Abstract
Stable adhesion of leukocytes to endothelium is crucial for transendothelial migration (TEM) of leukocytes evoked during inflammatory responses, immune surveillance, and homing and mobilization of hematopoietic progenitor cells. The basis of stable adhesion involves expression of intercellular adhesion molecule-1 (ICAM-1), an inducible endothelial adhesive protein that serves as a counter-receptor for β2-integrins on leukocytes. Interaction of ICAM-1 with β2-integrins enables leukocytes to adhere firmly to the vascular endothelium and subsequently, to migrate across the endothelial barrier. The emerging paradigm is that ICAM-1, in addition to firmly capturing leukocytes, triggers intracellular signaling events that may contribute to active participation of the endothelium in facilitating the TEM of adherent leukocytes. The nature, duration, and intensity of ICAM-1-dependent signaling events may contribute to the determination of the route (paracellular vs. transcellular) of leukocyte passage; these aspects of ICAM-1 signaling may in turn be influenced by density and distribution of ICAM-1 on the endothelial cell surface, the source of endothelial cells it is present on, and the type of leukocytes with which it is engaged. This review summarizes our current understanding of the “ICAM-1 paradigm” of TEM with an emphasis on the signaling events mediating ICAM-1 expression and activated by ICAM-1 engagement in endothelial cells. Antioxid. Redox Signal. 11, 823–839.
Introduction
A hallmark of inflammation is the recruitment of leukocytes, particularly polymorphonuclear leukocytes (PMN), to the site of injury or infection. To reach an inflammatory site in the interstitium, the circulating PMN must first traverse the endothelial barrier, a process referred to as transendothelial migration (TEM) or diapedesis. The TEM of leukocytes is also critical for immune surveillance, and homing and mobilization of hematopoietic progenitor cells (21, 27, 131, 147, 177). The TEM of leukocytes is a complex but highly ordered multi-step process involving sequential activation of adhesive proteins on endothelial cells and their counter receptors on the surface of leukocytes, as depicted in Fig. 1. The first step comprises the rolling of PMN along the vessel wall during which PMN make brief adhesive contacts (i.e., <25 ms) with the endothelium (138). It begins with the capture of PMN from the blood stream by tethering via l-selectin (CD62L), a constitutively expressed selectin on leukocytes that interacts with their glycoprotein ligands expressed on endothelial cells (1). PMN rolling is also supported by binding of endothelial-bound E-selectin and P-selectin (CD62E and CD62P) to their prototypic ligand platelet sialoglycoprotein ligand-1 (PSGL-1) on the surface of leukocytes (76, 79, 88). PMN rolling on activated endothelium can lead to secondary capture of PMN through homotypic interactions mediated by binding of L-selectin to PSGL-1 between a free-stream PMN and one adherent to endothelium (13, 140). Another mechanism implicated in supporting the leukocyte recruitment involves heterotypic adhesion to activated platelets forming a thrombus on the blood vessel wall (86). The second step, characterized by the transition from PMN rolling to arrest, is contingent upon the upregulation of intercellular adhesion molecule-1 (ICAM-1; CD54) on surface of inflamed endothelium (4, 97) and the activation of its counter receptor β2-integrins, LFA-1 (αLβ2, CD11a/CD18) and Mac-1 (αMβ2, CD11b/CD18) on the surface of leukocytes (38, 39, 43, 100). An emerging view of integrin activation is that selectin-mediated cell tethering and rolling positions leukocyte in such close proximity that endothelial-bound chemokine ligation can trigger the activation of β2-integrins (61). Interaction of ICAM-1 with activated β2-integrins ensures a stable shear resistant adhesion of PMN to the vascular endothelium (139, 142, 143) and thereby enables them to migrate across the endothelial cells to the underlying tissue (19, 56, 59). This review begins with an overview of the routes taken by migrating leukocytes to cross the endothelium, and then discusses the current state of knowledge about the role of ICAM-1 in leukocyte TEM especially in the context of the signaling events mediating ICAM-1 expression and activated by ICAM-1 engagement in endothelial cells.
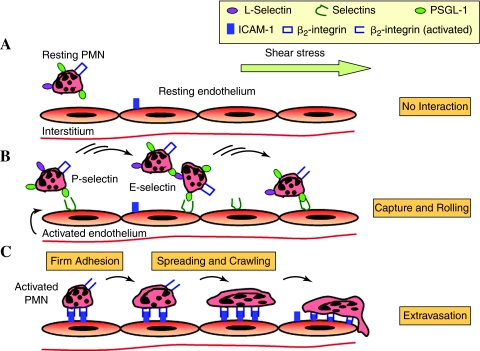
FIG. 1.
The multistep model of PMN capture, rolling, firm adhesion, and transendothelial migration. (A) The resting PMN express selectin ligands, but selectins are not present on the resting endothelium. Hence, PMN do not interact with the endothelium in resting stage. (B) Stimulation of the endothelium by proinflammatory mediators triggers the expression of selectins. The tethering on selectins and selectin ligands facilitates primary PMN capture to the endothelium and secondary PMN–PMN recruitment. Tethering transitions to rolling and results in activation of β2-integrins. (C) The interaction of β2-integrins with ICAM-1 upregulated on the activated endothelium causes the arrest, followed by spreading and then crawling, and finally trans endothelial migration of PMN. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
Leukocyte TEM: Transcellular or Paracellular?
The route taken by leukocytes during TEM is of fundamental importance from both the mechanistic and regulatory standpoints, and consequently has been a subject of active investigation for more than a century. Whereas the steps involving selectin-mediated rolling and ICAM-1-mediated firm adhesion during leukocyte TEM are now well accepted, the fundamental issue of whether leukocytes take a paracellular or transcellular route to migrate across endothelium remains poorly understood and a matter of debate. Until recently, the most accepted view was that shortly after arrest leukocytes begin to crawl over the luminal surface of endothelium and squeeze through the intercellular clefts between endothelial cells to the underlying tissues (19, 56, 59) (Fig. 2). It has now become increasingly clear that in addition to this “paracellular” route, there exists a “transcellular” route whereby leukocytes migrate through the endothelial cell body (Fig. 2). In fact, in vivo evidence indicating transcellular TEM of leukocytes existed as early as the 1960s and was further supported by a series of subsequent studies (32, 44, 47, 49, 50, 64, 99). Pioneering studies by Marchesi and Florey (99) showed the penetration of leukocytes through the endothelial cytoplasm, and those by Williamson and Grisham (173) documented the presence of leukocytes in large endothelial cytoplasmic vacuoles. These findings suggested the possibility that engulfment of leukocytes by endothelial cells could be a means of their migration through the endothelial cell body. In later studies, Feng et al. (49) demonstrated transcellular TEM of PMN through the microvasculature of the skin exposed to the chemotactic agent N-formyl-methionyl-leucyl-phenylalanine (fMLP). However, the idea of transcellular TEM of leukocytes was not viewed favorably until recently when a series of in vitro studies provided unambiguous proof of the existence of transcellular route for leukocyte TEM (24, 34, 103, 108, 181). These studies provide new insights into the mechanism of leukocyte TEM and give fresh impetus to the idea that transcellular and paracellular pathways may coexist.
FIG. 2.
Alternative routes of leukocyte passage across the endothelium. Following β2-integrin-mediated arrest of PMN, the endothelium proactively forms an ICAM-1 enriched “cuplike” structure (transmigratory cup) comprising microvilli-like projections which surround the adherent PMN. The PMN in the transmigratory cup can take two different pathways to migrate across the endothelium. PMN can either squeeze through the intercellular clefts between endothelial cells (“paracellular” TEM) or migrate through the endothelial cell body (“transcellular” TEM). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
The relative contribution of transcellular TEM in this coexistence model varies considerably and appears to rely on several factors including the type of leukocytes, the overall signaling, and state of activation of the leukocyte and the endothelium (71, 114). For example, Yang et al. (181) reported a robust transcellular TEM of PMN (∼ 50% of the total transmigration events) in an in vitro flow model of TNF-α-activated human umbilical vein endothelium cells (HUVEC). In contrast, primary T lymphocytes in this model exclusively used a paracellular route (181). Intriguingly, Nieminen et al. (108) made a paradoxical observation; these studies used endothelial cells isolated from fresh mouse skin preparations to report that lymphocytes preferentially use the transcellular route, whereas PMN migrate through inter-endothelial junctions. In another study, Cinamon et al. (34) showed that depending on the state of endothelial activation, and the type of activating chemoattractants displayed on the apical aspect of the endothelial barrier, PMN translate shear stress signals into transcellular TEM. The preference of one pathway over the other appears to be also influenced by the origin or source of endothelial cells. This possibility is highlighted by the studies of Millan et al. (103) that showed a marked increase in the percentage of lymphocytes migrating via transcellular route in experiments involving microvascular endothelium. Similarly, the migration of lymphocytes across the inflamed blood–brain barrier occurs via a transcellular route (44, 63, 114), and thus allowing the endothelial tight junctions remain intact. These observations point to the possibility that in the regions of vascular bed where endothelial cell–cell junctions are particularly tight and well organized such as blood–brain barrier, leukocytes might preferentially use the transcellular route for their transmigration. Contrary to this, the regions with relatively less tight endothelial junctions such as postcapillary venules might be the preferred sites for paracellular TEM of leukocytes. Collectively, the above findings support the notion that the choice of migratory pathway for leukocytes may be influenced by the type of stimuli, activation state and source of endothelial cells, and the type of leukocytes. However, additional studies are required to definitively establish these parameters as determinants of the route taken by migrating leukocytes.
Dual Role of ICAM-1 in Leukocyte TEM
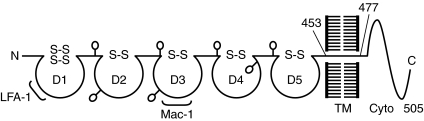
ICAM-1 is a cell surface glycoprotein of 505 amino acids with a molecular weight ranging from 76 to 114 kDa, depending upon extent of tissue-specific glycosylation (146, 148). It is a member of the immunoglobulin superfamily (IgSF) of adhesion molecules and is characterized by the presence of five extracellular Ig-like domains (domains 1–5, D1–5), a hydrophobic transmembrane domain, and a short cytoplasmic domain of 28 amino acids (Fig. 3) (146, 148, 149). The binding site for LFA-1 is present in D1 whereas that of Mac-1 maps to D3 of ICAM-1 (39, 40, 146, 148). The extracellular domains of ICAM-1 are essential and sufficient to mediate firm adhesion of leukocytes to the endothelium. Consistent with this, studies showed that expression of ICAM-1 mutant lacking the cytoplasmic domain was capable of supporting firm adherence of T lymphocytes to endothelial cells (44, 95).
FIG. 3.
Schematic of ICAM-1 protein structure and binding sites for leukocytes. ICAM-1 contains five extracellular Ig-like domains (D1through D5), a hydrophobic transmembrane domain, and a short cytoplasmic domain. D1 and D3 harbor the binding sites for LFA-1 and Mac-1, respectively.
The cytoplasmic domain of ICAM-1 interacts with the actin cytoskeleton via α-actinin, ezrin, and moesin, localizing it to cell surface microvilli (25, 68, 153). Increasing evidence indicate that the cytoplasmic domain of ICAM-1 plays a crucial role in facilitating the TEM of leukocytes (62, 95, 181). In support of this notion, studies have shown that inhibition of ICAM-1 cytoplasmic tail function or expression of truncated ICAM-1 mutant lacking cytoplasmic domain, each was effective in preventing the TEM without affecting the adhesion of leukocytes to endothelial cells (62, 95, 181). Intriguingly, interfering with ICAM-1 cytoplasmic domain function preferentially reduced the transcellular TEM of PMN (181). Similarly, an important role of ICAM-1 cytoplasmic tail in preferentially promoting transcellular TEM of lymphocytes has been documented in earlier studies (62, 95). Thus, the function of ICAM-1 is not restricted only to support the firm adhesion of leukocytes but also to facilitate the migration of the adherent leukocytes across the endothelium, preferentially via transcellular route, and this dual role of ICAM-1 can be assigned to the different domains of the molecule (62, 95, 181). Because ICAM-1 is also required for paracellular leukocyte TEM, its remains to be determined how ICAM-1 cytoplasmic tail preferentially contributes to transcellular TEM events.
The level of ICAM-1 expression is also implicated in determination of the route for leukocyte TEM. Studies by Yang et al. (181) showed that duration and intensity of ICAM-1 expression favors the transcellular TEM of PMN. These investigators found that prolonged exposure (24 h) of endothelial cells to TNFα was associated with a robust expression of ICAM-1 and increased proportion (∼20%) of PMN migrating via transcellular route compared to predominantly paracellular TEM of PMN when endothelial cells were challenged with TNFα for a short period (4 h) (181). In addition to the density, distribution of ICAM-1 on endothelial cell surface contributes to leukocyte TEM. Engagement of endothelial ICAM-1 with leukocyte β2-integrins alters the distribution dynamics of ICAM-1, leading to its clustering on endothelial cell surface. Carman et al. (23) demonstrated that in response to LFA-1 engagement, the endothelium proactively forms an ICAM-1 enriched “cuplike” structure (Fig. 2). In subsequent studies, they provided evidence for transcellular TEM in vitro and showed that virtually all, both para- and transcellular, TEM takes place in the context of this “cuplike” transmigratory structure (24). This docking structure is enriched not only in adhesive proteins but also in cytoskeletal components, and forms microvilli-like projections that surrounds the migrating leukocytes (Fig. 2). The observation of similar endothelial microvilli embracing transmigrating leukocytes in previous EM studies (47, 55, 127, 173) is consistent with formation of transmigratory cup in vivo. Additionally, this endothelial structure causes redistribution of leukocyte integrins into linear tracks that are aligned perpendicular to the plane of endothelium and parallel to the direction of TEM (24). Moreover, preventing the formation of these projections impairs the leukocyte TEM (24). Based on these findings, Carman et al. (24) proposed a novel mechanism of transmigration in which endothelium by virtue of forming the “transmigratory cup” provides directional guidance to leukocytes for their transmigration.
To clarify the role of the 28-amino acid cytoplasmic domain of ICAM-1 in the formation of such transmigratory cup, Oh et al. (110) recently showed that C-terminal 21 amino acids are dispensable whereas a segment of 5 amino acids 507RKIKK511 in the NH-terminal third of intracellular domain is required for the proper localization and dynamic distribution of ICAM-1 and the association of ICAM-1 with F-actin, ezrin, and moesin. Deletion of 507RKIKK511 motif markedly delays LFA-1-dependent membrane projection and decreases leukocyte adhesion and subsequent TEM (110). Moreover, exposure of endothelial cells with cell-permeant peptide comprising RKIKK sequences causes inhibition of leukocyte TEM (110). These findings indicate an essential role of 507RKIKK511 motif in the microvillus presentation of ICAM-1 and suggest a novel regulatory role for ICAM-1 topography in leukocyte TEM. Another mechanism by which cytoplasmic domain of ICAM-1 may contribute to leukocyte TEM involves its phosphorylation at Tyr474 and Tyr485 upon ICAM-1 ligation by fibrinogen (115, 141). Phosphorylation of these residues results in increased interaction of ICAM-1 with membrane type1-matrix metalloproteinases (MT1-MMP) and appears to play an important role in mediating the ectodomain cleavage of ICAM-1 and leukocyte TEM. Consistent with this, expression of phosphorylation-defective ICAM-1 mutants (ICAM-1Y474A and ICAM-1Y485A) in endothelial cells reduced the interaction of MT1-MMP with ICAM-1 and also inhibited monocyte TEM. Additionally, overexpression of MT1-MMP promoted ICAM-1 cleavage and monocyte TEM whereas RNAi knockdown of MT1-MMP suppressed monocyte TEM (141). Thus, these results show that tyrosine phosphorylation of ICAM-1 in the cytoplasmic domain controls leukocyte TEM through interaction with MT1-MMP. However, it remains to be clarified whether Tyr474 and/or Tyr485 phosphorylation also influences 507RKIKK511-dependent proper localization of ICAM-1 and LFA-1-dependent membrane projection and leukocyte TEM.
Clustering of cell surface ICAM-1 occurs following its engagement with leukocyte β2 integrins and serves to promote leukocyte adhesion to the endothelium and subsequent TEM (26, 175). It can also be induced by specific antibodies that mimic leukocyte binding to ICAM-1 or exposure of endothelial cells to TNFα (74, 153, 175). Clustering of constitutively expressed cell surface ICAM-1 plays an important role in mediating the rapid-onset of endothelial adhesivity toward PMN (74). The clustering leading to the induction of binding activity of the constitutive cell surface ICAM-1 is secondary to the phosphorylation of ICAM-1 by the atypical protein kinase Cζ (PKCζ). Inhibition of PKCζ activity by pharmacological and genetic approaches prevented the TNFα-induced ICAM-1 clustering and the early-onset of endothelial adhesivity. Similarly, studies by Wojciak–Stothard et al. (175) also showed that clustering of ICAM-1 is crucial for endothelial adhesivity, although in contrast to the above study, Wojciak–Stothard et al. (175) focused on the role of de novo expression of ICAM-1. In support of ICAM-1 clustering being a critical determinant of endothelial adhesivity, Jun et al. (77) showed that dimerized ICAM-1 has ∼1.5- to 3-fold greater affinity toward the I-domain of LFA-1 than monomeric ICAM-1. These studies also postulated that dimers and W-shaped tetramers form oligomers of ICAM-1, which could have important implications for regulating leukocyte adhesion (77). Collectively, these findings point to an important role of clustering in increased binding activity of cell surface ICAM-1 to PMN. The rapid clustering of constitutive cell surface ICAM-1 represents a novel mechanism for promoting the stable adhesion of PMN to endothelial cells that is needed to facilitate the early-onset TEM of PMN. The clustering of de novo synthesized ICAM-1 may be a mechanism of amplifying and sustaining the PMN adhesion and migration responses. These findings reveal ICAM-1 clustering as a central element contributing to both adhesion and TEM of leukocytes.
Milan et al. (103) recently showed that ICAM-1 clustering causes the translocation of apical ICAM-1 into caveolin-1-rich regions close to the ends of actin stress fibers. In these F-actin-rich areas, ICAM-1 is internalized and transcytosed to the basal plasma membrane through caveolae (103). This is followed by fusion of ICAM-1-containing caveolae into multivesicular structures, leading to formation of transcellular pores, characterized by a ring of F-actin and caveolin-1, through which leukocytes squeeze and cross the endothelial cell body for their transcellular TEM. Depletion of caveolin-1 with short-interfering RNA (siRNA) only inhibits the transcellular, not the paracellular, pathway of leukocyte migration (103). Given that the ICAM-1-enriched cuplike structures are associated both with the paracellular and transcellular TEM, these findings suggest the existence of additional mechanisms that may be engaged to initiate transcellular, rather than paracellular, TEM, and may involve association of ICAM-1 with F-actin and its translocation into caveolae. In brief, the findings of Milan et al. (103) suggest a model wherein binding of lymphocytes to endothelial cells induces ICAM-1 clustering and recruitment to caveolae, and subsequently, fusion of these caveolae into multivesicular structures, resulting in formation of a transcellular pore. The adherent lymphocytes, through their projections, will presumably scan endothelial surface for such sites for their transcytosis. This model finds further support from a previous report showing that leukocytes migrate through the endothelial cell cytoplasm in vivo through multivesicular structures called vesiculo-vacuolar organelles (50). However, it remains to be clarified what directs ICAM-1 to interact with F-actin and translocate into caveolae, and how the recruitment of ICAM-1 into caveolae contributes to transcellular TEM of leukocytes.
ICAM-1 as a Signaling Molecule
ICAM-1 has now emerged as a key signaling molecule; engagement of ICAM-1 triggers signaling events in endothelial cells that are critical for TEM of leukocytes (8, 26, 73, 166) (Fig. 4). The signaling capacity of ICAM-1 resides in the cytoplasmic domain, which, as discussed above, interacts with F-actin (159) and the cytoskeleton-associated proteins α-actinin (25) and ezrin (68). The emerging paradigm is that interaction of ICAM-1 with the actin cytoskeleton provides the structural framework for efficient signal transduction to occur. Indeed, destabilization of actin cytoskeleton by cytochalasin D and microtubule by nocodazole inhibited ERK1/2 phosphorylation, indicating the requirement of intact cytoskeleton architecture for ERK1/2 signaling (116). Ligation of endothelial ICAM-1 by leukocytes, antibodies, or fibrinogen causes increases in Ca2+, cytoskeletal changes, and gene expression (154, 166). Huang et al. (72) observed that transient increases in endothelial Ca2+ occur in response to PMN adhesion to and transmigration across fMLP- or IL-1-activated endothelial cells. Preventing the Ca2+ increase inhibits the PMN transmigration, but not PMN adhesion. PMN adhesion and transmigration induced by chemoattractants fMLP or LTB4 also causes phosphorylation of endothelial myosin light chain (MLC), and inhibition of MLC kinase (MLCK) decreases transmigration (57, 70, 132). PMN adhesion to TNFα-activated pulmonary microvascular endothelial cells induces cytoskeletal changes and these changes can be prevented by an anti-ICAM-1 antibody (166). Furthermore, these changes induced by PMN adhesion can be mimicked by cross-linking ICAM-1 with antibodies (163, 164). Ligation of ICAM-1 during PMN adhesion also induces ROS generation in endothelial cells (161, 164). Intriguingly, ligating either constitutive or induced ICAM-1 on the endothelial cell surface, or exposing endothelial cells to soluble ICAM-1 (sICAM-1), also increases GSH concentrations (117). The effect of ICAM-1 ligation on ROS generation and GSH levels suggest a tight regulation of endothelial redox status under basal and inflammatory conditions. It is likely that increasing GSH levels may limit the ROS accumulation upon ICAM-1 ligation and thus, may serve to restrict the inflammatory response (117). Unlike ROS generation, the mechanism of GSH increase by ICAM-1 ligation remains unclear.
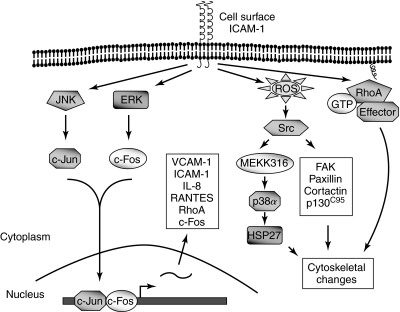
FIG. 4.
Signaling events activated by ICAM-1 engagement during leukocyte adhesion. Ligation of ICAM-1 induces a variety of responses in endothelial cells. These include RhoA activation, ERK, and JNK activation, ROS generation, and Src activation. Activated RhoA and ROS/Src, through activation of downstream effectors, induce reorganization of the actin cytoskeleton. ERK and JNK causes AP-1 (Jun/Fos) activation and gene expression including ICAM-1, suggesting the possibility of a feed-forward mechanism whereby ligation of ICAM-1 increases its own expression to amplify the adhesion and TEM of leukocytes to the site of inflammation.
Generation of ROS upon ICAM-1 ligation is mediated by xanthine oxidase, and ROS thus generated triggers Src tyrosine kinase activity and induces the tyrosine phosphorylation of the cytoskeleton-associated proteins cortactin (a substrate of Src kinase), focal adhesion kinase, paxillin, and the adaptor protein p130Cas (42, 45, 166, 167) (Fig. 4). Phosphorylation of cortactin at Tyr421, Tyr466, and Tyr482 plays a significant role in PMN transmigration (182). RNAi knockdown of cortactin in HUVEC-impaired TEM of PMN which was rescued by reexpression of wild-type cortactin, but not by a mutant of cortactin, in which tyrosine phosphorylation sites were mutated to phenylalanine (cortactin3F). Activated Src kinases also stimulate p38 mitogen-activated protein kinase, which in turn contributes to the cytoskeletal remodeling via phosphorylation of the heat shock protein 27 (HSP27), an actin-binding protein implicated in cytoskeletal rearrangement. Accordingly, inhibition of p38 MAP kinase was effective in preventing PMN adhesion-induced ICAM-1-dependent cytoskeletal changes in endothelial cells (165). Further studies led to the identification of p38α as the critical isoform that is activated by the upstream kinases MAP kinase kinase 3 and 6 (MKK-3 and -6) and mediates HSP27 phosphorylation, cytoskeletal changes, and PMN migration to the endothelial junctions in response to ICAM-1 ligation (168). Together, these studies unveil ROS/Src/MEKK(3/6)/ p38 MAPK/HSP27 and ROS/Src/cytoskeleton-associated proteins (Fig. 4) as important signaling cascades that are activated by PMN adhesion to mediate the cytoskeletal changes and subsequent PMN TEM. Ligation of ICAM-1 can also cause activation of extracellular signal-regulated kinase 1/2 (ERK1/2) and/or c-Jun N terminal kinase (JNK) depending on the experimental system (89, 134, 154). Additionally, ICAM-1 ligation promotes RhoA activation and the formation of actin stress fibers (2, 45) (Fig. 4). Activated Rho GTPase by this mechanism is implicated in facilitating lymphocyte TEM (26, 181). The Rho-dependent leukocyte migration is impaired when ICAM-1 cytoplasmic tail is deleted (135, 181). Future studies are required to decipher how these signaling networks are integrated to regulate leukocyte TEM.
Induction of endothelial cell gene expression represents another important consequence of ICAM-1 engagement. Antibody cross-linking of ICAM-1 results in activation of ERK-1 and the AP-1 transcription factor complex, without any increase in NF-κB activity, and transcription of VCAM-1 gene in HUVEC (89). Pretreatment of cells with MEK inhibitor PD98059 inhibited expression of VCAM-1 in response to ICAM-1 cross-linking, suggesting that the ERK pathway is involved in ICAM-1-mediated VCAM-1 expression. Activation of ERK1/2 is also required for the production of IL-8 and RANTES (134) and expression of ICAM-1 (35) in response to ICAM-1 cross-linking. In addition, ICAM-1 ligation induces the transcription of c-fos and rhoA expression in endothelial cells (153) (Fig. 4). Given that RhoA plays a crucial role in mediating ICAM-1 expression in endothelial cells (7), these findings raise the possibility of a positive feedback loop between RhoA and ICAM-1 that may serve to amplify expression of RhoA and ICAM-1, and thereby promote the sustained adhesion and TEM of leukocytes to the site of inflammation.
Signaling Mechanisms of ICAM-1 Expression in Endothelial Cells
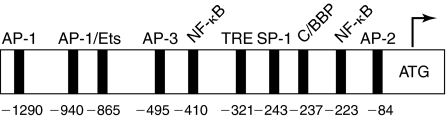
The human ICAM-1 gene is located on chromosome 19 and comprises seven exons and six introns with each of the five Ig-like domains encoded by a separate exon (129). Expression of ICAM-1 gene in endothelial cells can be induced by a variety of mediators including thrombin, TNFα, IL-1β, lipopolysaccharide (LPS), phorbol esters (PMA), vascular endothelial growth factor (VEGF), sheer stress, oxidants, infectious agents, and high glucose (20, 66, 76, 82, 87, 105, 119, 123, 125, 129, 150, 158, 176). The molecular cloning of ICAM-1 gene and functional dissection of ICAM-1 promoter showed that ICAM-1 expression is regulated mainly at the level of transcription (36, 37, 160, 170). Analysis of the 5′ flanking region of ICAM-1 gene revealed that ICAM-1 promoter contains binding sites for several transcription factors including two NF-κB sites; an upstream NF-κB site (-533 bases from translation start site) and a downstream NF-κB site (-223 bases from translation start site) (160) (Fig. 5). Site-directed mutagenesis of this region indicated that activation of the downstream NF-κB site is essential for ICAM-1 transcription (36, 90, 123). Gel supershift assays demonstrated that ICAM-1 expression requires NF-κB p65 (RelA/p65) binding to the downstream NF-κB site of the ICAM-1 promoter (90, 123). Activation of AP-1 is also implicated in some cases to mediate ICAM-1 expression in endothelial cells (85, 98, 128).
FIG. 5.
Schematic of ICAM-1 promoter structure. Rectangles indicate the location of the potential binding sites for transcription factors AP-1, AP-2, and AP-3 (activating protein-1, -2, and -3), Ets, NF-κB, TRE (tetradecanoyl phorbol acetate responsive element), Sp-1 (promoter selective-1), C/EBP (CAAT enhancer binding protein). The arrow above the initiation codon ATG indicates the translation start site.
NF-κB, typically a heterodimer of 50 kDa (p50) and 65 kDa (RelA) subunits, is sequestered in the cytoplasm in an inactive form by its association with IκBα, the prototype of a family of inhibitory proteins termed IκB proteins (12, 172). Activation of NF-κB requires the degradation of IκBα achieved through serine phosphorylation (Ser32 and Ser36) of IκBα by a macromolecular IκB kinase (IKK) complex (101, 185). Phosphorylation targets IκBα for polyubiquitination by the E3-SCFβ-TrCP ubiquitin ligase, and subsequently its degradation by the 26 S proteasome (80). The released NF-κB rapidly translocates to the nucleus to activate the transcription of target genes such as ICAM-1. Another important mechanism regulating NF-κB activity is through modulation of its transcriptional function by phosphorylation of RelA/p65 (41, 67, 133, 157, 162, 180). Studies have shown that phosphorylation of RelA/p65 at serines 276, 311, 529, or 536 increases the transcriptional capacity of NF-κB in the nucleus (41, 67, 133, 157, 162, 180). However, unlike IκBα phosphorylation, RelA/p65 phosphorylation site and the kinase involved vary in a stimulus- and cell type-specific manner (41, 67, 133, 157, 162, 180).
The majority of extracellular signals causing activation of ICAM-1 gene converge on NF-κB. The molecular mechanisms by which signaling from cell surface receptors lead to activation of NF-κB and thereby expression of ICAM-1 is still largely unknown. The following section will summarize the signaling mechanisms regulating NF-κB activation and the resultant ICAM-1 gene expression in endothelial cells, especially in the context of TNFα and thrombin, two well-studied activators of ICAM-1 gene in these cells.
Oxidant Signaling of ICAM-1
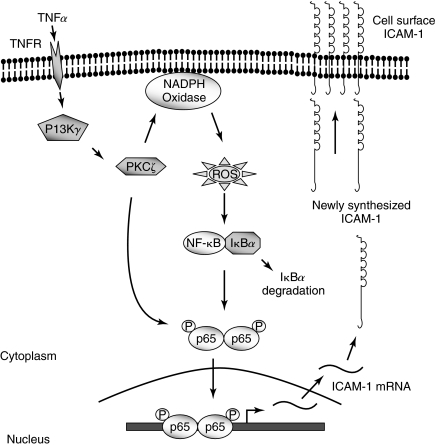
Several lines of evidence indicate that ROS, generated either extracellularly or intracellularly through ligand–receptor interactions, serve important signaling function in the mechanism of ICAM-1 expression in endothelial cells. Exposure of endothelial cells to exogenous H2O2 induced ICAM-1 expression via activation of AP-1/Ets elements within the ICAM-1 promoter (94, 130) (Fig. 5). The elevation of intracellular ROS by a variety of inducers such as cytokines, LPS, angiotensin II (Ag-II), cyclic strain, and shear stress, high glucose, homocysteine, and iron, is implicated in activating ICAM-1 gene transcription in endothelial cells (9, 18, 29–31, 60, 111, 112, 125, 145, 183). Accordingly, overexpression of Cu/Zn superoxide dismutase (SOD) in endothelial cells attenuates TNFα-induced superoxide anion (O2−.) production and ICAM-1 expression, and that this protective effect is mediated, in part, by inhibition of NF-κB (93). Recent evidence implicate that NADPH oxidase is the major source of ROS generation in endothelial cells (3, 5, 33, 54, 58, 92, 169) and ROS thus generated are critically involved in the ICAM-1 expression (14, 52, 53, 91, 112, 145). In particular, the role of NADPH oxidase in TNF-α signaling of ICAM-1 expression has been studied in some detail (46, 52, 91). It was found that TNF-α-induced oxidant signaling via NADPH oxidase leads to IκBα-degradation-dependent NF-κB activation and subsequently, ICAM-1 expression in endothelial cells. A crucial upstream event controlling TNFα-induced NADPH oxidase activation resulting in oxidant generation and NF-κB-dependent ICAM-1 expression involves activation PKCζ (52, 54, 121, 125) (Fig. 6).
FIG. 6.
Signaling events mediating TNFαa-induced ICAM-1 expression in endothelial cells. Stimulation of TNF receptor triggers the activation of PI3Kγ, which in turn activates PKCζ. Activated PKCζ induces the activation of NADPH oxidase resulting in ROS generation, which in turn promotes RelA/p65 homodimer binding to the ICAM-1 promoter through phosphorylation and degradation of IκBα. PKCζ also phosphorylates, and thereby amplifies the transactivation potential of the bound RelA/p65. The resultant ICAM-1 expression promotes the stable adhesion of leukocytes to endothelial cells.
PKC Signaling of ICAM-1
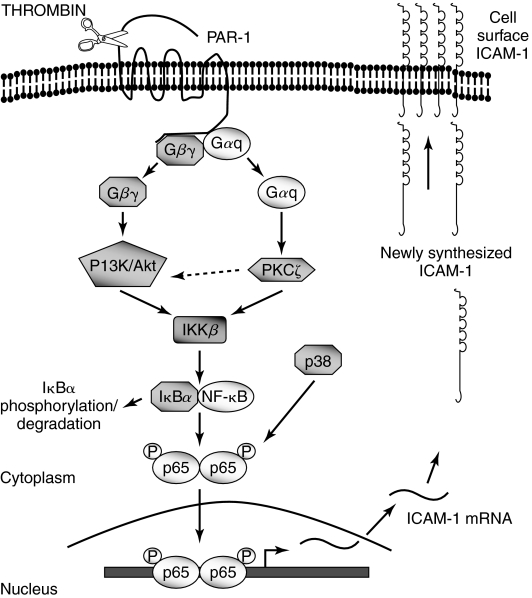
PKC plays a crucial role in promoting ICAM-1 expression in endothelial cells. Evidence implicating PKC in ICAM-1 expression was provided by the ability of PKC activators such as phorbol esters and phorbol dibutyrate to induce ICAM-1 expression (87, 150). The protective effect of PKC inhibitors suggested that PKC is also required for ICAM-1 expression induced by a variety of other proinflammatory mediators (82, 87, 104, 124, 150). While these studies point to the participation of PKC in the response, they do not identify the specific PKC isoforms that may be activated in a stimulus-specific manner to promote ICAM-1 expression. Using a combination of isoform-specific inhibitors and genetic approaches, studies have established that atypical PKCζ occupies a central position in TNF-α signaling of ICAM-1 expression by virtue of activating NF-κB in endothelial cells (52, 121, 125) (Fig. 6). These studies further showed that PKCζ controls NF-κB activation by promoting its DNA binding function secondary to degradation of IκBα. Studies by Anrather et al. (6) demonstrated that PKC-ζ contributes to the transcriptional activity of NF-κB by a mechanism involving phosphorylation of RelA/p65. Together, these findings indicate that PKCζ signals TNF-α-induced ICAM-1 expression by facilitating the binding of NF-κB to ICAM-1 promoter and enhancing the transcriptional activity of the bound RelA/p65 (Fig. 6). In other studies, it was found that sphingosine 1-phosphate mediates ICAM-1 expression through PKCα, whereas histamine response requires the participation of PKCδ (137). Similarly, studies have identified PKCδ as a critical isoform that signals thrombin-induced ICAM-1 expression in endothelial cells (124). These studies demonstrate that thrombin-induced expression of ICAM-1 is regulated by PKCδ through a dual mechanism involving the activation of IKKβ and p38 MAP kinase. Activation of IKKβ contributes to thrombin-induced ICAM-1 expression by promoting IκBα degradation and thereby promoting NF-κB binding to the ICAM-1 promoter, whereas p38 MAP kinase activity contributes to the response by increasing the transactivation potential of the bound NF-κB through phosphorylation of RelA/p65 (Fig. 7). These two mechanisms may operate in a synergistic fashion to sustain ICAM-1 expression in endothelial cells and thereby promote stable endothelial adhesivity.
FIG. 7.
Signaling events mediating thrombin-induced ICAM-1 expression in endothelial cells. Activation of Gαq and dissociation of Gβγ complex after thrombin stimulation of protease activated receptor-1 (PAR-1) results in the parallel activation of PKCδ and PI3 kinase and the downstream kinase Akt-dependent pathways. These pathways converge to activate IKKβ, which in turn promotes RelA/p65 homodimer binding to the ICAM-1 promoter secondary to phosphorylation and degradation of IκBα. PKC-δ also activates p38 MAP kinase, which induces phosphorylation of RelA/p65, and thereby enhances the transcriptional capacity of the bound RelA/p65. The resultant ICAM-1 expression promotes the stable adhesion of leukocytes to endothelial cells.
PI3K/Akt Signaling of ICAM-1
Several groups have demonstrated a role for PI3 kinase (PI3K) and its downstream target Akt in regulating ICAM-1 expression in endothelial cells. Studies involving a combination of dominant negative and constitutively active mutants of PI3K and Akt indicated the contribution of PI3K/Akt pathway in thrombin-induced NF-κB activation and ICAM-1 expression (126). Activation of PI3K/Akt pathway serves to link the signaling from the thrombin receptor, protease-activated receptor-1 (PAR-1), to IKK/NF-κB and thereby ICAM-1 (Fig. 7). Antisense oligonucleotide directed against PI3K prevents ICAM-1 expression induced by 2-fucosyllactose (H-2g), a glucose analogue of blood group H antigen (187). Arnold and Konig (10) have demonstrated that respiratory syncytial virus (RSV)-induced ICAM expression is dependent on PI3K. Using lung microvascular endothelial cells isolated from mice with targeted deletion of the p110γ catalytic subunit of PI3Kγ, Frey et al. (52) have recently established an important role of PI3Kγ in signaling TNF-α-induced ICAM-1 expression. This study extends the previous findings (54, 121, 125) by showing that PI3Kγ lies upstream of PKC-ζ in the endothelium, and its activation is crucial in signaling NADPH oxidase-dependent oxidant production and subsequent NF-κB activation and ICAM-1 expression (Fig. 6).
There are, however, some conflicting reports concerning the requirement of PI3K and Akt in the mechanism of ICAM-1 expression, especially in the context of VEGF signaling. For example, Kim et al. (82) have demonstrated that inhibition of PI3K by wortmannin promotes both basal and VEGF-induced ICAM-1 (as well as VCAM-1 and E-selectin) expression in endothelial cells. In another report, Kim et al. (83) have shown that adrenomedullin reduces VEGF-induced endothelial ICAM-1 expression through a PI3K-dependent pathway. This is in contrast to the findings of Radisavljevic et al. (120) showing that VEGF upregulates ICAM-1 expression via the PI3K/Akt pathway. PI3K also contributes to thrombin-induced ICAM-1 expression via activation of Akt (126). The exact reasons for the contrasting observations are not clear; one notable difference between these studies, however, is the use of pharmacological vs. genetic approaches to address the role of PI3K/Akt pathway.
MAP Kinase Signaling of ICAM-1
MAP kinases represent another important family of serine/threonine kinases involved in the regulation of ICAM-1 expression in endothelial cells. Studies have implicated a role of p38 MAP kinase in the regulation of ICAM-1 expression by various inducers, such as TNF-α, LPS, RSV, bile acids, nicotine, advanced oxidation protein products (AOPPs), sphingosine 1-phosphate (S1P), and thrombin (10, 28, 65, 75, 106, 118, 124, 137, 152, 156, 179). Consistent with the requirement of p38 MAP kinase, a number of agents suppress ICAM-1 expression by targeting p38 MAP kinase (11, 93, 109, 122). Other MAP kinases, JNK and ERK1/2, have also been reported to mediate ICAM-1 expression. Inhibition of JNK prevents thrombin-induced ICAM-1 expression in endothelial cells (102). Further evidence supporting a role JNK in ICAM-1 expression is provided by the ability of various compounds to inhibit ICAM-1 expression by interfering with the activation JNK (28, 84, 85, 93, 98, 144, 184). Studies have also shown a role of ERK1/2 in controlling ICAM-1 expression (11, 78, 96, 106). The mechanisms by which p38 MAPK, JNK, and ERK1/2 promote ICAM-1 expression involve activation of NF-κB or in some cases AP-1 (11, 28, 78, 84, 85, 96, 98, 109, 122, 124, 144, 156). However, in contrast to the above findings, there are reports that argue against the involvement of p38 MAPK, JNK, and ERK1/2 in signaling ICAM-1 expression in endothelial cells (51, 102, 158, 176, 186). It is unclear whether the observed differences reflect the differences in the experimental conditions/technique used in these studies.
Small GTPase Signaling of ICAM-1
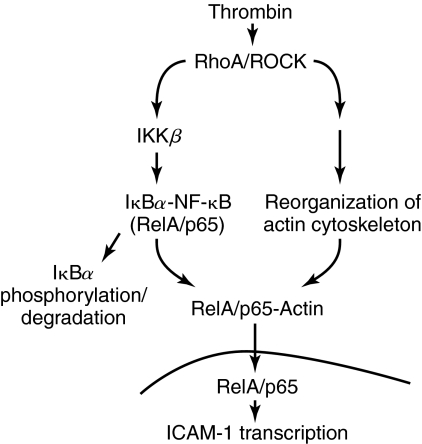
In addition to regulating cytoskeletal dynamics, cell adhesion, and migration, Rho GTPases can also affect gene expression (22, 69, 107). Indeed, GTPases have been shown to be critical determinants of ICAM-1 expression in endothelial cells. Chen et al. (29) used adenoviral-mediated expression of dominant negative mutant of Rac1 (N17Rac1) to address the role of this GTPase in mediating the expression of ICAM gene in endothelial cells. Expression of N17Rac1 by this approach inhibited TNFα-induced ICAM-1 expression by suppressing the transcriptional activity of NF-κB (29). Expression of N17Rac1 was also effective in preventing ICAM-1 expression by IL-6 (178). In another study, it was found that activation of Rac1 by shear stress is crucial for NF-κB-dependent ICAM-1 expression (155). RhoA is also implicated as a critical mediator of ICAM-1 expression in endothelial cells. Inhibition of RhoA by Clostridium botulinum C3 transferase or dominant negative form of RhoA (RhoAT19N) markedly decreased LPS-induced ICAM-1 expression in endothelial cells (151). In addition, RhoA is required for 12/15-lipoxygenase-induced ICAM-1 expression via activation of NF-κB in endothelial cells (17). Considered together, these findings suggest that RhoA and Rac1 regulate ICAM-1 expression in a stimulus-specific manner. This possibility became more evident in studies by Anwar et al. (7) that investigated the involvement RhoA/ROCK pathway in the mechanism of thrombin- and TNF-α-induced ICAM-1 expression in endothelial cells. Results showed that RhoA/ROCK pathway mediates thrombin- but not TNF-α-induced induced ICAM-1 expression. RhoA/ROCK signals thrombin-induced ICAM-1 expression by promoting RelA/p65 binding to the ICAM-1 promoter secondary to phosphorylation and degradation of IκBα (Fig. 8) as well as enhancing the transactivation capacity of the bound NF-κB through Ser536 phosphorylation of RelA/p65 (7). Other studies also found that TNF-α-induced ICAM-1 expression occurs independently of RhoA/ROCK pathway (26, 175). Importantly, RhoA/ROCK has been shown to mediate TNFα-induced reorganization of the actin cytoskeleton and endothelial cell apoptosis (113, 174, 175). Hence, the lack of involvement of the RhoA/ROCK pathway in TNFα-induced ICAM-1 expression cannot be ascribed to the inability of TNFα to activate RhoA/ROCK pathway in endothelial cells. The reasons for the different effects of RhoA/ROCK inhibition on thrombin- vs. TNFα-induced ICAM-1 expression are not clear. A possible explanation is that there may be distinct mechanisms of thrombin and TNFα activation of RhoA/ROCK as well as a specialized set of downstream effectors activated by each agonist. Consistent with these findings, Fazal et al. (48) recently demonstrated an important role of actin cytoskeleton downstream of RhoA in mediating thrombin- but not TNF–induced ICAM-1 expression (48). Results showed that actin cytoskeleton selectively contributes to thrombin-induced ICAM-1 expression by facilitating the nuclear translocation of RelA/p65 (Fig. 8). These findings support the notion that each receptor system utilizes a distinct set of adaptor molecules and signaling enzymes to construct a unique pathway that mediates NF-κB activation and ICAM-1 expression activation in a signal- and cell-specific manner.
FIG. 8.
Role of RhoA/ROCK pathway and the actin cytoskeleton in thrombin-induced ICAM-1 expression in endothelial cells. Thrombin challenge of endothelial cells results in activation of RhoA/ROCK, which activates IKKβ to mediate the release of RelA/p65 secondary to phosphorylation and degradation of IκBα. RhoA/ROCK, in addition to activating IKKβ, induces a dynamic reorganization of the actin cytoskeleton. This event serves to promote the association of actin with the liberated RelA/p65, and this interaction, in turn, facilitates the translocation of RelA/p65 to the nucleus. The nuclear RelA/p65 binds to the promoter and induces the expression of ICAM-1.
Tyrosine Kinase Signaling of ICAM-1
Clues for a role of tyrosine kinases in ICAM-1 expression were provided by the protective effect of tyrosine kinase inhibitors on the response. Pretreatment of endothelial cells with genistein or tyrphostin AG126 results in decreased expression of ICAM-1 in response to TNFα (81, 171). Tyrosine kinase signaling is also required for induction of ICAM-1 expression by glycosylphosphatidyinositol (GPI) toxin of malaria parasite (136). Similarly, activation of ICAM-1 promoter and subsequent transcription of ICAM-1 gene by lysophosphatidylcholine (lyso-PC) is dependent on tyrosine kinases (188). The expression of ICAM-1 in these cases is ascribed to the ability of tyrosine kinases to induce NF-κB DNA binding (136, 171, 188). Recently, Bijli et al. (15, 16) have identified c-Src and Syk (spleen tyrosine kinase) as critical regulators of ICAM-1 expression by thrombin. The above studies demonstrate that thrombin activates c-Src and Syk to phosphorylate RelA/p65. The tyrosine phosphorylation of RelA/p65 in turn promotes ICAM-1 expression by increasing the transcriptional activity of NF-κB. These findings highlight the importance of tyrosine kinases in controlling the expression of ICAM-1 by increasing NF-κB binding to the promoter or transcriptional capacity of the bound NF-κB.
Conclusions
In recent years, it has become clear that the role of ICAM-1 in leukocyte TEM is not restricted only to firm adhesion of leukocytes to the endothelium. An important consequence of ICAM-1 engagement during adhesion is the activation of signaling events that are believed to facilitate the active participation of endothelial cells in leukocyte TEM. The dual role of ICAM-1 in leukocyte TEM derives, at least in part, from its structure, whereas the binding to leukocytes is mediated by extracellular Ig-like domains, signaling capacity resides in the short cytoplasmic domain. It still remains unclear how the cytoplasmic domain is activated upon ICAM-1 ligation and how the activation of this domain induces signaling events in endothelial cells to facilitate TEM of leukocytes. One possibility is that it can be phosphorylated by protein kinases. In support of this concept, PKC-ζ has been shown to phosphorylate ICAM-1, leading to increased endothelial adhesivity toward PMN (74). Future studies are required to identify the phosphorylation sites and the kinases involved in order to understand the way ICAM-1 cytoplsamic domain is activated to initiate intracellular signaling. Furthermore, delineation of the complete spectrum of the signaling pathways activated by ICAM-1 engagement and those responsible for the upregulation of ICAM-1 expression in endothelial cells is critical to the understanding of the role played by the endothelium in leukocyte TEM. These studies may also enable us to identify the specific signals that are devoted to paracellular versus transcellular TEM of leukocytes. Additionally, these studies may identify the signals that amplify the leukocyte TEM by virtue of participating in upregulation of ICAM-1 expression and being activated upon ICAM-1 engagement. For example, ligation of ICAM-1 activates both RhoA and c-Src in endothelial cells (26, 166), and the activation of each has been shown to promote the ICAM-1 expression in these cells (7, 16). These findings suggest a model wherein RhoA and ICAM-1 or c-Src and ICAM-1 may function in a feed-forward manner to enhance and sustain the inflammation by facilitating the further waves of leukocyte TEM. By systematically elucidating the signaling pathways mediating upregulation of ICAM-1 expression and those activated downstream of ICAM-1 engagement, especially in the context of endothelial heterogeneity and the type of adherent leukocyte, it would be possible to selectively prevent or limit leukocyte TEM associated with various immune and inflammatory disease states.
Abbreviations
AP-1, activating protein-1; C/EBP, CAAT enhancer binding protein; ERK1/2; extracellular signal-regulated kinase 1/2; fMLP, N-formyl-methionyl-leucyl-phenylalanine; HSP27, heat shock protein 27; ICAM-1, intercellular adhesion molecule-1; IgSF, immunoglobulin superfamily; IKK, IκB kinase; IL-1, interleukin-1; JNK, c-Jun N terminal kinase; LFA-1, leukocyte function-associated antigen-1; LPS, lipopolysaccharide; LTB4, leukotriene B4; lyso-PC, lysophosphatidylcholine; MAPK, mitogen-activated protein kinase, MKK-3/6 , MAP kinase kinase 3/6; MLCK, myosin light chain kinase; NF-κB, nuclear factor-kappa B; PAR-1, protease-activated receptor-1; PKC, protein kinase C; PMN, polymorphonuclear leukocytes; PSGL-1, platelet sialoglycoprotein ligand-1; ROS, reactive oxygen species; RSV, respiratory syncytial virus; S1P, sphingosine 1-phosphate; SHP-2 Src homology 2-containing protein-tyrosine phosphatase-2; SOD, superoxide dismutase; Sp-1, promoter selective-1; TEM, transendothelial migration; TNFα, tumor necrosis factor alpha; TRE, tetradecanoyl phorbol acetate responsive element; VCAM-1, vascular cell adhesion molecule-1; VEGF, vascular endothelial growth factor.
Acknowledgments
We thank Matthew Murrill for Fig. 4. This work was supported by National Heart, Lung, and Blood Institute Grant HL67424 (A. Rahman) and a Biomedical Research Grant from the American Lung Association (F. Fazal).
Disclosure Statement
No competing financial interests exist.
References
- 1.Abbassi O. Lane CL. Krater S. Kishimoto TK. Anderson DC. McIntire LV. Smith CW. Canine neutrophil margination mediated by lectin adhesion molecule-1 in vitro. J Immunol. 1991;147:2107–2115. [PubMed] [Google Scholar]
- 2.Adamson P. Etienne S. Couraud PO. Calder V. Greenwood J. Lymphocyte migration through brain endothelial cell monolayers involves signaling through endothelial ICAM-1 via a rho-dependent pathway. J Immunol. 1999;162:2964–2973. [PubMed] [Google Scholar]
- 3.Al-Mehdi AB. Zhao G. Dodia C. Tozawa K. Costa K. Muzykantov V. Ross C. Blecha F. Dinauer M. Fisher AB. Endothelial NADPH oxidase as the source of oxidants in lungs exposed to ischemia or high K+ Circ Res. 1998;83:730–737. doi: 10.1161/01.res.83.7.730. [DOI] [PubMed] [Google Scholar]
- 4.Albelda SM. Smith CW. Ward PA. Adhesion molecules and inflammatory injury. FASEB J. 1994;8:504–512. [PubMed] [Google Scholar]
- 5.Alom-Ruiz SP. Anilkumar N. Shah AM. Reactive oxygen species and endothelial activation. Antioxid Redox Signal. 2008;10:1089–1100. doi: 10.1089/ars.2007.2007. [DOI] [PubMed] [Google Scholar]
- 6.Anrather J. Csizmadia V. Soares MP. Winkler H. Regulation of NF-kappaB RelA phosphorylation and transcriptional activity by p21(ras) and protein kinase Czeta in primary endothelial cells. J Biol Chem. 1999;274:13594–13603. doi: 10.1074/jbc.274.19.13594. [DOI] [PubMed] [Google Scholar]
- 7.Anwar KN. Fazal F. Malik AB. Rahman A. RhoA/Rho-associated kinase pathway selectively regulates thrombin-induced intercellular adhesion molecule-1 expression in endothelial cells via activation of I kappa B kinase beta and phosphorylation of RelA/p65. J Immunol. 2004;173:6965–6972. doi: 10.4049/jimmunol.173.11.6965. [DOI] [PubMed] [Google Scholar]
- 8.Aplin AE. Howe AK. Juliano RL. Cell adhesion molecules, signal transduction and cell growth. Curr Opin Cell Biol. 1999;11:737–744. doi: 10.1016/s0955-0674(99)00045-9. [DOI] [PubMed] [Google Scholar]
- 9.Arai T. Kelly SA. Brengman ML. Takano M. Smith EH. Goldschmidt–Clermont PJ. Bulkley GB. Ambient but not incremental oxidant generation effects intercellular adhesion molecule 1 induction by tumour necrosis factor alpha in endothelium. Biochem J. 1998;331:853–861. doi: 10.1042/bj3310853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arnold R. Konig W. Respiratory syncytial virus infection of human lung endothelial cells enhances selectively intercellular adhesion molecule-1 expression. J Immunol. 2005;174:7359–7367. doi: 10.4049/jimmunol.174.11.7359. [DOI] [PubMed] [Google Scholar]
- 11.Bai YP. Liu YH. Chen J. Song T. You Y. Tang ZY. Li YJ. Zhang GG. Rosiglitazone attenuates NF-kappaB-dependent ICAM-1 and TNF-alpha production caused by homocysteine via inhibiting ERK1/2/p38MAPK activation. Biochem Biophys Res Commun. 2007;360:20–26. doi: 10.1016/j.bbrc.2007.05.222. [DOI] [PubMed] [Google Scholar]
- 12.Baldwin AS., Jr. The NF-kappa B and I kappa B proteins: New discoveries and insights. Annu Rev Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 13.Bargatze RF. Kurk S. Butcher EC. Jutila MA. Neutrophils roll on adherent neutrophils bound to cytokine-induced endothelial cells via L-selectin on the rolling cells. J Exp Med. 1994;180:1785–1792. doi: 10.1084/jem.180.5.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhunia AK. Arai T. Bulkley G. Chatterjee S. Lactosylceramide mediates tumor necrosis factor-alpha-induced intercellular adhesion molecule-1 (ICAM-1) expression and the adhesion of neutrophil in human umbilical vein endothelial cells. J Biol Chem. 1998;273:34349–34357. doi: 10.1074/jbc.273.51.34349. [DOI] [PubMed] [Google Scholar]
- 15.Bijli KM. Fazal F. Minhajuddin M. Rahman A. Activation of Syk by protein kinase C-delta regulates thrombin-induced intercellular adhesion molecule-1 expression in endothelial cells via tyrosine phosphorylation of RelA/p65. J Biol Chem. 2008;283:14674–14684. doi: 10.1074/jbc.M802094200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bijli KM. Minhajuddin M. Fazal F. O'Reilly MA. Platanias LC. Rahman A. c-Src interacts with and phosphorylates RelA/p65 to promote thrombin-induced ICAM-1 expression in endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2007;292:L396–L404. doi: 10.1152/ajplung.00163.2006. [DOI] [PubMed] [Google Scholar]
- 17.Bolick DT. Orr AW. Whetzel A. Srinivasan S. Hatley ME. Schwartz MA. Hedrick CC. 12/15-lipoxygenase regulates intercellular adhesion molecule-1 expression and monocyte adhesion to endothelium through activation of RhoA and nuclear factor-kappaB. Arterioscler Thromb Vasc Biol. 2005;25:2301–2307. doi: 10.1161/01.ATV.0000186181.19909.a6. [DOI] [PubMed] [Google Scholar]
- 18.Brocq ML. Leslie SJ. Milliken P. Megson IL. Endothelial dysfunction: from molecular mechanisms to measurement, clinical implications, and therapeutic opportunities. Antioxid Redox Signal. 2008;10:1631–1674. doi: 10.1089/ars.2007.2013. [DOI] [PubMed] [Google Scholar]
- 19.Burns AR. Walker DC. Brown ES. Thurmon LT. Bowden RA. Keese CR. Simon SI. Entman ML. Smith CW. Neutrophil transendothelial migration is independent of tight junctions and occurs preferentially at tricellular corners. J Immunol. 1997;159:2893–2903. [PubMed] [Google Scholar]
- 20.Burns LJ. Pooley JC. Walsh DJ. Vercellotti GM. Weber ML. Kovacs A. Intercellular adhesion molecule-1 expression in endothelial cells is activated by cytomegalovirus immediate early proteins. Transplantation. 1999;67:137–144. doi: 10.1097/00007890-199901150-00023. [DOI] [PubMed] [Google Scholar]
- 21.Butcher EC. Leukocyte-endothelial cell recognition: Three (or more) steps to specificity and diversity. Cell. 1991;67:1033–1036. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- 22.Cammarano MS. Minden A. Dbl and the Rho GTPases activate NF kappa B by I kappa B kinase (IKK)-dependent and IKK-independent pathways. J Biol Chem. 2001;276:25876–25882. doi: 10.1074/jbc.M011345200. [DOI] [PubMed] [Google Scholar]
- 23.Carman CV. Jun CD. Salas A. Springer TA. Endothelial cells proactively form microvilli-like membrane projections upon intercellular adhesion molecule 1 engagement of leukocyte LFA-1. J Immunol. 2003;171:6135–6144. doi: 10.4049/jimmunol.171.11.6135. [DOI] [PubMed] [Google Scholar]
- 24.Carman CV. Springer TA. A transmigratory cup in leukocyte diapedesis both through individual vascular endothelial cells and between them. J Cell Biol. 2004;167:377–388. doi: 10.1083/jcb.200404129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carpen O. Pallai P. Staunton DE. Springer TA. Association of intercellular adhesion molecule-1 (ICAM-1) with actin-containing cytoskeleton and alpha-actinin. J Cell Biol. 1992;118:1223–1234. doi: 10.1083/jcb.118.5.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cernuda-Morollon E. Ridley AJ. Rho GTPases and leukocyte adhesion receptor expression and function in endothelial cells. Circ Res. 2006;98:757–767. doi: 10.1161/01.RES.0000210579.35304.d3. [DOI] [PubMed] [Google Scholar]
- 27.Chavakis E. Aicher A. Heeschen C. Sasaki K. Kaiser R. El Makhfi N. Urbich C. Peters T. Scharffetter–Kochanek K. Zeiher AM. Chavakis T. Dimmeler S. Role of beta2-integrins for homing and neovascularization capacity of endothelial progenitor cells. J Exp Med. 2005;201:63–72. doi: 10.1084/jem.20041402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Che W. Lerner–Marmarosh N. Huang Q. Osawa M. Ohta S. Yoshizumi M. Glassman M. Lee JD. Yan C. Berk BC. Abe J. Insulin-like growth factor-1 enhances inflammatory responses in endothelial cells: role of Gab1 and MEKK3 in TNF-alpha-induced c-Jun and NF-kappaB activation and adhesion molecule expression. Circ Res. 2002;90:1222–1230. doi: 10.1161/01.res.0000021127.83364.7d. [DOI] [PubMed] [Google Scholar]
- 29.Chen XL. Zhang Q. Zhao R. Ding X. Tummala PE. Medford RM. Rac1 and superoxide are required for the expression of cell adhesion molecules induced by tumor necrosis factor-alpha in endothelial cells. J Pharmacol Exp Ther. 2003;305:573–580. doi: 10.1124/jpet.102.047894. [DOI] [PubMed] [Google Scholar]
- 30.Cheng JJ. Wung BS. Chao YJ. Wang DL. Cyclic strain-induced reactive oxygen species involved in ICAM-1 gene induction in endothelial cells. Hypertension. 1998;31:125–130. doi: 10.1161/01.hyp.31.1.125. [DOI] [PubMed] [Google Scholar]
- 31.Chiu JJ. Wung BS. Shyy JY. Hsieh HJ. Wang DL. Reactive oxygen species are involved in shear stress-induced intercellular adhesion molecule-1 expression in endothelial cells. Arterioscler Thromb Vasc Biol. 1997;17:3570–3577. doi: 10.1161/01.atv.17.12.3570. [DOI] [PubMed] [Google Scholar]
- 32.Cho Y. De Bruyn PP. Internal structure of the postcapillary high-endothelial venules of rodent lymph nodes and Peyer's patches and the transendothelial lymphocyte passage. Am J Anat. 1986;177:481–490. doi: 10.1002/aja.1001770406. [DOI] [PubMed] [Google Scholar]
- 33.Chowdhury AK. Watkins T. Parinandi NL. Saatian B. Kleinberg ME. Usatyuk PV. Natarajan V. Src-mediated tyrosine phosphorylation of p47phox in hyperoxia-induced activation of NADPH oxidase and generation of reactive oxygen species in lung endothelial cells. J Biol Chem. 2005;280:20700–20711. doi: 10.1074/jbc.M411722200. [DOI] [PubMed] [Google Scholar]
- 34.Cinamon G. Shinder V. Shamri R. Alon R. Chemoattractant signals and beta 2 integrin occupancy at apical endothelial contacts combine with shear stress signals to promote transendothelial neutrophil migration. J Immunol. 2004;173:7282–7291. doi: 10.4049/jimmunol.173.12.7282. [DOI] [PubMed] [Google Scholar]
- 35.Clayton A. Evans RA. Pettit E. Hallett M. Williams JD. Steadman R. Cellular activation through the ligation of intercellular adhesion molecule-1. J Cell Sci. 1998;111(( Pt 4)):443–453. doi: 10.1242/jcs.111.4.443. [DOI] [PubMed] [Google Scholar]
- 36.Collins T. Read MA. Neish AS. Whitley MZ. Thanos D. Maniatis T. Transcriptional regulation of endothelial cell adhesion molecules: NF-kappa B and cytokine-inducible enhancers. FASEB J. 1995;9:899–909. [PubMed] [Google Scholar]
- 37.Degitz K. Li LJ. Caughman SW. Cloning and characterization of the 5′-transcriptional regulatory region of the human intercellular adhesion molecule 1 gene. J Biol Chem. 1991;266:14024–14030. [PubMed] [Google Scholar]
- 38.Diacovo TG. Roth SJ. Buccola JM. Bainton DF. Springer TA. Neutrophil rolling, arrest, and transmigration across activated, surface-adherent platelets via sequential action of P-selectin and the beta 2-integrin CD11b/CD18. Blood. 1996;88:146–157. [PubMed] [Google Scholar]
- 39.Diamond MS. Staunton DE. de Fougerolles AR. Stacker SA. Garcia–Aguilar J. Hibbs ML. Springer TA. ICAM-1 (CD54): A counter-receptor for Mac-1 (CD11b/CD18) J Cell Biol. 1990;111:3129–3139. doi: 10.1083/jcb.111.6.3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Diamond MS. Staunton DE. Marlin SD. Springer TA. Binding of the integrin Mac-1 (CD11b/CD18) to the third immunoglobulin-like domain of ICAM-1 (CD54) and its regulation by glycosylation. Cell. 1991;65:961–971. doi: 10.1016/0092-8674(91)90548-d. [DOI] [PubMed] [Google Scholar]
- 41.Duran A. Diaz–Meco MT. Moscat J. Essential role of RelA Ser311 phosphorylation by zetaPKC in NF-kappaB transcriptional activation. EMBO J. 2003;22:3910–3918. doi: 10.1093/emboj/cdg370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Durieu-Trautmann O. Chaverot N. Cazaubon S. Strosberg AD. Couraud PO. Intercellular adhesion molecule 1 activation induces tyrosine phosphorylation of the cytoskeleton-associated protein cortactin in brain microvessel endothelial cells. J Biol Chem. 1994;269:12536–12540. [PubMed] [Google Scholar]
- 43.Dustin ML. Springer TA. Lymphocyte function-associated antigen-1 (LFA-1) interaction with intercellular adhesion molecule-1 (ICAM-1) is one of at least three mechanisms for lymphocyte adhesion to cultured endothelial cells. J Cell Biol. 1988;107:321–331. doi: 10.1083/jcb.107.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Engelhardt B. Wolburg H. Mini-review: Transendothelial migration of leukocytes: through the front door or around the side of the house? Eur J Immunol. 2004;34:2955–2963. doi: 10.1002/eji.200425327. [DOI] [PubMed] [Google Scholar]
- 45.Etienne S. Adamson P. Greenwood J. Strosberg AD. Cazaubon S. Couraud PO. ICAM-1 signaling pathways associated with Rho activation in microvascular brain endothelial cells. J Immunol. 1998;161:5755–5761. [PubMed] [Google Scholar]
- 46.Fan J. Frey RS. Rahman A. Malik AB. Role of neutrophil NADPH oxidase in the mechanism of tumor necrosis factor-alpha -induced NF-kappa B activation and intercellular adhesion molecule-1 expression in endothelial cells. J Biol Chem. 2002;277:3404–3411. doi: 10.1074/jbc.M110054200. [DOI] [PubMed] [Google Scholar]
- 47.Faustmann PM. Dermietzel R. Extravasation of polymorphonuclear leukocytes from the cerebral microvasculature. Inflammatory response induced by alpha-bungarotoxin. Cell Tissue Res. 1985;242:399–407. doi: 10.1007/BF00214554. [DOI] [PubMed] [Google Scholar]
- 48.Fazal F. Minhajuddin M. Bijli KM. McGrath JL. Rahman A. Evidence for actin cytoskeleton-dependent and -independent pathways for RelA/p65 nuclear translocation in endothelial cells. J Biol Chem. 2007;282:3940–3950. doi: 10.1074/jbc.M608074200. [DOI] [PubMed] [Google Scholar]
- 49.Feng D. Nagy JA. Dvorak HF. Dvorak AM. Ultrastructural studies define soluble macromolecular, particulate, and cellular transendothelial cell pathways in venules, lymphatic vessels, and tumor-associated microvessels in man and animals. Microsc Res Tech. 2002;57:289–326. doi: 10.1002/jemt.10087. [DOI] [PubMed] [Google Scholar]
- 50.Feng D. Nagy JA. Pyne K. Dvorak HF. Dvorak AM. Neutrophils emigrate from venules by a transendothelial cell pathway in response to FMLP. J Exp Med. 1998;187:903–915. doi: 10.1084/jem.187.6.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fichtner F. Koslowski R. Augstein A. Hempel U. Rohlecke C. Kasper M. Bleomycin induces IL-8 and ICAM-1 expression in microvascular pulmonary endothelial cells. Exp Toxicol Pathol. 2004;55:497–503. doi: 10.1078/0940-2993-00345. [DOI] [PubMed] [Google Scholar]
- 52.Frey RS. Gao X. Javaid K. Siddiqui SS. Rahman A. Malik AB. Phosphatidylinositol 3-kinase gamma signaling through protein kinase Czeta induces NADPH oxidase-mediated oxidant generation and NF-kappaB activation in endothelial cells. J Biol Chem. 2006;281:16128–16138. doi: 10.1074/jbc.M508810200. [DOI] [PubMed] [Google Scholar]
- 53.Frey RS. Malik AB. Oxidant signaling in lung cells. Am J Physiol Lung Cell Mol Physiol. 2004;286:L1–L3. doi: 10.1152/ajplung.00337.2003. [DOI] [PubMed] [Google Scholar]
- 54.Frey RS. Rahman A. Kefer JC. Minshall RD. Malik AB. PKCzeta regulates TNF-alpha-induced activation of NADPH oxidase in endothelial cells. Circ Res. 2002;90:1012–1019. doi: 10.1161/01.res.0000017631.28815.8e. [DOI] [PubMed] [Google Scholar]
- 55.Fujita S. Puri RK. Yu ZX. Travis WD. Ferrans VJ. An ultrastructural study of in vivo interactions between lymphocytes and endothelial cells in the pathogenesis of the vascular leak syndrome induced by interleukin-2. Cancer. 1991;68:2169–2174. doi: 10.1002/1097-0142(19911115)68:10<2169::aid-cncr2820681014>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 56.Furie MB. McHugh DD. Migration of neutrophils across endothelial monolayers is stimulated by treatment of the monolayers with interleukin-1 or tumor necrosis factor-alpha. J Immunol. 1989;143:3309–3317. [PubMed] [Google Scholar]
- 57.Garcia JG. Verin AD. Herenyiova M. English D. Adherent neutrophils activate endothelial myosin light chain kinase: role in transendothelial migration. J Appl Physiol. 1998;84:1817–1821. doi: 10.1152/jappl.1998.84.5.1817. [DOI] [PubMed] [Google Scholar]
- 58.Gertzberg N. Neumann P. Rizzo V. Johnson A. NAD(P)H oxidase mediates the endothelial barrier dysfunction induced by TNF-alpha. Am J Physiol Lung Cell Mol Physiol. 2004;286:L37–L48. doi: 10.1152/ajplung.00116.2003. [DOI] [PubMed] [Google Scholar]
- 59.Gopalan PK. Burns AR. Simon SI. Sparks S. McIntire LV. Smith CW. Preferential sites for stationary adhesion of neutrophils to cytokine-stimulated HUVEC under flow conditions. J Leukoc Biol. 2000;68:47–57. [PubMed] [Google Scholar]
- 60.Gorbunov NV. Das DK. Goswami SK. Gurusamy N. Atkins JL. Spatial coordination of cell-adhesion molecules and redox cycling of iron in the microvascular inflammatory response to pulmonary injury. Antioxid Redox Signal. 2007;9:483–495. doi: 10.1089/ars.2006.1296. [DOI] [PubMed] [Google Scholar]
- 61.Green CE. Pearson DN. Camphausen RT. Staunton DE. Simon SI. Shear-dependent capping of L-selectin and P-selectin glycoprotein ligand 1 by E-selectin signals activation of high-avidity beta2-integrin on neutrophils. J Immunol. 2004;172:7780–7790. doi: 10.4049/jimmunol.172.12.7780. [DOI] [PubMed] [Google Scholar]
- 62.Greenwood J. Amos CL. Walters CE. Couraud PO. Lyck R. Engelhardt B. Adamson P. Intracellular domain of brain endothelial intercellular adhesion molecule-1 is essential for T lymphocyte-mediated signaling and migration. J Immunol. 2003;171:2099–2108. doi: 10.4049/jimmunol.171.4.2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Greenwood J. Etienne–Manneville S. Adamson P. Couraud PO. Lymphocyte migration into the central nervous system: Implication of ICAM-1 signalling at the blood-brain barrier. Vascul Pharmacol. 2002;38:315–322. doi: 10.1016/s1537-1891(02)00199-4. [DOI] [PubMed] [Google Scholar]
- 64.Greenwood J. Howes R. Lightman S. The blood-retinal barrier in experimental autoimmune uveoretinitis. Leukocyte interactions and functional damage. Lab Invest. 1994;70:39–52. [PubMed] [Google Scholar]
- 65.Guo ZJ. Niu HX. Hou FF. Zhang L. Fu N. Nagai R. Lu X. Chen BH. Shan YX. Tian JW. Nagaraj RH. Xie D. Zhang X. Advanced oxidation protein products activate vascular endothelial cells via a RAGE-mediated signaling pathway. Antioxid Redox Signal. 2008;10:1699–1712. doi: 10.1089/ars.2007.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Harcourt BH. Rota PA. Hummel KB. Bellini WJ. Offermann MK. Induction of intercellular adhesion molecule 1 gene expression by measles virus in human umbilical vein endothelial cells. J Med Virol. 1999;57:9–16. doi: 10.1002/(sici)1096-9071(199901)57:1<9::aid-jmv2>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 67.Hayden MS. Ghosh S. Signaling to NF-kappaB. Genes Dev. 2004;18:2195–2224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- 68.Heiska L. Alfthan K. Gronholm M. Vilja P. Vaheri A. Carpen O. Association of ezrin with intercellular adhesion molecule-1 and -2 (ICAM-1 and ICAM-2). Regulation by phosphatidylinositol 4, 5-bisphosphate. J Biol Chem. 1998;273:21893–21900. doi: 10.1074/jbc.273.34.21893. [DOI] [PubMed] [Google Scholar]
- 69.Hill CS. Wynne J. Treisman R. The Rho family GTPases RhoA, Rac1, and CDC42Hs regulate transcriptional activation by SRF. Cell. 1995;81:1159–1170. doi: 10.1016/s0092-8674(05)80020-0. [DOI] [PubMed] [Google Scholar]
- 70.Hixenbaugh EA. Goeckeler ZM. Papaiya NN. Wysolmerski RB. Silverstein SC. Huang AJ. Stimulated neutrophils induce myosin light chain phosphorylation and isometric tension in endothelial cells. Am J Physiol. 1997;273:H981–H988. doi: 10.1152/ajpheart.1997.273.2.H981. [DOI] [PubMed] [Google Scholar]
- 71.Hordijk PL. Endothelial signalling events during leukocyte transmigration. FEBS J. 2006;273:4408–4415. doi: 10.1111/j.1742-4658.2006.05440.x. [DOI] [PubMed] [Google Scholar]
- 72.Huang AJ. Manning JE. Bandak TM. Ratau MC. Hanser KR. Silverstein SC. Endothelial cell cytosolic free calcium regulates neutrophil migration across monolayers of endothelial cells. J Cell Biol. 1993;120:1371–1380. doi: 10.1083/jcb.120.6.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hubbard AK. Rothlein R. Intercellular adhesion molecule-1 (ICAM-1) expression and cell signaling cascades. Free Radic Biol Med. 2000;28:1379–1386. doi: 10.1016/s0891-5849(00)00223-9. [DOI] [PubMed] [Google Scholar]
- 74.Javaid K. Rahman A. Anwar KN. Frey RS. Minshall RD. Malik AB. Tumor necrosis factor-alpha induces early-onset endothelial adhesivity by protein kinase Czeta-dependent activation of intercellular adhesion molecule-1. Circ Res. 2003;92:1089–1097. doi: 10.1161/01.RES.0000072971.88704.CB. [DOI] [PubMed] [Google Scholar]
- 75.Jersmann HP. Hii CS. Ferrante JV. Ferrante A. Bacterial lipopolysaccharide and tumor necrosis factor alpha synergistically increase expression of human endothelial adhesion molecules through activation of NF-kappaB and p38 mitogen-activated protein kinase signaling pathways. Infect Immun. 2001;69:1273–1279. doi: 10.1128/IAI.69.3.1273-1279.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jones DA. Abbassi O. McIntire LV. McEver RP. Smith CW. P-selectin mediates neutrophil rolling on histamine-stimulated endothelial cells. Biophys J. 1993;65:1560–1569. doi: 10.1016/S0006-3495(93)81195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jun CD. Carman CV. Redick SD. Shimaoka M. Erickson HP. Springer TA. Ultrastructure and function of dimeric, soluble intercellular adhesion molecule-1 (ICAM-1) J Biol Chem. 2001;276:29019–29027. doi: 10.1074/jbc.M103394200. [DOI] [PubMed] [Google Scholar]
- 78.Kang JS. Yoon YD. Han MH. Han SB. Lee K. Lee KH. Park SK. Kim HM. Glabridin suppresses intercellular adhesion molecule-1 expression in tumor necrosis factor-alpha-stimulated human umbilical vein endothelial cells by blocking sphingosine kinase pathway: implications of Akt, extracellular signal-regulated kinase, and nuclear factor-kappaB/Rel signaling pathways. Mol Pharmacol. 2006;69:941–949. doi: 10.1124/mol.105.017442. [DOI] [PubMed] [Google Scholar]
- 79.Kansas GS. Selectins and their ligands: Current concepts and controversies. Blood. 1996;88:3259–3287. [PubMed] [Google Scholar]
- 80.Karin M. Ben–Neriah Y. Phosphorylation meets ubiquitination: The control of NF-[kappa]B activity. Annu Rev Immunol. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- 81.Kelly SA. Goldschmidt–Clermont PJ. Milliken EE. Arai T. Smith EH. Bulkley GB. Protein tyrosine phosphorylation mediates TNF-induced endothelial-neutrophil adhesion in vitro. Am J Physiol. 1998;274:H513–H519. doi: 10.1152/ajpheart.1998.274.2.H513. [DOI] [PubMed] [Google Scholar]
- 82.Kim I. Moon SO. Kim SH. Kim HJ. Koh YS. Koh GY. Vascular endothelial growth factor expression of intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), and E-selectin through nuclear factor-kappa B activation in endothelial cells. J Biol Chem. 2001;276:7614–7620. doi: 10.1074/jbc.M009705200. [DOI] [PubMed] [Google Scholar]
- 83.Kim W. Moon SO. Lee S. Sung MJ. Kim SH. Park SK. Adrenomedullin reduces VEGF-induced endothelial adhesion molecules and adhesiveness through a phosphatidylinositol 3′-kinase pathway. Arterioscler Thromb Vasc Biol. 2003;23:1377–1383. doi: 10.1161/01.ATV.0000081740.65173.D1. [DOI] [PubMed] [Google Scholar]
- 84.Kim YS. Ahn Y. Hong MH. Kim KH. Park HW. Hong YJ. Kim JH. Kim W. Jeong MH. Cho JG. Park JC. Kang JC. Rosuvastatin suppresses the inflammatory responses through inhibition of c-Jun N-terminal kinase and Nuclear Factor-kappaB in endothelial cells. J Cardiovasc Pharmacol. 2007;49:376–383. doi: 10.1097/FJC.0b013e31804a5e34. [DOI] [PubMed] [Google Scholar]
- 85.Kobuchi H. Roy S. Sen CK. Nguyen HG. Packer L. Quercetin inhibits inducible ICAM-1 expression in human endothelial cells through the JNK pathway. Am J Physiol. 1999;277:C403–C411. doi: 10.1152/ajpcell.1999.277.3.C403. [DOI] [PubMed] [Google Scholar]
- 86.Konstantopoulos K. Neelamegham S. Burns AR. Hentzen E. Kansas GS. Snapp KR. Berg EL. Hellums JD. Smith CW. McIntire LV. Simon SI. Venous levels of shear support neutrophil-platelet adhesion and neutrophil aggregation in blood via P-selectin and beta2-integrin. Circulation. 1998;98:873–882. doi: 10.1161/01.cir.98.9.873. [DOI] [PubMed] [Google Scholar]
- 87.Lane TA. Lamkin GE. Wancewicz EV. Protein kinase C inhibitors block the enhanced expression of intercellular adhesion molecule-1 on endothelial cells activated by interleukin-1, lipopolysaccharide and tumor necrosis factor. Biochem Biophys Res Commun. 1990;172:1273–1281. doi: 10.1016/0006-291x(90)91587-i. [DOI] [PubMed] [Google Scholar]
- 88.Lawrence MB. Springer TA. Neutrophils roll on E-selectin. J Immunol. 1993;151:6338–6346. [PubMed] [Google Scholar]
- 89.Lawson C. Ainsworth M. Yacoub M. Rose M. Ligation of ICAM-1 on endothelial cells leads to expression of VCAM-1 via a nuclear factor-kappaB-independent mechanism. J Immunol. 1999;162:2990–2996. [PubMed] [Google Scholar]
- 90.Ledebur HC. Parks TP. Transcriptional regulation of the intercellular adhesion molecule-1 gene by inflammatory cytokines in human endothelial cells. Essential roles of a variant NF-kappa B site and p65 homodimers. J Biol Chem. 1995;270:933–943. doi: 10.1074/jbc.270.2.933. [DOI] [PubMed] [Google Scholar]
- 91.Li JM. Fan LM. Christie MR. Shah AM. Acute tumor necrosis factor alpha signaling via NADPH oxidase in microvascular endothelial cells: Role of p47phox phosphorylation and binding to TRAF4. Mol Cell Biol. 2005;25:2320–2330. doi: 10.1128/MCB.25.6.2320-2330.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li JM. Mullen AM. Yun S. Wientjes F. Brouns GY. Thrasher AJ. Shah AM. Essential role of the NADPH oxidase subunit p47(phox) in endothelial cell superoxide production in response to phorbol ester and tumor necrosis factor-alpha. Circ Res. 2002;90:143–150. doi: 10.1161/hh0202.103615. [DOI] [PubMed] [Google Scholar]
- 93.Lin SJ. Shyue SK. Hung YY. Chen YH. Ku HH. Chen JW. Tam KB. Chen YL. Superoxide dismutase inhibits the expression of vascular cell adhesion molecule-1 and intracellular cell adhesion molecule-1 induced by tumor necrosis factor-alpha in human endothelial cells through the JNK/p38 pathways. Arterioscler Thromb Vasc Biol. 2005;25:334–340. doi: 10.1161/01.ATV.0000152114.00114.d8. [DOI] [PubMed] [Google Scholar]
- 94.Lum H. Roebuck KA. Oxidant stress and endothelial cell dysfunction. Am J Physiol Cell Physiol. 2001;280:C719–C741. doi: 10.1152/ajpcell.2001.280.4.C719. [DOI] [PubMed] [Google Scholar]
- 95.Lyck R. Reiss Y. Gerwin N. Greenwood J. Adamson P. Engelhardt B. T-cell interaction with ICAM-1/ICAM-2 double-deficient brain endothelium in vitro: The cytoplasmic tail of endothelial ICAM-1 is necessary for transendothelial migration of T cells. Blood. 2003;102:3675–3683. doi: 10.1182/blood-2003-02-0358. [DOI] [PubMed] [Google Scholar]
- 96.Maeng YS. Min JK. Kim JH. Yamagishi A. Mochizuki N. Kwon JY. Park YW. Kim YM. Kwon YG. ERK is an anti-inflammatory signal that suppresses expression of NF-kappaB-dependent inflammatory genes by inhibiting IKK activity in endothelial cells. Cell Signal. 2006;18:994–1005. doi: 10.1016/j.cellsig.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 97.Malik AB. Lo SK. Vascular endothelial adhesion molecules and tissue inflammation. Pharmacol Rev. 1996;48:213–229. [PubMed] [Google Scholar]
- 98.Manduteanu I. Dragomir E. Voinea M. Capraru M. Simionescu M. Enoxaparin reduces H2O2-induced activation of human endothelial cells by a mechanism involving cell adhesion molecules and nuclear transcription factors. Pharmacology. 2007;79:154–162. doi: 10.1159/000098952. [DOI] [PubMed] [Google Scholar]
- 99.Marchesi VT. Gowans JL. The migration of lymphocytes through the endothelium of venules in lymph nodes: An electron microscope study. Proc R Soc Lond B Biol Sci. 1964;159:283–290. doi: 10.1098/rspb.1964.0002. [DOI] [PubMed] [Google Scholar]
- 100.Marlin SD. Springer TA. Purified intercellular adhesion molecule-1 (ICAM-1) is a ligand for lymphocyte function-associated antigen 1 (LFA-1) Cell. 1987;51:813–819. doi: 10.1016/0092-8674(87)90104-8. [DOI] [PubMed] [Google Scholar]
- 101.Mercurio F. Zhu H. Murray BW. Shevchenko A. Bennett BL. Li J. Young DB. Barbosa M. Mann M. Manning A. Rao A. IKK-1 and IKK-2: cytokine-activated IkappaB kinases essential for NF-kappaB activation. Science. 1997;278:860–866. doi: 10.1126/science.278.5339.860. [DOI] [PubMed] [Google Scholar]
- 102.Miho N. Ishida T. Kuwaba N. Ishida M. Shimote–Abe K. Tabuchi K. Oshima T. Yoshizumi M. Chayama K. Role of the JNK pathway in thrombin-induced ICAM-1 expression in endothelial cells. Cardiovasc Res. 2005;68:289–298. doi: 10.1016/j.cardiores.2005.05.029. [DOI] [PubMed] [Google Scholar]
- 103.Millan J. Hewlett L. Glyn M. Toomre D. Clark P. Ridley AJ. Lymphocyte transcellular migration occurs through recruitment of endothelial ICAM-1 to caveola- and F-actin-rich domains. Nat Cell Biol. 2006;8:113–123. doi: 10.1038/ncb1356. [DOI] [PubMed] [Google Scholar]
- 104.Min JK. Kim YM. Kim SW. Kwon MC. Kong YY. Hwang IK. Won MH. Rho J. Kwon YG. TNF-related activation-induced cytokine enhances leukocyte adhesiveness: induction of ICAM-1 and VCAM-1 via TNF receptor-associated factor and protein kinase C-dependent NF-kappaB activation in endothelial cells. J Immunol. 2005;175:531–540. doi: 10.4049/jimmunol.175.1.531. [DOI] [PubMed] [Google Scholar]
- 105.Minami T. Aird WC. Endothelial cell gene regulation. Trends Cardiovasc Med. 2005;15:174–184. doi: 10.1016/j.tcm.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 106.Modur V. Zimmerman GA. Prescott SM. McIntyre TM. Endothelial cell inflammatory responses to tumor necrosis factor alpha. Ceramide-dependent and -independent mitogen-activated protein kinase cascades. J Biol Chem. 1996;271:13094–13102. doi: 10.1074/jbc.271.22.13094. [DOI] [PubMed] [Google Scholar]
- 107.Montaner S. Perona R. Saniger L. Lacal JC. Activation of serum response factor by RhoA is mediated by the nuclear factor-kappaB and C/EBP transcription factors. J Biol Chem. 1999;274:8506–8515. doi: 10.1074/jbc.274.13.8506. [DOI] [PubMed] [Google Scholar]
- 108.Nieminen M. Henttinen T. Merinen M. Marttila–Ichihara F. Eriksson JE. Jalkanen S. Vimentin function in lymphocyte adhesion and transcellular migration. Nat Cell Biol. 2006;8:156–162. doi: 10.1038/ncb1355. [DOI] [PubMed] [Google Scholar]
- 109.Nizamutdinova IT. Oh HM. Min YN. Park SH. Lee MJ. Kim JS. Yean MH. Kang SS. Kim YS. Chang KC. Kim HJ. Paeonol suppresses intercellular adhesion molecule-1 expression in tumor necrosis factor-alpha-stimulated human umbilical vein endothelial cells by blocking p38, ERK and nuclear factor-kappaB signaling pathways. Int Immunopharmacol. 2007;7:343–350. doi: 10.1016/j.intimp.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 110.Oh HM. Lee S. Na BR. Wee H. Kim SH. Choi SC. Lee KM. Jun CD. RKIKK motif in the intracellular domain is critical for spatial and dynamic organization of ICAM-1: Functional implication for the leukocyte adhesion and transmigration. Mol Biol Cell. 2007;18:2322–2335. doi: 10.1091/mbc.E06-08-0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Papatheodorou L. Weiss N. Vascular oxidant stress and inflammation in hyperhomocysteinemia. Antioxid Redox Signal. 2007;9:1941–1958. doi: 10.1089/ars.2007.1750. [DOI] [PubMed] [Google Scholar]
- 112.Park HS. Chun JN. Jung HY. Choi C. Bae YS. Role of NADPH oxidase 4 in lipopolysaccharide-induced proinflammatory responses by human aortic endothelial cells. Cardiovasc Res. 2006;72:447–455. doi: 10.1016/j.cardiores.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 113.Petrache I. Crow MT. Neuss M. Garcia JG. Central involvement of Rho family GTPases in TNF-alpha-mediated bovine pulmonary endothelial cell apoptosis. Biochem Biophys Res Commun. 2003;306:244–249. doi: 10.1016/s0006-291x(03)00945-8. [DOI] [PubMed] [Google Scholar]
- 114.Petri B. Bixel MG. Molecular events during leukocyte diapedesis. FEBS J. 2006;273:4399–4407. doi: 10.1111/j.1742-4658.2006.05439.x. [DOI] [PubMed] [Google Scholar]
- 115.Pluskota E. Chen Y. D'Souza SE. Src homology domain 2-containing tyrosine phosphatase 2 associates with intercellular adhesion molecule 1 to regulate cell survival. J Biol Chem. 2000;275:30029–30036. doi: 10.1074/jbc.M000240200. [DOI] [PubMed] [Google Scholar]
- 116.Pluskota E. D'Souza SE. Fibrinogen interactions with ICAM-1 (CD54) regulate endothelial cell survival. Eur J Biochem. 2000;267:4693–4704. doi: 10.1046/j.1432-1327.2000.01520.x. [DOI] [PubMed] [Google Scholar]
- 117.Pruitt HM. Langston W. Kevil CG. Patel RP. ICAM-1 cross-linking stimulates endothelial glutathione synthesis. Antioxid Redox Signal. 2007;9:159–164. doi: 10.1089/ars.2007.9.159. [DOI] [PubMed] [Google Scholar]
- 118.Qin P. Tang X. Elloso MM. Harnish DC. Bile acids induce adhesion molecule expression in endothelial cells through activation of reactive oxygen species, NF-kappaB, and p38. Am J Physiol Heart Circ Physiol. 2006;291:H741–H747. doi: 10.1152/ajpheart.01182.2005. [DOI] [PubMed] [Google Scholar]
- 119.Quagliaro L. Piconi L. Assaloni R. Da Ros R. Maier A. Zuodar G. Ceriello A. Intermittent high glucose enhances ICAM-1, VCAM-1 and E-selectin expression in human umbilical vein endothelial cells in culture: The distinct role of protein kinase C and mitochondrial superoxide production. Atherosclerosis. 2005;183:259–267. doi: 10.1016/j.atherosclerosis.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 120.Radisavljevic Z. Avraham H. Avraham S. Vascular endothelial growth factor up-regulates ICAM-1 expression via the phosphatidylinositol 3 OH-kinase/AKT/Nitric oxide pathway and modulates migration of brain microvascular endothelial cells. J Biol Chem. 2000;275:20770–20774. doi: 10.1074/jbc.M002448200. [DOI] [PubMed] [Google Scholar]
- 121.Rahman A. Anwar KN. Malik AB. Protein kinase C-zeta mediates TNF-alpha-induced ICAM-1 gene transcription in endothelial cells. Am J Physiol Cell Physiol. 2000;279:C906–C914. doi: 10.1152/ajpcell.2000.279.4.C906. [DOI] [PubMed] [Google Scholar]
- 122.Rahman A. Anwar KN. Minhajuddin M. Bijli KM. Javaid K. True AL. Malik AB. cAMP targeting of p38 MAP kinase inhibits thrombin-induced NF-kappaB activation and ICAM-1 expression in endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2004;287:L1017–L1024. doi: 10.1152/ajplung.00072.2004. [DOI] [PubMed] [Google Scholar]
- 123.Rahman A. Anwar KN. True AL. Malik AB. Thrombin-induced p65 homodimer binding to downstream NF-kappa B site of the promoter mediates endothelial ICAM-1 expression and neutrophil adhesion. J Immunol. 1999;162:5466–5476. [PubMed] [Google Scholar]
- 124.Rahman A. Anwar KN. Uddin S. Xu N. Ye RD. Platanias LC. Malik AB. Protein kinase C-delta regulates thrombin-induced ICAM-1 gene expression in endothelial cells via activation of p38 mitogen-activated protein kinase. Mol Cell Biol. 2001;21:5554–5565. doi: 10.1128/MCB.21.16.5554-5565.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rahman A. Bando M. Kefer J. Anwar KN. Malik AB. Protein kinase C-activated oxidant generation in endothelial cells signals intercellular adhesion molecule-1 gene transcription. Mol Pharmacol. 1999;55:575–583. [PubMed] [Google Scholar]
- 126.Rahman A. True AL. Anwar KN. Ye RD. Voyno–Yasenetskaya TA. Malik AB. Galpha(q) and Gbetagamma regulate PAR-1 signaling of thrombin-induced NF-kappaB activation and ICAM-1 transcription in endothelial cells. Circ Res. 2002;91:398–405. doi: 10.1161/01.res.0000033520.95242.a2. [DOI] [PubMed] [Google Scholar]
- 127.Raine CS. Cannella B. Duijvestijn AM. Cross AH. Homing to central nervous system vasculature by antigen-specific lymphocytes. II. Lymphocyte/endothelial cell adhesion during the initial stages of autoimmune demyelination. Lab Invest. 1990;63:476–489. [PubMed] [Google Scholar]
- 128.Roebuck KA. Oxidant stress regulation of IL-8 and ICAM-1 gene expression: Differential activation and binding of the transcription factors AP-1 and NF-kappaB (Review) Int J Mol Med. 1999;4:223–230. doi: 10.3892/ijmm.4.3.223. [DOI] [PubMed] [Google Scholar]
- 129.Roebuck KA. Finnegan A. Regulation of intercellular adhesion molecule-1 (CD54) gene expression. J Leukoc Biol. 1999;66:876–888. doi: 10.1002/jlb.66.6.876. [DOI] [PubMed] [Google Scholar]
- 130.Roebuck KA. Rahman A. Lakshminarayanan V. Janakidevi K. Malik AB. H2O2 and tumor necrosis factor-alpha activate intercellular adhesion molecule 1 (ICAM-1) gene transcription through distinct cis-regulatory elements within the ICAM-1 promoter. J Biol Chem. 1995;270:18966–18974. doi: 10.1074/jbc.270.32.18966. [DOI] [PubMed] [Google Scholar]
- 131.Ross R. Atherosclerosis–an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 132.Saito H. Minamiya Y. Kitamura M. Saito S. Enomoto K. Terada K. Ogawa J. Endothelial myosin light chain kinase regulates neutrophil migration across human umbilical vein endothelial cell monolayer. J Immunol. 1998;161:1533–1540. [PubMed] [Google Scholar]
- 133.Sakurai H. Chiba H. Miyoshi H. Sugita T. Toriumi W. IkappaB kinases phosphorylate NF-kappaB p65 subunit on serine 536 in the transactivation domain. J Biol Chem. 1999;274:30353–30356. doi: 10.1074/jbc.274.43.30353. [DOI] [PubMed] [Google Scholar]
- 134.Sano H. Nakagawa N. Chiba R. Kurasawa K. Saito Y. Iwamoto I. Cross-linking of intercellular adhesion molecule-1 induces interleukin-8 and RANTES production through the activation of MAP kinases in human vascular endothelial cells. Biochem Biophys Res Commun. 1998;250:694–698. doi: 10.1006/bbrc.1998.9385. [DOI] [PubMed] [Google Scholar]
- 135.Sans E. Delachanal E. Duperray A. Analysis of the roles of ICAM-1 in neutrophil transmigration using a reconstituted mammalian cell expression model: Implication of ICAM-1 cytoplasmic domain and Rho-dependent signaling pathway. J Immunol. 2001;166:544–551. doi: 10.4049/jimmunol.166.1.544. [DOI] [PubMed] [Google Scholar]
- 136.Schofield L. Novakovic S. Gerold P. Schwarz RT. McConville MJ. Tachado SD. Glycosylphosphatidylinositol toxin of Plasmodium upregulates intercellular adhesion molecule-1, vascular cell adhesion molecule-1, and E-selectin expression in vascular endothelial cells and increases leukocyte and parasite cytoadherence via tyrosine kinase-dependent signal transduction. J Immunol. 1996;156:1886–1896. [PubMed] [Google Scholar]
- 137.Shimamura K. Takashiro Y. Akiyama N. Hirabayashi T. Murayama T. Expression of adhesion molecules by sphingosine 1-phosphate and histamine in endothelial cells. Eur J Pharmacol. 2004;486:141–150. doi: 10.1016/j.ejphar.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 138.Simon SI. Goldsmith HL. Leukocyte adhesion dynamics in shear flow. Ann Biomed Eng. 2002;30:315–332. doi: 10.1114/1.1467677. [DOI] [PubMed] [Google Scholar]
- 139.Simon SI. Green CE. Molecular mechanics and dynamics of leukocyte recruitment during inflammation. Annu Rev Biomed Eng. 2005;7:151–185. doi: 10.1146/annurev.bioeng.7.060804.100423. [DOI] [PubMed] [Google Scholar]
- 140.Simon SI. Rochon YP. Lynam EB. Smith CW. Anderson DC. Sklar LA. Beta 2-integrin and L-selectin are obligatory receptors in neutrophil aggregation. Blood. 1993;82:1097–1106. [PubMed] [Google Scholar]
- 141.Sithu SD. English WR. Olson P. Krubasik D. Baker AH. Murphy G. D'Souza SE. Membrane-type 1-matrix metalloproteinase regulates intracellular adhesion molecule-1 (ICAM-1)-mediated monocyte transmigration. J Biol Chem. 2007;282:25010–25019. doi: 10.1074/jbc.M611273200. [DOI] [PubMed] [Google Scholar]
- 142.Smith CW. Marlin SD. Rothlein R. Toman C. Anderson DC. Cooperative interactions of LFA-1 and Mac-1 with intercellular adhesion molecule-1 in facilitating adherence and transendothelial migration of human neutrophils in vitro. J Clin Invest. 1989;83:2008–2017. doi: 10.1172/JCI114111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Smith CW. Rothlein R. Hughes BJ. Mariscalco MM. Rudloff HE. Schmalstieg FC. Anderson DC. Recognition of an endothelial determinant for CD 18-dependent human neutrophil adherence and transendothelial migration. J Clin Invest. 1988;82:1746–1756. doi: 10.1172/JCI113788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Son EW. Mo SJ. Rhee DK. Pyo S. Inhibition of ICAM-1 expression by garlic component, allicin, in gamma-irradiated human vascular endothelial cells via downregulation of the JNK signaling pathway. Int Immunopharmacol. 2006;6:1788–1795. doi: 10.1016/j.intimp.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 145.Sorescu GP. Song H. Tressel SL. Hwang J. Dikalov S. Smith DA. Boyd NL. Platt MO. Lassegue B. Griendling KK. Jo H. Bone morphogenic protein 4 produced in endothelial cells by oscillatory shear stress induces monocyte adhesion by stimulating reactive oxygen species production from a nox1-based NADPH oxidase. Circ Res. 2004;95:773–779. doi: 10.1161/01.RES.0000145728.22878.45. [DOI] [PubMed] [Google Scholar]
- 146.Springer TA. Adhesion receptors of the immune system. Nature. 1990;346:425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- 147.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 148.Staunton DE. Marlin SD. Stratowa C. Dustin ML. Springer TA. Primary structure of ICAM-1 demonstrates interaction between members of the immunoglobulin and integrin supergene families. Cell. 1988;52:925–933. doi: 10.1016/0092-8674(88)90434-5. [DOI] [PubMed] [Google Scholar]
- 149.Staunton DE. Merluzzi VJ. Rothlein R. Barton R. Marlin SD. Springer TA. A cell adhesion molecule, ICAM-1, is the major surface receptor for rhinoviruses. Cell. 1989;56:849–853. doi: 10.1016/0092-8674(89)90689-2. [DOI] [PubMed] [Google Scholar]
- 150.Sung CP. Arleth AJ. Nambi P. Evidence for involvement of protein kinase C in expression of intracellular adhesion molecule-1 (ICAM-1) by human vascular endothelial cells. Pharmacology. 1994;48:143–146. doi: 10.1159/000139173. [DOI] [PubMed] [Google Scholar]
- 151.Takeuchi S. Kawashima S. Rikitake Y. Ueyama T. Inoue N. Hirata K. Yokoyama M. Cerivastatin suppresses lipopolysaccharide-induced ICAM-1 expression through inhibition of Rho GTPase in BAEC. Biochem Biophys Res Commun. 2000;269:97–102. doi: 10.1006/bbrc.2000.2238. [DOI] [PubMed] [Google Scholar]
- 152.Tamura DY. Moore EE. Johnson JL. Zallen G. Aiboshi J. Silliman CC. p38 mitogen-activated protein kinase inhibition attenuates intercellular adhesion molecule-1 up-regulation on human pulmonary microvascular endothelial cells. Surgery. 1998;124:403–407. discussion 408. [PubMed] [Google Scholar]
- 153.Thompson PW. Randi AM. Ridley AJ. Intercellular adhesion molecule (ICAM)-1, but not ICAM-2, activates RhoA and stimulates c-fos and rhoA transcription in endothelial cells. J Immunol. 2002;169:1007–1013. doi: 10.4049/jimmunol.169.2.1007. [DOI] [PubMed] [Google Scholar]
- 154.Tsakadze NL. Zhao Z. D'Souza SE. Interactions of intercellular adhesion molecule-1 with fibrinogen. Trends Cardiovasc Med. 2002;12:101–108. doi: 10.1016/s1050-1738(01)00157-8. [DOI] [PubMed] [Google Scholar]
- 155.Tzima E. Del Pozo MA. Kiosses WB. Mohamed SA. Li S. Chien S. Schwartz MA. Activation of Rac1 by shear stress in endothelial cells mediates both cytoskeletal reorganization and effects on gene expression. EMBO J. 2002;21:6791–6800. doi: 10.1093/emboj/cdf688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ueno H. Pradhan S. Schlessel D. Hirasawa H. Sumpio BE. Nicotine enhances human vascular endothelial cell expression of ICAM-1 and VCAM-1 via protein kinase C, p38 mitogen-activated protein kinase, NF-kappaB, and AP-1. Cardiovasc Toxicol. 2006;6:39–50. doi: 10.1385/ct:6:1:39. [DOI] [PubMed] [Google Scholar]
- 157.Vermeulen L. De Wilde G. Van Damme P. Vanden Berghe W. Haegeman G. Transcriptional activation of the NF-kappaB p65 subunit by mitogen- and stress-activated protein kinase-1 (MSK1) EMBO J. 2003;22:1313–1324. doi: 10.1093/emboj/cdg139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Vielma SA. Krings G. Lopes–Virella MF. Chlamydophila pneumoniae induces ICAM-1 expression in human aortic endothelial cells via protein kinase C-dependent activation of nuclear factor-kappaB. Circ Res. 2003;92:1130–1137. doi: 10.1161/01.RES.0000074001.46892.1C. [DOI] [PubMed] [Google Scholar]
- 159.Vogetseder W. Dierich MP. Intercellular adhesion molecule-1 (ICAM-1, CD 54) is associated with actin-filaments. Immunobiology. 1991;182:143–151. doi: 10.1016/S0171-2985(11)80198-1. [DOI] [PubMed] [Google Scholar]
- 160.Voraberger G. Schafer R. Stratowa C. Cloning of the human gene for intercellular adhesion molecule 1 and analysis of its 5′-regulatory region. Induction by cytokines and phorbol ester. J Immunol. 1991;147:2777–2786. [PubMed] [Google Scholar]
- 161.Wakabayashi Y. Fujita H. Morita I. Kawaguchi H. Murota S. Conversion of xanthine dehydrogenase to xanthine oxidase in bovine carotid artery endothelial cells induced by activated neutrophils:Involvement of adhesion molecules. Biochim Biophys Acta. 1995;1265:103–109. doi: 10.1016/0167-4889(94)00202-p. [DOI] [PubMed] [Google Scholar]
- 162.Wang D. Westerheide SD. Hanson JL. Baldwin AS., Jr. Tumor necrosis factor alpha-induced phosphorylation of RelA/p65 on Ser529 is controlled by casein kinase II. J Biol Chem. 2000;275:32592–32597. doi: 10.1074/jbc.M001358200. [DOI] [PubMed] [Google Scholar]
- 163.Wang Q. Chiang ET. Lim M. Lai J. Rogers R. Janmey PA. Shepro D. Doerschuk CM. Changes in the biomechanical properties of neutrophils and endothelial cells during adhesion. Blood. 2001;97:660–668. doi: 10.1182/blood.v97.3.660. [DOI] [PubMed] [Google Scholar]
- 164.Wang Q. Doerschuk CM. Neutrophil-induced changes in the biomechanical properties of endothelial cells: roles of ICAM-1 and reactive oxygen species. J Immunol. 2000;164:6487–6494. doi: 10.4049/jimmunol.164.12.6487. [DOI] [PubMed] [Google Scholar]
- 165.Wang Q. Doerschuk CM. The p38 mitogen-activated protein kinase mediates cytoskeletal remodeling in pulmonary microvascular endothelial cells upon intracellular adhesion molecule-1 ligation. J Immunol. 2001;166:6877–6884. doi: 10.4049/jimmunol.166.11.6877. [DOI] [PubMed] [Google Scholar]
- 166.Wang Q. Doerschuk CM. The signaling pathways induced by neutrophil-endothelial cell adhesion. Antioxid Redox Signal. 2002;4:39–47. doi: 10.1089/152308602753625843. [DOI] [PubMed] [Google Scholar]
- 167.Wang Q. Pfeiffer GR., 2nd Gaarde WA. Activation of SRC tyrosine kinases in response to ICAM-1 ligation in pulmonary microvascular endothelial cells. J Biol Chem. 2003;278:47731–47743. doi: 10.1074/jbc.M308466200. [DOI] [PubMed] [Google Scholar]
- 168.Wang Q. Yerukhimovich M. Gaarde WA. Popoff IJ. Doerschuk CM. MKK3 and -6-dependent activation of p38alpha MAP kinase is required for cytoskeletal changes in pulmonary microvascular endothelial cells induced by ICAM-1 ligation. Am J Physiol Lung Cell Mol Physiol. 2005;288:L359–L369. doi: 10.1152/ajplung.00292.2004. [DOI] [PubMed] [Google Scholar]
- 169.Watson T. Goon PK. Lip GY. Endothelial progenitor cells, endothelial dysfunction, inflammation, and oxidative stress in hypertension. Antioxid Redox Signal. 2008;10:1079–1088. doi: 10.1089/ars.2007.1998. [DOI] [PubMed] [Google Scholar]
- 170.Wawryk SO. Cockerill PN. Wicks IP. Boyd AW. Isolation and characterization of the promoter region of the human intercellular adhesion molecule-1 gene. Int Immunol. 1991;3:83–93. doi: 10.1093/intimm/3.1.83. [DOI] [PubMed] [Google Scholar]
- 171.Weber C. Negrescu E. Erl W. Pietsch A. Frankenberger M. Ziegler-Heitbrock HW. Siess W. Weber PC. Inhibitors of protein tyrosine kinase suppress TNF-stimulated induction of endothelial cell adhesion molecules. J Immunol. 1995;155:445–451. [PubMed] [Google Scholar]
- 172.Whiteside ST. Israel A. I kappa B proteins: structure, function and regulation. Semin Cancer Biol. 1997;8:75–82. doi: 10.1006/scbi.1997.0058. [DOI] [PubMed] [Google Scholar]
- 173.Williamson JR. Grisham JW. Electron microscopy of leukocytic margination and emigration in acute inflammation in dog pancreas. Am J Pathol. 1961;39:239–256. [PMC free article] [PubMed] [Google Scholar]
- 174.Wojciak–Stothard B. Entwistle A. Garg R. Ridley AJ. Regulation of TNF-alpha-induced reorganization of the actin cytoskeleton and cell-cell junctions by Rho, Rac, and Cdc42 in human endothelial cells. J Cell Physiol. 1998;176:150–165. doi: 10.1002/(SICI)1097-4652(199807)176:1<150::AID-JCP17>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 175.Wojciak–Stothard B. Williams L. Ridley AJ. Monocyte adhesion and spreading on human endothelial cells is dependent on Rho-regulated receptor clustering. J Cell Biol. 1999;145:1293–1307. doi: 10.1083/jcb.145.6.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wu SQ. Aird WC. Thrombin, TNF-alpha, and LPS exert overlapping but nonidentical effects on gene expression in endothelial cells and vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 2005;289:H873–H885. doi: 10.1152/ajpheart.00993.2004. [DOI] [PubMed] [Google Scholar]
- 177.Wu Y. Ip JE. Huang J. Zhang L. Matsushita K. Liew CC. Pratt RE. Dzau VJ. Essential role of ICAM-1/CD18 in mediating EPC recruitment, angiogenesis, and repair to the infarcted myocardium. Circ Res. 2006;99:315–322. doi: 10.1161/01.RES.0000235986.35957.a3. [DOI] [PubMed] [Google Scholar]
- 178.Wung BS. Ni CW. Wang DL. ICAM-1 induction by TNFalpha and IL-6 is mediated by distinct pathways via Rac in endothelial cells. J Biomed Sci. 2005;12:91–101. doi: 10.1007/s11373-004-8170-z. [DOI] [PubMed] [Google Scholar]
- 179.Yan W. Zhao K. Jiang Y. Huang Q. Wang J. Kan W. Wang S. Role of p38 MAPK in ICAM-1 expression of vascular endothelial cells induced by lipopolysaccharide. Shock. 2002;17:433–438. doi: 10.1097/00024382-200205000-00016. [DOI] [PubMed] [Google Scholar]
- 180.Yang F. Tang E. Guan K. Wang CY. IKK beta plays an essential role in the phosphorylation of RelA/p65 on serine 536 induced by lipopolysaccharide. J Immunol. 2003;170:5630–5635. doi: 10.4049/jimmunol.170.11.5630. [DOI] [PubMed] [Google Scholar]
- 181.Yang L. Froio RM. Sciuto TE. Dvorak AM. Alon R. Luscinskas FW. ICAM-1 regulates neutrophil adhesion and transcellular migration of TNF-alpha-activated vascular endothelium under flow. Blood. 2005;106:584–592. doi: 10.1182/blood-2004-12-4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Yang L. Kowalski JR. Zhan X. Thomas SM. Luscinskas FW. Endothelial cell cortactin phosphorylation by Src contributes to polymorphonuclear leukocyte transmigration in vitro. Circ Res. 2006;98:394–402. doi: 10.1161/01.RES.0000201958.59020.1a. [DOI] [PubMed] [Google Scholar]
- 183.Yoshimoto T. Gochou N. Fukai N. Sugiyama T. Shichiri M. Hirata Y. Adrenomedullin inhibits angiotensin II-induced oxidative stress and gene expression in rat endothelial cells. Hypertens Res. 2005;28:165–172. doi: 10.1291/hypres.28.165. [DOI] [PubMed] [Google Scholar]
- 184.Yoshizumi M. Fujita Y. Izawa Y. Suzaki Y. Kyaw M. Ali N. Tsuchiya K. Kagami S. Yano S. Sone S. Tamaki T. Ebselen inhibits tumor necrosis factor-alpha-induced c-Jun N-terminal kinase activation and adhesion molecule expression in endothelial cells. Exp Cell Res. 2004;292:1–10. doi: 10.1016/j.yexcr.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 185.Zandi E. Rothwarf DM. Delhase M. Hayakawa M. Karin M. The IkappaB kinase complex (IKK) contains two kinase subunits, IKKalpha and IKKbeta, necessary for IkappaB phosphorylation and NF-kappaB activation. Cell. 1997;91:243–252. doi: 10.1016/s0092-8674(00)80406-7. [DOI] [PubMed] [Google Scholar]
- 186.Zhou Z. Connell MC. MacEwan DJ. TNFR1-induced NF-kappaB, but not ERK, p38MAPK or JNK activation, mediates TNF-induced ICAM-1 and VCAM-1 expression on endothelial cells. Cell Signal. 2007;19:1238–1248. doi: 10.1016/j.cellsig.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 187.Zhu K. Amin MA. Kim MJ. Katschke KJ., Jr. Park CC. Koch AE. A novel function for a glucose analog of blood group H antigen as a mediator of leukocyte-endothelial adhesion via intracellular adhesion molecule 1. J Biol Chem. 2003;278:21869–21877. doi: 10.1074/jbc.M213052200. [DOI] [PubMed] [Google Scholar]
- 188.Zhu Y. Lin JH. Liao HL. Verna L. Stemerman MB. Activation of ICAM-1 promoter by lysophosphatidylcholine: possible involvement of protein tyrosine kinases. Biochim Biophys Acta. 1997;1345:93–98. doi: 10.1016/s0005-2760(96)00169-5. [DOI] [PubMed] [Google Scholar]