Abstract
Vesicle formation provides a means of cellular entry for extracellular substances and for recycling of membrane constituents. Mechanisms governing the two primary endocytic pathways (i.e., caveolae- and clathrin-mediated endocytosis, as well as newly emerging vesicular pathways) have become the focus of intense investigation to improve our understanding of nutrient, hormone, and drug delivery, as well as opportunistic invasion of pathogens. In this review of endocytosis, we broadly discuss the structural and signaling proteins that compose the molecular machinery governing endocytic vesicle formation (budding, invagination, and fission from the membrane), with some regard for the specificity observed in certain cell types and species. Important biochemical functions of endocytosis and diseases caused by their disruption also are discussed, along with the structures of key components of endocytic pathways and their known mechanistic contributions. The mechanisms by which principal components of the endocytic machinery are recruited to the plasma membrane, where they interact to induce vesicle formation, are discussed, together with computational approaches used to simulate simplified versions of endocytosis with the hope of clarifying aspects of vesicle formation that may be difficult to determine experimentally. Finally, we pose several unanswered questions intended to stimulate further research interest in the cell biology and modeling of endocytosis. Antioxid. Redox Signal. 11, 1301–1312.
Introduction
The current paradigm for the understanding of vesicle formation that initiates endocytosis in cells is that characteristic coat proteins induce curvature in membranes to form vesicles that exhibit distinct morphologies. Clathrin (62) and caveolin (54) are coat proteins that are well known to form vesicles. Additional coat proteins, for example the flotillins, have also been discovered (40) and shown to be less universally distributed than clathrin and caveolin proteins, which are observed in a wide variety of cells and species.
Coat proteins may interact directly or indirectly with molecular cargo being internalized. It is possible that cargo molecules stimulate vesicle formation, for instance, through specific receptors. Alternatively, invaginated “preformed” vesicles may selectively accept cargo before pinching off from the plasma membrane. The mechanisms by which coat proteins participate in vesicle formation are incompletely understood and thus an active area of biomedical research. One of the reasons for the lack of understanding is the diversity of vesicle behavior in different species and in different cell types within the same species. The literature abounds with a large number of independent experimental observations, many of which are so recent that it is difficult to piece all the results together.
This review aims to compare the underlying molecular participants and mechanistic steps of well-studied endocytic mechanisms and to infer general principles from the mass of biologic observations. We attempt to bring together the body of knowledge in a concise manner that will enable the reader to gain an overview of the topic. With this end in mind, we begin by briefly discussing the implications of evolutionary conservation of endocytic proteins. This is followed by a short discussion on the specificity of endocytic structures observed in certain cell types and species. We then list some of the important biochemical functions fulfilled by different endocytic mechanisms and diseases caused by their disruption. Next, we describe the structures of key components of endocytic pathways and their known mechanistic contributions. Based on the known functions and structural details of endocytic participants, we then describe some of the possible ways in which the principal components of endocytosis are recruited to certain sites at the plasma membrane where they interact to induce vesicle formation. Computational approaches can be used to simulate simplified models of endocytosis that consider only the predominant interactions between the most important players. We finally discuss how computational models such as these can assist in clarifying certain aspects of vesicle formation that may be inaccessible by following a purely experimental approach.
Evolution of the endocytic machinery
Proteins that are key components of the endocytic machinery are conserved to differing extents in species belonging to different kingdoms. Proteins that are highly conserved across different species belonging to the same or different kingdoms often have a generalized structure. Such proteins typically accomplish similar functions through similar biochemical mechanisms. Proteins that are not highly conserved have probably evolved from different evolutionary roots and may demonstrate varied biochemistry. The behavior of a protein observed in a system that mimics the biochemistry of a particular species can be generalized to other species in which the protein is highly conserved.
Clathrins—key protein components of clathrin-coated pits (CCPs)—are found in yeasts, plants, and animals. They have features that are conserved across mammals as different as rats, cows, and humans (25). The significant differences between mammalian and yeast clathrins are observed mainly in the light-chain clathrin component (70); whereas the heavy-chain clathrin component is highly conserved (29).
Caveolins—proteins that are structural markers of vesicle-forming cave-like invaginations of the plasma membrane known as caveolae—are found in vertebrate and invertebrate animals, but are absent in plants, yeasts, and amoebozoa (16). They are evolutionarily conserved in organisms ranging from Caenorhabditis elegans (C. elegans) through zebrafish, mice, and humans (18). Vertebrate and invertebrate caveolin isoforms stably expressed in caveolin-1–null mice form morphologically identical caveolae. Interestingly, the ability of caveolins to induce the formation of caveolae has not been conserved in the primitive organism C. elegans (31). This lack of caveolae-forming ability of caveolins in C. elegans indicates that caveolin may have had a non-endocytic function in the early stages of evolution.
Dynamins—GTPases that are involved in the scission of endocytic vesicles from membranes—are found in metazoa, fungi, amoebozoa, and plants (16). They are varied and specialized, to the extent that they are even expressed differentially in various mammalian tissues (38).
Complexity and functional specificity
The mechanisms that regulate the formation of endocytic vesicles and the internalization of bound stimulants (hormones, drugs, plasma macromolecules, and invading organisms) and/or unbound solutes vary depending on both the stimulus and the specific cell type. The picture is complicated by the coexistence of several endocytic routes within most cell types, as well as by the nonspecificity of some cargo. One of the key questions in the field is whether preexisting endocytic vesicles trap and internalize cargo or the presence of a stimulant induces de novo vesicle formation. In the case of clathrin-mediated endocytosis, it seems clear that it depends on the cargo: epidermal growth factor receptor (EGF-R) and certain pathogens stimulate the formation of new clathrin-coated vesicles, whereas transferrin receptor (Tf-R), low-density lipoprotein receptor (LDL-R), and some others stabilize preexisting CCPs and use them for internalization (4). Receptors that use preassembled CCPs are thought to internalize by constitutive endocytosis (i.e., regardless of whether they are bound to ligands). In ligand-induced endocytosis, ligand binding to its receptor stimulates formation of the endocytic vesicle and receptor internalization (4, 43). Caveolae, which represent another type of endocytic vesicle, are described as preassembled, stable units that can undergo internalization in the presence of stimuli. However, recent data suggest that new caveolae can be assembled on cell stimulation with EGF; furthermore, the formation of the new vesicles is dependent on caveolin-1 phosphorylation (45). Different caveolar morphologies are observed to predominate, depending on the cell type. In adipocytes, caveolae are frequently observed in clusters called rosettes, whereas in endothelial cells, elongated caveolae are seen to form transcellular channels (54). In contrast to CCPs, caveolae do not strip their caveolar coat on internalization, but fuse with endosomes or the abluminal membrane and release their cargo before recycling back to the cell surface.
Classes and Functions of Endocytic Vesicles
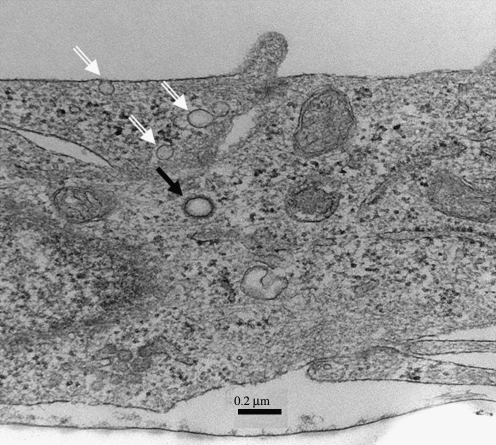
Vesicle formation is used as a gateway for the entry of extracellular substances into the cell or for recycling membrane components. Vesicle formation is thus related to various cellular functions that are accomplished through endocytosis. Based on the particular physical structure and chemical makeup involved, different vesicles carry out different functions. The two well-known endocytic invaginations, CCPs and caveolae, exhibit striking differences in their morphologies. CCPs are marked by their basket-like or soccer-ball appearance, whereas caveolae exhibit distinct spiral striations on their cytosolic surface. As shown in Fig. 1, which is an electron micrograph of an endothelial cell in cross section, caveolae (identified by open white arrows) and a clathrin-coated vesicle (CCV) (black arrow) are readily distinguishable. The other types of vesicles show apparently smooth surfaces and thus are devoid of the characteristic features of caveolae and CCPs.
FIG. 1.
Cross section of a human lung microvascular endothelial cell, showing caveolae (white arrows) and a CCV (black arrow). Human lung microvascular endothelial cells were rinsed once with PBS and fixed at room temperature in 2.5% glutaraldehyde in PBS, washed 3 times in PBS, and postfixed in 1% OsO4 in PBS. Samples were then embedded in Epoxy resin. Thin sections (83 nm) were placed on copper 200-mesh grids and stained with 3% uranyl acetate and lead citrate. Pictures were taken on a Jeol 1220 Transmission Electron Microscope with Gatan Erlangshen ESW1000W 785 Digital Camera with Gatan Digital Micrograph Software.
Clathrin mediated
CCPs were the first coated prevesicle invaginations to be recognized. These ∼200-nm structures are observed on the plasma membrane and internal organellar surfaces, such as the trans-Golgi network (TGN) (62). CCPs participate in clathrin-mediated endocytosis by detaching from the source membrane as CCVs that leave after selectively accepting molecular cargo. After transport through the cytosol, they dock at and fuse with target membranes to release the cargo (43).
CCVs participate in various cellular functions that are linked to vesicle formation and endocytosis. For instance, they are involved in the endocytosis of a wide variety of substances including nutrients, viruses, plasma membrane proteins and receptors, and extracellular ligands. Cell-surface receptors, including Tf-R, LDL-R, β2-adrenergenic receptors, CD4, insulin receptor, T-cell receptors, and B-cell receptors, are concentrated in CCPs. By controlling cell-surface receptors, clathrin structures accomplish endocytic regulation of cell signaling (references in 7, 62).
The internalization of pathogens such as the influenza virus, reovirus, and Listeria monocytogenes appears to depend on clathrin-coated structures that share many structural features and machinery with CCPs and CCVs (4). Defects in the formation of CCVs are implicated in diseases like familial hypercholesterolemia, hereditary hemochromatosis, leukemia, and muscle defects (references in 7). CCVs also carry out some functions that are related to intracellular transport, rather than endocytosis. For instance, CCVs are recruited to the reforming Golgi apparatus (GA) (during telophase), and clathrin is required for reassembly of the GA (53). Clathrin-coated structures also are involved in protein sorting at the TGN (7, 34). Although it is known that different cargos can be transported via the same CCP, it has been proposed that specialized types of CCPs also are specific to certain cargos (4).
Caveolae mediated
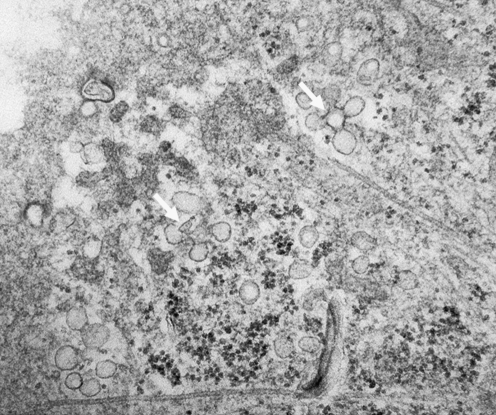
By the 1950s, in addition to clathrin-coated pits, electron microscopy had revealed apparently smooth 50- to 100-nm flask-like invaginations of the plasma membrane (47, 77). It was later realized that these invaginations, known as caveolae, could separate from the plasma membrane to form vesicles—singly, in groups (chains, grapelike clusters, rosettes; Fig. 2), or fused (transcellular channels, elongated chains, and tubules) (50, 54, 64, 71). Caveolae are found in almost all differentiated tissues but are most abundant in adipocytes, endothelial cells, and muscle cells (9). It was formerly thought that caveolae participated only in pinocytosis, but evidence gathered later indicated that caveolae also play roles in signaling, mechanotransduction, cholesterol trafficking, and cell growth (references in 63).
FIG. 2.
Horizontal section of a human lung microvascular endothelial cell showing caveolar clusters (white arrows). Cells were processed for TEM as in Fig. 1.
Caveolae have also been implicated in vesicular trafficking (transcytosis, endocytosis, and potocytosis) (1, 41, 54). The simian virus SV40 is internalized and delivered to the smooth endoplasmic reticulum by way of caveolae-mediated endocytosis, via caveolar aggregates called caveosomes (references in 33, 43). The entry of a wide variety of pathogens, ranging from viruses, bacteria, fungi, and prions, can be caveolae mediated. Studies in caveolin-1–null mice have linked the disruption of caveolin-1 expression with diseases such as cancer, diabetes, vascular abnormalities, urogenital abnormalities, eye disease, interstitial lung disease, muscular dystrophy, and cardiomyopathy (37, references in 9). Whether these pathologies are directly linked to the depletion of caveolin-1 per se or are related to disruption of caveolar formation is as yet unknown. Caveolae-mediated transcytosis was recently shown to be responsible for enhanced albumin permeability of pulmonary microvascular endothelial cells, in that polymorphonuclear neutrophil–induced activation of caveolae-mediated endocytosis and trafficking was linked to pulmonary edema formation (24).
Clathrin- and caveolin-independent endocytosis (raft-mediated)
Current research efforts are generating insight into alternative endocytic pathways that are clathrin and caveolin independent. Initially, all such pathways were lumped under the “clathrin-independent” descriptor, but as more information about the cellular machinery involved in such pathways is revealed, it has become increasingly apparent that distinct classes using different sets of endocytic participants exist. Flotillin-regulated, Cdc42-dependent, lipid raft–dependent, and macropinosome-mediated pathways are some of the known caveolin- and clathrin-independent endocytic pathways (19, 26, 40). Several examples are worth noting:
It is thought that the common cytokine receptor γ (γc) undergoes clathrin-independent endocytosis, because it has been shown that clathrin depletion by siRNA does not affect the uptake of γc (61).
The internalization of nonenveloped simian virus 40 (SV40) and the bacterial protein cholera toxin (CT) also are mediated by clathrin-independent pathways. Whereas SV40 is particularly enriched in caveolae, the endocytosis of CT has been demonstrated to be mediated by clathrin-dependent, caveolae-dependent, and clathrin- and caveolae-independent pathways that are sensitive to cholesterol depletion (30).
When stimulated with high doses of epidermal growth factor (EGF), the EGF receptor is ubiquitinated and is endocytosed through a clathrin-independent, lipid raft–dependent pathway involving epsin, eps15, and eps15R, which are recruited to the ubiquitinated EGF receptor (69).
Although the endocytosis of anthrax toxin is clathrin- and dynamin-dependent, it also requires lipid rafts, suggesting a parallel clathrin-independent pathway (40).
Endocytic Structures and Proteins of the Endocytic Machinery
Clathrin-coated pits
Clathrin, the primary coat protein found in CCPs, assembles into a mesh of tessellated polygons (pentagons and hexagons, like a soccer ball) that serves as the structural support for CCPs and CCVs. The edges of polygons in the mesh structure are formed by triskelions. Triskelions are composed of three heavy chains (clathrin heavy chains, CHCs) that form the core tri-legged structure, each leg being about 44 nm long. Additionally, triskelions have three light chains (clathrin light chain, CLC) that lie parallel to the heavy chains (4, 27, 74). Two kinds of CLCs have been identified in vertebrates, CLCa and CLCb (7). CCPs of ∼100 nm contain about 60 triskelions (11).
Although clathrin forms the tessellated polygonal scaffold that is the mechanical backbone of CCPs, it cannot adhere to the membrane components that form the pit or vesicle (references in 28 and 29). Adaptor proteins (APs) can attach to both clathrin and membrane components, enabling the clathrin scaffold to unite with the membrane. APs are also required to link clathrin with the cargo carried in the vesicle. About 20 different forms of clathrin adaptors are known (34, 46). Among them, AP1 and AP2 are the most widely recognized, and they stimulate the assembly of clathrin into the characteristic tri-legged hexameric triskelion structure (4, 29). AP1 is active at the TGN, and AP2 is active at the plasma membrane. AP2 has two phosphoinositide binding sites, one of which has a high affinity for phosphatidylinositol-4,5-bisphosphate (PtdIns[4,5]P2 or PIP2). The targeting of AP2 to the plasma membrane is mediated in part by the presence of PtdIns(4,5)P2 at the site of CCV assembly (5, 43). AP180 and auxilin are supporting APs that assist in both assembly and disassembly of clathrin structures (7).
In addition to clathrin and adaptor proteins, other proteins such as actin and dynamin, and phospholipids such as PtdIns(4,5)P2 play a role in forming clathrin structures. CCV formation is associated with actin polymerization (39). Disruption of actin polymerization inhibits the formation of CCPs, internalization of CCVs, and further restricts the limited lateral mobility of CCPs (79). The extent of inhibition of CCP and CCV formation is related to the extent of disruption of actin polymerization (5). The clathrin coat contains actin-binding proteins; however, CCV preparations do not contain actin or proteins that promote actin polymerization. Although none of the steps underlying CCV formation (nucleation, assembly, and scission) requires actin, sustained CCV formation grinds to a halt without an actin cytoskeleton. It is likely that the forced localization of clathrin structures due to the restricted mobility imposed by the cortical actin network aids vesicle formation by ensuring the association of all the participants necessary for vesicle formation (5). However, interference with actin polymerization does not seem to preclude clathrin-mediated endocytosis. Drugs that affect actin polymerization have varying effects on endocytosis and, in some cases, have no effect (references in 13). These apparently contradictory results suggest that although endocytosis is possible without actin, efficient clathrin-mediated endocytosis is possible only in the presence of actin.
The scission of a fully invaginated CCP to form a CCV is mediated by the 100-kDa GTPase, dynamin. Three dynamin isoforms have been found in mammalian cells: dynamin-1, dynamin-2, and dynamin-3. Dynamin-2 is expressed in most tissues (43).
Proteins that play important roles in clathrin-mediated endocytosis—for instance, AP2, dynamin, epsin, and AP180—have PtdIns(4,5)P2 binding domains. Impairing the binding of PtdIns(4,5)P2 to these proteins negatively affects endocytosis (72). Phosphatidylinositol 4-phosphate 5-kinase (PIP5K) is an enzyme that generates PtdIns(4,5)P2. Exposure to primary alcohols like 1-butanol decreases PIP5K activation, which leads to a decrease in the formation of PtdIns(4,5)P2. It has been shown that existing CCPs disappear, and no new CCPs are formed in cells treated with 1-butanol (5). Further, new CCPs were observed to form as soon as 1-butanol was removed or when PtdIns(4,5)P2 was reintroduced through liposomes. A quantitative analysis of PtdIns(4,5)P2 dynamics by using GFP-tagged protein probes at endocytic sites in budding yeasts indicates that recruitment of cargo is succeeded by PtdIns(4,5)P2 enrichment before CCP formation (72). PtdIns(4,5)P2 possibly plays a role in recruiting endocytic machinery to the plasma membrane and in promoting actin polymerization (72).
Caveolae
Caveolae are rich in the structural coat protein caveolin-1 that is known to be required for their formation. Caveolin-1 null mice lack caveolae (10, 54, 80), and expression of caveolin-1 can induce caveolar formation in cells that lack caveolae (17). Three caveolin proteins occur as the isoforms caveolin-1α, caveolin-1β, caveolin-2α, caveolin-2β, and caveolin-3 (71, 73). Caveolin-2 appears to have only a supporting role in caveolar formation (49), whereas caveolin-1 and caveolin-3 are essential for the formation of caveolae (23, 71). Caveolin-1 is expressed in endothelial cells (9) and nonstriated muscle cells (23), whereas caveolin-3 is muscle specific, and its deficiency has been linked to muscular dystrophy (21).
Oligomerized caveolin-1 is known to be the building block of caveolae (42, 60). High-resolution scanning electron microscopy has revealed that caveolar invaginations have a fine surface structure with ridges about 10 nm in diameter that spiral around the cytosolic surface (51, 57). Caveolin-1 oligomerizes into heptameric units that assemble to form the characteristic surface striations (15, 71).
Estimates for the number of caveolin-1 molecules in caveolae range from 100 to 200. The Pelkmans and Zerial (50) estimate of 144 caveolin molecules has been widely quoted in the literature, but it may be an underestimate. Each heptameric unit of caveolin-1 has a diameter of ∼10 nm, and a 100-nm planar caveolar structure having about three concentric filaments has ∼50 heptameric units (15). This suggests that, on average, caveolae can have as many as 350 caveolin-1 molecules, and possibly more.
It has been proposed over the last decade that the plasma membrane contains fluid-ordered detergent-insoluble glycolipid-rich domains referred to as lipid rafts. Lipid rafts were defined at the Keystone Symposium on Lipid Rafts and Cell Function (2006) as follows: Membrane rafts are small (10–200 nm), heterogeneous, highly dynamic, sterol- and sphingolipid-enriched domains that compartmentalize cellular processes. Small rafts can sometimes be stabilized to form larger platforms through protein–protein and protein–lipid interactions. Certain classes of lipid rafts are associated with specific structural protein components; changes in form and function of those classes of rafts may be attributed to such associations. Caveolin-1–enriched lipid raft domains invaginate to form caveolae. Electron microscopy of plasma membrane–associated caveolae revealed two pools of caveolae: shallow and deeply invaginated (20). This study suggests that the ratio of caveolin-1α to caveolin-1β is greater in deeply invaginated caveolae. The fact that lipid rafts can also form within intracellular organellar membranes such as the GA is suggestive of one potential mechanism for the biogenesis of caveolae. In this context, lipid rafts enriched with caveolin-1 at Golgi membranes form internal caveolae that are excised from the GA and transported to the plasma membrane where they dock, fuse, and appear as the familiar caveolar invaginations (2, 21). It is also known that caveolae are rich in cholesterol and that caveolin-1 binds cholesterol. Additionally, the caveolar structure is deformed by cholesterol removal (57). Thus, both caveolin-1 and cholesterol are thought to play critical roles in the formation and stability of caveolae.
Recently, proteins other than caveolins have been found to be integral to the caveolar structure. A 60-kDa protein was shown by immunoelectron microscopy to be present on the cytosolic surface of caveolin-1 containing caveolae in rat adipocytes and with caveolin-2 and -3 isoforms in other rat tissues as well (52, 75). Sequencing revealed the protein to be identical to polymerase I and transcript release factor (PTRF); however, it was named Cavin because of its association with caveolae (76). Cavin is known to colocalize with caveolin-1 and is sensitive to cholesterol depletion. Overexpression of cavin leads to an increase in the recruitment of caveolin-1 to lipid rafts in cultured cells. Cavin may serve also to connect caveolae with the cytoskeleton (36). Zebrafish and honeybee caveolin, both capable of inducing caveolae formation, recruit cavin to the plasma membrane, whereas C. elegans caveolin, which is unable to induce caveolae formation, does not recruit cavin to the plasma membrane. This indicates that cavin plays a role in the formation of caveolae. Furthermore, fluorescence lifetime imaging and fluorescence resonance energy transfer have shown that caveolin and cavin are closely associated in the plasma membrane (23). In prostate cancer cells, cavin is required for caveolae formation. In an NIH 3T3 fibroblast cell line with reduced cavin expression, caveolin was diffuse in the plasma membrane instead of aggregating, suggesting that cavin is essential for stabilization of caveolin in caveolae (23). Cavin is also essential for in vivo formation of caveolae in zebrafish notochord cells (23). The role of cavin in the formation of caveolae warrants further inquiry.
As in the case of clathrin-mediated endocytosis, the excision of surface caveolae to form endocytic vesicles is mediated by dynamin recruitment to budding caveolae (22, 44). Dynamin-2 strongly associates with invaginated caveolae, especially near constricted necks. It appears that direct binding occurs between dynamin-2 and caveolin-1 (78). Dynamin-2 is regulated by Src kinase–dependent phosphorylation, which stimulates its translocation to caveolin-1–rich membranes and promotes its association with caveolin-1 (68).
Electron-microscope tomography of endothelial cells and 3T3-L1 adipocytes has revealed that complex interconnections occur between clusters of caveolae and the actin cytoskeleton and microtubules (55). This finding suggests that actin and microtubules play roles in the formation and distribution of caveolar clusters, such as rosettes.
See Table 1 for a comparison of some of the important features of CCVs and caveolae.
Table 1.
Comparison of Caveolae and CCVs
| Feature | Clathrin | Caveolae |
|---|---|---|
| Oligomeric unit | Triskelions | Heptamers |
| Assembly unit | Polygons | Spiral striations |
| Cargo attachment proteins | Adaptors | Cavins (?) |
| Scission mechanism | Elongation | Constriction |
Clathrin and caveolin independent
Some endocytic mechanisms are independent of clathrin and caveolins, but share some of the endocytic participants. The familiar mechanism of recruitment of endocytic proteins, invagination, and scission is observed in these mechanisms. Sphingolipids and cholesterol may be involved in clathrin-independent pathways.
Specifically, in mutant Chinese hamster ovary cells, sphingolipid depletion disrupted clathrin-independent endocytosis but not clathrin-dependent endocytosis. The loss of sphingomyelin is particularly responsible for disruption of clathrin-independent RhoA- or Cdc42-dependent pathways (8).
The clathrin-independent endocytosis of γc receptor is dynamin and actin dependent and associated with flotillin-2 and lipid rafts (61).
CT is endocytosed by tubular 40- to 80-nm vesicles thought to be GPI-AP–enriched early endosomal compartments or GEECs (30).
The nontoxic homopentameric Gb3-binding B-subunit of Shiga toxin stimulates the formation of tubular membrane invaginations for its uptake. Dynamin inhibition leads to an increase in the appearance of these >200-nm tubular surface-connected invaginations. The invaginations are free of caveolin-1 and can form in caveolin-free cell systems (56).
Flotillin-1 and flotillin-2 are related proteins that coassemble into lipid raft–like microdomains. These laterally mobile domains have a morphology that is dissimilar to that of CCPs and somewhat similar to that caveolae. The similarity of flotillin vesicles and caveolar vesicles suggests that some structures thought to be caveolae may be invaginated flotillin microdomains (19).
Mechanisms of Vesicle Formation
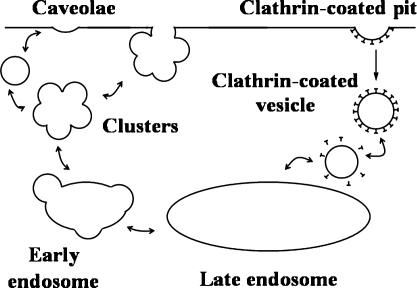
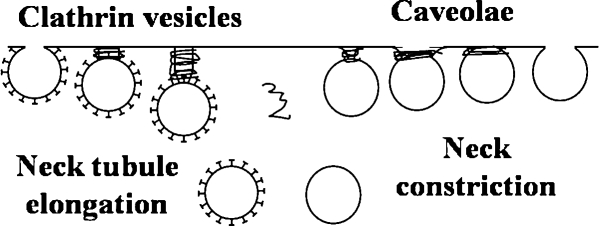
Currently, an incomplete understanding exists of the mechanisms of vesicle formation; however, some observations reveal some biologic elements of the mechanisms. A broad comparison of the processes of invagination, vesicle formation, and vesicle disassembly in the cases of caveolae and CCVs is shown in Fig. 3. At present, evidence of two major pathways is found. One is that the vesicle is formed on the membrane surface, either at the plasma membrane at the cell surface or at internal cell organelles like the TGN. Another possibility is that vesicles formed elsewhere dock at, fuse with, and then break off from membranes. Observations of intermediate structures suggest likely steps in the mechanisms for both paths.
FIG. 3.
Caveolae- and clathrin-mediated endocytic structures.
Stimulated vesicle formation; activated endocytosis
Clathrin
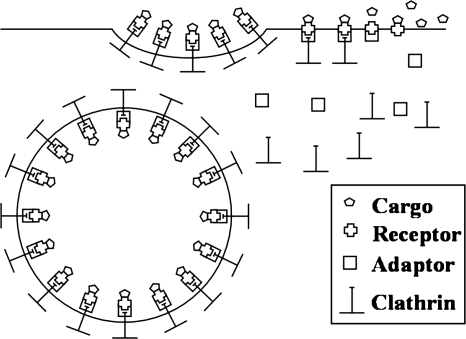
It is likely that CCV formation begins with AP1 and AP2 being recruited to and possibly self-assembling at receptor sites on the plasma membrane (7) (see Fig. 4). The adaptor proteins dock with receptors that may or may not be bound to cargo. This is followed by the recruitment of clathrin and attachment of clathrin molecules to adaptors. This is succeeded by the formation of triskelions followed by the assembly of triskelions into a curved polygonal mesh. The initial assembly of triskelions is seen to be in the form of hexagonal arrays, but the final clathrin-coated structure has both hexagonal and pentagonal cells (27, 62).
FIG. 4.
The process of CCV assembly. Once a cell-surface receptor is ligated by its specific ligand or cargo, intracellular adaptor protein and clathrin assemble on the membrane receptor, forming a clathrin-coated pit and, eventually, a clathrin-coated vesicle (CCV). Finally, the clathrin coat is removed, and the adaptor and clathrin are recycled.
Two different mechanisms have been proposed to explain how clathrin triskelions assemble into the final soccer-ball structure (43). One possibility is that triskelions first assemble into flat hexagonal assemblies. Then a lattice rearrangement occurs, in which some hexagons are replaced by pentagons to induce curvature. Geometric considerations suggest that to form a closed spherical structure, 12 pentagonal cells are required in the final curved tessellated polygonal assembly (28, 29). However, it is unclear how this substitution might take place. Possibly eps15 and amphiphysin are involved in the lattice rearrangement (7). Although auxilin and the ATPase Hsc70 are primarily involved in clathrin-coated vesicle disassembly, they may also play a role in the initial rearrangement of the clathrin lattice (12).
The second mechanism through which triskelions could assemble into CCPs is that the initial flat hexagonal assemblies serve as reservoirs for clathrin. Clathrin rapidly recruited from these reservoirs could assemble into triskelions that are inserted at edge positions in hexagonal cells or pentagonal cells directly as needed, avoiding the necessity of lattice rearrangement (43). Total internal reflective fluorescence (TIRF) microscopy of the membrane of isolated adipocytes that are not attached to solid substrate suggests that CCVs form and detach from stable patches of clathrin-coated membrane domains (3), wherein the clathrin patches are continuously replenished with clathrin from the cytosol. Whether the clathrin patches are aggregates of CCPs or clathrin arrays that form individual CCPs just before scission is not clear. This phenomenon may be related to the enrichment of clathrin and actin assembly-promoting phosphoinositides like PtdIns(4,5)P2 in specific membrane regions (3, 5, 72).
Preceding scission, constriction of the pits into necked structures with sequestration of cargo is observed. The narrow neck is then severed to complete vesicle formation. The sequence: constriction, neck elongation, scission is illustrated in Fig. 5. Dynamin is known to be involved in constriction and scission (7, 39). Dynamin self-assembles into helical ring structures in vitro and into stacks of connected rings under low-salt conditions (references in 38). Dynamin recruitment to the clathrin triskelion assembly is succeeded by GTP-triggered dynamin redistribution into helical rings at necks (38), with about 20 dynamin molecules in a spiral configuration in a helical ring (62). Evanescent wave microscopy combined with epifluorescence illumination has been used to image dynamically the process of internalization of CCPs. The process of internalization is marked by concurrent actin recruitment and assembly with a flurry of dynamin activity just before fission (39).
FIG. 5.
Comparison of scission in caveolae and CCVs. Cytoskeletal elements and dynamin-mediated “pinchase” activity are thought to induce vesicle fission from the membrane.
Caveolae
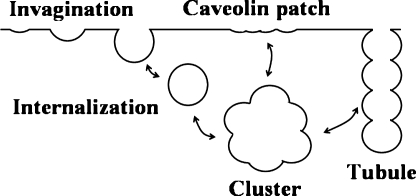
Although initial evidence indicated that caveolae form on the plasma membrane, recent studies point to Golgi complexes as being the original sites for caveolae formation (49). The synthesis of caveolin occurs in the ER, and caveolin molecules hook through the inner leaflet of the membrane in a hairpin loop–like manner (42). Soon after synthesis, the caveolin monomers oligomerize into heptamers, which are phosphorylated, it is thought, to prevent premature assembly (2). The phosphorylated oligomers are transported by the secretory pathway to the Golgi complex, where they are dephosphorylated (2). Cholesterol is hypothetically incorporated in membrane regions where the oligomers are attached, and the cholesterol-rich membrane domain with the caveolin heptamers curves to form caveolae (15, 21). It has been suggested that asymmetrically anchored caveolin oligomers exert forces on the underlying membrane, and that these forces exert bending moments that are responsible for deformation of the membrane into the characteristic flasklike shape (15). Internalized caveolae can then aggregate into clusters, as seen in Fig. 2. Figure 6 shows how caveolae can form caveolar clusters or fused caveolar structures.
FIG. 6.
Caveolae exist as individual caveolar buds, membrane-associated vesicles, or multiples of caveolae in the form of elongated channels or grapelike clusters.
Kiss-and-run mechanism
As an alternative to the invagination and pinching off of caveolae, a kiss-and-run mechanism has been proposed (50) from data showing that preexisting vesicles periodically undergo docking, fusion, and fission. While docked, the integrity of the caveolar coat is maintained. Between docking cycles, motility of vesicles is restricted within small volumes just beneath the cell-surface areas. Exposure to SV40 increases the frequency of surface docking events rather than the number of surface caveolae. The actin cytoskeleton may restrict caveolae from being fully internalized or from docking with internal organelles and cause them to conduct these kiss-and-run cycles.
It is thought that the way dynamin induces fission in the case of CCPs and caveolae differs in the constriction step (Fig. 5). In the case of CCPs, a long neck has been observed (35). Dynamin is thought to play a role in the elongation of the neck, which leads to the CCP pinching off from the membrane to form a CCV (38, 39). In the case of caveolae, neck formation has not been observed, and it is thought that dynamin constricts the neck of deeply invaginated caveolae (78).
Modeling Vesicle Formation
In silico studies can be viewed as theoretical or computational experiments conducted on simplified versions of real systems. The complexity of real systems can be reduced so that the interactions between principal components can be analyzed without having to consider numerous complicating factors present in vivo. By varying the features and parameters in a simulation, the basic structures and mechanisms involved in endocytosis can be isolated, analogous to a controlled experiment, although such experiments may not yet be feasible.
Many different mechanisms have been proposed to explain the possible steps underlying various kinds of endocytic routes (Fig. 3). It is precisely the quantitative aspects of modeling that help to distinguish the feasibility of mechanisms. This section organizes the essential questions that modeling can address and gives some examples of current approaches. Our aim is to identify generic aspects of mechanisms that can be the first focus of modeling.
The role of modeling
Modeling can be used to investigate the specific mechanisms suggested by the experimental observations outlined in this review. Although the structures associated with vesicle formation can be observed, it is not possible to determine experimentally which of these play necessary roles in the mechanism of endocytosis. In the case of caveolae, for instance, it is proposed that caveolin oligomers induce vesicle formation. The importance of the heptameric structure of these oligomers is unknown. Can the coat protein induce endocytosis if oligomerization does not occur? Is a critical oligomer size or optimal geometry of the associated surface striations necessary? The question of how the budding mechanism depends on quantitative parameters (such as the number of caveolins in each oligomer and the spatial distribution of oligomers) is one that can be directly addressed within a computational model—simply by specifying a desired configuration of structural elements and simulating the consequent evolution of the system. Similarly, forces can be added or removed as a means of investigating which are necessary and how they can be generated. For example, is an externally applied force required to deform a cell membrane (e.g., as a consequence of a signaling reaction or other stimulus), or can spontaneous curvature arise from the anisotropic composition of the lipid bilayer and resultant intrinsic bending stresses? If spontaneous curvature does arise, is this a sufficient condition for budding and invagination to occur? The literature presents a number of reports that begin to address these questions; some of these are described here. However, mechanistic models of increasing complexity will be required to unravel the tangle of observations that exists in the experimental literature.
Degrees of complexity in modeling
We have seen that endocytosis is a complex process and that the endocytic machinery has numerous interacting participants that fulfill various functions. Not only the mechanical properties of embedded proteins, but also those of the specific lipids enriched in lipid rafts may contribute to membrane deformation and hence vesicle formation. Accordingly, we must ask what degree of detail is required in a computational model. That is, what biologic elements are critical to represent the essential features of the mechanism for vesicle formation? It is difficult to say a priori what details can be omitted and which should be conserved at all costs. However, some general comments can be made. First, the number of structures and interactions included in the simulation will depend on the particular phenomena being investigated. In the case of small-scale spontaneous membrane curvature, it is possible to draw some conclusions based even on highly simplified models. A model that considers only the volume contributions of molecular constituents to the overall volume of one leaflet in a lipid bilayer has been proposed (49). The volumes corresponding to the standard amounts of caveolin-1 and cholesterol molecules are known. It is possible to estimate the volume contributed by cholesterol and caveolin-1 molecules to a known span of lipid bilayer, by referring to these standard volumes. Thus, it is possible to estimate the extent of expansion induced in a particular leaflet by the caveolin-1 and cholesterol molecules it contains. The resultant curvature caused by the expansion of one leaflet against the other can be then modeled through a truncated ellipsoid.
Much effort has been expended in modeling the lipid bilayer and its response to inclusions such as the proteins that are the focus of this review. These models fall into two broad classes: (a) continuum models, and (b) particulate or discrete models. Continuum models typically treat the bilayer as a two-dimensional linear-elastic sheet with surface tension (58, 66). Parameters like the bending modulus, the surface-tension coefficient, and spontaneous curvature are typically used to characterize the membrane properties. Forces on the membrane are usually modeled as entropic forces that arise from proteins attached to the surface or as stochastic forces due to the brownian fluctuations of the surrounding medium (6). However, because of the typical formulation of these models, only small displacements and small surface curvatures can be accurately modeled. The most detailed particulate models attempt to account for the details of the individual atoms making up the amphiphilic molecules of the lipid bilayer. Simulation methods such as molecular dynamics (MD) and Monte Carlo (MC) are used to address inter- and intramolecular interactions between all atoms in the membrane and the surrounding fluid. The time and length scales relevant to vesicle formation present an intractable problem for these computationally expensive fully atomistic models. Indeed, assuming computational technology advances according to Moore's law, Brannigan et al. (6) optimistically estimate a time of 46 years before a 1-μm-square patch of membrane can be modeled over an evolution time of 1 ms. By coarse-graining (grouping together) atoms into larger pseudo-particles, a mesoscopic description of the membrane is obtained that can be used to simulate efficiently the large-scale deformations of the lipid bilayer. At each level of coarse-graining, a stylized effective potential or entropic contribution is used to represent the detailed intramolecular or intermolecular effects discarded from the finer scale of description. Protein inclusions can similarly be represented as coarse-grained particles or bead-spring assemblies, such as those used in polymer kinetic theory. In extreme cases (such as contracting all the degrees of freedom of a stylized bead-spring model of an amphiphile down to just the position and direction of a rodlike particle), the underlying concept is so far removed from the atomistic fundamentals that the simplified geometry and physicochemical parameters are justified empirically by the overall membrane behavior being simulated. This is the approach of empirical models.
Continuum models
Given the enormous atomic scale complexity and degree of coordination between many elements, early attempts to model vesicle formation consisted of a simple continuum picture. The dynamics of a continuum system can be analyzed in terms of bending and stretching stresses and strains. Membrane deformations have been studied in terms of parameters such as deformed membrane surface shape, curvature, and neck radius. In the work of Sens (65), a budding parameter is defined in terms of these descriptors and used to describe the mathematical solutions to nonlinear dynamic equations that underlie vesicle formation in lipid membranes. Vesicles are known to regulate tension in membranes, and membrane tension in turn affects the morphology of vesicles (67). Membrane tension in cells is known to be affected by the anchoring of the cytoskeleton to the membrane. Physicochemical arguments support the idea that an energy barrier between flat and invaginated membrane domains facilitates their coexistence. It is thought that proteins like dynamin increase this energy barrier and thus deter membrane flattening (67). Thus, the role of the cytoskeleton in vesicle formation is an important open question.
Sens and Turner (66) modeled caveolin-1 oligomers as flexible bunches of polymeric chains that exert mechanical bending and stretching forces on an idealized lipid membrane modeled as an elastic or viscoelastic continuum. Continuum models that study the effect of caveolin aggregates on membranes by treating them as rigid or semirigid membrane inclusions indicate that higher local membrane rigidity should lead to an increased bud radius (66).
Chirality-induced budding has also been considered (59). In a multicomponent bilayer with a major and a minor component, the minor species may have an intrinsic tilt with respect to the membrane or may acquire one by association with additional components like cholesterol. The chirality of lipids will be expressed at length scales larger than molecular dimensions on account of this tilt (59). This kind of curvature could account for vesicle formation in the absence of the usual membrane-deforming proteins. Further, in the case of caveolae, this mechanism in conjunction with cholesterol-binding ability of caveolin-1 may serve to stabilize raft domains recruited into caveolae (58).
Theoretical models that consider interactions between membrane-bound proteins and the effect of membrane tension and bending modulus suggest that the appearance of striations on caveolae could be the result of membrane-mediated repulsion between proteins (14). Models that study the effects of membrane curvature and in-plane shear elasticity on resting shapes of vesicles suggest that neck formation is integral to stability of vesicles. Changing the opening radius of vesicles induces spontaneous curvature and has a dramatic effect on vesicle shape. The in-plane shear modulus affects the resting shape of vesicles in areas with significant changes in curvature (32).
Particulate models
An alternative to describing the lipid bilayer as a continuous sheet is to model it as a discrete assembly of particles. This approach has some advantages over the continuum model. Particulate models require no mathematical parameterization of the bilayer geometry and hence are not restricted to minor deformations of near-planar configurations or to constraints imposed by topology or numerical gridding, as is the case with most continuum models. They also enable the study of membranes of heterogeneous composition. However, rather than tracking the surface through a single equation, the location and interactions of every particle must be stored during the simulation. Given current computational technology, this requirement limits the size of systems that can be investigated with fully atomistic models to ∼1 nm for evolution times of ∼1 ns. Coarse-grained models can be used to address the evolution of larger membrane units for longer times by reducing the degrees of freedom included in the model. At the lowest level of coarse graining, neighboring atoms are grouped into larger pseudo-atoms, but the lipid unit still consists of a number of bonded particles. At a higher level of coarse graining, each lipid may be represented as an anisotropic particle (e.g., rod or ellipsoid). The use of an internal director enforces the molecular orientation that—in a fully atomistic or pseudo-atomistic simulation—would normally result from hydrophobic interactions with the surrounding solvent. As the level of coarse graining is increased, detail is systematically removed from the model, and suitable interparticle potentials of the simplified system are parameterized to mimic the mesoscopic effect of the missing degrees of freedom.
A novel coarse-grained approach to modeling membrane curvature and the forces responsible for bending is proposed by Akpa et al. (unpublished work). In this empirical model, the coarse-grained particles that make up the membrane are actually isotropic (i.e., each individual particle does not in and of itself embody any intrinsic directionality). Rather, a near-neighbor interaction potential is formulated to energetically favor a sheetlike relative arrangement of these particles, which collectively exhibit two-dimensional fluid behavior, while resisting bending deformations. Specifically, the relative location of particles is used to extract an effective normal direction by suitable near-neighbor summation. This vector figures in a soft potential that confines particles in a roughly planar arrangement, and a supplementary Lennard-Jones pair pseudo-potential operates in the plane to maintain cohesion. Involving only translational degrees of freedom (i.e., no directors or orientational variables), the resulting simulated construct behaves like an elastic membrane, in which particles automatically redistribute themselves in response to local forces. The parameters in the interaction potentials are tuned to the collective elastic and bending properties.
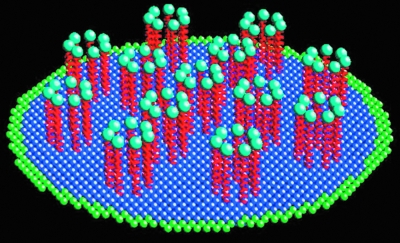
Having established a versatile computational framework for the lipid bilayer, the model goes on to address how changes in caveolin-1 oligomerization and phosphorylation affect membrane invagination, budding, and internalization by controlling (a) the stability of caveolin oligomers in the plasma membranes, and (b) their ability to facilitate changes in membrane curvature. A coarse-grained atomistic (CGA) approach is used to simulate key mechanistic features of the budding of vesicles from caveolae. It is postulated that coulombic interchain repulsion resulting from phosphorylation-induced charge creates asymmetric forces that cause the formation of curvature in the cell membrane. By combining Brownian dynamics of heptamers anchored to the membrane with the elastic membrane response, a model of caveolin-mediated curvature can be obtained (Fig. 7).
FIG. 7.
Discrete computational representation of caveolin molecules as bead-spring assemblies attached to the underlying membrane. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
Computational models feed back into biologic experiments. A computational model can be tweaked to evaluate “what if” scenarios. For instance, the effect of altering the physicomechanical properties of proteins on overall vesicle formation can be ascertained. This can be used to predict abnormal vesicle formation in diseased cells. The behavior of individual coat proteins dispersed on the membrane surface can be contrasted with that of oligomers of coat proteins. The role of external/cytosolic assemblies (actin cytoskeleton) can be modeled. The results of such computer experiments can suggest laboratory experiments that might be done to test/confirm mechanisms.
Conclusions and Future Directions
In this review, we highlight cellular signaling mechanisms that temporally and spatially regulate clathrin-coated vesicle-, caveolae-, and raft-mediated vesicle formation and endocytosis, pointing to several unanswered questions in these fields. Receptor- or cargo-specific endocytosis can uniquely activate signaling cascades that are cell-type specific and thus important for organ-specific functions. By controlling cell-surface receptors and scaffolded proteins, vesicle-associated structural proteins such as clathrin and caveolin-1 accomplish endocytic regulation of cell signaling. The molecular mechanisms of vesicle formation and trafficking, including coat protein assembly, disassembly, and interaction with cytoskeletal elements, are also at the forefront of research efforts, largely because of recent technicologic advances in the speed and resolution of light and electron microscopy. For example, it is now possible to distinguish between preexisting endocytic vesicle internalization of cargo and de novo vesicle formation on receptor activation, as well as the role of the cytoskeleton in vesicle formation, with such new instrumentation. Furthermore, as outlined herein, the ability to formulate and quickly modify testable hypotheses with the aid of computer simulations should bring into focus the feasibility of critical mechanisms governing the primary endocytic pathways as well as their potential affect on cell signaling. Research efforts that combine the use of molecular genetic approaches, proteomic profiling, live-cell imaging, ultrastructural analysis, and computer modeling will improve our understanding of the critical determinants of endocytic vesicle formation and trafficking with reference to cell-type, cargo, and disease specificity. Currently, state-of-the-art high-throughput, high-resolution, fast-acquisition live-cell fluorescent imaging platforms are being used to screen siRNA libraries (for example, to characterize the role of specific kinases in endocytosis and vesicle trafficking) (50). It is anticipated that similar studies will be used to elucidate the roles of the hundreds if not thousands of signaling molecules and protein–protein interactions involved in endocytosis, with the ultimate goal of understanding the specific compartmentalized events required for internalization and trafficking of cargo and drug molecules.
Abbreviations
AP, adaptor protein; CCP, clathrin-coated pit; CCV, clathrin-coated vesicle; CGA, coarse-grained atomistic; EGF, epidermal growth factor; ER, endoplasmic reticulum; GA, Golgi apparatus; PtdIns(4,5)P2, phosphatidylinositol-4,5-bisphosphate; TGN, trans-Golgi network; SV40, simian virus 40; TIRF, total internal reflection fluorescence.
References
- 1.Anderson R. Kamen B. Rothberg K. Lacey S. Potocytosis: sequestration and transport of small molecules by caveolae. Science. 1992;255:410–411. doi: 10.1126/science.1310359. [DOI] [PubMed] [Google Scholar]
- 2.Bauer M. Pelkmans L. A new paradigm for membrane-organizing and -shaping scaffolds. FEBS Lett. 2006;580:5559–5564. doi: 10.1016/j.febslet.2006.08.077. [DOI] [PubMed] [Google Scholar]
- 3.Bellve KD. Leonard D. Standley C. Lifshitz LM. Tuft RA. Hayakawa A. Corvera S. Fogarty KE. Plasma membrane domains specialized for clathrin-mediated endocytosis in primary cells. J Biol Chem. 2006;281:16139–16146. doi: 10.1074/jbc.M511370200. [DOI] [PubMed] [Google Scholar]
- 4.Benmerah A. Lamaze C. Clathrin-coated pits: vive la différence? Traffic. 2007;8:970–982. doi: 10.1111/j.1600-0854.2007.00585.x. [DOI] [PubMed] [Google Scholar]
- 5.Boucrot E. Saffarian S. Massol R. Kirchhausen T. Ehrlich M. Role of lipids and actin in the formation of clathrin-coated pits. Exp Cell Res. 2006;312:4036–4048. doi: 10.1016/j.yexcr.2006.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brannigan G. Lawrence C-L L. Brown FLH. Implicit solvent simulation models for biomembranes. Eur Phys J. 2006;35:104–124. doi: 10.1007/s00249-005-0013-y. [DOI] [PubMed] [Google Scholar]
- 7.Brodsky FM. Chen CY. Knuehl C. Towler MC. Wakeham DE. Biological basket weaving: formation and function of clathrin-coated vesicles. Annu Rev Cell Dev Biol. 2001;17:517–568. doi: 10.1146/annurev.cellbio.17.1.517. [DOI] [PubMed] [Google Scholar]
- 8.Cheng ZJ. Singh RD. Sharma DK. Holicky EL. Hanada K. Marks DL. Pagano RE. Distinct mechanisms of clathrin-independent endocytosis have unique sphingolipid requirements. Mol Biol Cell. 2006;17:3197–3210. doi: 10.1091/mbc.E05-12-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen AW. Hnasko R. Schubert W. Lisanti MP. Role of caveolae and caveolins in health and disease. Physiol Rev. 2004;84:1341–1379. doi: 10.1152/physrev.00046.2003. [DOI] [PubMed] [Google Scholar]
- 10.Drab M. Verkade P. Elger M. Kasper M. Lohn M. Lauterbach B. Menne J. Lindschau C. Mende F. Luft FC. Schedl A. Haller H. Kurzchalia TV. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science. 2001;293:2449–2452. doi: 10.1126/science.1062688. [DOI] [PubMed] [Google Scholar]
- 11.Ehrlich M. Boll W. van Oijen A. Hariharan R. Chandran K. Nibert ML. Kirchhausen T. Endocytosis by random initiation and stabilization of clathrin-coated pits. Cell. 2004;118:591–605. doi: 10.1016/j.cell.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 12.Eisenberg E. Greene L. Multiple roles of auxilin and Hsc70 in clathrin-mediated endocytosis. Traffic. 2007;8:640–646. doi: 10.1111/j.1600-0854.2007.00568.x. [DOI] [PubMed] [Google Scholar]
- 13.Engqvist-Goldstein AEY. Drubin DG. Actin assembly and endocytosis: from yeast to mammals. Annu Rev Cell Dev Biol. 2003;19:287–332. doi: 10.1146/annurev.cellbio.19.111401.093127. [DOI] [PubMed] [Google Scholar]
- 14.Evans RE. Turner MS. Sens P. Interactions between proteins bound to biomembranes. Physiol Rev. 2003;67:041907. doi: 10.1103/PhysRevE.67.041907. [DOI] [PubMed] [Google Scholar]
- 15.Fernandez I. Ying Y. Albanesi J. Anderson RG. Mechanism of caveolin filament assembly. Proc Natl Acad Sci USA. 2002;99:11193–11198. doi: 10.1073/pnas.172196599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Field MC. Gabernet-Castello C. Dacks JB. Reconstructing the evolution of the endocytic system: insights from genomics and molecular cell biology. Adv Exp Med Biol. 2007;607:85–96. doi: 10.1007/978-0-387-74021-8_7. [DOI] [PubMed] [Google Scholar]
- 17.Fra AM. Williamson E. Simons K. Parton RG. De novo formation of caveolae in lymphocytes by expression of VIP21-caveolin. Proc Natl Acad Sci U S A. 1995;92:8655–8659. doi: 10.1073/pnas.92.19.8655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frank PG. Lisanti MP. Zebrafish as a novel model system to study the function of caveolae and caveolin-1 in organismal biology. Am J Pathol. 2006;169:1910–1912. doi: 10.2353/ajpath.2006.060923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frick M. Bright NA. Riento K. Bray A. Christien M. Nichols BJ. Coassembly of flotillins induces formation of membrane microdomains, membrane curvature, and vesicle budding. Curr Biol. 2007;17:1151–1156. doi: 10.1016/j.cub.2007.05.078. [DOI] [PubMed] [Google Scholar]
- 20.Fujimoto T. Kogo H. Nomura RM. Une T. Isoforms of caveolin-1 and caveolar structure. J Cell Sci. 2000;113:3509–3517. doi: 10.1242/jcs.113.19.3509. [DOI] [PubMed] [Google Scholar]
- 21.Galbiati F. Razani B. Lisanti MP. Emerging themes in lipid rafts and caveolae. Cell. 2001;106:403–411. doi: 10.1016/s0092-8674(01)00472-x. [DOI] [PubMed] [Google Scholar]
- 22.Henley JR. Krueger EW. Oswald BJ. McNiven MA. Dynamin-mediated internalization of caveolae. J Cell Biol. 1998;141:85–99. doi: 10.1083/jcb.141.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hill MH. Bastiani M. Luetterforst R. Kirkham M. Kirkham A. Nixon SJ. Walser P. Abankwa D. Oorschot VMJ. Martin S. Hancock JF. Parton RG. PTRF-Cavin, a conserved cytoplasmic protein required for caveola formation and function. Cell. 2008;132:113–124. doi: 10.1016/j.cell.2007.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu G. Vogel SM. Schwartz DE. Malik AB. Minshall RD. Intercellular adhesion molecule-1 dependent neutrophil adhesion to endothelial cells induces caveolae-mediated pulmonary vascular hyperpermeability. Circ Res. 2008;102:e120–e131. doi: 10.1161/CIRCRESAHA.107.167486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jackson AP. Parham P. Structure of human clathrin light chains: conservation of light chain polymorphism in three mammalian species. J Biol Chem. 1988;263:16688–16695. [PubMed] [Google Scholar]
- 26.Johannes L. Lamaze C. Clathrin-dependent or not: is it still the question? Traffic. 2002;3:443–451. doi: 10.1034/j.1600-0854.2002.30701.x. [DOI] [PubMed] [Google Scholar]
- 27.Kirchhausen T. Harrison SC. Protein organization in clathrin trimers. Cell. 1981;23:755–761. doi: 10.1016/0092-8674(81)90439-6. [DOI] [PubMed] [Google Scholar]
- 28.Kirchhausen T. Coated pits and coated vesicles—sorting it all out. Curr Opin Struct Biol. 1993;3:182–188. [Google Scholar]
- 29.Kirchhausen T. Clathrin. Annu Rev Biochem. 2000;69:699–727. doi: 10.1146/annurev.biochem.69.1.699. [DOI] [PubMed] [Google Scholar]
- 30.Kirkham M. Fujita A. Chadda R. Nixon SJ. Kurzchalia TV. Sharma DK. Pagano RE. Hancock JF. Mayor S. Parton RG. Ultrastructural identification of uncoated caveolin independent early endocytic vehicles. J Cell Biol. 2005;168:465–476. doi: 10.1083/jcb.200407078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kirkham M. Nixon SJ. Howes MT. Abi-Rached L. Wakeham DE. Hanzal-Bayer M. Ferguson C. Hill MM. Fernandez-Rojo M. Brown DA. Hancock JF. Brodsky FM. Parton RG. Evolutionary analysis and molecular dissection of caveola biogenesis. J Cell Sci. 2008;121:2075–2086. doi: 10.1242/jcs.024588. [DOI] [PubMed] [Google Scholar]
- 32.Kosawada T. Inoue K. Schmid-Schönbein GW. Mechanics of curved plasma membrane vesicles: resting shapes, membrane curvature, and in-plane shear elasticity. J Biomech Eng. 2005;127:229–236. doi: 10.1115/1.1865197. [DOI] [PubMed] [Google Scholar]
- 33.Lajoie P. Nabi IR. Regulation of raft-dependent endocytosis. J Cell Mol Med. 2007;11:644–653. doi: 10.1111/j.1582-4934.2007.00083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Le Roy C. Wrana JL. Clathrin- and non-clathrin-mediated endocytic regulation of cell signaling. Nat Rev Mol Cell Biol. 2005;6:112–126. doi: 10.1038/nrm1571. [DOI] [PubMed] [Google Scholar]
- 35.Liu J. Kaksonen M. Drubin DG. Oster G. Endocytic vesicle scission by lipid phase boundary forces. Proc Natl Acad Sci U S A. 2006;103:10277–10282. doi: 10.1073/pnas.0601045103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu L. Pilch PF. A critical role of cavin (polymerase I and transcript release factor) in caveolae formation and organization. J Biol Chem. 2008;283:4314–4322. doi: 10.1074/jbc.M707890200. [DOI] [PubMed] [Google Scholar]
- 37.Maniatis NA. Shinin V. Schraufnagel DE. Okada S. Vogel SM. Malik AB. Minshall RD. Increased pulmonary vascular resistance and defective pulmonary artery filling in caveolin-1-/- mice. Am J Physiol Lung Cell Mol Physiol. 2008;294:L865–L873. doi: 10.1152/ajplung.00079.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McNiven MA. Cao H. Pitts KR. Yoon Y. The dynamin family of mechanoenzymes: pinching in new places. Trends Biochem Sci. 2000;25:115–120. doi: 10.1016/s0968-0004(99)01538-8. [DOI] [PubMed] [Google Scholar]
- 39.Merrifield CJ. Feldman ME. Wan L. Aimers W. Imaging actin and dynamin recruitment during invagination of single clathrin-coated pits. Nat Cell Biol. 2002;4:691–698. doi: 10.1038/ncb837. [DOI] [PubMed] [Google Scholar]
- 40.Miaczynska M. Stenmark H. Mechanisms and functions of endocytosis. J Cell Biol. 2008;180:7–11. doi: 10.1083/jcb.200711073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Minshall RD. Tiruppathi C. Vogel SM. Malik AB. Vesicle formation and trafficking and its role in regulation of endothelial barrier function. Histochem Cell Biol. 2002;117:105–112. doi: 10.1007/s00418-001-0367-x. [DOI] [PubMed] [Google Scholar]
- 42.Monier S. Parton RG. Vogel F. Behlke J. Henske A. Kurzchalia TV. VIP21-caveolin, a membrane protein constituent of the caveolar coat, oligomerizes in vivo and in vitro. Mol Biol Cell. 1995;6:911–927. doi: 10.1091/mbc.6.7.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mousavi SA. Malerød L. Berg T. Kjeken R. Clathrin-dependent endocytosis. Biochem J. 2004;377:1–16. doi: 10.1042/BJ20031000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oh P. McIntosh DP. Schnitzer JE. Dynamin at the neck of caveolae mediates their budding to form transport vesicles by GTP-driven fission from the plasma membrane of endothelium. J Cell Biol. 1998;141:101–114. doi: 10.1083/jcb.141.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Orlichenko L. Huang B. Krueger E. McNiven MA. Epithelial growth factor-induced phosphorylation of caveolin 1 at tyrosine 14 stimulates caveolae formation in epithelial cells. J Biol Chem. 2006;281:4570–4579. doi: 10.1074/jbc.M512088200. [DOI] [PubMed] [Google Scholar]
- 46.Owen DJ. Collins BM. Evans PR. Adaptors for clathrin coats: structure and function. Annu Rev Cell Dev Biol. 2004;20:153–191. doi: 10.1146/annurev.cellbio.20.010403.104543. [DOI] [PubMed] [Google Scholar]
- 47.Palade GE. Fine structure of blood capillaries. J Appl Physiol. 1953;24:1424. [Google Scholar]
- 48.Parton RG. Caveolae: from ultrastructure to molecular mechanisms. Nat Rev Mol Cell Biol. 2003;4:162–167. doi: 10.1038/nrm1017. [DOI] [PubMed] [Google Scholar]
- 49.Parton RG. Hanzal-Bayer M. Hancock JF. Biogenesis of caveolae: a structural model for caveolin-induced domain formation. J Cell Sci. 2006;119:787–796. doi: 10.1242/jcs.02853. [DOI] [PubMed] [Google Scholar]
- 50.Pelkmans L. Zerial M. Kinase-regulated quantal assemblies and kiss-and-run recycling of caveolae. Nature. 2005;436:128–133. doi: 10.1038/nature03866. [DOI] [PubMed] [Google Scholar]
- 51.Peters KR. Carley WW. Palade GE. Endothelial plasmalemmal vesicles have a characteristic striped bipolar surface structure. J Cell Biol. 1985;101:2233–2238. doi: 10.1083/jcb.101.6.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pilch PF. Souto RP. Liu L. Jedrychowski MP. Berg EA. Costello CE. Gygi SP. Cellular spelunking: exploring adipocyte caveolae. J Lipid Res. 2005;48:2103–2111. doi: 10.1194/jlr.R700009-JLR200. [DOI] [PubMed] [Google Scholar]
- 53.Radulescu AE. Siddhanta A. Shields D. A role for clathrin in reassembly of the Golgi apparatus. Mol Biol Cell. 2007;18:94–105. doi: 10.1091/mbc.E06-06-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Razani B. Woodman S. Lisanti M. Caveolae: From cell biology to animal physiology. Pharmacol Rev. 2002;54:431–467. doi: 10.1124/pr.54.3.431. [DOI] [PubMed] [Google Scholar]
- 55.Richter T. Floetenmeyer M. Ferguson C. Galea J. Goh J. Lindsay MR. Morgan GP. Marsh BJ. Parton RG. High-resolution 3D quantitative analysis of caveolar ultrastructure and caveola–cytoskeleton interactions. Traffic. 2008;9:893–909. doi: 10.1111/j.1600-0854.2008.00733.x. [DOI] [PubMed] [Google Scholar]
- 56.Römer W. Berland L. Chambon V. Gaus K. Windschieg B. Tenza D. Aly MRE. Fraisier V. Florent JC. Perrais D. Lamaze C. Raposo G. Steinem C. Sens P. Bassereau P. Johannes L. Shiga toxin induces tubular membrane invaginations for its uptake into cells. Nature. 2007;450:670–678. doi: 10.1038/nature05996. [DOI] [PubMed] [Google Scholar]
- 57.Rothberg KG. Heuser JE. Donzell WC. Ying YS. Glenney JR. Anderson RG. Caveolin, a protein component of caveolae membrane coats. Cell. 1992;68:673–682. doi: 10.1016/0092-8674(92)90143-z. [DOI] [PubMed] [Google Scholar]
- 58.Sarasij R. Mayor S. Rao M. Chirality-induced budding: a raft-mediated mechanism for endocytosis and morphology of caveolae. Biophys J. 2007;92:3140–3158. doi: 10.1529/biophysj.106.085662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sarasij R. Rao M. Tilt texture domains on a membrane and chirality induced budding. Phys Rev Lett. 2002;88:088101. doi: 10.1103/PhysRevLett.88.088101. [DOI] [PubMed] [Google Scholar]
- 60.Sargiacomo M. Scherer PE. Tang Z. Kubler E. Song KS. Sanders MC. Lisanti MP. Oligomeric structure of caveolin: implications for caveolae membrane organization. Proc Natl Acad Sci U S A. 1995;92:9407–9411. doi: 10.1073/pnas.92.20.9407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sauvonnet N. Dujeancourt A. Dautry-Varsat A. Cortactin and dynamin are required for the clathrin-independent endocytosis of γc cytokine receptor. J Cell Biol. 2005;168:155–163. doi: 10.1083/jcb.200406174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schmid SL. clathrin-coated vesicle formation and protein sorting: an integrated process. Annu Rev Biochem. 1997;66:511–548. doi: 10.1146/annurev.biochem.66.1.511. [DOI] [PubMed] [Google Scholar]
- 63.Schnitzer JE. Caveolae: from basic trafficking mechanisms to targeting transcytosis for tissue-specific drug and gene delivery in vivo. Adv Drug Deliv Rev. 2001;49:265–280. doi: 10.1016/s0169-409x(01)00141-7. [DOI] [PubMed] [Google Scholar]
- 64.Schnitzer JE. Oh P. McIntosh DP. Role of GTP hydrolysis in fission of caveolae directly from plasma membranes. Science. 1996;274:239–242. doi: 10.1126/science.274.5285.239. [DOI] [PubMed] [Google Scholar]
- 65.Sens P. Dynamics of nonequilibrium membrane bud formation. Phys Rev Lett. 2004;93:108103. doi: 10.1103/PhysRevLett.93.108103. [DOI] [PubMed] [Google Scholar]
- 66.Sens P. Turner M. Theoretical model for the formation of caveolae and similar membrane invaginations. Biophys J. 2004;86:2049–2057. doi: 10.1016/S0006-3495(04)74266-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sens P. Turner M. Budded membrane microdomains as tension regulators. Phys Rev E. 2006;73:31918. doi: 10.1103/PhysRevE.73.031918. [DOI] [PubMed] [Google Scholar]
- 68.Shajahan AN. Timblin BK. Sandoval R. Tiruppathi C. Malik AB. Minshall RD. Role of Src-induced dynamin-2 phosphorylation in caveolae-mediated endocytosis in endothelial cells. J Biol Chem. 2004;279:20392–20400. doi: 10.1074/jbc.M308710200. [DOI] [PubMed] [Google Scholar]
- 69.Sigismund S. Woelk T. Puri C. Maspero E. Tacchetti C. Transidico P. Di Fiore PP. Polo S. Clathrin-independent endocytosis of ubiquinated cargos. Proc Natl Acad Sci U S A. 2005;102:2760–2765. doi: 10.1073/pnas.0409817102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Silveira LA. Wong DH. Masiarz FR. Schekman R. Yeast clathrin has a distinctive light chain that is important for cell growth. J Cell Biol. 1990;111:1437–1449. doi: 10.1083/jcb.111.4.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stan RV. Structure of caveolae. Biochim Biophys Acta. 2005;1746:334–348. doi: 10.1016/j.bbamcr.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 72.Sun Y. Carroll S. Kaksonen M. Toshima JY. Drubin DG. PtdIns(4,5)P2 turnover is required for multiple stages during clathrin- and actin-dependent endocytic internalization. J Cell Biol. 2007;177:355–367. doi: 10.1083/jcb.200611011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sverdlov M. Shajahan AN. Minshall RD. Tyrosine phosphorylation-dependence of caveolae-mediated endocytosis. J Cell Mol Med. 2007;11:1239–1250. doi: 10.1111/j.1582-4934.2007.00127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ungewickell E. Branton D. Triskelions: the building blocks of clathrin coats. Trends Biochem Sci. 1982;7:358–361. [Google Scholar]
- 75.Vinten J. Voldstedlund M. Clausen H. Christiansen K. Carlsen J. Tranum-Jensen J. A 60-kDa protein abundant in adipocyte caveolae. Cell Tissue Res. 2001;305:99–106. doi: 10.1007/s004410100389. [DOI] [PubMed] [Google Scholar]
- 76.Vinten J. Johnsen AH. Roepstorff P. Harpøth J. Tranum-Jensen J. Identification of a major protein on the cytosolic face of caveolae. Biochim Biophys Acta. 2005;1717:34–40. doi: 10.1016/j.bbamem.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 77.Yamada E. The fine structures of the gall bladder epithelium of the mouse. J Biophys Biochem Cytol. 1955;1:445–458. doi: 10.1083/jcb.1.5.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yao Q. Chen J. Cao H. Orth JD. McCaffery JM. Stan RV. McNiven MA. Caveolin-1 interacts directly with dynamin-2. J Mol Biol. 2005;348:491–501. doi: 10.1016/j.jmb.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 79.Yarar D. Waterman-Storer CM. Schmid SL. A dynamic actin cytoskeleton functions at multiple stages of clathrin-mediated endocytosis. Mol Biol Cell. 2005;16:964–975. doi: 10.1091/mbc.E04-09-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhao Y-Y. Liu Y. Stan R-V. Fan L. Gu Y. Dalton N. Chu P-H. Peterson K. Ross J. Chien KR. Defects in caveolin-1 cause dilated cardiomyopathy and pulmonary hypertension in knockout mice. Proc Natl Acad Sci U S A. 2002;99:11375–11380. doi: 10.1073/pnas.172360799. [DOI] [PMC free article] [PubMed] [Google Scholar]