Abstract
Maintenance of ocular viability is one of the major impediments to successful whole-eye transplantation. This review provides a comprehensive understanding of the current literature to help guide future studies in order to overcome this hurdle. A systematic multistage review of published literature was performed. Three specific questions were addressed: (1) Is recovery of visual function following eye transplantation greater in cold-blooded vertebrates when compared with mammals? (2) Is outer retina function following enucleation and reperfusion improved compared with enucleation alone? (3) Following optic-nerve transection, is there a correlation between retinal ganglion cell (RGC) survival and either time after transection or proximity of the transection to the globe? In a majority of the studies performed in the literature, recovery of visual function can occur after whole-eye transplantation in cold-blooded vertebrates. Following enucleation (and reperfusion), outer retinal function is maintained from 4 to 9 h. RGC survival following optic-nerve transection is inversely related to both the time since transection and the proximity of transection to the globe. Lastly, neurotrophins can increase RGC survival following optic-nerve transection. This review of the literature suggests that the use of a donor eye is feasible for whole-eye transplantation.
Approximately 37 million people worldwide suffer from blindness, with up to 20% or 7.4 million having vision of only light perception or less.1, 2 Much of this irreversible blindness is due to age-related diseases such as macular degeneration, diabetic retinopathy and glaucoma,3–5 as well as trauma and ocular tumours.6–8 The irreversible nature of these diseases is a result of permanent optic-nerve damage. Irreversibly damaged axons of retinal ganglion cells (RGCs)—the output neurons from the retina that pass through the optic nerve—do not regain their function. Potentially, whole-eye transplantation can provide a blind recipient with viable RGCs capable of regeneration, as well as the optical system necessary for forming a retinal image.
In 1977, a 17-member advisory council for the National Eye Institute (NEI) called for a “limited and thoughtful laboratory effort” in the area of eye transplantation. However, the council acknowledged that “at present, any effort to transplant a mammalian eye is doomed to failure by the ganglion cell axon’s inability to withstand cutting, by the difficulty of insuring adequate circulation of blood to the transplanted eye during or shortly after operation, and lastly by immune rejection of foreign tissue.”9
Here we provide a comprehensive understanding of the current literature regarding ocular viability of the donor eye. Throughout this review, ocular viability will be defined in one of three ways: capacity for visual recovery, maintenance of outer retina function (measured using an electroretinogram (ERG)) or RGC survival. The following three questions relating to ocular viability were addressed: (1) Is recovery of visual function following eye transplantation greater in cold-blooded vertebrates when compared with mammals? (2) Is outer retina function following enucleation and reperfusion improved compared with enucleation alone? (3) Following optic-nerve transection, is there a correlation between RGC survival and either time after transection or proximity of the transection to the globe? To answer these questions, we systematically reviewed the published literature to determine the outcome of whole-eye transplantation in animals, outer retina function after enucleation and RGC survival after optic-nerve transection.
LITERATURE SOURCES AND EVALUATION
Pertinent articles were identified through a multistage systematic approach. In the first stage, a computerised search of MEDLINE database (National Library of Medicine, Bethesda, Maryland) was performed. The search-terms “optic nerve regeneration,” “eye transplantation,” “isolated perfused eye,” retinal ganglion cell survival axotomy” and “circulatory revascularisation of the eye” from the Medical Subject Headings (MeSH0 supplement to Index Medicus (National Library of Medicine, Bethesda, Maryland) were used for a broad search. This search produced 702 unique citations. Commercial internet search engines were queried for additional unique references. In the second stage, all abstracts were carefully scanned to identify articles that pertained to ocular viability. Whole copies of 153 articles were obtained. Bibliographies of the retrieved articles were manually searched for additional articles. In the third stage, complete articles were reviewed to identify those that discussed eye transplantation, ocular viability, retinal function in the isolated perfused eye or RGC survival following optic-nerve transection. The search was limited to English language articles.
Articles were grouped into three categories for further data abstraction: (1) articles that described in vivo whole-eye transplantation, (2) articles that described in vivo and ex vivo whole-eye reperfusion and (3) articles that described in vivo RGC survival and factors that increase RGC survival. Data were abstracted from identified articles to determine ocular viability in each of the above three categories and for factors that improve ocular viability.
For evidence of visual function, only articles in which the eye was completely enucleated and reimplanted were included. Articles in which the optic nerve was transected but the eye was not enucleated were excluded. We relied on each identified article’s conclusion to assess evidence of visual function. Outcome of whole-eye transplantation in cold-blooded vertebrate was compared with outcome in mammals. In total, 17 articles regarding whole-eye transplantation in both cold-blooded vertebrate and mammals satisfied our inclusion criteria.
For evidence of preserved outer retina function, only articles that evaluated outer retina function (with ERG) at greater than one time point were included. Articles, in which an enucleated eye was perfused, but maintenance of ocular viability at greater than one time point was not evaluated, were excluded. Articles in which there was no attempt to evaluate retina function were also excluded. In total, eight articles regarding ex vivo perfused eyes satisfied our inclusion criteria.
For RGC survival, articles were divided into two categories based upon the location of optic-nerve transection—intraorbital or intracranial. Articles that evaluated RGC survival at (at least) two of the following three time points were included: 1–3 days post-transection, 10–15 days post-transection and 26–30 days post-transection. Only articles in which complete optic-nerve transection (one optic-nerve crush article was included) was performed and in which quantification of RGC survival at more than one time point was assessed were included. In total, 12 articles regarding survival of RGC after optic-nerve transection satisfied our inclusion criteria.
Finally, articles that demonstrated factors that improved RGC survival were included. The effect of the positive factors is reported as the percentage difference of RGC survival between the eye that was treated with the factor, and the eye that was not treated. This percentage difference was calculated by subtracting the percentage (of healthy control) of RGC survival in the untreated eye from the percent (of healthy control) of RGC survival in the treated eye. The result of this calculation yielded the “percentage survival difference.” In total, 45 articles regarding factors that increase RGC survival satisfied our inclusion criteria.
A total of 82 articles were included in the study, and a total of 93 articles are referenced. A total of 60 articles were excluded based on our exclusion criteria.
RESULTS
Whole-eye transplantation
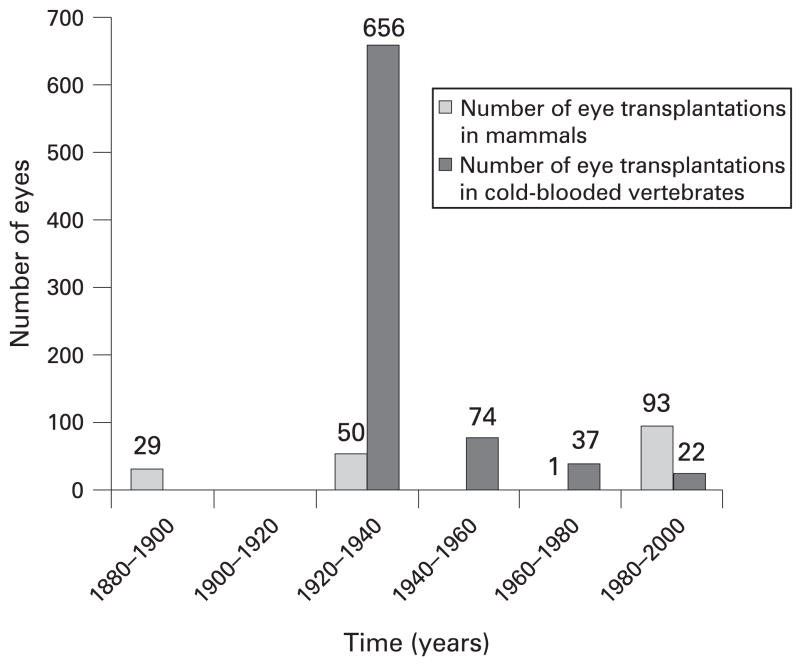
In defining ocular viability as recovery of visual function following transplantation, it is necessary to review attempts at whole-eye transplantation, which date back to 1885 when a rabbit eye was transplanted into a human orbit.10 Over the subsequent 10 years, several attempts at mammalian eye transplantation followed;10, 11 however, by the early to mid 20th century, most of the attempts at eye transplantation were performed in cold-blooded vertebrates, and much of our knowledge is obtained from these studies. In total, we have reviewed 17 articles regarding whole-eye transplantation.10–26 Figure 1 outlines eye transplantations performed from the years 1880 until 2000 (962 in total).
Figure 1.
Timeline of whole-eye transplantations performed between the years 1880 and 2000.
A total of seven articles describing 173 mammalian eye transplantations were reviewed.10–13, 15, 17, 18 Of the seven studies, only two have demonstrated recovery of visual function.13, 15 In 1925, Koppanyi and Baker reported the recovery of visual function in three out of 25 rats in which autograft transplantation (excision and reimplantation) was performed.15 Doubts have since been raised regarding the accuracy of the tests Koppanyi used to demonstrate visual function and whether the rats ever truly recovered vision.9, 14 The only other study that shows recovery of visual function in mammals was performed by Freed and Wyatt in 1980.13 They demonstrated that following transplantation of fetal eyes directly into the brains of adult rats, surviving ocular tissue could be identified. Visual evoked responses were positive in nine out of 10 rats in which surviving ocular tissue was identified. Although some of the remaining studies establish “success” in other capacities, no visual function was recovered following transplantation.
We have reviewed a total of 10 articles,15, 16, 19–26 eight of which describe 789 eye transplantations in cold-blooded vertebrates.16, 19–25 In the remaining two articles, recovery of visual function was reported, but the number of experimental models was not given.15, 26 One of the eight articles describes 296 transplantations, but the specific number in which recovery of visual function was tested is not given.23 In the remaining seven articles,16, 19–22, 24, 25 of the 493 transplantations that were recorded, 180 were tested for visual recovery, and 95 demonstrated recovery of visual function. Table 1 describes the 10 articles in greater detail.
Table 1.
Whole-eye transplantation in cold-blooded vertebrates
| Animal | No of eyes transplanted | No of eyes tested for vision | Method of testing | No of eyes that recovered visual function | Study |
|---|---|---|---|---|---|
| Bombinator (European toad) | NR | NR | Skin colour (visual controlled) | “Many” | Koppany and Baker15 |
| Salamander | NR | NR | Behavioural response | “Many” | Stone and Ussher26 |
| Salamander | 82 | 6 | Behavioural response | All 6 | Stone20 |
| Salamander | 296 | NR | Behavioural response | “Many specimens” | Stone and Cole23 |
| Salamander | 186 | 31 | Behavioural response | All 31 | Stone et al25 |
| Salamander | 33 | 9 | Behavioural response | 6 of 9 | Stone and Zaur24 |
| Salamander | 59 | 4 | Behavioural response. | NR | |
| Frog | 32 (16 animals) | All (16 animals) | Behavioural response | 2 of 16 frogs | Sperry19 |
| Salamander | 42 (21 animals) | All (21 animals) | Behavioural response | 12 of 21 salamanders | |
| Salamander | 11 eyes | 10 | Behavioural response | 7 of 10 | Stone22 |
| Salamander | 26 (13 animals) | 24 (12 animals) | Behavioural response | 9 of 12 salamanders | Stone21 |
| Salamander | 22 | 22 | Recovery of visual activated skin camouflage | 18 of 22 | Pietsch and Schneider16 |
NR, not recorded.
Many more eye transplantations were performed in cold-blooded vertebrate than in mammals. Furthermore, maintenance of ocular viability was more successful in cold-blooded vertebrates when compared with mammals.
Outer retina function after enucleation
In assessing ocular viability of the donor eye as it pertains to whole-eye transplantation, one of the primary concerns is the ability for the retina to maintain function following enucleation. Throughout this section, ocular viability will be defined as maintenance of outer retina function following enucleation as measured using ERG. An excellent model for assessing retina function after enucleation ex vivo is the isolated, perfused eye that was originally used in 1970.27 Even though this model was employed prior to 1970, it was not for the purposes of assessing retinal function.28, 29 In this system, the eye is enucleated and immediately (usually within less than 10 min) reperfused with an artificial perfusate designed to maintain retina function for as long as possible. In this review, a total of eight articles describing outer retina function in a perfused eye are reviewed.27, 30–36
All eight of the articles regarding outer retina function in an isolated perfused eye identified a period of time in which outer retina function was maintained after enucleation and reperfusion.27, 30–36 Maintenance of outer retina function was determined by the presence of a stable ERG response as determined by each individual study. Of the eight studies, seven used flash ERG,27, 30–33, 35, 36 and one used multifocal ERG (mfERG).34 Two of the eight studies reported that without reperfusion, ERG activity is greatly decreased or absent within 5 min after enucleation.27, 32 In the eight studies, the period in which outer retina function was maintained following enucleation and reperfusion ranged from 4 h to more than 9 h. Table 2 outlines these eight studies in greater detail.
Table 2.
Eye viability and retinal function in the perfused eye
| Animal | Eye viability (hours postenucleation) | Measurement of eye viability | Study |
|---|---|---|---|
| Cat | 6–8 | Stable ERG response | Gouras and Hoff27 |
| Bovine | 5–6* (>10) | Stable ERG response (small negative deflection) | Tazawa and Seaman35 |
| Cat | 8–10 | Stable ERG response | Niemeyer31 |
| Frog | >9 | Stable ERG response | Friedman and Marchese30 |
| Cat | 4.5 | Stable ERG response | Sandberg et al33 |
| Bovine | 8* | Stable ERG response | Tseng et al36 |
| Cat | 7–9 | Stable ERG response | Peachey et al32 |
| Bovine | 4 | mfERG (multifocal) response | Shahidullah et al34 |
Oxygenated blood was used instead of artificial perfusate.
RGC survival following optic-nerve transection
The third area of importance regarding maintaining ocular viability is RGC survival as assessed by histological analysis of the retina following optic-nerve transection. Specifically, we evaluated the correlation between RGC survival and both time after optic-nerve transection and distance of transection from the globe. We also evaluated factors that increase RGC survival after optic-nerve transection. In total, 12 articles describing RGC survival after optic-nerve transection were reviewed.37–48 An additional 45 articles identifying factors that increase RGC survival were reviewed.38, 39, 42, 43, 46, 49–88
In nine of the 12 studies that evaluated RGC survival at the above-mentioned time points, an intraorbital injury was performed,38–40, 42–46, 48 in two studies, both an intraorbital and intracranial injury was performed,37, 47 and in one study, only an intracranial injury was performed.41 The injuries were divided into two categories—intraorbital injury (11 studies) and intracranial injury (three studies).
The results of the studies using intraorbital injuries were as follows: eight studies evaluated RGC survival at the 1–3 day time point with survival ranging from 72 to 100%;37, 40, 42–46, 48 11 studies evaluated RGC survival at the 10–15 day time point with results ranging from 5.8 to 27%;37–40, 42–48 and six studies evaluated RGC survival at the 26–30 day time point with results ranging from “negligible” to 7.5%.37–40, 46, 47
The results of the intracranial injuries were as follows: two studies evaluated RGC survival at the 1–3 day time point with survival ranging from 90 to 100%;37, 41 four studies (three articles) evaluated RGC survival at the 10–15 day time point with results ranging from 57.4 to 68%;37, 41, 47 and four studies (three articles) evaluated RGC survival at the 26–30 day time point with results ranging from 49 to 71.4%.37, 41, 47 Table 3 outlines these studies in greater detail. An inverse relationship between RGC survival following optic-nerve transection, and both time after transection and proximity of transection to the globe is clearly demonstrated.
Table 3.
Retinal ganglion cell survival following axotomy in mammalian eyes
| Distance from eye | 1–3 days postinjury (%) | 10–15 days postinjury (%) | 26–30 days postinjury (%) | Study |
|---|---|---|---|---|
| Intraorbital (<5 mm) | NA | 24.7 | 18.2 | Villegas-Perez et al47 |
| 72 | 23.6 | NA | Takano and Horie45 | |
| 100 | 5.8 | Negligible | Berkelaar et al37 | |
| NA | 26 | 5 | Di Polo et al39 | |
| 100 | 19 | NA | Watanabe et al48 | |
| 85 | 24 | NA | Manabe et al44 | |
| NA | 9.6 | 2 | Cheng et al38 | |
| 97 | 14.9 | 7 | van Adel et al46 | |
| 88 | 27 | 7.54 | Germain et al40 | |
| 100 | 19 | NA | Hou et al42 | |
| 98 | 16.3 | NA | Kretz et al43 | |
| Intracranial (>8 mm) | 90 | 68 | 64 | Grafstein and Ingoglia41 |
| NA | 57.4 | 54.5 | Villegas-Perez et al47 | |
| NA | 65.6 | 71.4 | ||
| 100 | 63 | 49 | Berkelaar et al37 |
NA, not applicable.
A total of 45 articles that describe factors that increase RGC survival after optic-nerve transection were reviewed.38, 39, 42, 43, 46, 49–88 In these articles, 31 individual factors, six combined factors and 17 gene modifications are discussed. In the 45 articles, some of the factors that are studied include brain-derived neurotrophic factor (BDNF),39, 61, 68, 74, 76, 78, 85 ciliary neurotrophic factor (CNTF),46, 82, 83 glial-derived neurotrophic factor (GDNF),64, 66, 68, 82, 85 neurturin,68 nerve growth factor (NGF)51 and many others. Tables 4–6 list the factors along with details for each study.
Table 4.
Factors and details for each study: single factors that have been shown to increase retinal ganglion cell (RGC) survival following axotomy
| Factor* | Animal | No of days postaxotomy | Percentage survival difference† | Administration |
|---|---|---|---|---|
| aFGF80—postinjury | Rat | Day 30 | 24.5% | PN |
| Antisemaphorin 3A Ab79—postinjury | Rat | Day 8 | 79% | IO |
| Aurintricarboxylic acid(ATA)58—postinjury | Rat | Day 14 | 45% | IO |
| BDNF74, 78, 85, 68, 82—postinjury | Rat | Day 7–14 | 51.1–30% – | IO |
| Cat | Day 14 | 12% | IO | |
| bFGF80—postinjury | Rat | Day 30 | 20.5% | PN |
| CNTF82, 46—postinjury | Cat | Day 14 | 14% | IO |
| Rat | Day 14 | 14.8% | IO | |
| Collicular proteoglycan60—postinjury— | Rat | Day 7, 14 | 26%, 48% | IO |
| intraocular | ||||
| Cortisol59—postinjury | Rat | Day 14 | 33% | IO |
| Donepezil (AchE inhibitor)75—postinjury | Rat | Day 7 | 9.5% | Orally |
| Electrical stimulation77—postinjury | Rat | Day 7 | 29% | Nerve stump |
| Erythropoietin (EPO)84—postinjury | Rat | Day 14 | 872 and 455 RGC/mm2‡ | IO |
| Fibroblasts72—preinjury | Rat | Day 7 | 11.8% | IO |
| Flunarizine57—postinjury | Rat | Day 14 | 5% | SubQ |
| GDNF64, 66, 68, 82, 85—postinjury | Rat | Day 7–14 | 37–18% | IO |
| Cat | Day 14 | 13% | IO | |
| GM154—preinjury | Rat | Day 14 | 18.87% | IO |
| IGF-162—postinjury | Rat | Day 14 | 14% | IO |
| IL-1β55—postinjury | Rat | Day 14 | 39.4% | IO |
| Inosine42—postinjury | Rat | Day 14 | 5.9% | Intraperitoneal |
| Latanoprost70—preinjury | Rat | Day 10 | 31.8% | IO |
| Leukaemia inhibitory factor46—postinjury | Rat | Day 14 | 9% | IO |
| L-NAME (NO inhibitor)67—postinjury | Rat | Day 10, 14 | 21%, 22.4% | IO |
| Minocycline49—pre- and postinjury | Rat | Day 7 | 23% | Intraperitoneal |
| Neurturin68—postinjury | Rat | Day 14 | 16% | IO |
| NGF51—postinjury | Rat | Week 5, 7 | 16.7%, 16.9% | IO |
| NOLA (NO inhibitor)67—postinjury | Rat | Day 10, 14 | 29.2%, 32% | IO |
| NT-4 (neurotrophin)78, 82—postinjury | Rat | Day 7, 14 | 38.2%, 13.2% | IO |
| Cat | Day 14 | 8% | IO | |
| Optic-nerve graft53—preinjury implantation | Hamster | Day 7 | 15% | IO |
| Rifampicin63—postinjury | Mice | Day 14 | 25.9% | Intraperitoneal |
| Schwann cells72—pre injury | Rat | Day 7 | 16.1% | IO |
| Simvastatin43—postinjury | Rat | Day 7, 14 | 40.9%, 16.2% | IO |
| TNF-α56—postinjury | Rat | Day 14 | 35.1% | IO |
The time the factor was given is listed along with the factor.
Results are calculated as the percentage difference of RGC survival (compared with healthy retina) in the retina of treated eyes versus the untreated eye (ie, percentage of surviving RGC in the treated eye minus the percentage of surviving RGC in the untreated eye).
No controls were used numbers are reported as actual numbers.
Ab, antibodies; aFGF, acidic fibroblast growth factor; BDNF, brain-derived neurotrophic factor; bFGF, basic fibroblast growth factor; CNTF, ciliary neurotrophic factor; GDNF, glial derived neurotrophic factor; GM1, monosialotetrahexosylganglioside; IGF, insulin like growth factor; IL, interleukin; IO, intraocular; L-NAME, N-nitro-L-arginine methyl ester; NGF, nerve growth factor; NOLA, Nω-nitro-L-arginine; PN, perineural; TNF, tumour necrosis factor.
Table 6.
Factors and details for each study: gene modifications that have been shown to increase retinal ganglion cell (RGC) survival following axotomy
| Factor* | Animal | No of days postaxotomy | Percentage survival difference† | Administration |
|---|---|---|---|---|
| Anti-Apaf-1 RNA inhibitor73—postinjury | Rat | Day 14 | 200 and 423 RGC/mm2‡ | Nerve stump |
| Anti-c-Jun RNA inhibitor73—postinjury | Rat | Day 14 | 200 and 520 RGC/mm‡ | Nerve stump |
| Bcl-2 overexpression50 | Neonatal mice | 24 h | 50% | Transgenic mice |
| Bcl-2 overexpression52 | Mice | 2–3.5 months‡ | 58% | Transgenic mice |
| Ad-BDNF—postinjury39, 61 | Rat | Day 10, 14 | 39%, 7% | IO-Ad vector |
| BDNF cDNA+electroporation76 —postinjury | Rat | Day 14 | 57.9% | IO cDNA injection |
| Ad-CNTF83, 46—postinjury | Rat | Day 7–21 | 40.1–7% | IO-Ad vector |
| Ad-CNTF71—postinjury | Rat | Day 14 | 19.5% | Nerve stump |
| Lv-CNTF81—postinjury | Rat | Day 14 | 53.1% | IO-Lv vector |
| Ad-IL-1069—postinjury | Rat | Day 14 | 18% | IO-Ad vector |
| Ad-IL-469—postinjury | Rat | Day 14 | 5.4% | IO-Ad vector |
| Ad-p5371—postinjury | Rat | Day 14 | 17.4% | Nerve stump |
| Trk oncogene88—preinjury | Rat | Day 7 | 37% | Superior colliculus |
| TrkB gene38—preinjury | Rat | Day 7, 14, 28 | 25%, 17%, 5% | IO |
| TrkB gene transfer (preinjury)+BDNF (postinjury)38 | Rat | Day 7, 14, 28 | 47%, 66%, 15% | IO |
| Ad-XIAP87—postinjury | Rat | Day 14 | 16.9% | Nerve stump |
| VEGF overexpression86 | Mice | Day 14 | 13.3% | Transgenic mice |
The time the factor was given is listed along with the factor.
Results are calculated as the percentage difference of RGC survival (compared with healthy retina) in the retina of treated eyes versus the untreated eye (ie, percentage of surviving RGC in the treated eye minus the percentage of surviving RGC in the untreated eye).
Wild type was evaluated at 2 months and Transgenic was evaluated at 3.5 months.
Ad, adenoviral; BDNF, brain-derived neurotrophic factor; CNTF, ciliary neurotrophic factor; IL, interleukin; IO, intraocular; Lv, lentiviral; VEGF, vascular endothelial growth factor; XIAP, X-linked inhibitor of apoptosis.
DISCUSSION
Whole-eye transplantation may prove to be the ultimate treatment for irreversible causes of blindness. The three main impediments to transplanting a human eye are:9, 89 maintenance of donor eye viability, optic-nerve regeneration and restoration of topographic organisation, and avoidance of immunological rejection. We have systematically reviewed the first impediment to eye transplantation, which is maintenance of donor eye viability. Three measurable characteristics defining ocular viability that are of particular importance to whole-eye transplantation are recovery of visual function after eye transplantation, outer retina function after enucleation, and RGC survival after optic-nerve transection. The method in which ocular viability was determined and defined was specific to the individual factors and is clearly delineated in the introduction of this article. Our review of the literature concluded that recovery of visual function can occur after whole-eye transplantation in cold-blooded vertebrates. However, limited information is available regarding recovery of visual function after whole-eye transplantation in mammals. In mammals, following enucleation and reperfusion, outer retinal function is maintained from 4 to 9 h. RGC survival following optic-nerve transection is inversely related to both the time since transection and the proximity of transection to the globe. Neurotrophins can increase RGC survival following optic-nerve transection. Taken together, these findings suggest the feasibility of using the donor eye for possible whole-eye transplantation.
One of the primary concerns regarding maintenance of ocular viability is the ability to preserve or recover retinal function of the donor eye following enucleation. Of particular concern are the photoreceptor cells that are responsible for absorbing the light and initiating signal transduction to the higher visual centres. An ideal system for assessing this function is the isolated perfused eye.27 Following enucleation of a cat eye, the ophthalmic artery was immediately cannulated, and artificial perfusion was initiated. ERG responses were obtained over several hours in order to demonstrate that the eye remained viable. The presence of ERG responses demonstrated that the photoreceptors were still functioning. Several additional researchers adopted and improved the perfused eye technique.30, 31, 33, 35, 36 Although most researchers used this method to assess the toxicity of certain substances, its use provides excellent information regarding the length of time an eye can remain viable following enucleation. This is of particular importance with regards to eye transplantation, as it would be absolutely necessary to maintain the retina function of the donor eye in order to allow transplantation to the recipient.
RGC survival after optic-nerve transection is another major concern when evaluating ocular viability. It is well known that following optic-nerve transection, RGCs undergo degeneration.89 It is important, however, to identify the extent of RGC loss and survival as well as what additional factors affect RGC survival. For successful transplantation to occur, RGCs must survive in order to regenerate the optic nerve. We conclude from the literature review that transection of the optic nerve at a point that is more distal from the globe produces far less RGC loss than an injury that is proximal to the globe. Additionally, we were able to summarise many factors that are useful in augmenting RGC survival after optic-nerve transection. Another promising finding regarding RGC survival was demonstrated in cold-blooded vertebrates. In 1985, Scalia et al performed a quantitative analysis of RGC survival following optic-nerve injury and regeneration. Scalia was able to demonstrate that even though the animal fully recovered vision, only 29% of the RGCs remained after 50 weeks.90 Others have since noted survivals of 60%,91 50%92 and 40%93 at different time points after injury. Although these results are somewhat variable, we can easily conclude that considerably less than 100% RGC survival is sufficient for visual recovery. The evidence from cold-blooded vertebrates along with the increasing ability to maintain RGC survival in mammals provides us with very promising evidence regarding RGC survival in eye transplantation.
The ability to successfully reperfuse the enucleated eye after reanastomosis to a different blood supply is integral to maintaining ocular viability following enucleation and transplantation. Herman Sher was the first and one of the only researchers to report the surgical feasibility of reanastomosing the enucleated eye and to assess the presence of reperfusion following the reanastomosis. In one experiment, Sher attempted to reanastomose the ciliary artery of dogs to the femoral artery in rats.18 In a second experiment, Sher contralaterally transplanted sheep eyes.17 For each transplanted eye, eight vascular anastomoses were performed (two arterial and six venous). In both experiments, anastomotic patency and reperfusion were demonstrated by microscopic examination and fluorescein angiography, respectively.
Although much significant research with regards to whole-eye transplantation has been performed, much additional research is still necessary. Future research on eye transplantation should focus on promoting optic-nerve regeneration, as well as further enhancing ocular viability.
Table 5.
Factors and details for each study: combined factors that have been shown to increase retinal ganglion cell (RGC) survival following axotomy
| Factor* | Animal | No of days postaxotomy | Percentage survival difference† | Administration |
|---|---|---|---|---|
| Aurintricarboxylic acid+cortisol59—postinjury | Rat | Day 14 | 44 | IO |
| BDNF+CNTF82—postinjury | Cat | Day 14 | 12 | IO |
| BDNF+GDNF68, 85—postinjury | Rat | Day 14 | 65.2–59.6 | IO |
| BDNF+Neurturin68—postinjury | Rat | Day 14 | 65.1 | IO |
| BDNF+S-PBN (N-tert-butyl-(2-sulfophenyl)-nitrone)65—postinjury | Rat | Day 14 | 56.4 | IO |
| GDNF+Neurturin68—postinjury | Rat | Day 14 | 37 | IO |
The time the factor was given is listed along with the factor.
Results are calculated as the percentage difference of RGC survival (compared with healthy retina) in the retina of treated eyes versus the untreated eye (ie, percentage of surviving RGC in the treated eye minus the percentage of surviving RGC in the untreated eye).
BDNF, brain-derived neurotrophic factor; CNTF, ciliary neurotrophic factor; GDNF, glial derived neurotrophic factor.
Acknowledgments
Funding: Supported by: NIH P30-001792 (DTA) and an unrestricted departmental support from Research to Prevent Blindness (New York).
Footnotes
Competing interests: None.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Resnikoff S, Pascolini D, Etya’ale D, et al. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004;82:844–51. [PMC free article] [PubMed] [Google Scholar]
- 2.American Foundation for the Blind. Facts and figures on Americans with vision loss. [accessed 8 Apr 2009];Statistics and sources for professionals. 2008 September; http://www.afb.org/section.asp?sectionID=15&DocumentID=4398.
- 3.Owen CG, Carey IM, De Wilde S, et al. The epidemiology of medical treatment for glaucoma and ocular hypertension in the United Kingdom: 1994 to 2003. Br J Ophthalmol. 2006;90:861–8. doi: 10.1136/bjo.2005.088666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Owen CG, Fletcher AE, Donoghue M, et al. How big is the burden of visual loss caused by age related macular degeneration in the United Kingdom? Br J Ophthalmol. 2003;87:312–17. doi: 10.1136/bjo.87.3.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scotland GS, McNamee P, Philip S, et al. Cost-effectiveness of implementing automated grading within the national screening programme for diabetic retinopathy in Scotland. Br J Ophthalmol. 2007;91:1518–23. doi: 10.1136/bjo.2007.120972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Atkins EJ, Newman NJ, Biousse V. Post-traumatic visual loss. Rev Neurol Dis. 2008;5:73–81. [PMC free article] [PubMed] [Google Scholar]
- 7.Hollander DA, Jeng BH, Stewart JM. Penetrating ocular injuries in previously injured blind eyes: should we consider primary enucleation? Br J Ophthalmol. 2004;88:438. doi: 10.1136/bjo.2003.027508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dutton JJ. Optic nerve sheath meningiomas. Surv Ophthalmol. 1992;37:167–83. doi: 10.1016/0039-6257(92)90135-g. [DOI] [PubMed] [Google Scholar]
- 9.Anon Scientists urged to hold firm to eye transplant goal. JAMA. 1978;240:1227. [PubMed] [Google Scholar]
- 10.May CH. Enucleation with transplantation and reimplantation of eyes. Med Rec Weekly J Med Surg. 1886;29:613–20. [Google Scholar]
- 11.Bradford HW. A case of enucleatin with replacement of the human globe by that of a rabbit. Boston Med Surg J. 1885;113:269–70. [Google Scholar]
- 12.Anon . Northwest Arkansas Times. Vol. 265. Fayetteville: 1969. First human eye transplant performed. [Google Scholar]
- 13.Freed WJ, Wyatt RJ. Transplantation of eyes to the adult rat brain: histological findings and light-evoked potential response. Life Sci. 1980;27:503–10. doi: 10.1016/0024-3205(80)90132-0. [DOI] [PubMed] [Google Scholar]
- 14.Keeler CE. The functional capacity of transplanted adult frog eyes. J Exp Zool. 1929;54:462–72. [Google Scholar]
- 15.Koppanyi T, Baker C. Further studies on eye transplantation in the spotted rat. Am J Physiol. 1925;71:344–8. [Google Scholar]
- 16.Pietsch P, Schneider CW. Transplanted eyes of foreign donors can reinstate the optically activated skin camouflage reactions in bilaterally enucleated salamanders (Ambystoma) Brain Behav Evol. 1988;32:364–70. doi: 10.1159/000116563. [DOI] [PubMed] [Google Scholar]
- 17.Sher H. Revascularization of autotransplanted ovine eyes by microsurgical anastomosis. J Microsurg. 1981;2:269–72. doi: 10.1002/micr.1920020408. [DOI] [PubMed] [Google Scholar]
- 18.Sher H, Cohen RJ. Revascularization of isolated extracorporeal canine eyes by direct microsurgical anastomosis. J Microsurg. 1980;1:399–402. doi: 10.1002/micr.1920010512. [DOI] [PubMed] [Google Scholar]
- 19.Sperry RW. Restoration of vision after crossing of optic nerves and after contralateral transplantation of eye. J Neurophysiol. 1945;8:18–28. [Google Scholar]
- 20.Stone LS. Heteroplastic transplantation of the eyes between the larvae of two species of Amblystoma. J Exp Zool. 1930;55:193–261. [Google Scholar]
- 21.Stone LS. Return of vision in larval eyes exchanged between Ambystoma punctatum and the cave salamander, Tryphlotriton spelaeus. Invest Ophthalmol. 1964;3:555–65. [PubMed] [Google Scholar]
- 22.Stone LS. Return of vision in transplanted larval eyes of cave salamanders. J Exp Zool. 1964;156:219–27. doi: 10.1002/jez.1401560208. [DOI] [PubMed] [Google Scholar]
- 23.Stone LS, Cole CH. Grafting of larval and adult eyes in Amblystoma punctatum. Proc Soc Exp Biol Med. 1931;29:176–9. [Google Scholar]
- 24.Stone LS, Zaur IS. Reimplantation and transplantation of asult eyes in the salamander (Triturus viridescens) with return of vision. J Exp Zool. 1940;85:243–69. [Google Scholar]
- 25.Stone LS, Ussher NT, Beers DN. Reimplantation and transplantation of laval eyes in the salamander Amblystoma punctatum. J Exp Zool. 1937;77:13–47. [Google Scholar]
- 26.Stone LS, Ussher NT. Return of vision and other observations in replanted amphibian eyes. Proc Soc Exp Biol Med. 1927;25:213–15. [Google Scholar]
- 27.Gouras P, Hoff M. Retinal function in an isolated, perfused mammalian eye. Invest Ophthalmol. 1970;9:388–99. [PubMed] [Google Scholar]
- 28.Lele PP, Grimes P. The role of neural mechanisms in the regulation of intraocular pressure in the cat. Exp Neurol. 1960;2:199–220. doi: 10.1016/0014-4886(60)90009-1. [DOI] [PubMed] [Google Scholar]
- 29.Macri FJ. Acetazolamide and the nervous pressure of the eye. Arch Ophthalmol. 1960;63:953. doi: 10.1001/archopht.1960.00950020955010. [DOI] [PubMed] [Google Scholar]
- 30.Friedman AH, Marchese AL. The isolated perfused frog eye: a useful preparation for the investigation of drug effects on retinal function. J Pharmacol Meth. 1981;5:215–34. doi: 10.1016/0160-5402(81)90089-9. [DOI] [PubMed] [Google Scholar]
- 31.Niemeyer G. The function of the retina in the perfused eye. Doc Ophthalmol. 1975;39:53–116. doi: 10.1007/BF00578759. [DOI] [PubMed] [Google Scholar]
- 32.Peachey NS, Green DJ, Ripps H. Ocular ischemia and the effects of allopurinol on functional recovery in the retina of the arterially perfused cat eye. Invest Ophthalmol Vis Sci. 1993;34:58–65. [PubMed] [Google Scholar]
- 33.Sandberg MA, Pawlyk BS, Crane WG, et al. Effects of IBMX on the ERG of the isolated perfused cat eye. Vision Res. 1987;27:1421–30. doi: 10.1016/0042-6989(87)90152-0. [DOI] [PubMed] [Google Scholar]
- 34.Shahidullah M, Chan HH, Yap MK, et al. Multifocal electroretinography in isolated arterially perfused bovine eye. Ophthalmic Physiol Opt. 2005;25:27–34. doi: 10.1111/j.1475-1313.2004.00241.x. [DOI] [PubMed] [Google Scholar]
- 35.Tazawa Y, Seaman AJ. The electroretinogram of the living extracorporeal bovine eye. The influence of anoxia and hypothermia. Invest Ophthalmol. 1972;11:691–8. [PubMed] [Google Scholar]
- 36.Tseng MT, Liu KN, Radtke ND. Isolated, perfused bovine eye a model for acute retinal toxicity screening. Lens Eye Toxic Res. 1989;6:241–51. [PubMed] [Google Scholar]
- 37.Berkelaar M, Clarke DB, Wang YC, et al. Axotomy results in delayed death and apoptosis of retinal ganglion cells in adult rats. J Neurosci. 1994;14:4368–74. doi: 10.1523/JNEUROSCI.14-07-04368.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheng L, Sapieha P, Kittlerova P, et al. TrkB gene transfer protects retinal ganglion cells from axotomy-induced death in vivo. J Neurosci. 2002;22:3977–86. doi: 10.1523/JNEUROSCI.22-10-03977.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Di Polo A, Aigner LJ, Dunn RJ, et al. Prolonged delivery of brain-derived neurotrophic factor by adenovirus-infected Muller cells temporarily rescues injured retinal ganglion cells. Proc Natl Acad Sci USA. 1998;95:3978–83. doi: 10.1073/pnas.95.7.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Germain F, Calvo M, de la Villa P. Rabbit retinal ganglion cell survival after optic nerve section and its effect on the inner plexiform layer. Exp Eye Res. 2004;78:95–102. doi: 10.1016/j.exer.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 41.Grafstein B, Ingoglia NA. Intracranial transection of the optic nerve in adult mice: preliminary observations. Exp Neurol. 1982;76:318–30. doi: 10.1016/0014-4886(82)90212-6. [DOI] [PubMed] [Google Scholar]
- 42.Hou B, You SW, Wu MM, et al. Neuroprotective effect of inosine on axotomized retinal ganglion cells in adult rats. Invest Ophthalmol Vis Sci. 2004;45:662–7. doi: 10.1167/iovs.03-0281. [DOI] [PubMed] [Google Scholar]
- 43.Kretz A, Schmeer C, Tausch S, et al. Simvastatin promotes heat shock protein 27 expression and Akt activation in the rat retina and protects axotomized retinal ganglion cells in vivo. Neurobiol Dis. 2006;21:421–30. doi: 10.1016/j.nbd.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 44.Manabe S, Kashii S, Honda Y, et al. Quantification of axotomized ganglion cell death by explant culture of the rat retina. Neurosci Lett. 2002;334:33–6. doi: 10.1016/s0304-3940(02)01047-9. [DOI] [PubMed] [Google Scholar]
- 45.Takano M, Horie H. Critical period for degradation of adult rat retinal ganglion cells and their regeneration capability after axotomy. Neurosci Lett. 1994;175:129–32. doi: 10.1016/0304-3940(94)91096-0. [DOI] [PubMed] [Google Scholar]
- 46.van Adel BA, Arnold JM, Phipps J, et al. Ciliary neurotrophic factor protects retinal ganglion cells from axotomy-induced apoptosis via modulation of retinal glia in vivo. J Neurobiol. 2005;63:215–34. doi: 10.1002/neu.20117. [DOI] [PubMed] [Google Scholar]
- 47.Villegas-Perez MP, Vidal-Sanz M, Rasminsky M, et al. Rapid and protracted phases of retinal ganglion cell loss follow axotomy in the optic nerve of adult rats. J Neurobiol. 1993;24:23–36. doi: 10.1002/neu.480240103. [DOI] [PubMed] [Google Scholar]
- 48.Watanabe M, Inukai N, Fukuda Y. Survival of retinal ganglion cells after transection of the optic nerve in adult cats: a quantitative study within two weeks. Vis Neurosci. 2001;18:137–45. doi: 10.1017/s0952523801181137. [DOI] [PubMed] [Google Scholar]
- 49.Baptiste DC, Powell KJ, Jollimore CA, et al. Effects of minocycline and tetracycline on retinal ganglion cell survival after axotomy. Neuroscience. 2005;134:575–82. doi: 10.1016/j.neuroscience.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 50.Bonfanti L, Strettoi E, Chierzi S, et al. Protection of retinal ganglion cells from natural and axotomy-induced cell death in neonatal transgenic mice overexpressing bcl-2. J Neurosci. 1996;16:4186–94. doi: 10.1523/JNEUROSCI.16-13-04186.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carmignoto G, Maffei L, Candeo P, et al. Effect of NGF on the survival of rat retinal ganglion cells following optic nerve section. J Neurosci. 1989;9:1263–72. doi: 10.1523/JNEUROSCI.09-04-01263.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cenni MC, Bonfanti L, Martinou JC, et al. Long-term survival of retinal ganglion cells following optic nerve section in adult bcl-2 transgenic mice. Eur J Neurosci. 1996;8:1735–45. doi: 10.1111/j.1460-9568.1996.tb01317.x. [DOI] [PubMed] [Google Scholar]
- 53.Cho KS, Chung SK, Yip HK, et al. Differential effects of intravitreal optic nerve and sciatic nerve grafts on the survival of retinal ganglion cells and the regeneration of their axons. J Neurocytol. 2001;30:983–91. doi: 10.1023/a:1021884606771. [DOI] [PubMed] [Google Scholar]
- 54.Choi JS, Kim JA, Joo CK. Activation of MAPK and CREB by GM1 induces survival of RGCs in the retina with axotomized nerve. Invest Ophthalmol Vis Sci. 2003;44:1747–52. doi: 10.1167/iovs.01-0886. [DOI] [PubMed] [Google Scholar]
- 55.Diem R, Hobom M, Grotsch P, et al. Interleukin-1 beta protects neurons via the interleukin-1 (IL-1) receptor-mediated Akt pathway and by IL-1 receptor-independent decrease of transmembrane currents in vivo. Mol Cell Neurosci. 2003;22:487–500. doi: 10.1016/s1044-7431(02)00042-8. [DOI] [PubMed] [Google Scholar]
- 56.Diem R, Meyer R, Weishaupt JH, et al. Reduction of potassium currents and phosphatidylinositol 3-kinase-dependent AKT phosphorylation by tumor necrosis factor-(alpha) rescues axotomized retinal ganglion cells from retrograde cell death in vivo. J Neurosci. 2001;21:2058–66. doi: 10.1523/JNEUROSCI.21-06-02058.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Eschweiler GW, Bahr M. Flunarizine enhances rat retinal ganglion cell survival after axotomy. J Neurol Sci. 1993;116:34–40. doi: 10.1016/0022-510x(93)90086-e. [DOI] [PubMed] [Google Scholar]
- 58.Heiduschka P, Thanos S. Aurintricarboxylic acid promotes survival and regeneration of axotomised retinal ganglion cells in vivo. Neuropharmacology. 2000;39:889–902. doi: 10.1016/s0028-3908(99)00245-2. [DOI] [PubMed] [Google Scholar]
- 59.Heiduschka P, Thanos S. Cortisol promotes survival and regeneration of axotomised retinal ganglion cells and enhances effects of aurintricarboxylic acid. Graefes Arch Clin Exp Ophthalmol. 2006;244:1512–21. doi: 10.1007/s00417-005-0164-7. [DOI] [PubMed] [Google Scholar]
- 60.Huxlin KR, Dreher B, Schulz M, et al. Effect of collicular proteoglycan on the survival of adult rat retinal ganglion cells following axotomy. Eur J Neurosci. 1995;7:96–107. doi: 10.1111/j.1460-9568.1995.tb01024.x. [DOI] [PubMed] [Google Scholar]
- 61.Isenmann S, Klocker N, Gravel C, et al. Short communication: protection of axotomized retinal ganglion cells by adenovirally delivered BDNF in vivo. Eur J Neurosci. 1998;10:2751–6. doi: 10.1046/j.1460-9568.1998.00325.x. [DOI] [PubMed] [Google Scholar]
- 62.Kermer P, Klocker N, Labes M, et al. Insulin-like growth factor-I protects axotomized rat retinal ganglion cells from secondary death via PI3-K-dependent Akt phosphorylation and inhibition of caspase-3 In vivo. J Neurosci. 2000;20:2–8. [PubMed] [Google Scholar]
- 63.Kilic U, Kilic E, Lingor P, et al. Rifampicin inhibits neurodegeneration in the optic nerve transection model in vivo and after 1-methyl-4-phenylpyridinium intoxication in vitro. Acta Neuropathol. 2004;108:65–8. doi: 10.1007/s00401-004-0867-6. [DOI] [PubMed] [Google Scholar]
- 64.Klocker N, Braunling F, Isenmann S, et al. In vivo neurotrophic effects of GDNF on axotomized retinal ganglion cells. Neuroreport. 1997;8:3439–42. doi: 10.1097/00001756-199711100-00005. [DOI] [PubMed] [Google Scholar]
- 65.Klocker N, Cellerino A, Bahr M. Free radical scavenging and inhibition of nitric oxide synthase potentiates the neurotrophic effects of brain-derived neurotrophic factor on axotomized retinal ganglion cells In vivo. J Neurosci. 1998;18:1038–46. doi: 10.1523/JNEUROSCI.18-03-01038.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Koeberle PD, Ball AK. Effects of GDNF on retinal ganglion cell survival following axotomy. Vision Res. 1998;38:1505–15. doi: 10.1016/s0042-6989(97)00364-7. [DOI] [PubMed] [Google Scholar]
- 67.Koeberle PD, Ball AK. Nitric oxide synthase inhibition delays axonal degeneration and promotes the survival of axotomized retinal ganglion cells. Exp Neurol. 1999;158:366–81. doi: 10.1006/exnr.1999.7113. [DOI] [PubMed] [Google Scholar]
- 68.Koeberle PD, Ball AK. Neurturin enhances the survival of axotomized retinal ganglion cells in vivo: combined effects with glial cell line-derived neurotrophic factor and brain-derived neurotrophic factor. Neuroscience. 2002;110:555–67. doi: 10.1016/s0306-4522(01)00557-7. [DOI] [PubMed] [Google Scholar]
- 69.Koeberle PD, Gauldie J, Ball AK. Effects of adenoviral-mediated gene transfer of interleukin-10, interleukin-4, and transforming growth factor-beta on the survival of axotomized retinal ganglion cells. Neuroscience. 2004;125:903–20. doi: 10.1016/S0306-4522(03)00398-1. [DOI] [PubMed] [Google Scholar]
- 70.Kudo H, Nakazawa T, Shimura M, et al. Neuroprotective effect of latanoprost on rat retinal ganglion cells. Graefes Arch Clin Exp Ophthalmol. 2006;244:1003–9. doi: 10.1007/s00417-005-0215-0. [DOI] [PubMed] [Google Scholar]
- 71.Kugler S, Klocker N, Kermer P, et al. Transduction of axotomized retinal ganglion cells by adenoviral vector administration at the optic nerve stump: an in vivo model system for the inhibition of neuronal apoptotic cell death. Gene Ther. 1999;6:1759–67. doi: 10.1038/sj.gt.3301000. [DOI] [PubMed] [Google Scholar]
- 72.Li S, Hu B, Tay D, et al. Intravitreal transplants of Schwann cells and fibroblasts promote the survival of axotomized retinal ganglion cells in rats. Brain Res. 2004;1029:56–64. doi: 10.1016/j.brainres.2004.09.038. [DOI] [PubMed] [Google Scholar]
- 73.Lingor P, Koeberle P, Kugler S, et al. Down-regulation of apoptosis mediators by RNAi inhibits axotomy-induced retinal ganglion cell death in vivo. Brain. 2005;128:550–8. doi: 10.1093/brain/awh382. [DOI] [PubMed] [Google Scholar]
- 74.Mansour-Robaey S, Clarke DB, Wang YC, et al. Effects of ocular injury and administration of brain-derived neurotrophic factor on survival and regrowth of axotomized retinal ganglion cells. Proc Natl Acad Sci USA. 1994;91:1632–6. doi: 10.1073/pnas.91.5.1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Miki A, Otori Y, Morimoto T, et al. Protective effect of donepezil on retinal ganglion cells in vitro and in vivo. Curr Eye Res. 2006;31:69–77. doi: 10.1080/02713680500477438. [DOI] [PubMed] [Google Scholar]
- 76.Mo X, Yokoyama A, Oshitari T, et al. Rescue of axotomized retinal ganglion cells by BDNF gene electroporation in adult rats. Invest Ophthalmol Vis Sci. 2002;43:2401–5. [PubMed] [Google Scholar]
- 77.Morimoto T, Miyoshi T, Fujikado T, et al. Electrical stimulation enhances the survival of axotomized retinal ganglion cells in vivo. Neuroreport. 2002;13:227–30. doi: 10.1097/00001756-200202110-00011. [DOI] [PubMed] [Google Scholar]
- 78.Peinado-Ramon P, Salvador M, Villegas-Perez MP, et al. Effects of axotomy and intraocular administration of NT-4, NT-3, and brain-derived neurotrophic factor on the survival of adult rat retinal ganglion cells. A quantitative in vivo study. Invest Ophthalmol Vis Sci. 1996;37:489–500. [PubMed] [Google Scholar]
- 79.Shirvan A, Kimron M, Holdengreber V, et al. Anti-semaphorin 3A antibodies rescue retinal ganglion cells from cell death following optic nerve axotomy. J Biol Chem. 2002;277:49799–807. doi: 10.1074/jbc.M204793200. [DOI] [PubMed] [Google Scholar]
- 80.Sievers J, Hausmann B, Unsicker K, et al. Fibroblast growth factors promote the survival of adult rat retinal ganglion cells after transection of the optic nerve. Neurosci Lett. 1987;76:157–62. doi: 10.1016/0304-3940(87)90708-7. [DOI] [PubMed] [Google Scholar]
- 81.van Adel BA, Kostic C, Deglon N, et al. Delivery of ciliary neurotrophic factor via lentiviral-mediated transfer protects axotomized retinal ganglion cells for an extended period of time. Hum Gene Ther. 2003;14:103–15. doi: 10.1089/104303403321070801. [DOI] [PubMed] [Google Scholar]
- 82.Watanabe M, Tokita Y, Kato M, et al. Intravitreal injections of neurotrophic factors and forskolin enhance survival and axonal regeneration of axotomized beta ganglion cells in cat retina. Neuroscience. 2003;116:733–42. doi: 10.1016/s0306-4522(02)00562-6. [DOI] [PubMed] [Google Scholar]
- 83.Weise J, Isenmann S, Klocker N, et al. Adenovirus-mediated expression of ciliary neurotrophic factor (CNTF) rescues axotomized rat retinal ganglion cells but does not support axonal regeneration in vivo. Neurobiol Dis. 2000;7:212–23. doi: 10.1006/nbdi.2000.0285. [DOI] [PubMed] [Google Scholar]
- 84.Weishaupt JH, Rohde G, Polking E, et al. Effect of erythropoietin axotomy-induced apoptosis in rat retinal ganglion cells. Invest Ophthalmol Vis Sci. 2004;45:1514–22. doi: 10.1167/iovs.03-1039. [DOI] [PubMed] [Google Scholar]
- 85.Yan Q, Wang J, Matheson CR, et al. Glial cell line-derived neurotrophic factor (GDNF) promotes the survival of axotomized retinal ganglion cells in adult rats: comparison to and combination with brain-derived neurotrophic factor (BDNF) J Neurobiol. 1999;38:382–90. doi: 10.1002/(sici)1097-4695(19990215)38:3<382::aid-neu7>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 86.Kilic U, Kilic E, Jarve A, et al. Human vascular endothelial growth factor protects axotomized retinal ganglion cells in vivo by activating ERK-1/2 and Akt pathways. J Neurosci. 2006;26:12439–46. doi: 10.1523/JNEUROSCI.0434-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kugler S, Straten G, Kreppel F, et al. The X-linked inhibitor of apoptosis (XIAP) prevents cell death in axotomized CNS neurons in vivo. Cell Death Differ. 2000;7:815–24. doi: 10.1038/sj.cdd.4400712. [DOI] [PubMed] [Google Scholar]
- 88.Garcia Valenzuela E, Sharma SC. Rescue of retinal ganglion cells from axotomy-induced apoptosis through TRK oncogene transfer. Neuroreport. 1998;9:3165–70. doi: 10.1097/00001756-199810050-00008. [DOI] [PubMed] [Google Scholar]
- 89.MacLaren RE. Regeneration and transplantation of the optic nerve: developing a clinical strategy. Br J Ophthalmol. 1998;82:577–83. doi: 10.1136/bjo.82.5.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Scalia F, Arango V, Singman EL. Loss and displacement of ganglion cells after optic nerve regeneration in adult Rana pipiens. Brain Res. 1985;344:267–80. doi: 10.1016/0006-8993(85)90804-2. [DOI] [PubMed] [Google Scholar]
- 91.Humphrey MF, Beazley LD. Retinal ganglion cell death during optic nerve regeneration in the frog Hyla moorei. J Comp Neurol. 1985;236:382–402. doi: 10.1002/cne.902360307. [DOI] [PubMed] [Google Scholar]
- 92.Beazley LD, Darby JE, Perry VH. Cell death in the retinal ganglion cell layer during optic nerve regeneration for the frog Rana pipiens. Vision Res. 1986;26:543–56. doi: 10.1016/0042-6989(86)90003-9. [DOI] [PubMed] [Google Scholar]
- 93.Stelzner DJ, Strauss JA. A quantitative analysis of frog optic nerve regeneration: is retrograde ganglion cell death or collateral axonal loss related to selective reinnervation? J Comp Neurol. 1986;245:83–106. doi: 10.1002/cne.902450107. [DOI] [PubMed] [Google Scholar]