Abstract
Mutations in human copper-zinc superoxide dismutase (SOD1) cause an inherited form of amyotrophic lateral sclerosis (ALS, Lou Gehrig’s disease, motor neuron disease). Insoluble forms of mutant SOD1 accumulate in neural tissues of human ALS patients and in spinal cords of transgenic mice expressing these polypeptides, suggesting that SOD1-linked ALS is a protein misfolding disorder. Understanding the molecular basis for how the pathogenic mutations give rise to SOD1 folding intermediates, which may themselves be toxic, is therefore of keen interest. A critical step on the SOD1 folding pathway occurs when the copper chaperone for SOD1 (CCS) modifies the nascent SOD1 polypeptide by inserting the catalytic copper cofactor and oxidizing its intrasubunit disulfide bond. Recent studies reveal that pathogenic SOD1 proteins coming from cultured cells and from the spinal cords of transgenic mice tend to be metal-deficient and/or lacking the disulfide bond, raising the possibility that the disease-causing mutations may enhance levels of SOD1-folding intermediates by preventing or hindering CCS-mediated SOD1 maturation. This mini-review explores this hypothesis by highlighting the structural and biophysical properties of the pathogenic SOD1 mutants in the context of what is currently known about CCS structure and action. Other hypotheses as to the nature of toxicity inherent in pathogenic SOD1 proteins are not covered.
Keywords: superoxide dismutase, SOD1, amyotrophic lateral sclerosis, motor neuron disease, protein misfolding, protein aggregation, protofibrils, amyloid
SOD1 and Familial ALS
ALS, the most frequently occurring adult motor neuron disease, is a fatal, late-onset, paralytic disorder first described in the late 1800s by the French neurologist Jean-Martin Charcot (1). The hallmarks of ALS are spasticity, hyperreflexia, muscle atrophy, and paralysis (2). Death usually occurs in within five years of symptom onset, typically from respiratory failure. The majority of ALS cases are termed “sporadic” (sALS), meaning that the afflicted individual has no family history, while the remaining cases are termed “familial” (fALS), meaning that a genetic lesion is passed from generation to generation (3, 4).
In 1993, eleven families with histories of ALS were found to possess dominant mutations in the gene encoding the cytosolic antioxidant enzyme copper-zinc superoxide dismutase (SOD1) (5, 6). These findings generated enormous excitement in the ALS research community because the structure and action of SOD1 were fairly well characterized, and it was hoped that understanding the molecular basis for how the pathogenic SOD1 mutations exert their toxic effects in motor neurons would illuminate novel avenues of therapeutic intervention. In addition, because sALS and fALS are similar clinically, it is possible that the underlying molecular causes for the two forms of the disease could be related and therapeutics effective for SOD1-linked ALS might prove effective for the more prevalent sporadic forms of the disease. Today, nearly 16 years after lesions in the gene encoding SOD1 were first linked to fALS, the number of distinct ALS-SOD1 mutations published in the literature has risen to ~100 (Table 1) (7, 8). However, an effective treatment has still not yet been identified and acquiring an understanding of the molecular basis for SOD1-linked ALS has proven elusive.
Table 1.
Published ALS-SOD1 Proteins
| Mutations | Class | Principal references |
|---|---|---|
| Exon 1 | ||
| 1. A4→S, T, or V | B | 89–92 |
| 2. C6→F or G | B | 94, 95 |
| 3. V7→E | B | 97 |
| 4. L8→Q or V | B | 98, 99 |
| 5. G10→V | B | 100 |
| 6. G12→R | B | 105 |
| 7. V14→G or M | B | 107, 108 |
| 8. G16→A or S | B | 98, 109 |
| 9. N19→S | B | 98 |
| 10. F20→C | B | 98 |
| 11. E21→G or K | B | 99, 112 |
| 12. Q22→L | B | 98 |
| Exon 2 | ||
| 13. G37→R | B | 6 |
| 14. L38→R or V | B | 6, 116 |
| 15. G41→D or S | B | 6 |
| 16. H43→R | B | 6 |
| 17. F45→C | B | 93 |
| 18. H46→R | M | 121 |
| 19. V47→F | B | 98 |
| 20. H48→Q or R | M | 98, 123 |
| 21. E49→K | B | 116 |
| 22. T54→R | D | 98 |
| 23. C57→R | D | 118 |
| Exon 3 | ||
| 24. S59→I | D | 98 |
| 25. N65→S | M | 115 |
| 26. L67→R | M | 116 |
| 27. G72→C or S | M | 60, 127 |
| 28. D76→V or Y | M | 107, 129 |
| Exon 4 | ||
| 29. H80→R | M | 130 |
| 30. L84→F or V | M | 127, 131 |
| 31. G85→R | M | 6 |
| 32. N86→D, K, or S | B | 118, 132, 133 |
| 33. V87→A | B | 98 |
| 34. T88delTAD* | B | 98 |
| 35. A89→T or V | B | 98, 135 |
| 36. D90→A or V | B | 136, 137 |
| 37. G93→A, C, D, R, S, or V | B | 6, 99, 114, 122, 138 |
| Exon 4 | ||
| 38. A95→T | B | 93 |
| 39. D96→N | B | 96 |
| 40. V97→M | B | 98 |
| 41. E100→G or K | B | 6, 99 |
| 42. D101→G, H, N or Y | B | 101–104 |
| 43. I104→F | B | 106 |
| 44. S105→L or delSL | B | 98 |
| 45. L106→V | B | 6 |
| 46. G108→V | B | 110 |
| 47. D109→Y | B | 111 |
| 48. C111→Y | B | 113 |
| 49. I112→M or T | B | 114, 115 |
| 50. I113→F or T | B | 6, 98 |
| 51. G114→A | B | 98 |
| 52. R115→G | B | 117 |
| 53. T116→R | B | 118 |
| 54. V118→L or L ins (stop 122) | B | 98, 119, 120 |
| Exon 5 | ||
| 55. D124→G or V | M | 98, 122 |
| 56. D125→H | M | 123 |
| 57. L126→S or stop or del (stop 131) | B | 62, 99, 124 |
| 58. G127ins (stop 133) | B | 107 |
| 59. E132ins (stop 133) | B | 110 |
| 60. E133del* | B | 122 |
| 61. S134→N | M | 125 |
| 62. N139→H or K | B | 124, 126 |
| 63. A140→G | B | 111 |
| 64. G141→E or stop | B | 98, 102 |
| 65. L144→F or S | B | 5, 128 |
| 66. A145→G or T | B | 98, 128 |
| 67. C146→R | D | 99 |
| 68. G147→R | B | 98 |
| 69. V148→G or I | B | 5, 106 |
| 70. I149→T | B | 124 |
| 71. I151→S or T | B | 98, 134 |
B, β-barrel mutants; M, metal-binding region mutants; D, disulfide loop mutants.
Pathogenic SOD1 Proteins Have Acquired a Toxic Property
SOD1 detoxifies reactive superoxide anion, a normal byproduct of cellular respiration, to molecular oxygen and water [2O2− +2H+ → H2O2 + O2] (9). In mammals, SOD1 is ubiquitously expressed in all tissues and within cells it is primarily localized to the cytosol, although lesser amounts are found in the nucleus, peroxisomes, and mitochondria (10). The enzyme is particularly plentiful in the spinal cord and brain, where it has been estimated to comprise between 0.1% and 2.0% of the detergent-soluble protein (11, 12). This abundance likely reflects the copious superoxide generated by these highly respiring tissues. The fundamental role of SOD1 as an antioxidant protein, combined with its abundance in neural tissue, suggested an initial hypothesis that the pathogenic SOD1 mutations might result in an enzyme that is unable to detoxify reactive oxygen species. Over time, this loss of enzymatic function could lead to oxidative damage and death of neural cells. However, mice lacking SOD1 do not develop motor neuron disease (13) and transgenic mice expressing human fALS SOD1 mutants in addition to their own endogenous SOD1 develop paralytic symptoms strikingly similar to those observed in human patients (14–16). Together, these observations imply that pathogenic SOD1 molecules act through the gain of a cytotoxic property and not a loss of function (see below).
Genetics and Models of SOD1-Linked fALS
The human SOD1 gene, located on chromosome 21 (17), is comprised of 5 exons that are spliced to produce mRNA that, when translated, produces a single species of SOD1 protein. In other words, there is no evidence of alternative splicing to produce functionally distinct SOD1 isoforms. The mature mRNA codes for a protein of 154 amino acids, which is post-translationally modified by removal of the initiating methionine, followed by N-terminal acetylation. The mature protein consists of 153 amino acids, and the numbering system used to identify sites of mutation is based on the amino acid sequence of the mature polypeptide. Missense mutations that cause fALS have been documented at 68 positions in the SOD1 protein (Table 1 and Fig. 1). With more than 100 missense mutations at these 68 positions, it is obvious that multiple amino acid substitutions at a given position can cause disease (e.g., Gly93 to Ala, Cys, Asp, Arg, Ser, or Val). In initial studies of SOD1-linked fALS (5, 6), the evidence indicated that the disease was completely penetrant. However, as the identification of new families led to additional mutations, it has become clear that some are not (see http://alsod.iop.kcl.as.uk), meaning that a given individual inheriting a mutant SOD1 allele may live a normal lifespan, with the disease appearing in the next generation. The most common mutation in North America is a substitution of Val for Ala at position 4 (A4V); this mutation appears to be fully penetrant, producing a disease of relatively short duration.
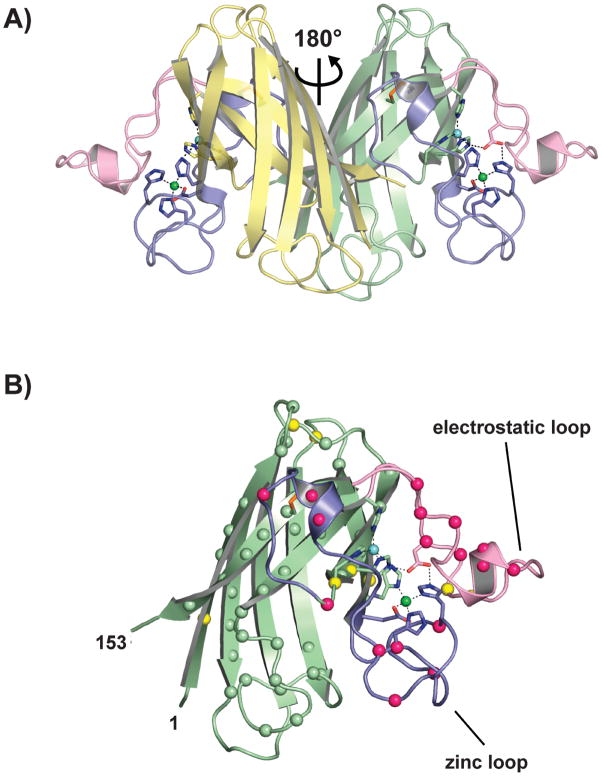
Figure 1.
SOD1 structure. (A) Human Cu-Zn superoxide dismutase [pdb code 2C9V (29)]. The relationship of the two monomers is indicated. Intrasubunit disulfide bonds are shown as orange sticks, the metal-binding loops (loop IV and VII) are shown in blue and pink, respectively. Copper and zinc ions are shown as cyan and green spheres, respectively. (B) The spatial distribution of the known pathogenic SOD1 mutations. A monomer of SOD1 is shown in the same orientation as the rightmost subunit in Figure 1A. The α-carbon positions of fALS mutations falling in the β-barrel and in the metal-binding loop elements are shown as green and hot pink spheres, respectively. The α-carbon positions of pathogenic SOD1 mutants for which there are mouse models are shown as yellow spheres.
Across species, the amino acid sequence of SOD1 is highly conserved; 112 of 153 residues are conserved in mammals, with 70 invariant across eukaryotic phyla (18). Sixty-one of the pathogenic mutations occur at residues conserved in mammals, with 49 occurring at positions that are extremely conserved (18). To our knowledge, there are no reports in the veterinary literature of spontaneous mutations in SOD1 that are associated with a neuromuscular disorder in domesticated or captive animals. However, it has been demonstrated that transgenic over-expression of the mouse SOD1 protein encoding a mutation associated with human fALS (G86R) induces a neuromuscular disorder remarkably similar to fALS (19). Apart from the experimental murine models, however, SOD1-linked ALS appears to be a uniquely human disease.
The discovery of mutations in SOD1 as a cause of ALS provided the first opportunity to produce a genetically faithful model of the disease. Transgenic mice that over-express human SOD1-harboring mutations linked to fALS develop muscle loss and paralysis characteristic of human ALS. Almost all of the published models were built by injecting a 12-kb fragment of human genomic DNA containing all regulatory elements as the transgene vector (20). Mutant human SOD1 genes that have been introduced into mice include A4V (21), G37R (22), H46R (23), G85R (15), G93A (16), L126Z (14, 21), L126del(stop 131) (24), and Gins127TGGG (25). In addition to the mice, there are two examples of transgenic rats that harbor the human gene [H46R (26) and G93A (27)]. The mouse model most widely used by ALS researchers is the first model that was developed by Mark Gurney and colleagues (16). This model expresses the fALS variant SOD1-G93A at very high levels and has a disease onset marked by hindlimb weakness at 3–4 months of age, with death occurring by 4–5 months of age. In general, the phenotypic manifestation of disease in all of the mouse and rat models is similar; limb weakness (usually hindlimb) is the first sign, followed by generalized weakness in all limbs and the trunk. Pathologic features of the disease are among the most faithful of all models of human neurologic disease, including loss of large motor neurons of the spinal cord, robust astrogliosis and microgliosis, and inclusion pathology. Overall, it is widely accepted that the mutant SOD1 mouse models faithfully model human SOD1-linked fALS.
Structural Properties of SOD1
SOD1 is a 32-kDa homodimeric enzyme in which each subunit folds as an 8-stranded Greek key β-barrel, binds one copper and one zinc ion, and contains one intrasubunit disulfide bond (28). Figure 1A shows the mature wild type holoenzyme [pdb code 2C9V (29)]. Two lengthy loop elements project from the β-barrel that are important in metal ion binding and the formation of the active site. These are termed the “zinc loop” (loop IV, residues 50–83) and the “electrostatic loop” (loop VII, residues 121–142). In the mature enzyme, the “disulfide loop,” a substructure of loop IV (residues 50–62), is covalently linked to the β-barrel through a disulfide bond between C57 and C146 [for review, see (7)].
The pathogenic SOD1 mutations are grouped based on their positions in the structure (Fig. 1B). “β-barrel mutants” are isolated from their expression systems with metal content nearly identical to that found for the wild type SOD1 expressed in the same systems, while “metal binding mutants” tend to be deficient in copper and/or zinc (30, 31). Three-dimensional structures are known for β-barrel mutants A4V (32), G37R (33, 34), H43R (35), G93A (36), and I113T (32), and these metal-replete structures reveal only slight perturbations relative to the wild type enzyme. Structures of the metal-binding mutants H46R (37, 38), H46R/H48Q double mutant (39), G85R (40), D125H (41), and S134N (37, 42) have also been determined, and most of these are metal-deficient, which in turn results in conformational disorder of the electrostatic and zinc loop elements.
The biophysical properties of these two classes of pathogenic SOD1 mutants are dramatically different in their metal-free, disulfide-reduced (newly translated) forms. In differential scanning calorimetry experiments, nascent β-barrel mutants tend to be substantially destabilized relative to the wild type enzyme, while newly translated metal-binding mutants tend to retain thermal stability similar to the wild type enzyme (43, 44). The thermal stability of newly translated SOD1 proteins overall is significantly enhanced via posttranslational modification to the mature holoenzyme via the action of its copper chaperone (CCS) (45) (see below).
The mature human SOD1 holoenzyme is a remarkably stable dimer, retaining enzymatic activity at elevated temperatures and in the presence of denaturing agents (46, 47). Although the stabilizing effects of metal ion binding have long been known, recent studies have also illuminated the importance of the intrasubunit disulfide bond to dimer stability. The dimer interface is formed predominantly by reciprocal interactions of the disulfide loop and β-strand 8 across the molecular two-fold axis (Fig. 1A) (48). Protein folding (49), gel filtration (50), and analytical ultracentrifugation (48) analyses have revealed that reduction of the disulfide bond in the metal-free protein results in monomerization. As can be inferred from Figure 1A, a reduced disulfide bond will result in enhanced mobility of the disulfide loop, weakening the interactions across the dimer interface (51).
Soluble Oligomers and Insoluble Aggregates of Mutant SOD1 in fALS
One of the common features of the mouse models of SOD1-linked ALS is the accumulation of insoluble forms of mutant protein as the disease progresses (52). These insoluble mutant proteins are generally thought of as aggregates [for review, see Murphy R.M., 2002 (53)] that are composed of assemblies of protein that attain relatively high molecular weight (examples include filamentous aggregates as well as smaller oligomeric structures). The mutant proteins are far more prone to form these assemblies than is normal human SOD1 (52). Soluble higher-order oligomers of pathogenic SOD1 have also been detected (54), and it is generally presumed that these are on the pathway to the formation of insoluble aggregates. Whether it is the misfolded pathogenic SOD1 monomers, soluble oligomers, or insoluble aggregates that are the noxious entities in SOD1-linked ALS remains unclear. Importantly, in tissues from diseased mice, a majority of the mutant proteins fractionate to the soluble fraction (52), meaning that only a portion of the total mutant SOD1 in the tissue ends up in the insoluble aggregate.
In general, pathologic protein aggregates resist dissociation in detergent, and larger oligomers can be separated from smaller soluble species by ultracentrifugation or size exclusion chromatography. Forms of mutant SOD1 that are insoluble in non-ionic detergent have been detected in multiple mouse models including mice that express the following variants: A4V (21), G37R (52), G85R (52), mouse G86R (55), G93A (52), L126Z (14) (Fig. 2), and Gins127TGGG (25). Similar aggregates were found in spinal cord tissues of an fALS patient harboring the A4V mutation (52). Aggregation of the mutant protein does not appear to be entirely secondary to disease processes in tissues because high-level expression of the mutant protein in cultured cells can produce assemblies of mutant protein that are biochemically and biophysically similar to the aggregates formed in tissues (52, 55).
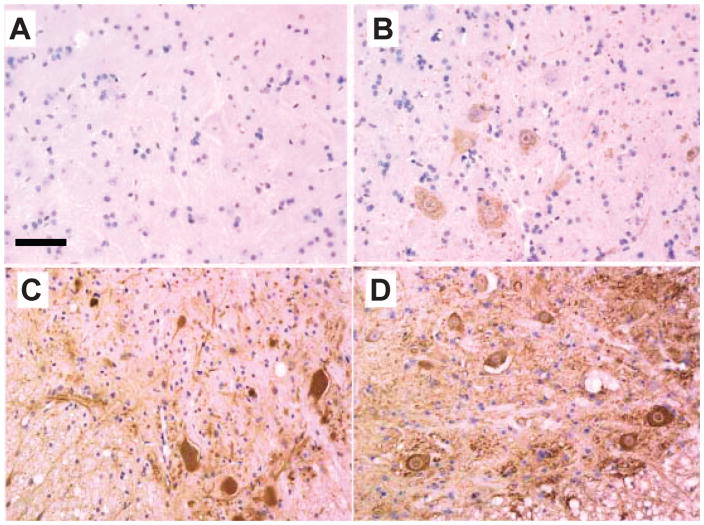
Figure 2.
Accumulation of SOD1-L126 truncation variant (L126Z) in somatodendritic compartments of spinal motor neurons. Tissue sections embedded in paraffin were deparaffinized and immunostained with hSOD1 anti-serum at a dilution of 1:500. (A) Non-transgenic littermate 9 months old. (B) Representative image from 3.5-month-old L126Z mice. (C) Image from a 7-month-old symptomatic SOD1-L126Z mouse shows longitudinal profiles of dendrites and motor neuron corpses filled with immunoreactivity. (D) Image from 9-month-old symptomatic SOD1-L126Z mouse shows intensifying of motor neuron soma and circular profiles resembling dendritic cross-sections. Scale bar = 50 mm [adapted from (14)].
In pathological examination of tissues from humans or from animal models, aggregates of mutant SOD1 are defined by the formation of macromolecular structures termed inclusion-bodies (Fig. 2). In human disease, the availability of autopsy cases from SOD1-linked cases has been limited, but there are a number of case reports in the literature. The most consistently reported pathologic structures are hyaline or Lewy-body like inclusions that are immunoreactive to SOD1 antibodies; mutants examined include A4V (56, 57); H46R (58); I113T (59); G72C (60); L126del (stop 131) (57, 61); and L126S (62). However, there have been reports of SOD1-linked ALS cases in which inclusion pathology was either absent or the inclusions that were unreactive with antibodies to SOD1 (56, 63–66). In the fALS mouse models, pathologic inclusions are not necessarily prominent pathologic features (14, 16, 22, 52), but have been observed as the major pathology of mice that express human SOD1-G85R (15).
The biophysical mechanisms of mutant SOD1 oligomerization/aggregation remain unclear. A number of recent studies provided evidence that aberrant intermolecular disulfide bonding of mutant SOD1 (cysteines at residues 6, 57, 111, 146) either promoted aggregation and/or stabilized aggregates generated by other mechanisms (18, 67–72). However, there have been reports of fALS mutations at all four cysteines residues in SOD1 (Table 1); recent studies demonstrated that SOD1 mutants encoding disease-linked mutations at these cysteine residues (e.g., C6G, C6F, C111Y, C146R) rapidly formed aggregates when expressed in cell culture (55, 69). Moreover, Karch and Borchelt demonstrated that experimental mutants that lack all four cysteine residues (C6F/C57S/C111Y/C146R), or which encode only a single cysteine at positions 6 or 111 (C6/C57S/C111Y/C146R or C6F/C57S/C111/C146R) rapidly aggregate when expressed in cultured cells (55). Recent in vitro studies by Chattopadhyay and colleagues (73) have demonstrated the aggregation of wild-type human SOD1 into amyloid-fibril–like structures via mechanisms that do not appear to involve intermolecular disulfide crosslinking. Collectively, these data suggest that extensive disulfide cross-linking is not required to either promote or stabilize mutant SOD1 oligomerization/aggregation.
Little is known of the structures of mutant SOD1 aggregates that form in vivo. In vitro, metal-depleted mutant forms of SOD1 can assemble into linear and helical filamentous arrays, based on the principle that their β-sheet edges have lost protection from the “negative design” inherent in the wild type enzyme (37, 74). The helical filamentous arrays of metal-depleted pathogenic SOD1 (Fig. 3) (37) have some similarity to the “amyloid pores” that have been observed in other neurodegenerative diseases in which protein aggregation is a characteristic feature (75). In most, but not all fALS mouse models, there is evidence of the accumulation of amyloid-like material; thioflavin-S positive structures (14, 52). As mentioned above, recent studies by Chattopadhyay and colleagues generated amyloid-like fibrils of wild-type human SOD1 in vitro. Overall, these studies indicate that SOD1 possesses structural features that impart an inherent propensity to oligomerize/aggregate; however, the precise nature of the higher order SOD1 species that form in vivo either early or late in disease remains poorly defined. Moreover, we lack a sufficient understanding of the role of specific SOD1 higher-order structures in disease pathogenesis to predict whether disruption of mutant SOD1 oligomerization/aggregation would be beneficial or detrimental. A recent study of forms of SOD1 engineered to produce stable dimeric enzyme suggested toxicity is not tied to aggregation (76). However, data from cell culture models suggest that formation of large SOD1 aggregates could be a primary mechanism of toxicity (77), although it remains possible that it is the soluble precursors of these large SOD1 aggregates and not the aggregates themselves that are toxic.
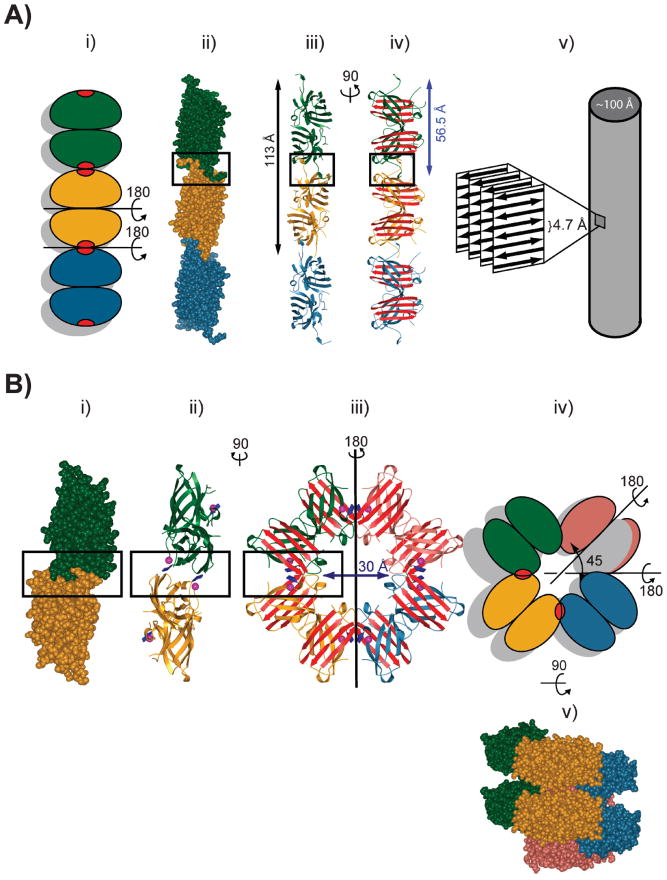
Figure 3.
Metal deficiency in fALS SOD1 gives rise to linear cross-β fibrils and helical filamentous arrays through loss of negative design (37, 74). (A) Linear, amyloid-like filaments formed by 3 dimers shown from top to bottom in green, gold, and blue. Nonnative SOD1-SOD1 interactions are shown as red patches in (i) and are boxed in (ii–iv). The “cross-β” structure observed in amyloid fibrils is shown schematically in (v). (B) Metal deficiency in pathogenic SOD1 also gives rise to water-filled helical filamentous arrays. (i) One-half of one turn of the helical filament is represented by the two dimers shown from top to bottom in green and gold. (ii–iii) Ribbon representation. The arrow indicates the diameter of the central cavity. The non-native interactions between SOD1 dimers are boxed. (iv) Schematic view of the helical filamentous array shown in (iii) with the new interdimer contacts shown as red patches. (v) This view of the helical filament is rotated 90° around a horizontal axis relative to the view in (iii) and (iv). Successive Zn–H46R dimers (green, yellow, blue, and red) comprise one turn of helical filament with a pitch of ~35 Å [adapted from (37)].
The Copper Chaperone for SOD1 (CCS)
Human CCS is a three-domain polypeptide that confers at least two critical stabilizing posttranslational modifications on newly synthesized SOD1: (i) the insertion of the catalytic copper ion (45), and (ii) the oxidation of the disulfide bond found within each SOD1 subunit (78). The presence of a disulfide bond is rare for cytosolic proteins given the strong reducing environment of the cytosol, and recent studies suggest that CCS-mediated oxidation of this disulfide bond occurs concomitant with copper delivery in an oxygen- or superoxide-dependent fashion (78). It is important to note that at the protein level, the ratio of SOD1 to CCS in the cytosol is estimated to be between 15:1 and 30:1 (79), meaning that CCS must cycle through the newly translated SOD1 pool to activate these molecules (68). It is presumed that upon copper delivery to SOD1, CCS becomes recharged with copper via the membrane-bound copper transporter CTR1, although to our knowledge, direct protein-protein interactions between CCS and CTR1 have not yet been demonstrated.
CCS domain I (residues 1–84) contains a copper-binding motif MXCXXC that is postulated to acquire copper ion from the membrane copper transporter CTR1 (68). Domain II (residues 85–233) is similar to human SOD1 and retains amino acid residues found at the SOD1 dimer interface (80). Because dimer interface residues are conserved, domain II is proposed to be responsible for the specificity of CCS/SOD1 interaction via the formation of a SOD1/CCS heterodimer (81). Domain III (residues 234–273) contains the copper-binding motif CXC, which is proposed to directly insert copper ion into nascent SOD1 (81). The “heterodimerization” model of CCS activation of SOD1 (Fig. 4) has endured for the last decade, although the spatial-temporal mechanistic details of the activation process have remained elusive. Human CCS itself dimerizes though its SOD1-like domain II, which contains a zinc binding site and disulfide bond analogous to those found in SOD1. Unanswered mechanistic questions include: How does CCS reorganize itself to utilize domain II as a nascent SOD1 recognition module? What conformational changes accompany the transfer of copper from CCS domain I to CCS domain III prior to delivery to SOD1? What amino acid residues of both proteins participate in copper delivery and disulfide bond oxidation? Perhaps most importantly, how might fALS mutations in SOD1 interfere with CCS action, and what are the properties of the resulting immature SOD1 proteins (see below)?
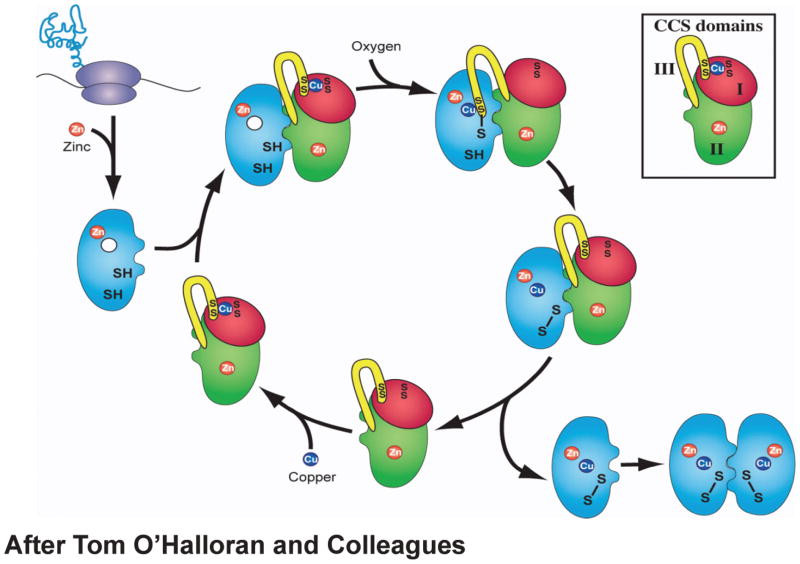
Figure 4.
Heterodimerization model of CCS action after O’Halloran and colleagues (68). Newly translated SOD1 monomers are shown in blue. CCS domain I is shown in red, CCS domain II is shown in green, and CCS domain III is shown in yellow (inset).
An Alternate Model of CCS Action
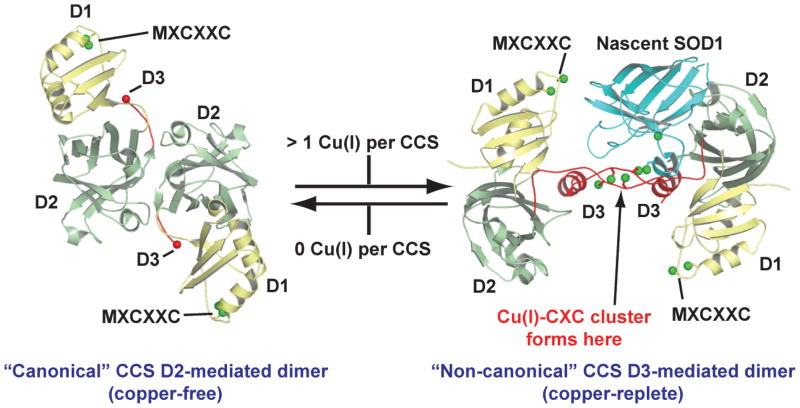
To probe various mechanistic aspects of CCS action, Blackburn and colleagues titrated purified human CCS with increasing concentrations of Cu(I) followed by EXAFS and gel filtration studies (82). Upon addition of a single equivalent of Cu(I) per CCS molecule, they observed that the “canonical” CCS dimer mediated by the SOD1-like domain II dissociated into monomers and that addition of additional equivalents resulted in the appearance of a “non-canonical” dimer mediated by a Cu4S6 copper cluster formed in part by the two CXC motifs of CCS domain III (Fig. 5). This canonical-to-noncanonical CCS dimer transition, if it occurs in vivo, would seem to act as a functional copper-sensing switch to make CCS domain II available to nascent SOD1 binding only when sufficient copper is available (82). Interestingly, a non-canonical domain III mediated CCS dimer similar to that shown in Figure 5 was also observed in the crystal structure of a yeast SOD1/yeast CCS complex, although no copper was present in the crystallization experiment (83). Taken together, these observations suggest an alternate model of CCS action shown in Figure 6. However, this new model has not been unambiguously validated and does not reveal precise mechanistic details of the posttranslational modification of SOD1 or how the binding of a single copper ion results in allosteric CCS dimer dissociation (82). The latter mechanism is particularly intriguing, given that CCS contains its own intrasubunit disulfide bond and bound zinc, which as described above, are both factors that are known to stabilize SOD1 dimers (48) and by extension, would be expected to stabilize CCS dimers.
Figure 5.
Human CCS quaternary structure as a function of copper loading. The CCS canonical dimer [pdb code 1QUP (80)] reorganizes to form the noncanonical dimer [pdb code 1JK9 (83)] upon the binding of Cu(I) (82), thereby freeing domain II to interact with nascent SOD1.
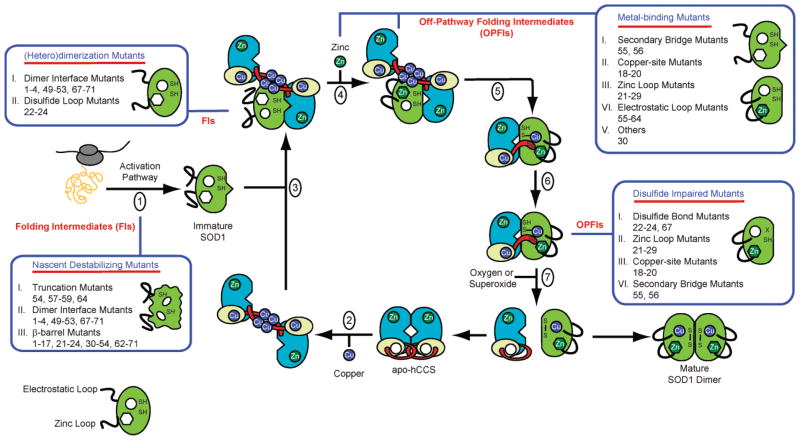
Figure 6.
An alternate model of CCS action and how the various pathogenic SOD1 mutations may hinder CCS-mediated SOD1 maturation. (1) SOD1 is translated. (2) The canonical CCS dimer is loaded with Cu(I) to generate the noncanonical CCS dimer mediated by a Cu4S6 cluster (82). (3) Nascent SOD1 binds to domain II of CCS in the noncanonical, Cu(I)-loaded CCS dimer. (4) Zinc is loaded into SOD1 (this could also occur as early as step 1). (5–7) Cu(I) from the Cu4S6 cluster is transferred to nascent SOD1 and the intrasubunit disulfide bond in nascent SOD1 is oxidized (68). Upon being depleted with Cu(I), CCS reforms the canonical CCS dimer and the cycle repeats. The pathogenic SOD1 mutations listed in Table 1 may interfere with CCS-mediated SOD1 maturation at the various positions indicated. The numbers of the pathogenic SOD1 mutants in the blue boxes correspond to their numbers in Table 1.
Immature Pathogenic SOD1 and Toxicity
We suspect that pathogenic SOD1 mutations may result in increased levels of immature SOD1 folding intermediates by hindering the action of CCS at various points in the SOD1 maturation cycle (Fig. 6). These immature SOD1 folding intermediates may lack some or all posttranslational modifications that are dependent upon CCS activity, including the insertion of copper co-factors and oxidation of the intrasubunit disulfide bond. Two recent in vitro studies indicate that failure to form the intrasubunit disulfide bond may be a key step (73, 84). Similarly, studies of aggregates isolated from cell culture and transgenic mouse models indicate that immature SOD1 molecules eventually end up in the insoluble inclusions (85).
One possible explanation for these observations is that mutant forms of SOD1 interact with CCS in a manner that inhibits normal posttranslational modifications (for example, the nascent metal-binding mutants). Alternatively, some mutant forms may be so destabilized that CCS encounters only a fraction of these variants before they exit the soluble fraction or are turned over by the protein quality control machinery (for example, the nascent β-barrel mutants). Indeed, some mutants, for example, the nascent L126 truncation variant, are so unstable that they would have little chance of normal interaction with CCS. Indeed, CCS would likely fail to bind to and stabilize the nascent L126 molecules it does encounter because the latter molecule is completely lacking a β-strand necessary for wild type SOD1 heterodimerization with CCS domain II. Although the nascent β-barrel mutants such as A4V, G37R, and G93A (among many others) are not quite as radically destabilized as L126Z, they remain significantly thermally destabilized relative to the nascent wild type enzyme (43, 44). As mentioned previously, because the SOD1:CCS ratio is between 15 and 30:1, we speculate that CCS is unable to cycle through the entire pool of these nascent SOD1 pathogenic mutants before they are degraded, oligomerize, or enter the insoluble fraction.
Metal binding mutants such as H46R, H48Q, H46R/H48Q, H80R, and D124V (among others), are not destabilized relative to the nascent wild type enzyme, but CCS is unable to convert these molecules to their mature forms via posttranslational modification because these mutations directly prevent metal binding. A recent study on the double copper-binding site mutant H46R/H48Q SOD1 revealed that this pathogenic variant forms quite stable 1:1 complexes with CCS that do not dissociate in analytical gel filtration, analytical ultracentrifugation, and native gel shift experiments (86), suggesting that this nonproductive SOD1:CCS complex may hinder CCS cycling through the newly translated pool of SOD1 molecules. Finally, CCS would be unable to fully stabilize the pathogenic SOD1 mutants C57R and C146R, which are incapable of forming the intrasubunit disulfide bond.
The notion that soluble immature pathogenic SOD1 molecules may be the noxious species in SOD1-linked fALS is supported by recent studies from the Culotta and Elliott laboratories. Over-expression of CCS was observed to greatly accelerate disease in a G93A SOD1 mouse model in the absence of visible proteinaceous inclusions (87). Surprisingly, CCS over-expression failed to enhance oxidation of the G93A SOD1 disulfide bond, and in fact, the population of disulfide-reduced G93A SOD1 in the soluble fraction of brain and spinal cord of these animals was elevated (88). In addition, CCS over-expression did not result in a larger fraction of active G93A SOD1 in these animals, suggesting that the SOD1 proteins also remain at least partially metal deficient (88). In this murine model of ALS, there appears to be augmentation of the mitochondrial pathology that is inherent in the G93A mice, but is not found in several other models (e.g., H46R/H48Q, G85R, L126Z, and G127insTGGG). These data suggest that CCS may be interacting with nascent G93A (these proteins are at an approximate stoichiometric ratio of 1:1 in these animals), preventing it from forming insoluble aggregates, but at the same time, failing to impart the stabilizing posttranslational modifications that transform the nascent SOD1 protein into the enormously stable, mature holoenzyme. It appears that the observed elevated levels of soluble, copper-depleted, disulfide-reduced G93A SOD1 augments the mitochondrial pathology, resulting in significantly earlier onset of paralytic symptoms in these animals. However, it remains unclear why CCS over-expression results in elevated levels of disulfide-reduced G93A SOD1, and additional studies aimed at understanding this phenomenon are needed.
Conclusions
More than 100 mutations in SOD1 have been identified as causing fALS. The precise role of mutant SOD1 oligomerization/aggregation in disease pathogenesis remains uncertain, although there is little dispute that insoluble aggregates of mutant SOD1 are found in all of the transgenic mouse models that have been generated thus far, except in one model where CCS is also over-expressed. Ultimately, the role of mutant SOD1 aggregation in disease may not be established until therapeutic compounds that specifically target mutant SOD1 aggregation are tested in clinical applications. We suggest a potential mechanism of toxicity involving reduced ability of the mutant proteins to interact properly with CCS, which mediates critical post-translational modification of the SOD1 as it folds into a stable dimeric enzyme. The resulting immature pathogenic SOD1 proteins are essentially folding intermediates that exert their toxic effects through their aggregated or soluble forms, or both.
Acknowledgments
This work has been supported over the years by the NIH/NINDS (PJH/DRB), the ALS Association (P.J.H./D.R.B.), the Robert A. Welch Foundation (P.J.H.), the Packard Foundation (D.R.B.), the William and Ella C. Owens Medical Research Foundation (P.J.H.), and the Judith and Jean Pape Adams Charitable Foundation (P.J.H.). Support for the X-ray Crystallography Core Laboratory by the Executive Research Council at the University of Texas Health Science Center is gratefully acknowledged.
References
- 1.Charcot JM, AJ Deux cas d’atrophie musculaire progressive avec lesions de la substance grise et de faisceaux anterolateraux de la moelle epiniere. Arch Physiol Norm Pathol. 1869;1:354–367. [Google Scholar]
- 2.Mulder DW, Kurland LT, Offord KP, Beard CM. Familial adult motor neuron disease: amyotrophic lateral sclerosis. Neurology. 1986;36:511–517. doi: 10.1212/wnl.36.4.511. [DOI] [PubMed] [Google Scholar]
- 3.Bruijn LI, Miller TM, Cleveland DW. Unraveling the mechanisms involved in motor neuron degeneration in ALS. Annu Rev Neurosci. 2004;27:723–749. doi: 10.1146/annurev.neuro.27.070203.144244. [DOI] [PubMed] [Google Scholar]
- 4.Valentine JS, Doucette PA, Zittin Potter S. Copper-zinc superoxide dismutase and amyotrophic lateral sclerosis. Annu Rev Biochem. 2005;74:563–593. doi: 10.1146/annurev.biochem.72.121801.161647. [DOI] [PubMed] [Google Scholar]
- 5.Deng HX, Hentati A, Tainer JA, Iqbal Z, Cayabyab A, Hung WY, Getzoff ED, Hu P, Herzfeldt B, Roos RP, et al. Amyotrophic lateral sclerosis and structural defects in Cu, Zn superoxide dismutase. Science. 1993;261:1047–1051. doi: 10.1126/science.8351519. [DOI] [PubMed] [Google Scholar]
- 6.Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, Donaldson D, Goto J, O’Regan JP, Deng HX, et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- 7.Hart PJ. Pathogenic superoxide dismutase structure, folding, aggregation and turnover. Curr Opin Chem Biol. 2006;10:131–138. doi: 10.1016/j.cbpa.2006.02.034. [DOI] [PubMed] [Google Scholar]
- 8.Winkler DD, Prudencio M, Karch C, Borchelt DR, Hart PJ. Copper-zinc superoxide dismutase, its copper chaperone, and familial amyotrophic lateral sclerosis. In: Dobson CM, Kelly JW, Ramirez-Alvarado M, editors. Protein Misfolding Diseases: Current and Emerging Prinicples. Hoboken, NJ: John Wiley & Sons, Inc; 2009. [Google Scholar]
- 9.Fridovich I. Superoxide dismutases. An adaptation to a paramagnetic gas. J Biol Chem. 1989;264:7761–7764. [PubMed] [Google Scholar]
- 10.Sturtz LA, Diekert K, Jensen LT, Lill R, Culotta VC. A fraction of yeast Cu, Zn-superoxide dismutase and its metallochaperone, CCS, localize to the intermembrane space of mitochondria. A physiological role for SOD1 in guarding against mitochondrial oxidative damage. J Biol Chem. 2001;276:38084–38089. doi: 10.1074/jbc.M105296200. [DOI] [PubMed] [Google Scholar]
- 11.Pardo CA, Xu Z, Borchelt DR, Price DL, Sisodia SS, Cleveland DW. Superoxide dismutase is an abundant component in cell bodies, dendrites, and axons of motor neurons and in a subset of other neurons. Proc Natl Acad Sci U S A. 1995;92:954–958. doi: 10.1073/pnas.92.4.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marklund SL. Extracellular superoxide dismutase in human tissues and human cell lines. J Clin Invest. 1984;74:1398–1403. doi: 10.1172/JCI111550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reaume AG, Elliott JL, Hoffman EK, Kowall NW, Ferrante RJ, Siwek DF, Wilcox HM, Flood DG, Beal MF, Brown RH, Jr, Scott RW, Snider WD. Motor neurons in Cu/Zn superoxide dismutase-deficient mice develop normally but exhibit enhanced cell death after axonal injury. Nat Genet. 1996;13:43–47. doi: 10.1038/ng0596-43. [DOI] [PubMed] [Google Scholar]
- 14.Wang J, Xu G, Li H, Gonzales V, Fromholt D, Karch C, Copeland NG, Jenkins NA, Borchelt DR. Somatodendritic accumulation of misfolded SOD1-L126Z in motor neurons mediates degeneration: alphaB-crystallin modulates aggregation. Hum Mol Genet. 2005;14:2335–2347. doi: 10.1093/hmg/ddi236. [DOI] [PubMed] [Google Scholar]
- 15.Bruijn LI, Becher MW, Lee MK, Anderson KL, Jenkins NA, Copeland NG, Sisodia SS, Rothstein JD, Borchelt DR, Price DL, Cleveland DW. ALS-linked SOD1 mutant G85R mediates damage to astrocytes and promotes rapidly progressive disease with SOD1-containing inclusions. Neuron. 1997;18:327–338. doi: 10.1016/s0896-6273(00)80272-x. [DOI] [PubMed] [Google Scholar]
- 16.Gurney ME, Pu H, Chiu AY, Dal Canto MC, Polchow CY, Alexander DD, Caliendo J, Hentati A, Kwon YW, Deng HX, et al. Motor neuron degeneration in mice that express a human Cu, Zn superoxide dismutase mutation. Science. 1994;264:1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- 17.Sherman L, Levanon D, Lieman-Hurwitz J, Dafni N, Groner Y. Human Cu/Zn superoxide dismutase gene: molecular characterization of its two mRNA species. Nucleic Acids Res. 1984;12:9349–9365. doi: 10.1093/nar/12.24.9349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang J, Xu G, Borchelt DR. Mapping superoxide dismutase 1 domains of non-native interaction: roles of intra- and intermolecular disulfide bonding in aggregation. J Neurochem. 2006;96:1277–1288. doi: 10.1111/j.1471-4159.2005.03642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ripps ME, Huntley GW, Hof PR, Morrison JH, Gordon JW. Transgenic mice expressing an altered murine superoxide dismutase gene provide an animal model of amyotrophic lateral sclerosis. Proc Natl Acad Sci U S A. 1995;92:689–693. doi: 10.1073/pnas.92.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Epstein CJ, Avraham KB, Lovett M, Smith S, Elroy-Stein O, Rotman G, Bry C, Groner Y. Transgenic mice with increased Cu/Zn-superoxide dismutase activity: animal model of dosage effects in Down syndrome. Proc Natl Acad Sci U S A. 1987;84:8044–8048. doi: 10.1073/pnas.84.22.8044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deng H-X, Shi Y, Furukawa Y, Zhai H, Fu R, Liu E, Gorrie GH, Khan MS, Hung WY, Bigio EH, Lukas T, Dal Canto MC, O’Halloran TV, Siddique T. Conversion to the amyotrophic lateral sclerosis phenotype is associated with intermolecular linked insoluble aggregates of SOD1 in mitochondria. Proc Natl Acad Sci U S A. 2006;103:7142–7147. doi: 10.1073/pnas.0602046103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong PC, Pardo CA, Borchelt DR, Lee MK, Copeland NG, Jenkins NA, Sisodia SS, Cleveland DW, Price DL. An adverse property of a familial ALS-linked SOD1 mutation causes motor neuron disease characterized by vacuolar degeneration of mitochondria. Neuron. 1995;14:1105–1116. doi: 10.1016/0896-6273(95)90259-7. [DOI] [PubMed] [Google Scholar]
- 23.Sasaki S, Nagai M, Aoki M, Komori T, Itoyama Y, Iwata M. Motor neuron disease in transgenic mice with an H46R mutant SOD1 gene. J Neuropathol Exp Neurol. 2007;66:517–524. doi: 10.1097/01.jnen.0000263868.84188.3b. [DOI] [PubMed] [Google Scholar]
- 24.Watanabe Y, Yasui K, Nakano T, Doi K, Fukada Y, Kitayama M, Ishimoto M, Kurihara S, Kawashima M, Fukuda H, Adachi Y, Inoue T, Nakashima K. Mouse motor neuron disease caused by truncated SOD1 with or without C-terminal modification. Brain Res Mol Brain Res. 2005;135:12–20. doi: 10.1016/j.molbrainres.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 25.Jonsson PA, Ernhill K, Andersen PM, Bergemalm D, Brannstrom T, Gredal O, Nilsson P, Marklund SL. Minute quantities of misfolded mutant superoxide dismutase-1 cause amyotrophic lateral sclerosis. Brain. 2004;127:73–88. doi: 10.1093/brain/awh005. [DOI] [PubMed] [Google Scholar]
- 26.Howland DS, Liu J, She Y, Goad B, Maragakis NJ, Kim B, Erickson J, Kulik J, DeVito L, Psaltis G, DeGennaro LJ, Cleveland DW, Rothstein JD. Focal loss of the glutamate transporter EAAT2 in a transgenic rat model of SOD1 mutant-mediated amyotrophic lateral sclerosis (ALS) Proc Natl Acad Sci U S A. 2002;99:1604–1609. doi: 10.1073/pnas.032539299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nagai M, Aoki M, Miyoshi I, Kato M, Pasinelli P, Kasai N, Brown RH, Jr, Itoyama Y. Rats expressing human cytosolic copper-zinc superoxide dismutase transgenes with amyotrophic lateral sclerosis: associated mutations develop motor neuron disease. J Neurosci. 2001;21:9246–9254. doi: 10.1523/JNEUROSCI.21-23-09246.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tainer JA, Getzoff ED, Beem KM, Richardson JS, Richardson DC. Determination and analysis of the 2 A-structure of copper, zinc superoxide dismutase. J Mol Biol. 1982;160:181–217. doi: 10.1016/0022-2836(82)90174-7. [DOI] [PubMed] [Google Scholar]
- 29.Strange RW, Antonyuk SV, Hough MA, Doucette PA, Valentine JS, Hasnain SS. Variable metallation of human superoxide dismutase: atomic resolution crystal structures of Cu-Zn, Zn-Zn and as-isolated wild-type enzymes. J Mol Biol. 2006;356:1152–1162. doi: 10.1016/j.jmb.2005.11.081. [DOI] [PubMed] [Google Scholar]
- 30.Hayward LJ, Rodriguez JA, Kim JW, Tiwari A, Goto JJ, Cabelli DE, Valentine JS, Brown RH., Jr Decreased metallation and activity in subsets of mutant superoxide dismutases associated with familial amyotrophic lateral sclerosis. J Biol Chem. 2002;277:15923–15931. doi: 10.1074/jbc.M112087200. [DOI] [PubMed] [Google Scholar]
- 31.Valentine JS, Hart PJ. Misfolded CuZnSOD and amyotrophic lateral sclerosis. Proc Natl Acad Sci U S A. 2003;100:3617–3622. doi: 10.1073/pnas.0730423100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hough MA, Grossmann JG, Antonyuk SV, Strange RW, Doucette PA, Rodriguez JA, Whitson LJ, Hart PJ, Hayward LJ, Valentine JS, Hasnain SS. Dimer destabilization in superoxide dismutase may result in disease-causing properties: Structures of motor neuron disease mutants. Proc Natl Acad Sci U S A. 2004;101:5976–5981. doi: 10.1073/pnas.0305143101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hart PJ, Liu H, Pellegrini M, Nersissian AM, Gralla EB, Valentine JS, Eisenberg D. Subunit asymmetry in the three-dimensional structure of a human CuZnSOD mutant found in familial amyotrophic lateral sclerosis. Protein Sci. 1998;7:545–555. doi: 10.1002/pro.5560070302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Banci L, Bertini I, D’Amelio N, Libralesso E, Turano P, Valentine JS. Metalation of the amyotrophic lateral sclerosis mutant glycine 37 to arginine superoxide dismutase (SOD1) apoprotein restores its structural and dynamical properties in solution to those of metalated wild-type SOD1. Biochemistry. 2007;46:9953–9962. doi: 10.1021/bi700620r. [DOI] [PubMed] [Google Scholar]
- 35.DiDonato M, Craig L, Huff ME, Thayer MM, Cardoso RM, Kassmann CJ, Lo TP, Bruns CK, Powers ET, Kelly JW, Getzoff ED, Tainer JA. ALS mutants of human superoxide dismutase form fibrous aggregates via framework destabilization. J Mol Biol. 2003;332:601–615. doi: 10.1016/s0022-2836(03)00889-1. [DOI] [PubMed] [Google Scholar]
- 36.Shipp EL, Cantini F, Bertini I, Valentine JS, Banci L. Dynamic properties of the G93A mutant of copper-zinc superoxide dismutase as detected by NMR spectroscopy: implications for the pathology of familial amyotrophic lateral sclerosis. Biochemistry. 2003;42:1890–1899. doi: 10.1021/bi026704y. [DOI] [PubMed] [Google Scholar]
- 37.Elam JS, Taylor AB, Strange R, Antonyuk A, Doucette PA, Rodriguez JA, Hasnain SS, Hayward LJ, Valentine JS, Yeates TO, Hart PJ. Amyloid-like filaments and water-filled nanotubes formed by SOD1 mutants linked to familial ALS. Nat Struct Biol. 2003;10:461–467. doi: 10.1038/nsb935. [DOI] [PubMed] [Google Scholar]
- 38.Antonyuk S, Elam JS, Hough MA, Strange RW, Doucette PA, Rodriguez JA, Hayward LJ, Valentine JS, Hart PJ, Hasnain SS. Structural consequences of the familial amyotrophic lateral sclerosis SOD1 mutant His46Arg. Protein Sci. 2005;14:1201–1213. doi: 10.1110/ps.041256705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang J. Disease-associated mutations at copper ligand histidine residues of superoxide dismutase 1 diminish the binding of copper and compromise dimer stability. J Biol Chem. 2007;282:345–352. doi: 10.1074/jbc.M604503200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cao X, Antonyuk SV, Seetharaman SV, Whitson LJ, Taylor AB, Holloway SP, Strange RW, Doucette PA, Valentine JS, Tiwari A, Hayward LJ, Padua S, Cohlberg JA, Hasnain SS, Hart PJ. Structures of the G85R variant of SOD1 in familial amyotrophic lateral sclerosis. J Biol Chem. 2008;283:16169–16177. doi: 10.1074/jbc.M801522200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elam JS, Malek K, Rodriguez JA, Doucette PA, Taylor AB, Hayward LJ, Cabelli DE, Valentine JS, Hart PJ. An alternative mechanism of bicarbonate-mediated peroxidation by copper-zinc superoxide dismutase. J Biol Chem. 2003;278:21032–21039. doi: 10.1074/jbc.M300484200. [DOI] [PubMed] [Google Scholar]
- 42.Banci L, Bertini I, D’Amelio N, Gaggelli E, Libralesso E, Matecko I, Turano P, Valentine JS. Fully metallated S134N Cu, Zn-superoxide dismutase displays abnormal mobility and intermolecular contacts in solution. J Biol Chem. 2005;280:35815–35821. doi: 10.1074/jbc.M506637200. [DOI] [PubMed] [Google Scholar]
- 43.Rodriguez JA, Shaw BF, Durazo A, Sohn SH, Doucette PA, Nersissian AM, Faull KF, Eggers DK, Tiwari A, Hayward LJ, Valentine JS. Destabilization of apoprotein is insufficient to explain Cu, Zn-superoxide dismutase-linked ALS pathogenesis. Proc Natl Acad Sci U S A. 2005;102:10516–10521. doi: 10.1073/pnas.0502515102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rodriguez JA, Valentine JS, Eggers DK, Roe JA, Tiwari A, Brown RH, Jr, Hayward LJ. Familial ALS-associated mutations decrease the thermal stability of distinctly metallated species of human copper-zinc superoxide dismutase. J Biol Chem. 2002;277:15932–15937. doi: 10.1074/jbc.M112088200. [DOI] [PubMed] [Google Scholar]
- 45.Culotta VC, Klomp LW, Strain J, Casareno RL, Krems B, Gitlin JD. The copper chaperone for superoxide dismutase. J Biol Chem. 1997;272:23469–23472. doi: 10.1074/jbc.272.38.23469. [DOI] [PubMed] [Google Scholar]
- 46.Forman HJ, Fridovich I. On the stability of bovine superoxide dismutase. The effects of metals. Histidine at the active site of superoxide dismutase. J Biol Chem. 1973;248:2645–2649. [PubMed] [Google Scholar]
- 47.Hartz JW, Deutsch HF. Subunit structure of human superoxide dismutase. J Biol Chem. 1972;247:7043–7050. [PubMed] [Google Scholar]
- 48.Doucette PA, Whitson LJ, Cao X, Schirf V, Demeler B, Valentine JS, Hansen JC, Hart PJ. Dissociation of human copper-zinc superoxide dismutase dimers using chaotrope and reductant. Insights into the molecular basis for dimer stability. J Biol Chem. 2004;279:54558–54566. doi: 10.1074/jbc.M409744200. [DOI] [PubMed] [Google Scholar]
- 49.Lindberg MJNJ, Holmgren A, Oliveberg M. Folding of human superoxide dismutase: disulfide reduction prevents dimerization and produces marginally stable monomers. Proc Natl Acad Sci U S A. 2004;101:15893–15898. doi: 10.1073/pnas.0403979101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arnesano F, Banci L, Bertini I, Martinelli M, Furukawa Y, O’Halloran TV. The unusually stable quaternary structure of human Cu, Zn-superoxide dismutase 1 is controlled by both metal occupancy and disulfide status. J Biol Chem. 2004;279:47998–48003. doi: 10.1074/jbc.M406021200. [DOI] [PubMed] [Google Scholar]
- 51.Banci L, Bertini I, Cantini F, D’Amelio N, Gaggelli E. Human SOD1 before harboring the catalytic metal: solution structure of copper-depleted, disulfide-reduced form. J Biol Chem. 2006;281:2333–2337. doi: 10.1074/jbc.M506497200. [DOI] [PubMed] [Google Scholar]
- 52.Wang J, Slunt H, Gonzales V, Fromholt D, Coonfield M, Copeland NG, Jenkins NA, Borchelt DR. Copper-binding-site-null SOD1 causes ALS in transgenic mice: aggregates of non-native SOD1 delineate a common feature. Hum Mol Genet. 2003;12:2753–2764. doi: 10.1093/hmg/ddg312. [DOI] [PubMed] [Google Scholar]
- 53.Murphy RM. Peptide aggregation in neurodegenerative disease. Annu Rev Biomed Eng. 2002;4:155–174. doi: 10.1146/annurev.bioeng.4.092801.094202. [DOI] [PubMed] [Google Scholar]
- 54.Ray SS, Nowak RJ, Strokovich K, Brown RH, Jr, Walz T, Lansbury PT., Jr An intersubunit disulfide bond prevents in vitro aggregation of a superoxide dismutase-1 mutant linked to familial amytrophic lateral sclerosis. Biochemistry. 2004;43:4899–4905. doi: 10.1021/bi030246r. [DOI] [PubMed] [Google Scholar]
- 55.Karch CM, Borchelt DR. A limited role for disulfide cross-linking in the aggregation of mutant SOD1 linked to familial amyotrophic lateral sclerosis. J Biol Chem. 2008;283:13528–13537. doi: 10.1074/jbc.M800564200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shibata N, Hirano A, Kobayashi M, Siddique T, Deng HX, Hung WY, Kato T, Asayama K. Intense superoxide dismutase-1 immunoreactivity in intracytoplasmic hyaline inclusions of familial amyotrophic lateral sclerosis with posterior column involvement. J Neuropathol Exp Neurol. 1996;55:481–490. doi: 10.1097/00005072-199604000-00011. [DOI] [PubMed] [Google Scholar]
- 57.Kato S, Sumi-Akamaru H, Fujimura H, Sakoda S, Kato M, Hirano A, Takikawa M, Ohama E. Copper chaperone for superoxide dismutase co-aggregates with superoxide dismutase 1 (SOD1) in neuronal Lewy body-like hyaline inclusions: an immunohistochemical study on familial amyotrophic lateral sclerosis with SOD1 gene mutation. Acta Neuropathol (Berl) 2001;102:233–238. doi: 10.1007/s004010000355. [DOI] [PubMed] [Google Scholar]
- 58.Ohi T, Nabeshima K, Kato S, Yazawa S, Takechi S. Familial amyotrophic lateral sclerosis with His46Arg mutation in Cu/Zn superoxide dismutase presenting characteristic clinical features and Lewy body-like hyaline inclusions. J Neurol Sci. 2004;225:19–25. doi: 10.1016/j.jns.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 59.Kokubo Y, Kuzuhara S, Narita Y, Kikugawa K, Nakano R, Inuzuka T, Tsuji S, Watanabe M, Miyazaki T, Murayama S, Ihara Y. Accumulation of neurofilaments and SOD1-immunoreactive products in a patient with familial amyotrophic lateral sclerosis with I113T SOD1 mutation. Arch Neurol. 1999;56:1506–1508. doi: 10.1001/archneur.56.12.1506. [DOI] [PubMed] [Google Scholar]
- 60.Stewart HG, Mackenzie IR, Eisen A, Brännström T, Marklund SL, Andersen PM. Clinicopathological phenotype of ALS with a novel G72C SOD1 gene mutation mimicking a myopathy. Muscle Nerve. 2006;33:701–706. doi: 10.1002/mus.20495. [DOI] [PubMed] [Google Scholar]
- 61.Kato S, Shimoda M, Watanabe Y, Nakashima K, Takahashi K, Ohama E. Familial amyotrophic lateral sclerosis with a two base pair deletion in superoxide dismutase 1 gene: Multisystem degeneration with intracytoplasmic hyaline inclusioins in astrocytes. J Neuropathol Exp Neurol. 1996;55:1089–1101. [PubMed] [Google Scholar]
- 62.Takehisa Y, Ujike H, Ishizu H, Terada S, Haraguchi T, Tanaka Y, Nishinaka T, Nobukuni K, Ihara Y, Namba R, Yasuda T, Nishibori M, Hayabara T, Kuroda S. Familial amyotrophic lateral sclerosis with a novel Leu126Ser mutation in the copper/zinc superoxide dismutase gene showing mild clinical features and lewy body-like hyaline inclusions. Arch Neurol. 2001;58:736–740. doi: 10.1001/archneur.58.5.736. [DOI] [PubMed] [Google Scholar]
- 63.Ince PG. Familial amyotrophic lateral sclerosis with a mutation in exon 4 of the Cu/Zn superoxide dismutase gene: pathological and immunocytochemical changes. Acta Neuropathol. 1996;92:395–403. doi: 10.1007/s004010050535. [DOI] [PubMed] [Google Scholar]
- 64.Shaw CE, Enayat ZE, Powell JF, Anderson VE, Radunovic A, al-Sarraj S, Leigh PN. Familial amyotrophic lateral sclerosis. Molecular pathology of a patient with a SOD1 mutation. Neurology. 1997;49:1612–1616. doi: 10.1212/wnl.49.6.1612. [DOI] [PubMed] [Google Scholar]
- 65.Kadekawa J, Fujimura H, Ogawa Y, Hattori N, Kaido M, Nishimura T, Yoshikawa H, Shirahata N, Sakoda S, Yanagihara T. A clinicopathological study of a patient with familial amyotrophic lateral sclerosis associated with a two base pair deletion in the copper/zinc superoxide dismutase (SOD1) gene. Acta Neuropathol. 1997;94:617–622. doi: 10.1007/s004010050758. [DOI] [PubMed] [Google Scholar]
- 66.Katayama S, Watanabe C, Noda K, Ohishi H, Yamamura Y, Nishisaka T, Inai K, Asayama K, Murayama S, Nakamura S. Numerous conglomerate inclusions in slowly progressive familial amyotrophic lateral sclerosis with posterior column involvement. J Neurol Sci. 1999;171:72–77. doi: 10.1016/s0022-510x(99)00252-x. [DOI] [PubMed] [Google Scholar]
- 67.Banci L, Bertini I, Durazo A, Girotto S, Gralla EB, Martinelli M, Valentine JS, Vieru M, Whitelegge JP. Metal-free superoxide dismutase forms soluble oligomers under physiological conditions: a possible general mechanism for familial ALS. Proc Natl Acad Sci U S A. 2007;104:11263–11267. doi: 10.1073/pnas.0704307104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Furukawa Y, O’Halloran TV. Posttranslational modifications in Cu, Zn-superoxide dismutase and mutations associated with amyotrophic lateral sclerosis. Antioxid Redox Signal. 2006;8:847–867. doi: 10.1089/ars.2006.8.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cozzolino M, Amori I, Pesaresi MG, Ferri A, Nencini M, Carrì MT. Cysteine 111 affects aggregation and cytotoxicity of mutant Cu, Zn-superoxide dismutase associated with familial amyotrophic lateral sclerosis. J Biol Chem. 2008;283:866–874. doi: 10.1074/jbc.M705657200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jonsson PA, Karin SG, Peter MA, Thomas Bnm, Mikael L, Mikael O. Disulphide-reduced superoxide dismutase-1 in CNS of transgenic amyotrophic lateral sclerosis models. Brain. 2006;129:451–464. doi: 10.1093/brain/awh704. [DOI] [PubMed] [Google Scholar]
- 71.Niwa J-i, Yamada S, Ishigaki S, Sone J, Takahashi M, Katsuno M, Tanaka F, Doyu M, Sobue G. Disulfide bond mediates aggregation, toxicity, and ubiquitylation of familial amyotrophic lateral sclerosis–linked mutant SOD1. J Biol Chem. 2007;282:28087–28095. doi: 10.1074/jbc.M704465200. [DOI] [PubMed] [Google Scholar]
- 72.Banci L, Bertini I, Boca M, Girotto S, Martinelli M, Valentine JS, Vieru M. SOD1 and amyotrophic lateral sclerosis: Mutations and oligomerization. PLoS ONE. 2008;3 doi: 10.1371/journal.pone.0001677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chattopadhyay M, Durazo A, Sohn SH, Strong CD, Gralla EB, Whitelegge JP, Valentine JS. Initiation and elongation in fibrillation of ALS-linked superoxide dismutase. Proc Natl Acad Sci U S A. 2008;105:18663–18668. doi: 10.1073/pnas.0807058105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Richardson JS, Richardson DC. Natural beta-sheet proteins use negative design to avoid edge-to-edge aggregation. Proc Natl Acad Sci U S A. 2002;99:2754–2759. doi: 10.1073/pnas.052706099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lashuel HA, Hartley D, Petre BM, Walz T, Lansbury PT., Jr Neurodegenerative disease: amyloid pores from pathogenic mutations. Nature. 2002;418:291. doi: 10.1038/418291a. [DOI] [PubMed] [Google Scholar]
- 76.Witan H, Kern A, Koziollek-Drechsler I, Wade R, Behl C, Clement AM. Heterodimer formation of wild-type and amyotrophic lateral sclerosis–causing mutant Cu/Zn-superoxide dismutase induces toxicity independent of protein aggregation. Hum Mol Genet. 2008;17:1373–1385. doi: 10.1093/hmg/ddn025. [DOI] [PubMed] [Google Scholar]
- 77.Matsumoto G, Stojanovic A, Holmberg CI, Kim S, Morimoto RI. Structural properties and neuronal toxicity of amyotrophic lateral sclerosis–associated Cu/Zn superoxide dismutase 1 aggregates. J Cell Biol. 2005;171:75–85. doi: 10.1083/jcb.200504050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brown NM, Torres AS, Doan PE, O’Halloran TV. Oxygen and the copper chaperone CCS regulate posttranslational activation of Cu, Zn superoxide dismutase. Proc Natl Acad Sci U S A. 2004;101:5518–5523. doi: 10.1073/pnas.0401175101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rothstein JD, Dykes-Hoberg M, Corson LB, Becker M, Cleveland DW, Price DL, Culotta VC, Wong PC. The copper chaperone CCS is abundant in neurons and astrocytes in human and rodent brain. J Neurochem. 1999;72:422–429. doi: 10.1046/j.1471-4159.1999.0720422.x. [DOI] [PubMed] [Google Scholar]
- 80.Lamb AL, Wernimont AK, Pufahl RA, Culotta VC, O’Halloran TV, Rosenzweig AC. Crystal structure of the copper chaperone for superoxide dismutase. Nat Struct Biol. 1999;6:724–729. doi: 10.1038/11489. [DOI] [PubMed] [Google Scholar]
- 81.Schmidt PJ, Rae TD, Pufahl RA, Hamma T, Strain J, O’Halloran TV, Culotta VC. Multiple protein domains contribute to the action of the copper chaperone for superoxide dismutase. J Biol Chem. 1999;274:23719–23725. doi: 10.1074/jbc.274.34.23719. [DOI] [PubMed] [Google Scholar]
- 82.Stasser JP, Siluvai GS, Barry AN, Blackburn NJ. A multinuclear copper(I) cluster forms the dimerization interface in copper-loaded human copper chaperone for superoxide dismutase. Biochemistry. 2007;46:11845–11856. doi: 10.1021/bi700566h. [DOI] [PubMed] [Google Scholar]
- 83.Lamb AL, Torres AS, O’Halloran TV, Rosenzweig AC. Heterodimeric structure of superoxide dismutase in complex with its metallochaperone. Nat Struct Biol. 2001;8:751–755. doi: 10.1038/nsb0901-751. [DOI] [PubMed] [Google Scholar]
- 84.Furukawa Y, Kaneko K, Yamanaka K, O’Halloran TV, Nukina N. Complete loss of post-translational modifications triggers fibrillar aggregation of SOD1 in the familial form of amyotrophic lateral sclerosis. J Biol Chem. 2008;283:24167–24176. doi: 10.1074/jbc.M802083200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Karch CM, Prudencio M, Winkler DD, Hart PJ, Borchelt DR. Role of mutant SOD1 disulfide oxidation and aggregation in the pathogenesis of familial ALS. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0902505106. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Winkler DD, Schuermann JP, Cao X, Holloway SP, Borchelt DR, Carroll MC, Proescher JB, Culotta VC, Hart PJ. Structural and biophysical properties of the pathogenic SOD1 variant H46R/H48Q. Biochemistry. 2009;48:3436–3447. doi: 10.1021/bi8021735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Son M, Puttaparthi K, Kawamata H, Rajendran B, Boyer PJ, Manfredi G, Elliott JL. Overexpression of CCS in G93A-SOD1 mice leads to accelerated neurological deficits with severe mitochondrial pathology. Proc Natl Acad Sci U S A. 2007;104:6072–6077. doi: 10.1073/pnas.0610923104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Proescher JB, Son M, Elliott JL, Culotta VC. Biological effects of CCS in the absence of SOD1 enzyme activation: implications for disease in a mouse model for ALS. Hum Mol Genet. 2008;17:1728–1737. doi: 10.1093/hmg/ddn063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nakano R, Sato S, Inuzuka T, Sakimura K, Mishina M, Takahashi H, Ikuta F, Honma Y, Fujii J, Taniguchi N, et al. A novel mutation in Cu/Zn superoxide dismutase gene in Japanese familial amyotrophic lateral sclerosis. Biochem Biophys Res Commun. 1994;200:695–703. doi: 10.1006/bbrc.1994.1506. [DOI] [PubMed] [Google Scholar]
- 90.Nakanishi T, Kishikawa M, Miyazaki A, Shimizu A, Ogawa Y, Sakoda S, Ohi T, Shoji H. Simple and defined method to detect the SOD-1 mutants from patients with familial amyotrophic lateral sclerosis by mass spectrometry. J Neurosci Methods. 1998;81:41–44. doi: 10.1016/s0165-0270(98)00012-0. [DOI] [PubMed] [Google Scholar]
- 91.Yim HS, Kang JH, Chock PB, Stadtman ER, Yim MB. A familial amyotrophic lateral sclerosis–associated A4V Cu, Zn- superoxide dismutase mutant has a lower Km for hydrogen peroxide. Correlation between clinical severity and the Km value. J Biol Chem. 1997;272:8861–8863. doi: 10.1074/jbc.272.14.8861. [DOI] [PubMed] [Google Scholar]
- 92.Rosen DR, Bowling AC, Patterson D, Usdin TB, Sapp P, Mezey E, McKenna-Yasek D, O’Regan J, Rahmani Z, Ferrante RJ, et al. A frequent ala 4 to val superoxide dismutase-1 mutation is associated with a rapidly progressive familial amyotrophic lateral sclerosis. Hum Mol Genet. 1994;3:981–987. doi: 10.1093/hmg/3.6.981. [DOI] [PubMed] [Google Scholar]
- 93.Gellera C, Castellotti B, Riggio MC, Silani V, Morandi L, Testa D, Casali C, Taroni F, Di Donato S, Zeviani M, Mariotti C. Superoxide dismutase gene mutations in Italian patients with familial and sporadic amyotrophic lateral sclerosis: Identification of three novel missense mutations. Neuromuscul Disord. 2001;11:404–410. doi: 10.1016/s0960-8966(00)00215-7. [DOI] [PubMed] [Google Scholar]
- 94.Kohno S, Takahashi Y, Miyajima H, Serizawa M, Mizoguchi K. A novel mutation (Cys6Gly) in the Cu/Zn superoxide dismutase gene associated with rapidly progressive familial amyotrophic lateral sclerosis. Neurosci Lett. 1999;276:135–137. doi: 10.1016/s0304-3940(99)00803-4. [DOI] [PubMed] [Google Scholar]
- 95.Morita M, Aoki M, Abe K, Hasegawa T, Sakuma R, Onodera Y, Ichikawa N, Nishizawa M, Itoyama Y. A novel two-base mutation in the Cu/Zn superoxide dismutase gene associated with familial amyotrophic lateral sclerosis in Japan. Neurosci Lett. 1996;205:79–82. doi: 10.1016/0304-3940(96)12378-8. [DOI] [PubMed] [Google Scholar]
- 96.Hand CK, Mayeux-Portas V, Khoris J, Briolotti V, Clavelou P, Camu W, Rouleau GA. Compound heterozygous D90A and D96N SOD1 mutations in a recessive amyotrophic lateral sclerosis family. Ann Neurol. 2001;49:267–271. doi: 10.1002/1531-8249(20010201)49:2<267::aid-ana51>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 97.Hirano M, Fujii J, Nagai Y, Sonobe M, Okamoto K, Araki H, Taniguchi N, Ueno S. A new variant Cu/Zn superoxide dismutase (Val7–>Glu) deduced from lymphocyte mRNA sequences from Japanese patients with familial amyotrophic lateral sclerosis. Biochem Biophys Res Commun. 1994;204:572–577. doi: 10.1006/bbrc.1994.2497. [DOI] [PubMed] [Google Scholar]
- 98.Andersen PM, Sims KB, Xin WW, Kiely R, O’Neill G, Ravits J, Pioro E, Harati Y, Brower RD, Levine JS, Heinicke HU, Seltzer W, Boss M, Brown RH., Jr Sixteen novel mutations in the Cu/Zn superoxide dismutase gene in amyotrophic lateral sclerosis: a decade of discoveries, defects and disputes. Amyotroph Lateral Scler Other Motor Neuron Disord. 2003;4:62–73. doi: 10.1080/14660820310011700. [DOI] [PubMed] [Google Scholar]
- 99.Siddique T, Deng HX. Genetics of amyotrophic lateral sclerosis. Hum Mol Genet. 1996;5(Spec):1465–1470. doi: 10.1093/hmg/5.supplement_1.1465. [DOI] [PubMed] [Google Scholar]
- 100.Kim NH, Kim HJ, Kim M, Lee KW. A novel SOD1 gene mutation in a Korean family with amyotrophic lateral sclerosis. J Neurol Sci. 2003;206:65–69. doi: 10.1016/s0022-510x(02)00338-6. [DOI] [PubMed] [Google Scholar]
- 101.Yulug IG, Katsanis N, de Belleroche J, Collinge J, Fisher EM. An improved protocol for the analysis of SOD1 gene mutations, and a new mutation in exon 4. Hum Mol Genet. 1995;4:1101–1104. doi: 10.1093/hmg/4.6.1101. [DOI] [PubMed] [Google Scholar]
- 102.Sato T, Yamamoto Y, Nakanishi T, Fukada K, Sugai F, Zhou Z, Okuno T, Nagano S, Hirata S, Shimizu A, Sakoda S. Identification of two novel mutations in the Cu/Zn superoxide dismutase gene with familial amyotrophic lateral sclerosis: mass spectrometric and genomic analyses. J Neurol Sci. 2004;218:79–83. doi: 10.1016/j.jns.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 103.Jones CT, Shaw PJ, Chari G, Brock DJ. Identification of a novel exon 4 SOD1 mutation in a sporadic amyotrophic lateral sclerosis patient. Mol Cell Probes. 1994;8:329–330. doi: 10.1006/mcpr.1994.1046. [DOI] [PubMed] [Google Scholar]
- 104.Tan CF, Piao YS, Hayashi S, Obata H, Umeda Y, Sato M, Fukushima T, Nakano R, Tsuji S, Takahashi H. Familial amyotrophic lateral sclerosis with bulbar onset and a novel Asp101Tyr Cu/Zn superoxide dismutase gene mutation. Acta Neuropathol. 2004;108:332–336. doi: 10.1007/s00401-004-0893-4. [DOI] [PubMed] [Google Scholar]
- 105.Penco S, Schenone A, Bordo D, Bolognesi M, Abbruzzese M, Bugiani O, Ajmar F, Garre C. A SOD1 gene mutation in a patient with slowly progressing familial ALS. Neurology. 1999;53:404–406. doi: 10.1212/wnl.53.2.404. [DOI] [PubMed] [Google Scholar]
- 106.Ikeda M, Abe K, Aoki M, Sahara M, Watanabe M, Shoji M, St George-Hyslop PH, Hirai S, Itoyama Y. Variable clinical symptoms in familial amyotrophic lateral sclerosis with a novel point mutation in the Cu/Zn superoxide dismutase gene. Neurology. 1995;45:2038–2042. doi: 10.1212/wnl.45.11.2038. [DOI] [PubMed] [Google Scholar]
- 107.Andersen PM, Nilsson P, Keranen ML, Forsgren L, Hagglund J, Karlsborg M, Ronnevi LO, Gredal O, Marklund SL. Phenotypic heterogeneity in motor neuron disease patients with CuZn-superoxide dismutase mutations in Scandinavia. Brain. 1997;120(Pt 10):1723–1737. doi: 10.1093/brain/120.10.1723. [DOI] [PubMed] [Google Scholar]
- 108.Deng HX, Tainer JA, Mitsumoto H, Ohnishi A, He X, Hung WY, Zhao Y, Juneja T, Hentati A, Siddique T. Two novel SOD1 mutations in patients with familial amyotrophic lateral sclerosis. Hum Mol Genet. 1995;4:1113–1116. doi: 10.1093/hmg/4.6.1113. [DOI] [PubMed] [Google Scholar]
- 109.Kawamata J, Shimohama S, Takano S, Harada K, Ueda K, Kimura J. Novel G16S (GGC-AGC) mutation in the SOD-1 gene in a patient with apparently sporadic young-onset amyotrophic lateral sclerosis. Hum Mutat. 1997;9:356–358. doi: 10.1002/(SICI)1098-1004(1997)9:4<356::AID-HUMU9>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 110.Orrell RW, Habgood JJ, Gardiner I, King AW, Bowe FA, Hallewell RA, Marklund SL, Greenwood J, Lane RJ, deBelleroche J. Clinical and functional investigation of 10 missense mutations and a novel frameshift insertion mutation of the gene for copper-zinc superoxide dismutase in UK families with amyotrophic lateral sclerosis. Neurology. 1997;48:746–751. doi: 10.1212/wnl.48.3.746. [DOI] [PubMed] [Google Scholar]
- 111.Naini A, Mehrazin M, Lu J, Gordon P, Mitsumoto H. Identification of a novel D109Y mutation in Cu/Zn superoxide dismutase (sod1) gene associated with amyotrophic lateral sclerosis. J Neurol Sci. 2007;254:17–21. doi: 10.1016/j.jns.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 112.Jones CT, Swingler RJ, Brock DJ. Identification of a novel SOD1 mutation in an apparently sporadic amyotrophic lateral sclerosis patient and the detection of Ile113Thr in three others. Hum Mol Genet. 1994;3:649–650. doi: 10.1093/hmg/3.4.649. [DOI] [PubMed] [Google Scholar]
- 113.Shibata N, Kawaguchi M, Uchida K, Kakita A, Takahashi H, Nakano R, Fujimura H, Sakoda S, Ihara Y, Nobukuni K, Takehisa Y, Kuroda S, Kokubo Y, Kuzuhara S, Honma T, Mochizuki Y, Mizutani T, Yamada S, Toi S, Sasaki S, Iwata M, Hirano A, Yamamoto T, Kato Y, Sawada T, Kobayashi M. Protein-bound crotonaldehyde accumulates in the spinal cord of superoxide dismutase-1 mutation-associated familial amyotrophic lateral sclerosis and its transgenic mouse model. Neuropathology. 2007;27:49–61. doi: 10.1111/j.1440-1789.2006.00746.x. [DOI] [PubMed] [Google Scholar]
- 114.Esteban J, Rosen DR, Bowling AC, Sapp P, McKenna-Yasek D, O’Regan JP, Beal MF, Horvitz HR, Brown RH., Jr Identification of two novel mutations and a new polymorphism in the gene for Cu/Zn superoxide dismutase in patients with amyotrophic lateral sclerosis. Hum Mol Genet. 1994;3:997–998. doi: 10.1093/hmg/3.6.997. [DOI] [PubMed] [Google Scholar]
- 115.Garcia-Redondo A, Bustos F, Juan YSB, Del Hoyo P, Jimenez S, Campos Y, Martin MA, Rubio JC, Canadillas F, Arenas J, Esteban J. Molecular analysis of the superoxide dismutase 1 gene in Spanish patients with sporadic or familial amyotrophic lateral sclerosis. Muscle Nerve. 2002;26:274–278. doi: 10.1002/mus.10193. [DOI] [PubMed] [Google Scholar]
- 116.Boukaftane Y, Khoris J, Moulard B, Salachas F, Meininger V, Malafosse A, Camu W, Rouleau GA. Identification of six novel SOD1 gene mutations in familial amyotrophic lateral sclerosis. Can J Neurol Sci. 1998;25:192–196. doi: 10.1017/s0317167100034004. [DOI] [PubMed] [Google Scholar]
- 117.Kostrzewa M, Burck-Lehmann U, Muller U. Autosomal dominant amyotrophic lateral sclerosis: a novel mutation in the Cu/Zn superoxide dismutase-1 gene. Hum Mol Genet. 1994;3:2261–2262. doi: 10.1093/hmg/3.12.2261. [DOI] [PubMed] [Google Scholar]
- 118.Andersen PM. Amyotrophic lateral sclerosis associated with mutations in the CuZn superoxide dismutase gene. Curr Neurol Neurosci Rep. 2006;6:37–46. doi: 10.1007/s11910-996-0008-9. [DOI] [PubMed] [Google Scholar]
- 119.Shimizu T, Kawata A, Kato S, Hayashi M, Takamoto K, Hayashi H. Autonomic failure in ALS with a novel SOD1 gene mutation. Neurology. 2000;54:1534–1537. doi: 10.1212/wnl.54.7.1534. [DOI] [PubMed] [Google Scholar]
- 120.Jackson M, Al-Chalabi A, Enayat ZE, Chioza B, Leigh PN, Morrison KE. Copper/zinc superoxide dismutase 1 and sporadic amyotrophic lateral sclerosis: analysis of 155 cases and identification of a novel insertion mutation. Ann Neurol. 1997;42:803–807. doi: 10.1002/ana.410420518. [DOI] [PubMed] [Google Scholar]
- 121.Aoki M, Ogasawara M, Matsubara Y, Narisawa K, Nakamura S, Itoyama Y, Abe K. Mild ALS in Japan associated with novel SOD mutation. Nat Genet. 1993;5:323–324. doi: 10.1038/ng1293-323. [DOI] [PubMed] [Google Scholar]
- 122.Hosler BA, Nicholson GA, Sapp PC, Chin W, Orrell RW, de Belleroche JS, Esteban J, Hayward LJ, McKenna-Yasek D, Yeung L, Cherryson AK, Dench JE, Wilton SD, Laing NG, Horvitz HR, Brown RH., Jr Three novel mutations and two variants in the gene for Cu/Zn superoxide dismutase in familial amyotrophic lateral sclerosis. Neuromuscul Disord. 1996;6:361–366. doi: 10.1016/0960-8966(96)00353-7. [DOI] [PubMed] [Google Scholar]
- 123.Enayat ZE, Orrell RW, Claus A, Ludolph A, Bachus R, Brockmuller J, Ray-Chaudhuri K, Radunovic A, Shaw C, Wilkinson J, et al. Two novel mutations in the gene for copper zinc superoxide dismutase in UK families with amyotrophic lateral sclerosis. Hum Mol Genet. 1995;4:1239–1240. doi: 10.1093/hmg/4.7.1239. [DOI] [PubMed] [Google Scholar]
- 124.Pramatarova A, Figlewicz DA, Krizus A, Han FY, Ceballos-Picot I, Nicole A, Dib M, Meininger V, Brown RH, Rouleau GA. Identification of new mutations in the Cu/Zn superoxide dismutase gene of patients with familial amyotrophic lateral sclerosis. Am J Hum Genet. 1995;56:592–596. [PMC free article] [PubMed] [Google Scholar]
- 125.Watanabe M, Aoki M, Abe K, Shoji M, Iizuka T, Ikeda Y, Hirai S, Kurokawa K, Kato T, Sasaki H, Itoyama Y. A novel missense point mutation (S134N) of the Cu/Zn superoxide dismutase gene in a patient with familial motor neuron disease. Hum Mutat. 1997;9:69–71. doi: 10.1002/(SICI)1098-1004(1997)9:1<69::AID-HUMU14>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 126.Nogales-Gadea G, Garcia-Arumi E, Andreu AL, Cervera C, Gamez J. A novel exon 5 mutation (N139H) in the SOD1 gene in a Spanish family associated with incomplete penetrance. J Neurol Sci. 2004;219:1–6. doi: 10.1016/j.jns.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 127.Shaw CE, Enayat ZE, Chioza BA, Al-Chalabi A, Radunovic A, Powell JF. Mutations in all five exons of SOD-1 may cause ALS. Ann Neurol. 1998;43:390–394. doi: 10.1002/ana.410430319. [DOI] [PubMed] [Google Scholar]
- 128.Sapp PC, Rosen DR, Hosler BA, Esteban J, McKenna-Yasek D, Regan JPO. Identification of three novel mutations in the gene for Cu/Zn superoxide dismutase in patients with familial amyotrophic lateral sclerosis. Neuromusc Disord. 1995;5:353–357. doi: 10.1016/0960-8966(95)00007-a. [DOI] [PubMed] [Google Scholar]
- 129.Segovia-Silvestre T, Andreu AL, Vives-Bauza C, Garcia-Arumi E, Cervera C, Gamez J. A novel exon 3 mutation (D76V) in the SOD1 gene associated with slowly progressive ALS. Amyotroph Lateral Scler Other Motor Neuron Disord. 2002;3:69–74. doi: 10.1080/146608202760196039. [DOI] [PubMed] [Google Scholar]
- 130.Alexander MD, Traynor BJ, Miller N, Corr B, Frost E, McQuaid S, Brett FM, Green A, Hardiman O. “True” sporadic ALS associated with a novel SOD-1 mutation. Ann Neurol. 2002;52:680–683. doi: 10.1002/ana.10369. [DOI] [PubMed] [Google Scholar]
- 131.Aoki M, Abe K, Houi K, Ogasawara M, Matsubara Y, Kobayashi T. Variance of age at onset in a Japanese family with amyotrophic lateral sclerosis associated with a novel Cu/Zn superoxide dismutase mutation. Ann Neurol. 1995;37:676–679. doi: 10.1002/ana.410370518. [DOI] [PubMed] [Google Scholar]
- 132.Beck M, Sendtner M, Toyka KV. Novel SOD1 N86K mutation is associated with a severe phenotype in familial ALS. Muscle Nerve. 2007;36:111–114. doi: 10.1002/mus.20756. [DOI] [PubMed] [Google Scholar]
- 133.Hayward C, Brock DJ, Minns RA, Swingler RJ. Homozygosity for Asn86Ser mutation in the CuZn-superoxide dismutase gene produces a severe clinical phenotype in a juvenile onset case of familial amyotrophic lateral sclerosis. J Med Genet. 1998;35:174. doi: 10.1136/jmg.35.2.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kostrzewa M, Damian MS, Muller U. Superoxide dismutase 1: identification of a novel mutation in a case of familial amyotrophic lateral sclerosis. Hum Genet. 1996;98:48–50. doi: 10.1007/s004390050157. [DOI] [PubMed] [Google Scholar]
- 135.Rezania K, Yan J, Dellefave L, Deng HX, Siddique N, Pascuzzi RT, Siddique T, Roos RP. A rare Cu/Zn superoxide dismutase mutation causing familial amyotrophic lateral sclerosis with variable age of onset, incomplete penetrance and a sensory neuropathy. Amyotroph Lateral Scler Other Motor Neuron Disord. 2003;4:162–166. doi: 10.1080/aml.4.3.162.166. [DOI] [PubMed] [Google Scholar]
- 136.Andersen PM, Nilsson P, Ala-Hurula V, Keranen ML, Tarvainen I, Haltia T, Nilsson L, Binzer M, Forsgren L, Marklund SL. Amyotrophic lateral sclerosis associated with homozygosity for an Asp90Ala mutation in CuZn-superoxide dismutase. Nat Genet. 1995;10:61–66. doi: 10.1038/ng0595-61. [DOI] [PubMed] [Google Scholar]
- 137.Chou CM, Huang CJ, Shih CM, Chen YP, Liu TP, Chen CT. Identification of three mutations in the Cu, Zn-superoxide dismutase (Cu, Zn-SOD) gene with familial amyotrophic lateral sclerosis: transduction of human Cu, Zn-SOD into PC12 cells by HIV-1 TAT protein basic domain. Ann N Y Acad Sci. 2005;1042:303–313. doi: 10.1196/annals.1338.053. [DOI] [PubMed] [Google Scholar]
- 138.Elshafey A, Lanyon WG, Connor JM. Identification of a new missense point mutation in exon 4 of the Cu/Zn superoxide dismutase (SOD-1) gene in a family with amyotrophic lateral sclerosis. Hum Mol Genet. 1994;3:363–364. doi: 10.1093/hmg/3.2.363. [DOI] [PubMed] [Google Scholar]