Abstract
During wound healing, some circulating monocytes enter the wound, differentiate into fibroblast-like cells called fibrocytes, and appear to then further differentiate into myofibroblasts, cells that play a key role in collagen deposition, cytokine release, and wound contraction. The differentiation of monocytes into fibrocytes is inhibited by the serum protein serum amyloid P (SAP). Depleting SAP at a wound site thus might speed wound healing. SAP binds to some types of agarose in the presence of Ca2+. We found that human SAP binds to an agarose with a KD of 7×10−8M and a Bmax of 2.1 μg SAP/mg wet weight agarose. Mixing this agarose 1: 5 w/v with 30 μg/mL human SAP (the average SAP concentration in normal serum) in a buffer containing 2mM Ca2+ reduced the free SAP concentration to ~0.02 μg/mL, well below the concentration that inhibits fibrocyte differentiation. Compared with a hydrogel dressing and a foam dressing, dressings containing this agarose and Ca2+ significantly increased the speed of wound healing in partial thickness wounds in pigs. This suggests that agarose/Ca2+ dressings may be beneficial for wound healing in humans.
During wound healing, some circulating monocytes present within the blood are attracted to the wound, where they differentiate into fibroblast-like cells called fibrocytes and at least in part mediate tissue repair.1–7 Fibrocytes express markers of both hematopoietic cells (CD45, MHC class II, CD34) and stromal cells (collagen I and III and fibronectin).1,8 Fibrocyte precursors appear to be a ~10% subpopulation of CD14+ peripheral blood monocytes.9–11
Fibrocytes are largely absent from normal skin but are present in scars in both humans and mice, suggesting that fibrocytes participate in wound healing.6,12,13 Interestingly, the number of fibrocytes in hypertrophic scars is higher than in normal scars.6 Mature fibrocytes exposed to transforming growth factor-β (TGF-β) in vitro are able to further develop into myofibroblasts, a population of fibroblast-like cells that are able to contract collagen gels, an in vitro model of wound contraction.9 Fibrocytes from burn patients secrete TGF-β to activate dermal fibroblasts, indicating that fibrocytes can have a multiplicative effect on wound healing.14
We found that a factor in serum inhibits the differentiation of monocytes into fibrocytes.11 We purified the component of human serum that inhibits human fibrocyte differentiation and identified it as serum amyloid P (SAP).11 SAP is a 27 kDa protein produced by the liver, secreted into the blood, and circulates as stable 135 kDa pentamers.15–17 SAP binds to apoptotic cells, DNA and some micro-organisms and is cleared by macrophages and other cells through Fc γ receptors.16,18–20 We were also able to show that a commercial preparation of SAP was able to inhibit fibrocyte differentiation, whereas the highly related protein C-reactive protein could not.11 To confirm that SAP is the active factor in serum that inhibits fibrocyte differentiation, we depleted SAP from serum using anti-SAP antibodies bound to protein G beads. The SAP-depleted serum had a poor ability to inhibit fibrocyte differentiation.11 Together with the ability of purified SAP to inhibit fibrocyte differentiation, these observations strongly suggested that SAP is the active factor in serum that inhibits fibrocyte differentiation.
Agarose is a polysaccharide polymer isolated from sea-weed. 21,22 Different preparations of agarose contain different amounts of pyruvate acetal adducts and covalently linked sulfate.23,24 SAP binds strongly to some, but not all, types of agarose in the presence of millimolar levels of Ca2+.25,26 A comparison of the SAP binding capacity of different commercial agarose preparations in the presence of 2mMCaCl2 showed a correlation with the pyruvate acetal content but no correlation with the agarose sulfate content.27
Wound fluid contains serum proteins.28 Since SAP is present in serum at a concentration of ~30 μg/mL 29 and inhibits fibrocyte differentiation at ~1 μg/mL or lower,11 there is a strong possibility that wound fluids initially contain enough SAP to inhibit fibrocyte differentiation. In support of this, we found that both systemic and local injections of murine SAP inhibit dermal wound healing in mice.30 Although it is unclear whether potentiating fibrocyte differentiation is beneficial to wound healing, we reasoned that a material that binds SAP might be able to deplete SAP at a wound site and thus potentiate fibrocyte differentiation, and that this might be beneficial to wound healing. In this report we show that a wound-healing dressing that contains a SAP-binding agarose and 2mM CaCl2 speeds wound healing in pigs.
MATERIALS AND METHODS
SAP binding
Prehydrated agarose beads (SP Sepharose FF, GE-Healthcare Biosciences, Uppsala, Sweden) were washed four times in 10 volumes of 10mM Tris pH 8, 140mM NaCl, and 2mMCaCl2 (TNC buffer), collecting the beads by centrifugation at 2,000×g for 1 minute. For each assay point, 20 mg of beads were placed in a 1.5mL Eppendorf tube. Hundred microliters of different concentrations of purified human SAP (EMD Biosciences, La Jolla, CA) in TNC was then added to the agarose. The tube was rotated end over end for 60 minutes at room temperature, and the agarose beads were collected by centrifugation at 2,000×g for 1 minute. Supernatants were collected, and SAP concentrations were determined by ELISA following Pilling et al.11 Because the free (supernatant) concentrations were equivalent to or less than the bound concentrations, bound amounts were calculated as total—free. Nonlinear regression to fit binding data to a standard one-site binding model was done with the Prism software package (Graph-Pad Software, San Diego, CA).
Binding specificity assay
SP agarose was washed in TNC four times as described above. To examine binding specificity, 200 μL of human serum (Sigma, St. Louis, MO) or pig serum (a gift from Dr. Oluyinka Olutoye, Baylor College of Medicine) was mixed with 200 μL of SP agarose slurry and 600 μL of TNC. The mixture was rotated overnight at 4 °C. The agarose beads were collected by centrifugation, washed five times in TNC, and bound material was eluted for 2 hours at room temperature with 200 μL of 10mM Tris pH 8, 140mM NaCl, 10mM EDTA. The supernatant was clarified by centrifugation and 10 μL of the eluate was mixed with SDS sample buffer and separated on a 4–20% Tris/glycine gel (Biorad, Hercules, CA). The gel was then stained with Coomassie. To detect SAP, Western blots were done following, Pilling et al.11 using a 1: 20,000 dilution of 1793-1 rabbit anti-SAP (Epitomics, Burlingame, CA) in TBS for the first antibody step.
Experimental animals
Fourteen young female Yorkshire specific pathogen free pigs (SPF: Ken-O-Kaw Farms, Windsor, IL) weighing 25–30 kg were kept in the University of Miami animal facility (meeting American Association for Accreditation of Laboratory Animal Care compliance) for 2 weeks before initiating the experiment. These animals were fed a basal diet ad libitum and were housed individually with controlled temperature (19–21°C) and lighting (12 hour/12 hour LD). The experimental animal protocols used for this study were approved by the University of Miami Institutional Animal Care and Use Committee and all the procedures followed the federal guidelines for the care and use of laboratory animals (US Department of Health and Human Services, US Department of Agriculture). The studies were conducted in compliance with the University of Miami’s Department of Dermatology and Cutaneous Surgery Standard Operating Procedures. Animals were monitored daily for any observable signs of pain or discomfort. In order to help minimize possible discomfort, 0.03 mg/kg buprenorphine (Buprenex injectable; Reckitt Benckiser, Hull, UK) was given to each animal on the first day, and every third day thereafter, and a 25 μg/hour fentanyl transdermal system (Duragesic; Alza Corp., Mountain View, CA) was used during the entire experiment.
Wounding technique
The flanks and backs of experimental animals were clipped with standard animal clippers on the day of the experiment. The skin on both sides of each animal was prepared for wounding by washing with a nonantibiotic soap (Neutrogena Soap Bar; Johnson and Johnson, Los Angeles, CA) and sterile water. Each animal was anesthetized intramuscularly with tiletamine HCl plus zolazepam (1.4 mg/kg) (Telazol; Lederle Parenterals Inc., Carolina, Puerto Rico), xylazine (2.0 mg/kg) (X-jet; Phoenix Scientific Inc., St. Joseph, MO), and atropine (0.04 mg/kg) (Atrojet SA; Phoenix Scientific Inc.) followed by mask inhalation of an isoflurane (Isothesia; Abbott Laboratories, Chicago, IL) and oxygen combination. Approximately 140 rectangular wounds measuring 10mm×7mm×0.5mm were made in the paravertebral and thoracic area of each pig with a specialized electrokeratome fitted with a 7mm blade. The wounds were separated from one another by 15mm of unwounded skin.
Treatments
Wounds on the 14 pigs were randomly assigned to each of six treatment groups with the exception of the untreated air exposed wounds, where each of the 14 pigs had at least five wounds for each day. The treatment groups were A, SP Sepharose in a proprietary carbomer vehicle containing 2mM Ca2+; B, the carbomer vehicle alone; C, Xeroform petrolatum gauze (Tyco Kendall, Seneca, SC); D, Intrasite hydrogel (Smith & Nephew, Largo, FL); E, Tielle polyurathane foam (Johnson & Johnson, New Brunswick, NJ); and F, untreated air-exposed control. There was a total of n= 30 wounds per treatment group/day, except for untreated air exposed where n was 70 for each day. In addition, there were three randomly assigned wounds for each treatment group for days 4, 7, and 10 that were used for histology. The hydrogels (groups A, B, and D) were applied over the wounds and surrounding normal skin with a sterile tongue depressor to the approximate thickness of ~1 mm. The wound dressing materials in groups A, B, and D were then covered with a Tegaderm polyurethane dressing (3 M, St. Paul, MN) to prevent desiccation. Since Tielle already has a polyurethane backing, and Xeroform is occlusive, no additional polyurethane was applied to these dressings. On day 1 after treatment, the animals were anesthetized and the dressings observed to make sure they were still intact. All materials were kept in place until wound evaluation unless it was observed that the materials needed to be replaced. In order to assess the wounds, a portion of the dressing material was removed to uncover wounds for evaluation. The animals were sacrificed on day 10.
Assessment of reepithelialization
Beginning on day 4 (after wounding on day 0), and on each day thereafter until all wounds were completely epithelialized, 30 wounds and the surrounding normal skin from each treatment group (with 70 wounds excised for the untreated air controls) were excised using an electrokeratome with a 22mm blade set at a depth of 0.7 mm. All specimens that were not excised intact were discarded. The resulting wound was not used for any further experiments. The excised skin containing the wound site was incubated in 0.5Msodium bromide at 37 °C for 24 hours, allowing for a separation of the dermis from the epidermis.31 After separation, the epidermal sheet was examined macroscopically for defects, and evaluations were performed blinded to treatment condition. Defects were defined as holes in the epidermal sheet or as a lack of epidermal continuity in the area of the wound. Epithelialization was considered complete (healed) if no defects were present; any defect in the wound area indicated that healing was incomplete. For each treatment group, on each day the number of wounds healed (completely epithelialized) was divided by the total number of wounds in that group sampled on that day, and multiplied by 100. Statistical analysis was done with chi square.
Histology and immunohistochemistry
Full-thickness 8mm biopsies were obtained through the center of the wounds used for histology. Skin sections were embedded in OCT (VWR, West Chester, PA), frozen on dry ice and then stored at −80°C. Ten micrometers cryosections were mounted on Superfrost Plus microscope slides (VWR). Sections were fixed in acetone for 10 minutes at room temperature, and then air-dried for 15 minutes. Slides were then rehydrated in water for 5 minutes. Sections were covered in Gill’s #3 Accustain Hematoxylin Solution (Sigma) diluted 1: 1 in water for 1 minute and were then rinsed with water for 3 minutes. Slides were dehydrated in 70% ethanol for 3 minutes, then 95% ethanol for 5 minutes. Sections were covered with 0.1% Eosin Y (Fisher Scientific, Pittsburgh, PA) in water for 1 minute. After rising off the Eosin Y, the slide was dehydrated in 100% ethanol for 5 minutes, then xylene for 10 minutes, and mounted with Permount (Fisher), as described previously.32
To detect cytokeratin and collagen-I, slides were fixed in acetone for 10 minutes, followed by a 60 minute incubation of the slide in 4% BSA in phosphate-buffered saline (PBS) to block nonspecific binding. Slides were then incubated for 60 minutes in PBS with 4% BSA containing either 5 μg/mL anti-cytokeratin monoclonal antibody (clone C-11, mouse IgG1, Sigma-Aldrich), or rabbit polyclonal anti-collagen-I antibodies (600-401-104, Rockland Inc., Gilbertsville, PA). Irrelevant mouse IgG1 monoclonal antibodies (BD Biosciences, San Jose, CA) or irrelevant rabbit polyclonal antibodies (Jackson ImmunoResearch, West Grove, PA) at 5 μg/mL were used as controls. Slides were then washed in six changes of PBS over 30 minutes. The slides were then incubated with either 2.5 μg/mL biotinylated rat F(ab′)2 anti-mouse IgG (Jackson ImmunoResearch,) or biotinylated goat F(ab′)2 anti-rabbit IgG (Southern Biotechnology, Birmingham, AL) with 4% BSA in PBS. After washing, the biotinylated antibodies were detected with a 1/200 dilution of ExtrAvidin alkaline phosphatase (Sigma-Aldrich) in PBS containing 4% BSA. Staining was developed with the Vector Red Alkaline Phosphatase kit (Vector Laboratories, Burlingame, CA) for 5 minutes and slides were then counter-stained for 10 seconds with Gill’s #3 Hematoxylin Solution (Sigma-Aldrich) diluted 1: 5 in water for 10 seconds. The slides were rinsed in water and were then dehydrated and mounted as above. All histology and immunocytochemistry evaluations were performed blinded to treatment condition.
RESULTS
High electroendosmosis (high EEO) agarose binds SAP in the presence of Ca2+.27 After testing 11 different agarose sources, we identified SP Sepharose FF as having the highest specific binding to human SAP from serum (data not shown). A binding curve fit with a classic one-site binding model indicated that SP Sepharose FF binds human SAP with a KD of ~9 μg/mL (7×10−8 M), and a Bmax of 42 μg SAP/20 mg wet weight agarose, or 2.1 μg SAP/mg wet weight agarose (Figure 1A). Fits to a model with cooperative binding gave a Hill coefficient of 0.95, indicating no cooperative binding, and an F-test comparison with a two-site binding model indicated that there was no significant evidence for two-site binding. To estimate the possible effect of adding agarose to wound fluid containing different amounts of SAP, we measured the SAP concentration in a buffer containing SAP before and after adding a 1: 5 w/v ratio of agarose beads to the solution. As shown in Figure 1B, adding agarose decreases the free SAP concentration. At an initial human SAP concentration of 30 μg/mL (approximately the average concentration in human serum29) the addition of 1: 5 w/v agarose lowered the free SAP concentration to ~0.02 μg/mL (arrow, Figure 1B), well below the concentration that inhibits fibrocyte differentiation. 11
Figure 1.
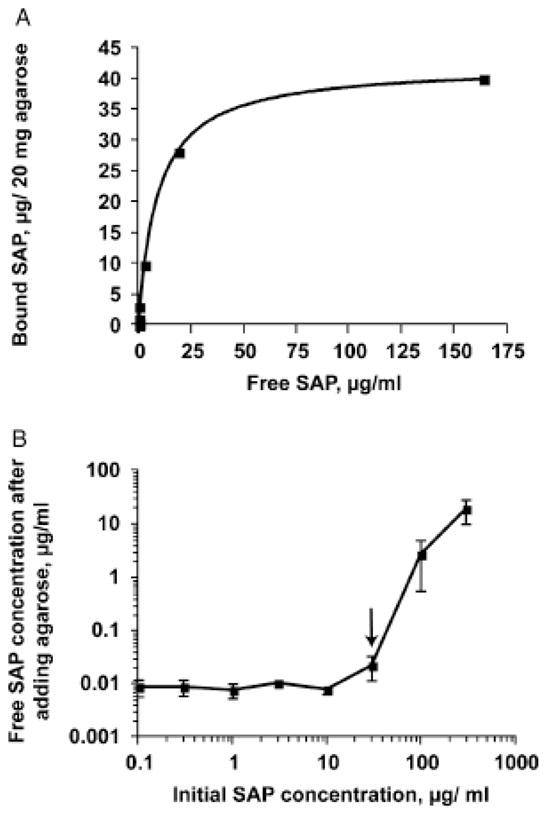
Binding of human SAP to SP agarose. (A) Binding data were plotted and a one-site binding model (solid line) was fitted to the data. (B) The SAP concentration before and after adding a 1: 5 w/v ratio of agarose beads to the SAP solution. Values are mean ± SEM (n= 3). The absence of an error bar indicates that the error was smaller than the plot symbol. Arrow indicates an input concentration of 30 μg/mL SAP on the X axis (roughly the normal serum concentration), which the addition of agarose then decreased to ~0.02 μg/mL (Y axis). SAP, serum amyloid P.
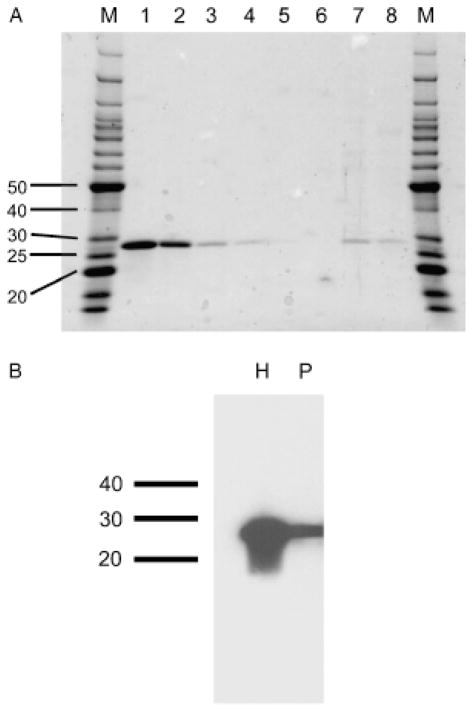
Porcine wounds are an excellent model for human wounds,33 and we envisioned using a topical application of high EEO agarose to deplete SAP from a porcine wound. High EEO agarose binds human SAP with high specificity, and in fact can be used to purify SAP from serum. 25,27,34 To determine if high EEO agarose will similarly bind porcine SAP, porcine serum was mixed with SP agarose, washed, and the bound material was eluted. As shown in Figure 2A, high EEO agarose showed high affinity binding of a single 27 kDa protein in porcine serum, and this protein had essentially the same molecular mass as human SAP. A Western blot stained with anti-human SAP antibodies suggested that the 27 kDa protein from porcine serum that bound to the SP agarose is SAP (Figure 2B). These results suggest that high EEO agarose can be used to specifically absorb porcine SAP from porcine serum.
Figure 2.
Specificity of the high-affinity binding of human and porcine serum proteins to SP agarose. (A) Human and porcine sera were incubated with SP agarose, washed, and bound material was eluted and separated on an SDS-polyacrylamide gel which was then stained with Coomassie. Lanes are M, molecular mass markers (molecular masses in kDa are indicated at left); (1) 1 μg human SAP; (2) 0.3 μg human SAP; (3) 0.1 μg human SAP; (4) 0.03 μg human SAP; (5) 0.01 μg human SAP; (6) 0.003 μg human SAP; (7) 10 μL of the eluted material from human serum, (8) 10 μL of the eluted material from porcine serum. (B) A Western blot of the protein eluted from SP agarose was stained with anti-human SAP antibodies. H is the material from human serum; P is the material from porcine serum. The position of molecular mass markers (in kDa) is indicated at left. SAP, serum amyloid P.
We then tested a proprietary hydrogel formulation containing SP agarose and 2mM Ca2+ in a carbomer vehicle (henceforth referred to as “agarose in carbomer”) on deep partial thickness porcine dermal wounds. These wounds heal by deposition of granulation tissue and epithelialization, and this model has been used for 30 years to evaluate a variety of topical agents, advanced occlusive dressings, topical growth factors, and other factors that can affect wound healing.31,35–38 As controls, we tested a variety of other treatments. The carbomer is a conventional hydrogel, with the carbomer vehicle having the appearance of a clear, thick viscous liquid, so we wanted to compare the effect with other wound hydrating materials. We chose three (Xeroform, Tielle, and Intrasite) that we considered representative of the diversity of such products on the market, in consultation with Roxane Chabot of RBC Consulting. The hydrogel treatments (carbomer, agarose in carbomer, and Intrasite) remained in place and were readily absorbed by the skin. The Xeroform gauze treatment did not remain in place throughout much of the study and needed to be changed frequently. The Tielle foam caused slight rewounding during the early assessment days. None of the wounds from any of the treatment groups showed any erythema or clinical signs of infection.
On day 4, wounds treated with agarose in carbomer showed the highest percentage of complete epithelialization (73%) (Table 1). This was followed by the Xeroform gauze (60%) IntraSite hydrogel (43%), and Tielle foam (20%). None of the untreated air exposed wounds epithelialized on this day. Statistical significance was observed between all groups and the untreated group (p < 0.001), between agarose in carbomer vs. carbomer (p < 0.05), IntraSite hydrogel (p < 0.05), or Tielle foam (p < 0.001); and between Xeroform gauze vs. Tielle foam (p < 0.01).
Table 1.
Epithelialization of partial thickness porcine skin wounds
| Complete epithelialization |
|||||
|---|---|---|---|---|---|
| Treatment | Day 4 | Day 5 | Day 6 | Day 7 | Day 8 |
| Untreated | 0/70 | 9/70 | 38/70 | 56/70 | 70/70 |
| 0% | 13% | 54% | 80% | 100% | |
| Agarose in carbomer* | 22/30 | 30/30 | 30/30 | 30/30 | 30/30 |
| 73% | 100% | 100% | 100% | 100% | |
| Carbomer* | 10/30 | 30/30 | 30/30 | 30/30 | 30/30 |
| 33% | 100% | 100% | 100% | 100% | |
| Xeroform Gauze | 18/30 | 29/30 | 30/30 | 30/30 | 30/30 |
| 60% | 97% | 100% | 100% | 100% | |
| IntraSite hydrogel* | 13/30 | 28/30 | 30/30 | 30/30 | 30/30 |
| 43% | 93% | 100% | 100% | 100% | |
| Tielle foam | 6/30 | 25/30 | 29/30 | 30/30 | 30/30 |
| 20% | 83% | 97% | 100% | 100% | |
Wounds were treated with the indicated dressings.
Indicates that the treatment additionally had a polyurethane dressing on top. On days 4 through 8 after wounding, 70 untreated wounds and 30 treated wounds per dressing type were excised and the number of epithelialized wounds was determined. The percentage of completely epithelialized wounds was then determined (shaded rows).
On day 5, wounds treated with agarose in carbomer or carbomer vehicle were all completely epithelialized (Table 1). Those treated with Xeroform gauze and IntraSite hydrogel were close behind (97% and 93%). Wounds treated with the Tielle foam and untreated air exposed wounds were 83% and 13% completely epithelialized. Statistical significance was observed only between all treatment groups and the untreated control group (p < 0.001).
On the sixth day, there was 100% complete epithelialization of wounds except for those treated with the Tielle foam (97% epithelialization) and the untreated wounds (54% epithelialization). All groups showed significant differences compared with the untreated group (p < 0.001); otherwise there were no intergroup differences. On the seventh day, all wounds that received treatment were 100% completely epithelialized. The untreated wounds were 80% epithelialized, and all treated groups showed significant differences compared with the untreated group (p < 0.01). On day 8 all wounds including the untreated wounds were completely epithelialized, and as a result there were no statistically significant differences.
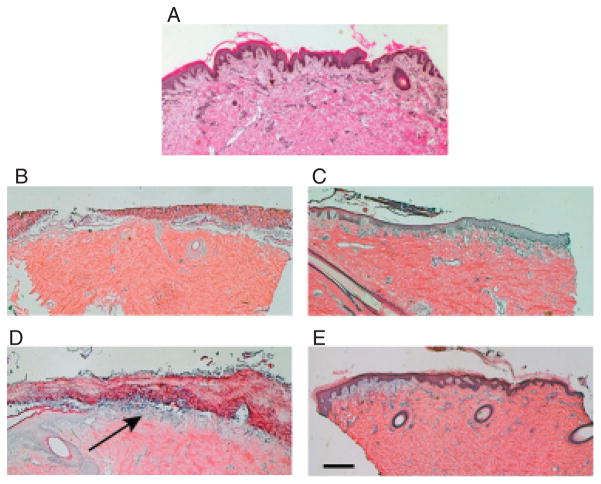
Hematoxylin and eosin-stained cryosections of porcine skin from an unwounded region showed a normal epidermis (dark purple) with the presence of rete ridges (Figure 3A). On the top surface, a thin stratum corneum was noted (bright mauve). As expected, the dermis has a normal basket weave collagen pattern. A section of an untreated day 4 wound (Figure 3B) showed significant crust formation (*, Figure 3B) separated in areas by an air gap over the dermis, with very little epidermis. A section of a wound at day 4 treated with agarose in carbomer (Figure 3C) showed a mature epidermis covering the entire dermis. At day 7, an untreated wound (Figure 3D) showed significant crust formation over the wound, with some epidermis migrating from a hair follicle (left side) (arrow, Figure 3D). At day 7, a wound treated with agarose in carbomer (Figure 3E) showed a mature epidermis over the wounded area. Together, the histology observations and percent epithelialization results indicate that treating porcine skin wounds with agarose in carbomer enhances epidermis formation sooner than control wounds.
Figure 3.
Sections of wounds stained with hematoxylin and eosin. (A) A section of skin before wounding. (B) A section of an untreated wound at day 4. The *shows an area of crust. (C) An agarose in carbomer-treated wound at day 4. (D) An untreated wound at day 7. The arrow shows a region of epidermis under the crust. (E) An agarose in carbomer-treated wound at day 7. Scale bar is 0.5mm.
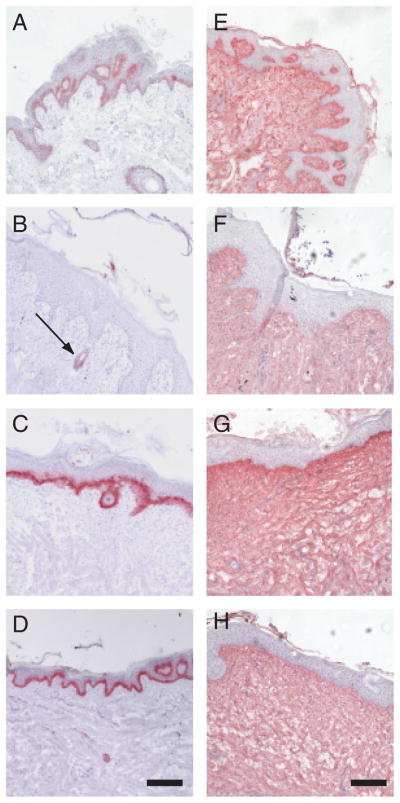
Cytokeratin is a marker of epithelial tissue.39 A section of unwounded porcine skin showed staining with anti-cytokeratin antibodies in a layer between the epidermis and dermis (Figure 4A). Similar staining was observed at day 10 for wounds treated with agarose in carbomer (Figure 4C) and wounds treated with IntraSite hydrogel (Figure 4D). This indicated that after healing with these two treatments, the skin formed a layer of cytokeratin-positive cells. However, at day 10, an untreated wound showed little expression of cytokeratin in the epithelial tissue, indicating that the untreated wounds were less mature than the treated wounds. The expression of cytokeratin around hair follicles in the untreated wounds served as an internal control of cytokeratin staining (arrow, Figure 4B). We also stained sections with anti-collagen I antibodies as a marker for dermal integrity and remodeling. Normal porcine skin, and untreated, agarose in carbomer-treated, and IntraSite hydrogel-treated wounds at day 10 (Figure 4E–H) all showed staining with anti-collagen I antibodies in the dermis. Together, the data suggest that at day 10, agarose in carbomer and IntraSite hydrogel treatments do not appear to affect the amount of collagen in the dermis, but increase the amount of epithelial cytokeratin staining compared with untreated wounds.
Figure 4.
Detection of cytokeratin and collagen-I in day 10 wounds. (A–D) Cryosections were stained with anti-cytokeratin antibodies to show reepithelialization. (A) Normal skin, (B) day 10 untreated wound (the arrow shows a hair follicle), (C) agarose in carbomer-treated wound, and (D) IntraSite hydrogel-treated wound. (E–H) Sections were stained with anti-collagen-I antibodies to show dermal remodeling. (E) Normal skin, (F) day 10 untreated-wounds compared with wounds treated with (G) agarose in carbomer, or (H) IntraSite hydrogel. Scale bars are 0.2mm.
DISCUSSION
Work from other groups showed that SAP binds to high EEO agarose in the presence of calcium, although the binding characteristics were never reported.25–27,34 In agreement with this, we found that several agarose samples bind SAP. We chose an agarose that showed high binding, and found that in vitro this material can bind 2.1 μg SAP/mg wet weight agarose. Human serum contains 5–60 μg/mL SAP, with an average of ~30 μg/mL.29,40 If we assume that the wound fluid after blood clotting contains a similar concentration of SAP, then our in vitro binding observations would indicate that placing 200 μg wet weight of agarose in a buffer containing 2mM calcium on a wound containing 1mL of wound fluid would reduce the SAP concentration in the wound fluid from ~30 to ~0.02 μg/mL (Figure 1). We previously observed that in vitro 30 μg/mL SAP completely inhibits fibrocyte differentiation, that the EC50 for SAP inhibition of fibrocyte differentiation is ~0.1 μg/mL, and that at 0.02 μg/mL there is no significant inhibitory effect of SAP on fibrocyte differentiation.11 This would then suggest that placing the same 200 μg wet weight of agarose in a buffer containing calcium on a wound containing 1mL of wound fluid would reduce the SAP concentration from one which inhibits fibrocyte differentiation to a SAP concentration which permits fibrocyte differentiation. It is thus possible that the effect of agarose in carbomer on the rate of wound epithelialization is due to its effect on fibrocyte differentiation.
The primary sequences and molecular masses of SAP are highly conserved across species,41 and in the presence of millimolar concentrations of Ca2+, high EEO agarose binds SAP from a variety of species including human, mouse, cow, fish, toad, and pig.26 We observed that in the presence of Ca2+, SP agarose binds a protein that we identified as porcine SAP based on its molecular mass on SDS-polyacrylamide gels, and cross-reactivity with anti-human SAP antibodies. According to the manufacturer, the anti-human SAP antibody we used is an affinity-purified rabbit monoclonal antibody against a domain in the N terminal region of human SAP. In the N-terminal 100 amino acids, porcine SAP has 81% identity and 94% similarity to human SAP, supporting the idea that an anti-human SAP antibody will cross-react with porcine SAP, and thus that the 27 kDa protein in porcine serum that binds to SP agarose in the presence of Ca2+ is porcine SAP.
Immediately after blood clotting in a wound, the wound fluid is by definition mostly blood serum, and will thus contain SAP. We envision that during normal wound healing, the concentration of SAP in the wound fluid decreases with time, due to some combination of degradation and ingestion by cells based on the opsonization of cell debris by SAP.18 Since SAP inhibits fibrocyte differentiation,11 and fibrocytes are observed in healing wounds,6,12,13 we speculate that at some point in normal wound healing, the free SAP concentration falls below the point at which it inhibits fibrocyte differentiation, allowing fibrocytes to participate in wound healing.
We observed that for the partial thickness porcine skin wounds, the agarose in carbomer dressing (with a polyurethane cover dressing) caused faster wound healing than Intrasite hydrogel (also with a polyurethane cover dressing) and Tielle foam. However, the faster wound healing of the agarose in carbomer was not significantly different from that of Xeroform gauze. Although the agarose in carbomer dressings could cause faster wound healing by an unknown mechanism, a possible explanation is that the agarose in the formulation was binding porcine SAP in the wound fluid, causing a rapid decrease in the free SAP concentration in the wound fluid and possibly in the upper cell layers of the wound. Compared with a normal wound, the rapid removal of free SAP would cause an equally rapid removal of the fibrocyte-inhibiting effect of SAP, allowing fibrocyte differentiation to occur earlier in the process of wound healing, thus speeding wound healing. Since the partial-thickness wounds used in this study heal by epithelialization and do not appear to require contraction, and the assay was a binary epithelialized/not fully epithelialized assay, further studies to elucidate the effect of agarose in carbomer dressings on myofibroblast-mediated contraction could be done in full-thickness wounds where contraction does occur, and with additional assays such as water-vapor transmission rates.
Fibrocytes participate in fibrotic lesions as well as wound healing.1–7 A systemic depletion of SAP thus might be deleterious. In these studies, we observed that the agarose in carbomer dressings caused faster healing only of the wounds to which they were applied, without speeding healing of the other wounds on the same pig. This result is consistent with the agarose in carbomer dressings causing a local but not a systemic depletion of SAP. In humans, the average serum SAP concentration is ~30 mg/L, with a range of 5–60 mg/L.29 If a wound had 10 g wet weight of SP agarose placed on it, our observed Bmax of 2.1 mgSAP bound/g agarose would predict a maximum of 21mg SAP bound by the 10 g of agarose. Assuming 5L of blood volume in an adult, and thus a total of 150 mg SAP, depleting 21 mg of SAP would reduce the total circulating SAP concentration by a maximum of 14%. At a free SAP concentration of 30 mg/L (30 μg/mL), SP agarose binds less SAP than the Bmax (Figure 1A), so the actual amount of SAP depleted by 10 g of agarose in an adult would be lower than 14% of the circulating SAP. Even a 14% depletion would reduce the serum SAP concentration to ~25 mg/L, well within the normal human range, so a 10 g agarose dressing should be safe to use on humans.
The ability of the agarose in carbomer dressings to speed wound healing in pigs suggests that these dressings might speed healing of human wounds. Fibrocytes are found in hypertrophic scars,6 and the ability to regulate fibrocyte differentiation by removal or addition of SAP might thus also allow a reduction in the formation of hypertrophic scars. Consistent with this hypothesis, local and systemic SAP injections slow wound healing in mice.30 Clinical investigation will be needed to establish the optimal balance and timing of pro- and anti-fibrotic activity regulated by SAP that might be useful to regulate wound healing.
Acknowledgments
We would like to thank Franco Pissani, Leonardo Real, and Fangcho Ma for their technical assistance and statistical analysis. This work was supported in part by NIH HL083029. RHG was an Investigator of the Howard Hughes Medical Institute.
- EEO
Electroendosmosis
- SAP
Serum mmyloid P
Footnotes
Financial Disclosure: Rice University has licensed intellectual property regarding the use of SAP-depleting compounds for use in wound healing to Trellis Bioscience. R. H. G. and D. P. are on the scientific advisory board of, and receive royalties from, Trellis. L. K. and S. E. are employees of Trellis.
References
- 1.Bucala R, Spiegel L, Chesney J, Hogan M, Cerami A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med. 1994;1:71–81. [PMC free article] [PubMed] [Google Scholar]
- 2.Majno G. Chronic inflammation: links with angiogenesis and wound healing. Am J Pathol. 1998;153:1035–9. doi: 10.1016/S0002-9440(10)65648-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Metz CN. Fibrocytes: a unique cell population implicated in wound healing. Cell Mol Life Sci. 2003;60:1342–50. doi: 10.1007/s00018-003-2328-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quan TE, Cowper S, Wu SP, Bockenstedt LK, Bucala R. Circulating fibrocytes: collagen-secreting cells of the peripheral blood. Int J Biochem Cell Biol. 2004;36:598–606. doi: 10.1016/j.biocel.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 5.Postlethwaite AE, Shigemitsu H, Kanangat S. Cellular origins of fibroblasts: possible implications for organ fibrosis in systemic sclerosis. Curr Opin Rheumatol. 2004;16:733–8. doi: 10.1097/01.bor.0000139310.77347.9c. [DOI] [PubMed] [Google Scholar]
- 6.Yang L, Scott PG, Dodd C, Medina A, Jiao H, Shankowsky HA, Ghahary A, Tredget EE. Identification of fibrocytes in postburn hypertrophic scar. Wound Repair Regen. 2005;13:398–404. doi: 10.1111/j.1067-1927.2005.130407.x. [DOI] [PubMed] [Google Scholar]
- 7.Bellini A, Mattoli S. The role of the fibrocyte, a bone marrow-derived mesenchymal progenitor, in reactive and reparative fibroses. Lab Invest. 2007;87:858–70. doi: 10.1038/labinvest.3700654. [DOI] [PubMed] [Google Scholar]
- 8.Chesney J, Metz C, Stavitsky AB, Bacher M, Bucala R. Regulated production of type I collagen and inflammatory cytokines by peripheral blood fibrocytes. J Immunol. 1998;160:419–25. [PubMed] [Google Scholar]
- 9.Abe R, Donnelly SC, Peng T, Bucala R, Metz CN. Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J Immunol. 2001;166:7556–62. doi: 10.4049/jimmunol.166.12.7556. [DOI] [PubMed] [Google Scholar]
- 10.Yang L, Scott PG, Giuffre J, Shankowsky HA, Ghahary A, Tredget EE. Peripheral blood fibrocytes from burn patients: identification and quantification of fibrocytes in adherent cells cultured from peripheral blood mononuclear cells. Lab Invest. 2002;82:1183–92. doi: 10.1097/01.lab.0000027841.50269.61. [DOI] [PubMed] [Google Scholar]
- 11.Pilling D, Buckley CD, Salmon M, Gomer RH. Inhibition of fibrocyte differentiation by serum amyloid P. J Immunol. 2003;17:5537–46. doi: 10.4049/jimmunol.171.10.5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chesney J, Bucala R. Peripheral blood fibrocytes: mesenchymal precursor cells and the pathogenesis of fibrosis. Curr Rheumatol Rep. 2000;2:501–5. doi: 10.1007/s11926-000-0027-5. [DOI] [PubMed] [Google Scholar]
- 13.Mori L, Bellini A, Stacey MA, Schmidt M, Mattoli S. Fibrocytes contribute to the myofibroblast population in wounded skin and originate from the bone marrow. Exp Cell Res. 2005;304:81–90. doi: 10.1016/j.yexcr.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 14.Wang JF, Jiao H, Stewart TL, Shankowsky HA, Scott PG, Tredget EE. Fibrocytes from burn patients regulate the activities of fibroblasts. Wound Repair Regen. 2007;15:113–21. doi: 10.1111/j.1524-475X.2006.00192.x. [DOI] [PubMed] [Google Scholar]
- 15.Steel DM, Whitehead AS. The major acute phase reactants: C-reactive protein, serum amyloid P component and serum amyloid A protein. Immunol Today. 1994;15:81–8. doi: 10.1016/0167-5699(94)90138-4. [DOI] [PubMed] [Google Scholar]
- 16.Pepys MB, Booth DR, Hutchinson WL, Gallimore JR, Collins PM, Hohenester E. Amyloid P component. A critical review Amyloid. 1997;4:274–95. [Google Scholar]
- 17.Hutchinson WL, Hohenester E, Pepys MB. Human serum amyloid P component is a single uncomplexed pentamer in whole serum. Mol Med. 2000;6:482–93. [PMC free article] [PubMed] [Google Scholar]
- 18.Mold C, Gresham H, Du Clos T. Serum amyloid P component and C-reactive protein mediate phagocytosis through murine Fc gamma Rs. J Immunol. 2001;166:1200–5. doi: 10.4049/jimmunol.166.2.1200. [DOI] [PubMed] [Google Scholar]
- 19.Bharadwaj D, Mold C, Markham E, Du Clos TW. Serum amyloid P component binds to Fc gamma receptors and opsonizes particles for phagocytosis. J Immunol. 2001;166:6735–41. doi: 10.4049/jimmunol.166.11.6735. [DOI] [PubMed] [Google Scholar]
- 20.Mortensen RF, Zhong W. Regulation of phagocytic leukocyte activities by C-reactive protein. J Leukoc Biol. 2000;67:495–500. doi: 10.1002/jlb.67.4.495. [DOI] [PubMed] [Google Scholar]
- 21.Araki C. Structure of the agarose constituent of agar-agar. Bull Chem Soc Jpn. 1956;29:543–5. [Google Scholar]
- 22.Araki CKA. Studies on the chemical constitution of agaragar: XX. Isolation of a tetrasaccharide by enzymatic hydrolysis of agar–agar. Bull Chem Soc Jpn. 1957;30:287–93. [Google Scholar]
- 23.Duckworth M, Yaphe W. The structure of Agar. I. Fractionation of a complex mixture of polysaccharides. Carbohydr Res. 1971;16:189–97. [Google Scholar]
- 24.Duckworth M, Yaphe W. The structure of Agar. III. Pyruvic acid, a common feature of agars from different agarophytes. Carbohydr Res. 1971;16:446–8. [Google Scholar]
- 25.Pepys MB, Dash AC. Isolation of amyloid P component (protein AP) from normal serum as a calcium-dependent binding protein. Lancet. 1977;1:1029–31. doi: 10.1016/s0140-6736(77)91260-0. [DOI] [PubMed] [Google Scholar]
- 26.Pepys MB, Dash AC, Fletcher TC, Richardson N, Munn EA, Feinstein A. Analogues in other mammals and in fish of human plasma proteins, C-reactive protein and amyloid P component. Nature. 1978;273:168–70. doi: 10.1038/273168a0. [DOI] [PubMed] [Google Scholar]
- 27.Hind CR, Collins PM, Renn D, Cook RB, Caspi D, Baltz ML, Pepys MB. Binding specificity of serum amyloid P component for the pyruvate acetal of galactose. J Exp Med. 1984;159:1058–69. doi: 10.1084/jem.159.4.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lehnhardt M, Jafari HJ, Druecke D, Steinstraesser L, Steinau HU, Klatte W, Schwake R, Homann HH. A qualitative and quantitative analysis of protein loss in human burn wounds. Burns. 2005;31:159–67. doi: 10.1016/j.burns.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 29.Nelson SR, Tennent GA, Sethi D, Gower PE, Ballardie FW, Amatayakul-Chantler S, Pepys MB. Serum amyloid P component in chronic renal failure and dialysis. Clin Chim Acta. 1991;200:191–9. doi: 10.1016/0009-8981(91)90090-y. [DOI] [PubMed] [Google Scholar]
- 30.Naik-Mathuria B, Pilling D, Crawford JR, Gay AN, Smith CW, Gomer RH, Olutoye OO. Serum amyloid P inhibits dermal wound healing. Wound Repair Regen. 2008;16:266–73. doi: 10.1111/j.1524-475X.2008.00366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eaglstein WH, Davis SC, Mehle AL, Mertz PM. Optimal use of an occlusive dressing to enhance healing. Effect of delayed application and early removal on wound healing. Arch Dermatol. 1988;124:392–5. [PubMed] [Google Scholar]
- 32.Pilling D, Roife D, Wang M, Ronkainen SD, Crawford JR, Travis EL, Gomer RH. Reduction of bleomycin-induced pulmonary fibrosis by serum amyloid P. J Immunol. 2007;179:4035–44. doi: 10.4049/jimmunol.179.6.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sullivan TP, Eaglstein WH, Davis SC, Mertz P. The pig as a model for human wound healing. Wound Repair Regen. 2001;9:66–76. doi: 10.1046/j.1524-475x.2001.00066.x. [DOI] [PubMed] [Google Scholar]
- 34.Urbanyi Z, Medzihradszky D. Rapid method to isolate serum amyloid P component from human plasma. Characterization of the isolated protein. J Chromatogr. 1992;578:130–3. doi: 10.1016/0378-4347(92)80235-i. [DOI] [PubMed] [Google Scholar]
- 35.Eaglstein WH, Mertz PM. New methods for assessing epidermal wound healing: the effects of triamcinolone acetonide and polyethylene film occlusion. J Invest Dermatol. 1978;71:382–4. doi: 10.1111/1523-1747.ep12556814. [DOI] [PubMed] [Google Scholar]
- 36.Sauder DN, Kilian PL, McLane JA, Quick TW, Jakubovic H, Davis SC, Eaglstein WH, Mertz PM. Interleukin-1 enhances epidermal wound healing. Lymphokine Res. 1990;9:465–73. [PubMed] [Google Scholar]
- 37.Kaiser MR, Davis SC, Mertz BA. Effect of ultraviolet radiation-induced inflammation on epidermal wound healing. Wound Repair Regen. 1995;3:311–5. doi: 10.1046/j.1524-475X.1995.30311.x. [DOI] [PubMed] [Google Scholar]
- 38.Davis SC, Eaglstein WH, Cazzaniga AL, Mertz PM. An octyl-2-cyanoacrylate formulation speeds healing of partial-thickness wounds. Dermatol Surg. 2001;27:783–8. doi: 10.1046/j.1524-4725.2001.00322.x. [DOI] [PubMed] [Google Scholar]
- 39.Bártek J, Vojtsek B, Stasková Z, Bártková J, Kerekés Z, Rejthar A, Kovarík J. A series of 14 new monoclonal antibodies to keratins: characterization and value in diagnostic histopathology. J Pathol. 1991;164:215–24. doi: 10.1002/path.1711640306. [DOI] [PubMed] [Google Scholar]
- 40.Bijl M, Bootsma H, Van Der Geld Y, Limburg PC, Kallenberg CG, Van Rijswijk MH. Serum amyloid P component levels are not decreased in patients with systemic lupus erythematosus and do not rise during an acute phase reaction. Ann Rheum Dis. 2004;63:831–5. doi: 10.1136/ard.2002.004796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rubio N, Sharp PM, Rits M, Zahedi K, Whitehead AS. Structure, expression, and evolution of guinea pig serum amyloid P component and C-reactive protein. J Biochem (Tokyo) 1993;113:277–84. doi: 10.1093/oxfordjournals.jbchem.a124039. [DOI] [PubMed] [Google Scholar]