Abstract
Increasing evidence suggests that circulating endothelial progenitor cells, which are a subpopulation of hematopoietic progenitor CD34+ cells, play a critical role in neovascularization and tissue repair. We have tested the hypothesis that traumatic brain injury (TBI) could mobilize CD34+ cells to peripheral blood and brain tissue, a process critical for vascular repair, in a rat model of TBI. Male Wistar rats were subjected to controlled fluid percussion. Blood and brain tissue were collected before and after TBI to measure the levels of CD34+ cells in peripheral blood and to detect their accumulation in the damaged cerebral tissue. Compared with surgery controls, CD34+ cells significantly increased in the peripheral blood and accumulated in the brain tissue of TBI rats. Immunohistochemistry detected new vessels with incomplete CD34+ endothelial-like cell lining and an increased number of microvessels in the injured and surrounding tissue. The results demonstrate a close correlation between an increase in circulating CD34+ cells in response to traumatic injury and angiogenesis in TBI rat brain. They also suggest that transplantation of CD34+ cells or augmentation of endogenous CD34+ cells may be a novel therapeutic approach for patients with TBI.
Key words: angiogenesis, CD34+ cells, endothelial progenitor cells, traumatic brain injury
Introduction
Neovascularization plays a critical role in tissue repair, wound healing, and tumor growth. Increasing evidence indicates that the bone marrow-derived hematopoietic progenitor CD34+ cells in peripheral blood are responsible for the process in ischemic or injured tissues (Asahara et al., 1997; Carmeliet, 2000; Tei et al., 2008). Early studies further suggest that CD34+ mononuclear cells maintain the vascular integrity not only by providing endothelial progenitor cells (EPCs), which are a subpopulation of CD34+ cells, but also as a source of growth/angiogenesis factors (Kocher et al., 2001; Rookmaaker et al., 2005; Kawamoto et al., 2006; Okada et al., 2008).
As a protective response, CD34+ cells are rapidly mobilized into peripheral blood during ischemic stroke and acute myocardial infarction (Paczkowska et al., 2005; Machaliński et al., 2006). The cells eventually home to the site of injured tissue (Beck et al., 2003; Assmus et al., 2006) to initiate and propagate angiogenesis (Krupinski et al., 2003; Morris et al., 2003). As a result, the levels of CD34+ cells in peripheral blood and injured tissue could be considered to reliably indicate the rate and extent of tissue repair. The phenotypic and biochemical properties of CD34+ cells also provide therapeutic potentials to promote angiogenesis and tissue repair (Kocher et al., 2001; Kawamoto et al., 2006). Such a potential has recently been demonstrated in a study showing that cultured human CD34+ cells injected into nude mice of hindlimb ischemia incorporate into new vasculature (Cho et al., 2007). CD34+ cells are also detected at the site of induced endothelial injury of carotid artery (Fujiyama et al., 2003) and found to improve ventricular function after myocardial infarction (Assmus et al., 2002, 2006).
There has been extensive research on how CD34+ cells contribute to tissue repair after myocardial infarction and stroke, but information on traumatic injury is very limited. The pathophysiological changes associated with traumatic brain injury (TBI) may not be the same as those with ischemic or hemorrhagic stroke because TBI occurs in patients who are healthy without predisposed atherosclerotic lesions. Here, we present experimental data that characterize the dynamic changes of CD34+ cells in peripheral blood and brain tissue in response to TBI in rats. The study was designed to test the hypothesis that CD34+ cells correlate with post-traumatic angiogenesis and tissue repair.
Materials and Methods
Experimental rats
A total of 98 adult (300–350 g) male Wistar rats (Military Medical Academy of China) were individually housed under a 12 h light/dark cycle with regular food and water supply. The rats were randomly assigned to control and TBI groups (49 rats in each group), each of which were further divided into seven subgroups (seven rats in each subgroup). The animal housing and care were maintained according to the guideline of animal care from the U.S. National Institute of Health.
Traumatic brain injury induced by fluid percussion
TBI was induced using a fluid percussion injury device (Model 01-B, New Sun, Health Products, Cedar Bluff, VA). Briefly, rats in control and TBI groups were anesthetized with 10% chloraldurat (3 mL/kg) and then placed in a stereotaxic frame. A 3.8 mm craniotomy was performed over the right parietal skull to expose the dura (4.0 mm posterior from bregma and 3.0 mm lateral to the sagittal suture). For TBI rats, a luer-loc connector (2.6 mm inner diameter) was cemented to the skull with cranioplastic cement. A syringe filled with sterile saline was inserted into the luer-loc syringe fitting and connected to the fluid percussion device. The pressure pulse (200–233 kPa) and pulse duration were measured by a transducer attached to the fluid percussion device. Immediately after TBI, incision was suture-closed and the rats were allowed to recover from anesthesia. Control rats underwent the same surgical procedure without being exposed to percussion injury.
Measurement of CD34+ cells by flow cytometry
Blood samples (0.5 mL) were collected from retro-orbital venous plexus at baseline, 24, 48, 72, 120, and 168 h after TBI and diluted with phosphate-buffered saline (PBS). Peripheral blood mononuclear cells were isolated by density-gradient centrifugation using Ficoll-Paque Plus (Amersham Pharmacia Biotech, Uppsala, Sweden). Isolated cells were washed twice with PBS and resuspended in 200 mL of PBS supplemented with 0.5% of bovine serum albumin and 2 mM of EDTA. CD34+ cells in peripheral blood were evaluated by immunostaining with PE-conjugated CD34 monoclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA) and detected by flow cytometry (FACScan, Becton-Dickinson, San Jose, CA). The isotype-matched mouse IgG was used as a control.
Brain autopsy
Rats in control and TBI groups were sacrificed before and after days 1, 3, 7, 14, and 21 of TBI (7 rats at each time point) and transcardially perfused with 0.9% NaCl to remove blood from the vasculature. After perfusion, brains were collected and fixed in 4% paraformaldehyde in PBS and processed for 5 mm coronal paraffin sections through the TBI zone and used for immunohistochemistry staining.
CD34 immunostaining
Five sections from each rat were cut at an interval of 50 mm and processed for CD34 staining. Briefly, after deparaffinization and redehydration, non-specific endogenous peroxidase activity was blocked by treating sections with 3% hydrogen peroxide in methanol for 30 min. Antigen was recovered by boiling the sections for 10 min in 10 mM citrate buffer (pH 6.0). Nonspecific binding was blocked with 1% non-immune serum in PBS for 30 min. The sections were then incubated with a monoclonal mouse CD34 antibody (1:100, Santa Cruz Biotechnology) overnight at 4°C. They were then washed with PBS, incubated with a biotinylated goat-anti-mouse IgG (1:100, Santa Cruz Biotechnology) for 2 h at 37°C, washed and incubated with an avidin peroxidase conjugate solution (1:100, Santa Cruz Biotechnology) for 1 h. Finally, the sections were developed with diaminobenzidine for 5 min. Negative controls were similarly processed without the primary antibody. The number of endothelial-like CD34+ cell singlet in each section was counted (per 100x, Leica, Wetzlar, Germany) in five fields by two independent observers who were blinded to the experimental conditions to obtain the average number of CD34+ cells per view field (per 100x).
Angiogenesis assay
Angiogenesis was measured by microvasculature density (MVD) in five sections at 50 mm intervals under a light microscope (100x, Leica) by two independent observers who were blinded to the experimental conditions. The brown-staining vessel-lumen structure with incomplete CD34+ endothelial cell–cell contact was defined as microvessel. The numbers of microvessels from each section were counted in the injured tissue and ipsilateral hippocampus, and calculated for the average number of microvessels per section.
Statistical analysis
The data were presented as mean ± SEM. Repeated measures ANOVA with post hoc LSD test was used to compare the number of CD34+ cells in peripheral blood samples collected at different time points. A two-way ANOVA with post hoc LSD test was used to compare the number of CD34+ cells and MVD in brain tissue at various time points. The Student t test was used to analyze the number of CD34+ cells in circulation and brain tissue (including MVD) between control and TBI at same time point. Statistical analysis was performed using SPSS system. A p value of <0.05 was considered to be statistically significant.
Results
CD34+ cells in peripheral blood
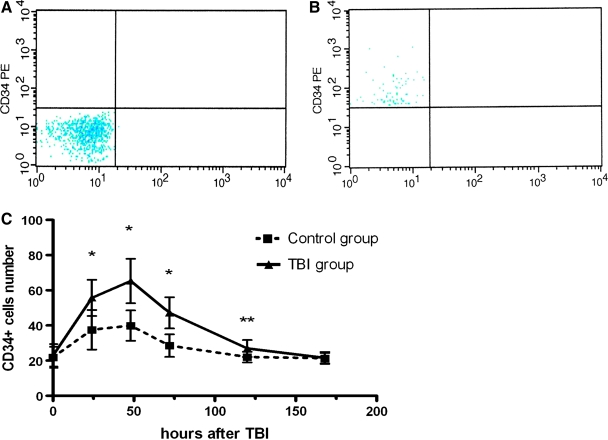
Circulating CD34+ cells were quantified as a subpopulation of isolated mononuclear cells by flow cytometry. Figure 1 shows a typical example of cells that were labeled with PE-conjugated CD34 antibody. The number of circulating CD34+ cells (1 × 104 mononuclear cells) increased 24 h in rats being exposed to TBI and those that underwent surgery only. It reached the peak level at 48 h and then gradually declined to baseline. Although following the same pattern, the number of circulating CD34+ cells was significantly higher in rats with TBI compared to controls that underwent the surgical procedure without actual percussion injury at 24, 48, 72, and 120 h after TBI. There was no difference between the two groups at baseline and 168 h after TBI (Fig. 1C).
FIG. 1.
Flow cytometry detection of CD34+ cells. (A) Isotype control antibody and (B) CD34-PE antibody: representative images of flow cytometrical detection of CD34+ cells (expressed as 1 × 104 mononuclear cells; mean ± SEM) in peripheral blood. (C) Number of circulating CD34+ cells at 0, 24, 48, 72, 120, and 168 h after TBI. Repeated measures ANOVA showed significant effects of injury (F = 27.34, p < 0.001), time (F = 39.58, p < 0.001) on the number of circulating CD34+ cells, and interaction between injury and time (F = 6.253, p < 0.001). The post hoc test (LSD) further indicated that CD34+ cell numbers in rats from TBI group were higher than those in controls except at baseline (0 h) and 168 h after TBI. *p < 0.01, **p < 0.05 compared with control at the same time point (Student t test; n = 14).
CD34+ cells in injured brain tissue
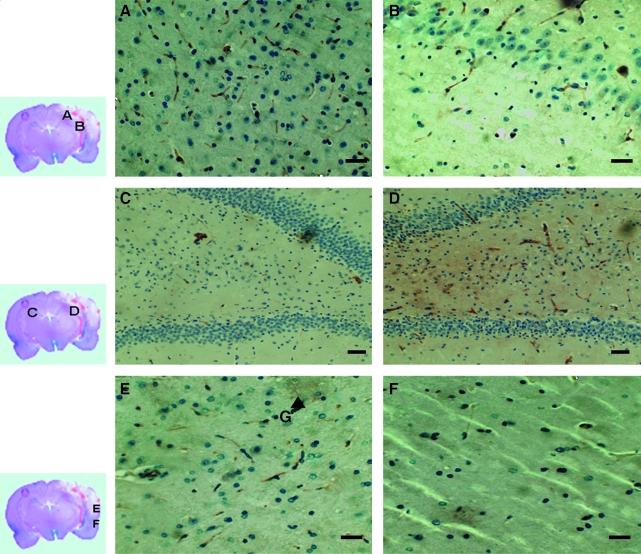
Twenty-four hours after TBI, CD34+ cells were detected in the injured tissue and ipsilateral hippocampus, but became significantly accumulated at 72 h after TBI (Fig. 2A and B). The number of CD34+ cells in ipsilateral hippocampus was greater than that in contralateral hippocampus (Fig. 2C and D). There were few CD34+ cells in areas remote from the injured zone (Fig. 2E and F). There was no significant change in CD34+ cell distribution in the control group during the same follow-up period.
FIG. 2.
Detection of CD34+ cells in the injured brain tissue: CD34+ cells that were spindle-shape cells and stained brown were counted in brain tissue from the injury zone (A) and ispilateral hippocampus (B) 24 h after TBI. (C and D) Representative images from the contralateral and ipsilateral hippocampus, respectively, showing significantly more CD34+ cells accumulated in ipsilateral hippocampus compared to that in the contralateral hippocampus. (E and F) Representative images from the injured tissue and tissue from the areas remote from the injury zone, showing fewer CD34+ cells in the remote tissue. Marker G indicated injury zone. The images were representative of CD34-stained tissue sections. Bars, 50 μm (A, B, E, F) or 100 μm (C and D).
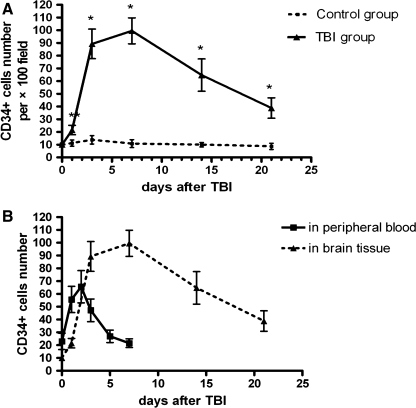
Quantitatively, the number of CD34+ cells in the injured tissue was 21.57 ± 1.71 per view field (100x) compared to 9.14 ± 2.47 in tissue from the control rats 24 h after TBI. The number increased rapidly thereafter and reached a plateau at approximately one week after TBI before declining to baseline. In comparison, CD34+ cells remained constantly lower in brain tissue from the control rats during the same period (Fig. 3A).
FIG. 3.
Quantification of CD34+ cells in brain tissue. (A) Changes in the number of CD34+ cells in brain tissue from TBI and control rats (per 100x). By two-way ANOVA, a significant difference was found in the effects of injury (F = 306.80, p < 0.001), time (F = 101.08, p < 0.001), and interaction (F = 88.46, p < 0.001) between injury and time. Post hoc test (LSD) further indicated that CD34+ cells number in TBI rats were higher than that in controls except at baseline (*p < 0.01, **p < 0.05 compared with control at the same time point; Student t test; n = 14). (B) Patterns of change in CD34+ cells in peripheral blood and brain tissue of TBI rats.
To investigate whether the level of circulating CD34+ cells indicates the accumulation of the same cells in the injured tissue, we correlated the levels of CD34+ cells in peripheral blood with the number of CD34+ cells in the injured and surrounding tissue. As shown in Figure 3B, the increase in circulating CD34+ cells appeared earlier than those detected in the injured tissue. The number of CD34+ cells in peripheral blood reached the maximum 48 h post-TBI, whereas it reached the maximum in the injured brain tissue approximately 7 days after TBI.
Detection of microvasculature in injured brain tissue
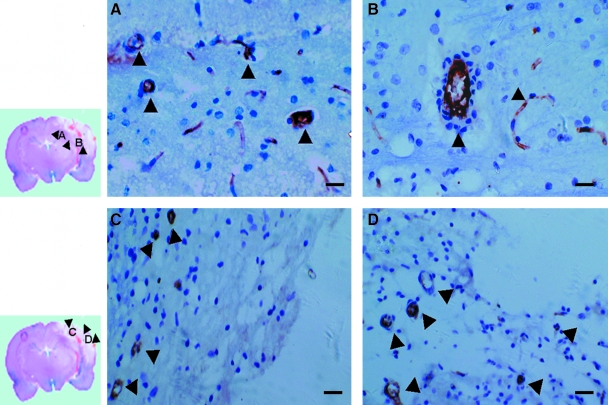
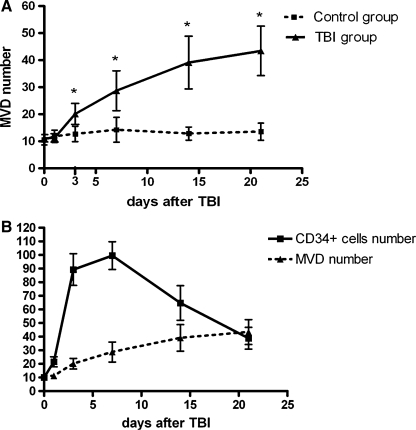
The vessel-lumen structure with CD34+ endothelial-like cell lining began to be detected in the area surrounding the injured tissue 72 h after TBI (Fig. 4A and B), but was detected in the injured tissue 14 days after TBI (Fig. 4C and D). The number of MVD was significantly higher in TBI rats (20.14 ± 3.78) compared to that of control rats (12.71 ± 2.86) at 72 h. More importantly, the number of MVD continued to increase to 28.71 ± 7.36 and 39.14 ± 9.74 when examined on days 7 and 14 in TBI rats, whereas it remained unchanged during the same period in control rats (Fig. 5A). We correlated the number of MVD with that of CD34+ cells in brain tissue from TBI rats. CD34+ cells increased rapidly, reaching plateau at about one week after TBI and then declining, whereas MVD showed a steady increase during the same period (Fig. 5B).
FIG. 4.
Detection of CD34+ microvasculature: brain tissues from indicated regions (small images, left) were immunochemically stained for CD34. (A and B) Representative of CD34 stained tissue from the boundary of the injured cerebral tissue, showing CD34+ endothelial-like lumen structures on day 3 after TBI. (C and D) Representative OF CD34 staining of tissue from the injured tissue, also showing CD34+ endothelial-like lumen structures, but only at approximately day 14 after TBI. Arrows indicated the vascular lumen-like structure. The images were representative from CD34-stained tissue sections. Bar, 30 μm (A and B) or 50 μm (C and D).
FIG. 5.
Changes in CD34+ cells and MVD after TBI. (A) Dynamic changes of post-traumatic MVD of TBI group and control group. By two-way ANOVA, significant difference was shown in the effects of injury (F = 116.94, p < 0.001) and time (F = 25.23, p < 0.001) on MVD number, and interaction (F = 19.20, p < 0.001) between injury and time. Post hoc test (LSD) further indicated that MVD number in TBI rats were significantly higher than that in controls except at baseline and 24 h after TBI (*p < 0.01 compared with control at the same time point; Student t test; n = 14). (B) Common pattern of changes in CD34+ cells and CD34+ MVD in the injured tissue.
Discussion
Dynamic changes in circulating CD34+ have been extensively studied in patients with cardiovascular and cerebrovascular diseases (Wojakowski et al., 2004; Paczkowska et al., 2005; Machaliński et al., 2006), but how circulating CD34+ cells change in response to acute traumatic brain injury remains largely unknown. The primary goal of the current study was to evaluate the effects of TBI on CD34+ cells in circulation and injured brain tissue, and to correlate such changes to the injury-induced angiogenesis. We showed that the levels of circulating CD34+ cells increase significantly upon TBI, reaching a plateau in approximately 48 h and then declining steadily (Fig. 1). In comparison to this early spike of circulating CD34+ cells, the highest number of CD34+ cells was detected in injured tissue 7 days after the injury. These cells first appear as single cells infiltrating the injured tissue and begin to accumulate (Figs. 2 and 3). The new vasculature (cell-cell tubular network) first appears in regions surrounding the injured tissue, then infiltrates the injured tissue. The finding is consistent in time with early reports that angiogenesis is first detected in the peri-infarct region 48 h after stroke in an experimental cerebral ischemia model (Wei et al., 2001; Shen et al., 2006). This time difference in reaching peak levels between circulating and infiltrated CD34+ cells suggests a clear sequence of multiple events. First, CD34+ cells are mobilized from the bone marrow to the peripheral blood in response to acute traumatic injury. Second, circulating CD34+ cells home to the injured vascular beds in the injured tissue. Finally, homed cells begin to proliferate and differentiate to form new vasculature, suggesting that the mobilized CD34+ cells eventually contribute to the formation of new vessels in the injured tissue, a critical event for neural recovery after traumatic injury (Ohab et al., 2004; Taguchi et al., 2004; Del Zoppo, 2006).
The data on the dynamic changes in circulating CD34+ cells are consistent with our early observation that the circulating endothelial progenitor cells (EPCs, defined as CD133 and CD34 double positive) increase at the acute phase of traumatic brain injury in patients (Liu et al., 2007). In the present study, CD34+ is used as the cell marker instead of CD34+ and CD133+ for two reasons. First, CD133 is an early marker for EPCs (Salven et al., 2003), so that it is not or only weakly expressed on the endothelial-like cells that form new vessels. Second, CD34 positivity includes a larger cell population that contains other hematopoietic progenitor cells critical for wound healing and tissue repair (Machaliński et al., 2006). One potential concern is that the activated microglia also expresses CD34 (Ladeby et al., 2005). To distinguish the two types of cells, we counted those cells that are not only CD34+, but also show endothelial-like spindle shape morphology.
Our data support a long notion that CD34+ cells play a pivotal role in forming new vasculature (Xu, 2005; Ohta et al., 2006). Our finding is also consistent with early reports that CD34+ cells selectively homed to the sites of angiogenesis, become fully integrated in the microvascular endothelium, and differentiate into mature endothelial cells (Krupinski et al., 2003; Morris et al., 2003). In addition to physical lining of vessel wall, the new endothelial cells have a significant stimulatory effect on the formation of new capillary (Zhang et al., 2002; Morris et al., 2003; Rookmaaker et al., 2005).
Finally, the results also suggest the therapeutic potential of CD34+ cells to promote neovascularization in the traumatically injured brain tissue. Such a potential has indeed been explored in a related model. Angiogenesis in the ischemic tissues and neuroplasticity are enhanced or improved by implantation of isolated CD34+ cells (Taguchi et al., 2004; Shyu et al., 2006) or ex vivo expanded endothelial progenitor cells (Kalka et al., 2000; Ohta et al., 2006). Furthermore, enhancing the mobilization of the bone marrow-derived CD34+ cells could provide another avenue for repairing the injured cerebral tissue.
Acknowledgment
This work was supported by the National Natural Science Foundation of China (30772229), the National 973 Project (2005CB522600), and an NIH grant (HL71895).
Author Disclosure Statement
No competing financial interests exist.
References
- Asahara T. Murohara T. Sullivan A. Silver M. Van der Zee R. Li T. Witzenbichler B. Schatteman G. Isner J.M. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- Assmus B. Honold J. Schachinger V. Britten M.B. Fischer-Rasokat U. Lehmann R. Teupe C. Pistorius K. Martin H. Abolmaali N.D. Tonn T. Dimmeler S. Zeiher A.M. Transcoronary transplantation of progenitor cells after myocardial infarction. N. Engl. J. Med. 2006;355:1222–1232. doi: 10.1056/NEJMoa051779. [DOI] [PubMed] [Google Scholar]
- Assmus B. Schachinger V. Teupe C. Britten M. Lehmann R. Dobert N. Grunwald F. Aicher A. Urbich C. Martin H. Hoelzer D. Dimmeler S. Zeiher A.M. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction (TOPCARE-AMI) Circulation. 2002;106:3009–3017. doi: 10.1161/01.cir.0000043246.74879.cd. [DOI] [PubMed] [Google Scholar]
- Beck H. Voswinckel R. Wagner S. Ziegelhoeffer T. Heil M. Helisch A. Schaper W. Acker T. Hatzopoulos A.K. Plate K.H. Participation of bone marrow-derived cells in long-term repair processes after experimental stroke. J. Cereb. Blood Flow Metab. 2003;23:709–717. doi: 10.1097/01.WCB.0000065940.18332.8D. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat. Med. 2000;6:389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- Cho S.W. Moon S.H. Lee S.H. Kang S.W. Kim J. Lim J.M. Kim H.S. Kim B.S. Chung H.M. Improvement of postnatal neovascularization by human embryonic stem cell derived endothelial-like cell transplantation in a mouse model of hindlimb ischemia. Circulation. 2007;116:2409–2419. doi: 10.1161/CIRCULATIONAHA.106.687038. [DOI] [PubMed] [Google Scholar]
- Del Zoppo G.J. Stroke and neurovascular protection. N. Engl. J. Med. 2006;354:553–555. doi: 10.1056/NEJMp058312. [DOI] [PubMed] [Google Scholar]
- Fujiyama S. Amano K. Uehira K. Yoshida M. Nishiwaki Y. Nozawa Y. Jin D. Takai S. Miyazaki M. Egashira K. Imada T. Iwasaka T. Matsubara H. Bone marrow monocyte lineage cells adhere on injured endothelium in a monocyte chemoattractant protein-1-dependent manner and accelerate reendothelialization as endothelial progenitor cells. Circ. Res. 2003;93:980–989. doi: 10.1161/01.RES.0000099245.08637.CE. [DOI] [PubMed] [Google Scholar]
- Kalka C. Masuda H. Takahashi T. Kalka-Moll W.M. Silver M. Kearney M. Li T. Isner J.M. Asahara T. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc. Natl. Acad. Sci. USA. 2000;97:3422–3427. doi: 10.1073/pnas.070046397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto A. Iwasaki H. Kusano K. Murayama T. Oyamada A. Silver M. Hulbert C. Gavin M. Hanley A. Ma H. Kearney M. Zak V. Asahara T. Losordo D.W. CD34-positive cells exhibit increased potency and safety for therapeutic neovascularization after myocardial infarction compared with total mononuclear cells. Circulation. 2006;114:2163–2169. doi: 10.1161/CIRCULATIONAHA.106.644518. [DOI] [PubMed] [Google Scholar]
- Kocher A.A. Schuster M.D. Szabolcs M.J. Takuma S. Burkhoff D. Wang J. Homma S. Edwards N.M. Itescu S. Neovascularization of ischemic myocardium by human BM-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat. Med. 2001;7:430–436. doi: 10.1038/86498. [DOI] [PubMed] [Google Scholar]
- Krupinski J. Stroemer P. Slevin M. Marti E. Kumar P. Rubio F. Three-dimensional structure and survival of newly formed blood vessels after focal cerebral ischemia. Neuroreport. 2003;14:1171–1176. doi: 10.1097/01.wnr.0000075304.76650.29. [DOI] [PubMed] [Google Scholar]
- Ladeby R. Wirenfeldt M. Dalmau I. Gregersen R. García-Ovejero D. Babcock A. Owens T. Finsen B. Proliferating resident microglia express the stem cell antigen CD34 in response to acute neural injury. Glia. 2005;50:121–131. doi: 10.1002/glia.20159. [DOI] [PubMed] [Google Scholar]
- Liu L. Liu H. Jiao J. Liu H. Bergeron A. Dong J.F. Zhang J.N. Changes in circulating human endothelial progenitor cells after brain injury. J. Neurotrauma. 2007;24:936–943. doi: 10.1089/neu.2006.0250. [DOI] [PubMed] [Google Scholar]
- Machaliński B. Paczkowska E. Koziarska D. Ratajczak M.Z. Mobilization of human hematopoietic stem/progenitor-enriched CD34+ cells into peripheral blood during stress related to ischemic stroke. Folia Histochem. Cytobiol. 2006;44:97–101. [PubMed] [Google Scholar]
- Morris D.C. Yeich T. Khalighi M.M. Soltanian-Zadeh H. Zhang Z.G. Chopp M. Microvascular structure after embolic focal cerebral ischemia in the rat. Brain Res. 2003;972:31–37. doi: 10.1016/s0006-8993(03)02433-8. [DOI] [PubMed] [Google Scholar]
- Ohab J.J. Fleming S. Blesch A. Carmichael S.T. A neurovascular niche for neurogenesis after stroke. J. Neurosci. 2006;26:13007–13016. doi: 10.1523/JNEUROSCI.4323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta T. Kikuta K. Imamura H. Takagi Y. Nishimura M. Arakawa Y. Hashimoto N. Nozaki K. Administration of ex vivo-expanded bone marrow-derived endothelial progenitor cells attenuates focal cerebral ischemia-reperfusion injury in rats. Neurosurgery. 2006;59:679–686. doi: 10.1227/01.NEU.0000229058.08706.88. [DOI] [PubMed] [Google Scholar]
- Okada S. Makino H. Nagumo A. Sugisawa T. Fujimoto M. Kishimoto I. Miyamoto Y. Kikuchi-Taura A. Soma T. Taguchi A. Yoshimasa Y. Circulating CD34-positive cell number is associated with brain natriuretic peptide level in type 2 diabetic patients. Diabetes Care. 2008;31:157–158. doi: 10.2337/dc07-1125. [DOI] [PubMed] [Google Scholar]
- Paczkowska E. Larysz B. Rzeuski R. Karbicka A. Kornacewicz-Jach Z. Ratajczak M.Z. Machaliński B. Human hematopoietic stem/progenitor-enriched CD34(+) cells are mobilized into peripheral blood during stress related to ischemic stroke or acute myocardial infarction. Eur. J. Haematol. 2005;75:461–467. doi: 10.1111/j.1600-0609.2005.00536.x. [DOI] [PubMed] [Google Scholar]
- Rookmaaker M.B. Verhaar M.C. Loomans C.J. Verloop R. Peters E. Westerweel P.E. Murohara T. Staal F.J. Van Zonneveld A.J. Koolwijk P. Rabelink T.J. Van Hinsbergh V.W. CD34+ cells home, proliferate, and participate in capillary formation, and in combination with CD34-cells enhance tube formation in a 3-dimensional matrix. Arterio. Thromb. Vasc. Biol. 2005;25:1–8. doi: 10.1161/01.ATV.0000177808.92494.14. [DOI] [PubMed] [Google Scholar]
- Salven P. Mustjoki S. Alitalo R. Alitalo K. Rafii S. VEGFR-3 and CD133 identify a population of CD34+ lymphatic/vascular endothelial precursor. Blood. 2003;101:168–172. doi: 10.1182/blood-2002-03-0755. [DOI] [PubMed] [Google Scholar]
- Shen F. Su H. Fan Y. Chen Y. Zhu Y. Liu W. Young W.L. Yang G.Y. Adeno-associated viral-vector-mediated hypoxia-inducible vascular endothelial growth factor gene expression attenuates ischemic brain injury after focal cerebral ischemia in mice. Stroke. 2006;37:2601–2606. doi: 10.1161/01.STR.0000240407.14765.e8. [DOI] [PubMed] [Google Scholar]
- Shyu W.C. Lin S.Z. Chiang M.F. Su C.Y. Li H. Intracerebral peripheral blood stem cell (CD34+) implantation induces neuroplasticity by enhancing beta1 integrin-mediated angiogenesis in chronic stroke rats. J Neurosci. 2006;26:3444–3453. doi: 10.1523/JNEUROSCI.5165-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi A. Soma T. Tanaka H. Kanda T. Nishimura H. Yoshikawa H. Tsukamoto Y. Iso H. Fujimori Y. Stern D.M. Naritomi H. Matsuyama T. Administration of CD34 cells after stroke enhances neurogenesis via angiogenesis in a mouse model. J. Clin. Invest. 2004;114:330–338. doi: 10.1172/JCI20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tei K. Matsumoto T. Mifune Y. Ishida K. Sasaki K. Shoji T. Kubo S. Kawamoto A. Asahara T. Kurosaka M. Kuroda R. Administrations of peripheral blood CD34-positive cells contribute to medial collateral ligament healing via vasculogenesis. Stem Cells. 2008;26:819–830. doi: 10.1634/stemcells.2007-0671. [DOI] [PubMed] [Google Scholar]
- Wei L. Erinjeri J.P. Rovainen C.M. Woolsey T.A. Collateral growth and angiogenesis around cortical stroke. Stroke. 2001;32:2179–2184. doi: 10.1161/hs0901.094282. [DOI] [PubMed] [Google Scholar]
- Wojakowski W. Tendera M. Michalowska A. Majka M. Kucia M. Maslankiewicz K. Wyderka R. Ochala A. Ratajczak M.Z. Mobilization of CD34/CXCR4+, CD34/CD117+, c-met + stem cells, and mononuclear cells expressing early cardiac, muscle, and endothelial markers into peripheral blood in patients with acute myocardial infarction. Circulation. 2004;110:3213–3220. doi: 10.1161/01.CIR.0000147609.39780.02. [DOI] [PubMed] [Google Scholar]
- Xu Q.-B. Endothelial progenitor cells in angiogenesis. Acta Physiol. Sinica. 2005;57:1–6. [PubMed] [Google Scholar]
- Zhang Z.G. Zhang L. Jiang Q. Chopp M. Bone marrow-derived endothelial progenitor cells participate in cerebral neovascularization after focal cerebral ischemia in the adult mouse. Circ. Res. 2002;90:284–288. doi: 10.1161/hh0302.104460. [DOI] [PubMed] [Google Scholar]