Abstract
Hippocalcin is a member of the neuronal Ca2+ sensor protein family. Among its many biochemical functions, its established physiological function is that via neuronal apoptosis inhibitory protein it protects the neurons from Ca2+-induced cell death. The precise biochemical mechanism/s through which hippocalcin functions, is not clear. In the present study, a new mechanism by which it functions is defined. The bovine form of hippocalcin (BovHpca) native to the hippocampus has been purified, sequenced, cloned and studied. The findings show that there is the evolutionary conservation of its structure. It is a Ca2+-sensor of a variant form of the ROS-GC subfamily of membrane guanylate cyclases, ONE-GC. It senses physiological increments of Ca2+ with a K1/2 of 0.5 μM and stimulates ONE-GC or ONE-GC-like membrane guanylate cyclase. The Hpca-modulated ONE-GC-like transduction system exists in the hippocampal neurons. And hippocalcin-modulated ONE-GC transduction system exists in the olfactory receptor neuroepithelium. The Hpca-gene knock out studies demonstrate that the portion of this is about 30% of the total membrane guanylate cyclase transduction system. The findings establish Hpca as a new Ca2+ sensor modulator of the ROS-GC membrane guanylate cyclase transduction subfamily. They support the concept on universality of the presence and operation of the ROS-GC transduction system in the sensory and sensory-linked neurons. They validate that the ROS-GC transduction system exists in multiple forms. And they provide an additional mechanism by which ROS-GC subfamily acts as a transducer of the Ca2+ signals originating in the neurons.
Keywords: membrane guanylate cyclase, calcium, calcium binding proteins, hippocalcin, hippocampus, olfactory neuroepithelium, signal transduction
INTRODUCTION
Cyclic GMP is an omnipresent intracellular second messenger of prokaryotes and eukaryotes [reviewed in: 1]. Generated by membrane-bound and soluble forms of guanylate cyclase, it plays a critical role in the control of physiological processes of cardiovasculature, smooth muscle relaxation, blood pressure, blood volume, cellular growth, sensory transduction, neural plasticity, learning and memory. Depending on its source, it is produced and functions through multiple pathways.
This paper deals with the pool of cyclic GMP that is catalyzed by the membrane bound guanylate cyclase transduction system. This transduction system is modulated by the Ca2+ spikes in the neuronal cells [reviewed in: 2]. It belongs to the ROS-GC subfamily, which is composed of three members—ROS-GC1, ROS-GC2 and ONE-GC, also termed GC-D [2].
The other membrane guanylate cyclase subfamily is also composed of three members: ANF-RGC, CNP-RGC and STa-RGC [reviewed in: 1]. Unlike ROS-GC, this subfamily does not respond to the intracellularly-generated Ca2+ signals. Its distinctive characteristic is that besides being a guanylate cyclase, it is also the surface receptor of its ligand, hormone. The ligands of ANF-RGC are ANF and BNP, of CNP-RGC is CNP and of STa-RGC are guanylin, uroguanylin and heat-stable enterotoxin.
A recent study shows that ONE-GC is a cross-over membrane guanylate cyclase; it responds to the extracellularly-generated peptide hormonal signal, uroguanylin and also to the intracellularly-generated neuronal Ca2+ signal modulated by neurocalcin δ [3].
To sense multiple forms of Ca2+ signals and yet to be specific to each of those, ROS-GC transduction system has evolved a remarkable structural design [reviewed in: 2]. It is a two-component transduction system: the Ca2+ sensor, GCAP (guanylate cyclase activating protein) or CD-(Ca2+-dependent) GCAP; and the transducer, ROS-GC membrane guanylate cyclase. Upon sensing progressive rises in free Ca2+, GCAPs proportionately decelerate and CD-GCAPs accelerate the ROS-GC activity. These properties make ROS-GC a bimodal Ca2+ signal transduction switch, turning itself “OFF” and “ON” with the intensity of the parent Ca2+ waves in the neurons. Each Ca2+ spike is faithfully translated into the production of cyclic GMP. Accompanied by a nearby cyclic GMP-gated channel, each parent spike is able to depolarize or polarize the membrane potential of the neuron. The generated action potential then becomes a general means of transmitting the Ca2+ signals across the neural sensory network [model in ref. 4].
In accordance with this model, LIGHT signal hyperpolarizes the rod outer segment membranes and the ODORANT signal, uroguanylin, depolarizes the olfactory ciliary neuroepithelial membranes [2, 4]. In the LIGHT signal GCAPs, 1 and 2, are the Ca2+ sensor components of ROS-GC1 and in the ODORANT signal the CD-GCAP, neurocalcin δ, is the Ca2+ sensor component of ONE-GC.
Six Ca2+-sensor components of the ROS-GC transduction system have been identified. Three are GCAPs: 1, 2 3; three are CD-GCAPs: S100B, neurocalcin δ and frequenin [reviewed in: 2]. GCAP1 and GCAP2 are expressed across species [reviewed in: 5] but the expression of GCAP3 is limited to the cones of human and zebra fish [6].
With the presence of ROS-GC in three forms and of universal GCAPs in two forms, 1 and 2, theoretically, with the individual pairing of a GCAP with each ROS-GC, the GCAP-modulated ROS-GC transduction machine can exist in multiple forms. This theoretical prediction has been validated by the studies on the GCAP-modulated ROS-GC1 transduction system. Three types of these machines have been characterized in the retinal neurons [7–10], one in the olfactory bulb [11], and one in the pinealocytes [12].
Similarly, paired with a CD-GCAP, ROS-GC1 transduction system can also exist in multiple forms. And this prediction has also been validated by demonstrating the presence of S100B-modulated ROS-GC1 in the photoreceptor-bipolar synapse [13], the pinealocytes [12], and in the gustatory epithelium [14]; neurocalcin δ-modulated ROS-GC1, in the inner retinal neurons [15].
The existence in the multiple forms may be the general property of the ROS-GC subfamily transduction system because the variant form of ROS-GC1, ONE-GC, is Ca2+-modulated through its Ca2+-senosr component neurocalcin δ in the native olfactory neuroepithelium [16]; and a ONE-GC-like transducer is frequenin-modulated in hippocampal neurons [17]. Another interesting GCAP1-modulated Ca2+ signaling ONE-GC transduction system exists in the olfactory neuroepithelium [18]. Remarkably, in this case, GCAP1, an inhibitory Ca2+ sensor of ROS-GC1 in photoreceptors, is a stimulatory Ca2+ sensor of ONE-GC.
In presence of the multiple forms of Ca2+ sensor components and the multiple forms of transduction components in a given neuron, how does that neuron achieve cellular specificity to the parent Ca2+ signal?
The emerging picture is that it is achieved through the elegant structural designs of the GCAPs/CD-GCAPs and of the domains of ROS-GC, which they modulate. Each ROS-GC module is crafted to fit with only a single Ca2+ sensor, and upon their union, the Ca2+-activated module of ROS-GC attains an independent status of being a sole messenger of that GCAP- or CD-GCAP-captured Ca2+ signal. In this manner, at one time, only one type of the Ca2+ signal controls the activity of the catalytic module of ROS-GC1. The net result is that each Ca2+ spike is faithfully translated into the production of cyclic GMP by the ROS-GC and cyclic GMP via its down stream signaling components precisely controls cellular activity.
The present study broadens this mode of signaling. The findings identify hippocalcin as a new Ca2+ sensor component of the transducer ONE-GC. They demonstrate that Ca2+-modulated hippocalcin-ONE-GC transduction system is present in the sensory and the sensory-linked neurons of olfaction. They, therefore, support the concept that the Ca2+-modulated ROS-GC transduction machinery in its variant forms is a central operational component of all sensory-linked networks of neurons [reviewed in: 2].
MATERIALS AND METHODS
Purification of hippocalcin from bovine hippocampus
All purification steps were conducted in cold room maintained at 8 °C unless stated otherwise. Partial purification with minor modifications of the bovine form of hippocalcin (Hpca) was carried out following the previous protocol used for purification of the rodent form [19].
The hippocampus proper and subiculum were dissected out from the fresh bovine brain and homogenized in 6 volumes of 20 mM Tris-HCl, pH 7.5, containing 1mM PMSF, 5 mM EDTA, 5 mM EGTA and protease inhibitors cocktail (Sigma). The homogenate was clarified by sequential centrifugation at 5000g to remove nuclei and cellular debris and 12,000g to remove the mitochondria. The post mitochondrial supernatant was centrifuged at 100,000g and the supernatant was subjected to ammonium sulfate fractionation. The pellet obtained at 40–70% ammonium sulfate concentration was dissolved in 20 mM Tris-HCl, pH 7.5 and dialyzed overnight against the same buffer with two buffer changes.
Phenyl-Sepharose column chromatography
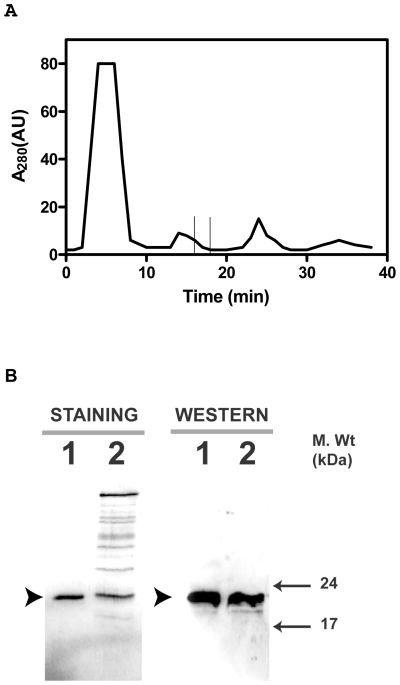
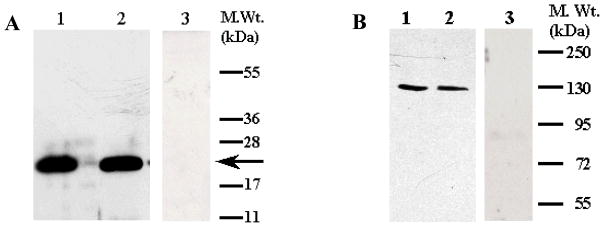
The dialysate was brought to 0.5 M NaCl and 2 mM CaCl2 concentration and centrifuged to remove any precipitate. The supernatant was loaded onto a phenyl-Sepharose column equilibrated with 10 column volumes of 20 mM Tris-HCl, pH 7.5, containing 0.5 M NaCl, and 2 mM CaCl2. The column was washed with 20 column volumes of the same buffer followed by 20 mM Tris-HCl, pH 7.5 containing 0.1 M NaCl and 2 mM CaCl2. Elution of the bound proteins was carried out by a linear gradient of 0.1–0 M NaCl, in 20mM Tris HCl, pH 7.5 containing 5 mM EGTA (Fig. 1A). Tightly-bound proteins were eluted by double distilled H2O followed by 6 M urea. All fractions were analyzed on SDS-12%PAGE, followed by Western blotting using specific anti-Hpca antibody (Abgent, CA) (Fig. 1B). The fractions containing Hpca immunoreactivity were pooled and concentrated using Amicon Ultra-4 centrifugal filtration units with 10 kDa cut-off.
Figure 1. Purification of Hpca from bovine hippocampus.
Bovine Hpca was partially purified by hydrophobic interaction chromatography on phenyl-Sepharose CL-4B column as described in the “Materials and Methods” section. A. Elution Profile. The absorbance of the eluted proteins at 280nm was plotted against the elution time. The time at which Hpca elutes is marked by the window. B. Fractions containing Hpca were concentrated, separated by SDS-12%PAGE and analyzed by Coomassie blue staining and Western blotting. Band corresponding to Hpca is indicated by solid arrowhead. Lane 1. Recombinant myr-Hpca. Lane 2. Eluted and concentrated fraction.
Protein identification
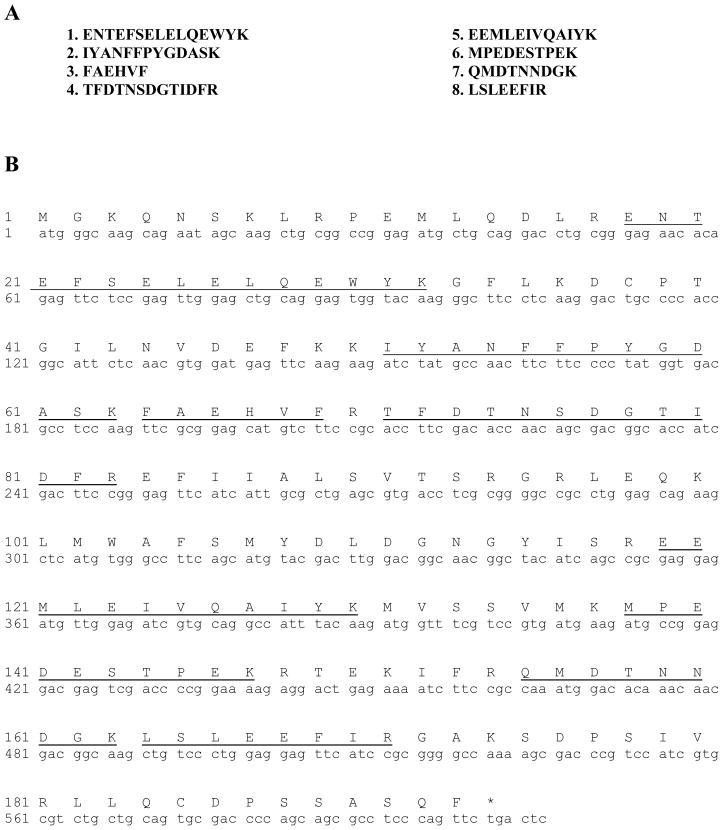
The pooled-fractions were separated by 12%-SDS-PAGE. The protein bands were visualized by Coomassie blue staining, de-stained and washed extensively in de-ionized water. The band corresponding to Hpca antibody reactivity on Western blot was excised and trypsin-digested. The resultant peptides were analyzed by high pressure liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS) (Howard Hughes Medical Institute Biopolymer Laboratory and W.M. Keck Foundation Biotechnology Resource Laboratory at Yale University New Haven, CT). The peptide masses were used to search the protein database using two independent programs: ProFound and Mascot. Eight tryptic-peptides were identified (Fig. 2A). Their sequences are present in the sequences of the recombinant forms of the human [20, 21], mouse [21] and rat [19] Hpca. It was thereby concluded that the purified hippocampal protein is the bovine form of Hpca and is referred as BovHpca.
Figure 2.
A. Amino acid sequence of tryptic peptides of native bovine Hpca. Sequences of internal peptides were determined by LC-MS/MS. B. Nucleotide and deduced amino acid sequence of the Hpca cloned from bovine hippocampus. Coding region of Hpca from bovine hippocampal RNA was amplified by RT-PCR as described in “Materials and Methods”. Eight peptides identified and sequenced from native bovine Hpca are underlined.
Cloning, expression and purification of recombinant BovHpca
BovHpca was cloned by amplifying the hippocampal RNA through PCR, using the forward primer: 5′-GTACCATGGGCAAGCAGAAYAGCAAG-3′ and the reverse primer: 5′-GTACTCGAGTCAGAACTGGGARGCGCT-3′ (GeneBank accession no. L27421). To facilitate cloning, Nco1 and Xho1 restriction sites (underlined) were introduced in the forward and reverse primers, respectively. The amplicon encompassing the entire BovHpca coding region (Fig. 2B) was cloned into the pET 21d bacterial expression vector and sequenced to confirm its identity. For expression of the protein, E. coli strain BL21-Codon Plus cells were transformed with the construct HCal/21d. To obtain N-myristoylated BovHpca, BL21-Codon Plus cells were co-transformed with the plasmid pBB131 harboring the N-myristoyl transferase from S. cerevisiae along with Hcal/21d. The bacterial cells were grown in LB medium at 37 °C and at OD of 0.6 at 600 nm they were induced with 1 mM IPTG. The myristoylated form was obtained by the addition of myristic acid (50 μg/ml) to the culture medium 30 min prior to the addition of IPTG. Cells were harvested 3 hrs after induction, suspended in 20 mM Tris-HCl/0.1 M NaCl, pH 7.5 and stored at −80 °C until use.
Purification of the myristoylated or non-myristoylated recombinant BovHpca from bacterial lysate was carried out on the phenyl-Sepharose column as described above for the native BovHpca.
Polyclonal antibodies
Characterization of highly specific antibodies raised in rabbits against ONE-GC has been described previously [16]. Anti-Hpca antibodies were either purchased (Abgent, CA) or custom raised in rabbits against bacterially expressed recombinant myristoylated BovHpca. The antibodies were affinity purified using Hpca coupled Sepharose beads (NHS activated Sepharose Fast Flow, Amersham Biosciences). Hpca coupled Sepharose beads were prepared by coupling 5 mg of purified recombinant Hpca to NHS Sepharose according to the manufacturer’s protocol. Antibody specificity for BovHpca was tested for its cross-reactivity against other calcium sensor proteins by Western blotting (Fig. 3).
Figure 3. Specificity of Hpca antibody.
2 μg of each: frequenin (FQ), GCAP1, GCAP2, Hpca, neurocalcin δ (Ncal δ), and S100β were separated by SDS-15%PAGE. One gel was stained with Coomassie blue (STAINING) and another gel containing the same set of proteins was transferred to PVDF membranes and probed with anti Hpca antibody (WESTERN). The band corresponding to Hpca is indicated by an arrow. Molecular size markers are given alongside.
Ca2+-dependent membrane association
To assess the presence of active Ca2+-myristoyl switch in BovHpca native to hippocampus, Ca2+-dependent association of Hpca to the membranes was investigated according to the earlier protocols [15]. The bovine hippocampal membranes were isolated in the presence of 2 mM EGTA (Ca2+-depleted condition), 50 μM Ca2+ (Ca2+-enriched condition), or without any additions (native conditions). They were then subjected to SDS-12% PAGE, transferred onto PVDF membranes and probed with the anti-BovHpca antibody.
In the recombinant heterologous system, the membranes of COS-7 cells were incubated with bacterially-expressed recombinant myristoylated BovHpca. The membranes were washed twice with 20 mM Tris-HCl/0.1 M NaCl, pH 8.0, and 1mM PMSF and re-suspended in the same buffer. 5 μg of recombinant myr-Hpca was incubated with COS cell membranes (equivalent of 15 μg protein) in the presence of 2 mM EGTA or 50 μM CaCl2 in a reaction volume of 25 μl at room temperature for 20 min. The reaction mixture was centrifuged at 14,000 rpm for 20 min. Both the pellet and the supernatant were subjected to SDS-12% PAGE. The proteins were transferred to PVDF membrane and probed with the anti-BovHpca antibody.
Coimmunoprecipitation
Affinity purified antibodies (against ONE-GC or Hpca) were individually coupled to AminoLink® coupling gel (Pierce) according to the manufacturer’s protocol. Membranes of the olfactory neuroepithelium were isolated in the presence of 100 μM Ca2+ and solubilized in a buffer containing 20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1% Triton X-100 and 2 mM PMSF. The solubilized membranes were incubated with AminoLink coupled antibodies in the presence of 1 mM Ca2+ overnight at 4 °C. The AminoLink-antibody-antigen complexes were spun down and washed several times with the 20 mM Tris-HCl/150 mM NaCl buffer pH 7.5 containing 1 mM CaCl2. Bound antigens were eluted using SDS-sample buffer, separated through SDS-polyacrylamide gel electrophoresis and transferred to nitrocellulose membrane. Samples were probed with antibodies against Hpca or ONE-GC.
Guanylate cyclase assay
Membrane fractions isolated from bovine hippocampus, mice olfactory neuroepithelium or transfected COS cells were assayed for guanylate cyclase activity as described previously [15–17]. Briefly, membranes were pre-incubated on ice with or without bovine Hpca in the assay system containing 10 mM theophylline, 15 mM phosphocreatine, 20 μg creatine kinase and 50 mM Tris-HCl, pH 7.5, adjusted to appropriate free Ca2+ concentrations with pre-calibrated Ca2+/EGTA solutions (Molecular Probes). The total assay volume was 25 μl. The reaction was initiated by addition of the substrate solution (4 mM MgCl2 and 1mM GTP, final concentration) and maintained by incubation at 37°C for 10 min. The reaction was terminated by the addition of 225 μl of 50 mM sodium acetate buffer, pH 6.2 followed by heating on a boiling water bath for 3 min. The amount of cyclic GMP formed was determined by radioimmunoassay [22].
Hpca knock out mice
Characterization of the Hpca−/− genotype mice is provided in reference [23].
RESULTS
Hpca native to bovine hippocampus
Purification
The protein purified from the bovine hippocampus was analyzed by SDS-PAGE and Western blot using anti-BovHippocampus antibody. The protein shows only one Coomassie blue stained band and its molecular mass is 22 kDa (Fig. 1B, panel “STAINING”). The protein reacts with the anti-BovHpca antibody (Abgent, CA) (Fig. 1B, panel “WESTERN”). These results show that the bovine hippocampal protein is homogeneous and is selectively recognized by the anti-BovHpca antibody. Thus the isolated protein is, or is immunologically similar to Hpca.
Microsequencing
The N-terminal sequencing of the protein was not successful, indicating that it was acylated, thus, blocked. The internal fragments generated by tryptic digestion were identified by mass spectrometry (LC-MS/MS). The masses were used to search the protein database using two independent programs, Profound and Mascot. All eight sequences (Fig. 2A) of the generated peptides were found in the sequences of Hpca cloned from human [20], mouse [21] and rat [19].
It is, therefore, concluded that the purified and characterized protein native to the bovine hippocampus is Hpca.
Molecular cloning
The full length bovine cDNA clone isolated from the bovine hippocampus library encoded all 8 tryptic peptides (Fig. 2B). This indicated that the mRNA encoding the native hippocampal BovHpca has been cloned.
The cDNA-based sequence of the protein was identical to the published sequences of the recombinant forms of human [20, 21], rat [19] and mouse [21] Hpca.
It is concluded that the Hpca native to the bovine hippocampus has been cloned, its structure is conserved throughout evolution and most probably the primary structures of all mammalian forms of Hpca are identical.
Like other forms, BovHpca contains four Ca2+-binding motifs (EF hands), yet only three--EF2, EF3, EF4—are predicted to be functional. This raises the possibility that the apparent mode of Ca2+ signaling modulated by Hpca is identical in all mammalian species.
Ca2+-myristoyl switch
Ca2+ myristoyl switch is an important biochemical property of several members of the neuronal Ca2+ sensor proteins. This property is bestowed upon the protein by its N-myristoyl group. In the absence of free Ca2+, myristoyl group is tethered into the hydrophobic pocket of the protein. Upon binding of Ca2+ to the protein’s EF-hands, the myristoyl group is extruded out of the pocket and anchors the protein to the membrane compartment of the cell. This property was originally established for recoverin [24], and subsequently has also been found for neurocalcin δ [25], frequenin [17, 26] and for the human and the rodent forms of Hpca [27–29].
Does BovHpca in native hippocampus contain an active Ca2+ myristoyl switch?
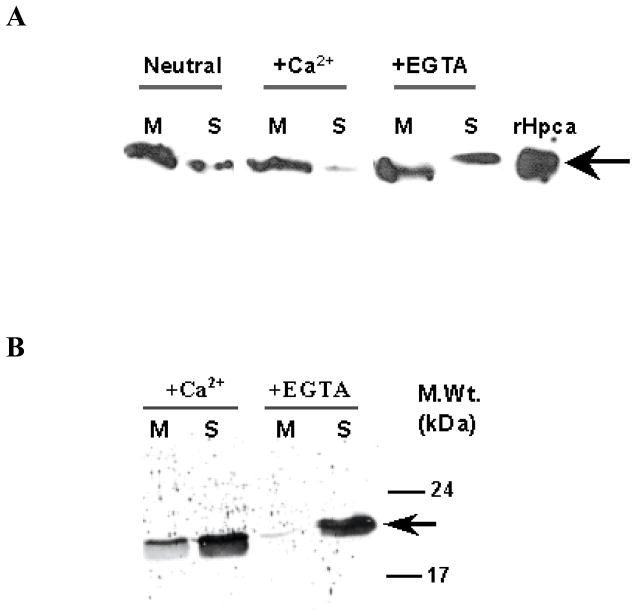
To address this issue the bovine hippocampal membranes were isolated under three conditions: 1) Neutral (no addition of Ca2+ or EGTA), 2) Ca2+-treated (50 μM CaCl2), 3) Ca2+-depleted (1mM EGTA). Attachment of the BovHpca to the membranes was assessed by Western blotting using the anti-BovHippocalin. The results are presented in figure 4A.
Figure 4. Calcium dependent distribution of Hpca in the membrane and soluble fractions of bovine hippocampus and COS cells.
(A) The membrane and soluble fractions of bovine hippocampus were prepared in the presence of 50 μM Ca2+ (+Ca2+), 2 mM EGTA (+EGTA) or in the absence of either (Neutral) as described in the “Materials and Methods” section. The membrane (M) and soluble (S) fractions (100 μg protein of each fraction) were individually separated by SDS-12%PAGE and subjected to Western blotting. 1 μg of recombinant Hpca was used as a positive control. (B) 5 μg of myristoylated Hpca was incubated with membrane fraction of COS cells (15 μg protein) in the presence of 50 μM Ca2+ (+Ca2+) or 1 mM EGTA (−Ca2+) as described in “Materials and Methods”. After 1 hr incubation the reaction mixture was centrifuged and the membrane (M) and soluble (S) fractions were analyzed by Western blotting using anti Hpca antibody. The position of the Hpca antibody immunoreactive band is indicated by an arrow. Molecular size markers are given alongside.
Under native conditions, significant portion of Hpca is present in the membrane fraction (Fig. 4A: Panel “Neutral”); in presence of the Ca2+ (50 μM) almost all Hpca is bound to the membranes (Fig. 4A: Panel “+Ca2+”); and in absence of the Ca2+ (1 mM EGTA) its considerable amount migrates to the soluble fraction (Fig. 4A: Panel “+EGTA”).
These results demonstrate that Ca2+ enhances membrane anchoring of Hpca and that under native conditions there is sufficient concentration of Ca2+ in the hippocampal membranes to keep most of the Hpca membrane-bound. Therefore, it is concluded that the bovine form of Hpca present in its native hippocampus contains an active Ca2+ myristoyl switch, a situation identical to the rodent and human forms of Hpca [27–29].
Similar results were obtained with the reconstituted system where membranes of COS cells and myristoylated Hpca were incubated in the presence or absence of Ca2+ (50 μM Ca2+ or 1 mM EGTA, respectively) and the membranes were probed for the presence of Hpca by Western blotting (Fig. 4B). Significantly more intense Hpca band was observed in the membrane fraction when the incubation was carried out in the presence of 1 mM Ca2+ than in the presence of 1 mM EGTA (Fig. 4B: compare the M lines in panels “+Ca2+” and “+EGTA”). It is noted than in both cases considerable amount of Hpca was also present in the soluble fraction (Fig. 4B: the S lines in panels “+Ca2+” and “+EGTA”). The reason for this is that, as compared with the native system, in the reconstituted system an excess of Hpca was present in the incubation mixture.
Ca2+-dependent Hpca-modulated membrane guanylate cyclase transduction system in hippocampus
Ca2+-modulated ROS-GC transduction system has been proposed to be a universal constituent of all sensory and sensory-linked neuronal networks [reviewed in: 2]. Because hippocampus is an important processing unit of the SENSES and also, it contains a Ca2+ signaling frequenin-modulated ONE-GC-like signal transduction system [17], a possibility of the presence of Hpca-modulated membrane guanylate cyclase transduction system in the bovine hippocampal neurons was investigated.
Step 1
Analysis of the particulate fraction of bovine hippocampus revealed that it contained a total guanylate cyclase activity of 7 pmol cyclic GMP/min/mg protein. This is a cumulative value representing all putative membrane guanylate cyclases present in this tissue. This information indicated that at least one member of the membrane guanylate cyclases family is expressed in the hippocampus.
Step 2
Between the two subfamilies of membrane guanylate cyclases, peptide hormone receptor and ROS-GC, only ROS-GC subfamily is Ca2+-modulated. To determine which of the three ROS-GC subfamily members--ROS-GC1, ROS-GC2, ONE-GC-- is a transducer of the Hpca-modulated Ca2+ signal in the hippocampus, each of the respective cyclases expressed in COS cells was tested for Ca2+-dependent Hpca-modulated activity. Because myristoylated form of the Hpca is the natural constituent of the hippocampus, this form was used in the experiments.
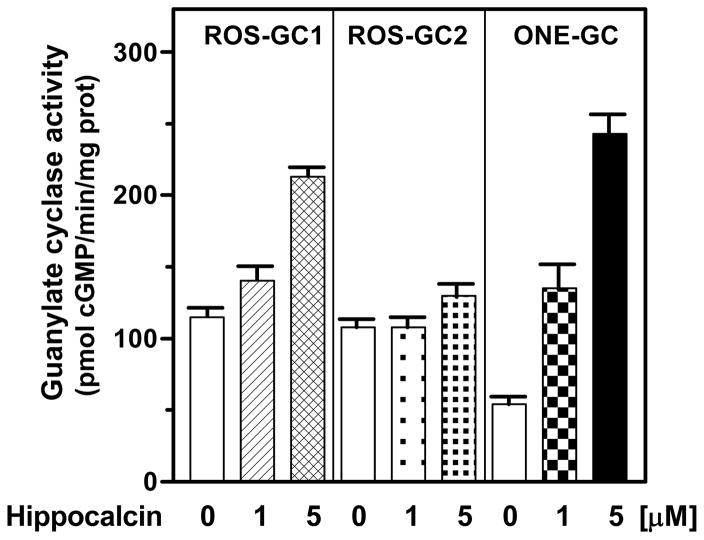
At the saturation concentration of free Ca2+ (100 μM), r-myr-Hpca stimulated robustly only ONE-GC activity, 4.5 fold over the basal level (from 54 to 243 pmol cyclic GMP/min/mg protein); there was no stimulation of ROS-GC2 and only marginal, less than 2-fold (from 115 to 213 pmol cyclic GMP/min/mg protein), of ROS-GC1 (Fig. 5). These results indicate that Hpca is the Ca2+ sensor component of solely ONE-GC.
Figure 5. Effect of myr-hyppocalcin on recombinant ROS-GCs.
COS cells were individually transfected with ROS-GC1, ROS-GC2 and ONE-GC cDNA, their membrane fractions prepared and assayed for guanylate cyclase activity in the presence of 100 μM Ca2+ and indicated concentrations of recombinant myr-Hpca. The experiment was done in triplicate and repeated four times with different myr-Hpca and COS cells preparation. The results presented (mean ± SE) are from one representative experiment.
ONE-GC gene, however, is rodent-specific; it does not exist in the cow [30]. Instead, a ONE-GC-like gene exists in the bovine hippocampus [17]. It is, therefore, deduced that Hpca in the hippocampus is a Ca2+ sensor component of this ONE-GC-like membrane guanylate cyclase.
Step 3
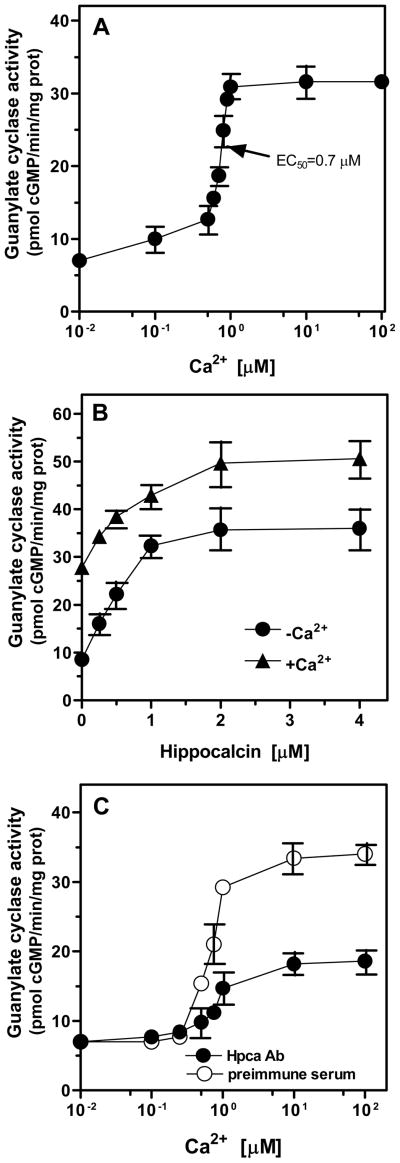
To determine Ca2+-dependent kinetics of the ONE-GC-like membrane guanylate cyclase in the hippocampus, the particulate fraction of the bovine hippocampus was exposed to the increasing concentrations of free Ca2+. A dose-dependent stimulation of the cyclase activity was observed (Fig. 6A). The maximal stimulation was ~4.5-fold over the basal value and the enzyme reached saturation at ~1 μM Ca2+. The K1/2 value for Ca2+ was ~ 0.7 μM. These results revealed that the hippocampus contains a membrane guanylate cyclase transduction system that is modulated by the physiological (submicromolar) levels of free Ca2+. Furthermore, they showed that that the native membrane fraction of the hippocampus is not fully saturated with Ca2+ and, thus, for its responses, is amenable to the fluctuating concentrations of free Ca2+.
Figure 6. Ca2+ dependent membrane guanylate cyclase system in bovine hippocampus.
A. Hippocampal membranes were assayed for guanylate cyclase activity in the presence of indicated concentrations of Ca2+. B. Response to recombinant myr-Hpca. Hippocampal membranes isolated in the presence of 50 μM Ca2+ (+Ca2+) or 2 mM EGTA (+EGTA) were assayed for guanylate cyclase activity in the presence of increasing concentrations of myr-Hpca and 100 μM Ca2+ as described in “Materials and Methods”. (C) Anti-Hpca antibody inhibits guanylate cyclase activity in the hippocampal membranes. Hippocampal membranes isolated in the presence of 50 μM Ca2+ were incubated with anti-Hpca antibody or preimmune serum (1:5 dilution for 1hr on ice) and assayed for guanylate cyclase activity under different Ca2+ concentrations. Cyclic GMP formed was measured by radioimmunoassay. The experiments were repeated two times with different preparations of hippocampal membranes. The results presented (mean ±SE) are from one representative experiment.
Step 4
To assess the extent of involvement of BovHpca in signaling of the membrane guanylate cyclase native to the hippocampus, the hippocampal particulate fraction was incubated with the exogenously supplied r-myr-BovHpca. Hpca caused dose-dependent increase of the cyclase activity with an EC50 of 0.5 μM and an overall stimulation of ~2-fold over the basal activity (Fig. 6B; triangles). The ability of Hpca to stimulate the cyclase activity beyond the point achieved through the addition of Ca2+ alone to these membranes indicates that the levels of Ca2+-dependent activators, Hpca and previously identified frequenin, in these membrane preparations are lower than those needed for maximal activation of the cyclase. The prior depletion of Ca2+ via the addition of EGTA during the membranes preparation resulted in ~4-fold stimulation of the cyclase activity by Hpca (Fig. 6B; circles). The stimulation was dose-dependent and the EC50 value for Hpca was also of ~0.5 μM. The difference between the maximal cyclase activity obtained with membranes prepared in the presence and absence of Ca2+ is attributed to the loss of Hpca, frequenin and possibly other Ca2+ dependent modulators caused by Ca2+ depletion. These results indicate that (a) the native hippocampal membranes contain a fraction of Ca2+-bound BovHpca; (b) the Ca2+-depletion removes membrane-bound BovHpca; and (c) this depletion results in higher stimulation of the guanylate cyclase by the exogenously supplied r-myr-BovHpca.
Step 5
To directly validate presence of the Ca2+-dependent Hpca-modulated transduction pathway native to the hippocampus, Hpca-specific antibody probe was used. The hippocampal membranes were pre-incubated with purified Hpca antibody, and the guanylate cyclase activity was measured at increasing Ca2+ concentrations. Membranes incubated with pre-immune serum served as the control. Fig 6C shows that the Ca2+-dependent guanylate cyclase activity was inhibited by 61%, validating presence of the Ca2+-dependent Hpca-modulated membrane guanylate cyclase transduction system in the hippocampal neurons. Because the ONE-GC-like membrane guanylate cyclase in hippocampus is also modulated by the Ca2+-sensor frequenin this transduction system is responsible for the cyclase activity not inhibited by Hpca antibody.
Through Steps 1 to 5 it is concluded that the hippocampal neurons contain a Ca2+-modulated membrane guanylate cyclase transduction system; that this system responds to the physiological submicromolar levels of free Ca2+; and the system is composed of the Ca2+ sensor component, Hpca and the transducer component ONE-GC-like membrane guanylate cyclase.
Hpca-modulated ONE-GC Ca2+ signal transduction system in the olfactory neurons
Guided by the results that Hpca is a Ca2+-sensor of ONE-GC and by the previous findings that the Ca2+-modulated ONE-GC transduction system is an important constituent of the odorant transduction, biochemical and physiological presence of the Hpca-modulated ONE-GC transduction system was investigated in the olfactory neuroepithelium of the rodents, mice and rat. The model system of the Hpca−/− (Hpca gene KO) [23] mice was used as one of the probes.
Biochemical presence
To establish the presence of Hpca in the rat and mouse olfactory neuroepithelium, Western blot analyses were performed according to the previous protocols [15–17]. The particulate fractions were isolated in the presence of 100 μM Ca2+, detergent-solubilized and probed with the affinity-purified Hpca antibody. The r-myristoylated Hpca served as a control. A single immunoreactive band was detected in the neuroepithelial membrane fractions of the rat and mouse (Fig. 7A). The electrophoretic mobility of this band was identical to that of the positive control, 22 kDa (Fig. 7A). No such band was detected in the identically prepared olfactory neuroepithelium membrane fraction from Hpca−/− mice.
Figure 7. Presence of Hpca and ONE-GC in the olfactory neuroepithelium.
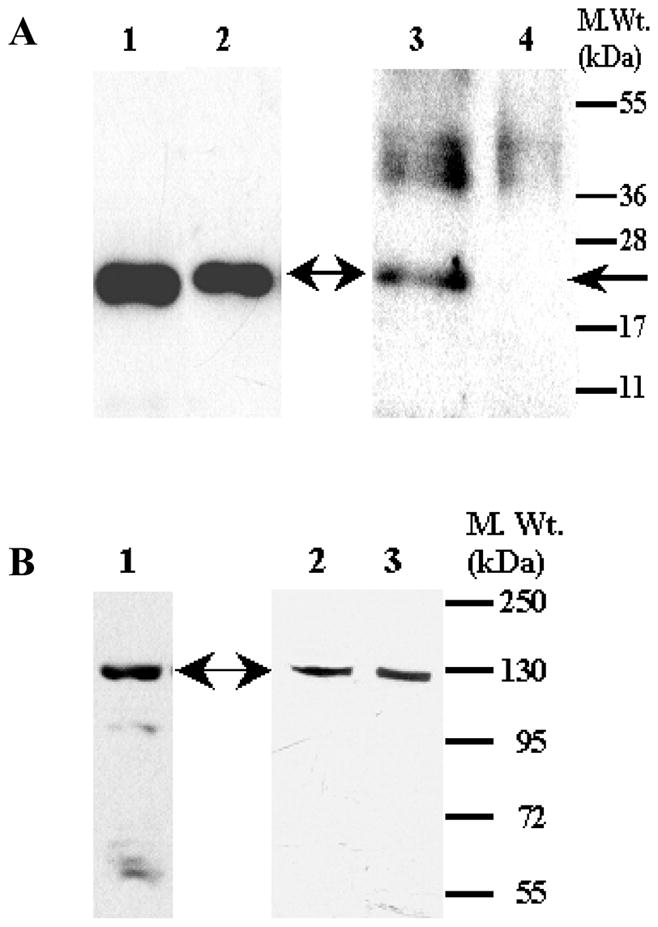
Membranes of rat and mice olfactory neuroepithelium were isolated in the presence of 100 μM Ca2+ as describd in the “Materials and Methods” section. The membranes were solubilized with 1% TritonX-100, the proteins separated through SDS-PAGE, transferred to the PVDF membranes and analyzed by Western blotting. (A) Hippocalcin. Lane 1-recombinant myr Hpca (control); Lane 2- rat olfactory neuroepithelium; Lane 3- mouse olfactory neuroepithelium; Lane 4- Hpca−/− mouse olfactory neuroepithelium. The position of the Hpca antibody immunoreactive band is indicated by an arrow. Positions of the molecular weight markers are given alongside. (B) ONE-GC. Lane 1 – rat olfactory neuroepithelium; Lane 2-mouse olfactory neuroepithelium; Lane 3- Hpca−/− mouse olfactory neuroepithelium. Positions of the molecular weight markers are marked and the position of the ONE-GC antibody immunoreactive band is indicated by an arrow.
These results demonstrate that the membrane portions of the olfactory neuroepithelial layers of the rat and mouse express Hpca, and, as expected, there is no such expression in the corresponding Hpca−/− membrane fractions.
The above experiments in the same fractions were repeated for the presence of ONE-GC. An immunoreactive band with mobility corresponding to the molecular weight of 126 kDa was present on Western blots of the olfactory neuroepithelial membranes from rat, the wild type and Hpca−/− mice (Fig. 7B). Intensities of these bands were comparable indicating that the absence of Hpca expression does not affect the expression of ONE-GC.
It is, thus, concluded that the Ca2+ sensor Hpca and the transducer ONE-GC are present in the membrane compartments of the rat and mouse olfactory neuroepithelium.
Physiological coupling
To determine the physiological coupling of Ca2+ sensor Hpca with its transducer component ONE-GC, the model systems of the wild type and Hpca gene KO (Hpca−/−) mouse were used.
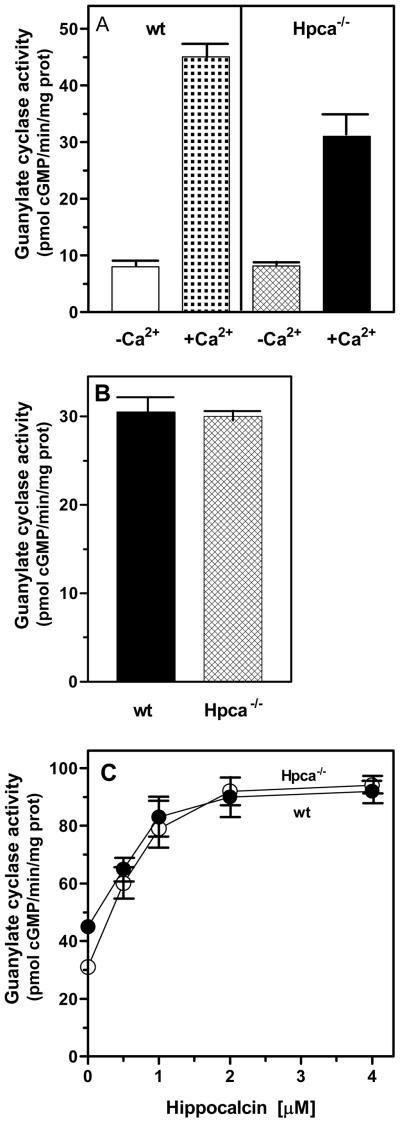
The olfactory neuroepithelial particulate fraction isolated from the wild type (wt, control) and Hpca−/− mice were assayed for the guanylate cyclase activity in the presence of 1 mM EGTA (Ca2+ depleted conditions) and 100 μM Ca2+. For the wt membranes the specific activity was ~8 pmol cyclic GMP/min/mg protein in the absence of Ca2+ and ~45 pmol cyclic GMP/min/mg protein in the absence of Ca2+ (Fig. 8A). The difference between these two activities reflects the combined contributions of Hpca and the already established Ca2+-dependent modulators of ONE-GC activity in the olfactory neuroepithelium, neurocalcin δ and GCAP1 [16, 18]. To directly assess the contribution of Hpca-modulated ONE-GC pathway the membranes of the Hpca−/− olfactory neuroepithelium were assayed under the same conditions. The specific activity in the absence of Ca2+ was identical to that of the wt membranes, ~8 pmol cyclic GMP/min/mg protein; in the presence of Ca2+ the activity was ~31 pmol cyclic GMP/min/mg protein (Fig. 8A). Thus, the input of Hpca modulated ONE-GC activity is reflected in the difference between activity of the wt and Hpca−/− neuroepithelial membranes; the combined input of neurocalcin δ and GCAP1 is described by the difference between the activities of Hpca−/− membranes in the absence and presence of Ca2+.
Figure 8. Hpca-modulated ONE-GC system in the olfactory neuroepithelium.
Particulate fractions of the olfactory neuroepithelium were isolated from the wild type and Hpca−/− mice in the presence of 100 μM Ca2+. Membranes were assayed for the guanylate cyclase activity in the presence of 1 mM EGTA or 100 μM Ca2+ (A), in the presence of 100 μM Ca2+ and Hpca antibody (1: 100 dilution) (B), and in the presence of 100 μM Ca2+ and indicated concentrations of myr-Hpca (C). Cyclic GMP formed was measured by radioimmunoassay. The experiment was done in triplicate and repeated two times with separate membrane preparations. The results presented (mean ± SE) are from one experiment.
This conclusion was validated by two sets of experiments, one with the use of antibodies against Hpca and the second, reconstitution experiment.
First, membranes of wt and Hpca−/− olfactory neuroepithelium were preincubated with antibodies against Hpca in the presence of 100 μM Ca2+ prior to guanylate cyclase activity assay. It was reasoned that if, indeed, Hpca modulates ONE-GC activity, anti-Hpca antibody should prevent this modulation in the wt membranes and the resulting cyclase activity should be approximately the same as in Hpca−/− membranes. The results are presented in figure 8B. The guanylate cyclase activity in the wt membranes preincubated with antibodies was 30.5 pmol cyclic GMP/min/mg protein and was virtually identical to the activity in the Hpca−/− membranes, 30 pmol cyclic GMP/min/mg protein.
Second, membranes of the wt and Hpca−/− olfactory neuroepithelial membranes were reconstituted with exogenous myr-Hpca. The rationale for the experiment was that exogenous Hpca should bring the cyclase activity to approximately the same maximal level in both types of membranes. And, indeed, Hpca stimulated guanylate cyclase activity in a dose dependent manner (Fig. 8C). The activity of the wt membranes was stimulated 2-fold above the basal level and of the Hpca−/− membranes, 3-fold reaching the maximal activity of ~90 pmol cyclic GMP/min/mg protein.
Together, these results show that Hpca-ONE-GC transduction system is functional in the olfactory neuroepithelium.
Coimmunoprecipitation
To further validate the above-described conclusions that Hpca and ONE-GC form a transduction system in the olfactory neuroepithelium, coimmunoprecipitation experiments were carried out. To obtain more experimental material rat olfactory neuroepithelium was used. Membranes of the olfactory neuroepithelium were isolated in the presence of 100 μM Ca2+, solubilized and incubated with affinity purified ONE-GC or Hpca antibody coupled to AminoLink® coupling gel. The immunoprecipitated complexes were separated from the coupling gel and analyzed by Western blotting using anti-Hpca or anti-ONE-GC antibody. The results are presented in figure 9. A single Hpca immunoreactive band is present in complexes precipitated with ONE-GC antibody (Fig. 9A) and ONE-GC immunoreactive band is present in complexes precipitated with Hpca antibodies (Fig. 9B). To verify the specificity of the coimmunoprecipitation, control experiments were performed which involved incubation of the solubilized membranes with AminoLink coupling gel alone followed by Western blotting. There was no interaction between either ONE-GC or Hpca with the gel (Fig. 9A lane 3 and 9B lane 3). Therefore, because both ONE-GC and Hpca were co-precipitated with both antibodies, the results demonstrate that ONE-GC and Hpca exist as a complex in membranes of the olfactory neuroepithelium.
Figure 9. ONE-GC and Hpca exist as a complex in the olfactory neuroepithelium.
(A) ONE-GC antibody coprecipitates Hpca. Membranes of the rat olfactory neuroepithelium were solubilized and incubated (4 mg protein) with AminoLink® coupled ONE-GC antibody as described in “Materials and Methods”. The immunoprecipitated complexes were analyzed by Western blotting with anti Hpca antibody. Lane 1- Hpca coprecipitated with ONE-GC antibody; Lane 2- control – Hpca bound to Hpca antibody column; Lane 3- control – Solubilized membranes of rat olfactory neuroepithelium were incubated with the AminoLink® coupling gel alone and the reaction mixture was processed as for lanes 1 and 2. (B) Hpca antibody coprecipitates ONE-GC. The experiment was done as described in (A) except that Hpca antibody was coupled to AminoLink® and the complexes were analyzed by Western blotting using anti ONE-GC antibody. Lane 1- ONE-GC coprecipitated with Hpca antibody; Lane 2- control ONE-GC bound to ONE-GC antibody column; Lane 3- control - Solubilized membranes of rat olfactory neuroepithelium were incubated with the AminoLink® coupling gel alone and the reaction mixture was processed as for lanes 1 and 2.
The results of the above experiments establish that in the olfactory neuroepithelium ONE-GC and Hpca exist as a functional complex and Hpca directly stimulates ONE-GC activity. These results are in contrast to the conclusion of another group that “Hippocalcin did not affect the activity of GCs at higher Ca2+ concentrations, suggesting that it is not involved in regulation of particulate GCs in the cilia upon odorant stimulation and subsequent increases in intracellular Ca2+” [31].
DISCUSSION
The present study documents the biochemical and physiological identity of a new Ca2+-sensor of the Ca2+-modulated ROS-GC subfamily member of the membrane guanylate cyclases. The Ca2+ sensor is Hpca. It exists in the hippocampal and the olfactory neuroepithelium neurons. With these findings Hpca becomes the sixth Ca2+ sensor of the general ROS-GC transduction machinery, the other five being GCAPs, 1 and 2, S100B, neurocalcin δ and frequenin.
Among the three members of the ROS-GC subfamily, ONE-GC is the only one modulated by the Ca2+ signals transmitted through Hpca. ROS-GC2 does not respond to Hpca and ROS-GC1 responds only minimally. Therefore Hpca is a Ca2+ sensor of ONE-GC. And together Hpca and ONE-GC constitute the Ca2+-modulated ONE-GC transduction machinery.
ONE-GC gene is rodent-specific [30], it does not occur in the bovine. The bovine species instead has developed a parallel ONE-GC-like Ca2+ signal transduction system [17]. In the hippocampal neurons, Hpca is the Ca2+-modulator of ONE-GC-like membrane guanylate cyclase. It is anticipated that in the rodent hippocampus Hpca-modulated Ca2+ signal will cause the activation of ONE-GC. For this reason, in the presented report, in functional terms no distinction is made in the common usage of the ONE-GC transduction system for both the hippocampal and the rat neuroepithelial neurons.
In the hippocampal and the olfactory neuroepithelium neurons, Hpca modulates the native ONE-GC activity within the physiological concentrations of Ca2+. The K1/2 of Ca2+ is 0.5 to 0.7 μM, and EC50 of Hpca is 0.7 μM. These conclusions are supported by the Hpca-specific antibody study. The antibody blocks Hpca-modulated guanylate cyclase activity and co-immunoprecipitates ONE-GC from the neuroepithelial membranes. This evidence demonstrates that in the native states Hpca and ONE-GC physically interact. This interaction is suitable for the rapid transduction of Ca2+ signals originating in the neuroepithelium. The signals are captured by Hpca and transduced by the physically linked ONE-GC into the production of cyclic GMP.
The conclusion that Hpca and ONE-GC are physiologically coupled in the olfactory neuroepithelium is further supported by the Hpca−/− mouse model studies. Hpca−/− mice lose the ONE-GC/Hpca transduction system and the exogenously supplied Hpca restores it. Contribution of this signaling system in the wild-type mice is 30% of the total ONE-GC transduction system.
What is the nature of the remainder 70% of the Ca2+-modualted ONE-GC transduction system in the rodent olfactory neuroepithelium?
While direct answer to this question is not available, it can be deduced from the prior findings. Besides Hpca, ONE-GC is modulated through two additional Ca2+ sensors, neurocalcin δ and GCAP1 [16, 18]. Both Ca2+-sensors are physically linked with ONE-GC and present in the rat neuroepithelium [16, 18]. It, therefore, appears that 70 % of the total ONE-GC transduction activity is controlled by its Ca2+ sensor components, neurocalcin δ and GCAP1; and remainder 30% by Hpca.
It is, now, concluded that ONE-GC in the olfactory neuroepithelium is modulated through three different types of Ca2+ signals: neurocalcin δ, GCAP1 and Hpca.
In relation to the hippocampal neurons, ONE-GC senses Ca2+ signals through frequenin [17] and Hpca. This begins to suggest a pattern. Like ROS-GC1 Ca2+ signal transduction system [reviewed in: 2], ONE-GC transduction system is also multi-modulated by Ca2+. However, between them exists a remarkable contrast. ROS-GC1 is both inversely, through GCAPs, and positively, through CD-GCAPS, locked in with the intensity of the Ca2+ waves whereas ONE-GC appears to be locked only positively through the CD-GCAPs. And intriguing aspect of the Ca2+ sensor GCAP1 is that it functions as a typical GCAP1 with the ROS-GC1 transduction system and as a CD-GCAP with the ONE-GC transduction system [3].
Presence of the Hpca-modulated Ca2+ signaling of ONE-GC may have physiological implications in the odorant signal transduction. It has recently been realized that besides cyclic AMP [32–35], cyclic GMP signaling pathway is also linked with the odorant signal transduction [3, 16, 36–38]. The source of cyclic GMP in the cyclic GMP signal transduction is ONE-GC. Through patch clamp and ONE-GC targeted gene analysis it has been demonstrated that the odorant, uroguanylin, a urine constituent, functions through ONE-GC [37]. The biochemical analysis has supported this contention and shown that the odorant uroguanylin, indeed, functions through the surface receptor of ONE-GC [3]. Although the details of the cyclic GMP-linked odorant transduction process are not known, the process appears to be more complex. Besides its direct linkage with the odorant through its surface receptor, the catalytic module of the ONE-GC is directly linked with the Ca2+ sensor neurocalcin δ [39] and, through its yet undefined domain also with GCAP1. These two Ca2+ sensors modulate ONE-GC activity in the physiological micromolar ranges of Ca2+. With the present findings that Hpca is also the Ca2+-modulator of ONE-GC, there are four modes of ONE-GC modulation. Each mode needs to be sorted out individually to ascertain its role in the odorant signal transduction.
Finally, this study broadens the emerging general Ca2+ signal transduction concept proposed to be applicable in all sensory neurons and the sensory-connected secondary neurons [reviewed in: 2, 4, 40]. The central theme of the concept is that Ca2+ signals through a delicately controlled ROS-GC transduction machinery. ROS-GC, in turn, generates pulsated levels of cyclic GMP. Cyclic GMP then serves as a Ca2+ second messenger. The delicacy and specificity of the transduction machinery is achieved through its unique composition and structural design present in that particular neuron. To date this concept was based mostly on the model system of ROS-GC1, which in different neurons ROS-GC1 was linked with different Ca2+ sensors. The present study shows that this may hold true also with the ONE-GC transduction system. In the neuroepithelium, this transduction system exists in four different forms and each form is able to sense and define only a specified Ca2+ signal. In this mode, Ca2+, the Ca2+ sensor and ROS-GC are the symbiotic signaling partners.
Acknowledgments
This research was supported by USPHS awards: DC 005349 (R. K. S.) and HL084584 (T. D.).
Abbreviations
- DTT
1,4-dithiothreitol
- EDTA
ethylene diamine tetraacetic acid
- EGTA
ethylene glycol bis(2-aminoethyl ether)-N,N,N′,N′-tetraacetic acid
- GCAP
guanylate cyclase activating protein
- HEPES
1-Piperazineethane sulfonic acid, 4-(2-hydroxyethyl)-monosodium salt
- ONE-GC
olfactory neuroepithelial membrane guanylate cyclase
- PMSF
Phenylmethylsulphonylfluoride
- ROS-GC
rod outer segment membrane guanylate cyclase
- SDS-PAGE
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
References
- 1.Sharma RK. Evolution of the membrane guanylate cyclase transduction system. Mol Cell Biochem. 2002;230:3–30. [PubMed] [Google Scholar]
- 2.Sharma RK, Duda T, Venkataraman V, Koch K-W. Calcium-modulated membrane guanylate cyclase, ROS-GC transduction machinery in sensory neurons: a universal concept. Curr Topics Biochem Res. 2004;6:111–144. [Google Scholar]
- 3.Duda T, Sharma RK. ONE-GC membrane guanylate cyclase, a trimodal odorant signal transducer. Biochem Biophys Res Commun. 2008;367:440–445. doi: 10.1016/j.bbrc.2007.12.153. [DOI] [PubMed] [Google Scholar]
- 4.Sharma RK, Duda T. Calcium sensor neurocalcin δ-modulated ROS-GC transduction machinery in the retinal and olfactory neurons. Calcium Binding Proteins. 2006;1:7–11. [Google Scholar]
- 5.Palczewski K, Sokal I, Baehr W. Guanylate cyclase-activating proteins: structure, function, and diversity. Biochem Biophys Res Commun. 2004;322:1123–1130. doi: 10.1016/j.bbrc.2004.07.122. [DOI] [PubMed] [Google Scholar]
- 6.Imanishi Y, Li N, Sokal I, Sowa ME, Lichtarge O, Wensel TG, Saperstein DA, Baehr W, Palczewski K. Characterization of retinal guanylate cyclase-activating protein 3 (GCAP3) from zebrafish to man. Eur J Neurosci. 2002;15:63–78. doi: 10.1046/j.0953-816x.2001.01835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dizhoor AM, Olshevskaya EV, Henzel WJ, Wong SC, Stults JT, Ankoudinova I, Hurley JB. Cloning, sequencing, and expression of a 24-kDa Ca2+-binding protein activating photoreceptor guanylyl cyclase. J Biol Chem. 1995;270:25200–25206. doi: 10.1074/jbc.270.42.25200. [DOI] [PubMed] [Google Scholar]
- 8.Lowe DG, Dizhoor AM, Liu K, Gu Q, Spencer M, Laura R, Lu L, Hurley JB. Cloning and expression of a second photoreceptor-specific membrane retina guanylyl cyclase (RetGC), RetGC-2. Proc Natl Acad Sci USA. 1995;92:5535–9553. doi: 10.1073/pnas.92.12.5535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dizhoor AM, Lowe DG, Olshevskaya EV, Laura RP, Hurley JB. The human photoreceptor membrane guanylyl cyclase, RetGC, is present in outer segments and is regulated by calcium and a soluble activator. Neuron. 1994;12:1345–1352. doi: 10.1016/0896-6273(94)90449-9. [DOI] [PubMed] [Google Scholar]
- 10.Duda T, Goraczniak R, Surgucheva I, Rudnicka-Nawrot M, Gorczyca WA, Palczewski K, Sitaramayya A, Baeh W, Sharma RK. Calcium modulation of bovine photoreceptor guanylate cyclase. Biochemistry. 1996;35:8478–8482. doi: 10.1021/bi960752z. [DOI] [PubMed] [Google Scholar]
- 11.Duda T, Venkataraman V, Krishnan A, Nagele RG, Sharma RK. Negatively calcium-modulated membrane guanylate cyclase signaling system in the rat olfactory bulb. Biochemistry. 2001;40:4654–4662. doi: 10.1021/bi0027985. [DOI] [PubMed] [Google Scholar]
- 12.Venkataraman V, Nagele R, Duda T, Sharma RK. Rod outer segment membrane guanylate cyclase type 1-linked stimulatory and inhibitory calcium signaling systems in the pineal gland: biochemical, molecular, and immunohistochemical evidence. Biochemistry. 2000;39:6042–6052. doi: 10.1021/bi9929960. [DOI] [PubMed] [Google Scholar]
- 13.Duda T, Koch K-W, Venkataraman V, Lange C, Beyermann M, Sharma RK. Ca(2+) sensor S100beta-modulated sites of membrane guanylate cyclase in the photoreceptor-bipolar synapse. The EMBO J. 2002;21:2547–2556. doi: 10.1093/emboj/21.11.2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duda T, Sharma RK. S100B-modulated Ca2+-dependent ROS-GC1 transduction machinery in the gustatory epithelium: a new mechanism in gustatory transduction. FEBS Lett. 2004;577:393–398. doi: 10.1016/j.febslet.2004.09.089. [DOI] [PubMed] [Google Scholar]
- 15.Krishnan A, Venkataraman V, Fik-Rymarkiewicz E, Duda T, Sharma RK. Structural, biochemical, and functional characterization of the calcium sensor neurocalcin delta in the inner retinal neurons and its linkage with the rod outer segment membrane guanylate cyclase transduction system. Biochemistry. 2004;43:2708–2723. doi: 10.1021/bi035631v. [DOI] [PubMed] [Google Scholar]
- 16.Duda T, Jankowska A, Venkataraman V, Nagele R, Sharma RK. A novel calcium-regulated membrane guanylate cyclase transduction system in the olfactory neuroepithelium. Biochemistry. 2001;40:12067–12077. doi: 10.1021/bi0108406. [DOI] [PubMed] [Google Scholar]
- 17.Fik-Rymarkiewicz E, Duda T, Sharma RK. Novel frequenin-modulated Ca2+-signaling membrane guanylate cyclase (ROS-GC) transduction pathway in bovine hippocampus. Mol Cell Biochem. 2006;291:187–204. doi: 10.1007/s11010-006-9215-6. [DOI] [PubMed] [Google Scholar]
- 18.Duda T, Krishnan R, Sharma RK. GCAP1: Antithetical calcium sensor of ROS-GC transduction machinery. Calcium Binding Protein. 2006;1:102–107. [Google Scholar]
- 19.Kobayashi M, Takamatsu K, Saitoh S, Miura M, Noguchi T. Molecular cloning of Hpca, a novel calcium-binding protein of the recoverin family exclusively expressed in hippocampus. Biochem Biophys Res Commun. 1992;189:511–517. doi: 10.1016/0006-291x(92)91587-g. Erratum in: Biochem Biophys Res Commun 196, 1017, 1993. [DOI] [PubMed] [Google Scholar]
- 20.Takamatsu K, Kobayashi M, Saitoh S, Fujishiro M, Noguchi T. Molecular cloning of human hippocalcin cDNA and chromosomal mapping of its gene. Biochem Biophys Res Commun. 1994;200:606–611. doi: 10.1006/bbrc.1994.1491. [DOI] [PubMed] [Google Scholar]
- 21.Masaki T, Sakai E, Furuta Y, Kobayashi M, Tkamatsu K. Genomic structure and chromosomal mapping of the human and mouse hippocalcin genes. Gene. 1998:117–124. doi: 10.1016/s0378-1119(98)00526-5. [DOI] [PubMed] [Google Scholar]
- 22.Nambi P, Aiyar NV, Sharma RK. Adrenocorticotropin-dependent particulate guanylate cyclase in rat adrenal and adrenocortical carcinoma: comparison of its properties with soluble guanylate cyclase and its relationship with ACTH-induced steroidogenesis. Arch Biochem Biophys. 1982;217:638–646. doi: 10.1016/0003-9861(82)90545-8. [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi M, Masaki T, Hori K, Masuo Y, Miyamoto M, Tsubokawa H, Noguchi H, Nomura M, Takamatsu K. Hippocalcin-deficient mice display a defect in cAMP response element-binding protein activation associated with impaired spatial and associative memory. Neuroscience. 2005;133:471–484. doi: 10.1016/j.neuroscience.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 24.Zozulya S, Stryer L. Calcium-myristoyl protein switch. Proc Natl Acad Sci U S A. 1992;89:11569–11573. doi: 10.1073/pnas.89.23.11569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ladant D. Calcium and membrane binding properties of bovine neurocalcin delta expressed in Escherichia coli. J Biol Chem. 1995;270:3179–3185. [PubMed] [Google Scholar]
- 26.McFerran BW, Weiss JL, Burgoyne RD. Neuronal Ca(2+) sensor 1. Characterization of the myristoylated protein, its cellular effects in permeabilized adrenal chromaffin cells, Ca(2+)-independent membrane association, and interaction with binding proteins, suggesting a role in rapid Ca(2+) signal transduction. J Biol Chem. 1999;274:30258–30265. doi: 10.1074/jbc.274.42.30258. [DOI] [PubMed] [Google Scholar]
- 27.Kobayashi M, Takamatsu K, Saitoh S, Noguchi T. Myristoylation of hippocalcin is linked to its calcium-dependent membrane association properties. J Biol Chem. 1993;268:18898–18904. [PubMed] [Google Scholar]
- 28.O’Callaghan DW, Ivings L, Weiss JL, Ashby MC, Tepikin AV, Burgoyne RD. Differential use of myristoyl groups on neuronal calcium sensor proteins as a determinant of spatio-temporal aspects of Ca2+ signal transduction. J Biol Chem. 2002;277:14227–14237. doi: 10.1074/jbc.M111750200. [DOI] [PubMed] [Google Scholar]
- 29.O’Callaghan DW, Tepikin AV, Burgoyne RD. Dynamics and calcium sensitivity of the Ca2+/myristoyl switch protein hippocalcin in living cells. J Cell Biol. 2003;163:715–721. doi: 10.1083/jcb.200306042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caenepeel S, Charydczak G, Sudarsanam S, Hunter T, Manning G. The mouse kinome: discovery and comparative genomics of all mouse protein kinases. Proc Natl Acad Sci USA. 2004;101:11707–11712. doi: 10.1073/pnas.0306880101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mammen A, Simpson PJ, Nighorn A, Imanishi Y, Palczewski K, Ronnett GV, Moon C. Hippocalcin in the olfactory epithelium: a mediator of second messenger signaling. Biochem Biophys Res Commun. 2004;322:1131–1139. doi: 10.1016/j.bbrc.2004.07.123. Erratum in: Biochem Biophys Res Commun. 326, 2005. [DOI] [PubMed] [Google Scholar]
- 32.Reisert J, Yau KW, Margolis FL. Olfactory marker protein modulates the cAMP kinetics of the odour-induced response in cilia of mouse olfactory receptor neurons. J Physiol. 2007;585:731–740. doi: 10.1113/jphysiol.2007.142471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bakalyar HA, Reed RR. Identification of a specialized adenylyl cyclase that may mediate odorant detection. Science. 1990;250:1403–1406. doi: 10.1126/science.2255909. [DOI] [PubMed] [Google Scholar]
- 34.Dhallan RS, Yau KW, Schrader KA, Reed RR. Primary structure and functional expression of a cyclic nucleotide-activated channel from olfactory neurons. Nature. 1990;347(6289):184–187. doi: 10.1038/347184a0. [DOI] [PubMed] [Google Scholar]
- 35.Sklar PB, Anholt RR, Snyder SH. The odorant-sensitive adenylate cyclase of olfactory receptor cells. Differential stimulation by distinct classes of odorants. J Biol Chem. 1986;261:15538–15543. [PubMed] [Google Scholar]
- 36.Juilfs DM, Fulle HJ, Zhao AZ, Houslay MD, Garbers DL, Beavo JA. A subset of olfactory neurons that selectively express cGMP-stimulated phosphodiesterase (PDE2) and guanylyl cyclase-D define a unique olfactory signal transduction pathway. Proc Natl Acad Sci U S A. 1997;94:3388–3395. doi: 10.1073/pnas.94.7.3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leinders-Zufall T, Cockerham RE, Michalakis S, Biel M, Garbers DL, Reed RR, Zufall F, Munger SD. Contribution of the receptor guanylyl cyclase GC-D to chemosensory function in the olfactory epithelium. Proc Natl Acad Sci U S A. 2007;104:14507–14512. doi: 10.1073/pnas.0704965104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meyer MR, Angele A, Kremmer E, Kaupp UB, Muller F. A cGMP-signaling pathway in a subset of olfactory sensory neurons. Proc Natl Acad Sci U S A. 2000;97:10595–10600. doi: 10.1073/pnas.97.19.10595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duda T, Fik-Rymarkiewicz E, Venkataraman V, Krishnan A, Sharma RK. Calcium-modulated ciliary membrane guanylate cyclase transduction machinery: constitution and operational principles. Mol Cell Biochem. 2004;267:107–122. doi: 10.1023/b:mcbi.0000049372.33965.4f. Erratum in: Mol Cell Biochem. 273, 225–226, 2005. [DOI] [PubMed] [Google Scholar]
- 40.Duda T, Venkataraman V, Sharma RK. In: Vision and odorant-linked neurocalcin δ-dependent Ca2+-modulated machinery: Constitution and operational principles in “Neuronal calcium sensor proteins”. Phillippov P, Koch K-W, editors. Nova Science Publishers; Hauppage, NY, USA: 2007. pp. 91–113. [Google Scholar]