2003; Rhee et al. 2003; Wilhelmsen & Ehlers 2005; Agrawal & Lynskey 2006; Hopfer et al. 2007; Agrawal et al. 2008). Hopfer and colleagues (2007) conducted a genome wide scan for loci influencing adolescent cannabis dependence in 324 sibling pairs from 192 families. In that study, probands (52.1% of whom were EuroAmerican, 36.5% of whom were Hispanic, and 7.8% of whom were African-American) were identified from consecutive admissions to substance abuse treatment facilities. The authors found evidence for suggestive linkage on chromosomes 3q21 (LOD = 2.61) and 9q34 (LOD = 2.57). These areas of the genome were not identified in the current linkage scan for Cannabis dependence II/B, however, a region on chromosome 3 was previously identified in a genome-wide linkage analysis for loci associated with the syndromic diagnoses of childhood conduct disorder (CD), ASPD, and the combined phenotype ASPD/CD in this Native American community. Agrawal and colleagues (2008) conducted a linkage scan in a sample from the COGA study for six DSM-IV cannabis dependence criteria considered as a continuous variable (0–6) and found a maximum LOD score of 1.9 at 95 cM on chromosome 14. A “region of interest” on chromosome 14 at 122 cM was also found for the type B/II cannabis subtype in the present study. This region on chromosome 14 was also identified previously in a genome-wide linkage analysis for ASPD/CD in this Native American community (Ehlers et al., 2008).
Two areas of the genome were identified in the present study with LOD scores that provide evidence for linkage on chromosome 16 (@139 cM, LOD score= 4.4), and on chromosome 19 (@74 cM, LOD score= 6.4). There are no published studies that have identified a region on chromosome 16 for cannabis dependence. However, there have been several reports of linkage findings within a 35 cM region on chromosome 16 for other drug dependence phenotypes. In this American Indian sample a region of interest was identified on chromosome 16 at 95 cM for a phenotype of “Any drug dependence /regular tobacco usage”. In this general area of chromosome 16, three other studies have also found regions of interest for alcohol dependence (Sheffield et al. 1999; Hill et al. 2004), as well as for the number of grams of alcohol consumed a day (Ma et al. 2003). There have been some findings in genome scans for cannabis dependence and other drug use related phenotypes on chromosome 19. A region has been identified on chromosome 19 at 17 cM for early-onset cannabis use in a study of 2314 Australian families (Agrawal et al. 2008). Closer to the region identified in the present study were the findings from the COGA study for a genome-wide screen for genes influencing conduct disorder where a region was identified on chromosome 19 at 35 cM.
Taken together the results of our study suggest that a Type B/II cannabis dependence phenotype can be identified in this Indian population that it is in part heritable. Additionally, a genome-wide linkage analysis has identified regions of the genome that are linked to this cannabis dependence phenotype in this population. Some of the locations identified for the TypeB/II cannabis dependence subtype on chromosomes 14, 16 and 22 were previously found to be associated with drug/tobacco dependence and/or ASPD/CD, but not alcohol dependence, phenotypes in this Indian population. Additionally, all of the sites identified have some supportive data for substance dependence related phenotypes in published genome scans in other population samples. The results of this study should, however, be assessed in the light of several limitations. Our venue-based and respondent driven ascertainment strategies may not have yielded a sample representative of the Indian population assessed. Given the diversity of Native American populations, our findings may not generalize to other Native Americans. Our study consisted of a cross-sectional sample, which contained individuals who may not have yet passed through the age of risk for developing cannabis dependence and the clinical symptoms used in the cluster analysis. A prospective study would address these limitations. It is important to point out that heritability of a cannabis dependence cluster does not necessarily arise from genetic variation since shared family environments may have contributed to its heritability. Comparisons of linkage findings to non-Indian populations may be limited by differences in a host of potential genetic and environmental variables. The underlying assumption that these phenotypes are normally distributed, an assumption of variance component analyses, may not be warranted. Finally, because this population has significant admixture, estimates of allele frequencies may produce biased LOD scores.
Despite these limitations, we believe that this study illustrates a potentially useful approach to characterizing heritable vs. non-heritable subtypes of cannabis dependence whose differentiation is useful for genotyping for genetic studies and may also be important for characterizing clinical course, and matching treatment and prevention strategies.
Table 1.
Average count of cluster variables and age onset of use in each cluster and comparison of variables in each pair.
| Variable | Cluster 1 | Cluster 2 | Cluster 3 | 1 vs 2 p-value |
1 vs 3 p-value |
2 vs 3 p-value |
|---|---|---|---|---|---|---|
| Adult antisocial symptoms |
2.84 | 5.03 | 3.06 | 0.001 | 0.89 | 0.001 |
| CD symptoms | 1.29 | 3.84 | 0.83 | 0.001 | 0.45 | 0.001 |
| Psychiatric Disorders |
0.39 | 0.53 | 0.28 | 0.49 | 0.86 | 0.48 |
| Cannabis use severity count |
2.61 | 2.73 | 2.89 | 0.45 | 0.23 | 0.64 |
| Withdrawal symptoms |
1.10 | 1.80 | 1.61 | 0.01 | 0.37 | 0.88 |
| Dependence symptoms |
4.77 | 6.21 | 4.83 | 0.001 | 0.99 | 0.01 |
| Age onset use (yrs) |
13.94 | 11.46 | 19.44 | 0.001 | 0.001 | 0.001 |
ACKNOWLEDGEMENTS
Supported in part by the National Institute on Drug Abuse Grant: DA019333, National Institute on Alcohol Abuse and Alcoholism Grants: AA00269, AA010201, the National Center on Minority Health and Health Disparities (NCMHD), and the Stein Endowment Fund. The authors thank Linda Corey, Michelle Dixon, Abigail Gross, Lilach Harris, Philip Lau, Susan Lopez, Evelyn Phillips, Shirley Sanchez, Gina Stouffer, Derek Wills, and Vincent Wong for assistance in data collection and analysis.
REFERENCES
- Agosti V, Nunes E, Levin F. Rates of psychiatric comorbidity among U.S. residents with lifetime cannabis dependence. Am J Drug Alcohol Abuse. 2002;28:643–652. doi: 10.1081/ada-120015873. [DOI] [PubMed] [Google Scholar]
- Agrawal A, Hinrichs AL, Dunn G, Bertelsen S, Dick DM, Saccone SF, et al. Linkage scan for quantitative traits identifies new regions of interest for substance dependence in the Collaborative Study on the Genetics of Alcoholism (COGA) sample. Drug Alcohol Depend. 2008;93:12–20. doi: 10.1016/j.drugalcdep.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal A, Lynskey MT. The genetic epidemiology of cannabis use, abuse and dependence. Addiction. 2006;101:801–812. doi: 10.1111/j.1360-0443.2006.01399.x. [DOI] [PubMed] [Google Scholar]
- Almasy L, Blangero J. Multipoint quantitative-trait linkage analysis in general pedigrees. Am J Hum Genet. 1998;62:1198–1211. doi: 10.1086/301844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnosis and statistical manual of mental disorders (DSM-III-R) Washington, DC: American Psychiatric Association; 1987. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-IV) Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-IV-TR) Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- Anthony JC, Warner LA, Kessler RC. Comparative epidemiology of dependence on tobacco, alcohol, controlled substances, and inhalants: basic findings from the National Comorbididty Survey. Exp Clin Psychopharmacol. 1994;2:244–268. [Google Scholar]
- Babor TF, Caetano R. Subtypes of substance dependence and abuse: implications for diagnostic classification and empirical research. Addiction. 2006;101 Suppl 1:104–110. doi: 10.1111/j.1360-0443.2006.01595.x. [DOI] [PubMed] [Google Scholar]
- Babor TF, Hofmann M, DelBoca FK, Hesselbrock V, Meyer RE, Dolinsky ZS, et al. Types of alcoholics, I. Evidence for an empirically derived typology based on indicators of vulnerability and severity. Arch Gen Psychiatry. 1992;49:599–608. doi: 10.1001/archpsyc.1992.01820080007002. [DOI] [PubMed] [Google Scholar]
- Ball SA, Carroll KM, Babor TF, Rounsaville BJ. Subtypes of cocaine abusers: support for a type A-type B distinction. J Consult Clin Psychol. 1995;63:115–124. doi: 10.1037//0022-006x.63.1.115. [DOI] [PubMed] [Google Scholar]
- Blangero J, Williams JT, Almasy L. Robust LOD scores for variance component-based linkage analysis. Genet Epidemiol. 2000;19 Suppl:S8–S14. doi: 10.1002/1098-2272(2000)19:1+<::AID-GEPI2>3.0.CO;2-Y. 1. [DOI] [PubMed] [Google Scholar]
- Brown J, Babor TF, Litt MD, Kranzler HR. The type A/type B distinction. Subtyping alcoholics according to indicators of vulnerability and severity. Ann N Y Acad Sci. 1994;708:23–33. doi: 10.1111/j.1749-6632.1994.tb24695.x. [DOI] [PubMed] [Google Scholar]
- Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI, Jr, et al. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. J Stud Alcohol. 1994;55:149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- Cloninger CR, Bohman M, Sigvardsson S. Inheritance of alcohol abuse. Cross-fostering analysis of adopted men. Arch Gen Psychiatry. 1981;38:861–868. doi: 10.1001/archpsyc.1981.01780330019001. [DOI] [PubMed] [Google Scholar]
- Conway KP, Compton W, Stinson FS, Grant BF. Lifetime comorbidity of DSM-IV mood and anxiety disorders and specific drug use disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry. 2006;67:247–257. doi: 10.4088/jcp.v67n0211. [DOI] [PubMed] [Google Scholar]
- Dick DM, Li TK, Edenberg HJ, Hesselbrock V, Kramer J, Kuperman S, et al. A genome-wide screen for genes influencing conduct disorder. Mol Psychiatry. 2004;9:81–86. doi: 10.1038/sj.mp.4001368. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Gilder DA, Slutske WS, Lind PA, Wilhelmsen KC. Externalizing disorders in American Indians: comorbidity and a genome wide linkage analysis. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:690–698. doi: 10.1002/ajmg.b.30666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Gilder DA, Wall TL, Phillips E, Feiler H, Wilhelmsen KC. Genomic screen for loci associated with alcohol dependence in Mission Indians. Am J Med Genet B Neuropsychiatr Genet. 2004;129B:110–115. doi: 10.1002/ajmg.b.30057. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Wall TL, Corey L, Lau P, Gilder DA, Wilhelmsen K. Heritability of illicit drug use and transition to dependence in Southwest California Indians. Psych Genet. 2007;17:171–176. doi: 10.1097/01.ypg.0000242201.56342.1a. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Wilhelmsen KC. Genomic scan for alcohol craving in Mission Indians. Psychiatr Genet. 2005;15:71–75. doi: 10.1097/00041444-200503000-00012. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Wilhelmsen KC. Genomic screen for loci associated with tobacco usage in Mission Indians. BMC Med Genet Feb 10. 2006;7:9. doi: 10.1186/1471-2350-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Wilhelmsen KC. Genomic screen for substance dependence and body mass index in Southwest California Indians. Genes Brain Behav. 2007;6:184–191. doi: 10.1111/j.1601-183X.2006.00246.x. [DOI] [PubMed] [Google Scholar]
- Feingold A, Ball SA, Kranzler HR, Rounsaville BJ. Generalizability of the type A/type B distinction across different psychoactive substances. Am J Drug Alcohol Abuse. 1996;22:449–462. doi: 10.3109/00952999609001671. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Horwood LJ. Does cannabis use encourage other forms of illicit drug use? Addiction. 2000;95:505–520. doi: 10.1046/j.1360-0443.2000.9545053.x. [DOI] [PubMed] [Google Scholar]
- Fisher BA, Ghuran A, Vadamalai V, Antonios TF. Cardiovascular complications induced by cannabis smoking: a case report and review of the literature. Emerg Med J. 2005;22:679–680. doi: 10.1136/emj.2004.014969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foroud T, Bucholz KK, Edenberg HJ, Goate A, Neuman RJ, Porjesz B, et al. Linkage of an alcoholism-related severity phenotype to chromosome 16. Alcohol Clin Exp Res. 1998;22:2035–2042. [PubMed] [Google Scholar]
- Gelernter J, Panhuysen C, Weiss R, Brady K, Hesselbrock V, Rounsaville B, et al. Genomewide linkage scan for cocaine dependence and related traits: significant linkages for a cocaine-related trait and cocaine-induced paranoia. Am J Med Genet B Neuropsychiatr Genet. 2005;136B:45–52. doi: 10.1002/ajmg.b.30189. [DOI] [PubMed] [Google Scholar]
- Gelernter J, Panhuysen C, Wilcox M, Hesselbrock V, Rounsaville B, Poling J, et al. Genomewide linkage scan for opioid dependence and related traits. Am J Hum Genet. 2006;78:759–769. doi: 10.1086/503631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilder DA, Lau P, Corey L, Ehlers CL. Factors associated with remission from cannabis dependence in Southwest California Indians. J Addict Dis. 2007;26:23–30. doi: 10.1300/J069v26n04_04. [DOI] [PubMed] [Google Scholar]
- Gilder DA, Lau P, Dixon M, Corey L, Phillips E, Ehlers CL. Comorbidity of select anxiety, affective, and psychotic disorders with cannabis dependence in Southwest California Indians. J Addict Dis. 2006;25:67–79. doi: 10.1300/J069v25n04_07. [DOI] [PubMed] [Google Scholar]
- Gilder DA, Wall TL, Ehlers CL. Comorbidity of select anxiety and affective disorders with alcohol dependence in Southwest California Indians. Alcohol Clin Exp Res. 2004;28:1805–1813. doi: 10.1097/01.alc.0000148116.27875.b0. [DOI] [PubMed] [Google Scholar]
- Grant BF. Comorbidity between DSM-IV drug use disorders and major depression: results of a national survey of adults. J Subst Abuse. 1995;7:481–497. doi: 10.1016/0899-3289(95)90017-9. [DOI] [PubMed] [Google Scholar]
- Grant BF, Pickering R. The relationship between cannabis use and DSM-IV cannabis abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. J Subst Abuse. 1998;10:255–264. doi: 10.1016/s0899-3289(99)00006-1. [DOI] [PubMed] [Google Scholar]
- Hashibe M, Straif K, Tashkin DP, Morgenstern H, Greenland S, Zhang ZF. Epidemiologic review of marijuana use and cancer risk. Alcohol. 2005;35:265–275. doi: 10.1016/j.alcohol.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Heckathorn DD. Respondent-driven sampling: a new approach to the study of hidden populations. Soc Probl. 1997;44:174–199. [Google Scholar]
- Hesselbrock M, Easton C, Bucholz KK, Schuckit M, Hesselbrock V. A validity study of the SSAGA--a comparison with the SCAN. Addiction. 1999;94:1361–1370. doi: 10.1046/j.1360-0443.1999.94913618.x. [DOI] [PubMed] [Google Scholar]
- Hesselbrock MN, Hesselbrock VM, Segal B, Schuckit MA, Bucholz K. Ethnicity and psychiatric comorbidity among alcohol-dependent persons who receive inpatient treatment: African Americans, Alaska Natives, Caucasians and Hispanics. Alcohol Clin Exp Res. 2003;27:1368–1373. doi: 10.1097/01.ALC.0000080164.21934.F9. [DOI] [PubMed] [Google Scholar]
- Hesselbrock VM, Hesselbrock MN.Are there empirically supported and clinically useful subtypes of alcohol dependence?Addiction 2006101Suppl 197–103. [DOI] [PubMed] [Google Scholar]
- Hesselbrock VM, Segal B, Hesselbrock MN. Alcohol dependence among Alaska Natives entering alcoholism treatment: a gender comparison. J Stud Alcohol. 2000;61:150–156. doi: 10.15288/jsa.2000.61.150. [DOI] [PubMed] [Google Scholar]
- Hill SY, Shen S, Zezza N, Hoffman EK, Perlin M, Allan W. A genome wide search for alcoholism susceptibility genes. Am J Med Genet B Neuropsychiatr Genet. 2004;128B:102–113. doi: 10.1002/ajmg.b.30013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopfer CJ, Lessem JM, Hartman CA, Stallings MC, Cherny SS, Corley RP, et al. A genome-wide scan for loci influencing adolescent cannabis dependence symptoms: evidence for linkage on chromosomes 3 and 9. Drug Alcohol Depend. 2007;89:34–41. doi: 10.1016/j.drugalcdep.2006.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalton G, Anderson DW. Sampling rare populations. J Roy Stat Soc. 1986;149:65–82. [Google Scholar]
- Kandel DB, Chen K. Types of marijuana users by longitudinal course. J Stud Alcohol. 2000;61:367–378. doi: 10.15288/jsa.2000.61.367. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, Neale MC, Prescott CA. Illicit psychoactive substance use, heavy use, abuse, and dependence in a U.S. population-based sample of male twins. Arch Gen Psychiatry. 2000;57:261–269. doi: 10.1001/archpsyc.57.3.261. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Myers J, Prescott CA. Specificity of genetic and environmental risk factors for symptoms of cannabis, cocaine, alcohol, caffeine, and nicotine dependence. Arch Gen Psychiatry. 2007;64:1313–1320. doi: 10.1001/archpsyc.64.11.1313. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA. Cannabis use, abuse, and dependence in a population-based sample of female twins. Am J Psychiatry. 1998;155:1016–1022. doi: 10.1176/ajp.155.8.1016. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Arch Gen Psychiatry. 2003;60:929–937. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- Kranzler HR, Wilcox M, Weiss RD, Brady K, Hesselbrock V, Rounsaville B, et al. The validity of cocaine dependence subtypes. Addict Behav. 2008;33:41–53. doi: 10.1016/j.addbeh.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynskey M, Hall W. The effects of adolescent cannabis use on educational attainment: a review. Addiction. 2000;95:1621–1630. doi: 10.1046/j.1360-0443.2000.951116213.x. [DOI] [PubMed] [Google Scholar]
- Lynskey MT, Heath AC, Bucholz KK, Slutske WS, Madden PA, Nelson EC, et al. Escalation of drug use in early-onset cannabis users vs co-twin controls. JAMA. 2003;289:427–433. doi: 10.1001/jama.289.4.427. [DOI] [PubMed] [Google Scholar]
- Lynskey MT, Heath AC, Nelson EC, Bucholz KK, Madden PA, Slutske WS, et al. Genetic and environmental contributions to cannabis dependence in a national young adult twin sample. Psychol Med. 2002;32:195–207. doi: 10.1017/s0033291701005062. [DOI] [PubMed] [Google Scholar]
- Ma JZ, Zhang D, Dupont RT, Dockter M, Elston RC, Li MD. Mapping susceptibility loci for alcohol consumption using number of grams of alcohol consumed per day as a phenotype measure. BMC Genet. 2003;4 Suppl 1:1–5. doi: 10.1186/1471-2156-4-S1-S104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes HH, Woodard CE, Murrelle L, Meyer JM, Silberg JL, Hewitt JK, et al. Tobacco, alcohol and drug use in eight- to sixteen-year-old twins: the Virginia Twin Study of Adolescent Behavioral Development. J Stud Alcohol. 1999;60:293–305. doi: 10.15288/jsa.1999.60.293. [DOI] [PubMed] [Google Scholar]
- Miles DR, van den Bree MB, Gupman AE, Newlin DB, Glantz MD, Pickens RW. A twin study on sensation seeking, risk taking behavior and marijuana use. Drug Alcohol Depend. 2001;62:57–68. doi: 10.1016/s0376-8716(00)00165-4. [DOI] [PubMed] [Google Scholar]
- Mitchell CM, Beals J, Novins DK, Spicer P. Drug use among two American Indian populations: prevalence of lifetime use and DSM-IV substance use disorders. Drug Alcohol Depend. 2003;69:29–41. doi: 10.1016/s0376-8716(02)00253-3. [DOI] [PubMed] [Google Scholar]
- Mittleman MA, Lewis RA, Maclure M, Sherwood JB, Muller JE. Triggering myocardial infarction by marijuana. Circulation. 2001;103:2805–2809. doi: 10.1161/01.cir.103.23.2805. [DOI] [PubMed] [Google Scholar]
- Muhib FB, Lin LS, Stueve A, Miller RL, Ford WL, Johnson WD, et al. A venue-based method for sampling hard-to-reach populations. Public Health Rep. 2001;116 Suppl 1:216–222. doi: 10.1093/phr/116.S1.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope HG, Jr., Ionescu-Pioggia M, Pope KW. Drug use and life style among college undergraduates: a 30-year longitudinal study. Am J Psychiatry. 2001;158:1519–1521. doi: 10.1176/appi.ajp.158.9.1519. [DOI] [PubMed] [Google Scholar]
- Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, et al. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study. JAMA. 1990;264:2511–2518. [PubMed] [Google Scholar]
- Rhee SH, Hewitt JK, Young SE, Corley RP, Crowley TJ, Stallings MC. Genetic and environmental influences on substance initiation, use, and problem use in adolescents. Arch Gen Psychiatry. 2003;60:1256–1264. doi: 10.1001/archpsyc.60.12.1256. [DOI] [PubMed] [Google Scholar]
- Saccone NL, Kwon JM, Corbett J, Goate A, Rochberg N, Edenberg HJ, et al. A genome screen of maximum number of drinks as an alcoholism phenotype. Am J Med Genet B Neuropsychiatr Genet. 2000;96B:632–637. doi: 10.1002/1096-8628(20001009)96:5<632::aid-ajmg8>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. The clinical implications of primary diagnostic groups among alcoholics. Arch Gen Psychiatry. 1985;42:1043–1049. doi: 10.1001/archpsyc.1985.01790340021003. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. Drug and alcohol abuse: a clinical guide to diagnosis and treatment. 6th ed. New York: Springer; 2006. [Google Scholar]
- Schuckit MA, Tipp JE, Bergman M, Reich W, Hesselbrock VM, Smith TL. Comparison of induced and independent major depressive disorders in 2,945 alcoholics. Am J Psychiatry. 1997a;154:948–957. doi: 10.1176/ajp.154.7.948. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Tipp JE, Bucholz KK, Nurnberger JI, Jr, Hesselbrock VM, Crowe RR, et al. The life-time rates of three major mood disorders and four major anxiety disorders in alcoholics and controls. Addiction. 1997b;92:1289–1304. [PubMed] [Google Scholar]
- Schuckit MA, Tipp JE, Smith TL, Shapiro E, Hesselbrock VM, Bucholz KK, et al. An evaluation of type A and B alcoholics. Addiction. 1995;90:1189–1203. doi: 10.1046/j.1360-0443.1995.90911894.x. [DOI] [PubMed] [Google Scholar]
- Sharma BP. Cannabis and its users in Nepal. Br J Psychiatry. 1975;127:550–552. doi: 10.1192/bjp.127.6.550. [DOI] [PubMed] [Google Scholar]
- Sheffield LJ, Knauert MP, Pakstis AJ, Zhao H, Kidd KK. Analyses of the COGA data set in one ethnic group with examinations of alternative definitions of alcoholism. Genet Epidemiol. 1999;17 Suppl 1:S319–S324. doi: 10.1002/gepi.1370170754. [DOI] [PubMed] [Google Scholar]
- SOLAR. Sequential Oligogenic Linkage Analysis Routines. San Antonio, TX: Southwest Foundation for Biomedical Research; http://solar.sfbrgenetics.org/ [Google Scholar]
- Solowij N, Stephens RS, Roffman RA, Babor T, Kadden R, Miller M, et al. Cognitive functioning of long-term heavy cannabis users seeking treatment. JAMA. 2002;287:1123–1131. doi: 10.1001/jama.287.9.1123. [DOI] [PubMed] [Google Scholar]
- Stinson FS, Ruan WJ, Pickering R, Grant BF. Cannabis use disorders in the USA: prevalence, correlates and comorbidity. Psychol Med. 2006;36:1447–1460. doi: 10.1017/S0033291706008361. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA) Results from the 2003 national survey on drug use and health: National findings. Rockville, MD: NSDUH Series H-25, DHHS Publication No. SMA 04–3964, U.S.DHHS, Office of Applied Studies; 2003. [Google Scholar]
- Swift W, Hall W, Teesson M. Cannabis use and dependence among Australian adults: results from the National Survey of Mental Health and Wellbeing. Addiction. 2001;96:737–748. doi: 10.1046/j.1360-0443.2001.9657379.x. [DOI] [PubMed] [Google Scholar]
- Tashkin DP. Smoked marijuana as a cause of lung injury. Monaldi Arch Chest Dis. 2005;63:93–100. doi: 10.4081/monaldi.2005.645. [DOI] [PubMed] [Google Scholar]
- Taylor DR, Poulton R, Moffitt TE, Ramankutty P, Sears MR. The respiratory effects of cannabis dependence in young adults. Addiction. 2000;95:1669–1677. doi: 10.1046/j.1360-0443.2000.951116697.x. [DOI] [PubMed] [Google Scholar]
- Troisi A, Pasini A, Saracco M, Spalletta G. Psychiatric symptoms in male cannabis users not using other illicit drugs. Addiction. 1998;93:487–492. doi: 10.1046/j.1360-0443.1998.9344874.x. [DOI] [PubMed] [Google Scholar]
- Tsuang MT, Lyons MJ, Eisen SA, Goldberg J, True W, Lin N, et al. Genetic influences on DSM-III-R drug abuse and dependence: a study of 3,372 twin pairs. Am J Hum Genet. 1996;67:473–477. doi: 10.1002/(SICI)1096-8628(19960920)67:5<473::AID-AJMG6>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Tsuang MT, Lyons MJ, Meyer JM, Doyle T, Eisen SA, Goldberg J, et al. Co-occurrence of abuse of different drugs in men: the role of drug-specific and shared vulnerabilities. Arch Gen Psychiatry. 1998;55:967–972. doi: 10.1001/archpsyc.55.11.967. [DOI] [PubMed] [Google Scholar]
- Uhl GR, Liu QR, Walther D, Hess J, Naiman D. Polysubstance abuse-vulnerability genes: genome scans for association, using 1,004 subjects and 1,494 single-nucleotide polymorphisms. Am J Hum Genet. 2001;69:1290–1300. doi: 10.1086/324467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Bureau of the Census. Characteristics of American Indians by Tribe and Language. Washington, D.C: United States Department of Commerce, Economics and Statistics Administration; 1990. [Google Scholar]
- Vega WA, Aguilar-Gaxiola S, Andrade L, Bijl R, Borges G, Caraveo-Anduaga JJ, et al. Prevalence and age of onset for drug use in seven international sites: results from the international consortium of psychiatric epidemiology. Drug Alcohol Depend. 2002;68:285–297. doi: 10.1016/s0376-8716(02)00224-7. [DOI] [PubMed] [Google Scholar]
- Weber JL, May PE. Abundant class of human DNA polymorphisms which can be typed using the polymerase chain reaction. Am J Hum Genet. 1989;44:388–396. [PMC free article] [PubMed] [Google Scholar]
- Wilhelmsen KC, Ehlers C. Heritability of substance dependence in a Native American population. Psychiatr Genet. 2005;15:101–107. doi: 10.1097/00041444-200506000-00006. [DOI] [PubMed] [Google Scholar]
- Wilhelmsen KC, Schuckit M, Smith TL, Lee JV, Segall SK, Feiler HS, et al. The search for genes related to a low-level response to alcohol determined by alcohol challenges. Alcohol Clin Exp Res. 2003;27:1041–1047. doi: 10.1097/01.ALC.0000075551.02714.63. [DOI] [PubMed] [Google Scholar]