Abstract
A reversed-phase high-performance liquid chromatographic technique was developed to separate cadmium–phytochelatin complexes (Cd-PC2, Cd-PC3, and Cd-PC4) of interest in the plant Arapidopsis thaliana. High-performance liquid chromatography (HPLC) was coupled to an inductively coupled plasma mass spectrometric (ICP-MS) system with some modification to the interface. This was done in order to sustain the plasma with optimum sensitivity for cadmium detection in the presence of the high methanol loads used in the gradient elution of the reversed-phase separation. The detection limits were found to be 91.8 ng l−1, 77.2 ng l−1 and 49.2 ng l−1 for Cd-PC2, Cd-PC3, and Cd-PC4 respectively. The regression coefficients (r2) for Cd-PC2 to Cd-PC4 detection ranged from 0.998 to 0.999. The method was then used to investigate the occurrence and effect of cadmium–phytochelatin complexes in wild-type Arabidopsis and a phytochelatin-deficient mutant cad1-3 that had been genetically modified to ectopically express the wheat TaPCS1 phytochelatin synthase enzyme. The primary complex found in both wild-type and transgenic plants was Cd-PC2. In both lines, higher levels of Cd-PC2 were found in shoots than in roots, showing that phytochelatin synthases contribute to the accumulation of cadmium in shoots, in the Cd-PC2 form. Genetic modification did, however, impact the overall accumulation of Cd. Transgenic plants contained almost two times more cadmium in the form of Cd-PC2 in their roots than did the corresponding wild-type plants. Similarly, the shoot samples of the modified species also contained more (by 1.6 times) cadmium in the form of Cd-PC2 than the wild type. The enhanced role of PC2 in the transgenic Arabidopsis correlates with data showing long-distance transport of Cd in transgenic plants. Targeted transgenic expression of non-native phytochelatin synthases may contribute to improving the efficiency of plants for phytoremediation.
Keywords: Phytoremediation, HPLC-ICP-MS, Arabidopsis thaliana, Genetic modification, Cadmium
1. Introduction
Phytochelatins (PCs) are a class of polydispersive, cysteine-rich, thiol-reactive oligopeptides having the general formula (γ-GluCys)nGly, where n can range from 2 to 11. They are enzymatically biosynthesized in plants as a defense mechanism to cope with the stress of toxic heavy metal ions such as Cd2+, Pb2+, Ag+, and Hg2+,[1] as well as metalloid species such as Sb3+, AsO4−, and SeO32−. Originally, biosynthesis of PCs was thought to be activated by the binding of heavy metal ions to the enzyme PC synthase, a heavy metal-activated enzyme, and terminated by sequestration of heavy metal ions by the PCs [2,3]. However, recent findings show that heavy metal/glutathione or heavy metal/PC complexes can serve as co-substrates for catalysis via a substituted enzyme mechanism [2,4]. Despite the details of the biosynthesis mechanism, it is generally accepted that phytochelatins play an essential role in detoxification and homeostatis of heavy metals in plants [5].
Cadmium is a heavy metal widely released to the environment by power stations, heating systems, metal working industries, waste incinerators, urban traffic, cement factories, phosphate fertilizer factories and other sources [6]. Since cadmium is a relatively immobile element (except in acidic soil), it can accumulate in soil and become dangerous to living systems. Though its toxicity is classified as intermediate, high to very high cadmium concentrations can be carcinogenic, mutagenic, and teratogenic [7]. When plants are exposed to cadmium in the environment, phytochelatin–Cd complexes are formed. Since metals are often sequestered in plant vacuoles, it has generally been presumed that Cd complexation with a phytochelatin aided in its movement across the vascular membrane. More recent studies have shown that in addition to vacuolar sequestration, phytochelations also undergo long-distance root-to-shoot transport in plants [8]. An improved grafting technique for mature Arabidopsis plants demonstrates long-distance root-to-shoot transport of phytochelatins in Arabidopsis [8,9]. This function may increase a plant's potential to assist in the remediation of metal-contaminated soil. While metal uptake by plants is a widespread phenomenon, much of the absorbed metal usually remains in the root. For efficient soil remediation, phytoextraction must be coupled to translocation. Metals within aerial tissues are more readily accessed and removed.
Genetic modification of organisms to express desirable traits – for a variety of purposes – has become an area of intense interest. For phytoremediation of metals, focus has been on characteristics that allow some species to hyperaccumulate, transport, or transform inorganic contaminants [10,11]. Transgenic expression of a wheat cDNA, TaPCS1 in Saccharomyces cerevisiae, results in a dramatic increase in cadmium tolerance in this species [12]. The cad1-3 mutant is a recessive, loss-of-function mutation in the Arabidopsis AtPCS1 gene and shows no detectable PC levels under Cd2+ stress [13]. In studying cadmium tolerance in genetically modified A. thaliana, Gong et al. showed that both root-specific and ectopic expression (expression of a gene in an abnormal place in an organism) of the TaPCS1 gene complements the cadmium-sensitive phenotype of mature cad1-3 plants [9]. PCS genes were isolated in Arabidopsis and wheat and shown to encode the enzyme phytochelatin synthases (PCS) that catalyze the final step in the biosynthesis of phytochelatins [12]. Compared to the wild type, transgenic plants expressing the wheat TaPCS1 phytochelatin synthase exhibited increased cadmium accumulation in rosette leaves. This was attributed to enhanced long-distance root-to-shoot cadmium transport. However, genetic modification did not increase PC levels significantly compared with those in the wild type. Therefore, to better understand if the long-distance transport of cadmium is PC-mediated or not, a direct analysis of cadmium–phytochelatin complexes in roots and shoots is necessary.
Most of the analytical techniques developed to date for cadmium–phytochelatin speciation use a fractionation scheme mainly with size exclusion chromatography (SEC) followed by either offline or online detection of cadmium by atomic absorption spectrometry (AAS), inductively coupled plasma atomic emission spectrometry (ICP-AES), or ICP-MS [12,13-21]. Some have also employed electrospray mass spectrometry (ESI-MS) for characterization [22-24]. However, the current separation schemes often yield inadequate resolution to separate individual cadmium–phytochelatin complexes. The cadmium-containing fractions are therefore collected and the phytochelatins are further separated by a reversed-phase high-performance liquid chromatography (RP-HPLC) with pre-column or post-column derivatization using UV–vis or fluorescence detection. Loreti et al. recently showed this second separation coupled to ICP-MS [25]. The separation conditions used in the RP-HPLC, as well as the derivatization protocol used in these techniques, require an acidification step wherein the cadmium–phytochelatin complexes are dissociated and are rather used to characterize the apo-phytochelatins.
An SEC-ICP-MS technique developed by Vacchina et al. showed, for the first time, the separation of individual cadmium–phytochelatin standards with satisfactory figures of merit and was applied to cadmium–phytochelatin speciation in plant tissue [26]. However, the residual anionic charges on the stationary phase of the size-exclusion column compete for the metal ions from the cadmium–phytochelatin complexes and they are irreversibly adsorbed onto the stationary phase. The authors had to undertake a rigorous and time-consuming (>1 h) column-conditioning protocol between each injection. Furthermore, care had to be taken to avoid traces of metals in the conditioning solution to prevent their accumulation at the column head during the conditioning phase.
The objective of the present study is to develop an ion-pair reversed-phase high-performance liquid chromatographic (IP-RP-HPLC) technique for separating cadmium–phytochelatin species and detecting them by inductively coupled plasma mass spectrometry for cadmium-specific detection. The high efficiency and resolution capability of RP-HPLC combined with the excellent sensitivity and low detection capability of ICP-MS in cadmium-selective detection facilitate the speciation of cadmium–phytochelatin complexes and accelerate the investigation of long-distance root-to-shoot transport of cadmium mediated by phytochelatins or other cadmium chaperones in A. thaliana.
2. Experimental
2.1. Reagents and standards
Deionized water (18MΩ2cm) was prepared by passing doubly distilled water through a NanoPure treatment system (Barnstead, Boston, MA, USA) and was used in all standards and buffer preparation. Commercial chemicals were of analytical reagent grade and were used without further purification. Phytochelatin standards, (γ-GluCys)2Gly (PC2), (γ-GluCys)3Gly (PC3), and (γ-GluCys)4Gly (PC4), were synthesized and purchased from New England Peptides (Gardner, MA, USA). The purity of PC standards were >95% (according to the manufacturer's certificates of analysis) and were also characterized by mass spectrometric methods. Ammonium acetate, tris(hydroxymethyl)-aminomethane, tetraethy-lammonium hydroxide, and tetrabutylammonium hydroxides were purchased from Sigma–Aldrich (St. Louis, MO, USA).
2.2. HPLC conditions
The Agilent 1100 liquid chromatograph was equipped with the following: a binary HPLC pump, an autosampler, a vacuum degasser system, a thermostated column compartment and a diode array detector. The HPLC system was connected through a remote cable that allowed the simultaneous start of the chromatographic run on both instruments. A reversed-phase C18 column (Vydac 218TP, 250 mm × 4.6 mm, 5 μm, 300 Å, Grace Vydac, Hesperia, CA, USA) was used for separation of the cadmium–phytochelatin complexes. The optimized gradient elution used was: 0–5 min 0% mobile phase B, 5–6 min 20% mobile phase B, 6–50 min 50% mobile phase B, 50–60 min 50% mobile phase B, and 60–65 min 0% mobile phase B, where, mobile phase A is 20 mM ammonium acetate/acetic acid buffer, 0.04% tetrabutylammonium hydroxide (pH = 7.3), and solvent B is 20 mM ammonium acetate/acetic acid buffer, 0.04% tetrabutylammonium hydroxide, 80% methanol, pH 7.3. The details of the HPLC separation conditions are given in Table 1.
Table 1.
Instrumental operating conditions for HPLC and ICP-MS
| HPLC Parameters | |
| Column: | VYDAC® 218TP, 250 mm × 4.6 mm × 5 μm, 300 Å |
| Mobile phase: | A: 20 mM ammonium acetate/acetic acid buffer, 0.04% tetrabutylammonium hydroxide, pH = 7.3 |
| B: 20 mM ammonium acetate/acetic acid buffer, 0.04% tetrabutylammonium hydroxide, 80% methanol, pH 7.3 | |
| Flow rate: | 1.0 ml min−1 |
| Injection volume: | 50 μl |
|
| |
| ICP-MS Parameters | |
| Forward power: | 1500 W |
| Plasma gas flow rate: | 15.0 l min−1 |
| Carrier gas flow rate: | 0.85 l min−1 |
| Optional gas (Oxygen) flow rate: | 5 % |
| Sampling depth: | 7.5 mm |
| Sampling and Skimmer cones: | Platinum |
| Skimmer base: | Brass |
| Dwell time: | 0.1 s per isotope |
| Isotopes monitored: | 111Cd, and 114Cd |
| Nebulizer: | Micromist |
| Spray chamber: | Scott double-pass with a Peltier cooling device |
2.3. ICP-MS procedures
An Agilent 7500ce ICP-MS (Agilent Technologies, Tokyo, Japan) was employed for cadmium-specific detection; this instrument is equipped with an octapole ion guide operated in the rf only mode. The instrument operating conditions are shown in Table 1. 111Cd+ and 114Cd + ions were monitored as the element-specific signal for cadmium. A platinum shield plate and bonnet, also known as Agilent's Shield Torch System, was used. This system is comprised of a grounded metal plate, which lies between the plasma rf load coil and the torch and has the effect of removing the capacitive coupling between them to effect a narrow ion energy spread through the mass spectrometer interface.
2.4. Coupling of HPLC to ICP-MS: interface modification
ICP-MS instrument fittings such as sample uptake and drain tubing, connector and spray chamber O-rings were replaced with organic solvent resistant substitutes constructed of PTFE material. The plasma-torch used was of high-purity and narrow I.D. (1.5 mm) quartz. Platinum sampler and skimmer cones were employed in order to withstand the high organic solvent load. A peltier cooling device was used to cool the spray chamber to −5 °C to reduce the vapor pressure of volatile solvents. Oxygen for carbon decomposition is provided by addition of a small percentage of oxygen (as an optional gas) directly into the Ar carrier gas through a T-connector before the torch. A default flow of 5% oxygen was added to the carrier gas flow through the T-piece connector and the organic solvent is aspirated through the nebulizer. The oxygen flow was reduced until a buildup of carbon on the sampling cone was observed. The oxygen flow was then increased until the carbon deposits were decomposed and the green C2 emission “tongue” visible in the central channel of the plasma was seen to stop well before the sample cone orifice [26].
2.5. Plant culturing and cadmium exposure
Seeds of a wild-type A. thaliana and transgenic seeds of the cad1-3 mutant expressing the wheat TaPCS1 cDNA were used in the present study. The transgenic Arabidopsis cad1-3 mutant is disrupted in the native Arabidopsis phytochelatin synthase gene AtPCS1 [13] and was modified to express the wheat phytochelatin synthases (PCS) under the control of the ectopic cauliflower mosaic virus 35S promoter. Phytochelatin synthases catalyze the final step in the biosynthesis of phytochelatins. Transgenic cad1-3 ectopically expressing TaPCS1 has been shown to exhibit enhanced cadmium accumulation in leaves compared to wild-type plants [9]. For this study, both the transgenic and wild-type seeds were first germinated on agar in conical centrifuge tubes (one seed per tube) that were placed in a water bath to maintain moisture. After germination, plants were grown hydroponically in 25% Hoagland's solution and maintained in a controlled growth room (15/20 °C, 8 h dark/16h light, 520 lux) for 4 weeks. Following this, five plants (of each type) were exposed to a 10 μM solution of Cd2+ (prepared from CdCl2 salt) for a period of 4 days. Five control plants (grown in unamended Hoagland's solution) were also established for comparison. Following the exposure period, all plants were harvested and treated as follows.
2.6. Sample and cadmium–phytochelatin standards preparation procedures
At harvest, both the wild-type and the transgenic plants were rinsed with distilled deionized water to remove cadmium from the cell walls, separated into roots and shoots, frozen, and lyophilized. Samples (50 mg) of freeze-dried material were frozen in liquid nitrogen (−196 °C) in order to break the cell walls and then ground to a homogeneous mixture using 20 mg quartz sand and a mortar and pestle. The ground homogeneous samples were transferred to small polyethylene Ziploc bags and were stored at −20 °C until further treatment. The water-soluble cadmium complexes were extracted from the homogenized sample using 1 ml portions of 10 mM Tris–HCl (pH 7.5) followed by ultrasonication for 1 h [8]. After extraction the solution was ultra-centrifuged (Beckman Coulter, Fullerton, CA, USA) for 30 min at 10 000 × g at 4 °C and filtered through a 0.45 μm syringe filter. The filtrates were injected on the HPLC column with 10 times dilution using mobile phase solvent A. Cadmium–phytochelatin complexes (Cd-PC2, Cd-PC3, and Cd-PC4) were used as standards for HPLC-ICP-MS method development and were prepared following the method of Vacchina et al. [26].
3. Results and discussion
3.1. ICP-MS interface modification
In order to sustain the plasma with optimum analyte sensitivity at the high organic solvent loading typical for reversed-phase chromatographic separation, three major modifications were performed. These included the use of solvent resistant hardware, control of vapor pressure and removal of organic carbon. The solvent-resistant hardware used in the modification is described in the experimental section. As the high vapor pressure of organic solvent, even at room temperature, can disrupt or even extinguish the plasma, it is essential to control the vapor pressure by cooling the spray chamber at the point of sample aerosol generation. The presence of high level of organic solvent in the sample aerosol can lead to deposition of carbon (soot) on the sampling cone resulting in clogging of the cone orifice and reduction in sensitivity. To prevent carbon deposition, the carbon in the sample undergoes reaction with oxygen to form CO2. Oxygen at 5% of the total Ar carrier flow was found to be optimal to decompose the organic matrix.
To optimize the analyte sensitivity in ICP-MS in the presence of high organic solvent, a 1 μg l−1 solution of Li, Y, Ce, Tl, and Co in 20 mM ammonium acetate, 0.04% tetrabutylammonium hydroxide, 50% methanol (pH 7.3) was used as a tuning solution. In the organic mode, the carrier gas flow had to be lowered for optimum signal intensity and was found to be at 0.85 l min−1 in comparison to 1.24 l min−1 in the absence of any organic modifier. The ICP-MS conditions were finally optimized using a 1 μg l−1 solution of Cd2+ in 20 mM ammonium acetate, 0.04% tetrabutylammonium hydroxide, and 50% methanol (pH 7.3). The optimized ICP-MS conditions are outlined in Table 1.
3.2. HPLC-ICP-MS method development
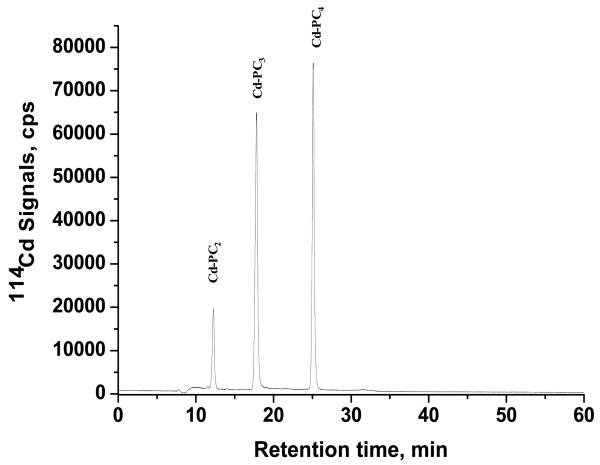
Cadmium–phytochelatin (Cd-PC) complexes are strongly hydrophilic and anionic in nature and thus will not be retained in a reversed-phase column as such, even in the presence of organic modifiers [26]. Moreover, trifluoroacetic acid, acetic acid or formic acid (at concentrations ≤0.1%) used in the mobile phase in a typical reversed-phase separation can dissociate the Cd-PC complexes as they are known to be unstable at lower pH values. Furthermore, the physiological pH within the cytoplasm of higher plant cells where the Cd-PC complexes are bio-synthesized is ~7.3. From pH distribution of the fractionally ionized forms of PC2 calculated from the micro-constants, Dorcak and Krezel showed that, at this pH range, the ligand practically exists as a single isoform out of 15 possible species [27]. Based on these considerations, the pH of the mobile phase was established to be 7.3. Both tris (30 mM) and ammonium acetate (20 mM) were considered as buffering agents to achieve the pH; ammonium acetate was ultimately chosen due to its volatile nature and lower background metal concentrations, which is more suitable for coupling to ICP-MS. The pH was adjusted with concentrated acetic acid. A cationic ion pair agent (0.04%) was used in the mobile phase to neutralize the negative charges on the Cd-PC complexes, followed by their retention on the reversed-phase column by the ion pair reversed-phase mechanism. Three cationic ion pair agents (tetramethylammonium hydroxide, tetraethylammonium hydroxide, and tetrabutylammonium hydroxide) of increasing cation size were evaluated for the most favorable results with respect to two parameters: (1) neutralization of the solute charge and (2) achievement of adequate hydrophobicity (and subsequent retention) of the ion pairs formed. The reversed-phase HPLC separation conditions are outlined in Table 1. Both tetramethylammonium hydroxide and tetraethylammonium hydroxide as ion pair agents did not provide adequate resolution for the separation of Cd-PC complexes (data not shown). However, with tetraethylammonium hydroxide, there appears to be an increase in interaction of the Cd-PC complexes with the stationary phase. As shown in Fig. 1, with tetrabutylammonium hydroxide, satisfactory separation was achieved for the standard mixture of three Cd-PC complexes and this compound was used as the ion pair agent for the subsequent analyses. Quantitation was performed using the most abundant 114Cd isotope. However, both 111Cd and 114Cd signals were monitored in order to check the ratio of the two signal intensities (compared to the ratio of their natural abundance) to ensure that none of the signals were artificially represented by isobaric or polyatomic interferences.
Fig. 1.
RP-HPLC-ICP-MS chromatogram for the separation of standard mixture of Cd-PC complexes; ion pair reagent: tetrabutylammonium hydroxide. A 50 μl of standard mixture of 3.26 μM Cd-PC2 (as PC2), 3.51 μM Cd-PC3 (as PC3), and 4.48 μM Cd-PC4 (as PC4) was used.
3.3. Analytical figures of merit
Cadmium–phytochelatin complexes (Cd-PC2, Cd-PC3, and Cd-PC4) were used as standards to prepare calibration curves for each of the complexes and ranged from 0.7 to 65.0 μM for Cd-PC2 (as PC2), 0.9 to 89.0 μM for Cd-PC3 (as PC3), and 0.7 to 70.0 μM for Cd-PC4 (as PC4). The regression coefficients were >0.998 for all the three complexes. The detection limits were 91.8 ng l−1, 77.2 ng l−1, and 49.2 ng l−1 for Cd-PC2, Cd-PC3, and Cd-PC4, respectively. Detection limits were calculated based on three times the standard deviation of seven replicates of the blank mobile-phase buffer solvent A (under the same separation conditions as standards/samples) divided by the slope of the calibration curve (IUPAC). Percent extraction efficiency for sample preparation and separation technique (column recovery) was determined by comparison of Cd present in chromatographed extracts versus that present in total Cd analysis of the same extract and was found to range from 84 to 90%. The combined recovery of these two parameters demonstrated results to be highly accurate. Reproducibility was evaluated for both signal and retention time by the analysis of seven replicate mid-level standards. The analytical figures of merit are summarized in Table 2.
Table 2.
Analytical figures of merit
| Figures of Merit | Cd-PC2 | Cd-PC3 | Cd-PC4 |
|---|---|---|---|
| Regression coefficient (r2) | 0.999 | 0.998 | 0.999 |
| LOD (concentration, n = 7) | 91.8 ng l−1 | 77.2 ng l−1 | 49.2 ng l−1 |
| LOD (amount, n = 7) | 4.6 pg | 3.9 pg | 2.5 pg |
| RSD, retentions time, n = 7 | 1.3 % | 2.1 % | 0.4 % |
| RSD, signal, n = 7 | 2.9 % | 2.4 % | 3.2 % |
| Column recovery | 90 % | 88 % | 84 % |
3.4. Analysis of Arabidopsis thaliana tissues
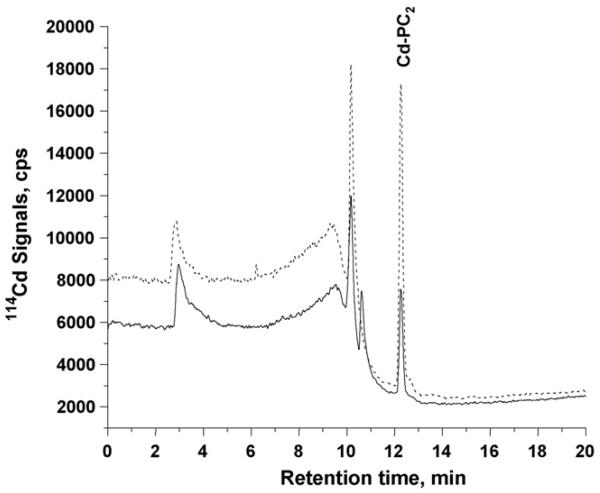
The presence of Cd-PC complexes in the Tris–HCl extracts of Arabidopsis roots and shoots were identified by retention times matching to those of the Cd-PC standards. Further, an aliquot of the standard was added to each type (root and shoot) of the sample extracts to ensure that the plant matrix did not cause a shift in retention time. Control plants did not show the presence of Cd–phytochelatins. With respect to the Cd-supplemented plants and as shown in Fig. 2, Cd-PC2 was present in the roots of wild-type and genetically modified A. thaliana plants. Based on the area counts under the peaks, the roots of genetically modified plants accumulated about two times more Cd-PC2 than the wild type; however, no Cd-PC3 or Cd-PC4 were detected. In addition to Cd-PC2, roots of the wild-type plants had two unidentified species at retention times of 10.15 min and 10.62 min, respectively. The species at 10.15 min was present in both plant types, but was more concentrated (1.9×) in transgenic plants than in the wild type. Peak areas were calculated by baseline-to-baseline integration as performed by the Agilent Chemstation software. In addition to the organically bound species, what is assumed to be inorganic cadmium, eluting at the void volume of the column (small tailing peak at 2.85 min), was also found in the root samples of both plant types.
Fig. 2.
RP-HPLC-ICP-MS chromatograms for the root samples of Arabidopsis thaliana plants; the solid line represents wild-type species, the dashed line represents genetically modified species. A 50 μl of 10 times diluted (using mobile phase solvent A) extract of the root sample was used.
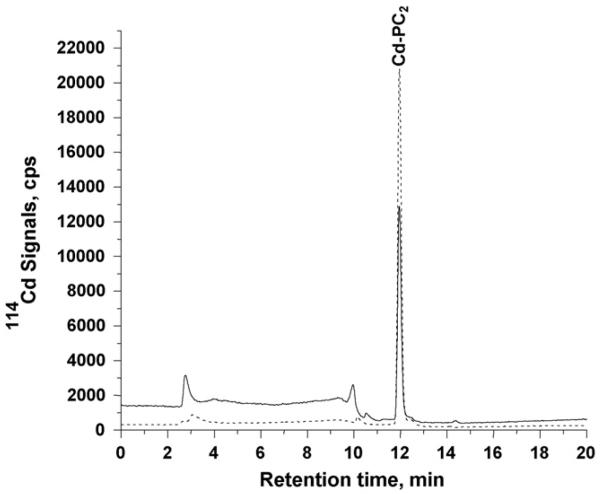
The HPLC-ICP-MS chromatograms for the Tris–HCl extract of the shoot tissues are shown in Fig. 3. The major species found in the shoot of both plant types was also Cd-PC2. Similar to the root, the shoots of transgenic cad1-3 35S::TaPCS1 plants accumulated more cadmium as Cd-PC2 (about 1.6 times more).
Fig. 3.
RP-HPLC-ICP-MS chromatograms for the shoot samples of Arabidopsis thaliana plants; solid line represents wild-type species, dashed line represents genetically modified species. A 50 μl of 10 times diluted (using mobile phase solvent A) extract of the shoot sample was used.
Cadmium accumulation as cadmium–phytochelatin complexes was greater in both the root and the shoot tissues of the transgenic plants as compared to the wild type. More Cd-PC2 was found in the shoots than in the roots. These data suggest that phytochelatins contribute to accumulation of cadmium in aerial parts of the plants. Moreover, genetic modification increased the accumulation of cadmium in shoots as Cd-PC2.
A previous study using these transgenic lines showed enhanced accumulation of Cd in shoots of cad1-3 35S:TaPCS1 plants [9], consistent with the present analyses, which showCd is complexed to PC2. This previous study, however, showed similar cadmium accumulation levels in roots of wild-type and cad1-3 35S::TaPCS1 plants, which can be due to different growth conditions used in the two studies. Note that in two other studies, transgenic overexpression of the native Arabidopsis AtPCS1 cDNA in wild-type Arabidopsis resulted in cadmium hypersensitivity with respect to wild-type controls [28]. Two main differences exist for these transgenic plants compared to those analyzed in the present study. Here we used the cad1-3 mutant rather than wild-type plants to express the non-native wheat TaPCS1 cDNA rather than the native Arabidopsis AtPCS1 cDNA. Several reasons for the differential effects can be considered, including that expression of a non-native wheat TaPCS1 may avoid native protein complex (down) regulation, as previously discussed [9] and/or the present conditions may avoid negative feedback regulation of the glutathione PC biosynthesis pathway, as shown for the histidine pathway in Arabidospsis [29].
Another possibility is that overexpression of the native AtPCS1 protein [28] may cause deregulation of the glutathione PC biosynthesis pathway, or reduction of glutathione levels under stress such that these plants are more susceptible to cadmium-induced reactive oxygen (e) species-induced stress. Interestingly, in one of these studies ACT2:AtPCS1 plants were more resistant to arsenic than wild-type plants showing an interesting difference between arsenic and cadmium resistance [28]. Further studies will be needed to determine the molecular and biochemical mechanisms underlying the regulation of the glutathione PC biosynthesis network. The present study shows that transgenic expression of a non-native wheat phytochelatin synthase in Arabidopsis enhances the accumulation of cadmium as Cd-PC2.
4. Conclusions
A reversed-phase high-performance liquid chromatographic method was developed to separate intact cadmium–phytochelatin complexes, namely, Cd-PC2, Cd-PC3, and Cd-PC4. An ion pair agent, tetrabutylammonium hydroxide, was used to neutralize the negative charges on the Cd-PC complexes at physiological pH values (7.3) followed by their retention on a C18 reversed-phase column using a water–methanol solvent gradient prepared in ammonium acetate buffer. In order to withstand the high organic solvent load, the interface between the HPLC and ICP-MS was modified by replacing the nickel sampler and skimmer cones with platinum cones. The vapor pressure of the organic solvent in the spray chamber was reduced by cooling it to −5 °C. The carbon from the high organic load of the mobile phase was reacted in the plasma by addition of 5% oxygen to the carrier gas flow through a T-connector before the torch. Cd-PC2 was found to be present in both wild-type and transgenic A. thaliana. The roots of the genetically modified plants were found to accumulate about two times more Cd-PC2 than the wild type. Similar to the root results, the shoot samples contained more cadmium as Cd-PC2 (about 1.6 times more) than the wild type. In general, in both types of the plants, more Cd-PC2 was found to be present in the shoots than in the roots, showing that transgenic expression of a wheat phytochelatin synthase cDNA in Arabidopsis aids in enhancing accumulation of cadmium and that genetic modification may be an effective means of altering plant traits to enhance phytoextraction and phytoremediation of heavy metals.
Acknowledgment
We would like to thank Dr. David Mendoza-Cozatl for his helpful comments on the manuscript.
Footnotes
Publisher's Disclaimer: This article was published in an Elsevier journal. The attached copy is furnished to the author for non-commercial research and education use, including for instruction at the author's institution, sharing with colleagues and providing to institution administration.
Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited.
References
- 1.Grill E, Winnacker EL, Zenk MH. Science. 1985;230:674. doi: 10.1126/science.230.4726.674. [DOI] [PubMed] [Google Scholar]
- 2.Spain SM, Rabenstein DL. Anal. Chem. 2003;75:3712. doi: 10.1021/ac0207426. [DOI] [PubMed] [Google Scholar]
- 3.Zenk MH. Gene. 1996;179:21. doi: 10.1016/s0378-1119(96)00422-2. [DOI] [PubMed] [Google Scholar]
- 4.Vatamaniuk OK, Mari S, Lu YP, Rea PA. J. Biol. Chem. 2000;275:31451. doi: 10.1074/jbc.M002997200. [DOI] [PubMed] [Google Scholar]
- 5.Cobbett C, Goldsbrough P. Ann. Rev. Plant Biol. 2002;53:159. doi: 10.1146/annurev.arplant.53.100301.135154. [DOI] [PubMed] [Google Scholar]
- 6.Polec-Pawlak K, Ruzik R, Abramski K, Ciurzynska M, Gawronska H. Anal. Chim. Acta. 2005;540:61. [Google Scholar]
- 7.Toppi L.S.d., Gabbrielli R. Environ. Exp. Bot. 1999;41:105. [Google Scholar]
- 8.Chen A, Komives EA, Schroeder JI. Plant Physiol. 2006;141:108. doi: 10.1104/pp.105.072637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gong JM, Lee DA, Schroeder JI. Proc. Natl. Acad. Sci. USA. 2003;100:10118. doi: 10.1073/pnas.1734072100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu YL, Pilon-Smits EAH, Jouanin L, Terry N. Plant Physiol. 1999;119:73. doi: 10.1104/pp.119.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Larsson EH, Asp H, Bornman JF. J. Exp. Bot. 2002;53:447. doi: 10.1093/jexbot/53.368.447. [DOI] [PubMed] [Google Scholar]
- 12.Clemens S, Kim EJ, Neumann D, Schroeder JI. EMBO J. 1999;18:3325. doi: 10.1093/emboj/18.12.3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ha SB, Smith AP, Howden R, Dietrich WM, Bugg S, O'Connell MJ, Goldsbrough PB, Cobbett CS. Plant Cell. 1999;11:1153. doi: 10.1105/tpc.11.6.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kubota H, Sato K, Yamada T, Maitani T. Plant Sci. 1995;106:157. [Google Scholar]
- 15.Maitani T, Kubota H, Sato K, Yamada T. Plant Physiol. 1996;110:1145. doi: 10.1104/pp.110.4.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leopold I, Gunther D. Anal. Bioanal. Chem. 1997;359:364. [Google Scholar]
- 17.Kubota H, Sato K, Yamada T, Maitani T. J. Chromatogr. A. 1998;803:315. [Google Scholar]
- 18.Jemal F, Didierjean L, Ghrir R, Ghorbal MH, Burkard G. Plant Sci. 1998;137:143. [Google Scholar]
- 19.Chassaigne H, Vacchina V, Kutchan TM, Zenk MH. Phytochemistry. 2001;56:657. doi: 10.1016/s0031-9422(00)00489-1. [DOI] [PubMed] [Google Scholar]
- 20.Scarano G, Morelli E. Biometals. 2002;15:145. doi: 10.1023/a:1015288000218. [DOI] [PubMed] [Google Scholar]
- 21.Wei Z, Wong JW, Chen D. Microchem. J. 2003;74:207. [Google Scholar]
- 22.Yen TY, Villa JA, DeWitt JG. J. Mass Spectrom. 1999;34:930. doi: 10.1002/(SICI)1096-9888(199909)34:9<930::AID-JMS853>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 23.Chekmeneva E, Diaz-Cruz JM, Arino C, Esteban M. Electroanalysis. 2007;19:310. [Google Scholar]
- 24.Navaza AP, Bayon MM, LeDuc DL, Terry N, Sanz-Medal A. J. Mass Spectrom. 2006;41:323. doi: 10.1002/jms.992. [DOI] [PubMed] [Google Scholar]
- 25.Loreti V, Toncelli D, Morelli E, Scarano G, Bettmer J. Anal. Bioanal. Chem. 2005;383:398. doi: 10.1007/s00216-005-3385-z. [DOI] [PubMed] [Google Scholar]
- 26.Vacchina V, Polec K, Szpunar J. J. Anal. Atom. Spectrom. 1999;14:1557. [Google Scholar]
- 27.Dorcak V, Krezel A. Dalton Trans. 2003;11:2253. [Google Scholar]
- 28.Li Y, Dhankher OP, Carreira L, Lee D, Chen A, Schroeder JI, Balish RS, Meagher RB. Plant Cell Physiol. 2004;45:1787. doi: 10.1093/pcp/pch202. [DOI] [PubMed] [Google Scholar]
- 29.Wycisk K, Kim EJ, Schroeder JI, Kramer U. FEBS Lett. 2004;578:128. doi: 10.1016/j.febslet.2004.10.086. [DOI] [PubMed] [Google Scholar]