Abstract
The importance of evidence-based health policy is widely acknowledged among health care professionals, patients and politicians. Health care resources available for medical procedures, including pharmaceuticals, are limited all over the world. Economic evaluations help to alleviate the burden of scarce resources by improving the allocative efficiency of health care financing. Reimbursement of new medicines is subject to their cost-effectiveness and affordability in more and more countries.
There are three major approaches to calculate the cost-effectiveness of new pharmaceuticals. Economic analyses alongside pivotal clinical trials are often inconclusive due to the suboptimal collection of economic data and protocol-driven costs. The major limitation of observational naturalistic economic evaluations is the selection bias and that they can be conducted only after registration and reimbursement.
Economic modelling is routinely used to predict the cost-effectiveness of new pharmaceuticals for reimbursement purposes. Accuracy of cost-effectiveness estimates depends on the quality of input variables; validity of surrogate end points; and appropriateness of modelling assumptions, including model structure, time horizon and sophistication of the model to differentiate clinically and economically meaningful outcomes. These economic evaluation methods are not mutually exclusive; in practice, economic analyses often combine data collection alongside clinical trials or observational studies with modelling.
The need for pharmacoeconomic evidence has fundamentally changed the strategic imperatives of research and development (R&D). Therefore, professionals in pharmaceutical R&D have to be familiar with the principles of pharmacoeconomics, including the selection of health policy-relevant comparators, analytical techniques, measurement of health gain by quality-adjusted life-years and strategic pricing of pharmaceuticals.
Keywords: pharmacoeconomics, reimbursement, economic evaluation alongside clinical trials, naturalistic health economic study, economic modelling, cost-effectiveness, full economic evaluation, strategic pricing
Introduction
The importance of evidence-based medicine and evidence-based health policy is widely acknowledged among health care professionals, patients and politicians all over the world. Expectations for evidence base of different health care interventions, however, are not equal. The registration criteria for pharmaceuticals are strict, the efficacy and safety of new medicines have to be proven in randomized controlled trials (RCTs) and the quality of products has to be guaranteed by well-defined methods and regular monitoring.
The criteria for registration are not so strict for surgical interventions, diagnostic equipment, medical devices and appliances. Furthermore, the rules of evidence-based medicine are not routinely applied in the area of complementary medicine.
In spite of evidence from RCTs, we are aware of several cases, when the real-world health benefit of drugs which otherwise met the strict requirements of the registration procedure is still doubtful. In the case of some pharmaceuticals, the intermediate or surrogate health benefit observed in pivotal clinical trials eventually does not result in survival and/or clinically meaningful quality-of-life (QoL) benefit in real-world situations. When the application of a pharmaceutical therapy does not result in benefit in real world, health care resources are wasted. To minimize this welfare loss, societies need an objective list of health care technologies with real-world health benefit, so we have to exclude the utilization of ineffective health care procedures.
If this very important step is implemented, public resources are still not enough to cover the provision of all effective health care technologies. When a physician indicates a therapy for a patient, this means that at the end of the day another patient will miss the appropriate treatment due to the scarcity of resources. If in the later case the missed treatment would have resulted in more health benefit than for the former patient, the opportunity cost of the decision – the amount of the missed health gain – is greater than the proceeds of the decision. This is the reason why we have to spend public resources only on the most cost-effective health care technologies, and we have to sacrifice those treatments that are effective, but too expensive. It would be complicated and time consuming to rank all available health care technologies according to their cost-effectiveness (Mauskopf et al., 2003); therefore, the cost-effectiveness criteria are assessed mainly for the new and expensive therapies in the majority of countries.
This paper summarizes the principles of pharmacoeconomics and the impact of cost-effectiveness criteria on the pharmaceutical research and development (R&D), including the strategic pricing of medicines.
Classification of pharmacoeconomic evaluations according to the method of data collection
There are several different approaches to assess the cost-effectiveness of pharmaceuticals. Pharmacoeconomic evaluations can be classified based upon their method of data collection (see Table 1).
Table 1.
Classification of economic evaluations according to the method of data collection
| Advantages | Disadvantages | |
|---|---|---|
| Economic evaluation alongside clinical trials | Randomization (internal validity) Low cost of economic data collection Economic results are available before reimbursement decisions | Selected patient population Protocol-induced costs Limited time horizon Monitoring of economic data is less strict than of clinical variables Calculation of statistical power is based on efficacy end points Economically meaningful events after clinical end point and study drug discontinuation |
| Naturalistic pharmacoeconomic studies | Non-selected ordinary patients in routine care settings (external validity) Real-world resource utilization and costs independent from the study protocol Easy monitoring if individual patient records in payers or managed care database can be linked based upon individual patient ID Large patient population | Unpredictable data collection, complicated study administration, lack of data monitoring Selection bias (if no randomization) Limited time horizon Economic results available only after reimbursement decisions |
| Economic modelling on the basis of prospectively collected clinical trial data | Economic modelling results available before major decisions (e.g. reimbursement) Generalizable results, adjusted to local medical practice and patient population | Results depend on appropriateness of modelling assumptions (e.g. model structure) Known uncertainty in input parameters reduces the clarity of conclusions |
Pharmacoeconomic evaluations conducted alongside clinical trials are often called piggy-back analysis (O'Sullivan et al., 2005). In these studies, the incremental cost of pharmacoeconomic data collection is less than if it is done separately in stand-alone health economic trials (Hlatky et al., 2006). Prospective follow-up of patients in randomized trials reduces diversity of patient groups. Therefore, perceived differences in pharmacoeconomic parameters are not attributable to differences between patient groups, and so the internal validity of cost-effectiveness conclusions is strong.
Economic analyses are mostly carried out alongside the pivotal clinical trials for registration purposes. Thus, pharmacoeconomic results are available before the submission of full documentation for reimbursement purposes.
The piggy-back approach, however, has several caveats (Ramsey et al., 2005). In prospective clinical trials, the time horizon of data collection is limited. As the inclusion criteria for selecting patient population are strict, it is difficult to generalize health economic results for ordinary patients. The lack of external validity can be illustrated with the example on how we value the cost-effectiveness of statins over the age of 80, as patients over the age of 70 are usually excluded from clinical trials.
It is difficult to prove in economic analyses alongside pivotal clinical trials that a new medicine reduces the number of outpatient visits or diagnostics, as the clinical trial protocol requires strictly scheduled meetings with the investigator or diagnostic procedures to record efficacy end points. Regular protocol-driven consultations with physicians or diagnostic tests reduce the chance of unexpected outpatient visits and the need for symptom-driven diagnostic procedures.
Furthermore, much greater attention is paid for the monitoring of efficacy and safety variables than for the validity of health economic data; therefore, the quality of economic data collection may not always meet the expectations.
In pivotal clinical trials, the calculation of statistical power is based on primary efficacy end points. As usual, a larger sample size is needed to achieve confirmatory evidence for economic end points than for efficacy parameters; economic analyses alongside pivotal clinical trials often show no difference or only trends, but rarely statistically significant differences.
The final methodological reason for inconclusive piggy-back analyses stems from the problem of study drug discontinuation. When patients reach major clinical study end points (e.g. heart attack in cardiovascular trials), they usually discontinue the study drug and they are switched to non-blinded, efficacious, safe and adjustable therapy for ethical reasons. After study drug discontinuation, the thorough data collection is stopped, and further data collection is restricted only to fatal events, malignancies and pregnancies. Strange but true, the real health economic story starts only after the detailed data collection ends. Patients are relatively problem-free before they reach major clinical end points, the majority of non-scheduled resource utilization occurs only after these events. This problem is especially valid in the field of prevention and chronic maintenance therapies, consequently economic analyses alongside pivotal clinical trials are rarely decisive for these therapies.
In naturalistic health economic analyses, health gain and resource utilization of patients are measured in routine care settings; there are no protocol-driven outpatient visits or diagnostic procedures. Health economic results represent real-world benefits and costs in non-selected patient population (Garrison et al., 2007). This approach improves the generalizability, in other words the external validity of health economic conclusions, but the implementation of such trial is very difficult. Appointments with the investigators cannot be planned in advance, monitoring of collected data is complicated unless the medical history and the resource utilization of patients are recorded in a validated database. Feasibility of naturalistic trials is easier in managed care settings or in health care systems with unique patient ID that can link resource utilization events of patients.
Naturalistic pharmacoeconomic studies are mostly non-interventional. As opposed to interventional clinical trials information on patient compliance can be collected in observational studies. However, the selection bias may significantly reduce the confirmatory evidence from non-interventional trials, as there is no classical randomization of patients, therefore selection among treatment options can be influenced by the clinical and socioeconomic status of patients. Statistical techniques, such as multivariate regression analysis, can help to reduce the impact of selection bias.
Time horizon of naturalistic studies is also limited, similarly to economic analyses alongside clinical trials. As data are collected in routine care settings, naturalistic trials are appropriate to investigate only registered and reimbursed medical technologies. Non-registered investigational medical therapies cannot be administered without strict clinical trial settings, including mandated outpatient visits to assess at least the safety of patients. Without reimbursement, only the most affluent patients can afford to pay for the investigational therapy in observational trials, which represents an obvious selection bias.
Pharmacoeconomic analyses are most useful to substantiate the reimbursement decisions of new pharmaceuticals. As results of naturalistic studies are available mainly after reimbursement decisions, their applicability for decision-making purposes is limited, mostly restricted for the revision of the initial decision on reimbursement and formulary listing.
Economic modelling on the basis of prospectively collected clinical trial data is more and more accepted by decision makers. Decision tree, Markov and discrete event simulation models are the most frequently applied techniques. In economic modelling, clinical data from RCTs are synthesized with information from epidemiological and cost-of-illness studies and other retrospective data sources to assess the potential economic benefit of new medical technologies.
If we rely only on prospective data collection, we can just partially understand the true pharmacoeconomic benefits of preventive medicines or maintenance therapies for chronic diseases. Reimbursement decisions of new anti-hypertensive medicines are not applicable only for a limited period, as the reimbursement is usually granted for the entire lifetime of patients. Cost-effectiveness analysis of new anti-hypertensives with 3 year time horizon is certainly not sufficient to capture the avoided mortality and major complications due to better blood pressure control, even if only 3 year data are available from pivotal registration trials. Economic modelling enables us to project intermediate results to longer time horizon, and thus estimate the true value of preventive medicines and the lifetime administration of pharmaceuticals for chronic conditions. Validation of surrogate end points, however, is a prerequisite for modelling beyond trial duration (Weinstein et al., 2003).
In economic models, we can assess the impact of those important parameters (e.g. patient compliance or burden of caregivers) on the cost-effectiveness of new medical technologies that cannot be investigated in pivotal clinical trials (e.g. patient compliance or burden of caregivers). If we follow certain rules and guidelines, we can apply pharmacoeconomic results to specific patient groups or local settings, which is crucial to ensure the relevancy of national reimbursement decisions (Sculpher and Drummond, 2006). Cost-effectiveness results by economic modelling can be presented before decision of payers on reimbursement and formulary listing.
Economic models should deliver the expected costs and health effects for all options relating to appropriate population and subpopulations, based on full range of existing evidence. Economic models have to include quantification of decision uncertainty, and may represent the valuation of future research. Accuracy of cost-effectiveness estimates based upon economic models depends on the appropriateness of modelling assumptions, including the model structure, time horizon and sophistication of the model to differentiate clinically and economically meaningful outcomes (Weinstein et al., 2003). Known uncertainty in input parameters reduces the clarity of conclusions; the reliability of input parameters is the most crucial factor in the adaptation of models to local settings. Thorough sensitivity analyses help us to understand the impact of uncertain input parameters on decisions.
In practice, economic models cannot be absolutely accurate. The search for absolute accuracy increases complexity, induces costs of gathering evidence and computation time, complicates the communication of results and creates potential modelling errors. Therefore, more complicated models need justifications in terms of better decisions.
Criteria of full economic evaluations
Two analytical conditions have to be fulfilled to assess the cost-effectiveness of medical procedures. It is not meaningful to state that a new drug is cost-effective. Cost-effectiveness of a medical technology is a relative category that can be examined in comparison with another therapeutic alternative. If we compare a bad drug to an even worse therapy, the bad drug still can be cost-effective. In full economic evaluations, the selection of the relevant comparator is one of the most crucial factors in estimating the cost-effectiveness of a medical technology.
In the case of new pharmaceuticals, the comparator can be the current gold standard therapy if the new medicine competes with the most widely used first-line technology. The comparator, however, can be the second or third line therapy, if the new medicine is used only when the first line therapy failed. Even placebo, palliative care or no treatment can be appropriate comparators, if there is no available effective therapy, or if the new drug can be used only for patients not responding or intolerant to current therapies. In case of diagnostic procedures, the new diagnostics and the subsequent treatments have to be compared with the old diagnostics and consequent therapy. In the economic evaluation of screening programmes, the screening and subsequent treatment are compared with treatment without screening, unless two screening techniques compete with each other.
Beyond the relativity of cost-effectiveness, there is an additional important criterion to assess the economic value of any health care procedure. If health care costs related to the use of a drug are lower than treatment costs related to another therapy, it may not be more cost-effective. In full economic evaluations, both cost of care and health gain, have to be examined. If we restricted our analysis to costs, selling ambulance cars would be the preferred option to modern emergency care, which is an obviously false conclusion. Therefore, cost of care cannot be interpreted without understanding changes in health status.
In summary, full economic evaluations compare at least two alternative medical technologies by examining both costs and consequences (Drummond et al., 1997).
Classification of pharmacoeconomic evaluations according to the measurement of health gain
We can differentiate four types of full economic evaluation (Drummond et al., 1997) according to the measurement of health gain (see Table 2). It is important to clarify the two different meanings of cost-effectiveness. In a wider sense, we can assess the cost-effectiveness of a medical procedure by any full economic evaluation method. In a narrow sense, cost-effectiveness analysis denotes a specific case of the four different types of full economic evaluation.
Table 2.
Classification of full economic evaluations according to the measurement of health gain
| Type of evaluation | Measurement of health gain | Measurement of costs | Applicability |
|---|---|---|---|
| Cost-minimization analysis | Non-specified (equal health gain) | Monetary value | Comparison of medical procedures with equal health gain |
| Cost-effectiveness analysis | Natural units (traditional clinical trial end points) | Monetary value | Comparison of medical procedures with non-equal health gain measurable in the same health dimension |
| Cost-utility analysis | Quality-adjusted life-years | Monetary value | Comparison of any medical procedures |
| Cost-benefit analysis | Monetary value | Monetary value | Comparison of any medical and non-medical procedures and investment options |
In cost-minimization analysis, the measurement of health gain is not specified; however, the health benefit of competing medical technologies has to be equal. If the therapeutic equivalence of pharmaceutical products within an ATC group is proven, the product with the lowest treatment cost is the most cost-effective alternative among them.
In cost-effectiveness analysis, we measure the health benefit by natural units and traditional clinical trial end points. Clinical diagnostic or device outcomes, such as Hgmm blood pressure reduction or mmol·L−1 LDL cholesterol reduction can be used in cost-effectiveness analysis, only if improvement in these intermediate outcomes results in similar improvement in hard end points. Assessment of health status by physicians (such as ulcer-free days, symptom-free days), major clinical events or hard end points (such as amputation, heart attack, stroke or death) are frequently used outcomes in cost-effectiveness analyses. The economic value of medical procedures with non-equal health gain can be compared in cost-effectiveness analysis, if the health benefit of alternative technologies can be measured in the same health dimension. Therefore, we cannot apply this method for the comparison of any health care technologies (e.g. we cannot judge whether a new prothesis in hip replacement, HPV vaccination, a new oral antidiabetic drug or a new lung cancer therapy should receive priority if resources are limited), as their health benefits are measured in different dimensions.
In cost-utility analysis, health gain is measured by an artificial unit, which combines the QoL and the survival benefit of patients. The most frequently used measure is called quality-adjusted life-years (QALYs) (Weinstein et al., 2009). In the calculation of QALYs, the expected life-years are weighted by their quality. The quality weight of perfect health is 1; no one can have better QoL than patients in perfect health. The utility weight of death is 0; however, there are potentially several health status with worse QoL (e.g. coma). In general, we can transform direct utility measures, self-reported patient preferences and QoL measures to quality weights for QALY calculations.
Several reasons justify the use of QALYs in evidence-based health policy. First, interpretability of perceived health gain in clinical trials requires such a specific measure, otherwise we cannot judge how meaningful a health benefit is a 5-point improvement on ADAS-Cog scale for an Alzheimer patient or 1 day reduction of influenza symptoms. Second, the comparability of health technologies is an important necessity in priority setting; we need to rank objectively the benefits of medical technologies with health gain in different dimensions. Finally, the majority of medical procedures result in both health gain and side effects. We need to summarize the health benefits to assess whether the application of the technology eventually results in positive health gain.
In comprehensive treatment of malignancies, patients may receive surgical care, radiotherapy, chemotherapy and maintenance pharmaceutical therapy. The initial phase of care might result in fatal consequences; surgery, radiotherapy and chemotherapy usually have serious side effects. Long-term maintenance pharmaceutical therapy may also result in side effects. On the contrary, the progression of the disease may stop or slow down due to the therapy, and consequently the life expectancy improves in addition to the less rapid deterioration of QoL. The area between the treatment and palliative care curves represents the QALY gain attributable to the comprehensive oncology therapy (see Figure 1).
Figure 1.
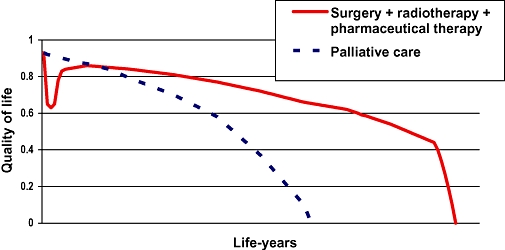
QALY gain of a comprehensive oncology therapy.
In cost-utility analysis, we can compare the economic value of any medical procedures, as the health benefit of alternative technologies can be measured in the same unit. The incremental cost of a QALY gain achievable by the investigated medical procedure is the final outcome of a cost-utility analysis. In theory, we can rank all available medical procedures according to their cost-effectiveness by applying cost-utility analyses; however, this project may require sufficient time, significant investment and strict methodological rules (Fox and Leichter, 1993). Alternatively, we can test whether the cost-effectiveness ratio of any new technologies exceeds the threshold acceptable for the society. This is how cost-utility analyses are used in more and more countries by health care payers.
In a cost-benefit analysis, not only costs but also health gain is measured by monetary values. A cost-benefit analysis is applicable to compare any health technologies even with non-medical investment opportunities. The validity, reliability and acceptance of converting health benefits into monetary values are low; therefore, cost-benefit analyses are rarely used for reimbursement and formulary listing decisions nowadays.
Impact of pharmacoeconomics on pharmaceutical R&D
Since the beginning of the 1990s, the scope of evidence base required for the market access of new pharmaceuticals has been reassessed. For registration purposes, innovators have to prove that the efficacy of the investigational medical product meets the requested threshold in RCTs, its risk/benefit ratio is acceptable and its quality is permanent.
For reimbursement and formulary listing purposes, drug manufacturers need to convince payers that the drug results in considerable health benefit in real world; its cost-effectiveness ratio does not exceed the threshold accepted by the society, and its financing is affordable, fits into the budget.
Pharmaceutical R&D faces the need for a new type of strategy: evidence base for registration and reimbursement has to be created at high scientific standards. This is a difficult task, as compliance with the different requirements needs different approaches. Evidences for registration have to be achieved with cheap, quick and low-risk clinical programme, because R&D resources are limited, and due to the capped patent protection period, a shorter clinical development phase results in a longer return phase. Simple clinical trials with suboptimal comparators support these objectives.
Evidences for reimbursement and formulary listing require another approach; innovators should prove in real-world situation that their new product is worth public financing against the best and most widely used alternative procedures. These clinical trials are longer and more expensive, as sample size has to be greater to power these studies adequately. In addition to efficacy and safety end points, a new type of data must be collected alongside clinical trials, which increases the risks and the expected budget of the R&D programme.
Role of health economists in the pharmaceutical R&D process
The role of health economists in the pharmaceutical R&D process is to initiate and optimize pharmacoeconomic data collection during the clinical development program so that the cost-effectiveness of investigational medical products can be presented at the time of launch. The armamentarium of health economists includes burden of disease, cost of illness studies, collection of resource utilization and QoL data prospectively alongside clinical trials, naturalistic health economic studies and economic modelling.
Appropriate timing of health economic activities is essential. During the early development phase, the health economic profile of target indications has to be established. Economic modelling could provide reasonable estimate about the economic value of the target product profile, which is a key input into the expected net present value calculations at internal R&D decision points (Pandey, 2003). Economic modelling may contribute to ‘go-no-go’ decisions (including business development and licensing, selection of the order of target indications and strategic pricing of new drugs).
The health economic profile defines the major value drivers of the investigational product, based upon which the health economic plan can be established. The health economic plan determines activities during the clinical development programme between phases I and III. Before launch, the global health economic dossier is prepared by the global health economic team in parallel with the adaptation of results to most important countries or markets. Local health economists in affiliates prepare country-specific health economic dossiers in order to obtain reimbursement and formulary listing in all countries. Health economists in the global teams and affiliates are also involved into the planning and pharmacoeconomic assessment of major phase IV, and observational studies to extend the economic evidence base in a constantly changing clinical and economic environment. Finally, in the case of new indications, a new health economic dossier has to be prepared.
Use of economic modelling for strategic pricing of pharmaceuticals
As already mentioned, market access of new prescription medicines is not only subject to approval by regulatory agencies, but also depends on the reimbursement by payers. Pricing of new pharmaceuticals has been constantly challenged by payers and economists (Newhouse, 2004). The optimal price for the company and their shareholders may not be acceptable for the society. Consequently, the reimbursement of new pharmaceuticals is subject to fulfilling the criteria of cost-effectiveness and affordability in more and more countries.
Therefore, traditional techniques, such as price elasticity curves elicited by market researchers, are not sufficient to establish pricing strategies of future drugs. Determining the right pricing and reimbursement strategy in the drug development process is critical. The question, ‘What is the highest price I can charge for this product’ is obviously incorrect. The right question is, ‘What pricing strategy meets the business objectives of the company or the product’ or ‘What price results in fast reimbursement or formulary listing in major markets’.
Strategic pricing includes strong health economic elements. The economic value of new medications can be calculated based upon the comparator price and the differential value (see Figure 2). The comparator is usually the most likely gold standard therapy at the time of the expected launch of the product.
Figure 2.
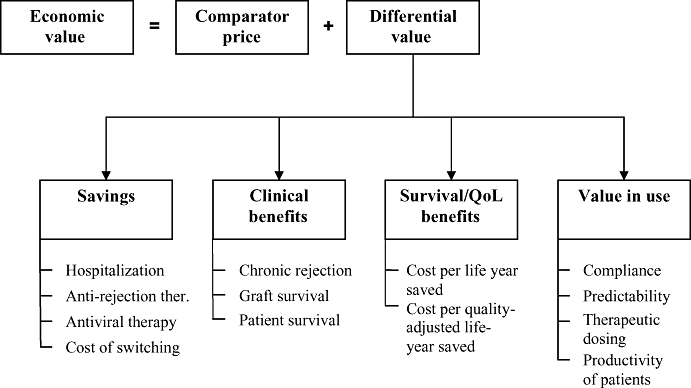
Economic value of a new immunosuppressive medicine.
Differential value includes cost savings; economic value of clinical, survival and QoL benefits; and the value in use, such as ease of administration, improved compliance, etc.
Figure 3 depicts a hypothetical example of how an innovative adjunct immunosuppressant with sufficient clinical benefit can be priced. The new drug improves graft survival, which results in avoided dialysis costs compared to the gold standard therapy. The new adjunct medicine allows reduction in baseline drug therapy; therefore, the total cost of the immunosuppressive regimen is not increased even if the new drug is more expensive than the old adjunct medicine. The incidence of some adverse events may be reduced while other events are more frequent; in our example, a net positive balance for avoided serious adverse event is depicted. Finally, each society is willing to pay a certain amount for QALY benefits. In the United States, Medicare pays approximately $80 000 for a QALY gain for patients on dialysis therapy.
Figure 3.
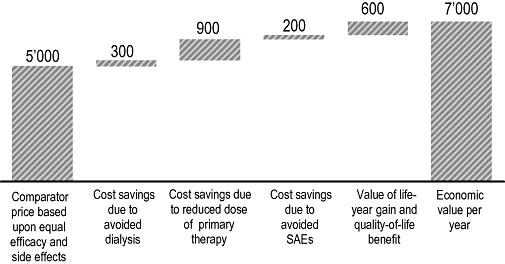
Economic value of a new immunosuppressive medicine – a theoretical example.
Although the price of a new medicine can be established independently from its economic value, this calculation is still a very important input into strategic pricing decisions. If the price of a new medicine is equal or less than its economic value, the cost-effectiveness of the product can be demonstrated easily, which is essential to obtain reimbursement and formulary listing in many countries.
Conclusion
Health care resources available for medical procedures, including pharmaceuticals, are limited all over the world. Economic evaluations help to alleviate the burden of scarce resources by improving the allocative efficiency of health care financing. Reimbursement and formulary listing of new medicines are subject to their cost-effectiveness and affordability in more and more countries. The need for pharmacoeconomic evidence has fundamentally changed the strategic imperatives of pharmaceutical R&D.
Professionals in the pharmaceutical R&D process have to be familiar with the principles of pharmacoeconomics, including selection of health policy-relevant comparators, analytical techniques and measurement of heath gain by QALYs.
There are three major approaches to calculate the economic value of new pharmaceuticals. Economic analyses alongside pivotal clinical trials are often inconclusive due to the suboptimal collection of economic data and protocol-driven costs. The major limitation of observational naturalistic economic evaluations is the selection bias and that they can be conducted only after registration and reimbursement.
Economic modelling has become a routine approach to predict the cost-effectiveness of new pharmaceuticals for reimbursement purposes. Accuracy of cost-effectiveness estimates based upon economic models depends on the quality of input variables; validity of surrogate end points; and appropriateness of modelling assumptions, including model structure, time horizon and sophistication of the model to differentiate clinically and economically meaningful outcomes. In addition to supporting reimbursement claims, economic modelling may also contribute to ‘go-no-go’ decisions (including business development and licensing), selecting the order of target indications and strategic pricing of new drugs.
These economic evaluation methods are not mutually exclusive; in practice, economic analyses often combine data collection alongside clinical trials or observational studies with modelling.
Glossary
Abbreviations:
- ENPV
expected net present value
- QALY
quality-adjusted life-years
- QoL
quality of life
- RCT
randomized controlled trial
- R&D
research and development
References
- Drummond MF, O'Brien BJ, Stoddart GL, Torrance GW. Basic types of economic evaluation. In: Drummond MF, O'Brien BJ, Stoddart GL, Torrance GW, editors. Methods for the Economic Evaluation of Health Care Programmes. New York: Oxford University Press; 1997. pp. 6–26. [Google Scholar]
- Fox DM, Leichter HM. State model: Oregon. The ups and downs of Oregon's rationing plan. Health Aff (Millwood) 1993;12:66–70. doi: 10.1377/hlthaff.12.2.66. [DOI] [PubMed] [Google Scholar]
- Garrison LP, Neumann PJ, Erickson P, Marshall D, Mullins CD. Using real-world data for coverage and payment decisions: the ISPOR Real-World Data Task Force Report. Value Health. 2007;10:326–335. doi: 10.1111/j.1524-4733.2007.00186.x. [DOI] [PubMed] [Google Scholar]
- Hlatky MA, Owens DK, Sanders GD. Cost-effectiveness as an outcome in randomized clinical trials. Clin Trials. 2006;3:543–551. doi: 10.1177/1740774506073105. [DOI] [PubMed] [Google Scholar]
- Mauskopf J, Rutten F, Schonfeld W. Cost-effectiveness league tables: valuable guidance for decision makers? Pharmacoeconomics. 2003;21:991–1000. doi: 10.2165/00019053-200321140-00001. [DOI] [PubMed] [Google Scholar]
- Newhouse JP. How much should Medicare pay for drugs? Health Aff. 2004;23:89–102. doi: 10.1377/hlthaff.23.1.89. [DOI] [PubMed] [Google Scholar]
- O'Sullivan AK, Thompson D, Drummond MF. Collection of health-economic data alongside clinical trials: is there a future for piggyback evaluations? Value Health. 2005;8:67–79. doi: 10.1111/j.1524-4733.2005.03065.x. [DOI] [PubMed] [Google Scholar]
- Pandey M. Investment decisions in pharmaceutical R&D projects. Drug Discov Today. 2003;8:968–971. doi: 10.1016/s1359-6446(03)02731-4. [DOI] [PubMed] [Google Scholar]
- Ramsey S, Willke R, Briggs A, Brown R, Buxton M, Chawla A, et al. Good research practices for cost-effectiveness analysis alongside clinical trials: the ISPOR RCT-CEA Task Force report. Value Health. 2005;8:521–533. doi: 10.1111/j.1524-4733.2005.00045.x. [DOI] [PubMed] [Google Scholar]
- Sculpher MF, Drummond MJ. Analysis sans frontières: can we ever make economic evaluations generalisable across jurisdictions? Pharmacoeconomics. 2006;24:1087–1099. doi: 10.2165/00019053-200624110-00006. [DOI] [PubMed] [Google Scholar]
- Weinstein MC, O'Brien B, Hornberger J, Jackson J, Johannesson M, McCabe C, et al. Principles of good practice for decision analytic modeling in health-care evaluation: report of the ISPOR Task Force on Good Research Practices – Modeling Studies. Value Health. 2003;6:9–17. doi: 10.1046/j.1524-4733.2003.00234.x. [DOI] [PubMed] [Google Scholar]
- Weinstein MC, Torrance G, McGuire A. QALYs: the basics. Value Health. 2009;12(Suppl. 1):S5–9. doi: 10.1111/j.1524-4733.2009.00515.x. [DOI] [PubMed] [Google Scholar]


