Abstract
Objective
Behavioral conditioning as an inherent component of many pharmacotherapeutic protocols has never been examined. We hypothesized that psoriasis patients treated under a partial schedule of pharmacologic (corticosteroid) reinforcement would show less severe symptoms and relapse than those given the same amount of drug under standard conditions.
Methods
A double blind, simple randomization intervention was conducted with 46 patients from California and New York. Initially, lesions were treated with 0.1% acetonide triamcinolone under standard treatment conditions. Thereafter, a Standard Therapy group continued on continuous reinforcement (active drug every treatment) with 100% of the initial dose; Partial Reinforcement patients received a full dose 25-50% of the time and placebo medication other times; Dose Control patients received continuous reinforcement with 25-50% of the initial dose.
Results
Severity of disease scores in CA neither supported nor refuted the hypothesis. In NY, where there was no difference between Partial Reinforcement and Dose Control groups at baseline, partial reinforcement effected a greater reduction in lesion severity than Dose Control conditions and did not differ from Standard Therapy patients receiving 2-4 times more drug. For the entire population, the incidence of relapse under partial reinforcement (26.7%) was lower than in Dose Control patients (61.5%) and did not differ from full-dose treatment (22.2%).
Conclusions
A partial schedule of pharmacotherapeutic reinforcement could maintain psoriasis patients with a cumulative amount of corticosteroid that was relatively ineffective when administered under standard treatment conditions. Conceivably, corticosteroid administration only one quarter or half as frequently as currently prescribed is sufficient to treat psoriasis. We posit, however, that these preliminary observations implicate conditioning processes in—and for the design of—regimens of pharmacotherapy.
Keywords: conditioning, partial reinforcement, pharmacotherapy, psoriasis
INTRODUCTION
Clinical research and drug evaluation studies have adhered to the model in which a drug or placebo is administered in order to evaluate the efficacy of pharmacotherapies or to define the pharmacological (as opposed to the psychological) action of a drug. There have been repeated but unanswered calls for studies of the placebo effect as a phenomenon that may have important clinical implications in its own right (2). Still, it is only the initial, nonspecific response to a placebo that is studied in the majority of placebo research. Here, we address placebo effects as they apply to chronic drug treatment conditions.
The response to a placebo “looks like” the response to a conditioned stimulus. In behavioral terms, physiological effects elicited by pharmacologic agents are unconditioned responses (UCRs), the drug itself being the unconditioned stimulus (UCS). Events or stimuli that are coincidentally or purposely associated with and reliably predict the voluntary or involuntary receipt of drug—but are neutral with respect to eliciting the unconditioned effects of the active drug—are conditioned stimuli (CSs). These could include the environment in which medication is taken or administered (and by whom) and characteristics of the “pill” or injection, itself. Repeated associations of CS and UCS eventually enable the CS to elicit a conditioned response—an approximation of the response unconditionally elicited by the UCS. Thus, the response to an inert or therapeutically irrelevant substance or placebo has been described as a conditioned response.
There is a substantial literature in humans and lower animals supporting the proposition that the response to a placebo is a learned response (3)—and one that is specific rather than nonspecific (4). The substitution of conditioned stimuli for a proportion of active immunosuppressive drug treatments delayed the onset of proteinuria and mortality in lupus prone mice using a cumulative amount of drug that was not, by itself, sufficient to influence progression of the autoimmune disorder (5), suggesting that there might be some heuristic value in viewing pharmacotherapeutic protocols as a series of conditioning trials. This strategy was also effective in the treatment of a child with systemic lupus erythematosus (6). Other studies have also shown the salutary effects of exposure to conditioned stimuli previously paired with therapeutic agents in animals (7-11) and in humans (12). The response to a placebo is influenced by the sequence in which drug and placebo are administered (13-15), and relapse is delayed among patients who are given placebos upon withdrawal of active medication (e.g.,16). Also, conditioning is a parsimonious explanation of the delayed relapse that occurs among patients treated with drug and then switched to placebo (CSs) in double-blind crossover studies (17,18).
The role of conditioning in placebo responding has been questioned on the grounds that some conditioned pharmacologic responses, compensatory or paradoxical conditioned responses, are opposite in direction to the effects of the drug used as the unconditioned stimulus (19). However, there is an operational difference between conditioned pharmacologic and conditioned pharmacotherapeutic responses (3). In the former, the UCS delivered to normal subjects elicits a physiological response that represents some deviation from some homeostatic level and there are occasions when the UCS elicits a compensatory response. In the case of conditioned pharmacotherapeutic responses, a therapeutic agent delivered to a subject (patient) is calculated to correct a naturally occurring or experimentally induced physiologic imbalance. We are unaware of any direct evidence of compensatory conditioned pharmacotherapeutic responses.
Currently, research designed to evaluate drug effects involves two distinct groups: an experimental group that receives active drug and a control group that does not---receiving, instead, an inert or chemically irrelevant substance (the placebo). In all other respects, the stimuli that attend drug or placebo administration are, presumably, “identical.” Experimental subjects receive medication that is invariably followed (reinforced) by the unconditioned effects of the drug (in behavioral terms, a continuous or 100% reinforcement schedule). In contrast, control subjects who engage in the same behaviors, are subject to the same environmental conditions, and receive placebo medication are never therapeutically reinforced; they are on a 0% reinforcement schedule. One is therefore prompted to ask about schedules of reinforcement between 0 and 100%? There is an alternative to evaluating drug effects by administering drug or placebo: one can administer drug and placebo. One can introduce partial schedules of reinforcement in which “medication” and the attendant environmental cues are therapeutically reinforced on some occasions but not on others. Thus, by capitalizing on conditioning effects, it might be possible to approximate the therapeutic effects of a continuous schedule of reinforcement, that is, suppress symptoms or maintain some physiologic homeostatic limits, using lower cumulative amounts of drug.
We explore this possibility by attempting to reduce the amount of corticosteroid medication required for the maintenance of patients with mild to moderate psoriasis. Psoriasis is not a fatal disease, but usually requires life long treatment and can become a source of significant morbidity. There is strong evidence that immune regulation plays a pathophysiologic role in the development of this disease (20) and there is striking evidence for the involvement of neurogenic inflammation as well (21,22). There is also literature implicating neuroendocrine factors in the inflammatory and proliferate processes of psoriasis (23,24). It is not surprising, then, that affective states and stressful life experiences have been associated with the appearance or exacerbation of psoriasis (25,26), and it would not, therefore, be unlikely that conditioned pharmacotherapeutic responses could affect the course of disease.
We propose, then, to capitalize on conditioned pharmacotherapeutic responses to reduce the cumulative amount of corticosteroid medication used in the treatment of psoriasis. Specifically, we will test the prediction derived from a conditioning model of pharmacotherapy (3) that patients treated under an intermittent schedule of corticosteroid medication will have a lower incidence of relapse and less severe symptoms of disease compared to patients treated with that same (reduced) amount of drug administered under a continuous schedule of reinforcement.
METHODS
Participants
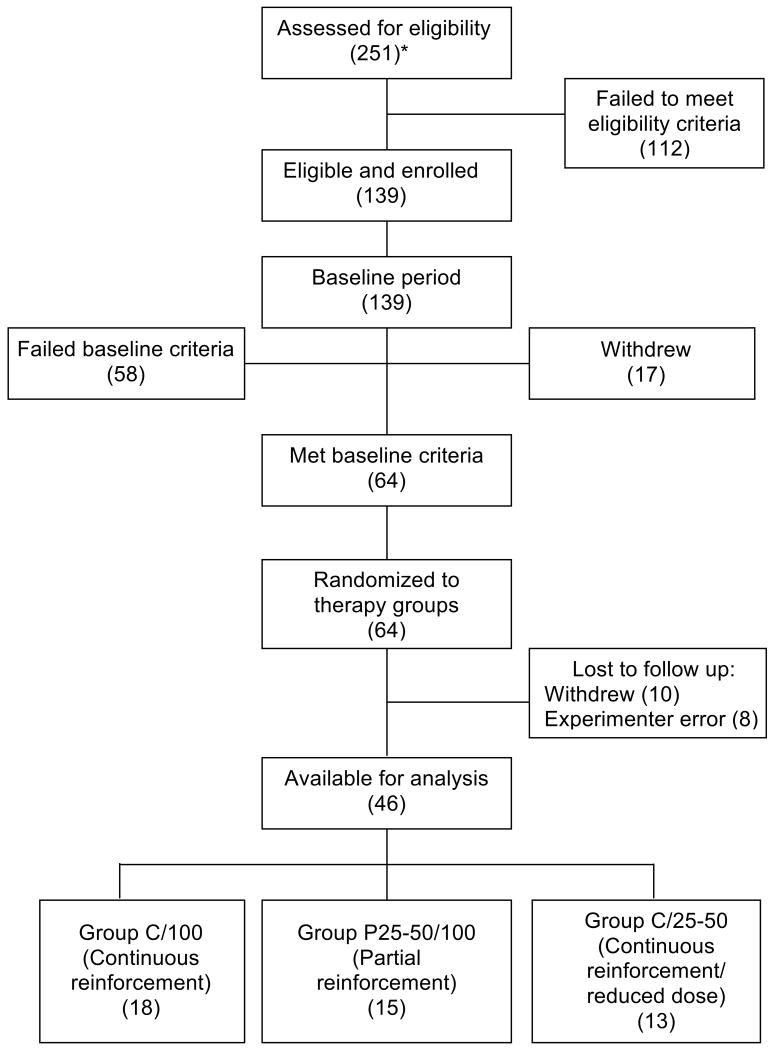
Patients with mild to moderate psoriasis, 19-70 years of age, were recruited from newspaper and television advertisements and paid for their participation. Patients reported that they had not been treated with topical or systemic psoriasis medication in the previous two months and agreed to refrain from using any other psoriasis treatments during the course of the study. A total of 251 subjects were screened and 139 satisfied inclusion criteria. Of these, 58 failed to meet baseline period criteria, 27 withdrew from the study (17 before completing the baseline period) for a variety of personal reasons or, most often (70%), without providing reasons, and 8 were victims of protocol errors (e.g., remained under baseline conditions after attaining the inclusion criterion) providing a population of 46 patients (83% were white and 56% were male) for analysis (Figure 1). Approximately half the patients were studied at the University of Rochester School of Medicine and Dentistry and half at the Stanford University Medical School throughout the calendar year between 2001 and 2006. The protocol was approved by the Institutional Review Board of both universities. Patients signed a consent form indicating that this study was an attempt to determine if their psoriasis could be managed with smaller amounts of corticosteroid and, at some point in the course of the study, we might reduce the amount of medication they were receiving and that the chance of being in such a group was completely random.
Figure 1.
Flow of participants through each stage of the study. *(N).
Procedures
During an initial screening, two comparable psoriatic plaques were selected and clinically evaluated with respect to erythema, induration and scaling on a 9-point modified Psoriasis Severity Scale (PSS) (27). Only patients with a PSS score of ≥7 were enrolled. The majority of lesions (approximately 70%) were on elbows or knees and the target and control lesions were on contralateral sides. There were no group differences in the location of lesions. At this same time, participants also completed several brief questionnaires: the Psoriasis Life Stress Inventory (28.29), Hassle Scale (30), an Impact of Events Scale (31) and the Interpersonal Support Evaluation List (32).
For the next 3-6 weeks, each patient applied his or her distinctively fragrant and colored medication, 0.1% triamcinolone acetonide in aquaphor (Aristocort A), twice daily, to the randomly selected “target” lesion. A commercial moisturizing cream was applied to the “control” lesion. Medication was packaged in a connected strip of daily application syringes sufficient for one week. Evaluations of the psoriatic lesions were made weekly throughout the maintenance (conditioning) and experimental periods by a dermatologist blinded to the group to which patients belonged. Patients who did not show evidence of improvement within six weeks (a ≥ 3-point decline in PSS score) or showed an equal PSS decline in the target and control lesions were excused from further participation in the study.
Following the baseline period, patients were randomly assigned to one of three groups (Table 1):
TABLE 1.
Experimental Protocol
| BASELINE PERIOD (3 to 6 weeks) |
EXPERIMENTAL PERIOD (8 weeks) |
|||
|---|---|---|---|---|
| GROUP | Drug Dose* | Reinforcement Schedule** | Drug Dose | Reinforcement Schedule |
| Standard Therapy (N = 18) | 100 | 100 | 100 | 100 |
| Partial Reinforcement (N = 15) | 100 | 100 | 100 | 25 to 50 |
| Dose Control (N = 13) | 100 | 100 | 25 to 50 | 100 |
Percentage of 0.1% Aristocort A
Percentage of treatment occasions when active drug is received.
Standard Therapy patients continued to receive a full dose of medication on the same continuous (100%) reinforcement schedule received during the baseline (maintenance) period. Corticosteroid was applied to the selected lesion twice daily for as many as eight additional weeks. This is a continuation of their standard pharmacotherapeutic regimen.
Partial Reinforcement patients, the experimental group, were treated under a partial schedule of reinforcement. That is, patients received the same 0.1% dose of Aristocort A but only a portion of the syringes contained active drug; the remaining syringes contained a placebo ointment (aquaphor with the same embellished fragrance and color as the corticosteroid ointment, absent the corticosteroid)—a CS. Initially, patients in the Partial Reinforcement (n = 6) and Dose Control (n = 4) groups were treated under a 50% reinforcement schedule or dose of triamcinolone acetonide. It appeared, however, that the incidence of relapse might be insufficient to discriminate among the groups, so the protocol was amended and the reinforcement schedule was reduced to 25% for the remaining experimental patients (n = 9) and adjusted accordingly in the Dose Control group (n = 9). The sequence of medication was random with the restriction that one of every two or four randomly selected applications of salve contained active drug.
Dose Control patients served as a control for the cumulative amount of drug received. They were treated twice daily under a continuous reinforcement schedule, but each syringe contained only 25 or 50% of the dose of corticosteroid received during the baseline period. Thus, patients in the Partial Reinforcement and Dose Control groups treated under different schedules of pharmacologic reinforcement received the same cumulative amount of active drug.
The primary outcome measures were based on PSS scores. “Relapse,” defined a priori as a return to a PSS score within 2 PSS units of the individual patient’s initial score is an arbitrary criterion but does signify an inability to maintain the therapeutic effects achieved during the standard pharmacotherapeutic regimen imposed during the baseline period. Additionally, we analyzed changes in PSS scores over time. Per-protocol analyses were limited to participants who met the baseline criteria and provided at least four data points during the 8-wk treatment period.
It was considered possible that a noncontinuous or intermittent schedule of pharmacologic reinforcement (and the concomitant reduced amount of active drug) could exert effects indistinguishable from a continuous (standard) regimen of pharmacotherapy (a higher cumulative amount of drug). That outcome or comparison, however, is not critical for evaluating the role of conditioning in the pharmacotherapy of psoriasis. Specifically, we tested the prediction that patients treated under a partial schedule of corticosteroid medication would show a greater amelioration of symptoms and a reduced incidence and rate of relapse than that achieved by patients treated with that same (reduced) amount of drug administered under a continuous schedule of reinforcement.
Statistical Methods
The incidence of relapse was analyzed with nonparametric tests and a repeated measures ANOVA (Group X Week X Site) was applied to the PSS data. Based on the specificity of the a priori hypothesis, the available n for these preliminary observations, and the significant effects found in a preliminary, unpublished experiment, we used post-hoc one-tailed tests for the planned comparisons of the incidence of relapse of the Standard Therapy vs. the Dose Control group (a dose effect) and the Partial Reinforcement vs. the Dose Control group (the predicted experimental effect).
RESULTS
There were no differences in initial Psoriasis Severity Scale (PSS) scores or between the target and control lesions of the groups of patients who did and did not remain in the study, and neither age nor sex were related to either PSS scores or relapse. Further, neither group comparisons nor correlation analyses uncovered any associations between any of the psychometric instruments used to gauge the level or manner of dealing with the life stressors accompanying psoriasis at the time of enrollment and either subsequent relapse or PSS scores.
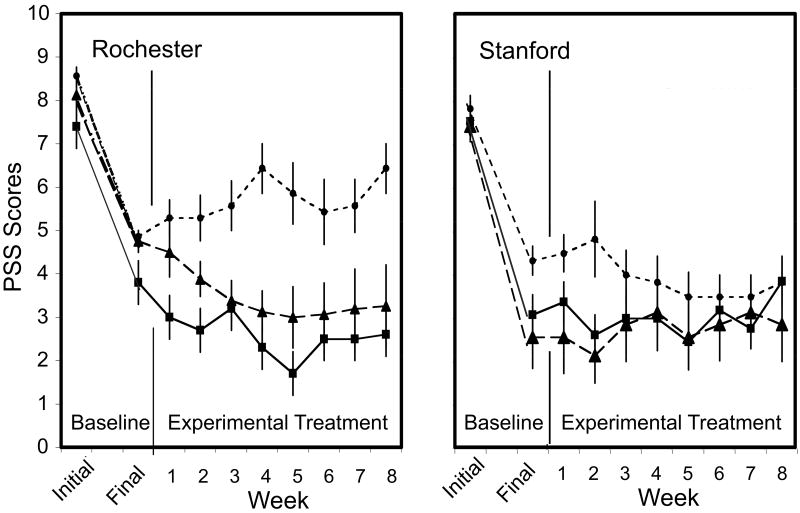
PSS scores (including the final baseline values) yielded a significant Group X Week X Site interaction (F(16/320) = 2.47, p = .002). Differences among treatment groups varied at the two study sites (Fig. 2). At Stanford, PSS scores remained at the approximate level of the final baseline scores and there were no group differences (F(16,184) = 1.14, p = .33). In Rochester, there was no difference between the final baseline PSS values of the Partial Reinforcement and Dose Control groups. Under these circumstances, partial reinforcement effected a greater reduction in lesion severity during the experimental period than continuous reinforcement with the same cumulative amount of drug (F(16,136) = 2.29, p =.005). The Partial Reinforcement Group did not differ from the Standard Therapy group that received 2-4 times more drug.
Figure 2.
Weekly Psoriasis Severity Scale (PSS) scores (mean ± S.E.) as a function of reinforcement schedule and amount of drug at each of the study sites.■ = Standard Therapy (Rochester n = 5; Stanford n = 13); ▲ = Partial Reinforcement (Rochester n = 8; Stanford n = 7); ● = Dose Control (Rochester n = 7; Stanford n = 6).
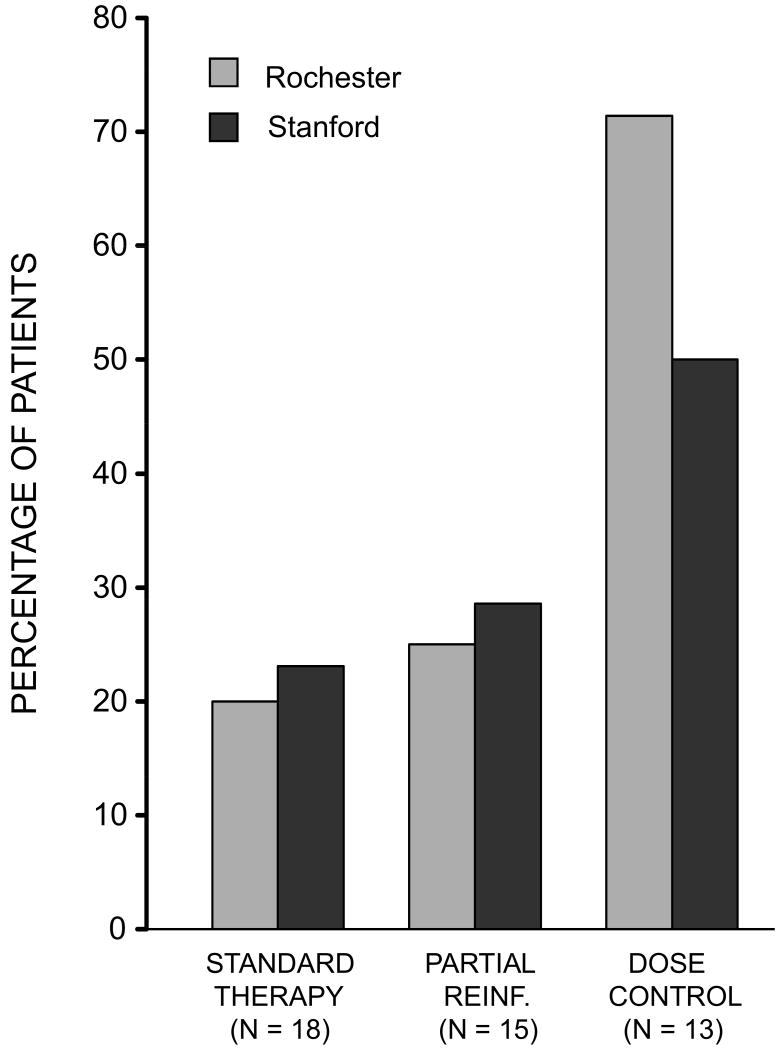
With respect to relapse, there were no differences between the 25 and 50% reinforcement schedules or between patients studied in New York and California. These groups were therefore combined. The incidence of relapse is shown in Figure 3. An overall Chi square analysis revealed differences in the frequency with which relapse occurred in the three differentially treated groups (X2 = 5.81, p < .05, 1 tail). Among those treated under a standard regimen of corticosteroid therapy, 4 of 18 patients (22.2%) relapsed within the 8-wk experimental period. Among patients treated under a partial schedule of reinforcement, 4 of 15 (26.7%) relapsed. In contrast, 8 of the 13 dose control patients (61.5%) that received the same reduced amount of drug relapsed in the same period of time. Planned comparisons showed that the incidence of relapse in the Partial Reinforcement group was lower than in Dose Control patients treated with the same amount of corticosteroid (X2 = 3.45, p < .05) and did not differ from patients receiving a full dose of drug all the time X2 = 0.09, p >.10).
Figure 3.
Incidence of “relapse” as a function of reinforcement schedule and amount of drug. Planned comparisons showed incidence of relapse in the Partial Reinforcement group to be lower than in Dose Control patients treated with the same amount of corticosteroid.
The control lesion, which defines the effects of emollient alone, provides an approximation of a within-subject control for the natural history of disease. Therefore, we compared the difference between the PSS scores of the target and control lesions for each patient to determine the effect of treatment over and above the effects of emollient. There were Group differences (F(2/43) = 3.94, p =.027), but there were no interaction effects. Overall, the difference between target and control lesions among patients in the Dose Control group was significantly (44%) less (i.e., the severity of the psoriatic lesions was greater) than among patients in the Partial Reinforcement Group (PLSD Critical Difference = 1.23. p < .014). There was no difference between the Partial Reinforcement and the Standard Therapy groups (p = .39). Again, however, these differences paralleled differences seen at the final baseline week and cannot be used to support the hypothesis.
DISCUSSION
Based on a learning model of placebo effects under chronic drug treatment conditions (3) the hypothesis that psoriasis patients treated under a partial schedule of pharmacologic reinforcement would show greater amelioration of symptoms and less relapse than patients treated with the same cumulative amount of drug under a standard pharmacotherapeutic regimen—a continuous schedule of pharmacologic reinforcement was—confirmed. These observations are consistent with the results obtained in the animal research (3,5,7-10) from which the present study was derived. At one of the study sites, however, there were no group differences in PSS scores that could be attributed to the different treatments. This may have been related to the (not to be expected) high baseline values in the randomly selected Dose Control subjects. It might even reflect the amount of sunshine available in Stanford relative to Rochester. This subset of data for this outcome measure, then, neither supports nor refutes the hypothesis. The elevated PSS baseline in the Dose Control group cannot, however, explain the difference in the incidence of relapse between the Partial Reinforcement and Dose Control groups since the somewhat higher incidence of relapse occurred in Dose Control subjects in Rochester where there was no difference in baseline PSS scores.
Among psoriasis patients who showed comparable levels of improvement under standard treatment conditions, the subsequent imposition of a partial schedule of pharmacologic reinforcement enabled these patients to be maintained with a cumulative amount of topical corticosteroid that was, under standard treatment regimens, relatively ineffective in treating the disease. The fact that there were no differences between patients treated under a schedule of partial reinforcement and those treated under a standard treatment regimen who received 2-4 times more drug is another interesting feature of these results. We cannot conclude, however, that the partial and continuous reinforcement treatments were equivalent. Nonetheless, these results suggest that a parametric examination of the interactions among drug dose, frequency and schedule of reinforcement would yield interesting results with important implications for the design of pharmacotherapeutic regimens.
While the feasibility of incorporating a behavioral strategy in the titration of prescribed medications has been established here, we can only infer, based on the operations performed, that conditioning processes were responsible for the observed effects. In this initial study, we did not have an independent measure of conditioned physiological responses relevant to the amelioration of symptoms of psoriasis. Also, other predictions that can be derived from the model have yet to be tested, e.g., would pre-exposure to the CS or UCS attenuate learning and would reinforcement schedule predict resistance to extinction (the partial reinforcement effect)?
Similarly, alternative hypotheses need to be addressed. Multiple doses of .025 or .05% Aristocort A may not be pharmacodynamically equivalent to 0.1% Aristocort A and, while repeated low doses are relatively ineffective, a bolus of corticosteroid once every other day or every four days (without intervening conditioned stimuli) may be sufficient to maintain reduced symptoms of psoriasis. If true, however, the standard qday or bid dosing regimen for treating psoriasis may be prescribing 2-4 times more corticosteroid than necessary. One might (should) consider a comparison group maintained on a continuous schedule of reinforcement that receives only a full dose of drug once every 2 or 4 days. Experimentally, the data from such a group would be suspect, however, since, unlike all other study subjects, these patients could not be blinded. Even so, such a group will need to be studied.
Another variable to consider is that the “side” effects of drug treatments could be reduced under a partial schedule of reinforcement (less drug is being administered) resulting in greater adherence to the treatment regimen than occurs under a continuous, full dose regimen. This beneficial side effect may play some role in the therapeutic equation in humans. But, it could not explain the comparable results obtained in lupus-prone mice treated under the same conditions described here (5).
It has been shown that, even in the case of an autoimmune disorder, animals behave in their biological interests. For example, unlike normal mice that develop an aversion to flavors associated with the effects of cyclophosphamide, lupus-prone mice do not display an aversion to a novel taste associated with the immunosuppressive drug (33). The possibility that a conditioned compensatory response could emerge in a pharmacotherapeutic situation, a biologically maladaptive outcome, seems unlikely, but could be examined in subhuman animals using a pharmacologic agent that reliably elicits a compensatory response in normal animals and has therapeutic effects in animals with a spontaneously occurring or experimentally-induced pathologic condition.
There are, in addition, several methodological issues that will need to be addressed. Thus far, the selection of reinforcement schedule has been essentially arbitrary and will need to be established (and is likely to be different) for different medical conditions. The duration of the baseline period the—period during which conditioning occurs—has yet to be examined. Given a sufficient number of treatment trials (each medication instance constituting a single trial), we would predict that all patients (not just a subgroup of placebo “responders”) would eventually acquire the conditioned pharmacotherapeutic response.
Psoriasis was chosen for this first study because neither the disease nor treatment with topical corticosteroid was considered a major health risk, there were measurable clinical outcomes and, presumably, a sufficient number of qualified participants. This model, however, was not ideal for examining possible biological mechanisms. Corticosteroids are assumed to influence the expression of psoriasis through its immunosuppressive and anti-inflammatory actions, but which specific aspects of immune function are responsible for the alleviation of symptoms of psoriasis and which of these are capable of being conditioned remains to be determined. It seems unlikely that a single biological mechanism will be found to explain conditioned pharmacotherapeutic effects. Hypothetically, an elaboration of the biological mechanisms underlying conditioned pharmacotherspeutic responses will depend on the ability to condition those effects of specific drugs that are directly or indirectly responsible for alleviating the symptoms of specific disease processes.
While there are several research strategies that need to be pursued, these initial data limited by a smaller than expected or planned number of patients may reasonably be construed as providing a proof-of-principle in illustrating the feasibility and potential clinical impact of designing drug treatment protocols that consider a behavioral dimension that is an inherent component of many pharmacotherapeutic regimens. Operationally, it is not possible to administer a therapeutic agent that is not accompanied by conditioned stimuli. One can choose to ignore the learning component of pharmacotherapies. Alternatively, one can explore ways to exploit conditioning in designing drug treatment regimens that consider both the behavioral and the pharmacologic components of the response to medications. While these strategies would not apply in the case of replacement therapies, the adoption of a conditioning perspective suggests testable hypotheses and innovative strategies for the experimental analysis of drug and placebo effects and for the design of pharmacotherapeutic regimens in a variety of other disorders. The present results support the proposition, based on principles of learning, that the imposition of partial schedules of reinforcement in a pharmacotherapeutic protocol might: reduce the total amount of drug required for the treatment of some pathophysiological conditions, thereby maximizing benefits and reducing risks; reduce (deleterious or noxious) side effects (and thereby increase adherence to a treatment protocol); extend the effects of pharmacotherapy (i.e., increase resistance to extinction); and last, but by no means least, reduce substantially the cost of long-term drug treatments.
Acknowledgments
The authors acknowledge with gratitude the contributions of Stephen Bean, Director of Investigational Drug Studies and his staff for the preparation and coding of the blister strips provided to study patients. We also thank Dr. Xin Tu, Department of Biostatistics, for statistical advice and Dr. Alice Pentland for conducting occasional clinical evaluations.
Supported by grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (R01 AR46825) to the senior author and from the National Center for Complementary and Alternative Medicine (R24 AG031089) to the Rochester Center for Mind-Body Research.
- PSS
Psoriasis Severity Scale
- CR
conditioned response
- UCR
unconditioned response
- CS
conditioned stimulus
- UCS
unconditioned stimulus
Footnotes
Presented, in part, at the annual meetings of the American Psychosomatic Society, Baltimore, MD, March 14, 2008.
Contributor Information
Robert Ader, Department of Psychiatry, University of Rochester School of Medicine and Dentistry, Rochester, NY.
Mary Gail Mercurio, Department of Dermatology, University of Rochester School of Medicine and Dentistry, Rochester, NY.
James Walton, Department of Psychiatry, University of Rochester School of Medicine and Dentistry, Rochester, NY.
Deborra James, Department of Dermatology, University of Rochester School of Medicine and Dentistry, Rochester, NY.
Michael Davis, Department of Dermatology, Stanford University School of Medicine, Stanford, CA.
Valerie Ojha, Department of Dermatology, Stanford University School of Medicine, Stanford, CA.
Alexa Boer Kimball, Department of Dermatology , Harvard University Medical School, Cambridge, MA.
David Fiorentino, Department of Dermatology, Stanford University School of Medicine, Stanford, CA.
References
- 1.Tilburt JC, Emanuel EJ, Kaptchuk TJ, Curlin FA, Miller FG. Prescribing “placebo treatments”: results of national survey of US internists and rheumatologists. BMJ. 2008;337:a1938. doi: 10.1136/bmj.a1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guess HA, Kleinman A, Kusel, Engel LW, editors. The science of the placebo. London: BMJ Books; 2002. [Google Scholar]
- 3.Ader R. The role of conditioning in pharmacotherapy. In: Harrington A, editor. The Placebo Effect: An Interdisciplinary Exploration. Cambridge: Harvard Univ. Press; 1997. pp. 138–65. [Google Scholar]
- 4.Amanzio M, Benedetti F. Neuropharmacological dissection of placebo analgesia: expectation-activated upload systems versus conditioning-activated specific subsystems. J Neurosci. 1999;19:484–94. doi: 10.1523/JNEUROSCI.19-01-00484.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ader R, Cohen N. Behaviorally conditioned immunosuppression and murine systemic lupus erythematosus. Science. 1982;215:1534–6. doi: 10.1126/science.7063864. [DOI] [PubMed] [Google Scholar]
- 6.Olness K, Ader R. Conditioning as an adjunct in the pharmacotherapy of lupus erythematosus. J Dev Behav Pediatr. 1992;13:124–5. doi: 10.1097/00004703-199204000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Klosterhalfen W, Klosterhalfen S. Pavlovian conditioning of immunosuppression modifies adjuvant arthritis in rats. Behav Neurosci. 1983;97:663–6. doi: 10.1037//0735-7044.97.4.663. [DOI] [PubMed] [Google Scholar]
- 8.Gorczynski RM. Conditioned enhancement of skin allografts in mice. Brain Behav Immun. 1990;4:85–92. doi: 10.1016/0889-1591(90)90011-e. [DOI] [PubMed] [Google Scholar]
- 9.Grochowicz PM, Schedlowski M, Husband AJ, King MG, Hibberd AD, Bowen KM. Behavioral conditioning prolongs heart allograft survival in rats. Brain Behav Immun. 1991;5:349–56. doi: 10.1016/0889-1591(91)90030-e. [DOI] [PubMed] [Google Scholar]
- 10.Exton MS, von Hörsten SB, Schultz M, Vöge J, Strubel T, Donath S, Steinmüller C, Seelinger H, Nagel E, Westermann J, Schedlowski M. Behaviourally conditioned immunosuppression using cyclosporin A: Central nervous system reduces IL-2 production via splenic innervation. J Neuroimmunol. 1998;88:182–91. doi: 10.1016/s0165-5728(98)00122-2. [DOI] [PubMed] [Google Scholar]
- 11.Jones RE, Moes NM, Zwickey H, Cunningham CL, Gregory WL, Oken B. Treatment of experimental autoimmune encephalomyelitis with alpha lipoic acid and associative conditioning. Brain Behav Immun. 2008;22:538–43. doi: 10.1016/j.bbi.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castes M, Palenque M, Canelones P, Hagel I, Lynch N. Classic conditioning and placebo effects in the bronchodilator response of asthmatic children. Neuroimmunomodulation. 1998;5:70. [Google Scholar]
- 13.Batterman RC. Methodology of analgesic evaluation: experience with orphenadrine citrate compound. Curr Ther Res Clin Exp. 1965;7:639–47. [PubMed] [Google Scholar]
- 14.Sunshine A, Laska E, Meisner M, Morgan S. Analgesic studies of indomethacin as analyzed by computer techniques. Clin Pharmacol Ther. 1964;5:699–707. doi: 10.1002/cpt196456part1699. [DOI] [PubMed] [Google Scholar]
- 15.Moertel CG, Taylor WF, Roth A, Tyce FA. Who responds to sugar pills? Mayo Clin Proc. 1976;51:96–100. [PubMed] [Google Scholar]
- 16.Greenberg LM, Roth S. Differential effects of abrupt versus gradual withdrawal of chlorpromazine in hospitalized chronic schizophrenic patients. Am J Psychiat. 1966;123:221–6. doi: 10.1176/ajp.123.2.221. [DOI] [PubMed] [Google Scholar]
- 17.Ader R. Conditioning effects in pharmacotherapy and the incompleteness of the double-blind, crossover design. Integ Psychiat. 1989;69:165–70. [Google Scholar]
- 18.Suchman AL, Ader R. Classic conditioning and placebo effects in crossover studies. Clin Pharmacol Ther. 1992;52:372–7. doi: 10.1038/clpt.1992.157. [DOI] [PubMed] [Google Scholar]
- 19.Kirsch I. Specifying nonspecifics: Psychological mechanisms of placebo effects. In: Harrington A, editor. The Placebo Effect: An Interdisciplinary Exploration. Cambridge: Harvard Univ Press; 1997. pp. 166–186. [Google Scholar]
- 20.Farber EM, Lanigan SW, Boer J. The role of cutaneous sensory nerves in the maintenance of psoriasis. Int J Dermatol. 1990;29:418–420. doi: 10.1111/j.1365-4362.1990.tb03825.x. [DOI] [PubMed] [Google Scholar]
- 21.Raychaudhuri SP, Farber EM. Are sensory nerves essential for the development of psoriatic lesions? J Am Acad Dermatol. 1993;28:488–489. doi: 10.1016/s0190-9622(08)81760-4. [DOI] [PubMed] [Google Scholar]
- 22.Raychaudhuri SP, Farber EM. Neuroendocrine influences on the pathogenesis of psoriasis. In: Ader R, Cohen N, Felten D, editors. Psychoneuroimmunology. 3. New York, NY: Academic Press; 2000. pp. 471–482. [Google Scholar]
- 23.Farber EM, Nickoloff BJ, Recht B, Fraki JE. Stress, symmetry, and psoriasis: possible role of neuropeptides. J Am Acad Dermatol. 1986;114:305–311. doi: 10.1016/s0190-9622(86)70034-0. [DOI] [PubMed] [Google Scholar]
- 24.Fava GA, Perini GI, Santonastaso P, Fornasa CV. Life events and psychological distress in dermatologic disorders: psoriasis, chronic urticaria and fungal infections. Br J Med Psychol. 1980;53:277–82. doi: 10.1111/j.2044-8341.1980.tb02551.x. [DOI] [PubMed] [Google Scholar]
- 25.Seville RH. Psoriasis and stress. Br J Dermatol. 1977;97:297–302. doi: 10.1111/j.1365-2133.1977.tb15186.x. [DOI] [PubMed] [Google Scholar]
- 26.Raychaudhuri SP, Gross J. Psoriasis risk factors: role of lifestyle practices. Cutis. 2000;66:348–52. [PubMed] [Google Scholar]
- 27.Emtestam L, Bergland L, Angelin B, Drummond GS, Kappas A. Tin-proroporphyrin and long wave length ultraviolet light in treatment of psoriasis. Lancet. 1989;1:1231–1233. doi: 10.1016/s0140-6736(89)92331-3. [DOI] [PubMed] [Google Scholar]
- 28.Gupta MA, Gupta AK. The psoriasis life stress inventory: A preliminary index of psoriasis-related stress. Arch Dermatol Venerol (Stockh) 1995;75:240–3. doi: 10.2340/0001555575240243. [DOI] [PubMed] [Google Scholar]
- 29.Fortune D, Main CJ, O’Sullivan TM, Griffiths CE. Assessing illness-related stress in psoriasis: The psychometric properties of the psoriasis life stress inventory. J Psychosom Res. 1997;42:467–75. doi: 10.1016/s0022-3999(97)00036-6. [DOI] [PubMed] [Google Scholar]
- 30.Kanner D, Coyne JHC, Schaefer C, Lazarus RS. Comparison of two modes of stress measurement: Daily hassles and uplifts versus major life events. J Behav Med. 1981;4:1–39. doi: 10.1007/BF00844845. [DOI] [PubMed] [Google Scholar]
- 31.Horowitz M, Wilner N, Alvarez W. Impact of event scale: A measure of subjective stress. Psychosom Med. 1979;41:209–18. doi: 10.1097/00006842-197905000-00004. [DOI] [PubMed] [Google Scholar]
- 32.Cohen S, Mermelstein R, Kamarack T, Hoberman HM. Measuring the functional components of social support. In: Sarason G, Sarason BR, editors. Social support: Theory, research and applications. Martinus Niijhof; The Hague: 1985. pp. 73–94. [Google Scholar]
- 33.Ader R, Grota LJ, Moynihan JA, Cohen N. Behavioral adaptations in autoimmune disease-susceptible mice. In: Ader R, Felten DL, Cohen N, editors. Psychoneuroimmunology. Second Edition. San Diego: Academic Press; 1991. pp. 685–708. [Google Scholar]