Abstract
Background
African-Americans remain underrepresented in clinical research despite experiencing a higher burden of disease compared to all other ethnic groups in the United States. The purpose of this article is to describe the study design and discuss strategies used to recruit and retain African-American smokers in a pharmacokinetic study.
Methods
The parent study was designed to evaluate the differences in the steady-state concentrations of bupropion and its three principal metabolites between African-American menthol and non-menthol cigarette smokers. Study participation consisted of four visits at a General Clinical Research Center (GCRC) over six weeks. After meeting telephone eligibility requirements, phone-eligible participants underwent additional screening during the first two GCRC visits. The last two visits (pharmacokinetic study phase) required repeated blood draws using an intravenous catheter over the course of 12 hours.
Results
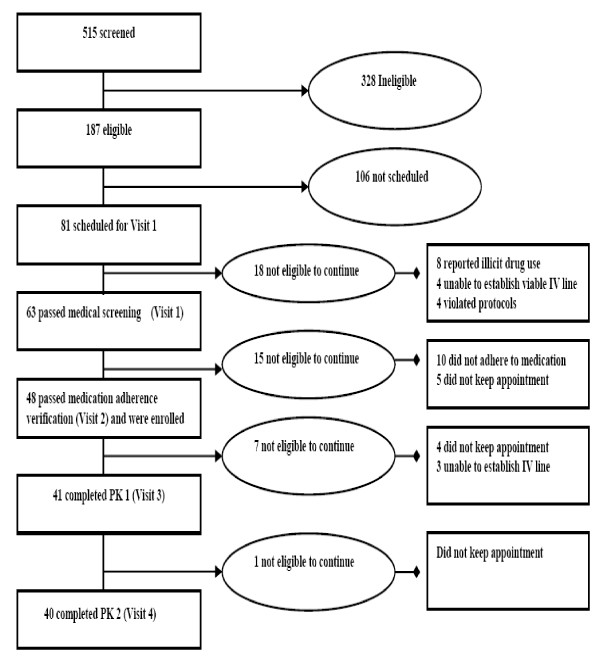
Five hundred and fifteen African-American smokers completed telephone screening; 187 were phone-eligible and 92 were scheduled for the first GCRC visit. Of the 81 who attended the first visit, 48 individuals were enrolled in the pharmacokinetic study, and a total of 40 individuals completed the study (83% retention rate).
Conclusions
Although recruitment of African-American smokers into a non-treatment, pharmacokinetic study poses challenges, retention is feasible. The results provide valuable information for investigators embarking on non-treatment laboratory-based studies among minority populations.
Background
Although African-Americans experience a disproportionately high burden of disease [1,2], they remain underrepresented in clinical research [3-14]. Clinical research studies traditionally have included predominantly White samples and only a small percentage of minority participants. Therefore, findings of these studies have limited generalizability to African-Americans or other racial/ethnic minority populations [5,9,15-18]. Reasons for low levels of participation of African-Americans in research studies have included mistrust of the medical and research communities, lack of awareness of potential benefits of study programs, and barriers related to low economic status [12,19]. While some investigators are beginning to address these barriers [20-25], recruitment and retention of African-Americans in clinical research studies remain a critical challenge [26].
Tobacco-related morbidity and mortality is higher among African-Americans compared to other racial/ethnic groups in the United States [27]. However, African-Americans remain underrepresented in smoking cessation and tobacco research [28]. While about 80% of African-American smokers smoke menthol cigarettes [29-31], smoking menthol cigarettes is associated with decreased abstinence following bupropion treatment in African-American smokers [32,33]. The purpose of the parent study was to evaluate the differences in the steady-state concentrations of bupropion and its three principal metabolites between African-American menthol and non-menthol cigarette smokers. Understanding the effect of menthol on bupropion metabolism could inform the choice of smoking cessation medications to African-American smokers. This study is unique and presents recruitment and retention challenges for the following reasons: 1) unlike clinical trials, this study was laboratory-based and involved a non-treatment-seeking study population; 2) participants were neither required to be interested in quitting nor motivated to quit; 3) participants were not in need of clinical investigation or treatment for any physical illness that would motivate them to visit the hospital multiple times and stay in the hospital for long hours of investigation. This article describes the design, successful recruitment and retention of African-Americans in our pharmacokinetic study. We will discuss challenges and solutions regarding recruitment and retention issues in the study, which could provide useful information for investigators embarking on similar research among minority populations.
Methods
The study protocol was reviewed and approved by the University of Kansas Medical Center Human Subjects Committee. This study was conducted within the General Clinical Research Center (GCRC) at the University of Kansas Medical Center.
Study Design
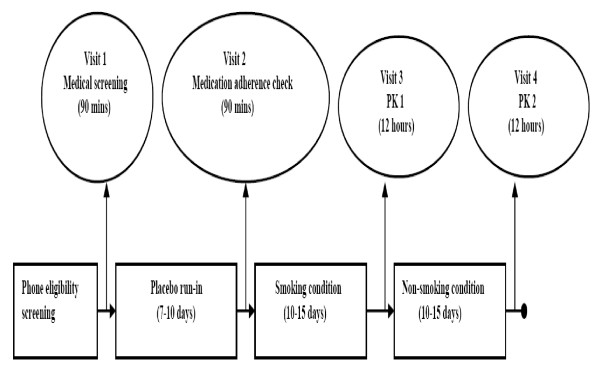
Figure 1 provides an overview of the study that included screening and four visits to the GCRC. The study was designed to provide information about possible interaction between menthol in mentholated cigarettes and bupropion (Zyban®, Glaxo SmithKline) by evaluating the effects of menthol in mentholated cigarettes on the pharmacokinetic (PK) profiles of bupropion and its three principal metabolites: hydroxybupropion, threohydrobupropion, and erythrohydrobupropion. The study aimed to recruit and enroll 20 African-American smokers of mentholated cigarettes (menthol smokers) matched 1:1 with 20 African-American smokers of non-mentholated cigarettes (non-menthol smokers) by gender, number of cigarettes per day smoked (CPD), and body mass index (BMI). Pharmacokinetic (PK) parameters of bupropion and its three principal metabolites were assessed at steady state under smoking and non-smoking conditions. During the smoking condition which lasted 10-15 days, subjects smoked their usual brand of cigarettes. This period was followed by a non-smoking condition lasting another 10-15 days. The PK parameters were then assessed between 1) menthol and non-menthol smokers, and 2) smoking and non-smoking conditions.
Figure 1.
Overview of the study design to evaluate the effect of menthol on the pharmacokinetics of bupropion and its metabolites.
Preparatory Phase
Recruitment
Participants were recruited using clinic-based and community-based strategies. Clinic-based strategies included informing medical center staff about the study through flyers, broadcast emails, departmental meetings, and employee newsletters. Posters and flyers were distributed at a community health center that serves a predominantly African-American patient population. Invitation letters were sent to former research participants who gave written consent to be contacted in the future. Community strategies included paid advertisements in neighborhood newspapers, the major city paper, and on two radio stations with large African-American audiences. Research staff provided flyers to African-American business owners and religious organizations. Those interested in the study were asked to call the study office and speak with a study coordinator who assessed their eligibility for the study.
Eligibility criteria
Eligible individuals identified themselves as African-American or Black, aged 18 years or older. They smoked at least 10 cigarettes per day and had a BMI between 18 and 45 kg/m2. Additionally, each participant must have smoked either mentholated or non-mentholated cigarettes exclusively for the past year. Consistent with contraindications for bupropion use, exclusion criteria included predisposition to seizures, a diagnosis of bulimia or anorexia nervosa in the past year, an unstable medical or psychiatric illness, alcohol dependency within the last year, or a myocardial infarction in the last month. In addition, individuals who planned to move from the metropolitan area in the next month, or who had used other forms of tobacco in the past 30 days, or had used bupropion in the past 30 days were excluded from participation, as were those currently using any prescription or other medications contraindicated or with known interaction with bupropion, those reporting illicit drug use and women who were pregnant, breastfeeding, or contemplating pregnancy in the next month.
Eligibility Screening
Eligibility screening was multi-staged: telephone eligibility screening was followed by medical screening, and then, lastly, by screening for adverse events with medication and adherence to medication.
Telephone Screening
Study staff specifically informed callers that this was a "non-treatment study to find out how the body breaks down a medication called Zyban". Research staff verified individuals' willingness to adhere to study instructions. Interested individuals were screened on the phone according to the eligibility criteria described above. Those eligible were informed that medical screening would include an additional medical history, a physical examination and laboratory tests to rule out contraindications to bupropion use. They were instructed to fast overnight prior to the medical screening visit. A written copy of these instructions was mailed to all individuals who had passed the telephone screening.
Medical Screening
Phone-eligible individuals came to the General Clinical Research Center (GCRC) after an overnight fast. The study staff discussed the details of the study with participants during the informed consent process. Individuals signed a written informed consent form. They provided a 20 ml blood sample for a complete blood count, a complete metabolic profile, and cotinine analysis. Women also gave a urine sample for a pregnancy test. The GCRC nurse took a medical history and examined the participants. Participants completed a baseline smoking history questionnaire and also completed a smoking topography using a CreSSmicro smoking topography device (Plowshare Technologies, Baltimore, MD). The study staff and the study physician reviewed the results of the laboratory tests, medical history and physical examination findings to determine eligibility. Eligible participants were given a sterile container for collecting urine beginning 24 hours prior to the next visit to verify their menthol status. They were also given a special cigarette carrying container (Smartpak) to use during the study.
Reporting adverse events and verifying medication adherence
Medically eligible participants were given a 7-10 day supply of placebo in a container with a Medication Event Monitoring System (MEMS) cap, an adherence verification device (AARDEX, Union City, CA). On their second visit, participants were asked about adverse events using the Common Terminology Criteria for Adverse Events, version 3.0 (National Cancer Institute). Unused study medication was collected and counted, and data were downloaded from the MEMS cap. Individuals who did not tolerate the placebo medication or used less than 75% of the prescribed dose were excluded from continued participation. Such individuals were excluded with the rationale that they were unlikely to take the active medication as prescribed during the entire study. Participants were enrolled in the pharmacokinetics phase of the study after they passed the medical adherence eligibility screening (Visit 2), and were scheduled for Visit 3. The participants continued to use the MEMS cap for the entire study to monitor bupropion use. The study staff instructed participants to bring their MEMS cap and all medications to each GCRC visit.
Pharmacokinetic Study Phase
Procedure overview
After a seven-day placebo run-in period and an initial three-day dosing period of 150 mg/day, participants were given 300 mg/day (150 mg 2×/day) sustained-release bupropion for 20-25 days. Participants were asked to smoke their usual brand of cigarettes ad lib for the first 10-15 days (smoking condition) and to quit smoking for the remaining 10-15 days of the study (non-smoking condition). Blood samples were drawn for pharmacokinetics (PK) analysis on two occasions, 10-15 days after the commencement of bupropion while participants were still smoking (PK 1), and again at days 20-25 (PK 2) when they were required not to smoke (non-smoking condition). The blood samples at Visit 3 (PK 1) provided PK parameters when participants were exposed to both bupropion and menthol in cigarettes. Samples at Visit 4 (PK 2) provided PK parameters when participants were exposed only to bupropion but not to menthol. At each PK visit, approximately 10 ml of blood specimens for PK were taken through an intravenous line inserted into the participant's arm prior to ingestion of 150 mg bupropion-SR and at 1, 2, 2.5, 3, 3.5, 4, 5, 6, 8, and 12 hours after ingestion of the first dose of 150 mg bupropion-SR. Use of the second dose of bupropion for the day was delayed until after all blood draws were completed.
Retention Strategies
Reminders
Once participants were scheduled for visits, reminder letters were mailed one week before each visit, and reminder telephone calls were placed two days and one day before each visit. The study staff provided information about the procedures that participants would undergo in the upcoming visit and answered questions. Participants who missed an appointment were contacted by telephone to reschedule the appointment, provided they were still within the study visit window. The research team met weekly to review recruitment and retention progress and to provide feedback to the study staff.
Incentives
Monetary compensation was discussed with participants during the informed consent process. Participants were given $50 Visa gift cards for each screening visit and $150 Visa gift cards for each pharmacokinetic study visit. Fifty dollar Visa gift cards were given to the participants for the use of Smartpak and MEMS devices. Additional $50 Visa gift cards were given to participants who were able to abstain from smoking during the non-smoking phase (verified with Nic check).
Data Analysis
This is a descriptive summary and, as such, we summarized categorical variables by frequencies and percentages and continuous variables by means and standard deviations using SAS statistical package, version 9.1.
Results
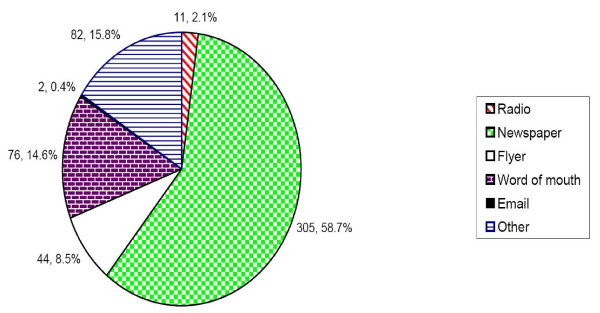
Recruitment was conducted from February 2006 to February 2007, during which time a total of 515 African-American smokers were screened via telephone. Interested individuals learned about the study from newspapers (58.7%), word of mouth (14.6%), and flyers (8.5%) See Figure 2. Similarly, newspaper advertisement produced the highest yield of participants enrolled in the pharmacokinetic study. Of the 515 screened, 187 were phone-eligible (Figure 3). Some of the reasons for ineligibility during phone screening included: smoking fewer than 10 cigarettes per day; not smoking menthol or non-menthol cigarettes exclusively; smoking other tobacco products such as "Black and Mild" cigars; excessive drinking of alcohol; and inability to attend 12-hour appointments.
Figure 2.
Sources of information about study. *The distribution represents 520 responses from 515 individuals screened on the phone.
Figure 3.
Flow chart showing the recruitment and retention of participants in the pharmacokinetics study.
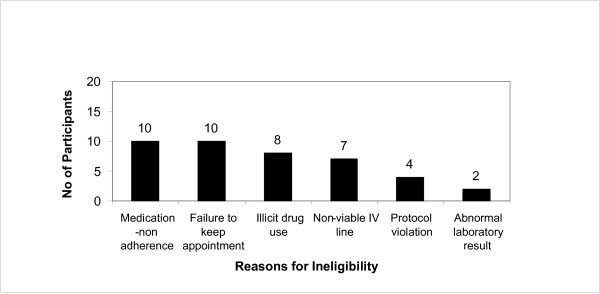
In total, 92 smokers were scheduled for Visit 1, and 81 kept their appointments. Figure 3 presents an overview of participant retention, which shows 40 participants completing all study visits. Figure 4 provides a summary of the primary reasons for exclusion. Of the 81 smokers who attended Visit 1, 41 (50.6%) were excluded due to: medication non-adherence (12.3%); failure to keep appointments (12.3%); illicit drug use (9.9%); failure to establish IV lines (8.7%); protocol violations (4.9%); or abnormal test results (2.5%).
Figure 4.
Primary reasons for excluding participants from study after attending medical screening visit (n = 41).
Table 1 presents the characteristics of the participants who were enrolled in the pharmacokinetic study (n = 48). Participants were approximately 51 (SD = 9.7) years old and smoked an average of 15.1 (SD = 4.6) cigarettes per day for a mean duration of 33 years. Fifty-eight percent were females. The average BMI was 29.9 (SD = 7.0), and the mean age of smoking initiation was 17.6 (SD = 4.4) years.
Table 1.
Baseline characteristics of participants enrolled in the PK study (n = 48)
| All | Menthol (n = 29) | Non-Menthol (n = 19) | P value | |
|---|---|---|---|---|
| Mean age (SD) | 50.9 (9.7) | 47.4 (9.3) | 56.3 (7.9) | <0.0011 |
| Gender, n (% female) | 28 (58.3%) | 16 (57.1%) | 12 (42.9%) | 0.772 |
| BMI* | 29.9 (7.0) | 30.5 (7.0) | 29.0 (7.1) | 0.48 |
| Mean age of smoking initiation | 17.6 (4.4) | 18.0 (5.1) | 16.9 (3.1) | 0.34 |
| Mean age of regular smoking | 19.7 (5.0) | 19.9 (5.9) | 20.9 (4.5) | 0.70 |
| No. of years smoked | 33.4 (10.1) | 29.4 (9.4) | 39.4 (7.8) | <0.001 |
| Mean CPD at baseline | 15.1 (4.6) | 15.3 (5.2) | 16.1 (3.7) | 0.331 |
| FTND Score** | 4.8 (1.7) | 4.5 (1.6) | 5.2 (1.8) | 0.18 |
1Wilcoxon rank sum test; 2Fisher's exact test; all other p-values calculated with t -tests;
*Body Mass Index. **Fagerström Test of Nicotine Dependence.
Discussion
This study demonstrated that African-American smokers can be successfully recruited to participate in an intensive GCRC-based non-treatment study. Given the intensity of participation required for the study, including 24-hour urine collection, the use of cigarette and pill monitoring devices, the number and duration of study visits, and multiple blood draws, achieving this recruitment level was considered a success. This outcome supports the feasibility of including African-Americans in laboratory-based research. Showing that African-Americans will volunteer to enroll in this type of research is important, given the persistent national problem of underrepresentation of African-Americans in clinical studies [10]. The recruitment goal of the study was to have 40 African-Americans complete the pharmacokinetic study. We screened 515 and identified 187 eligible African-American smokers. Of 81 individuals scheduled for the medical screening (Visit 1) at the GCRC, 49.4% completed the full PK study. It should be noted that it took approximately 12 months to meet this enrollment goal, a much longer time than is needed to recruit the same number of smokers in most smoking cessation studies [34-36]. This discrepancy is likely due to the intensive nature of the protocol as well as the location of the study. This study was located in an academic medical center and most of our participants heard about the study through newspaper advertisement. This is a departure from our previous study in a community health center serving a predominantly low-income African-American population. In that study, word of mouth yielded more participants [37]. Our advertisement strategies also played a role. Periodically, we reviewed progress with recruitment methods and invested more in newspaper advertisement, which proved to be more effective compared to other sources.
Multiple factors contributed to recruitment success. Our research team has a track record of successfully engaging African-Americans in research studies, and we applied this experience to our recruitment efforts [32,34,37-53]. We emphasized cultural competence and cultural sensitivity training within our research team. We involved the African-American community and health care providers through a Community Advisory Board in the planning and execution of our projects [34]. Our efforts in the community have progressively built trust, which is critical given that lack of trust is a major impediment to the participation of African-Americans in medical research studies [12]. We integrated recruitment tracking mechanisms to maximize retention of our participants. In this study, we achieved a relatively high retention rate of 83%. Of the 48 participants who met final eligibility criteria and enrolled in the pharmacokinetic study, 40 participants completed the study. This rate is similar to the retention rates of 84% we obtained in our previous clinical trials [52,54], but lower than 90%, reported in a more intensive long-term clinical trial involving African-Americans with renal insufficiency [55]. This implies that retention might be better in a treatment-seeking population than in a non-treatment-seeking population.
The recruitment of African-American non-menthol smokers into our study was particularly challenging. Only about 20% of African-American smokers smoke non-menthol cigarettes [29-32,56]. Because of the lower prevalence of African-American non-menthol smokers, we could not enroll eligible menthol smokers until we found non-menthol smokers who could be matched with menthol smokers. Consequently, we had a long waiting list of menthol smokers who, although eligible for the study, could not be enrolled due to lack of a non-menthol match. This sampling technique is prone to selection bias and may limit the generalization of our study findings. This limitation speaks to methodological challenges pertaining to research involving menthol and non-menthol African-American smokers.
The majority of the participants who were excluded from this study after the informed consent process either did not adhere to medication regimen or did not keep the scheduled appointment. We do not know if failure to attend visit during the abstinence phase was due to the fact that some of the smokers could not stop smoking during this phase. While the exclusion criteria were carefully chosen to remove individuals who seemed unlikely to comply with our protocol, this study also provided insight into issues that warrant further investigation, such as adherence to medication among minority populations, and factors that influence minority participants to not keep the study appointments. Illicit drug use (primarily marijuana) accounted for 19.5% of those who were excluded from study after consenting to participate. Although use of illicit drugs was a clear exclusion criterion, we administered this question in person because of its sensitive nature and the need to get more accurate information along with a certificate of confidentiality on their behalf from the federal government agency for their protection.
Conclusions
Our study demonstrates the feasibility of recruiting African-American smokers to a pharmacokinetic study, and identifies unique challenges to recruitment and retention. These challenges include intense eligibility criteria with multiple levels of eligibility, rigorous protocol, and invasive procedures. Despite these, we have demonstrated that recruiting African-Americans in non-treatment studies involving multiple blood draws and follow-up visits can be accomplished effectively. This paper provides valuable information for investigators embarking on non-treatment laboratory-based studies among minority populations.
Competing interests
Dr. Ahluwalia is a consultant to Pfizer Inc.
Authors' contributions
All authors have made substantial contributions to the intellectual content of the manuscript as follows: BF, CAB and IO participated in the acquisition of data; BF, LSC and CAB participated in drafting the manuscript; all authors participated in the concept and design of the study, interpretation of data, review and approval of the final version of the manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
Babalola Faseru, Email: bfaseru@kumc.edu.
Lisa S Cox, Email: lcox@kumc.edu.
Carrie A Bronars, Email: cbronars@kumc.edu.
Isaac Opole, Email: iopole@kumc.edu.
Gregory A Reed, Email: greed@kumc.edu.
Matthew S Mayo, Email: mmayo@kumc.edu.
Jasjit S Ahluwalia, Email: jahluwal@umn.edu.
Kolawole S Okuyemi, Email: kokuyemi@umn.edu.
Acknowledgements
The authors would like to thank Michelle Boyd, Qingjiang Hou, Tricia Snow, Dawn Dillinger, Diana Stewart, Jean Sunega, and the KUMC GCRC Staff for their efforts on this project. We are grateful to the volunteers who participated in this study. This work was performed at the University of Kansas Medical Center, Kansas City, Kansas, with support from the National Institute on Drug Abuse (R21 DA 018720) and National Cancer Institute (R01 CA091912). KUMC GCRC is funded by the National Center for Research Resources (M01 RR0239410). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- USDHHS. Healthy people 2000: National health promotion and disease prevention objectives full report with commentary. Washington, D.C.: Government Printing Office; 1991. [Google Scholar]
- CDC. Health Disparities Experienced by Black or African-Americans - United States. MMWR. 2005;54(01):1–3. [PubMed] [Google Scholar]
- Roberson NL. Clinical trial participation: Viewpoints from racial/ethnic groups. Cancer. 1994;74(Suppl 9):2687–2691. doi: 10.1002/1097-0142(19941101)74:9+<2687::AID-CNCR2820741817>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Holcombe RF, Jacobson J, Li A, Moinpour CM. Inclusion of black Americans in oncology clinical trials: the Louisiana State University Medical Center experience. Am J Clin Oncol. 1999;22(1):18–21. doi: 10.1097/00000421-199902000-00005. [DOI] [PubMed] [Google Scholar]
- Swanson GM, Ward AJ. Recruiting minorities into clinical trials: toward a participant-friendly system. J Natl Cancer Inst. 1995;87(23):1747–1759. doi: 10.1093/jnci/87.23.1747. [DOI] [PubMed] [Google Scholar]
- Dennis BP, Neese JB. Recruitment and retention of African American elders into community-based research: lessons learned. Arch Psychiatr Nurs. 2000;14(1):3–11. doi: 10.1016/S0883-9417(00)80003-5. [DOI] [PubMed] [Google Scholar]
- Evelyn B, Toigo T, Banks D, Pohl D, Gray K, Robins B, Ernat J. Participation of racial/ethnic groups in clinical trials and race-related labeling: a review of new molecular entities approved 1995-1999. J Natl Med Assoc. 2001;93(Suppl 12):S18–S24. [PMC free article] [PubMed] [Google Scholar]
- Ganz PA. Clinical trials. Concerns of the patient and the public. Cancer. 1990;65(Suppl 10):2394–2399. doi: 10.1002/1097-0142(19900515)65:10+<2394::AID-CNCR2820651509>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Giuliano AR, Mokuau N, Hughes C, Tortolero-Luna G, Risendal B, Ho RCS, Prewitt TE, McCaskill-Stevens WJ. Participation of minorities in cancer research: the influence of structural, cultural, and linguistic factors. Ann Epidemiol. 2000;10(Suppl 8):S22–S34. doi: 10.1016/S1047-2797(00)00195-2. [DOI] [PubMed] [Google Scholar]
- King TE Jr. Racial disparities in clinical trials. N Engl J Med. 2002;346(18):1400–1402. doi: 10.1056/NEJM200205023461812. [DOI] [PubMed] [Google Scholar]
- Powell JH, Fleming Y. Making medicines for America: the case for clinical trial diversity. J Natl Med Assoc. 2000;92(11):507–514. [PMC free article] [PubMed] [Google Scholar]
- Shavers-Hornaday VL, Lynch CF, Burmeister LF, Torner JC. Why are African Americans under-represented in medical research studies? Impediments to participation. Ethn Health. 1997;2(1-2):31–45. doi: 10.1080/13557858.1997.9961813. [DOI] [PubMed] [Google Scholar]
- Stark N, Paskett E, Bell R, Cooper MR, Walker E, Wilson A, Tatum C. Increasing participation of minorities in cancer clinical trials: summary of the "Moving Beyond the Barriers" Conference in North Carolina. J Natl Med Assoc. 2002;94(1):31–39. [PMC free article] [PubMed] [Google Scholar]
- Svensson CK. Representation of American blacks in clinical trials of new drugs. JAMA. 1989;261(2):263–265. doi: 10.1001/jama.261.2.263. [DOI] [PubMed] [Google Scholar]
- El-Sadr W, Capps L. The challenge of minority recruitment in clinical trials for AIDS. JAMA. 1992;267(7):954–957. doi: 10.1001/jama.267.7.954. [DOI] [PubMed] [Google Scholar]
- Moyé LA, Powell JH. Evaluation of ethnic minorities and gender effects in clinical trials: opportunities lost and rediscovered. J Natl Med Assoc. 2001;93(Suppl 12):29S–34S. [PMC free article] [PubMed] [Google Scholar]
- Nicholson WK, Brown AF, Gathe J, Grumbach K, Washington AE, Pérez-Stable EJ. Hormone replacement therapy for African American women: missed opportunities for effective intervention. Menopause. 1999;6(2):147–155. doi: 10.1097/00042192-199906020-00012. [DOI] [PubMed] [Google Scholar]
- D'Agostino RB Sr, Grundy S, Sullivan LM, Wilson P. CHD Risk Prediction Group. Validation of the Framingham coronary heart disease prediction scores: results of a multiple ethnic groups investigation. JAMA. 2001;286(2):180–187. doi: 10.1001/jama.286.2.180. [DOI] [PubMed] [Google Scholar]
- Bonner GJ, Miles TP. Participation of African Americans in clinical research. Neuroepidemiology. 1997;16(6):281–284. doi: 10.1159/000109698. [DOI] [PubMed] [Google Scholar]
- Gorelick PB, Harris Y, Burnett B, Bonecutter FJ. The recruitment triangle: reasons why African Americans enroll refuse to enroll, or voluntarily withdraw from a clinical trial. J Natl Med Assoc. 1998;90(3):141–145. [PMC free article] [PubMed] [Google Scholar]
- Paskett ED, DeGraffinreid C, Tatum CM, Margitić SE. The recruitment of African Americans to cancer prevention and control studies. Prev Med. 1996;25(5):547–553. doi: 10.1006/pmed.1996.0088. [DOI] [PubMed] [Google Scholar]
- Escobar-Chaves SL, Tortolero SR, Mâsse LC, Watson KB, Fulton JE. Recruiting and retaining minority women: findings from the Women on the Move study. Ethn Dis. 2002;12(2):242–251. [PubMed] [Google Scholar]
- Corbie-Smith G, Thomas SB, Williams MV, Moody-Ayers S. Attitudes and beliefs of African Americans toward participation in medical research. J Gen Intern Med. 1999;14(9):537–546. doi: 10.1046/j.1525-1497.1999.07048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbie-Smith G, Thomas SB, St George DM. Distrust, race, and research. Arch Intern Med. 2002;162(21):2458–2463. doi: 10.1001/archinte.162.21.2458. [DOI] [PubMed] [Google Scholar]
- Mouton CP, Harris S, Rovi S, Solorzano P, Johnson MS. Barriers to black women's participation in cancer clinical trials. J Natl Med Assoc. 1997;89(11):721–727. [PMC free article] [PubMed] [Google Scholar]
- Stoy DB, Curtis RC, Dameworth KS, Dowdy AA, Hegland J, Levin JA, Sousoulas BG. The successful recruitment of elderly black subjects in a clinical trial: the CRISP experience. Cholesterol Reduction in Seniors Program. J Natl Med Assoc. 1995;87(4):280–287. [PMC free article] [PubMed] [Google Scholar]
- Harris RE, Zang EA, Anderson JI, Wynder EL. Race and sex differences in lung cancer risk associated with cigarette smoking. Int J Epidemiol. 1993;22(4):592–599. doi: 10.1093/ije/22.4.592. [DOI] [PubMed] [Google Scholar]
- Okuyemi K, Cox L, Choi WS, Ahluwalia JS. In: VA in the Vanguard: Building on Success in Smoking Cessation. Isaacs SL, editor. 2005. Smoking cessation in U.S. ethnic minority populations. [Google Scholar]
- Orleans CT, Schoenbach VJ, Salmon MA, Strecher VJ, Kalsbeek W, Quade D, Brooks EF, Konrad TR, Blackmon C, Watts CD. A survey of smoking and quitting patterns among black Americans. Am J Public Health. 1989;79(2):176–181. doi: 10.2105/AJPH.79.2.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidney S, Tekawa I, Friedman GD. Mentholated cigarette use among multiphasic examinees 1979-86. Am J Public Health. 1989;79(10):1415–6. doi: 10.2105/AJPH.79.10.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings KM, Giovino G, Mendicino AJ. Cigarette advertising and black-white differences in brand preference. Public Health Rep. 1987;102(6):698–701. [PMC free article] [PubMed] [Google Scholar]
- Okuyemi KS, Ahluwalia JS, Ebersole-Robinson M, Catley D, Mayo MS, Resnicow K. Does menthol attenuate the effect of bupropion among African American smokers? Addiction. 2003;98(10):1387–1393. doi: 10.1046/j.1360-0443.2003.00443.x. [DOI] [PubMed] [Google Scholar]
- Okuyemi KS, Faseru B, Sanderson Cox L, Bronars CA, Ahluwalia JS. Relationship between menthol cigarettes and smoking cessation among African American light smokers. Addiction. 2007;102(12):1979–1986. doi: 10.1111/j.1360-0443.2007.02010.x. [DOI] [PubMed] [Google Scholar]
- Okuyemi KS, Cox LS, Nollen NL, Snow TM, Kaur H, Choi W, Nazir N, Mayo MS, Ahluwalia JS. Baseline characteristics and recruitment strategies in a randomized clinical trial of African-American light smokers. Am J Health Promot. 2007;21(3):183–191. doi: 10.4278/0890-1171-21.3.183. [DOI] [PubMed] [Google Scholar]
- Cox LS, Cupertino AP, Mussulman LM, Nazir N, Greiner KA, Mahnken JD, Ahluwalia JS, Ellerbeck EF. Design and baseline characteristics from the KAN-QUIT disease management intervention for rural smokers in primary care. Prev Med. 2008;47(2):200–205. doi: 10.1016/j.ypmed.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cupertino PA, Richter KP, Cox LS, Nazir N, Greiner AK, Ahluwalia JS, Ellerbeck EF. Smoking cessation pharmacotherapy preferences in rural primary care. Nicotine Tob Res. 2008;10(2):301–307. doi: 10.1080/14622200701825817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KJ, Ahluwalia JS, Catley D, Okuyemi KS, Mayo MS, Resnikow K. Successful recruitment of minorities into clinical trials: The Kick It at Swope project. Nicotine Tob Res. 2003;5(4):575–84. doi: 10.1080/1462220031000118540. [DOI] [PubMed] [Google Scholar]
- Ahluwalia JS, Resnicow K, Clark WS. Knowledge about smoking reasons for smoking, and reasons for wishing to quit in inner-city African Americans. Ethn Dis. 1998;8(3):385–393. [PubMed] [Google Scholar]
- Ahluwalia JS, McNagny SE, Clark WS. Smoking cessation among inner-city African Americans using the nicotine transdermal patch. J Gen Intern Med. 1998;13(1):1–8. doi: 10.1046/j.1525-1497.1998.00001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahluwalia JS, Richter K, Mayo MS, Ahluwalia HK, Choi WS, Schmelzle KH, Resnicow K. African American smokers interested and eligible for a smoking cessation clinical trial: predictors of not returning for randomization. Ann Epidemiol. 2002;12(3):206–212. doi: 10.1016/S1047-2797(01)00305-2. [DOI] [PubMed] [Google Scholar]
- Woods MN, Harris KJ, Mayo MS, Catley D, Scheibmeir M, Ahluwalia JS. Participation of African Americans in a smoking cessation trial: a quantitative and qualitative study. J Natl Med Assoc. 2002;94(7):609–618. [PMC free article] [PubMed] [Google Scholar]
- Ahluwalia JS, Harris KJ, Catley D, Okuyemi KS, Mayo MS. Sustained-release bupropion for smoking cessation in African Americans: a randomized controlled trial. JAMA. 2002;288(4):468–474. doi: 10.1001/jama.288.4.468. [DOI] [PubMed] [Google Scholar]
- Okuyemi KS, Richter KP, Ahluwalia JS, Mosier MC, Nazir N, Resnicow K. Smoking reduction practices among African American smokers. Nicotine Tob Res. 2002;4(Suppl 2):S167–173. doi: 10.1080/1462220021000032744. [DOI] [PubMed] [Google Scholar]
- Okuyemi KS, Scheibmeir M, Butler J, Ahluwalia JS. Perceptions of smoking among African American light smokers. Subst Abus. 2003;24(3):191–193. doi: 10.1080/08897070309511548. [DOI] [PubMed] [Google Scholar]
- Okah FA, Okuyemi KS, McCarter KS, Harris KJ, Catley D, Kaur H, Ahluwalia JS. Predicting adoption of home smoking restriction by inner-city black smokers. Arch Pediatr Adolesc Med. 2003;157(12):1202–1205. doi: 10.1001/archpedi.157.12.1202. [DOI] [PubMed] [Google Scholar]
- Okuyemi KS, Ahluwalia JS, Banks R, Harris KJ, Mosier MC, Nazir N, Powell J. Differences in smoking and quitting experiences by levels of smoking among African Americans. Ethn Dis. 2004;14(1):127–133. [PubMed] [Google Scholar]
- Harris KJ, Okuyemi KS, Catley D, Mayo MS, Ge B, Ahluwalia JS. Predictors of smoking cessation among African-Americans enrolled in a randomized controlled trial of bupropion. Prev Med. 2004;38(4):498–502. doi: 10.1016/j.ypmed.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Nollen NL, Catley D, Davies G, Hall M, Ahluwalia JS. Religiosity, social support, and smoking cessation among urban African American smokers. Addict Behav. 2005;30(6):1225–1229. doi: 10.1016/j.addbeh.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Okuyemi KS, Faseru B, Sanderson Cox L, Bronars CA, Ahluwalia JS. Relationship between menthol cigarettes and smoking cessation among African American light smokers. Addiction. 2007;102(12):1979–1986. doi: 10.1111/j.1360-0443.2007.02010.x. [DOI] [PubMed] [Google Scholar]
- Cox LS, Bronars CA, Thomas JL, Okuyemi KS, King G, Mayo MS, Ahluwalia JS. Achieving high rates of consent for genetic testing among African American smokers. Nicotine Tob Res. 2007;9(6):711–716. doi: 10.1080/14622200701365228. [DOI] [PubMed] [Google Scholar]
- Okuyemi KS, Powell JN, Savage CR, Hall SB, Nollen N, Holsen LM, McClernon FJ, Ahluwalia JS. Enhanced cue-elicited brain activation in African American compared with Caucasian smokers: an fMRI study. Addict Biol. 2006;11(1):97–106. doi: 10.1111/j.1369-1600.2006.00007.x. [DOI] [PubMed] [Google Scholar]
- Ahluwalia JS, Okuyemi K, Nollen N, Choi WS, Kaur H, Pulvers K, Mayo MS. The effects of nicotine gum and counseling among African American light smokers: a 2 × 2 factorial design. Addiction. 2006;101(6):883–891. doi: 10.1111/j.1360-0443.2006.01461.x. [DOI] [PubMed] [Google Scholar]
- Woods MN, Harris KJ, Ahluwalia JS, Schmelzle KH, Mayo MS. Smoking in urban African Americans: behaviors, gender differences and motivation to quit. Ethn Dis. 2001;11(3):532–539. [PubMed] [Google Scholar]
- Nollen NL, Mayo MS, Sanderson Cox L, Okuyemi KS, Choi WS, Kaur H, Ahluwalia JS. Predictors of quitting among African American light smokers enrolled in a randomized, placebo-controlled trial. J Gen Intern Med. 2006;21(6):590–595. doi: 10.1111/j.1525-1497.2006.00404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks D, Charleston J, Dowie D, Gabriel A, Hall YB, Hiremath L, Lightfoot T, Sika M, Smith WC, Wang X. Predictors of participant adherence and retention in the African American Study of Kidney Disease and Hypertension. Nephrol Nurs J. 2008;35(2):133–42. [PubMed] [Google Scholar]
- Kabat GC, Hebert JR. Use of mentholated cigarettes and lung cancer risk. Cancer Res. 1991;51(24):6510–3. [PubMed] [Google Scholar]