Summary
Enteropathogenic Escherichia coli (EPEC) is a diarrheal pathogen that adheres to epithelial cells of the small intestine and uses a type III secretion system to inject effector proteins into host cells. EPEC infection leads to disruption of host intestinal tight junctions that are important for maintaining intestinal barrier function. This disruption is dependent on the bacterial type III secretion system, as well as the translocated effectors EspF and Map. Here we show that a third type III translocated bacterial effector protein, NleA, is also involved in tight junction disruption during EPEC infection. Using the drug Brefeldin A, we demonstrate that the effect of NleA on tight junction integrity is related to its inhibition of host cell protein trafficking through COPII dependent pathways. These results suggest that NleA's striking effect on virulence is mediated, at least in part, via its role in disruption of intestinal barrier function.
Introduction
Enteropathogenic E. coli (EPEC) is a diarrheal pathogen of major medical significance in developing countries, where it is estimated to cause 30 – 40% of all cases of infantile diarrhea. With a mortality rate of approximately 1/3, EPEC infections are estimated to kill several hundred thousand children each year (Deborah Chen and Frankel, 2005). EPEC is spread through the fecal/oral route and once ingested it adheres to epithelial cells of the small intestine. EPEC have a number of virulence-associated genes that are absent from non-pathogenic E. coli strains and have been associated with virulence in animal models and in human challenge studies. However, despite recent progress, the mechanisms leading to illness during EPEC infection are not completely characterized. Important virulence determinants of EPEC and related pathogens are the type III secretion system (TTSS), encoded within the locus for enterocyte effacement pathogenicity island (LEE) and its effector proteins which are translocated into host cells.
One important TTSS-dependent virulence strategy of EPEC is the disruption of intestinal tight junctions. Tight junctions form a belt-like structure that forms a regulated seal at the apical region between adjacent polarized cells. Tight junctions have a dual role in polarized epithelia: acting as a “gate” to selectively restrict movement across the paracellular space between cells and as a “fence” by limiting diffusion of molecules between the apical and basolateral domains of cells (reviewed in (Gruenheid and Finlay, 2003)). Tight junctions are a common target of many bacterial and viral pathogens. Whereas some pathogens bind and modify junction components directly, others exert their effects through the actin cytoskeleton, which controls the integrity of tight junctions (reviewed in (Gruenheid and Finlay, 2003)). EPEC-mediated disruption of tight junctions was first described in the mid-1990s and has since been studied in considerable detail. This disruption has been correlated with a number of events within the host cells, including a decrease in the transepithelial resistance of polarized monolayers, phosphorylation of myosin light chain and ezrin, dephosphorylation of occludin, dissociation of occludin and ZO-1 from tight junctions, and the migration of normally basolateral proteins such as β-1 integrin to the apical membrane (Spitz et al., 1995; Canil et al., 1993; Yuhan et al., 1997; Simonovic et al., 2001; Simonovic et al., 2000; Philpott et al., 1996; Muza-Moons et al., 2003). The LEE-encoded TTSS effectors EspF and Map are necessary for EPEC-mediated tight junction disruption (Dean and Kenny, 2004; McNamara et al., 2001) as is the bacterial outer membrane protein intimin, but surprisingly not its cognate receptor, the LEE-encoded TTSS effector Tir (Dean and Kenny 2004). Furthermore, two other TTSS-translocated bacterial effector proteins, EspG and EspG2 play a role in altering paracellular permeability but do so without disrupting tight junction architecture (Matsuzawa et al., 2005; Tomson et al., 2005). Despite the identification of these bacterial proteins required for the disruption and alteration of tight junctions, the relationships between them as well as their mechanisms of action are still uncharacterized.
One of the first-described non-LEE encoded effectors of the TTSS of EPEC and related pathogens is the protein NleA (non-LEE encoded effector A), also called EspI. The gene encoding NleA is absent from non-pathogenic E. coli strains and is preferentially found in LEE-containing E. coli strains that are associated with outbreaks of human disease (Coombes et al., 2008; Mundy et al., 2004a). NleA plays a critical role in disease development and transmission in the murine model for EPEC infections, Citrobacter rodentium infection of mice (Wickham et al., 2007; Gruenheid et al., 2004; Mundy et al., 2004b). We have previously shown that NleA binds and inhibits mammalian COPII through direct interaction with the COPII component Sec24 (Kim et al., 2007). COPII is a protein complex that plays an important role in protein trafficking. COPII produces vesicles at ER exit sites containing integral membrane proteins, as well as proteins destined for secretion or localization to “post-Golgi” compartments such as lysosomes. Expression of an NleA-GFP fusion protein reduces the efficiency of secretion of a reporter protein by 50%, and secretion is also ~50% inhibited in EPEC-infected cells (Kim et al., 2007). In biochemical assays, NleA inhibits in vitro cargo packaging and/or vesicle budding by COPII (Kim et al., 2007). The link between the manipulation of these pathways by NleA and NleA's role in virulence remains unclear. Here we show that NleA participates in the perturbation of intestinal tight junctions by EPEC and that its role in this is related to the disruption of cellular protein trafficking. Together, these results suggest that NleA's effect on virulence is mediated, at least in part, via its role in disruption of intestinal barrier function.
Results and Discussion
NleA is implicated in alteration of epithelial barrier function during EPEC infection
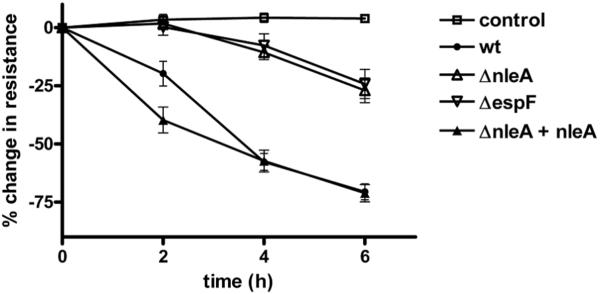
To test if NleA was involved in the disruption of epithelial barrier function during EPEC infection, T84 and Caco2 intestinal epithelial cells were grown on semi-permeable inserts at confluency until polarized and infected with wildtype EPEC, EPECΔespF, EPECΔnleA, and EPECΔnleA complemented with plasmid-encoded NleA. Epithelial barrier integrity was assessed by measuring the transepithelial resistance of the monolayers before and at various times post-infection. Figure 1 shows the combined data from 15 replicates in 5 independent experiments in T84 cells. Similar results were seen in Caco2 cells (data not shown). As expected, the transepithelial resistance of the wildtype-infected samples showed a striking and progressive decrease which was evident at 2 hours post-infection, when the cells displayed a 24% +/− 5% decrease in resistance compared to the value before infection (mean +/− SEM). The resistance decreased progressively and at 6 hours post-infection the cells displayed a 71% +/− 3% decrease in resistance compared to the value before infection (mean +/− SEM). In contrast, EPECΔnleA was severely attenuated in its ability to cause a decrease in transepithelial resistance of the infected cells. At 2 hours post-infection the resistance of EPECΔnleA–infected monolayers was not significantly different from uninfected cells (P=0.6). By 6 hours, the resistance of EPECΔnleA–infected monolayers had only decreased by 27% +/− 5%. The kinetics and extent of the decrease in transepithelial resistance of EPECΔnleA–infected monolayers were virtually identical to that seen in cells infected with an EPEC mutant lacking EspF (Figure 1: EPECΔespF), a TTSS effector that has previously been shown to be critically important for the loss of epithelial barrier function during infection (McNamara et al., 2001), indicating that NleA is as important as EspF in this process. When cells were infected with the ΔnleA strain complemented by the expression of nleA on a plasmid, the transepithelial resistance of the monolayers decreased the same amount as wildtype-infected cells, indicating that the inability of the ΔnleA strain to fully disrupt epithelial barrier integrity is specific and directly caused by the absence of nleA expression. Adherence assays were performed and indicated that EPEC wt, EPECΔnleA, and the complemented strain adhered to the monolayers equally (data not shown). Closer examination of the curves of the wildtype and complemented strain-infected monolayers reveals that in the cells infected with the ΔnleA strain complemented by the expression of nleA on a plasmid, the decrease in transepithelial resistance occurs somewhat more rapidly than in cells infected with EPEC wildtype, with a significantly lower value at 2 hours post-infection (P=0.0145). This may reflect the fact that the complemented strain over-expresses NleA, due to its expression on a multicopy number plasmid, leading to an exaggeration of the effect of NleA.
Figure 1. NleA contributes to the decrease in transepithelial resistance of polarized monolayers observed during EPEC infection.
T84 cells were polarized on semi-permeable filter inserts and then infected with the EPEC strains indicated. Transepithelial resistance was measured before infection and at 2, 4 and 6 hours post infection. Data is expressed and the % change in resistance compared with resistance at time 0. Each point represents the mean +/− the standard error of the mean from 15 biological replicates in three separate experiments. Control = uninfected cells processed in parallel. Similar results were also seen in Caco2 cells (data not shown).
NleA contributes to the disruption of tight junctions during EPEC infection
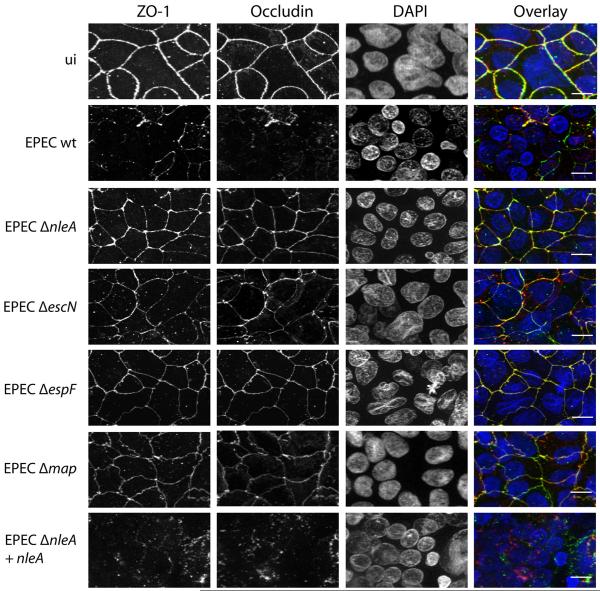
The decreases in transepithelial resistance of polarized epithelial monolayers seen during EPEC infection have been correlated with disruption of tight junctions, but other causes of decreased transepithelial resistance are possible. In order to investigate whether the effect of NleA on transepithelial resistance was due to a role in the disruption of tight junctions, localization of tight junction proteins was determined in polarized epithelial cells infected with wildtype and mutant EPEC strains. As seen in Figure 2 (top panels: ui), the tight junction proteins occludin and ZO-1 assume a typical pattern at the periphery of uninfected cells, indicating their localization at tight junctions. In the wildtype EPEC-infected samples, disruption of occludin and ZO-1 localization was seen (Figure 2: EPEC wt). In the cells infected with EPECΔnleA, ZO-1 and occludin localization was undisrupted and remained at the periphery of cells, resembling the localization in uninfected cells (Figure 2: EPECΔnleA). As expected, tight junction protein localization was also undisrupted in cells infected with an EPEC mutant defective in type III secretion (Figure 2: EPECΔescN) and in cells infected with EPECΔespF or EPECΔmap (Figure 2). Infection of cells with EPECΔnleA complemented with plasmid-encoded NleA lead to the disruption of occludin and ZO-1 localization, similar to that seen in wildtype EPEC-infected cells (Figure 2, bottom panel). These results indicate that, in addition to EspF and Map, NleA is necessary for the disruption of intestinal tight junctions by EPEC.
Figure 2. NleA is required for disruption of tight junction proteins during EPEC infection.
Caco2 cells were polarized on semi-permeable filter inserts and then infected with the EPEC strains indicated for 6 hours. Uninfected cells were processed in parallel (ui). Samples were fixed and immunostained for ZO-1 (left panel, green in the overlay), occludin (2nd panel from left, red in the overlay) and nuclei were stained with DAPI (3rd panel from left, blue in the overlay). An overlay is shown in the right panel. Scale bar = 20 micrometers.
NleA does not affect the secretion or translocation of EspF or Map
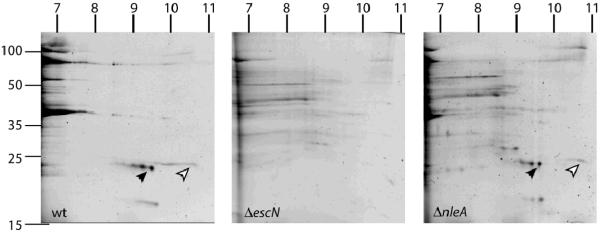
Since the TTSS effectors EspF and Map are known to be required for tight junction disruption by EPEC, we investigated whether these proteins were secreted and translocated into host cells by EPECΔnleA. In order to rule out a direct effect of NleA on EspF or Map secretion, we investigated protein secretion in EPECΔnleA. To do this, EPEC wildtype, EPECΔescN, and EPECΔnleA strains were grown in type III secretion-inducing conditions (Deng et al., 2004), and secreted proteins were assessed using 2D gel analysis and total protein staining, as we have done previously (Deng et al., 2004; Gruenheid et al., 2004). Since EspF and Map are both extremely basic proteins (pI 10.61 and 9.08 respectively), a basic range first dimension gradient was employed (pI 6–11). Based on the predicted migration and on our previous experiments mapping EHEC secreted proteins on 2D gels (Deng et al., 2004; Gruenheid et al., 2004), we identified the spots on the gel predicted to correspond to EspF and Map on the gels of wildtype and ΔnleA secreted proteins (Figure 3: EspF: Theoretical pI / mw : 10.61 / 20977.68; Map: Theoretical pI / mw : 9.08 / 22.568.69). These spots were present in equivalent abundance in both the wildtype and ΔnleA samples, and were absent from the sample prepared from the type III secretion mutant EPECΔescN (Figure 3). To confirm the identity of the proteins, the spots were excised from the ΔnleA gel and analyzed using ion trap mass spectroscopy. Each spot yielded a single match in Mascot corresponding to the expected protein (EspF: Mascot score 399; 7 unique peptides identified; 47% coverage; Map: Mascot score 548; 10 unique peptides identified; 67% coverage). EspF secretion levels were also confirmed by Western blot analysis (supplemental figure S1). Thus both EspF and Map are secreted at wildtype levels in EPECΔnleA.
Figure 3. Map and EspF are secreted at wildtype levels by EPECΔnleA.
Sypro-stained 2-dimensional gels of secreted proteins from EPEC wildtype (left panel), EPEC ΔescN (center panel), and EPECΔnleA (right panel). Migration of molecular weight markers (in kiloDaltons) is indicated to the left of the first gel. Estimated migration of the pI gradients are indicated on the top of each gel. Map and EspF are indicated by the black and white arrowheads respectively. The identity of the proteins was verified by ion trap mass spectroscopy (see text).
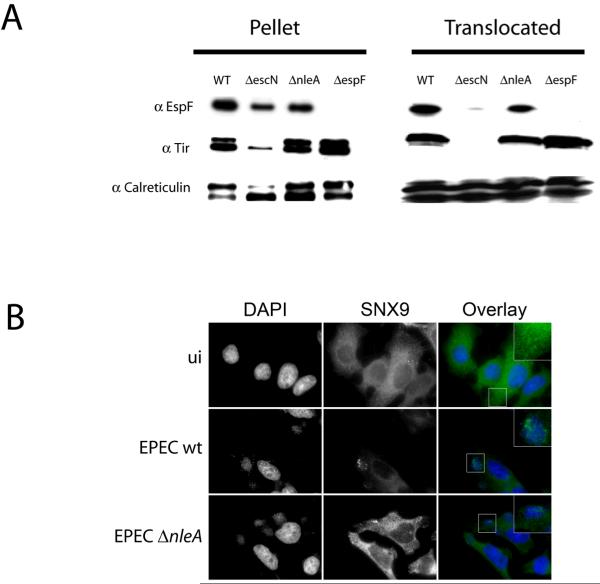
Next, we assessed whether EspF was translocated in host cells infected with EPECΔnleA. HeLa cells were infected for 3 h with EPEC wildtype, EPECΔnleA, EPECΔespF, and EPECΔescN and were fractionated using an established protocol (Figure 4A; Gruenheid et al. 2004). This separates the sample into a low speed pellet fraction containing bacteria and unbroken host cells and a supernatant fraction containing host cell cytosol and membranes and bacterial proteins that have been translocated into the host cell. Probing the fractions with anti-EspF antiserum revealed the presence of a strong EspF signal in the host cell (translocated) fractions from EPEC wildtype and EPECΔnleA-infected cells. In contrast, there was no EspF signal detected in either the bacterial or host cell fraction in cells infected with EPECΔespF, and only a faint background band of EspF present in the host cell fraction of cells infected with EPECΔescN. To confirm that the host cell fraction was not non-specifically contaminated with non-translocated bacterial proteins, fractions were probed with anti-Tir antiserum. Bacterially-expressed Tir migrates as an approximately 78 kDa protein, but is modified upon host cell translocation, leading to its migration at 90 kDa. As expected, both forms of Tir were present in the pellet fraction, but the 90 kDa form of Tir was predominant in the host cell fraction, indicating that the bacterial proteins present in these fractions represent bona fide translocated proteins only. Equal loading of fractions was assessed by blotting with anti-Calreticulin.
Figure 4. EspF is translocated and functional in cells infected with EPECΔnleA.
A. Translocation assay: HeLa cells were infected with the strains indicated and subjected to subcellular fractionation. Fractions were probed with antisera against EspF, Tir, and Calreticulin. B. HeLa cells seeded onto glass coverslips were infected for 90 minutes with wt EPEC or EPEC ΔnleA , or left uninfected (ui). Cells were stained with DAPI (left panels, blue in the overlay) and anti-SNX9 antibodies (center panels, green in the overlay). Areas demonstrating changes in SNX9 localization after wt EPEC or ΔnleA infection as compared to uninfected are enlarged in the overlay panels.
To assess whether translocated EspF was functional in EPECΔnleA-infected cells we took advantage of the fact that EspF is a multifunctional effector, with cellular effects distinct from its role in tight junction disruption. EspF binds sorting nexin 9 (SNX9) in epithelial cells, leading to the formation of SNX9-containing tubule structures (Alto et al., 2007; Marches et al., 2006). This process is unrelated to EspF's role in tight junction disruption, as EspF with a mutated SNX9-binding domain no longer binds SNX9 or induces tubule formation, but still affects tight junctions (Alto et al., 2007). SNX9 tubule formation was examined in cells infected with EPEC wildtype and EPECΔnleA strains. As shown in figure 4B, SNX9 tubule formation occured in cells infected with EPEC wildtype and EPECΔnleA, providing evidence that translocated EspF is functional in cells infected with EPECΔnleA.
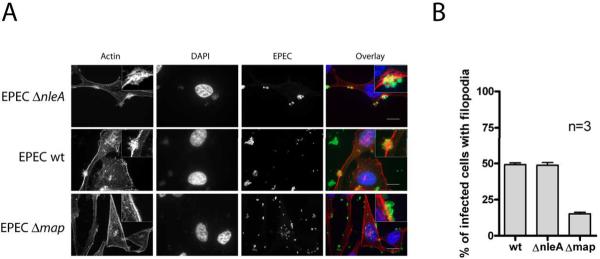
In a similar fashion, we assessed Map function by examining the formation of filopodia at the site of bacterial attachment early in infection in the presence or absence of NleA. Map induces the transient formation of actin-rich filopodia structures in cells during the first 30 minutes of infection (Kenny et al., 2002). To assess filopodia formation in the presence or absence of NleA, HeLa cells were infected for 15 minutes with EPEC wildtype, EPECΔnleA and EPECΔmap strains, followed by fixation and phalloidin staining to visualize filamentous actin (Figure 5). Filopodia were readily apparent in cells infected with EPECΔnleA and EPEC wildtype, but not in cells infected with EPECΔmap. Quantification of the percent of infected cells displaying filopodia-like actin staining in the vicinity of bacteria indicated that cells infected with EPECΔnleA displayed the same extent of filopodia formation as those infected with EPEC wildtype, providing evidence that Map is still translocated and functional in the absence of NleA.
Figure 5. NleA does not affect Map-dependent filopodia formation by EPEC.
HeLa cells were infected with EPECΔnleA (A); EPEC wildtype (B); and EPECΔmap (C.) strains for 15 minutes. Actin filopodia at the site of bacterial attachment were visualized using Alexa-568-conjugated phalloidin (left panels and red in the overlay). Bacterial and eukaryotic DNA was stained with DAPI (left center panels and blue in the overlay), and EPEC bacteria were visualized with anti-EPEC antibody followed by an Alexa 488-conjugated secondary (right center panels and green in the overlay). Scale bar = 20 micrometers.
Furthermore, to determine whether the role of NleA in tight junction disruption involved directly binding to either Map or EspF, we used yeast 2-hybrid to test for direct interactions between NleA and EspF as well as NleA and Map. Using this assay we found no evidence that NleA directly binds either Map or EspF (data not shown).
NleA and EspF mutants can be complemented in trans
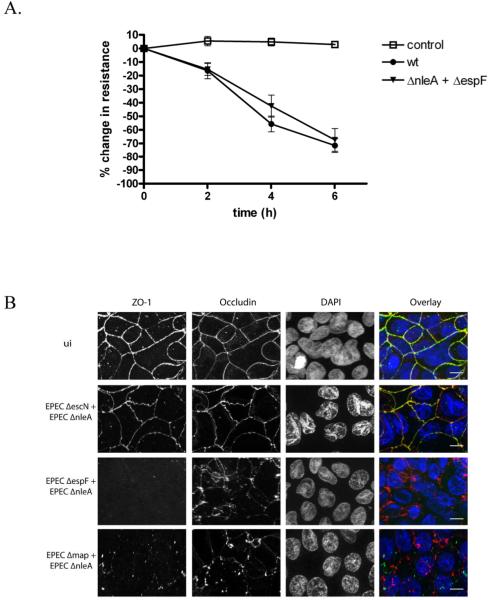
Based on the above results, we concluded that the effects of NleA on tight junctions were unlikely to be due to a direct effect of NleA on EspF or Map. We further hypothesized that if the deficiencies in these effectors were complemented in trans, tight junction disruption would occur. To test this, we simultaneously co-infected cells with EPEC ΔnleA and EPEC ΔespF strains or EPEC ΔnleA and EPEC Δmap strains. As opposed to the cells infected with either mutant alone, cells co-infected with EPEC ΔnleA and EPEC ΔespF had disrupted tight junctions (Figure 6). A similar result was observed in cells co-infected with EPEC ΔnleA and EPEC Δmap (Figure 6). As a negative control, cells were co-infected with or EPEC ΔnleA and EPEC ΔescN and in these samples cross-complementation was not observed. Together, these results provide further evidence against a non-specific effect of the ΔnleA mutation on the expression or secretion of EspF or Map. It also indicates that in a mixed infection, when NleA and the other effectors are translocated into host cells (e.g. EspF and Map by ΔnleA; NleA by ΔespF or Δmap), these effectors cooperate to disrupt tight junctions.
Figure 6. The inability of a NleA mutant to disrupt tight junctions can be complemented in trans.
A. T84 cells were polarized on semi-permeable filter inserts and then infected with the wildtype EPEC or a 1:1 mixture of EPECΔnleA and EPECΔespF. Transepithelial resistance was measured before infection and at 2, 4 and 6 hours post infection. Data is expressed and the % change in resistance compared with resistance at time 0. Each point represents the mean +/− the standard error of the mean from 9 biological replicates from 3 separate experiments. Control = uninfected cells processed in parallel. B. Caco2 cells were polarized on semi-permeable filter inserts and then infected with 1:1 mixtures of the EPEC strains indicated for 6 hours. Uninfected cells were processed in parallel (ui). Samples were fixed and immunostained for ZO-1 (left panel, green in the overlay), occludin (2nd panel from left, red in the overlay) and nuclei were stained with DAPI (3rd panel from left, blue in the overlay). An overlay is shown in the right panel. Scale bar = 20 micrometers.
The absence of tight junction disruption by EPECΔnleA can be functionally complemented by disruption of ER to Golgi trafficking
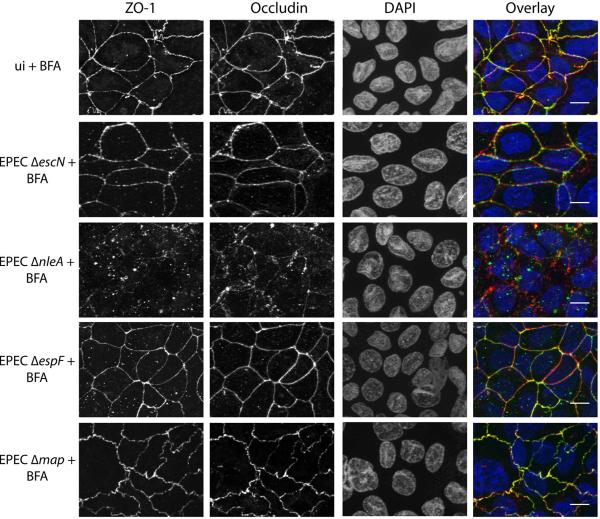
We have previously shown that NleA binds and inhibits COPII, a protein complex involved in trafficking of integral membrane proteins, proteins destined for secretion, or localization to “post-Golgi” compartments such as lysosomes (Kim et al., 2007). We hypothesized that the role of NleA in tight junction disruption might be related to its role in the inhibition of COPII-dependent protein trafficking. To test this, we examined the ability of EPECΔnleA to disrupt tight junctions in the presence of the drug Brefeldin A. Brefeldin A is a fungal metabolite that inhibits trafficking of vesicles between the ER and the Golgi, and thus functionally shuts down secretion in host cells. As shown in Figure 7, tight junctions were disrupted in cells infected with EPEC ΔnleA and simultaneously treated with Brefeldin A, indicating that Brefeldin A treatment functionally complements the absence of NleA. Notably, tight junctions were not disrupted in cells treated with Brefeldin A alone, or infected with EPEC ΔescN, EPECΔespF or EPECΔmap in the presence of Brefeldin A. This indicates that Brefeldin A treatment alone is not sufficient to disrupt tight junctions, and also that Brefeldin A treatment does not functionally complement the absence of EspF or Map. Taken together, these results support a model where tight junction disruption by EPEC requires the inhibition of COPII function by NleA as well as at least another cellular event, mediated by EspF and Map. It is tempting to speculate that EspF and Map induce the disassembly of tight junctions, whereas NleA disruption of COPII function blocks the delivery of new tight junction proteins which would otherwise repair the disassembled tight junctions. However, the direct action of EspF and Map in the dissassembly of tight junctions, as well as the direct action of NleA in blocking traffic of tight junction proteins remain to be demonstrated. Alternative hypotheses that are also consistent with the data presented here include the possibility that NleA blocks the trafficking of an as-of-yet unidentified factor which is required by host cells to maintain tight junction integrity but whose function is only critical when EspF and Map are present or which is directly required by EspF and Map in order to disrupt tight junctions. Our current findings provide a starting point for future investigations into the actions of all three bacterial effectors in tight junction disruption which should provide further insight into this complex process.
Figure 7. The absence of NleA can be functionally complemented by disruption of ER-Golgi trafficking.
Caco2 cells were polarized on semi-permeable filter inserts and then infected with the EPEC strains indicated for 6 hours in the presence of 10 micrograms per ml Brefeldin A. Uninfected cells were processed in parallel (ui). Samples were fixed and immunostained for ZO-1 (left panel, green in the overlay), occludin (2nd panel from left, red in the overlay) and nuclei were stained with DAPI (3rd panel from left, blue in the overlay). An overlay is shown in the right panel. Scale bar= 20 micrometers.
Our results indicate that disruption of tight junctions by EPEC is a complex event, requiring the concerted effort of at least three TTSS-translocated bacterial effector proteins: EspF, Map, and NleA. The observation that NleA inhibits protein trafficking through COPII-dependent pathways, which are important in numerous cellular processes, suggests that NleA may have multiple effects on host cell biology in addition to its effects on tight junctions. Support for this is seen in the mouse model of EPEC and related infections, Citrobacter rodentium infection of mice, in which bacteria lacking nleA are severely attenuated (Gruenheid et al., 2004), with a level of virulence much lower than either ΔespF or Δmap strains (Deng et al., 2004; Gruenheid et al., 2004). Future investigations into other cell biological effects of NleA should provide further insight into other roles of NleA.
Experimental Procedures
Bacterial Strains
The following strains were used in this study: EPEC wildtype E2348//69 (Levine et al., 1978); EPECΔnleA (Kim et al., 2007); EPECΔnleA complemented with plasmid-encoded NleA (Kim et al., 2007); EPECΔescN (Gauthier et al., 2003), EPECΔmap (provided by Brett Finlay), EPECΔespF (McNamara and Donnenberg, 1998).
Cell culture and EPEC infection
T84 cells were grown in a 1:1 (vol/vol) mixture of Dulbecco-Vogt modified Eagle's medium and Ham's F-12 supplemented with 6% newborn calf serum (Invitrogen). For the electrophysiological studies cells were grown to confluence on 33-cm2 collagen coated permeable supports (Transwell; Costar/Corning, Wilkes Barre, PA). Overnight bacterial cultures grown in Luria-Bertani broth with appropriate antibiotics, were diluted (1:33) in serum and antibiotic free tissue culture medium containing 0.5% mannose and grown at 37° C to mid-log growth phase. Monolayers were infected at a multiplicity of infection (MOI) of 100. For adherence assays, monolayers were washed three times after 1 hour of infection to remove the non- adherent bacteria. Cells were lysed with PBS+0.5% Triton X-100 and serial dilutions were plated on Miller's LB Agar (Research Products International Corp.).
Caco-2 cells were cultured in Alpha minimal Eagle's Medium (αMEM) supplemented with 10% (v/v) heat-inactivated fetal calf serum at 37°C in a humidified atmosphere of 5% CO2. Before confluence, Caco-2 cells were trypsinized for ≈ 20min and the resulting suspension was diluted 1:5 in culture medium. This was added to plastic translucent inserts for 24-well dishes containing a 2×106 cm2 pore density (3μm pore size; Greiner bio-one, Ultident Scientific). Monolayers were given fresh medium every 2 days and used in infection assays at 10 days post seeding.
For infection of Caco-2 monolayers, the cells were incubated overnight in serum-free αMEM with supplemented with 0.5% D(+)-mannose (Acros Organics). Overnight bacterial cultures (grown at 37°C with shaking) were diluted in αMEM supplemented with 0.5% D(+)-mannose at a 1:3 dilution of media . Bacteria were then grown (at 37°C with shaking) for ~1.5h before being added to the apical surface of Caco-2 monolayers (16.5uL of 0.45OD600 bacteria per well). Cells were infected for 6 hrs. In experiments with Brefeldin A treatment, Brefeldin A was added at a concentration of 10 micrograms per ml at the beginning of the infection.
HeLa cells were cultured in Dulbecco's minimal Eagles Medium (DMEM) supplemented with 10% (v/v) heat inactivated fetal calf serum at 37°C in a humidified atmosphere of 5% CO2. . At confluence, HeLa cells were trypsinized for 5min and the suspension was diluted 1:5 in culture medium. This was added to 24 well plates containing glass cover slips making a final cell concentration of 5×104cells/well. For infection of HeLa cells, bacteria were grown overnight at 37°C without shaking. The suspensions were then diluted 1:50 into DMEM without supplements and bacteria were pre-induced by growing for another 3hr at 37°C in a humidified atmosphere of 5% CO2 without shaking. The medium from wells of 24-well plates was then replaced with 3hr pre-induced bacteria. Cells were infected for 15mins at which point the media was removed and cells were washed with phosphate-buffered saline (PBS) and processed for immunoflurorescence (see below).
Transepithelial electrical resistance measurements
Transepithelial electrical resistance (TER) was measured before infection and at two hour intervals during infection using an epithelial Voltohmmeter(World Precision Instruments) Values were calculated to reflect Ohm's x cm2.
Immunofluoresence microscopy
Caco-2 monolayers grown on membrane filters were infected as described above. After 6hr infection cells were washed gently with PBS containing MgCl2 (0.1mg/mL) and CaCl2 (0.1 mg/mL). Cells were then fixed with 100% ethanol for 30mins at 4°C followed by treatment with ice cold acetone for 1 min. Acetone was removed completely and cells were then permeabilized and blocked overnight at 4°C in PBS containing 0.1% Triton X-100 and 10% normal goat serum. Cells were incubated for 60mins in staining solution containing 1:100 dilution of anti-occludin monoclonal antibody (Zymed Laboratories). The bound anti-occludin antibody was detected using an Alexa 568-conjugated secondary antibody (Invitrogen, Molecular Probes) at a 1:300 dilution. Cells were also stained with anti-(ZO)-1 polyclonal antibody (Zymed Laboratories) which had been pre-absorbed with fixed EPEC wildtype bacteria. The bound anti-(ZO)-1 antibody was detected using an Alexa 488-conjugated secondary antibody. After immunostaining, cellular and bacterial DNA were visualized by staining with 4'-6-Diamidino-2-phenylindole (DAPI). Membrane filters were excised, mounted in Prolong Gold Antifade Reagent (Invitrogen) and sealed beneath a coverslip with nail varnish. Monolayers were viewed using a Zeiss Axioplan 2 fluoresence microscope equipped with an apotome. Serial sections were taken and then combined into a 2-dimensional projection using Zeiss Axiovision software.
For assessment of filopodia, HeLa cells were washed several times post-infection in PBS supplemented with MgCl2 (0.1 mg/mL) and CaCl2 (0.1 mg/mL). Cells were then fixed with 2.5% paraformaldehyde for 15mins. Cells were permeabilized in 0.1% Triton X-100 in PBS for 5mins and blocked for 30mins in 10% NGS in PBS. Cells were then incubated for 60min in a staining solution containing a 1:100 dilution of tetramethylrhodamine isothiocyanate (TRITC)-conjugated phalloidin (to stain polymerized actin) and anti-EPEC polyclonal antibody. The bound anti-EPEC antibody was detected using Alexa-488 conjugated secondary antibody at a dilution of 1:300 (Invitrogen, Molecular Probes). After staining, coverslips were mounted in Prolong Gold Antifade Reagent (Invitrogen) and sealed with nail varnish. Cells were viewed using a Zeiss Axioplan Fluoresence microscope equipped with an apotome. Serial sections were taken and then combined into a 2-dimensional projection using Zeiss Axiovision software.
For assessment of Sorting Nexin 9 tubule formation, HeLa cells were seeded onto glass coverslips and infected the following day with EPEC for 90 minutes at an MOI of 100. The cells were then rinsed twice in PBS and fixed for 20 minutes in 3.7% formaldehyde in PBS. Cells were rinsed, permeabilized in 0.5% Triton X-100 in PBS for 10 minutes, then blocked in 2.5% BSA in PBS for one hour. Polyclonal anti-SNX9 antibodies raised in rabbit, a generous gift from Neal M. Alto, were diluted in blocking solution and incubated with the cells for one hour. Cells were washed 5 times in blocking solution and incubated for 30 minutes with Alexa Flour 568-conjugated anti-rabbit IgG antibodies raised in goat (Invitrogen, Carlsbad, Ca.). Cells were rinsed in PBS and mounted on slides using ProLong Gold antifade reagent (Invitrogen, Carlsbad, Ca.). All manipulations were performed at room temperature.
Preparation of secreted proteins, 2-dimensional gels, and ion trap mass spectroscopy analysis
Secreted proteins were prepared as described previously (Deng et al., 2004; Gruenheid et al., 2004). Trichloroacetic acid-precipitated proteins from 15 ml cultures were separated overnight on 7 cm immobilized pH gradients (pH 6–11, GE Healthcare) using an IPGPhor apparatus (GE Healthcare) according to the manufacturer's instructions. Following the first dimension separation, equilibration was performed as described previously (Deng et al., 2004; Gruenheid et al., 2004), and samples were loaded onto SDS-PAGE mini gels containing 15% acrylamide. Gels were stained with Sypro Ruby (Invitrogen), and visualized using a UV transilluminator. Spots of interest were excised from the gel, and subjected to in gel tryptic digestion. The digested sample peptides were separated by HPLC reverse phase chromatography and subjected to mass spectrometry (ion trap HCT ultra; Bruker Daltonics). Data were formatted, using DataAnalysis (Brukert Daltonics) and searched against E. coli sequences in the NCBI database, using the Mascot search engine (v. 2.2; Matrix Sciences).
Yeast 2-hybrid analysis
EPEC genomic DNA was used as a template for polymerase chain reaction amplification of nleA, espF, and map with High fidelity Taq (Fermentas). PCR products were subcloned into TOPO vectors (Invitrogen) and sequenced. Following sequence confirmation, inserts were subcloned into yeast expression plasmids pGBKT7 and pGADT7 (Clontech). Interaction between NleA/EspF and NleA/Map was assessed using a commercially available yeast two-hybrid system (MatchMaker system 3, Clontech). Individual pGBKT7 and pGADT7 constructs were transformed into yeast cells using a lithium acetate transformation procedure, and expression of the corresponding recombinant GAL4 proteins was confirmed by immunoblotting as per the manufacturer's instructions. Positive transformants were mated and the resulting diploids were tested for reconstitution of GAL4 activity by plating on synthetic medium (SD) of various stringencies: low stringency lacking tryptophan and leucine (SD-Trp/-Leu), medium stringency lacking histidine, tryptophan, and leucine (SD-His/-Trp/-Leu), and high stringency lacking adenine, histidine, tryptophan, and leucine (SD-Ade/-His/-Trp/-Leu). Protein-protein interactions were also evaluated by alpha- and beta-gal assays as per the manufacturer's instructions. NleA/Sec24 interaction was used as a positive control in all assays.
Supplementary Material
Bacterial strains were grown overnight in LB broth with antibiotics where appropriate. Overnight cultures were diluted 1:30 into serum-free DMEM and grown to an OD600 of approximately 0.5. Bacteria were pelleted by centrifugation at 4000 rcf and supernatants decanted and passed through 0.22μm filters. Bacterial cells were resuspended in 1x Laemmli buffer and lysed by boiling for 10 minutes. Sodium deoxycholate was added to 0.2% and supernatants incubated on ice for 30 minutes. Trichloroacetic acid was then added to 10% and supernatants incubated overnight at 4°C. Precipitated proteins were pelleted by centrifugation at 20,000 rcf for 30 minutes at 4°C. Protein pellets were resuspended in cold acetone and pelleted by centrifugation for 20 minutes at 12,000 rcf. Acetone was decanted and pellets air dried for 5 minutes at room temperature. Pellets were dissolved in 150mM Tris pH 8.8 diluted 1:1 with 2x Laemmli buffer, boiled and loaded onto SDS-PAGE gels along with bacterial cell lysates. Proteins were detected by Western blotting with mouse monoclonal anti-RpoA antibodies (NeoClone, Madison, Wi) and rabbit polyclonal anti-EspF antibodies (Covance, Princeton, NJ).
Acknowledgements
The authors wish to thank Rebekah DeVinney for helpful discussions and for providing the ZO-1 antibody. This work was supported by a CIHR Operating Grant awarded to SG. SG is a Canada Research Chair in Bacterial Pathogenesis. MM was supported by a studentship from the Canadian Association of Gastroenterology and the CIHR. Work was also supported by DK50694 and DK58964 from NIH and a VA Merit to GH. Mass spectrometry was performed by Dr. Kurt Dejgaard at the McGill Life Sciences Complex core facility.
References
- Alto NM, Weflen AW, Rardin MJ, Yarar D, Lazar CS, Tonikian R, et al. The type III effector EspF coordinates membrane trafficking by the spatiotemporal activation of two eukaryotic signaling pathways. J Cell Biol. 2007;178:1265–1278. doi: 10.1083/jcb.200705021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campellone KG, Robbins D, Leong JM. EspFU is a translocated EHEC effector that interacts with Tir and N-WASP and promotes Nck-independent actin assembly. Dev Cell. 2004;7:217–228. doi: 10.1016/j.devcel.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Canil C, Rosenshine I, Ruschkowski S, Donnenberg MS, Kaper JB, Finlay BB. Enteropathogenic Escherichia coli decreases the transepithelial electrical resistance of polarized epithelial monolayers. Infect Immun. 1993;61:2755–2762. doi: 10.1128/iai.61.7.2755-2762.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombes BK, Wickham ME, Mascarenhas M, Gruenheid S, Finlay BB, Karmali MA. Molecular analysis as an aid to assess the public health risk of non-O157 Shiga toxin-producing Escherichia coli strains. Appl Environ Microbiol. 2008;74:2153–2160. doi: 10.1128/AEM.02566-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean P, Kenny B. Intestinal barrier dysfunction by enteropathogenic Escherichia coli is mediated by two effector molecules and a bacterial surface protein. Mol Microbiol. 2004;54:665–675. doi: 10.1111/j.1365-2958.2004.04308.x. [DOI] [PubMed] [Google Scholar]
- Dean P, Kenny B. The effector repertoire of enteropathogenic E. coli: ganging up on the host cell. Curr Opin Microbiol. 2009 doi: 10.1016/j.mib.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deborah Chen H, Frankel G. Enteropathogenic Escherichia coli: unravelling pathogenesis. FEMS Microbiol Rev. 2005;29:83–98. doi: 10.1016/j.femsre.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Deng W, Li Y, Vallance BA, Finlay BB. Locus of enterocyte effacement from Citrobacter rodentium: sequence analysis and evidence for horizontal transfer among attaching and effacing pathogens. Infect Immun. 2001;69:6323–6335. doi: 10.1128/IAI.69.10.6323-6335.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Li Y, Hardwidge PR, Frey EA, Pfuetzner RA, Lee S, et al. Regulation of type III secretion hierarchy of translocators and effectors in attaching and effacing bacterial pathogens. Infect Immun. 2005;73:2135–2146. doi: 10.1128/IAI.73.4.2135-2146.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Puente JL, Gruenheid S, Li Y, Vallance BA, Vazquez A, et al. Dissecting virulence: systematic and functional analyses of a pathogenicity island. Proc Natl Acad Sci U S A. 2004;101:3597–3602. doi: 10.1073/pnas.0400326101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott SJ, Wainwright LA, McDaniel TK, Jarvis KG, Deng YK, Lai LC, et al. The complete sequence of the locus of enterocyte effacement (LEE) from enteropathogenic Escherichia coli E2348/69. Mol Microbiol. 1998;28:1–4. doi: 10.1046/j.1365-2958.1998.00783.x. [DOI] [PubMed] [Google Scholar]
- Garmendia J, Phillips AD, Carlier MF, Chong Y, Schuller S, Marches O, et al. TccP is an enterohaemorrhagic Escherichia coli O157:H7 type III effector protein that couples Tir to the actin-cytoskeleton. Cell Microbiol. 2004;6:1167–1183. doi: 10.1111/j.1462-5822.2004.00459.x. [DOI] [PubMed] [Google Scholar]
- Gauthier A, Puente JL, Finlay BB. Secretin of the enteropathogenic Escherichia coli type III secretion system requires components of the type III apparatus for assembly and localization. Infect Immun. 2003;71:3310–3319. doi: 10.1128/IAI.71.6.3310-3319.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenheid S, Finlay BB. Microbial pathogenesis and cytoskeletal function. Nature. 2003;422:775–781. doi: 10.1038/nature01603. [DOI] [PubMed] [Google Scholar]
- Gruenheid S, Sekirov I, Thomas NA, Deng W, O'Donnell P, Goode D, et al. Identification and characterization of NleA, a non-LEE-encoded type III translocated virulence factor of enterohaemorrhagic Escherichia coli O157:H7. Mol Microbiol. 2004;51:1233–1249. doi: 10.1046/j.1365-2958.2003.03911.x. [DOI] [PubMed] [Google Scholar]
- Kenny B, Ellis S, Leard AD, Warawa J, Mellor H, Jepson MA. Co-ordinate regulation of distinct host cell signalling pathways by multifunctional enteropathogenic Escherichia coli effector molecules. Mol Microbiol. 2002;44:1095–1107. doi: 10.1046/j.1365-2958.2002.02952.x. [DOI] [PubMed] [Google Scholar]
- Kim J, Thanabalasuriar A, Chaworth-Musters T, Fromme JC, Frey EA, Lario PI, et al. The bacterial virulence factor NleA inhibits cellular protein secretion by disrupting mammalian COPII function. Cell Host Microbe. 2007;2:160–171. doi: 10.1016/j.chom.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Lee MC, Miller EA, Goldberg J, Orci L, Schekman R. Bi-directional protein transport between the ER and Golgi. Annu Rev Cell Dev Biol. 2004;20:87–123. doi: 10.1146/annurev.cellbio.20.010403.105307. [DOI] [PubMed] [Google Scholar]
- Lee SF, Kelly M, McAlister A, Luck SN, Garcia EL, Hall RA, et al. A C-terminal class I PDZ binding motif of EspI/NleA modulates the virulence of attaching and effacing Escherichia coli and Citrobacter rodentium. Cell Microbiol. 2008;10:499–513. doi: 10.1111/j.1462-5822.2007.01065.x. [DOI] [PubMed] [Google Scholar]
- Levine MM, Bergquist EJ, Nalin DR, Waterman DH, Hornick RB, Young CR, Sotman S. Escherichia coli strains that cause diarrhoea but do not produce heat-labile or heat-stable enterotoxins and are non-invasive. Lancet. 1978;1:1119–1122. doi: 10.1016/s0140-6736(78)90299-4. [DOI] [PubMed] [Google Scholar]
- Marches O, Ledger TN, Boury M, Ohara M, Tu X, Goffaux F, et al. Enteropathogenic and enterohaemorrhagic Escherichia coli deliver a novel effector called Cif, which blocks cell cycle G2/M transition. Mol Microbiol. 2003;50:1553–1567. doi: 10.1046/j.1365-2958.2003.03821.x. [DOI] [PubMed] [Google Scholar]
- Marches O, Batchelor M, Shaw RK, Patel A, Cummings N, Nagai T, et al. EspF of enteropathogenic Escherichia coli binds sorting nexin 9. J Bacteriol. 2006;188:3110–3115. doi: 10.1128/JB.188.8.3110-3115.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzawa T, Kuwae A, Abe A. Enteropathogenic Escherichia coli type III effectors EspG and EspG2 alter epithelial paracellular permeability. Infect Immun. 2005;73:6283–6289. doi: 10.1128/IAI.73.10.6283-6289.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara BP, Donnenberg MS. A novel proline-rich protein, EspF, is secreted from enteropathogenic Escherichia coli via the type III export pathway. FEMS Microbiol.Lett. 1998;166:71–78. doi: 10.1111/j.1574-6968.1998.tb13185.x. [DOI] [PubMed] [Google Scholar]
- McNamara BP, Koutsouris A, O'Connell CB, Nougayrede JP, Donnenberg MS, Hecht G. Translocated EspF protein from enteropathogenic Escherichia coli disrupts host intestinal barrier function. J Clin Invest. 2001;107:621–629. doi: 10.1172/JCI11138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy R, Jenkins C, Yu J, Smith H, Frankel G. Distribution of espI among clinical enterohaemorrhagic and enteropathogenic Escherichia coli isolates. J Med Microbiol. 2004a;53:1145–1149. doi: 10.1099/jmm.0.45684-0. [DOI] [PubMed] [Google Scholar]
- Mundy R, Petrovska L, Smollett K, Simpson N, Wilson RK, Yu J, et al. Identification of a novel Citrobacter rodentium type III secreted protein, EspI, and roles of this and other secreted proteins in infection. Infect Immun. 2004b;72:2288–2302. doi: 10.1128/IAI.72.4.2288-2302.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muza-Moons MM, Koutsouris A, Hecht G. Disruption of cell polarity by enteropathogenic Escherichia coli enables basolateral membrane proteins to migrate apically and to potentiate physiological consequences. Infect Immun. 2003;71:7069–7078. doi: 10.1128/IAI.71.12.7069-7078.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perna NT, Mayhew GF, Posfai G, Elliott S, Donnenberg MS, Kaper JB, Blattner FR. Molecular evolution of a pathogenicity island from enterohemorrhagic Escherichia coli O157:H7. Infect Immun. 1998;66:3810–3817. doi: 10.1128/iai.66.8.3810-3817.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philpott DJ, McKay DM, Sherman PM, Perdue MH. Infection of T84 cells with enteropathogenic Escherichia coli alters barrier and transport functions. Am J Physiol. 1996;270:G634–645. doi: 10.1152/ajpgi.1996.270.4.G634. [DOI] [PubMed] [Google Scholar]
- Simonovic I, Rosenberg J, Koutsouris A, Hecht G. Enteropathogenic Escherichia coli dephosphorylates and dissociates occludin from intestinal epithelial tight junctions. Cell Microbiol. 2000;2:305–315. doi: 10.1046/j.1462-5822.2000.00055.x. [DOI] [PubMed] [Google Scholar]
- Simonovic I, Arpin M, Koutsouris A, Falk-Krzesinski HJ, Hecht G. Enteropathogenic Escherichia coli activates ezrin, which participates in disruption of tight junction barrier function. Infect Immun. 2001;69:5679–5688. doi: 10.1128/IAI.69.9.5679-5688.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitz J, Yuhan R, Koutsouris A, Blatt C, Alverdy J, Hecht G. Enteropathogenic Escherichia coli adherence to intestinal epithelial monolayers diminishes barrier function. Am J Physiol. 1995;268:G374–379. doi: 10.1152/ajpgi.1995.268.2.G374. [DOI] [PubMed] [Google Scholar]
- Tomson FL, Viswanathan VK, Kanack KJ, Kanteti RP, Straub KV, Menet M, et al. Enteropathogenic Escherichia coli EspG disrupts microtubules and in conjunction with Orf3 enhances perturbation of the tight junction barrier. Mol Microbiol. 2005;56:447–464. doi: 10.1111/j.1365-2958.2005.04571.x. [DOI] [PubMed] [Google Scholar]
- Wickham ME, Brown NF, Boyle EC, Coombes BK, Finlay BB. Virulence is positively selected by transmission success between mammalian hosts. Curr Biol. 2007;17:783–788. doi: 10.1016/j.cub.2007.03.067. [DOI] [PubMed] [Google Scholar]
- Yuhan R, Koutsouris A, Savkovic SD, Hecht G. Enteropathogenic Escherichia coli-induced myosin light chain phosphorylation alters intestinal epithelial permeability. Gastroenterology. 1997;113:1873–1882. doi: 10.1016/s0016-5085(97)70006-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Bacterial strains were grown overnight in LB broth with antibiotics where appropriate. Overnight cultures were diluted 1:30 into serum-free DMEM and grown to an OD600 of approximately 0.5. Bacteria were pelleted by centrifugation at 4000 rcf and supernatants decanted and passed through 0.22μm filters. Bacterial cells were resuspended in 1x Laemmli buffer and lysed by boiling for 10 minutes. Sodium deoxycholate was added to 0.2% and supernatants incubated on ice for 30 minutes. Trichloroacetic acid was then added to 10% and supernatants incubated overnight at 4°C. Precipitated proteins were pelleted by centrifugation at 20,000 rcf for 30 minutes at 4°C. Protein pellets were resuspended in cold acetone and pelleted by centrifugation for 20 minutes at 12,000 rcf. Acetone was decanted and pellets air dried for 5 minutes at room temperature. Pellets were dissolved in 150mM Tris pH 8.8 diluted 1:1 with 2x Laemmli buffer, boiled and loaded onto SDS-PAGE gels along with bacterial cell lysates. Proteins were detected by Western blotting with mouse monoclonal anti-RpoA antibodies (NeoClone, Madison, Wi) and rabbit polyclonal anti-EspF antibodies (Covance, Princeton, NJ).