Abstract
Background and purpose:
5-Hydroxytryptamine (5-HT) has been shown to control and modulate many physiological and behavioural functions in insects. In this study, we report the cloning and pharmacological properties of a 5-HT1 receptor of an insect model for neurobiology, physiology and pharmacology.
Experimental approach:
A cDNA encoding for the Periplaneta americana 5-HT1 receptor was amplified from brain cDNA. The receptor was stably expressed in HEK 293 cells, and the functional and pharmacological properties were determined in cAMP assays. Receptor distribution was investigated by RT-PCR and by immunocytochemistry using an affinity-purified polyclonal antiserum.
Key results:
The P. americana 5-HT1 receptor (Pea5-HT1) shares pronounced sequence and functional similarity with mammalian 5-HT1 receptors. Activation with 5-HT reduced adenylyl cyclase activity in a dose-dependent manner. Pea5-HT1 was expressed as a constitutively active receptor with methiothepin acting as a neutral antagonist, and WAY 100635 as an inverse agonist. Receptor mRNA was present in various tissues including brain, salivary glands and midgut. Receptor-specific antibodies showed that the native protein was expressed in a glycosylated form in membrane samples of brain and salivary glands.
Conclusions and implications:
This study marks the first pharmacological identification of an inverse agonist and a neutral antagonist at an insect 5-HT1 receptor. The results presented here should facilitate further analyses of 5-HT1 receptors in mediating central and peripheral effects of 5-HT in insects.
Keywords: biogenic amine, constitutive activity, cellular signalling, G-protein-coupled receptor, insect, methiothepin, salivary gland, 5-HT, serotonin, WAY 100635
Introduction
The biogenic amine 5-hydroxytryptamine (5-HT) acts as a chemical messenger in most animal phyla in which it controls and modulates a great variety of important physiological and behavioural processes (Weiger, 1997). Disruption of the 5-hydroxytryptaminergic system has been linked to several human disease states, such as schizophrenia, migraine, depression, suicidal behaviour, infantile autism, eating disorders and obsessive–compulsive disorder (Jones and Blackburn, 2002). In insects, 5-HT signalling has been ascribed as being involved in the modulation of the heart rate (Zornik et al., 1999), secretory processes (Just and Walz, 1996), development (Colas et al., 1999), circadian rhythms (Page, 1987; Yuan et al., 2005), aggression (Dierick and Greenspan, 2007), behavioural gregarization in locusts (Anstey et al., 2009) and learning and memory (Sitaraman et al., 2008). The multifaceted actions of 5-HT are mediated by binding to integral membrane receptors, most of which, except for the 5-HT3 receptor ion channel, belong to the superfamily of G-protein-coupled receptors (GPCRs). In vertebrates, six main classes of G-protein-coupled 5-HT receptors have been classified on the basis of their sequence similarities, gene organization, second-messenger coupling pathways and pharmacological characteristics (Hoyer et al., 2002; nomenclature follows Alexander et al., 2009). The 5-HT1 and 5-HT5 receptors couple preferentially to Gi/o proteins, and inhibit cAMP synthesis. The 5-HT2 receptors couple preferentially to Gq/11 proteins, which mediate the hydrolysis of inositol phosphates and a subsequent increase in cytosolic Ca2+ levels. The 5-HT4, 5-HT6 and 5-HT7 receptors all couple preferentially to Gs proteins, and promote cAMP formation. In invertebrates, the 5-hydroxytryptaminergic system might be similarly complex (Hauser et al., 2006). For example, the fruit fly Drosophila melanogaster is known to express at least four 5-HT receptor subtypes that are predicted to be orthologs of the mammalian 5-HT1A, 5-HT2 and 5-HT7 receptors. These are the Dm5-HT1A and Dm5-HT1B (Saudou et al., 1992), Dm5-HT2 (Colas et al., 1995) and Dm5-HT7 (Witz et al., 1990) receptors, respectively (see Nichols, 2006; Nichols and Nichols, 2008). The sequences and the signal transduction mechanisms of 5-HT receptors are generally highly conserved between vertebrates and invertebrates (Hen, 1992). In contrast, the pharmacological properties of vertebrate and invertebrate receptors vary significantly in many cases (see Blenau and Baumann, 2001; Tierney, 2001). The 5-HT1 receptors form the largest class of 5-HT receptors (Gerhardt and van Heerikhuizen, 1997). In vertebrates, several members of this class show agonist-independent activation of associated G proteins, and thus are constitutively active (McLoughlin and Strange, 2000; Martel et al., 2007). Constitutive activity is now accepted as being a common property of many GPCRs (Seifert and Wenzel-Seifert, 2002), but has not, as yet, been shown for any insect 5-HT1 receptor.
In the present study, we have cloned and characterized a 5-HT receptor of the cockroach Periplaneta americana with significant homologies to members of the 5-HT1 receptor class. Cockroaches have been widely used as a model organism for basic research in physiology and neurobiology (Downer, 1990; Watanabe and Mizunami, 2007). In particular, the salivary gland of P. americana is a well-established model system for studying excitation–secretion coupling in epithelia and aminergic signal transduction (see House and Ginsborg, 1985; Walz et al., 2006). Information has thus been accumulated on the pharmacology of amine receptors in the salivary glands and other tissues of cockroaches (Downer, 1990; Walz et al., 2006; Troppmann et al., 2007). Comparatively little is known, however, concerning the exact repertoire and molecular properties of amine receptors in P. americana (Bischof and Enan, 2004; Rotte et al., 2009), and, until this study, no molecular data on 5-HT receptors have been available.
In this investigation, we show that the mRNA encoding a cockroach 5-HT1 receptor is expressed in the brain, salivary gland and midgut tissue. Immunohistochemical analysis has revealed the presence of the receptor protein in a specific subset of pars intercerebralis cells of the cockroach brain. When stably expressed in HEK 293 cells, the receptor inhibits the formation of cAMP with an EC50 of ∼130 nM for serotonin. The receptor shows constitutive activity, which can be blocked by the 5-HT1A receptor antagonist WAY 100635. Our study has therefore elucidated unique molecular and pharmacological details of an insect 5-HT1 receptor, and advances our knowledge concerning the complexity of the 5-hydroxytryptaminergic system in insects.
Methods
Cloning of Pea5-ht1 cDNA
Degenerate primers (DF1: 5′-TGYTGGBTICCITTYTT-3′; DR1: 5′-TTDATISHRTADATIAYIGGRTT-3′) corresponding to highly conserved amino acid sequences in TM 6 and TM 7 of biogenic amine receptors were designed to amplify receptor fragments (Walz et al., 2006). The PCR was performed on a P. americana brain cDNA library (Blenau and Baumann, 2005). Amplification was carried out for 2.5 min at 94°C (one cycle), followed by 35 cycles of 40 s at 94°C, 40 s at 55–65°C and 30 s at 72°C, and a final extension of 10 min at 72°C. The PCR product was cloned into pGEM-T vector (Promega, Mannheim, Germany), and subsequently analysed by DNA sequencing (AGOWA, Berlin, Germany). Based on this sequence information, specific primers for rapid amplification of cDNA ends (RACE) PCR experiments were designed. To amplify the missing 5′-region of the cDNA, two consecutive 5′ RACE experiments were performed with specific reverse primers (S5-1: 5′-GAGTTGAAATAGCCGAGCC-3′, S5-2: 5′-CACTAGGAGCGTTGTGTCC-3′). Amplification of the 3′ end was performed by 3′ RACE by using a specific forward primer (S3: 5′-GGAGAGCTTCTTTCTGTGG-3′). Finally, a PCR was performed on single-stranded P. americana brain cDNA to amplify the entire coding region of Pea5-ht1 by using two gene-specific primers annealing in the 5′- and 3′-untranslated regions (SF1: 5′-GTGCGGTGCTGTCGACGCC-3′; SR1: 5′-CTCCGTTAATATAGCGCAC-3′). The nucleotide sequence of Pea5-ht1 has been submitted to the EBI database (accession no. FN298392).
Multiple sequence alignment and phylogenetic analysis
Amino acid sequences used for phylogenetic analyses were identified by protein–protein BLAST searches of the NCBI database with the deduced amino acid sequence of Pea5-ht1 (Pea5-HT1) as ‘bait’. Multiple sequence alignments of the complete amino acid sequences were performed with ClustalW. Values for identity (ID) and similarity (S) were calculated by using the BLOSUM62 substitution matrix in BioEdit 7.0.5 (Hall, 1999). MEGA 4 (Tamura et al., 2007) was used to calculate the genetic distances between the core sequences, and to construct neighbour-joining trees with 2000-fold bootstrap resampling. The D. melanogaster ninaE-encoded rhodopsin 1, and the D. melanogaster FMRFamide receptor were used as outgroups.
RT-PCR amplification of Pea5-ht1 fragments
Total RNA was isolated from brain, salivary glands, midgut, Malpighian tubules and flight muscle of adult male cockroaches by using TRIZOL LS (Invitrogen, Karlsruhe, Germany). The samples were either digested with DNase I (Ambion, Huntingdon, UK) to degrade contaminating genomic DNA or with DNase I and an RNase Cocktail (Ambion) for negative controls. Pea5-ht1-specific fragments were amplified from 100 ng total RNA by using the SuperScript One-Step RT-PCR System (Invitrogen). The sense primer was 5′-GACACTAGTGGTGCTTCTGG-3′, and the antisense primer was 5′-GTCATGGGACCTACGCCATC-3′. Amplification resulted in a fragment of 243 bp. RT-PCR was also performed with primers for the P. americana actin gene (accession no. AY116670) as an internal control (ActinF: 5′-CGAGTAGCTCCTGAAGAGC-3′; ActinR: 5′-GGCCTCTGGACAACGGAACC-3′). cDNA was synthesized for 30 min at 50°C, followed by a single denaturation step at 94°C for 2 min. Amplification of Pea5ht-1 or Peaactin fragments was performed for 30 cycles at 94°C for 40 s, 60°C for 40 s and 72°C for 40 s, followed by a final extension at 72°C for 10 min.
Antibody production and purification
The anti-Pea5-HT1 receptor polyclonal rabbit antiserum was produced commercially (Pineda-Antikörper-Service, Berlin, Germany). Antibodies were raised against a synthetic peptide (NH2-CFITKRRFRRMKSNKKSS-CONH2) corresponding to a region within the 3rd cytoplasmic loop of the Pea5-HT1 receptor (Figure 1). A cysteine residue was added N-terminally to the peptide for coupling to the protein carrier, viz., keyhole limpet haemocyanin. The monospecific IgG fraction was purified via affinity chromatography.
Figure 1.

Amino acid sequence alignment of Pea5-HT1 and orthologous receptors from Drosophila melanogaster (Dm5-HT1A; accession no. CAA77570), Dm5-HT1B (no. CAA77571), Papilio xuthus (Pxu5-HT1, no. BAD72868) and Penaeus monodon (Pem5-HT1, no. AAV48573). Identical residues (≥80%) between the receptors are shown as white letters against black, whereas conservatively substituted residues are shaded. Putative transmembrane domains (TM 1–TM 7) are indicated by grey bars. Potential N-glycosylation sites (+) and putative palmitoylation sites (*) of Pea5-HT1 are labelled. Underlined letters represent the region within the third cytoplasmic loop from which the Pea5-HT1-specific peptide antigen was derived. The amino acid position is indicated on the right.
Western blot analysis
Entire cockroach brains were homogenized in 150 µL Roti-load sample buffer (Roth, Karlsruhe, Germany), and incubated for 5 min at 95°C, or membrane proteins were isolated and incubated for 5 min at 60°C. Proteins were separated by sodium dodecyl sulphate–polyacrylamide gel electrophoresis on 12% gels. Approximately 10 µg total protein, as determined by a modified Bradford assay, was run per lane. Proteins were transferred to polyvinylidene fluoride membranes (Roth). Membranes were blocked with 5% dry milk in Tris-buffered saline containing Tween 20 (TBS-T; 10 mM Tris–HCl, pH 7.5, 150 mM NaCl, 0.01% Tween 20) for 30 min at room temperature. Membranes were probed with affinity-purified anti-Pea5-HT1 antibodies (dilution 1:20 000 in TBS-T). For controls, antibodies were pre-absorbed to the synthetic peptide (15 µg·mL–1). Membranes were washed with TBS-T, followed by incubation with a secondary antibody conjugated to horseradish peroxidase (1:20 000; American Qualex, La Mirada, CA, USA). Signals were visualized with an enhanced chemiluminescence detection system (Super Signal West Pico Chemiluminescent Substrate, Pierce, Rockford, IL, USA). Each analysis was performed three times with independently isolated protein samples.
Immunofluorescence staining of brain sections
Cockroach brains were dissected, fixed overnight at 4°C in 4% paraformaldehyde in phosphate-buffered saline (pH 7.4) and washed 3 × 5 min with buffer 1 (50 mM Tris–HCl, pH 7.5). Brains were embedded in 18% gelatin (250 g Bloom, Fluka, Buchs, Switzerland), and cut into 50 µm thick serial sections on a vibratome (Pelco 101, series 1000; Pelco, St Louis, MO, USA). Mildly agitated, free-floating sections were subsequently exposed to the following steps: (i) de-gelatinization in warm buffer 1 for 2 × 10 min; (ii) rinsing in buffer 1 for 2 × 15 min; (iii) incubation in 50 mM NH4Cl in buffer 1 for 10 min; (iv) washing in buffer 1 for 3 × 10 min; (v) incubation for 90 min in a blocking solution (buffer 1 containing 3% normal goat serum and 0.5% Triton X-100), which was also used as antibody diluent (AD); (vi) overnight incubation at 4°C in primary antisera (rabbit anti-Pea5-HT1 1:1000 and rat anti-5-HT 1:100 (Chemicon, Temecula, CA, USA) in AD; (vii) washing in buffer 2 (50 mM Tris, pH 7.5; 145 mM NaCl) for 3 × 15 min; (viii) incubation in Alexa Fluor 488 goat anti-rabbit IgG 1:100 (Molecular Probes, Eugene, OR, USA) and Cy3-conjugated AffiniPure goat anti-rat IgG 1:400 (Jackson Immunoresearch, West Grove, PA, USA) diluted in AD for 3 h at room temperature; (ix) washing in buffer 2 for 3 × 15 min; and (x) mounting on slides with Mowiol 4.88 (Farbwerke Hoechst, Frankfurt, Germany) containing 2% n-propyl-gallate as an anti-fading reagent. Sections from 15 individual brains were treated with the Pea5-HT1 receptor antiserum and sections from five brains with pre-absorbed antiserum. Fluorescence images were recorded with a Zeiss LSM 510 confocal microscope (Carl Zeiss, Jena, Germany).
Construction of pcPea5-ht1-HA expression vector
An expression-ready construct of Pea5-ht1 cDNA was generated by PCR. To monitor transfection efficiency and receptor protein expression, a hemagglutinin (HA) epitope tag was engineered onto the 3′ end of the cDNA. PCR was performed with a sense primer 5′-TATGATGTGCGGCCGCCCACCATGGATCTCCTGAGC-3′ and the antisense primers, 5′-TGGGACGTCGTatGGGTaTCTAAGCTTTCCCGGCCTG-3′ (first-round PCR) and 5′-TTTTCTAGATTAAGCGTAGTCTGGGACGTCGTATGGGTA-3′ (second-round PCR). The PCR product was digested with Not I and Xba I, and subcloned into pcDNA3.1(+) vector (Invitrogen) yielding pcPea5-ht1-HA. The correct insertion was confirmed by DNA sequencing.
Functional expression of the Pea5-HT1-HA receptor
Approximately 8 µg pcPea5-ht1-HA vector was introduced into exponentially growing HEK 293 cells (∼4 × 105 cells per 5 cm Petri dish) by a modified calcium phosphate method (Chen and Okayama, 1987). Stably transfected cells were selected in the presence of the antibiotic G418 at 0.8 mg·mL−1. Isolated foci were propagated and analysed for the expression of Pea5-HT1-HA by immunocytochemistry and Western blot with a commercial anti-HA antibody (Anti-HA High Affinity, Roche, Penzberg, Germany).
Functional characterization of Pea5-HT1 receptors
Assays to determine the ability of Pea5-HT1-HA receptors to attenuate adenylyl cyclase activity were performed as described earlier (Grohmann et al., 2003). Pea5-HT1-expressing cells were grown in minimal essential medium with GlutaMAX™I, 10% (v/v) fetal bovine serum (FBS), 1% (v/v) non-essential amino acids and antibiotics (all from Invitrogen). Cells were incubated with ligands for 30 min at 37°C in the presence of the phosphodiesterase inhibitor, isobutylmethylxanthine (IBMX; final concentration 100 µM) and lysed by adding 0.5 mL ice-cold ethanol. After 1 h at 4°C, the lysate was transferred to a reaction tube, and lyophilized. The amount of cAMP produced was determined with the TRK 432 cyclic AMP assay kit (GE Healthcare, Freiburg, Germany). Mean values of cAMP·mg−1 protein were determined from four independent measurements, performed in duplicate to quadruplicate.
In earlier experiments with a HEK 293 cell line expressing an insect 5-HT7 receptor (Am5-HT7, Schlenstedt et al., 2006), we observed desensitization effects of Am5-HT7, most likely due to the presence of 5-HT in FBS. For that reason, we determined dose–response curves for 5-HT with Pea5-HT1 receptor-expressing cells in medium supplemented with either 10% FBS or 2% Ultroser G (Pall Bioserpa, Cergy-Saint-Christophe, France). As EC50 values were not significantly different under these conditions (data not shown), all experiments were performed with cells grown in medium containing 10% (v/v) FBS as indicated above.
Data analysis
Data shown represent the mean values ± SEM, and were analysed (sigmoidal dose–response curves, calculation of EC50 values, statistics) and displayed by using PRISM 4.01 software (GraphPad, San Diego, CA, USA). Statistical significance of differences between means was determined by using one-way anova followed by Dunnett's multiple comparison test with P values ≤ 0.05 being considered significant.
Materials
The pharmacological ligands 5-carboxamidotryptamine maleate salt (5-CT), 5-HT hydrochloride, 5-methoxytryptamine (5-MeOT) (+/−)-8-hydroxy-2-(dipropylamino)tetralin hydrobromide (8-OH-DPAT), buspirone hydrochloride, dopamine hydrochloride, histamine dihydrochloride, lysergic acid diethylamide (LSD), methiothepin mesylate salt, mianserin hydrochloride (+/−)-octopamine hydrochloride, spiperone, sumatriptan succinate, tyramine hydrochloride and WAY 100635 maleate salt were all purchased from Sigma-Aldrich Chemie GmbH (Taufkirchen, Germany).
Results
Cloning and sequence analysis of a 5-HT1 receptor from P. americana
Initially, a 97 base pair long cDNA fragment of a putative 5-HT receptor of P. americana was amplified by using degenerate primers (see Methods). The full-length cDNA was obtained by 5′ and 3′ RACE experiments. Sequence analysis revealed that the P. americana receptor had highest similarity to 5-HT receptors of the 5-HT1 subtype. The deduced amino acid sequence is characterized by a long third cytoplasmic loop and a short C-terminal region, consistent with the structure of 5-HT1 receptors, which are coupled to Gi proteins (Gerhardt and van Heerikhuizen, 1997). Accordingly, the P. americana receptor was named Pea5-HT1. The nucleotide sequence of Pea5-ht1 consists of 2564 nucleotides. The longest open reading frame comprises 2049 nucleotides encoding a predicted protein of 683 amino acids with a calculated molecular weight of 74 kDa (Pea5-HT1). Upstream of the translation initiation codon (ATG, position 193–195), stop codons were identified in all three reading frames. The flanking sequence of this ATG triplet agrees well with the consensus sequence for the eukaryotic translation start site (CCACCATGG; Kozak, 1984). Analysis of the deduced amino acid sequence with the topology predictor Phobius (Käll et al., 2004) led to the prediction that the polypeptide contains an extracellular N-terminus, a cytoplasmic C-terminal region and seven hydrophobic helical domains. Within these transmembrane segments, the typical sequence motifs of 5-HT1 receptors were well conserved (Figure 1). 5-HT receptors are known for their numerous post-translational modifications. The N-terminal region of the Pea5-HT1 receptor contains seven consensus sites for N-linked glycosylation (Figure 1). The third cytoplasmic loop comprises four consensus sites for phosphorylation by protein kinase C ([S/T]-x-[R/K]). The C-terminus of Pea5-HT1 receptors harbours a cysteine residue (C666), a potential target for palmitoylation (Papoucheva et al., 2004). Anchorage of palmitic acid in the membrane would create a fourth intracellular loop, which is believed to stabilize the structure of the receptor. Together, these results demonstrate that the deduced amino acid sequence of the Pea5-HT1 receptor displays the characteristic features of seven-transmembrane-domain receptors with qualities appropriate to 5-HT receptors.
BLAST searches with the Pea5-HT1 sequence indicated that it shared pronounced homology with 5-HT1 receptors of crustaceans and insects. High amino acid identity (ID)/similarity (S) was found with the 5-HT1 receptors of the holometabolous insects Papilio xuthus (Px5-HT1; ID 44%, S 53%) and D. melanogaster (Dm5-HT1A; ID 39%, S 50%; Dm5-HT1B; ID 39%, S 52%) and for the crustaceans Penaeus monodon (Pem5-HT1; ID 42%, S 52%), Procambarus clarkii (Pro5-HT1; ID 45%, S 54%) and Panulirus interruptus (Pan5-HT1; ID 43%, S 51%) (Figure 1). A multiple amino acid sequence comparison within the conserved transmembrane domains and short linker regions of Pea5-HT1 with invertebrate and human 5-HT receptors was used to calculate a phylogenetic tree (Figure 2). The Pea5-HT1 receptor was incorporated into the comprehensive branch of the 5-HT1 receptor class, and was robustly placed in a clade of invertebrate 5-HT1 receptors.
Figure 2.
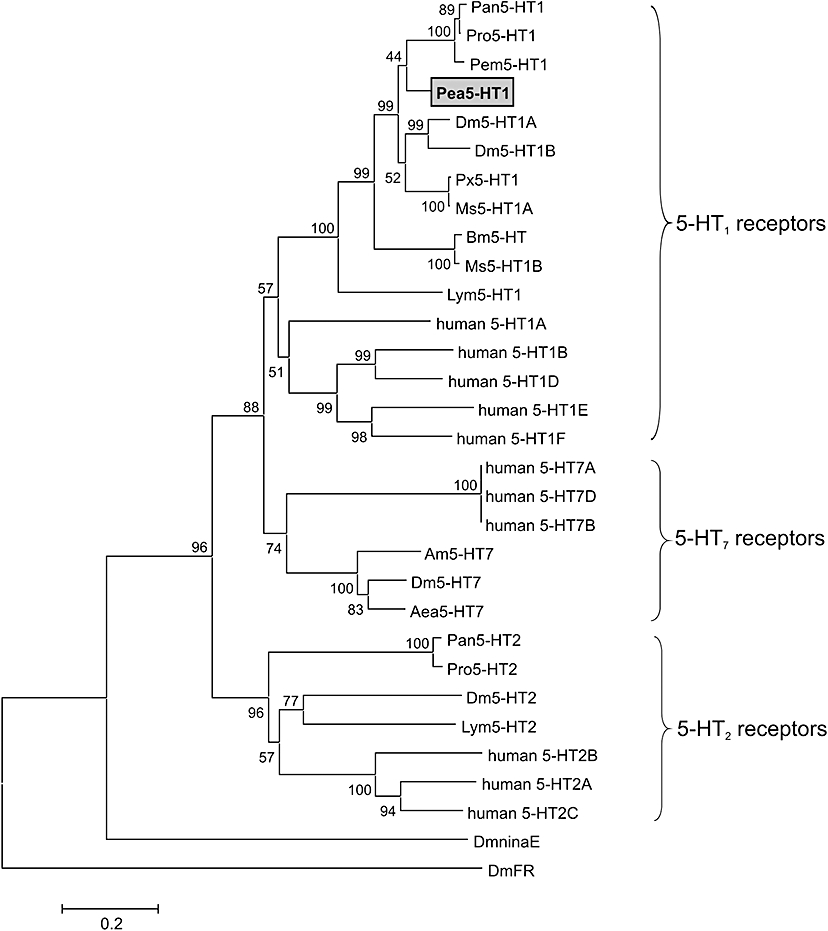
Phylogenetic analysis of Pea5-HT1 and various 5-HT receptors. Alignments were performed with BioEdit (version 7.0.5; Hall, 1999) using the core amino acid sequences lacking the variable regions of the amino and carboxyl terminus, and the third cytoplasmic loop. The genetic distance was calculated with MEGA4 (Tamura et al., 2007). The receptor sequences followed by their accession numbers are listed in the order illustrated: Panulirus interruptus (Pan5-HT1, no. AY528822); Procambarus clarkii (Pro5-HT1, no. ABX10973), Penaeus monodon (Pem5-HT1, no. AAV48573), Periplaneta americana (Pea5-HT1, no. FN298392), Drosophila melanogaster (Dm5-HT1A, no. CAA77570), Dm5-HT1B (no. CAA77571), Papilio xuthus (Pxu5-HT1, no: BAD72868), Manduca sexta (Ms5-HT1A, no. DQ840515), Bombyx mori (Bm5-HT, no. CAA64862), Ms5-HT1B (no. DQ840516), Lymnaea stagnalis (Lym5-HT1, no. L06803), human 5-HT1A (no. NP_000515), human 5-HT1B (no. NP_000854), human 5-HT1D (no. NP_000855), human 5-HT1E (no. NP_000856), human 5-HT1F (no. NP_000857), human 5-HT7 isoform a (no. NP_000863), human 5-HT7 isoform d (no. NP_062873), human 5-HT7 isoform b (no. NP_062874), Apis mellifera (Am5-HT7, no. AM076717), Dm5-HT7 (no. A38271), Aedes aegypti (Aae5-HT7, no. AAG49292), Pan5-HT2 (no. AY550910), Pro5-HT2 (no. ABX10972), Dm5-HT2 (no. CAA57429), Lym5-HT2 (no. U50080), human 5-HT2B (no. NP_000858), human 5-HT2A (no. NP_000612), human 5-HT2C (no. NP_000859), D. melanogaster ninaE-encoded rhodopsin 1 (DmninaE, no. NM_079683) and D. melanogaster FMRFamide receptor (DmFR, no. AAF47700). The numbers at the nodes of the branches represent the percentage bootstrap support for each branch. The scale bar allows conversion of branch lengths in the dendrogram to genetic distance between clades.
Tissue distribution of Pea5-ht1 mRNA
The expression pattern of Pea5-ht1 mRNA in various tissues of P. americana was investigated by RT-PCR with specific primers corresponding to sequences within the third cytoplasmic loop. The transcript of the Pea5-HT1 gene was detected in samples of the brain, salivary glands and midgut (Figure 3). Conversely, no receptor mRNA expression was detected in samples of Malpighian tubules and leg muscle. To ensure that the fragments had not been amplified from genomic DNA, samples were treated with DNase I. The negative control PCR on samples treated additionally with an RNase cocktail did not result in the amplification of any PCR product (data not shown).
Figure 3.
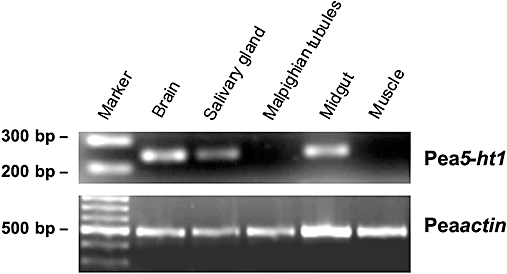
Tissue distribution of Pea5-ht1 mRNA. The 100 bp DNA ladder (marker) is shown on the left. Detection of PCR products amplified on total RNA isolated from brain, salivary glands, Malpighian tubules, midgut and muscle. Amplification failed when samples were digested with an RNase cocktail prior to RT-PCR (data not shown). The lower panel shows RT-PCR products amplified by using actin (accession no. AY116670)-specific primers as a control.
Generation of an anti-Pea5-HT1 antibody and immunohistochemical localization of Pea5-HT1 receptors
We generated a polyclonal antiserum directed against a part of the third cytoplasmic loop of Pea5-HT1 receptors, as described in Methods. Western blots of P. americana brain proteins showed that the antibody recognized a single band of ∼80 kDa (Figure 4A). After pre-absorption with the peptide (15 µg·mL−1) used for immunization, the signal was completely lost. This result demonstrated that the antibody specifically recognized the Pea5-HT1 receptor protein. In addition to its presence in brain tissue, the 5-HT1 receptor was detected on Western blots with membrane proteins from salivary gland tissue. Here, the receptor protein was less abundantly expressed. Furthermore, we studied whether the receptor protein was glycosylated in these tissues. De-glycosylation of membrane proteins from brain and salivary gland tissue by PNGase F digest resulted in a slight shift of the apparent molecular weight from ∼80 to ∼75 kDa (Figure 4B). This molecular weight was in good accordance with the predicted molecular weight of 74 kDa for the Pea5-HT1 receptor.
Figure 4.
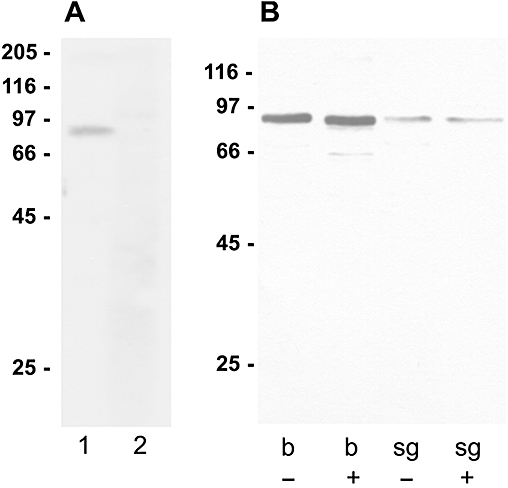
Western blot analysis with the anti-Pea5-HT1 receptor antibody. Molecular weight marker in kDa. (A) Specificity of anti-Pea5-HT1 receptor antibody tested on Periplaneta americana brain proteins. Lane 1: Western blot analysis with anti-Pea5-HT1 antibody (1:20 000). A single band of ∼80 kDa was detected. Lane 2: Western blot analysis with anti-Pea5-HT1 antibody (1:20 000) pre-absorbed with 15 µg·mL−1 of the peptide used for immunization; no band was detected. (B) Western blot of membrane proteins (10 µg per lane) from brain tissue (b) and salivary glands (sg) treated without (–) or with (+) PNGase F. De-glycosylation resulted in a slight shift of the protein band from ∼80 to ∼75 kDa.
To determine the cellular distribution of the Pea5-HT1 receptor within the brain of P. americana, vibratome sections were immunostained with the anti-Pea5-HT1 antibody and with an anti-5-HT antibody for orientation purposes. The receptor antibody specifically labelled some large somata in the pars intercerebralis (Figure 5A and C). Labelled axons of these neurons pass down the anterior surface of the brain and cross over in the chiasma region of the corpora cardiaca nerve 1 (NCC 1). Extensive arborization is observed in the protocerebral region ventral of the pars intercerebralis. The labelled axons then proceed posteriorly and continue to the corpora cardiaca complex. Accordingly, in more posterior frontal sections of the brain, the cell cluster in the pars intercerebralis and NCC 1 profiles were stained (Figure 5B and C).
Figure 5.
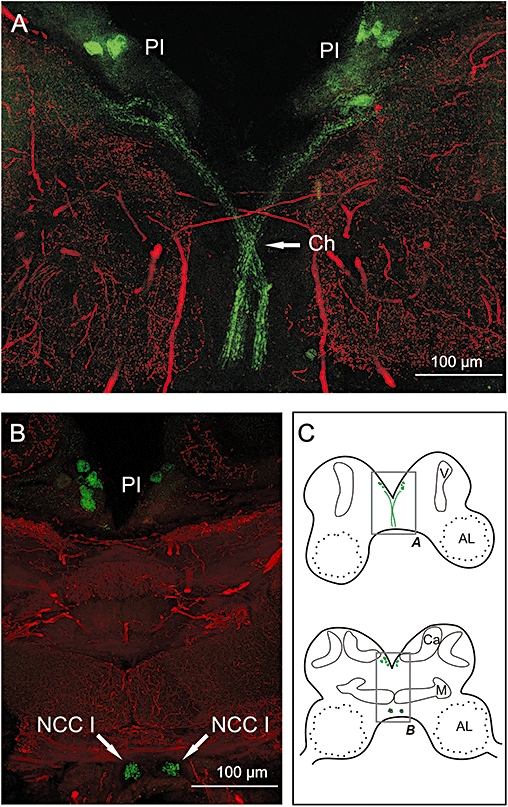
Immunohistochemical localization of Pea5-HT1 receptors in brain sections. Vibratome sections of brains were double labelled with anti-5-HT (red) and anti-Pea5-HT1 (green), and imaged by confocal microscopy. Each image shows the sum of multiple consecutive optical sections representing a total thickness of ∼20 µM. (A) Central part of an anterior frontal section of the cockroach brain (for schematic drawing, see C). (B) Central part of a more posterior frontal section of the cockroach brain. (C) Schematic drawings of sections of the cockroach brain. Grey boxes highlight the details displayed in A + B. Abbreviations: V, vertical lobe; AL, antennal lobe; Ca, calyx; Ch, chiasm region of nervi coporis cardiaci I; M, medial lobe; NCC I, nervus corporis carciaci I; PI, pars intercerebralis.
Functional analyses of Pea5-HT1 receptors in HEK 293 cells
For functional and pharmacological characterization, we generated a HEK 293 cell line stably expressing the Pea5-HT1 receptor (see Methods). Expression of Pea5-HT1 receptors was confirmed by Western blotting and immunocytochemistry (data not shown). Basal levels of intracellular cAMP ([cAMP]i) were not significantly different in non-transfected (12.9 ± 0.5 pmol cAMP·mg−1 protein, n= 4) and Pea-5-HT1-expressing cells (16.3 ± 0.7 pmol cAMP·mg−1 protein, n= 4). However, Pea5-HT1-expressing cells treated with a non-saturating concentration of NKH-477 (10 µM; Wachten et al., 2006) exhibited significantly higher levels of cAMP (154.4 ± 7.3 pmol cAMP·mg−1 protein, n= 4) than NKH-477-treated controls (76.7 ± 2.3 pmol cAMP·mg−1 protein, n= 4). To analyse the ligand specificity of the cloned receptor, various biogenic amines (5-HT, dopamine, tyramine, octopamine and histamine, each at a concentration of 10 µM) were tested for the inhibition of NKH-477-stimulated cAMP production in non-transfected and Pea5-HT1-expressing cells. Only 5-HT significantly decreased the NKH-477-induced production of cAMP in Pea5-HT1-expressing cells (Figure 6A). No effect of 5-HT was observed in non-transfected cells. The dose–response relationship of 5-HT on the cAMP level was examined for 5-HT concentrations ranging from 1 nM to 30 µM. In Pea5-HT1-expressing cells, the 5-HT effect was concentration dependent and saturable, resulting in a sigmoidal dose–response curve (Figure 6B). Half-maximal reduction of cAMP production (EC50) was observed with ∼130 nM 5-HT (logEC50=−6.89 ± 0.08, mean ± SEM). Maximal attenuation of cAMP synthesis (by ∼75%) was attained with 5-HT concentrations of ≥10 µM.
Figure 6.
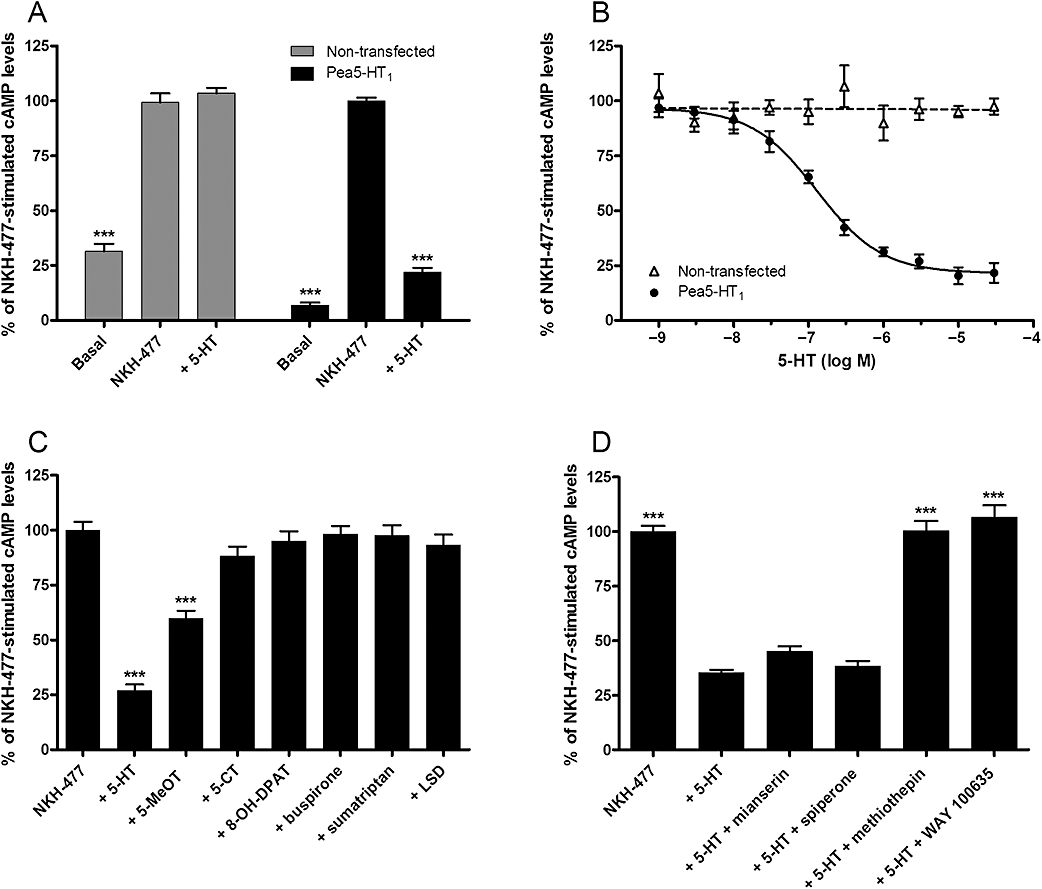
Modulation of intracellular cAMP levels in HEK 293 cells stably expressing the Pea5-HT1 receptor and in non-transfected cells. The amount of cAMP is given as the percentage of the value obtained after incubation with 10 µM NKH-477 (100%), a water-soluble forskolin analogue. Error bars indicate SEM and are in some cases too small to be represented. The statistical analysis is based on a one-way anova followed by Dunnett's multiple comparison test; ***P < 0.0001. (A) Effect of NKH-477 and 5-HT (10 µM) on cAMP levels in non-transfected cells and in Pea5-HT1-expressing cells. To determine the basal [cAMP]i, cells were incubated with 100 µM IBMX only (basal). Data represent the mean ± SEM of 16 values from four experiments each performed in quadruplicate. Asterisks indicate statistically significant differences for drug versus NKH-477 (100%) for a given cell line. (B) Dose-dependent effect of 5-HT (10−9–3 × 10−5 M) on [cAMP]i. Data represent the mean ± SEM of eight replicates from four experiments each performed in duplicate. (C) Effects of 5-HT receptor agonists (10 µM) on NKH-477-stimulated cAMP production in Pea5-HT1-expressing cells. Data represent the mean ± SEM of 14 values from four experiments each performed in either triplicate or quadruplicate. Asterisks indicate statistically significant differences for drug versus NKH-477. (D) Effects of putative antagonists (10 µM) on 5-HT-mediated (500 nM) inhibition of NKH-477-stimulated cAMP production in Pea5-HT1-expressing cells. Data represent the mean ± SEM of 16 values from four experiments each performed in quadruplicate. Asterisks indicate statistically significant differences for drug versus 5-HT.
To characterize the pharmacological profile of Pea5-HT1 receptors in detail, the effect of various 5-HT receptor agonists and antagonists on NKH-477-induced cAMP production was investigated. The 5-HT derivative 5-MeOT, a non-selective 5-HT receptor agonist, acted as partial agonist and decreased the NKH-477-mediated cAMP production by ∼40% (Figure 6C). 5-CT and 8-OH-DPAT, selective agonists for mammalian 5-HT1 and 5-HT7 receptors, respectively, showed no significant effect on [cAMP]i. Furthermore, the selective 5-HT1A receptor agonist buspirone, the 5-HT1B/D/F agonist sumatriptan and the ergoline LSD (a 5-HT2 receptor agonist) also showed no detectable effect. In addition, the ability of four putative antagonists to inhibit the 5-HT effect on NKH-477-stimulated cAMP production was assayed. Mianserin and spiperone showed no antagonistic activity at the tested concentration of 10 µM (Figure 6D). On the contrary, the inhibitory effect of 5-HT (500 nM) was antagonized by methiothepin, a non-selective antagonist at mammalian 5-HT receptors (Hoyer et al., 1994), and by WAY 100635, a potent and selective 5-HT1A receptor antagonist (Fletcher et al., 1996). The effect of both substances was dose dependent (Figure 7A). Methiothepin precisely compensated for the 5-HT effect, which resulted in a [cAMP]i of ∼100%. In contrast, WAY 100635 not only compensated for the 5-HT effect, but increased [cAMP]i even further to more than 100%. These results indicated that methiothepin acted as a neutral antagonist, whereas WAY 100635 acted as an inverse agonist on the Pea5-HT1 receptor, which is constitutively active when expressed in HEK 293 cells (see above). To confirm this hypothesis, the effect of both substances on the NKH-477-induced cAMP production was examined in the absence of 5-HT. As expected, the neutral antagonist methiothepin did not affect [cAMP]i. WAY 100635, in contrast, increased [cAMP]i in a dose-dependent manner, thus antagonizing the constitutive activity of Pea5-HT1 receptors by acting as an inverse agonist (Figure 7B). In non-transfected control cells, WAY 100635 (30 µM) did not significantly influence the NKH-477-stimulated cAMP level (data not shown). Finally, the neutral antagonist methiothepin was able to block the effect of the inverse agonist WAY 100635 in Pea5-HT1-expressing cells (Figure 7C).
Figure 7.
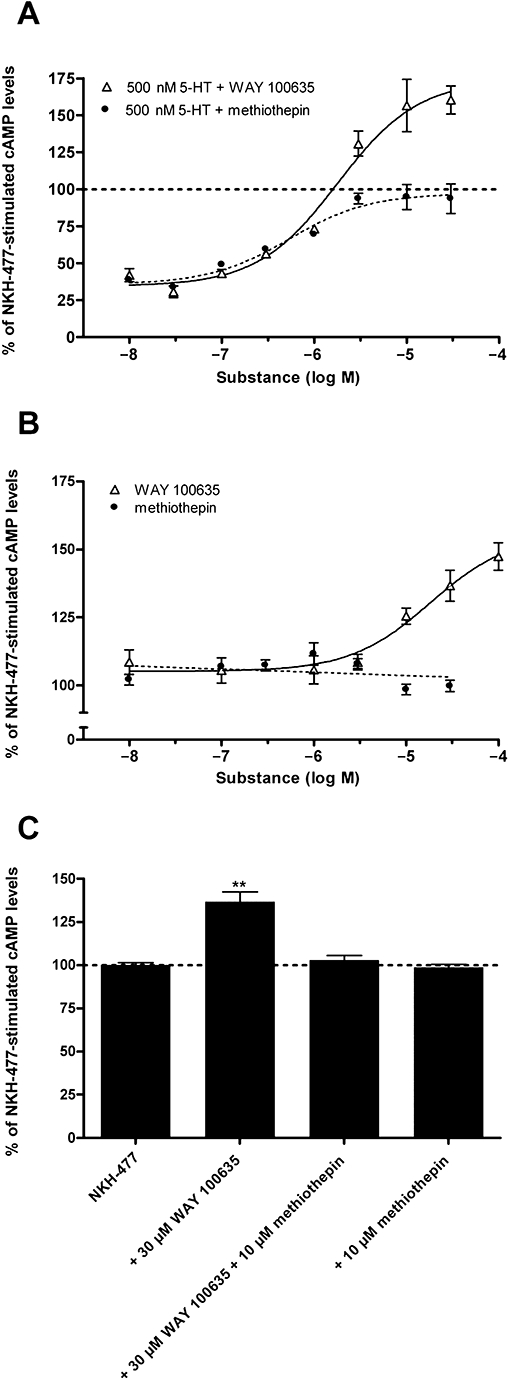
Modulation of intracellular cAMP levels in HEK 293 cells stably expressing the Pea5-HT1 receptor by WAY 100635 and methiothepin. (A) Dose-dependent effects of the neutral antagonist methiothepin and the inverse agonist WAY 100635 on NKH-477-stimulated cAMP production in the presence of 500 nM 5-HT. Data represent the mean ± SEM of eight replicates from two experiments representative of four similar experiments. (B) Dose-dependent effects of methiothepin and WAY 100635 on NKH-477-stimulated cAMP production in the absence of 5-HT. Data represent the mean ± SEM of eight replicates from two experiments representative of four similar experiments. (C) Inhibition of the effect of WAY 100635 (30 µM) on the NKH-477-stimulated cAMP production by methiothepin (10 µM). Data represent the mean ± SEM of eight replicates from two experiments representative of four similar experiments. Asterisks indicate statistically significant differences for drug versus NKH-477 (one-way anova followed by Dunnett's multiple comparison test; **P < 0.01).
HEK 293 cells expressing the Pea5-HT1 receptor did not show 5-HT-dependent Ca2+ signals (data not shown). Application of the Ca2+ ionophore, ionomycin (2 µM), served as a positive control to demonstrate that the Ca2+ detection system worked properly.
Discussion and conclusions
In the present study, we have characterized a 5-HT1 receptor of the American cockroach, P. americana. Orthologous receptors have been reported from several other invertebrate species, ranging from insects to molluscs (Saudou et al., 1992; Olde and McCombie, 1997; Barbas et al., 2002; Spitzer et al., 2008). These invertebrate receptors share pronounced sequence and functional similarity with mammalian 5-HT1 receptors. However, not all of these invertebrate receptors have been characterized in detail.
Structural characteristics of Pea5-HT1 receptors
The amino acid sequence of Pea5-HT1 receptors displays characteristic properties of amine-activated GPCRs in general (Strader et al., 1995) and 5-HT1 receptors in particular (Kroeze et al., 2002; Nichols and Nichols, 2008). The presence of a large third cytoplasmic loop and a short C-terminal region is typical for the 5-HT1 receptors and for other biogenic amine receptors that couple to Gi proteins (Kroeze et al., 2002; Nichols and Nichols, 2008). Furthermore, signature sequence motifs for co- and post-translational modifications, ligand binding and receptor activation are well conserved in Pea5-HT1 receptors
Functional and pharmacological properties of Pea5-HT1 receptors
We have established a HEK 293 cell line that stably expresses the cloned Pea5-HT1 receptor in order to examine the functional and pharmacological properties of the receptor. When compared with non-transfected cells, Pea5-HT1-expressing cells show an increased sensitivity to NKH-477, a direct activator of adenylyl cyclase. This supersensitization of adenylyl cyclase is a typical property of cells expressing constitutively active Gi/o-coupled receptors (Johnston and Watts, 2003; Beggs et al., 2005). Constitutive activity has been described for mammalian and crustacean 5-HT1 receptors (Newman-Tancredi et al., 1997; Spitzer et al., 2008) and for various other GPCRs (see Seifert and Wenzel-Seifert, 2002). Interestingly, mammalian 5-HT1 receptors display constitutive activity not only in heterologous expression systems, but also in vivo (Martel et al., 2007). High levels of GPCR constitutive activity are assumed to be associated with certain human pathological states, including metabolic diseases and some forms of cancer (Seifert and Wenzel-Seifert, 2002). In insects, constitutive activity has been reported only for a few amine receptors so far, namely, for a D1-like dopamine receptor, a D2-like dopamine receptor and a 5-HT7 receptor of the honeybee Apis mellifera (Mustard et al., 2003; Beggs et al., 2005; Schlenstedt et al., 2006).
Activation of 5-HT1 receptors, including Pea5-HT1, results in the inhibition of cAMP accumulation. Application of 5-HT to Pea5-HT1-expressing HEK 293 cells attenuated the NKH-477-induced cAMP formation by 67%. Two D. melanogaster 5-HT1 receptors (Dm5-HT1A and Dm5-HT1B) inhibit adenylyl cyclase in a comparable manner (Saudou et al., 1992). The EC50 of 5-HT for Pea5-HT1 receptors was 130 nM, thus demonstrating a relatively low potency compared with other arthropod orthologues, for example, the 5-HT1 receptors of D. melanogaster (30 nM for Dm5-HT1A, 18 nM for Dm5-HT1B; Saudou et al., 1992) and Boophilus microplus (83 nM; Chen et al., 2004).
Our attempts to identify full agonists that mimic the inhibitory effect of 5-HT have revealed that the receptor is resistant to most of the tested compounds. Only 5-MeOT, which is a non-selective agonist of various vertebrate and invertebrate 5-HT receptors, inhibited cAMP production and acted as a partial agonist. Our search for substances that could counteract the effect of 5-HT led to the discovery that both methiothepin and WAY 100635 display this property. Interestingly, the selective 5-HT1A receptor ligand WAY 100635 (Fletcher et al., 1996; Newman-Tancredi et al., 1997) blocked not only the 5-HT-induced inhibition of adenylyl cyclase activity via Pea5-HT1 receptors, but also agonist-independent activity of this receptor, resulting in cAMP levels above 100%. This result confirms our assumption that Pea5-HT1 receptors are constitutively active, and demonstrates that WAY 100635 acts as an inverse agonist on this insect 5-HT1 receptor. The latter is surprising, as WAY 100635 has been shown to act as a neutral antagonist on mammalian 5-HT1A receptors in most studies (Fletcher et al., 1996; Newman-Tancredi et al., 1997; Martel et al., 2007). However, under certain conditions, it can also display inverse agonist properties in mammals (Cosi and Koek, 2000). Methiothepin has been described as an inverse agonist at mammalian 5-HT1 receptors (McLoughlin and Strange, 2000; Martel et al., 2007). With respect to the Pea5-HT1 receptor, however, methiothepin is a neutral agonist that is able to compensate for the effects of the full agonist 5-HT and the inverse agonist WAY 100635.
Expression pattern of Pea5-HT1 receptors
Clues to the possible functions of a receptor might be obtained from its cellular localization. In D. melanogaster, the Dm5-HT1A receptor is predominantly expressed in the mushroom bodies (Yuan et al., 2006), whereas Dm5-HT1B is expressed not only in the mushroom bodies, but also strongly in pars intercerebralis neurons and certain clock neurons (Yuan et al., 2005). Nothing is known regarding the expression of 5-HT1 receptors in other insects. In P. americana, we have been able to show expression of Pea5-HT1 receptors in a subset of pars intercerebralis cells and a neural tract connecting these cells with the retro-cerebral complex and the stomatogastric nervous system. The labelling of only a few cells within the brain of P. americana by the Pea5-HT1-specific antibody might be considered surprising with respect to the widespread distribution of 5-HT. Two explanations for this apparent discrepancy come to mind. First, additional 5-HT receptors probably exist that might also be expressed in the brain in P. americana. Second, the anti-Pea5-HT1 receptor antibody might only label cells that express the receptor polypeptide at high density. Our inability to detect the receptor immunocytochemically in the salivary glands, where its expression has been established by RT-PCR and Western blotting, argues in favour of this option.
Possible physiological roles of Pea5-HT1 receptors
As Pea5-HT1 receptors are expressed in the salivary glands of P. americana, these receptors probably participate in the control of 5-HT-stimulated salivary secretion. In physiological experiments investigating the pharmacology of 5-HT-induced salivary secretion, we have established a pharmacological profile similar to that of the heterologously expressed Pea5-HT1 receptor: 5-MeOT acts as a partial agonist and provokes saliva secretion, whereas 5-CT and 8-OH-DPAT show only minor effects (Troppmann et al., 2007). Furthermore, the non-selective antagonist methiothepin blocks 5-HT-induced saliva secretion. However, we have also shown that mianserin inhibits salivary secretion, whereas it is not a potent antagonist at the Pea5-HT1 receptor expressed in HEK 293 cells. In the salivary gland, 5-HT induces the secretion of proteins from a specific cell type, viz., the central cells (Just and Walz, 1996; Walz et al., 2006). However, because this effect is mimicked by interventions that elevate intracellular cAMP levels and are modulated by increased intracellular Ca2+ (Rietdorf et al., 2005), the Pea5-HT1 receptor is unlikely to be the mediator of this effect. For these reasons, we expect that one or more additional 5-HT receptors (5-HT7 and/or 5-HT2) are expressed in P. americana salivary glands. We have meanwhile cloned a putative 5-HT7 receptor fragment of the cockroach, and have detected expression of the respective mRNA in salivary gland tissue by RT-PCR (Troppmann and Blenau, unpublished data). This receptor and 5-HT2 receptors of P. americana remain to be characterized in the future in order to complete our understanding of the complex effects of 5-HT in the control and modulation of salivary secretion in this insect. From the expression of Pea5-HT1 in pars intercerebralis neurons projecting to the retrocerebral complex and stomatogastric nervous system, we conclude that the Pea5-HT1 receptor is probably involved in the 5-HT-mediated control or modulation of neuroendocrine secretion processes and/or the motion of the gut and foregut. 5-HT stimulates both the fore- and hindgut of cockroaches (Brown, 1965, 1975; Cook et al., 1969). As the effect has been observed in denervated preparations, this action of 5-HT appears to be directly on the gut muscle (Cook et al., 1969; Brown, 1975). As the Pea5-HT1 receptor is also expressed in the gut, it is a likely candidate in mediating this effect of 5-HT on visceral muscle activity.
Considerably, more is known about the functions of the two 5-HT1 receptors in the genetic model organism D. melanogaster. Dm5-HT1A mRNA has been found to oscillate with a phase of ZT18 (Claridge-Chang et al., 2001), whereas there is no circadian variation in mRNA or protein levels of Dm5-HT1B (Yuan et al., 2005). Furthermore, the Dm5-HT1B receptor is expressed in the clock network, and affects circadian light sensitivity by decreasing the activity of the protein kinase SHAGGY, which, in turn, produces increased stability of the transcription factor TIMELESS (Yuan et al., 2005). In contrast, the Dm5-HT1A receptor seems to have a sleep-regulating role, because Dm5-HT1A mutant flies have short and fragmented sleep patterns (Yuan et al., 2006). Recently, Johnson et al. (2009) have postulated a role of 5-HT1 receptors in the modulation of aggressive behaviour in D. melanogaster. Interestingly, these authors postulate a ‘certain constitutive level of activation’ of 5-HT1-like receptors (Dm5-HT1A and Dm5-HT1B) such that increased activation of these circuits (by 8-OH-DPAT) increases certain forms of aggressive behaviour, whereas inactivation of these circuits (by WAY 100635) decreases this behaviour (Johnson et al., 2009).
Cockroaches are also established model organisms for studying both circadian rhythms (Helfrich-Förster et al., 1998) and aggressive behaviour (Bell and Sams, 1973). The pharmacological characterization and localization of the Pea5-HT1 receptor now provide the basis for new studies regarding the significance of this particular receptor for cockroach behaviour and physiology. For example, the consequences of interference with Pea5-HT1 expression (application of the RNAi technique) or receptor activation (application of identified Pea5-HT1 receptor ligands) for rhythmic and aggressive behavioural patterns can now be analysed.
Acknowledgments
This study was supported by a grant from the German Research Foundation (BL 469/4).
Glossary
Abbreviations:
- [cAMP]I
intracellular concentration of 3′-5′-cyclic adenosine monophosphate
- 5-CT
5-carboxamidotryptamine
- 5-MeOT
5-methoxytryptamine
- 8-OH-DPAT
(+/−)-8-hydroxy-2-(dipropylamino)tetralin
- AD
antibody diluent
- Dm5-HT1A/1B/2/7
Drosophila melanogaster 5-HT1A/1B/2/7 receptor
- GPCRs
G-protein-coupled receptors
- HA
hemagglutinin epitope
- IBMX
isobutylmethylxanthine
- NCC 1
corpora cardiaca nerve 1
- Pea5-ht1, gene
mRNA or cDNA of the Periplaneta americana 5-HT1 receptor
- Pea5-HT1
P. americana Pea5-HT1 receptor
- RACE
rapid amplification of cDNA ends
- TBS-T
Tris-buffered saline containing Tween 20
- TM
transmembrane domain
- ZT
zeitgeber time
Conflict of interest
The authors state no conflict of interest.
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 4th edition. Br J Pharmacol. 2009;158(Suppl. 1):S1–S254. doi: 10.1111/j.1476-5381.2009.00499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anstey ML, Rogers SM, Ott SR, Burrows M, Simpson SJ. 5-HT mediates behavioral gregarization underlying swarm formation in desert locusts. Science. 2009;323:627–630. doi: 10.1126/science.1165939. [DOI] [PubMed] [Google Scholar]
- Barbas D, Zappulla JP, Angers S, Bouvier M, Castellucci VF, DesGroseillers L. Functional characterization of a novel 5-HT receptor (5-HTap2) expressed in the CNS of Aplysia californica. J Neurochem. 2002;80:335–345. doi: 10.1046/j.0022-3042.2001.00703.x. [DOI] [PubMed] [Google Scholar]
- Beggs KT, Hamilton IS, Kurshan PT, Mustard JA, Mercer AR. Characterization of a D2-like dopamine receptor (AmDOP3) in honey bee, Apis mellifera. Insect Biochem Mol Biol. 2005;35:873–882. doi: 10.1016/j.ibmb.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Bell WJ, Sams GR. Aggressiveness in the cockroach Periplaneta americana (Orthoptera, Blattidae) Behav Biol. 1973;9:581–593. doi: 10.1016/s0091-6773(73)80052-5. [DOI] [PubMed] [Google Scholar]
- Bischof LJ, Enan EE. Cloning, expression and functional analysis of an octopamine receptor from Periplaneta americana. Insect Biochem Mol Biol. 2004;34:511–521. doi: 10.1016/j.ibmb.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Blenau W, Baumann A. Molecular and pharmacological properties of insect biogenic amine receptors: lessons from Drosophila melanogaster and Apis mellifera. Arch Insect Biochem Physiol. 2001;48:13–38. doi: 10.1002/arch.1055. [DOI] [PubMed] [Google Scholar]
- Blenau W, Baumann A. Molecular characterization of the ebony gene from the American cockroach, Periplaneta americana. Arch Insect Biochem Physiol. 2005;59:184–195. doi: 10.1002/arch.20064. [DOI] [PubMed] [Google Scholar]
- Brown BE. Pharmacologically active constituents of the cockroach corpus cardiacum: resolution and some characteristics. Gen Comp Endocrinol. 1965;5:387–401. doi: 10.1016/0016-6480(65)90099-7. [DOI] [PubMed] [Google Scholar]
- Brown BE. Proctolin: a peptide transmitter candidate in insects. Life Sci. 1975;17:1241–1252. doi: 10.1016/0024-3205(75)90133-2. [DOI] [PubMed] [Google Scholar]
- Chen C, Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987;7:2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen A, Holmes SP, Pietrantonio PV. Molecular cloning and functional expression of a serotonin receptor from the Southern cattle tick, Boophilus microplus (Acari: Ixodidae) Insect Mol Biol. 2004;13:45–54. doi: 10.1111/j.1365-2583.2004.00457.x. [DOI] [PubMed] [Google Scholar]
- Claridge-Chang A, Wijnen H, Naef F, Boothroyd C, Rajewsky N, Young MW. Circadian regulation of gene expression systems in the Drosophila head. Neuron. 2001;32:657–671. doi: 10.1016/s0896-6273(01)00515-3. [DOI] [PubMed] [Google Scholar]
- Colas JF, Launay JM, Kellermann O, Rosay P, Maroteaux L. Drosophila 5-HT2 5-HT receptor: coexpression with fushi-tarazu during segmentation. Proc Natl Acad Sci USA. 1995;92:5441–5445. doi: 10.1073/pnas.92.12.5441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colas JF, Launay JM, Vonesch JL, Hickel P, Maroteaux L. 5-HT synchronises convergent extension of ectoderm with morphogenetic gastrulation movements in Drosophila. Mech Dev. 1999;87:77–91. doi: 10.1016/s0925-4773(99)00141-0. [DOI] [PubMed] [Google Scholar]
- Cook BJ, Eraker J, Anderson GR. The effect of various biogenic amines on the activity of the foregut of the cockroach, Blaberus giganteus. J Insect Physiol. 1969;15:445–455. doi: 10.1016/0022-1910(69)90293-5. [DOI] [PubMed] [Google Scholar]
- Cosi C, Koek W. The putative ‘silent’ 5-HT1A receptor antagonist, WAY 100635, has inverse agonist properties at cloned human 5-HT1A receptors. Eur J Pharmacol. 2000;401:9–15. doi: 10.1016/s0014-2999(00)00410-6. [DOI] [PubMed] [Google Scholar]
- Dierick HA, Greenspan RJ. 5-HT and neuropeptide F have opposite modulatory effects on fly aggression. Nat Genet. 2007;39:678–682. doi: 10.1038/ng2029. [DOI] [PubMed] [Google Scholar]
- Downer RGH. Octopamine, dopamine, and 5-hydroxytryptamine in the cockroach nervous system. In: Huber I, Masler EP, Rao BR, editors. Cockroaches as Models for Neurobiology: Application in Biomedical Research. Boca Raton, FL: CRC Press; 1990. pp. 103–124. [Google Scholar]
- Fletcher A, Forster EA, Bill DJ, Brown G, Cliffe IA, Hartle JE, et al. Electrophysiological, biochemical, neurohormonal and behavioural studies with WAY-100635, a potent, selective and silent 5-HT1A receptor antagonist. Behav Brain Res. 1996;73:337–353. doi: 10.1016/0166-4328(96)00118-0. [DOI] [PubMed] [Google Scholar]
- Gerhardt CC, van Heerikhuizen H. Functional characteristics of heterologously expressed 5-HT receptors. Eur J Pharmacol. 1997;334:1–23. doi: 10.1016/s0014-2999(97)01115-1. [DOI] [PubMed] [Google Scholar]
- Grohmann L, Blenau W, Erber J, Ebert PR, Strünker T, Baumann A. Molecular and functional characterization of an octopamine receptor from honeybee (Apis mellifera) brain. J Neurochem. 2003;86:725–735. doi: 10.1046/j.1471-4159.2003.01876.x. [DOI] [PubMed] [Google Scholar]
- Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser. 1999;41:95–98. [Google Scholar]
- Hauser F, Cazzamali G, Williamson M, Blenau W, Grimmelikhuijzen CJP. A review of neurohormone GPCRs present in the fruitfly Drosophila melanogaster and the honey bee Apis mellifera. Prog Neurobiol. 2006;80:1–19. doi: 10.1016/j.pneurobio.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Helfrich-Förster C, Stengl M, Homberg U. Organization of the circadian system in insects. Chronobiol Int. 1998;15:567–594. doi: 10.3109/07420529808993195. [DOI] [PubMed] [Google Scholar]
- Hen R. Of mice and flies: commonalities among 5-HT receptors. Trends Pharmacol Sci. 1992;13:160–165. doi: 10.1016/0165-6147(92)90054-a. [DOI] [PubMed] [Google Scholar]
- House CR, Ginsborg BL. Salivary gland. In: Kerkut GA, Gilbert LI, editors. Comprehensive Insect Physiology, Biochemistry and Pharmacology. Oxford: Pergamon Press; 1985. pp. 195–224. Vol. 11. [Google Scholar]
- Hoyer D, Clarke DE, Fozard JR, Hartig PR, Martin GR, Mylecharane EJ, et al. International union of pharmacology classification of receptors for 5-hydroxytryptamine (serotonin) Pharmacol Rev. 1994;46:157–203. [PubMed] [Google Scholar]
- Hoyer D, Hannon JP, Martin GR. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol Biochem Behav. 2002;71:533–554. doi: 10.1016/s0091-3057(01)00746-8. [DOI] [PubMed] [Google Scholar]
- Johnson O, Becnel J, Nichols CD. 5-HT 5-HT2 and 5-HT1A-like receptors differentially modulate aggressive behaviors in Drosophila melanogaster. Neuroscience. 2009;158:1292–1300. doi: 10.1016/j.neuroscience.2008.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston CA, Watts VJ. Sensitization of adenylate cyclase: a general mechanism of neuroadaptation to persistent activation of Gai/o-coupled receptors? Life Sci. 2003;73:2913–2925. doi: 10.1016/s0024-3205(03)00703-3. [DOI] [PubMed] [Google Scholar]
- Jones BJ, Blackburn TP. The medical benefit of 5-HT research. Pharmacol Biochem Behav. 2002;71:555–568. doi: 10.1016/s0091-3057(01)00745-6. [DOI] [PubMed] [Google Scholar]
- Just F, Walz B. The effects of 5-HT and dopamine on salivary secretion by isolated cockroach salivary glands. J Exp Biol. 1996;199:407–413. doi: 10.1242/jeb.199.2.407. [DOI] [PubMed] [Google Scholar]
- Käll L, Krogh A, Sonnhammer EL. A combined transmembrane topology and signal peptide prediction method. J Mol Biol. 2004;338:1027–1036. doi: 10.1016/j.jmb.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984;12:857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroeze WK, Kristiansen K, Roth BL. Molecular biology of 5-HT receptors – structure and function at the molecular level. Curr Top Med Chem. 2002;2:507–528. doi: 10.2174/1568026023393796. [DOI] [PubMed] [Google Scholar]
- Martel JC, Ormière AM, Leduc N, Assié MB, Cussac D, Newman-Tancredi A. Native rat hippocampal 5-HT1A receptors show constitutive activity. Mol Pharmacol. 2007;71:638–643. doi: 10.1124/mol.106.029769. [DOI] [PubMed] [Google Scholar]
- McLoughlin DJ, Strange PG. Mechanisms of agonism and inverse agonism at 5-HT 5-HT1A receptors. J Neurochem. 2000;74:347–357. doi: 10.1046/j.1471-4159.2000.0740347.x. [DOI] [PubMed] [Google Scholar]
- Mustard JA, Blenau W, Hamilton IS, Ward VK, Ebert PR, Mercer AR. Analysis of two D1-like dopamine receptors from the honey bee Apis mellifera reveals agonist-independent activity. Brain Res Mol Brain Res. 2003;113:67–77. doi: 10.1016/s0169-328x(03)00091-3. [DOI] [PubMed] [Google Scholar]
- Newman-Tancredi A, Conte C, Chaput C, Spedding M, Millan MJ. Inhibition of the constitutive activity of human 5-HT1A receptors by the inverse agonist, spiperone but not the neutral antagonist, WAY 100,635. Br J Pharmacol. 1997;120:737–739. doi: 10.1038/sj.bjp.0701025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols CD. Drosophila melanogaster neurobiology, neuropharmacology, and how the fly can inform central nervous system drug discovery. Pharmacol Ther. 2006;112:677–700. doi: 10.1016/j.pharmthera.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Nichols DE, Nichols CD. 5-HT receptors. Chem Rev. 2008;108:1614–1641. doi: 10.1021/cr078224o. [DOI] [PubMed] [Google Scholar]
- Olde B, McCombie WR. Molecular cloning and functional expression of a 5-HT receptor from Caenorhabditis elegans. J Mol Neurosci. 1997;8:53–62. doi: 10.1007/BF02736863. [DOI] [PubMed] [Google Scholar]
- Page TL. 5-HT phase-shifts the circadian rhythm of locomotor activity in the cockroach. J Biol Rhythms. 1987;2:23–34. doi: 10.1177/074873048700200103. [DOI] [PubMed] [Google Scholar]
- Papoucheva E, Dumuis A, Sebben M, Richter DW, Ponimaskin EG. The 5-hydroxytryptamine(1A) receptor is stably palmitoylated, and acylation is critical for communication of receptor with Gi protein. J Biol Chem. 2004;279:3280–3291. doi: 10.1074/jbc.M308177200. [DOI] [PubMed] [Google Scholar]
- Rietdorf K, Blenau W, Walz B. Protein secretion in cockroach salivary glands requires an increase in intracellular cAMP and Ca2+ concentrations. J Insect Physiol. 2005;51:1083–1091. doi: 10.1016/j.jinsphys.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Rotte C, Krach C, Balfanz S, Baumann A, Walz B, Blenau W. Molecular characterization and localization of the first tyramine receptor of the American cockroach (Periplaneta americana) Neuroscience. 2009;162:1120–1133. doi: 10.1016/j.neuroscience.2009.05.066. [DOI] [PubMed] [Google Scholar]
- Saudou F, Boschert U, Amlaiky N, Plassat JL, Hen R. A family of Drosophila 5-HT receptors with distinct intracellular signalling properties and expression patterns. EMBO J. 1992;11:7–17. doi: 10.1002/j.1460-2075.1992.tb05021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlenstedt J, Balfanz S, Baumann A, Blenau W. Am5-HT7: molecular and pharmacological characterization of the first 5-HT receptor of the honeybee (Apis mellifera) J Neurochem. 2006;98:1985–1998. doi: 10.1111/j.1471-4159.2006.04012.x. [DOI] [PubMed] [Google Scholar]
- Seifert R, Wenzel-Seifert K. Constitutive activity of G-protein-coupled receptors: cause of disease and common property of wild-type receptors. Naunyn Schmiedebergs Arch Pharmacol. 2002;366:381–416. doi: 10.1007/s00210-002-0588-0. [DOI] [PubMed] [Google Scholar]
- Sitaraman D, Zars M, Laferriere H, Chen YC, Sable-Smith A, Kitamoto T, et al. 5-HT is necessary for place memory in Drosophila. Proc Natl Acad Sci USA. 2008;105:5579–5584. doi: 10.1073/pnas.0710168105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer N, Edwards DH, Baro DJ. Conservation of structure, signaling and pharmacology between two 5-HT receptor subtypes from decapod crustaceans, Panulirus interruptus and Procambarus clarkii. J Exp Biol. 2008;211:92–105. doi: 10.1242/jeb.012450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strader CD, Fong TM, Graziano MP, Tota MR. The family of G-protein-coupled receptors. FASEB J. 1995;9:745–754. [PubMed] [Google Scholar]
- Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- Tierney AJ. Structure and function of invertebrate 5-HT receptors: a review. Comp Biochem Physiol A Mol Integr Physiol. 2001;128:791–804. doi: 10.1016/s1095-6433(00)00320-2. [DOI] [PubMed] [Google Scholar]
- Troppmann B, Walz B, Blenau W. Pharmacology of serotonin-induced salivary secretion in Periplaneta americana. J Insect Physiol. 2007;53:774–781. doi: 10.1016/j.jinsphys.2007.02.020. [DOI] [PubMed] [Google Scholar]
- Wachten S, Schlenstedt J, Gauss R, Baumann A. Molecular identification and functional characterization of an adenylyl cyclase from the honeybee. J Neurochem. 2006;96:1580–1590. doi: 10.1111/j.1471-4159.2006.03666.x. [DOI] [PubMed] [Google Scholar]
- Walz B, Baumann O, Krach C, Baumann A, Blenau W. The aminergic control of cockroach salivary glands. Arch Insect Biochem Physiol. 2006;62:141–152. doi: 10.1002/arch.20128. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Mizunami M. Pavlov's cockroach: classical conditioning of salivation in an insect. PLoS ONE. 2007;2:e529. doi: 10.1371/journal.pone.0000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiger WA. Serotonergic modulation of behaviour: a phylogenetic overview. Biol Rev Camb Philos Soc. 1997;72:61–95. doi: 10.1017/s0006323196004975. [DOI] [PubMed] [Google Scholar]
- Witz P, Amlaiky N, Plassat JL, Maroteaux L, Borrelli E, Hen R. Cloning and characterization of a Drosophila 5-HT receptor that activates adenylate cyclase. Proc Natl Acad Sci USA. 1990;87:8940–8944. doi: 10.1073/pnas.87.22.8940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Q, Joiner WJ, Sehgal A. A sleep-promoting role for the Drosophila 5-HT receptor 1A. Curr Biol. 2006;16:1051–1062. doi: 10.1016/j.cub.2006.04.032. [DOI] [PubMed] [Google Scholar]
- Yuan Q, Lin F, Zheng X, Sehgal A. 5-HT modulates circadian entrainment in Drosophila. Neuron. 2005;47:115–127. doi: 10.1016/j.neuron.2005.05.027. [DOI] [PubMed] [Google Scholar]
- Zornik E, Paisley K, Nichols R. Neural transmitters and a peptide modulate Drosophila heart rate. Peptides. 1999;20:45–51. doi: 10.1016/s0196-9781(98)00151-x. [DOI] [PubMed] [Google Scholar]


